Anti-Melanogenic and Anti-Inflammatory Effects of 2′-Hydroxy-4′,6′-dimethoxychalcone in B16F10 and RAW264.7 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
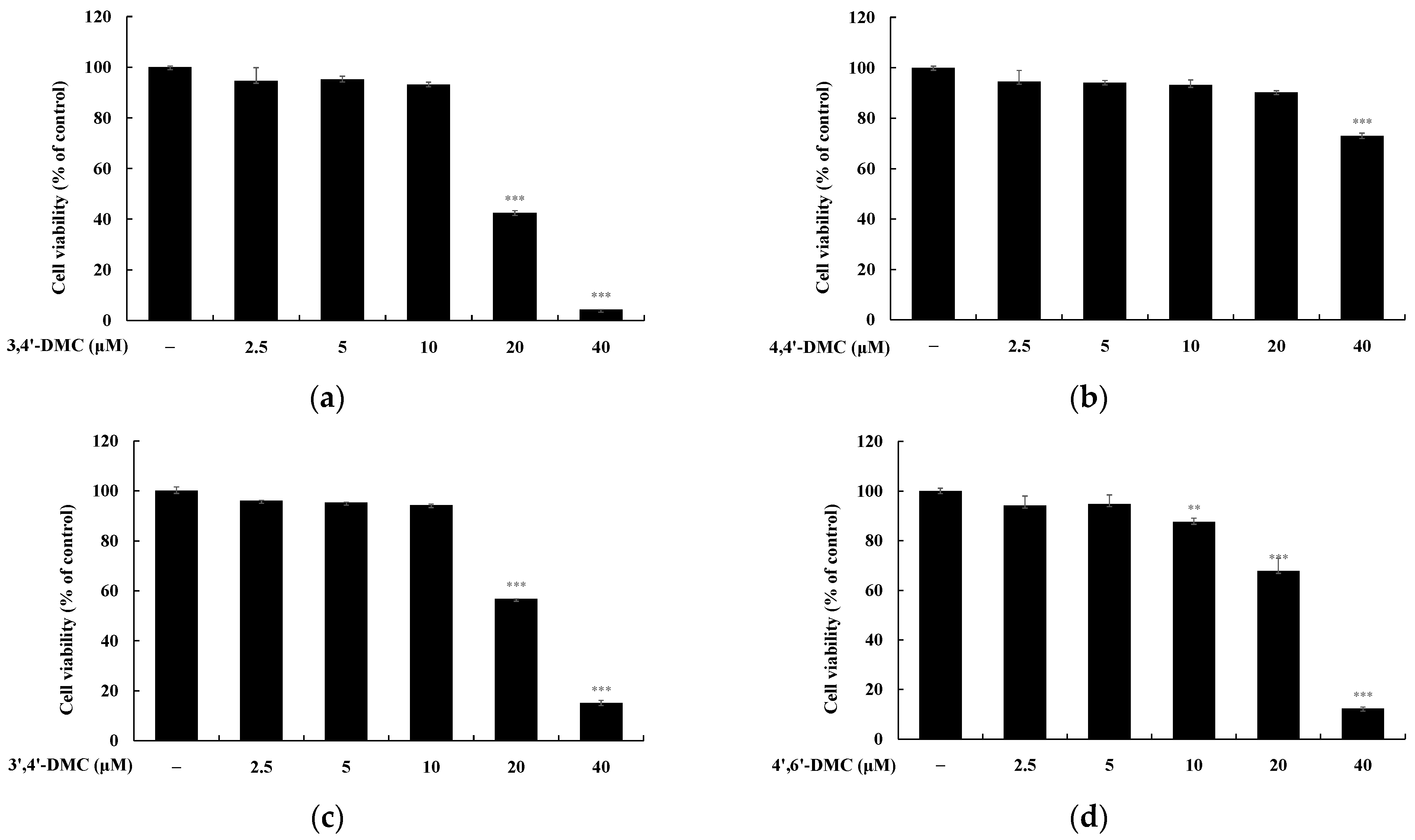
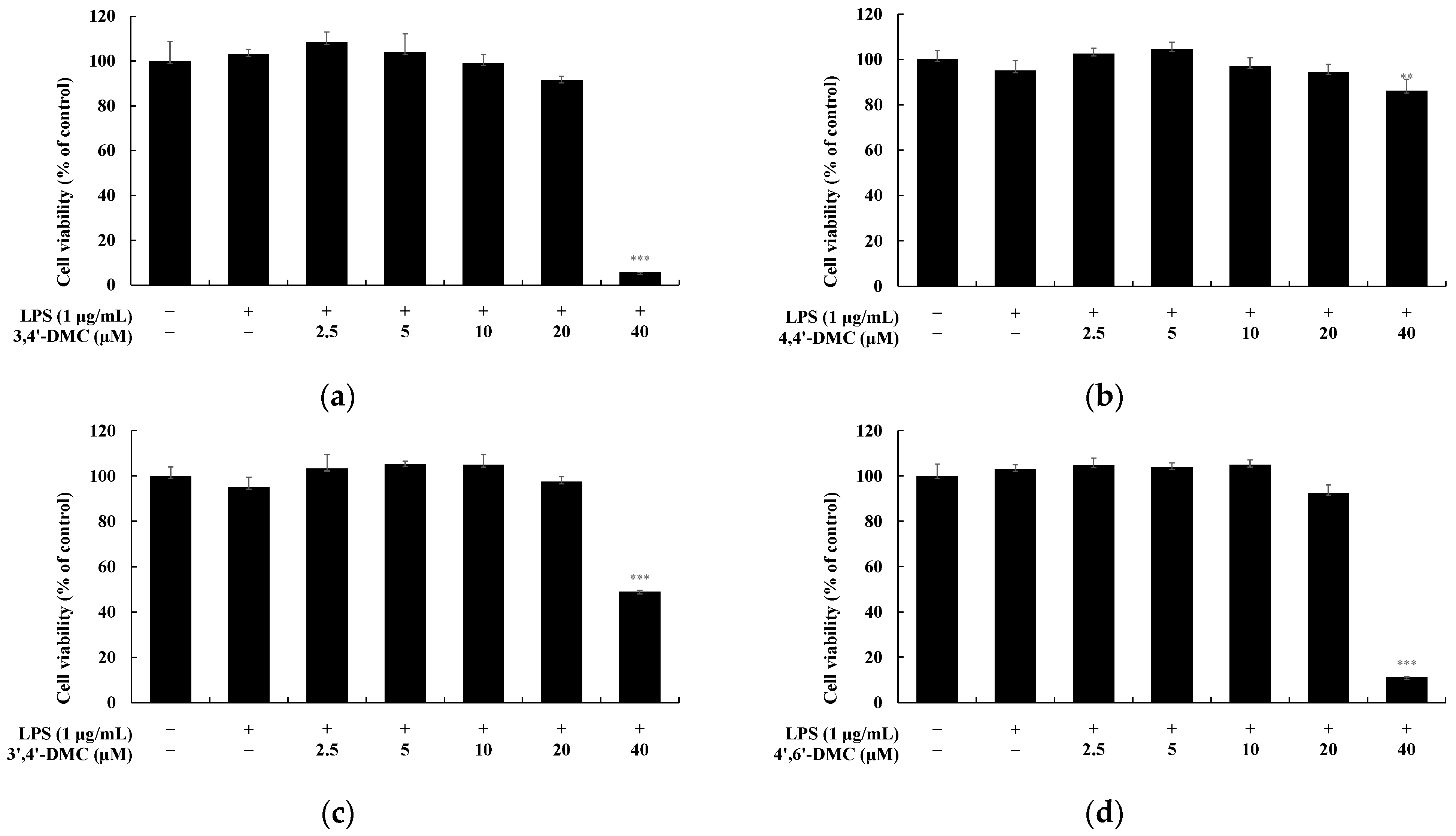
2.2. Cell Viabilities
2.3. Measurement of Nitric Oxide (NO) Production
2.4. Measurement of Pro-Inflammatory Cytokine Production
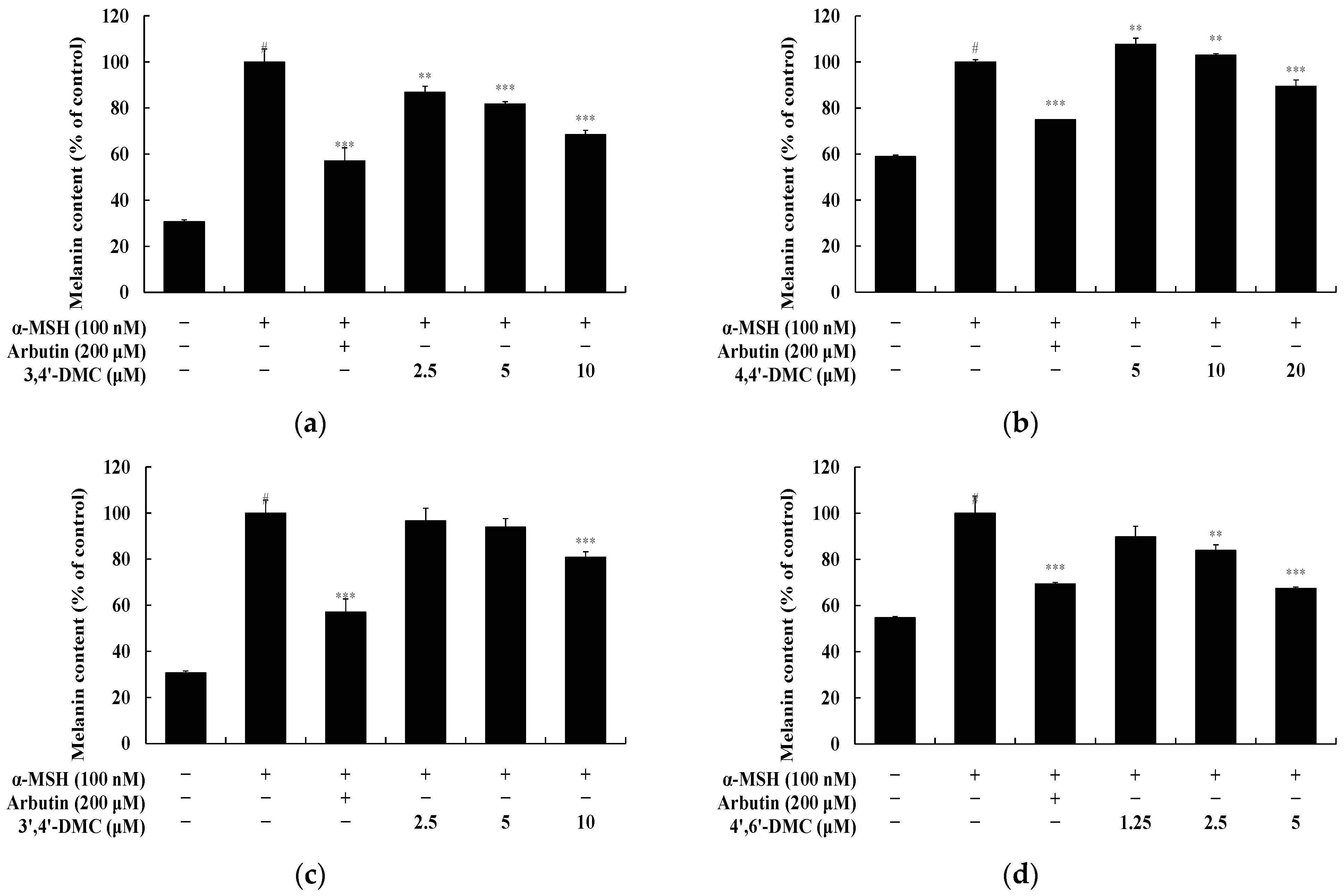
2.5. Measurement of Melanin Contents
2.6. Measurement of Intracellular Tyrosinase Activity
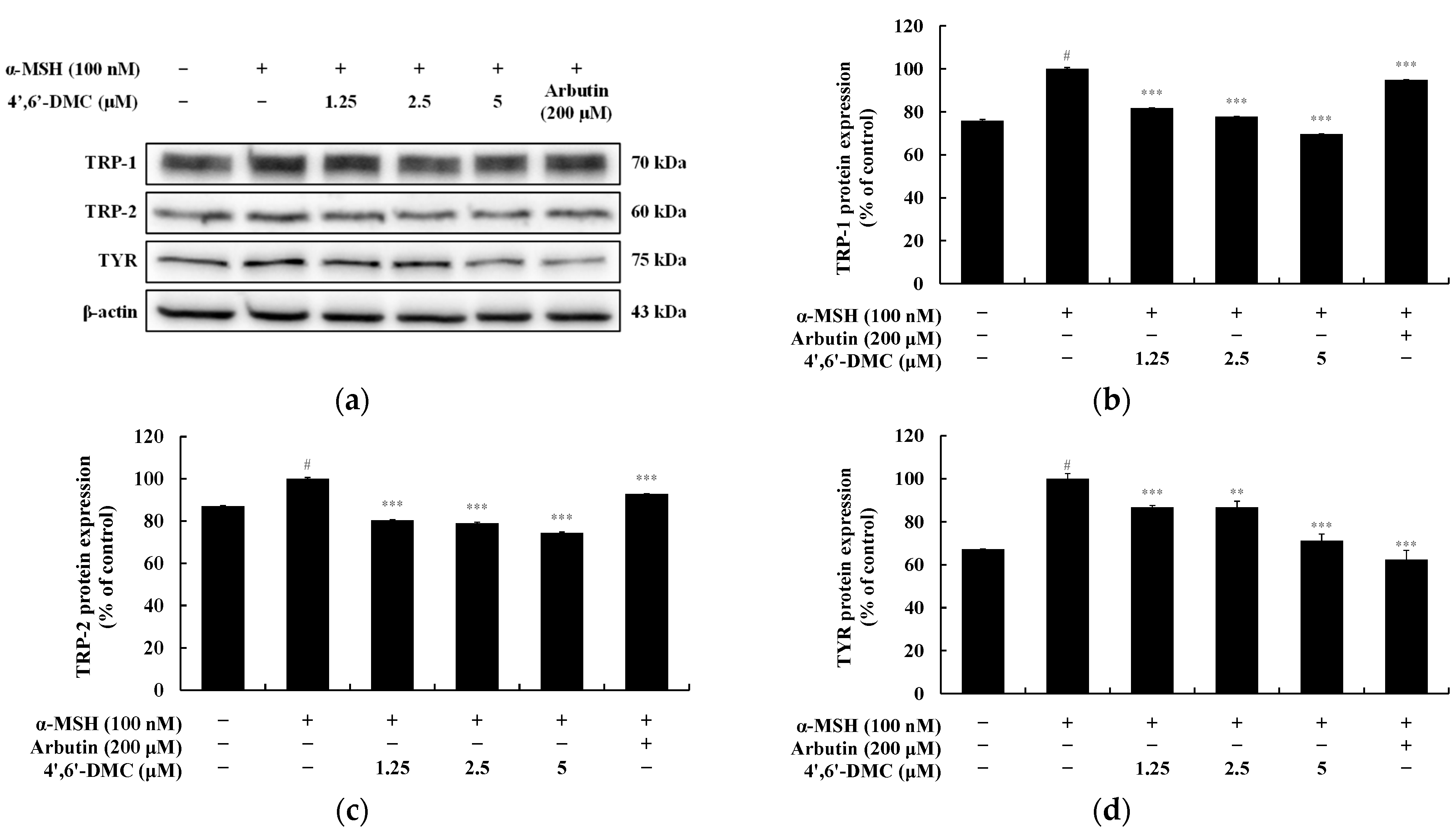
2.7. Western Blot
2.8. Human Skin Patch Test
2.9. Statistical Analyses
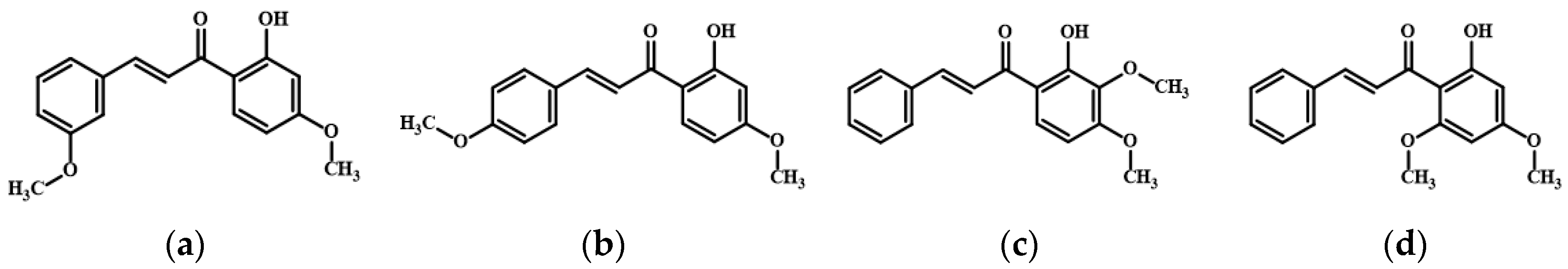
3. Results
3.1. 2′-Hydroxy-4′-Methoxychalcone Derivatives Inhibited Melanin Content and Tyrosinase Activity in B16F10 Cells
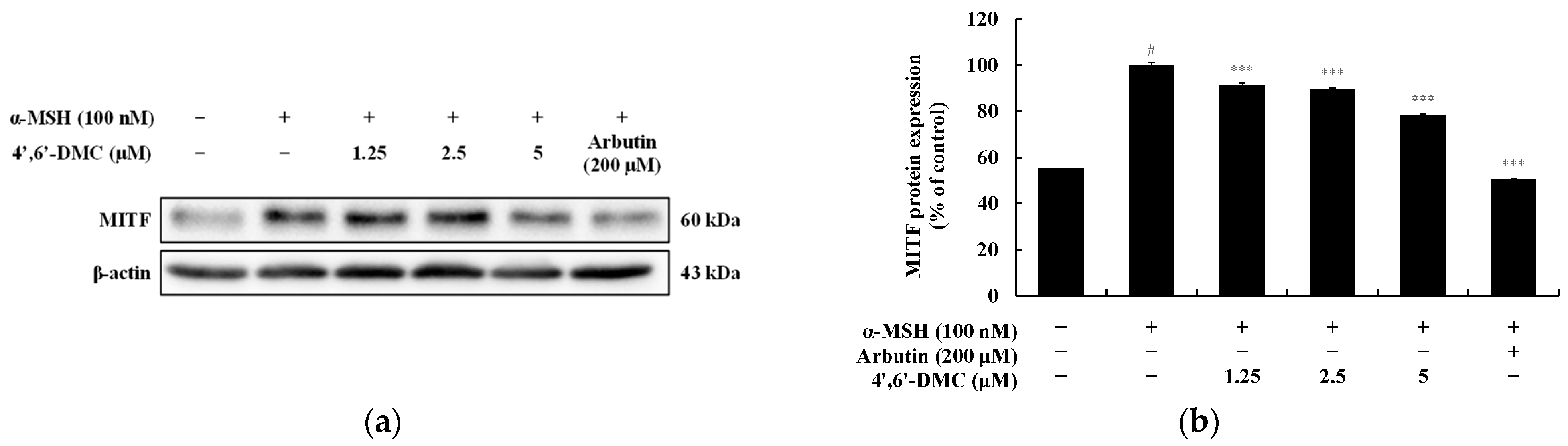
3.2. 4′,6′-DMC Regulated the Expression of Melanogenesis-Related Proteins in B16F10 Cells
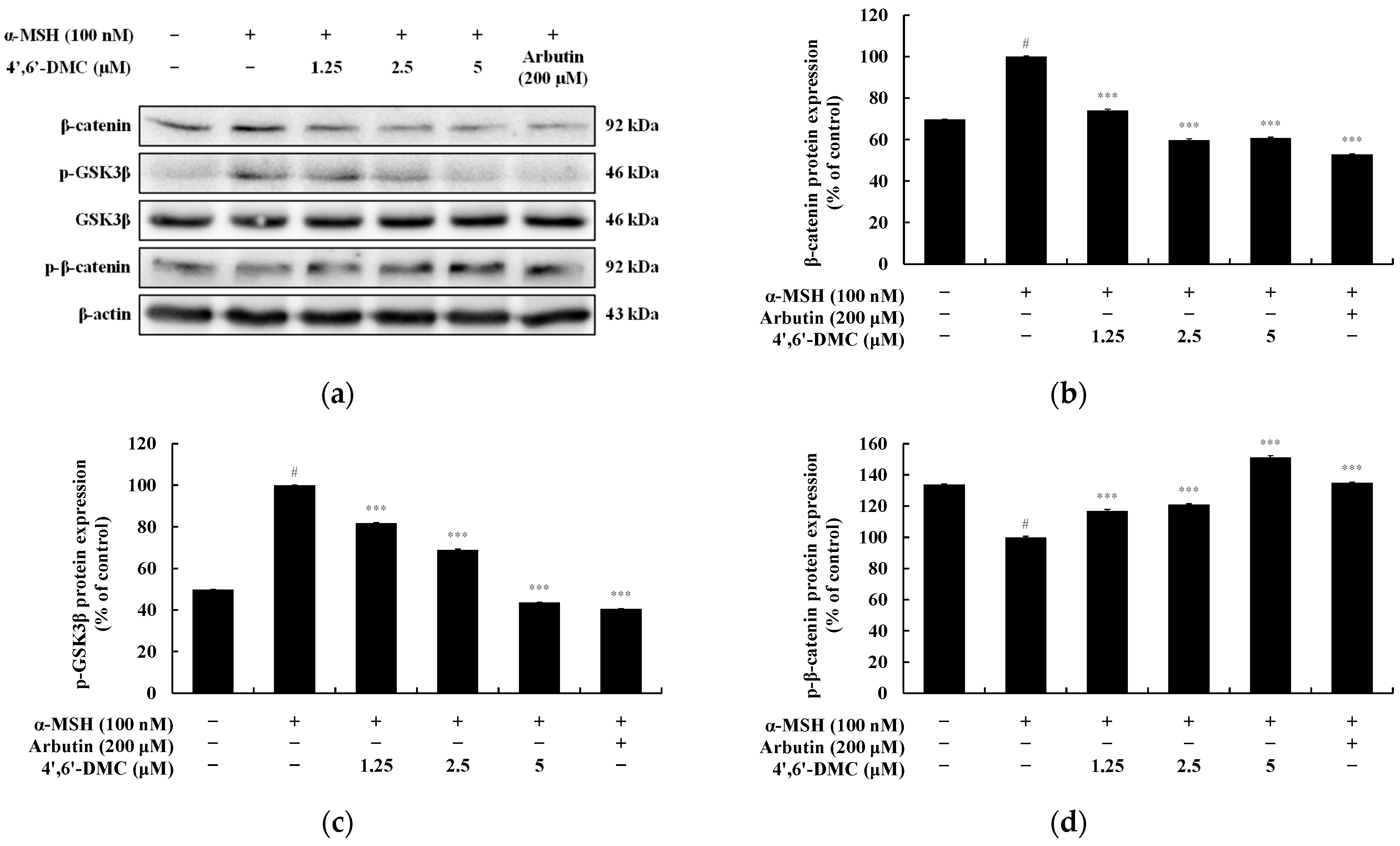
3.3. 4′,6′-DMC Inhibits Melanogenesis through the GSK-3β/β-Catenin Pathway in B16F10 Cells
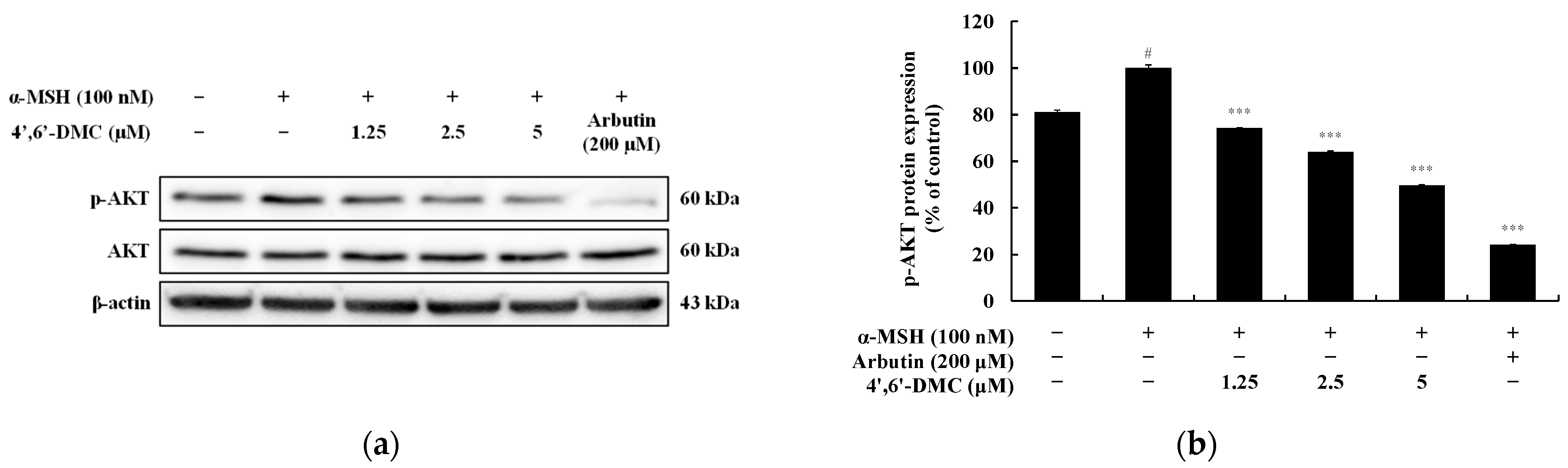
3.4. 4′,6′-DMC Inhibits Melanogenesis through the PI3K/Akt Pathway in B16F10 Cells
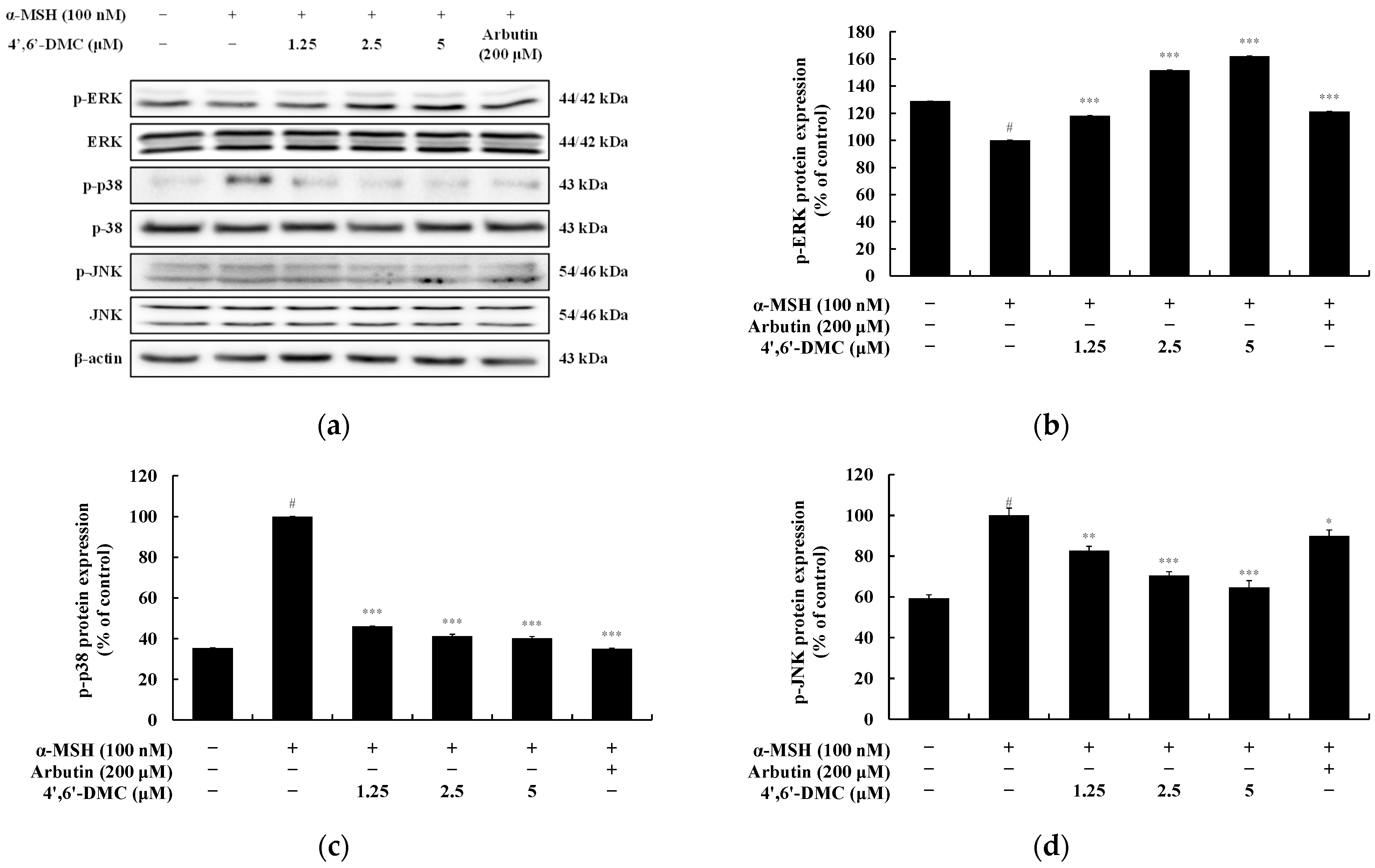
3.5. 4′,6′-DMC Inhibits Melanogenesis through the MAPK Pathway in B16F10 Cells
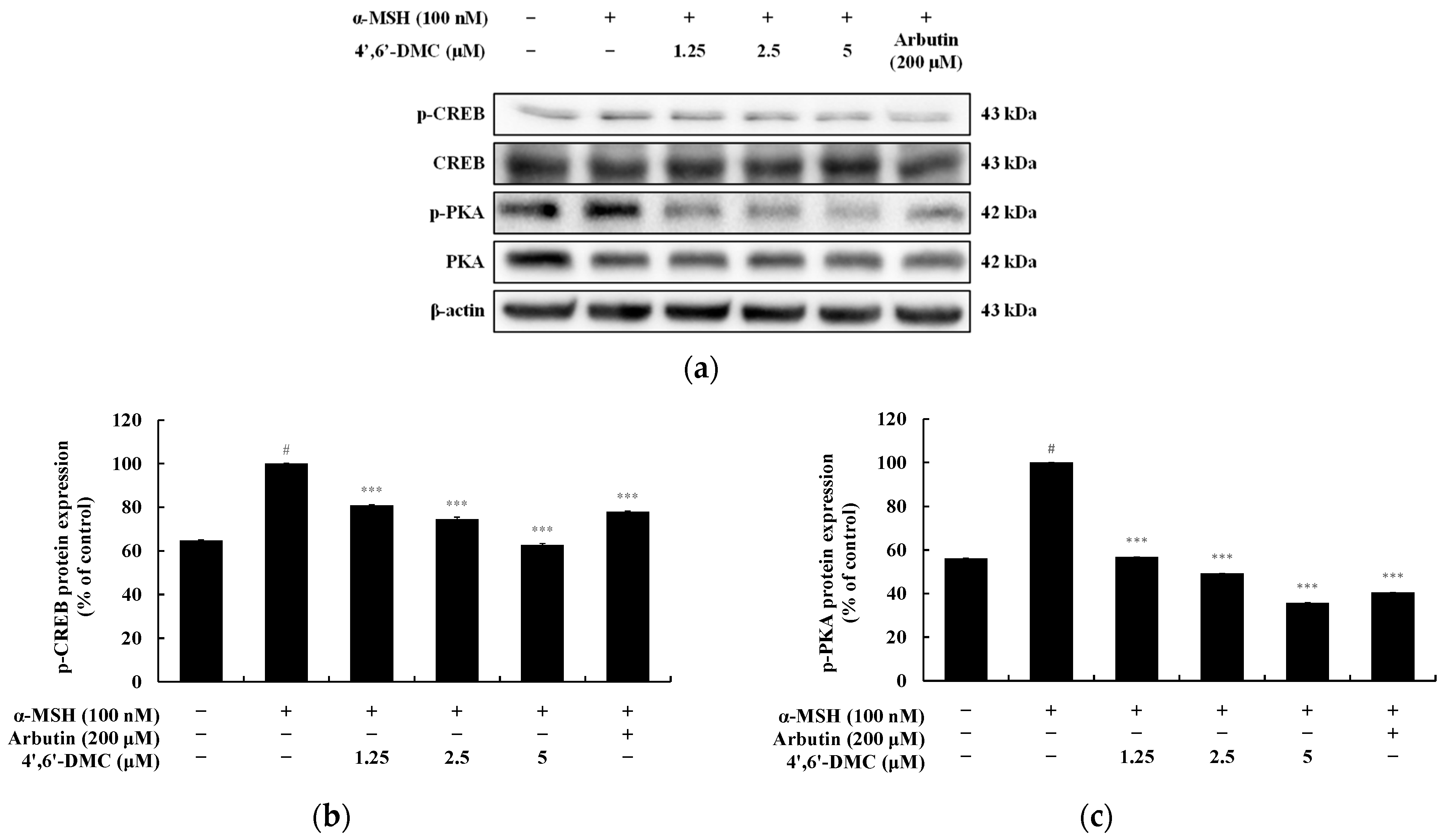
3.6. 4′,6′-DMC Inhibits Melanogenesis through the cAMP/PKA Pathway in B16F10 Cells
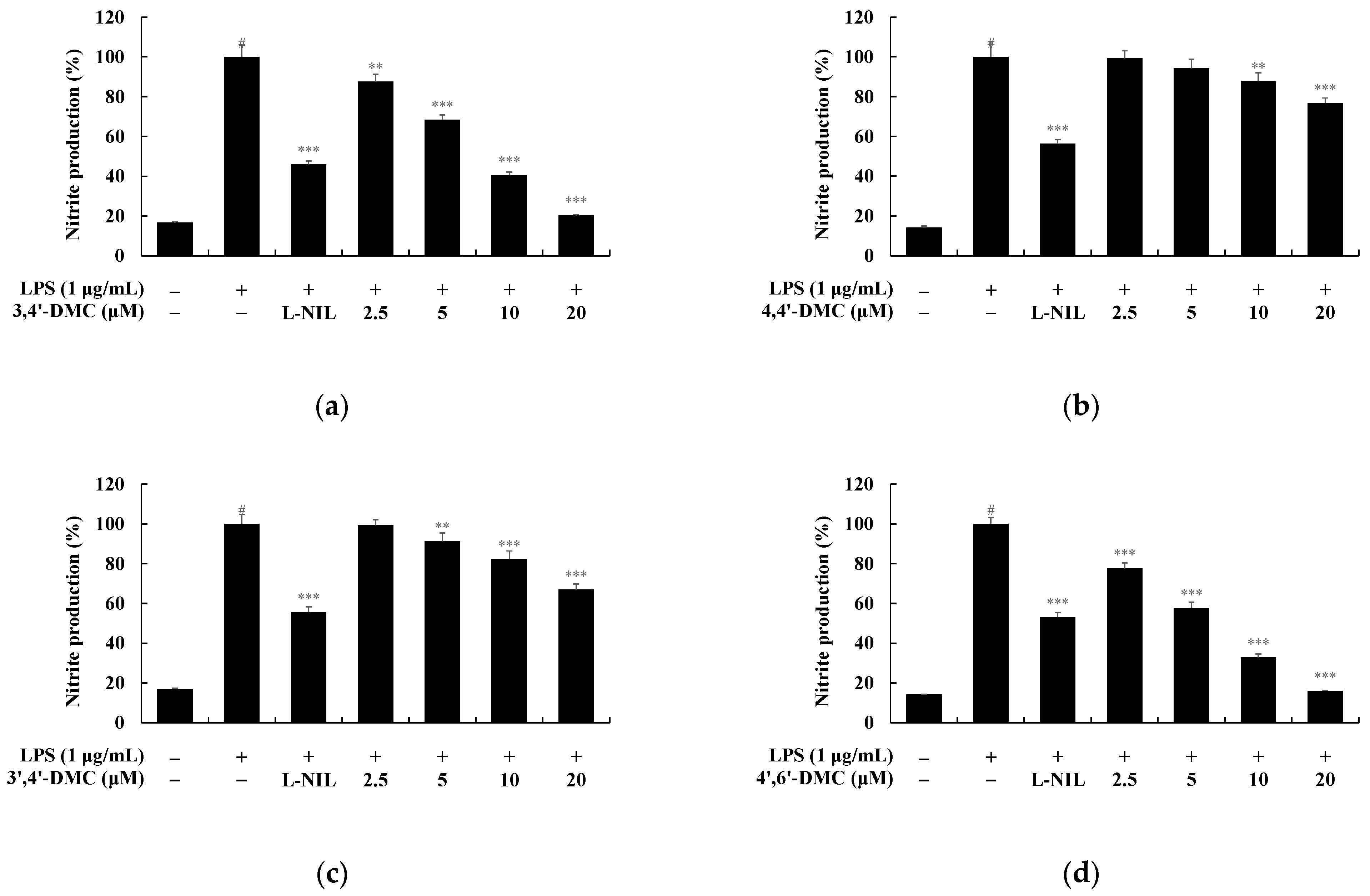
3.7. 2′-Hydroxy-4′-Methoxychalcone Derivatives Inhibited the Nitric Oxide Production in RAW 264.7 Cells
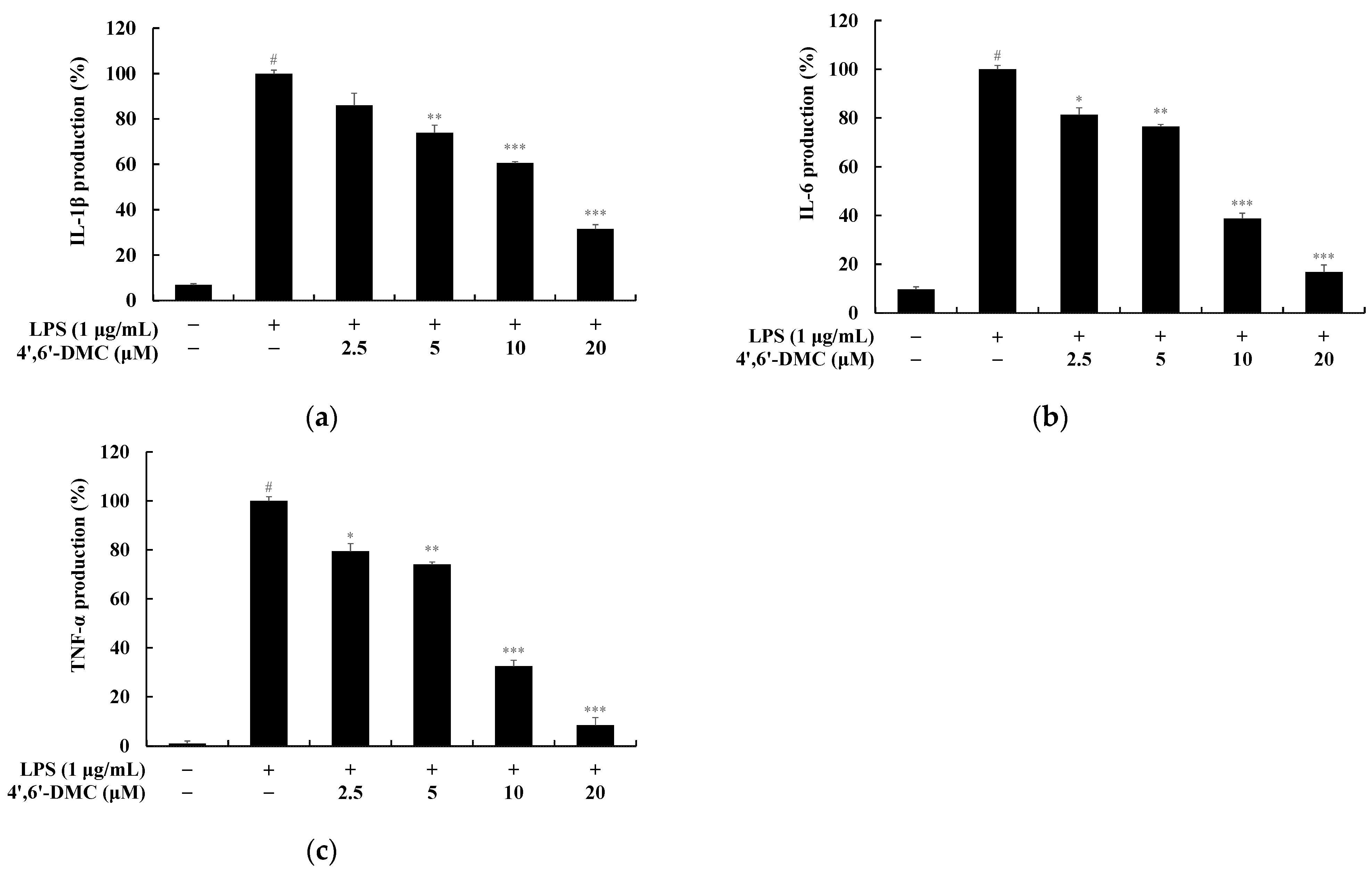
3.8. 4′,6′-DMC Inhibited the Production of Pro-Inflammatory Cytokines in RAW 264.7 Cells
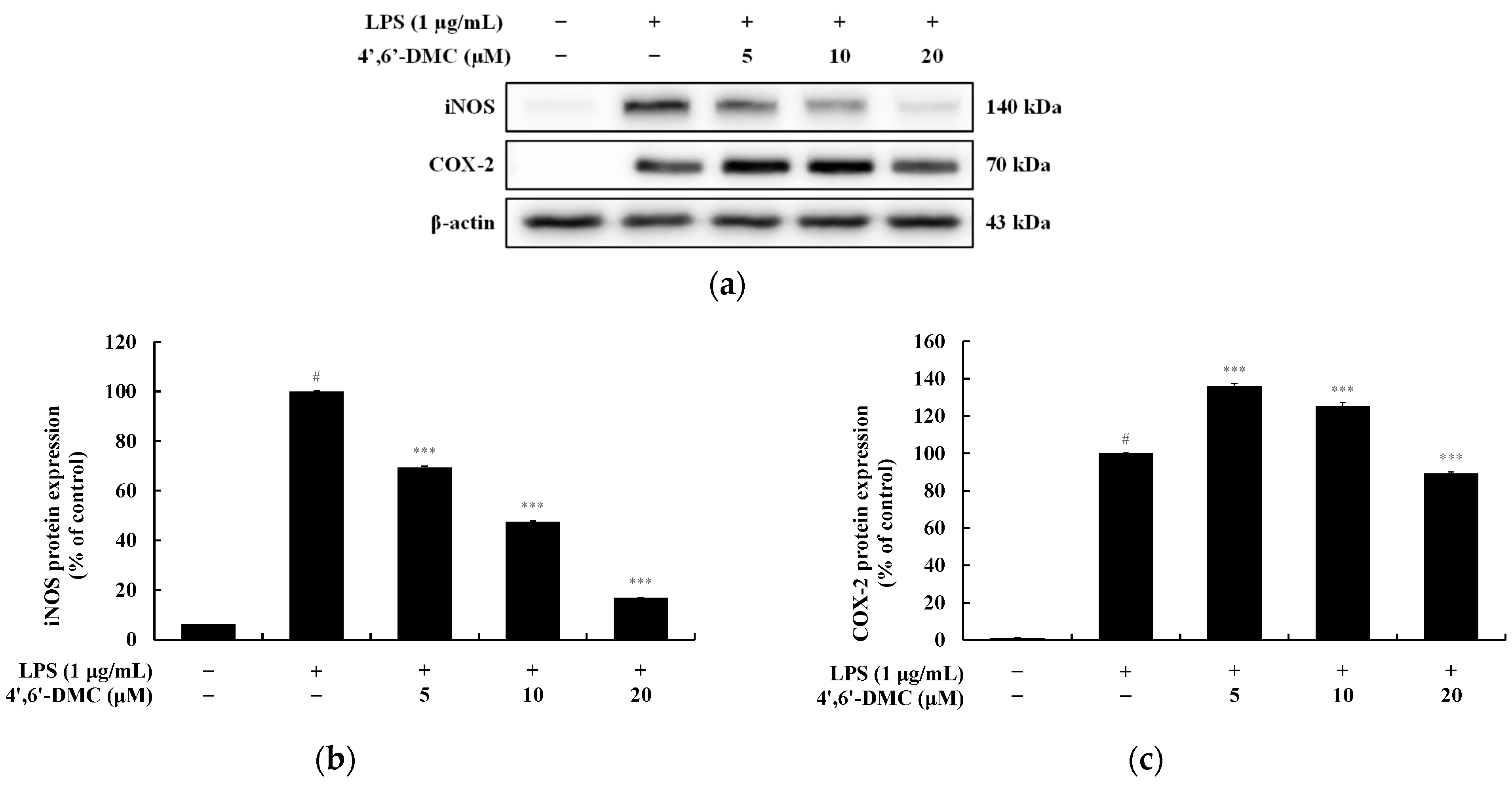
3.9. 4′,6′-DMC Inhibited the Expression of iNOS and COX-2 Proteins in RAW 264.7 Cells
3.10. 4′,6′-DMC Inhibited Inflammation in RAW 264.7 Cells through the MAPK Signaling Pathway
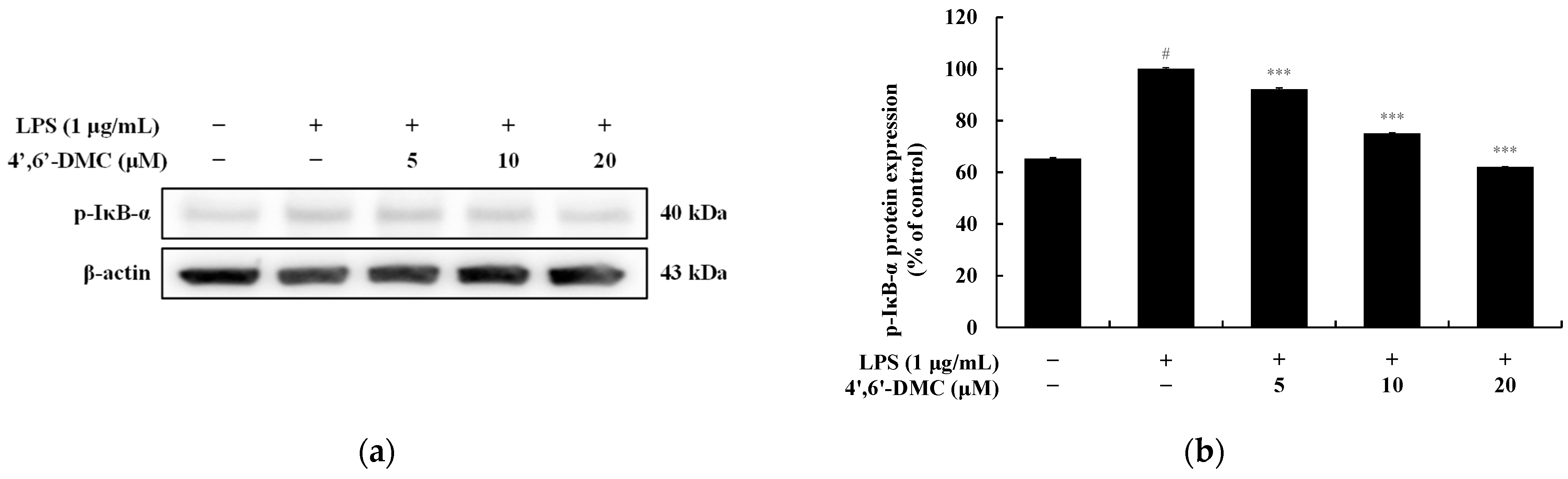
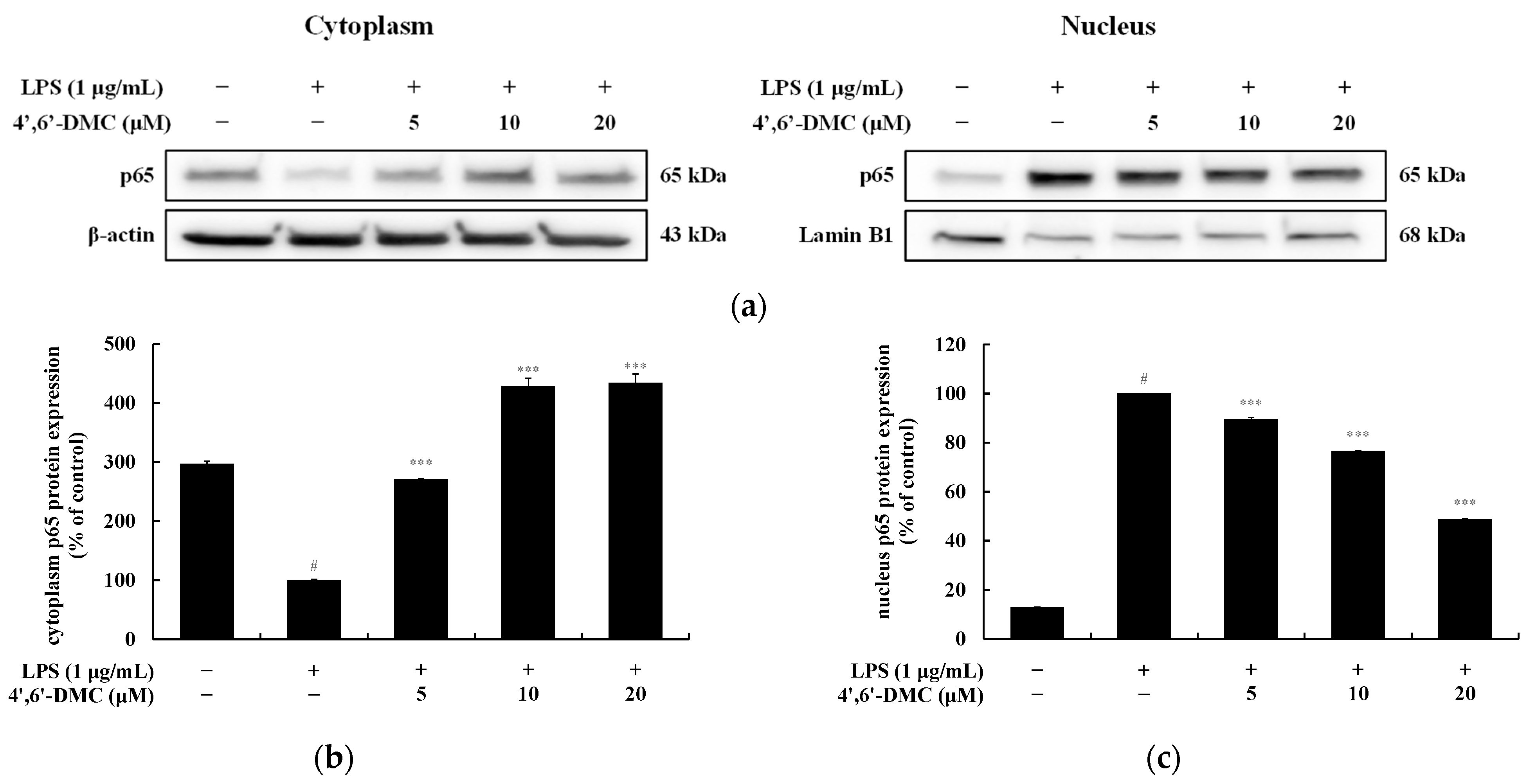
3.11. 4′,6′-DMC Repressed Inflammation in RAW 264.7 Cells through NF-kB Signaling Pathways
