Abstract
At high elevations, the human body experiences a number of pathological, physiological, and biochemical changes, all of which have adverse impacts on human health and organ vitality. This study aimed to investigate the alterations in the liver and kidney biomarkers, oxidative stress markers, gene expression, and cellular histology of rats maintained at high altitudes and normal sea level. A total of twenty male Wistar rats at 2 months of age were randomly assigned to two groups. The rats in group A were maintained at normal sea level in Jeddah, whereas rats in group B were maintained in an area in Taif 2600 m above sea level. After 2 months of housing, orbital blood samples were collected for the analysis of significant biochemical indicators of oxidative stress biomarkers of the liver and kidneys. Liver and kidney tissues from both groups were taken to examine the hepatorenal changes occurring at the biochemical, histological, immunohistochemical, and genetic levels. The results revealed substantial increases in the serum levels of liver and kidney biomarkers (GPT, GOT, urea, and creatinine) and decreases in the serum levels of antioxidant biomarkers (SOD, catalase, GSH, and NO). In parallel, the levels of the malondialdehyde (MDA) tissue damage marker and inflammatory cytokines (IL-1β, TNF-α, and IFN-γ) were increased in the high-altitude group compared to the normal sea level group. In addition, there were significant alterations in the oxidative and inflammatory status of rats that lived at high altitude, with considerable upregulation in the expression of hepatic VEGF, type 1 collagen, Cox-2, TNF-α, and iNOS as well as renal EPASI, CMYC, HIF-α, and EGLN-2 genes in the high-altitude group compared with controls housed at normal sea level. In conclusion, living at high altitude induces hepatorenal damage and biochemical and molecular alterations, all of which may serve as critical factors that must be taken into account for organisms living at high altitudes.
1. Introduction
Hypoxia is defined as a state in which an organism, its organs, or its cells cannot readily obtain the oxygen they need. Hypoxia causes different negative impacts on organs, including physiological, biochemical, hematological, and molecular changes in a way to adapt to the extreme environmental conditions [1]. A variety of mechanisms must be activated to ensure the subject’s energy level remains stable under these condition. Hypoxia is associated with a state of high blood pressure, which helps in improving oxygen distribution across red blood cells [2]. Heart function suffers as a result of oxidative stress caused by intermittent hypercapnic hypoxia [3]. When an organism is exposed to hypoxic circumstances, systemic and cellular responses are activated in an attempt to meet oxygen demands [4].
Hypoxia has a significant impact on people’s health and daily activities at high altitudes because it profoundly affects physiology and causes pathological alterations in the body [5]. Drug absorption and distribution are closely linked to these changes induced by hypoxia [6]. As a result, pharmacokinetics cannot function without arterial blood gas analysis. Blood rheology, distribution, medication metabolism, and excretion are all affected [7]. As evidenced by growing studies, hypoxia appears to trigger an inflammatory response [8]. More than 140 million individuals around the world live at high altitudes [1], and living at high altitude has the potential to induce organ dysfunction syndromes that are caused by hypoxia. Organ dysfunction syndromes can cause severe intestinal barrier dysfunction, encourage bacterial and endotoxic translocation, and trigger an inflammatory response throughout the body [9]. Short- or long-term exposure at high altitudes induces respiratory, cardiovascular, and endocrine system dysfunction [10]. The deiodination pathway, which regulates thyroid hormone levels in the blood, can be impacted by hypoxia [11].
The environmental characteristics at high altitude include, but are not limited to, decreases in ambient oxygen tension, increase in solar radiation, extreme diurnal ranges in temperature, arid climate, and poor soil quality, in which hypoxia is a significant factor affecting the activity of human life and organs vitality [12]. The effects of hypoxia on both animals’ and humans’ biochemical, physiological, and metabolic systems is a topic of interest for further investigation through research [13].
Elevations above sea level are classified as high altitude (2000 to 4000 m), very high altitude (4000 to 5500 m), and extremely high altitude (>5500 m). These altitudes affect normal physiology and health due to the low partial pressure of oxygen [14]. Hypoxia develops due to this low oxygen pressure [15]; therefore, the body adapts through several physiological and molecular strategies. The blood pressure is increased to provide more oxygen to the tissues [16]. On the other hand, rapid changes in blood flow cause fluid leakage from the capillaries, leading to the development of serious conditions such as high-altitude pulmonary edema and high-altitude cerebral edema [17]. Cells can sense hypoxia via oxygen sensor prolyl hydroxylases [16], which activate the transcription factor hypoxia-inducible factor 1 (HIF-1) [18]. HIF-1 can relay the hypoxia signal and trigger adaptive hypoxic responses throughout the body [16,19].
Taif is located about 1700–2500 m above sea level and hosts resorts for national and international tourists. The impact of high altitude on global gene expression and the levels of biochemical and oxidative stress biomarkers must be characterized to provide residents with relevant information about the effects of hypoxia on their health. Therefore, the present study aimed to examine and compare the impacts of living at a high altitude (Taif) and at sea levels (Jeddah) on Wistar rats in terms of oxidative stress and inflammatory, antioxidant, and hepatorenal biomarkers. The changes in both the histology and immunohistochemistry of genes associated with hypoxia and oxidative stress in the liver and kidney were compared in groups housed at either high or normal sea levels.
2. Materials and Methods
2.1. Animal Handling and Experimental Design
Twenty albino rats were kept at room temperature with free access to food and water at the Biotechnology Department, Taif (High altitude), and King Fahad Institute of Research in Jeddah (sea level) following instructions from Taif University’s Institutional Animal Care and Use Committee, who authorized all animal use. Rats were birthed at normal sea levels at Jeddah. Rats were maintained for 2 months and weighed 150–170 g. Next rats were divided into two groups: group A (normal sea level group) was kept at Jeddah (sea level) for an additional 2 months; group B (high-altitude group) was taken to Taif (high altitude) and maintained for an extra 2 months. At the end of experimental study (2 months), rats were euthanized by decapitation following anesthetization. A non-heparinized Vacutainer tube was used to collect orbital blood, which was then centrifuged at 3000× g for 10 min. Samples were stored at −20 °C before being analyzed for blood chemistry and metabolite changes. Sliced and cleaned liver and kidney samples were then rinsed in cold saline to remove any remaining debris. For histological and immunohistochemical examination, tissue samples were stored in a 10% neutral buffer formalin solution or QIAzol for RNA extraction and real-time PCR.
