Abstract
The management of rheumatoid arthritis (RA) has undergone several practice-defining evolutions, beginning with the approval of low-dose methotrexate and continuing through the introduction of numerous disease-modifying antirheumatic drugs (DMARDs). With increasing capability to target pro-inflammatory pathways, successive therapeutics have carried the promise of improved disease control for patients with RA; however, many patients still fail to meet treatment objectives, leading to the recognition of clinical phenotypes that remain therapeutically challenging under the current treat-to-target standard of care, including preclinical inflammatory arthritis, late-onset RA, and treatment-resistant RA. Precision medicine approaches are beginning to characterize the pathogenesis of RA in such populations, and to inform effective tailoring of DMARD therapy to individual patients. Simultaneously, observational data derived from clinical practice are increasingly being used to understand the risks and benefits of long-term DMARD therapy under real-world conditions of use, with registries and other observational sources confirming long-term effectiveness, revising safety profiles, and estimating the costs of treatment for approved therapies. Together, these strategies offer opportunities to address unmet needs in the care of patients with RA. In this review of peer-reviewed clinical and translational research in RA, we identify several clinical phenotypes that demonstrate inadequate response to guideline-directed therapy and review frontiers in clinical research in RA emerging over the last decade, highlighting the use of precision medicine and real-world evidence-based approaches to advance individualized, patient-centered care.
1. Introduction
Rheumatoid arthritis (RA) is the most common type of autoimmune arthritis, with an estimated prevalence of up to 1% of the global population [1]. Since the approval of low-dose methotrexate for the treatment of RA in 1988 [2], numerous disease-modifying antirheumatic drugs (DMARDs) have been approved for use in RA in the United States, including conventional synthetic DMARDs (csDMARDs), targeted synthetic DMARDs (tsDMARDs), and biological DMARDs (bDMARDs). The American College of Rheumatology (ACR) recently updated guidelines for the management of RA in 2021 [3], and consensus goals for pharmacotherapy have prioritized the reduction in disease activity. However, despite sustained research in RA management, a substantial proportion of patients with RA still do not achieve low disease activity or remission and/or lack a durable response to DMARD therapy. Several clinical phenotypes have been described, most notably “difficult-to-treat” RA, for which ongoing research is aimed at improving therapeutic strategies. The objective of this review is to synthesize frontiers in clinical and translational research in RA, emphasizing findings with the potential to refine the assessment of disease state and the optimization of clinical management using existing pharmacologic strategies.
2. Current Treatment Paradigms in RA
2.1. Guideline-Directed Treatment
In the United States, the standard of care for pharmacologic management in RA is largely informed by the 2021 ACR guidelines, developed by a voting panel comprising clinicians and patients, which strongly recommend initiation and optimization of methotrexate monotherapy for DMARD-naive patients with moderate or greater disease activity. Escalation of therapy by adding or switching to bDMARDs (e.g., tumor necrosis factor-alpha [TNF-a] inhibitor, T cell costimulatory inhibitor, interleukin [IL]-6 inhibitor, or anti-CD20 antibody therapy) or tsDMARDs (i.e., Janus kinase [JAK] inhibitors) is considered if disease control is not achieved with methotrexate alone [3]. DMARD-naïve patients with low disease activity at diagnosis may alternatively be treated with hydroxychloroquine or sulfasalazine instead of methotrexate. TNF-a inhibitors are widely accepted as first-line bDMARDs, although approximate therapeutic equivalence has been demonstrated across classes of United States Food and Drug Administration (FDA)-approved bDMARDs and tsDMARDs [4,5,6]. Combination therapy with csDMARDs represents another therapeutic strategy, most commonly triple therapy with methotrexate (or leflunomide), hydroxychloroquine, and sulfasalazine [7]; given tradeoffs between benefits (e.g., lower costs and less risk of adverse events) and drawbacks (e.g., high pill burden, longer lag time to take effect, and poor retention) [8], guidelines conditionally recommend escalation to b/tsDMARD over triple therapy, in most cases prioritizing response to therapy as quickly as possible. Glucocorticoid avoidance is recommended for most patients.
International guidelines for RA treatment are similar to current ACR guidelines, with some key differences. Methotrexate is universally preferred as first-line csDMARD therapy for patients, although ACR permits alternative csDMARD treatment in cases of low disease activity, with hydroxychloroquine preferred [9]. In contrast to ACR guidelines, which recommend against the routine short-term or long-term use of glucocorticoids when initiating DMARD therapy, the European League of Associations for Rheumatology (EULAR) and Asia Pacific League of Associations for Rheumatology (APLAR) allow for the use of tapering glucocorticoids at the time of diagnosis, for up to 3 or 6 months, respectively, as a bridge to therapeutic efficacy after initiation of DMARD therapy [9]. Amongst all guidelines, subsequent treatment escalation, including to b/tsDMARD therapy, is informed not only by disease activity but also by considering patient preferences, comorbidity, and risk for adverse events, as well as clinician experience, local DMARD availability, and cost (Table 1).

Table 1.
Pharmacologic management of rheumatoid arthritis (RA) recommended by major international guidelines [9].
2.2. Treat-to-Target
Current guidelines recommend the adoption of a treat-to-target (T2T) strategy over usual care for RA management. This paradigm involves regular reassessment of disease activity with validated measures to guide adjustment and/or escalation of treatment, with the goal of achieving low disease activity or clinical remission [10]. Options for adjusting therapy include escalation of existing DMARD therapy (i.e., increasing dose or switching route of administration from oral to injection), combination therapy (i.e., adding DMARDs), or switching to a different class of DMARDs, with holistic reassessment of disease activity using composite measures of disease activity to drive treatment decisions. Although criteria for remission have been developed, an initial target of low disease activity may be preferred, as many patients may struggle to achieve strict targets due to comorbidities, treatment intolerance, or other patient-specific factors [11]. Overall, treatment goals should be individualized and based on clinical presentations as well as shared decision-making between patients and clinicians to ensure that the target is remission whenever feasible.
There is substantial evidence supporting the T2T approach, which demonstrates improved likelihood of remission, radiographic stability, and function and quality of life as compared to usual care [12]. Longitudinal analysis of patients with RA demonstrated an association of the T2T strategy with remission across disease activity indices [13]. Despite the proven benefits of T2T, adoption in real-world clinical settings is inconsistent. A systematic review of T2T literature has identified a variety of factors as potential barriers to uptake, including awareness of treatment goals, differences between clinician and patient perceptions of goal disease activity, perceived risks of escalating therapy, and resource constraints limiting possible DMARD choices [14]. Questions remain over what the optimal target is for individual patients [15], including preferred disease activity measures, or even who (i.e., clinicians or patients) is best positioned to evaluate longitudinal treatment outcomes [16]. Moreover, adherence is limited in real-world settings due to a variety of clinician, patient, and systemic factors [14,17], and optimal treatment targets have not been established [18]. Despite such implementation challenges, T2T remains the standard for care for RA.
3. Identifying Unmet Needs in the Management of RA
Despite the effectiveness of T2T as a treatment strategy for RA, many patients do not achieve remission in routine clinical practice [19], with a substantial proportion of patients having suboptimal responses to conventional guideline-directed therapies [20,21]. Several clinical phenotypes have been identified in which existing therapeutic strategies may be insufficient to fully address patient needs.
3.1. Treatment-Resistant RA
Recognition that a subset of patients fail to achieve treatment targets despite multiple lines of therapy led to the characterization of treatment-resistant RA phenotypes. Clinical heterogeneity among patients with treatment-resistant RA contributes to therapeutic and prognostic differences within this disease subpopulation (Table 2).

Table 2.
Defining treatment-refractory rheumatoid arthritis (RA).
There is growing recognition of the difficult-to-treat (D2T) RA concept, defined as follows: (1) failure of 2 or more bDMARDs or tsDMARDs after initial treatment with csDMARDs per guidelines, (2) signs suggesting active or progressive disease, and (3) management of signs and symptoms considered problematic by the patient and treating clinician, such as intolerable side effects of treatment or complications of chronic glucocorticoid use [24]. Prevalence estimates for D2T RA vary significantly, with a recent review suggesting between 5.5% and 27.5% of patients may meet these criteria [25]. Numerous clinical features have been associated with D2T status, including younger age at diagnosis, female sex, seropositivity, and delayed treatment initiation [22]. Patients who progress to D2T status are more likely to have had high disease activity and radiographic damage at baseline [26,27]. Comorbidity relating to persistent inflammatory state, extra-articular disease, and the effects of long-term immunosuppressive therapy have also been described in D2T cohorts [28]. Pain and fatigue syndromes, including fibromyalgia, are more common amongst patients with D2T disease and are posited to contribute to suboptimal treatment outcomes [29]. DMARD failure in D2T RA may reflect some or all these elements, with a combination of biological processes (e.g., accumulated joint damage, immunogenicity, and inflammatory drivers such as obesity) and psychosocial factors (e.g., treatment adherence, comorbid anxiety and depression, limited coping with pain and disability) contributing to suboptimal treatment outcomes [30].
In addition, patients with treatment-resistant RA can be broadly characterized by an inflammatory state. Patients with persistent inflammatory refractory RA (PIRRA), despite multiple lines of therapy, may continue to present with polyarthritis or may resolve to one or a few joints with refractory synovitis [23]. In contrast, non-inflammatory refractory RA (NIRRA) is characterized by persistent arthralgia despite low swollen joint counts and inflammatory markers and is associated with coincident obesity and fibromyalgia [31]. The PIRRA and NIRRA phenotypes underscore the spectrum of potential causes underlying treatment failure in RA, ranging from refractory inflammation to accumulated damage associated with progressive arthritis to non-inflammatory comorbidities contributing to chronic pain. Accordingly, differentiation of patients by inflammatory state can inform goals for treatment, such as the use of intra-articular steroids or synovectomy referral for persistent synovitis in a solitary joint after DMARD therapy, or prioritization of the treatment of comorbidities in the absence of overt inflammation.
3.2. Late-Onset RA
Late-onset RA (LORA) refers to patients developing new inflammatory arthritis after the age of 60 years, with several distinctions between LORA and conventional presentations of RA, such as acute onset of symptoms, approximately equal sex distribution, and more frequent involvement of large proximal joints (e.g., shoulders and knees) [32]. Presentations with prominent bursal involvement resembling polymyalgia rheumatica, or related remitting seronegative symmetrical synovitis with pitting edema (RS3PE), have also been described [33]. Patients with LORA are less likely to be seropositive than younger patients [34]. Comorbid musculoskeletal conditions, including osteoarthritis and osteoporosis, are common and may confound assessment.
Treatment of LORA requires consideration of aging-related comorbidities that may contribute to increased treatment toxicity. Although response rates are comparable to patients with earlier-onset disease [35], LORA is associated with a greater incidence of adverse events during treatment, including declining physical function [36]. For patients aged 75 years and older, targeting low-disease activity may be more appropriate than remission, as it is imperative to balance the risks and benefits of treatment escalation in this population [37].
3.3. Preclinical and Early RA
Over time, increasing attention has been focused on identifying patients at risk of developing RA and developing preventative strategies for those with preclinical or early disease. The concept of preclinical RA represents the period of time during which patients begin to develop circulating rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs) that precede the development of inflammatory arthritis [38], with titers rising alongside increased systemic inflammation and early joint symptoms immediately prior to the onset of sustained synovitis consistent with diagnosis [39]. Although genetic risk for RA may be conferred by factors such as the HLA-DRB1 shared epitope, several modifiable risk factors have also been associated with the development of these pathogenic antibodies, including cigarette smoking, occupational exposures, obesity, and vitamin D deficiency [40]. Microbiome dysregulation, in particular overrepresentation of P. gingivalis and associated periodontitis, has also been identified as a risk factor for progression to RA in ACPA-positive individuals [41,42]. Other implicated microorganisms, including P. copri, S. didolesgii, and S. parasanguinis, contribute to RA pathogenesis through a variety of mechanisms, including the production of citrullinated or RA-mimicking (i.e., presented on HLA molecules containing the shared epitope) antigens, as well as induction of metabolic changes contributing to increased inflammation [43]. Of note, while genetic and environmental factors are predictive of the risk of developing RA, and in some cases of future disease severity, their utility for guiding preventative efforts or selection of DMARD therapy has not been clarified [44,45,46].