3.12. 4′,6′-DMC Is Safe for Human Skin
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naik, P.P.; Farrukh, S.N. Influence of Ethnicities and Skin Color Variations in Different Populations: A Review. Skin Pharmacol. Physiol. 2022, 35, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Hearing, V.J. Melanocytes and their diseases. Cold Spring Harb. Perspect. Med. 2014, 4, a017046. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Ko, W.S.; Yoon, H.J. A Study on Correlation of Melanin and Pigmentation Disorder and Viscera and Bowels. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2016, 29, 27–41. [Google Scholar] [CrossRef]
- Manvar, D.; Mishra, M.; Kumar, S.; Pandey, V.N. Identification and evaluation of anti hepatitis C virus phytochemicals from Eclipta Alba. J. Ethnopharmacol. 2012, 144, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: A comparative review. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Alaluf, S.; Atkins, D.; Barrett, K.; Blount, M.; Carter, N.; Heath, A. The impact of epidermal melanin on objective measurements of human skin colour. Pigment Cell Res. 2002, 15, 119–226. [Google Scholar] [CrossRef] [PubMed]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Sun, H.T.; Lin, I.P.; Kuo, P.C.; Li, J.C. The functional property of royal jelly 10-hydroxy-2-decenoic acid as a melanogenesis inhibitor. BMC Complement. Altern. Med. 2017, 17, 392. [Google Scholar] [CrossRef] [PubMed]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Postepy. Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Ali, S.A.; Naaz, I. Current challenges in understanding the story of skin pigmentation—Bridging the morpho-anatomical and functional aspects of mammalian melanocytes. In Muscle Cell and Tissue; IntechOpen: London, UK, 2015; pp. 262–285. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef]
