Donor Variability Alters the Characteristics of Human Brain Microvascular Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Immunocytochemistry
2.3. Western Blotting
2.4. Assessment of In Vitro Blood–Brain Barrier Integrity and Function
2.5. Tube Formation Assay
2.6. Senescence-Associated β-Galactosidase Activity
2.7. Statistical Analyses
3. Results
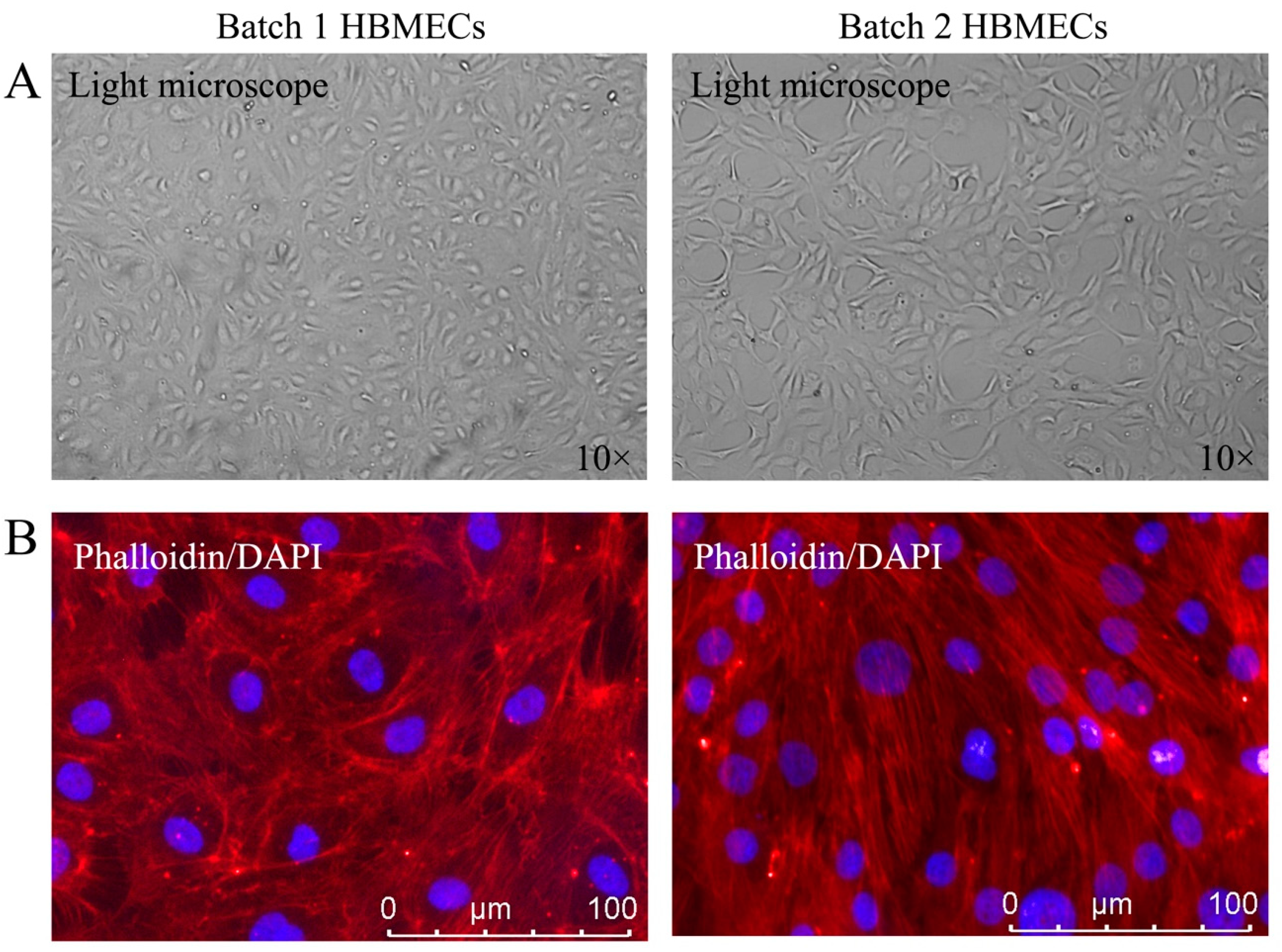
3.1. HBMECs Display Different Phenotypes
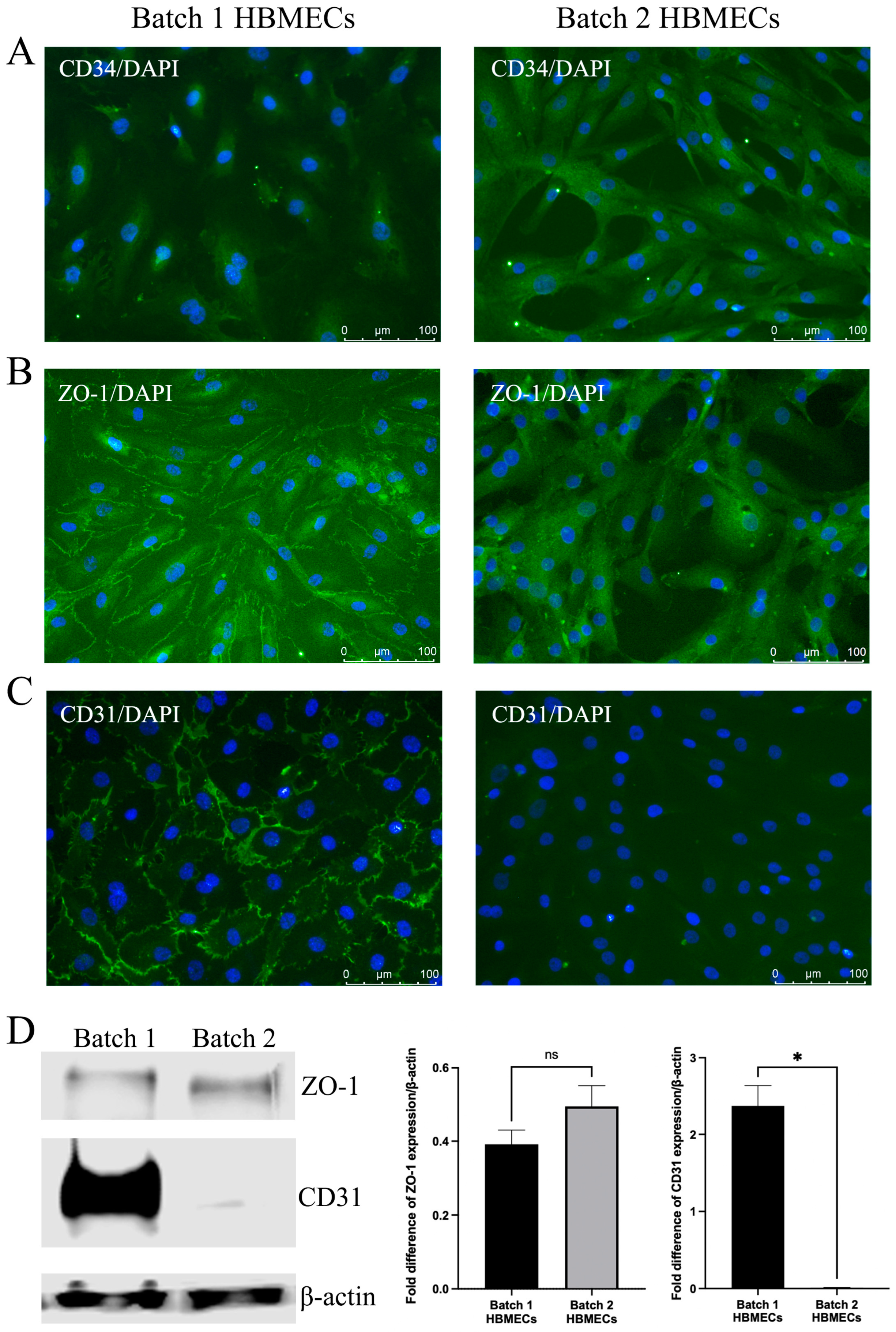
3.2. HBMECs Express Endothelial Markers Differently
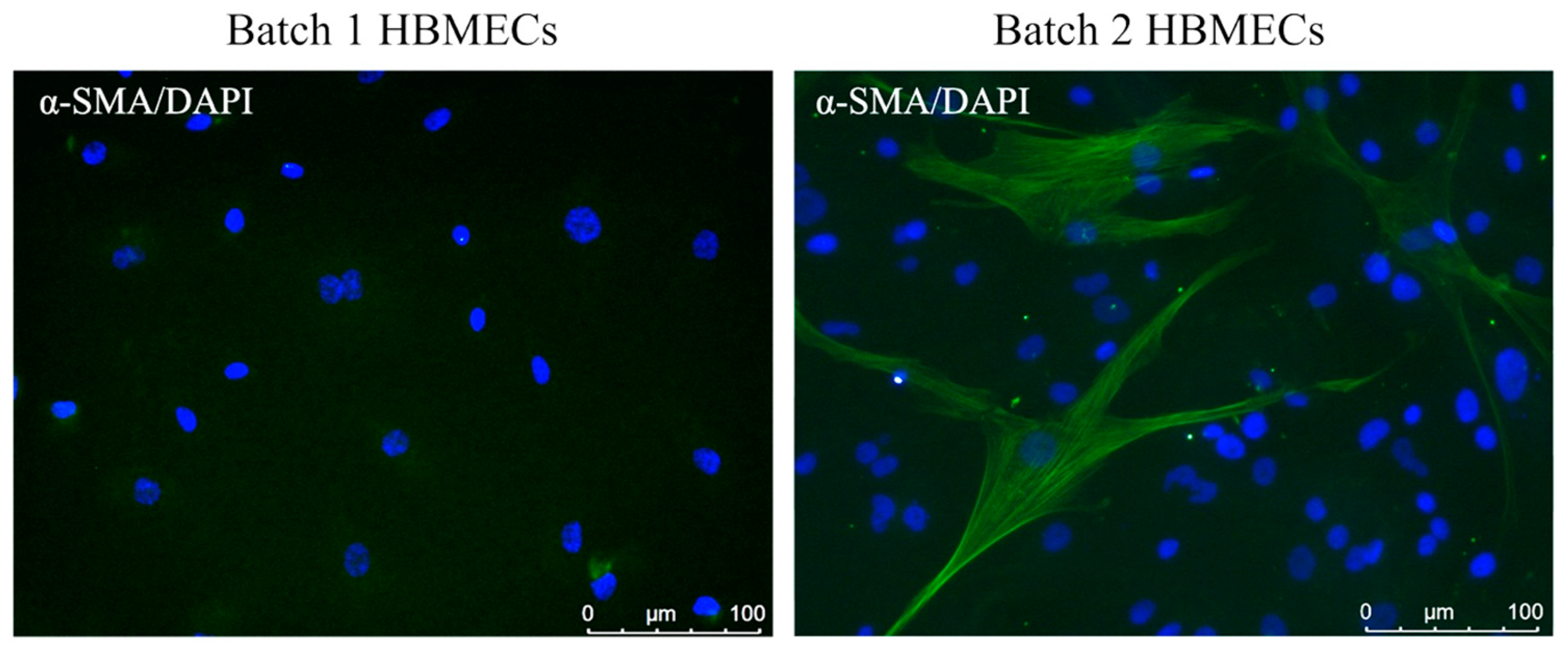
3.3. Scrutiny of Alpha-Smooth Muscle Actin Expression in Endothelial Cells
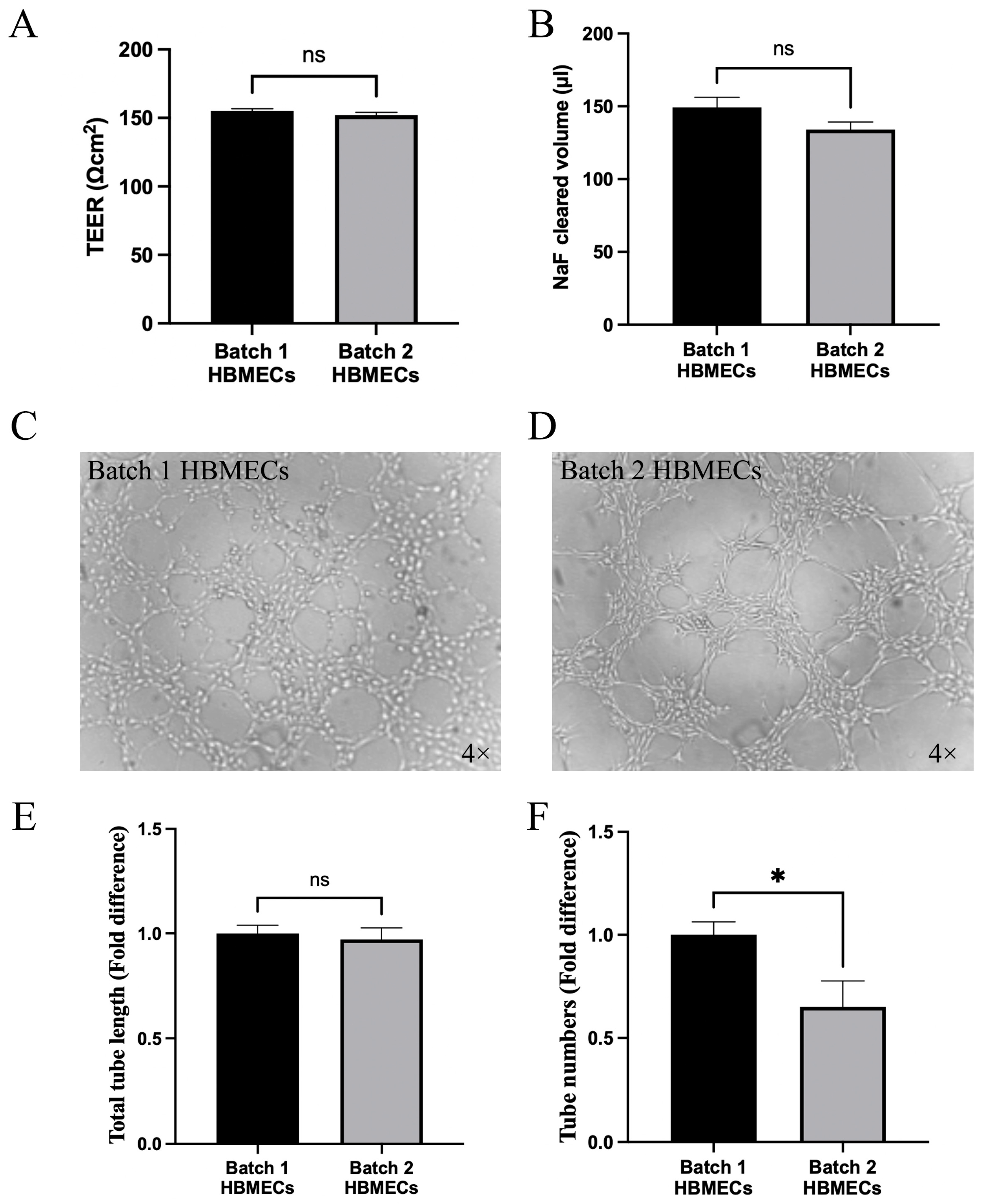
3.4. Effect of Changes in Endothelial Cell Characteristics on Functional Activity
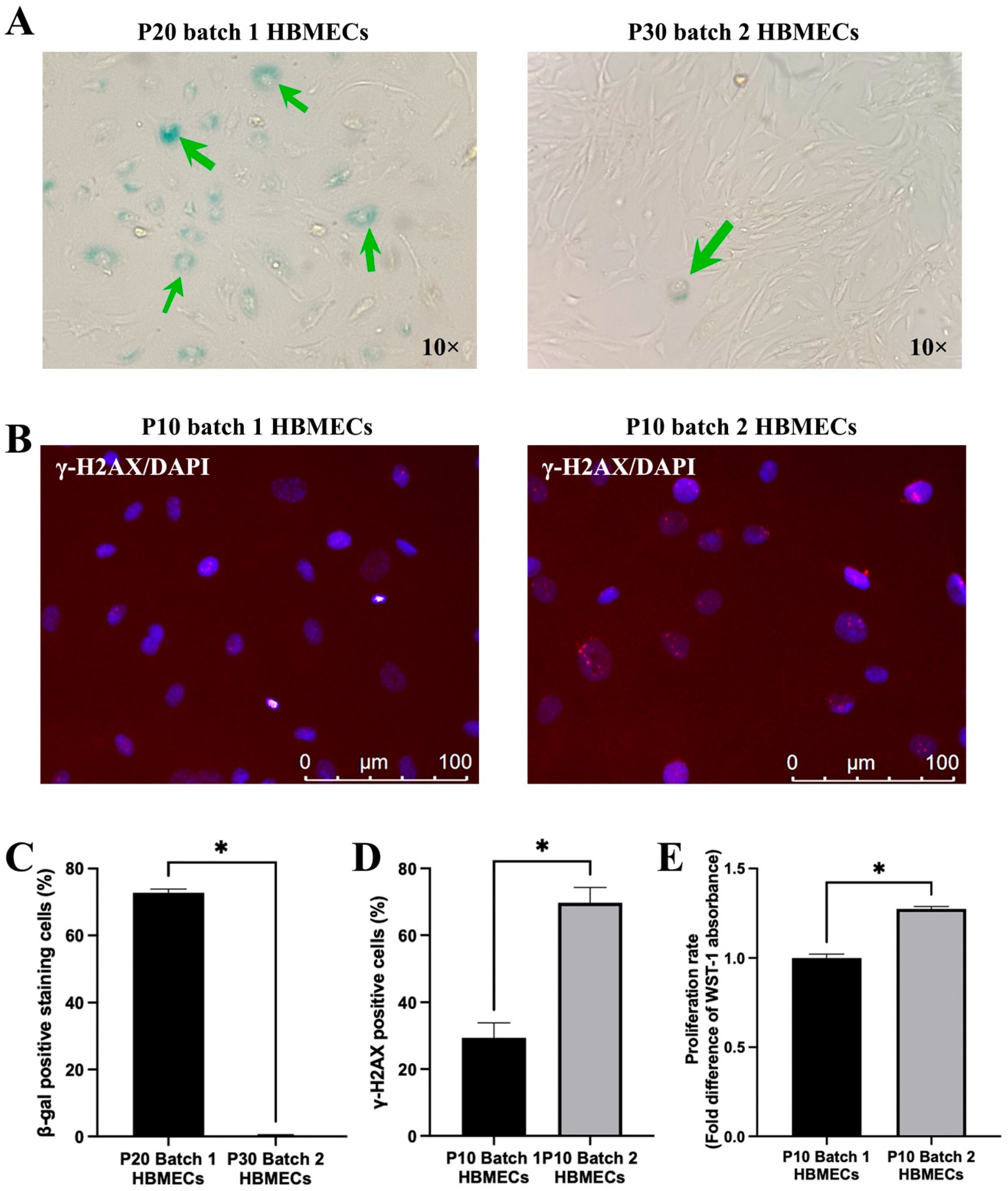
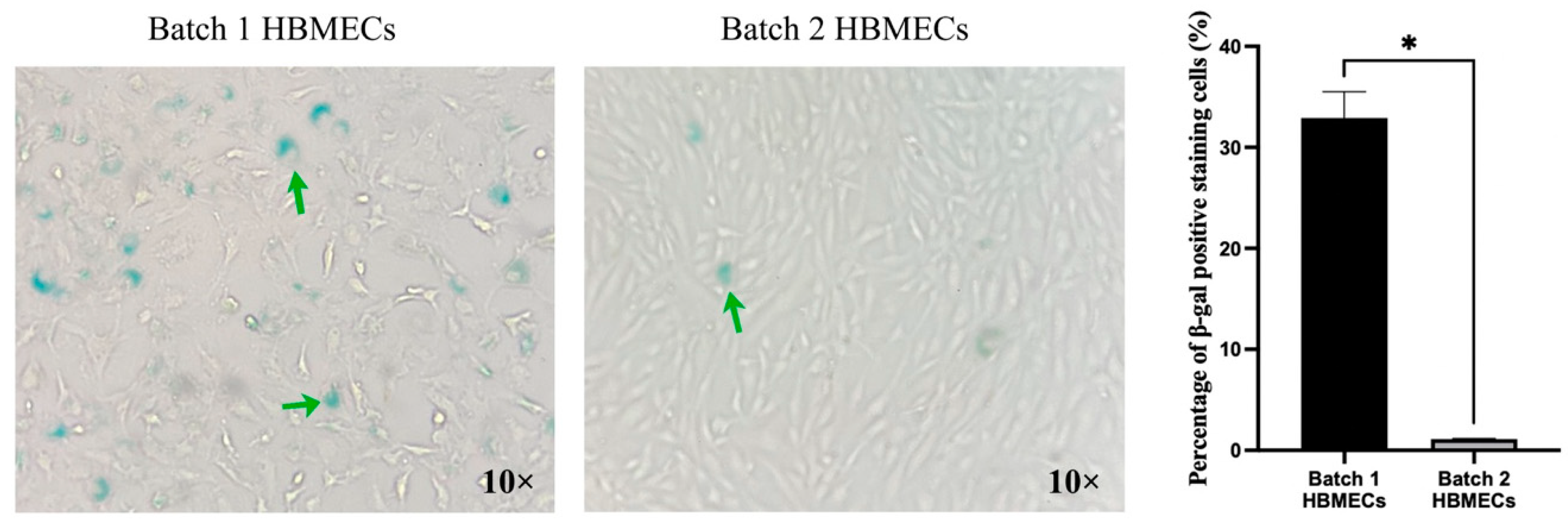
3.5. Endothelial Cells Respond to Senescence-Inducing Stimuli Differently
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sandoo, A.; van Zanten, J.J.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Dessalles, C.A.; Leclech, C.; Castagnino, A.; Barakat, A.I. Integration of substrate- and flow-derived stresses in endothelial cell mechanobiology. Commun. Biol. 2021, 4, 764. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Shao, B.; Bayraktutan, U. PKC-beta exacerbates in vitro brain barrier damage in hyperglycemic settings via regulation of RhoA/Rho-kinase/MLC2 pathway. J. Cereb. Blood Flow Metab. 2013, 33, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Suchorska, W.M.; Trzeciak, T. From Donor to the Lab: A Fascinating Journey of Primary Cell Lines. Front. Cell Dev. Biol. 2021, 9, 711381. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef]
- Hashmat, A.; Ya, J.; Kadir, R.; Alwjwaj, M.; Bayraktutan, U. Hyperglycaemia perturbs blood-brain barrier integrity through its effects on endothelial cell characteristics and function. Tissue Barriers 2024, 2350821. [Google Scholar] [CrossRef] [PubMed]
- Ya, J.; Whitby, A.; Bayraktutan, U. Metabolites and Metabolic Functional Changes-Potential Markers for Endothelial Cell Senescence. Biomolecules 2024, 14, 1476. [Google Scholar] [CrossRef]
- Supp, D.M.; Hahn, J.M.; Combs, K.A.; McFarland, K.L.; Powell, H.M. Isolation and feeder-free primary culture of four cell types from a single human skin sample. STAR Protoc. 2022, 3, 101172. [Google Scholar] [CrossRef]
- Kadir, R.R.A.; Alwjwaj, M.; Bayraktutan, U. Establishment of an In Vitro Model of Human Blood-Brain Barrier to Study the Impact of Ischemic Injury. Methods Mol. Biol. 2022, 2492, 143–155. [Google Scholar] [CrossRef]
- Moonen, J.R.; Lee, E.S.; Schmidt, M.; Maleszewska, M.; Koerts, J.A.; Brouwer, L.A.; van Kooten, T.G.; van Luyn, M.J.; Zeebregts, C.J.; Krenning, G.; et al. Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc. Res. 2015, 108, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Qadan, M.A.; Piuzzi, N.S.; Boehm, C.; Bova, W.; Moos, M., Jr.; Midura, R.J.; Hascall, V.C.; Malcuit, C.; Muschler, G.F. Variation in primary and culture-expanded cells derived from connective tissue progenitors in human bone marrow space, bone trabecular surface and adipose tissue. Cytotherapy 2018, 20, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Chan, Y.K.; Goodman, D.B.; Guo, X.; Chavez, A.; Lim, E.T.; Church, G.M. Enabling multiplexed testing of pooled donor cells through whole-genome sequencing. Genome Med. 2018, 10, 31. [Google Scholar] [CrossRef]
