Characterization of Netrin-1 and Its Receptors UNC5B and Neogenin-1 in a Rat Rotator Cuff Tear Model: Associations with Inflammatory Mediators and Neurite Extension
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Rat Rotator Cuff Tear Model
2.3. Quantitative Real-Time PCR
2.4. Isolation and Culture of Rotator Cuff–Derived Tenocytes
2.5. Effect of Netrin-1 on Sensory Neurons
3. Results
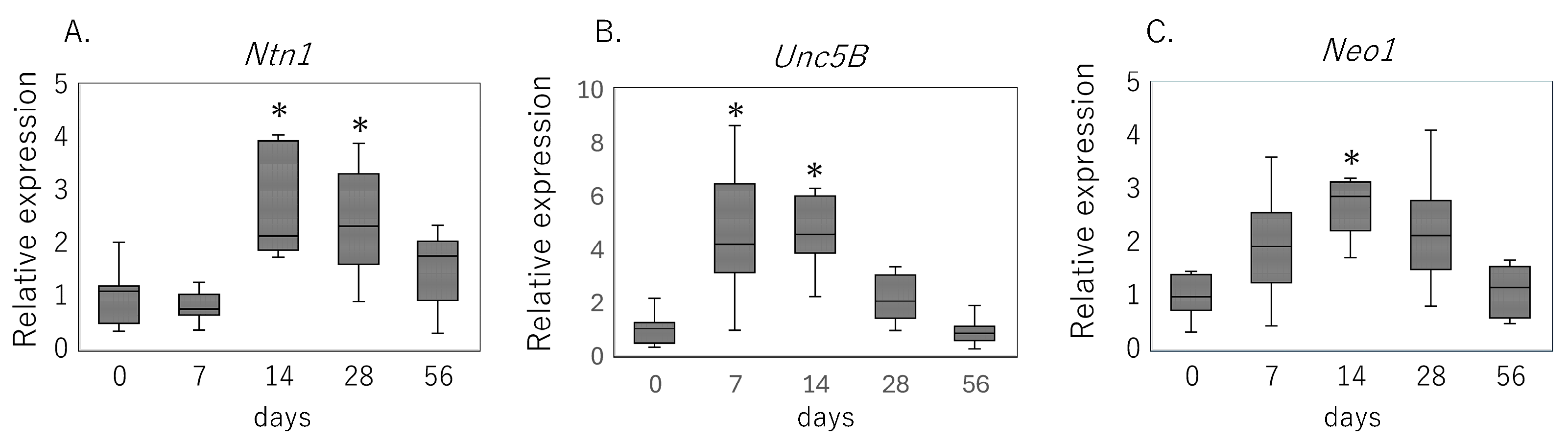
3.1. Expression of Netrin-1 and Its Receptors Following Rotator Cuff Injury
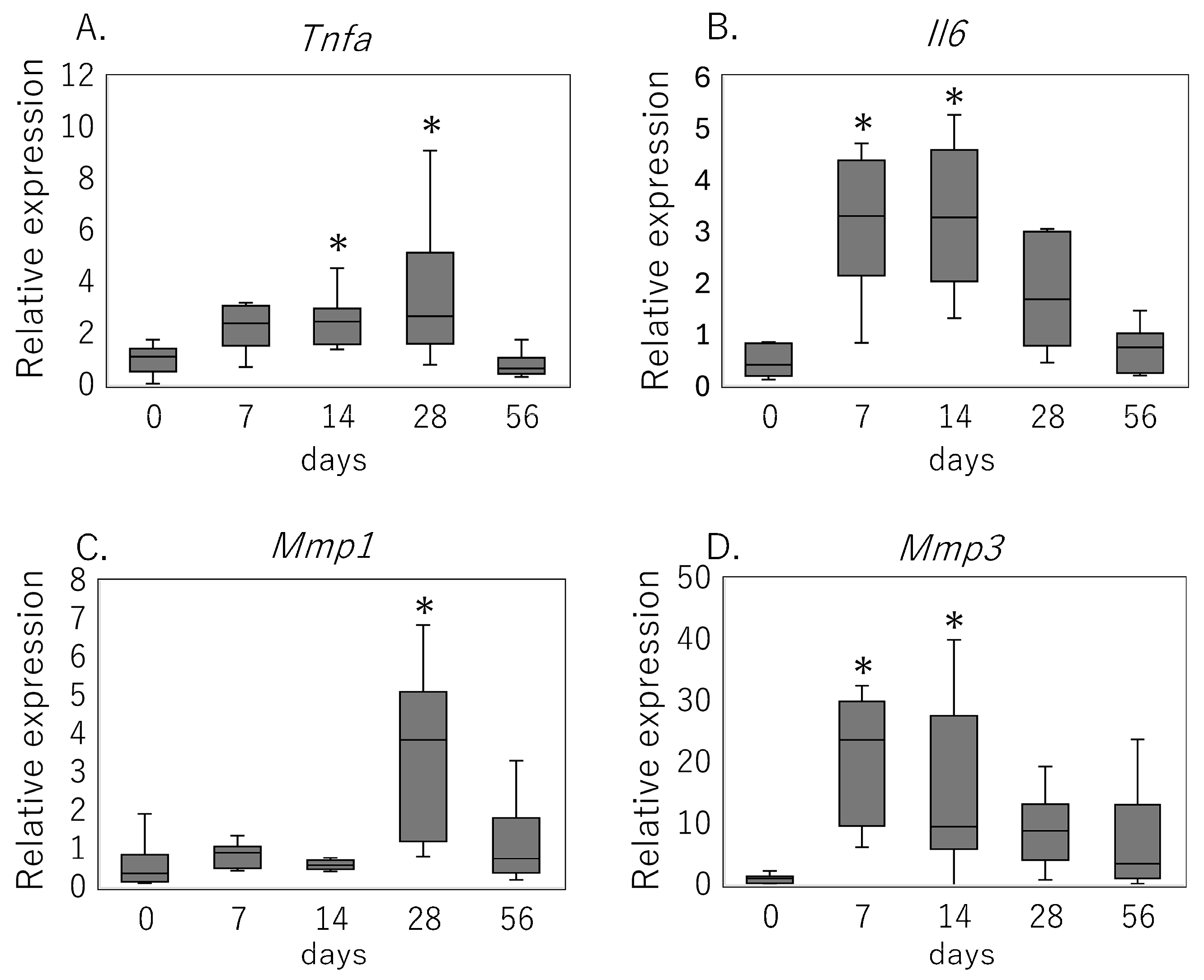
3.2. Inflammatory Cytokine and MMP Expression Following Rotator Cuff Injury
3.3. Netrin-1–Induced Expression of Cytokines and MMPs in Primary Tenocytes
3.4. Effect of Netrin-1 on Neurite Outgrowth in Human iPSC-Derived Sensory Neurons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IL-6 | Interleukin-6 |
| MMP | Matrix metalloproteinases |
| TNF-α | Tumor necrosis factor--α |
References
- Tempelhof, S.; Rupp, S.; Seil, R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J. Shoulder Elbow Surg. 1999, 8, 296–299. [Google Scholar] [CrossRef]
- Altamimi, T.A.; Alkathami, A.A.; Al-Awn, R.M.M.; Alkhaldi, M.H.E.; Alhudaithi, M.H.M.; Alqahtani, A.A.; Alzahrani, A.A.S.; Aladwani, S.S.M.; Abdulrahman, A.F.; Almutawah, A.N.A. A Narrative Review of Rotator Cuff Tear Management: Surgery Versus Conservative Treatment. Cureus 2024, 16, e74988. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Facchinetti, G.; Marchetti, A.; Candela, V.; Risi Ambrogioni, L.; Faldetta, A.; De Marinis, M.G.; Denaro, V. Sleep Disturbance and Rotator Cuff Tears: A Systematic Review. Medicina 2019, 55, 453. [Google Scholar] [CrossRef]
- HajAssaad, A.; Willacy, R.; Wilson, R. A Systematic Review of the Histological and Molecular Changes in the Subacromial Bursa in Rotator Cuff Disease. J. Surg. Orthop. Adv. 2020, 29, 1–4. [Google Scholar]
- Voloshin, I.; Gelinas, J.; Maloney, M.D.; O’Keefe, R.J.; Bigliani, L.U.; Blaine, T.A. Proinflammatory cytokines and metalloproteases are expressed in the subacromial bursa in patients with rotator cuff disease. Arthroscopy 2005, 21, 1076.e1–1076.e9. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Ochiai, N.; Kenmoku, T.; Ohtori, S.; Sasho, T.; Miyagi, M.; Ishikawa, T.; Kamoda, H.; Orita, S.; Sasaki, Y.; et al. Assessment of pain-related behavior and pro-inflammatory cytokine levels in the rat rotator cuff tear model. J. Orthop. Res. 2014, 32, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Nagura, N.; Kenmoku, T.; Uchida, K.; Nakawaki, M.; Inoue, G.; Takaso, M. Nerve growth factor continuously elevates in a rat rotator cuff tear model. J. Shoulder Elbow Surg. 2019, 28, 143–148. [Google Scholar] [CrossRef]
