Abstract
In recent years, the research community is tremendously investigating unexplored plants and herbals as they represent a potential source of various biomolecules, which not only contribute to nutrition but also to human health. In fact, Muicle (Justicia spicigera) has attracted the attention of scientists thanks to its multiple biological activities associated with the phytochemicals and specific biomolecules present in this plant. In this review, an evidence on current development works assaying the potential biological properties of Muicle is given. Here, we introduce the key biologically active molecules ascribed to such properties, along with the mechanism of action and interaction. Although the utilization of this plant has been majorly focused on traditional medicine, specific applications in terms of production of new feedstocks and nanomaterials, and developments of functional foods and formulations, are also a current direction towards the exploitation of this natural source. Therefore, this review reports the main outcomes of current research towards the utilization of biomolecules and other elements of the plant in new fields of research such as materials chemistry.
1. Introduction
It is reported that around 85% of the worldwide population uses various plants and herbals to treat and face several diseases, known as “traditional medicine” [1]. Traditional medicine, particularly herbal medicine, plays a fundamental role in providing culturally pertinent and accessible health coverage. Medicinal plants are indeed a relevant source of biologically active compounds to design and fabricate new drugs, around 25% of the commercially available drugs and medicines come from natural plants [2]. In addition to this, according to one study by the Plant Based Foods Association, economic activity in the US’s plant-based foods industry alone amounts to sales of approximately USD13.7 billion a year. At current growth rates, the plant-based food industry is predicted to generate USD13.3 billion in tax revenues over the next 10 years. Sales of plant-based foods in the United States is increasing by an average of 8% per year.
Thanks to the advances in analytical chemistry, quantitative and identification tools have been conducted to identify a large number of plants based on their content of biomolecules. This has driven the right selection of the most relevant plants to initiate not only pharmacological research but also to extend the application of these natural sources. One of these selected plants with great potential as a source of phytochemicals is Justicia spicigera Schltdl, known as “muicle”, “hierba delimalin”, “muhuite”, “expaxihuitl”, “moyotle”, “moyotli”, “trompetilla”, “huitzixochitl”, among others, which belongs to the Acanthaceae family. It is believed that J. spicigera is native to Latin America countries including Belize, Costa Rica, Mexico, Honduras, among others. This plant can be grown under full sun or partial shade. Preferentially, it grows in warm weather but also can stand cold temperatures (−3 °C). J. spicigera commonly reaches 2–5 feet high and its leaves can reach about 0.02 m long. This plant presents a color varying from red to dark blue, and it has been extensively used in traditional medicine against infectious diseases and several other homemade treatments (e.g., shadow lifting) [3]. Various reports have indeed performed pharmacological studies with promising preliminary outcomes without knowing precisely the phytochemical composition of the plant. Nowadays, it is known that several types of compounds, including simple carbohydrates; pectins; glycoproteins; phenolic compounds (such as glycosides of kaempferol) and anthocyanins (such as cyanidin 3-O-glucoside and cyanidin 3,5-di glucoside); resin; and essential oils and minerals (e.g., potassium, calcium acetate and oxalate, sulfate and sodium chloride), are present in the plant. Interestingly, specific phenolic compounds of the plant have been determined, such as kaempferitrin and kaempferol trirhamnoside [4]. The latter molecules have been isolated from leaves, while tannins have been extracted from flowers [5]. In current studies, many types of phytochemicals have been identified in J. spicigera, such as 4-methyl-3-pentanal, methyl 2-hydroxy-2-methyl-butanoate, 3,4-epoxy-2-hexanone, 2-hydroxy-2-methylbutyric acid, ethyl 2-hexenoate, 3-thiophene-acetic acid, phthalic acid and butyl 2-ethylbutyl ester, to mention just a few [6]. Such compounds, which were also extracted mainly from the leaves, have been ascribed to specific biological properties, such as antibacterial, antifungal [6] and antidiabetic activities [7], along with other pharmacological properties against gonorrhea, oral diseases, dysentery, parasites, anticancer, antileukemia and antianemia [2,5]. Due to these attributes, the research behind the compounds contained in J. spicigera has been intensified. In this review, we elucidate the current research dealing with the key biomolecules responsible for such properties, as well as the main mechanisms of action and interaction. However, the exploration of this plant has been extended to the production of new feedstocks and nanomaterials, and developments of functional foods and formulations. Here, the main outcomes of current research towards the utilization of biomolecules and other elements of the plant in new fields of research are also provided.
2. Towards the Identification and Related Biological Activities of Biomolecules Contained in Muicle (Justicia spicigera)
2.1. Anti-Diabetic Properties
Two decades ago, pioneering investigations were inspired keeping in mind two crucial aims, (i) to establish the complete chemical composition of the plant, identifying its phytochemicals, and (ii) to find the related biological properties to specific biomolecules. As mentioned earlier, Vega-Avila et al. [6] identified several compounds in the hexane fraction obtained from ethanolic extract from J. spicigera, which later exhibited antibacterial and antifungal properties against Candida albicans, Staphylococcus aureus, Shigella flexneri, Salmonella typhi and Escherichia coli. Based on this study, such biological properties and the related molecules helped to understand the role of the plant in traditional medicine towards infectious diseases. Concurrently, hydroalcoholic extracts of J. spicigera were evaluated for their potential antidiabetic activity in vitro assays [7]. In that research, glucosidase and lipase inhibitory activities were apparently attributed to the plant-based extract, documenting 0.4 and 12.7% inhibition in glucosidase and lipase activity, respectively. Thanks to the stimulation of glucose uptake in both insulin-sensitive and insulin-resistant murine and human adipocytes, Ortiz-Andrade et al. [8] associated the possible anti-diabetic effect of J. spicigera ethanolic extracts. Moreover, the extracts displayed glucose-lowering effect in normoglycemic and STZ-induced diabetic rats.