2.2. Serum Biochemical Parameters
Superoxide dismutase (SOD), glutathione (GSH), catalase, and malondialdehyde (MDA) were measured using a colorimetric spectrophotometer based on the instructions provided by the manufacturers from Biodiagnostic Co. (Dokki, Giza, Egypt). Nitric oxide (NO) quantities were measured following the method of [20]. The activity of the glutamate pyruvate transaminase (GOT), glutamate oxaloacetate transaminase (GOT), and gamma-glutamyl transferase (GGT) enzymes in serum were tested using the corresponding kits from Spectrum Diagnostics, Obour City, Egypt (http://www.spectrum-diagnostics.com/new/p_01_02_Enzymes.php, accessed on 4 March 2022) according to the manufacturer’s instructions [21,22]. Urea levels were evaluated as described earlier [23]. Serum creatinine was assessed using modified Jaffe’s reaction as described earlier [24]. Uric acid was evaluated using Trinder’s enzymatic reaction as described in this study [25]. The Rat IL-6 ELISA Kits (ab100772) were obtained from Abcam Co. (Tokyo, Japan). The serum interferon-γ (IFN-γ) and tumor necrosis factor (TNF-α) levels were analyzed using specific ELISA kits (MyBioSource, San Diego, CA 92195-3308, USA) according to the manufacturer’s instructions.
2.3. Molecular Analysis Using qRT-PCR
Total RNA was extracted from liver and kidney tissues of groups A and B using an RNeasy Mini Kit (Cat# 74104, Hilden, Germany). RNA purity was measured using the A260/A280 ratio. RNA samples with A260/A280 ratios between 1.8 and 2 indicated high purity and were used in cDNA synthesis. The complementary DNA (cDNA) was synthesized with the HiSenScript kit by mixing 10 µL of 2 RT reaction solution, 1 µL of the enzyme, and 1 µg of total RNA and made up to final volume of 20 µL with RNase-free water. The reverse transcription reaction was made by incubating the mixture at 50 °C for 30 min and then at 85 °C for 10 min to inactivate the enzyme. The qRT-PCR was carried out on the PCR thermal cycler machine (iQ5 Real-time PCR, Bio-Rad, Hercules City, CA, USA) using a Quanti Fast SYBR Green PCR kit. The information for the primers, sequence, and product size is listed in Table 1. Each PCR reaction consisted of 1 μL cDNA and 10μL SYBR Green PCR Master Mix (Quanti Tect SYBR Green PCR Kit, Qiagen, Valencia, CA, USA), along with 1 μM of forward and reverse primer for each examined gene and nuclease-free H2O to a final volume of 20 μL. Reactions were run and analyzed in a Bio-Rad iQ5 real-time PCR machine. Real-time PCR conditions were: first denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s (second denaturation), then annealing as shown in Table 1 for 60 stages. The critical threshold (Ct) of the target gene was normalized with quantities (Ct) of the housekeeping gene (β-actin) and GAPDH using the formula x = 2 − ΔΔCt, where there x = the fold difference.

Table 1.
Primer names, gene accession numbers, and sequences used for quantitative real-time PCR in rats.
2.4. Histopathological Evaluation of Hepatic and Renal Tissues
All rats were decapitated and necropsied at the end of the experiment [26,27], and representative hepatic and renal tissue was taken from each rat then incubated in 10% neutral buffered formalin for 24 h for preservation. After fixation, dehydration in ethanol, removal of impurities, impregnation, and embedding in paraffin wax were all carried out on the specimens before they were finally stained with hematoxylin and eosin and sectioned at a thickness of 4 µm [28]. The stained sections were evaluated microscopically. A multiparametric quantitative lesion assessments were separately performed on 40 images (8 images/5 rats per each group), as shown in last table.
2.5. Immunohistochemical Investigation of Heme Oxygenase-1 (HO-1) and Nuclear Factor Erythroid Factor 2-Related Factor-2 (Nrf-2)
For immunohistochemical analysis, slices were embedded in paraffin then deparaffinized and rehydrated. They were then soaked for 15 min in 2% H2O2 then washed in PBS to inhibit peroxidase activity. Non-specific binding sites were blocked using 5% bovine serum albumin. HO-1 antibody (ab13243) and Nrf-2 (ab31163) antibody were obtained from Abcam Corporation, Cambridge, MA, USA. These antibodies were diluted to 1:500 and added to the prepared slices from liver and kidney tissues. Sample slides were incubated overnight at 4 °C. The slides were then washed three times with PBS and incubated with a 1:2000 dilution of biotin-conjugated secondary antibody. These were developed using 3,3-diaminobezidine tetrahydrochloride and counterstained with hematoxylin [29]. In five non-overlapping, randomly selected microscopic fields, eight different snapshots were taken (8 shots per field for 5 different fields) for each gene to examine the immunoreactivity between groups A (sea level) and B (high altitude). These images were examined to measure the degree of immunoreactivity for the changes in the expression of Nrf-2 and HO-1 in the liver and kidneys, respectively [30]. The collective results for the immunoreactivity of the examined genes can be seen in last table of this study.
2.6. Statistical Analysis
The current data were analyzed using SPSS software for Windows, including a one-way ANOVA and Dunnett’s post hoc descriptive test (SPSS, IBM, Chicago, IL, USA). The data are presented as means with standard error (SEM). Here, p < 0.05 is considered to indicate statistically significant differences between the examined groups.
3. Results
3.1. Effects of High Altitude on Hepatorenal Function in Rats
The results in Table 2 reveal significant increases in the levels of liver biomarkers. There were substantial elevations in the GPT, GOT, and GGT in the high-altitude group compared with the normal sea level group. In addition, there were significant increases in the levels of the kidney injury markers creatinine, urea, and uric acid compared to the normal sea level group.

Table 2.
Alterations in serum liver and kidney biomarkers in groups living at normal sea levels and at high altitude.
3.2. Effects of High Altitude on Serum MDA, Catalase, SOD, NO, and GSH Levels
Table 3 indicates significant increases in the MDA levels with prominent and significant decreases in the serum SOD, catalase, GSH, and NO levels in the high-altitude group relative to the normal sea level group.

Table 3.
Alterations in serum MDA, catalase, SOD, NO, and GSH levels between groups living at normal sea level and at high altitude.
3.3. Effects of High Altitude on Inflammatory Cytokine Biomarkers
The data presented in Table 4 show significant increases in the serum levels of IL-6, TNF-α, and IFN-γ for high-altitude rats compared to the normal sea level group. This confirms that high altitude (HA) is a stress factor that mediates inflammation and bodily responses in the subjects living in the Taif region.

Table 4.
Alterations in serum IL-6, TNF-α, and IFNγ concentrations in groups living at normal sea level and at high altitude.
3.4. Effects of High Altitude on Hepatorenal Gene Expression
The data presented in Figure 1 show the changes in the expression of genes in the livers of rats that lived at sea level (group A) and at high altitude (group B). There was significant upregulation in the mRNA expression levels of hepatic VEGF, type 1 collagen, Cox-2, TNF-α, and iNOS in group B compared to group A. However, there was downregulation in the expression of AMPK (Figure 1). Regarding the effect of high altitude on the expression of renal genes, the data in Figure 2 show that there was considerable upregulation in the expression of renal EPASI, CMYC, HIF-α, and EGLN-2 in the high-altitude group (group B) compared with the normal sea level group (group A). There was no change in VHL mRNA expression between the groups.
