1. Introduction
Severe trauma is still the leading cause of death in industrialized countries in patients under the age of 45. In its “Injury and Fatality Report”, the World Health Organization stated that, worldwide, the number of deaths from unintentional injuries was approximately three times higher than from HIV, tuberculosis, and malaria combined [
1].
The classic trimodal model of trauma mortality was described by Trunkey and coworkers as early as 1983 [
2]. In this model, 50–60% of the patients who die after trauma die within the first few minutes (immediate death). They therefore do not survive the so-called “first hit”, mostly a combination of the trauma itself and concomitant tissue disruption. The second peak of death occurs within the first 24 h after trauma and is considered an early death. Since early deaths regularly occur inside the hospital, they can possibly be reduced by optimal early trauma care. However, studies have shown that the proportion of early deaths following trauma has remained unchanged in recent years [
3]. The third group, that of late deaths, was originally classified as deaths that occurred more than one week after trauma, but more recent studies also include deaths happening after the first 24 h after trauma. Even though these late deaths could have been significantly reduced over the last decades, they still make up 10–20% of trauma deaths [
4,
5]. The main causes of late deaths are sepsis and multiple organ dysfunction syndrome, leading to multiple organ failure (MOF) [
6]. An important role in those cascades is played by post-traumatic coagulation disorders.
Trauma-induced coagulopathy, influenced by platelet function, is a critical part of the “lethal triad” in traumatic shock, alongside hypothermia and acidosis. Studies indicate that coagulopathy increases mortality risk significantly after trauma, underscoring the platelets’ influence on both immediate and long-term survival outcomes [
7,
8]. Standard tests, such as the prothrombin time or the activated partial thromboplastin time, provide important information and are part of routine clinical blood tests. However, a comprehensive assessment of coagulation dynamics, including information on clot formation, stability, and fibrinolysis, can only be carried out using more comprehensive testing methods such as thromboelastometry.
Following severe trauma, the innate immune system is rapidly activated. Circulating leukocytes, particularly neutrophils and monocytes, are rapidly mobilized and contribute to the early inflammatory response by releasing pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β [
9]. This immediate immune response plays a critical role in pathogen defense and tissue repair. However, an excessive or unbalanced activation may also promote systemic inflammation and contribute to post-traumatic complications. While the first hit normally leads to a limited activation of the systemic inflammatory response syndrome (SIRS) and the concomitant compensatory anti-inflammatory response syndrome (CARS), subsequent second hits can lead to excessive immune reactions and immunological imbalances. Typically, second hits are caused by operations, hypoxia, cardiovascular instability, and infections [
10,
11]. The immunologic reaction to the second hit consists of complex interactions between pro- and anti-inflammatory immune cells. Due to immune conditioning from previous exposure, exaggerated immune responses are more likely upon subsequent exposure. This imbalance of SIRS and CARS increases the susceptibility to organ failure or systemic infection. At present, the body’s immune response to trauma is not fully understood. There are no standardized methods to precisely monitor these immune reactions, nor are there established interventions targeting the immunologic pathways. Since the first hit in trauma can almost never be altered, focusing on second hits and the resulting immunologic reaction is essential for improving trauma care and reducing mortality in this field.
Therefore, investigating the molecular pathways has received increasing attention in recent years. Platelets have recently been ascribed an increasingly important role as immune mediators. Since it was discovered that platelets express all nine Toll-like receptors (TLRs) and other signaling surface proteins, like the tetraspanin family, it has been clear that platelets not only play a central role in hemostasis, but also work as active agents within the immune system. Upon activation, platelets release various pro-inflammatory cytokines, including IL-1β, TNF-α, IL-1, and IL-6, along with lipid mediators. These TLRs enable the platelets to recognize pathogen-associated molecular patterns, allowing them to bind and neutralize antigens either through encapsulation or by releasing antimicrobial peptides [
12]. In addition, platelets communicate with and influence other immune cells.
Following severe trauma, platelets assume a dual role. In addition to their primary function in clotting, they release both pro- and anti-inflammatory mediators, which significantly impact the immune environment [
13]. Preliminary studies by our research group have shown that platelet activity can directly influence CD4+ regulatory T cells (T
reg) [
14]. An imbalance in these responses may lead to complications such as sepsis or MOF.