2.2. Anti-Hypertensive Properties
Euler and Alam [9] have previously analyzed J. spicigera extracts by means of nuclear magnetic resonance spectroscopy (1H-NMR) and chromatographic techniques, identifying trirhamnosides of kaempferol and kaempferitrin, which can be utilized to control hypertension [10]. In the light of this latter disease, various extracts, based on hexane, methanol, chloroform and water, demonstrated antihypertensive effect in L-NAME-induced hypertensive rats [11]. The outcomes revealed that hexane and methanol extracts did not have any effect in lowering blood pressure, while the aqueous extract only decreased the diastolic blood pressure (from 163/148 ± 6.65/6.64 mmHg to 156/128 ± 11.8/4.51 mmHg) in the hypertensive rats. Surprisingly, chloroform-based extract also reduced the arterial blood pressure (approximately 180/164 ± 1.7/3.2 mmHg) down to values similar to those of the normotensive rats (ca. 149/133 mmHg). By analyzing chloroform-based extract via liquid chromatography, researchers noticed chloroform yielded in major quantity flavonoids, such as hesperidin, naringenin and kaempferol [11]. Importantly, the authors also concluded that these results open a new window of application of J. spicigera extracts; however, these results cannot be extrapolated to humans, pointing out further research in the field. Apart from kaempferitrin, Dominguez et al. [12], for instance, produced hexane-isopropyl ether-methanol extracts of J. spicigera, determining the presence of β-sitosterol, 3-O-glucoside of β-sitosterol and cryptoxanthin I, this latter compound is recognized as a very polar anthocyanin that conferred fluorescence to the extracts.
J. spicigera has been part of various Mexican medicinal plants, such as Papaveraceae Argemone mexicana L., Burseraceae Bursera simaruba (L.) Sarg. and Selaginellaceae Selaginella lepidophylla, among others, towards the treatment of cardiovascular issues. As assayed by Esquivel-Gutiérrez et al. [11], the cardiovascular effect of J. spicigera in male Wistar rats was studied by Magos-Guerrero et al. [13], who preliminary prepared a methanolic extract and later administrated via intravenous injection to the rats with and without glucose-induced hypertension. In this study, the blood pressure and heart rate were monitored revealing that such methanolic extracts decreased the mean arterial pressure, in which the effect displayed a dose-dependent behavior. Moreover, J. spicigera extracts decreased the heart rate in rats without sugar-induced hypertension while the heart rate in rats with hypertension or blood pressure was not affected.
2.3. Anti-Depressant and Antianxiety Properties
A few years later, Cassani et al. [14] demonstrated that kaempferitrin (see Figure 1), which is identified as a metabolite of kaempferol, displayed an antidepressant-like effect in mice. Here, the researchers associated the mechanism of action due to the interaction of the flavonoid with the 5-HT1A receptor as a mediator of its effect. In another investigation, López-Rubalcava and Estrada-Camarena [3] also claimed possible mitigation of the anxiety and depressive behavior in a typical rodent model of menopause. Supporting previous studies, García-Ríos et al. [15] analyzed the anxiolytic-like effect of J. spicigera aqueous extracts presenting flavonoids, sterols and terpenes. Experimentally, it was just needed to administrate 12 mg/kg in female Wistar rats to reduce the anxiety index, which was comparable with the effect released by a commercial anxiolytic (diazepam).
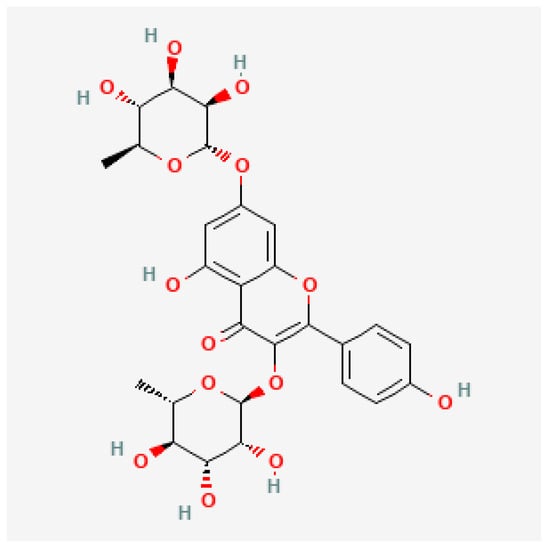
Figure 1.
Chemical structure of kaempferitrin.
2.4. Anti-Cancer Properties
In a series of studies, Alonso-Castro et al. [16,17] have documented the promising activity of kaempferitrin towards chronic diseases. As for extract preparation, powdered dried leaves of Justicia spicigera were extracted with ethanol using a Soxtherm apparatus (Soxtherm automatic, Gerhardt, Königswinter, Germany) for 2 h. Initially, researchers have reported the apoptotic effect of kaempferitrin on human cancer cells and human noncancerous cells, where the administration of 10, 50 and 100 mg/kg by intraperitoneal route inhibited the tumor growth by 28, 41 and 53%, respectively, in mice bearing HeLa tumor, while a lack of effect for normal cells was observed [16]. In general, the study demonstrated that J. spicigera extracts fostered the proliferation of murine splenocytes and induced the activity of natural killer cells.
In subsequent research, it was also pointed out that kaempferitrin induced a high cytotoxicity effect in vitro and in vivo against HeLa cells [17]. Such an effect was ascribed to the cell cycle arrest in the G1 phase and apoptosis via an intrinsic pathway in a caspase-dependent pathway. Towards new relevant biological properties of J. spicigera extracts, Juárez-Vázquez et al. [18] assayed the extracts, containing mainly kaempferitrin, for immunostimulatory activity in terms of proliferation of murine splenocytes and macrophages, and human peripheral blood mononuclear cells (PBMC). In fact, kaempferitrin was credited to promote the proliferation of macrophages (ca. 23%), splenocytes (ca. 17%) and PBMC (ca. 24%). Additionally, an enhanced in pinocytosis (ca. 25%) and lysosomal activity (ca. 57%) in macrophages was observed. Fernández-Pomares et al. [19] also found out that a hydroalcoholic extract (ethanol:water 50:50 v/v) of J. spicigera displayed an effect on LNCaP prostate cancer cells. The study was inspired by the evidence that androgen-independent DU-145 prostate cancer cell line may resist the cytotoxic mechanism of J. spicigera [20]. Thanks to the deep research and analysis, Fernández-Pomares et al. [19] confirmed that hydroalcoholic extract influences the proliferation of the cells via cytostatic effect, which prevents and delays the progression of mitosis in the cell cycle.