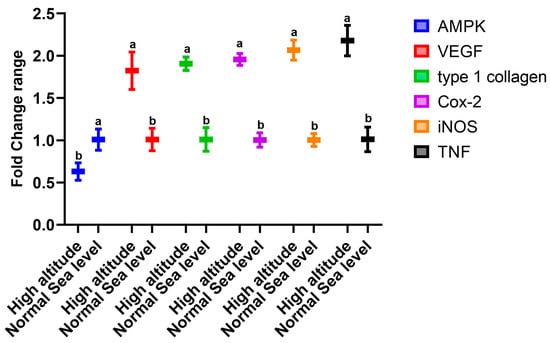
Figure 1.
Impacts of high altitude on the expression levels of hepatic oxidative stress marker genes associated with hypoxia in the liver as assessed using quantitative real-time PCR. Bars indicate densitometric analysis of the expression levels of the examined genes for 10 different rats per group. Bars with different letters indicate significant differences between groups A (normal sea level) and B (high altitude) at p < 0.05.
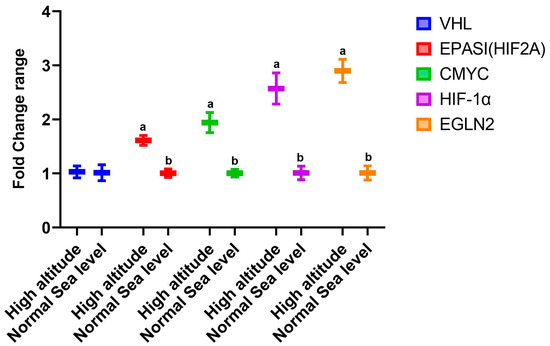
Figure 2.
Impacts of high altitude on the expression levels of renal oxidative stress marker genes associated with hypoxia in the kidneys as assessed using quantitative real-time PCR. Bars indicate densitometric analysis of the expression levels of the examined genes for 10 different rats per group. Bars with different letters indicate significant differences between groups A (normal sea level) and B (high altitude) at p < 0.05.
3.5. Effects of High Altitude on Hepatorenal Histological Architect
The histology of the hepatic tissue of the control group (Figure 3A) shows the presence of intact polyhedral hepatocytes arranged in a cord-like pattern radiating from the central vein, where each cord is separated from the others by hepatic sinusoids. The hepatic tissue samples of rats from the high-altitude group showed marked congestion of the central vein, nuclear pyknosis, swelling, vacuolar degeneration, and areas of hepatocyte necrosis (Figure 3B). The Nrf-2 immunostaining expression was markedly decreased in the high-altitude group compared with the control group (Figure 3C,D), confirming the occurrence of hepatic oxidative stress. The renal cortex of the sea level group shows intact glomeruli with narrow capsular space in addition to intact proximal and distal convoluted tubules (Figure 4A). The kidney samples of the high-altitude group show marked degenerative changes in renal glomeruli and renal tubules (Figure 4B). HO-1 immunostaining was markedly decreased in the high-altitude group compared with the control group, showing mild positive HO-1 expression (Figure 4C,D). The immunoreactivity and lesion scoring results for both liver and kidney samples are presented in Table 5.
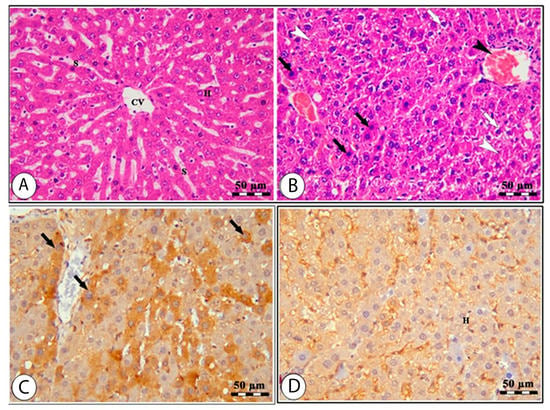
Figure 3.
Liver sections of normal sea level (A,C) and high altitude (B,D) groups stained by H&E (A,B) and Nrf-2 IHC (C,D). The H&E-stained liver sample from the normal sea level group (A) shows normal hepatocytes (H), hepatic sinusoids (S), and the central vein (CV). The H&E-stained liver sample from the high-altitude group (B) shows a congested central vein (black arrowhead), areas of necrosis (white arrowheads), vacuolar degeneration (white arrows), pyknotic nuclei, and swelling of hepatocytes (black arrows). The Nrf-2 IHC-stained liver sample from the normal sea level group (C) shows hepatocytes with Nrf-2 positive staining (H). The Nrf-2 IHC-stained liver sample from the high-altitude group (D) shows hepatocytes with less Nrf-2 staining (black arrows).
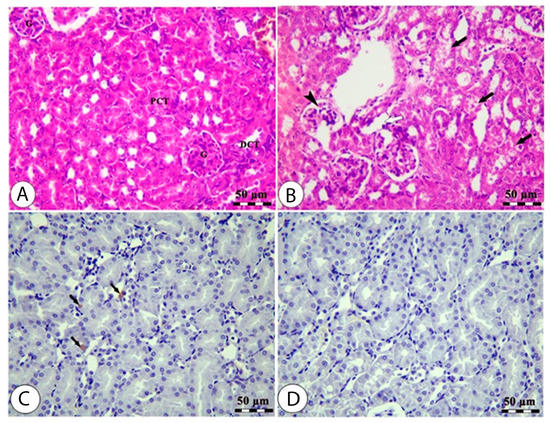
Figure 4.
Kidney sections of normal sea level (A,C) and high altitude (B,D) groups stained by H&E (A&B) and HO-1 IHC (C,D). The H&E-stained kidney sample from the normal sea level group (A) shows normal glomeruli (G), normal proximal (PCT), and distal (DCT) convoluted tubules. The H&E-stained kidney sample from the high-altitude group (B) shows degenerated glomeruli (black arrowhead), degenerated renal tubules (black arrows), and interstitial edema (white arrow). The HO-1 IHC-stained kidney sample from the normal sea level group (C) shows renal tubules with mildly positive HO-1 staining (black arrows). The HO-1 IHC-stained kidney sample from the high-altitude group (D) shows renal tubules with less HO-1 staining.

Table 5.
Histopathological changes as described by H&E and immunoexpression of HO-1 and Nrf-2 in the hepatic and renal tissues of rats who lived at sea and high altitude levels.
4. Discussion
The current study confirmed the feedback of living at high altitude in Taif compared to normal sea level at Jeddah for 2 months. We confirmed the changes in liver and kidney biomarkers at biochemical, cellular, and molecular levels. There were increases in the levels of oxidative stress markers for rats living at high altitudes compared to the normal sea level control group and decreases in Nrf2 and HO-1 immunohistochemistry in rats who lived at high altitude. The expression levels of hepatic Nrf-2, HO-1, VEGF, type 1 collagen, Cox-2, TNF-α, and iNOS together with renal EPASI, CMYC, HIF-α, and EGLN-2 all showed upregulation. There were more changes in liver and kidney morphology in the high-altitude group than in the normal sea level group. In parallel, living in Taif induced a state of inflammation as reported by the increases in IL-1β, TNF-α, and IFN-γ levels at high altitudes compared to normal sea level.