One of the less extensively studied surface markers on platelets is the tetraspanin CD63. It is expressed on various cell types, including immune cells such as mast cells, dendritic cells, T
regs, and platelets. By clustering with other cell surface receptors, CD63 is involved in multiple physiological pathways of cell signaling, adhesion, and molecular trafficking. After trauma, CD63 can modulate inflammatory responses by regulating the activation, migration, and proliferation of immune cells to the injured tissue. Those processes are realized through the release of inflammatory markers and cytokines, such as TNF-alpha [
15].
On platelets, CD63 is located in the endosomal system of the alpha granula and on the cell surface [
16]. The surface expression is increased with platelet activation, enabling CD63 to be used as an activity marker of platelets [
17]. CD63 is involved in the signaling pathways that mediate platelet activation and contribute to their amplification process [
18]. However, the full extent of the involvement of the tetraspanin family after trauma is not fully understood, highlighting the need for further research in this field. Understanding the immunological activation of platelets and their concomitant hemostatic dysfunction in the setting of severe trauma is crucial for improving therapeutic strategies.
We hypothesized that the activation level of CD63 transmitters increases significantly after trauma and, at the same time, increases the activation and hemostatic power of platelets.
2. Materials and Methods
2.1. Study Population and Sampling Protocol
In this prospective, non-interventional trial, 20 severely injured patients were enrolled at the trauma room of a German Level I trauma center between February and September 2021. Enrollment was based on clinical presentation, injury pattern, and the need for interdisciplinary trauma care. Final study inclusion required a retrospectively calculated Injury Severity Score (ISS) of ≥16, in accordance with established criteria for severe trauma used by the TraumaRegister DGU®. Written informed consent was obtained from all patients or their legal representatives.
Patients were excluded when the time from trauma to presentation in the ER exceeded 12 h or when patients were under 18 years of age. Also, secondary transfers from other hospitals were excluded. Further exclusion criteria were pregnancy and incarceration. Enrollment also depended on the availability of the study team and capacity in the research laboratory. Pre-existing antithrombotic medication was not defined as an exclusion criterion, as reliable medication histories are often not available in acute emergency settings involving severely injured patients.
Nine sequential blood samples were taken and analyzed: in the trauma room (with a maximum of 60 min post-admission); at 6, 12, 24, 48, and 72 h; and 5, 7, and 10 days after trauma. The trauma-room samples were obtained prior to any transfusion. Blood was drawn and collected in commercially available monovettes (Sarstedt AG & Co., Nümbrecht, Germany) either laced with ethylenediaminetetraacetic acid for flow cytometry or citrate for thromboelastometry.
Clinical data on patients, such as age, gender, trauma mechanism, and the exact injury pattern with the severity of the injuries, classified according to the AIS, were collected. Additionally, the ISS was calculated. Data on the course of treatment, including the number of surgical interventions, time spent in the ICU, and mortality, were also noted.
2.2. CD63 Expression on Platelets
The MACSQuant
® 9 Analyzer from Miltenyi Biotec (Bergisch Gladbach, Germany) was used to determine the surface expression markers of platelets. The platelets were isolated, fixed, stained, and then measured by flow cytometry. For sample collection, the patients’ blood was drawn using the standard venipuncture technique or by laying central venous catheters and collected in a 2.9 mL Sarstedt S-Monovette
® with 3.2% citrate (Sarstedt AG & Co. KG, Nümbrecht, Germany). First, 1.5 mL of the blood sample was transferred to a 2 mL SafeSeal reaction tube (Sarstedt AG & Co. KG, Nümbrecht, Germany) and centrifuged at 500×
g and 21 °C for 10 min (Centrifuge 5415 R, Eppendorf SE, Hamburg, Germany) in an inclined suspension. The platelet-rich plasma (PRP) formed at the boundary zone between the centrifugate and serum was then pipetted off and transferred to another 2 mL SafeSeal reaction tube (Sarstedt AG & Co. KG, Nümbrecht, Germany). After renewed centrifugation at 200×
g and 21 °C for 10 min (Centrifuge 5415 R, Eppendorf SE, Hamburg, Germany), the supernatant was decanted, and the platelets were mixed with 620 µL of phosphate-buffered saline with 0.3% paraformaldehyde (PFA 0.3%, see
Table A1) for fixation. In each case, 100 µL was distributed in 6 wells of a 96-well round-bottom plate (Sarstedt AG & Co. KG, Nümbrecht, Germany) and incubated at room temperature under light protection for 30 min.
