Abstract
Vascular endothelial growth factor (VEGF) plays a crucial role in angiogenesis and placental development, which are vital for a healthy pregnancy. Preeclampsia (PE), a hypertension condition that can cause major difficulties for both the mother and the fetus, has been linked to VEGF gene polymorphisms in several studies. PE susceptibility has been associated with several VEGF polymorphisms, including VEGF −2578C/A, −634G/C, +936C/T, and +405G/C, with differing outcomes in various ethnicities. Some polymorphisms, like VEGF −2578C/A, are linked to the disease’s progression, whereas others, like VEGF +405G/C, may protect severe PE. The findings are still uncertain, though, with some studies reporting noteworthy outcomes and others finding no correlation. Further complicating our knowledge of VEGF’s role in PE is the possibility that the interaction between maternal and fetal VEGF polymorphisms may affect PE risk. Studies on environmental variables and placental and fetal VEGF gene polymorphisms point to a complicated interaction in influencing the severity and susceptibility of PE. The precise genetic processes behind PE are still unknown, despite the mounting evidence, necessitating additional research to confirm possible biomarkers and treatment targets. In at-risk pregnancies, a better understanding of the connection between VEGF polymorphisms and PE may help with risk assessment and management techniques.
1. Introduction
Globally, the maternal mortality ratio has dropped by about 34% in the last 20 years, according to the World Health Organization (WHO) [1]. Preeclampsia (PE), which affects 2 to 5% of pregnancies, is still a serious pregnancy condition that greatly increases maternal and perinatal morbidity and mortality [2]. New-onset hypertension (systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg) that manifests after the 20th week of pregnancy, frequently close to term, is what defines it as a hypertensive condition of pregnancy. A dipstick reading of ≥2+, a protein:creatinine ratio of ≥30 mg/mmol, or proteinuria (≥300 mg in a 24-h urine collection) are commonly used to make the diagnosis. Acute kidney injury, hepatic impairment (elevated aspartate aminotransferase/alanine aminotransferase), neurological symptoms (altered mental status, severe headaches, or vision disturbances), hematologic abnormalities (thrombocytopenia or hemolysis), and uteroplacental dysfunction—which can lead to fetal growth restriction, an abnormal umbilical artery Doppler waveform, or stillbirth—are some of the systemic maternal organ dysfunctions that PE may present within the absence of proteinuria [2,3].
Oliguria, upper abdominal pain, severe headaches, visual disturbances, or pulmonary edema are clinical manifestations of severe preeclampsia, which is defined as a systolic blood pressure of 160 mmHg and/or a diastolic blood pressure of 110 mmHg. These symptoms may progress to eclampsia, a potentially fatal condition marked by convulsions [4,5]. HELLP syndrome (Hemolysis, Elevated Liver Enzymes, and Low Platelet count) is a serious complication of preeclampsia (PE). PE can lead to HELLP syndrome or other severe consequences, often beginning with prenatal hypertension [6,7].
The precise pathophysiology of PE is still unclear [8]. It is generally agreed to be a two-stage condition, with poor placentation in the first stage and systemic disease symptoms in the second stage caused by extensive maternal vascular inflammation [9]. Numerous factors, including immunologic dysregulation, genetic susceptibility, and inadequate placentation, have been implicated. There is mounting evidence that both angiogenic and antiangiogenic elements are important in PE formation [10]. In particular, PE pregnancies have been linked to changes in the levels of circulating vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and soluble fms-like tyrosine kinase-1 (sFlt-1) [11,12].
In terms of structure, VEGF is a 40 kDa heterodimeric glycoprotein with a cystine-knot motif, which is distinguished by a particular configuration of disulfide linkages [13]. The VEGF family consists of placental growth factor (PlGF), VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E (viral VEGF), VEGF-F (snake venom), and other isoforms made by alternative exon splicing [14]. By attaching itself to the VEGFR-1, VEGFR-2, and VEGFR-3 receptors, VEGF produces its biological effects [15]. Vascular growth, endothelial integrity, and vascular permeability are all significantly impacted by VEGF, which is mostly expressed in placental syncytiotrophoblasts and invasive chorionic trophoblasts during pregnancy [16,17].
