Abstract
Venous thromboembolism (VTE) is a challenging clinical obstacle in oncological settings, marked by elevated incidence rates and resulting morbidity and mortality. In the context of cancer-associated thrombosis (CAT), endothelial dysfunction (ED) plays a crucial role in promoting a pro-thrombotic environment as endothelial cells lose their ability to regulate blood flow and coagulation. Moreover, emerging research suggests that this disorder may not only contribute to CAT but also impact tumorigenesis itself. Indeed, a dysfunctional endothelium may promote resistance to therapy and favour tumour progression and dissemination. While extensive research has elucidated the multifaceted mechanisms of ED pathogenesis, the genetic component remains a focal point of investigation. This comprehensive narrative review thus delves into the genetic landscape of ED and its potential ramifications on cancer progression. A thorough examination of genetic variants, specifically polymorphisms, within key genes involved in ED pathogenesis, namely eNOS, EDN1, ACE, AGT, F2, SELP, SELE, VWF, ICAM1, and VCAM1, was conducted. Overall, these polymorphisms seem to play a context-dependent role, exerting both oncogenic and tumour suppressor effects depending on the tumour and other environmental factors. In-depth studies are needed to uncover the mechanisms connecting these DNA variations to the pathogenesis of malignant diseases.
1. Introduction
Venous thromboembolism (VTE), also referred to as venous thrombosis, is a prevalent and intricate cardiovascular condition. The disease has two main manifestations: when the thrombus first forms in a deep vein—deep vein thrombosis (DVT)—followed by its migration into the bloodstream and subsequent lodging in the lungs—pulmonary embolism (PE) [1]. In Europe, VTE affects around one to two individuals per 1000 annually [2]. Although the incidence rate in the United States of America (USA) is greatly similar, it varies significantly from a global perspective, indicating a potential regional influence on the occurrence of thrombotic events. Indeed, VTE has been associated with multiple risk determinants, comprising acute (e.g., surgery) and subacute (e.g., oral contraceptive use) triggers; basal/genetic (e.g., genetic polymorphisms, such as Factor V Leiden (F5 rs6025) and Prothrombin G20210A (F2 rs1799963)) and acquired (e.g., autoimmune diseases) risk factors [2,3]. One acquired risk factor of VTE that warrants prominent consideration is cancer. With an estimated annual incidence of VTE at 0.5% among cancer patients, compared to 0.1% in the general population, these statistics underscore the markedly heightened vulnerability to venous thrombogenesis among individuals with malignant diseases [4]. In recent years, the link between cancer physiopathology and VTE has attained increasing attention, leading to the emergence of the concept of cancer-associated thrombosis (CAT). This constitutes a bidirectional relationship, wherein both cancer and VTE serve as mutual risk factors for each other, as well as exert a significant impact on each other’s mortality rates [5]. Compared to VTE in the general population, CAT seems to be a distinct and more complex disorder. Mechanistically, tumour cells produce pro-coagulant, anti-fibrinolytic and pro-inflammatory substances, which trigger pro-thrombotic and pro-inflammatory cascades leading to venous thrombogenesis [4].
The pathogenesis of VTE, both in the general population and among cancer subjects, can be explained by the Virchow Triad, which integrates three promoting factors: stasis of blood flow, blood hypercoagulability, and endothelial dysfunction (ED) (Figure 1) [4,6]. ED refers to an alteration in the normal function of the endothelial cells (ECs) lining the interior of blood vessels. The first step of this disorder is endothelial stimulation (type I activation) followed by delayed endothelial activation (type II activation), both reversible upon cessation of the stimulus. In advanced stages, ED also encompasses EC apoptosis and necrosis, which leads to endothelial detachment, giving rise to circulating endothelial cells (CECs) [7]. Apart from contributing to VTE, ED is a critical factor in the pathogenesis of other cardiovascular and metabolic diseases, including atherosclerosis, hypertension, coronary artery disease, chronic heart failure, peripheral artery disease, diabetes, and chronic renal failure [8,9,10]. Importantly, a relevant bridge to cancer is also formed as the pro-inflammatory state of ED promotes tumour growth and progression. Additionally, the inhibition of vasodilation (characteristic of ED) supports cell proliferation and anti-apoptotic responses, reinforcing its association with cancer [11,12].
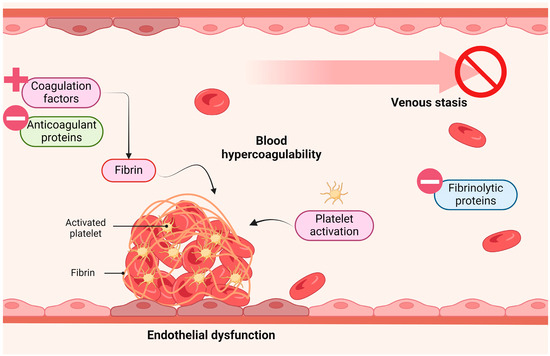
Figure 1.
Virchow triad. Venous thrombosis is suggested to be promoted by three important factors: blood hypercoagulability, venous stasis and endothelial dysfunction. Figure created with Biorender.com (accessed on 13 April 2024).
Like VTE, ED presentation can also be influenced by genetic polymorphisms, which are DNA variations present in greater than 1% of a given population. These variations include single-nucleotide polymorphisms (SNPs), copy number variations (CNVs), insertions and deletions (Indels), and tandem repeats [13,14]. Starting with SNPs, they represent genetic alterations characterised by single nucleotide substitutions [15]. CNVs arise from the deletion or duplication of DNA segments, ranging from kilobases to megabases, leading to a varied number of copies of a specific DNA sequence on homologous chromosomes [16]. In contrast, indels are small insertions or deletions of nucleotides in the DNA sequence [17]. Regarding tandem repeats, these genetic variations comprise repetitive DNA sequences spanning one or more nucleotides within both coding and non-coding regions. Their classification depends on the length of the repeated sequence. Namely, simple sequence repeats (SSRs), also known as short tandem repeats (STRs), consist of short repeating units (two to six nucleotides). SSRs represent a subset of a variable number of tandem repeats (VNTRs), characterised by their varying lengths [18]. Overall, genetic polymorphisms have the potential to modulate gene expression, disrupt gene function and alter protein-coding sequences, thereby affecting protein levels and/or activity. Consequently, these DNA variations can modulate the susceptibility to several disorders, including ED and its manifestations (e.g., VTE) [14].
Considering the roles of ED in cancer-related thrombogenesis and tumorigenesis, it is important to explore how genetic determinants implicated in this disorder could aid in the identification of at-risk populations and pinpoint potential therapeutic targets for a more personalised treatment in Oncology [14]. Given the implications for clinical application, this thorough narrative review seeks to delve into the influence of genetic variations linked to ED on tumorigenesis and cancer patient’s prognosis. The review concentrates on examining polymorphisms in pivotal ED-related genes such as endothelial nitric oxide synthase (eNOS), endothelin 1 (EDN1), angiotensin I converting enzyme (ACE), angiotensinogen (AGT), coagulation factor 2 (F2), selectin P (SELP), selectin E (SELE), von Willebrand factor (VWF), intercellular adhesion molecule 1 (ICAM1), and vascular cell adhesion molecule 1 (VCAM1). A thoughtful research was conducted by reviewing the PubMed database’s occurrences until 6th March 2024 using different combinations of keywords: “SNP”, “SNPs”, “polymorphism”, “polymorphisms”, “cancer”, “eNOS”, Endothelin-1”, “ET-1”, “Angiotensin II”, “AGT”, “ACE” and “Angiotensin Converting Enzyme”, “F2”, “Prothrombin”, “SELP”, “P-selectin”, “SELE”, “E-selectin”, “E-selectin”, “Von Willebrand factor”, “VWF”, “CD54”, “ICAM1”, “ICAM-1”, “VCAM1”, “VCAM-1” and “CD106”. Only studies with significant associations were selected. Additionally, matching publications were cross-referenced and screened for pertinent bibliographic references. Studies were excluded if the polymorphisms lacked functional relevance and/or the associations were observed solely considering specific therapeutic interventions. A total of 826 articles underwent review, resulting in the selection of 149 papers that met the inclusion and exclusion criteria.
2. Vascular Homeostasis
Gatekeeping the integrity of ECs is crucial for human well-being and illness management as these cells are responsible for vascular tone regulation, haemostasis and thrombosis control, cellular adhesion, smooth muscle cell proliferation, and vascular inflammation [6,19]. Under physiological conditions, ECs mediate multiple anti-coagulant and anti-platelet aggregation processes, restricting coagulation to only vascular sites where needed, thus preventing disseminated thrombotic complications (Figure 2) [20]. They do so by continuously expressing and/or releasing components that block platelet activity (prostacyclin (prostaglandin I2 (PGI2), nitric oxide (NO) and ectonucleoside triphosphate diphosphohydrolase-1 (E-NTPDase1)), inhibit coagulation progression (antithrombin III (ATIII)), thrombomodulin (TM) and tissue factor pathway inhibitor 1 (TFPI1)) and promote fibrinolysis (urokinase-type plasminogen activator (u-PA) and tissue-type plasminogen activator (tPA)) [21,22,23,24]. In opposition, when the vascular endothelium is disrupted, ECs shift to an adhesive, pro-inflammatory and pro-clotting phenotype [21]. The initial response to vascular damage is vasoconstriction, which slows the blood flow to prevent excessive blood loss. This mechanism is the basis for blood coagulation [25]. Parallelly, induced by pro-inflammatory cytokines, ECs express cell-surface adhesion molecules essential for the recruitment and attachment of immune cells against possible pathogens [26]. Once an immune barrier is established and haemostasis is restored, a process of vascular repair is initiated [27]. However, under pathological conditions (such as hyperhomocysteinaemia, hyperglycaemia, hypercholesterolaemia and accumulation of NO inhibitors), the endothelium loses its natural properties, shifting towards reduced vasodilation, inflammation and thrombosis, which overall defines ED [28]. Essentially, a dysfunctional endothelium arises when there is an imbalance between endothelium-derived relaxation (EDRFs) and constriction (EDCFs) factors. The former includes NO, prostacyclin and endothelium-derived hyperpolarizing factor (EDHF), while endothelin (ET-1), angiotensin II (Ang II), thrombin and thromboxane A2 (TXA2) represent EDCFs [6]. It is worth noting that prostacyclin, EDHF and TXA2 fall outside the scope of this review.
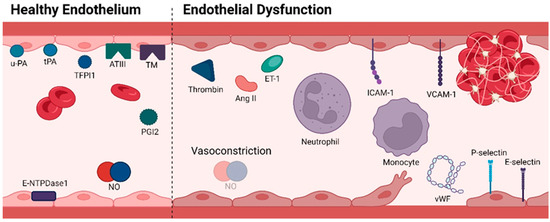
Figure 2.
Molecular profile of a healthy endothelium characterized by anti-thrombotic mechanisms (left); Molecular profile of endothelial dysfunction with the display of adhesive, pro-inflammatory, and pro-clotting properties (right). Abbreviations: ATIII, antithrombin III; ET-1, Endothelin-1; E-NTPDase1, ectonucleoside triphosphate diphosphohydrolase-1; ICAM-1, intercellular adhesion molecule-1; NO, nitric oxide; PGI2, prostaglandin I2; TFPI1, tissue factor pathway inhibitor 1; TM, thrombomodulin; tPA, tissue-type plasminogen activator; u-PA, urokinase-type plasminogen activator; VCAM-1, vascular cell adhesion molecule-1; vWF, von Willebrand factor. Figure created with Biorender.com (accessed on 13 April 2024).
Nitric Oxide (NO)
The most well-defined EDRF is NO, which is the protector of the vascular wall, with anti-inflammatory and antioxidant properties [6,10,29,30]. In addition to being a potent vasodilator gatekeeping endothelial health, NO is also a concentration-dependent cell proliferation and apoptosis modulator, as low relative concentrations appear to promote cell proliferation and anti-apoptotic responses and vice-versa [12,31,32]. Furthermore, as previously mentioned, it possesses platelet inhibitor properties [21]. Consequently, a decrease in NO bioavailability usually occurs in tandem with a pro-thrombotic and pro-inflammatory cascade and a less flexible endothelial state [6,28,30].
Deficiencies of NO can be caused by alterations in nitric oxide synthase 3 (NOS3), also known as eNOS [33]. The linkage between NO, ED and cancer is reinforced by the cell proliferation and anti-apoptotic pathways activated when this vasodilator is reduced, which enables tumour spread, angiogenesis and metastasis [34]. According to the literature, there is a total of 168 genetic polymorphisms located within or close to NOS3, of which three have emerged as particularly noteworthy due to their shared impact on reducing NO levels and their established associations with cancer: rs2070744, rs1799983, and rs869109213 (Table 1) [33].
Regarding rs2070744 (T>C), this intronic variant consists of the substitution of thymine (T) to cytosine (C) at codon -786 in the 5′-flanking region of NOS3. This alternation leads to diminishing gene promoter activity, with consequent serum NO reduction, enabling proliferation pathways and inhibiting tumour cell apoptosis [12,35,36]. To date, many meta-analyses associated rs2070744 with the risk of overall cancer, particularly among individuals of Caucasian descent. Further clustering by cancer type links the C allele (the minor and also ancestral allele) to a higher risk of breast (BC), prostate (PCa), and bladder (BLCA) cancers [33,36,37,38,39,40,41]. In a study regarding oral squamous cell carcinoma (OSCC), individuals with the TC genotype faced an increased likelihood of progressing to an advanced clinical stage (III/IV) compared to those with the TT genotype [42]. Similarly, BC patients carrying the C allele exhibited a significantly higher risk of disease recurrence or mortality compared to those with the TT genotype [12]. Carriers of the C allele are also more prone to colorectal cancer (CRC) [43]. Likewise, the CC genotype was associated with a five-fold increased risk for gastric cancer (GC) development [34]. On the other hand, regarding PCa in the Turkish population, the C allele was found to be less prevalent among patients compared to healthy controls, suggesting a protective effect of this allele [44,45]. Moreover, the C allele among uterine cervical cancer (UCC) patients was associated with a reduced risk of advancing to later disease stages, invasion of the parametrium, and metastasis to pelvic lymph nodes [46].
Another important polymorphism of NOS3 is rs1799983 (G>T). This missense SNP leads to a glutamate-to-aspartate (Glu-to-Asp) substitution at position 298 in exon 7 [36,47]. This variant is linked to a substantial reduction in eNOS enzyme activity. Notably, this SNP exhibited associations with PCa, BLCA, and BC [33,36,38,39,40,45]. Concerning BC development, the effect of the T allele (minor allele) depends on the menopause status, exerting a protective effect on postmenopausal women [48]. Contrariwise, the presence of this allele was associated with an increased susceptibility to CRC [43]. Different populational studies have suggested a negative effect of the T allele concerning CRC, BLCA and endometrial carcinoma (EMCA) [47,49,50]. Furthermore, two investigations have identified noteworthy associations between this SNP and lung cancer (LC) and urothelial cell carcinoma (UC), respectively. The first one demonstrated a link to EGFR-mutated lung adenocarcinomas, particularly with exon 19 in-frame deletions, suggesting this SNP as a potential predictor of tumour invasiveness and responsiveness to therapy [51]. The second study denoted a propensity for increased tumour size development among UC patients carrying the rs1799983 T allele [52].
The variant rs869109213 (4a/b) is a VNTR polymorphism (27 bp) in the intron 4 of NOS3 consisting of two alleles: 4a (with four repeats) and 4b (with five repeats). This DNA variation is linked to modified eNOS activity, affecting the baseline production of plasma NO. Specifically, the 4a allele carries present lower NO levels compared to those with the 4b/4b genotype [53]. Similarly to rs2070744, rs869109213 is associated with overall cancer risk in Caucasians, particularly PCa [33,37,38,40]. The 4a allele is linked to a higher risk of CRC in an early-onset (under 60 years old) [54]. Moreover, the heterozygous genotype (4a/4b) was found to be more common in BC patients when compared with a control group [55]. In the context of LC, a noteworthy association was also identified, however, linking the 4b allele to a higher risk for disease development [56]. In another study, the rs869109213 4a/4b genotype in combination with the rs2070744 CC genotype, as well as the C/G/4b haplotype for rs2070744/rs1799983/rs869109213 exhibited a 21-fold and 11-fold escalation in the risk of developing OSCC, respectively [57].

