Abstract
The dried root extract of Astragalus membranaceus, also known as Astragali radix, is widely used in traditional Chinese medicine for its multiple health benefits and well-established safety profile. Astragalus root extract exhibits several bioactive properties, including anti-inflammatory, antioxidant, antiviral and hepatoprotective effects. Due to its unique features, it is being investigated in a novel application as a complementary remedy in the management of joint disorders. In this study, we evaluated the effect of Astragalus membranaceus hydroalcoholic root extract (0.01 and 0.1 mg/mL) in vitro on the HTB-94 cell line, a well-known model for studying inflammatory pathways in human chondrocytes. The mRNA modulation levels were measured by quantitative real-time polymerase chain reaction (qRT-PCR), while the protein secretion levels were assessed using an Enzyme-Linked Immunosorbent Assay (ELISA). Results obtained demonstrated that this extract is able to decrease the tumor necrosis factor-α (TNF-α)-induced inflammatory response by downregulating both the mRNA expression and release of the pro-inflammatory mediators Interleukin-6 (IL-6), Interleukin-1β (IL-1β) and Interelukin-8 (IL-8), as well as matrix metalloproteases, including Matrix Metalloprotease-3 (MMP-3), Matrix Metalloprotease-13 (MMP-13) and A disintegrin, and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5). Moreover, the interleukin and matrix metalloprotease production was also assessed in non-TNF-α-stimulated cells, revealing that the extract did not alter the basal levels of these mediators. Finally, our findings highlight the potential benefits of Astragalus membranaceus extract, both in terms of its favorable safety profile and its efficacy mitigating joint inflammatory responses. These results support the potential of this extract as a nutraceutical agent for joint health support.
1. Introduction
Joints are anatomical structures that connect bones within the skeleton, providing both stability and mobility. These structures are frequently affected by painful and sometimes disabling conditions, largely driven by inflammatory processes that result in the degradation of articular cartilage []. Inflammation is a hallmark of various joint disorders, which are broadly classified under the term arthritis. The causes of joint inflammation are multifactorial []. It may be due to autoimmune mechanisms, as observed in rheumatoid arthritis (RA), juvenile idiopathic arthritis, neuropathic arthropathy and seronegative spondyloarthropathies, or it may be associated with aging, as in the case of osteoarthritis (OA) [,]. Among these conditions, OA is the most common joint disorder, affecting several diarthrodial joints, such as the hands, knees and hips []. Due to the growing proportion of elderly individuals, its medical relevance is increasing, particularly in Western countries. OA affects 7.6% of the global population and is now considered a disease of the entire joint structure, including the articular cartilage, subchondral bone, ligaments, capsule and synovial membrane []. OA is typically classified into two forms: primary OA, which is idiopathic and associated with aging, obesity, genetic predisposition and low-grade systemic inflammation; and secondary OA, which results from trauma, surgical interventions and congenital joint abnormalities [,]. If left untreated, inflammatory arthropathies result in progressive joint damage and deformity [,], leading to a chronic and irreversible degenerative process. Since inflammation is the mechanism driving both the onset and progression of degenerative joint diseases, early and appropriate anti-inflammatory intervention is essential to prevent structural damage, disease advancement and disability. The structure of cartilage tissue, which is mainly constituted by glycosaminoglycans, proteoglycans and collagens, depends on a homeostasis due to the balance between anabolic and catabolic processes. Pro-inflammatory stimulation can alter this balance, causing the hyperproduction of joint-degrading enzymes, matrix metalloproteases (MMPs) and articular damages [,]. For this reason, the current pharmacological treatments are mainly based on the administration of steroidal or nonsteroidal anti-inflammatory drugs (NSAIDs) []. However, the long-term administration of these agents is associated with significant side effects, including gastrointestinal [], renal [], cardiovascular [] and hepatic [] toxicity. In addition, NSAIDs may also cause allergic reactions [] and increase the risk of hypertension and fluid retention []. Given these limitations, there is growing interest in identifying novel approaches capable not only of controlling symptoms but also of modifying the progression of chronic joint diseases. In this context, nutraceuticals, particularly those derived from botanicals and plant extracts, have emerged as promising sources of bioactive compounds with anti-inflammatory properties [,].
Among these, Astragalus, a genus belonging to the Fabaceae (or Leguminosae) family, is widely distributed throughout the temperate regions of the world from Central and Southwestern Asia to South America and Africa, and it is known for its broad spectrum of biological effects [,,]. The dried root of Astragalus membranaceus (Fisch.) Bunge var. Mongolicus (Bunge) Hisao, or Astragalus membranaceus (Fisch.) Bunge, commonly referred to as Astragalus radix, is a key component in traditional Chinese medicine and is widely used in functional food, health products, cosmetics and veterinary applications [,,]. Several studies have demonstrated that A. membranaceus extract possesses well-known anti-inflammatory [], immunomodulatory [,] and antioxidant [,] properties, therefore making it particularly suitable for the treatment of those pathologies with inflammatory etiologies. In addition, emerging studies have demonstrated its antiviral, anti-aging, anti-tumor, hepatoprotective, nephroprotective and cardiovascular protective properties [,]. A. membranaceus extract has also shown anti-inflammatory efficacy in intestinal and allergic pathologies [,,,]. A summary of clinical trials reporting activities for A. membranaceus can be found in the review by Durazzo et al. []. A. membranaceus extract has also been shown to reduce the adverse side effects of specific therapies used to treat diseases, particularly those related to food intake problems []. Furthermore, according to the Chinese Food and Drug Administration (FDA), A. membranaceus extract can also be utilized as a functional food []. Given its favorable safety profile, further investigation into the potential anti-inflammatory action of A. membranaceus extract in joint-related diseases is warranted.