- Lee, A.; Kim, J.Y.; Heo, J.; Cho, D.H.; Kim, H.S.; An, I.S.; Bae, S. The inhibition of melanogenesis via the PKA and ERK signaling pathways by Chlamydomonas reinhardtii extract in B16F10 melanoma cells and artificial human skin equivalents. J. Microbiol. Biotechnol. 2018, 28, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Su, T.R.; Lin, J.J.; Tsai, C.C.; Huang, T.K.; Yang, Z.Y.; Wu, M.O.; Wu, Y.J. Inhibition of melanogenesis by gallic acid: Possible involvement of the PI3K/Akt, MEK/ERK and Wnt/β-catenin signaling pathways in B16F10 cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Beer, J.Z.; Hearing, V.J. Melanin mediated apoptosis of epidermal cells damaged by ultraviolet radiation: Factors influencing the incidence of skin cancer. Arch. Dermatol. Res. 2008, 300, S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pig. Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Hearing, V.J. Physiological factors that regulate skin pigmentation. Biofactors 2009, 35, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Matsui, M.S.; Ichihashi, M. Quasi-drugs developed in Japan for the prevention or treatment of hyperpigmentary disorders. Int. J. Mol. Sci. 2010, 11, 2566–2575. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.I.; Lee, Y.M.; Lim, B.O.; Lim, J.H. Inhibition of NAT10 suppresses melanogenesis and melanoma growth by attenuating microphthalmia-associated transcription factor (MITF) expression. Int. J. Mol. Sci. 2017, 18, 1924. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.J.; Fisher, D.E. The roles of microphthalmia-associated transcription factor and pigmentation in melanoma. Arch. Biochem. Biophys. 2014, 563, 28–34. [Google Scholar] [CrossRef]
- Zaidi, K.U.; Ali, S.; Ali, A.; Thawani, V. Natural melanogenesis stimulator a potential tool for the treatment of hypopigmentation disease. Int. J. Mol. Biol. 2017, 2, 37–40. [Google Scholar] [CrossRef][Green Version]
- Truong, X.T.; Park, S.H.; Lee, Y.G.; Jeong, H.Y.; Moon, J.H.; Jeon, T.I. Protocatechuic acid from pear inhibits melanogenesis in melanoma cells. Int. J. Mol. Sci. 2017, 18, 1809. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.I.; Yokoyama, K.; Takahashi, K.; Tomita, Y.; Shibahara, S. Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J. Biol. Chem. 1997, 272, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, C.; Buscà, R.; Abbe, P.; Bille, K.; Aberdam, E.; Ortonne, J.P.; Ballotti, R. Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: Pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol. Cell Biol. 1998, 18, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Suzuki, H.; Yasumoto, K.I.; Tomita, Y.; Shibahara, S. Molecular cloning and functional analysis of a cDNA coding for human DOPAchrome tautomerase/tyrosinase-related protein-2. Biochim. Biophys. Acta. 1994, 1217, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Bessa, J.; Meyer, C.A.; de Vera Mudry, M.C.; Schlicht, S.; Smith, S.H.; Iglesias, A.; Cote-Sierra, J. Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation. J. Autoimmun. 2014, 55, 33–41. [Google Scholar] [CrossRef]
- White, G.E.; Iqbal, A.J.; Greaves, D.R. CC chemokine receptors and chronic inflammation—Therapeutic opportunities and pharmacological challenges. Pharmacol. Rev. 2013, 65, 47–89. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, T.; Cao, Q.; Chen, X.; Chu, Z.; Zhang, Z. Anti-inflammatory effects of Torin2 on lipopolysaccharide-treated RAW264. 7 murine macrophages and potential mechanisms. Heliyon 2022, 8, e09917. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.R., Jr.; Goldberg, J.B. Purification and visualization of lipopolysaccharide from Gram-negative bacteria by hot aqueous-phenol extraction. J. Vis. Exp. 2012, 28, 3916. [Google Scholar] [CrossRef]
- Kawahara, M.; Nemoto, M.; Nakata, T.; Kondo, S.; Takahashi, H.; Kimura, B.; Kuda, T. Anti-inflammatory properties of fermented soy milk with Lactococcus lactis subsp. lactis S-SU2 in murine macrophage RAW264. 7 cells and DSS-induced IBD model mice. Int. Immunopharmacol. 2015, 26, 295–303. [Google Scholar] [CrossRef]
- McCoy, C.E.; O′neill, L.A. The role of toll-like receptors in macrophages. Front Biosci. 2008, 13, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Jeon, Y.J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef]