Table 1.
Epidemiological studies on the role of NOS3 polymorphisms on cancer susceptibility and progression.
Table 1.
Epidemiological studies on the role of NOS3 polymorphisms on cancer susceptibility and progression.
| First Author (Year) | Country/Ethnic Background | Population Characteristics | Study Design | Studied Polymorphisms |
|---|---|---|---|---|
| Choi et al. (2006) [12] | South Korea/Unclear | 1039 BC patients 995 non-cancer controls | Cohort study | rs2070744 rs1799983 |
| Lu et al. (2006) [41] | USA/non-Hispanic Caucasian | 421 BC patients 423 non-cancer controls | Case–control study | rs2070744 rs1799983 rs869109213 |
| Yeh et al. (2009) [54] | Taiwan/Taiwanese | 727 CRC patients 736 healthy controls | Case–control study | rs2070744 rs1799983 rs869109213 |
| Oztürk et al. (2011) [47] | Turkey/Turkish | 89 EMCA patients 60 total hysterectomy controls | Case–control study | rs1799983 rs869109213 |
| Arıkan et al. (2012) [49] | Turkey/Turkish | 84 CRC patients 99 healthy controls | Case–control study | rs1799983 |
| Jang et al. (2013) [43] | South Korea/Korean | 528 CRC patients 509 healthy controls | Case–control study | rs2070744 rs1799983 rs869109213 |
| Ramírez-Patiño et al. (2013) [55] | Mexico/Mexican | 429 BC patients 281 healthy women | Case–control study | rs869109213 |
| Wu et al. (2014) [36] | Mixed | 4169 cancer cases and 4185 controls (rs2070744) 7775 cancer cases and 7817 controls (rs1799983) 3430 cancer cases and 3842 controls (rs869109213) | Meta-analysis | rs2070744 rs1799983 rs869109213 |
| Zhang et al. (2014) [37] | Mixed | 4220 cancer cases and 4016 controls (rs2070744) 8359 cancer cases and 9575 controls (rs1799983) 2873 cancer cases and 3338 controls (rs869109213) | Meta-analysis | rs2070744 rs1799983 rs869109213 |
| Gao et al. (2015) [33] | Mixed (meta-analysis) Han Chinese (case–control) | 873 BC patients 1034 healthy women (case–control) | Meta-analysis Case–control study | rs2070744 rs1799983 rs869109213 |
| Krishnaveni et al. (2015) [34] | India/South Indian | 150 GC patients 150 healthy controls | Case–control study | rs2070744 |
| Polat et al. (2015) [50] | Turkey/Turkish | 75 BLCA patients 143 healthy controls | Case–control study | rs2070744 rs1799983 rs869109213 |
| Diler et al. (2016) [45] | Turkey/Turkish | 84 PCa patients 116 healthy controls | Case–control study | rs2070744 rs1799983 rs869109213 |
| Polat et al. (2016) [39] | Turkey/Turkish | 50 PCa patients 50 healthy controls | Case–control study | rs2070744 rs1799983 rs869109213 |
| Chen et al. (2018) [48] | Taiwan/Taiwanese | 139 premenopausal and 144 postmenopausal BC patients 100 premenopausal and 100 postmenopausal healthy women | Case–control study | rs2070744 rs1799983 rs869109213 |
| Huang et al. (2018) [51] | Taiwan/Taiwanese | 277 LC patients | Cohort study | rs2070744 rs1799983 |
| Su et al. (2018) [42] | Taiwan/Taiwanese | 1044 OSCC patients 1200 healthy controls | Case–control study | rs2070744 rs1799983 |
| Hung et al. (2019) [46] | Taiwan/Taiwanese | 117 UCC patients 95 patients with cervical precancerous lesions 330 healthy controls | Case–control study | rs2070744 rs1799983 |
| Nan et al. (2019) [38] | Mixed | 41 case–control studies | Meta-analysis | rs2070744 rs1799983 rs869109213 |
| Tsay et al. (2019) [52] | Taiwan/Taiwanese | 431 UC patients 862 healthy controls | Case–control study | rs2070744 rs1799983 |
| Abedinzadeh et al. (2020) [40] | Mixed | 4464 cancer cases and 4347 controls (rs1799983) 589 cancer cases and 789 controls (rs869109213) 588 cancer cases and 692 controls (rs2070744) | Meta-analysis | rs2070744 rs1799983 rs869109213 |
| Carkic et al. (2020) [57] | Serbia/Serbian | 50 OSCC patients 110 healthy controls | Case–control study | rs2070744 rs1799983 rs869109213 |
| Koçer et al. (2020) [56] | Turkey/Turkish | 107 LC patients 100 healthy controls | Case–control study | rs1799983 rs869109213 |
| Balci et al. (2023) [44] | Turkey/Unclear | 48 PCa patients 42 biopsy individuals 27 healthy controls | Case–control study | rs2070744 |
Abbreviations: BC, breast cancer; BLCA; bladder cancer; CRC, colorectal cancer; EMCA, endometrial carcinoma; GC, gastric cancer; LC, lung cancer; PCa, prostate cancer; OSCC, oral squamous cell carcinoma; UC, urothelial cell carcinoma; UCC, uterine cervical cancer; USA, United States of America.
3. Consequences of ED
As previously mentioned, ET-1, Ang II, and thrombin constitute EDCFs. Impaired vasodilation from ED implies the release and action of these vasoconstrictors inhibiting the anti-inflammatory and anti-coagulant attributes of healthy ECs [6]. Various genetic variations have been documented to influence the expression and/or activity of these molecules during ED. Consequently, investigating the role of these polymorphisms holds promise for elucidating the molecular mechanisms underlying ED and may offer novel therapeutic targets for managing ED and controlling cancer growth and progression (Table 2).
3.1. Endothelin-1 (ET-1)
ET-1, a potent vasoconstrictor peptide, is crucial in regulating vascular tone and endothelial function. Genetic variations within the ET-1 gene (EDN1) have been suggested to modulate the protein expression and/or activity, impacting vascular homeostasis and predisposing individuals to ED-related pathologies [58,59].
The association between EDN1 and cancer has been documented with three distinct SNPs: rs5370 (C>A), a missense variation encoding an aspargine (N) instead of a lysine (K) [60]; rs1800541 (T>G), an alteration located at the gene promotor; and rs2070699 (G>T), an intronic variation [59]. Concerning rs5370, the presence of the A allele (forward strand) was associated with papillary thyroid cancer in individuals over 40 years, notably in men [61]. In contrast, the minor alleles of rs1800541 and rs2070699 seem to confer protection regarding osteosarcoma prognosis. Likewise, the rs1800541 G allele was associated with a reduced risk for pulmonary metastasis and chemoresistance. For the latter condition, the rs2070699 T allele was also related to a decreased risk [58,62]. Concordantly, a haplotype with a greater risk for hormone-refractory PCa was established with the ancestral alleles—rs1800541 T and rs2070699 G [63].
3.2. Angiotensin II (Ang II)
Ang II plays a pivotal role in vascular homeostasis. It is synthesized from angiotensinogen, encoded by the AGT gene, and further processed by the angiotensin-converting enzyme (ACE), encoded by a gene with the same name [64,65,66,67,68]. Variations in these genes may influence Ang II levels, impacting endothelial function.
An indel (I/D) polymorphism in ACE intron 16 is extensively documented in the literature. Current evidence indicates that the D allele carriers have increased ACE expression and activity [64,69]. Furthermore, this indel is referred to as influencing the risk of cancer [70,71,72]. Regarding GC, the DD genotype was associated with a higher risk of gastric tumorigenesis, lymph node metastasis and advanced clinical stages [64,69,73,74,75]. However, according to a 2015 meta-analysis, these associations are only observed in population-based studies, as the I allele seems to confer an increased risk to GC in hospital-based studies [76]. The ACE Indel is also referred to as contributing to the PCa risk in Latino and Asian ethnic groups [77,78]. Moreover, DD carriers also present more advanced stages of the disease and early-age diagnostics [79,80,81,82,83]. The same genotype seems to be related to an increased risk of oral precancerous lesions in betel quid chewers and OSCC and lymph node metastasis in men [84,85]. In disagreement, the II genotype was also associated with a three-fold risk of OSCC development [86,87,88]. The I allele could also be linked to the occurrence of EMCA (particularly in normotensive women under 63 years old), endometriosis and leiomyomas [67,89]. Regarding CRC, this indel is considered to have a gender-dependent effect. While D male carriers present larger tumours than those with the II genotype, females carrying the DD genotype have higher survival rates when compared to I carriers [90]. Another study showed an increased risk of early relapse and higher TNM stage for I allele carriers [91]. Contrariwise, the D allele correlates with poor differentiation and lymph node metastasis [92,93]. Regarding LC development, the I allele has a negative effect, particularly when combined with smoking habits in the older population [94,95,96]. In opposition, DD carriers have an increased susceptibility to squamous cell carcinoma development and smoking-related cancer death [97,98]. On the other hand, compared to the other genotypes, heterozygous individuals are suggested to have a raised non-small cell lung cancer (NSCLC) predisposition [99]. Moreover, the ID genotype seems to be related to adrenal incidentalomas compared to the controls [100]. Additionally, II and DD genotypes confer susceptibility to pancreatic cancer (PC) and chronic pancreatitis, respectively [101]. Regarding BC, the I allele carriers show a decreased risk [66,102,103,104,105]. Those with the I allele have a greater expression of HER2 [106], while DD genotype carriers present a better disease-free survival rate [106,107]. On the contrary, the DD genotype seems to be concomitant with worse prognostic factors in premenopausal women and decreased cancer-free survival in postmenopausal women [108,109,110,111]. Regarding hepatocellular carcinoma (HCC) progress, two different studies demonstrated conflicting results, showing decreased and increased risk for DD carriers, respectively [112,113]. Additionally, the ID genotype is also suggested to exert a protective role against BC [114]. The D allele presence is associated with an increased risk of uterine leiomyoma [115]; gall bladder carcinoma (GBC) [116], and glioma [117], while the homozygous D genotype is associated with increased susceptibility to glioma development and low overall survival [118,119]; renal cell carcinoma (RCC) [120]; BLCA [121]; basal cell carcinoma (BCC) [122,123,124]; poor leukaemia survival rates [125]; lymph nodes metastasis in laryngeal cancer (LaC) [126]; pituitary adenomas development and progression [127]; and EMCA [128]. Regarding cancer patients’ prognosis, while a direct impact is not described, the ACE ID genotype was associated with higher haemoglobin levels and overall lower fat mass and muscle strength in patients at advanced stages compared to the II genotype [129].
The ACE rs4291 (T>A) is an alteration in the promoter region referred to confer susceptibility to cancer in the Asian and Caucasian ethnic groups and specifically to BC in Latino populations [68,103]. This SNP seems to be in linkage disequilibrium (LD) with the ACE indel among women. Those with the low-activity alleles (A and I of each polymorphism, respectively) showed decreased BC risk [66]. Moreover, women present a greater risk of BC when carrying the ACE rs4291 T allele and rs4343 (G>A) G allele concurrently [130].
The D allele carriers aged between 36 to 54 years old are reported to present a greater risk of BC, whereas a reduced risk was associated with the II/AG and II/CC of ACE indel/AGT rs699 (A>G) and ACE indel/AGT rs4762 (G>A) haplotypes, respectively [131].
The AGT rs699 and rs4762 are two missense variants. The first one implicates a replacement of methionine by threonine in exon 2, whereas rs4762 represents a substitution of threonine with methionine at position 174 in the amino acid sequence [130]. Furthermore, nodal spread in intestinal-type GC correlates with the combined expression of this Indel and angiotensin II receptor type I (AT1R) [132]. Also, regarding Helicobacter pylori (HP) status, negative individuals seem to present a decreased risk of GC [133], whereas, in the HP-positive group with atrophy, the ID genotype seems to confer an increased risk [134].
The G allele of AGT rs699 was suggested to be associated with an increased risk of BCC [135], BLCA [136] and CRC [137]. However, in a 2023 study of the same population, the heterozygous genotype was significantly more frequent in the BCC patient group than in the controls [122]. The AA genotype was associated with decreased disease-free survival of BC [138]. Furthermore, the rs699 in the AGT gene showed reduced prevalence in Australian EMCA women [65]. Moreover, several AGT SNPs, namely rs7539020 (C>T), rs3889728 (C>G), rs3789662 (A>G), rs1326889 (C>T), and rs2493137 (T>C), are suggested to modulate renal cell cancer susceptibility among hypertensive or overweight individuals [139]. Regarding CRC, a greater prevalence of the AG/AG haplotype for rs699/rs5051 (C>T) was found in men [140].
3.3. Thrombin
Thrombin, a serine protease originating from prothrombin (its inactive precursor, encoded by coagulation factor 2 (F2)), is a key player in haemostasis, coordinating platelet aggregation and blood coagulation. Its impact extends to diverse cellular functions, including chemotaxis, proliferation, extracellular matrix remodelling, and cytokine release. Just as Factor V Leiden, F2 rs1799963 (G>A) is well-established as a risk factor for VTE [141,142]. This SNP is located at nucleotide position 20210 within the promoter region. The A allele leads to elevated levels of prothrombin, consequently increasing thrombin generation and favouring thrombogenesis [3,143]. Female carriers of the F2 rs1799963 A allele with gynaecological malignancies are suggested to show advanced cancer stages at the time of surgery [144]. The rs1799963 AG genotype was also associated with a five-fold increased risk for HCC in subjects with hepacivirus [145]. Regarding CRC, whereas an increased susceptibility was correlated with the AA genotype, the AG genotype presented 30% less predisposition for its development [146,147].

Table 2.
Epidemiological studies on the role of polymorphisms in vasoconstrictors-encoding genes on cancer susceptibility and progression.
Table 2.