In this study, we employed the in vitro HTB-94 cell line, an established experimental model for studying inflammatory pathways in human chondrocytes, to evaluate whether A. membranaceus extract can inhibit the TNF-α-induced expression of pro-inflammatory interleukins and downregulate MMP production. Previously, three different A. membranaceus extracts were analyzed and compared to natural A. membranaceus extract, finding that one of the three, the commercial sample named Axtragyl®, showed a higher content of bioactive compounds [], prompting us to use this extract. Moreover, Durazzo et al. found that several compounds present in A. membranaceus extract have anti-inflammatory properties []. The aim of this study was to explore the potential of commercial A. membranaceus extract (Axtragyl®) as a safe and effective nutraceutical candidate for modulating joint inflammation and preventing cartilage degradation.
2. Materials and Methods
2.1. Cells
The human chondrosarcoma cell line HTB-94 (SW1353; American Type Culture Collection) was cultured at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) (HyClone, Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen, Paisley, UK), 2 mM glutamine, nonessential amino acids, 100 IU/mL penicillin and 100 μg/mL streptomycin.
2.2. Materials
The hydroalcoholic extract derived from the dried roots of Astragalus membranaceus (marketed as Axtragyl®) was generously supplied by Giellepi (Milan, Italy). Recombinant tumor necrosis factor-α (TNF-α) was obtained from PeproTech EC Ltd. (29 Margravine Road, London, UK). The ELISA Pro kits for human IL-6, IL-1β and IL-8 (CXCL8) were purchased from Mabtech AB (SE-131 28, Nacka Strand, Sweden). The SensiFAST™ cDNA Synthesis Kit and SensiFAST™ SYBR Hi-ROX Kit were acquired from Bioline Meridian Biosciences (5171 Wilfong Road, Memphis, TN, USA). ELISA kits for human ADAMTS5, MMP-13 and MMP-3 were sourced from Fine Biotech Co., Ltd. (Wuhan, China).
2.3. Assessment of Cell Viability (MTS Assay)
To assess any potential cytotoxic effects of A. membranaceus extract, the viability of HTB-94 cells exposed to this compound was analyzed using an MTS 3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium)-based colorimetric assay (Promega Corporation, Madison, WI, USA) in accordance with the manufacturer’s protocols.
In brief, 1.5 × 104 cells per well were plated in a 96-well plate with 100 μL of medium. Cells were either left untreated (control, CTL) or exposed to A. membranaceus extract at concentrations of 0.01, 0.1, 0.5 and 1 mg/mL for 24, 48 and 72 h. After each incubation period, a 100 μL of MTS reagent was added to each well. Absorbance was measured at 492 nm after a 3 h incubation using a microplate reader (NB-12-0035, NeBiotech, Holden, MA, USA). Cell viability was expressed as a percentage of dead cells using the following formula: % dead cells = 100 × [(OD treated/OD control) × 100].
2.4. Cell Treatment
HTB-94 cells were plated at the required density and allowed to adhere. After adhesion, the cells were serum-starved (DMEM containing 1% FBS) before stimulation and/or other treatments.
After 24 h of serum starvation, cells were untreated (CTL) or treated with A. membranaceus extract (0.01 and 0.1 mg/mL). After 1 h of incubation at 37 °C, cells were left untreated (CTL) or exposed to TNF-α 10 (ng/mL) in the presence or absence of the A. membranaceus extract (0.01 and 0.1 mg/mL) for the required time.
In order to analyze the pro-inflammatory cytokine or MMP mRNA expression levels, the cells were harvested after 30 min of stimulation with TNF-α, and then the mRNAs were processed for qRT-PCR.
To quantify the pro-inflammatory cytokine and MMP protein secretion, cell supernatants were collected and analyzed by ELISA, after 1 h or 24 h of TNF-α stimulation, respectively.
2.5. RNA Extraction and Reverse Transcription
Total RNA was isolated from both untreated and A. membranaceus extract-treated HTB-94 cells using the Blood/Tissues Total RNA extraction kit (Fisher Molecular Biology, Trevose, PA, USA). Reverse transcription was then performed using OneScript Hot Reverse Transcriptase (Applied Biological Materials Inc., Richmond, BC, Canada) in accordance with the manufacturer’s guidelines.
2.6. Quantitative Real-Time Polymerase Chain Reaction
qRT-PCR analysis was conducted using the ABI Prism 7300 system (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). Amplification reactions were carried out with the SensimixPlus SYBR Master mix (Bioline, London, UK). The primers listed in Table 1 were synthesized by Bio-Fab research (Via di Castel Romano 100, Rome, Italy) and designed using Primer Express software v1.4.0 (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). Gene expression levels were calculated using the 2−ΔΔCt method, with normalization to the 18S rRNA housekeeping gene as the internal control.

Table 1.
List of primers used for RT-PCR. Accession numbers are indicated.
2.7. ELISA
The concentrations of pro-inflammatory cytokines and MMP proteins in the supernatants of both A. membranaceus extract-treated and untreated HTB-94 cells were quantified using ELISA kits, following the protocols provided by the manufacturers. The Optical Density (OD) was measured at 450 nm using a microplate reader (NB-12-0035, NeBiotech, Holden, MA, USA).
2.8. Statistical Analysis
Results were expressed as mean ± standard error of the mean (SEM) from a minimum of at least three independent experiments, each performed in duplicate or triplicate. Statistical analysis was carried out using Prism 5.0 software (GraphPad Software, San Diego, CA, USA). A two-way repeated measures analysis of variance (ANOVA) was applied, followed by Bonferroni’s post hoc test for multiple comparisons. A p-value below 0.05 was considered statistically significant.
3. Results
3.1. Cytotoxicity Test
In order to evaluate the potential cytotoxic effect of Astragalus membranaceus extract, HTB-94 cells were exposed to increasing concentrations of the extract, ranging from 1 to 0.01 mg/mL, for 24, 48 and 72 h. The results obtained are shown in Figure 1. Under our experimental conditions, no cytopathic effect was observed.
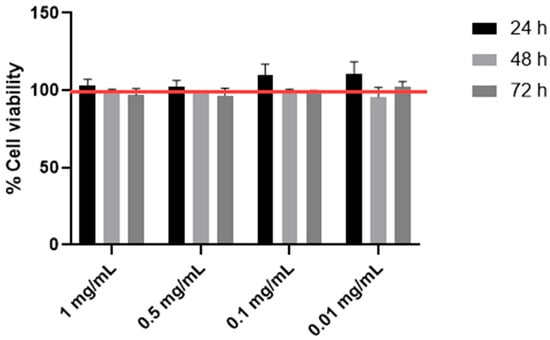
Figure 1.