Several studies indicate that PE, especially in the third trimester, is linked to decreased maternal VEGF levels and elevated sFlt-1 levels [6,18,19]. The complexity of its action is highlighted by contradictory data that suggest increased VEGF levels may also play a role in the illness [20]. VEGF polymorphisms, or genetic variants in VEGF, have drawn attention as possible causes of PE vulnerability considering these disparities [21]. Examining the relationship between VEGF polymorphisms found in placental tissue or maternal peripheral blood and the onset of preeclampsia, with an emphasis on their possible diagnostic and prognostic implications, is the goal of this review.
2. Materials and Methods
The relevance of VEGF gene polymorphisms in preeclampsia (PE) is examined in this review, which solely focuses on VEGF and leaves out other family members, including PlGF and KDR. The PubMed database was used to perform a thorough search of the literature. “Vascular Endothelial Growth Factor polymorphisms”, “VEGF polymorphisms”, and “preeclampsia” were the MeSH terms used. This search retrieved 54 articles published over the past 21 years.
Studies published from 2004 to the present were included because we did not strictly enforce a chronological limit in order to guarantee thorough coverage. Only primary, peer-reviewed research articles that were pertinent to human studies were taken into account. Studies that used animal models (e.g., mice) or that concentrated on non-VEGF polymorphisms were not included, nor were meta-analyses, reviews, or systematic reviews. Following screening, 32 studies were deemed eligible for analysis after 5 reviews, 5 meta-analyses, and 12 other articles were eliminated.
Furthermore, two studies were disqualified because of language obstacles; their English abstracts were insufficient for a thorough analysis, and their complete texts were only available in Chinese and Portuguese. Of the included studies, 26 examined VEGF polymorphisms in maternal peripheral blood, 7 used placental blood or tissue, and 2 examined HELLP syndrome. Figure 1 illustrates the study selection process.
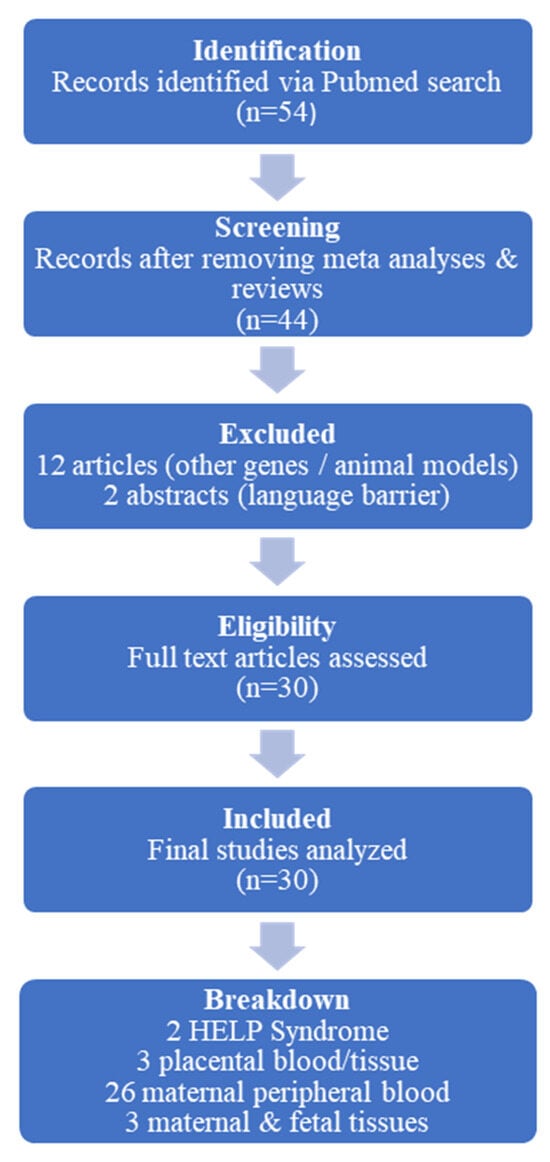
Figure 1.
Schematic presentation of the study selection process in the review. Out of 54 initially identified articles, 30 were included after screening for relevance, with exclusions based on study type, focus on non-VEGF genes, use of animal models, or language barriers.
