Abstract
There has been debate about whether individuals with different color phenotypes should have different taxonomic status. In order to determine whether the different color phenotypes of Nedyopus patrioticus require separate taxonomic status or are simply synonyms, here, the complete mitochondrial genomes (mitogenomes) of two different colored N. patrioticus, i.e., red N. patrioticus and white N. patrioticus, are presented. The two mitogenomes were 15,781 bp and 15,798 bp in length, respectively. Each mitogenome contained 13 PCGs, 19 tRNAs, 2 rRNAs, and 1 CR, with a lack of trnI, trnL2, and trnV compared to other Polydesmida species. All genes were located on a single strand in two mitogenomes. Mitochondrial DNA analyses revealed that red N. patrioticus and white N. patrioticus did not show clear evolutionary differences. Furthermore, no significant divergence was discovered by means of base composition analysis. As a result, we suggest that white N. patrioticus might be regarded as a synonym for red N. patrioticus. The current findings confirmed the existence of color polymorphism in N. patrioticus, which provides exciting possibilities for future research. It is necessary to apply a combination of molecular and morphological methods in the taxonomy of millipedes.
1. Introduction
The existence of two or more distinctly colored phenotypes among individuals of an interbreeding population is known as color polymorphism [1]. Color polymorphism is common in many animals, occurring from invertebrates to vertebrates [2,3,4]. It is an ideal model system to investigate and understand fundamental evolutionary processes [5]. However, whether different phenotypes truly require separate taxonomic status, or whether these co-occur and belong to a single taxon, has been a subject of debate and requires more biological information to provide a basis for establishing the answer [1].
The mitochondrial genome (mitogenome) is widely used in the research of the evolutionary origin and genetic diversity of organisms due to its fast evolution rate, simplified structure, and efficient genetic information [6,7,8]. The mitogenome of animals is usually a circular, double-stranded molecule, typically containing a standard set of 13 protein-coding genes (PCGs), two ribosomal RNA genes (rRNAs), 22 transfer RNA genes (tRNAs), and one control region (CR) [9]. This set of 37 genes is conserved across bilaterian metazoans, with only a few exceptions, such as a small number of genes lost in some derived groups [10]. In arthropods, the mitogenome exhibits diverse structures, and aberrant genomic systems are present. For example, each tRNA gene has been severely truncated in some species of the order Diptera [11]. Additionally, the mitogenomes of some species in the order Anoplura have been observed to split into several chromosomes [12]. Previous studies have found differences in the mitogenome among species, subspecies, and geographic populations of invertebrates, which can be used to explore the genetic diversity and evolution of invertebrates [13,14].
The tribe Nedyopodini is one of the most characteristic elements in the paradoxosomatid fauna of east and southeast Asia [15]. However, the tribe Nedyopodini is perhaps one of the most confused tribes of Paradoxosomatidae in taxonomy. The existing descriptions of its genera, species, and subspecies are often very poor. Even a few actual morphological keys are too shallow to be meaningful [16]. In order to better understand the classification relationship of the tribe Nedyopodini, a classification method based on its mitogenome should be adopted to enhance the results of morphological methods. Research on the complete mitogenome can increase the opportunity to identify taxonomic relationships [17].
Nedyopus patrioticus belongs to the class Diplopoda, order Polydesmida, family Paradoxosomatidae [18]. So far, there are two subspecies recognized in N. patrioticus: N. patrioticus patriotocus and N. patrioticus unicolor [15]; however, there are very few descriptions available of them. The homology of the two subspecies is also disputed. These two color variations of N. patrioticus can be used as an example to study the taxonomic relationships of color-polymorphic species.
In this study, we present the complete mitogenomes of red N. patrioticus and white N. patrioticus. We try to verify the taxonomic relationship of these two color variations of N. patrioticus based on their mitogenomes. The results of this study provide new insights into the color polymorphism of N. patrioticus and the phylogenetic relationships of Diplopoda. Our study makes a certain contribution to the determination of the taxonomic relationships of color-polymorphic species. Our results could also lay the foundation for research on color polymorphism.
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
A total of 17 individuals, including 10 red N. patrioticus and 7 white N. patrioticus, used in this paper were captured on 24 May 2023 in the moist section of a deciduous forest on Mufu Mountain (32°7′ N, 118°47′ E) in Nanjing, Jiangsu, China. After species diagnosis performed based on morphological features given in previous research [16] and the distribution area provided by the Global Biodiversity Information Facility website (GBIF, available at https://www.gbif.org, accessed on 9 October 2023) [19], the specimens were stored in a −80 °C refrigerator at the Nanjing Forestry University Animal Molecular Evolution Laboratory. Due to the lack of relevant previous research, we could not determine which subspecies the red N. patrioticus and white N. patrioticus belong to. The collection of the specimens was reviewed and approved by Nanjing Forestry University. The specimens used in this study were collected and studied in accordance with Chinese laws. Total genomic DNA of two samples was extracted using a FastPure Cell/Tissue DNA Isolation Mini Kit (Vazyme, Nanjing, China), and stored at −20 °C for the follow-up investigation.