2.5. Anti-Oxidant Properties
To date, solvent extraction has been the main pathway of separating the compounds from J. spicigera; however, a particular emphasis should be paid to the type of solvent used, together with the extraction conditions. For instance, García-Márquez et al. [21] investigated the influence of solvent (such as water, ethanol, glycerol and propylene glycol) and temperature (in the range of 25–60 °C) on the phenolic content and antioxidant activity of extracts obtained from leaves of J. spicigera. The antioxidant activity was determined via DPPH and ABTS radical scavenging activity. After experimentation, it was found out that the extracts based on solvents with higher polarity offered more effective radical-scavenging activity compared with less polar solvents. Additionally, the authors demonstrated that the pH impacted significantly on the total phenolic contents and, thus, the antioxidant activity of the extracts. By using ultrasound-assisted extraction, Anaya-Esparza et al. [22] also corroborated that the extraction conditions dictate to some extent the recovery yields in extracts from leaves of J. spicigera. This was demonstrated when recovering phenolic compounds from the plant at different conditions (such as extraction time and sonication amplitude). Herein, three methods for antioxidant activity were employed such as DPPH and ABTS radical scavenging activity and Ferric-ion reducing antioxidant power (FRAP). The obtained extracts, containing up to 54 mg/g, also showed significant antioxidant activity, i.e., 294 Trolox mmol/g extract dry basis, determined via ABTS technique. Importantly, long-time extraction can lower the antioxidant activity of Muicle extracts, as recently reported by Martinez-Morales et al. [23].
2.6. Anti-Inflammatory, Anti-Obesity Properties and Other Health Benefits
Using ethyl acetate as a solvent, Awad et al. [24] extracted up to 42.94 mg/g of phenolic compounds in which 12 anthocyanins, including peonidin 3,5-diglucoside (ca. 64%), malvidin 3,5-diglucoside (ca. 10%) and petunidin 3,5-diglucoside (4%), were identified. Such extract apparently exhibited improvements in liver functions and oxidative stress markers in induced hepatic fibrosis rats. The same authors also observed an improvement in several liver function enzymes, such as aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase and gamma-glutamyl trans-peptidase, using an ethanolic extract of J. spicigera [25]. Real-Sandoval et al. [26] referred that kaempferitrin apparently decreased the weight gain as a result of decreasing fat accumulation. Furthermore, when assaying in obesity-induced male Wistar rats, a decrease in serum triglyceride concentration was observed. In the same work, kaempferitrin also demonstrated to negatively modulate the expression of pro-anti-inflammatory genes (e.g., Tlr4, Tnf-α, Nlrp3, Caspase-1, Il-18, Il-1β, Srebp-1c, Ppar-γ and Ucp2) while overexpressing Ppar-α. It is known that the expression of such transcription factors regulates the lipidic metabolism in a state of obesity. On the other hand, in an inflammatory stage, Ppar-α fosters an anti-inflammatory mechanism inactivating NF-κB and its related target genes.
In more recent studies, kaempferitrin was used to cope with other ailments, Zapata-Morales et al. [27], for instance, utilized an ethanolic extract to assay its antinociceptive and sedative effect. Particularly, an extract containing 200 mg/kg by mouth displayed an analgesic effect in mice. Alternatively, the extract was inactive in the hot plate in a ketamine-induced sleeping time test, exhibiting no sedative effects. Even in this report, it was recommended that further research is needed to better understand such mechanisms of action. Ángeles-López et al. [28], for instance, attributed the antinociceptive and spasmolytic-like effects of the J. spicigera ethanol extract in mice to the presence of 5-HT1A receptor antagonist (WAY100635, 0.1mg/kg, subcutaneous) and partially by blocking opioid receptors (NX, 1mg/kg, intraperitoneal). Furthermore, the inhibition of nitric oxide production conducted antinociception of the extract.
Based on empirical knowledge that J. spicigera is used to mediate epilepsy, González-Trujano et al. [29] investigated the anticonvulsant-like activity of J. spicigera aqueous extract containing mainly kaempferitrin. The extract and a reference drug (ethosuximide) were separately administrated in rats via intracerebroventricular, delaying the onset of seizures and reduced the frequency of tonic convulsion and mortality in mice. On the other hand, kaempferitrin was also able to decrease the frequency and amplitude of the electrographic spike in a similar way to ethosuximide in rats. In theory, it is believed that the mechanism of action could be related to the antidepressant activity, in which kaempferitrin acts in the serotoninergic neurotransmission similar to agonistic 5-HT1A activity [14].
Until now, kaempferitrin has been the most studied molecule and responsible for most of the biological properties; however, procumbenoside B (i.e., 4-O-beta-D-glucopyranosyl-(1‴->2″)-beta-D-apiofuranosyldiphyllin, see Figure 2), which is identified as an arylnaphthalide lignan [30], has been very recently associated with a potential anti-inflammatory effect on lipopolysaccharide-stimulated RAW 264.7 macrophages and Zebrafish models [31]. To purify such a bioactive compound, a methanolic extract was made and subsequently concentrated under reduced pressure. Afterward, the final concentration was subjected to chromatography using a silica gel column assisted by n-hexane/ethyl acetate (4:2). Finally, the active fraction was processed by Sephadex LH-20. In terms of bioactivity, procumbenoside B significantly inhibited the secretion of pro-anti-inflammatory mediators test while increasing the production of interleukin-10 and blocking the nuclear factor-kappa B. This latter finding is recognized as the main mechanism of action. According to the authors, the biomolecule also suppressed the production of lipopolysaccharide-stimulated nitric oxide and reactive oxygen species in the zebrafish model.
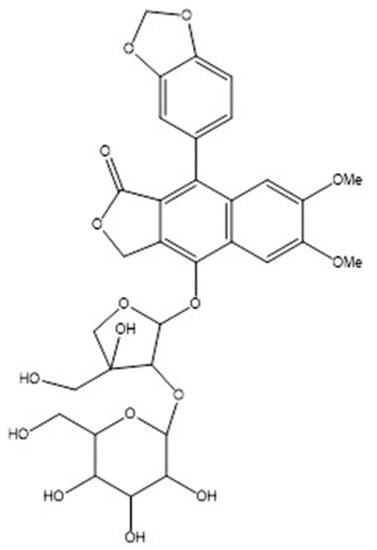
Figure 2.
Chemical structure of procumbenoside B.
