Abstract
Epilepsy affects approximately 50 million people worldwide, with nearly one-third of patients experiencing inadequate seizure control with conventional anti-epileptic drugs. The GABAergic system, responsible for inhibitory neurotransmission in the central nervous system, represents a critical target for seizure management. GABA aminotransferase (GABA-T), the enzyme responsible for GABA catabolism, has emerged as a particularly attractive therapeutic target. Inhibition of GABA-T increases synaptic GABA availability, enhancing inhibitory neurotransmission and raising the seizure threshold. Vigabatrin, an irreversible GABA-T inhibitor, has demonstrated remarkable efficacy in specific epilepsy syndromes, particularly infantile spasms and refractory partial seizures. However, its clinical utility is tempered by the risk of irreversible visual field defects, necessitating careful patient selection and monitoring. This review examines the molecular biology of GABA-T, the mechanisms of action of its inhibitors, clinical applications, safety considerations, and emerging developments in this therapeutic area. We discuss the structure–function relationships of GABA-T, the pharmacology of vigabatrin and experimental inhibitors, clinical efficacy across various epilepsy syndromes, adverse effect profiles, and future directions including novel inhibitors with improved safety profiles. Understanding the role of GABA-T in epilepsy pathophysiology and the therapeutic potential of its inhibitors provides insights into rational drug design and personalized treatment strategies for epilepsy management.
1. Introduction
Epilepsy is one of the most common serious neurological disorders, characterized by recurrent, unprovoked seizures resulting from abnormal, excessive, or synchronous neuronal activity in the brain [1,2,3,4]. According to the World Health Organization, epilepsy affects approximately 50 million individuals worldwide, imposing substantial burdens on patients, families, and healthcare systems [5,6]. Despite the availability of numerous anti-epileptic drugs (AEDs), approximately 30% of epilepsy patients continue to experience inadequate seizure control, defining the population with drug-resistant or refractory epilepsy [7,8].
The fundamental pathophysiology of epilepsy involves an imbalance between excitatory and inhibitory neurotransmission in the central nervous system (CNS) [9,10]. While glutamate serves as the primary excitatory neurotransmitter, γ-aminobutyric acid (GABA) functions as the principal inhibitory neurotransmitter in the mammalian brain. The GABAergic system plays a crucial role in modulating neuronal excitability, and its dysfunction has been implicated in various forms of epilepsy. Consequently, enhancing GABAergic inhibition represents a rational therapeutic strategy for seizure control [11,12,13].
Multiple approaches have been developed to enhance GABAergic neurotransmission, including direct GABA receptor agonists, modulators of GABA receptor function (such as benzodiazepines and barbiturates), inhibitors of GABA reuptake, and inhibitors of GABA metabolism. Among these strategies, the inhibition of GABA aminotransferase (GABA-T), the primary enzyme responsible for GABA catabolism, has proven particularly effective in specific clinical contexts [14,15,16].
GABA-T catalyzes the conversion of GABA to succinic semialdehyde, representing the rate-limiting step in GABA degradation [17,18]. By inhibiting this enzyme, GABA-T inhibitors increase GABA concentrations in the synaptic cleft and throughout the CNS, thereby enhancing inhibitory neurotransmission [19]. Vigabatrin (γ-vinyl-GABA), an irreversible GABA-T inhibitor, was developed in the 1970s and has since become an established treatment for specific epilepsy syndromes, particularly infantile spasms (West syndrome) and refractory complex partial seizures [20,21].
The clinical success of vigabatrin has validated GABA-T as a therapeutic target, yet its use has been limited by significant adverse effects, most notably irreversible bilateral concentric visual field defects [21,22]. This safety concern has motivated ongoing research into alternative GABA-T inhibitors with improved therapeutic indices, as well as refined patient selection criteria and monitoring strategies.
This comprehensive review examines the role of GABA-T in epilepsy pathophysiology and treatment. We begin by reviewing GABAergic neurotransmission and its relevance to epilepsy, then explore the molecular biology, structure, and function of GABA-T. Subsequently, we examine the pharmacology and clinical applications of GABA-T inhibitors, with particular emphasis on vigabatrin. We discuss the safety profile and monitoring requirements for these agents, genetic considerations affecting drug response, and emerging research directions. Finally, we consider the challenges and future prospects for GABA-T inhibition as an anti-epileptic strategy.
2. GABA and GABAergic Neurotransmission
2.1. The Central Role of GABA in Neuronal Inhibition
γ-Aminobutyric acid (GABA) is the predominant inhibitory neurotransmitter in the mammalian central nervous system, playing a fundamental role in regulating neuronal excitability and maintaining the balance between excitation and inhibition [23,24]. Discovered in 1950 by Eugene Roberts and Sam Frankel, GABA is present in high concentrations throughout the brain, with GABAergic neurons comprising approximately 20–30% of all cortical neurons [25,26]. The GABAergic system is essential for numerous physiological processes, including motor control, sensory perception, cognitive function, anxiety regulation, and sleep–wake cycles [27,28,29].
GABA mediates its inhibitory effects through two major classes of receptors: ionotropic GABAA receptors and metabotropic GABAB receptors [30]. GABAA receptors are ligand-gated chloride channels that mediate fast inhibitory neurotransmission. Upon GABA binding, these receptors undergo conformational changes that open the chloride channel, allowing chloride ions to flow into the neuron [31,32]. This influx of negatively charged chloride ions hyperpolarizes the neuronal membrane, moving the membrane potential away from the threshold for action potential generation and thereby reducing neuronal excitability [33].
GABAA receptors are heteromeric pentamers typically composed of two α subunits, two β subunits, and one γ subunit, though considerable subunit diversity exists [25,34]. This heterogeneity contributes to the pharmacological and functional diversity of GABAergic inhibition across brain regions. GABAA receptors are also the primary targets for several important classes of psychoactive drugs, including benzodiazepines, barbiturates, neurosteroids, and certain anesthetics, which act as positive allosteric modulators to enhance GABAergic inhibition [25,35].
GABAB receptors, in contrast, are G-protein-coupled receptors that mediate slower, prolonged inhibitory responses [36,37]. These receptors function as obligate heterodimers composed of GABA-B1 and GABA-B2 subunits [38,39,40]. Activation of GABAB receptors leads to several downstream effects: activation of inwardly rectifying potassium channels (causing hyperpolarization), inhibition of voltage-gated calcium channels (reducing neurotransmitter release), and modulation of various intracellular signaling pathways [41,42]. GABAB receptors are located both postsynaptically, where they mediate slow inhibitory postsynaptic potentials, and presynaptically, where they function as autoreceptors to regulate GABA release [30].
2.2. GABA Synthesis and Metabolism
GABA is synthesized from glutamate by the enzyme glutamic acid decarboxylase (GAD), which exists in two isoforms: GAD65 and GAD67. This reaction requires pyridoxal 5′-phosphate (vitamin B6) as a cofactor [43,44,45]. GAD65 is primarily localized to nerve terminals and is associated with vesicular GABA synthesis for neurotransmission, while GAD67 is distributed throughout the neuron and may contribute more to metabolic GABA pools [43,46,47,48]. The synthesis of GABA from glutamate represents a unique metabolic feature whereby the principal excitatory neurotransmitter is converted into the principal inhibitory neurotransmitter (Figure 1).
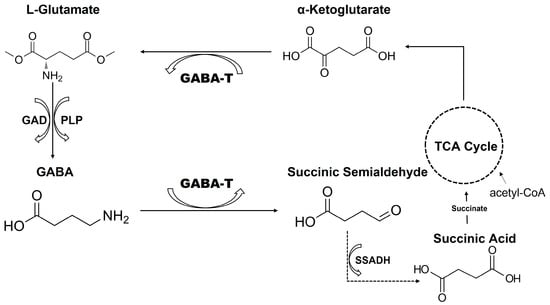
Figure 1.
Schematic representation of the catalytic mechanism of GABA. Glutamic acid decarboxylase (GAD) converts L-glutamate to GABA using PLP (pyridoxal 5′-phosphate) as a cofactor. GABA is then metabolized by GABA aminotransferase (GABA-T) to succinic semialdehyde, which is subsequently oxidized by succinic semialdehyde dehydrogenase (SSADH) to succinic acid, linking GABA metabolism to the tricarboxylic acid (TCA) cycle.
Following its release into the synaptic cleft and receptor binding, GABA is rapidly removed from the extracellular space by high-affinity GABA transporters (GATs). Four subtypes of GABA transporters have been identified in mammals: GAT-1, GAT-2, GAT-3, and the betaine/GABA transporter (BGT-1) [49,50]. GAT-1 is the predominant neuronal transporter and represents the primary target for GABA reuptake inhibitors such as tiagabine. These transporters are expressed on both neurons and astrocytes, enabling efficient clearance of synaptic GABA and termination of inhibitory signaling [51].
Once taken up into neurons or glia, GABA undergoes metabolic degradation primarily through the GABA shunt pathway, an alternative metabolic route that bypasses two steps of the tricarboxylic acid (TCA) cycle. The first and rate-limiting step of GABA catabolism is catalyzed by GABA aminotransferase (GABA-T) [52,53].
2.3. GABAergic Dysfunction in Epilepsy
The central role of γ-aminobutyric acid (GABA)-mediated inhibition in regulating neuronal excitability makes the GABAergic system a critical element in the pathogenesis of epilepsy [54]. Epileptic seizures arise when the balance between excitation and inhibition is disrupted, leading to excessive synchronous neuronal firing that overwhelms inhibitory control mechanisms [55]. Increasing evidence from clinical, genetic, and experimental studies has established that impairments in GABAergic signaling are a major contributor to seizure generation and epileptogenesis.
Clinical studies of epileptic brain tissue, particularly from patients with temporal lobe epilepsy (TLE), focal cortical dysplasia, and drug-resistant epilepsies, have identified multiple alterations in GABAergic markers [56,57,58,59]. These include reduced GABA concentrations, selective loss of GABAergic interneuron subtypes such as parvalbumin- and somatostatin-positive interneurons, and region-specific reductions in GABAergic synaptic density within the hippocampus, dentate gyrus, and temporal neocortex [58,60,61]. Changes in the expression and subunit composition of GABAA and GABAB receptors have also been documented, with several studies reporting downregulation of α1-containing GABAA receptors and compensatory increases in α4-containing subunits, which exhibit altered pharmacological properties and reduced inhibitory strength. These receptor remodeling events may contribute to decreased seizure threshold and benzodiazepine resistance in chronic epilepsy [62,63].
Genetic evidence further supports a direct link between GABAergic dysfunction and seizure susceptibility. Mutations in genes encoding GABAA receptor subunits, such as GABRA1, GABRB3, GABRG2, and GABRD, have been identified in patients with idiopathic generalized epilepsies, Dravet syndrome, childhood absence epilepsy, and febrile seizures [64,65]. Many of these mutations result in reduced receptor trafficking to the membrane, impaired channel gating, or dominant-negative effects that substantially diminish inhibitory tone. Additionally, variants in genes encoding GABA transporters (e.g., SLC6A1) and enzymes involved in GABA metabolism have broadened recognition of genetic GABAergic defects in epileptogenesis.
Experimental models have provided further mechanistic insights. Pharmacological blockade of GABAA receptors using bicuculline, picrotoxin, or PTZ reliably induces seizures across species, confirming the necessity of intact GABAergic inhibition for maintaining normal neuronal activity [66,67]. Conversely, many clinically effective antiseizure medications, including benzodiazepines, barbiturates, vigabatrin, and tiagabine, act by enhancing GABAergic signaling, underscoring the therapeutic relevance of this pathway [68]. Genetic mouse models with targeted disruption of GABAA receptor subunits or interneuron development pathways (e.g., Arx, Dlx, or Gabrb3 knockout lines) frequently exhibit spontaneous seizures and behavioral phenotypes analogous to human epileptic syndromes, reinforcing the causal role of impaired inhibition [69,70].
The temporal dynamics of GABAergic changes in epilepsy are complex. Acute seizures may transiently enhance GABA release or upregulate certain GABA receptor subunits as an adaptive response, whereas chronic epilepsy is commonly associated with interneuron loss, impaired GABA synthesis, deficits in chloride homeostasis (e.g., NKCC1/KCC2 imbalance), and synaptic reorganization that collectively reduce inhibitory efficacy. Such long-term alterations contribute to hyperexcitable circuits capable of generating spontaneous recurrent seizures [54].
Importantly, different forms of epilepsy may involve distinct mechanisms of GABAergic dysfunction. For example, Temporal lobe epilepsy (TLE): marked loss of hippocampal interneurons, mossy fiber sprouting, and altered δ-subunit-containing GABAA receptors; Absence epilepsy: disturbances in thalamocortical oscillations involving enhanced GABAB receptor-mediated inhibition and altered tonic GABA currents in thalamic relay neurons; Focal cortical dysplasia: disrupted interneuron migration and abnormal lamination result in spatially heterogeneous inhibitory deficits; and Neonatal seizures: immature chloride transporter expression renders GABA depolarizing rather than inhibitory, contributing to seizure susceptibility [71]. Understanding these distinct patterns of GABAergic impairment is crucial for the rational design of targeted therapies. Emerging research aims to develop strategies that restore inhibitory balance, including interneuron transplantation, modulation of chloride transport, and selective targeting of GABA metabolic enzymes such as GABA-T.
2.4. Therapeutic Implications
The central role of GABA in controlling neuronal excitability has made the GABAergic system a major target for anti-epileptic drug development. Multiple mechanisms for enhancing GABAergic inhibition have been successfully exploited therapeutically. These include positive allosteric modulation of GABAA receptors (benzodiazepines, barbiturates), inhibition of GABA reuptake (tiagabine), and inhibition of GABA catabolism (vigabatrin) [72,73,74,75].
Each approach has distinct advantages and limitations. Positive allosteric modulators of GABAA receptors are highly effective for acute seizure control but are associated with tolerance, dependence, and sedation [68,76]. GABA reuptake inhibitors increase synaptic GABA availability but may have limited efficacy due to compensatory mechanisms. GABA-T inhibitors offer the advantage of increasing GABA levels throughout all compartments of the CNS, potentially providing broader therapeutic effects [77].
The success of GABAergic AEDs in clinical practice validates this therapeutic strategy while highlighting the importance of developing agents with improved selectivity, efficacy, and safety profiles. Understanding the detailed molecular mechanisms of GABAergic neurotransmission and its metabolism continues to inform drug discovery efforts aimed at optimizing seizure control while minimizing adverse effects.
3. GABA Aminotransferase: Structure, Function, and Mechanism
3.1. Molecular Structure and Organization
GABA aminotransferase (GABA-T; EC 2.6.1.19), also known as γ-aminobutyrate:α-ketoglutarate aminotransferase, is a pyridoxal 5′-phosphate (PLP)-dependent enzyme that catalyzes the initial step in GABA catabolism (Table 1). The enzyme is encoded by the ABAT gene in humans, located on chromosome 16p13.2. The gene consists of 16 exons and spans approximately 100 kilobases of genomic DNA [52,78,79].
GABA-T is a homodimeric protein with a molecular mass of approximately 109 kDa (54.5 kDa per monomer). Crystal structures of GABA-T from various species, including pig, mouse, and bacterial sources, have been solved, providing detailed insights into its three-dimensional architecture. Each monomer consists of approximately 500 amino acids and adopts a characteristic fold found in PLP-dependent aminotransferases [80].
The structure of GABA-T can be divided into two distinct domains: a large domain containing the active site and a small domain involved in dimerization and substrate binding. The large domain displays a typical α/β architecture with a central β-sheet surrounded by α-helices. The active site is located at the interface between the two domains and is formed by residues from both monomers of the homodimer, explaining the functional importance of the dimeric quaternary structure [25,52].

Table 1.
Structural and Functional Characteristics of GABA-T.
Table 1.
