USP30-AS1 Dictates Breast Cancer Cell Fate and Chemoresistance via a miR-3646/FZD7/Wnt/β-Catenin Circuit
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection
2.2. Tumor Spheroid Formation Assay
2.3. Quantitative Real-Time PCR (qRT-PCR)
2.4. Transwell Assay
2.5. Western Blot
2.6. CCK-8 Proliferation Assay
2.7. Luciferase Reporter Assay
2.8. Detection of ALDH Activity Using ALDEFLUOR Assay and Flow Cytometry
2.9. Statistical Analysis
3. Results
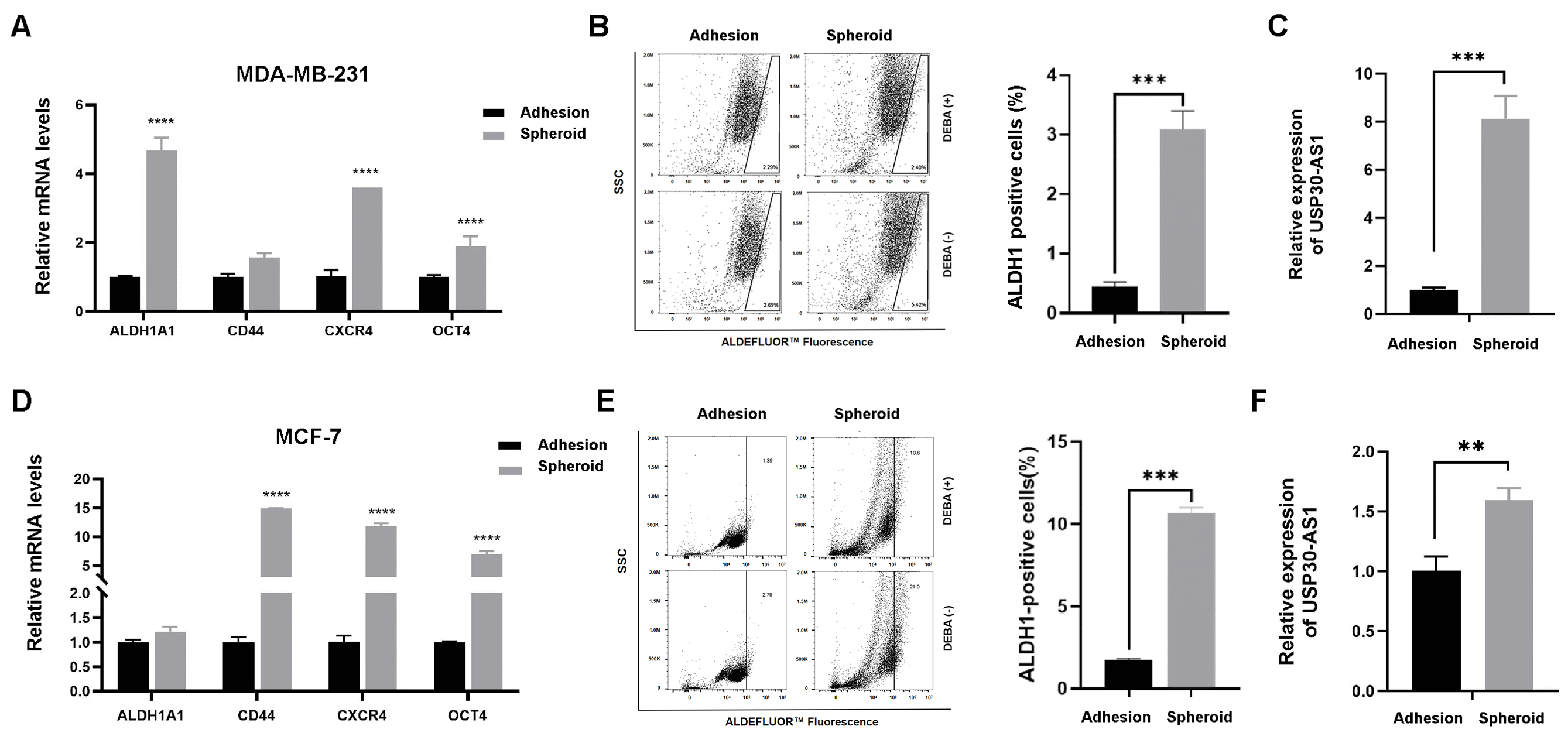
3.1. Elevated Expression of USP30-AS1 in Breast Cancer Stem Cells
3.2. USP30-AS1 Modulates Breast Cancer Stem Cell Properties
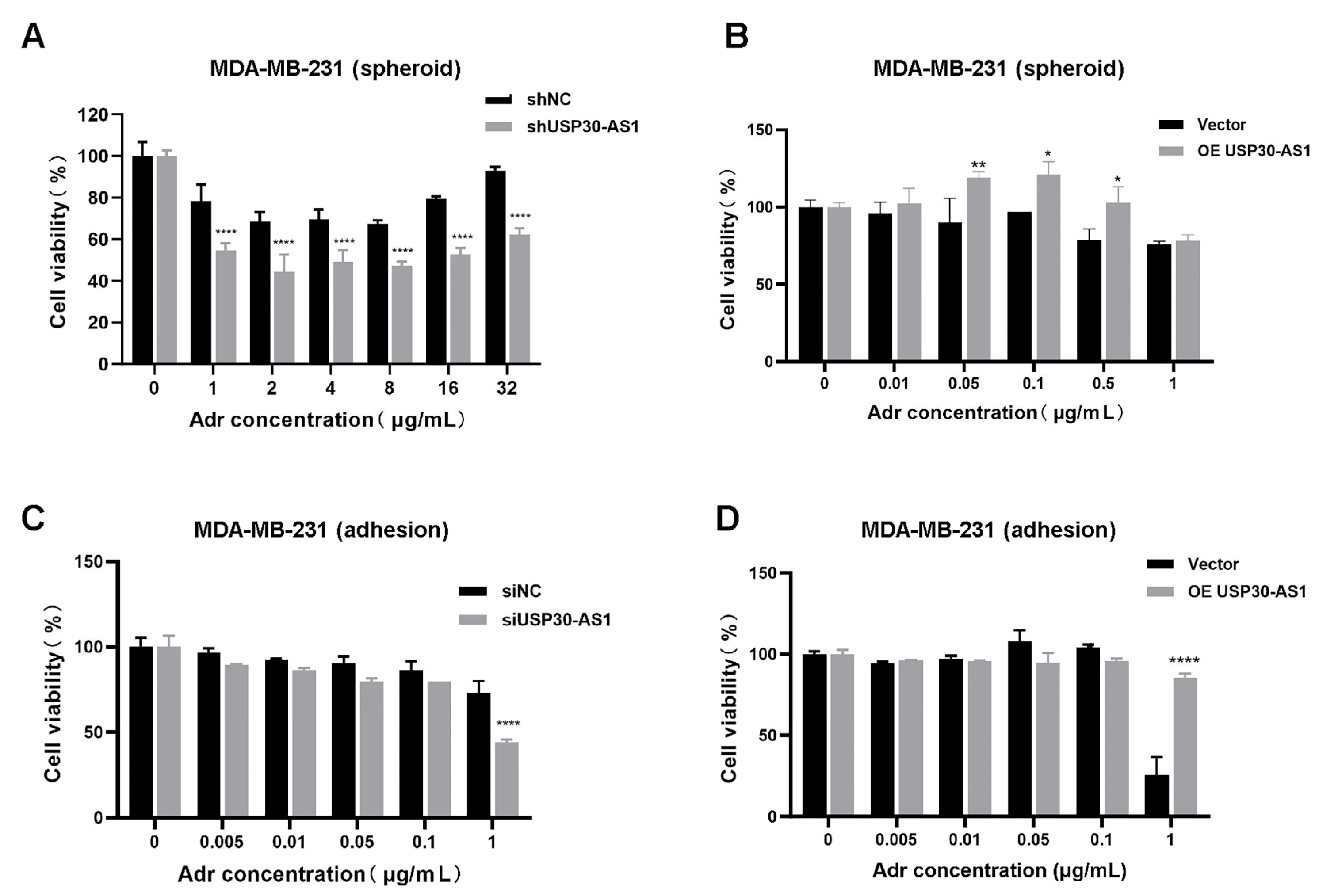
3.3. USP30-AS1 Confers Chemoresistance in Breast Cancer Stem Cells
3.4. USP30-AS1 Enhances Migratory, Invasive Abilities and WNT Transcriptional Activity
3.5. USP30-AS1 Modulates Expression of Key Wnt Pathways Components
3.6. USP30-AS1 Regulates WNT Signaling via Sponging miR-3646
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene | Sense (5′-3′) | Antisense (5′-3′) |
|---|---|---|
| USP30-AS1 | CCAGAGTGGAAATAGGTCGCA | GGCACCCAAGTAAACAATAAGT |
| CD44 | CTGCCGCTTTGCAGGTGTA | CATTGTGGGCAAGGTGCTATT |
| MDR1 | GCTCCTGACTATGCCAAAGCC | CTTCACCTCCAGGCTCAGTCCC |
| OCT4 | GGGAGATTGATAACTGGTGTGTT | GTGTATATCCCAGGGTGATCCTC |
| CXCR4 | ACTACACCGAGGAAATGGGCT | CCCACAATGCCAGTTAAGAAGA |
| ALDH1A1 | GCACGCCAGACTTACCTGTC | CCTCCTCAGTTGCAGGATTAAAG |
| C-myc | CTTCTCTCCGTCCTCGGATTCT | GAAGGTGATCCAGACTCTGACCTT |
| Cyclin D1 | GGCGGATTGGAAATGAACTT | TCCTCTCCAAAATGCCAGAG |
| TCF4 | GCCTCTTATCACGTACAGCAAT | GCCAGGCGATAGTGGGTAAT |
| Axin2 | ATTCGGCCACTGTTCAGACG | GACAACCAACTCACTGGCCTG |
| FZD7 | TTCTCGGACGATGGCTACC | GAACCAAGTGAGAGACAGAATGACC |
| β-catenin | TGGATTGATTCGAAATCTTGC | GAACAAGCAACTGAACTAGT |
| Wnt3a | GCACCACCGTCCACGACAGC | CCTCGCTACAGCCACCCCAC |
| Wnt9a | TGGAGGCCGTGAGCATGAGT | CTTAAGGTTGTCTCCGCAGC |
| GAPDH | UGAGGCAGUAGAUUGAAU | UCAAUCUACUGCCUCAUU |
References
- Butti, R.; Gunasekaran, V.P.; Kumar, T.V.S.; Banerjee, P.; Kundu, G.C. Breast cancer stem cells: Biology and therapeutic implications. Int. J. Biochem. Cell Biol. 2019, 107, 38–52. [Google Scholar] [CrossRef]