3. Application of Muicle (J. spicigera) Biomolecules in Food and Bioproducts
Belonging to the class of flavonoids, J. spicigera also presents significant content of anthocyanins, which are considered natural colorants with antioxidant activity as well [32]. Anthocyanins from the plant have even been used in prehispanic manuscript fabrication, such as Codex Borbonicus, in which hybrid dyes were identified [33]. In addition, some other high-added-value molecules, such as eucalyptol, phytol and azulene, were also identified in the plant exhibiting different color and tonalities including green-iridescent, green-yellow or pink [34]. Baqueiro and co-workers confirmed the antioxidant properties of different water-ethanol extracts produced via ultrasound extraction technique. When dealing with anthocyanins, Pavón-García et al. [35] evaluated their storage stability when encapsulated by aqueous extract via spray drying. For this, two different wall materials, such as gum Arabic-mesquite gum and mesquite gum-maltodextrin DE10 mixtures, were applied. In general, the microcapsules had a water activity lower than 0.7, representing an optimal value for meeting maximum storage stability. After storage stability evaluation, mesquite gum-maltodextrin DE10 blend mixture protected and extended the stability of the anthocyanins for a longer time. In a similar manner, Castro-Alatorre et al. [36] recently used DE10 maltodextrin and soy protein isolate to microencapsulate phenolic compounds (e.g., tannins and monomeric anthocyanins) from the plant. The compounds were effectively extracted from the plant using simple water:ethanol mixtures, followed by concentration using vacuum evaporation. After microencapsulation via spray drying, the microcapsules were employed to enrich functional foods such as yogurt and jelly. Particularly, the microcapsules based on soy protein isolate demonstrated better retention of bioactive compounds and antioxidant properties than maltodextrin when embedded in yogurt, but maltodextrin had better acceptance through the sensory evaluation.
Phenolic compounds extracted via water solutions (at 50 °C) from Muicle were used to enrich maize tortillas by Alvarez-Poblano et al. [37]. Once incorporated into nixtamalized maize flour to elaborate tortillas, the J. spicigera extract reflected a decrease in viscoelastic properties which was dependent on the concentration of extract in the range of 0.7 and 2.7 g/100 g; also, it improved the hydration of tortillas (starch structures). Of course, the phenolic content, together with antioxidant properties, was improved in the resulting tortillas while lowering the enzymatic hydrolysis rate. Concerning the physical aspect, color properties were very comparable with the typical blue maize tortillas, as shown in Figure 3. This study also demonstrated the improvement of starch digestibility without changing the hardness of the tortilla.
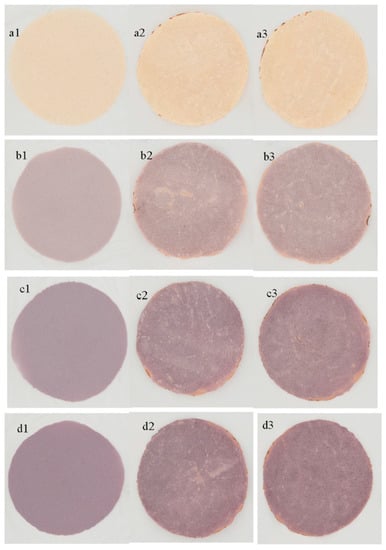
Figure 3.
Real images of masa and tortillas containing different concentrations of J. spicigera aqueous extracts. Masa containing 0 (a1), 0.7 (b1), 1.7 (c1) and 2.7 (d1) g/100 g. The subsequent images from left to right correspond to their tortillas of both sides. Reprinted from Ref. [37].
4. Application of Muicle (J. spicigera) Biomolecules as Colorants and Synthesis of Materials
Due to the multiple phytochemicals contained in J. spicigera, a new scope of research relies on the extraction of the compounds and their application as natural colorants. Regarding the separation, Chan-Bacab et al. [38] extracted the flavonoids of the plant through several methods, e.g., decoction using distilled water and 10% ethanol, and microwave-assisted extraction (with distilled water and 10% ethanol). Using all parts of the plant (seeds, bracts and leaves), the extraction yields were in the order of 31, 34, 32 and 32% per 20 g of plant, respectively. Such extracts were later applied to ordinary cotton cloth (i.e., manta), which demonstrated to be adjectives with no need for mordant (i.e., adjunct agents). Therefore, the pigments of the plant represent an eco-friendly alternative against chemically synthesized dyes, with a great potential of application in the textile industry. It is worth mentioning that J. spicigera extracts potentially exhibited toxicity towards Artemia salina and Lactuca sativa.
J. spicigera aqueous extracts (water at 60 °C) from leaves have been utilized towards the synthesis of silver (Ag) nanoparticles [39]. The extract was basically mixed with silver nitrate and then heated to 60 °C for 15 min. To some extent, such generated nanoparticles, having a spherical morphology with particle size between 86 and 100 nm, were evaluated in terms of antimicrobial properties. In fact, they were effective against Alternaria alternata (a), Colletotrichum sp. (b), Macrophomina phaseolina (c) and Fusarium solani (d), as illustrated in Figure 4. The radial growth inhibition was of about 60, 40, 70 and 35%, respectively, during nine days of incubation. When dealing with the mechanism of action, it has been widely documented that Ag nanoparticles can display an oxidative and non-oxidative stress induction and metal ion release [40]. Ultimately, there is evidence that Ag nanoparticles can stick onto the bacterial cell, negatively affecting the permeability and respiration of the bacteria. The nanostructured particles can also affect the cell membrane provoking cell lysis and they can go further, damaging at the DNA level [41,42]. In further investigations, the research team also reported a significant reduction in chlorophyll, epidermal polyphenol content and photochemical efficiency of Prosopis glandulosa after Ag nanoparticles exposure (over 72 h) [43]. In general, metal ions are able to form intermediate complexes with different biomolecules, especially containing functional groups such as polyphenols. Such complexes, thus, reduce the metal ions acting as capping agents. On the contrary, bimetallic nanoparticles (Cu/Ag) exhibited a positive effect on such physiological parameters.