Cell viability by MTS. The viability of the HTB-94 cells treated with 0.01, 0.1, 0.5 and 1 mg/mL of Astragalus membranaceus extract was tested following 24, 48 and 72 h of treatment. Cell viability was normalized to that of untreated control cells, which was set at 100%, and is indicated by the horizontal red line. Data are presented as the mean ± standard deviation, based on results from three different experiments.
3.2. Effects of Astragalus membranaceus Extract on the Modulation of Pro-Inflammatory Genes
To evaluate the anti-inflammatory effectiveness of A. membranaceus extract, HTB-94 chondrocytes were pretreated with 0.01 and 0.1 mg/mL of the extract and subsequently stimulated with TNF-α to mimic an inflamed-joint environment. As shown in Figure 2, TNF-α stimulation increased the mRNA expression levels of IL-6, IL-8 and IL-1 β, confirming the activation of a pro-inflammatory response. Pretreatment with A. membranaceus extract was able to attenuate this upregulation. In particular, IL-6 expression was inhibited only at the higher concentration of A. membranaceus extract, while IL-8 and IL-1 β were decreased at both concentrations (0.01 and 0.1 mg/mL). Notably, treatment with A. membranaceus extract alone, in the absence of TNF-α stimulation, did not result in any statistically significant changes compared to the untreated control cells (CTL) (Figure 2).
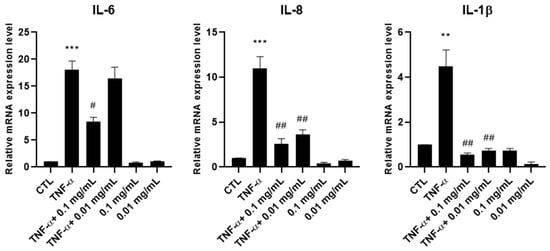
Figure 2.
Effects of Astragalus membranaceus extract on interleukin mRNA expression levels. Cells were either left untreated (CTL), were exposed to 10 ng/mL TNF-α for 30 min or were pretreated with 0.01 and 0.1 mg/mL of the extract for 1 h prior to TNF-α (10 ng/mL for 30 min). Following treatment, total RNA was isolated and analyzed by RT-PCR. The mRNA expression levels of IL-6, IL-8 and IL-1β were quantified relative to 18S rRNA using the 2−ΔΔCt method. Data are presented as mean ± standard deviation (SD) from three independent experiments. ** p < 0.01, TNF-α vs. CTL; *** p < 0.005, TNF-α vs. CTL; # p < 0.05, treated vs. TNF-α; ## p < 0.01, treated vs. TNF-α.
3.3. Effects of Astragalus membranaceus Extract on Pro-Inflammatory Cytokine Secretion
To measure the effect of A. membranaceus extract on pro-inflammatory cytokines, ELISAs were performed on culture supernatants collected from HTB-94 cells stimulated with TNF-α and treated with 0.01 or 0.1 mg/mL A. membranaceus extract. Treatment with both concentrations of A. membranaceus extract decreased IL-6 and IL-8 production, bringing their levels below those observed in the CTL. Regarding IL-1β, treatment with 0.1 mg/mL A. membranaceus extract reduced the cytokine secretion to CTL levels, while 0.01 mg/mL also exerted a significant inhibitory effect, although to a slightly lesser extent (Figure 3).
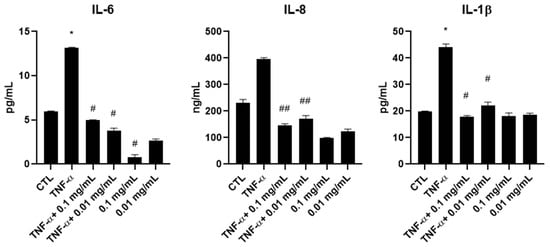
Figure 3.
Effects of Astragalus membranaceus extract on interleukin secretion in the culture medium. Cells were either left untreated (CTL), were exposed to 10 ng/mL TNF-α for 1 h or were pretreated with two concentrations of extract for 1 h followed by stimulation with 10 ng/mL TNF-α for an additional hour. After the treatments, cell culture supernatants were collected and analyzed using ELISAs. Results are presented as pg/mL and expressed as mean ± SD from three independent experiments. * p < 0.05, TNF-α vs. CTL; # p < 0.05, treated vs. TNF-α; ## p < 0.01, treated vs. TNF-α.
Interestingly, in the absence of TNF-α stimulation, A. membranaceus extract at 0.1 mg/mL induced a significant reduction in IL-6 secretion below the basal CTL level. A similar trend was observed for IL-8, although the decrease was not statistically significant. In contrast, the IL-1β levels remained comparable to those of untreated cells following A. membranaceus extract treatment (Figure 3).
3.4. Effects of Astragalus membranaceus Extract on the Modulation of Metalloprotease Genes
We then evaluated the ability of A. membranaceus extract to inhibit the mRNA expression of metalloproteases (MMPs). HTB-94 cells were pretreated with 0.01 or 0.1 mg/mL of A. membranaceus extract, followed by stimulation with TNF-α. RNA was extracted, and the expression levels of MMP-3, MMP-13 and ADAMTS-5 were quantified by RT-PCR. The results obtained showed that, at both concentrations, the A. membranaceus extract significantly downregulated the mRNA expression of all the enzymes studied (Figure 4). In cells not stimulated with TNF-α, the A. membranaceus extract had no significant effect on the MMP-13 mRNA expression, which remained comparable to the CTL level. However, both the MMP-3 and ADAMTS-5 mRNA levels were significantly reduced below the CTL levels (Figure 4).