Meanwhile, the antibody mixture was prepared for staining. CD41 (anti-human, HIP8, PacificBlue
TM, BioLegend, San Diego, CA, USA) was used for identification, and CD63 (anti-human, H5C6, PE, BioLegend, San Diego, CA, USA), CD62P (anti-human, Psel.KO2.3, APC, eBioscience, San Diego, CA, USA), and TLR9 (anti-human, 5G5, FITC, abcam, Cambridge, UK) were used as activity markers and mixed together under light protection. The inclusion of CD62P and TLR9 antibodies in the staining panel reflects a standard protocol for platelet immunophenotyping, which was used by our study group in preliminary studies. These markers were not analyzed in the context of the present study but are reported in
Section 2 to ensure full transparency regarding the composition of the antibody mixture.
A total of 1.5 µL of each antibody was used, and the mixture was diluted with 60 µL of phosphate-buffered saline (PBS, see
Table A1), resulting in 66 µL of antibody mixture. With an additional 3 wells, each containing approximately 3 mL of platelets to be stained, the recommended antibody dosage of 1:50 was obtained in the test sample.
Once fixation was complete, the platelets were washed with 100 µL PBS, and the round-bottom plate was centrifuged at 500× g and 4 °C for 10 min (Centrifuge 5810 R, Eppendorf SE, Hamburg, Germany). The supernatant was then decanted, and the platelets were stained.
In three wells labeled “Thrombo +”, the platelets were resuspended and stained with 20 µL of the antibody mixture, and in the other three wells, labeled “Thrombo −”, the platelets were resuspended with 20 µL of PBS. Staining and subsequent incubation at 4 °C were carried out under light protection. After 45 min of staining, the staining was terminated by adding 200 µL of phosphate-buffered saline with bovine serum albumin (PBA, see
Table A1) per well and centrifugation at 500×
g and 4 °C for 10 min (Centrifuge 5810 R, Eppendorf SE, Hamburg, Germany). After removal of the supernatant, the samples were carefully resuspended in 100 µL PBS, and the round-bottom plate was stored on a Chill 96 Rack (Miltenyi Biotec, Bergisch Gladbach, Germany). The subsequent measurement of the samples with the MACSQuant
TM 9 Analyzer (Miltenyi Biotec, Bergisch Gladbach, Germany) was performed after calibration of the device with MACSQuant
TM Calibration Beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. The voltages listed in
Table A2 were determined using preliminary tests and set for measurement. Further settings were a low flow rate, a medium resuspension of the samples before uptake, and a washing step between each measurement. A total of 75 µL per well was measured at a rate of up to 10,000 events per second. This resulted in approximately 3 million recognized events per well. All measurements were performed as triplets. For statistical analyses, the mean values of those triplets were used.
2.3. Platelet Function
The blood remaining in the 2.9 mL Sarstedt S-Monovette
® with 3.2% citrate (Sarstedt AG & Co. KG, Nümbrecht, Germany) from the flow cytometric measurement of platelets was used to determine platelet function. It should be emphasized that the blood samples were analyzed within 10 min after collection. Platelet activation and hemostatic function were measured using ROTEM
® (Tem International GmbH, Munich, Germany). The ROTEM
® assay involves the continuous rotation of a pin immersed in the blood sample, allowing for the real-time measurement of clot formation and fibrinolysis parameters. Each blood sample was analyzed using the EX-TEM
®, IN-TEM
®, FIB-TEM
®, and NA-TEM
® pathways. The blood and coagulation activators used were warmed to 37 °C before the experiment. In the first step, the starting reagents EX-TEM
®, IN-TEM
®, and FIB-TEM
® (see
Table A3) were mixed with calcium (STAR-TEM
®, see
Table A3) in the prepared measuring cuvettes (Werfen GmbH, Munich, Germany). For the NA-TEM
® measurement, only the STAR-TEM
® reagent was added to the cuvette. The blood sample was then added, the plunger inserted into the cuvette, and the measurement recording started. The quantity of reagents and blood sample used is automatically determined by the electronic pipette of the ROTEM
® delta (Werfen GmbH, Munich, Germany). While additional ROTEM
® channels (IN-TEM
® and NA-TEM
®) were also recorded for completeness, the analysis focused exclusively on the comparison of EX-TEM
® and FIB-TEM
® to isolate the platelet-specific contribution to clot firmness [
19].