- Kent, D.G. Battle of the sexes: Understanding donor:recipient sex differences in transplantation biology. Hemasphere 2024, 8, e70000. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. Fully-automated and ultra-fast cell-type identification using specific marker combinations from single-cell transcriptomic data. Nat. Commun. 2022, 13, 1246. [Google Scholar] [CrossRef]
- Alizadeh, E.; Castle, J.; Quirk, A.; Taylor, C.D.L.; Xu, W.; Prasad, A. Cellular morphological features are predictive markers of cancer cell state. Comput. Biol. Med. 2020, 126, 104044. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.M.; Izumo, S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J. Cell Sci. 1996, 109 Pt 4, 713–726. [Google Scholar] [CrossRef]
- Vion, A.C.; Perovic, T.; Petit, C.; Hollfinger, I.; Bartels-Klein, E.; Frampton, E.; Gordon, E.; Claesson-Welsh, L.; Gerhardt, H. Endothelial Cell Orientation and Polarity Are Controlled by Shear Stress and VEGF Through Distinct Signaling Pathways. Front. Physiol. 2020, 11, 623769. [Google Scholar] [CrossRef]
- Liang, J.; Gu, S.; Mao, X.; Tan, Y.; Wang, H.; Li, S.; Zhou, Y. Endothelial Cell Morphology Regulates Inflammatory Cells Through MicroRNA Transferred by Extracellular Vesicles. Front. Bioeng. Biotechnol. 2020, 8, 369. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.; Gossen, M.; Lendlein, A.; Jung, F. Venous and Arterial Endothelial Cells from Human Umbilical Cords: Potential Cell Sources for Cardiovascular Research. Int. J. Mol. Sci. 2021, 22, 978. [Google Scholar] [CrossRef] [PubMed]
- Wijelath, E.S.; Carlsen, B.; Cole, T.; Chen, J.; Kothari, S.; Hammond, W.P. Oncostatin M induces basic fibroblast growth factor expression in endothelial cells and promotes endothelial cell proliferation, migration and spindle morphology. J. Cell Sci. 1997, 110 Pt 7, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Clere, N.; Renault, S.; Corre, I. Endothelial-to-Mesenchymal Transition in Cancer. Front. Cell Dev. Biol. 2020, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Gibieza, P.; Petrikaite, V. The regulation of actin dynamics during cell division and malignancy. Am. J. Cancer Res. 2021, 11, 4050–4069. [Google Scholar] [PubMed]