- Ramirez, D.C.; Gimenez, M.S. Induction of redox changes, inducible nitric oxide synthase and cyclooxygenase-2 by chronic cadmium exposure in mouse peritoneal macrophages. Toxicol. Lett. 2003, 145, 121–132. [Google Scholar] [CrossRef]
- Shirato, K.; Kizaki, T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon 2021, 7, e06187. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Shin, Y.K.; Lee, S.H. Anti-inflammatory activity of patchouli alcohol in RAW264. 7 and HT-29 cells. Food Chem. Toxicol. 2013, 55, 229–233. [Google Scholar] [CrossRef]
- Abraham, E. Nuclear factor—κB and its role in sepsis-associated organ failure. J. Infect. Dis. 2003, 187 (Suppl. S2), S364–S369. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwak, C.H.; Lee, S.K.; Ha, S.H.; Park, J.; Chung, T.W.; Kim, C.H. Anti-inflammatory effect of ascochlorin in LPS-stimulated RAW 264.7 macrophage cells is accompanied with the down-regulation of iNOS, COX-2 and proinflammatory cytokines through NF-κB, ERK1/2, and p38 signaling pathway. J. Cell Biochem. 2016, 117, 978–987. [Google Scholar] [CrossRef]
- Cho, W.; Nam, J.W.; Kang, H.J.; Windono, T.; Seo, E.K.; Lee, K.T. Zedoarondiol isolated from the rhizoma of Curcuma heyneana is involved in the inhibition of iNOS, COX-2 and pro-inflammatory cytokines via the downregulation of NF-κB pathway in LPS-stimulated murine macrophages. Int. Immunopharmacol. 2009, 9, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Henklewska, M.; Hernández Suárez, B.; Łużny, M.; Kozłowska, E.; Obmińska-Mrukowicz, B.; Janeczko, T. Chalcone methoxy derivatives exhibit antiproliferative and proapoptotic activity on canine lymphoma and leukemia cells. Molecules 2020, 25, 4362. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wu, S.N.; Gao, J.M.; Liao, Z.Y.; Tseng, Y.T.; Fülöp, F.; Lo, Y.C. The antioxidant, anti-inflammatory, and neuroprotective properties of the synthetic chalcone derivative AN07. Molecules 2020, 25, 2907. [Google Scholar] [CrossRef]