3. Results
PE and VEGF polymorphisms have been extensively researched, with numerous studies reporting differing conclusions [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. Several investigations have examined polymorphisms such as NG_008732.1:g.3222C>A, NG_008732.1:g.5383G>C, NG_008732.1:g.8331C>T, and NG_008732.1:g.6075G>C, often yielding inconsistent findings across populations.
Papazoglou et al. (2004), in Greece, analyzed VEGF −2578C/A, NG_008732.1:g.5383G>C, and NG_008732.1:g.8331C>T in 42 women with PE (22 mild, 20 severe) and 73 controls, identifying NG_008732.1:g.8331C>T as a possible biomarker for severe PE [22]. Similarly, a Hungarian study (2006), involving 84 nulliparous women with severe PE (including 12 with HELLP syndrome) and 96 controls, found NG_008732.1:g.3222C>A to be associated with disease progression, while +405G/C appeared protective [23]. In Korea, studies yielded conflicting results regarding NG_008732.1:g.8331C>T and NG_008732.1:g.5383G>C [24,25].
Ethnic variability has been highlighted in research. A Brazilian study (2009) suggested that haplotypes C-2578, G-1154, and C-634 might be protective against PE [26], whereas a USA study found VEGF-C alleles NC_000006.12:g.43715473G>A and NC_000006.12:g.43720123C>T in Black women and rs7664413 in White women to be linked to increased PE risk [27]. In contrast, studies in Mexico, Brazil, Sri Lanka, and Egypt reported no significant associations for several VEGF polymorphisms [28,29,30,31,32,33,34,35,36].
Further investigations in Iran and Sudan identified VEGF-634CC as a risk factor for severe PE [30,31], while studies in the Philippines and Uganda suggested NG_008732.1:g.8331C>T could be linked to PE, though results varied [32,40]. Similarly, studies analyzing VEGF polymorphisms in different biological samples, such as umbilical cord blood and placental tissue, showed mixed findings [43,44,45,46]. Table 1 summarizes the results of the included studies regarding the association between VEGF polymorphisms in maternal peripheral blood and PE, whereas Table 2 presents the included studies exploring the association between VEGF polymorphisms in fetal blood circulation and PE.

Table 1.
This table summarizes various studies exploring the association between VEGF polymorphisms in maternal peripheral blood and PE, including details on the citation, country, study participants, study type, VEGF polymorphisms investigated, tissue samples, extraction method, and key findings.

Table 2.
This table summarizes various studies exploring the association between VEGF polymorphisms in fetal circulation and PE, including details on the citation, country, study participants, study type, VEGF polymorphisms investigated, tissue samples, extraction method, and key findings.
HELLP syndrome, a severe PE complication, has been less studied. A Hungarian study linked NG_008732.1:g.6263C>T and NG_008732.1:g.6075G>C polymorphisms to increased risk [42]. Meanwhile, maternal-fetal genetic interactions have also been explored, with studies indicating that fetal VEGF genotypes, such as NG_008732.1:g.6075G>C, may contribute to maternal PE risk [47,48]. Table 3 summarizes studies investigating the relationship between various VEGF polymorphisms and HELLP syndrome.

Table 3.
This table summarizes studies investigating the relationship between various VEGF polymorphisms and HELLP, including details such as citation, country of the study, participant groups, study type, VEGF polymorphisms analyzed, tissue samples used, extraction method, and key findings.
A study in Iran analyzed VEGF NG_008732.1:g.3251_?ins/del, NG_008732.1:g.5383G>C, and NG_008732.1:g.4878A>G polymorphisms in maternal blood and placental tissue, identifying NG_008732.1:g.5383G>C and CC as significantly associated with PE severity [49]. Additionally, a unique study by Sandrim et al. (2015) explored whether or not VEGF SNPs influenced the response to antihypertensive treatment in PE but found no statistically significant results [50]. Table 4 compiles the results of multiple investigations into the relationship between different VEGF polymorphisms, both maternal and fetal, and PE.

Table 4.
This table compiles the results of multiple investigations into the relationship between different VEGF polymorphisms, both maternal and fetal, and PE.