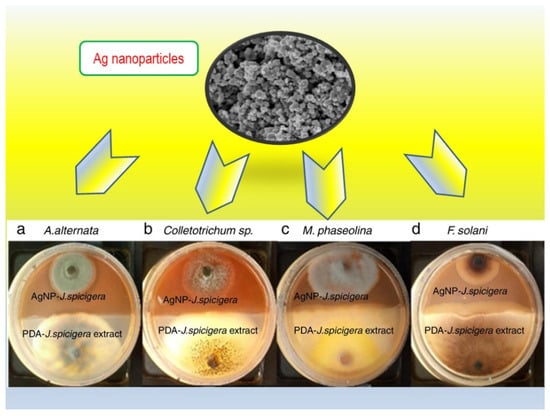
Figure 4.
Ag nanoparticles based on J. spicigera aqueous extracts and their antifungal effect.
A current breakthrough relies on the green synthesis of Fe3O4-Ag nanoalloys using J. spicigera. This research was proposed by Ruíz-Baltazar [44], who utilized the aqueous extracts as a reducing agent of metallic salts of Fe and Ag. By applying sonication, the ion dispersion was favored in the solution and thus promoted the efficient nucleation and grown of the Fe3O4-Ag nanostructures, as illustrated in Figure 5. The resulting nanoparticles exhibited a promising photocatalytic activity when degrading red Congo. As a concluding remark from this study, the green synthesis of nano-sized particles represents an eco-friendly approach meeting with the current requirements of green chemistry [45]. This process facilitates a simple, non-toxic, low-cost alternative to manufacturing Fe3O4 nanoalloys with well-defined morphology.
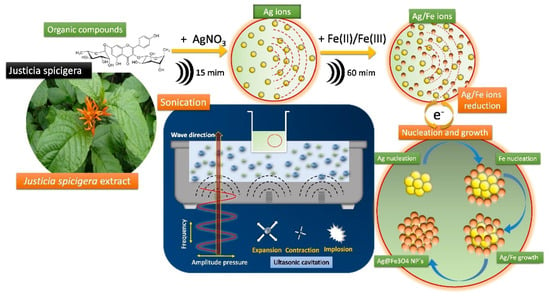
Figure 5.
Green synthesis route of Fe3O4-Ag nanoparticles mediated by sonication using J. spicigera aqueous extracts. Reprinted with permission from Ref. [44]. Copyright 2020 Elsevier.
Very recently, zinc oxide (ZnO) nanoparticles were biosynthesized by Soto-Robles et al. [46]. In a similar way compared with Ruíz-Baltazar [44], the authors evaluated the potentiality of J. spicigera extracts towards the sustainable preparation of nanoparticles. In fact, the implementation of the twelve principles of green chemistry in nanoparticle synthesis is a new trend in nanotechnology concerning sustainability. This field has a promising impact thanks to its ability to design alternative, safer, energy-efficient and less toxic routes towards synthesis [47]. As can be seen in Figure 6, Soto-Robles et al. [46] used the flavonoids contained in the Muicle extract, and their related hydroxyl and amino groups, to form the ZnO nanoparticles with an average size of ca. 22 nm. The hypothetic mechanism has been presented, dealing with an interaction between the precursor salt (i.e., Zn (NO3)2) and water. After this, the hydrolysis separated free zinc ions as they come in contact with the phenolic compounds that belong to the extract. As a final step, calcination was used for the removal of organic material and concurrently produced the ZnO particles. Interestingly, the catalytic activity presented degradation rates as high as 92% towards methylene blue after 90 min of irradiation. According to the authors’ findings, such degradation rate depended on the concentration of the extract.
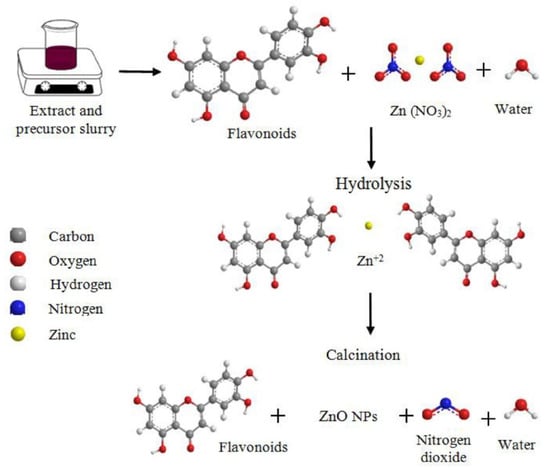
Figure 6.
Synthesis route of ZnO nanoparticles. Reprinted with permission from Ref. [44]. Copyright 2021 Elsevier.
5. Concluding Remarks, Future Perspectives and Suggestions for the New Researchers in the Field
In this review, an update on current research towards Muicle (J. spicigera) has been given. Muicle (J. spicigera) has been a core of study since it displays multiple biological properties such as anti-diabetic, anti-hypertensive, anti-depressant, anti-cancer, anti-oxidant properties, anti-inflammatory and anti-obesity, among many other attributes. Today, it is known that kaempferitrin is responsible for such biological properties and specific mechanisms of action are revealed; however, most of the research evaluated the biological properties of the entire J. spicigera extract, which presents a wide type of biologically active compounds (e.g., phenolic compounds). Obviously, such bioactivity may be a result of a synergistic effect of many biomolecules. Therefore, as a suggestion for the new researchers in the field, a huge need of knowing completely the presence and types of all biomolecules present in the plant. Additionally, some of the research also associated the bioactivity to kaempferitrin by determining its presence in the obtained extracts; however, more importantly, there is a big need in determining quantitatively the concentration of such a compound in the extract. This latter suggestion will contribute to making a fair comparison among the different studies and the related biological effects. Great advances in chemistry have contributed to identifying other valuable phytochemicals in the plant, including such phenolic compounds as anthocyanins and lignan glycosides, for example, procumbenoside B. This latter compound, which is an arylnaphthalide lignan, has been extensively pointed out as a pro-anti-inflammatory mediator [31]. As a research gap in this field, the research community is still investigating further mechanisms of action of the multiple compounds.