Structural and Functional Characteristics of GABA-T.
| Feature | Description | References |
|---|---|---|
| Enzyme name | GABA aminotransferase (GABA-T); EC 2.6.1.19 | [80,81] |
| Gene | ABAT (chromosome 16p13.2, 16 exons) | |
| Molecular mass | ~109 kDa (homodimer; ~54.5 kDa per monomer) | |
| Amino acid residues | ~500 amino acids per monomer | |
| Domain structure | Large catalytic domain (residues 48–374, active site Lys329); small domain (residues 1–47 and 375–500) involved in dimerization and substrate binding | |
| Cofactor | Pyridoxal 5′-phosphate (PLP) covalently bound to Lys329 via Schiff base | |
| Substrate specificity | Prefers GABA and α-ketoglutarate; catalyzes formation of succinic semialdehyde and glutamate | |
| Subcellular localization | Mitochondrial matrix (post-cleavage of targeting sequence) | |
| Physiological function | Catalyzes rate-limiting step in GABA degradation; links neurotransmitter metabolism to energy production |
The PLP cofactor is covalently bound to a conserved lysine residue (Lys329 in human GABA-T) through a Schiff base linkage, forming an internal aldimine. The PLP-binding site is highly conserved among aminotransferases and includes residues that properly orient and stabilize the cofactor [82,83,84]. The pyridine ring of PLP is held in position through interactions with aromatic residues and hydrogen bonding networks, ensuring optimal geometry for catalysis (Figure 2).
A distinctive feature of GABA-T is its relatively narrow substrate-binding pocket, which accommodates the short, four-carbon structure of GABA while excluding larger amino acids [85]. Key residues lining the substrate-binding site include Glu270, Arg192, and Phe189, which interact with the amino and carboxyl groups of GABA. This specificity is crucial for the enzyme’s physiological function and has important implications for inhibitor design.
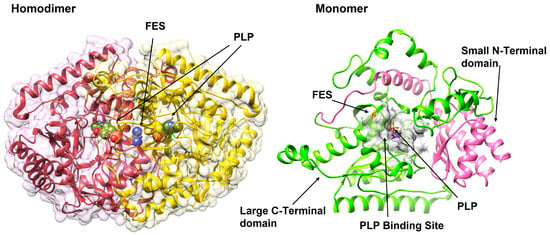
Figure 2.
The 3D structural overview of GABA-T (PDB ID: 1OHW). The enzyme exists as a functional homodimer (left), with each monomer (right) comprising a large C-terminal catalytic domain (green) and a small N-terminal domain involved in dimerization and substrate binding (pink). The PLP cofactor and FES (2Fe-2S; iron-sulfur cluster), an intrinsic cofactor, are shown at the active site within the PLP-binding pocket (grey).
3.2. Catalytic Mechanism
GABA-T catalyzes the reversible transamination of GABA and α-ketoglutarate to produce succinic semialdehyde and glutamate. The reaction proceeds through the classic ping-pong bi-bi mechanism characteristic of PLP-dependent aminotransferases, involving two half-reactions [86,87,88].
In the first half-reaction, GABA binds to the active site and forms an external aldimine with the PLP cofactor, displacing the lysine residue. A catalytic base (typically Lys329) abstracts the α-proton from GABA, and the resulting carbanion intermediate is stabilized by the electron-withdrawing PLP system. The Schiff base then undergoes transamination, releasing succinic semialdehyde and leaving the PLP in its pyridoxamine 5′-phosphate (PMP) form, with the amino group from GABA now attached to the cofactor. In the second half-reaction, α-ketoglutarate binds to the active site and reacts with PMP to form a Schiff base. Through the reverse sequence of steps, the amino group is transferred from PMP to α-ketoglutarate, producing glutamate and regenerating the PLP-enzyme complex. The enzyme is now ready for another catalytic cycle [80].
The catalytic efficiency of GABA-T is impressive, with kcat values ranging from 10 to 50 s−1 and Km values for GABA typically between 1 and 7 mM, depending on the species and experimental conditions. The enzyme shows high specificity for GABA as the amino donor, though it can also accept β-alanine at much lower efficiency. For the amino acceptor, α-ketoglutarate is strongly preferred, although other α-keto acids can serve as substrates at reduced rates [80,89].
3.3. Cellular Localization and Physiological Function
A critical aspect of GABA-T biology is its subcellular localization. The enzyme is localized to the mitochondrial matrix, where it associates with the outer surface of the inner mitochondrial membrane. This localization is directed by an N-terminal mitochondrial targeting sequence that is cleaved upon import into mitochondria. The mature, active form of GABA-T lacks this targeting peptide [90,91].
The mitochondrial localization of GABA-T has several important implications. First, it couples GABA metabolism directly to cellular energy production, as the product succinic semialdehyde is further metabolized to succinate, which enters the tricarboxylic acid cycle. This integration of neurotransmitter metabolism with energy metabolism may have important regulatory functions [91].
Second, the compartmentalization of GABA-T means that GABA must be transported into mitochondria before degradation can occur. This transport process represents an additional regulatory point in GABA homeostasis. The mitochondrial GABA transporter has not been fully characterized, but its activity may influence the rate of GABA catabolism [91,92,93].
Third, the mitochondrial location affects the pharmacokinetics and pharmacodynamics of GABA-T inhibitors. Inhibitors must cross not only the plasma membrane but also the mitochondrial membranes to reach their target enzyme. This requirement has influenced inhibitor design strategies [94].
GABA-T is expressed throughout the brain, with particularly high levels in regions rich in GABAergic neurons, including the cerebral cortex, hippocampus, cerebellum, basal ganglia, and hypothalamus. The enzyme is present in both neurons and astrocytes, reflecting the distributed nature of GABA metabolism. However, the relative contributions of neuronal versus glial GABA-T to overall GABA catabolism remain incompletely understood [95].
3.4. GABA-T in the GABA Shunt Pathway
The GABA shunt represents an alternative route for metabolizing α-ketoglutarate to succinate, bypassing two steps of the citric acid cycle. This pathway consists of three enzymes: glutamate decarboxylase (GAD), GABA-T, and succinic semialdehyde dehydrogenase (SSADH). The net reaction of the shunt converts α-ketoglutarate and glutamate to succinate and GABA, with no net production of ATP or reducing equivalents in the bypass portion (Figure 3) [86,88,91,96].
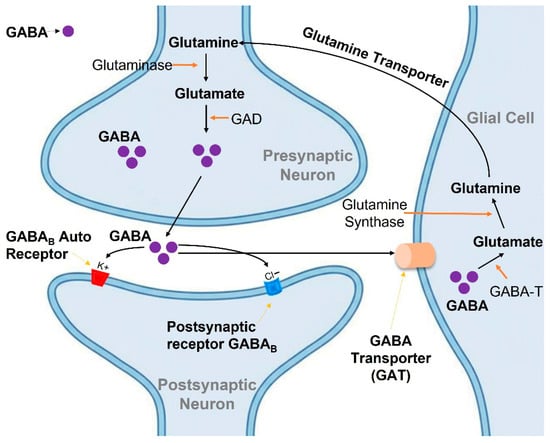
Figure 3.
Schematic illustration of GABAergic neurotransmission and metabolism in the GABA shunt pathway. GABA is synthesized from glutamate by glutamic acid decarboxylase (GAD) in the presynaptic neuron and released into the synaptic cleft to activate GABAB receptors. GABA is then taken up by glial cells and neurons via GABA transporters (GAT) and metabolized by GABA-T, with the resulting glutamate converted to glutamine and recycled back to neurons.
The physiological significance of the GABA shunt has been debated. While initially considered primarily a neurotransmitter metabolism pathway, evidence suggests the shunt may play important roles in cellular energy metabolism, particularly under conditions of metabolic stress. The shunt can function as an anaplerotic pathway, replenishing TCA cycle intermediates, and may help maintain the NADH/NAD+ ratio under certain conditions [97,98].
Importantly, the GABA shunt provides a metabolic link between GABAergic neurotransmission and cellular bioenergetics. Alterations in either neurotransmitter function or energy metabolism can therefore affect the other. This coupling may be particularly relevant in epilepsy, where both excessive neuronal activity and metabolic dysfunction can contribute to pathophysiology [93,94,99].
3.5. GABA-T Regulation, Expression, and Enzymatic Activity
The expression of GABA-T is regulated at multiple levels, including transcriptional control, post-transcriptional modifications, and post-translational regulation. The ABAT gene promoter contains binding sites for several transcription factors, allowing for tissue-specific and developmentally regulated expression [100,101]. GABA-T expression shows developmental changes, generally increasing during postnatal brain maturation. This developmental pattern parallels the maturation of GABAergic systems and may contribute to age-dependent differences in seizure susceptibility and response to GABAergic drugs [102,103,104].
Several studies have examined factors that regulate GABA-T expression. Neuronal activity, hormones, and metabolic status can all influence GABA-T levels. For example, chronic seizure activity has been reported to alter GABA-T expression in some brain regions, potentially representing a compensatory response to altered GABA metabolism [105].
Post-translational modifications of GABA-T, including phosphorylation, may modulate enzyme activity, though these regulatory mechanisms are not fully characterized. The stability of the GABA-T protein and its turnover rate in cells are areas requiring further investigation.
3.6. Pathological Alterations in GABA-T Function
While increased GABA-T activity has been hypothesized to contribute to epilepsy by accelerating GABA catabolism, direct evidence for this mechanism in human epilepsy is limited. More commonly, alterations in GABA-T expression or activity in epilepsy appear to be secondary consequences of seizure activity rather than primary causative factors.
Conversely, loss-of-function mutations in the ABAT gene cause GABA-T deficiency, a rare autosomal recessive neurometabolic disorder. This condition is characterized by psychomotor retardation, hypotonia, hyperreflexia, seizures, and accelerated linear growth [106,107]. The paradoxical occurrence of seizures in patients with GABA-T deficiency, despite presumed elevation of GABA levels, remains incompletely explained but may relate to altered neuronal development, receptor downregulation, or other compensatory mechanisms.
The study of GABA-T deficiency has provided insights into the physiological roles of this enzyme beyond simple GABA catabolism [86,108]. The diverse clinical manifestations of GABA-T deficiency suggest that the enzyme plays important roles in brain development, metabolic regulation, and perhaps other cellular processes.
4. GABA-T as a Therapeutic Target in Epilepsy
4.1. Rationale for GABA-T Inhibition
The concept of inhibiting GABA-T to enhance GABAergic neurotransmission and thereby suppress seizures is based on solid theoretical foundations. By blocking the primary catabolic pathway for GABA, GABA-T inhibitors increase GABA concentrations throughout the brain [93], including in the synaptic cleft, neuronal cytoplasm, and glial cells. This broad elevation of GABA levels enhances inhibitory tone across neural networks, counteracting the excessive excitability characteristic of epilepsy.
Several features make GABA-T an attractive target for anti-epileptic therapy. First, GABA-T inhibition affects GABA metabolism systemically throughout the CNS rather than modulating individual synapses, potentially providing more comprehensive seizure control [77]. Second, unlike direct receptor agonists, GABA-T inhibitors work by preserving endogenously released GABA, maintaining the spatial and temporal specificity of physiological GABAergic signaling while amplifying its magnitude [17]. Third, GABA-T inhibition may be less likely to cause receptor desensitization or tolerance compared to direct receptor activation [109], though clinical experience suggests some tolerance can still develop.
The elevation of GABA levels following GABA-T inhibition has been convincingly demonstrated through multiple experimental approaches. Magnetic resonance spectroscopy (MRS) studies in humans have shown substantial increases in brain GABA concentrations following vigabatrin treatment, with increases of 100–200% commonly observed [110,111]. These elevations correlate with the degree of GABA-T inhibition and persist for extended periods due to the irreversible nature of vigabatrin’s mechanism.
Animal studies have similarly demonstrated elevated GABA levels in brain tissue, cerebrospinal fluid, and extracellular fluid following GABA-T inhibition [112,113]. Microdialysis studies have shown increased basal GABA levels and enhanced GABA release following neuronal stimulation in vigabatrin-treated animals [114]. These findings confirm that GABA-T inhibition effectively increases GABA availability for neurotransmission.
4.2. Effects on Seizure Threshold and Epileptic Activity
Extensive preclinical studies in animal models have validated the anti-epileptic efficacy of GABA-T inhibition [115,116]. Vigabatrin and other GABA-T inhibitors demonstrate broad-spectrum activity in acute chemical seizure models induced by pentylenetetrazol, picrotoxin, and bicuculline, where elevated extracellular GABA effectively counteracts the reduced inhibition produced by GABAA receptor antagonists, resulting in increased seizure threshold and attenuation of seizure severity [117,118]. Similar effects are observed in electrical seizure models, including maximal electroshock and kindling paradigms, where GABA-T inhibitors reliably raise seizure threshold, reduce after discharge duration, and suppress seizure generalization [68,116,119]. In kindling models that recapitulate progressive aspects of epileptogenesis, vigabatrin has been shown to slow kindling acquisition and delay secondary generalization, indicating potential disease-modifying effects rather than merely symptomatic seizure suppression [120].
Genetic models of epilepsy provide important insights into syndrome-specific responses to GABA-T inhibition. In mice carrying mutations in GABRA1 or GABRG2, which model generalized epilepsies associated with impaired GABAA receptor function, vigabatrin markedly reduces seizure frequency and improves survival, likely by compensating for reduced synaptic inhibition through sustained increases in extracellular GABA [121,122]. In the absence of epilepsy models such as Stargazer and Tottering mice, vigabatrin decreases spike-and-wave discharges, though responsiveness is strain-dependent and influenced by thalamocortical GABAergic mechanisms [123]. Conversely, models such as SCN1A mutant mice (Dravet syndrome) demonstrate limited or variable responses to GABA-T inhibition because their underlying pathology reflects sodium channel dysfunction rather than primary deficits in GABAergic inhibition [119]. These findings underscore that GABA-T inhibitors are most effective in epilepsy types where impaired GABAergic signaling is a central pathogenic mechanism.
Mechanistic studies using hippocampal slices and neuronal cultures show that GABA-T inhibition enhances both phasic and tonic GABAergic inhibition. Elevation of tonic inhibitory currents, mediated by high-affinity extrasynaptic GABAA receptors containing δ subunits, appears especially important for reducing network excitability because tonic inhibition provides a persistent, non-desensitizing inhibitory influence on neuronal firing [77,112,124,125]. Electrophysiological recordings further demonstrate that GABA-T inhibition prolongs inhibitory postsynaptic currents, elevates ambient GABA levels, decreases population spike amplitudes, and suppresses epileptiform burst activity in hyperexcitable hippocampal networks [14,126,127,128]. These cellular and network-level effects collectively support the conclusion that GABA-T inhibitors help restore a more physiological balance between excitation and inhibition. Rather than indiscriminately suppressing all neural activity, GABA-T inhibition selectively enhances inhibitory tone in overactive circuits, thereby normalizing firing patterns and reducing pathological synchronization.
4.3. Advantages over Alternative GABAergic Strategies
Compared to other approaches for enhancing GABAergic function, GABA-T inhibition offers several potential advantages. Unlike benzodiazepines and barbiturates, which directly enhance GABAA receptor function, GABA-T inhibitors work by increasing the concentration of endogenous GABA [129,130]. This mechanism preserves the normal spatial and temporal patterns of GABAergic signaling, potentially reducing side effects related to excessive, non-physiological receptor activation.
GABA-T inhibitors differ from GABA reuptake inhibitors in their site of action. While reuptake inhibitors like tiagabine primarily increase GABA concentrations in the synaptic cleft, GABA-T inhibitors elevate GABA throughout all cellular compartments [93,131]. This broader distribution may provide advantages in some clinical contexts, though it may also contribute to adverse effects.