2.2. Sequence Analysis
Library construction and sequencing were carried out using the Illumina platform (Personal, Shanghai, China) with an insert size of 300 bp (about 4 Gb of raw data). To generate clean data, low-quality sequences were removed. The mitogenome of Asiomorpha coarctata (accession no. KU721885.1) was used as a template for assembly using Geneious Prime 2023 software [20]. The medium sensitivity/speed option was used for the assembly. Two consensus sequences were generated with a 50% base call threshold, obtaining the complete mitogenomes of red N. patrioticus and white N. patrioticus.
The preliminary examination of the two mitogenomes was conducted using DNASTAR Lasergene 7.1 and the MITOS Web Server (available at https://usegalaxy.eu/root?tool_id=tooshed.g2.bx.psu.edu%2Frepos%2Fiuc%2Fmtos%2Fmtos%2F1.1.1%20galaxy0, assessed on 20 October 2023) for sequence alignment and gene recognition [21,22,23]. The MITOS Web Server was utilized to locate RNA genes. The PCGs were predicted using both MITOS and the CD-search tool on the NCBI website (available at https://www.ncbi.nlm.nih.gov/, assessed on 22 October 2023). The correct mitogenomes were submitted to GenBank (accession numbers: OR755973.1 for red Nedyopus patrioticus; OR777861.1 for white Nedyopus patrioticus). The composition skew was calculated based on the following formula: AT-skew = (A − T)/(A + T) and GC-skew = (G − C)/(G + C) [24]. MEGA X software was utilized to calculate the relative synonymous codon usage (RSCU) and the non-synonymous (Ka) and synonymous substitutions (Ks) [25]. The ggplot2 and aplot packages, implemented in R v.4.3.1, were employed to produce visual representations of the data [26]. The prediction of protein secondary structures was conducted using the GOR4 secondary structure prediction method (available at https://npsa-prabi.ibcp.fr/, accessed on 2 November 2023) [27].
2.3. Phylogenetic Analysis
A total of 24 species of Diplopoda with complete mitogenomes, representing 11 families and 7 orders, were included in the phylogenetic analyses (Table 1). Additionally, a Chilopoda species, Cermatobius longicornis, was utilized as an outgroup. Phylogenetic analyses were conducted using sequences of amino acids. All procedures were carried out using the PhyloSuite v1.2.3 software package [28]. Multiple sequence alignments were performed using MAFFT v7.313 “Normal” mode, and the sequence pruning of amino acids was executed using the “Protein” pattern of Gblock. With BIC as the standard criterion, partition analysis was performed for IQ-TREE and Mrbayes using ModelFinder’s edge-unlinked mode [29]. A Bayesian inference (BI) tree was reconstructed using MrBayes with four Markov Chain Monte Carlo chains (three hot chains and one cold chain) [30]. Two independent runs of 1,000,000 generations were conducted with sampling every 1000 generations, and the first 25% of samples were discarded as burn-ins to reduce simulation error. The best-fit models obtained using ModelFinder for MrBayes were as follows: partition 1 (CYTB, ATP6, ATP8, COXII, COXIII, ND1, ND2, ND3, ND4, ND4L, ND5, ND6), model mtREV+F+I+G4; partition 2 (COXI), model mtMAM+I+G4. A maximum likelihood (ML) tree was reconstructed using IQ-TREE with 1000 bootstrap replicates [31]. The best-fit models obtained using ModelFinder for IQ-TREE were as follows: partition 1 (CYTB, ATP6, ATP8, COXII, COXIII, ND1, ND2, ND3, ND4, ND4L, ND5, ND6), model mtZOA+F+R5; partition 2 (COXI), model LG+F+I+G4. Phylogenetic trees were visualized and edited using the Interactive Tree of Life Web Server (iTOL, available at https://itol.embl.de, accessed on 9 November 2023) [32].

Table 1.
The mitogenomes used in phylogenetic analyses.
3. Results and Discussion
3.1. Genome Organization and Composition
The mitogenome lengths of red N. patrioticus and white N. patrioticus were 15,781 bp and 15,798 bp, respectively (Table 2 and Figure 1). These lengths were well within the range found in other species of Polydesmida. Each mitogenome contained 13 PCGs, 19 tRNAs, 2 rRNAs, and one CR, which was different from typical sets of genes found in invertebrate mitogenomes in terms of the lack of trnI, trnL2, and trnV [33]. The genes of both mitogenomes were all situated on the minor strand (N-stand), a characteristic shared with other species of Polydesmida [20,33].