Although the percentage of oxygen in inspired air remains constant at different altitudes, the partial pressure of inspired oxygen and the driving pressure for gas exchange in the lungs drops with the fall in atmospheric pressure at higher altitudes [31]. Living at high altitudes causes oxidative damage mediated by free radicals, which is a significant factor in the development of certain diseases and induction of undesirable metabolic reactions [32]. Proteins are inactivated by free radical reactions resulting from oxidative stress caused by high altitude living and carbonyls are formed. Carbonyls may cause cell and organ malfunction, particularly in the liver, due to functional impairment and cell death [33]. An organism’s physiological system undergoes a series of structural and functional alterations resulting from hypoxia. As a result, several pathophysiological diseases have been linked to HA-induced hypobaric hypoxia. The liver becomes more prone to oxidative stress when oxygen delivery is reduced due to hypoxia caused by high altitude [34]. Physiologically, this impairs the liver’s ability to detoxify drugs and impairs the functioning of liver cells, thereby explaining the reported alterations in liver activity in the group living in Taif.
Our data reveal that there were significant increases in the liver injury markers GPT, GOT, and GGT in the high-altitude group compared to the normal sea level group, and these results are in agreement with another study [35] in which increased levels of GPT, GOT, and lactate dehydrogenase (LDH) were observed in hypoxic rats, which was attributed to the disruption of lysosomes, an effect of hypoxia that leads to alterations in the permeability of cell membranes and subsequent enzyme release. Alterations in liver function considerably impact enzyme production [36] and activity, since the liver is a primary organ for drug metabolism [37]. In addition, another [36] report on the biochemical study of BUN, uric acid, and creatinine levels showed their concentrations were substantially increased at high altitudes. In that study, hypobaric hypoxia was found to reduce the glomerular filtration rate and renal blood flow at the same time, as it altered cell membranes and impacted the excretion of furosemide from the kidneys. Kidney cells were more vulnerable to oxidative stress and damage when the O2 concentration gradient was lower at high altitudes. The renal histology of rats confirmed this biochemical conclusion. These observations support our findings concerning the kidney injury markers creatinine, urea, and uric acid related to normal sea levels. High-altitude hypoxia damages renal cells by altering energy metabolism and membrane transport system performance, releasing free radicals, catalyzing enzymes, and damaging the cytoskeleton [38].
Data in the present study revealed that there were significant decreases in the SOD levels in the high-altitude compared to the normal sea level group; this result was consistent with [39], in which a decrease in the SOD enzymatic activity was reported to be due to decrease in oxidative phosphorylation of O2 in the mitochondrial respiratory chain, due to its limited availability at high altitudes [40].
Rats at high altitudes exhibited more significant levels of thiobarbituric acid reactive substances (TBARS). They had lower activities of SOD and CAT and lower levels of GSH than rats at low altitudes. Still, lower levels of TBARS, SOD, and CAT activities and higher GSH levels have been reported in kidney homogenates [41], findings that are in agreement with our results concerning the oxidative injury induced by hypoxic conditions. These findings show that the livers of animals living at high altitudes have higher levels of lipid peroxidation, and as a result more free radicals. Hypoxia has been shown to induce the expression of the steroidogenic acute regulatory protein and increase glucocorticoid release [42]. In the brains of rats treated with glucocorticoids, researchers detected decreased activity of antioxidant enzymes (systemic SOD and CAT) [43]. This could account for the drop in tissue levels of SOD and CAT that we observed in our research.
Hypobaric hypoxia is a symptom of high altitude considered an acute physiological stressor and a source of oxidative stress and oxidative damage caused by reactive oxygen species (ROS) in cells, tissues, or organs. ROS initiate lipid peroxidation, a cyclic event that generates free radicals by oxidizing polyunsaturated fatty acids in membranes [44], while SOD, GSR, and GPX also appear to be affected by hypoxia, which is known to impair enzyme activity [45]
Concerning the NO data, our results reveal that rats at high altitude showed decreased NO levels compared with those at normal sea levels, a result consistent with [46], where the authors reported that chronic hypoxia reduces NO concentrations in the serum of rats due to decreased NO bioavailability in the systemic circulation, which is a major contributor to hypoxia-induced endothelial dysfunction [47].
Hypoxia is associated with inflammation [44]. Inflammatory cytokines can activate nuclear factor kappa-B (NFκB), leading to increased inflammation and interleukin generation, suppressing the elevated pro-inflammatory markers (NF-κB, TNFα, and IL6) that might be responsible for adaptogenic potential during exposure to hypoxia [48]. In addition, cytokines such as IL-1, IL-2, IL-4, IL-5, IL-6, IFN-γ, and TNF-α are secreted in response to hypoxia [49]. These findings explain our obtained results concerning the cytokines in which there were significant increases in the serum IL-6, TNF-α, and IFN-γ levels of high-altitude rats compared to those in the normal sea level group. There are numerous molecular events and pathways that are associated with living at high altitude; our findings show that there was significant upregulation of hepatic VEGF, type 1 collagen, Cox-2, TNF-α, and iNOS in the high-altitude group compared with the normal sea level group and significant downregulation in hepatic AMPK mRNA expression. In parallel, the high-altitude group showed significant upregulation of renal EPASI, CMYC, HIF-α, and EGLN-2 levels compared with the normal sea level group, and there were no changes in the VHL expression between the examined groups. In the cirrhotic liver, angiogenesis is mostly dependent on VEGF [50]. The expression of VEGF and VEGF receptors is upregulated in the regenerating rat liver and plays a significant role in the proliferation of sinusoidal endothelial cells, making it clear why induced VEGF expression is a frequent response to liver injury [51]. Collagen 1 expression might be directly induced by hypoxia in the initial stages [52].
Our findings are in harmony with others [53] that concluded that hypoxia enhances VEGF expression and collagen I expression in hepatic cells and suggested that it may play a role in advancing chronic liver disorders through its involvement in angiogenesis and fibrogenesis. A rise in HIF-1 protein levels is a direct result of hypoxia, indicating that in the presence of intermittent hypoxic circumstances, systemic adaptation occurs gradually at the cellular level. The adaptation of cells or tissues to hypoxic conditions is assumed to be responsible for this rise [54]. In addition, it was found that green tea extract significantly inhibits hypoxia- and HIF-1α-related protein accumulation and the corresponding expression of VEGF at both mRNA and protein levels in HeLa and HepG2 cells. These findings suggest that the suppression of hypoxia-induced VEGF expression by green tea extract and EGCG is due, at least in part, to their inhibitory effects on HIF-1α transactivation of the VEGF gene in HeLa cells [55].
Consistent with this, in rats vulnerable to hypoxia, the level of HIF-1 expression is statistically higher [56], which is accordance with the statement in [57] that inflammatory disorders are more likely to occur in animals that suffer from hypoxia. The results of our molecular pathway analysis are consistent with those of other studies in which HIF-mediated gene expression and caspase-3 were induced in hypoxia [58,59]. When hypoxia is detected, the body responds by producing HIF, a protein crucial for adaptation [60]. In addition, the authors of [61] reported the increased expression of VEGF receptors in prolonged exposure to hypoxia. In cells lacking the VHL gene, HIF-1 suppresses c-Myc activity, decreasing mitochondrial biogenesis and O2 consumption [62].