- Yadunandanan Nair, N.; Samuel, V.; Ramesh, L.; Marib, A.; David, D.T.; Sundararaman, A. Actin cytoskeleton in angiogenesis. Biol. Open 2022, 11, bio058899. [Google Scholar] [CrossRef]
- Weber, C.R. Dynamic properties of the tight junction barrier. Ann. N. Y. Acad. Sci. 2012, 1257, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Pusztaszeri, M.P.; Seelentag, W.; Bosman, F.T. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J. Histochem. Cytochem. 2006, 54, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Coracina, A.; Baesso, I.; Agostini, C.; Tiengo, A.; Avogaro, A.; de Kreutzenberg, S.V. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke 2006, 37, 2277–2282. [Google Scholar] [CrossRef]
- Zhang, Z.; Gan, Q.; Han, J.; Tao, Q.; Qiu, W.Q.; Madri, J.A. CD31 as a probable responding and gate-keeping protein of the blood-brain barrier and the risk of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2023, 43, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Kuffer, S.; Cheung, K.C.P.; Jiang, X.; Trumper, L.; Wulf, G.G.; Strobel, P. CD31 Expression Determines Redox Status and Chemoresistance in Human Angiosarcomas. Clin. Cancer Res. 2018, 24, 460–473. [Google Scholar] [CrossRef]