Table 2.
General features of the mitogenomes of red Nedyopus patrioticus and white Nedyopus patrioticus.
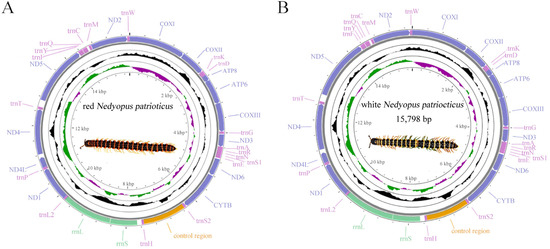
Figure 1.
Circular map of the mitogenomes of red Nedyopus patrioticus (A) and white Nedyopus patrioticus (B). Yellow blocks: control region; green blocks: rRNAs; light purple blocks: tRNAs; dark purple blocks: PCGs.
The lengths of every gene in the two mitogenomes were mostly identical. The base composition of red N. patrioticus was A 25.11%, T 43.12%, G 23.21%, and C 8.54%, whereas the base composition of white N. patrioticus was A 25.21%, T 43.07%, G 23.20%, and C 8.52%. The base compositions of the two genomes were almost the same. Base composition analysis showed that the whole mitogenomes of Polydesmida species were biased towards A and T (Table 3), from 64.04% for Appalachioria falcifera to 75.11% for Epanerchodus koreanus, which was the same as in previous studies [33]. Additionally, the A + T% of PCGs, rRNAs, and tRNAs in Polydesmida species were also higher than the G + C%. Skewness analysis based on base composition was used to estimate the relative numbers of A to T and G to C. The results of skewness showed that the AT-skews of Polydesmida species were negative, and the GC-skews were positive, which was consistent with other millipedes [34]. The A + T% and skewness of red N. patrioticus and white N. patrioticus were all nearly identical.

Table 3.
Base compositions of the whole genomes, PCGs, rRNAs, and tRNAs of the six Polydesmida mitogenomes.
These two mitogenomes had two identical overlapping regions: one between ATP8 and ATP6 and the other between rrnL and trnL1. In addition, the mitogenome of white N. patrioticus had an extra overlapping region between ATP6 and COX3. The longest overlapping region of the two mitogenomes was found between rrnL and trnL1, measuring 11 bp in length.
3.2. Protein-Coding Genes
The lengths of the PCGs in the two mitogenomes were both 10,767 bp, which was slightly lower than in other Polydesmida species [20,33]. The A + T content of Polydesmida species ranged from 63.08% (A. falcifera) to 73.87% (E. koreanus). In the mitogenome of red N. patrioticus, seven PCGs (COXI, COXII, ATP8, COXIII, ND4L, ND4, ND5) used ATG as the initiation codon; four PCGs (ATP6, ND6, CYTB, ND1) used ATA as the initiation codon; and two PCGs (ND3, ND2) used ATT as the initiation codon. In the mitogenome of white N. patrioticus, six PCGs (COXI, ATP8, COXIII, ND4L, ND4) used ATG as the initiation codon; four PCGs (ATP6, ND6, CYTB, ND5) used ATA as the initiation codon; and two PCGs (ND3, ND2) used ATT as the initiation codon. A nonstandard initiation codon GTA was observed in ND1 in the mitogenome of white N. patrioticus. Unusual initiation codons have previously been reported in many animals, including ND2 of Sellanucheza jaegeri, which starts with TTG, and COXI of Botyodes diniasalis, which starts with CGA [35,36]. The termination codons of the two mitogenomes are identical; 10 PCGs (COXI, COXII, ATP8, ATP6, COXIII, ND6, CYTB, ND4L, ND4, ND2) used TAN (TAA, TAG) as termination codon, and the other PCGs (ND3, ND1, ND5) used T as the termination codon. These special termination codons are also found in other arthropods [37], and these codons might be transformed into TAA or TAG for formal functions [38].
The RSCU values of six millipede species from Polydesmida were summarized to determine the frequency of synonymous codon usage (Figure 2). The three most commonly used amino acids were Leu2, Val, and Gly, whereas the three least used codon families were Cys, Arg, and His. Analogously, the biased use of A + T nucleotides was reflected in the codon frequencies. The usage of codons ending in A/U was significantly higher than that of codons ending in C/G, reflecting the strong AT bias of the third codon, a finding consistent with previous studies on the class Myriapoda [35,37].