The ROTEM® parameters of clotting time (CT), clot-formation time, MCF, and lysis index were measured. CT refers to the time it takes for the blood to begin clot formation after the initiation of coagulation. CT is measured from the start of the analysis until a predetermined level of clot firmness is reached, which in the case of ROTEM® analysis is set at 20 mm. A prolonged CT may indicate deficiencies in clotting factors, impaired platelet function, or the presence of anticoagulant medications. Conversely, a shortened CT may suggest hypercoagulability or increased thrombin generation.
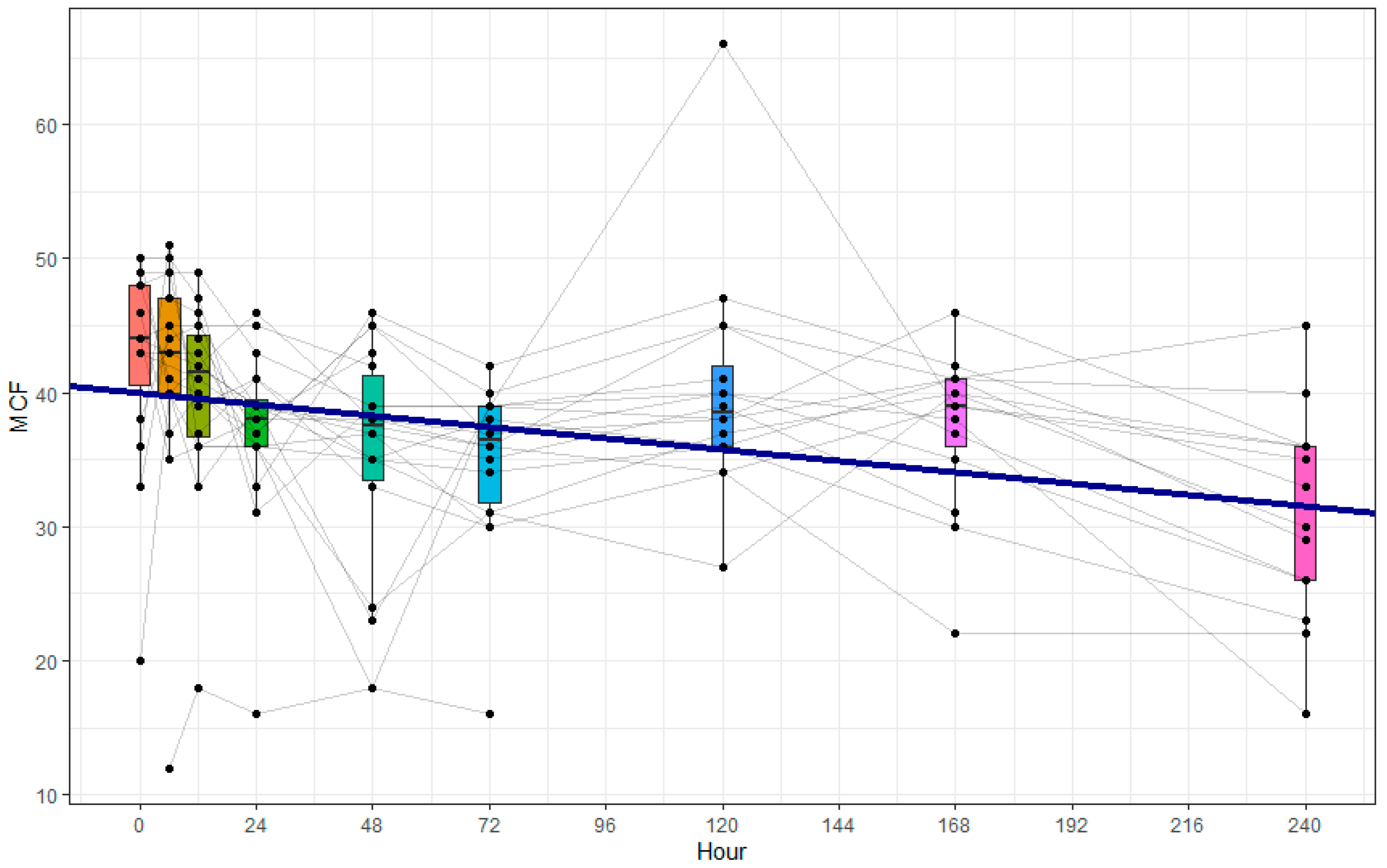
MFC represents the maximum strength or firmness of the blood clot formed during the coagulation process. It is determined by the maximum amplitude or elasticity of the clot measured on the ROTEM® curve. This point represents the peak of clot firmness reached during the coagulation process. The higher the MCF value, the stronger and more stable the clot is considered to be.