- Nagura, N.; Uchida, K.; Kenmoku, T.; Inoue, G.; Nakawaki, M.; Miyagi, M.; Takaso, M. IL-1beta mediates NGF and COX-2 expression through transforming growth factor-activating kinase 1 in subacromial bursa cells derived from rotator cuff tear patients. J. Orthop. Sci. 2019, 24, 925–929. [Google Scholar] [CrossRef]
- Castagna, A.; Cesari, E.; Gigante, A.; Conti, M.; Garofalo, R. Metalloproteases and their inhibitors are altered in both torn and intact rotator cuff tendons. Musculoskelet. Surg. 2013, 97 (Suppl. 1), 39–47. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, A.P.; Debs, L.H.; Nguyen, D.; Patel, K.; Grati, M.; Mittal, J.; Yan, D.; Chapagain, P.; Liu, X.Z. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J. Cell Physiol. 2016, 231, 2599–2621. [Google Scholar] [CrossRef]
- Lakemeier, S.; Braun, J.; Efe, T.; Foelsch, C.; Archontidou-Aprin, E.; Fuchs-Winkelmann, S.; Paletta, J.R.; Schofer, M.D. Expression of matrix metalloproteinases 1, 3, and 9 in differing extents of tendon retraction in the torn rotator cuff. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Selman, M. MMP-1: The elder of the family. Int. J. Biochem. Cell Biol. 2005, 37, 283–288. [Google Scholar] [CrossRef]
- Garofalo, R.; Cesari, E.; Vinci, E.; Castagna, A. Role of metalloproteinases in rotator cuff tear. Sports Med. Arthrosc. Rev. 2011, 19, 207–212. [Google Scholar] [CrossRef]
- Nabeel Mustafa, A.; Salih Mahdi, M.; Ballal, S.; Chahar, M.; Verma, R.; Ali Al-Nuaimi, A.M.; Kumar, M.R.; Kadhim, A.A.-H.R.; Adil, M.; Jasem Jawad, M. Netrin-1: Key insights in neural development and disorders. Tissue Cell 2025, 93, 102678. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, Y.; Wang, W.; Li, X.; Wang, K.; You, X.; Wu, J. Netrin-1 Role in Nociceptive Neuron Sprouting through Activation of DCC Signaling in a Rat Model of Bone Cancer Pain. J. Integr. Neurosci. 2024, 23, 47. [Google Scholar] [CrossRef]
- Ly, N.P.; Komatsuzaki, K.; Fraser, I.P.; Tseng, A.A.; Prodhan, P.; Moore, K.J.; Kinane, T.B. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 14729–14734. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, M.; Cyr, Y.; Newman, A.A.C.; Schreyer, K.; Barcia Duran, J.G.; Sharma, M.; Bozal, F.K.; Gourvest, M.; La Forest, M.; Afonso, M.S.; et al. Targeting Unc5b in macrophages drives atherosclerosis regression and pro-resolving immune cell function. Proc. Natl. Acad. Sci. USA 2024, 121, e2412690121. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.L.; Zhu, J.W.; Gao, X.M. Netrin-1 promotes the vasculogenic capacity of human adipose-derived stem cells. Cell Tissue Bank. 2023, 24, 357–367. [Google Scholar] [CrossRef]