Epidemiological studies on the role of polymorphisms in vasoconstrictors-encoding genes on cancer susceptibility and progression.
| First Author (Year) | Country/Ethnic Background | Population Characteristics | Study Design | Studied Polymorphisms |
|---|---|---|---|---|
| Hajek et al. (2003) [125] | Czech Republic/ Unclear | 25 leukaemia patients | Cohort study | ACE indel |
| Koh et al. (2003) [66] | Singapore/Singaporean | 189 BC patients 671 healthy controls | Nested case–control study | ACE indel ACE rs4291 |
| Tormene et al. (2003) [144] | Italy/Unclear | 52 women operated for gynaecological malignancy 198 women operated for gynaecological non-malignant disease | Case–control study | F2 rs1799963 |
| Freitas-Silva et al. (2004) [67] | Portugal/Portuguese | 70 EMCA patients 101 healthy controls | Case–control study | ACE indel |
| Medeiros et al. (2004) [80] | Portugal/Portuguese | 170 PCa patients 30 healthy controls | Case–control study | ACE indel |
| Chung et al. (2005) [84] | Taiwan/Taiwanese | 61 OPL betel quid chewers 61 asymptomatic betel quid chewers | Case–control study | ACE indel |
| Ebert et al. (2005) [73] | Germany/Caucasian | 88 GC patients 145 healthy controls | Case–control study | ACE indel |
| González-Zuloeta Ladd et al. (2005) [111] | Netherland/Unclear | 4878 female postmenopausal total participants 114 BC patients | Cohort study | ACE indel |
| Goto et al. (2005) [134] | Japan/Japanese | 454 GC patients 202 healthy controls | Case–control study | ACE indel |
| Röcken et al. (2005) [75] | Germany/Unclear | 113 GC patients 189 healthy controls | Case–control study | ACE indel |
| Arima et al. (2006) [98] | Japan/Japanese | 937 total participants 176 subjects died of malignant neoplasm | Cohort study | ACE indel |
| Yaren et al. (2006) [109] | Turkey/Turkish | 44 BC patients 46 healthy premenopausal women | Case–control study | ACE indel |
| Carl-McGrath et al. (2007) [69] | Germany/Unclear | 45 GC patients | Cohort study | ACE indel |
| González-Zuloeta Ladd et (2007) [138] | Netherlands/Unclear | 203 BC cases 3323 controls | Case–control study | AGT rs699 |
| Hsieh et al. (2007) [89] | Taiwan/Taiwanese | 120 UL patients 125 endometriosis patients 128 healthy controls | Case–control study | ACE indel |
| Röcken et al. (2007) [132] | Germany/Unclear | 100 GC patients | Cohort study | ACE indel |
| Röcken et al. (2007) [90] | Germany/Unclear | 141 CRC patients 189 healthy controls | Case–control study | ACE indel |
| Vairaktaris et al. (2007) [86] | Greece/Greek and German | 60 OSCC patients 153 healthy controls | Case–control study | ACE indel |
| Yaren et al. (2007) [108] | Turkey/Turkish | 57 BC patients 52 healthy controls | Case–control study | ACE indel |
| Yigit et al. (2007) [83] | Turkey/Turkish | 48 PCa patients 51 healthy controls | Case–control study | ACE indel |
| van der Knaap et al. (2008) [110] | Netherland/Unclear | 7679 participants * | Cohort study | ACE indel |
| Alves Corrêa et al. (2009) [114] | Brazil/Brazilian | 101 BC patients 307 healthy controls | Case–control study | ACE indel |
| Harman et al. (2009) [100] | Turkey/Turkish | 50 adrenal mass patients 30 healthy controls | Case–control study | ACE indel NOS3 rs1799983 |
| Loh et al. (2009) [71] | Mixed/Asian and Caucasian | 203 case–control studies | Meta-analysis | ACE indel |
| Vairaktaris et al. (2009) [88] | Mixed/Greek and German | 162 OSCC patients 168 healthy controls | Case–control study | ACE indel |
| Vasků et al. (2009) [140] | Czech Republic/Czech | 102 CRC patients 101 healthy controls | Case–control study | AGT rs699 AGT rs5051 |
| Vigano et al. (2009) [129] | Canada/Unclear | 72 GC and NSCLC advanced cancer patients | Cohort study | ACE indel |
| Andreotti et al. (2010) [139] | Mixed | 1035 RCC patients 777 controls | Case–control study | AGT rs7539020 AGT rs3889728 AGT rs3789662 AGT rs1326889 AGT rs2493137 |
| Nacak et al. (2010) [95] | Turkey/Turkish | 25 LC patients 165 healthy controls | Case–control study | ACE indel |
| Namazi et al. (2010) [106] | Iran/Iranian | 70 BC patients 70 healthy controls | Case–control study | ACE indel |
| Srivastava et al. (2010) [116] | India/North Indian | 233 GBC patients 260 non-cancer controls | Case–control study | ACE indel |
| Liu et al. (2011) [92] | China/Chinese | 241 CRC patients 299 non-cancer controls | Case–control study | ACE indel |
| Lukic et al. (2011) [101] | Serbia/Unclear | 45 PC patients 55 chronic pancreatitis patients 128 healthy controls | Case–control study | ACE indel |
| De Martino et al. (2011) [120] | Austria/Unclear | 10 RCC patients 173 healthy controls | Case–control study | ACE indel |
| Mendizábal-Ruiz et al. (2011) [104] | Mexico/Mexican | 65 BC patients 40 benign breast disease patients | Case–control study | ACE indel AGT rs699 |
| Vossen et al. (2011) [147] | Germany/German | 1801 CRC patients 1853 healthy controls | Case–control study | F2 rs1799963 |
| Dević Pavlić et al. (2012) [97] | Croatia/Croatian | 308 LC patients 353 healthy controls | Case–control study | ACE indel |
| Correa-Noronha et al. (2012) [128] | Brazil/Brazilian | 74 EMCA patients and 228 controls 83 EOC patients and 297 controls | Case–control study | ACE indel |
| Huhn et al. (2012) [137] | Mixed/Czech and German | 1025 Czech cancer cases and 787 Czech controls 1798 German cancer cases and 1810 German controls | Case–control study | AGT rs699 |
| Liu et al. (2012) [85] | Taiwan/Taiwanese | 205 male oral cancer patients 88 Oral precancerous lesions patients 120 healthy controls | Case–control study | ACE indel |
| Wang et al. (2012) [79] | China/Han Chinese | 189 PCa patients 290 non-cancer controls | Case–control study | ACE indel |
| Altas et al. (2013) [127] | Turkey/Unclear | 21 hypophyseal adenoma patients 20 healthy controls | Case–control study | ACE indel |
| Fishchuk et al. (2013) [131] | Ukraine/Ukrainian | 131 BC patients 102 healthy women | Case–control study | ACE indel AGT rs699 AGT rs4762 |
| Namazi et al. (2013) [107] | Iran/Iranian | 110 BC patients | Prospective study | ACE indel |
| Vylliotis et al. (2013) [87] | Mixed/Greek and German | 160 OSCC patients 168 healthy controls | Case–control study | ACE indel F2 rs1799963 AGT rs699 |
| Yapijakis et al. (2013) [123] | Greece/Greek | 92 BCC patients 103 healthy controls | Case–control study | ACE indel |
| Yuan et al. (2013) [112] | China/Chinese | 293 HCC patients 384 healthy controls | Case–control study | ACE indel NOS3 rs869109213 |
| Zang et al. (2013) [62] | China/Han Chinese | 260 pulmonary metastatic stage III osteosarcoma patients 260 matched pulmonary metastatic stage IIB osteosarcoma patients | Case–control study | EDN1 rs1800541 EDN1 rs2070699 EDN1 rs5370 |
| Phukan et al. (2014) [94] | India/Northeast Indian | 151 LC patients 151 controls | Case–control study | ACE indel |
| Xie et al. (2014) [82] | Mixed | 7025 cancer cases 34,911 controls | Meta-analysis | ACE indel |
| Zhang et al. (2014) [70] | Mixed | 5007 cancer cases 8173 controls | Meta-analysis | ACE indel |
| Zhou et al. (2014) [58] | China/Han Chinese | 350 Paediatric osteosarcoma patients with <90% tumour necrosis 350 matched osteosarcoma patients with ≥90% tumour necrosis | Case–control study | EDN1 rs1800541 EDN1 rs2070699 EDN1 rs5370 |
| Ding et al. (2015) [130] | China/Han Chinese | 606 BC patients 633 healthy controls | Case–control study | ACE rs4291 ACE rs4343 |
| Gan et al. (2015) [133] | Mixed/Asian and Caucasian | 1480 GC cases 3773 non-cancer controls | Meta-analysis | ACE indel |
| Lian et al. (2015) [119] | China/Chinese | 800 glioma patients 800 healthy controls | Case–control study | ACE indel |
| Pabalan et al. (2015) [74] | Mixed | 1459 cancer cases 2581 controls | Meta-analysis | ACE indel |
| Wei et al. (2015) [64] | Mixed | 1392 cancer cases 2951 controls | Meta-analysis | ACE indel |
| Yang et al. (2015) [76] | Mixed/Asian and White | 2903 GC cases 10,833 controls | Meta-analysis | ACE indel |
| Zha et al. (2015) [113] | China/Dai Chinese | 210 HCC patients 206 healthy controls | Case–control study | ACE indel |
| Hanafy et al. (2016) [145] | Egypt/Egyptian | 280 HCV-infected patients 100 healthy controls | Case–control study | F2 rs1799963 |
| Pringle et al. (2016) [65] | Australia/Mixed | 184 type 1 endometrioid cancer women 153 healthy controls | Case–control study | AGT rs699 ACE rs4291 |
| Ali et al. (2017) [121] | Pakistan/Pakistani | 200 BLCA patients 200 healthy controls | Case–control study | ACE indel |
| Baghad et al. (2017) [146] | Morocco/Moroccan | 76 CRC patients 182 healthy controls | Case–control study | F2 rs1799963 |
| Marques et al. (2017) [91] | Brazil/Admixed Brazilian | 140 CRC patients 140 non-cancer controls | Case–control study | ACE indel |
| Xu et al. (2017) [63] | China/Han Chinese | 234 PCa patients with HRPC within six years after androgen deprivation therapy 234 matched PCa patients without HRPC within six years after androgen deprivation therapy | Case–control study | EDN1 rs1800541 EDN1 rs2070699 EDN1 rs5370 |
| Zheng et al. (2017) [93] | China/Chinese | 146 CRC patients 106 healthy controls | Case–control study | ACE indel |
| Moghimi et al. (2018) [102] | Mixed | 2846 BC cases 9299 controls | Meta-analysis | ACE indel |
| Pandith et al. (2018) [118] | India/Indian | 12 glioma patients 141 non-cancer controls | Case–control study | ACE indel |
| Peddireddy et al. (2018) [99] | India/South Indian | 246 NSCLC patients 250 healthy controls | Case–control study | ACE indel NOS3 rs869109213 |
| Singh et al. (2018) [105] | India/North Indian | 161 BC patients 152 healthy women | Case–control study | ACE indel |
| Wang et al. (2018) [77] | Mixed | 1098 PCa cases 12,960 controls | Meta-analysis | ACE indel |
| Aydin et al. (2019) [61] | Turkey/Unclear | 113 PTC patients 185 healthy controls | Case–control study | EDN1 rs1800541 EDN1 rs5370 |
| Benenemissi et al. (2019) [117] | Algeria/Algerian | 36 glioma patients 195 healthy controls | Case–control study | ACE indel |
| Keshavarzi et al. (2019) [115] | Iran/Iranian | 202 UL patients 211 healthy controls | Case–control study | ACE indel |
| Papaggelopoulos et al. (2019) [135] | Greece/Greek | 190 BCC patients 99 healthy controls | Case–control study | AGT rs699 |
| Xiao et al. (2019) [68] | Mixed | 8 case–control studies | Meta-analysis | ACE rs4291 |
| Banerjee et al. (2021) [96] | India/North Indian | 154 LC patients 205 healthy controls | Case–control study | ACE indel |
| Dastgheib et al. (2021) [103] | Mixed | 35 case–control studies | Meta-analysis | ACE indel ACE rs4291 |
| Koronellos et al. (2021) [124] | Greece/Greek | 104 BCC patients 111 healthy controls | Case–control study | ACE indel |
| Samara et al. (2021) [136] | Greece/Caucasian | 73 BLCA patients 73 healthy controls | Case–control study | AGT rs699 |
| Du et al. (2022) [81] | Mixed | 817 PCa patients 917 controls | Meta-analysis | ACE indel |
| Said et al. (2022) [78] | Tunisia/Tunisian | 124 PCa patients 143 healthy controls | Case–control study | ACE indel |
| Kumbul et al. (2023) [126] | Turkey/Unclear | 44 LaC patients 61 healthy controls | Case–control study | ACE indel |
| Yapijakis et al. (2023) [122] | Greece/Greek | 100 BCC patients 103 healthy controls | Case–control study | AGT rs699 ACE indel |
* Cancer specification not available. Abbreviations: BC, breast cancer; BCC, basal cell carcinoma; BLCA, bladder cancer; CRC, colorectal cancer; EMCA, endometrial cancer; EOC, epithelial ovarian cancer; GBC, gall bladder cancer; GC, gastric cancer; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HRPC, hormone-refractory prostate cancer; LaC, laryngeal cancer; LC, lung cancer; NSCLC, non-small cell lung cancer; OSCC, oral squamous cell carcinoma; OPL, oral precancerous lesions; PC, pancreatic cancer; PCa, prostate cancer; PTC, papillary thyroid cancer; RCC, Renal Cell Carcinoma; UL, uterine leiomyoma.
4. Adhesion Molecules
The ability of ECs to induce vasodilation mediated by NO is the most common way to measure endothelial function using the flow-mediated vasodilation (FMD) test. However, this ultrasound imaging-based test has poor reproducibility due to operational and patient cardiac function variability [6,8,148]. In this context, the use of ED-circulating biomarkers may present a more reliable alternative [7,26].
In addition to endothelial permeability, decreased NO bioavailability induces the expression of important adhesion molecules, namely P-selectin, E-selectin, vWF, ICAM-1 and VCAM-1, which facilitate cell-to-cell interaction, promoting the migration and adhesion of leucocytes [149]. These molecules are indicative of the pro-thrombotic environment that precedes the development of cardiovascular conditions, with the extent of ED serving as a valuable prognostic indicator [7,8,26]. These well-characterized markers can be measured in circulation with readily available commercial immunoassays, exceeding at least four indicative criteria of an ideal marker/test [149]. Specifically, they demonstrate ease of use, cost-effectiveness, operator independence, and superior reproducibility. Nevertheless, as not all markers exhibit high sensitivity, combining various methodologies, such as microparticles and CECs, would be advocated [150].
Given the implications of ED in cancer pathways, exploring genetic polymorphisms within genes encoding for adhesion molecules takes on paramount significance (Table 3).
4.1. P-Selectin
P-selectin is a product of SELP, and a member of the selectin protein family found on the outer membrane of activated ECs. The crucial function of P-selectin in facilitating leukocyte recruitment to the site of inflammation has been proposed as a driver of tumour aggressiveness and a contributing factor to the onset of cancer cachexia [151]. Two SELP SNPs are highlighted in this context: the intergenic variant rs3917647 (G>A) and the missense SNP rs6136 (T>G). Regarding the former, the GG and AA genotypes were linked to high and low P-selectin plasma levels, respectively. The unfavourable nature of the rs3917647 GG genotype in patients with head and neck cancer (HNC) suggests this alternation as a protective factor against cancer malnutrition and potential cachexia [151]. Likewise, the rs6136 T allele was linked to increased expression of SELP mRNA, while the G allele was associated with reduced serum P-selectin levels. The evidence pinpoints the rs6136 T allele as a protective factor for cancer cachexia in HNC patients, as well [151,152]. These findings were concordant in locally advanced and metastatic PC [153].
4.2. E-Selectin
E-selectin, encoded by SELE, is another significant member of the selectin family. This protein plays a fundamental role in promoting tumour angiogenesis and cancer progression, facilitating interactions between cancer cells and endothelial monolayers, especially during the metastatic process, underlining its importance in early metastasis stages [154]. Concordantly, some tumours, particularly BC and CRC, were found to express E-selectin ligands [155,156].
Four SNPs of SELE are described in the literature. Starting with rs5361 (T>G), this missense polymorphism causes the exchange of an uncharged serine with a positively charged arginine within the epidermal growth factor domain. This alteration possesses the capability to alter ligand affinity [156]. The ancestral T allele was demonstrated to be a protective factor against BC [157,158]. The negative impact of the SNP G allele is confirmed among GC patients, with the allele being associated with disease development and poor prognosis [159,160]. The same allele was also associated with an increased risk of PC and ovarian cancer (OC) and a worsened prognosis for BC patients [161,162,163]. Likewise, this allele was also linked to an elevated risk of relapse, metastasis and mortality among CRC patients [154,155,156]. A meta-analysis suggested rs5361 as an overall cancer risk factor among Caucasian and Asian ethnic groups [164]. In the same fashion as rs5361, rs5362 (A>G), rs5367 (A>G), and rs5368 (G>A) variant alleles were demonstrated to be associated with an increased risk of BC. Among them, only rs5368 causes a change in the amino acid sequence from histidine to tyrosine [154].
4.3. Von Willebrand Factor (vWF)
Besides P-selectin, vWF emerges as one of the molecules meeting the criteria for robust biomarkers of ED [148]. Only one VWF polymorphism is identified to influence cancer pathways, namely the intronic SNP rs73049469 (C>A). The variant A allele is linked to lower VWF expression at the transcription levels, and it is shown to be associated with worse overall survival among NSCLC patients [165,166].
4.4. ICAM-1
This cell adhesion molecule, a member of the Ig-superfamily, serves as a crucial factor in the recruitment, activation, and facilitation of leukocyte functions at inflammatory sites. As a result of proteolytic cleavage, its soluble form becomes notably elevated in both inflammatory and malignant conditions [167,168]. Six polymorphisms within ICAM1 have been associated with tumorigenic roles: rs5498 (A>G), rs1799969 (G>A), rs281437(C>T), rs1437 (A>G), rs923366 (C>T) and rs3093030 (C>T).