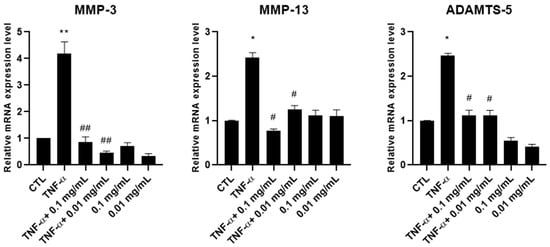
Figure 4.
Effects of Astragalus membranaceus extract on MMP mRNA expression levels. Cells were either left untreated (CTL), were exposed to10 ng/mL TNF-α for 30 min or were pretreated with 0.01 and 0.1 mg/mL of the extract for 1 h prior to TNF-α stimulation (10 ng/mL for 30 min). Following treatment, total RNA was isolated and analyzed by RT-PCR. The mRNA expression levels of MMP-3, MMP-13 and ADAMTS-5 were quantified relative to 18S rRNA using the 2−ΔΔCt method. Data are presented as mean ± standard deviation (SD) from three independent experiments. * p < 0.05, TNF-α vs. CTL; ** p < 0.01, TNF-α vs. CTL; # p < 0.05, treated vs. TNF-α; ## p < 0.01, treated vs. TNF-α.
3.5. Effects of Astragalus membranaceus Extract on MMP Production
To assess whether the reduction in MMP gene expression observed upon A. membranaceus extract treatment was also reflected at the protein level, the production of MMP-3, MMP-13 and ADAMTS-5 was evaluated via ELISAs. Supernatants were collected from HTB-94 cells stimulated with TNF-α and treated with 0.01 or 0.1 mg/mL of A. membranaceus extract. Consistent with the mRNA expression data, treatment with both concentrations of A. membranaceus extract effectively reduced the production of all the examined MMPs comparable to the CTL levels (Figure 5). In this case, the TNF-α-induced increase in MMP-13 and ADAMTS-5 secretion was not statistically significant. Interestingly, in cells treated with A. membranaceus extract without TNF-α stimulation, no significant changes in MMP secretion were observed (Figure 5).
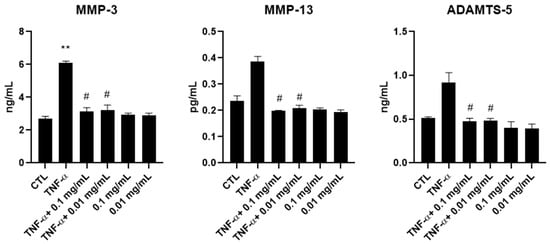
Figure 5.
Effects of Astragalus membranaceus on MMP secretion in the culture medium. Cells were either left untreated (CTL), were exposed to 10 ng/mL TNF-α for 24 h or were treated with two concentrations of A. membranaceus extract and simultaneously stimulated with 10 ng/mL TNF-α for 24 h. Following treatment, cell culture supernatants were collected and analyzed using ELISAs. Data are presented as mean ± SD from three independent experiments. # p < 0.05, treated vs. TNF-α; ** p < 0.01, TNF-α vs. CTL.
4. Discussion
Astragalus membranaceus is a medicinal herb that is widely used in traditional Chinese medicine as an adaptogen and immunomodulator, and for the treatment of various diseases, including joint-related disorders. In recent years, its use has been investigated in the context of inflammatory and tumor diseases [,], with growing evidence supporting its ability to mitigate drug-induced toxicity when used in combination with conventional pharmacological treatments.
Joints are frequently affected by arthropathies, including rheumatoid arthritis, juvenile idiopathic arthritis, neuropathic arthropathy, seronegative spondyloarthropathies and OA [,]. These disabling pathologies are primarily driven by inflammatory processes that promote the degradation of articular cartilage, leading to pain, stiffness and the progressive loss of joint function [].
In this study, we investigated the effects of a hydroalcoholic extract of Astragalus membranaceus roots on the HTB-94 cell line, demonstrating that this extract is capable of inhibiting both the gene expression and protein production of key pro-inflammatory cytokines and metalloproteases in chondrocytes under inflammatory conditions induced by TNF-α stimulation.
TNF-α was used to stimulate HTB-94 chondrocytes to mimic the inflammation typical of degenerative joint disease progression. Under inflammatory conditions, HTB-94 cells exhibited a significant increase in mRNA expression and secretion of IL-6, IL-8 and IL-1β. Treatment with A. membranaceus extract at 0.01 and 0.1 mg/mL significantly reduced the mRNA expression of IL-8 and IL-1β, while IL-6 was significantly decreased only at the higher concentration (0.1 mg/mL). These results suggest that A. membranaceus extract, by acting against inflammation of articular cartilage, may exert a protective role in joint health. These findings are of particular interest considering that the pro-inflammatory cytokines IL-6 and IL-8 are consistently found to be overexpressed in chronic joint diseases, such as RA and OA, where they play an important role in inflammation and subsequent joint damage []. Notably, the A. membranaceus extract treatment did not alter the interleukin levels in unstimulated cells, with the exception of IL-6, whose secretion was significantly reduced at 0.1 mg/mL. This finding is especially relevant, considering that IL-6 is one of the earliest mediators released in inflammatory conditions and plays a pivotal role in the activation of several pro-inflammatory pathways [,,].
Articular cartilage is constituted by the extracellular matrix (ECM), consisting mainly of proteoglycans, collagen, elastin, gelatin and matrix glycoproteins [], and contains only one cell type: chondrocytes []. The homeostasis of articular cartilage is tightly regulated by the balance between anabolic and catabolic processes, which is mainly mediated by matrix metalloproteases (MMPs) and their endogenous tissue inhibitors (TIMPs) [].
Inflammatory cytokines in joints act as key mediators of MMP gene expression, stimulating both transcriptional activity and subsequent enzyme production [].