The irreversible mechanism of vigabatrin produces long-lasting GABA elevation even after the drug is cleared from the body. This extended duration of action means that once-daily or even less frequent dosing may be sufficient for seizure control [132,133,134]. However, this irreversibility also means that adverse effects may persist long after drug discontinuation. GABA-T inhibitors may have effects beyond the simple elevation of GABA levels. Some evidence suggests that chronic GABA-T inhibition may induce compensatory changes in GABAergic systems, including alterations in GABA receptor expression or function [134]. These adaptive responses may contribute to both therapeutic effects and the development of tolerance or adverse effects.
4.4. Limitations and Considerations
Despite the strong rationale for GABA-T inhibition, several limitations must be acknowledged. First, the paradox of GABA-T deficiency, in which genetic absence of GABA-T leads to seizures rather than seizure protection [60,68], suggests that the relationship between GABA levels and seizure control is complex. Excessive GABA elevation during critical developmental periods may disrupt normal brain maturation, and chronic supraphysiological GABA levels may trigger compensatory downregulation of inhibitory function [135,136]. Second, GABA-T inhibition affects GABA metabolism throughout the body, not just in epileptic foci. This systemic effect may contribute to adverse effects unrelated to seizure control. The lack of regional or circuit-specific selectivity limits the therapeutic index of current GABA-T inhibitors. Third, individual variability in response to GABA-T inhibitors is substantial, with some patients showing excellent seizure control while others derive minimal benefit [137]. The molecular basis for this variability is incompletely understood but likely involves differences in epilepsy etiology, GABAergic system function, and pharmacogenomic factors. Fourth, the development of tolerance to GABA-T inhibitors has been observed in some patients, potentially limiting long-term efficacy [138,139]. The mechanisms underlying tolerance development, whether pharmacokinetic adaptation, pharmacodynamic compensation, or disease progression, require further elucidation.
Despite these limitations, the clinical success of vigabatrin in specific epilepsy syndromes has validated GABA-T as a therapeutic target. Ongoing research aims to develop next-generation GABA-T inhibitors that retain efficacy while overcoming the limitations of current agents, particularly the risk of visual field defects.
5. Molecular and Genetic Considerations
5.1. GABA-T Gene Mutations and Deficiency Phenotypes
Loss-of-function mutations in the ABAT gene, which encodes GABA transaminase (GABA-T), cause an autosomal recessive disorder known as GABA-T deficiency. Fewer than a hundred cases have been reported, typically presenting in infancy with severe psychomotor retardation, hypotonia, seizures, movement disorders, and variable hepatic dysfunction.
Distinctive systemic findings include accelerated linear growth, advanced bone age, and elevated growth hormone and IGF-1 levels. Neuroimaging often reveals cerebral atrophy, delayed myelination, and hyperintense T2 signals in the globus pallidus and thalamus. Biochemically, cerebrospinal fluid (CSF) GABA concentrations are markedly elevated, up to twentyfold higher than normal, accompanied by increases in β-alanine and homocarnosine [140,141].
5.2. Genetic Spectrum and Mutation Types
Pathogenic ABAT variants include nonsense, missense, frameshift, and splice-site mutations, with most patients being compound heterozygotes. These mutations disrupt enzyme function, but genotype–phenotype correlations remain unclear due to the rarity and heterogeneity of reported cases [142].
5.3. The GABA-T Deficiency Paradox
Despite profound elevations in GABA levels, many GABA-T-deficient patients experience seizures, a paradox that challenges the assumption that increased GABA is uniformly protective. Proposed mechanisms include developmental interference of excess GABA on neuronal maturation and circuit formation, compensatory downregulation of GABA receptors, mitochondrial dysfunction due to disruption of the GABA shunt, and altered chloride homeostasis leading to excitatory GABAergic signaling during early brain development. Structural brain abnormalities seen in these patients may also contribute to epileptogenesis. This paradox underscores the context-dependent nature of GABAergic neurotransmission [143,144].
5.4. Pharmacogenomics of GABA-T Inhibitor Response
Although routine pharmacogenomic testing is not yet integrated into clinical use of vigabatrin, genetic factors may influence both efficacy and toxicity. Variations within ABAT could modify baseline enzyme activity, vigabatrin binding affinity, or enzyme resynthesis rate following inactivation [78]. Additionally, polymorphisms in other GABAergic system genes, including GABAA receptor subunits (e.g., GABRA1, GABRB3, GABRG2), glutamic acid decarboxylase (GAD1, GAD2), and GABA transporters (SLC6A1), may modulate the drug’s pharmacodynamic effects [145,146].
5.5. Genetic Factors and Retinal Toxicity
Understanding genetic susceptibility to vigabatrin-induced visual field defects remains a key unmet need. Candidate genes under investigation include those involved in retinal oxidative stress defense, taurine metabolism, GABA receptor signaling, and mitochondrial bioenergetics [147]. However, no validated genetic markers predicting retinal toxicity have yet been identified.
5.6. Epilepsy-Associated Gene Interactions
The genetic background of the underlying epilepsy may also influence vigabatrin response. Its exceptional efficacy in tuberous sclerosis complex (TSC), related infantile spasms suggests that TSC1/TSC2 pathway dysregulation may enhance responsiveness to GABAergic modulation [148]. Conversely, in SCN1A-related epilepsies such as Dravet syndrome, vigabatrin’s effects are variable. Mutations in GABAA receptor genes may similarly alter drug response [149], though supporting data remain limited.
6. GABA-T Inhibitors: Development and Mechanisms
6.1. Vigabatrin (γ-vinyl-GABA)
6.1.1. Discovery and Development History
Vigabatrin, chemically known as γ-vinyl-GABA or 4-amino-5-hexenoic acid, represents one of the most successful examples of rational drug design based on enzyme mechanism [150,151]. The compound was first synthesized in the 1970s by researchers at Merrell Dow Pharmaceuticals as part of a program aimed at developing irreversible inhibitors of GABA-T [152,153]. The design strategy was based on the concept of mechanism-based enzyme inactivation, also known as “suicide inhibition” [154].
The structural design of vigabatrin was inspired by the observation that vinyl-substituted amino acids could serve as mechanism-based inhibitors of PLP-dependent enzymes [155]. The molecule consists of GABA with a vinyl group attached to the γ-carbon, creating a structural analog that undergoes the initial steps of normal catalysis but then irreversibly inactivates the enzyme. Initial preclinical studies in the late 1970s demonstrated potent GABA-T inhibition, substantial increases in brain GABA levels, and broad-spectrum anti-seizure activity in animal models [156,157,158,159].
Clinical development of vigabatrin began in the early 1980s [160]. The drug was first approved in the United Kingdom in 1989 as adjunctive therapy for refractory epilepsy [161,162,163]. Subsequently, vigabatrin received regulatory approval in numerous countries throughout Europe, South America, Asia, and other regions. However, approval in the United States was delayed by safety concerns, particularly regarding retinal toxicity observed in preclinical studies and visual field defects reported in clinical trials [161].
The recognition of vigabatrin’s particular efficacy in infantile spasms (West syndrome) emerged from clinical observations in the 1990s. This indication became especially important because infantile spasms are notoriously difficult to treat, and vigabatrin demonstrated response rates superior to many alternative therapies. Based on this evidence, the US Food and Drug Administration (FDA) ultimately approved vigabatrin in 2009 for two specific indications: infantile spasms and refractory complex partial seizures in adults, with a Risk Evaluation and Mitigation Strategy (REMS) program due to the risk of permanent vision loss [164,165].
6.1.2. Mechanism of Irreversible Inhibition
Vigabatrin functions as a mechanism-based irreversible inhibitor, or “suicide substrate,” of GABA-T. This type of inhibition represents an elegant pharmacological strategy in which the inhibitor molecule is a substrate analog that undergoes the initial steps of normal enzyme catalysis. However, during the catalytic process, a highly reactive intermediate is generated that forms a covalent bond with the enzyme, resulting in permanent inactivation [166,167].
The mechanism of vigabatrin’s action begins with the drug entering the GABA-T active site and binding to the PLP cofactor, forming an external aldimine similar to what occurs with the natural substrate GABA. The catalytic base then abstracts the α-proton from vigabatrin, forming a carbanion intermediate stabilized by the PLP system. At this point, the mechanism diverges from normal GABA metabolism. The vinyl group on vigabatrin is positioned such that it can participate in Michael addition chemistry. Following formation of the carbanion, the activated intermediate undergoes an internal rearrangement in which the double bond of the vinyl group becomes conjugated with the PLP system. This creates an α,β-unsaturated imine, a highly electrophilic species capable of reacting with nucleophilic residues in the active site. A nucleophilic amino acid residue in the active site (likely a lysine or cysteine) then attacks the activated intermediate, forming a covalent adduct. This covalent modification irreversibly inactivates the enzyme (Figure 4). Crystallographic studies have confirmed the formation of stable covalent complexes between vigabatrin-derived intermediates and GABA-T. The specific residues modified and the exact structure of the covalent adduct have been characterized through X-ray crystallography and mass spectrometry studies [20,168,169].
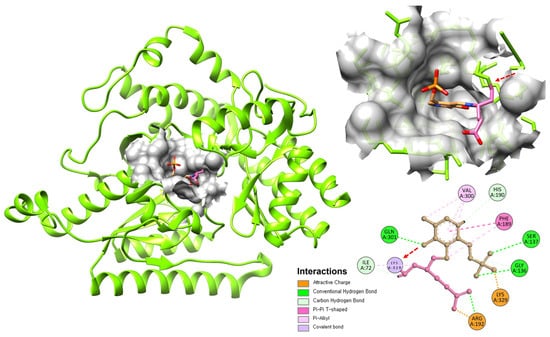
Figure 4.
Binding interaction of GABA-T monomer with PLP and Tiagabine. The structure shows PLP (Pink) and Tiagabine (Brown) bound at the active site of GABA-T. A covalent bond between PLP and Lys329 is highlighted by the red arrow, with the 2D interaction map showing additional hydrogen bonding and hydrophobic interactions stabilizing the complex.
The irreversibility of vigabatrin’s inhibition has profound pharmacological implications. Once GABA-T is inactivated, the enzyme cannot be reactivated. Recovery of GABA-T activity depends entirely on the synthesis of new enzyme protein, a process that takes several days to weeks. This means that the duration of vigabatrin’s pharmacodynamic effect far exceeds its pharmacokinetic half-life in plasma. Even after vigabatrin is eliminated from the body (typically within 24–48 h), GABA levels remain elevated and seizure control persists [80,170].
Vigabatrin is administered as a racemic mixture of S(+) and R(−) enantiomers. The S(+) enantiomer is the active form and is approximately 200-fold more potent as a GABA-T inhibitor than the R(−) enantiomer. However, both enantiomers are absorbed and distributed similarly, and the inactive R(−) enantiomer may contribute to some of the drug’s adverse effects [171,172,173]. The development of enantiopure formulations was explored but ultimately not pursued commercially.
6.1.3. Pharmacokinetics and Pharmacodynamics
Vigabatrin exhibits favorable pharmacokinetic properties that contribute to its clinical utility. The drug is administered orally and is rapidly and almost completely absorbed from the gastrointestinal tract, with peak plasma concentrations typically achieved within 1–2 h after dosing. Bioavailability is approximately 60–80% and is not significantly affected by food, though meals may slightly delay absorption [174,175].
Vigabatrin displays linear pharmacokinetics across the therapeutic dose range, with dose-proportional increases in plasma concentrations. The volume of distribution is approximately 0.8 L/kg, suggesting distribution throughout total body water with minimal tissue binding. Importantly, vigabatrin readily crosses the blood–brain barrier, achieving brain concentrations comparable to plasma levels. This efficient CNS penetration is essential for the drug’s therapeutic action [168].
One of the most distinctive pharmacokinetic features of vigabatrin is its lack of significant protein binding. Approximately 95–99% of vigabatrin in plasma is in the free, unbound form. This characteristic minimizes the potential for protein-binding displacement interactions with other drugs and ensures predictable free drug concentrations [176].
Vigabatrin undergoes minimal metabolism, with approximately 95% of an administered dose excreted unchanged in urine. The small amount of metabolism that occurs produces inactive products and does not involve cytochrome P450 enzymes. This lack of hepatic metabolism has important clinical implications: vigabatrin does not induce or inhibit drug-metabolizing enzymes, resulting in minimal pharmacokinetic interactions with other medications. This is a significant advantage in epilepsy treatment, where polypharmacy is common [177]. The elimination half-life of vigabatrin from plasma is approximately 5–8 h in adults, with renal clearance being the primary route of elimination. In patients with impaired renal function, vigabatrin clearance is reduced proportionally to the decrease in creatinine clearance, necessitating dose adjustments. Age also affects pharmacokinetics: elimination is slower in elderly patients (half-life 12–13 h) and faster in children (half-life 4–6 h), requiring age-appropriate dosing strategies [178]. Despite the relatively short plasma half-life, the pharmacodynamic effects of vigabatrin are prolonged due to irreversible enzyme inhibition. Studies using PET imaging and biochemical markers have shown that GABA-T activity remains suppressed for weeks after vigabatrin discontinuation. Brain GABA levels, measured by magnetic resonance spectroscopy, remain elevated for 4–8 weeks after stopping treatment, gradually returning to baseline as new GABA-T enzyme is synthesized [179].
This dissociation between pharmacokinetics and pharmacodynamics has clinical implications for dosing strategies. Vigabatrin is typically administered once or twice daily, and steady-state GABA elevation is achieved within several days. When discontinuing vigabatrin, gradual tapering is recommended not because of withdrawal effects related to drug elimination, but rather to minimize the risk of seizure exacerbation as GABA levels gradually decline.
6.2. Development of Second-Generation Inhibitors
The proven efficacy of vigabatrin, coupled with its risk of visual toxicity, has prompted extensive research into safer and more selective GABA-T inhibitors. Current strategies focus on optimizing pharmacodynamic and pharmacokinetic properties to maintain efficacy while reducing adverse effects. Efforts include developing reversible inhibitors, prodrug formulations, and modified GABA analogs that enhance central nervous system (CNS) selectivity and minimize peripheral exposure.
Unlike vigabatrin’s irreversible mechanism, reversible inhibitors interact non-covalently with GABA-T, allowing temporary enzyme inhibition (Table 2). New strategies involve developing reversible GABA-T inhibitors, such as cyclic GABA analogs and ethanolamine O-sulfate, which interact non-covalently with the enzyme [180,181,182,183]. These compounds offer potential benefits such as dose flexibility, predictable pharmacokinetics, and faster recovery after treatment cessation. Although they have shown modest activity in preclinical models, achieving sufficient potency and brain penetration remains challenging.
CPP-115, a rationally designed analog of vigabatrin (Figure 5), represents a significant advancement in this direction. With enhanced lipophilicity and improved CNS selectivity, CPP-115 achieves greater central GABA-T inhibition at lower systemic concentrations, theoretically reducing retinal exposure. Preclinical results are promising, but clinical development remains in its early stages, and further evaluation is needed to confirm its safety and efficacy [158,184,185,186]. Similarly, γ-acetylenic GABA, another mechanism-based inactivator, has shown high in vitro potency, though its safety has not yet been established.
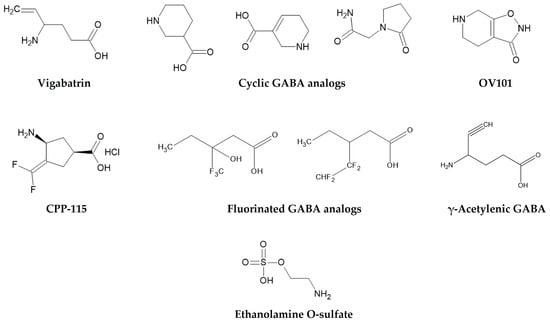
Figure 5.
Chemical structures of vigabatrin and representative GABA-T inhibitors, including cyclic, fluorinated, acetylenic GABA analogs and ethanolamine O-sulfate.