- Wang, Y.H.; Dong, H.H.; Zhao, F.; Wang, J.; Yan, F.; Jiang, Y.Y.; Jin, Y.S. The synthesis and synergistic antifungal effects of chalcones against drug resistant Candida albicans. Bioorg. Med. Chem. Lett. 2016, 26, 3098–3102. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Batista, A.D.J.; Philipon, C.I.S.; de Souza Albernaz, M.; Pinto, S.R.; Rossi-Bergmann, B.; Santos-Oliveira, R. New chalcone compound as a promising antileishmanial drug for an old neglected disease: Biological evaluation using radiolabelled biodistribution. J. Global Antimicrob. Res. 2018, 13, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Borsari, C.; Santarem, N.; Torrado, J.; Olías, A.I.; Corral, M.J.; Baptista, C.; Costi, M.P. Methoxylated 2′-hydroxychalcones as antiparasitic hit compounds. Eur. J. Med. Chem. 2017, 126, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.; Neves, B.J. Chalcone derivatives: Promising starting points for drug design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.M.I.I.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoon, H.S.; Lee, S.R.; Ko, H.C.; Choi, S.Y.; Park, J.G.; Kim, J.K.; Kim, S.J. Involvement of extracellular signal-regulated kinase in nobiletin-induced melanogenesis in murine B16/F10 melanoma cells. Biosci Biotechnol Biochem. 2007, 71, 1781–1784. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Ko, H.C.; Kim, S.S.; Park, K.J.; An, H.J.; Choi, Y.H.; Kim, S.J.; Lee, N.H.; Hyun, C.G. Tangeretin triggers melanogenesis through the activation of melanogenic signaling proteins and sustained extracellular signal- regulated kinase in B16/F10 murine melanoma cells. Nat. Prod. Commun. 2015, 10, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Hyun, C.G. The Effects of 2′-Hydroxy-3,6′-Dimethoxychalcone on Melanogenesis and Inflammation. Int. J. Mol. Sci. 2023, 24, 10393. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, K.B.; Hyun, C.G. A 7-Hydroxy 4-Methylcoumarin Enhances Melanogenesis in B16-F10 Melanoma Cells. Molecules 2023, 28, 3039. [Google Scholar] [CrossRef]
- Han, H.J.; Hyun, C.G. Acenocoumarol Exerts Anti-Inflammatory Activity via the Suppression of NF-κB and MAPK Pathways in RAW 264.7 Cells. Molecules 2023, 28, 2075. [Google Scholar] [CrossRef]