Overall, while VEGF polymorphisms show potential in influencing PE susceptibility and severity, the inconsistencies among studies suggest that genetic variations interact with multiple factors, warranting further research to determine their clinical relevance.
4. Discussion
The normal course of pregnancy depends on both angiogenesis and placental development, both of which are significantly influenced by VEGF. A higher risk of PE, a pregnancy condition that can cause serious difficulties for both the mother and the fetus, has been associated with variations in VEGF gene polymorphisms. VEGF polymorphisms and PE have been the subject of numerous investigations, which have provided insight into how these genetic variations may affect the disease’s risk and severity.
PE has been linked to several VEGF polymorphisms, including VEGF +405G/C, NG_008732.1:g.3222C>A, NG_008732.1:g.8331C>T, and NG_008732.1:g.5383G>C. Some VEGF genotypes may be protective, as evidenced by the NG_008732.1:g.6075G>C polymorphism, which has been associated with a lower incidence of severe PE in nulliparous women [23]. However, in women with severe PE, the NG_008732.1:g.3222C>A variant has been linked to a quicker rate of illness development [23], suggesting that certain polymorphisms may make the situation worse. Furthermore, the development of HELLP syndrome [42], a serious complication of PE, has been associated with the NG_008732.1:g.3222C>A, NG_008732.1:g.6263C>T, and NG_008732.1:g.6075G>C polymorphisms, demonstrating how distinct genetic variants might affect the course of pregnancy problems.
The connection between the severity of PE and placental VEGF polymorphisms has been the subject of numerous investigations. For instance, Keshavarzi et al. discovered that serious PE was linked to the placental NG_008732.1:g.2549_2550del/del genotype, whereas both PE and severe PE were linked to the −634GC and CC genotypes [49]. According to these results, placental VEGF polymorphisms may be useful indicators for determining which women are more likely to have severe PE. VEGF expression may be upregulated in response to PE, possibly as part of the body’s attempt to compensate for impaired placental angiogenesis, according to further research by Keshavarzi et al. [45], which showed that women with PE, especially those with the NG_008732.1:g.5383C/C genotype, had higher mRNA expression of the placental VEGF gene. VEGF polymorphisms may play a different function in controlling VEGF levels depending on the particular variant and tissue context, as evidenced by the lack of correlation between VEGF NG_008732.1:g.4878A>G and NG_008732.1:g.3251_?ins/del polymorphisms and VEGF mRNA expression.
Another important element in the development of PE has been identified as the interaction between maternal and fetal VEGF polymorphisms. The angiogenic balance during pregnancy may be impacted by maternal and fetal VEGF polymorphisms, according to research by Procopciuc et al. [47] and Chen et al. [48]. The rs2010963 polymorphism in maternal VEGF-A has been linked to a higher incidence of PE. In particular, children with the rs2010963 polymorphism’s CC or GC genotype may be more susceptible to PE than children with the GG genotype. Furthermore, Chen et al. found a strong correlation between passive smoking and the maternal NG_008732.1:g.6075G>C polymorphism, underscoring the complexity of PE development, in which environmental factors and genetic susceptibility combine to affect pregnancy outcomes [48].
When paired with other risk variables, including maternal age, parity, and environmental exposures, VEGF polymorphisms may be useful biomarkers for identifying women who are at high risk for PE, according to the mounting body of research. Identifying particular VEGF genotypes associated with protective benefits (e.g., NG_008732.1:g.6075G and NG_008732.1:g.3222A) or increased risk (e.g., NG_008732.1:g.5383C/C and NG_008732.1:g.2549_2550del/del) may aid doctors in better managing and predicting PE. Additionally, by comprehending the function of VEGF polymorphisms, tailored treatment plans that reduce the risk of PE and enhance the health of both the mother and the fetus may be developed. To rebalance pro- and anti-angiogenic factors in preeclamptic pregnancies, this may entail angiogenesis pathway-targeting therapies. Figure 2 outlines the interplay between the genetic and environmental factors influencing angiogenesis, leading to placental dysfunction and various clinical outcomes of PE.