MMPs are a class of proteolytic enzymes, classified according to the nature of their active sites and the substrates they degrade []. Their catalytic mechanism requires the presence of metal ions as cofactors []. The metalloprotease superfamily consists of different subfamilies: the MMP family, the A Disintegrin Metalloproteinase (ADAM) family and the ADAM with Thrombospondin type 1 Motif (ADAMTS) family []. These zinc-dependent endopeptidases are primarily responsible for the remodeling and degradation of the ECM and play a major role in the development and progression of several joint diseases []. While MMPs contribute to tissue homeostasis under normal physiological conditions, their expression is significantly upregulated in inflamed joints, as seen in rheumatoid arthritis and OA. In particular, MMPs may damage both collagen and proteoglycans [,,], the major component of cartilage, disrupting the balance between synthesis and degradation of the ECM. The excessive enzymatic activity of MMPs and ADAMTSs leads to the progressive loss of the cartilage matrix, contributing to joint space narrowing, pain and reduced mobility, hallmarks of degenerative joint diseases []. Thus, strategies aimed at suppressing MMP overexpression and activity are of major interest.
In the present study, we investigated the effects of A. membranaceus extract on the mRNA expression levels and protein production of MMP-3, MMP-13 and ADAMTS-5 in human HTB-94 cells stimulated with TNF- α. To the best of our knowledge, this is the first manuscript studying the effects of an A. membranaceus extract on a chondrocyte model. Previous studies have been focused on several intracellular pathways, such as inflammatory and oxidative ones, using intestinal epithelium cells or RAW64.7 macrophages [,,,]. Moreover, A. membranaceus extract has also been studied as a cardioprotective and anti-diabetic agent in rat and mouse models, respectively [,]. To analyze the effects on the intracellular pathways involved in joint disorders, we utilized the HTB-94 cell line, which closely mimics the behavior of primary chondrocytes in terms of the MMP expression induced by inflammatory cytokines and is a well-established model for studying inflammatory pathways in chondrocytes []. We focused particularly on these MMPs due to their essential role in maintaining the ECM balance. MMP-3, also known as stromelysin-1, is an endopeptidase belonging to the MMP enzyme family. In normal physiological processes, MMP-3 plays a key role in the degradation of ECM proteins during tissue remodeling []; however, it also contributes to exacerbation of inflammation in several pathological conditions []. MMP-3 has been identified as a potential therapeutic target in various inflammatory diseases, including joint inflammation and OA, as well as obesity, pulmonary inflammation and periodontitis [,,]. Regarding joint disease, this enzyme represents a marker of synovial inflammation and cartilage degradation, with elevated serum MMP-3 levels being closely related to the disease severity []. MMP-3 thus serves not only as a biomarker for monitoring disease progression but also as an indicator of therapeutic efficacy [,]. Like MMP-3, MMP-13 is an important mediator of cartilage degradation [,,,]. It belongs to a subgroup of MMPs known as collagenases, which are among the most effective enzymes capable of initiating the cleavage of native collagen []. MMP-13, also referred to as collagenase-3, is localized in articular cartilage and plays a central role in osteoarthritic joints by cleaving type II collagen, the predominant collagen in cartilage []. ADAMTS-5, also known as aggrecanase-2, is another key protease involved in the turnover of proteoglycans such as aggrecan and versican. Its overexpression is considered a pivotal risk factor in the progression of degenerative joint diseases []. Both aggrecan and versican interact with hyaluronic acid and other ECM components to form a hydrated, resilient cartilage matrix []. In addition, aggrecan modulates the inflammatory response through interactions with growth factors, cytokines and chemokines. Inhibiting aggrecanase activity may offer advantages over collagenase inhibition [], as the loss of aggrecan in cartilage is one of the earliest events in the onset of joint diseases such as OA []. Considering the crucial role of ADAMTS-5 in the pathogeneses of joint diseases, inhibition of its expression and production could represent a promising therapeutic strategy for joint inflammatory diseases [].
Taken together, our in vitro results demonstrate that Astragalus membranaceus protects cartilage by suppressing the production of pro-inflammatory cytokines and MMPs. These effects, however, are specifically attributable to the particular extract of Astragalus membranaceus tested in this study and cannot be generalized to other preparations without further investigation.
5. Conclusions
To the best of our knowledge, this is the first study demonstrating that treatment of chondrocytes with Astragalus membranaceus inhibits the expression and production of not only inflammatory cytokines but also of MMP-3, MMP-13 and ADAMTS-5, which play a key role in articular cartilage degradation. These findings suggest that the specific A. membranaceus extract used in this study may represent a promising nutraceutical approach for mitigating joint inflammation and cartilage degeneration. Our findings also suggest that this extract could be considered as a supportive strategy to maintain joint health and prevent inflammation-related damage. Further in vivo studies and clinical trials are warranted to confirm these results and explore the full potential of this extract in different populations.
Author Contributions
Conceptualization, A.S.d. and F.S.; investigation, A.M. and A.S.d.; writing—original draft preparation, A.S.d., A.M. and F.S.; writing—review and editing, A.S.d., A.M. and F.S.; visualization, R.R.; supervision, A.S.d. and F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The HTB-94 human chondrosarcoma cell line (SW1353) was obtained from the American Type Culture Collection. Data are contained within the article and are available upon request.