Beyond direct enzyme inhibition, alternative pharmacological strategies have been explored. OV101 (Gaboxadol), a selective agonist at extrasynaptic GABAA receptors containing δ-subunits, enhances tonic inhibition independently of GABA-T. Initially developed for insomnia, Gaboxadol was later evaluated for epilepsy, Angelman syndrome, and Fragile X syndrome, but clinical results were mixed, and development for epilepsy was discontinued [187,188]. Although its mechanism is distinct from GABA-T inhibition, OV101 illustrates how enhancing tonic GABAergic inhibition can be achieved at the receptor level, providing a useful comparator for GABA-T-targeting approaches.
Additionally, fluorinated GABA analogs have been designed to modulate lipophilicity, enzyme affinity, and metabolic stability, with some analogs showing enhanced inhibitory activity in vitro [80,189]. These structural modifications aim to balance potency, selectivity, and safety, although advanced clinical evaluation still remains to be proven.

Table 2.
Comparative Overview of GABA-T Inhibitors.
Table 2.
Comparative Overview of GABA-T Inhibitors.
| Inhibitor | Mechanism of Action | Reversibility | Key Features | Clinical/Development Status | References |
|---|---|---|---|---|---|
| Vigabatrin (γ-vinyl-GABA) | Mechanism-based irreversible inhibition (suicide substrate forming covalent adduct with PLP) | Irreversible | Potent CNS penetration; elevates GABA levels 100–200%; risk of retinal toxicity | FDA-approved for infantile spasms and refractory partial seizures | [190,191] |
| Cyclic GABA analogs | Conformationally restricted inhibitors | Reversible | Modest activity; low potency | Experimental | [112,192,193] |
| OV101 (Gaboxadol) | Selective agonist at extrasynaptic GABAA receptors containing the δ-subunit; enhances tonic inhibition without directly affecting synaptic (phasic) transmission. | Reversible | Acts independently of GABA-T inhibition; increases inhibitory tone through sustained activation of high-affinity GABAA receptors; provides a novel approach to restoring inhibitory balance. | Originally developed for insomnia; later investigated for epilepsy, Angelman syndrome, and Fragile X syndrome. Phase II clinical trials showed mixed efficacy; development for epilepsy was discontinued, but research in neurodevelopmental disorders continues. | [194,195,196] |
| CPP-115 | Vigabatrin analog with improved CNS selectivity and reduced peripheral exposure | Irreversible | Designed to lower retinal toxicity; greater potency at lower doses | Early clinical stage | [197,198,199] |
| Ethanolamine O-sulfate | GABA analog; competitive inhibition of GABA-T | Reversible | Mild anticonvulsant effect; limited potency | Preclinical | [200,201,80] |
| γ-Acetylenic GABA | Mechanism-based inactivator similar to vigabatrin | Irreversible | High in vitro potency; safety not established | Experimental | [202,203] |
| Fluorinated GABA analogs | Modified lipophilicity and enzyme affinity | Reversible or irreversible (depending on analog) | Potentially enhanced selectivity | Preclinical | [204,205,206] |
6.3. Comparative Pharmacology of Reversible and Irreversible Inhibition
Irreversible GABA-T inhibitors such as vigabatrin provide sustained enzyme inactivation, producing long-lasting effects even after treatment cessation. This confers dosing convenience and robust efficacy but also prolongs adverse outcomes, particularly visual toxicity [32,112]. In contrast, reversible inhibitors allow finer dose control and rapid offset of action, theoretically improving safety. However, achieving durable seizure suppression with reversible inhibition remains difficult because of limited residence time and rapid GABA turnover [112,207]. To date, reversible inhibitors have not demonstrated clinical superiority over vigabatrin; however, they may offer an improved side effect profile, particularly with potentially reduced risk of irreversible visual toxicity, though clinical data remain limited.
6.4. Structure–Activity Relationships of GABA-T Inhibitors
Medicinal chemistry efforts have revealed key structural determinants governing GABA-T inhibition. The amino acid backbone, containing both amino and carboxyl groups in specific spatial orientation, is essential for enzyme recognition. GABA’s four-carbon chain provides an optimal fit within the active site, whereas shorter or longer analogs show reduced activity. Substituents at the γ-position, such as the vinyl group in vigabatrin, act as reactive centers enabling mechanism-based inactivation, and variations in these groups markedly influence potency.
Stereochemistry plays a critical role, as only the S(+) enantiomer of vigabatrin exhibits significant activity, approximately 200-fold higher than the R(–) form, highlighting the enzyme’s strict stereospecificity. Lipophilicity determines CNS penetration but must be balanced against solubility and peripheral distribution. Incorporation of heterocycles or ring systems has been employed to optimize binding affinity and pharmacokinetics, provided that the essential pharmacophoric elements are retained [208].
6.5. Experimental and Dual-Action GABA-T Inhibitors
Several experimental compounds have demonstrated promising GABA-T inhibitory activity in preclinical studies. Ethanolamine O-sulfate shows moderate inhibition and anticonvulsant effects but lacks sufficient potency for clinical utility [209,210]. γ-Acetylenic GABA derivatives act via mechanism-based enzyme inactivation similar to vigabatrin, with some analogs displaying superior in vitro potency; however, their in vivo safety remains uncertain [211]. Cyclic GABA analogs designed to restrict conformational flexibility have shown reversible inhibition but modest activity overall [212].
Dual-action molecules that combine GABA-T inhibition with other mechanisms, such as GAT-1 inhibition or GABAA receptor modulation, are also under exploration [68]. These agents aim to enhance efficacy through synergistic effects but introduce additional challenges in selectivity and safety assessment.
6.6. Outlook and Challenges in GABA-T Inhibitor Development
Developing next-generation GABA-T inhibitors require demonstrating clear clinical advantages over vigabatrin, a difficult benchmark given its established efficacy in select epilepsies. New agents must achieve comparable seizure control while markedly reducing retinal toxicity, a task requiring extensive preclinical safety validation. Moreover, the relatively small patient population for conditions such as infantile spasms limit commercial incentives. Nonetheless, advances in structural biology, computational design, and targeted delivery systems offer promising avenues for generating safer and more effective GABA-T inhibitors.
7. Emerging Research and Future Directions
7.1. Innovation in Inhibitor Design and Discovery Strategies
The therapeutic success of vigabatrin has spurred ongoing interest in developing next-generation GABA-T inhibitors with improved safety and selectivity. Advances in structural biology and computational chemistry have enabled more precise structure-based drug design, helping to elucidate key molecular interactions required for selective inhibition [213,214]. Current efforts focus on optimizing CNS specificity while reducing retinal exposure by modulating lipophilicity, utilizing CNS-specific prodrug strategies, and leveraging selective transporters at the blood–brain barrier. Additionally, reversible inhibitors with tunable kinetics are being explored to maintain therapeutic efficacy while allowing faster enzyme recovery upon treatment withdrawal, thereby mitigating cumulative toxicity [215,216,217].
Complementary to these developments, computer-aided drug discovery methods, particularly molecular docking, virtual screening, and related simulation-based approaches, have broadly facilitated the identification and optimization of novel molecular scaffolds across diverse therapeutic targets [218,219,220,221,222,223,224,225], supporting the discovery of candidates with improved pharmacokinetic and pharmacodynamic potential, including emerging compounds designed to modulate GABA metabolism and selectively inhibit GABA-T [213,226,227,228,229]. Fragment-based drug discovery has further expanded chemical diversity and enabled the rational assembly of small fragments into more potent and selective inhibitors. Together, these integrative discovery approaches provide promising starting points for designing safer and more effective GABA-T-targeted therapies [230,231].
7.2. Targeted Delivery and Multi-Mechanistic Therapeutic Strategies
Efforts to improve the clinical safety of GABA-T inhibitors have also turned toward advanced drug delivery and therapeutic combination strategies. Innovative nanocarrier systems aim to enhance brain selectivity while reducing peripheral accumulation, particularly in ocular tissues implicated in vigabatrin-associated retinal toxicity [232]. Localized CNS delivery methods, though largely preclinical, are being explored to restrict drug exposure to epileptogenic brain regions and minimize systemic adverse effects [233,234].
At the same time, rational polytherapy is gaining interest as a strategy to enhance efficacy while lowering individual drug burden. Combining GABA-T inhibitors with agents targeting complementary pathways, such as GABAA receptors, glutamatergic signaling, or neuroprotective mechanisms, may produce synergistic effects at reduced doses. Multi-target drug designs that integrate transporter modulation or receptor interactions with GABA-T inhibition also show promise for achieving more balanced neurochemical regulation. Adjunctive use of antioxidants, mitochondrial stabilizers, or taurine has been proposed as a means to mitigate retinal toxicity associated with long-term vigabatrin therapy [235,236,237].
7.3. Biomarkers, Neuroprotection, and Expanding Clinical Applications
A major future direction in the field involves identifying reliable biomarkers to guide individualized therapy and predict treatment response. Neuroimaging tools such as magnetic resonance spectroscopy can quantify brain GABA levels, whereas functional MRI and PET imaging offer complementary insights into GABAergic network activity and receptor occupancy [238,239]. EEG-based biomarkers, particularly gamma oscillations and event-related potentials, are being investigated for early prediction of therapeutic response in conditions such as infantile spasms. Molecular and genetic markers reflecting GABA metabolism, neuroinflammation, or retinal vulnerability may ultimately support more personalized treatment strategies [240,241].
Beyond seizure suppression, emerging evidence suggests that GABA-T inhibition may exert neuroprotective or anti-epileptogenic effects. Preclinical models show that early intervention can modulate neuroinflammation, reduce aberrant synaptic remodeling, and attenuate neuronal injury, raising the possibility of delaying or preventing chronic epilepsy development. Clinically, early vigabatrin use—especially in infants with tuberous sclerosis complex—has been associated with improved developmental outcomes, although the degree to which this reflects true anti-epileptogenic activity remains under investigation [242,243,244].
Finally, modulation of GABA metabolism is under exploration for therapeutic applications beyond epilepsy. Preliminary studies have assessed vigabatrin and related compounds in substance-use disorders, particularly cocaine and methamphetamine dependence, where enhancing GABAergic inhibition may dampen dopaminergic reward pathways. Additional early investigations have considered potential benefits in neuropathic pain, movement disorders, anxiety, and depression. Continued development of safer, reversible, and more targeted GABA-T inhibitors could revive and expand research into these alternative clinical indications [245,246].
8. Challenges and Limitations
Current monitoring of vigabatrin therapy primarily relies on visual field testing, which can detect retinal damage only after it has occurred and depends heavily on patient cooperation. This makes it particularly unreliable for children, infants, and individuals with cognitive impairment. The test is also subject to variability, learning effects, and significant resource and time demands, limiting its practicality for preventive screening. In pediatric cases, especially in infantile spasms, the urgency of rapid seizure control must be balanced against the risk of potentially irreversible visual toxicity, with treatment decisions often relying on caregiver consent because early ophthalmologic assessment is difficult.
A key challenge arises from the mechanism underlying vigabatrin-associated visual toxicity. Vigabatrin irreversibly inhibits GABA transaminase (GABA-T), increasing GABA concentrations systemically, including in the retina. Excessive retinal GABA may disrupt normal neurotransmission, alter photoreceptor function, and contribute to mitochondrial stress and oxidative damage in retinal cells. Additionally, vigabatrin has been shown to accumulate in the retina at higher levels than in the brain, possibly due to distinct transporter activity, further amplifying toxicity risk. Because the drug binds irreversibly, both therapeutic and adverse effects persist long after dosing, complicating adjustments and making washout studies difficult.
Interindividual responses to vigabatrin vary widely, and no reliable biomarkers exist to predict patients who are most susceptible to visual field loss. Long-term data on visual, cognitive, and developmental outcomes remain limited, highlighting the need for extended and systematic follow-up. Required ophthalmologic monitoring increases the cost and reduces accessibility of vigabatrin therapy, particularly in low-resource settings.
These limitations emphasize the urgent need for safer, more selective GABA-T inhibitors. Novel compounds with improved tissue selectivity, reduced retinal accumulation, reversible binding mechanisms, or lower propensity to elevate retinal GABA levels may retain therapeutic efficacy while significantly reducing toxicity risk. Advances in rational drug design, retinal-specific pharmacokinetic modeling, and biomarker discovery may help guide the development of next-generation GABA-T-targeted therapies that overcome the major barriers associated with vigabatrin.
9. Conclusions
GABA aminotransferase remains a well-validated and clinically important therapeutic target in epilepsy, as demonstrated by the success of vigabatrin in treating infantile spasms and refractory partial seizures. Its role in GABA catabolism makes it ideal for enhancing inhibitory neurotransmission, and vigabatrin’s mechanism-based irreversible inhibition provides sustained seizure control through long-lasting elevation of brain GABA levels. However, this same property underlies its major limitation, the risk of irreversible visual field defects, necessitating cautious and selective clinical use. Despite these drawbacks, the overall therapeutic principle of GABA-T inhibition remains robust. Vigabatrin’s clinical impact highlights the potential of mechanism-driven drug design, while also underscoring the critical need for improved safety and individualized treatment strategies. Future research is focused on developing reversible or more selective GABA-T inhibitors that maintain efficacy but reduce retinal toxicity, as well as innovative delivery methods to enhance CNS targeting while minimizing peripheral exposure. Furthermore, integrating pharmacogenomic and biomarker-based approaches may help identify patients most likely to benefit with minimal risk, paving the way for precision medicine in epilepsy care. The ongoing exploration of combination therapies and neuroprotective adjuncts also offers opportunities to optimize outcomes and mitigate adverse effects.
Ultimately, the legacy of vigabatrin extends beyond its own clinical utility, it exemplifies how a deep understanding of neurochemical pathways can yield transformative therapies while revealing the complexities of balancing efficacy and safety. Continued research into GABA metabolism, enzyme regulation, and patient-specific factors will likely sustain GABA-T inhibition as a cornerstone concept in antiepileptic drug development, guiding the creation of next-generation treatments that offer the benefits of enhanced GABAergic function with improved safety and tolerability.