High-altitude exposure also stimulates the expression of enzymes that regulate fatty acid production. High-altitude exposure could significantly increase the expression of hepatic acetyl-CoA carboxylase-1 (ACC-1), a rate-limiting enzyme in fatty acid biosynthesis [63], with a significant reduction in the expression of AMPK, an enzyme reported to inhibit lipogenesis through suppressing the activity of ACC-1 and malonyl CoA (M-CoA) [64], which supports our finding concerning hepatic AMPK mRNA expression. Renal inflammation and interstitial fibrosis are both exacerbated by intra-renal hypoxia. Renal tubular cells may release inflammatory cytokines or interact with macrophages, explaining the connection between HIF-1 and renal interstitial inflammation [65]. Cell metabolism and proliferation are thought to be accelerated and induced by the transcription factor c-Myc [66]. Evidence suggests that several animal models of renal fibrosis show markedly elevated c-Myc protein expression [67]. The mRNA expression of HIF-2A increased in mice exposed to chronic and intermittent hypoxia [68].
Hypoxic conditions also increase HIF-1α and VEGF levels [69]. Studies have shown that HIF-1 is an excellent indicator of hypoxia. Multiple genes are activated when hypoxia and other stimuli activate the HIF-1 signaling cascade. Hypoxia-induced ROS production is a signaling chain involving HIF-1 [70,71]. Free radicals are being produced due to chronic hypoxia [72], activating the transcriptional activity of NFkB in the nucleus by increasing its nuclear translocation. Pro-inflammatory cytokines, TNF, interleukins, COX-2, iNOS, as well as adhesion molecules such as vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and E-selectin are produced when NFkB is activated [73]. Aside from endothelial cells, EPAS1 expression has also been observed in the lungs, placenta, kidneys, heart, liver, small intestine, and other organs that regulate oxygen metabolism [74]. In genome-wide association studies, EPAS1 was found to be linked to hypoxia adaption and decreased hemoglobin concentration in Tibetans [75]. The expression levels of HIF-1 and iNOS are significantly linked. In a separate investigation, hypoxia boosted the expression of the iNOS gene in the pulmonary artery endothelial cells of rats through activating HIF-1 [76].
Thus, HIF-1 and iNOS may be necessary for the pathophysiology of hypoxia damage, and HIF-1 controls iNOS expression [77]. Rats exposed to high altitude had higher EGLN-1 and PPAR gene expression levels in their heart, liver, and kidneys. In addition, at high altitudes, the expression of the HIF-2 protein in the liver, brain, and renal tissues was dramatically elevated. In the heart, liver, and kidneys, expression of prolyl hydroxylase domain-containing protein 2 (PHD2) and PPAR increased [78], which may be utilized as indicators of hypoxia. Hypobaric hypoxia significantly reduces Nrf-2 expression. After being exposed to oxidative stress, the level of HO-1 showed a comparable reaction consistent with that of Nrf-2. Nrf-2 has been shown to function as a transcription factor that regulates cellular redox balance and promotes the production of antioxidant enzymes to protect cells from oxidative damage [79]. Under normal conditions, Nrf-2 is sequestered in the cytoplasm by binding to the cysteine-rich Kelch-like ECH-associated protein 1 (Keap1), which is involved in the detoxification of reactive oxidants, the maintenance of cellular homeostasis, and the removal of a variety of exogenous and endogenous chemicals [80]. This confirms the downregulation in Nrf-2 and HO-1 immunoreactivity seen in liver and kidney immunohistochemistry.
5. Conclusions
In summary, our results demonstrate that a hypoxia environment causes pathological changes in the liver and kidney that are evident from an increase in hepatic and renal injury markers and an increase in the levels of oxidative stress markers, together with the increased expression of cytokines and the activation of genes related to adaptation to hypoxia (namely hepatic VEGF, type 1 collagen, Cox-2, TNF-α, and iNOS) and renal EPASI, CMYC, HIF-α, and EGLN-2 levels at high altitude. The altered biochemical and molecular markers are potentially significant therapeutic targets for the prevention or treatment of hepatorenal injury induced by living at high altitude. The collective impacts of living at high altitude are summarized in Figure 5.
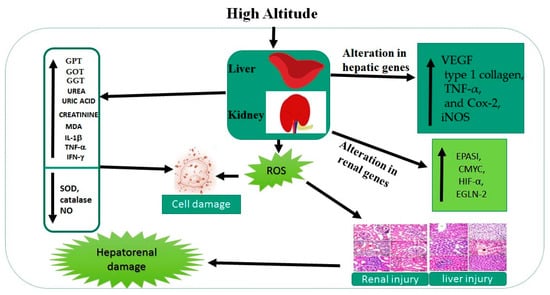
Figure 5.
Collective impacts of living at high altitude on liver and kidney markers.
Author Contributions
M.M.S., A.A., A.M.E.-S., A.M.E.-S. and S.S. designed the study plan; M.M.S., A.M.E.-S., S.S., F.A., S.A., M.S. and M.M.A. drafted the manuscript; M.M.S., A.M.E.-S., S.S., F.A., S.A., M.S. and M.M.A. helped in conducting the research work, data analysis, and assisted in the writing of the manuscript; M.M.S., A.M.E.-S., S.S., F.A., S.A., A.A., M.S. and M.M.A. provided technical help in writing the manuscript; writing—review and editing, M.M.S., A.M.E.-S., S.S., F.A., S.A., M.S. and M.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by High Altitude Research Center, Taif University, Saudi Arabia. Grant No 1-442-44.
Institutional Review Board Statement
All of the experimental procedures in this study were carried out under the National Institutes of Health Guidelines and regulations for the care and use of laboratory animals. All steps to decrease or minimize the suffering of the experimental animals were followed.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data sets obtained and analyzed during the current study are available in the manuscript.
Acknowledgments
The authors would like to extend their sincere thanks to High Altitude Research Center, Taif University for its funding of this research through the research group (project number: 1-442-44).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ACC-1: Acetyl-CoA carboxylase-1; AMPK: activated mitogen protein kinase; CAT: catalase; cDNA: complementary DNA; C-MYC: c-myelocytomatosis; Cox-2: cyclooxygenase-2; CT: cycle threshold; EGLN2: Egl-9 family hypoxia-inducible factor 2; EPASI(HIF2A): hypoxia-inducible factor 2A; GOT: glutamate oxaloacetate transaminase; GPT: glutamate pyruvate transaminase; GPX: glutathione peroxidase; GSR: glutathione reductase; HACE: high-altitude cerebral edema; HIF-1: hypoxia-inducible factor-1; HIF-1α: hypoxia-inducible factor 1 alpha; HO-1: heme oxygenase; iNOS: inducible nitrous oxide; Keap1: Kelch-like ECH-associated protein 1; LDH: lactate dehydrogenase; MDA: malondialdehyde; M-CoA: malonyl CoA; NF-κB: nuclear factor kappa-B; Nrf-2: nuclear factor-erythroid factor 2-related factor 2; PHD2: prolyl hydroxylase domain-containing protein 2; PPAR: peroxisome proliferator-activated receptor; qRT-PCR: quantitative real-time PCR; ROS: reactive oxygen species; SOD: superoxide dismutase; VEGF: vascular endothelial growth factor; TBARS: thiobarbituric acid reactive substances; TNF-α: tumor necrosis factor alpha; VHLEL: Von Hippel–Lindau tumor suppressor.