The rs5498 polymorphism represents a missense variant within exon 6, giving rise to an amino acid substitution from glutamine (E) to lysine (K). This shift affects the splicing of ICAM1 mRNA, leading to a higher concentration of the soluble protein [168,169,170]. Beyond its well-established association with atherosclerosis, this SNP has garnered attention for its diverse implications in various cancer types. However, its effects remain the subject of debate, as it can either confer risk or protection depending on the tumour [171]. Namely, the homozygous minor allele (G allele) genotype was associated with an increased risk of cancer in the Asian ethnic group but decreased risk in Europeans [172,173]. The G allele was also related to an increased risk of OSCC but diminished for CRC and melanoma [168,173,174]. Furthermore, this allele seems to increase the susceptibility to CRC, especially for older individuals [175,176,177,178]. When in homozygosity, the presence of the lysine correlates with well-differentiated CRC [179]. The G variant allele is also suggested to be a protective factor for cervical adenocarcinoma [169]. In GC, the AA genotype was associated with an augmented risk and a higher likelihood of metastasis compared to the G allele [168,180]. Likewise, the A allele showed an association with advanced stages and poorer survival rates among NSCLC patients [181]. In opposition, the G allele was related to the risk of OC (especially for those with first-degree hereditary tumours or precocious menarche), UC development and invasive stages, HCC in smokers, PCa development, precancerous lesions in uterine cervical carcinogenesis and gliomas development [182,183,184,185,186,187].
The rs1799969 SNP results in an exchange of a glycine for an arginine in exon 4, at codon 241, with the ability to alter the functional activity of ICAM-1 and consequently grant the capacity to recruit and activate immune cells. The variant A allele was shown to be associated with higher cancer risk [172,188]. The presence of the A allele was linked to gliomas and CRC and the GA genotype to BC [168,177]. The A/G haplotype for rs1799969/rs5498 is associated with an increased risk of BC, while it is suggested to exert a protective effect on primary brain tumours [167,168].
The variant T alleles of rs281437 and rs923366 ICAM1 SNPs, two 3′ UTR variants, were associated with increased and reduced risk of primary HCC, respectively [189]. Nonetheless, the CC genotype of rs281437 seems to be related to a higher risk of BC development when compared to the other genotypes [190]. As for rs1437, a 3′ UTR located SNP, the variant G allele was linked to OC augmented risk [191].
Although the functional consequence of rs3093030 is unknown, a protective effect of the variant T allele was found for UCC and primary HCC [169,189]. In contrast, in a different population, women seem to be more susceptible to invasive uterine cervical carcinogenesis when the variant allele is in homozygosity. The C/G, T/A and T/G haplotypes of rs3093030/rs5498 were shown to increase the risk of precancerous lesions and invasive UCC [186]. For UCC, a reduced risk C/T/G haplotype of rs281432(G>C)/rs3093030/rs5498 was discovered [169].
4.5. VCAM-1
Similarly to ICAM-1, VCAM-1 acts in the immune-endothelial communication system, contributing to inflammatory and immune processes and cancer metastasis [192]. Numerous VCAM1 polymorphisms have been linked to cancer. The intronic variation rs3176861 (C>T) currently has an unknown functional consequence. Nevertheless, the presence of the T allele relates to a substantial decrease in the odds of developing lymphedema after BC surgery [193]. The polymorphism rs1041163 (T>C) is an intergenic variant located within exon 9. The SNP C allele was deemed a protective factor for non-Hodgkin lymphoma (NHL) [194]. In opposition, for the synonymous variant rs3176879 (G>A), the variant allele seems to confer susceptibility to recurrent BLCA in patients submitted to immunotherapy [195,196].

Table 3.
Epidemiological studies on the role of ED-related adhesion molecules gene polymorphisms on cancer susceptibility and progression.
Table 3.
Epidemiological studies on the role of ED-related adhesion molecules gene polymorphisms on cancer susceptibility and progression.
| First Author (Year) | Country/Ethnic Background | Population Characteristics | Study Design | Studied Polymorphisms |
|---|---|---|---|---|
| Chen et al. (2006) [185] | USA/African-American | 286 PCa patients 391 healthy controls | Case–control study | ICAM1 rs5498 |
| Theodoropoulos et al. (2006) [177] | Greece/Greek | 222 CRC patients 200 healthy controls | Case–control study | ICAM1 rs5498 ICAM1 rs1799969 |
| Alessandro et al. (2007) [156] | Italy/Caucasian | 172 CRC patients 80 healthy controls | Case–control study | SELE rs5361 |
| Arandi et al. (2008) [167] | Iran/southern Iranian | 276 BC patients and 235 healthy controls 264 BC patients and 200 healthy controls | Case–control study | ICAM1 rs1799969 ICAM1 rs5498 |
| Burim et al. (2009) [187] | Brazil/Unclear | 158 astrocytoma patients and 162 controls | Case–control study | ICAM1 rs5498 ICAM1 rs1799969 |
| Wang et al. (2009) [179] | China/Chinese | 87 CRC patients 102 non-CRC controls | Case–control study | ICAM1 rs5498 ICAM1 rs1799969 |
| Wang et al. (2009) [194] | Jamaica/Jamaican | 395 NHL patients 309 non-NHL controls | Case–control study | VCAM1 rs1041163 |
| Panoussopoulos et al. (2010) [161] | Greece/Unclear | 80 PC patients 160 healthy controls | Case–control study | SELE rs5361 |
| Naidu et al. (2011) [157] | Malaysia/Malaysian | 387 BC patients 252 healthy controls | Case–control study | SELE rs5361 |
| Tan et al. (2012) [152] | Scotland and Canada/Unclear | 775 cancer patients 101 validation cohort patients | Cohort study | SELP rs6136 ICAM1 rs281432 |
| Thanopoulou et al. (2012) [181] | Greece/Unclear | 203 NSCLC patients 175 healthy controls | Case–control study | ICAM1 rs5498 |
| Tian et al. (2012) [180] | China/Chinese | 332 GC patients 380 healthy controls | Case–control study | ICAM1 rs5498 |
| Xia et al. (2012) [159] | China/Chinese | 311 GC patients 425 controls | Case–control study | SELE rs5361 |
| Kontogianni et al. (2013) [163] | Greece/Unclear | 261 BC patients 480 healthy controls | Case–control study | SELE rs5361 |
| Liarmakopoulos et al. (2013) [160] | Greece/Greek | 88 GC patients 480 healthy controls | Case–control study | SELE rs5361 |
| Lin et al. (2013) [174] | Taiwan/Unclear | 595 OSCC patients 561 healthy controls | Case–control study | ICAM1 rs5498 |
| Miaskowski et al. (2013) [193] | Mixed | 155 BC patients with lymphedema 387 BC patients without lymphedema | Case–control study | VCAM1 rs3176861 |
| Yilmaz et al. (2013) [168] | Turkey/Turkish | 92 primary brain tumour patients 92 healthy controls | Case–control study | ICAM1 rs5498 ICAM1 rs1799969 |
| Avan et al. (2014) [153] | Italy/Unclear | 303 locally advanced or metastatic PC | Cohort study | SELP rs6136 |
| Cai et al. (2014) [182] | China/Northern Han Chinese | 408 OC patients 520 healthy controls | Case–control study | ICAM1 rs5498 |
| Cheng et al. (2014) [164] | Mixed/Asian and Caucasian | 1675 cancer patients 2285 controls | Meta-analysis | SELE rs5361 |
| Wang et al. (2014) [183] | Taiwan/Taiwanese | 279 UC patients 279 healthy controls | Case–control study | ICAM1 rs5498 |
| Andrew et al. (2015) [196] | USA/Caucasian | 783 UC patients | Cohort study | VCAM1 rs3176879 |
| Cheng et al. (2015) [172] | Mixed | 4844 cancer patients 5618 healthy controls | Meta-analysis | ICAM1 rs5498 ICAM1 rs1799969 |
| Tang et al. (2015) [173] | Mixed | 5528 cancer patients and 6173 controls for rs5498 3138 cancer cases and 3699 controls for rs3093030 | Meta-analysis | ICAM1 rs5498 ICAM1 rs3093030 |
| Chen et al. (2016) [184] | Taiwan/Taiwanese | 305 HCC patients 613 healthy controls | Case–control study | ICAM1 rs5498 |
| Ghazy et al. (2016) [191] | Egypt/Unclear | 60 mixed-type OC patients 20 healthy controls | Case–control study | ICAM1 rs1437 |
| Golnarnik et al. (2016) [158] | Iran/Northern Iranian | 100 BC patients 120 healthy controls | Case–control study | SELE rs5361 |
| Lu et al. (2016) [162] | China/Chinese | 687 OC patients 687 healthy controls | Case–control study | SELE rs5361 |
| Novikov et al. (2016) [178] | Russia/unclear | 49 CRC patients 30 BC patients 33 controls | Case–control study | ICAM1 rs5498 |
| Sun et al. (2016) [186] | Taiwan/Taiwanese | 91 UCC patients 63 patients with precancerous lesions 290 healthy controls | Case–control study | ICAM1 rs5498 ICAM1 rs3093030 ICAM1 rs281432 |
| Zhang et al. (2016) [188] | Mixed | 4608 cancer patients 4913 controls | Meta-analysis | ICAM1 rs1799969 ICAM1 rs3093030 |
| Liu et al. (2017) [175] | China/Chinese | 195 CRC patients 188 healthy controls | Case–control study | ICAM1 rs5498 |
| Powrózek et al. (2019) [151] | Poland/Unclear | 62 HNC patients | Cohort study | SELP rs3917647 SELP rs6136 |
| Qian et al. (2019) [166] | Mixed European/Caucasian | 948 NSCLC patients | Cohort study | VWF rs73049469 |
| Ghazy et al. (2020) [190] | Egypt/Egyptian | 40 BC patients 40 healthy controls | Case–control study | ICAM1 rs281437 |
| Feng et al. (2021) [169] | China/Northern Chinese Han | 488 UCC patients 684 patients with cervical precancerous lesions 510 healthy females | Case–control study | ICAM1 rs5498 ICAM1 rs3093030 ICAM1 rs281432 |
| He et al. (2021) [189] | China/Unclear | 290 HCC patients 290 healthy controls | Case–control study | ICAM1 rs281437 ICAM1 rs923366 ICAM1 rs3093030 |
| Qiu et al. (2021) [176] | Mixed | 1003 CRC patients 1303 healthy controls | Case–control study | ICAM1 rs5498 ICAM1 rs3093030 |
| Zakariya et al. (2022) [154] | Iraq/Iraqi | 60 BC patients 40 healthy controls | Case–control study | SELE rs5361 SELE rs5368 SELE rs5362 |
Abbreviations: BC, breast cancer; CRC, colorectal cancer; GC, gastric cancer; HCC, hepatocellular carcinoma; HNC, head and neck cancer; OC, ovarian cancer; OSCC, oral squamous cell carcinoma; PCa, prostate cancer; PC, pancreatic cancer; NHL, non-Hodgkin lymphoma; NSCLC, non-small cell lung carcinoma; UC, urothelial cell carcinoma; UCC, uterine cervical cancer.
5. ED-Related Proteins and Cancer Hallmarks
Overall, despite data inconsistencies, ED-related genetic polymorphisms appear to impact the tumorigenic process. Literature suggests a complex relationship between ED and cancer, with the former playing a multifaceted role in the risk and progression of the latter [11,197,198]. By examining the specific contributions of proteins associated with ED to different hallmarks of cancer, the molecular mechanisms underlying tumour formation and dissemination can be further dissected. This knowledge lays the groundwork for validating the role of ED-related genetic polymorphisms (Table 4) in cancer biology, enriching our comprehension of the intricate interplay between the two conditions.
From a protein standpoint, as already discussed, NO can exert a dual role in cancer, modulating cell proliferation and apoptosis in a concentration-dependent manner, with low concentrations promoting cell proliferation and anti-apoptotic responses and vice versa [12]. Furthermore, NO dysregulation can foster a pro-thrombotic and pro-inflammatory environment, which promotes tumour proliferation, limits immune response and facilitates angiogenesis and metastasis [199]. Impaired vasodilation raises the action of vasoconstrictors, such as ET-1, Ang II and thrombin. Besides thrombosis, these molecules play a role in tumorigenesis by promoting cellular proliferation, angiogenesis, and metastasis. Regarding ET-1, it triggers sustained proliferative signalling, apoptosis evasion, and migration and invasion, through its receptor ETA [61,200]. Additionally, it promotes angiogenesis by fibroblast stimulation, resulting in remodelling and deposition of the extracellular matrix (ECM) and consequent release of angiogenic factors [200]. Similarly to ET-1, Ang II is a mitogenic and pro-angiogenic vasoconstrictor that promotes tumour angiogenesis and inflammation through the upregulation of vascular endothelial growth factor (VEGF) and prostaglandins [201]. Moreover, upon binding to its receptors, AT1R and AT2R, Ang II activates signalling pathways of cell proliferation. Interestingly, AT1R (unlike AT2R) exhibits anti-apoptotic properties [202,203]. Lastly, thrombin can stimulate DNA synthesis and upregulate several growth and angiogenesis-related genes by activating the protease-activated receptor 1 (PAR-1) pathway [204,205]. Furthermore, by promoting the overexpression of adhesion molecules, these vasoconstrictors may facilitate immune evasion and tumour invasion and metastasis [206,207]. Indeed, the levels of selectins and CAMs in the serum of cancer patients correlate with tumour dissemination [208]. Furthermore, vWF, in combination with thrombin, contributes to the formation of tumour-platelet aggregates, enabling tumour cell survival and their successful metastasis [198]. In summary, proteins associated with ED play a pivotal role in cancer initiation and progression, contributing to various hallmarks of the disease (Figure 3). Thus, polymorphisms within their coding genes may contribute to alterations in cancer susceptibility and progression in patients carrying these variants. Understanding the impact of these DNA variations might enhance our comprehension of cancer development and open avenues for targeted interventions to disrupt these pathways and hinder disease development and progression.

Figure 3.
Endothelial dysfunction-related proteins and their respective contribution to cancer hallmarks. Hallmarks depicted with transparency denote the absence of documented endothelial dysfunction-related proteins contributing to them. Abbreviations: Ang II, Angiotensin II; ET-1, Endothelin-1; ICAM-1, intercellular adhesion molecule-1; NO, nitric oxide; VCAM-1, vascular cell adhesion molecule-1; vWF, von Willebrand factor. Figure created with Biorender.com (accessed on 13 April 2024).

Table 4.
Characterization of ED-related Genetic Polymorphisms via Ensembl.
Table 4.