Author Contributions
Conceptualization, M.Y. and W.C.; methodology, M.Y.; resources, W.C.; data curation, W.C.; writing—original draft preparation, M.Y. and W.C.; writing—review and editing, W.C., J.C. and J.-H.H.; supervision, W.C.; project administration, W.C., J.C. and J.-H.H.; funding acquisition, W.C., J.C. and J.-H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2025-23323186, W.C.) and by the Regional Innovation System & Education (RISE) program through the Gangwon RISE Center, funded by the Ministry of Education (MOE) and the Gangwon State (G.S.), Republic of Korea (2025-RISE-10-002, W.C.), and by Korea Basic Science Institute (National Research Facilities and Equipment Center) grants funded by the Ministry of Education (RS-2020-NF000330, J.C.).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Giourou, E.; Stavropoulou-Deli, A.; Giannakopoulou, A.; Kostopoulos, G.K.; Koutroumanidis, M. Introduction to epilepsy and related brain disorders. In Cyberphysical Systems for Epilepsy and Related Brain Disorders: Multi-Parametric Monitoring and Analysis for Diagnosis and Optimal Disease Management; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–38. [Google Scholar]
- Anwar, H.; Khan, Q.U.; Nadeem, N.; Pervaiz, I.; Ali, M.; Cheema, F.F. Epileptic seizures. Discoveries 2020, 8, e110. [Google Scholar] [CrossRef]
- Potnis, V.; Albhar, K.G.; Nanaware, P.A.; Pote, V.S. A review on epilepsy and its management. J. Drug Deliv. Ther. 2020, 10, 273–279. [Google Scholar] [CrossRef]
- Sazgar, M.; Young, M.G. Seizures and epilepsy. In Absolute Epilepsy and EEG Rotation Review: Essentials for Trainees; Springer: Berlin/Heidelberg, Germany, 2019; pp. 9–46. [Google Scholar]
- De Boer, H.M.; Mula, M.; Sander, J.W. The global burden and stigma of epilepsy. Epilepsy Behav. 2008, 12, 540–546. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Improving the Lives of People with Epilepsy: A Technical Brief; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Trinka, E.; Kwan, P.; Lee, B.; Dash, A. Epilepsy in Asia: Disease burden, management barriers, and challenges. Epilepsia 2019, 60, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Niriayo, Y.L.; Gebregziabher, T.; Demoz, G.T.; Tesfay, N.; Gidey, K. Drug therapy problems and contributing factors among patients with epilepsy. PLoS ONE 2024, 19, e0299968. [Google Scholar] [CrossRef]
- Engelborghs, S.; D’hooge, R.; De Deyn, P. Pathophysiology of epilepsy. Acta Neurol. Belg 2000, 100, 201–213. [Google Scholar]
- Cavazos, J.E.; Sanchez, R. Pathophysiology of seizures and epilepsy. In Epilepsy; CRC Press: Boca Raton, FL, USA, 2004; pp. 23–39. [Google Scholar]
- Walls, A.B.; Waagepetersen, H.S.; Bak, L.K.; Schousboe, A.; Sonnewald, U. The glutamine–glutamate/GABA cycle: Function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem. Res. 2015, 40, 402–409. [Google Scholar] [CrossRef]
- Patri, M. Synaptic transmission and amino acid neurotransmitters. In Neurochemical Basis of Brain Function and Dysfunction; IntechOpen: London, UK, 2019. [Google Scholar]
- Sears, S.M.; Hewett, S.J. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp. Biol. Med. 2021, 246, 1069–1083. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, S.; Arora, A.; Ram, P.; Kumar, S.; Kumar, P.; Abed, S.N. Pharmacology of GABA and its receptors. In Frontiers in Pharmacology of Neurotransmitters; Springer: Berlin/Heidelberg, Germany, 2020; pp. 241–292. [Google Scholar]
- Enna, S.J. The GABA receptors. In The GABA Receptors; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–21. [Google Scholar]
- Murala, S.; Yelam, A.; Ismail, M.M.; Bollu, P.C. Gaba. In Neurochemistry in Clinical Practice; Springer: Berlin/Heidelberg, Germany, 2022; pp. 73–89. [Google Scholar]
- Kabała, K.; Janicka, M. Relationship between the GABA pathway and signaling of other regulatory molecules. Int. J. Mol. Sci. 2024, 25, 10749. [Google Scholar] [CrossRef]
- Jalil, S.U.; Ansari, S.A.; Ansari, M.I. GABA Genetic Engineering/Genome Editing Aspects. In GABA Signaling System and Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2025; pp. 365–377. [Google Scholar]
- Tette, F.-M.; Kwofie, S.K.; Wilson, M.D. Therapeutic anti-depressant potential of microbial GABA produced by Lactobacillus rhamnosus strains for GABAergic signaling restoration and inhibition of addiction-induced HPA axis hyperactivity. Curr. Issues Mol. Biol. 2022, 44, 1434–1451. [Google Scholar] [CrossRef]
- Krämer, G.; Wohlrab, G.C. Vigabatrin. In The Treatment of Epilepsy; Wiley: Hoboken, NJ, USA, 2015; pp. 667–679. [Google Scholar]
- Grant, S.M.; Heel, R.C. Vigabatrin: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in epilepsy and disorders of motor control. Drugs 1991, 41, 889–926. [Google Scholar] [CrossRef] [PubMed]
- Pathan, U.H.A.; Shetty, N.; Anhar, S.; Mayya, R. Peripheral visual field defect of vigabatrin in pediatric epilepsy: A review. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 95. [Google Scholar] [CrossRef]
- Li, K.; Xu, E. The role and the mechanism of γ-aminobutyric acid during central nervous system development. Neurosci. Bull. 2008, 24, 195–200. [Google Scholar] [CrossRef]
- Avoli, M.; Krnjević, K. The long and winding road to gamma-amino-butyric acid as neurotransmitter. Can. J. Neurol. Sci. 2016, 43, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Schinella, G.R.; Moragrega, I. Phenolics as GABAA receptor ligands: An updated review. Molecules 2022, 27, 1770. [Google Scholar] [CrossRef]
- Möhler, H. Physiology and pharmacology of the GABA system: Focus on GABA receptors. In GABA and Sleep: Molecular, Functional and Clinical Aspects; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–23. [Google Scholar]
- Almutairi, S.; Sivadas, A.; Kwakowsky, A. The effect of oral GABA on the nervous system: Potential for therapeutic intervention. Nutraceuticals 2024, 4, 241–259. [Google Scholar] [CrossRef]
- Oishi, Y.; Saito, Y.C.; Sakurai, T. GABAergic modulation of sleep-wake states. Pharmacol. Ther. 2023, 249, 108505. [Google Scholar] [CrossRef]
- Benarroch, E.E. GABAB receptors: Structure, functions, and clinical implications. Neurology 2012, 78, 578–584. [Google Scholar] [CrossRef]
- Michałowski, M.; Kraszewski, S.; Mozrzymas, J. Binding site opening by loop C shift and chloride ion-pore interaction in the GABA A receptor model. Phys. Chem. Chem. Phys. 2017, 19, 13664–13678. [Google Scholar]
- Bryson, A.; Reid, C.; Petrou, S. Fundamental neurochemistry review: GABAA receptor neurotransmission and epilepsy: Principles, disease mechanisms and pharmacotherapy. J. Neurochem. 2023, 165, 6–28. [Google Scholar] [CrossRef] [PubMed]
- Hübner, C.A.; Holthoff, K. Anion transport and GABA signaling. Front. Cell. Neurosci. 2013, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Sieghart, W. Subunit composition and structure of GABAA-receptor subtypes. In The GABA Receptors; Springer: Berlin/Heidelberg, Germany, 2007; pp. 69–86. [Google Scholar]
- Antkowiak, B.; Rammes, G. GABA (A) receptor-targeted drug development-New perspectives in perioperative anesthesia. Expert Opin. Drug Discov. 2019, 14, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Padgett, C.L.; Slesinger, P.A. GABAB receptor coupling to G-proteins and ion channels. Adv. Pharmacol. 2010, 58, 123–147. [Google Scholar]
- Shen, C.; Mao, C.; Xu, C.; Jin, N.; Zhang, H.; Shen, D.-D.; Shen, Q.; Wang, X.; Hou, T.; Chen, Z. Structural basis of GABAB receptor–Gi protein coupling. Nature 2021, 594, 594–598. [Google Scholar] [CrossRef]
- Castelli, M.P.; Gessa, G.L. Distribution and localization of the GABAB receptor. In GABAB Receptor; Springer: Berlin/Heidelberg, Germany, 2016; pp. 75–92. [Google Scholar]
- Evenseth, L.S.M.; Gabrielsen, M.; Sylte, I. The GABAB receptor—Structure, ligand binding and drug development. Molecules 2020, 25, 3093. [Google Scholar] [CrossRef]
- Papasergi-Scott, M.M.; Robertson, M.J.; Seven, A.B.; Panova, O.; Mathiesen, J.M.; Skiniotis, G. Structures of metabotropic GABAB receptor. Nature 2020, 584, 310–314. [Google Scholar] [CrossRef]
- Kantamneni, S. Modulation of neurotransmission by the GABAB receptor. In GABAB Receptor; Springer: Berlin/Heidelberg, Germany, 2016; pp. 109–128. [Google Scholar]
- Brown, D.A. Neurons, receptors, and channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 9–30. [Google Scholar] [CrossRef]
- Lee, S.-E.; Lee, Y.; Lee, G.H. The regulation of glutamic acid decarboxylases in GABA neurotransmission in the brain. Arch. Pharmacal Res. 2019, 42, 1031–1039. [Google Scholar] [CrossRef]
- Wu, H.; Jin, Y.; Buddhala, C.; Osterhaus, G.; Cohen, E.; Jin, H.; Wei, J.; Davis, K.; Obata, K.; Wu, J.-Y. Role of glutamate decarboxylase (GAD) isoform, GAD65, in GABA synthesis and transport into synaptic vesicles—Evidence from GAD65-knockout mice studies. Brain Res. 2007, 1154, 80–83. [Google Scholar] [CrossRef]
- Ueno, H. Enzymatic and structural aspects on glutamate decarboxylase. J. Mol. Catal. B Enzym. 2000, 10, 67–79. [Google Scholar] [CrossRef]
- Buddhala, C.; Hsu, C.-C.; Wu, J.-Y. A novel mechanism for GABA synthesis and packaging into synaptic vesicles. Neurochem. Int. 2009, 55, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Bolneo, E.; Chau, P.Y.S.; Noakes, P.G.; Bellingham, M.C. Investigating the role of GABA in neural development and disease using mice lacking GAD67 or VGAT genes. Int. J. Mol. Sci. 2022, 23, 7965. [Google Scholar] [CrossRef] [PubMed]
- Kanaani, J.; Cianciaruso, C.; Phelps, E.A.; Pasquier, M.; Brioudes, E.; Billestrup, N.; Baekkeskov, S. Compartmentalization of GABA synthesis by GAD67 differs between pancreatic beta cells and neurons. PLoS ONE 2015, 10, e0117130. [Google Scholar] [CrossRef]
- Zhu, X.M.; Ong, W.Y. Changes in GABA transporters in the rat hippocampus after kainate-induced neuronal injury: Decrease in GAT-1 and GAT-3 but upregulation of betaine/GABA transporter BGT-1. J. Neurosci. Res. 2004, 77, 402–409. [Google Scholar] [CrossRef]
- Alekseeva, O.; Gerda, B.; Zhilyaeva, A.; Demchenko, I. Anticonvulsant Efficacy of Inhibition of Synaptic and Extrasynaptic GABA-Transporters in the Prevention of Hyperbaric Oxygen Seizures. J. Evol. Biochem. Physiol. 2023, 59, 709–718. [Google Scholar] [CrossRef]
- Łątka, K.; Bajda, M. Analysis of different binding modes for tiagabine within the GAT-1 transporter. Biomolecules 2022, 12, 1663. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, L.; Li, H.; Chen, Q.; Li, N.; Li, J.; Zhao, Z.; Xiao, D.; Tang, T.; Bi, C. Insights and progress on the biosynthesis, metabolism, and physiological functions of gamma-aminobutyric acid (GABA): A review. PeerJ 2024, 12, e18712. [Google Scholar] [CrossRef]
- Jalil, S.U.; Ansari, S.A.; Ansari, M.I. GABA Transporters and Their Function in Stress Tolerance. In GABA Signaling System and Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2025; pp. 89–105. [Google Scholar]
- Marafiga, J.R.; Pasquetti, M.V.; Calcagnotto, M.E. GABAergic interneurons in epilepsy: More than a simple change in inhibition. Epilepsy Behav. 2021, 121, 106935. [Google Scholar] [CrossRef]
- Rho, J.M.; Boison, D. The metabolic basis of epilepsy. Nat. Rev. Neurol. 2022, 18, 333–347. [Google Scholar] [CrossRef]
- Sperk, G.; Furtinger, S.; Schwarzer, C.; Pirker, S. GABA and its receptors in epilepsy. Adv. Exp. Med. Biol. 2004, 548, 92–103. [Google Scholar]
- Treiman, D.M. GABAergic mechanisms in epilepsy. Epilepsia 2001, 42, 8–12. [Google Scholar] [CrossRef]
- Sarlo, G.L.; Holton, K.F. Brain concentrations of glutamate and GABA in human epilepsy: A review. Seizure 2021, 91, 213–227. [Google Scholar] [CrossRef]
- Li, R.; Xiao, L.; Han, H.; Long, H.; Liao, W.; Yang, Z.; Zhu, H.; Wang, X.; Zou, T.; Huang, Y. Transcriptionally downregulated GABAergic genes associated with synaptic density network dysfunction in temporal lobe epilepsy. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 1970–1988. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wei, Z.-H.; Liu, C.; Li, G.-Y.; Qiao, X.-Z.; Gan, Y.-J.; Zhang, C.-C.; Deng, Y.-C. Genetic variations in GABA metabolism and epilepsy. Seizure 2022, 101, 22–29. [Google Scholar] [CrossRef]
- Scheper, M.; Sørensen, F.N.; Ruffolo, G.; Gaeta, A.; Lissner, L.J.; Anink, J.J.; Korshunova, I.; Jansen, F.E.; Riney, K.; van Hecke, W. Impaired GABAergic regulation and developmental immaturity in interneurons derived from the medial ganglionic eminence in the tuberous sclerosis complex. Acta Neuropathol. 2024, 147, 80. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.; King, S.; Lambert, J.; Belelli, D.; Duka, T. GABAA receptor subtype involvement in addictive behaviour. Genes Brain Behav. 2017, 16, 149–184. [Google Scholar] [CrossRef] [PubMed]
- Engin, E.; Liu, J.; Rudolph, U. α2-containing GABAA receptors: A target for the development of novel treatment strategies for CNS disorders. Pharmacol. Ther. 2012, 136, 142–152. [Google Scholar] [CrossRef]
- Zhu, X.; Li, P. GABA (A) Receptor Subunit (γ2, δ, β1-3) Variants in Genetic Epilepsy: A Comprehensive Summary of 206 Clinical Cases. J. Child Neurol. 2024, 39, 354–370. [Google Scholar] [CrossRef]
- Absalom, N.L.; Lin, S.X.; Liao, V.W.; Chua, H.C.; Møller, R.S.; Chebib, M.; Ahring, P.K. GABAA receptors in epilepsy: Elucidating phenotypic divergence through functional analysis of genetic variants. J. Neurochem. 2024, 168, 3831–3852. [Google Scholar] [CrossRef]
- Parodi, G.; Zanini, G.; Collo, L.; Impollonia, R.; Cervetto, C.; Frega, M.; Chiappalone, M.; Martinoia, S. In vitro electrophysiological drug testing on neuronal networks derived from human induced pluripotent stem cells. Stem Cell Res. Ther. 2024, 15, 433. [Google Scholar] [CrossRef] [PubMed]