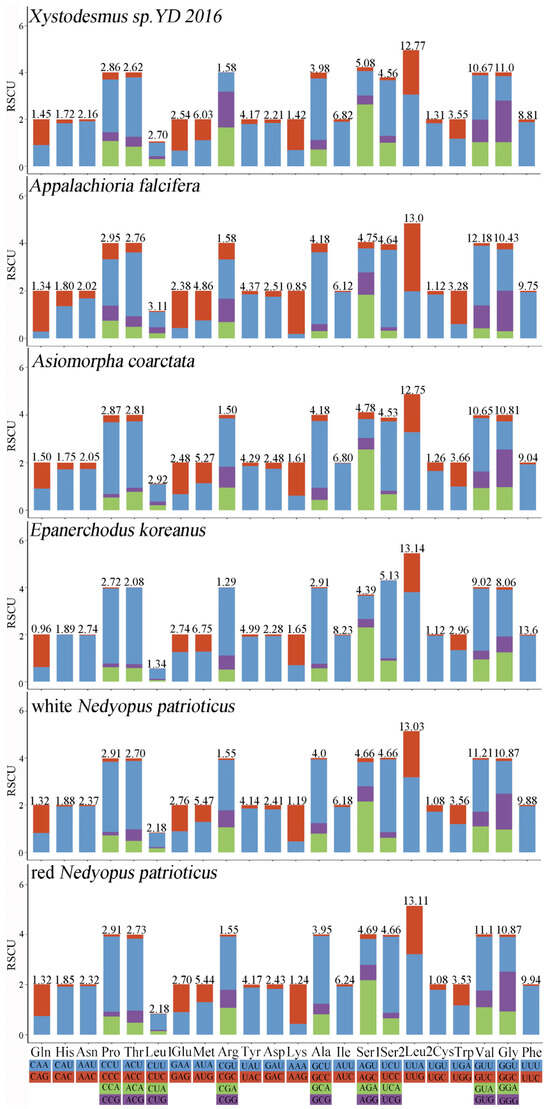
Figure 2.
RSCU of Polydesmida. Different colors correspond to different third codons.
To analyze the evolutionary pattern of PCGs in polynemid species, the Ka/Ks values were assessed (Figure 3). Under the assumption of neutral protein-level evolution, the ratio of Ka to Ks should be equal, resulting in a Ka/Ks ratio of 1. A Ka/Ks ratio below 1 indicates the presence of purifying or stabilizing selection, which suggests a resistance to change. On the other hand, a ratio above 1 implies positive or Darwinian selection, which drives evolutionary change. The ND4 gene (1.55) and the ND5 gene (2.48) had an average Ka/Ks of more than 1, which suggests that the two genes experienced positive selection [39,40]. The COXI gene (0.17) had the lowest average Ka/Ks, suggesting a low evolution rate because of high selection pressure [39,40].
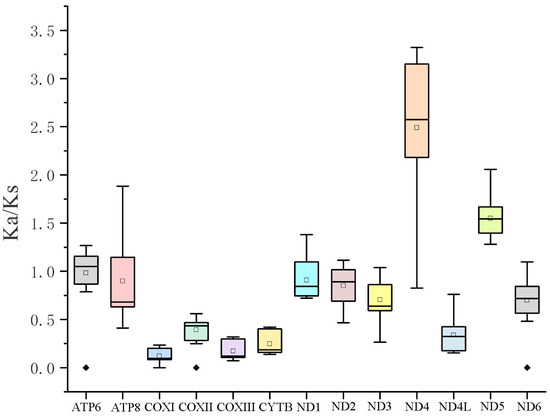
Figure 3.
Ka/Ks values for the 13 PCGs of the order Polydesmida.
To enhance the examination of genetic variances between red N. patrioticus and white N. patrioticus, we employed GOR4 for the anticipation of the secondary structure of the polypeptide sequences (Figure 4). The location of variations in the secondary structure is marked by black block. Our findings revealed alterations in the secondary structure of seven proteins, with variations spanning from 1 position to 11 positions. The detailed analysis revealed that the variations in ATP6 (1 position), ATP8 (3 positions), and COXIII (1 position) lead to an increase in the random coil (Figure 4A–C). The variations in ND3 (2 positions) lead to an increase in the alpha helix (Figure 4F). The variations in CYTB (11 positions), ND1 (9 positions), and ND5 (8 positions) are more complex, including transformations in three secondary structures (Figure 4D,E,G). In summary, the genetic variance between red N. patrioticus and white N. patrioticus was small. This small genetic variation could be one reason for the color polymorphism.