ROTEM® data were acquired and analyzed using the ROTEM® software (Version LibROTEM 20070213 Rawlog {Default-1000}) provided by the manufacturer. Clot formation curves were generated, and ROTEM® parameters were calculated automatically based on the curve characteristics.
2.4. Statistical Analysis
Data were analyzed using GraphPad Prism 8 (version 8.0.2.263, GraphPad Software Inc., Boston, MA, USA) and RStudio IDE (version 2024.12.1+563, Posit PBC, Boston, MA, USA). Patients’ characteristics were described using the mean and standard deviation (SD) for continuous variables. Absolute numbers and relative percentages were used for categorical variables. CD63 expression and platelet function were analyzed using linear mixed-effects model analysis (LMM), since this method accounts for both fixed and random effects in repeated-measures data and is robust to missing values. p-values <0.05 were considered statistically significant.
4. Discussion
Trauma-induced coagulopathy (TIC) is a well-documented phenomenon, characterized by a complex interplay among systemic inflammation, endothelial dysfunction, and platelet alterations [
7]. Coagulopathies are of particular relevance in the context of polytrauma, as they are associated with an increased need for blood products, higher rates of organ failure, and elevated overall mortality [
20]. The mean ISS in our study cohort was 28.1, with most of our patients suffering from multiple injuries. Head and chest injuries, which were among the most frequent injuries, are known to have significant effects on systemic inflammation and coagulation pathways [
21]. The high prevalence of extremity and pelvic injuries may further contribute to thromboinflammatory processes due to extensive soft tissue damage and prolonged immobilization. The observed study population is therefore well-suited to conduct observations on post-traumatic platelet activation and TIC.