The association between palindromic rheumatism (PR) and RA has offered potential insights into the pathogenesis of RA. PR is an episodic arthritis characterized by flares of inflammatory arthritis that may last for days, resolving without incurring irreversible joint damage. Similarities in autoantibody profiles and patterns of joint involvement have been noted in PR and RA [47], with imaging studies that suggest that PR flares are characterized by increased extracapsular inflammation, as opposed to the synovitis characteristic of RA [48]. Although the relationship of PR and RA remains unclear, historical reports note a high (>50%) rate of progression to RA, and PR is sometimes considered to exist on the spectrum of seropositive arthralgia with other precursors to clinical RA [47].
Numerous therapies have been evaluated for use in RA prevention, although the results have been inconsistent. A systematic review of clinical studies evaluating prevention strategies in individuals at high risk of developing RA, or with undifferentiated inflammatory arthritis, did not identify any DMARD-based strategies that successfully prevented the onset of disease, although treatment with abatacept or rituximab was associated with a delayed onset of RA by up to 18 months [49]. While early treatment with methotrexate does not prevent progression to clinical arthritis, long-term outcomes with regard to inflammation and functional status were improved [50]. Trials of glucocorticoids and other csDMARDs such as hydroxychloroquine have been less successful [51]. Lifestyle interventions, including physical activity, stress reduction, and avoidance of environmental irritants (e.g., cigarette smoke or occupational exposures), are posited to reduce the risk of developing RA, although most evidence is derived from observational sources with limited ability to determine causality [52]. While RA prevention strategies have generally failed to prevent the onset of disease thus far, data show promise for modifying disease trajectory, suggesting potential utility for specific cohorts such as patients at risk for developing severe disease.
4. RA Research Frontiers
Improved understanding of clinical phenotypes with suboptimal response to existing therapeutic strategies has fueled interest in developing precision medicine approaches to RA management. Molecular insights into the pathogenesis of the disease, as well as evidence drawn from large cohorts and other sources of real-world data, can aid in tailoring DMARD therapy at treatment initiation and/or at junctions when treatment changes are being considered (Figure 1).
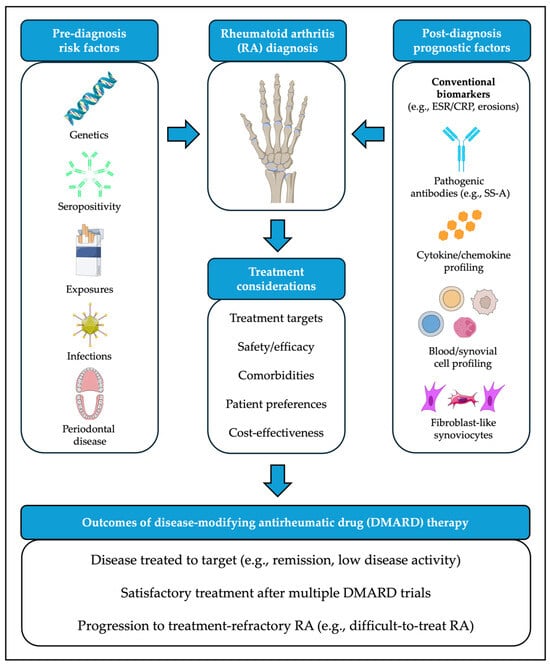
Figure 1.
Clinical contributors to pathogenesis and treatment outcomes for rheumatoid arthritis (RA). Images adapted from bioicons (https://bioicons.com/, accessed on 20 January 2026), licensed under CC BY 3.0 (https://creativecommons.org/licenses/by/3.0/, accessed on 20 January 2026): “cigarette”, “teeth-gum-adult”, “fibroblast-4”, “adeno-virus” icons by Servier) or CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/, accessed on 20 January 2026): “Posterior_view_of_bones_of_right_hand”, “B-DNA” icons by DBCLS; “Rubisco” icon by Margot Riggi; “B-cell-1”, “B-cell-4”, “cancer_cell” icons by El-Jayawant; “epithelial_cell”, “neutrophil”, “immuneglobuline_igG_blue”, “immuneglobuline_igG_green”, icons by Helicase 11).
4.1. Biomarkers
Biomarkers, a term encompassing a variety of entities derived from blood or other tissues usable to study biological processes, have long been utilized in the diagnosis and clinical evaluation of RA. Traditional laboratory evaluation in RA includes measurement of acute phase reactants (e.g., C-reactive protein, CRP, and erythrocyte sedimentation rate, ESR) for assessment of active inflammation, as well as characteristic autoantibodies (i.e., RF and ACPA) conventionally defining seropositivity. The history of RF and ACPA testing has been reviewed extensively in the literature. Summarized briefly, these autoantibodies, respectively, directed against the Fc region of immunoglobulin G and citrullinated proteins, both serve as useful biomarkers in the management of RA; ACPAs are commonly observed in early disease, and both are associated with higher disease activity, with RF predictive of extraarticular disease and ACPA commonly associated with more severe erosive arthritis (and, conversely, improved response to DMARD therapy) [53]. While not necessary for RA diagnosis, both autoantibodies, when present, can aid in risk stratification among patients. Only recently have additional biomarkers offered opportunities to further characterize individual patients and to inform the selection of therapy. While numerous RA biomarkers remain under varying stages of development [54], we have highlighted several that have shown promise for clinical implementation.
4.1.1. Autoantibodies
In addition to RF and ACPAs, patients with RA may present with additional autoantibodies against proteins that have undergone non-citrullination post-translational modifications, including anti-carbamylated protein, anti-acetylated protein, and malondialdehyde-acetaldehyde antibodies observed in early clinical disease [55]. Antibodies against native proteins, including PTX3, DUSP11, and PAD4, have also been identified [56]. These more recently described autoantibodies frequently correlate with the development of conventional ACPAs and can be associated with radiographic progression. Although highly specific, they are less sensitive and not yet widely adopted in routine clinical practice.
Further serological testing may also enhance patient assessment. Most notably, while up to 7.5% of patients with RA demonstrate clinical evidence of co-occurring (“secondary”) Sjogren’s disease, rates of positivity for antibodies to SS-A are substantially higher, being noted in up to 15% of patients [57]. Secondary Sjogren’s disease is associated with higher RA disease activity [58,59,60] and is more commonly identified amongst patients with D2T-RA [61]. Anti-SS-A antibodies, even in the absence of sicca or other Sjogren’s disease manifestations, are associated with reduced response to methotrexate and TNF inhibitors [62,63], highlighting the potential need for alternative first-line DMARDs. Specifically, hydroxychloroquine and rituximab, both commonly used in the management of primary Sjogren’s disease, may offer theoretical benefit for such patients [57,58,59], although evidence from long-term studies in RA patients with positive anti-SS-A antibodies without overt Sjogren’s disease is lacking. Small studies have also shown beneficial effects of tocilizumab and abatacept for patients with RA with positive anti-SS-A antibodies not responding to TNF inhibition [58,64], with the latter also ameliorating symptoms of secondary Sjogren’s disease [65]. Regarding JAK inhibitors, the known association between Sjogren’s disease and lymphoma suggests careful consideration must be taken when selecting therapies to minimize malignancy risk. In the clinical development program for tofacitinib, incident lymphoma was observed amongst participants, with numerically greater rates of concomitant Sjogren’s disease in those developing malignancy, underscoring the need for careful monitoring in this population [66]. Additional cardiovascular comorbidities and serologies (e.g., antiphospholipid antibodies) are not only associated with an increased risk of treatment complications but may also give pause to consideration for treatment with JAK inhibitors.
While the presence of anti-SS-A antibodies in RA patients may aid prognostication and inform early treatment with non-TNF bDMARDs, their utility as a biomarker for RA remains incompletely understood. Although testing is readily available in the US and other developed nations, global differences in accessibility and cost are likely to persist. The interpretation of results also requires nuance; there are no established standards for the interpretation of titers, with major international RA guidelines not addressing the clinical relevance of anti-SS-A antibodies, and seropositivity does not differentiate between patients with and without secondary Sjogren’s disease or other overlapping connective tissue diseases. Finally, the relatively low prevalence of anti-SS-A antibodies in the overall RA population suggests that serostatus is unlikely to completely capture patients at risk of severe disease or failure of traditional treatment paradigms, with associations between seropositivity and other prognostic factors, such as D2T status, remaining incompletely understood. Further research is required to fully clarify the significance of anti-SS-A antibody testing for the clinical management of RA.
4.1.2. Cytokine Profiles
Deconvolution of convergent pathways in the RA inflammatory cascade offers another approach to guide treatment decisions, with TNF-a, IL-1, IL-6, and other pro-inflammatory cytokines well-described as key drivers in the pathogenesis of RA [67]. Novel disease activity scores derived from cytokine profiling correlate with conventional measures and offer a more nuanced insight into inflammation than ESR or CRP [68]. Despite the increased sensitivity of cytokine-based testing, its utility in disease management for individual patients is poorly understood. For example, treatment with methotrexate is associated with reductions in TNF-a, IL-17, and IFN gamma [69], but bDMARD treatment results in less consistent cytokine responses [70]. Discovering how to target dominant pro-inflammatory pathways remains a goal of cytokine-based molecular phenotyping, especially for patients with D2T. For a subset of patients, profiling may also support combination therapy; in small studies, the addition of rituximab or anti-IL-17 to TNF inhibitor therapy showed promise as a therapeutic strategy for treatment-resistant patients, although the safety of dual bDMARDs requires further investigation [71]. In the future, cytokine profiling may offer clinicians additional insight into selecting between approved therapeutic mechanisms, with serial testing providing early evidence of secondary treatment failure to anticipate the need to change therapy.
4.2. Cellular Profiling
Characterization of cell types driving RA pathogenesis has focused on the synovium and peripheral blood, providing insights into disease mechanisms and potential differences in therapeutic response.
4.2.1. Synovium
Translational research indicates that features of the synovial microenvironment may predict disease trajectory. Spatial transcriptomics have revealed tissue-resident macrophages expressing the cell surface receptor LYVE1 as critical to synovial homeostasis; these macrophages are lost in early RA, but successful treatment with csDMARDs is associated with restoration of macrophage networks [72]. Their role in more advanced disease remains unknown. In addition to macrophages, the synovial cell repertoire includes substantial populations of T cells, fibroblasts, and myeloid cells, among which increased T cells, in conjunction with fibroblasts, are most commonly associated with seropositivity and higher baseline disease activity [73]. The composition of synovial cell populations varies independently of patient factors such as age and sex but is correlated with current therapy, suggesting DMARDs may influence the synovial microenvironment. By describing cellular changes developing in the setting of therapy, such analyses suggest explanations for primary and secondary treatment failure and potential therapeutic avenues based on dominant synovial phenotypes after initial treatment [74]. Through gene expression and histological analyses, four synovial phenotypes for RA have been characterized, each with distinct clinical implications. Patients with the myeloid phenotype were more likely to respond to TNF inhibitor therapy, whereas those with the lymphoid phenotype had better responses to tocilizumab; low inflammatory (pauci-immune) and fibroid phenotypes, characterized by minimal or low evidence of pro-inflammatory pathway activation, tended to demonstrate lower response rates to DMARD therapy [75]. Fibroblast-like synoviocytes (FLS) are recognized as a key driver in chronic inflammation in RA and are often associated with seronegativity and D2T status; while there is interest in developing FLS-targeted medications, such therapeutic strategies remain an unmet need [76].
4.2.2. Peripheral Blood
The accessibility of circulating immune cells has also facilitated investigation into their roles in systemic inflammation. Immunophenotyping of peripheral blood cells offers an alternative to clinical phenotyping or classical serologies to classify patients with RA. Notably, clustering patients by T cell and B cell abundance yields distinct clusters predicting differential responses to DMARD classes; treatment with the putative bDMARD or tsDMARD of choice is expected to provide the most beneficial results, including higher rates of low disease activity or remission than other agents [77]. Other studies have further characterized T cells in RA, with seropositive patients demonstrating increased dysregulation of Th17 and regulatory T (Treg) cells as compared to seronegative patients, a pathophysiologic process potentially mediated by IL-4 and associated with increased systemic inflammation [78]. T cell subsetting is under investigation for potential use in predicting response to specific therapies such as tocilizumab and abatacept [79]. In parallel, prognostic and therapeutic roles of autoreactive B cells [80,81], NK cells [82], circulating macrophages [83], and additional cell types continue to emerge. Finally, clonal hematopoiesis is increasingly recognized as contributing to immune dysregulation, not only in aging but also in RA. A large Finnish cohort study identified unique clonal pathways for seropositive patients, characterized by DNMT3A mutations associated with higher disease activity, as well as seronegative patients, who have an increased presence of TET2 mutations thought to be implicated in pro-inflammatory signaling via IL-1β [84]. Despite observed associations, the mechanistic contribution of clonal hematopoiesis to systemic inflammation in RA remains incompletely clarified.