References
- Khurana, P.; Gupta, A.; Sugadev, R.; Sharma, Y.K.; Kumar, B. HAHmiR. DB: A server platform for high-altitude human miRNA–gene coregulatory networks and associated regulatory circuits. Database 2020, 2020, baaa101. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J. The cellular response to hypoxia: Tuning the system with microRNAs. J. Clin. Investig. 2010, 120, 3815–3817. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.A.; Farías, J.G.; González-Candia, A.; Short, S.E.; Carrasco-Pozo, C.; Castillo, R.L. Ω3 Supplementation and intermittent hypobaric hypoxia induce cardioprotection enhancing antioxidant mechanisms in adult rats. Mar. Drugs 2015, 13, 838–860. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. O2-regulated gene expression: Transcriptional control of cardiorespiratory physiology by HIF-1. J. Appl. Physiol. 2004, 96, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chai, W.; Gao, W.; Xu, L.; Zhang, H.; Yang, Y. Hyperoxygenated solution: Effects on acute hypobaric hypoxia-induced oxidative damage in rabbits. High Alt. Med. Biol. 2009, 10, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.; Welford, S.M.; Haaga, J.; LaManna, J.; Strohl, K.P. Hypoxia—Implications for pharmaceutical developments. Sleep Breath. 2010, 14, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Han, E.J.; Lee, D.S. Significance of metabolites in the environmental risk assessment of pharmaceuticals consumed by human. Sci. Total Environ. 2017, 592, 600–607. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, D.; Zhou, Q. The SEM observation of small intestinal mucosa in the rabbits under simulated high altitude hypoxia. Chin. J. Gastroenterol. Hepatol. 2009, 18, 751–753. [Google Scholar]
- Zhou, Q.-Q.; Yang, D.-Z.; Luo, Y.-J.; Li, S.-Z.; Liu, F.-Y.; Wang, G.-S. Over-starvation aggravates intestinal injury and promotes bacterial and endotoxin translocation under high-altitude hypoxic environment. World J. Gastroenterol. WJG 2011, 17, 1584. [Google Scholar] [CrossRef]
- Pialoux, V.; Hanly, P.J.; Foster, G.E.; Brugniaux, J.V.; Beaudin, A.E.; Hartmann, S.E.; Pun, M.; Duggan, C.T.; Poulin, M.J. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am. J. Respir. Crit. Care Med. 2009, 180, 1002–1009. [Google Scholar] [CrossRef]
- Sandoval, D.A.; Matt, K.S. Gender differences in the endocrine and metabolic responses to hypoxic exercise. J. Appl. Physiol. 2002, 92, 504–512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Wang, Y.; Liu, X. Mountain Sickness; Publishing House of Qinghai People: Xining, China, 1984. [Google Scholar]
- Brahmachari, H.; Malhotra, M.; Joseph, S.; Ramachandran, K. Effects of stay at high altitude on the serum proteins of man. Indian J. Physiol. Pharmacol. 1973, 17, 4. [Google Scholar]
- Peacock, A.J. Oxygen at high altitude. BMJ 1998, 317, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Irarrázaval, S.; Allard, C.; Campodónico, J.; Pérez, D.; Strobel, P.; Vásquez, L.; Urquiaga, I.; Echeverría, G.; Leighton, F. Oxidative stress in acute hypobaric hypoxia. High Alt. Med. Biol. 2017, 18, 128–134. [Google Scholar] [CrossRef]
- Giaccia, A.J.; Simon, M.C.; Johnson, R. The biology of hypoxia: The role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004, 18, 2183–2194. [Google Scholar] [CrossRef]
- Mehta, S.; Chawla, A.; Kashyap, A. Acute mountain sickness, high altitude cerebral oedema, high altitude pulmonary oedema: The current concepts. Med. J. Armed Forces India 2008, 64, 149. [Google Scholar] [CrossRef]
- Bishop, T.; Ratcliffe, P.J. Signaling hypoxia by hypoxia-inducible factor protein hydroxylases: A historical overview and future perspectives. Hypoxia 2014, 2, 197. [Google Scholar]
- Hopfl, G.; Ogunshola, O.; Gassmann, M. Hypoxia and high altitude. The molecular response. Adv. Exp. Med. Biol. 2003, 543, 89–115. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Murray, R.; Kaplan, A. Alanine aminotransferase. In Clinical Chemistry: Theory, Analysis and Correlation; Kaplan, L.A., Pesce, A.J., Eds.; CV Mosby: St. Louis, MO, USA, 1984; Volume 1090. [Google Scholar]
- Szasz, G. New substrates for measuring gamma-glutamyl transpeptidase activity. Z. Klin. Chem. Klin. Biochem. 1974, 12, 228. [Google Scholar]
- Talke, H.; Schubert, G.E. Enzymatic Urea Determination in the Blood and Serum in the Warburg Optical Test. Klin. Wochenschr. 1965, 43, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.D. Kinetic serum creatinine assays I. The role of various factors in determining specificity. Clin. Chem. 1980, 26, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Barham, D.; Trinder, P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Ruehl-Fehlert, C.; Kittel, B.; Morawietz, G.; Deslex, P.; Keenan, C.; Mahrt, C.R.; Nolte, T.; Robinson, M.; Stuart, B.P.; Deschl, U. Revised guides for organ sampling and trimming in rats and mice—Part 1: A joint publication of the RITA and NACAD groups. Exp. Toxicol. Pathol. 2003, 55, 91–106. [Google Scholar] [CrossRef]
- Fiette, L.; Slaoui, M. Necropsy and sampling procedures in rodents. In Drug Safety Evaluation; Springer: Berlin/Heidelberg, Germany, 2011; pp. 39–67. [Google Scholar]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hsu, S.-M.; Raine, L.; Fanger, H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 1981, 29, 577–580. [Google Scholar] [CrossRef]
- Metwally, M.M.; Ebraheim, L.L.; Galal, A.A. Potential therapeutic role of melatonin on STZ-induced diabetic central neuropathy: A biochemical, histopathological, immunohistochemical and ultrastructural study. Acta Histochem. 2018, 120, 828–836. [Google Scholar] [CrossRef]
- Mazzeo, R.S.; Reeves, J.T. Adrenergic contribution during acclimatization to high altitude: Perspectives from Pikes Peak. Exerc. Sport Sci. Rev. 2003, 31, 13–18. [Google Scholar] [CrossRef]
- Yu, L.; Cao, X.; Tao, W.; Li, M.; Li, X.; Chen, L. Antioxidant activity and potential ameliorating effective ingredients for high altitude-induced fatigue from Gansu Maxianhao (Pedicularis Kansuensis Maxim.). J. Tradit. Chin. Med. Chung I Tsa Chih Ying Wen Pan 2020, 40, 83–93. [Google Scholar]