- Dahn, M.L.; Marcato, P. Targeting the Roots of Recurrence: New Strategies for Eliminating Therapy-Resistant Breast Cancer Stem Cells. Cancers 2020, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, K.; Bolliger, M.; Bago-Horvath, Z.; Steger, G.; Kauer-Dorner, D.; Helfgott, R.; Gruber, C.; Moinfar, F.; Mittlbock, M.; Fitzal, F. Impact of Surgical Margins in Breast Cancer After Preoperative Systemic Chemotherapy on Local Recurrence and Survival. Ann. Surg. Oncol. 2020, 27, 1700–1707. [Google Scholar] [CrossRef]
- Ye, S.; Ding, Y.F.; Jia, W.H.; Liu, X.L.; Feng, J.Y.; Zhu, Q.; Cai, S.L.; Yang, Y.S.; Lu, Q.Y.; Huang, X.T.; et al. SET Domain-Containing Protein 4 Epigenetically Controls Breast Cancer Stem Cell Quiescence. Cancer Res. 2019, 79, 4729–4743. [Google Scholar] [CrossRef] [PubMed]
- Shima, H.; Yamada, A.; Ishikawa, T.; Endo, I. Are breast cancer stem cells the key to resolving clinical issues in breast cancer therapy? Gland Surg. 2017, 6, 82–88. [Google Scholar] [CrossRef]
- Gillespie, M.S.; Chiang, K.; Regan-Mochrie, G.L.; Choi, S.Y.; Ward, C.M.; Sahay, D.; Garcia, P.; Arnold, R.; Davies, C.C. PRMT5-regulated splicing of DNA repair genes drives chemoresistance in breast cancer stem cells. Oncogene 2025, 44, 862–876. [Google Scholar] [CrossRef]
- Xiang, L.; Semenza, G.L. Hypoxia-inducible factors promote breast cancer stem cell specification and maintenance in response to hypoxia or cytotoxic chemotherapy. Adv. Cancer Res. 2019, 141, 175–212. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Zhang, R.; Tu, J.; Liu, S. Novel molecular regulators of breast cancer stem cell plasticity and heterogeneity. Semin. Cancer Biol. 2022, 82, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, Q.; Jiang, X.; Wang, T.; Li, Z.; Qing, H.; Dong, Y.; Ma, Y.; Zhao, B.; Zhang, J.; et al. Targeting UBE2T suppresses breast cancer stemness through CBX6-mediated transcriptional repression of SOX2 and NANOG. Cancer Lett. 2024, 611, 217409. [Google Scholar] [CrossRef]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef]
- Xu, J.; Wu, K.J.; Jia, Q.J.; Ding, X.F. Roles of miRNA and lncRNA in triple-negative breast cancer. J. Zhejiang Univ. Sci. B 2020, 21, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, S.T.; Orre, C.; Bertevello, P.S.; Mirebeau-Prunier, D.; Dumas, J.F.; Desquiret-Dumas, V. Breast Cancer Chemoresistance: Insights into the Regulatory Role of lncRNA. Int. J. Mol. Sci. 2023, 24, 15897. [Google Scholar] [CrossRef]