4.2.3. Chemokines and Other Proteins
Increased expression of the B cell signaling molecule CXCL13 has been observed in patients with early RA, regardless of seropositivity, who do not respond to methotrexate, suggesting that earlier treatment with bDMARD or tsDMARD therapy may be warranted [85]. Likewise, upregulation of the vascular homeostasis protein ANGPTL4 has also been identified as a moderator of erosive disease via TNF-related pathways [86] and is being explored as a therapeutic target. However, not all efforts have led to the development of novel therapies. CCR5, a chemokine receptor expressed by synoviocytes, has been implicated in pro-inflammatory pathways via the activation of Th1 cells [87], but antagonizing therapeutic antibodies failed to demonstrate benefit [88]. To date, hundreds of putative biomarkers have been described, although few have defined roles in the evaluation or management of RA [89]. As new molecular signatures are identified, their utility as RA biomarkers or therapeutic targets must be clarified prior to clinical uptake.
4.3. Personalized DMARD Selection
A corollary of precision DMARD selection is the likelihood that patients may be exposed to therapeutics at earlier stages of disease, in novel combinations, and for prolonged periods of time. Currently, rates of DMARD discontinuation or switches are high, with median retention times of 24 to 36 months or less across DMARDs in most cohorts [90,91,92,93]. As we approach 30 years since the FDA approved the first bDMARD (etanercept, in 1998), clinicians will increasingly face decisions regarding treatment strategies for patients who have been exposed to decades of immunosuppressive therapy (Table 3). In addition to therapeutic safety monitoring, understanding the real-world effectiveness of b/tsDMARDs is essential to pharmacologic selection over long disease trajectories.

Table 3.
Pharmacotherapy for the management of rheumatoid arthritis in the United States.
4.3.1. Real-World Safety of Biologic and Targeted Synthetic DMARDs
Clinicians seeking to escalate DMARD therapy beyond methotrexate must navigate safety profiles arising from randomized trials, observational studies, analyses derived from administrative claims data, and other sources. Over time, large registries and population-based studies have refined assessments of b/tsDMARD safety, with several themes arising under real-world conditions.
Although a serious consideration, infection risk is comparable for most approved RA therapies, with fewer than 10 serious infections per 100 treatment years [95,96]. For TNF inhibitor therapies, risk appears to decline after the initial 6–12 months of treatment [97,98], a pattern that has been extrapolated for other therapeutic mechanisms, including rituximab [99]. While close monitoring for infection is warranted, evidence to date does not suggest that infection risk precludes long-term treatment for most patients with RA.
Malignancy remains a concern for patients exposed to prolonged immunosuppression, especially when increased systemic inflammation and advancing age are considered as additional risk factors. Population-based studies have reassuringly demonstrated no increased risk of malignancy overall for long-term TNF inhibition, anti-IL-6, or anti-CD20 therapy as compared to b/tsDMARD-naïve patients or age-matched controls [100]. Given the biological role of CTLA4, concern persists over abatacept’s effects on anti-tumoral T cell immunity; in a recent meta-analysis, pooled data from observational studies supported increased risk of malignancy for abatacept [101], although postmarketing safety data reveals no greater risk of malignancy for abatacept compared to other therapeutic mechanisms [102]. Studies on JAK inhibitors have inconsistently associated use with increased risk of malignancy [103], notably non-melanoma skin cancer [104], and decreased risk of other cancers, including those of GI origin [105]. Given persistent uncertainty over risk, cautious use is recommended, with careful surveillance for evidence of malignancy.
Real-world data also informs estimates of risk for characteristic adverse events first observed in the development of b/tsDMARDs. TNF inhibitor therapies remain contraindicated for patients with symptomatic heart failure [106], as well as for those who have or are at risk of developing multiple sclerosis [107,108], but are otherwise comparatively safe [109]. Although abatacept was initially suspected to exacerbate chronic obstructive pulmonary disease (COPD), this signal has not been observed in analyses of administrative data [110]. IL-6 inhibitors increase lipid levels, but their use has not been associated with increased risk of cardiovascular disease as compared to tsDMARDs [111,112]. Previous diverticulitis, on the other hand, remains a contraindication, with higher intestinal perforation rates than any other DMARD class corroborated in a large RA registry [113]. Despite findings in postapproval clinical trials that JAK inhibitors are associated with an increased risk of major adverse cardiovascular events (MACE) and venous thromboembolism (VTE) [114], only a class-wide increased risk of VTE has been consistently identified in subsequent analyses [115]. Notably, rates of MACE have proven comparable to those of TNF inhibitors [116], even for patients with a history of cardiovascular disease [117,118], raising uncertainty over the contribution of DMARD therapy to risk independent of that conferred by the hyperinflammatory state of RA, a risk that is only partially abrogated by effective treatment. Finally, other than rare reports of progressive multifocal leukoencephalopathy associated with rituximab, estimated to occur at a rate of 2.6 cases per 100,000 treated patients with RA [119], long-term use is generally well tolerated and is not associated with increased risk of infection [120,121].
An important caveat to the safety of b/tsDMARDs must be reserved for elderly patients with RA, including the LORA subpopulation. Major international guidelines do not differentiate between elderly and non-elderly patients with regard to treatment initiation or goals, including T2T [3,10]. However, patients with LORA are less likely to be started on DMARD therapy, with an analysis of Medicare beneficiaries estimating less than one-third of patients newly diagnosed with RA receive a DMARD [122]. Elderly patients receiving DMARD therapy are likely at greater risk of infection, as well as herpes zoster reactivation, compared to younger patients; other adverse events, including malignancies and cardiovascular disease, have been inconsistently demonstrated across studies [123]. Comorbidities associated with advancing age, such as renal insufficiency or cardiovascular disease, complicate therapy planning via narrowed therapeutic windows and perceived risk of adverse events, while concerns over adherence to complex regimens by elderly patients with mechanical limitations or cognitive impairment also contribute to prescriber hesitancy [124]. Patient perceptions of disease burden and expected benefits of treatment are likely to contribute to under-treatment as well, based on the de-prioritization of RA treatment as compared to other comorbidities by many elderly patients [125]. Despite perceived risks, elderly patients can be effectively and longitudinally treated with DMARDs of all classes [126,127], suggesting that safety concerns should not preclude treatment and rather can be considered on a personalized basis.
Although special considerations may be required for at-risk populations, real-world analyses have largely confirmed the tolerability of b/tsDMARDs. However, clinicians must weigh individual patient factors against continuously evolving clinical data when making treatment decisions.
4.3.2. Anticipating Clinical Trajectories for Patients Treated with b/tsDMARDs
As conventional clinical trials only provide information on the outcomes of treatment over relatively short time periods, additional sources of clinical evidence are critical to understanding responses to treatment over intervals that more accurately reflect the natural course of RA. Clinical data derived from multiple observational sources now provide opportunities to evaluate b/tsDMARD performance in real-world settings.
Rheumatology registries have existed since the mid-twentieth century, yielding invaluable insights into the epidemiology of RA, comorbidities, and treatment outcomes [128]. Their presence at the forefront of computerized health information systems has allowed registries to capture increasingly granular data, facilitating therapeutic assessment via target trials and other novel designs. Registry-based research efforts continue globally to this day. For example, the “JAK-pot” collaboration leverages data from tens of thousands of b/tsDMARD treatment courses to explore the safety and comparative effectiveness of JAK inhibitors across clinical settings [129,130], including for patients with D2T disease. Although registry analyses require careful consideration of the generalizability of findings beyond enrolled populations, these curated sources of real-world data can provide insights into the selection and sequencing of DMARD therapy [131].
In addition to registries, large-scale observational studies increasingly answer clinical questions in RA, driven by the availability of clinical documentation and expanded testing for secondary analyses [132]. Efforts such as the Rheumatoid Arthritis Real-world Cohort Study in China (ReALSA) aim to collect data on patients longitudinally, correlating clinical, laboratory, imaging, and pathological data to facilitate evolving analyses of real-world patients with RA [133]. Observational studies can also support therapeutic strategies, such as improved response rates with combined bDMARDs and csDMARDs [134], although few studies have demonstrated a consistent superior benefit of one therapeutic strategy over others [135]. In one notable exception, the Kyoto University Rheumatoid Arthritis Management Alliance (KURAMA) cohort documented improvement in functional outcomes associated with increasing b/tsDMARD use over 10 years of follow-up; researchers observed more rapid improvement in disease activity with TNF inhibitors, but greater sustained responses to IL-6 inhibitors, addressing a knowledge gap left by the absence of comparative efficacy trials with the potential to inform clinical decision-making [136]. The development of biobanks can extend the reach of such studies, enabling reanalysis of biological specimens in light of future technologies and validating translational insights into RA pathophysiology [73,137].
4.3.3. Outcome Measures
As observational studies have propagated, standardization of rheumatology outcomes assessments spurred by groups including ACR and the International Consortium for Health Outcomes Measurement (ICHOM) can facilitate meta-analysis of clinical outcomes across practice settings [138,139]. Others, including Outcome Measures in Rheumatology (OMERACT), have sought to describe and standardize outcome measures used in observational studies directly [140]. Given the marked heterogeneity of RA, no consensus has been reached on ideal outcome measures for the evaluation of RA, although the Clinical and Simplified Disease Activity Indices (CDAI/SDAI) and Disease Activity Score-28 (DAS28) are commonly used. Patient-reported outcome measures such as the Health Assessment Questionnaire (HAQ), Patient-Reported Outcomes Measurement Information System (PROMIS) score, and others also inform assessment by providing patient perspectives on function and disability [139]. As observational sources of data continue to develop, standardized outcome assessments are necessary to extend clinical trial findings and inform RA management beyond conventional drug development timelines.
4.3.4. Cost-Effectiveness
Cost-effectiveness represents another means of informing DMARD selection. Systematic reviews of cost-effectiveness for RA management have identified substantial heterogeneity among analyses, including in which DMARDs are included in comparisons, methodology for defining effective care in observational data sources, and thresholds for cost-effectiveness [141]. However, it can be generally stated that b/tsDMARD therapies cost more than csDMARD-based management strategies, with costs associated with one year of effective treatment frequently eclipsing USD 100,000 in the United States [142]. Specific cost estimates for DMARDs vary within and between countries based on complex factors, including regional differences in availability and market competition, insurance coverage and reimbursement structures for specialty medications, and, where applicable, national health technology assessments that inform reimbursement by payers. Accordingly, studies have drawn differing conclusions about the use of b/tsDMARDs in various practice settings [143,144,145,146]. Limited evidence supports the cost-effectiveness of b/tsDMARDs in the United States as compared to csDMARDs [147], with lower costs associated with switching between classes as compared to trialing multiple agents in the same class (conventionally, TNF inhibitors) [148]. Earlier treatment with JAK inhibitors has been specifically identified, in one study, as a cost-effective strategy [149]. Of note, cost-effectiveness is not a static concept, as drug prices change over time. The advent of biosimilars for many bDMARDs has begun to reduce costs associated with the treatment of RA [150,151], with further cost savings likely as barriers to biosimilar prescribing and dispensing are eliminated [152]. Although cost-effectiveness may influence coverage decisions for b/tsDMARDs, uncertainty over the relative cost-effectiveness of various therapies suggests that clinical decisions are likely to rely primarily on individual patient factors for the foreseeable future.
4.3.5. Shared Decision Making
While significant emphasis has been placed on translational breakthroughs in the management of RA, research findings should be viewed in terms of their potential to support shared decision-making (SDM) between patients and clinicians. One of the overarching principles of RA management under the T2T framework is the prioritization of patient preference in treatment decisions through SDM [10]. Despite such recommendations, SDM frequently falls short in real-world practice [153]. Factors associated with poor SDM among patients with RA include low health literacy, limited English proficiency, older age, and lower trust in physicians [154], suggesting potential benefits for interventions reducing barriers to patients’ understanding treatment options and advocating for personal care preferences.
There is no single approach to SDM for the management of RA, although groups such as OMERACT have articulated ideals for SDM, including means of assessing the impact of interventions [155]. Within the field of rheumatology, SDM tools are most frequently created for use in RA [156], with examples including clinician education modules, decision aids, and structured dialog intended to elicit patient questions and values that inform preferences for treatment (Table 4) [156,157]. However, uptake remains inconsistent in rheumatology practice, due to factors including a lack of validation across practice settings, as well as time constraints in clinical care. Nonetheless, there is substantial ongoing research into new SDM approaches, with multimodal strategies to improve health literacy and elicit care preferences under investigation [158]. In the absence of a consensus approach, management decisions should be made with respect for patients’ expectations for treatment, risk tolerance for adverse events, medication burden, willingness to escalate or change therapy, and other personalized considerations. SDM should continue to be prioritized under precision medicine approaches, with clinicians providing decision support to patients by helping interpret the increasingly granular data available at the point of care. This approach ensures that care is truly individualized and that personal health information is used to inform patient choices, rather than preempt them.