- Kim, H.M.; Hyun, C.G. Miglitol, an oral antidiabetic drug, downregulates melanogenesis in B16F10 melanoma cells through the pka, mapk, and gsk3β/β-catenin signaling pathways. Molecules 2022, 28, 115. [Google Scholar] [CrossRef]
- Lee, Y.; Hyun, C.G. Anti-Inflammatory Effects of Psoralen Derivatives on RAW264. 7 Cells via Regulation of the NF-κB and MAPK Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 5813. [Google Scholar] [CrossRef] [PubMed]
- Nur, S.; Setiawan, H.; Hanafi, M.; Elya, B. Pharmacognostical and Phytochemical Studies and Biological Activity of Curculigo latifolia Plant Organs for Natural Skin-Whitening Compound Candidate. ScientificWorldJournal 2023, 2023, 5785259. [Google Scholar] [CrossRef] [PubMed]
- Suryaningsih, B.E. Melanogenesis and its associated signalings. Bali Med. J. 2020, 9, 327–331. [Google Scholar] [CrossRef]
- Chang, T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Liu, F.; Xu, T.; He, J.; Jiang, Y.; Qu, L.; Wang, L.; Ma, J.; Yang, Q.; Wu, W.; Sun, D.; et al. Exploring the potential of white birch sap: A natural alternative to traditional skin whitening agents with reduced side effects. Heliyon 2024, 10, e26715. [Google Scholar] [CrossRef] [PubMed]
- Veerichetty, V.; Saravanabavan, I. Molecular docking Study of Nuciferine as a Tyrosinase Inhibitor and Its Therapeutic Potential for Hyperpigmentation. Genomics Inform. 2023, 21, e43. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.M.P.F.; Moniz, T.; Leite, A.; Oliveira, A.; Fernandes, P.; Ramos, M.J.; Araújo, A.N.; Freitas, M.; Fernandes, E.; Rangel, M. A combined experimental and computational study to discover novel tyrosinase inhibitors. J. Inorg. Biochem. 2022, 234, 111879. [Google Scholar] [CrossRef] [PubMed]
- Ghayas, S.; Ali Masood, M.; Parveen, R.; Aquib, M.; Farooq, M.A.; Banerjee, P.; Sambhare, S.; Bavi, R. 3D QSAR pharmacophore-based virtual screening for the identification of potential inhibitors of tyrosinase. J. Biomol. Struct. Dyn. 2020, 38, 2916–2927. [Google Scholar] [CrossRef]