Characterization of ED-related Genetic Polymorphisms via Ensembl.
| Gene | Polymorphism | Substitution | Ancestral Allele | Global MAF (MA) | Most Severe Consequence |
|---|---|---|---|---|---|
| NOS3 | rs2070744 | C>G | C | 23% (C) | Intron variant |
| rs1799983 | T>G/A | G | 18% (T) | Missense variant | |
| rs869109213 | VNTR | NA | NA | Intron variant | |
| EDN1 | rs5370 | G>T | G | 25% (T) | Missense variant |
| rs1800541 | T>G | T | 28% (G) | Regulatory region variant | |
| rs2070699 | G>C/T | G | 36% (T) | Intron variant | |
| ACE | Indel | Indel | NA | NA | - |
| rs4291 | T>A/G | A | 35% (T) | Regulatory region variant | |
| rs4343 | G>A | A | 36% (G) | Synonymous variant | |
| AGT | rs699 | A>G | G | 29% (A) | Missense variant |
| rs4762 | G>A | G | 10% (A) | Missense variant | |
| rs1326889 | C>T/A | T | 22% (C) | Intron variant | |
| rs281432 | C>G | G | 48% (C) | Intron variant | |
| rs2493137 | T>C | T | 48% (T) | Intron variant | |
| rs5050 | T>C/G | G | 18% (G) | 5 prime UTR variant | |
| rs5051 | C>G/A/T | T | 29%(C) | 5 prime UTR variant | |
| rs7539020 | C>T | C | 49%(C) | Intron variant | |
| rs3889728 | C>G/T | C | 30%(T) | Intron variant | |
| rs3789662 | A>G | A | 34%(G) | 3 prime UTR variant | |
| F2 | rs1799963 | G>A | G | <1% (A) | 3 prime UTR variant |
| SELP | rs3917647 | G>A | G | 46% (A) | Intergenic variant |
| rs6136 | T>C/G | T | 4% (G) | Missense variant | |
| SELE | rs5361 | T>G/A | T | 5% (G) | Missense variant |
| rs5362 | A>G | G | 5% (G) | Non-coding transcript exon variant | |
| rs5367 | A>G | A | 5% (G) | Splice region variant | |
| rs5368 | G>A | G | 15% (A) | Missense variant | |
| VWF | rs73049469 | C>A | C | 13% (A) | Intron variant |
| ICAM1 | rs1437 | A>G/T | G | 37% (G) | 3 prime UTR variant |
| rs5498 | A>G | A | 36% (G) | Missense variant | |
| rs1799969 | G>A | G | 6% (A) | Missense variant | |
| rs281437 | C>G/T | C | 26% (T) | 3 prime UTR variant | |
| rs923366 | C>T/A | T | 35% (T) | 3 prime UTR variant | |
| rs3093030 | C>T | C | 32% (T) | Non-coding transcript exon variant | |
| VCAM1 | rs3176861 | C>T | C | 20% (T) | Intron variant |
| rs3176879 | G>A | A | 13% (G) | Synonymous variant | |
| rs1041163 | T>C | T | 18% (C) | Intergenic variant |
Abbreviations: MA, minor allele; MAF, minor allele frequency; NA, no data available; VNTR, variable number tandem repeats.
6. Conclusions
In this comprehensive narrative review, genetic polymorphisms implicated in ED were evaluated for their impact on cancer susceptibility and progression among distinct ethnic groups. Briefly, our examination reveals a tendency for BC as a primary focus in studies concerning multiple ED-related genetic polymorphisms, closely followed by CRC. Notably, BC has garnered widespread attention across various countries, particularly in China, where research efforts have been particularly pronounced. China also stands out for its extensive study of distinct SNPs, a trend also observed in Turkey. Moreover, among the polymorphisms examined, the ACE indel distinguishes itself as a frequently studied variant, suggesting its potential relevance in the tumorigenic process. The polymorphisms under study exhibit a clear tendency to modulate cancer risk. The ACE indel stands out with over 50 risk associations for cancer, especially for BC, followed by PCa. Across all cancer models, the D allele commonly emerges associated with risk, while inversely the I allele is reported to confer protection. Additionally, as many risk associations for CRC were found for ICAM1 rs5498 as for the ACE indel, despite being much less studied in the general population. Controversy surrounds this SNP, with the G allele being the most frequently associated with cancer risk and also the most frequently associated with protection. Protection against osteosarcoma was solely associated with EDN1 SNPs, while PCa was mainly studied in relation to NOS3 SNPs. Overall, BC, PCa, and CRC were the main tumour models in studies concerning ED-related genetic polymorphisms. Most of the variants seem to have a context-dependent role varying upon specific tumour and patient characteristics. It should be noted that many of the conducted studies exhibited significant flaws, such as failing to specify the risk/protection genotype, or not confirming the results with subsequent validation studies. Hence, future studies with larger sample sizes are warranted to elucidate these complexities. Since proteins associated with ED contribute to several hallmarks of cancer, a better understanding of these DNA variations holds promise for the development of precision medicine approaches to improve cancer patient care and enhance clinical outcomes. Inclusively, as a wide range of molecules play relevant roles in ED, the implications of other downstream proteins in tumorigenesis should be dissected. Likewise, given the central role of ED in CAT, the influence of the studied polymorphisms in CAT pathogenesis needs to be clarified.
Author Contributions
Conceptualisation, I.G.d.M.; Methodology, I.G.d.M.; Writing—Original Draft Preparation, I.G.d.M.; Writing—Review & Editing, I.G.d.M., V.T., D.P. and R.M.; Supervision, V.T., D.P. and R.M.; Funding Acquisition, V.T. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Portuguese Oncology Institute of Porto (IPO Porto) (CI-IPOP-22-2015) and Fundação para a Ciência e Tecnologia (FCT). V.T. is a Ph.D. scholarship holder (Grant reference: 2020.08969.BD) supported by FCT, co-financed by European Social Funds (FSE) and national funds of MCTES. The institutions had no implications for writing and publishing this manuscript.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Ministério da Saúde de Portugal, Portuguese Oncology Institute of Porto (IPO Porto), Portuguese League Against Cancer (NRNorte) and Fundação para a Ciência e Tecnologia (FCT).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Marques, I.S.; Tavares, V.; Neto, B.V.; Mota, I.N.R.; Pereira, D.; Medeiros, R. Long Non-Coding RNAs in Venous Thromboembolism: Where Do We Stand? Int. J. Mol. Sci. 2023, 24, 12103. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Zakai, N.A. Epidemiology and prevention of venous thromboembolism. Nat. Rev. Cardiol. 2023, 20, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Tavares, V.; Neto, B.V.; Vilas-Boas, M.I.; Pereira, D.; Medeiros, R. Impact of hereditary thrombophilia on cancer-associated thrombosis, tumour susceptibility and progression: A review of existing evidence. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188778. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.J.; Morinaga, L.T.K.; Alves, J.L.J.; Castro, M.A.; Calderaro, D.; Jardim, C.V.P.; Souza, R. Cancer-associated thrombosis: The when, how and why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef] [PubMed]
- Kacimi, S.E.O.; Moeinafshar, A.; Haghighi, S.S.; Saghazadeh, A.; Rezaei, N. Venous thromboembolism in cancer and cancer immunotherapy. Crit. Rev. Oncol. Hematol. 2022, 178, 103782. [Google Scholar] [CrossRef]
- Poredos, P.; Jezovnik, M.K. Endothelial Dysfunction and Venous Thrombosis. Angiology 2018, 69, 564–567. [Google Scholar] [CrossRef]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Migliacci, R.; Becattini, C.; Pesavento, R.; Davi, G.; Vedovati, M.C.; Guglielmini, G.; Falcinelli, E.; Ciabattoni, G.; Dalla Valle, F.; Prandoni, P.; et al. Endothelial dysfunction in patients with spontaneous venous thromboembolism. Haematologica 2007, 92, 812–818. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Franses, J.W.; Drosu, N.C.; Gibson, W.J.; Chitalia, V.C.; Edelman, E.R. Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis. Int. J. Cancer 2013, 133, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, K.M.; Noh, D.Y.; Ahn, S.H.; Lee, J.E.; Han, W.; Jang, I.J.; Shin, S.G.; Yoo, K.Y.; Hayes, R.B.; et al. Genetic polymorphisms of eNOS, hormone receptor status, and survival of breast cancer. Breast Cancer Res. Treat. 2006, 100, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Trent, R.J. Molecular Medicine, 4th ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 81–115. [Google Scholar]
- Chiarella, P.; Capone, P.; Sisto, R. Contribution of Genetic Polymorphisms in Human Health. Int. J. Environ. Res. Public Health 2023, 20, 912. [Google Scholar] [CrossRef] [PubMed]
- Leaché, A.D.; Oaks, J.R. The utility of single nucleotide polymorphism (SNP) data in phylogenetics. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 69–84. [Google Scholar] [CrossRef]
- Hovhannisyan, G.; Harutyunyan, T.; Aroutiounian, R.; Liehr, T. DNA copy number variations as markers of mutagenic impact. Int. J. Mol. Sci. 2019, 20, 4723. [Google Scholar] [CrossRef] [PubMed]
- Sehn, J.K. Insertions and deletions (indels). In Clinical Genomics; Elsevier: Amsterdam, The Netherlands, 2015; pp. 129–150. [Google Scholar]
- Gemayel, R.; Vinces, M.D.; Legendre, M.; Verstrepen, K.J. Variable tandem repeats accelerate evolution of coding and regulatory sequences. Annu. Rev. Genet. 2010, 44, 445–477. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Verhamme, P.; Hoylaerts, M. The pivotal role of the endothelium in haemostasis and thrombosis. Acta Clin. Belg. 2006, 61, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, K.; Zieger, B. Endothelial cells and coagulation. Cell Tissue Res. 2022, 387, 391–398. [Google Scholar] [CrossRef]
- Shibata, S.; Harpel, P.; Bona, C.; Fillit, H. Monoclonal antibodies to heparan sulfate inhibit the formation of thrombin-antithrombin III complexes. Clin. Immunol. Immunopathol. 1993, 67, 264–272. [Google Scholar] [CrossRef]
- Weiler, H.; Isermann, B. Thrombomodulin. J. Thromb. Haemost. 2003, 1, 1515–1524. [Google Scholar] [CrossRef]
- Sandset, P.M. Tissue factor pathway inhibitor (TFPI)–an update. Pathophysiol. Haemost. Thromb. 1996, 26, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.J.; Gutterman, D.D. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation 2013, 20, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tavares, V.; Pinto, R.; Assis, J.; Pereira, D.; Medeiros, R. Venous thromboembolism GWAS reported genetic makeup and the hallmarks of cancer: Linkage to ovarian tumour behaviour. Biochim. Biophys. Acta-Rev. Cancer 2020, 1873, 188331. [Google Scholar] [CrossRef]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Dardi, P.; Dos Reis Costa, D.E.F.; Assunção, H.C.R.; Rossoni, L.V. Venous endothelial function in cardiovascular disease. Biosci. Rep. 2022, 42, BSR20220285. [Google Scholar] [CrossRef]
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Paolisso, G.; Casamassimi, A.; Al-Omran, M.; Barbieri, M.; Sommese, L.; Infante, T.; Ignarro, L.J. Effects of nitric oxide on cell proliferation: Novel insights. J. Am. Coll. Cardiol. 2013, 62, 89–95. [Google Scholar] [CrossRef]
- Gao, X.; Wang, J.; Wang, W.; Wang, M.; Zhang, J. eNOS Genetic Polymorphisms and Cancer Risk: A Meta-Analysis and a Case-Control Study of Breast Cancer. Medicine 2015, 94, e972. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, D.; Amar Chand, B.; Shravan Kumar, P.; Uma Devi, M.; Ramanna, M.; Jyothy, A.; Pratibha, N.; Balakrishna, N.; Venkateshwari, A. Association of endothelial nitric oxide synthase gene T-786C promoter polymorphism with gastric cancer. World J. Gastrointest. Oncol. 2015, 7, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, H.; Qi, X.; Zhou, M. eNOS rs2070744 polymorphism might influence predisposition to hemorrhagic cerebral vascular diseases in East Asians: A meta-analysis. Brain Behav. 2020, 10, e01538. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Z.F.; Xu, Y.; Ren, R.; Heng, B.L.; Su, Z.X. Association between three eNOS polymorphisms and cancer risk: A meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 5317–5324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L.M.; Wang, M.N.; Chen, X.J.; Li, N.; Huang, Y.D.; Chen, M. The G894t, T-786c and 4b/a polymorphisms in Enos gene and cancer risk: A meta-analysis. J. Evid. Based Med. 2014, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Liu, Y.; Xu, C.; Ge, D. Effects of eNOS gene polymorphisms on individual susceptibility to cancer: A meta-analysis. Nitric Oxide 2019, 84, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Polat, F.; Turaçlar, N.; Yilmaz, M.; Bingöl, G.; Cingilli Vural, H. eNOS gene polymorphisms in paraffin-embedded tissues of prostate cancer patients. Turk. J. Med. Sci. 2016, 46, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Abedinzadeh, M.; Dastgheib, S.A.; Maleki, H.; Heiranizadeh, N.; Zare, M.; Jafari-Nedooshan, J.; Kargar, S.; Neamatzadeh, H. Association of Endothelial Nitric Oxide Synthase Gene Polymorphisms with Susceptibility to Prostate Cancer: A Comprehensive Systematic Review and Meta-Analysis. Urol. J. 2020, 17, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wei, Q.; Bondy, M.L.; Yu, T.K.; Li, D.; Brewster, A.; Shete, S.; Sahin, A.; Meric-Bernstam, F.; Wang, L.E. Promoter polymorphism (-786t>C) in the endothelial nitric oxide synthase gene is associated with risk of sporadic breast cancer in non-Hispanic white women age younger than 55 years. Cancer 2006, 107, 2245–2253. [Google Scholar] [CrossRef]
- Su, C.W.; Chien, M.H.; Lin, C.W.; Chen, M.K.; Chow, J.M.; Chuang, C.Y.; Chou, C.H.; Liu, Y.C.; Yang, S.F. Associations of genetic variations of the endothelial nitric oxide synthase gene and environmental carcinogens with oral cancer susceptibility and development. Nitric Oxide 2018, 79, 1–7. [Google Scholar] [CrossRef]
- Jang, M.J.; Jeon, Y.J.; Kim, J.W.; Chong, S.Y.; Hong, S.P.; Oh, D.; Cho, Y.K.; Chung, K.W.; Kim, N.K. Association of eNOS polymorphisms (-786T>C, 4a4b, 894G>T) with colorectal cancer susceptibility in the Korean population. Gene 2013, 512, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Balci, S.; Akbayir, S.; Bozlu, M.; Tamer, L. Investigation of the relationship between endothelial nitric oxide synthase T786C polymorphism and PSA, PSA derivatives, and prostate cancer in the Turkish population. J. Med. Biochem. 2023, 42, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Diler, S.B.; Öden, A. The T-786C, G894T, and Intron 4 VNTR (4a/b) Polymorphisms of the Endothelial Nitric Oxide Synthase Gene in Prostate Cancer Cases. Genetika 2016, 52, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.C.; Wu, T.F.; Ng, S.C.; Lee, Y.C.; Shen, H.P.; Yang, S.F.; Wang, P.H. Involvement of endothelial nitric oxide synthase gene variants in the aggressiveness of uterine cervical cancer. J. Cancer 2019, 10, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Oztürk, E.; Dikensoy, E.; Balat, O.; Uğur, M.G.; Balcı, S.O.; Aydın, A.; Kazancı, U.; Pehlivan, S. Association of endothelial nitric oxide synthase gene polymorphisms with endometrial carcinoma: A preliminary study. J. Turk. Ger. Gynecol. Assoc. 2011, 12, 229–233. [Google Scholar] [CrossRef]