Table 4.
Example shared decision-making (SDM) tools developed for use in patients with rheumatoid arthritis (RA) [157].
5. Conclusions
In this review, we discuss several unmet needs in RA management and recent developments in precision medicine approaches to RA. Advances in clinical and translational RA research can be understood along several pathways; improved description of RA phenotypes and an expanding body of evidence supporting the safety and effectiveness of approved DMARDs are converging to guide clinicians in the selection of personalized treatment regimens. Further work is needed to develop effective strategies across the clinical spectrum, including prevention in individuals at risk of developing RA and management of treatment-resistant RA, to integrate novel biomarkers into routine practice, and to inform novel uses of DMARDs such as dual biologic therapy. The intersection of such research objectives is in and of itself a priority of translational research in RA, as new technologies capable of anticipating the trajectory of disease offer opportunities to intervene with therapies tailored to individual patients, with the goal of preventing onset, reducing pain or the accrual of joint damage, and minimizing the complications of DMARD therapy. Where current strategies may not sufficiently control RA for all patients, emerging technologies such as neuromodulation, transdermal drug delivery systems, nanomedicines, cellular therapy, and others offer promise. Moreover, the importance of non-pharmacologic interventions, including weight loss, smoking cessation, and ensuring oral health, should not be deemphasized as new technologies and pharmacologic strategies enter routine clinical practice.
There are several limitations to the present work that warrant consideration. This review focuses on research addressing unmet needs in the pharmacologic management of RA, with an emphasis on optimizing the use of existing therapies for the management. While we have highlighted numerous approaches to clinical and biochemical phenotyping, it is not possible to provide a comprehensive historical overview of clinical and translational research findings in RA, nor to address every research finding with potential translational value for clinical management. Of note, uptake of new technologies is likely to differ across clinical settings, based on factors including regulatory approval, local expertise, and cost, such that their true impact on RA management cannot be predicted from preliminary feasibility assessments. Moreover, we do not specifically address novel therapeutic mechanisms for which no pharmacologic agents are currently approved. As of January 2026, there are more than 500 pending or ongoing clinical trials in RA registered on ClinicalTrials.gov, suggesting the scope of ongoing clinical development for the disease [159]. In addition, we did not include discussion of non-pharmacologic management for RA, including vagus nerve stimulation, which was notably granted FDA approval in 2025. Such interventions may offer benefits beyond those of conventional pharmacological management but are unfortunately beyond the scope of this review. Finally, it should be acknowledged that real-world data must be interpreted within the context of the studies, registries, and populations from which it is derived; the translation of safety and effectiveness findings between clinical practice settings and between observational and interventional sources of clinical evidence remains largely unvalidated. Of note, there is no central listing of global RA registries, nor a consensus standard for data collection, limiting our ability to meta-analyze findings or comprehensively assess the strengths and limitations of registries as a source of clinical evidence in RA. This non-systematic review should be viewed as an overview of emerging research of clinical interest, rather than as an exhaustive taxonomy of clinical and translational research in RA.
As we mark 75 years since the Nobel Prize was awarded for the discovery of cortisone, first used for the treatment of RA, advances in biomedical science continue to offer promise for individuals living with RA whose needs are not fully met by the standard of care.
Author Contributions
Conceptualization, J.J.S. and B.H.; methodology, J.J.S. and B.H.; writing—original draft preparation, J.J.S.; writing—review and editing, J.J.S. and B.H.; supervision, B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RA | Rheumatoid arthritis |
| DMARD | Disease-modifying antirheumatic drug |
| ACR | American College of Rheumatology |
| TNF-a | Tumor necrosis factor-alpha |
| IL | Interleukin |
| CD20 | Cluster of differentiation 20 |
| JAK | Janus kinase |
| FDA | (United States) Food and Drug Administration |
| EULAR | European Association of Alliances for Rheumatology (formerly European League Against Rheumatism) |
| APLAR | Asia-Pacific League of Associations for Rheumatology |
| T2T | Treat-to-target |
| D2T | Difficult-to-treat |
| PIRRA | Persistent inflammatory refractory rheumatoid arthritis |
| NIRRA | Non-inflammatory refractory rheumatoid arthritis |
| LORA | Late-onset rheumatoid arthritis |
| RS3PE | Remitting seronegative symmetrical synovitis with pitting edema |
| RF | Rheumatoid factor |
| ACPA | Anti-citrullinated protein antibody |
| HLA | Human leukocyte antigen |
| PR | Palindromic rheumatism |
| PTX3 | Pentraxin 3 |
| DUSP11 | Dual specificity phosphatase 11 |
| PAD4 | Peptidyl arginine deiminase type 4 |
| SS-A | Sjögren syndrome-related antigen A |
| CRP | C-reactive protein |
| ESR | Erythrocyte sedimentation rate |
| IFN | Interferon |
| LYVE1 | Lymphatic vessel endothelial hyaluronan receptor 1 |
| FLS | Fibroblast-like synoviocyte |
| NK | Natural killer |
| DNMT3 | DNA methyltransferase 3 |
| TET2 | Tet methylcytosine dioxygenase 2 |
| CXCL13 | C-X-C motif chemokine ligand 13 |
| ANGPTL4 | Angiopoietin-like 4 |
| CCR5 | C-C chemokine receptor type 5 |
| CTLA4 | Cytotoxic T-lymphocyte-associated protein 4 |
| GI | Gastrointestinal |
| COPD | Chronic obstructive pulmonary disease |
| MACE | Major adverse cardiovascular events |
| VTE | Venous thromboembolism |
| ReALSA | Rheumatoid Arthritis Real-world Cohort Study in China |
| KURAMA | Kyoto University Rheumatoid Arthritis Management Alliance |
| ICHOM | International Consortium for Health Outcomes Measurement |
| OMERACT | Outcome Measures in Rheumatology |
| CDAI | Clinical Disease Activity Index |
| SDAI | Simplified Disease Activity Index |
| DAS28 | Disease Activity Score-28 |
| HAQ | Health Assessment Questionnaire |
| PROMIS | Patient-Reported Outcomes Measurement Information System |
| SDM | Shared decision-making |
References
- Almutairi, K.B.; Nossent, J.C.; Preen, D.B.; Keen, H.I.; Inderjeeth, C.A. The Prevalence of Rheumatoid Arthritis: A Systematic Review of Population-Based Studies. J. Rheumatol. 2021, 48, 669–676. [Google Scholar] [CrossRef]
- Weinblatt, M.E. Methotrexate in Rheumatoid Arthritis: A Quarter Century of Development. Trans. Am. Clin. Clim. Assoc. 2013, 124, 16–25. [Google Scholar]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1108–1123. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D. Rheumatoid Arthritis Therapy Reappraisal: Strategies, Opportunities and Challenges. Nat. Rev. Rheumatol. 2015, 11, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Konzett, V.; Aletaha, D. Management Strategies in Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2024, 20, 760–769. [Google Scholar] [CrossRef]
- Konzett, V.; Kerschbaumer, A.; Smolen, J.S.; Aletaha, D. Determination of the Most Appropriate ACR Response Definition for Contemporary Drug Approval Trials in Rheumatoid Arthritis. Ann. Rheum. Dis. 2024, 83, 58–64. [Google Scholar] [CrossRef]
- O’Dell, J.R.; Mikuls, T.R.; Taylor, T.H.; Ahluwalia, V.; Brophy, M.; Warren, S.R.; Lew, R.A.; Cannella, A.C.; Kunkel, G.; Phibbs, C.S.; et al. Therapies for Active Rheumatoid Arthritis after Methotrexate Failure. N. Engl. J. Med. 2013, 369, 307–318. [Google Scholar] [CrossRef]
- Sparks, J.A.; Krumme, A.A.; Shrank, W.H.; Matlin, O.S.; Brill, G.; Pezalla, E.J.; Choudhry, N.K.; Solomon, D.H. Intensification to Triple Therapy Non-Biologic Disease-Modifying Antirheumatic Drugs for Rheumatoid Arthritis in the United States from 2009 to 2014. Arthritis Rheumatol. 2016, 68, 1588–1595. [Google Scholar] [CrossRef]
- Cubberley, C.; Maharaj, A. Global RA Treatment Recommendations: An Update from the Various International Societies. Best Pract. Res. Clin. Rheumatol. 2025, 39, 102019. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Bijlsma, J.W.J.; Breedveld, F.C.; Boumpas, D.; Burmester, G.; Combe, B.; Cutolo, M.; Wit, M.D.; Dougados, M.; et al. Treating Rheumatoid Arthritis to Target: Recommendations of an International Task Force. Ann. Rheum. Dis. 2010, 69, 631–637. [Google Scholar] [CrossRef]
- Felson, D.T.; Smolen, J.S.; Wells, G.; Zhang, B.; van Tuyl, L.H.D.; Funovits, J.; Aletaha, D.; Allaart, R.; Bathon, J.; Bombardieri, S.; et al. American College of Rheumatology/European League against Rheumatism Preliminary Definition of Remission in Rheumatoid Arthritis for Clinical Trials. Arthritis Rheum. 2011, 63, 573–586. [Google Scholar] [CrossRef]
- Hao, Y.; Oon, S.; Nikpour, M. Efficacy and Safety of Treat-to-Target Strategy Studies in Rheumatic Diseases: A Systematic Review and Meta-Analysis. Semin. Arthritis Rheum. 2024, 67, 152465. [Google Scholar] [CrossRef]
- Ramiro, S.; Landewé, R.B.; van der Heijde, D.; Sepriano, A.; FitzGerald, O.; Ostergaard, M.; Homik, J.; Elkayam, O.; Thorne, J.C.; Larche, M.; et al. Is Treat-to-Target Really Working in Rheumatoid Arthritis? A Longitudinal Analysis of a Cohort of Patients Treated in Daily Practice (RA BIODAM). Ann. Rheum. Dis. 2020, 79, 453–459. [Google Scholar] [CrossRef]
- Gossec, L.; Bessette, L.; Xavier, R.M.; Favalli, E.G.; Östör, A.; Buch, M.H. Barriers to, Facilitators of, and Interventions to Support Treat-to-Target Implementation in Rheumatoid Arthritis: A Systematic Review. Arthritis Care Res. 2024, 76, 1626–1636. [Google Scholar] [CrossRef]
- van Vollenhoven, R. Treat-to-Target in Rheumatoid Arthritis—Are We There Yet? Nat. Rev. Rheumatol. 2019, 15, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kakehasi, A.M.; Duarte, A.L.B.P.; Brenol, C.V.; Domiciano, D.S.; Laurindo, I.M.M.; Bonfiglioli, K.R.; da Mota, L.M.H.; Buch, M.H.; de Almeida Macêdo, E.; Xavier, R.M. Challenges in Implementing Treat-to-Target in Rheumatoid Arthritis: A Perspective from Brazilian Rheumatologists. Adv. Rheumatol. 2024, 64, 63. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.A.; Solomon, D.H. Challenges in Implementing Treat-to-Target Strategies in Rheumatology. Rheum. Dis. Clin. N. Am. 2019, 45, 101–112. [Google Scholar] [CrossRef]