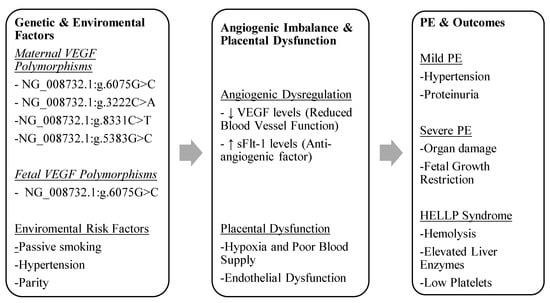
Figure 2.
The interplay between genetic and environmental factors influencing angiogenesis, leading to placental dysfunction and various clinical outcomes of PE.
These results highlight the need for more investigation into the molecular processes behind the association between VEGF and PE, in addition to genetic variables. Additional research may examine the possibility of VEGF-related medicines, such as angiogenesis-promoting medications or VEGF inhibitors, to treat PE or stop its progression.
5. Conclusions
VEGF polymorphisms are closely associated with the onset, severity, and progression of PE. Particularly, placental VEGF polymorphisms are associated with the risk and severity of PE; some genetic variations may have protective benefits, while others may increase the likelihood of severe illness. The complex pathophysiology of PE is emphasized by the significant role that the interaction between fetal and maternal VEGF genotypes plays in regulating the angiogenic balance in preeclamptic pregnancies. The potential of VEGF polymorphisms as prognostic biomarkers for PE and the creation of tailored therapy plans for pregnancies at risk are both supported by these findings. More research is necessary in order to completely comprehend these genetic markers’ function in the development and progression of PE.
Author Contributions
Conceptualization, A.P. and S.S.; methodology, I.Z. and E.M.; formal analysis, N.K. and C.T.; investigation, C.C., I.A. and E.D.; writing—original draft preparation, I.Z. and E.M.; writing—review and editing, A.P., C.C., I.A., N.K., C.T., E.D., P.P., P.D. and S.S.; visualization, E.M.; supervision, P.P. and P.D.; project administration, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Maternal Mortality. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/maternal-mortality (accessed on 30 January 2025).
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2019, 145 (Suppl. S1), 1–33. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef]
- Leveno, K.J.; Bloom, S.L.; Spong, C.Y.; Dashe, J.S.; Hoffman, B.L.; Casey, B.M.; Sheffield, J.S. Williams Obstetrics; Cunningham, F.G., Ed.; McGraw-Hill Medical: New York, NY, USA, 2014; Volume 7, pp. 28–1125. [Google Scholar]
- Chang, K.-J.; Seow, K.-M.; Chen, K.-H. Preeclampsia: Recent Advances in Predicting, Preventing, and Managing the Maternal and Fetal Life-Threatening Condition. Int. J. Environ. Res. Public Health 2023, 20, 2994. [Google Scholar] [CrossRef] [PubMed]
- Erez, O.; Romero, R.; Jung, E.; Chaemsaithong, P.; Bosco, M.; Suksai, M.; Gallo, D.M.; Gotsch, F. Preeclampsia and eclampsia: The conceptual evolution of a syndrome. Am. J. Obstet. Gynecol. 2022, 226, S786–S803. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef]
- Khalid, F.; Mahendraker, N.; Tonismae, T. HELLP Syndrome. In StatPearls [Internet]. Treasure Island (FL); StatPearls Publishing: Tampa, FL, USA, 2025. [Google Scholar]
- Chiang, Y.-T.; Seow, K.-M.; Chen, K.-H. The Pathophysiological, Genetic, and Hormonal Changes in Preeclampsia: A Systematic Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2024, 25, 4532. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Lee, E.S.; Oh, M.-J.; Jung, J.W.; Lim, J.-E.; Seol, H.-J.; Lee, K.-J.; Kim, H.-J. The Levels of Circulating Vascular Endothelial Growth Factor and Soluble Flt-1 in Pregnancies Complicated by Preeclampsia. J. Korean Med Sci. 2007, 22, 94–98. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endo-thelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar] [PubMed]