- Chen, C.H.; Wu, S.H.; Tseng, Y.M.; Hou, M.F.; Tsai, L.Y.; Tsai, S.M. Distinct role of endothelial nitric oxide synthase gene polymorphisms from menopausal status in the patients with sporadic breast cancer in Taiwan. Nitric Oxide 2018, 72, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Arıkan, S.; Cacina, C.; Guler, E.; Çulcu, S.; Tuna, G.; Yaylım-Eraltan, I. The effects of NOS3 Glu298Asp variant on colorectal cancer risk and progression in Turkish population. Mol. Biol. Rep. 2012, 39, 3245–3249. [Google Scholar] [CrossRef] [PubMed]
- Polat, F.; Diler, S.B.; Azazi, İ.; Öden, A. T-786C, G894T, and intron 4 VNTR (4a/b) polymorphisms of the endothelial nitric oxide synthase gene in bladder cancer cases. Asian Pac. J. Cancer Prev. 2015, 16, 2199–2202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, C.Y.; Hsieh, M.J.; Wu, W.J.; Chiang, W.L.; Liu, T.C.; Yang, S.F.; Tsao, T.C. Association of endothelial nitric oxide synthase (eNOS) polymorphisms with EGFR-mutated lung adenocarcinoma in Taiwan. J. Cancer 2018, 9, 2518–2524. [Google Scholar] [CrossRef]
- Tsay, M.D.; Hsieh, M.J.; Wang, S.S.; Wang, W.C.; Chou, Y.Y.; Shih, C.H.; Yang, S.F.; Chou, Y.E. Impact of endothelial nitric oxide synthase polymorphisms on urothelial cell carcinoma development. Urol. Oncol. 2019, 37, e291–e293. [Google Scholar] [CrossRef]
- Raina, P.; Sikka, R.; Gupta, H.; Matharoo, K.; Bali, S.K.; Singh, V.; Bhanwer, A. Association of eNOS and MCP-1 Genetic Variants with Type 2 Diabetes and Diabetic Nephropathy Susceptibility: A Case–Control and Meta-Analysis Study. Biochem. Genet. 2021, 59, 966–996. [Google Scholar] [CrossRef]
- Yeh, C.C.; Santella, R.M.; Hsieh, L.L.; Sung, F.C.; Tang, R. An intron 4 VNTR polymorphism of the endothelial nitric oxide synthase gene is associated with early-onset colorectal cancer. Int. J. Cancer 2009, 124, 1565–1571. [Google Scholar] [CrossRef]
- Ramírez-Patiño, R.; Figuera, L.E.; Puebla-Pérez, A.M.; Delgado-Saucedo, J.I.; Legazpí-Macias, M.M.; Mariaud-Schmidt, R.P.; Ramos-Silva, A.; Gutiérrez-Hurtado, I.A.; Gómez Flores-Ramos, L.; Zúñiga-González, G.M.; et al. Intron 4 VNTR (4a/b) polymorphism of the endothelial nitric oxide synthase gene is associated with breast cancer in Mexican women. J. Korean Med. Sci. 2013, 28, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Koçer, C.; Benlier, N.; Balci, S.O.; Pehlivan, S.; Şanli, M.; Nacak, M. The role of endothelial nitric oxide synthase gene polymorphisms in patients with lung cancer. Clin. Respir. J. 2020, 14, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Carkic, J.; Nikolic, N.; Nisevic, J.; Lazarevic, M.; Kuzmanovic-Pficer, J.; Jelovac, D.; Milasin, J. Endothelial nitric oxide synthase polymorphisms/haplotypes are strong modulators of oral cancer risk in Serbian population. J. Oral Sci. 2020, 62, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, B.; Wang, M.; Ni, J. Endothelin-1 gene polymorphisms and risk of chemoresistant pediatric osteosarcoma. Pediatr. Blood Cancer 2014, 61, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Rghigh, A. Polymorphism in Endothelin-1 Gene: An Overview. Curr. Clin. Pharmacol. 2016, 11, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Kirshbom, P.M.; Mahle, W.T.; Joyner, R.W.; Leong, T.; Wilson, M.; Kogon, B.E.; Kanter, K.R.; Bouzyk, M.M. The endothelin-1 G5665T polymorphism impacts transplant-free survival for single ventricle patients. J. Thorac. Cardiovasc. Surg. 2008, 136, 117–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aydin, A.F.; Vural, P.; Doğru-Abbasoğlu, S.; Çil, E. The endothelin 1 and endothelin receptor A gene polymorphisms increase the risk of developing papillary thyroid cancer. Mol. Biol. Rep. 2019, 46, 199–205. [Google Scholar] [CrossRef]
- Zang, X.; Zhou, Y.; Huang, Z.; Zhang, C. Endothelin-1 single nucleotide polymorphisms and risk of pulmonary metastatic osteosarcoma. PLoS ONE 2013, 8, e73349. [Google Scholar] [CrossRef]
- Xu, D.; Wang, X.; Lou, Y. Association of endothelin-1 gene single-nucleotide polymorphisms and haplotypes with risk of hormone refractory prostate cancer. Pharmazie 2017, 72, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.T.; Chen, N.; He, Y.Z.; Wang, J.R.; Yang, Y.; Guo, X.J.; Wang, Z.Q. Angiotensin-converting enzyme insertion/deletion polymorphism and gastric cancer: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Pringle, K.G.; Delforce, S.J.; Wang, Y.; Ashton, K.A.; Proietto, A.; Otton, G.; Blackwell, C.C.; Scott, R.J.; Lumbers, E.R. Renin-angiotensin system gene polymorphisms and endometrial cancer. Endocr. Connect. 2016, 5, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.P.; Yuan, J.M.; Sun, C.L.; van den Berg, D.; Seow, A.; Lee, H.P.; Yu, M.C. Angiotensin I-converting enzyme (ACE) gene polymorphism and breast cancer risk among Chinese women in Singapore. Cancer Res. 2003, 63, 573–578. [Google Scholar] [PubMed]
- Freitas-Silva, M.; Pereira, D.; Coelho, C.; Bicho, M.; Lopes, C.; Medeiros, R. Angiotensin I-converting enzyme gene insertion/deletion polymorphism and endometrial human cancer in normotensive and hypertensive women. Cancer Genet. Cytogenet. 2004, 155, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Dong, Z.; Zhu, J.; You, J.; Fan, J. Association between ACE A240T polymorphism and cancer risk: A meta-analysis. J. Int. Med. Res. 2019, 47, 5917–5925. [Google Scholar] [CrossRef] [PubMed]
- Carl-McGrath, S.; Ebert, M.P.; Lendeckel, U.; Röcken, C. Expression of the local angiotensin II system in gastric cancer may facilitate lymphatic invasion and nodal spread. Cancer Biol. Ther. 2007, 6, 1218–1226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, K.; Cheng, D.; Yi, L.; Shi, H.; Zhen, G. Association between angiotensin I-converting enzyme gene polymorphism and susceptibility to cancer: A meta analysis. Int. J. Clin. Exp. Pathol. 2014, 7, 6291–6300. [Google Scholar] [PubMed]
- Loh, M.; Koh, K.X.; Yeo, B.H.; Song, C.M.; Chia, K.S.; Zhu, F.; Yeoh, K.G.; Hill, J.; Iacopetta, B.; Soong, R. Meta-analysis of genetic polymorphisms and gastric cancer risk: Variability in associations according to race. Eur. J. Cancer 2009, 45, 2562–2568. [Google Scholar] [CrossRef]
- Gard, P.R. Implications of the angiotensin converting enzyme gene insertion/deletion polymorphism in health and disease: A snapshot review. Int. J. Mol. Epidemiol. Genet. 2010, 1, 145–157. [Google Scholar]
- Ebert, M.P.; Lendeckel, U.; Westphal, S.; Dierkes, J.; Glas, J.; Folwaczny, C.; Roessner, A.; Stolte, M.; Malfertheiner, P.; Röcken, C. The angiotensin I-converting enzyme gene insertion/deletion polymorphism is linked to early gastric cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2987–2989. [Google Scholar] [CrossRef] [PubMed]
- Pabalan, N.; Jarjanazi, H.; Ozcelik, H. Associations of the Insertion/Deletion Polymorphism in the ACE Gene and Risk of Gastric Cancer: A Meta-Analysis. J. Gastrointest. Cancer 2015, 46, 370–379. [Google Scholar] [CrossRef]
- Röcken, C.; Lendeckel, U.; Dierkes, J.; Westphal, S.; Carl-McGrath, S.; Peters, B.; Krüger, S.; Malfertheiner, P.; Roessner, A.; Ebert, M.P. The number of lymph node metastases in gastric cancer correlates with the angiotensin I-converting enzyme gene insertion/deletion polymorphism. Clin. Cancer Res. 2005, 11, 2526–2530. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cai, C.; Ye, L.; Rao, Y.; Wang, Q.; Hu, D.; Huang, X. The relationship between angiotensin-converting enzyme gene insertion/deletion polymorphism and digestive cancer risk: Insights from a meta-analysis. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 1306–1313. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Li, H.Y.; Jiang, Z.P.; Zhou, T.B. Relationship between angiotensin-converting enzyme insertion/deletion gene polymorphism and prostate cancer susceptibility. J. Cancer Res. Ther. 2018, 14, S375–S380. [Google Scholar] [CrossRef] [PubMed]
- Said, R.; Jenni, R.; Boussetta, S.; Ammous, F.; Zouari, S.; Zaghbib, S.; Chakroun, M.; Derouiche, A.; Chebil, M.; Ouerhani, S. Association of a common genetic variant (insertion/deletion) in ACE gene with prostate cancer susceptibility in a Tunisian population. J. Clin. Lab. Anal. 2022, 36, e24129. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Lin, Y.W.; Wu, J.; Chen, H.; Mao, Y.Q.; Zheng, X.Y.; Zhou, C.; Xie, L.P. Angiotensin-converting enzyme insertion/deletion polymorphism and the risk of prostate cancer in the Han population of China. Med. Oncol. 2012, 29, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Vasconcelos, A.; Costa, S.; Pinto, D.; Lobo, F.; Morais, A.; Oliveira, J.; Lopes, C. Linkage of angiotensin I-converting enzyme gene insertion/deletion polymorphism to the progression of human prostate cancer. J. Pathol. 2004, 202, 330–335. [Google Scholar] [CrossRef]
- Du, J.; Lan, J.; Yang, H.; Ying, Q.; Huang, G.; Mou, J.; Long, J.; Qiao, Z.; Hu, Q. Association of angiotensin-converting enzyme insertion/deletion (ACE I/D) gene polymorphism with susceptibility to prostate cancer: An updated meta-analysis. World J. Surg. Oncol. 2022, 20, 354. [Google Scholar] [CrossRef]
- Xie, Y.; You, C.; Chen, J. An updated meta-analysis on association between angiotensin I-converting enzyme gene insertion/deletion polymorphism and cancer risk. Tumour Biol. 2014, 35, 6567–6579. [Google Scholar] [CrossRef]
- Yigit, B.; Bozkurt, N.; Narter, F.; Yilmaz, H.; Yucebas, E.; Isbir, T. Effects of ACE I/D polymorphism on prostate cancer risk, tumor grade and metastatis. Anticancer Res. 2007, 27, 933–936. [Google Scholar] [PubMed]
- Chung, F.M.; Yang, Y.H.; Chen, C.H.; Lin, C.C.; Shieh, T.Y. Angiotensin-converting enzyme gene insertion/deletion polymorphism is associated with risk of oral precancerous lesion in betel quid chewers. Br. J. Cancer 2005, 93, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Lin, L.W.; Chen, C.Y.; Wang, C.P.; Liu, H.P.; Houng, J.Y.; Chung, F.M.; Shieh, T.Y. Polymorphism of angiotensin I-converting enzyme gene is related to oral cancer and lymph node metastasis in male betel quid chewers. Oral Oncol. 2012, 48, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Vairaktaris, E.; Yapijakis, C.; Tsigris, C.; Vassiliou, S.; Derka, S.; Nkenke, E.; Spyridonidou, S.; Vylliotis, A.; Vorris, E.; Ragos, V.; et al. Association of angiotensin-converting enzyme gene insertion/deletion polymorphism with increased risk for oral cancer. Acta Oncol. 2007, 46, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Vylliotis, A.; Yapijakis, C.; Nkenke, E.; Nisyrios, T.; Avgoustidis, D.; Adamopoulou, M.; Ragos, V.; Vassiliou, S.; Koronellos, N.; Vairaktaris, E. Effect of thrombosis-related gene polymorphisms upon oral cancer: A regression analysis. Anticancer Res. 2013, 33, 4033–4039. [Google Scholar]
- Vairaktaris, E.; Serefoglou, Z.; Avgoustidis, D.; Yapijakis, C.; Critselis, E.; Vylliotis, A.; Spyridonidou, S.; Derka, S.; Vassiliou, S.; Nkenke, E.; et al. Gene polymorphisms related to angiogenesis, inflammation and thrombosis that influence risk for oral cancer. Oral Oncol. 2009, 45, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.Y.; Lee, C.C.; Chang, C.C.; Wang, Y.K.; Yeh, L.S.; Lin, C.S. Angiotensin I-converting enzyme insertion-related genotypes and allele are associated with higher susceptibility of endometriosis and leiomyoma. Mol. Reprod. Dev. 2007, 74, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Röcken, C.; Neumann, K.; Carl-McGrath, S.; Lage, H.; Ebert, M.P.; Dierkes, J.; Jacobi, C.A.; Kalmuk, S.; Neuhaus, P.; Neumann, U. The gene polymorphism of the angiotensin I-converting enzyme correlates with tumor size and patient survival in colorectal cancer patients. Neoplasia 2007, 9, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.; Ferreira-Costa, L.R.; Ferreira-Costa, L.L.; Correa, R.D.S.; Borges, A.M.P.; Ito, F.R.; Ramos, C.C.O.; Bortolin, R.H.; Luchessi, A.D.; Ribeiro-Dos-Santos, Â.; et al. Association of insertion-deletions polymorphisms with colorectal cancer risk and clinical features. World J. Gastroenterol. 2017, 23, 6854–6867. [Google Scholar] [CrossRef]
- Liu, S.Y.; Sima, X.; Wang, C.H.; Gao, M. The association between ACE polymorphism and risk of colorectal cancer in a Chinese population. Clin. Biochem. 2011, 44, 1223–1226. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, G.; Cui, G.; Cheng, M.; Zhang, N.; Hu, S. Angiotensin-Converting Enzyme Gene Deletion Polymorphism is Associated with Lymph Node Metastasis in Colorectal Cancer Patients in a Chinese Population. Med. Sci. Monit. 2017, 23, 4926–4931. [Google Scholar] [CrossRef] [PubMed]
- Phukan, R.K.; Borah, P.K.; Saikia, B.J.; Das, M.; Sekhon, G.S.; Mahanta, J. Interaction of tobacco smoking and chewing with Angiotensin converting enzyme (insertion/deletion) gene polymorphisms and risk of lung cancer in a high risk area from northeast India. Asian Pac. J. Cancer Prev. 2014, 15, 10691–10695. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nacak, M.; Nacak, I.; Sanli, M.; Ozkur, M.; Pektaş, M.; Aynacioğlu, A.S. Association of angiotensin converting enzyme gene insertion/deletion polymorphism with lung cancer in Turkey. Cancer Genet. Cytogenet. 2010, 198, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Gupta, A.; Agnihotri, V.; Pradhan, R.; Kandel, R.; Upadhyay, A.D.; Dwivedi, S.; Kumar, L.; Dey, S.; Dey, A.B. Lung cancer in the older population: Interactive effects of angiotensin converting enzyme gene polymorphism (rs 4340 ID) and tobacco addiction in risk assessment. Indian J. Cancer 2021, 60, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Dević Pavlić, S.; Ristić, S.; Flego, V.; Kapović, M.; Radojčić Badovinac, A. Angiotensin-converting enzyme insertion/deletion gene polymorphism in lung cancer patients. Genet. Test. Mol. Biomark. 2012, 16, 722–725. [Google Scholar] [CrossRef]