Conflicts of Interest
Rosario Russo was employed by the company Giellepi S.p.A. and was not involved in the data analysis or interpretation of the results. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Di Nicola, V. Degenerative osteoarthritis a reversible chronic disease. Regen. Ther. 2020, 15, 149–160. [Google Scholar] [CrossRef]
- Glocker, M.O.; Guthke, R.; Kekow, J.; Thiesen, H.J. Rheumatoid arthritis, a complex multifactorial disease: On the way toward individualized medicine. Med. Res. Rev. 2006, 26, 63–87. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef]
- Helmick, C.G.; Felson, D.T.; Lawrence, R.C.; Gabriel, S.; Hirsch, R.; Kwoh, C.K.; Liang, M.H.; Kremers, H.M.; Mayes, M.D.; Merkel, P.A.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008, 58, 15–25. [Google Scholar] [CrossRef]
- Martel-Pelletier, J. Pathophysiology of osteoarthritis. Osteoarthr. Cartil. 2004, 12, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, Z.; Li, Y.; Zhang, J.; Li, C.; Lv, N. Exploration beyond osteoarthritis: The association and mechanism of its related comorbidities. Front. Endocrinol. 2024, 15, 1352671. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, X.; Zhang, Y.; Tian, Z.; Wang, M.; Chen, Z. Advances in the pathology and treatment of osteoarthritis. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef]
- Berman, S.; Bucher, J.; Koyfman, A.; Long, B.J. Emergent Complications of Rheumatoid Arthritis. J. Emerg. Med. 2018, 55, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Krenn, V.; Waldstein, W.; Najm, A.; Perino, G.; Gaulke, R. Histopathological classification principles of rheumatic joint diseases: Contribution of pathology to the diagnosis. Orthopade 2018, 47, 941–948. [Google Scholar] [CrossRef]
- Pérez-García, S.; Carrión, M.; Gutiérrez-Cañas, I.; Villanueva-Romero, R.; Castro, D.; Martínez, C.; González-álvaro, I.; Blanco, F.J.; Juarranz, Y.; Gomariz, R.P. Profile of matrix-remodeling proteinases in osteoarthritis: Impact of fibronectin. Cells 2020, 9, 40. [Google Scholar] [CrossRef]
- Steinmeyer, J. Pharmacological basis for the therapy of pain and inflammation with nonsteroidal anti-inflammatory drugs. Arthritis Res. 2000, 2, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Whelton, A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: Physiologic foundations and clinical implications. Am. J. Med. 1999, 106, 13S–24S. [Google Scholar] [CrossRef]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef]
- Sriuttha, P.; Sirichanchuen, B.; Permsuwan, U. Hepatotoxicity of Nonsteroidal Anti-Inflammatory Drugs: A Systematic Review of Randomized Controlled Trials. Int. J. Hepatol. 2018, 2018, 5253623. [Google Scholar] [CrossRef]
- Berkes, E.A. Anaphylactic and anaphylactoid reactions to aspirin and other NSAIDs. Clin. Rev. Allergy Immunol. 2003, 24, 137–148. [Google Scholar] [CrossRef]
- Warner, T.D.; Mitchell, J.A. COX-2 selectivity alone does not define the cardiovascular risks associated with non-steroidal anti-inflammatory drugs. Lancet 2008, 371, 270–273. [Google Scholar] [CrossRef]
- Gonfa, Y.H.; Tessema, F.B.; Bachheti, A.; Rai, N.; Tadesse, M.G.; Nasser Singab, A.; Chaubey, K.K.; Bachheti, R.K. Anti-inflammatory activity of phytochemicals from medicinal plants and their nanoparticles: A review. Curr. Res. Biotechnol. 2023, 6, 100152. [Google Scholar] [CrossRef]
- Mariano, A.; Bigioni, I.; Misiti, F.; Fattorini, L.; Scotto d’Abusco, A.; Rodio, A. The Nutraceuticals as Modern Key to Achieve Erythrocyte Oxidative Stress Fighting in Osteoarthritis. Curr. Issues Mol. Biol. 2022, 44, 3481–3495. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Yang, X.; Wei, J.-R.; Chen, N.-M.; Xu, J.-P.; Bi, Y.-Q.; Yang, M.; Gong, X.; Li, Z.-Y.; Ren, K.; et al. Ethnopharmacology, Phytochemistry, Pharmacology, Toxicology and Clinical Applications of Radix Astragali. Chin. J. Integr. Med. 2021, 27, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Z.; Zhang, Z.; Cao, H.; Kong, L.; Ma, W.; Ren, W. A review of the botany, phytochemistry, traditional uses, pharmacology, toxicology, and quality control of the Astragalus memeranaceus. Front. Pharmacol. 2023, 14, 1242318. [Google Scholar] [CrossRef]
- Yao, J.; Peng, T.; Shao, C.; Liu, Y.; Lin, H.; Liu, Y. The Antioxidant Action of Astragali radix: Its Active Components and Molecular Basis. Molecules 2024, 29, 1691. [Google Scholar] [CrossRef]
- Wang, Q.; Zhen, W.; Lippi, G.; Liu, Q. The effect of Astragali Radix-Radix Angelica Sinensis on acute kidney injury: A network pharmacology and molecular docking study. Transl. Androl. Urol. 2024, 13, 91–103. [Google Scholar] [CrossRef]
- Huang, L.; He, C.; Ma, P.; Xiao, P. Strategic Thinking on the Development of Food-Medicine Industry. Chinese J. Eng. Sci. 2022, 24, 81. [Google Scholar] [CrossRef]
- Ghabeshi, S.; Mousavizadeh, L.; Ghasemi, S. Enhancing the Antiviral Potential and Anti-inflammatory Properties of Astragalus membranaceus: A Comprehensive Review. Antiinflamm. Antiallergy. Agents Med. Chem. 2023, 22, 211–219. [Google Scholar] [CrossRef]