- Messelink, M.A.; den Broeder, A.A.; Marinelli, F.E.; Michgels, E.; Verschueren, P.; Aletaha, D.; Tekstra, J.; Welsing, P.M.J. What Is the Best Target in a Treat-to-Target Strategy in Rheumatoid Arthritis? Results from a Systematic Review and Meta-Regression Analysis. RMD Open 2023, 9, e003196. [Google Scholar] [CrossRef] [PubMed]
- Mierau, M.; Schoels, M.; Gonda, G.; Fuchs, J.; Aletaha, D.; Smolen, J.S. Assessing Remission in Clinical Practice. Rheumatology 2007, 46, 975–979. [Google Scholar] [CrossRef]
- Thomas, K.; Lazarini, A.; Kaltsonoudis, E.; Drosos, A.; Papalopoulos, I.; Sidiropoulos, P.; Tsatsani, P.; Gazi, S.; Pantazi, L.; Boki, K.A.; et al. Treatment Patterns and Achievement of the Treat-to-Target Goals in a Real-Life Rheumatoid Arthritis Patient Cohort: Data from 1317 Patients. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20937132. [Google Scholar] [CrossRef]
- Scott, I.C.; Ibrahim, F.; Panayi, G.; Cope, A.P.; Garrood, T.; Vincent, A.; Scott, D.L.; Kirkham, B.; TITRATE Programme Investigators. The Frequency of Remission and Low Disease Activity in Patients with Rheumatoid Arthritis, and Their Ability to Identify People with Low Disability and Normal Quality of Life. Semin. Arthritis Rheum. 2019, 49, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, S.; Kaneko, Y. Unmet Needs and Current Challenges of Rheumatoid Arthritis: Difficult-to-Treat Rheumatoid Arthritis and Late-Onset Rheumatoid Arthritis. J. Clin. Med. 2024, 13, 7594. [Google Scholar] [CrossRef]
- Buch, M.H.; Eyre, S.; McGonagle, D. Persistent Inflammatory and Non-Inflammatory Mechanisms in Refractory Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2021, 17, 17–33. [Google Scholar] [CrossRef]
- Nagy, G.; Roodenrijs, N.M.; Welsing, P.M.; Kedves, M.; Hamar, A.; van der Goes, M.C.; Kent, A.; Bakkers, M.; Blaas, E.; Senolt, L.; et al. EULAR Definition of Difficult-to-Treat Rheumatoid Arthritis. Ann. Rheum. Dis. 2021, 80, 31–35. [Google Scholar] [CrossRef]
- Hofman, Z.L.M.; Roodenrijs, N.M.T.; Nikiphorou, E.; Kent, A.L.; Nagy, G.; Welsing, P.M.J.; van Laar, J.M. Difficult-to-Treat Rheumatoid Arthritis: What Have We Learned and What Do We Still Need to Learn? Rheumatology 2025, 64, 65–73. [Google Scholar] [CrossRef]
- Natalucci, F.; Triaille, C.; Sapart, E.; Dierckx, S.; Van Mullem, C.; De Montjoye, S.; Sokolova, T.; Avramovska, A.; Durez, P. Evolution from Early to Difficult-to-Treat Rheumatoid Arthritis: Incidence and Risk Factors in the ERA Uclouvain Brussels Cohort. Arthritis Res. Ther. 2025, 27, 182. [Google Scholar] [CrossRef]
- Garcia-Salinas, R.; Sanchez-Prado, E.; Mareco, J.; Ronald, P.; Ruta, S.; Gomez, R.; Magri, S. Difficult to Treat Rheumatoid Arthritis in a Comprehensive Evaluation Program: Frequency According to Different Objective Evaluations. Rheumatol. Int. 2023, 43, 1821–1828. [Google Scholar] [CrossRef]
- Dey, M.; Nagy, G.; Nikiphorou, E. Comorbidities and Extra-Articular Manifestations in Difficult-to-Treat Rheumatoid Arthritis: Different Sides of the Same Coin? Rheumatology 2023, 62, 1773–1779. [Google Scholar] [CrossRef]
- Roodenrijs, N.M.T.; van der Goes, M.C.; Welsing, P.M.J.; Tekstra, J.; Lafeber, F.P.J.G.; Jacobs, J.W.G.; van Laar, J.M. Difficult-to-Treat Rheumatoid Arthritis: Contributing Factors and Burden of Disease. Rheumatology 2021, 60, 3778–3788. [Google Scholar] [CrossRef] [PubMed]
- Roodenrijs, N.M.T.; Welsing, P.M.J.; van Roon, J.; Schoneveld, J.L.M.; van der Goes, M.C.; Nagy, G.; Townsend, M.J.; van Laar, J.M. Mechanisms Underlying DMARD Inefficacy in Difficult-to-Treat Rheumatoid Arthritis: A Narrative Review with Systematic Literature Search. Rheumatology 2022, 61, 3552–3566. [Google Scholar] [CrossRef] [PubMed]
- David, P.; Di Matteo, A.; Hen, O.; Dass, S.; Marzo-Ortega, H.; Wakefield, R.J.; Bissell, L.-A.; Nam, J.; Mankia, K.; Emery, P.; et al. Poly-Refractory Rheumatoid Arthritis: An Uncommon Subset of Difficult to Treat Disease With Distinct Inflammatory and Noninflammatory Phenotypes. Arthritis Rheumatol. 2024, 76, 510–521. [Google Scholar] [CrossRef]
- Zimba, O.; Baimukhamedov, C.; Kocyigit, B.F. Late-Onset Rheumatoid Arthritis: Clinical Features, Diagnostic Challenges, and Treatment Approaches. Rheumatol. Int. 2025, 45, 152. [Google Scholar] [CrossRef]
- Pavlov-Dolijanovic, S.; Bogojevic, M.; Nozica-Radulovic, T.; Radunovic, G.; Mujovic, N. Elderly-Onset Rheumatoid Arthritis: Characteristics and Treatment Options. Medicina 2023, 59, 1878. [Google Scholar] [CrossRef]
- Kobak, S.; Bes, C. An Autumn Tale: Geriatric Rheumatoid Arthritis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cesta, A.; Movahedi, M.; Bombardier, C. Late-Onset Rheumatoid Arthritis Has a Similar Time to Remission as Younger-Onset Rheumatoid Arthritis: Results from the Ontario Best Practices Research Initiative. Arthritis Res. Ther. 2022, 24, 255. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, N.; Tanaka, E.; Inoue, E.; Abe, M.; Sugano, E.; Saka, K.; Ochiai, M.; Yamaguchi, R.; Ikari, K.; Yamanaka, H.; et al. Higher Risk of Poor Functional Outcome and Unfavourable Clinical Events for Late-Onset Rheumatoid Arthritis: Results from the IORRA Cohort. Rheumatology 2025, 64, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Harigai, M.; Sugihara, T. Management of Late-Onset Rheumatoid Arthritis with Treat-to-Target Strategy. Drugs Aging 2025, 42, 413–433. [Google Scholar] [CrossRef]
- Deane, K.D.; Holers, V.M. Rheumatoid Arthritis: Pathogenesis, Prediction and Prevention—An Emerging Paradigm Shift. Arthritis Rheumatol. 2021, 73, 181–193. [Google Scholar] [CrossRef]
- Kelmenson, L.B.; Wagner, B.D.; McNair, B.K.; Frazer-Abel, A.; Demoruelle, M.K.; Bergstedt, D.T.; Feser, M.L.; Moss, L.K.; Parish, M.C.; Mewshaw, E.A.; et al. Timing of Elevations of Autoantibody Isotypes Prior to Diagnosis of Rheumatoid Arthritis. Arthritis Rheumatol. 2020, 72, 251–261. [Google Scholar] [CrossRef]
- Maisha, J.A.; El-Gabalawy, H.S.; O’Neil, L.J. Modifiable Risk Factors Linked to the Development of Rheumatoid Arthritis: Evidence, Immunological Mechanisms and Prevention. Front. Immunol. 2023, 14, 1221125. [Google Scholar] [CrossRef]
- Cheng, Z.; Do, T.; Mankia, K.; Meade, J.; Hunt, L.; Clerehugh, V.; Speirs, A.; Tugnait, A.; Emery, P.; Devine, D. Dysbiosis in the Oral Microbiomes of Anti-CCP Positive Individuals at Risk of Developing Rheumatoid Arthritis. Ann. Rheum. Dis. 2021, 80, 162–168. [Google Scholar] [CrossRef]
- Mankia, K.; Cheng, Z.; Do, T.; Hunt, L.; Meade, J.; Kang, J.; Clerehugh, V.; Speirs, A.; Tugnait, A.; Hensor, E.M.A.; et al. Prevalence of Periodontal Disease and Periodontopathic Bacteria in Anti-Cyclic Citrullinated Protein Antibody-Positive At-Risk Adults Without Arthritis. JAMA Netw. Open 2019, 2, e195394. [Google Scholar] [CrossRef]
- Seymour, B.J.; Allen, B.E.; Kuhn, K.A. Microbial Mechanisms of Rheumatoid Arthritis Pathogenesis. Curr. Rheumatol. Rep. 2024, 26, 124–132. [Google Scholar] [CrossRef]
- Jiang, X.; Askling, J.; Saevarsdottir, S.; Padyukov, L.; Alfredsson, L.; Viatte, S.; Frisell, T. A Genetic Risk Score Composed of Rheumatoid Arthritis Risk Alleles, HLA-DRB1 Haplotypes, and Response to TNFi Therapy—Results from a Swedish Cohort Study. Arthritis Res. Ther. 2016, 18, 288. [Google Scholar] [CrossRef] [PubMed]
- Sieberts, S.K.; Zhu, F.; García-García, J.; Stahl, E.; Pratap, A.; Pandey, G.; Pappas, D.; Aguilar, D.; Anton, B.; Bonet, J.; et al. Crowdsourced Assessment of Common Genetic Contribution to Predicting Anti-TNF Treatment Response in Rheumatoid Arthritis. Nat. Commun. 2016, 7, 12460. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, J.; Meng, Q. From Genetic Variants to Therapeutic Targets: Insights into Understanding Rheumatoid Arthritis. Front. Immunol. 2025, 16, 1556971. [Google Scholar] [CrossRef]
- Mankia, K.; Emery, P. Palindromic Rheumatism as Part of the Rheumatoid Arthritis Continuum. Nat. Rev. Rheumatol. 2019, 15, 687–695. [Google Scholar] [CrossRef]
- Mankia, K.; D’Agostino, M.-A.; Wakefield, R.J.; Nam, J.L.; Mahmood, W.; Grainger, A.J.; Emery, P. Identification of a Distinct Imaging Phenotype May Improve the Management of Palindromic Rheumatism. Ann. Rheum. Dis. 2019, 78, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Frazzei, G.; Musters, A.; de Vries, N.; Tas, S.W.; van Vollenhoven, R.F. Prevention of Rheumatoid Arthritis: A Systematic Literature Review of Preventive Strategies in at-Risk Individuals. Autoimmun. Rev. 2023, 22, 103217. [Google Scholar] [CrossRef]
- Krijbolder, D.I.; Verstappen, M.; van Dijk, B.T.; Dakkak, Y.J.; Burgers, L.E.; Boer, A.C.; Park, Y.J.; de Witt-Luth, M.E.; Visser, K.; Kok, M.R.; et al. Intervention with Methotrexate in Patients with Arthralgia at Risk of Rheumatoid Arthritis to Reduce the Development of Persistent Arthritis and Its Disease Burden (TREAT EARLIER): A Randomised, Double-Blind, Placebo-Controlled, Proof-of-Concept Trial. Lancet 2022, 400, 283–294. [Google Scholar] [CrossRef]
- Deane, K.D.; Holers, V.M.; Emery, P.; Mankia, K.; El-Gabalawy, H.; Sparks, J.A.; Costenbader, K.H.; Schett, G.; van der Helm-van Mil, A.; van Schaardenburg, D.; et al. Therapeutic Interception in Individuals at Risk of Rheumatoid Arthritis to Prevent Clinically Impactful Disease. Ann. Rheum. Dis. 2025, 84, 14–28. [Google Scholar] [CrossRef]
- Schäfer, C.; Keyßer, G. Lifestyle Factors and Their Influence on Rheumatoid Arthritis: A Narrative Review. J. Clin. Med. 2022, 11, 7179. [Google Scholar] [CrossRef]
- Iyengar, K.P.; Vaish, A.; Nune, A. Anti-Cyclic Citrullinated Peptide Antibody (ACPA) and Rheumatoid Arthritis: Clinical Relevance. J. Clin. Orthop. Trauma. 2021, 24, 101729. [Google Scholar] [CrossRef]
- Aletaha, D. Precision Medicine and Management of Rheumatoid Arthritis. J. Autoimmun. 2020, 110, 102405. [Google Scholar] [CrossRef] [PubMed]
- Nijjar, J.S.; Morton, F.R.; Bang, H.; Buckley, C.D.; van der Heijde, D.; Gilmour, A.; Paterson, C.; McInnes, I.B.; Porter, D.; Raza, K.; et al. The Impact of Autoantibodies against Citrullinated, Carbamylated, and Acetylated Peptides on Radiographic Progression in Patients with New-Onset Rheumatoid Arthritis: An Observational Cohort Study. Lancet Rheumatol. 2021, 3, e284–e293. [Google Scholar] [CrossRef]
- Sokolova, M.V.; Schett, G.; Steffen, U. Autoantibodies in Rheumatoid Arthritis: Historical Background and Novel Findings. Clin. Rev. Allergy Immunol. 2022, 63, 138–151. [Google Scholar] [CrossRef]