- Bampali, K.; Koniuszewski, F.; Vogel, F.D.; Fabjan, J.; Andronis, C.; Lekka, E.; Virvillis, V.; Seidel, T.; Delaunois, A.; Royer, L. GABAA receptor-mediated seizure liabilities: A mixed-methods screening approach. Cell Biol. Toxicol. 2023, 39, 2793–2819. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E.; Bialer, M.; White, H.S. New GABA-Targeting Therapies for the Treatment of Seizures and Epilepsy: I. Role of GABA as a Modulator of Seizure Activity and Recently Approved Medications Acting on the GABA System. CNS Drugs 2023, 37, 755–779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, F.; Xu, H.; Chen, M.; Chen, X.; Guo, L.; Zhou, C.; Xu, Y.; Wang, F.; Yu, J. Dysregulation of REV-ERBα impairs GABAergic function and promotes epileptic seizures in preclinical models. Nat. Commun. 2021, 12, 1216. [Google Scholar] [CrossRef]
- Shen, D.; Wan, J.; Zhang, X.; Sui, J.; Zhan, L.; Zheng, Y.; Ni, Y.; Zhang, Q. A knock-in mouse model for GABRG2-related epileptic encephalopathy displays spontaneous generalized seizures and cognitive impairment. Cell Death Discov. 2025, 11, 443. [Google Scholar] [CrossRef]
- Han, H.A.; Cortez, M.A.; Snead, O.C., III. GABAB receptor and absence epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Croarkin, P.E.; Levinson, A.J.; Daskalakis, Z.J. Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci. Biobehav. Rev. 2011, 35, 818–825. [Google Scholar] [CrossRef]
- Pelkey, K.A.; Chittajallu, R.; Craig, M.T.; Tricoire, L.; Wester, J.C.; McBain, C.J. Hippocampal GABAergic inhibitory interneurons. Physiol. Rev. 2017, 97, 1619–1747. [Google Scholar] [CrossRef]
- Feng, H.-J. Allosteric modulation of αβδ GABAA receptors. Pharmaceuticals 2010, 3, 3461–3477. [Google Scholar] [CrossRef]
- Nieto, A.; Bailey, T.; Kaczanowska, K.; McDonald, P. GABAB receptor chemistry and pharmacology: Agonists, antagonists, and allosteric modulators. Curr. Top. Behav. Neurosci. 2021, 52, 81–118. [Google Scholar]
- Janković, S.M.; Dješević, M.; Janković, S.V. Experimental GABA A receptor agonists and allosteric modulators for the treatment of focal epilepsy. J. Exp. Pharmacol. 2021, 13, 235–244. [Google Scholar] [CrossRef]
- Koh, W.; Kwak, H.; Cheong, E.; Lee, C.J. GABA tone regulation and its cognitive functions in the brain. Nat. Rev. Neurosci. 2023, 24, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.; Floriani, F.; Manni, V.; Dando, I.; Montioli, R. Biochemical investigation of pathogenic missense mutations of human 4-amino butyrate aminotransferase towards the understanding of the molecular pathogenesis of GABA transaminase deficiency. Mol. Genet. Metab. 2025, 145, 109149. [Google Scholar] [CrossRef]
- Okuda, K.; Kato, S.; Ito, T.; Shiraki, S.; Kawase, Y.; Goto, M.; Kawashima, S.; Hemmi, H.; Fukada, H.; Yoshimura, T. Role of the aminotransferase domain in B acillus subtilis GabR, a pyridoxal 5′-phosphate-dependent transcriptional regulator. Mol. Microbiol. 2015, 95, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.B. Design and mechanism of GABA aminotransferase inactivators. Treatments for epilepsies and addictions. Chem. Rev. 2018, 118, 4037–4070. [Google Scholar] [CrossRef] [PubMed]
- Park, M.G.; Han, A.-r.; Kim, S.Y.; Kim, T.Y.; Kim, H.M.; Lee, C.J. High-yield synthesis and purification of recombinant human GABA transaminase for high-throughput screening assays. J. Enzym. Inhib. Med. Chem. 2021, 36, 2016–2024. [Google Scholar] [CrossRef]
- Tramonti, A.; Ghatge, M.S.; Babor, J.T.; Musayev, F.N.; di Salvo, M.L.; Barile, A.; Colotti, G.; Giorgi, A.; Paredes, S.D.; Donkor, A.K. Characterization of the Escherichia coli pyridoxal 5′-phosphate homeostasis protein (YggS): Role of lysine residues in PLP binding and protein stability. Protein Sci. 2022, 31, e4471. [Google Scholar] [CrossRef]
- Tran, J.U.; Brown, B.L. Structural basis for allostery in PLP-dependent enzymes. Front. Mol. Biosci. 2022, 9, 884281. [Google Scholar] [CrossRef]
- Roura Padrosa, D.; Alaux, R.; Smith, P.; Dreveny, I.; López-Gallego, F.; Paradisi, F. Enhancing PLP-binding capacity of class-III ω-transaminase by single residue substitution. Front. Bioeng. Biotechnol. 2019, 7, 282. [Google Scholar] [CrossRef]
- Giraudo, A. Development of Innovative GABAa Receptor Ligands Using a Bioisosteric Approach. Ph.D. Thesis, University of Turin, Torino, Italy, 2018. [Google Scholar]
- Andersen, J.V. The Glutamate/GABA-Glutamine Cycle: Insights, Updates, and Advances. J. Neurochem. 2025, 169, e70029. [Google Scholar] [CrossRef]
- Ravasz, D.; Kacso, G.; Fodor, V.; Adam-Vizi, V.; Chinopoulos, C. Catabolism of GABA, succinic semialdehyde or gamma-hydroxybutyrate through the GABA shunt impair mitochondrial substrate-level phosphorylation. Neurochem. Int. 2017, 109, 41–53. [Google Scholar] [CrossRef]
- Hertz, L.; Rothman, D.L. Glucose, lactate, β-hydroxybutyrate, acetate, GABA, and succinate as substrates for synthesis of glutamate and GABA in the glutamine–glutamate/GABA cycle. In The Glutamate/GABA-Glutamine Cycle: Amino Acid Neurotransmitter Homeostasis; Springer: Berlin/Heidelberg, Germany, 2016; pp. 9–42. [Google Scholar]
- Brikis, C. Characterization of Glyoxylate/Succinic Semialdehyde Reductases in Plants and Impact of Elevated CO2 on γ-Aminobutyrate Metabolism in ‘Empire’ Apple Fruit Stored Under Controlled Atmosphere Conditions; University of Guelph: Guelph, ON, Canada, 2015. [Google Scholar]
- Nesterov, S.V.; Skorobogatova, Y.A.; Panteleeva, A.A.; Pavlik, L.L.; Mikheeva, I.B.; Yaguzhinsky, L.S.; Nartsissov, Y.R. NMDA and GABA receptor presence in rat heart mitochondria. Chem.-Biol. Interact. 2018, 291, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti-de-Albuquerque, J.P.; de-Souza-Ferreira, E.; de Carvalho, D.P.; Galina, A. Coupling of GABA metabolism to mitochondrial glucose phosphorylation. Neurochem. Res. 2022, 47, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Scimemi, A. Structure, function, and plasticity of GABA transporters. Front. Cell. Neurosci. 2014, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Schousboe, A. Milestone Review: Metabolic dynamics of glutamate and GABA mediated neurotransmission—The essential roles of astrocytes. J. Neurochem. 2023, 166, 109–137. [Google Scholar] [CrossRef]
- Andersen, J.V.; Schousboe, A.; Wellendorph, P. Astrocytes regulate inhibitory neurotransmission through GABA uptake, metabolism, and recycling. Essays Biochem. 2023, 67, 77–91. [Google Scholar] [CrossRef]
- Si, W.; Li, H.; Kang, T.; Ye, J.; Yao, Z.; Liu, Y.; Yu, T.; Zhang, Y.; Ling, Y.; Cao, H. Effect of gaba-t on reproductive function in female rats. Animals 2020, 10, 567. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Anokhina, G.B.; Shakhov, Z.N.; Moskvina, P.P.; Igamberdiev, A.U. The Role of Glutamate Metabolism and the GABA Shunt in Bypassing the Tricarboxylic Acid Cycle in the Light. Int. J. Mol. Sci. 2024, 25, 12711. [Google Scholar] [CrossRef]
- Ansari, M.I.; Jalil, S.U.; Ansari, S.A.; Hasanuzzaman, M. GABA shunt: A key-player in mitigation of ROS during stress. Plant Growth Regul. 2021, 94, 131–149. [Google Scholar] [CrossRef]
- Jalil, S.U.; Ansari, S.A.; Ansari, M.I. GABA and Physiological Response Against Hypoxia Stress. In GABA Signaling System and Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2025; pp. 253–267. [Google Scholar]
- Schousboe, A.; Bak, L.K.; Waagepetersen, H.S. Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front. Endocrinol. 2013, 4, 102. [Google Scholar] [CrossRef]
- Bellieny-Rabelo, D.; De Oliveira, E.A.G.; Ribeiro, E.d.S.; Costa, E.P.; Oliveira, A.E.A.; Venancio, T.M. Transcriptome analysis uncovers key regulatory and metabolic aspects of soybean embryonic axes during germination. Sci. Rep. 2016, 6, 36009. [Google Scholar] [CrossRef]
- Deng, C.-X.; Wu, Z.-B.; Chen, Y.; Yu, Z.-M. Pinellia total alkaloids modulate the GABAergic system in hippocampal formation on pilocarpine-induced epileptic rats. Chin. J. Integr. Med. 2020, 26, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Kilb, W. Development of the GABAergic system from birth to adolescence. Neuroscientist 2012, 18, 613–630. [Google Scholar] [CrossRef]
- Kilb, W.; Kirischuk, S.; Luhmann, H.J. Role of tonic GABAergic currents during pre-and early postnatal rodent development. Front. Neural Circuits 2013, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Deidda, G.; Bozarth, I.F.; Cancedda, L. Modulation of GABAergic transmission in development and neurodevelopmental disorders: Investigating physiology and pathology to gain therapeutic perspectives. Front. Cell. Neurosci. 2014, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, A.L. Neuroactive amino acids in focally epileptic human brain: A review. Neurochem. Res. 1999, 24, 1385–1395. [Google Scholar] [CrossRef]
- Braat, S.; Kooy, R.F. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron 2015, 86, 1119–1130. [Google Scholar] [CrossRef]
- García-Cazorla, Á.; Artuch, R.; Pearl, P.L. Disorders of neurotransmission. In Inborn Metabolic Diseases: Diagnosis and Treatment; Springer: Berlin/Heidelberg, Germany, 2022; pp. 547–570. [Google Scholar]
- Parviz, M.; Vogel, K.; Gibson, K.M.; Pearl, P.L. Disorders of GABA metabolism: SSADH and GABA-transaminase deficiencies. J. Pediatr. Epilepsy 2014, 3, 217–227. [Google Scholar] [CrossRef]
- Hashimoto, K. The role of glutamate on the action of antidepressants. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1558–1568. [Google Scholar] [CrossRef]
- Levy, L.M.; Degnan, A.J. GABA-based evaluation of neurologic conditions: MR spectroscopy. Am. J. Neuroradiol. 2013, 34, 259–265. [Google Scholar] [CrossRef]
- Chowdhury, F.A.; O’Gorman, R.L.; Nashef, L.; Elwes, R.D.; Edden, R.A.; Murdoch, J.B.; Barker, G.J.; Richardson, M.P. Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. J. Magn. Reson. Imaging 2015, 41, 694–699. [Google Scholar] [CrossRef]
- Park, M.G.; Lim, J.; Kim, D.; Lee, W.-S.; Yoon, B.-E.; Lee, C.J. Suppressing astrocytic GABA transaminase enhances tonic inhibition and weakens hippocampal spatial memory. Exp. Mol. Med. 2025, 57, 379–389. [Google Scholar] [CrossRef]
- Morland, C.; Frøland, A.-S.; Pettersen, M.N.; Storm-Mathisen, J.; Gundersen, V.; Rise, F.; Hassel, B. Propionate enters GABAergic neurons, inhibits GABA transaminase, causes GABA accumulation and lethargy in a model of propionic acidemia. Biochem. J. 2018, 475, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.G.; Weber, O.M.; Duc, C.O.; Weber, B.; Meier, D.; Russ, W.; Boesiger, P.; Wieser, H.G. Effects of vigabatrin on brain GABA+/CR signals in patients with epilepsy monitored by 1H-NMR-spectroscopy: Responder characteristics. Epilepsia 2001, 42, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Sherif, F.M. Pharmacological profile of the GABA-transaminase inhibitor vigabatrin. World J. Pharm. Pharm. Sci. 2015, 4, 139–148. [Google Scholar]
- Wu, P.-P.; Cao, B.-R.; Tian, F.-Y.; Gao, Z.-B. Development of SV2A ligands for epilepsy treatment: A review of levetiracetam, brivaracetam, and padsevonil. Neurosci. Bull. 2024, 40, 594–608. [Google Scholar] [CrossRef]
- Gajcy, K.; Lochynski, S.; Librowski, T. A role of GABA analogues in the treatment of neurological diseases. Curr. Med. Chem. 2010, 17, 2338–2347. [Google Scholar] [CrossRef]
- Kamieniak, M.; Kośmider, K.; Miziak, B.; Czuczwar, S.J. The Oxidative Stress in Epilepsy—Focus on Melatonin. Int. J. Mol. Sci. 2024, 25, 12943. [Google Scholar] [CrossRef]
- Bialer, M.; Johannessen, S.I.; Koepp, M.J.; Levy, R.H.; Perucca, E.; Tomson, T.; White, H.S. Progress report on new antiepileptic drugs: A summary of the Fourteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XIV). I. Drugs in preclinical and early clinical development. Epilepsia 2018, 59, 1811–1841. [Google Scholar] [CrossRef]
- Maragakis, N.J. The challenges of translational medicine. In Translational Neuroscience: Applications in Psychiatry, Neurology, and Neurodevelopmental Disorders; Cambridge University Press: Cambridge, UK, 2012; p. 214. [Google Scholar]
- Zhang, Q.; Forster-Gibson, C.; Bercovici, E.; Bernardo, A.; Ding, F.; Shen, W.; Langer, K.; Rex, T.; Kang, J.-Q. Epilepsy plus blindness in microdeletion of GABRA1 and GABRG2 in mouse and human. Exp. Neurol. 2023, 369, 114537. [Google Scholar] [CrossRef]
- Łukawski, K.; Czuczwar, S.J. Emerging therapeutic targets for epilepsy: Preclinical insights. Expert Opin. Ther. Targets 2022, 26, 193–206. [Google Scholar] [CrossRef]
- Maheshwari, A.; Nahm, W.K.; Noebels, J.L. Paradoxical proepileptic response to NMDA receptor blockade linked to cortical interneuron defect in stargazer mice. Front. Cell. Neurosci. 2013, 7, 156. [Google Scholar] [CrossRef]
- Pakkhesal, S. GABAA receptor modulation by the endocannabinoid system: Insights into the regulatory mechanisms involving glutamine synthetase and MAPK mediators. Hum. Cell 2024, 37, 1751–1753. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-T.; Chang, Y.-G.; Chern, Y. Insights into GABAAergic system alteration in Huntington’s disease. R. Soc. Open Biol. 2018, 8, 180165. [Google Scholar] [CrossRef] [PubMed]
- Branchereau, P.; Cattaert, D.; Delpy, A.; Allain, A.-E.; Martin, E.; Meyrand, P. Depolarizing GABA/glycine synaptic events switch from excitation to inhibition during frequency increases. Sci. Rep. 2016, 6, 21753. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U.; Wildner, H.; Yévenes, G.E. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 2012, 92, 193–235. [Google Scholar] [CrossRef]
- Madsen, K.K.; White, H.S.; Schousboe, A. Neuronal and non-neuronal GABA transporters as targets for antiepileptic drugs. Pharmacol. Ther. 2010, 125, 394–401. [Google Scholar] [CrossRef]
- Yang, J.; Shen, J. Elevated endogenous GABA concentration attenuates glutamate-glutamine cycling between neurons and astroglia. J. Neural Transm. 2009, 116, 291–300. [Google Scholar] [CrossRef]