- Nadhan, R.; Isidoro, C.; Song, Y.S.; Dhanasekaran, D.N. LncRNAs and the cancer epigenome: Mechanisms and therapeutic potential. Cancer Lett. 2024, 605, 217297. [Google Scholar] [CrossRef]
- Jin, H.; Du, W.; Huang, W.; Yan, J.; Tang, Q.; Chen, Y.; Zou, Z. lncRNA and breast cancer: Progress from identifying mechanisms to challenges and opportunities of clinical treatment. Mol. Ther. Nucleic Acids 2021, 25, 613–637. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, S.S.; Aure, M.R.; Häkkinen, J.; Vallon-Christersson, J.; Kumar, S.; Evensen, K.B.; Fleischer, T.; Tost, J.; Osbreac; Sahlberg, K.K.; et al. Subtype and cell type specific expression of lncRNAs provide insight into breast cancer. Commun. Biol. 2022, 5, 834. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, K.; Wang, J.; Wang, X.; Cheng, K.; Shi, F.; Jiang, L.; Zhang, Y.; Dou, J. MiR-7, inhibited indirectly by lincRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of breast cancer stem cells by downregulating the STAT3 pathway. Stem Cells 2014, 32, 2858–2868. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, P.; Wang, J.; Shu, Y.; Zhong, X.; Gao, Z.; Yang, J.; Jiang, Y.; Zhou, X.; et al. Long noncoding RNA HOTAIR regulates the stemness of breast cancer cells via activation of the NF-kappaB signaling pathway. J. Biol. Chem. 2022, 298, 102630. [Google Scholar] [CrossRef]
- Kallen, A.N.; Zhou, X.B.; Xu, J.; Qiao, C.; Ma, J.; Yan, L.; Lu, L.; Liu, C.; Yi, J.S.; Zhang, H.; et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell 2013, 52, 101–112. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, Y.; Shang, L.; Qiu, Y.; Shen, N.; Wang, J.; Adam, T.; Wei, W.; Song, Q.; Li, J.; et al. LncRNA XIST regulates breast cancer stem cells by activating proinflammatory IL-6/STAT3 signaling. Oncogene 2023, 42, 1419–1437. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Jing, C.; Xiao, C.; Li, T. An autophagy-related long non-coding RNA prognostic signature accurately predicts survival outcomes in bladder urothelial carcinoma patients. Aging 2020, 12, 15624–15637. [Google Scholar] [CrossRef]