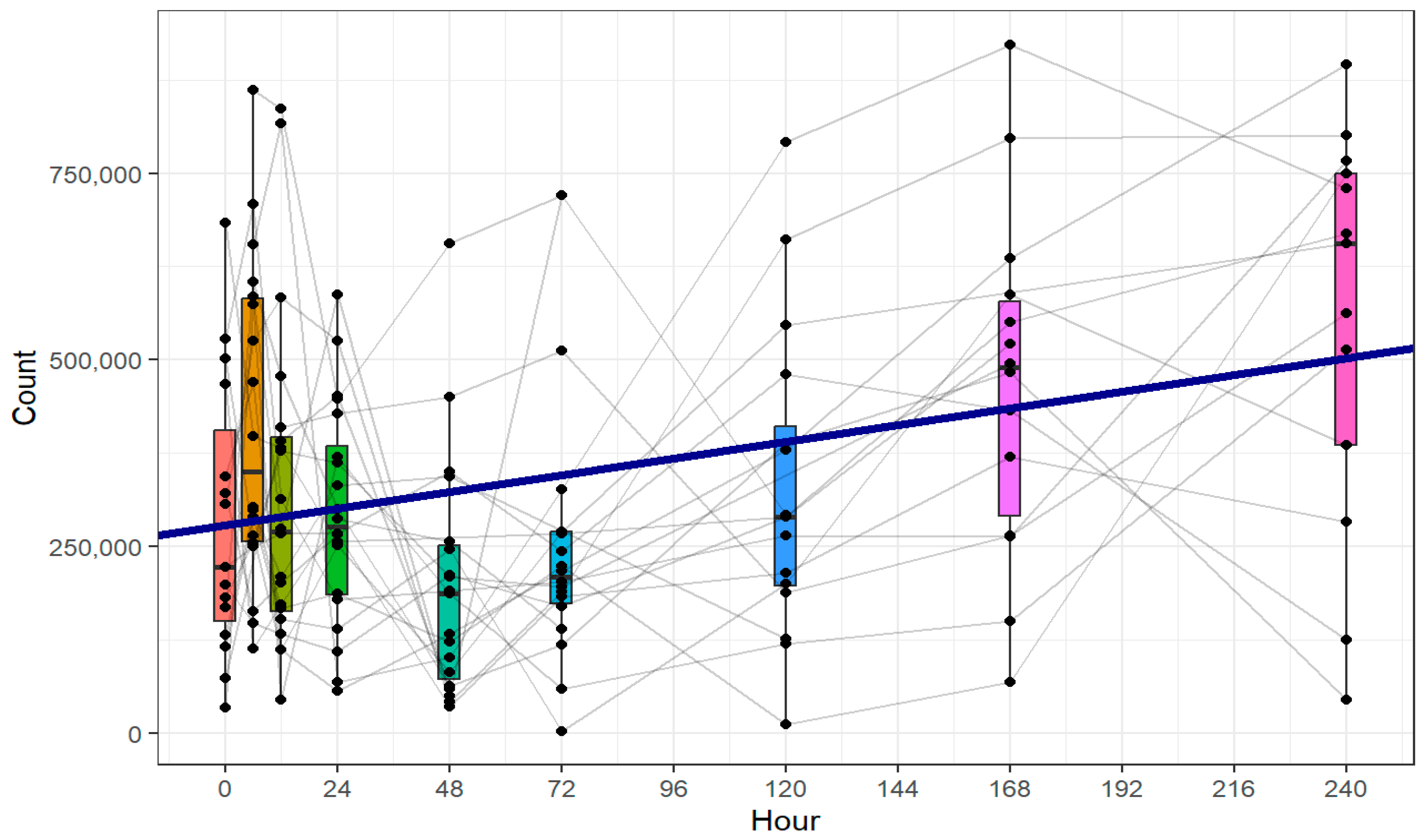
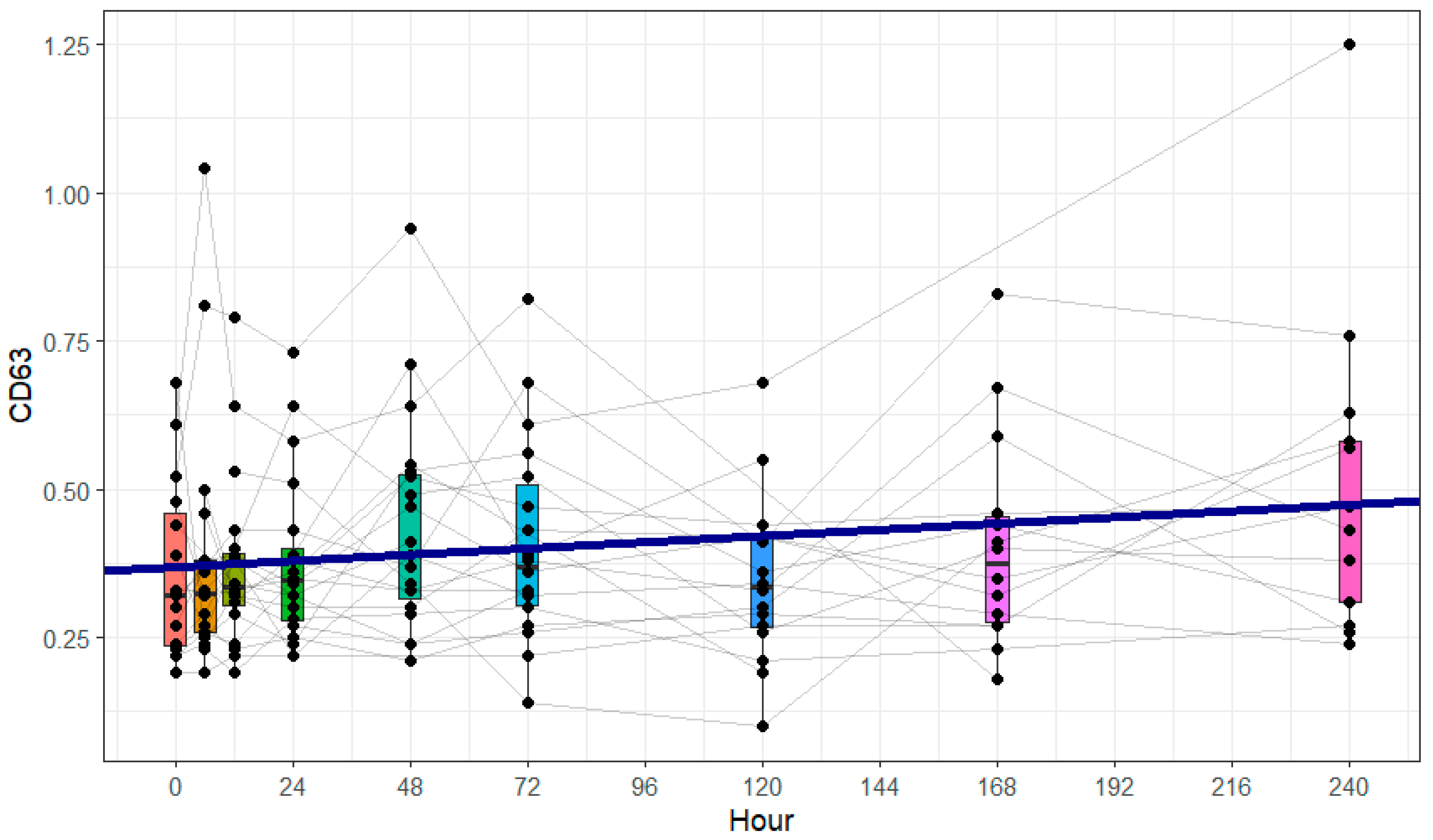
One central observation of our study was that increased CD63 expression, indicating immunological platelet activation, was paralleled by a decrease in clot firmness attributable to platelets, as assessed by ROTEM®. Furthermore, we observed a significant increase in the absolute platelet count.
Flow cytometry was used not only to determine platelet numbers but, more importantly, to assess immunological activation at the single-cell level. This method allowed us to directly evaluate the surface expression of CD63 on platelet populations, which would not have been possible using standard hematology analyzers. Although flow cytometric platelet counting may be affected by sample preparation, its ability to provide detailed functional phenotyping made it the method of choice for this study. We acknowledge that the EX-TEM
® minus FIB-TEM
® MCF difference is only an indirect estimate of platelet function and is subject to several confounding factors. It lacks diagnostic specificity and is not established as an outcome predictor. In a recent study, global coagulation parameters such as clotting times remained within normal ranges, while platelet dysfunction was clearly detectable only through targeted assessments. These findings emphasize the need for platelet-specific testing, such as the ROTEM
®-based differential approach used in our study, to accurately detect functional impairments after trauma [
22].
CD63 is a well-established marker of platelet activation. It is translocated to the platelet surface upon α-granule release and has been widely used in flow cytometric studies to quantify platelet degranulation. Its expression correlates with activation status in both experimental and clinical settings, and it plays a role in tetraspanin–integrin signaling complexes [