- Arima, H.; Kiyohara, Y.; Tanizaki, Y.; Nakabeppu, Y.; Kubo, M.; Kato, I.; Sueishi, K.; Tsuneyoshi, M.; Fujishima, M.; Iida, M. Angiotensin I-converting enzyme gene polymorphism modifies the smoking-cancer association: The Hisayama Study. Eur. J. Cancer Prev. 2006, 15, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Peddireddy, V.; Badabagni, S.P.; Gundimeda, S.D.; Mundluru, H.P. Association of eNOS and ACE gene polymorphisms and plasma nitric oxide with risk of non-small cell lung cancer in South India. Clin. Respir. J. 2018, 12, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Harman, E.; Karadeniz, M.; Biray, C.; Zengi, A.; Cetinkalp, S.; Ozgen, A.G.; Saygili, F.; Berdeli, A.; Gündüz, C.; Yilmaz, C. The relation of adiponectin and tumor necrosis factor alpha levels between endothelial nitric oxide synthase, angiotensin-converting enzyme, transforming growth factor beta, and tumor necrosis factor alpha gene polymorphism in adrenal incidentalomas. J. Endocrinol. Investig. 2009, 32, 881–888. [Google Scholar] [CrossRef]
- Lukic, S.; Nikolic, A.; Alempijevic, T.; Popovic, D.; Sokic Milutinovic, A.; Ugljesic, M.; Knezevic, S.; Milicic, B.; Dinic, D.; Radojkovic, D. Angiotensin-converting enzyme gene insertion/deletion polymorphism in patients with chronic pancreatitis and pancreatic cancer. Dig. Surg. 2011, 28, 258–262. [Google Scholar] [CrossRef]
- Moghimi, M.; Kargar, S.; Jafari, M.A.; Ahrar, H.; Jarahzadeh, M.H.; Neamatzadeh, H.; Sadeghizadeh- Yazdi, J. Angiotensin Converting Enzyme Insertion/Deletion Polymorphism is Associated with Breast Cancer Risk: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2018, 19, 3225–3231. [Google Scholar] [CrossRef][Green Version]
- Dastgheib, S.A.; Asadian, F.; Farbod, M.; Karimi-Zarchi, M.; Meibodi, B.; Akbarian, E.; Neamatzadeh, H. Association of ACE I/D, -240A > T and AT1R A1166C polymorphisms with susceptibility to breast cancer: A systematic review and meta-analysis based on 35 case-control studies. Nucleosides Nucleotides Nucleic Acids 2021, 40, 117–135. [Google Scholar] [CrossRef]
- Mendizábal-Ruiz, A.P.; Morales, J.; Castro Martinez, X.; Gutierrez Rubio, S.A.; Valdez, L.; Vásquez-Camacho, J.G.; Sanchez Corona, J.; Moran Moguel, M.C. RAS polymorphisms in cancerous and benign breast tissue. J. Renin Angiotensin Aldosterone Syst. 2011, 12, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Srivastava, N.; Amit, S.; Prasad, S.N.; Misra, M.P.; Ateeq, B. Association of AGTR1 (A1166C) and ACE (I/D) Polymorphisms with Breast Cancer Risk in North Indian Population. Transl. Oncol. 2018, 11, 233–242. [Google Scholar] [CrossRef]
- Namazi, S.; Monabati, A.; Ardeshir-Rouhani-Fard, S.; Azarpira, N. Association of angiotensin I converting enzyme (insertion/deletion) and angiotensin II type 1 receptor (A1166C) polymorphisms with breast cancer prognostic factors in Iranian population. Mol. Carcinog. 2010, 49, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Namazi, S.; Daneshian, A.; Mohammadianpanah, M.; Jafari, P.; Ardeshir-Rouhani-Fard, S.; Nasirabadi, S. The impact of renin-angiotensin system, angiotensin I converting enzyme (insertion/deletion), and angiotensin II type 1 receptor (A1166C) polymorphisms on breast cancer survival in Iran. Gene 2013, 532, 125–131. [Google Scholar] [CrossRef]
- Yaren, A.; Turgut, S.; Kursunluoglu, R.; Oztop, I.; Turgut, G.; Degirmencioglu, S.; Kelten, C.; Erdem, E. Insertion/deletion polymorphism of the angiotensin I-converting enzyme gene in patients with breast cancer and effects on prognostic factors. J. Investig. Med. 2007, 55, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Yaren, A.; Turgut, S.; Kursunluoglu, R.; Oztop, I.; Turgut, G.; Kelten, C.; Erdem, E. Association between the polymorphism of the angiotensin-converting enzyme gene and tumor size of breast cancer in premenopausal patients. Tohoku J. Exp. Med. 2006, 210, 109–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van der Knaap, R.; Siemes, C.; Coebergh, J.W.; van Duijn, C.M.; Hofman, A.; Stricker, B.H. Renin-angiotensin system inhibitors, angiotensin I-converting enzyme gene insertion/deletion polymorphism, and cancer: The Rotterdam Study. Cancer 2008, 112, 748–757. [Google Scholar] [CrossRef]
- González-Zuloeta Ladd, A.M.; Arias Vásquez, A.; Sayed-Tabatabaei, F.A.; Coebergh, J.W.; Hofman, A.; Njajou, O.; Stricker, B.; van Duijn, C. Angiotensin-converting enzyme gene insertion/deletion polymorphism and breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2143–2146. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zhang, L.S.; Li, H.Y.; Liao, M.; Lv, M.; Zhang, C. Influence of angiotensin I-converting enzyme gene polymorphism on hepatocellular carcinoma risk in China. DNA Cell Biol. 2013, 32, 268–273. [Google Scholar] [CrossRef]
- Zha, Y.; Gan, P.; Liu, Q.; Tan, J. Relationship between polymorphism of angiotensin-converting enzyme gene insertion/deletion and risk of hepatocellular carcinoma in a Chinese Dai population. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Alves Corrêa, S.A.; Ribeiro de Noronha, S.M.; Nogueira-de-Souza, N.C.; Valleta de Carvalho, C.; Massad Costa, A.M.; Juvenal Linhares, J.; Vieira Gomes, M.T.; Guerreiro da Silva, I.D. Association between the angiotensin-converting enzyme (insertion/deletion) and angiotensin II type 1 receptor (A1166C) polymorphisms and breast cancer among Brazilian women. J. Renin Angiotensin Aldosterone Syst. 2009, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, F.; Teimoori, B.; Farzaneh, F.; Mokhtari, M.; Najafi, D.; Salimi, S. Association of ACE I/D and AGTR1 A1166C Gene Polymorphisms and Risk of Uterine Leiomyoma: A Case-Control Study. Asian Pac. J. Cancer Prev. 2019, 20, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Srivastava, A.; Mittal, B. Angiotensin I-converting enzyme insertion/deletion polymorphism and increased risk of gall bladder cancer in women. DNA Cell Biol. 2010, 29, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Benenemissi, I.H.; Sifi, K.; Sahli, L.K.; Semmam, O.; Abadi, N.; Satta, D. Angiotensin-converting enzyme insertion/deletion gene polymorphisms and the risk of glioma in an Algerian population. Pan Afr. Med. J. 2019, 32, 197. [Google Scholar] [CrossRef]
- Pandith, A.A.; Qasim, I.; Zahoor, W.; Shah, P.; Bhat, A.R. ACE I/D sequence variants but not MTHFR C677T, is strongly linked to malignant glioma risk and its variant DD genotype may act as a promising predictive biomarker for overall survival of glioma patients. Gene 2018, 639, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Jiang, H.; Wang, H.; Guo, S. Angiotensin-converting enzyme insertion/deletion gene polymorphisms is associated with risk of glioma in a Chinese population. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 443–447. [Google Scholar] [CrossRef]
- De Martino, M.; Klatte, T.; Schatzl, G.; Waldert, M.; Remzi, M.; Haitel, A.; Kramer, G.; Marberger, M. Insertion/deletion polymorphism of angiotensin I-converting enzyme gene is linked with chromophobe renal cell carcinoma. Urology 2011, 77, e1005.e9–e1005.e13. [Google Scholar] [CrossRef]
- Ali, S.H.B.; Bangash, K.S.; Rauf, A.; Younis, M.; Anwar, K.; Khurram, R.; Khawaja, M.A.; Azam, M.; Qureshi, A.A.; Akhter, S.; et al. Identification of novel potential genetic predictors of urothelial bladder carcinoma susceptibility in Pakistani population. Fam. Cancer 2017, 16, 577–594. [Google Scholar] [CrossRef]
- Yapijakis, C.; Gintoni, I.; Charalampidou, S.; Angelopoulou, A.; Papakosta, V.; Vassiliou, S.; Chrousos, G.P. Angiotensinogen, Angiotensin-Converting Enzyme, and Chymase Gene Polymorphisms as Biomarkers for Basal Cell Carcinoma Susceptibility. Adv. Exp. Med. Biol. 2023, 1423, 175–180. [Google Scholar] [CrossRef]
- Yapijakis, C.; Koronellos, N.; Spyridonidou, S.; Vylliotis, A.; Avgoustidis, D.; Goutas, N.; Vlachodimitropoulos, D.; Vairaktaris, E. Association of angiotensin-converting enzyme gene insertion/deletion polymorphism with decreased risk for basal cell carcinoma. Arch. Dermatol. Res. 2013, 305, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Koronellos, N.; Yapijakis, C.; Katoulis, A.; Avgoustidis, D.; Vylliotis, A.; Papakosta, V.; Diamantopoulou, S.; Zografos, O.; Vairaktari, G.; Vairaktaris, E.; et al. Association study indicates combined effect of interleukin-10 and angiotensin-converting enzyme in basal cell carcinoma development. Arch. Dermatol. Res. 2021, 313, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Hajek, D.; Tomiska, M.; Krahulcova, E.; Druckmuller, M.; Florianova, M.; Izakovicova-Holla, L.; Vacha, J. I/D ACE gene polymorphism in survival of leukemia patients—Hypothesis and pilot study. Med. Hypotheses 2003, 61, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kumbul, Y.; Hekimler Öztürk, K.; Yasan, H.; Akın, V.; Sivrice, M.E.; Caner, F. Angiotensin-converting enzyme insertion/deletion gene polymorphism in patients with laryngeal cancer. Acta Otorhinolaryngol. Ital. 2023, 43, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Altas, M.; Bayrak, O.F.; Serefhan, A.; Silav, G.; Coskun, K.K.; Cerci, A.; Isik, N.; Elmaci, I. Investigation of ACE genome insertion/deletion correlation with immunohistochemical profile in pituitary adenomas. Turk. Neurosurg. 2013, 23, 464–469. [Google Scholar] [CrossRef]
- Correa-Noronha, S.A.; Noronha, S.M.; Alecrim, C.; Mesquita Ade, C.; Brito, G.S.; Junqueira, M.G.; Leite, D.B.; Carvalho, C.V.; Silva, I.D. Association of angiotensin-converting enzyme I gene I/D polymorphism with endometrial but not with ovarian cancer. Gynecol. Endocrinol. 2012, 28, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Vigano, A.; Trutschnigg, B.; Kilgour, R.D.; Hamel, N.; Hornby, L.; Lucar, E.; Foulkes, W.; Tremblay, M.L.; Morais, J.A. Relationship between angiotensin-converting enzyme gene polymorphism and body composition, functional performance, and blood biomarkers in advanced cancer patients. Clin. Cancer Res. 2009, 15, 2442–2447. [Google Scholar] [CrossRef]
- Ding, P.; Yang, Y.; Ding, S.; Sun, B. Synergistic association of six well-characterized polymorphisms in three genes of the renin-angiotensin system with breast cancer among Han Chinese women. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 1232–1239. [Google Scholar] [CrossRef]
- Fishchuk, L.E.; Gorovenko, N.G. Genetic polymorphisms of the renin-angiotensin system in breast cancer patients. Exp. Oncol. 2013, 35, 101–104. [Google Scholar]
- Röcken, C.; Röhl, F.W.; Diebler, E.; Lendeckel, U.; Pross, M.; Carl-McGrath, S.; Ebert, M.P. The angiotensin II/angiotensin II receptor system correlates with nodal spread in intestinal type gastric cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1206–1212. [Google Scholar] [CrossRef]
- Gan, L.; Liu, X.; Wu, Z.; Huang, M.; Zhang, X.; Guo, W. Angiotensin-converting enzyme insertion/deletion polymorphism and gastric cancer: A systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 5788–5793. [Google Scholar] [PubMed]
- Goto, Y.; Ando, T.; Nishio, K.; Ishida, Y.; Kawai, S.; Goto, H.; Hamajima, N. The ACE gene polymorphism is associated with the incidence of gastric cancer among H. pylori seropositive subjects with atrophic gastritis. Asian Pac. J. Cancer Prev. 2005, 6, 464–467. [Google Scholar] [PubMed]
- Papaggelopoulos, J.; Angelopoulou, A.; Avgoustidis, D.; Koronellos, N.; Derka, S.; Vassiliou, S.; Yapijakis, C. Association of Polymorphisms in the Genes of Angiotensinogen and Angiotensin Receptors with Risk for Basal Cell Carcinoma. Anticancer Res. 2019, 39, 5525–5530. [Google Scholar] [CrossRef]
- Samara, M.; Papathanassiou, M.; Farmakioti, I.; Anagnostou, M.; Satra, M.; Mitrakas, L.; Anastasiou, D.; Chasiotis, G.; Christopoulos, A.; Anagnostou, A.; et al. Renin-Angiotensin System Single Nucleotide Polymorphisms Are Associated with Bladder Cancer Risk. Curr. Oncol. 2021, 28, 4702–4708. [Google Scholar] [CrossRef]
- Huhn, S.; Bevier, M.; Rudolph, A.; Pardini, B.; Naccarati, A.; Hein, R.; Hoffmeister, M.; Vodickova, L.; Novotny, J.; Brenner, H.; et al. Shared ancestral susceptibility to colorectal cancer and other nutrition related diseases. BMC Med. Genet. 2012, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- González-Zuloeta Ladd, A.M.; Arias Vásquez, A.; Siemes, C.; Yazdanpanah, M.; Coebergh, J.W.; Hofman, A.; Stricker, B.H.; van Duijn, C.M. Differential roles of Angiotensinogen and Angiotensin Receptor type 1 polymorphisms in breast cancer risk. Breast Cancer Res. Treat. 2007, 101, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, G.; Boffetta, P.; Rosenberg, P.S.; Berndt, S.I.; Karami, S.; Menashe, I.; Yeager, M.; Chanock, S.J.; Zaridze, D.; Matteev, V.; et al. Variants in blood pressure genes and the risk of renal cell carcinoma. Carcinogenesis 2010, 31, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Vasků, A.; Vokurka, J.; Bienertová-Vasků, J. Obesity-related genes variability in Czech patients with sporadic colorectal cancer: Preliminary results. Int. J. Color. Dis. 2009, 24, 289–294. [Google Scholar] [CrossRef] [PubMed]
- League, S.; Hooper, W.C. Molecular diagnostics of inherited thrombosis. Clin. Lab. Sci. 2005, 18, 271–279. [Google Scholar]
- Lim, M.Y.; Deal, A.M.; Kim, S.; Musty, M.D.; Conard, J.; Simioni, P.; Dutrillaux, F.; Eid, S.S.; Middeldorp, S.; Halbmayer, W.M.; et al. Thrombophilic risk of individuals with rare compound factor V Leiden and prothrombin G20210A polymorphisms: An international case series of 100 individuals. Eur. J. Haematol. 2016, 97, 353–360. [Google Scholar] [CrossRef]
- Poort, S.R.; Rosendaal, F.R.; Reitsma, P.H.; Bertina, R.M. A common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 1996, 88, 3698–3703. [Google Scholar] [CrossRef]
- Tormene, D.; Beltramello, P.; Perlati, M.; Brandolin, B.; Barbar, S.; De Toffoli, G.; Simioni, P. The risk of cancer progression in women with gynecological malignancies and thrombophilic polymorphisms: A pilot case-control study. Clin. Appl. Thromb. Hemost. 2009, 15, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.S.; Alaa, F.A.; Randa, M.H. Association of thrombogenic genes polymorphisms with hepatocellular carcinoma in HCV Egyptian patients. Gene 2016, 580, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Baghad, I.; Erguibi, D.; Chehab, F.; Nadifi, S. Risk of colorectal cancer and clotting factor gene polymorphisms in Moroccan Population. Int. J. Adv. Res. 2017, 5, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Vossen, C.Y.; Hoffmeister, M.; Chang-Claude, J.C.; Rosendaal, F.R.; Brenner, H. Clotting factor gene polymorphisms and colorectal cancer risk. J. Clin. Oncol. 2011, 29, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.R.; Borges-Canha, M.; Cardoso, R.; Neves, J.S.; Castro-Ferreira, R.; Leite-Moreira, A. Novel Biomarkers for Evaluation of Endothelial Dysfunction. Angiology 2020, 71, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.Y.; Teo, A.; Yeo, T.W. Overview of the Assessment of Endothelial Function in Humans. Front. Med. 2020, 7, 542567. [Google Scholar] [CrossRef] [PubMed]