- Wang, P.; Eshaq, R.S.; Meshul, C.K.; Moore, C.; Hood, R.L.; Leidenheimer, N.J. Neuronal gamma-aminobutyric acid (GABA) type A receptors undergo cognate ligand chaperoning in the endoplasmic reticulum by endogenous GABA. Front. Cell. Neurosci. 2015, 9, 188. [Google Scholar] [CrossRef]
- Marvin, J.S.; Shimoda, Y.; Magloire, V.; Leite, M.; Kawashima, T.; Jensen, T.P.; Kolb, I.; Knott, E.L.; Novak, O.; Podgorski, K. A genetically encoded fluorescent sensor for in vivo imaging of GABA. Nat. Methods 2019, 16, 763–770. [Google Scholar] [CrossRef]
- Perucca, E.; Brodie, M.J.; Kwan, P.; Tomson, T. 30 years of second-generation antiseizure medications: Impact and future perspectives. Lancet Neurol. 2020, 19, 544–556. [Google Scholar] [CrossRef]
- Koshal, P.; Jamwal, S.; Akula, K.K.; Bansal, P.K. Animal Models of Epilepsy. In Animal Models of Neurological Disorders: Principle and Working Procedure for Animal Models of Neurological Disorders; Springer: Berlin/Heidelberg, Germany, 2018; pp. 59–78. [Google Scholar]
- Rissardo, J.P.; Fornari Caprara, A.L. Cenobamate (YKP3089) and drug-resistant epilepsy: A review of the literature. Medicina 2023, 59, 1389. [Google Scholar] [CrossRef] [PubMed]
- Kirmse, K.; Hübner, C.A.; Isbrandt, D.; Witte, O.W.; Holthoff, K. GABAergic transmission during brain development: Multiple effects at multiple stages. Neuroscientist 2018, 24, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Gillard, M.; Sands, Z.A.; Kaminski, R.M.; Klitgaard, H. Synaptic vesicle glycoprotein 2A ligands in the treatment of epilepsy and beyond. CNS Drugs 2016, 30, 1055–1077. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Y.; Wei, P.; Feng, K.; Niu, J.; Shen, G.; Lu, W.; Xiao, W.; Wang, J.; Smagghe, G.J. High gama-aminobutyric acid contents involved in abamectin resistance and predation, an interesting phenomenon in spider mites. Front. Physiol. 2017, 8, 216. [Google Scholar] [CrossRef]
- Geisler, C.E.; Ghimire, S.; Bruggink, S.M.; Miller, K.E.; Weninger, S.N.; Kronenfeld, J.M.; Yoshino, J.; Klein, S.; Duca, F.A.; Renquist, B.J. A critical role of hepatic GABA in the metabolic dysfunction and hyperphagia of obesity. Cell Rep. 2021, 35, 109301. [Google Scholar] [CrossRef]
- Besse, A.; Wu, P.; Bruni, F.; Donti, T.; Graham, B.H.; Craigen, W.J.; McFarland, R.; Moretti, P.; Lalani, S.; Scott, K.L. The GABA transaminase, ABAT, is essential for mitochondrial nucleoside metabolism. Cell Metab. 2015, 21, 417–427. [Google Scholar] [CrossRef]
- Louro, P.; Ramos, L.; Robalo, C.; Cancelinha, C.; Dinis, A.; Veiga, R.; Pina, R.; Rebelo, O.; Pop, A.; Diogo, L.; et al. Phenotyping GABA transaminase deficiency: A case description and literature review. J. Inherit. Metab. Dis. 2016, 39, 743–747. [Google Scholar] [CrossRef]
- Nagappa, M.; Bindu, P.S.; Chiplunkar, S.; Govindaraj, P.; Narayanappa, G.; Krishnan, A.; Bharath, M.S.; Swaminathan, A.; Saini, J.; Arvinda, H.R. Hypersomnolence-hyperkinetic movement disorder in a child with compound heterozygous mutation in 4-aminobutyrate aminotransferase (ABAT) gene. Brain Dev. 2017, 39, 161–165. [Google Scholar] [CrossRef]
- Pontes, A.; Zhang, Y.; Hu, W. Novel functions of GABA signaling in adult neurogenesis. Front. Biol. 2013, 8, 496–507. [Google Scholar] [CrossRef]
- Barker-Haliski, M.L.; Löscher, W.; White, H.S.; Galanopoulou, A.S. Neuroinflammation in epileptogenesis: Insights and translational perspectives from new models of epilepsy. Epilepsia 2017, 58, 39–47. [Google Scholar] [CrossRef]
- Shen, W.; Nwosu, G.; Honer, M.; Clasadonte, J.; Schmalzbauer, S.; Biven, M.; Langer, K.; Flamm, C.; Poliquin, S.; Mermer, F.; et al. γ-Aminobutyric acid transporter and GABA(A) receptor mechanisms in Slc6a1(+/A288V) and Slc6a1(+/S295L) mice associated with developmental and epileptic encephalopathies. Brain Commun. 2024, 6, fcae110. [Google Scholar] [CrossRef] [PubMed]
- Randhave, K.; Zavalin, K.; Kang, J.-Q. 4-Phenylbutyrate Rescues Neurobehavioral Phenotypes in SLC6A1-Related Encephalopathy. Epilepsy Res. 2025, 107708. [Google Scholar] [CrossRef]
- Na, J.-H.; Lee, Y.-M. Therapeutic approach to epilepsy in patients with mitochondrial diseases. Yonsei Med. J. 2025, 66, 131. [Google Scholar] [CrossRef]
- Conte, E.; Boccanegra, B.; Dinoi, G.; Pusch, M.; De Luca, A.; Liantonio, A.; Imbrici, P. Therapeutic approaches to tuberous sclerosis complex: From available therapies to promising drug targets. Biomolecules 2024, 14, 1190. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Huang, S.; Wei, M.; Wu, Y.; Xie, Z. Dravet syndrome: Novel insights into SCN1A-mediated epileptic neurodevelopmental disorders within the molecular diagnostic-therapeutic framework. Front. Neurosci. 2025, 19, 1634718. [Google Scholar]
- Kostić, N.; Dotsikas, Y.; Malenović, A. Critical review on the analytical methods for the determination of zwitterionic antiepileptic drugs—Vigabatrin, pregabalin, and gabapentin—In bulk and formulations. Instrum. Sci. Technol. 2014, 42, 486–512. [Google Scholar] [CrossRef]
- Mandrioli, R.; Mercolini, L. Metabolism of drugs used in the therapy of seizures: An analytical point of view. Part 1. Curr. Drug Metab. 2017, 18, 735–756. [Google Scholar] [CrossRef]
- Ben-Menachem, E. Vigabatrin. Epilepsia 1995, 36, S95–S104. [Google Scholar] [CrossRef]
- Connelly, J.F. Vigabatrin. Ann. Pharmacother. 1993, 27, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, R.; Advani, M.; Mali, S.; Pawar, S. Enzymes as targets of Drug Action: An Overview. Int. J. 2020, 3, 114. [Google Scholar]
- Moschitto, M.J.; Doubleday, P.F.; Catlin, D.S.; Kelleher, N.L.; Liu, D.; Silverman, R.B. Mechanism of Inactivation of Ornithine Aminotransferase by (1 S, 3 S)-3-Amino-4-(hexafluoropropan-2-ylidenyl) cyclopentane-1-carboxylic Acid. J. Am. Chem. Soc. 2019, 141, 10711–10721. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.P. GABA receptors and GABAergic synapses as targets for drug development. Drug Dev. Res. 1990, 21, 151–160. [Google Scholar] [CrossRef]
- Waschkies, C.F.; Bruns, A.; Müller, S.; Kapps, M.; Borroni, E.; Von Kienlin, M.; Rudin, M.; Künnecke, B. Neuropharmacological and neurobiological relevance of in vivo 1H-MRS of GABA and glutamate for preclinical drug discovery in mental disorders. Neuropsychopharmacology 2014, 39, 2331–2339. [Google Scholar] [CrossRef]
- Lou, S.; Cui, S. Drug treatment of epilepsy: From serendipitous discovery to evolutionary mechanisms. Curr. Med. Chem. 2022, 29, 3366–3391. [Google Scholar] [CrossRef]
- Löscher, W.; Jäckel, R.; Müller, F. Anticonvulsant and proconvulsant effects of inhibitors of GABA degradation in the amygdala-kindling model. Eur. J. Pharmacol. 1989, 163, 1–14. [Google Scholar] [CrossRef]
- Shorvon, S. History of the Drug Treatment of Epilepsy Between 1955 and 1989 with Special Reference to the Role of the International League Against Epilepsy (ILAE). Treat. Epilepsy 2009. [Google Scholar] [CrossRef]
- Foroozan, R. Vigabatrin: Lessons learned from the United States experience. J. Neuro-Ophthalmol. 2018, 38, 442–450. [Google Scholar] [CrossRef]
- Faught, E. Vigabatrin therapy for refractory complex partial seizures: Review of clinical trial experience in the United States. Acta Neurol. Scand. 2011, 124, 29–35. [Google Scholar] [CrossRef]
- Chiron, C. Stiripentol and vigabatrin current roles in the treatment of epilepsy. Expert Opin. Pharmacother. 2016, 17, 1091–1101. [Google Scholar] [CrossRef]
- Pesaturo, K.A.; Spooner, L.M.; Belliveau, P. Vigabatrin for infantile spasms. Pharmacotherapy 2011, 31, 298–311. [Google Scholar] [CrossRef]
- Granström, M.L.; Gaily, E.; Liukkonen, E. Treatment of infantile spasms: Results of a population-based study with vigabatrin as the first drug for spasms. Epilepsia 1999, 40, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Tuley, A.; Fast, W. The taxonomy of covalent inhibitors. Biochemistry 2018, 57, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- Raboni, S.; Spyrakis, F.; Campanini, B.; Amadasi, A.; Bettati, S.; Peracchi, A.; Mozzarelli, A.; Contestabile, R. 7.10-pyridoxal 5′-phosphate-dependent enzymes: Catalysis, conformation, and genomics. Compr. Nat. Prod. II 2010, 7, 273–350. [Google Scholar]
- Ben-Menachem, E. Mechanism of action of vigabatrin: Correcting misperceptions. Acta Neurol. Scand. 2011, 124, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sills, G.J. Mechanisms of action of antiepileptic drugs. Epilepsy 2011, 25, 295–303. [Google Scholar]
- Asif, M. A review on antiepileptic drug and their uses, mechanism of actions, adverse effects and drug interaction. Curr. Sci. Perspect. 2016, 2, 19–38. [Google Scholar]
- Zheng, Q.; He, S.; Xu, S.-L.; Ma, M.-D.; Fan, M.; Ge, J.-F. Pharmacokinetics and tissue distribution of vigabatrin enantiomers in rats. Saudi Pharm. J. 2024, 32, 101934. [Google Scholar] [CrossRef]
- Duhamel, P.; Ounissi, M.; Le Saux, T.; Bienayme, H.; Chiron, C.; Jullien, V. Determination of the R (−) and S (+)-enantiomers of vigabatrin in human plasma by ultra-high-performance liquid chromatography and tandem mass-spectrometry. J. Chromatogr. B 2017, 1070, 31–36. [Google Scholar] [CrossRef]
- Rasmussen, A.D.; Truchot, N.; Dyssegaard, A.; Fant, P. Retinal and systemic toxicity of vigabatrin is driven by the S-enantiomer in the long Evans rat. Toxicol. Pathol. 2023, 51, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, M.K.; Frølund, S.; Holm, R.; Nielsen, C.U. Pharmacokinetic aspects of the anti-epileptic drug substance vigabatrin: Focus on transporter interactions. Ther. Deliv. 2014, 5, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, M.K.; Thale, Z.I.; Brodin, B.; Hansen, S.H.; Holm, R.; Nielsen, C.U. Intestinal absorption of the antiepileptic drug substance vigabatrin is altered by infant formula in vitro and in vivo. Pharmacol. Res. Perspect. 2014, 2, e00036. [Google Scholar] [CrossRef] [PubMed]
- Kalasapur, A.S. A Comparative Study of the Efficacy and Safety of Sodium Valproate V/S Magnesium Valproate in Generalized Tonic Clonic Seizures in Neurology OPD; Rajiv Gandhi University of Health Sciences: Bengaluru, India, 2018. [Google Scholar]
- Anderson, G.D.; Hakimian, S. Pharmacokinetic of antiepileptic drugs in patients with hepatic or renal impairment. Clin. Pharmacokinet. 2014, 53, 29–49. [Google Scholar] [CrossRef]
- Patsalos, P.N. Vigabatrin. In Antiepileptic Drug Interactions: A Clinical Guide; Springer International Publishing: Cham, Switzerland, 2016; pp. 141–144. [Google Scholar]
- Lerner, J.T.; Salamon, N.; Sankar, R. Clinical profile of vigabatrin as monotherapy for treatment of infantile spasms. Neuropsychiatr. Dis. Treat. 2010, 6, 731–740. [Google Scholar] [CrossRef][Green Version]
- Bhagat, K.; Singh, J.V.; Pagare, P.P.; Kumar, N.; Sharma, A.; Kaur, G.; Kinarivala, N.; Gandu, S.; Singh, H.; Sharma, S. Rational approaches for the design of various GABA modulators and their clinical progression. Mol. Divers. 2021, 25, 551–601. [Google Scholar] [CrossRef]
- Rodríguez-Lozada, J.; Tovar-Gudiño, E.; Guevara-Salazar, J.A.; Razo-Hernández, R.S.; Santiago, Á.; Pastor, N.; Fernández-Zertuche, M. QSAR and molecular docking studies of the inhibitory activity of novel heterocyclic GABA analogues over GABA-AT. Molecules 2018, 23, 2984. [Google Scholar] [CrossRef]
- Tovar-Gudiño, E.; Guevara-Salazar, J.A.; Bahena-Herrera, J.R.; Trujillo-Ferrara, J.G.; Martínez-Campos, Z.; Razo-Hernández, R.S.; Santiago, Á.; Pastor, N.; Fernández-Zertuche, M. Novel-substituted heterocyclic GABA analogues. Enzymatic activity against the GABA-AT enzyme from Pseudomonas fluorescens and in silico molecular modeling. Molecules 2018, 23, 1128. [Google Scholar] [CrossRef]
- Puranik, N.; Song, M. Therapeutic role of heterocyclic compounds in neurodegenerative diseases: Insights from Alzheimer’s and Parkinson’s diseases. Neurol. Int. 2025, 17, 26. [Google Scholar] [CrossRef]
- Zaccara, G.; Schmidt, D. Antiepileptic drugs in clinical development: Differentiate or die? Curr. Pharm. Des. 2017, 23, 5593–5605. [Google Scholar] [CrossRef]
- Silverman, R.B. The 2011 EB Hershberg Award for important discoveries in medicinally active substances:(1 S, 3 S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a GABA aminotransferase inactivator and new treatment for drug addiction and infantile spasms. J. Med. Chem. 2012, 55, 567–575. [Google Scholar] [CrossRef]
- Pejčić, A.; Janković, S.M.; Đešević, M.; Gojak, R.; Lukić, S.; Marković, N.; Milosavljević, M. Novel and emerging therapeutics for genetic epilepsies. Expert Rev. Neurother. 2021, 21, 1283–1301. [Google Scholar] [CrossRef] [PubMed]
- Khorkova, O.; Hsiao, J.; Wahlestedt, C. Nucleic acid–based therapeutics in orphan neurological disorders: Recent developments. Front. Mol. Biosci. 2021, 8, 643681. [Google Scholar] [CrossRef] [PubMed]
- Maranga, C.; Fernandes, T.G.; Bekman, E.; da Rocha, S.T. Angelman syndrome: A journey through the brain. FEBS J. 2020, 287, 2154–2175. [Google Scholar] [CrossRef]
- Borisova, T.; Pozdnyakova, N.; Shaitanova, E.; Gerus, I.; Dudarenko, M.; Mironets, R.; Haufe, G.; Kukhar, V. Synthesis of new fluorinated analogs of GABA, Pregabalin bioisosteres, and their effects on [3H] GABA uptake by rat brain nerve terminals. Bioorg. Med. Chem. 2015, 23, 4316–4323. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Doud, E.H.; Wu, R.; Sanishvili, R.; Juncosa, J.I.; Liu, D.; Kelleher, N.L.; Silverman, R.B. Mechanism of Inactivation of γ-Aminobutyric Acid Aminotransferase by (1S,3S)-3-Amino-4-difluoromethylene-1-cyclopentanoic Acid (CPP-115). J. Am. Chem. Soc. 2015, 137, 2628–2640. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Hoon, M.; Pattnaik, B.R.; Ver Hoeve, J.N.; Wahlgren, B.; Gloe, S.; Williams, J.; Wetherbee, B.; Kiland, J.A.; Vogel, K.R.; et al. Vigabatrin-Induced Retinal Functional Alterations and Second-Order Neuron Plasticity in C57BL/6J Mice. Investig. Ophthalmol. Vis. Sci. 2020, 61, 17. [Google Scholar] [CrossRef]