Apart from the testing of J. spicigera extracts in bioassays, the presence of various compounds has extended the exploration of the plant in other fields, such as food and bioproducts, where the extracted compounds have been used to enrich functional foods (such as yogurt, jelly and tortillas) [36,37]. It is well known that anthocyanins are among the main nutraceuticals with well-established properties, such as antioxidant and coloring effects. As a current trend in the field, the phenolic compounds, specially flavonoids, of Muicle have been involved within the green routes to fabricate nanostructures, such as ZnO [46] and Ag nanoparticles [44], among others. By using the analytes of the plant, safer and eco-friendly biosynthesis protocols have been established by the research community. Therefore, due to the current principles of green chemistry and sustainability, it is likely that the biomolecules present in the plant will be continuously explored within the synthesis of new nanostructured particles to be applied not only in catalysis but also in the synthesis of biocomposites, membranes [48], etc.
Over the course of this review, it has been identified that solvent extraction (e.g., ethanol, water, methanol) has been the main pathway to separate the compounds from J. spicigera. However, according to the current principles of green chemistry, there is a big need of applying eco-friendly solvents for the extraction, such as ethyl acetate [24], deep eutectic solvents (DESs) [49], etc. It is worth mentioning that there is no report, until now, utilizing DES in the extraction of compounds from the plant. Additionally, particular attention should be paid to the optimization of the operating extraction conditions since they dictate the extraction yield, purity and biological activity of the compounds. Importantly, the composition of the plant (depending on the age), as well as the operating conditions of the extraction (e.g., pH, solvent type and temperature, among others), play an important role on the final bioactivity of the extract. To some extent, some emerging extraction technologies, such as ultrasound-assisted extraction [22], have been initiated to be used in the extraction of bioactives from Muicle; at this point, it is likely that some other emerging technologies, including microwave-assisted extraction [50], pressurized liquid extraction [51], supercritical fluid extraction [52], electric field based-techniques [53] and membrane technologies [54], among others, could be explored in the near future.
Author Contributions
All authors contributed equally into this work (writing—original draft preparation, writing—review and editing). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Financial support from the Polish National Agency for Academic Exchange (NAWA) under Ulam Programme (Agreement No. PPN/ULM/2020/1/00005/U/00001) is gratefully acknowledged. R. Castro-Muñoz also acknowledges the School of Engineering and Science and the FEMSA-Biotechnology Center at Tecnológico de Monterrey for their support through the Bioprocess (0020209I13) Focus Group. This work was also supported by the Regional Government of Castilla y León and the EU-FEDER (CLU 2017-09, CL-EI-2021-07, UIC 338.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Geck, M.S.; Cristians, S.; Berger-González, M.; Casu, L.; Heinrich, M.; Leonti, M. Traditional Herbal Medicine in Mesoamerica: Toward Its Evidence Base for Improving Universal Health Coverage. Front. Pharmacol. 2020, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Maldonado-Miranda, J.J.; Zarate-Martinez, A.; Jacobo-Salcedo, M.D.R.; Fernández-Galicia, C.; Figueroa-Zuñiga, L.A.; Rios-Reyes, N.A.; de León-Rubio, M.A.; Medellín-Castillo, N.A.; Reyes-Munguia, A.; et al. Medicinal plants used in the Huasteca Potosina, México. J. Ethnopharmacol. 2012, 143, 292–298. [Google Scholar] [CrossRef] [PubMed]
- López-Rubalcava, C.; Estrada-Camarena, E. Mexican medicinal plants with anxiolytic or antidepressant activity: Focus on preclinical research. J. Ethnopharmacol. 2016, 186, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Baqueiro-Peña, I.; Guerrero-Beltrán, J.A. Uses of Justicia spicigera in medicine and as a source of pigments. Funct. Foods Health Dis. 2014, 4, 401–414. [Google Scholar] [CrossRef]
- Vega-Avila, E.; Tapia-Aguilar, R.; Reyes-Chilpa, R.; Guzmán-Gutiérrez, S.L.; Pérez-Flores, J.; Velasco-Lezama, R. Actividad antibacteriana y antifúngica de Justicia spicigera. Rev. Latinoam. Quim. 2012, 40, 75–82. [Google Scholar]
- Ramírez, G.; Zavala, M.; Pérez, J.; Zamilpa, A. In VitroScreening of Medicinal Plants Used in Mexico as Antidiabetics with Glucosidase and Lipase Inhibitory Activities. Evid.-Based Complement. Altern. Med. 2012, 2012, 701261. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Andrade, R.; Cabañas-Wuan, A.; Argaez, V.E.A.; Alonso-Castro, A.J.; Zapata-Bustos, R.; Salazar-Olivo, L.A.; Dominguez, F.; Chávez, M.; Carranza-Álvarez, C.; García-Carrancá, A. Antidiabetic effects of Justicia spicigera Schltdl (Acanthaceae). J. Ethnopharmacol. 2012, 143, 455–462. [Google Scholar] [CrossRef]
- Euler, K.L.; Alam, M. Isolation of Kaempferitrin From Justicia spicigera. J. Nat. Prod. 1982, 45, 220–221. [Google Scholar] [CrossRef]
- Yadav, N.; Vasudeva, N.; Singh, S.; Sharma, S.K. Medicinal properties of genus Chenopodium linn. Indian J. Nat. Prod. Resour. 2007, 6, 131–134. [Google Scholar]
- Esquivel-Gutiérrez, E.R.; Noriega-Cisneros, R.; AreIIano-PIaza, M.; Ibarra-Barajas, M.; Salgado-Garciglia, R.; Saave-dra-Molina, A. Antihypertensive effect of Justicia spicigera in L-NAME-induced hypertensive rats. Pharmacologyonline 2013, 2, 120–127. [Google Scholar]