- Horai, Y.; Kurushima, S.; Shimizu, T.; Nakamura, H.; Kawakami, A. A Review of the Impact of Sjögren’s Syndrome and/or the Presence of Anti-Ro/SS-A Antibodies on Therapeutic Strategies for Rheumatoid Arthritis. J. Clin. Med. 2025, 14, 568. [Google Scholar] [CrossRef]
- Christ, L.; Kissling, S.; Finckh, A.; Fisher, B.A.; Adler, S.; Maurer, B.; Möller, B.; Kollert, F. Concomitant Sjögren’s Disease as a Biomarker for Treatment Effectiveness in Rheumatoid Arthritis—Results from the Swiss Clinical Quality Management Cohort. Arthritis Res. Ther. 2024, 26, 68. [Google Scholar] [CrossRef] [PubMed]
- Laroche, M.; Degboe, Y.; Constantin, A. Sjögren’s Syndrome Associated with Erosive Rheumatoid Arthritis Alters Its Prognosis and Long-Term Therapeutic Response: A Case-Control Study. Rheumatol. Int. 2023, 43, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, T.; Cox, T.; Kollert, F.; Bowman, S.J.; Ito, H.; Matsuda, S.; Fisher, B.A. The Impact of Concomitant Sjögren’s Disease on Rheumatoid Arthritis Disease Activity: A Systematic Review and Meta-Analysis. Clin. Exp. Rheumatol. 2023, 41, 2484–2492. [Google Scholar] [CrossRef]
- Alp, G.; Cinakli, H.; Kurut Aysin, İ.; Solmaz, D.; Akar, S. Challenges and Insights in Managing Difficult-to-Treat Rheumatoid Arthritis: Real-World Clinical Perspectives. Clin. Exp. Rheumatol. 2024, 42, 1398–1406. [Google Scholar] [CrossRef]
- Matsudaira, R.; Tamura, N.; Sekiya, F.; Ogasawara, M.; Yamanaka, K.; Takasaki, Y. Anti-Ro/SSA Antibodies Are an Independent Factor Associated with an Insufficient Response to Tumor Necrosis Factor Inhibitors in Patients with Rheumatoid Arthritis. J. Rheumatol. 2011, 38, 2346–2354. [Google Scholar] [CrossRef]
- Waki, D.; Tamai, H.; Yokochi, R.; Kido, T.; Yagyu, Y.; Yanai, R.; Sada, K.-E. Effects of Anti-SSA Antibodies on the Response to Methotrexate in Rheumatoid Arthritis: A Retrospective Multicenter Observational Study. PLoS ONE 2022, 17, e0271921. [Google Scholar] [CrossRef]
- Hagiwara, S.; Tsuboi, H.; Honda, F.; Takahashi, H.; Kurata, I.; Ohyama, A.; Yagishita, M.; Abe, S.; Kurashima, Y.; Kaneko, S.; et al. Association of Anti-Ro/SSA Antibody with Response to Biologics in Patients with Rheumatoid Arthritis. Mod. Rheumatol. 2016, 26, 857–862. [Google Scholar] [CrossRef]
- Tsuboi, H.; Toko, H.; Honda, F.; Abe, S.; Takahashi, H.; Yagishita, M.; Hagiwara, S.; Ohyama, A.; Kondo, Y.; Nakano, K.; et al. Abatacept Ameliorates Both Glandular and Extraglandular Involvements in Patients with Sjögren’s Syndrome Associated with Rheumatoid Arthritis: Findings from an Open-Label, Multicentre, 1-Year, Prospective Study: The ROSE (Rheumatoid Arthritis with Orencia Trial Toward Sjögren’s Syndrome Endocrinopathy) and ROSE II Trials. Mod. Rheumatol. 2023, 33, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Chen, C.; Biswas, P.; Kwok, K.; Boy, M.G. Lymphoma in the Tofacitinib Rheumatoid Arthritis Clinical Development Program. Arthritis Care Res. 2018, 70, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Hu, W.; Wang, R.; Yang, Q.; Zhu, M.; Li, M.; Cai, J.; Rose, P.; Mao, J.; Zhu, Y.Z. Signaling Pathways in Rheumatoid Arthritis: Implications for Targeted Therapy. Sig. Transduct. Target. Ther. 2023, 8, 68. [Google Scholar] [CrossRef]
- Avouac, J.; Kay, J.; Choy, E. Personalised Treatment of Rheumatoid Arthritis Based on Cytokine Profiles and Synovial Tissue Signatures: Potentials and Challenges. Semin. Arthritis Rheum. 2025, 73, 152740. [Google Scholar] [CrossRef] [PubMed]
- Lama, M.; Sarkar, R.; Ghosh, B. Serum Cytokine Profiles in Patients with Rheumatoid Arthritis Before and After Treatment with Methotrexate. J. Interferon Cytokine Res. 2023, 43, 344–350. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, B.-H.; Hu, L.; Li, M.-C.; Chang, D.; Dou, X. Relationship between Biologic Therapy and Cytokine Levels in Patients with Inflammatory Arthritis. Medicine 2025, 104, e42953. [Google Scholar] [CrossRef]
- Furer, V.; Elkayam, O. Dual Biologic Therapy in Patients with Rheumatoid Arthritis and Psoriatic Arthritis. Rambam Maimonides Med. J. 2023, 14, e0007. [Google Scholar] [CrossRef]
- De Lima, J.; Boutet, M.-A.; Bortolotti, O.; Chépeaux, L.-A.; Glasson, Y.; Dumé, A.-S.; Lau, R.; Humbert, P.; Allain, S.; Le Pluart, A.; et al. Spatial Mapping of Rheumatoid Arthritis Synovial Niches Reveals a LYVE1+ Macrophage Network Associated with Response to Therapy. Ann. Rheum. Dis. 2025, 84, 1955–1967. [Google Scholar] [CrossRef]
- Weisenfeld, D.; Zhang, F.; Donlin, L.; Jonsson, A.H.; Apruzzese, W.; Campbell, D.; Accelerating Medicines Partnership Program: RA/SLE Network; Rao, D.A.; Wei, K.; Holers, V.M.; et al. Associations Between Rheumatoid Arthritis Clinical Factors and Synovial Cell Types and States. Arthritis Rheumatol. 2024, 76, 356–362. [Google Scholar] [CrossRef]
- Humby, F.; Durez, P.; Buch, M.H.; Lewis, M.J.; Rizvi, H.; Rivellese, F.; Nerviani, A.; Giorli, G.; Mahto, A.; Montecucco, C.; et al. Rituximab versus Tocilizumab in Anti-TNF Inadequate Responder Patients with Rheumatoid Arthritis (R4RA): 16-Week Outcomes of a Stratified, Biopsy-Driven, Multicentre, Open-Label, Phase 4 Randomised Controlled Trial. Lancet 2021, 397, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G.; Holweg, C.T.J.; Kummerfeld, S.K.; Choy, D.F.; Setiadi, A.F.; Hackney, J.A.; Haverty, P.M.; Gilbert, H.; Lin, W.Y.; Diehl, L.; et al. Synovial Phenotypes in Rheumatoid Arthritis Correlate with Response to Biologic Therapeutics. Arthritis Res. Ther. 2014, 16, R90. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Mease, P.; Kerschbaumer, A.; Voll, R.E.; Breedveld, F.C.; Smolen, J.S.; Gottenberg, J.-E.; Baraliakos, X.; Kiener, H.P.; Aletaha, D.; et al. Unmet Need in Rheumatology: Reports from the Advances in Targeted Therapies Meeting, 2023. Ann. Rheum. Dis. 2024, 83, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Miyazaki, Y.; Nishino, T.; Fujita, Y.; Kono, M.; Kawashima, T.; Ishigaki, K.; Kusaka, K.; Tanaka, H.; Ueno, M.; et al. Peripheral Blood Immunophenotypic Diversity in Patients with Rheumatoid Arthritis and Its Impact on Therapeutic Responsiveness. Ann. Rheum. Dis. 2025, 84, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Su, R.; Guo, Q.; Su, R.; Gao, C.; Li, X.; Wang, C. Differential Immunological Profiles in Seronegative versus Seropositive Rheumatoid Arthritis: Th17/Treg Dysregulation and IL-4. Front. Immunol. 2024, 15, 1447213. [Google Scholar] [CrossRef]
- Nakayamada, S.; Kubo, S.; Yoshikawa, M.; Miyazaki, Y.; Yunoue, N.; Iwata, S.; Miyagawa, I.; Hirata, S.; Nakano, K.; Saito, K.; et al. Differential Effects of Biological DMARDs on Peripheral Immune Cell Phenotypes in Patients with Rheumatoid Arthritis. Rheumatology 2018, 57, 164–174. [Google Scholar] [CrossRef]
- Blomberg, N.J.; Kristyanto, H.; Verstappen, M.; Neppelenbroek, S.; van der Helm-van Mil, A.H.M.; Toes, R.E.M.; Scherer, H.U. Autoreactive B Cells in Extremes of Rheumatoid Arthritis Disease Phenotypes. Ann. Rheum. Dis. 2025. [Google Scholar] [CrossRef]
- Neppelenbroek, S.; Blomberg, N.J.; Kampstra, A.S.B.; van der Hem, J.G.K.; Huizinga, T.W.J.; Toes, R.E.M.; Scherer, H.U. Autoreactive B Cells Remain Active despite Clinical Disease Control in Rheumatoid Arthritis. J. Autoimmun. 2024, 149, 103320. [Google Scholar] [CrossRef]
- Coyle, C.; Ma, M.; Abraham, Y.; Mahony, C.B.; Steel, K.; Simpson, C.; Guerra, N.; Croft, A.P.; Rapecki, S.; Cope, A.; et al. NK Cell Subsets Define Sustained Remission in Rheumatoid Arthritis. JCI Insight 2024, 9, e182390. [Google Scholar] [CrossRef] [PubMed]
- de Maleprade, B.; Gérard, B.; Brevet, P.; Riou, G.; Candon, S.; Boyer, O.; Vittecoq, O.; Lequerré, T.; Fréret, M. Macrophage Requirements for Abatacept Response in Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2025, 43, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Hiitola, E.; Korhonen, J.; Kokkonen, H.; Koskela, J.; Kankainen, M.; Alakuijala, M.; Liu, A.; Lundgren, S.; Häppölä, P.; Almusa, H.; et al. Clonal Hematopoiesis Is Associated with Distinct Rheumatoid Arthritis Phenotypes. Sci. Adv. 2025, 11, eadt9846. [Google Scholar] [CrossRef]
- De Stefano, L.; Bozzalla Cassione, E.; Sammali, Y.; Luvaro, T.; Montecucco, C.; Manzo, A.; Bugatti, S. High Serum Levels of CXCL13 Predict Lower Response to csDMARDs in Both ACPA-Positive and ACPA-Negative Early Rheumatoid Arthritis. Rheumatology 2025, 64, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; He, Q.; Qu, J.; Wang, X.; Li, K.; Gong, X.; Li, L.; Xu, J.; Yu, Q.; Yu, H.; et al. Bone-Protective Effects of Neutralizing Angiopoietin-like Protein 4 Monoclonal Antibody in Rheumatoid Arthritis. Mol. Ther. 2024, 32, 4497–4513. [Google Scholar] [CrossRef]
- Miao, J.; Zhang, B.; Sun, H.; Zhang, P.; Shen, H.; Wang, J.; Jia, J.; Zhang, K.; Zheng, Z.; Zhu, P. CCR5 Mediates Rheumatoid Arthritis Progression by Promoting the Activation and Proliferation of Non-Classical Th1 Cells. Int. J. Rheum. Dis. 2024, 27, e15370. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kameda, H. What Is the Future of CCR5 Antagonists in Rheumatoid Arthritis? Arthritis Res. Ther. 2012, 14, 114. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Siebuhr, A.S.; Damgaard, D.; Drobinski, P.; Thudium, C.; Mortensen, J.; Nielsen, C.H. Objective and Noninvasive Biochemical Markers in Rheumatoid Arthritis: Where Are We and Where Are We Going? Expert Rev. Proteom. 2021, 18, 159–175. [Google Scholar] [CrossRef]
- Strand, V.; Miller, P.; Williams, S.A.; Saunders, K.; Grant, S.; Kremer, J. Discontinuation of Biologic Therapy in Rheumatoid Arthritis: Analysis from the Corrona RA Registry. Rheumatol. Ther. 2017, 4, 489–502. [Google Scholar] [CrossRef]
- Agarwal, S.; Zaman, T.; Handa, R. Retention Rates of Disease-Modifying Anti-Rheumatic Drugs in Patients with Rheumatoid Arthritis. Singap. Med. J. 2009, 50, 686–692. [Google Scholar]
- Westerlind, H.; Glintborg, B.; Hammer, H.B.; Saevarsdottir, S.; Krogh, N.S.; Hetland, M.L.; Hauge, E.-M.; Martinez Tejada, I.; Sexton, J.; Askling, J. Remission, Response, Retention and Persistence to Treatment with Disease-Modifying Agents in Patients with Rheumatoid Arthritis: A Study of Harmonised Swedish, Danish and Norwegian Cohorts. RMD Open 2023, 9, e003027. [Google Scholar] [CrossRef]
- Ebina, K.; Hashimoto, M.; Yamamoto, W.; Ohnishi, A.; Kabata, D.; Hirano, T.; Hara, R.; Katayama, M.; Yoshida, S.; Nagai, K.; et al. Drug Retention and Discontinuation Reasons between Seven Biologics in Patients with Rheumatoid Arthritis—The ANSWER Cohort Study. PLoS ONE 2018, 13, e0194130. [Google Scholar] [CrossRef]
- United States Food and Drug Administration (FDA). Drugs@FDA: FDA Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 12 January 2026).