- Wang, N.; Li, J.; Xin, Q.; Xu, N. USP30-AS1 contributes to mitochondrial quality control in glioblastoma cells. Biochem. Biophys. Res. Commun. 2021, 581, 31–37. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, S.; Deng, T.; Zhou, R.; Wang, C. LncRNA USP30-AS1 promotes the survival of acute myeloid leukemia cells by cis-regulating USP30 and ANKRD13A. Hum. Cell 2022, 35, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liang, X.; Liu, Y. lncRNA USP30-AS1 sponges miR-765 and modulates the progression of colon cancer. World J. Surg. Oncol. 2022, 20, 73. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, J.; Sun, X.; Zhao, R.; Gao, K. Prognostic role of long non-coding RNA USP30-AS1 in ovarian cancer: Insights into immune cell infiltration in the tumor microenvironment. Aging 2023, 15, 13776–13798. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chi, Y.; Chen, H.; Zhao, L. Long non-coding RNA USP30-AS1 aggravates the malignant progression of cervical cancer by sequestering microRNA-299-3p and thereby overexpressing PTP4A1. Oncol. Lett. 2021, 22, 505. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Jiang, Y.; Zhang, H.; Yang, S.; Xie, W.; Xu, N. USP30-AS1 Suppresses Colon Cancer Cell Inflammatory Response Through NF-κB/MYBBP1A Signaling. Inflammation 2025, 48, 11. [Google Scholar] [CrossRef]
- Jiang, Y.; Liao, W.; Xin, Q.; Wang, R.; Lin, G.; Li, J.; Yang, Z.; Yang, S.; Zhang, H.; Li, X.; et al. Nuclear and cytoplasmic USP30-AS1 coordinately regulate breast cancer progression through HnRNPF/p21 and EZH2/c-Myc/p21 axes. Genes Dis. 2025, 101684. [Google Scholar] [CrossRef]
- Castagnoli, L.; Cancila, V.; Cordoba-Romero, S.L.; Faraci, S.; Talarico, G.; Belmonte, B.; Iorio, M.V.; Milani, M.; Volpari, T.; Chiodoni, C.; et al. WNT signaling modulates PD-L1 expression in the stem cell compartment of triple-negative breast cancer. Oncogene 2019, 38, 4047–4060. [Google Scholar] [CrossRef]
- Yousefnia, S.; Seyed Forootan, F.; Seyed Forootan, S.; Nasr Esfahani, M.H.; Gure, A.O.; Ghaedi, K. Mechanistic Pathways of Malignancy in Breast Cancer Stem Cells. Front. Oncol. 2020, 10, 452. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Xu, F.; Jiang, S. Wnt signaling in breast cancer: Biological mechanisms, challenges and opportunities. Mol. Cancer 2020, 19, 165. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Wu, J.F.; Luo, Q.C.; Liu, Q.F.; Wu, Q.W.; Ye, G.D.; She, H.Q.; Li, B.A. Pygo2 activates MDR1 expression and mediates chemoresistance in breast cancer via the Wnt/beta-catenin pathway. Oncogene 2016, 35, 4787–4797. [Google Scholar] [CrossRef]