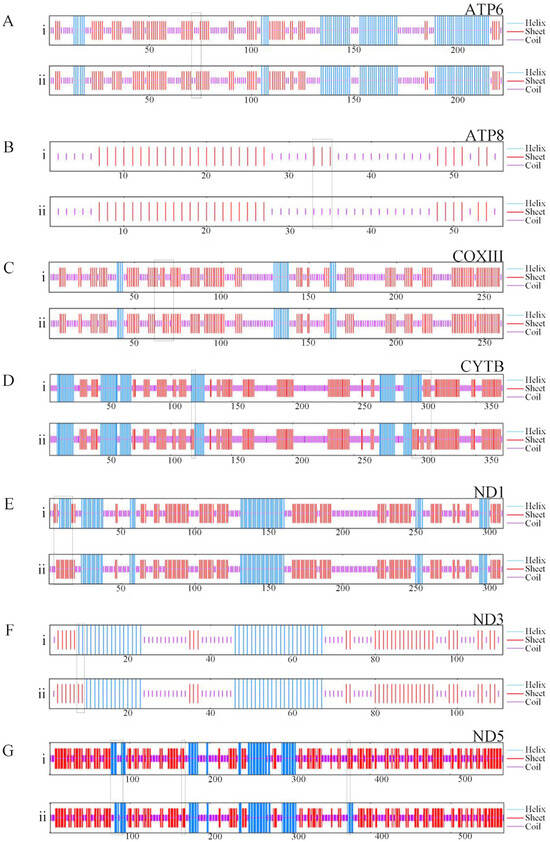
Figure 4.
Effect of amino acid substitutions on the protein secondary structure encoded by mitogenomes of red Nedyopus patrioticus (i) and white Nedyopus patrioticus (ii). (A–G) Individual proteins; the area in the box represents the site of secondary structure change.
3.3. rRNAs, tRNAs, and CR
rrnS and rrnL were located between trnH and trnL1 (Table 2). The rrnS of two mitogenomes were both 737 bp in length. The length of rrnL was 1360 bp in red N. patrioticus and 1359 bp in white N. patrioticus.
There were 19 tRNAs in the two mitogenomes, respectively (Table 2), with a lack of trnI, trnL2, and trnV compared to other Polydesmida species [20,33]. The lengths of the two mitogenomes were 1203 bp and 1208 bp, which were comparatively smaller than those of other Polydesmida species, attributed to the lack of three tRNAs.
One CR was found between trnS2 and trnH in the two mitogenomes, respectively (Table 2). The lengths of CR were 1168 bp and 1154 bp, with a difference of 14 bp, which was the primary reason for the variation in the lengths of the whole mitogenomes between red N. patrioticus and white N. patrioticus.
3.4. Gene Order
The arrangement of the mitogenome is considered a crucial tool for studying deep phylogenetic relationships because of its low rate of homoplasy [41]. Gene order arrangements were compared with mitogenome organization in other Diplopoda species (Figure 5). The gene order of the mitogenomes varies significantly in Diplopoda. For some Diplopoda species, mitochondrial gene order (MGO) patterns are shared at the family level (e.g., Spirostreptidae), whereas for other species, MGO patterns can differ within the same family. All genes were located on a single strand in two mitogenomes, which is consistent with the other species of Polydesmida [20,33]. Compared with other species of Polydesmida, three tRNAs (trnV, trnL2, and trnI) were lost in the two mitogenomes. And trnH underwent short-distance movements, resulting in the formation of trnS2-trnH gene clusters. The duplication–random loss (TDRL) model could potentially provide an explanation for this arrangement [42]. Based on this model, the replication process involves the duplication of specific DNA segments at homologous sites during replication, followed by their subsequent removal. This process ultimately leads to either the restoration of the original genomic organization or a rearrangement of the genome [43]. The gene order of Polydesmida is more susceptible to gene rearrangement between trnS2 and trnM (Figure 5). To enhance our understanding of the evolutionary implications associated with gene arrangements in Diplopoda, it is essential to conduct further research on mitogenomes, covering a wider taxonomic range.
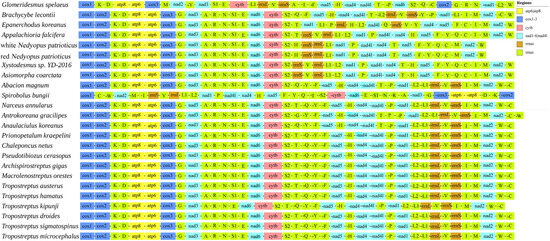
Figure 5.
Gene arrangement of Diplopoda mitogenomes.