- Block, K.I.; Mead, M.N. Immune system effects of echinacea, ginseng, and astragalus: A review. Integr. Cancer Ther. 2003, 2, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Bival Štefan, M. Astragalus membranaceus, Nigella sativa, and Perilla frutescens as Immunomodulators—Molecular Mechanisms and Clinical Effectiveness in Allergic Diseases. Curr. Issues Mol. Biol. 2024, 46, 9016–9032. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Zhao, C.J.; Wang, W.; Zhao, B.S.; Li, J.; Efferth, T. Preparation and antioxidant activity of Radix Astragali residues extracts rich in calycosin and formononetin. Biochem. Eng. J. 2011, 56, 84–93. [Google Scholar] [CrossRef]
- Xiang, L.; Zhang, Q.Y.; Zhao, Q.M.; Qin, L.P.; Gong, W. Research progress on chemical constituents, pharmacological effects and clinical applications of Astragali Radix-Angelicae Sinensis Radix. Chinese Tradit. Herb. Drugs 2022, 53, 2196–2213. [Google Scholar] [CrossRef]
- Cao, L.; Wang, M.; Zhao, J.; Peng, L.-H.; Cheng, J.-L.; Qiu, S.; Khan, I.A.; Li, X.-C. Comparative analysis of chemical profiles of Radix Astragali between ultrafine granular powder and sliced traditional material. Med. Plant Biol. 2022, 1, 4. [Google Scholar] [CrossRef]
- Adesso, S.; Russo, R.; Quaroni, A.; Autore, G.; Marzocco, S. Astragalus membranaceus extract attenuates inflammation and oxidative stress in intestinal epithelial cells via NF-κB activation and Nrf2 response. Int. J. Mol. Sci. 2018, 19, 800. [Google Scholar] [CrossRef]
- D’Avino, D.; Cerqua, I.; Ullah, H.; Spinelli, M.; Di Matteo, R.; Granato, E.; Capasso, R.; Maruccio, L.; Ialenti, A.; Daglia, M.; et al. Beneficial Effects of Astragalus membranaceus (Fisch.) Bunge Extract in Controlling Inflammatory Response and Preventing Asthma Features. Int. J. Mol. Sci. 2023, 24, 10954. [Google Scholar] [CrossRef]
- Bunddulam, P.; Nakamura, M.; Zorig, A.; Hinata, Y.; Takasugi, M.; Feng, C.-H.; Sato, T.; Arai, H. Effects of Astragalus membranaceus Leaf Extract on Allergic Inflammation in Immune Cell Lines. Prev. Nutr. Food Sci. 2025, 30, 68–80. [Google Scholar] [CrossRef]
- Durazzo, A.; Nazhand, A.; Lucarini, M.; Silva, A.M.; Souto, S.B.; Guerra, F.; Severino, P.; Zaccardelli, M.; Souto, E.B.; Santini, A. Astragalus (Astragalus membranaceus Bunge): Botanical, geographical, and historical aspects to pharmaceutical components and beneficial role. Rend. Lincei. Sci. Fis. Nat. 2021, 32, 625–642. [Google Scholar] [CrossRef]
- Ny, V.; Houška, M.; Pavela, R.; Tříska, J. Potential benefits of incorporating Astragalus membranaceus into the diet of people undergoing disease treatment: An overview. J. Funct. Foods 2021, 77, 104339. [Google Scholar] [CrossRef]
- Chau, C.-F.; Wu, S.-H. The development of regulations of Chinese herbal medicines for both medicinal and food uses. Trends Food Sci. Technol. 2006, 17, 313–323. [Google Scholar] [CrossRef]
- Santoro, V.; Parisi, V.; D’Ambola, M.; Sinisgalli, C.; Monné, M.; Milella, L.; Russo, R.; Severino, L.; Braca, A.; Tommasi, N. De Chemical Profiling of Astragalus membranaceus Roots (Fish.) Bunge Herbal Preparation and Evaluation of Its Bioactivity. Nat. Prod. Commun. 2020, 15, 1934578X2092415. [Google Scholar] [CrossRef]
- Borowicz, K.K.; Jach, M.E. Astragalus membranaceus—Can It Delay Cellular Aging? Nutrients 2025, 17, 1299. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.-B.; Ko, J.K. Astragalus membranaceus: A Review of its Protection Against Inflammation and Gastrointestinal Cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Anderson, D.D.; Brown, T.D.; Tochigi, Y.; Martin, J.A. The Roles of Mechanical Stresses in the Pathogenesis of Osteoarthritis: Implications for Treatment of Joint Injuries. Cartilage 2013, 4, 286–294. [Google Scholar] [CrossRef]
- Islam, S.; Kermode, T.; Sultana, D.; Moskowitz, R.W.; Mukhtar, H.; Malemud, C.J.; Goldberg, V.M.; Haqqi, T.M. Expression profile of protein tyrosine kinase genes in human osteoarthritis chondrocytes. Osteoarthr. Cartil. 2001, 9, 684–693. [Google Scholar] [CrossRef]
- Barnes, T.C.; Anderson, M.E.; Moots, R.J. The many faces of interleukin-6: The role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int. J. Rheumatol. 2011, 2011, 721608. [Google Scholar] [CrossRef]
- Forcina, L.; Franceschi, C.; Musarò, A. The hormetic and hermetic role of IL-6. Ageing Res. Rev. 2022, 80, 101697. [Google Scholar] [CrossRef]
- Jarlborg, M.; Gabay, C. Systemic effects of IL-6 blockade in rheumatoid arthritis beyond the joints. Cytokine 2022, 149, 155742. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Hoffman, B.E.; Newman-Tarr, T.M.; Gibbard, A.; Wang, S.; Hanning, C.; Pratta, M.A.; Boyle, R.J.; Kumar, S.; Majumdar, M.K. Development and characterization of a human articular cartilage-derived chondrocyte cell line that retains chondrocyte phenotype. J. Cell. Physiol. 2010, 222, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.J.; Yu, W.B.; Luo, W.; Gao, S.G.; Li, Y.S.; Lei, G.H. Effect of osteopontin on TIMP-1 and TIMP-2 mRNA in chondrocytes of human knee osteoarthritis in vitro. Exp. Ther. Med. 2014, 8, 391–394. [Google Scholar] [CrossRef]
- Mengshol, J.A.; Vincenti, M.P.; Coon, C.I.; Barchowsky, A.; Brinckerhoff, C.E. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor κB: Differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000, 43, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Favero, M.; Belluzzi, E.; Trisolino, G.; Goldring, M.B.; Goldring, S.R.; Cigolotti, A.; Pozzuoli, A.; Ruggieri, P.; Ramonda, R.; Grigolo, B.; et al. Inflammatory molecules produced by meniscus and synovium in early and end-stage osteoarthritis: A coculture study. J. Cell. Physiol. 2019, 234, 11176–11187. [Google Scholar] [CrossRef]