- Beltran, E.; Serafini, M.R.; Alves, I.A.; Aragón Novoa, D.M. Novel Synthesized Tyrosinase Inhibitors: A Systematic Patent Review (2012–Present). Curr. Med. Chem. 2024, 31, 308–335. [Google Scholar] [CrossRef]
- Boo, Y.C. Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef] [PubMed]


| Grade | Description of Clinical Observation |
|---|---|
| +1 | Slight erythema |
| +2 | Moderate erythema, possibly with barely perceptible edema at the margin, papules may be present |
| +3 | Moderate erythema, with generalized edema |
| +4 | Severe erythema with severe edema, with or without vesicles |
| +5 | Severe reaction spread beyond the area of the patch |
| No | Test Samples | No. of Responder | 20 min after Patch Removal | 24 h after Patch Removal | Reaction Grade (R) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| +1 | +2 | +3 | +4 | +1 | +2 | +3 | +4 | ||||
| 1 | 4′,6′-DMC(10 μM) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 4′,6′-DMC(5 μM) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.; Lee, J.-N.; Hyun, C.-G. Anti-Melanogenic and Anti-Inflammatory Effects of 2′-Hydroxy-4′,6′-dimethoxychalcone in B16F10 and RAW264.7 Cells. Curr. Issues Mol. Biol. 2024, 46, 6018-6040. https://doi.org/10.3390/cimb46060359
Bae S, Lee J-N, Hyun C-G. Anti-Melanogenic and Anti-Inflammatory Effects of 2′-Hydroxy-4′,6′-dimethoxychalcone in B16F10 and RAW264.7 Cells. Current Issues in Molecular Biology. 2024; 46(6):6018-6040. https://doi.org/10.3390/cimb46060359
Chicago/Turabian StyleBae, Sungmin, Jung-No Lee, and Chang-Gu Hyun. 2024. "Anti-Melanogenic and Anti-Inflammatory Effects of 2′-Hydroxy-4′,6′-dimethoxychalcone in B16F10 and RAW264.7 Cells" Current Issues in Molecular Biology 46, no. 6: 6018-6040. https://doi.org/10.3390/cimb46060359
APA StyleBae, S., Lee, J.-N., & Hyun, C.-G. (2024). Anti-Melanogenic and Anti-Inflammatory Effects of 2′-Hydroxy-4′,6′-dimethoxychalcone in B16F10 and RAW264.7 Cells. Current Issues in Molecular Biology, 46(6), 6018-6040. https://doi.org/10.3390/cimb46060359