- Althobaiti, F.; Hassan, M.M.; El-Shehawi, A.M.; Alotaibi, S.S.; Youssef, G.B.; Soliman, M.M.; Sayed, S.; Aldhahrani, A. The Protective Impact of Salsola imbricata Leaf Extract From Taif Against Acrylamide-Induced Hepatic Inflammation and Oxidative Damage: The Role of Antioxidants, Cytokines, and Apoptosis-Associated Genes. Front. Vet. Sci. 2022, 8, 817183. [Google Scholar]
- Jun, J.; Savransky, V.; Nanayakkara, A.; Bevans, S.; Li, J.; Smith, P.L.; Polotsky, V.Y. Intermittent hypoxia has organ-specific effects on oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1274–R1281. [Google Scholar] [CrossRef]
- Gola, S.; Gupta, A.; Keshri, G.K.; Nath, M.; Velpandian, T. Evaluation of hepatic metabolism and pharmacokinetics of ibuprofen in rats under chronic hypobaric hypoxia for targeted therapy at high altitude. J. Pharm. Biomed. Anal. 2016, 121, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, J.; Yang, T.; Li, W.; Zhang, J.; Wang, C.; Zhao, A.; Wang, R. Evaluation of renal excretion and pharmacokinetics of furosemide in rats after acute exposure to high altitude at 4300 m. Biopharm. Drug Dispos. 2018, 39, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, R.; Xie, H.; Zhang, J.; Jia, Z. Changes of pathological and physiological indicators affecting drug metabolism in rats after acute exposure to high altitude. Exp. Ther. Med. 2015, 9, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Miyata, T.; Inagi, R.; Kurokawa, K.; Adler, S.; Fujita, T.; Nangaku, M. Hypoxia-induced apoptosis in cultured glomerular endothelial cells: Involvement of mitochondrial pathways. Kidney Int. 2003, 64, 2020–2032. [Google Scholar] [CrossRef]
- Radak, Z.; Lee, K.; Choi, W.; Sunoo, S.; Kizaki, T.; Oh-Ishi, S.; Suzuki, K.; Taniguchi, N.; Ohno, H.; Asano, K. Oxidative stress induced by intermittent exposure at a simulated altitude of 4000 m decreases mitochondrial superoxide dismutase content in soleus muscle of rats. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 392–395. [Google Scholar] [CrossRef]
- de Groot, H.; Littauer, A. Hypoxia, reactive oxygen, and cell injury. Free Radic. Biol. Med. 1989, 6, 541–551. [Google Scholar] [CrossRef]
- AL-Hashem, F.H.; Shatoor, A.S.; Sakr, H.F.; Al–Daghri, N.; Khalil, M.; Alkhateeb, M. Co-administration of Vitamins E and C protects against stress-induced hepatorenal oxidative damage and effectively improves lipid profile at both low and high altitude. Afr. J. Biotechnol. 2012, 11, 10416–10423. [Google Scholar]
- Raff, H.; Hong, J.J.; Oaks, M.K.; Widmaier, E.P. Adrenocortical responses to ACTH in neonatal rats: Effect of hypoxia from birth on corticosterone, StAR, and PBR. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R78–R85. [Google Scholar] [CrossRef]
- McIntosh, L.J.; Hong, K.E.; Sapolsky, R.M. Glucocorticoids may alter antioxidant enzyme capacity in the brain: Baseline studies. Brain Res. 1998, 791, 209–214. [Google Scholar] [CrossRef]
- Bailey, D.M.; Davies, B. Acute mountain sickness; prophylactic benefits of antioxidant vitamin supplementation at high altitude. High Alt. Med. Biol. 2001, 2, 21–29. [Google Scholar] [CrossRef]
- Maiti, P.; Singh, S.B.; Sharma, A.K.; Muthuraju, S.; Banerjee, P.K.; Ilavazhagan, G. Hypobaric hypoxia induces oxidative stress in rat brain. Neurochem. Int. 2006, 49, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Kang, J.; Li, X.; Wang, M.; Shang, M.; Luo, Y.; Xiong, M.; Hu, K. Chronic intermittent hypoxia vs chronic continuous hypoxia: Effects on vascular endothelial function and myocardial contractility. Clin. Hemorheol. Microcirc. 2020, 74, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Hesse, C.; Dehnert, C.; Siedler, H.; Kleinbongard, P.; Bardenheuer, H.J.; Kelm, M.; Bärtsch, P.; Haefeli, W.E. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 2005, 172, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, M.H.; Neurath, M.F. Differential TNF-signaling in chronic inflammatory disorders. Curr. Mol. Med. 2004, 4, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Naldini, A.; Carraro, F.; Silvestri, S.; Bocci, V. Hypoxia affects cytokine production and proliferative responses by human peripheral mononuclear cells. J. Cell. Physiol. 1997, 173, 335–342. [Google Scholar] [CrossRef]
- El-Assal, O.N.; Yamanoi, A.; Soda, Y.; Yamaguchi, M.; Igarashi, M.; Yamamoto, A.; Nabika, T.; Nagasue, N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: Possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology 1998, 27, 1554–1562. [Google Scholar] [CrossRef]
- Mochida, S.; Ishikawa, K.; Inao, M.; Shibuya, M.; Fujiwara, K. Increased Expressions of Vascular Endothelial Growth Factor and Its Receptors, flt-1 andKDR/flk-1, in Regenerating Rat Liver. Biochem. Biophys. Res. Commun. 1996, 226, 176–179. [Google Scholar] [CrossRef]
- Orphanides, C.; Fine, L.G.; Norman, J.T. Hypoxia stimulates proximal tubular cell matrix production via a TGF-β1-independent mechanism. Kidney Int. 1997, 52, 637–647. [Google Scholar] [CrossRef]
- Corpechot, C.; Barbu, V.; Wendum, D.; Kinnman, N.; Rey, C.; Poupon, R.; Housset, C.; Rosmorduc, O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology 2002, 35, 1010–1021. [Google Scholar] [CrossRef]