- Yamazaki, Y.; Morita, T. Molecular and functional diversity of vascular endothelial growth factors. Mol. Divers. 2006, 10, 515–527. [Google Scholar] [CrossRef]
- Patel, S.A.; Nilsson, M.B.; Le, X.; Cascone, T.; Jain, R.K.; Heymach, J.V. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin. Cancer Res. 2023, 29, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Bolatai, A.; He, Y.; Wu, N. Vascular endothelial growth factor and its receptors regulation in gestational diabetes mellitus and eclampsia. J. Transl. Med. 2022, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, H.; Qin, H.; Yang, J.; Wang, Y.; Jiang, S.; Pan, Y. Vascular Endothelial Growth Factor expression in peripheral blood of patients with pregnancy induced hypertension syndrome and its clinical significance. Pak. J. Med Sci. 1969, 30, 634–637. [Google Scholar] [CrossRef]
- Das, U.N. Cytokines, angiogenic, and antiangiogenic factors and bioactive lipids in preeclampsia. Nutrition 2015, 31, 1083–1095. [Google Scholar] [CrossRef]
- Lam, C.; Lim, K.-H.; Karumanchi, S.A. Circulating Angiogenic Factors in the Pathogenesis and Prediction of Preeclampsia. Hypertension 2005, 46, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.; Aitkenhead, M.; Caldwell, C.; McCracken, G.; Wilson, D.; McClure, N. Serum Levels of Vascular Endothelial Growth Factor in Preeclamptic and Normotensive Pregnancy. Hypertension 2000, 36, 965–969. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, M.; Bi, X.; Fu, Y.; Jing, X.; Zhang, H.; Cao, B.; Wang, C. A systematic review on the application of vascular endothelial growth factors in preeclampsia. Ann. Palliat. Med. 2021, 10, 9259–9266. [Google Scholar] [CrossRef]
- Papazoglou, D.; Galazios, G.; Koukourakis, M.I.; Panagopoulos, I.; Kontomanolis, E.N.; Papatheodorou, K.; Maltezos, E. Vascular endothelial growth factor gene polymorphisms and pre-eclampsia. Mol. Hum. Reprod. 2004, 10, 321–324. [Google Scholar] [CrossRef]
- Bányász, I.; Szabó, S.; Bokodi, G.; Vannay, Á.; Vásárhelyi, B.; Szabó, A.; Tulassay, T.; Rigo, J. Genetic polymorphisms of vascular endothelial growth factor in severe pre-eclampsia. Mol. Hum. Reprod. 2006, 12, 233–236. [Google Scholar] [CrossRef]
- Shim, J.-Y.; Jun, J.K.; Jung, B.-K.; Kim, S.H.; Won, H.-S.; Lee, P.R.; Kim, A. Vascular endothelial growth factor gene +936 C/T polymorphism is associated with preeclampsia in Korean women. Am. J. Obstet. Gynecol. 2007, 197, 271.e1–271.e4. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, B.H.; Park, H.; Jung, S.-C.; Pang, M.-G.; Ryu, H.-M.; Lee, K.-S.; Eom, S.-M.; Park, H.-Y. No Association of the Genetic Polymorphisms of Endothelial Nitric Oxide Synthase, Dimethylarginine Dimethylaminohydrolase, and Vascular Endothelial Growth Factor with Preeclampsia in Korean Populations. Twin Res. Hum. Genet. 2008, 11, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Sandrim, V.C.; Palei, A.C.T.; Cavalli, R.C.; Araújo, F.M.; Ramos, E.S.; Duarte, G.; Tanus-Santos, J.E. Vascular endothelial growth factor genotypes and haplotypes are associated with pre-eclampsia but not with gestational hypertension. Mol. Hum. Reprod. 2008, 15, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.K.; Morrison, A.C.; Andrela, C.M.; Elovitz, M.A. Allelic variations in angiogenic pathway genes are associated with preeclampsia. Am. J. Obstet. Gynecol. 2010, 202, 445.e1–445.e11. [Google Scholar] [CrossRef]