- Thomas, K.; Vassilopoulos, D. Infections in Patients with Rheumatoid Arthritis in the Era of Targeted Synthetic Therapies. Mediterr. J. Rheumatol. 2020, 31, 129–136. [Google Scholar] [CrossRef]
- Rutherford, A.I.; Subesinghe, S.; Hyrich, K.L.; Galloway, J.B. Serious Infection across Biologic-Treated Patients with Rheumatoid Arthritis: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann. Rheum. Dis. 2018, 77, 905–910. [Google Scholar] [CrossRef]
- Askling, J.; Fored, C.M.; Brandt, L.; Baecklund, E.; Bertilsson, L.; Feltelius, N.; Cöster, L.; Geborek, P.; Jacobsson, L.T.; Lindblad, S.; et al. Time-Dependent Increase in Risk of Hospitalisation with Infection among Swedish RA Patients Treated with TNF Antagonists. Ann. Rheum. Dis. 2007, 66, 1339–1344. [Google Scholar] [CrossRef]
- Galloway, J.B.; Hyrich, K.L.; Mercer, L.K.; Dixon, W.G.; Fu, B.; Ustianowski, A.P.; Watson, K.D.; Lunt, M.; Symmons, D.P.M.; BSRBR Control Centre Consortium; et al. Anti-TNF Therapy Is Associated with an Increased Risk of Serious Infections in Patients with Rheumatoid Arthritis Especially in the First 6 Months of Treatment: Updated Results from the British Society for Rheumatology Biologics Register with Special Emphasis on Risks in the Elderly. Rheumatology 2011, 50, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Silva-Fernández, L.; De Cock, D.; Lunt, M.; Low, A.S.; Watson, K.D.; Symmons, D.P.M.; Hyrich, K.L. Serious Infection Risk after 1 Year between Patients with Rheumatoid Arthritis Treated with Rituximab or with a Second TNFi after Initial TNFi Failure: Results from The British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology 2018, 57, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Huss, V.; Bower, H.; Wadström, H.; Frisell, T.; Askling, J. Short-and Longer-Term Cancer Risks with Biologic and Targeted Synthetic Disease-Modifying Antirheumatic Drugs as Used against Rheumatoid Arthritis in Clinical Practice. Rheumatology 2021, 61, 1810–1818. [Google Scholar] [CrossRef]
- Zuckerman, B.P.; Gibson, M.; Roy, R.; Hughes, M.; Mehta, D.; Yang, Z.; Adas, M.; Ng, K.; Russell, M.D.; Cope, A.; et al. Abatacept and the Risk of Malignancy: A Meta-Analysis across Disease Indications. Rheumatology 2025, 64, 3280–3287. [Google Scholar] [CrossRef]
- Simon, T.A.; Suissa, S.; Boers, M.; Hochberg, M.C.; Skovron, M.L.; Askling, J.; Michaud, K.; Strangfeld, A.; Pedro, S.; Frisell, T.; et al. Malignancy Outcomes in Patients with Rheumatoid Arthritis Treated with Abatacept and Other Disease-Modifying Antirheumatic Drugs: Results from a 10-Year International Post-Marketing Study. Semin. Arthritis Rheum. 2024, 64, 152240. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Purschke, A.; Zietemann, V.; Rudi, T.; Meissner, Y.; Richter, A.; Berger, S.; Rockwitz, K.; Krüger, K.; Schneider, K.M.; et al. Comparative Risk of Incident Malignancies in Rheumatoid Arthritis Patients Treated with Janus Kinase Inhibitors or bDMARDs: Observational Data from the German RABBIT Register. Ann. Rheum. Dis. 2025, 84, 1779–1790. [Google Scholar] [CrossRef]
- Westermann, R.; Cordtz, R.L.; Duch, K.; Mellemkjaer, L.; Hetland, M.L.; Burden, A.M.; Dreyer, L. Cancer Risk in Patients with Rheumatoid Arthritis Treated with Janus Kinase Inhibitors: A Nationwide Danish Register-Based Cohort Study. Rheumatology 2024, 63, 93–102. [Google Scholar] [CrossRef]
- Xu, C.; Wang, S.-I.; Leung, Y.Y.; Wei, J.C.-C. Comparative Cancer Incidence by Organ Site in Rheumatoid Arthritis Treated with Janus Kinase Inhibitors versus Tumor Necrosis Factor Inhibitors: A Retrospective Real-World Cohort Analysis. Biologics 2025, 19, 525–538. [Google Scholar] [CrossRef]
- Avouac, J.; Ait-Oufella, H.; Habauzit, C.; Benkhalifa, S.; Combe, B. The Cardiovascular Safety of Tumour Necrosis Factor Inhibitors in Arthritic Conditions: A Structured Review with Recommendations. Rheumatol. Ther. 2025, 12, 211–236. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Aviña-Zubieta, J.A.; Bernstein, C.N.; Kaplan, G.G.; Tremlett, H.; Xie, H.; Peña-Sánchez, J.-N.; Marrie, R.A.; Etminan, M. Risk of Multiple Sclerosis Among Users of Antitumor Necrosis Factor α in 4 Canadian Provinces. Neurology 2023, 100, e558–e567. [Google Scholar] [CrossRef]
- Delcoigne, B.; Kopp, T.I.; Arkema, E.V.; Hellgren, K.; Provan, S.A.; Relas, H.; Aaltonen, K.; Trokovic, N.; Gudbjornsson, B.; Grondal, G.; et al. Exposure to Specific Tumour Necrosis Factor Inhibitors and Risk of Demyelinating and Inflammatory Neuropathy in Cohorts of Patients with Inflammatory Arthritis: A Collaborative Observational Study across Five Nordic Rheumatology Registers. RMD Open 2023, 9, e002924. [Google Scholar] [CrossRef]
- Xiao, R.; Tang, P.; Zhou, J.; Zheng, W.; Cao, Y.; Zhu, Y.; Xiao, W.; Tan, H.; Wen, T.; Abdirahman, A.; et al. Safety of TNF-α Inhibitors Therapy in Patients with Rheumatoid Arthritis: An Umbrella Review. eClinicalMedicine 2025, 88, 103488–103496. [Google Scholar] [CrossRef]
- Suissa, S.; Hudson, M.; Dell’Aniello, S.; Shen, S.; Simon, T.A.; Ernst, P. Comparative Safety of Abatacept in Rheumatoid Arthritis with COPD: A Real-World Population-Based Observational Study. Semin. Arthritis Rheum. 2019, 49, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Yun, H.; Levitan, E.B.; Muntner, P.; Curtis, J.R. Tocilizumab and the Risk of Cardiovascular Disease: Direct Comparison Among Biologic Disease-Modifying Antirheumatic Drugs for Rheumatoid Arthritis Patients. Arthritis Care Res. 2019, 71, 1004–1018. [Google Scholar] [CrossRef]
- Castagné, B.; Viprey, M.; Martin, J.; Schott, A.-M.; Cucherat, M.; Soubrier, M. Cardiovascular Safety of Tocilizumab: A Systematic Review and Network Meta-Analysis. PLoS ONE 2019, 14, e0220178. [Google Scholar] [CrossRef]
- Strangfeld, A.; Richter, A.; Siegmund, B.; Herzer, P.; Rockwitz, K.; Demary, W.; Aringer, M.; Meißner, Y.; Zink, A.; Listing, J. Risk for Lower Intestinal Perforations in Patients with Rheumatoid Arthritis Treated with Tocilizumab in Comparison to Treatment with Other Biologic or Conventional Synthetic DMARDs. Ann. Rheum. Dis. 2017, 76, 504–510. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Solitano, V.; Ahuja, D.; Lee, H.H.; Gaikwad, R.; Yeh, K.-H.; Facciorusso, A.; Singh, A.G.; Ma, C.; Ananthakrishnan, A.N.; Yuan, Y.; et al. Comparative Safety of JAK Inhibitors vs TNF Antagonists in Immune-Mediated Inflammatory Diseases: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2025, 8, e2531204. [Google Scholar] [CrossRef]
- Aymon, R.; Mongin, D.; Guemara, R.; Salis, Z.; Askling, J.; Choquette, D.; Codreanu, C.; Di Giuseppe, D.; Flouri, I.; Huschek, D.; et al. Incidence of Major Adverse Cardiovascular Events in Patients with Rheumatoid Arthritis Treated with JAK Inhibitors Compared with Biologic Disease-Modifying Antirheumatic Drugs: Data from an International Collaboration of Registries. Arthritis Rheumatol. 2025, 77, 1194–1204. [Google Scholar] [CrossRef]
- Mok, C.C.; So, H.; Yim, C.W.; To, C.H.; Lao, W.N.; Wong, S.P.Y.; Ng, H.Y.; Lee, J.M.Y.; Lee, P.M.L.; Ying, S.K.Y.; et al. Safety of the JAK and TNF Inhibitors in Rheumatoid Arthritis: Real World Data from the Hong Kong Biologics Registry. Rheumatology 2024, 63, 358–365. [Google Scholar] [CrossRef] [PubMed]
- You, S.-H.; Cho, S.-K.; Kim, J.-Y.; Song, Y.-J.; Jung, S.-Y.; Sung, Y.-K. Risk of Major Adverse Cardiovascular Events Following Targeted Therapy in Patients with Rheumatoid Arthritis: A Real-World Analysis Stratified by Cardiovascular Risk. Semin. Arthritis Rheum. 2025, 73, 152721. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.R.; Malik, V.; Lacey, S.; Brunetta, P.; Lehane, P.B. Progressive Multifocal Leukoencephalopathy in Rituximab-Treated Rheumatic Diseases: A Rare Event. J. Neurovirol. 2018, 24, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L.; Saag, K.; Cascino, M.D.; Pei, J.; John, A.; Jahreis, A.; Haselkorn, T.; Furst, D.E.; Abdulky, M.; Abeles, M.; et al. Long-Term Safety of Rituximab in Patients With Rheumatoid Arthritis: Results of a Five-Year Observational Study. Arthritis Care Res. 2019, 71, 993–1003. [Google Scholar] [CrossRef]
- van Vollenhoven, R.F.; Fleischmann, R.M.; Furst, D.E.; Lacey, S.; Lehane, P.B. Longterm Safety of Rituximab: Final Report of the Rheumatoid Arthritis Global Clinical Trial Program over 11 Years. J. Rheumatol. 2015, 42, 1761–1766. [Google Scholar] [CrossRef]
- Lee, J.; Martindale, J.; Makris, U.E.; Singh, N.; Yung, R.; Bynum, J.P.W. Initiation of Disease-Modifying Antirheumatic Drugs in Older Medicare Beneficiaries With New Diagnosis of Late-Onset Rheumatoid Arthritis. ACR Open Rheumatol. 2023, 5, 694–700. [Google Scholar] [CrossRef]
- Lahaye, C.; Tatar, Z.; Dubost, J.-J.; Tournadre, A.; Soubrier, M. Management of Inflammatory Rheumatic Conditions in the Elderly. Rheumatology 2019, 58, 748–764. [Google Scholar] [CrossRef]
- Lehoczki, A.; Ungvari, Z.; Szappanos, Á.; Fekete, M.; Gunkl-Tóth, L.; Nagy, G. Geroscience Insights into Difficult-to-Treat Rheumatoid Arthritis: The Role of Unhealthy Aging, Comorbidity, and Therapeutic Complexity. GeroScience 2025, 1–25. [Google Scholar] [CrossRef]
- van Onna, M.; Öztürk, B.; Starmans, M.; Peeters, R.; Boonen, A. Disease and Management Beliefs of Elderly Patients with Rheumatoid Arthritis and Comorbidity: A Qualitative Study. Clin. Rheumatol. 2018, 37, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Ishchenko, A.; Lories, R.J. Safety and Efficacy of Biological Disease-Modifying Antirheumatic Drugs in Older Rheumatoid Arthritis Patients: Staying the Distance. Drugs Aging 2016, 33, 387–398. [Google Scholar] [CrossRef]