3.5. Phylogenetic Analysis
Because of the limited mitogenome sequences of Diplopoda species, we included only 24 species with credible annotations from 11 families of Diplopoda in the Phylogenetic analysis and selected one species in Chilopoda (C. longicornis) as an outgroup to root the phylogenetic trees. The results from both the BI and ML trees showed remarkable similarities and mutually supported each other (Figure 6). There is controversy about the sister-group relationship between A. coarctata and Xystodesmus sp. 2016. Yan Dong’s study suggested that X. sp. 2016 had a sister-group relationship with A. falcifera [20], while other studies proposed that X. sp. 2016 had a sister-group relationship with A. coarctata [35,44]. Our results provide strong support for the sister-group relationship between X. sp. 2016 and A. coarctata (posterior probability, PP = 1; bootstrap, BS = 100). This result reflects a potential flaw in the morphology-based species classification of Xystodesmidae and Paradoxosomah. The combination of molecular and morphological methods can lead to more accurate classification results. The phylogenetic analyses provided strong statistical support for the relationship between red N. patrioticus and white N. patrioticus (posterior probability, PP = 1; bootstrap, BS = 100). This result supports Attem’s hypothesis of conspecificity between N. patrioticus patriotocus and N. patrioticus unicolor [15]. The phylogenetic analyses provided strong support for the various families and orders within the Diplopoda. Our findings demonstrate that mitogenome sequences serve as effective molecular markers for examining the systematic relationships among Diplopoda species. However, it is important to note that our dataset included only 24 species, indicating its limited scope. To address the existing taxonomic debates and elucidate the higher-level phylogeny within Diplopoda species, it would be beneficial to expand sequencing efforts to encompass a greater number of taxa.
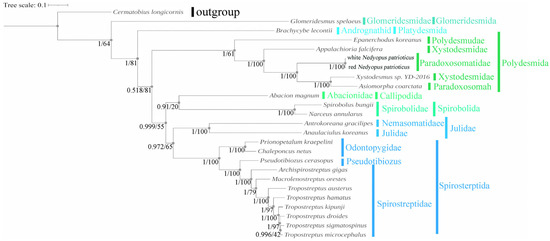
Figure 6.
Amino acid-based phylogenetic tree of 24 Diplopoda for 13 PCGs. Numbers at nodes represent the posterior probability and bootstrap values for BI and ML analyses.
4. Conclusions
In summary, we show that the mitogenomes of two color variations of N. patrioticus exhibited high similarity in base composition, protein secondary structure, and gene order. In addition, there was a closer genetic relationship between red N. patrioticus and white N. patrioticus compared to other millipedes. Based on these foundations, we consider white N. patrioticus to be the same species as red N. patrioticus. In other words, N. patrioticus patriotocus and N. patrioticus unicolor are synonyms. Phylogenetic analysis has shown that mitogenomes can be a reliable tool for analyzing the phylogenetic relationships of Diplopoda species. This study is the first to report the complete mitogenomes of N. patrioticus, which will further enhance our understanding of the genetics, evolution, and taxonomy of the tribe Nedyopodini. In addition, previous studies have shown that different phenotypes exhibited due to color polymorphism may also belong to a synonym [1,45]. Our results indicate that classifying species with color polymorphism solely based on morphological characteristics is imperfect. It is necessary to apply a combination of molecular and morphological methods in the taxonomy of millipedes. In addition, this study has also demonstrated the necessity of integrating molecular and morphological methods in the taxonomy of millipedes.
Since mitochondrial genes serve the mitochondria themselves and their own protein synthesis and do not directly influence the expression of genes related to pigment composition in millipedes, our study is unable to explore the intricacies of biochemical genetics to reveal the molecular mechanisms of the inheritance of traits such as color variation. In order to explore the causes of color polymorphism and delve deeper into the intricacies of biochemical genetics, further studies based on nuclear data are needed.
Author Contributions
H.L. and H.R. conceived the study. G.Z., T.X. and Y.C. conducted the sampling. G.Z., W.X., Y.L. and Y.W. carried out the bioinformatics analysis. G.Z. drafted the manuscript. F.Z., H.R. and H.L. reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 32101339) and the Innovation and Entrepreneurship Training Program for College Students of China (202310298062Z).
Institutional Review Board Statement
This study was approved by the Ethics Committee of Nanjing Forestry University (ID: 2023020). All methods were in accordance with the relevant guidelines and regulations.
Informed Consent Statement
Not applicable.
Data Availability Statement
DNA sequences: GenBank accession number OR755973 for red Nedyopus patrioticus and OR777861 for white Nedyopus patrioticus.
Conflicts of Interest
All authors declare no conflicts of interest.