- Garza-Veloz, I.; la Rosa, C.C.-D.; Cortes-Flores, R.; Martinez-Gaytan, V.; E Rivera-Muñoz, J.; A Garcia-Mayorga, E.; Meza-Lamas, E.; Rojas-Martinez, A.; Ortiz-Lopez, R.; Martinez-Fierro, M.L. No association between polymorphisms/haplotypes of the vascular endothelial growth factor gene and preeclampsia. BMC Pregnancy Childbirth 2011, 11, 35. [Google Scholar] [CrossRef]
- Luizon, M.R.; Sandrim, V.C.; Palei, A.C.; Lacchini, R.; Cavalli, R.C.; Duarte, G.; E Tanus-Santos, J. Epistasis among eNOS, MMP-9 and VEGF maternal genotypes in hypertensive disorders of pregnancy. Hypertens. Res. 2012, 35, 917–921. [Google Scholar] [CrossRef]
- Salimi, S.; Yaghmaei, M.; Tabatabaei, E.; Mokhtari, M.; Naghavi, A. Vascular endothelial growth factor (VEGF)-634G/C polymorphism was associated with severe pre-eclampsia and lower serum VEGF level. J. Obstet. Gynaecol. Res. 2015, 41, 1877–1883. [Google Scholar] [CrossRef]
- Hamid, H.M.; Abdalla, S.E.; Sidig, M.; Adam, I.; Hamdan, H.Z. Association of VEGFA and IL1β gene polymorphisms with preeclampsia in Sudanese women. Mol. Genet. Genom. Med. 2020, 8, e1119. [Google Scholar] [CrossRef]
- Amosco, M.D.; Villar, V.A.M.; Naniong, J.M.A.; David-Bustamante, L.M.G.; Jose, P.A.; Palmes-Saloma, C.P. VEGF-A and VEGFR1 SNPs associate with preeclampsia in a Philippine population. Clin. Exp. Hypertens. 2016, 38, 578–585. [Google Scholar] [CrossRef]
- Niktalab, R.; Piravar, Z.; Behzadi, R. Different Polymorphisms of Vascular Endothelial Growth Factor Gene in Patients with Pre-Eclampsia among the Iranian Women Population. Int. J. Fertil. Steril. 2020, 14, 41–45. [Google Scholar] [CrossRef]
- Silva, V.R.S.; Soardi, F.C.; Tanaka, S.C.S.V.; da Silva-Grecco, R.L.; Paschoini, M.C.; Balarin, M.A.S. Investigation of polymorphisms in pre-eclampsia related genes VEGF and IL1A. Arch. Gynecol. Obstet. 2014, 291, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Andraweera, P.H.; Dekker, G.A.; Dissanayake, V.H.; Bianco-Miotto, T.; Jayasekara, R.W.; Roberts, C.T. Vascular endothelial growth factor family gene polymorphisms in preeclampsia in Sinhalese women in Sri-Lanka. J. Matern. Neonatal Med. 2012, 26, 532–536. [Google Scholar] [CrossRef]
- Mowad, H.H.; Abougabal, K.M.; Fahim, A.S.; Shehata, N.A.; Ali, H.A.; Nasser, M.Z. Vascular endothelial growth factor C/A 2578 gene polymorphism and umbilical artery Doppler in preeclamptic women. Pregnancy Hypertens. 2019, 18, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Gannoun, M.B.A.; Al-Madhi, S.A.; Zitouni, H.; Raguema, N.; Meddeb, S.; Ben Ali, F.H.; Mahjoub, T.; Almawi, W.Y. Vascular endothelial growth factor single nucleotide polymorphisms and haplotypes in pre-eclampsia: A case-control study. Cytokine 2017, 97, 175–180. [Google Scholar] [CrossRef]
- Pacheco-Romero, J.; Conchucos, O.A.; Canales, D.H.; Ramos, S.C.; Chávez, M.V.; Sánchez, P.M.; Guerrero, M.H.; Paredes, J.S.; Gabriel, R.L.; Mateus, J.; et al. Genetic markers for preeclampsia in Peruvian women. Colomb. Medica 2021, 52, e2014437. [Google Scholar] [CrossRef]