- Kadir, R.R.A.; Alwjwaj, M.; Bayraktutan, U. Protein kinase C-beta distinctly regulates blood-brain barrier-forming capacity of Brain Microvascular endothelial cells and outgrowth endothelial cells. Metab. Brain Dis. 2022, 37, 1815–1827. [Google Scholar] [CrossRef]
- Lu, X.; Gong, J.; Dennery, P.A.; Yao, H. Endothelial-to-mesenchymal transition: Pathogenesis and therapeutic targets for chronic pulmonary and vascular diseases. Biochem. Pharmacol. 2019, 168, 100–107. [Google Scholar] [CrossRef]
- Reskiawan, A.K.R.; Alwjwaj, M.; Ahmad Othman, O.; Rakkar, K.; Sprigg, N.; Bath, P.M.; Bayraktutan, U. Inhibition of oxidative stress delays senescence and augments functional capacity of endothelial progenitor cells. Brain Res. 2022, 1787, 147925. [Google Scholar] [CrossRef] [PubMed]
- Mukai, N.; Akahori, T.; Komaki, M.; Li, Q.; Kanayasu-Toyoda, T.; Ishii-Watabe, A.; Kobayashi, A.; Yamaguchi, T.; Abe, M.; Amagasa, T.; et al. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp. Cell Res. 2008, 314, 430–440. [Google Scholar] [CrossRef]