- Nandi, S.K.; Roychowdhury, T.; Chattopadhyay, S.; Basu, S.; Chatterjee, K.; Choudhury, P.; Banerjee, N.; Saha, P.; Mukhopadhyay, S.; Mukhopadhyay, A.; et al. Deregulation of the CD44-NANOG-MDR1 associated chemoresistance pathways of breast cancer stem cells potentiates the anti-cancer effect of Kaempferol in synergism with Verapamil. Toxicol. Appl. Pharmacol. 2022, 437, 115887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.M.; Zhang, J.G.; Zhang, X.; Li, Q. Targeting cancer stem cells for reversing therapy resistance: Mechanism, signaling, and prospective agents. Signal Transduct. Target. Ther. 2021, 6, 62. [Google Scholar] [CrossRef]
- Paul, R.; Dorsey, J.F.; Fan, Y. Cell plasticity, senescence, and quiescence in cancer stem cells: Biological and therapeutic implications. Pharmacol. Ther. 2022, 231, 107985. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, B. MicroRNA miR-3646 promotes malignancy of lung adenocarcinoma cells by suppressing sorbin and SH3 domain-containing protein 1 via the c-Jun NH2-terminal kinase signaling pathway. Bioengineered 2022, 13, 4869–4884. [Google Scholar] [CrossRef]
- Tao, S.; Liu, Y.B.; Zhou, Z.W.; Lian, B.; Li, H.; Li, J.P.; Zhou, S.F. miR-3646 promotes cell proliferation, migration, and invasion via regulating G2/M transition in human breast cancer cells. Am. J. Transl. Res. 2016, 8, 1659–1677. [Google Scholar] [PubMed]
- Zhang, X.; Zhong, S.; Xu, Y.; Yu, D.; Ma, T.; Chen, L.; Zhao, Y.; Chen, X.; Yang, S.; Wu, Y.; et al. MicroRNA-3646 Contributes to Docetaxel Resistance in Human Breast Cancer Cells by GSK-3beta/beta-Catenin Signaling Pathway. PLoS ONE 2016, 11, e0153194. [Google Scholar] [CrossRef]
- Venkatesh, J.; Wasson, M.D.; Brown, J.M.; Fernando, W.; Marcato, P. LncRNA-miRNA axes in breast cancer: Novel points of interaction for strategic attack. Cancer Lett. 2021, 509, 81–88. [Google Scholar] [CrossRef]
- Entezari, M.; Taheriazam, A.; Orouei, S.; Fallah, S.; Sanaei, A.; Hejazi, E.S.; Kakavand, A.; Rezaei, S.; Heidari, H.; Behroozaghdam, M.; et al. LncRNA-miRNA axis in tumor progression and therapy response: An emphasis on molecular interactions and therapeutic interventions. Biomed. Pharmacother. 2022, 154, 113609. [Google Scholar] [CrossRef]