- Ding, G.; Li, Y.; Gao, J.; Wang, W.; Wang, H.; Bai, G. Associations between AGT, MTHFR, and VEGF gene polymorphisms and preeclampsia in the Chinese population. Placenta 2022, 118, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Nabweyambo, S.; Kanyerezi, S.; Petterson, J.H.-O.; Katabazi, F.A.; Ssekagiri, A.; Mwesigwa, S.; Mboowa, G.; Nakazzi, F.; Keesiga, A.; Adroma, M.; et al. No association of a Vascular endothelial growth factor A (VEGFA) gene polymorphism with pre-eclampsia among pregnant women in Uganda. BMC Genom. 2023, 24, 132. [Google Scholar] [CrossRef]
- Saw, K.E.E.; Thann, T.S.A.M. Association Between Vascular Endothelial Growth Factor (VEGF) +936C/T Polymorphism (rs3025039) and Preeclampsia Among Myanmar Pregnant Women. J. Pregnancy 2024, 2024, 7608096. [Google Scholar] [CrossRef]
- Nagy, B.; Savli, H.; Molvarec, A.; Várkonyi, T.; Rigó, B.; Hupuczi, P.; Rigó, J. Vascular endothelial growth factor (VEGF) polymorphisms in HELLP syndrome patients determined by quantitative real-time PCR and melting curve analyses. Clin. Chim. Acta 2008, 389, 126–131. [Google Scholar] [CrossRef]
- Chedraui, P.; Solis, E.J.; Bocci, G.; Gopal, S.; Russo, E.; Escobar, G.S.; Hidalgo, L.; Pérez-López, F.R.; Genazzani, A.R.; Mannella, P.; et al. Feto-placental nitric oxide, asymmetric dimethylarginine and vascular endothelial growth factor (VEGF) levels and VEGF gene polymorphisms in severe preeclampsia. J. Matern. Neonatal Med. 2013, 26, 226–232. [Google Scholar] [CrossRef]
- Atis, A.; Oruc, O.; Aydin, Y.; Cetincelik, U.; Goker, N. Vascular endothelial growth factor gene +813CC polymorphism of foetus is associated with preterm labour but not with pre-eclampsia in Turkish pregnant women. Int. J. Immunogenet. 2012, 39, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, F.; Shahrakipoor, M.; Teimoori, B.; Yaghmaei, M.; Narooei-Nejad, M.; Rasooli, A.; Salimi, S. Association of the placental VEGF promoter polymorphisms and VEGF mRNA expression with preeclampsia. Clin. Exp. Hypertens. 2018, 41, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Macías-Salas, A.; Sosa-Macías, M.; Barragán-Zúñiga, L.J.; Blanco-Castañeda, R.; Damiano, A.; Garcia-Robles, R.; Ayala-Ramírez, P.; Bueno-Sánchez, J.; Giachini, F.R.; Escudero, C.; et al. Preeclampsia association of placental nucleotide variations in eNOS, VEGFA, and FLT-1 genes in Latin American pregnant women. Placenta 2023, 135, 1–6. [Google Scholar] [CrossRef]
- Procopciuc, L.M.; Caracostea, G.; Zaharie, G.; Stamatian, F. Maternal/newbornVEGF-C936Tinteraction and its influence on the risk, severity and prognosis of preeclampsia, as well as on the maternal angiogenic profile. J. Matern. Neonatal Med. 2014, 27, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Z.; Yu, S.J.; Wei, M.H.; Li, C.Y.; Yan, W.R. Effects of maternal and fetal vascular endothelial growth factor a single nucleotide polymorphisms on pre-eclampsia: A hybrid design study. Cytokine 2020, 127, 154995. [Google Scholar] [CrossRef]
- Keshavarzi, F.; Mohammadpour-Gharehbagh, A.; Shahrakipour, M.; Teimoori, B.; Yazdi, A.; Yaghmaei, M.; Naroeei-Nejad, M.; Salimi, S. The placental vascular endothelial growth factor polymorphisms and preeclampsia/preeclampsia severity. Clin. Exp. Hypertens. 2017, 39, 606–611. [Google Scholar] [CrossRef]
- Sandrim, V.C.; Palei, A.C.T.; Eleuterio, N.; Tanus-Santos, J.E.; Cavalli, R.C. Antihypertensive therapy in preeclampsia is not modulated by VEGF polymorphisms. Arch. Gynecol. Obstet. 2015, 291, 799–803. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).