- Katsuta, E.; Sawant Dessai, A.; Ebos, J.M.; Yan, L.; Ouchi, T.; Takabe, K. H2AX mRNA expression reflects DNA repair, cell proliferation, metastasis, and worse survival in breast cancer. Am. J. Cancer Res. 2022, 12, 793–804. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ya, J.; Bayraktutan, U. Donor Variability Alters the Characteristics of Human Brain Microvascular Endothelial Cells. Curr. Issues Mol. Biol. 2025, 47, 73. https://doi.org/10.3390/cimb47020073
Ya J, Bayraktutan U. Donor Variability Alters the Characteristics of Human Brain Microvascular Endothelial Cells. Current Issues in Molecular Biology. 2025; 47(2):73. https://doi.org/10.3390/cimb47020073
Chicago/Turabian StyleYa, Jingyuan, and Ulvi Bayraktutan. 2025. "Donor Variability Alters the Characteristics of Human Brain Microvascular Endothelial Cells" Current Issues in Molecular Biology 47, no. 2: 73. https://doi.org/10.3390/cimb47020073
APA StyleYa, J., & Bayraktutan, U. (2025). Donor Variability Alters the Characteristics of Human Brain Microvascular Endothelial Cells. Current Issues in Molecular Biology, 47(2), 73. https://doi.org/10.3390/cimb47020073