- Tsukada, A.; Uekusa, Y.; Ohta, E.; Hattori, A.; Mukai, M.; Iwase, D.; Aikawa, J.; Ohashi, Y.; Inoue, G.; Takaso, M.; et al. Association Between Synovial NTN4 Expression and Pain Scores, and Its Effects on Fibroblasts and Sensory Neurons in End-Stage Knee Osteoarthritis. Cells 2025, 14, 395. [Google Scholar] [CrossRef]
- Tadagavadi, R.K.; Wang, W.; Ramesh, G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J. Immunol. 2010, 185, 3750–3758. [Google Scholar] [CrossRef]
- Schlegel, M.; Korner, A.; Kaussen, T.; Knausberg, U.; Gerber, C.; Hansmann, G.; Jonasdottir, H.S.; Giera, M.; Mirakaj, V. Inhibition of neogenin fosters resolution of inflammation and tissue regeneration. J. Clin. Investig. 2018, 128, 4711–4726. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.M.; Chen, C.T.; Shindle, M.K.; Cordasco, F.A.; Rodeo, S.A.; Warren, R.F. Failed healing of rotator cuff repair correlates with altered collagenase and gelatinase in supraspinatus and subscapularis tendons. Am. J. Sports Med. 2012, 40, 1993–2001. [Google Scholar] [CrossRef]
- Peng, L.; Bin, J.; Ou, Y.C.; Zhu, L.X.; Lu, J.P. Lack of association between matrix metalloproteinase-1 gene rs1799750 polymorphism and osteoarthritis susceptibility: A meta-analysis. Biosci. Rep. 2019, 39, BSR20181960. [Google Scholar] [CrossRef]
- Assuncao, J.H.; Godoy-Santos, A.L.; Dos Santos, M.; Malavolta, E.A.; Gracitelli, M.E.C.; Ferreira Neto, A.A. Matrix Metalloproteases 1 and 3 Promoter Gene Polymorphism Is Associated With Rotator Cuff Tear. Clin. Orthop. Relat. Res. 2017, 475, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Konig, K.; Gatidou, D.; Granja, T.; Meier, J.; Rosenberger, P.; Mirakaj, V. The axonal guidance receptor neogenin promotes acute inflammation. PLoS ONE 2012, 7, e32145. [Google Scholar] [CrossRef]
- Neumann, H.; Schweigreiter, R.; Yamashita, T.; Rosenkranz, K.; Wekerle, H.; Barde, Y.A. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J. Neurosci. 2002, 22, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Davis, D.A.; Hasheminassab, S.; Sioutas, C.; Morgan, T.E.; Finch, C.E. Urban traffic-derived nanoparticulate matter reduces neurite outgrowth via TNFalpha in vitro. J. Neuroinflamm. 2016, 13, 19. [Google Scholar] [CrossRef]