- Liu, Y.V.; Hubbi, M.E.; Pan, F.; McDonald, K.R.; Mansharamani, M.; Cole, R.N.; Liu, J.O.; Semenza, G.L. Calcineurin promotes hypoxia-inducible factor 1α expression by dephosphorylating RACK1 and blocking RACK1 dimerization. J. Biol. Chem. 2007, 282, 37064–37073. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, X.; Lu, Q.; Zhang, Z.; Rao, J.; Le, A.D. Green tea extract and (−)-epigallocatechin-3-gallate inhibit hypoxia-and serum-induced HIF-1α protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol. Cancer Ther. 2006, 5, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Dzhalilova, D.S.; Kosyreva, A.M.; Diatroptov, M.E.; Ponomarenko, E.A.; Tsvetkov, I.S.; Zolotova, N.A.; Mkhitarov, V.A.; Khochanskiy, D.N.; Makarova, O.V. Dependence of the severity of the systemic inflammatory response on resistance to hypoxia in male Wistar rats. J. Inflamm. Res. 2019, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Kirova, Y.I.; Germanova, E.; Lukyanova, L. Phenotypic features of the dynamics of HIF-1α levels in rat neocortex in different hypoxia regimens. Bull. Exp. Biol. Med. 2013, 154, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Cimino, F.; Balestra, C.; Germonpre, P.; De Bels, D.; Tillmans, F.; Saija, A.; Speciale, A.; Virgili, F. Pulsed high oxygen induces a hypoxic-like response in human umbilical endothelial cells and in humans. J. Appl. Physiol. 2012, 113, 1684–1689. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, M.; Park, K.; Kim, T.-H.; Seol, D.-W. Blockade of processing/activation of caspase-3 by hypoxia. Biochem. Biophys. Res. Commun. 2008, 375, 684–688. [Google Scholar] [CrossRef]
- Pereira, E.R.; Frudd, K.; Awad, W.; Hendershot, L.M. Endoplasmic reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (HIF-1) transcriptional activity on targets like vascular endothelial growth factor (VEGF). J. Biol. Chem. 2014, 289, 3352–3364. [Google Scholar] [CrossRef]
- Tuder, R.M.; Flook, B.E.; Voelkel, N.F. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J. Clin. Investig. 1995, 95, 1798–1807. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, P.; Fukuda, R.; Kumar, G.; Krishnamachary, B.; Zeller, K.I.; Dang, C.V.; Semenza, G.L. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 2007, 11, 407–420. [Google Scholar] [CrossRef]
- Viollet, B.; Mounier, R.; Leclerc, J.; Yazigi, A.; Foretz, M.; Andreelli, F. Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 2007, 33, 395–402. [Google Scholar] [CrossRef]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of AMP-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J. Physiol. 2006, 574, 41–53. [Google Scholar] [CrossRef]
- Li, Z.-L.; Lv, L.-L.; Tang, T.-T.; Wang, B.; Feng, Y.; Zhou, L.-T.; Cao, J.-Y.; Tang, R.-N.; Wu, M.; Liu, H. HIF-1α inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019, 95, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, A.; Mai, S. c-MYC-induced genomic instability. Cold Spring Harb. Perspect. Med. 2014, 4, a014373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ni, J.; Li, J.; Huo, C.; Miao, N.; Yin, F.; Cheng, Q.; Xu, D.; Xie, H.; Chen, P. RIG-I aggravates interstitial fibrosis via c-Myc-mediated fibroblast activation in UUO mice. J. Mol. Med. 2020, 98, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Kasparova, D.; Neckar, J.; Dabrowska, L.; Novotny, J.; Mraz, J.; Kolar, F.; Zurmanova, J. Cardioprotective and nonprotective regimens of chronic hypoxia diversely affect the myocardial antioxidant systems. Physiol. Genom. 2015, 47, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Flora, R.; Zulkarnain, M.; Sorena, E.; Deva, I.; Widowati, W. Correlation between hypoxia inducible factor-1α and vesicular endothelial growth factor in male Wistar rat brain tissue after anaerobic exercise. Trends Med. Res. 2016, 11, 35–41. [Google Scholar] [CrossRef]
- Jusman, S.W.; Halim, A.; Wanandi, S.I.; Sadikin, M. Expression of hypoxia-inducible factor-1alpha (HIF-1alpha) related to oxidative stress in liver of rat-induced by systemic chronic normobaric hypoxia. Acta Med. Indones 2010, 42, 17–23. [Google Scholar]
- Hermes-Lima, M. Oxygen in biology and biochemistry: Role of free radicals. In Functional Metabolism: Regulation and Adaptation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 319–368. [Google Scholar]
- Cummins, E.P.; Taylor, C.T. Hypoxia-responsive transcription factors. Pflügers Arch. 2005, 450, 363–371. [Google Scholar] [CrossRef]
- Sawada, H.; Mitani, Y.; Maruyama, J.; Jiang, B.H.; Ikeyama, Y.; Dida, F.A.; Yamamoto, H.; Imanaka-Yoshida, K.; Shimpo, H.; Mizoguchi, A. A nuclear factor-κB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest 2007, 132, 1265–1274. [Google Scholar] [CrossRef]
- Putra, A.C.; Eguchi, H.; Lee, K.L.; Yamane, Y.; Gustine, E.; Isobe, T.; Nishiyama, M.; Hiyama, K.; Poellinger, L.; Tanimoto, K. The A Allele at rs13419896 of EPAS1 is associated with enhanced expression and poor prognosis for non-small cell lung cancer. PLoS ONE 2015, 10, e0134496. [Google Scholar] [CrossRef]
- Tashi, T.; Scott Reading, N.; Wuren, T.; Zhang, X.; Moore, L.G.; Hu, H.; Tang, F.; Shestakova, A.; Lorenzo, F.; Burjanivova, T. Gain-of-function EGLN1 prolyl hydroxylase (PHD2 D4E: C127S) in combination with EPAS1 (HIF-2α) polymorphism lowers hemoglobin concentration in Tibetan highlanders. J. Mol. Med. 2017, 95, 665–670. [Google Scholar] [CrossRef]
- Strassheim, D.; Karoor, V.; Stenmark, K.; Verin, A.; Gerasimovskaya, E. A current view of G protein-coupled receptor-mediated signaling in pulmonary hypertension: Finding opportunities for therapeutic intervention. Vessel. Plus 2018, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wu, W.; Deng, Z.; Zheng, X.; Zhang, J.; Deng, S.; Chen, J.; Ma, Q.; Wang, Y.; Yu, X. High altitude increases the expression of hypoxia-inducible factor 1α and inducible nitric oxide synthase with intest-inal mucosal barrier failure in rats. Int. J. Clin. Exp. Pathol. 2015, 8, 5189. [Google Scholar] [PubMed]
- Xie, H.; Hao, Y.; Yin, Q.; Li, W.B.; Lu, H.; Jia, Z.P.; Wang, R. Expression of plateau adaptation gene of rat tissues after plain acute exposure to high altitude. Zhejiang Da Xue Xue Bao Yi Xue Ban 2015, 44, 571–577. [Google Scholar] [PubMed]
- Mann, G.E. Nrf2-mediated redox signalling in vascular health and disease. Free Radic. Biol. Med. 2014, 75 (Suppl. 1), S1. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Gambelunghe, A.; Talesa, V.N.; Muzi, G. Reactive oxygen species induce apoptosis in bronchial epithelial BEAS-2B cells by inhibiting the antiglycation glyoxalase I defence: Involvement of superoxide anion, hydrogen peroxide and NF-κB. Apoptosis Int. J. Program. Cell Death 2014, 19, 102–116. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).