- Gavande, N.; Yamamoto, I.; Salam, N.K.; Ai, T.H.; Burden, P.M.; Johnston, G.A.; Hanrahan, J.R.; Chebib, M. Novel Cyclic Phosphinic Acids as GABAC ρ Receptor Antagonists: Design, Synthesis, and Pharmacology. ACS Med. Chem. Lett. 2011, 2, 11–16. [Google Scholar] [CrossRef]
- Suemasa, A.; Watanabe, M.; Kobayashi, T.; Suzuki, H.; Fukuda, H.; Minami, M.; Shuto, S. Design and synthesis of cyclopropane-based conformationally restricted GABA analogues as selective inhibitors for betaine/GABA transporter 1. Bioorg. Med. Chem. Lett. 2018, 28, 3395–3399. [Google Scholar] [CrossRef]
- Brickley, S.G.; Mody, I. Extrasynaptic GABA(A) receptors: Their function in the CNS and implications for disease. Neuron 2012, 73, 23–34. [Google Scholar] [CrossRef]
- Bird, L.M.; Ochoa-Lubinoff, C.; Tan, W.-H.; Heimer, G.; Melmed, R.D.; Rakhit, A.; Visootsak, J.; During, M.J.; Holcroft, C.; Burdine, R.D. The STARS phase 2 study: A randomized controlled trial of gaboxadol in Angelman syndrome. Neurology 2021, 96, e1024–e1035. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.D.; Ling, L.L.; Uteshev, V.V.; Caspary, D.M. Extrasynaptic GABAA Receptors and Tonic Inhibition in Rat Auditory Thalamus. PLoS ONE 2011, 6, e16508. [Google Scholar] [CrossRef] [PubMed]
- Briggs, S.W.; Mowrey, W.; Hall, C.B.; Galanopoulou, A.S. CPP-115, a vigabatrin analogue, decreases spasms in the multiple-hit rat model of infantile spasms. Epilepsia 2014, 55, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Gerasimov, M.R.; Kvist, T.; Wellendorph, P.; Madsen, K.K.; Pera, E.; Lee, H.; Schousboe, A.; Chebib, M.; Bräuner-Osborne, H.; et al. (1S, 3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a potent γ-aminobutyric acid aminotransferase inactivator for the treatment of cocaine addiction. J. Med. Chem. 2012, 55, 357–366. [Google Scholar] [CrossRef]
- Doumlele, K.; Conway, E.; Hedlund, J.; Tolete, P.; Devinsky, O. A case report on the efficacy of vigabatrin analogue (1S, 3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115) in a patient with infantile spasms. Epilepsy Behav. Case Rep. 2016, 6, 67–69. [Google Scholar] [CrossRef]
- Qume, M.; Fowler, L. Effect of chronic treatment with the GABA transaminase inhibitors γ-vinyl GABA and ethanolamine O-sulphate on the in vitro GABA release from rat hippocampus. Br. J. Pharmacol. 1997, 122, 539. [Google Scholar] [CrossRef]
- John, R.A.; Rummer, E.M.; Williams, J.; Cole, G.; Fowler, L.J.; Richens, A. Micro-vacuolation in rat brains after long term administration of GABA-transaminase inhibitors: Comparison of effects of ethanolamine-O-sulphate and vigabatrin. Biochem. Pharmacol. 1987, 36, 1467–1473. [Google Scholar] [CrossRef]
- Abbot, E.L.; Grenade, D.S.; Kennedy, D.J.; Gatfield, K.M.; Thwaites, D.T. Vigabatrin transport across the human intestinal epithelial (Caco-2) brush-border membrane is via the H+ -coupled amino-acid transporter hPAT1. Br. J. Pharmacol. 2006, 147, 298–306. [Google Scholar] [CrossRef]
- Abdul-Ghani, A.S.; Coutinho-Netto, J.; Bradford, H.F. The action of γ-vinyl-GABA and γ-acetylenic-GABA on the resting and stimulated release of GABA in vivo. Brain Res. 1980, 191, 471–481. [Google Scholar] [CrossRef]
- Lu, H.; Silverman, R.B. Fluorinated conformationally restricted γ-aminobutyric acid aminotransferase inhibitors. J. Med. Chem. 2006, 49, 7404–7412. [Google Scholar] [CrossRef] [PubMed]
- Abdul Manan, M.A.F.; Cordes, D.B.; Slawin, A.M.; Bühl, M.; Liao, V.W.; Chua, H.C.; Chebib, M.; O’Hagan, D. The Synthesis and Evaluation of Fluoro-, Trifluoromethyl-, and Iodomuscimols as GABA Agonists. Chemistry 2017, 23, 10848–10852. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.; Valentine, H.; Dannals, R.F.; Wong, D.F.; Horti, A.G. Synthesis and Evaluation of a New (18)F-Labeled Radiotracer for Studying the GABA(B) Receptor in the Mouse Brain. ACS Chem. Neurosci. 2018, 9, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Maranchick, N.; Pomar-Forero, D.; Busl, K.; Hirsch, L.; Bruzzone, M.; Eisenschenk, S.; Peloquin, C.; Maciel, C. VIGAB-STAT—A Phase IIa Feasibility Trial of Irreversible GABA-transaminase Inhibition as Adjunct Treatment of Status Epilepticus After Cardiac Arrest (P4-2.003). Neurology 2024, 102, 3498. [Google Scholar] [CrossRef]
- Zhao, J.; Shin, Y.; Jin, Y.; Jeong, K.M.; Lee, J. Determination of enantiomeric vigabatrin by derivatization with diacetyl-L-tartaric anhydride followed by ultra-high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. J. Chromatogr. B 2017, 1040, 199–207. [Google Scholar] [CrossRef]
- Löscher, W. The epileptic gerbil. Neuronal networks and actions of antiepileptic drugs. In Drugs for the Control of Epilepsy; CRC Press: Boca Raton, FL, USA, 2019; pp. 309–324. [Google Scholar]
- Pathan, S.A.; Jain, G.K.; Akhter, S.; Vohora, D.; Ahmad, F.J.; Khar, R.K. Insights into the novel three ‘D’s of epilepsy treatment: Drugs, delivery systems and devices. Drug Discov. Today 2010, 15, 717–732. [Google Scholar] [CrossRef]
- Mehdi, S. Covalent Enzyme Inhibition in Drug Discovery and Development. Enz. Technol. 2013, 81–129. [Google Scholar] [CrossRef]
- Labouta, I.; Jacobsen, P.; Thorbek, P.; Krogsgaard-Larsen, P.; Hjeds, H. Syntheses of Some Cyclic Amino Acids Structurally Related to the GABA Analogue Homo-beta-Proline. Acta Chem. Scand. 1982, 36, 669–674. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Lee, Y.; Han, E.-T.; Park, W.S.; Han, J.-H.; Kwon, Y.-S.; Lee, H.-J.; Chun, W. Discovery of GABA Aminotransferase Inhibitors via Molecular Docking, Molecular Dynamic Simulation, and Biological Evaluation. Int. J. Mol. Sci. 2023, 24, 16990. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.-T.; Park, W.S.; Han, J.-H.; Kwon, Y.-S.; Lee, H.-J.; Chun, W. Computational exploration of the effects of mutations on GABA aminotransferase in GABA aminotransferase deficiency. Int. J. Mol. Sci. 2023, 24, 10933. [Google Scholar] [CrossRef]
- Belete, T.M. Recent progress in the development of new antiepileptic drugs with novel targets. Ann. Neurosci. 2023, 30, 262–276. [Google Scholar] [CrossRef]
- Tashima, T. Carrier-Mediated Delivery of Low-Molecular-Weight N-Containing Drugs across the Blood–Brain Barrier or the Blood–Retinal Barrier Using the Proton-Coupled Organic Cation Antiporter. Future Pharmacol. 2023, 3, 742–762. [Google Scholar] [CrossRef]
- Gentile, D. A Computational Approach for the Molecular Recognition of Novel Ophthalmic Prodrugs and Molecular Modelling of Sigma Receptor Ligands in Ocular Diseases. 2021. Available online: https://tesidottorato.depositolegale.it/handle/20.500.14242/73926 (accessed on 4 December 2025).
- Yasir, M.; Park, J.; Choe, J.; Han, J.-H.; Han, E.-T.; Park, W.S.; Chun, W. Structure-Guided Identification of JAK2 Inhibitors: From Similarity to Stability and Specificity. Future Pharmacol. 2025, 5, 66. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.-T.; Han, J.-H.; Park, W.S.; Choe, J.; Chun, W. Integration of Deep Learning with Molecular Docking and Molecular Dynamics Simulation for Novel TNF-α-Converting Enzyme Inhibitors. Future Pharmacol. 2025, 5, 55. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.-T.; Han, J.-H.; Park, W.S.; Choe, J.; Chun, W. Investigating Natural Product Inhibitors of IKKα: Insights from Integrative In Silico and Experimental Validation. Molecules 2025, 30, 2025. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Park, J.; Han, E.-T.; Park, W.S.; Han, J.-H.; Chun, W. Structural comparison of human and Plasmodium proteasome β5 subunits: Informing selective inhibitor design for anti-malaria agents. Malar. J. 2025, 24, 21. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.-T.; Han, J.-H.; Park, W.S.; Hassan, M.; Kloczkowski, A.; Chun, W. Discovery of novel TACE inhibitors using graph convolutional network, molecular docking, molecular dynamics simulation, and Biological evaluation. PLoS ONE 2024, 19, e0315245. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.-T.; Park, W.S.; Han, J.-H.; Chun, W. Identification of Potential Tryptase Inhibitors from FDA-Approved Drugs Using Machine Learning, Molecular Docking, and Experimental Validation. ACS Omega 2024, 9, 38820–38831. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Chun, W. Discovery of novel aldose reductase inhibitors via the integration of ligand-based and structure-based virtual screening with experimental validation. ACS Omega 2024, 9, 20338–20349. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.-T.; Han, J.-H.; Park, W.S.; Chun, W. Identification of malaria-selective proteasome β5 inhibitors through pharmacophore modeling, molecular docking, and molecular dynamics simulation. Int. J. Mol. Sci. 2024, 25, 11881. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, Y.; Lee, H.J.; Kwon, Y.-S.; Chun, W. In silico screening of GABA aminotransferase inhibitors from the constituents of Valeriana officinalis by molecular docking and molecular dynamics simulation study. J. Mol. Model. 2020, 26, 228. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, F.F. Integrated virtual screening, molecular modeling and machine learning approaches revealed potential natural inhibitors for epilepsy. Saudi Pharm. J. 2023, 31, 101835. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Dubey, M.; Misal, S.; Alavala, R.R.; CS, R. Computational Analyses of the Mechanism of Action of Antiepileptic Agents. In Applications of Computational Tools in Drug Design and Development; Springer: Berlin/Heidelberg, Germany, 2025; pp. 885–933. [Google Scholar]
- Yasir, M.; Park, J.; Han, E.-T.; Park, W.S.; Han, J.-H.; Choe, J.; Hassan, M.; Kloczkowski, A.; Chun, W. Computational Analysis of GAT1 Mutations: Functional Consequences from Molecular Dynamics and Binding Free Energy Calculations. Int. J. Mol. Sci. 2025, 26, 11339. [Google Scholar] [CrossRef]
- Chan, B.W.; Lynch, N.B.; Tran, W.; Joyce, J.M.; Savage, G.P.; Meutermans, W.; Montgomery, A.P.; Kassiou, M. Fragment-based drug discovery for disorders of the central nervous system: Designing better drugs piece by piece. Front. Chem. 2024, 12, 1379518. [Google Scholar] [CrossRef]
- Wasko, M.J.; Pellegrene, K.A.; Madura, J.D.; Surratt, C.K. A role for fragment-based drug design in developing novel lead compounds for central nervous system targets. Front. Neurol. 2015, 6, 197. [Google Scholar] [CrossRef]
- Li, C.; Yao, S.; Li, Z.; Gao, Y. Application of Novel Drug-Delivery Strategies in Neurological Disorders. Adv. Mater. 2025, 37, 2503646. [Google Scholar] [CrossRef]
- Gernert, M.; Feja, M. Bypassing the blood–brain barrier: Direct intracranial drug delivery in epilepsies. Pharmaceutics 2020, 12, 1134. [Google Scholar] [CrossRef]
- Boison, D. Cell and gene therapies for refractory epilepsy. Curr. Neuropharmacol. 2007, 5, 115–125. [Google Scholar] [CrossRef][Green Version]
- Police, A. Approaches to Alleviate the Vigabatrin Induced Retinal Toxicity. Ph.D. Thesis, The University of Mississippi, Oxford, MS, USA, 2020. [Google Scholar][Green Version]
- Heim, M.; Gidal, B. Vigabatrin-associated retinal damage–potential biochemical mechanisms. Acta Neurol. Scand. 2012, 126, 219–228. [Google Scholar] [CrossRef]
- Löscher, W. Single-target versus multi-target drugs versus combinations of drugs with multiple targets: Preclinical and clinical evidence for the treatment or prevention of epilepsy. Front. Pharmacol. 2021, 12, 730257. [Google Scholar] [CrossRef]
- Zhu, X.-H.; Lee, B.-Y.; Tuite, P.; Coles, L.; Sathe, A.G.; Chen, C.; Cloyd, J.; Low, W.C.; Steer, C.J.; Chen, W. Quantitative assessment of occipital metabolic and energetic changes in Parkinson’s patients, using in Vivo 31P MRS-based metabolic imaging at 7T. Metabolites 2021, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Auriat, A.M.; Neva, J.L.; Peters, S.; Ferris, J.K.; Boyd, L.A. A review of transcranial magnetic stimulation and multimodal neuroimaging to characterize post-stroke neuroplasticity. Front. Neurol. 2015, 6, 226. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.K.; Lenartowicz, A.; Makeig, S. Research review: Use of EEG biomarkers in child psychiatry research–current state and future directions. J. Child Psychol. Psychiatry 2016, 57, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Ewen, J.B.; Sweeney, J.A.; Potter, W.Z. Conceptual, regulatory and strategic imperatives in the early days of EEG-based biomarker validation for neurodevelopmental disabilities. Front. Integr. Neurosci. 2019, 13, 45. [Google Scholar] [CrossRef]
- Prezioso, G.; Chiarelli, F.; Matricardi, S. Efficacy and safety of vigabatrin in patients with tuberous sclerosis complex and infantile epileptic spasm syndrome: A systematic review. Expert Rev. Neurother. 2023, 23, 661–671. [Google Scholar] [CrossRef]
- Hussain, S.A.; Schmid, E.; Peters, J.M.; Goyal, M.; Bebin, E.M.; Northrup, H.; Sahin, M.; Krueger, D.A.; Wu, J.Y.; Pearson, D. High vigabatrin dosage is associated with lower risk of infantile spasms relapse among children with tuberous sclerosis complex. Epilepsy Res. 2018, 148, 1–7. [Google Scholar] [CrossRef]
- Jones, K.; Go, C.; Boyd, J.; Ochi, A.; McCoy, B.; Puka, K.; Snead, O.C., III. Vigabatrin as first-line treatment for infantile spasms not related to tuberous sclerosis complex. Pediatr. Neurol. 2015, 53, 141–145. [Google Scholar] [CrossRef]
- Li, X.; Slesinger, P.A. GABAB receptors and drug addiction: Psychostimulants and other drugs of abuse. In Behavioral Neurobiology of GABAB Receptor Function; Springer: Berlin/Heidelberg, Germany, 2021; pp. 119–155. [Google Scholar]
- Somoza, E.C.; Winship, D.; Gorodetzky, C.W.; Lewis, D.; Ciraulo, D.A.; Galloway, G.P.; Segal, S.D.; Sheehan, M.; Roache, J.D.; Bickel, W.K. A multisite, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of vigabatrin for treating cocaine dependence. JAMA Psychiatry 2013, 70, 630–637. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).