- Powrózek, T.; Mlak, R.; Brzozowska, A.; Mazurek, M.; Gołębiowski, P.; Małecka-Massalska, T. Relationship Between -2028 C/T SELP Gene Polymorphism, Concentration of Plasma P-Selectin and Risk of Malnutrition in Head and Neck Cancer Patients. Pathol. Oncol. Res. 2019, 25, 741–749. [Google Scholar] [CrossRef]
- Tan, B.H.; Fladvad, T.; Braun, T.P.; Vigano, A.; Strasser, F.; Deans, D.A.; Skipworth, R.J.; Solheim, T.S.; Damaraju, S.; Ross, J.A.; et al. P-selectin genotype is associated with the development of cancer cachexia. EMBO Mol. Med. 2012, 4, 462–471. [Google Scholar] [CrossRef]
- Avan, A.; Avan, A.; Le Large, T.Y.; Mambrini, A.; Funel, N.; Maftouh, M.; Ghayour-Mobarhan, M.; Cantore, M.; Boggi, U.; Peters, G.J.; et al. AKT1 and SELP polymorphisms predict the risk of developing cachexia in pancreatic cancer patients. PLoS ONE 2014, 9, e108057. [Google Scholar] [CrossRef] [PubMed]
- Zakariya, B.F.; Almohaidi, A.M.S.; Şimşek, S.A.; Al-Waysi, S.A.; Al-Dabbagh, W.H.; Kamal, A.M. The relationship of E-selectin singlenucleotide polymorphisms with breast cancer in Iraqi Arab women. Genom. Inform. 2022, 20, e42. [Google Scholar] [CrossRef] [PubMed]
- Turpin, A.; Labreuche, J.; Fléjou, J.F.; Andre, T.; de Gramont, A.; Hebbar, M. Prognostic factors in patients with stage II colon cancer: Role of E-selectin gene polymorphisms. Dig. Liver Dis. 2019, 51, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Alessandro, R.; Seidita, G.; Flugy, A.M.; Damiani, F.; Russo, A.; Corrado, C.; Colomba, P.; Gullotti, L.; Buettner, R.; Bruno, L.; et al. Role of S128R polymorphism of E-selectin in colon metastasis formation. Int. J. Cancer 2007, 121, 528–535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naidu, R.; Har, Y.C.; Taib, N.A. Polymorphic variant Ser128Arg of E-Selectin is associated with breast cancer risk and high grade tumors. Onkologie 2011, 34, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Golnarnik, G.; Mashayekhi, F.; Saedi, H.S. The E-selectin S149R polymorphisms in breast cancer in a northern Iran population. Cell. Mol. Biol. 2016, 62, 34–37. [Google Scholar] [PubMed]
- Xia, H.Z.; Du, W.D.; Wu, Q.; Chen, G.; Zhou, Y.; Tang, X.F.; Tang, H.Y.; Liu, Y.; Yang, F.; Ruan, J.; et al. E-selectin rs5361 and FCGR2A rs1801274 variants were associated with increased risk of gastric cancer in a Chinese population. Mol. Carcinog. 2012, 51, 597–607. [Google Scholar] [CrossRef]
- Liarmakopoulos, E.; Gazouli, M.; Aravantinos, G.; Theodoropoulos, G.; Rizos, S.; Vaiopoulou, A.; Kouraklis, G.; Nikiteas, N. E-Selectin S128R gene polymorphism in gastric cancer. Int. J. Biol. Markers 2013, 28, 38–42. [Google Scholar] [CrossRef]
- Panoussopoulos, G.S.; Theodoropoulos, G.; Michalopoulos, N.V.; Gazouli, M.; Flessas, J.; Taka, S.; Stamopoulos, P.; Manouras, A.; Zografos, G.C. Analysis of E-Selectin S128R gene polymorphism in pancreatic cancer. J. Surg. Oncol. 2010, 102, 604–607. [Google Scholar] [CrossRef]
- Lu, Z.H.; Gu, X.J.; Shi, K.Z.; Li, X.; Chen, D.D.; Chen, L. Association between genetic polymorphisms of inflammatory response genes and the risk of ovarian cancer. J. Formos. Med. Assoc. 2016, 115, 31–37. [Google Scholar] [CrossRef][Green Version]
- Kontogianni, P.; Zambirinis, C.P.; Theodoropoulos, G.; Gazouli, M.; Michalopoulos, N.V.; Flessas, J.; Liberi, M.; Zografos, G.C. The impact of the stromal cell-derived factor-1-3′A and E-selectin S128R polymorphisms on breast cancer. Mol. Biol. Rep. 2013, 40, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.Y.; Hao, Y.W.; Zhou, W.L.; Ma, Y.R. E-selectin S128R polymorphism is associated with cancer risk: A meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.; Gragnano, F.; Golia, E.; Grove, E.L. von Willebrand Factor and Venous Thromboembolism: Pathogenic Link and Therapeutic Implications. Semin. Thromb. Hemost. 2018, 44, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Liu, H.; Wang, X.; Ge, J.; Luo, S.; Patz, E.F., Jr.; Moorman, P.G.; Su, L.; Shen, S.; Christiani, D.C.; et al. Potentially functional genetic variants in the complement-related immunity gene-set are associated with non-small cell lung cancer survival. Int. J. Cancer 2019, 144, 1867–1876. [Google Scholar] [CrossRef]
- Arandi, N.; Talei, A.; Erfani, N.; Ghaderi, A. Intercellular adhesion molecule-1 genetic markers (+241G/A and +469A/G) in Iranian women with breast cancer. Cancer Genet. Cytogenet. 2008, 183, 9–13. [Google Scholar] [CrossRef]
- Yilmaz, U.; Zeybek, U.; Kahraman, O.T.; Kafadar, A.M.; Toptas, B.; Yamak, N.; Celik, F.; Yaylim, I. Investigation of ICAM-1 and β3 integrin gene variations in patients with brain tumors. Asian Pac. J. Cancer Prev. 2013, 14, 5929–5934. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, Y.; Li, X.; Ma, Q.; Zhang, S.; Zhu, M.; Li, S.; Fang, L.; Tian, J.; Sun, L. Intercellular Adhesion Molecule-1 Gene Polymorphisms and Susceptibility to Cervical Cancer in the Northern Chinese Han Population. Front. Genet. 2021, 12, 668539. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wan, A.; Feng, A.; Rui, R.; Zhou, B. ICAM-1 gene rs5498 polymorphism decreases the risk of coronary artery disease. Medicine 2018, 97, e12523. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Wang, Y.; Zhang, Z.; Qin, R.; Peng, Y.; Tang, W.; Xi, Y.; Tian, G.; Zhang, Y. Roles of intercellular cell adhesion molecule-1 (ICAM-1) in colorectal cancer: Expression, functions, prognosis, tumorigenesis, polymorphisms and therapeutic implications. Front. Oncol. 2022, 12, 1052672. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, B. Intercellular Adhesion Molecule-1 (ICAM-1) Polymorphisms and Cancer Risk: A Meta-Analysis. Iran. J. Public Health 2015, 44, 615–624. [Google Scholar]
- Tang, W.; Wang, Y.; Chen, Y.; Gu, H.; Chen, S.; Kang, M. Polymorphisms in the intercellular adhesion molecule 1 gene and cancer risk: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 11996–12008. [Google Scholar] [PubMed]
- Lin, C.W.; Chuang, C.Y.; Tang, C.H.; Chang, J.L.; Lee, L.M.; Lee, W.J.; Chow, J.M.; Yang, S.F.; Chien, M.H. Combined effects of icam-1 single-nucleotide polymorphisms and environmental carcinogens on oral cancer susceptibility and clinicopathologic development. PLoS ONE 2013, 8, e72940. [Google Scholar] [CrossRef]
- Liu, L.B.; Liu, T.; Xin, F.Z. Correlations of ICAM-1 gene polymorphisms with susceptibility and multidrug resistance in colorectal cancer in a Chinese population. Medicine 2017, 96, e7481. [Google Scholar] [CrossRef]
- Qiu, Z.; Xie, Z.; Qin, R.; Chen, M.; He, H.; Zhang, Z.; Wang, Y.; Hong, M.; Tang, W.; Xi, Y.; et al. Evaluation of ICAM-1 rs5498 and rs3093030 Polymorphisms in Chinese Patients with Colorectal Cancer. DNA Cell Biol. 2021, 40, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulos, G.; Papaconstantinou, I.; Felekouras, E.; Nikiteas, N.; Karakitsos, P.; Panoussopoulos, D.; Lazaris, A.; Patsouris, E.; Bramis, J.; Gazouli, M. Relation between common polymorphisms in genes related to inflammatory response and colorectal cancer. World J. Gastroenterol. 2006, 12, 5037–5043. [Google Scholar] [CrossRef]
- Novikov, V.V.; Shumilova, S.V.; Novikov, D.V.; Kalugin, A.V.; Fomina, S.G.; Karaulov, A.V. Genetic Instability in Locus rs5498 E469K (A/G) of ICAM-1 Gene in Patients with Colorectal Cancer and Breast Cancer. Bull. Exp. Biol. Med. 2016, 160, 811–813. [Google Scholar] [CrossRef]
- Wang, Q.L.; Li, B.H.; Liu, B.; Liu, Y.B.; Liu, Y.P.; Miao, S.B.; Han, Y.; Wen, J.K.; Han, M. Polymorphisms of the ICAM-1 exon 6 (E469K) are associated with differentiation of colorectal cancer. J. Exp. Clin. Cancer Res. 2009, 28, 139. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.M.; Sun, Y.; Li, Z.W.; Wu, Y.; Zhao, A.L.; Li, J.Y. Polymorphisms of ICAM-1 are associated with gastric cancer risk and prognosis. World J. Gastroenterol. 2012, 18, 368–374. [Google Scholar] [CrossRef]
- Thanopoulou, E.; Kotzamanis, G.; Pateras, I.S.; Ziras, N.; Papalambros, A.; Mariolis-Sapsakos, T.; Sigala, F.; Johnson, E.; Kotsinas, A.; Scorilas, A.; et al. The single nucleotide polymorphism g.1548A > G (K469E) of the ICAM-1 gene is associated with worse prognosis in non-small cell lung cancer. Tumor Biol. 2012, 33, 1429–1436. [Google Scholar] [CrossRef]
- Cai, G.; Ma, X.; Zou, W.; Huang, Y.; Zhang, J.; Wang, D.; Chen, B. Prediction value of intercellular adhesion molecule-1 gene polymorphisms for epithelial ovarian cancer risk, clinical features, and prognosis. Gene 2014, 546, 117–123. [Google Scholar] [CrossRef]
- Wang, S.S.; Hsieh, M.J.; Ou, Y.C.; Chen, C.S.; Li, J.R.; Hsiao, P.C.; Yang, S.F. Impacts of ICAM-1 gene polymorphisms on urothelial cell carcinoma susceptibility and clinicopathologic characteristics in Taiwan. Tumor Biol. 2014, 35, 7483–7490. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.P.; Lee, H.L.; Huang, Y.H.; Hsieh, M.J.; Chiang, W.L.; Kuo, W.H.; Chou, M.C.; Yang, S.F.; Yeh, C.B. Association of intercellular adhesion molecule-1 single nucleotide polymorphisms with hepatocellular carcinoma susceptibility and clinicopathologic development. Tumor Biol. 2016, 37, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hernandez, W.; Shriver, M.D.; Ahaghotu, C.A.; Kittles, R.A. ICAM gene cluster SNPs and prostate cancer risk in African Americans. Hum. Genet. 2006, 120, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.H.; Yang, S.F.; Liu, Y.F.; Ko, J.L.; Wu, C.H.; Wu, T.F.; Wang, P.H. Single-Nucleotide Polymorphisms and Haplotypes of Intercellular Adhesion Molecule-1 in Uterine Cervical Carcinogenesis in Taiwanese Women. Reprod. Sci. 2016, 23, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Burim, R.V.; Teixeira, S.A.; Colli, B.O.; Peria, F.M.; Tirapelli, L.F.; Marie, S.K.; Malheiros, S.M.; Oba-Shinjo, S.M.; Gabbai, A.A.; Lotufo, P.A.; et al. ICAM-1 (Lys469Glu) and PECAM-1 (Leu125Val) polymorphisms in diffuse astrocytomas. Clin. Exp. Med. 2009, 9, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, J.; Bai, J.; Lu, W.; Zhang, M.; Mei, H. Association of Polymorphisms in Intercellular Adhesion Molecule 1 (ICAM-1) Gene with Cancer Susceptibility: A Meta-Analysis of 14 Case-Control Studies. Med. Sci. Monit. 2016, 22, 569–579. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, S.; Ma, X. The Influence of ICAM1 3′UTR Gene Polymorphism on the Occurrence and Metastasis of Primary Liver Cancer. Biomed. Res. Int. 2021, 2021, 7377299. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, A.A.; Elsheredy, H.G.; Abouelella, A.M.; Rashwan, E.K.; Khaled, B.E.A.; Elsheredy, A.G. Significance of Intracellular Adhesion Molecule-1 Polymorphism and IP-10 among Breast Cancer Patients. Egypt. J. Immunol. 2020, 27, 187–195. [Google Scholar] [PubMed]
- Ghazy, A.A.; El-Etreby, N.M. Relevance of HLA-DP/DQ and ICAM-1 SNPs among Ovarian Cancer Patients. Front. Immunol. 2016, 7, 202. [Google Scholar] [CrossRef]
- Dadgar Pakdel, F.; Keramatipour, M.; Noorbakhsh, F.; Talebi, S.; Vodjgani, M. Investigating the Effect of rs3783605 Single-nucleotide Polymorphism on the Activity of VCAM-1 Promoter in Human Umbilical Vein Endohelial Cells. Iran. J. Allergy Asthma Immunol. 2015, 14, 179–187. [Google Scholar]
- Miaskowski, C.; Dodd, M.; Paul, S.M.; West, C.; Hamolsky, D.; Abrams, G.; Cooper, B.A.; Elboim, C.; Neuhaus, J.; Schmidt, B.L.; et al. Lymphatic and angiogenic candidate genes predict the development of secondary lymphedema following breast cancer surgery. PLoS ONE 2013, 8, e60164. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Carreon, J.D.; Hanchard, B.; Chanock, S.; Hisada, M. Common genetic variants and risk for non-Hodgkin lymphoma and adult T-cell lymphoma/leukemia in Jamaica. Int. J. Cancer 2009, 125, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.I.; Jun, S.E.; Shin, D.H. Associations of VCAM-1 gene polymorphisms with obesity and inflammation markers. Inflamm. Res. 2017, 66, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Andrew, A.S.; Gui, J.; Hu, T.; Wyszynski, A.; Marsit, C.J.; Kelsey, K.T.; Schned, A.R.; Tanyos, S.A.; Pendleton, E.M.; Ekstrom, R.M.; et al. Genetic polymorphisms modify bladder cancer recurrence and survival in a USA population-based prognostic study. BJU Int. 2015, 115, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, P.; Cordova, F.; Duran, W.N.; Sanchez, F.A. S-nitrosylation and its role in breast cancer angiogenesis and metastasis. Nitric Oxide 2019, 87, 52–59. [Google Scholar] [CrossRef]
- Nastri, C.O.; Martins Wde, P.; Reis, F.J.; Ferriani, R.A. Breast cancer and endothelial dysfunction. Rev. Assoc. Med. Bras. 2008, 54, 467–470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choudhari, S.K.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.; Loizidou, M.; Taylor, I. Endothelin-1 and angiogenesis in cancer. Curr. Vasc. Pharmacol. 2005, 3, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Cazaubon, S.; Deshayes, F.; Couraud, P.O.; Nahmias, C. Endothelin-1, angiotensin II and cancer. Med. Sci. 2006, 22, 416–422. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Cai, L.; Yang, Y.; Sui, G.; Fu, S. Angiotensin II/angiotensin II type I receptor (AT1R) signaling promotes MCF-7 breast cancer cells survival via PI3-kinase/Akt pathway. J. Cell. Physiol. 2010, 225, 168–173. [Google Scholar] [CrossRef]
- Deshayes, F.; Nahmias, C. Angiotensin receptors: A new role in cancer? Trends Endocrinol. Metab. 2005, 16, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Mannucci, P.M. Thrombin and cancer: From molecular basis to therapeutic implications. Semin. Thromb. Hemost. 2012, 38, 95–101. [Google Scholar] [CrossRef]
- Rickles, F.R.; Patierno, S.; Fernandez, P.M. Tissue factor, thrombin, and cancer. Chest 2003, 124, 58s–68s. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wei, W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin. Exp. Immunol. 2015, 179, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Kobayashi, H.; Boelte, K.C.; Lin, P.C. Endothelial cell adhesion molecules and cancer progression. Curr. Med. Chem. 2007, 14, 377–386. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).