- Dominguez, X.A.; Achenbach, H.; Gonzalez, C.; Ferre-D’Amare, A. Estudio Químico del “Muitle” (Justicia spicigera). Rev. Latinoam. Quim. 1990, 21, 142–143. [Google Scholar]
- MagosGuerrero, G.; SantiagoMeja, J.; Carrasco, O. Exploratory studies of some Mexican medicinal plants. Cardiovascular effects in rats with and without hypertension. J. Intercult. Ethnopharmacol. 2017, 6, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Cassani, J.; Dorantes-Barrón, A.M.; Novales, L.M.; Real, G.A.; Estrada-Reyes, R. Anti-Depressant-Like Effect of Kaempferitrin Isolated from Justicia spicigera Schltdl (Acanthaceae) in Two Behavior Models in Mice: Evidence for the Involvement of the Serotonergic System. Molecules 2014, 19, 21442–21461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Ríos, R.I.; Mora-Pérez, A.; González-Torres, D.; Carpio-Reyes, R.D.J.; Soria-Fregozo, C. Anxiolytic-like effect of the aqueous extract of Justicia spicigera leaves on female rats: A comparison to diazepam. Phytomedicine 2019, 55, 9–13. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Ortiz-Sánchez, E.; Domínguez, F.; Arana-Argáez, V.; Juárez-Vázquez, M.D.C.; Chávez, M.; Carranza-Álvarez, C.; Gaspar-Ramírez, O.; Espinosa-Reyes, G.; López-Toledo, G.; et al. Antitumor and immunomodulatory effects of Justicia spicigera Schltdl (Acanthaceae). J. Ethnopharmacol. 2012, 141, 888–894. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Ortiz-Sánchez, E.; García-Regalado, A.; Ruiz, G.; Núñez-Martínez, J.M.; González-Sánchez, I.; Quintanar-Jurado, V.; Morales-Sánchez, E.; Dominguez, F.; López-Toledo, G.; et al. Kaempferitrin induces apoptosis via intrinsic pathway in HeLa cells and exerts antitumor effects. J. Ethnopharmacol. 2013, 145, 476–489. [Google Scholar] [CrossRef]
- Juárez-Vázquez, M.D.C.; Alonso-Castro, A.J.; García-Carrancá, A. Kaempferitrin induces immunostimulatory effects in vitro. J. Ethnopharmacol. 2013, 148, 337–340. [Google Scholar] [CrossRef]
- Fernández-Pomares, C.; Juárez-Aguilar, E.; Domínguez-Ortiz, M.; Gallegos-Estudillo, J.; Herrera-Covarrubias, D.; Sánchez-Medina, A.; Aranda-Abreu, G.E.; Manzo, J.; Hernández, M.E. Hydroalcoholic extract of the widely used Mexican plant Justicia spicigera Schltdl. exerts a cytostatic effect on LNCaP prostate cancer cells. J. Herb. Med. 2018, 12, 66–72. [Google Scholar] [CrossRef]
- Jacobo-Salcedo, M.D.R.; Alonso-Castro, A.J.; Salazar-Olivo, L.A.; Carranza-Alvarez, C.; Gonzaĺez-Espińdola, L.A.; Domińguez, F.; Maciel-Torres, S.P.; Garciá-Lujan, C.; Gonzaĺez-Martińez, M.D.R.; Goḿez-Sańchez, M.; et al. Antimicrobial and cytotoxic effects of Mexican medicinal plants. Nat. Prod. Commun. 2011, 6, 1925–1928. [Google Scholar] [CrossRef] [Green Version]
- García-Márquez, E.; Román-Guerrero, A.; Pérez-Alonso, C.; Cruz-Sosa, F.; Jiménez-Alvarado, R.; Vernon-Carter, E.J. Effect of solvent-temperature extraction conditions on the initial antioxidant activity and total phenolic content of muitle extracts and their decay upon storage at different pH. Rev. Mex. Ing. Química 2012, 11, 1–10. [Google Scholar]
- Anaya-Esparza, L.M.; Ramos-Aguirre, D.; Zamora-Gasga, V.M.; Yahia, E.; Montalvo-González, E. Optimization of ultrasonic-assisted extraction of phenolic compounds from Justicia spicigera leaves. Food Sci. Biotechnol. 2018, 27, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Morales, F.; Alonso-Castro, A.J.; Zapata-Morales, J.R.; Carranza-Álvarez, C.; Aragon-Martinez, O.H. Use of standardized units for a correct interpretation of IC50 values obtained from the inhibition of the DPPH radical by natural antioxidants. Chem. Pap. 2020, 74, 3325–3334. [Google Scholar] [CrossRef]
- Awad, N.E.; Abdelkawy, M.A.; Hamed, M.A.; Souleman, A.M.A.; Abdelrahman, E.H.; Ramadan, N.S. Anti-oxidant and hepatoprotective effects of Justicia spicigera ethyl acetate fraction and characterization of its anthocyanin con-tent. Int. J. Pharm. Pharm. Sci. 2015, 7, 91–96. [Google Scholar]
- Awad, N.E.; Abdelkawy, M.A.; Rahman, E.H.A.; Hamed, M.A.; Ramadan, N.S. Phytochemical and in vitro Screening of Justicia spicigera Ethanol Extract for Antioxidant Activity and in vivo Assessment Against Schistosoma mansoni Infection in Mice. Anti-Infect. Agents 2018, 16, 49–56. [Google Scholar] [CrossRef]
- Real-Sandoval, S.A.; Gutiérrez-López, G.F.; Domínguez-López, A.; Paniagua-Castro, N.; Michicotl-Meneses, M.M.; Jaramillo-Flores, M.E. Downregulation of proinflammatory liver gene expression by Justicia spicigera and kaempferitrin in a murine model of obesity-induced by a high-fat diet. J. Funct. Foods 2020, 65, 103781. [Google Scholar] [CrossRef]
- Zapata-Morales, J.R.; Alonso-Castro, A.J.; Domínguez, F.; Carranza-Álvarez, C.; Castellanos, L.M.O.; Martínez-Medina, R.M.; Pérez-Urizar, J. Antinociceptive Activity of an Ethanol Extract of Justicia spicigera. Drug Dev. Res. 2016, 77, 180–186. [Google Scholar] [CrossRef]
- Ángeles-López, G.E.; González-Trujano, M.E.; Rodríguez, R.; Déciga-Campos, M.; Brindis, F.; Ventura-Martínez, R. Gastrointestinal activity of Justicia spicigera Schltdl. in experimental models. Nat. Prod. Res. 2021, 35, 1847–1851. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Domínguez, F.; Pérez-Ortega, G.; Aguillón, M.; Martínez-Vargas, D.; Almazán-Alvarado, S.; Martínez, A. Justicia spicigera Schltdl. and kaempferitrin as potential anticonvulsant natural products. Biomed. Pharmacother. 2017, 92, 240–248. [Google Scholar] [CrossRef]