- Shen, P.; Yu, Y.; Yan, Y.; Yu, B.; You, W. LncRNA CASC15 regulates breast cancer cell stemness via the miR-654-5p/MEF2D axis. J. Biochem. Mol. Toxicol. 2022, 36, e23023. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, R.; Wu, X.; Piao, Z.; Zhang, M.; Jin, T. LncRNA HAGLROS promotes breast cancer evolution through miR-135b-3p/COL10A1 axis and exosome-mediated macrophage M2 polarization. Cell Death Dis. 2024, 15, 633. [Google Scholar] [CrossRef]
- Sengupta, P.; Roy, A.; Roy, L.; Bose, D.; Halder, S.; Jana, K.; Mukherjee, G.; Chatterjee, S. Long non-coding intergenic RNA, LINC00273 induces cancer metastasis and stemness via miRNA sponging in triple negative breast cancer. Int. J. Biol. Macromol. 2024, 274 Pt 1, 132730. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Chen, B.; Zhu, Z.; Du, C.; Gao, S.; Zhao, G.; Zhao, P.; Wang, Y.; Wang, A.; Schwartz, Z.; et al. Long noncoding RNA (lncRNA) H19: An essential developmental regulator with expanding roles in cancer, stem cell differentiation, and metabolic diseases. Genes Dis. 2023, 10, 1351–1366. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Yang, S.; Xin, Q.; Jiang, Y.; Jiang, L.; Xu, N. USP30-AS1 Dictates Breast Cancer Cell Fate and Chemoresistance via a miR-3646/FZD7/Wnt/β-Catenin Circuit. Curr. Issues Mol. Biol. 2025, 47, 974. https://doi.org/10.3390/cimb47120974
He Q, Yang S, Xin Q, Jiang Y, Jiang L, Xu N. USP30-AS1 Dictates Breast Cancer Cell Fate and Chemoresistance via a miR-3646/FZD7/Wnt/β-Catenin Circuit. Current Issues in Molecular Biology. 2025; 47(12):974. https://doi.org/10.3390/cimb47120974
Chicago/Turabian StyleHe, Qian, Shiyue Yang, Qilei Xin, Yapei Jiang, Li Jiang, and Naihan Xu. 2025. "USP30-AS1 Dictates Breast Cancer Cell Fate and Chemoresistance via a miR-3646/FZD7/Wnt/β-Catenin Circuit" Current Issues in Molecular Biology 47, no. 12: 974. https://doi.org/10.3390/cimb47120974
APA StyleHe, Q., Yang, S., Xin, Q., Jiang, Y., Jiang, L., & Xu, N. (2025). USP30-AS1 Dictates Breast Cancer Cell Fate and Chemoresistance via a miR-3646/FZD7/Wnt/β-Catenin Circuit. Current Issues in Molecular Biology, 47(12), 974. https://doi.org/10.3390/cimb47120974