References
- Beukema, W.; Nicieza, A.G.; Lourenço, A.; Velo-Antón, G. Colour polymorphism in Salamandra salamandra (Amphibia: Urodela), revealed by a lack of genetic and environmental differentiation between distinct phenotypes. J. Zool. Syst. Evol. Res. 2016, 54, 127–136. [Google Scholar] [CrossRef]
- Galeotti, P.; Rubolini, D.; Dunn, P.O.; Fasola, M. Colour polymorphism in birds: Causes and functions. J. Evol. Biol. 2003, 16, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F. Revision of the genus Arthrotus Motschulsky, 1858 (Coleoptera, Chrysomelidae, Galerucinae) of Taiwan, with notes on color polymorphism. Zookeys 2022, 1091, 161–208. [Google Scholar] [CrossRef]
- Mulder, K.P.; Alarcón-Ríos, L.; Nicieza, A.G.; Fleischer, R.C.; Bell, R.C.; Velo-Antón, G. Independent evolutionary transitions to pueriparity across multiple timescales in the viviparous genus Salamandra. Mol. Phylogenet. Evol. 2022, 167, 107347. [Google Scholar] [CrossRef] [PubMed]
- Wellenreuther, M.; Svensson, E.I.; Hansson, B. Sexual selection and genetic colour polymorphisms in animals. Mol. Ecol. 2014, 23, 5398–5414. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, J.; Wang, R.; Xu, Y.; Zhang, D. Progress of insect mitochondrial genome. J. Insp. Quar. 2010, 3, 69–73. (In Chinese) [Google Scholar]
- Wei, M.; Liu, Y.; Guo, H.; Zhao, F.; Chen, S. Characterization of the complete mitochondrial genome of Cynoglossus gracilis and a comparative analysis with other Cynoglossinae fishes. Gene 2016, 591, 369–375. [Google Scholar] [CrossRef]
- Xu, W.; Lin, S.P.; Liu, H.Y. Mitochondrial genomes of five Hyphessobrycon tetras and their phylogenetic implications. Ecol. Evol. 2021, 11, 12754–12764. [Google Scholar] [CrossRef]
- Iwasaki, W.; Fukunaga, T.; Isagozawa, R.; Yamada, K.; Maeda, Y.; Satoh, T.P.; Sado, T.; Mabuchi, K.; Takeshima, H.; Miya, M. Mitofish and Mitoannotator: A mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol. Biol. Evol. 2013, 30, 2531–2540. [Google Scholar] [CrossRef]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Beckenbach, A.T.; Joy, J.B. Evolution of the mitochondrial genomes of Gall Midges (Diptera: Cecidomyiidae): Rearrangement and severe truncation of tRNA genes. Genome Biol. Evol. 2009, 1, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.F.; Kirkness, E.F.; Barker, S.C. The single mitochondrial chromosome typical of animals has evolved into 18 minichromsomes in the human body louse, Pediculus humanus. Genome Res. 2009, 19, 904–912. [Google Scholar] [CrossRef]
- Franck, P.; Garnery, L.; Solignac, M.; Cornuet, J.M. Molecular confirmation of a fourth lineage in honeybees from the near east. Apidologie 2000, 31, 167–180. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, S.; Zhu, X.; Xu, X.; Wang, W.; Zha, L.; Wang, P.; Wang, J.; Lai, K.; Wang, S.; et al. Genetic differentiation of eastern honey bee (Apis cerana) populations across Qinghai-Tibet plateau-valley landforms. Front. Genet. 2019, 10, 483. [Google Scholar] [CrossRef]
- Chen, C.C.; Golovatch, S.I.; Chang, H.W. The millipede tribe Nedyopodini, with special reference to the fauna of Taiwan (Diplopoda: Polydesmida: Paradoxosomatidae). J. Nat. Hist. 2005, 39, 3997–4030. [Google Scholar] [CrossRef]
- Wang, Y.M. Serica la: Records of Myriapods on Formosa with descriptions of new species. Q. J. Taiwan Mus. 1955, 8, 13–16. [Google Scholar]
- Ambriz-Morales, P.; Rosa-Reyna, X.F.D.L.; Sifuentes-Rincon, A.M.; Parra-Bracamonte, G.M.; Villa-Melchor, A.; Chassin-Noria, O.; Arellano-Vera, W. The complete mitochondrial genomes of nine white-tailed deer subspecies and their genomic differences. J. Mammal. 2016, 97, 234–245. [Google Scholar] [CrossRef]
- Noguchi, S.; Mori, N.; Higa, Y.; Kuwahara, Y. Identification of Nedyopus patrioticus (ATTEMS, 1898) (Polydesmida: Paradoxosomatidae) Secretions as Possible Defense Substances. Appl. Entomol. Zool. 1997, 32, 447–452. [Google Scholar] [CrossRef][Green Version]