- Maruyama, K.; Takemura, N.; Martino, M.M.; Kondo, T.; Akira, S. Netrins as prophylactic targets in skeletal diseases: A double-edged sword? Pharmacol. Res. 2017, 122, 46–52. [Google Scholar] [CrossRef]
- Enoki, Y.; Sato, T.; Tanaka, S.; Iwata, T.; Usui, M.; Takeda, S.; Kokabu, S.; Matsumoto, M.; Okubo, M.; Nakashima, K.; et al. Netrin-4 derived from murine vascular endothelial cells inhibits osteoclast differentiation in vitro and prevents bone loss in vivo. FEBS Lett. 2014, 588, 2262–2269. [Google Scholar] [CrossRef]
- Mirakaj, V.; Dalli, J.; Granja, T.; Rosenberger, P.; Serhan, C.N. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J. Exp. Med. 2014, 211, 1037–1048. [Google Scholar] [CrossRef]
- Mediero, A.; Wilder, T.; Ramkhelawon, B.; Moore, K.J.; Cronstein, B.N. Netrin-1 and its receptor Unc5b are novel targets for the treatment of inflammatory arthritis. FASEB J. 2016, 30, 3835–3844. [Google Scholar] [CrossRef]
- Mao, X.; Xing, H.; Mao, A.; Jiang, H.; Cheng, L.; Liu, Y.; Quan, X.; Li, L. Netrin-1 attenuates cardiac ischemia reperfusion injury and generates alternatively activated macrophages. Inflammation 2014, 37, 573–580. [Google Scholar] [CrossRef]
- Mirakaj, V.; Gatidou, D.; Potzsch, C.; Konig, K.; Rosenberger, P. Netrin-1 signaling dampens inflammatory peritonitis. J. Immunol. 2011, 186, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Jayakumar, C.; Santhakumar, M.; Ramesh, G. Netrin-1 regulates colon-kidney cross talk through suppression of IL-6 function in a mouse model of DSS-colitis. Am. J. Physiol. Renal Physiol. 2013, 304, F1187–F1197. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.V.; Jayakumar, C.; Mohamed, R.; Dong, Z.; Ramesh, G. Netrin-1 regulates the inflammatory response of neutrophils and macrophages, and suppresses ischemic acute kidney injury by inhibiting COX-2-mediated PGE2 production. Kidney Int. 2013, 83, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.V.; Jayakumar, C.; Ramesh, G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. Am. J. Physiol. Renal Physiol. 2013, 304, F948–F957. [Google Scholar] [CrossRef]
- van Gils, J.M.; Derby, M.C.; Fernandes, L.R.; Ramkhelawon, B.; Ray, T.D.; Rayner, K.J.; Parathath, S.; Distel, E.; Feig, J.L.; Alvarez-Leite, J.I.; et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat. Immunol. 2012, 13, 136–143. [Google Scholar] [CrossRef]