- Weng, J.-R.; Ko, H.-H.; Yeh, T.-L.; Lin, H.-C.; Lin, C.-N. Two New Arylnaphthalide Lignans and Antiplatelet Constituents from Justicia procumbens. Arch. Pharm. 2004, 337, 207–212. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P.; Flores, J.M.M.; Gonzalez, A.M.N. Anti-inflammatory effect of procumbenoside B from Justicia spicigera on lipopolysaccharide-stimulated RAW 264.7 macrophages and zebrafish model. Pharmacogn. Res. 2018, 10, 218–224. [Google Scholar] [CrossRef]
- Valencia-Arredondo, J.A.; Hernández-Bolio, G.I.; Cerón-Montes, G.I.; Castro-Muñoz, R.; Yáñez-Fernández, J. Enhanced process integration for the extraction, concentration and purification of di-acylated cyanidin from red cabbage. Sep. Purif. Technol. 2020, 238, 116492. [Google Scholar] [CrossRef]
- Arberet, L.; Pottier, F.; Michelin, A.; Nowik, W.; Bellot-Gurlet, L.; Andraud, C. Spectral characterisation of a traditional Mesoamerican dye: Relationship between in situ identification on the 16th century Codex Borbonicus manuscript and composition of Justicia spicigera plant extract. Analyst 2021, 146, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Baqueiro-Peña, I.; Guerrero-Beltrán, J. Physicochemical and antioxidant characterization of Justicia spicigera. Food Chem. 2017, 218, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Pavón-García, L.M.A.; Pérez-Alonso, C.; Orozco-Villafuerte, J.; Pimentel-González, D.J.; Huezo, M.E.R.; Vernon-Carter, E.J. Storage stability of the natural colourant from Justicia spicigera microencapsulated in protective colloids blends by spray-drying. Int. J. Food Sci. Technol. 2011, 46, 1428–1437. [Google Scholar] [CrossRef]
- Castro-Alatorre, N.; Gallardo-Velázquez, T.; Boyano-Orozco, L.; Téllez-Medina, D.; Meza-Márquez, O.; Osorio-Revilla, G. Extraction and Microencapsulation of Bioactive Compounds from Muicle (Justicia spicigera) and Their Use in the Formulation of Functional Foods. Foods 2021, 10, 1747. [Google Scholar] [CrossRef]
- Alvarez-Poblano, L.; Roman-Guerrero, A.; Vernon-Carter, E.; Alvarez-Ramirez, J. Exogenous addition of muicle (Justicia spicigera Schechtendal) extract to white maize tortillas affects the antioxidant activity, texture, color, and in vitro starch digestibility. LWT 2020, 133, 110120. [Google Scholar] [CrossRef]
- Chan-Bacab, M.J.; Sanmartín, P.; Camacho-Chab, J.C.; Palomo-Ascanio, K.B.; Huitz-Quimé, H.E.; Ortega-Morales, B.O. Characterization and dyeing potential of colorant-bearing plants of the Mayan area in Yucatan Peninsula, Mexico. J. Clean. Prod. 2015, 91, 191–200. [Google Scholar] [CrossRef]
- Bernardo-Mazariegos, E.; Valdez-Salas, B.; González-Mendoza, D.; Abdelmoteleb, A.; Camacho, O.T.; Duran, C.C.; Gutiérrez-Miceli, F. Silver nanoparticles from Justicia spicigera and their antimicrobial potentialities in the biocontrol of foodborne bacteria and phytopathogenic fungi. Rev. Argent. Microbiol. 2019, 51, 103–109. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 48, 53. [Google Scholar] [CrossRef] [Green Version]
- Castro-Muñoz, R. The Role of New Inorganic Materials in Composite Membranes for Water Disinfection. Membranes 2020, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.-S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Mendoza, D.; Valdez-Salas, B.; Bernardo-Mazariegos, E.; Camacho, O.T.; Gutiérrez-Miceli, F.; Ruíz-Valdiviezo, V.; Rodríguez-Hernández, L.; Sanchez-Viveros, G. Influence of monometallic and bimetallic phytonanoparticles on physiological status of mezquite. Open Life Sci. 2019, 14, 62–68. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, D.J. Green synthesis assisted by sonochemical activation of Fe3O4-Ag nano-alloys: Structural characterization and studies of sorption of cationic dyes. Inorg. Chem. Commun. 2020, 120, 108148. [Google Scholar] [CrossRef]
- Anastas, P.T.; Eghbali, N. Green Chesmitry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 201–312. [Google Scholar] [CrossRef] [PubMed]
- Soto-Robles, C.; Nava, O.; Cornejo, L.; Lugo, E.; Vilchis-Nestor, A.; Castro-Beltrán, A.; Luque, P. Biosynthesis, characterization and photocatalytic activity of ZnO nanoparticles using extracts of Justicia spicigera for the degradation of methylene blue. J. Mol. Struct. 2021, 1225, 129101. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; González-Melgoza, L.L.; García-Depraect, O. Ongoing progress on novel nanocomposite membranes for the separation of heavy metals from contaminated water. Chemosphere 2021, 270, 129421. [Google Scholar] [CrossRef]
- Serna-Vázquez, J.; Ahmad, M.Z.; Boczkaj, G.; Castro-Muñoz, R. Latest Insights on Novel Deep Eutectic Solvents (DES) for Sustainable Extraction of Phenolic Compounds from Natural Sources. Molecules 2021, 26, 5037. [Google Scholar] [CrossRef]
- Cienfuegos, N.; Santos, P.; García, A.; Soares, C.; Lima, A.; Souza, R. Integrated process for purification of capsaicin using aqueous two-phase systems based on ethanol. Food Bioprod. Process. 2017, 106, 1–10. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.D.M.; Espínola, F.; Moya, M.; de Torres, A.; Romero, I.; Castro, E. Extraction of oleuropein and luteolin-7-O-glucoside from olive leaves: Optimization of technique and operating conditions. Food Chem. 2019, 293, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Yan, L.-G.; Deng, Y.; Ju, T.; Wu, K.; Xi, J. Continuous high voltage electrical discharge extraction of flavonoids from peanut shells based on “annular gap type” treatment chamber. Food Chem. 2018, 256, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; Boczkaj, G.; Gontarek, E.; Cassano, A.; Fíla, V. Membrane technologies assisting plant-based and agro-food by-products processing: A comprehensive review. Trends Food Sci. Technol. 2020, 95, 219–232. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).