- García-Roselló, E.; Guisande, C.; Manjarrés-Hernández, A.; González-Dacosta, J.; Heine, J.; Pelayo-Villamil, P.; González-Vilas, L.; Vari, R.P.; Vaamonde, A.; Granado-Lorencio, C.; et al. Can we derive macroecological patterns from primary Global Biodiversity Information Facility data? Glob. Ecol. Biogeogr. 2015, 24, 335–347. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, L.X.; Bai, Y.; Ou, Y.Y.; Wang, C.B. Complete mitochondrial genomes of two flat-backed millipedes by next-generation sequencing (Diplopoda, Polydesmida). Zookeys 2016, 637, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Clewley, J.P. Macintosh sequence analysis software. Mol. Biotechnol. 1995, 3, 221–224. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo Metazoan Mitochondrial Genome Annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.H.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef] [PubMed]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Murphy, D. Application of ggplot2 to Pharmacometric Graphics. CPT-Pharmacomet. Syst. Pharmacol. 2013, 2, 1–16. [Google Scholar] [CrossRef]
- Suresh, V.; Parthasarathy, S. SVM-PB-Pred: SVM Based Protein Block Prediction Method Using Sequence Profiles and Secondary Structures. Protein Pept. Lett. 2014, 21, 736–742. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zhou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.; Lee, J.; Lee, D.Y.; Xi, H.; Park, J. The complete mitochondrial genome of the millipede Epanerchodus koreanus Verhoeff, 1937 collected in limestone cave of Korea (Polydesmidae: Polydesmida). Mitochondrial DNA Part B-Resour. 2020, 5, 3845–3847. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fang, Y.; Cao, G.; Shen, C.; Liu, H.; Ruan, H. The Complete Mitochondrial Genome of Spirobolus bungii (Diplopoda, Spirobolidae): The First Sequence for the Genus Spirobolus. Genes 2022, 13, 1587. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, X.J.; Yuan, H.; Shen, J.; Yang, M.X. Complete mitochondrial genome of Sellanucheza jaegeri Golovatch, 2013 by next generation sequencing (Polydesmida: Paradoxosomatidae) and phylogenetic analysis in Diplopoda. Mitochondrial DNA Part B-Resour. 2018, 3, 603. [Google Scholar] [CrossRef]
- Dai, X.; Deng, L.F.; Xiao, X.; Yu, X.J.; Lv, J.X.; Xiong, H.Y.; Ma, L.; Chen, Q.; Yang, L.Y.; Wang, X. First record of the complete mitochondrial genome of Botyodes diniasalis (Walker, 1859) (Lepidoptera: Crambidae). Mitochondrial DNA Part B-Resour. 2023, 8, 1401–1405. [Google Scholar] [CrossRef]
- Woo, H.J.; Lee, Y.S.; Park, S.J.; Lim, J.T.; Jang, K.H.; Choi, E.H.; Choi, Y.G.; Hwang, U.W. Complete mitochondrial genome of a troglobite millipede Antrokoreana gracilipes (Diplopoda, Juliformia, Julida), and juliformian phylogeny. Mol. Cells 2007, 23, 182–191. [Google Scholar] [CrossRef]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Chen, S.; Zan, R.; Xiao, H.; Zhang, Y.P. The complete mitochondrial genomes of two species from Sinocyclocheilus (Cypriniformes: Cyprinidae) and a phylogenetic analysis within Cyprininae. Mol. Biol. Rep. 2010, 37, 2163–2171. [Google Scholar] [CrossRef]
- Sun, Y.B.; Shen, Y.Y.; Irwin, D.M.; Zhang, Y.P. Evaluating the roles of energetic functional constraints on teleost mitochondrial-encoded protein evolution. Mol. Biol. Evol. 2011, 28, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.R.; Dowton, M. Mitochondrial genomes in the Hymenoptera and their utility as phylogenetic markers. Syst. Ento. 2007, 32, 60–69. [Google Scholar] [CrossRef]
- Dowton, M.; Castro, R.L.; Austin, D.A. Mitochondrial gene rearrangements as phylogenetic characters in the invertebrates: The examination of genome ‘morphology’. Invertebr. Syst. 2002, 16, 345–356. [Google Scholar] [CrossRef]
- Blanke, A.; Wesener, T. Revival of forgotten characters and modern imaging techniques help to produce a robust phylogeny of the Diplopoda (Arthropoda, Myriapoda). Arthropod Struct. Dev. 2014, 43, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Zhang, Z.S.; Shen, Y.J. Novel mitochondrial gene rearrangements pattern in the millipede Polydesmus sp. GZCS-2019 and phylogenetic analysis of the Myriapoda. Ecol. Evol. 2022, 12, e8764. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, G.; Tan, S.; Gong, S.; Yang, M.; Peng, Q.; Peng, R.; Zou, F. The complete mitochondrial genome sequence analysis of Tibetan argali (Ovis ammon hodgsoni): Implications of Tibetan argali and Gansu argali as the same subspecies. Gene 2013, 521, 24–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).