23,
24,
25]. Elevated CD63 expression after trauma indicates increased immunological platelet activation, consistent with the known systemic inflammatory response and thromboinflammatory processes [
17,
26]. This observation is in agreement with previous studies showing that trauma induces a hyperactive platelet phenotype [
27]. However, concurrent with the immunological activation, we observed a decrease in platelet coagulation function. The hypothesis of increased platelet coagulation activity after severe trauma must therefore be rejected. Our results emphasize the need to investigate the different platelet functions together in future studies in order to gain a more comprehensive understanding of post-traumatic platelet dysfunction.
Trauma-induced platelet dysfunction has been extensively described in the literature [
7,
28,
29,
30]. However, the exact mechanisms responsible for the observed decline in platelet hemostatic function remain unclear. Potential contributors include direct platelet exhaustion, interactions with leukocytes and the endothelium, and alterations in platelet receptor signaling [
31,
32,
33]. In this context, recent findings suggest that the trauma-induced loss of platelet surface receptors, particularly GPVI and GPIbα, may play a critical role in reducing platelet responsiveness during ongoing bleeding [
34]. Additionally, transcriptomic studies have identified trauma-associated changes in alternative RNA splicing within platelets, suggesting a possible molecular basis for impaired platelet function and TIC [
35]. Our findings expand on these observations by indicating a possible dissociation between immunological activation, reflected by increased CD63 expression, and hemostatic capacity, as measured by clot firmness.