- Paradisi, A.; Maisse, C.; Coissieux, M.M.; Gadot, N.; Lepinasse, F.; Delloye-Bourgeois, C.; Delcros, J.G.; Svrcek, M.; Neufert, C.; Flejou, J.F.; et al. Netrin-1 up-regulation in inflammatory bowel diseases is required for colorectal cancer progression. Proc. Natl. Acad. Sci. USA 2009, 106, 17146–17151. [Google Scholar] [CrossRef]


| Gene | Sequence | bp | |
|---|---|---|---|
| Ntn1 | sense | TTCTGAAGGCGGACAAAGCA | 86 |
| antisense | GCGTATACGACTTGTGCCCT | ||
| Unc5B | sense | GACTCCAAGAACTGCACCGA | 172 |
| antisense | TCCGGTACACGATCACTCCT | ||
| Neo1 | sense | GAACCCGAGGAAATGCTAGA | 105 |
| antisense | AGGAAGTCCTGAGGCAACAC | ||
| Tnfa | sense | CTCTTCTCATTCCCGCTCGT | 104 |
| antisense | GGGAGCCCATTTGGGAACTT | ||
| Il6 | sense | CCAGTTGCCTTCTTGGGACT | 224 |
| antisense | TCTGACAGTGCATCATCGCT | ||
| Mmp1 | sense | CTCACACATTCCCACCAGGC | 82 |
| antisense | TTGTCACTGTTGTCGGTCCA | ||
| Mmp3 | sense | TTGGCACAAAGGTGGATGCT | 103 |
| antisense | TGGGTCACTTTCCCTGCATT | ||
| Gapdh | sense | GACTCCAAGAACTGCACCGA | 172 |
| antisense | TCCGGTACACGATCACTCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, K.; Uchida, K.; Matsumoto, M.; Tazawa, R.; Ohta, E.; Hattori, A.; Kenmoku, T.; Ito, Y.; Uekusa, Y.; Inoue, G.; et al. Characterization of Netrin-1 and Its Receptors UNC5B and Neogenin-1 in a Rat Rotator Cuff Tear Model: Associations with Inflammatory Mediators and Neurite Extension. Curr. Issues Mol. Biol. 2025, 47, 511. https://doi.org/10.3390/cimb47070511
Inoue K, Uchida K, Matsumoto M, Tazawa R, Ohta E, Hattori A, Kenmoku T, Ito Y, Uekusa Y, Inoue G, et al. Characterization of Netrin-1 and Its Receptors UNC5B and Neogenin-1 in a Rat Rotator Cuff Tear Model: Associations with Inflammatory Mediators and Neurite Extension. Current Issues in Molecular Biology. 2025; 47(7):511. https://doi.org/10.3390/cimb47070511
Chicago/Turabian StyleInoue, Kosuke, Kentaro Uchida, Mitsuyoshi Matsumoto, Ryo Tazawa, Etsuro Ohta, Akito Hattori, Tomonori Kenmoku, Yuka Ito, Yui Uekusa, Gen Inoue, and et al. 2025. "Characterization of Netrin-1 and Its Receptors UNC5B and Neogenin-1 in a Rat Rotator Cuff Tear Model: Associations with Inflammatory Mediators and Neurite Extension" Current Issues in Molecular Biology 47, no. 7: 511. https://doi.org/10.3390/cimb47070511
APA StyleInoue, K., Uchida, K., Matsumoto, M., Tazawa, R., Ohta, E., Hattori, A., Kenmoku, T., Ito, Y., Uekusa, Y., Inoue, G., & Takaso, M. (2025). Characterization of Netrin-1 and Its Receptors UNC5B and Neogenin-1 in a Rat Rotator Cuff Tear Model: Associations with Inflammatory Mediators and Neurite Extension. Current Issues in Molecular Biology, 47(7), 511. https://doi.org/10.3390/cimb47070511