- Nooh, N.; Lwin, M.N.; Edwards, C. Considerations for the Use of Biological Therapies in Elderly Patients with Rheumatoid Arthritis. Expert Opin. Biol. Ther. 2024, 24, 1109–1117. [Google Scholar] [CrossRef]
- Studenic, P.; Meissner, Y.; Kearsley-Fleet, L.; De Cock, D. Role of Rheumatoid Arthritis Registries Worldwide: What Have They Taught Us? Best Pract. Res. Clin. Rheumatol. 2025, 39, 102017. [Google Scholar] [CrossRef] [PubMed]
- Lauper, K.; Iudici, M.; Mongin, D.; Bergstra, S.A.; Choquette, D.; Codreanu, C.; Cordtz, R.; De Cock, D.; Dreyer, L.; Elkayam, O.; et al. Effectiveness of TNF-Inhibitors, Abatacept, IL6-Inhibitors and JAK-Inhibitors in 31 846 Patients with Rheumatoid Arthritis in 19 Registers from the “JAK-Pot” Collaboration. Ann. Rheum. Dis. 2022, 81, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Aymon, R.; Mongin, D.; Bergstra, S.A.; Choquette, D.; Codreanu, C.; De Cock, D.; Dreyer, L.; Elkayam, O.; Huschek, D.; Hyrich, K.L.; et al. Evaluation of Discontinuation for Adverse Events of JAK Inhibitors and bDMARDs in an International Collaboration of Rheumatoid Arthritis Registers (the ‘JAK-Pot’ Study). Ann. Rheum. Dis. 2024, 83, 421–428. [Google Scholar] [CrossRef]
- Pope, J.E.; Fleischmann, R.M. Jack Pot! What Can We Learn about Registries with Respect to Treatment Cycling in Rheumatoid Arthritis? Ann. Rheum. Dis. 2023, 82, 161–163. [Google Scholar] [CrossRef]
- De Cock, D.; Myasoedova, E.; Aletaha, D.; Studenic, P. Big Data Analyses and Individual Health Profiling in the Arena of Rheumatic and Musculoskeletal Diseases (RMDs). Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221105978. [Google Scholar] [CrossRef]
- Lin, J.-Z.; Zhu, Y.; Li, Q.-H.; Ouyang, Z.-M.; Liu, H.-J.; Zou, Y.-W.; Yang, Y.; Yang, K.-M.; Yang, L.-J.; Yang, Z.-H.; et al. Rheumatoid Arthritis Real-World Cohort Study in China (ReALSA): Protocol for a Multicentre Prospective, Longitudinal Cohort Study. BMJ Open 2025, 15, e092583. [Google Scholar] [CrossRef]
- Larid, G.; Vix, J.; Garlantezec, R.; Loppin, E.; Gervais, E. Increased Remission with Fewer Corticosteroids and More Biologics in Rheumatoid Arthritis at 7-Year Follow-up in Real-Life Conditions. Sci. Rep. 2022, 12, 2563. [Google Scholar] [CrossRef]
- Sparks, J.A.; Harrold, L.R.; Simon, T.A.; Wittstock, K.; Kelly, S.; Lozenski, K.; Khaychuk, V.; Michaud, K. Comparative Effectiveness of Treatments for Rheumatoid Arthritis in Clinical Practice: A Systematic Review. Semin. Arthritis Rheum. 2023, 62, 152249. [Google Scholar] [CrossRef]
- Fujii, T.; Murata, K.; Onizawa, H.; Onishi, A.; Tanaka, M.; Murakami, K.; Nishitani, K.; Furu, M.; Watanabe, R.; Hashimoto, M.; et al. Management and Treatment Outcomes of Rheumatoid Arthritis in the Era of Biologic and Targeted Synthetic Therapies: Evaluation of 10-Year Data from the KURAMA Cohort. Arthritis Res. Ther. 2024, 26, 16. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-Y.; Zhang, J.; Qian, T.-T.; Gao, P.; Wu, Q.; Fang, Q.; Ke, S.-S.; Huang, R.-G.; Zhang, H.-C.; Qiao, N.-N.; et al. Metabolomic Profiles, Polygenic Risk Scores and Risk of Rheumatoid Arthritis: A Population-Based Cohort Study in the UK Biobank. RMD Open 2023, 9, e003560. [Google Scholar] [CrossRef] [PubMed]
- England, B.R.; Tiong, B.K.; Bergman, M.J.; Curtis, J.R.; Kazi, S.; Mikuls, T.R.; O’Dell, J.R.; Ranganath, V.K.; Limanni, A.; Suter, L.G.; et al. 2019 Update of the American College of Rheumatology Recommended Rheumatoid Arthritis Disease Activity Measures. Arthritis Care Res. 2019, 71, 1540–1555. [Google Scholar] [CrossRef] [PubMed]
- Oude Voshaar, M.A.H.; Das Gupta, Z.; Bijlsma, J.W.J.; Boonen, A.; Chau, J.; Courvoisier, D.S.; Curtis, J.R.; Ellis, B.; Ernestam, S.; Gossec, L.; et al. International Consortium for Health Outcome Measurement Set of Outcomes That Matter to People Living With Inflammatory Arthritis: Consensus From an International Working Group. Arthritis Care Res. 2019, 71, 1556–1565. [Google Scholar] [CrossRef]
- Lopez-Olivo, M.A.; Zogala, R.J.; des Bordes, J.; Zamora, N.V.; Christensen, R.; Rai, D.; Goel, N.; Carmona, L.; Pratt, G.; Strand, V.; et al. Outcomes Reported in Prospective Long-Term Observational Studies and Registries of Patients With Rheumatoid Arthritis Worldwide: An Outcome Measures in Rheumatology Systematic Review. Arthritis Care Res. 2021, 73, 649–657. [Google Scholar] [CrossRef]
- Joensuu, J.T.; Huoponen, S.; Aaltonen, K.J.; Konttinen, Y.T.; Nordström, D.; Blom, M. The Cost-Effectiveness of Biologics for the Treatment of Rheumatoid Arthritis: A Systematic Review. PLoS ONE 2015, 10, e0119683. [Google Scholar] [CrossRef]
- Gharaibeh, M.; Bonafede, M.; McMorrow, D.; Hernandez, E.J.M.; Stolshek, B.S. Effectiveness and Costs Among Rheumatoid Arthritis Patients Treated with Targeted Immunomodulators Using Real-World U.S. Data. J. Manag. Care Spec. Pharm. 2020, 26, 1039–1049. [Google Scholar] [CrossRef]
- Tanaka, E.; Inoue, E.; Yamaguchi, R.; Shimizu, Y.; Kobayashi, A.; Sugimoto, N.; Hoshi, D.; Shidara, K.; Sato, E.; Seto, Y.; et al. Pharmacoeconomic Analysis of Biological Disease Modifying Antirheumatic Drugs in Patients with Rheumatoid Arthritis Based on Real-World Data from the IORRA Observational Cohort Study in Japan. Mod. Rheumatol. 2017, 27, 227–236. [Google Scholar] [CrossRef]
- Cárdenas, M.; de la Fuente, S.; Font, P.; Castro-Villegas, M.C.; Romero-Gómez, M.; Ruiz-Vílchez, D.; Calvo-Gutiérez, J.; Escudero-Contreras, A.; Casado, M.A.; Del Prado, J.R.; et al. Real-World Cost-Effectiveness of Infliximab, Etanercept and Adalimumab in Rheumatoid Arthritis Patients: Results of the CREATE Registry. Rheumatol. Int. 2016, 36, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, J.T.; Aaltonen, K.J.; Aronen, P.; Sokka, T.; Puolakka, K.; Tuompo, R.; Korpela, M.; Vasala, M.; Ilva, K.; Nordström, D.; et al. Cost-Effectiveness of Biologic Compared with Conventional Synthetic Disease-Modifying Anti-Rheumatic Drugs in Patients with Rheumatoid Arthritis: A Register Study. Rheumatology 2016, 55, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Azizpoor, J.; Aflaki, E.; Rezaee, M.; Keshavarz, K. Cost-Effectiveness Analysis of Biopharmaceuticals for Treating Rheumatoid Arthritis: Infliximab, Adalimumab, and Etanercept. Biomed. Res. Int. 2021, 2021, 4450162. [Google Scholar] [CrossRef]
- Jansen, J.P.; Incerti, D.; Mutebi, A.; Peneva, D.; MacEwan, J.P.; Stolshek, B.; Kaur, P.; Gharaibeh, M.; Strand, V. Cost-Effectiveness of Sequenced Treatment of Rheumatoid Arthritis with Targeted Immune Modulators. J. Med. Econ. 2017, 20, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Chastek, B.; Chen, C.-I.; Proudfoot, C.; Shinde, S.; Kuznik, A.; Wei, W. Treatment Persistence and Healthcare Costs Among Patients with Rheumatoid Arthritis Changing Biologics in the USA. Adv. Ther. 2017, 34, 2422–2435. [Google Scholar] [CrossRef]
- Claxton, L.; Taylor, M.; Soonasra, A.; Bourret, J.A.; Gerber, R.A. An Economic Evaluation of Tofacitinib Treatment in Rheumatoid Arthritis After Methotrexate or After 1 or 2 TNF Inhibitors from a U.S. Payer Perspective. J. Manag. Care Spec. Pharm. 2018, 24, 1010–1017. [Google Scholar] [CrossRef]
- Chaplin, S.; van Stiphout, J.; Chen, A.; Li, E. Budget Impact Analysis of Including Biosimilar Adalimumab on Formulary: A United States Payer Perspective. J. Manag. Care Spec. Pharm. 2024, 30, 1226–1238. [Google Scholar] [CrossRef]
- Müskens, W.D.; Dartel, S.A.A.R.; van Riel, P.L.C.M.; Adang, E.M.M. Does Etanercept Biosimilar Prescription in a Rheumatology Center Bend the Medication Cost Curve? J. Rheumatol. 2021, 48, 1803–1809. [Google Scholar] [CrossRef]
- Jiang, T.E.; Ramachandran, R.; Skydel, J.J. Regulatory Change Could Improve Biosimilar Access in the US. BMJ 2025, 390, e084860. [Google Scholar] [CrossRef]
- Barton, J.L.; Décary, S. New Galaxies in the Universe of Shared Decision Making and Rheumatoid Arthritis. Curr. Opin. Rheumatol. 2020, 32, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.L.; Trupin, L.; Tonner, C.; Imboden, J.; Katz, P.; Schillinger, D.; Yelin, E. English Language Proficiency, Health Literacy, and Trust in Physician Are Associated with Shared Decision Making in Rheumatoid Arthritis. J. Rheumatol. 2014, 41, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Toupin-April, K.; Barton, J.; Fraenkel, L.; Li, L.C.; Brooks, P.; De Wit, M.; Stacey, D.; Légaré, F.; Meara, A.; Shea, B.; et al. Toward the Development of a Core Set of Outcome Domains to Assess Shared Decision-Making Interventions in Rheumatology: Results from an OMERACT Delphi Survey and Consensus Meeting. J. Rheumatol. 2017, 44, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.; Foster, E.; Dougherty, J.; Barton, J. Shared Decision Making in Rheumatology: A Scoping Review. Semin. Arthritis Rheum. 2022, 56, 152041. [Google Scholar] [CrossRef]
- Aref, H.A.T.; Turk, T.; Dhanani, R.; Xiao, A.; Olson, J.; Paul, P.; Dennett, L.; Yacyshyn, E.; Sadowski, C.A. Development and Evaluation of Shared Decision-Making Tools in Rheumatology: A Scoping Review. Semin. Arthritis Rheum. 2024, 66, 152432. [Google Scholar] [CrossRef]
- Barton, J.L.; Niederhausen, M.; Tuepker, A.; Schmajuk, G.; Baker, J.; Lovejoy, T.I.; Morasco, B.J.; Kunneman, M.; Scholl, I. Implementation of Shared Decision Making in Rheumatoid Arthritis: Study Protocol for RAiSeD (Rheumatoid Arthritis Shared Decision Making) Stepped Wedge, Cluster-Randomized Trial. Trials 2025, 26, 381. [Google Scholar] [CrossRef]
- Home—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 13 June 2021).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.