An alternative explanation for the observed reduction in clot firmness could be platelet exhaustion, a phenomenon in which sustained activation leads to a functional decline over time. This concept has been described in inflammatory and thromboinflammatory settings, where hyperactivated platelets progressively lose their hemostatic competence despite ongoing stimulation [
30,
36,
37]. However, in our study, the decline in ROTEM
®-based platelet-dependent clot firmness occurred in parallel with the early increase in CD63 expression, not as a delayed consequence. This temporal pattern suggests that functional impairment and immunological activation may occur simultaneously rather than sequentially and may reflect distinct regulatory mechanisms following trauma.
In recent years, the interplay between inflammation and thrombosis has garnered increasing attention under the concepts of “thromboinflammation” and “immunothrombosis”. However, the precise underlying mechanisms remain an active area of research, and there is a lack of pharmacological approaches to address the observed complications effectively [
38]. To the best of our knowledge, this is the first prospective study to simultaneously examine both the immunological activation of platelets and their hemostatic capacity in severely injured patients. Previous research has either focused on platelet activation markers or functional deficits separately.
Our data indicate that patient age, sex, and ISS had no significant influence on platelet activation or function. This finding suggests that trauma severity alone does not dictate platelet behavior and that other factors, such as immune status, immunological priming, or endothelial interactions, may play a more critical role. This contrasts with some prior reports suggesting that age and sex influence coagulation responses post-trauma, warranting further investigation into potential underlying mechanisms [
39,
40].
The nature of this prospective, non-interventional study imposes limitations on the scope and interpretability of its findings.
The study population of 20 patients is relatively small, especially for conducting subgroup analyses. This limitation results from the study’s complex design and the rarity of the target population: patients with severe trauma. Subgroup analyses resulted in cohorts of partly fewer than 10 patients, rendering the absence of statistically significant differences in CD63 expression and platelet hemostatic function likely attributable to insufficient statistical power and data variability. To overcome this problem, patients’ characteristics were analyzed as covariables in the LMM. The LMM offers significant advantages in handling missing data, accounting for individual variability through random effects, and modeling within-subject correlations. Unlike traditional methods, it provides robust statistical power and allows for the inclusion of covariates, making it a valuable tool for analyzing complex longitudinal data, especially when the focus is on overall trends rather than specific time points, as pointed out in our study. However, the use of LMM for statistical analyses has limitations, as it does not compare discrete time points directly or conduct analyses on an individual-patient level. Instead, the LMM identifies overall trends across the dataset, often assuming linear change over time. Consequently, transient or non-linear temporal dynamics may not be accurately detected.
An additional limitation lies in the potential influence of pre-injury antithrombotic medication, which could not be systematically excluded. In the acute emergency setting of severe trauma, reliable medication histories are often unavailable at the time of admission, and pre-existing antithrombotic use may therefore have introduced variability into the observed platelet phenotypes. Additionally, while no patients received blood products prior to the initial blood sampling, several required transfusions during the subsequent course of treatment. These interventions may have influenced later platelet phenotypes and ROTEM® measurements and represent a potential confounding factor.
We acknowledge that ROTEM® is not a direct measure of platelet function in the classical sense. However, the differential approach using EX-TEM® and FIB-TEM® is well established in trauma research and allows for a valid approximation of the platelet contribution to clot firmness. While not providing mechanistic insights into platelet signaling, this method enables the objective tracking of temporal changes in platelet-dependent coagulation activity.
A formal correlation analysis between CD63 expression and platelet-specific clot firmness (MCF) was not performed in this study. As an exploratory pilot study in a rare and critically injured patient population, our primary aim was to describe temporal changes in immunological and hemostatic platelet behavior rather than to test predefined mechanistic hypotheses. The observed divergence between CD63 upregulation and declining MCF represents a hypothesis-generating finding that could inform the design of future studies. Investigating potential mechanistic links, such as direct correlation or causal relationships, between platelet activation and functional impairment will require larger, targeted cohorts with broader analytical designs.
Nonetheless, the primary objective of this pilot study was to explore cellular mechanisms following polytrauma. The study design is well-suited for this exploratory purpose and serves as a starting point for future research projects. This publication aims to offer initial insights and lay the groundwork for future studies, rather than establishing causal or mechanistic relationships.