- Ra, H.J.; Parks, W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007, 26, 587–596. [Google Scholar] [CrossRef]
- Zhong, S.; Khalil, R.A. A Disintegrin and Metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) family in vascular biology and disease. Biochem. Pharmacol. 2019, 164, 188–204. [Google Scholar] [CrossRef]
- Wojtowicz-Praga, S.M.; Dickson, R.B.; Hawkins, M.J. Matrix metalloproteinase inhibitors. Investig. New Drugs 1997, 15, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Knäuper, V.; López-Otin, C.; Smith, B.; Knight, G.; Murphy, G. Biochemical characterization of human collagenase-3. J. Biol. Chem. 1996, 271, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Mengshol, J.A.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases as therapeutic targets in arthritic diseases: Bull’s-eye or missing the mark? Arthritis Rheum. 2002, 46, 13–20. [Google Scholar]
- Lai, P.K.; Chan, J.Y.; Wu, S.; Cheng, L.; Ho, G.K.; Lau, C.; Kennelly, E.J.; Leung, P.; Fung, K.; Lau, C.B. Anti-inflammatory Activities of an Active Fraction Isolated from the root of Astragalus membranaceus in RAW 264.7 Macrophages. Phyther. Res. 2014, 28, 395–404. [Google Scholar] [CrossRef]
- Park, H.M.; Lee, J.Y.; Kim, M.Y.; Kang, C.-H.; Hwang, H.S. Anti-Oxidative and Anti-Inflammatory Activities of Astragalus membranaceus Fermented by Lactiplantibacillus plantarum on LPS-Induced RAW 264.7 Cells. Fermentation 2021, 7, 252. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Noh, H.-J.; Choi, J.; Lee, K.-H.; Lee, M.-H.; Lee, J.-H.; Hong, Y.; Lee, S.-E.; Kim, S.-Y.; Kim, G.-S. Anti-Inflammatory Cycloartane-Type Saponins of Astragalus membranaceus. Molecules 2013, 18, 3725–3732. [Google Scholar] [CrossRef]
- Wang, D.; Zhuang, Y.; Tian, Y.; Thomas, G.N.; Ying, M.; Tomlinson, B. Study of the Effects of Total Flavonoids of Astragalus on Atherosclerosis Formation and Potential Mechanisms. Oxid. Med. Cell. Longev. 2012, 2012, 282383. [Google Scholar] [CrossRef]
- Agyemang, K.; Han, L.; Liu, E.; Zhang, Y.; Wang, T.; Gao, X. Recent Advances in Astragalus membranaceus Anti-Diabetic Research: Pharmacological Effects of Its Phytochemical Constituents. Evidence-Based Complement. Altern. Med. 2013, 2013, 654643. [Google Scholar] [CrossRef]
- Liacini, A.; Sylvester, J.; Li, W.Q.; Huang, W.; Dehnade, F.; Ahmad, M.; Zafarullah, M. Induction of matrix metalloproteinase-13 gene expression by TNF-α is mediated by MAP kinases, AP-1, and NF-κB transcription factors in articular chondrocytes. Exp. Cell Res. 2003, 288, 208–217. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, Q.; Rajasekaran, S.; Wu, R. MMP3 at the crossroads: Linking molecular pathways to disease diagnosis and therapy. Pharmacol. Res. 2025, 216, 107750. [Google Scholar] [CrossRef]
- Van Hove, I.; Lemmens, K.; Van de Velde, S.; Verslegers, M.; Moons, L. Matrix metalloproteinase-3 in the central nervous system: A look on the bright side. J. Neurochem. 2012, 123, 203–216. [Google Scholar] [CrossRef]
- Batool, A.; Vaithilingam, R.D.; Mohamad Hassan, N.H.; Safii, S.H.; Saub, R. Evaluating the potential of matrix metalloproteinase as a diagnostic biomarker in rheumatoid arthritis and periodontitis: A systematic review and meta-analysis. Medicine 2023, 102, e35340. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Patricia, H.R.; Timo, S.; Claudia, B.; Marcela, H. Matrix metalloproteinases as regulators of periodontal inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Bay-Jensen, A.-C.; Karsdal, M.A.; Siebuhr, A.S.; Zheng, Q.; Maksymowych, W.P.; Christiansen, T.G.; Henriksen, K. The active form of MMP-3 is a marker of synovial inflammation and cartilage turnover in inflammatory joint diseases. BMC Musculoskelet. Disord. 2014, 15, 93. [Google Scholar] [CrossRef]
- Reboul, P.; Pelletier, J.P.; Tardif, G.; Cloutier, J.M.; Martel-Pelletier, J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes: A role in osteoarthritis. J. Clin. Investig. 1996, 97, 2011–2019. [Google Scholar] [CrossRef]
- Mitchell, P.G.; Magna, H.A.; Reeves, L.M.; Lopresti-Morrow, L.L.; Yocum, S.A.; Rosner, P.J.; Geoghegan, K.F.; Hambor, J.E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Investig. 1996, 97, 761–768. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157. [Google Scholar]
- Santamaria, S.; Cuffaro, D.; Nuti, E.; Ciccone, L.; Tuccinardi, T.; Liva, F.; D’Andrea, F.; de Groot, R.; Rossello, A.; Ahnström, J. Exosite inhibition of ADAMTS-5 by a glycoconjugated arylsulfonamide. Sci. Rep. 2021, 11, 949. [Google Scholar] [CrossRef]
- Matsumoto, K.; Shionyu, M.; Go, M.; Shimizu, K.; Shinomura, T.; Kimata, K.; Watanabe, H. Distinct Interaction of Versican/PG-M with Hyaluronan and Link Protein. J. Biol. Chem. 2003, 278, 41205–41212. [Google Scholar] [CrossRef] [PubMed]
- Pratta, M.A.; Yao, W.; Decicco, C.; Tortorella, M.D.; Liu, R.Q.; Copeland, R.A.; Magolda, R.; Newton, R.C.; Trzaskos, J.M.; Arner, E.C. Aggrecan Protects Cartilage Collagen from Proteolytic Cleavage. J. Biol. Chem. 2003, 278, 45539–45545. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lin, J.; Zhao, S.; Wu, J.; Jin, Y.; Yu, L.; Wu, N.; Wu, Z.; Wang, Y.; Lin, M. ADAMTS5 in Osteoarthritis: Biological Functions, Regulatory Network, and Potential Targeting Therapies. Front. Mol. Biosci. 2021, 8, 703110. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).