Abstract
Background: This study aimed to evaluate the risk of upper gastrointestinal (UGI) adverse events (AEs) associated with oral anticoagulants (OACs) and identify potential interactions with cardiovascular (CV) drugs. Methods: Individual case safety reports (ICSRs) from the FDA Adverse Event Reporting System from July 2014 to December 2023 were analyzed. Dataset I was constructed to assess the associations between OACs and UGI AEs using disproportionality analysis. Dataset Ⅱ included OAC-related ICSRs to explore potential interactions with CV drugs through logistic regression. Positive signals were defined as potential associations identified by disproportionality analysis metrics, such as reporting odds ratios (RORs) or adjusted RORs (aRORs) accounting for confounders. Results: Dataset I included 12,905,290 ICSRs, and a positive signal for dabigatran was detected with an ROR of 1.19 (95% CI, 1.13–1.25). A total of 364,044 OAC-related ICSRs were included in dataset II. At the pharmacologic drug class level, several positive signals were identified, represented as aRORs with 95% CIs: for warfarin, amiodarone analogs (1.22; 1.04–1.43); for apixaban, angiotensin-converting enzyme inhibitors (1.34; 1.24–1.45), angiotensin receptor blockers (1.23; 1.14–1.33), dihydropyridine calcium channel blockers (1.30; 1.21–1.41), and digitalis glycosides (1.72; 1.49–2.00); and for edoxaban, angiotensin receptor blockers (1.88; 1.48–2.37), amiodarone analogs (1.73; 1.06–2.85), and anti-platelets (1.56; 1.20–2.03). No signals were observed for rivaroxaban or dabigatran. At the individual drug level, 62 OAC-CV pairs were identified as having potential interactions. Conclusions: Drug-specific interaction profiles should be considered to ensure safe and personalized use of OACs in clinical practice.
1. Introduction
Upper gastrointestinal (UGI) adverse events (AEs), such as abdominal discomfort, indigestion, ulcer-like symptoms, and gastritis, are frequently reported with a wide range of medications. While UGI AEs are generally not associated with life-threatening or clinically serious outcomes, they have the potential to reduce medication adherence and lead to discontinuation of treatment [1]. This, in turn, would negatively impact the overall treatment effectiveness. The risk of drug-induced UGI AEs may be increased in patients undergoing polypharmacy [2], and it is particularly pronounced in elderly patients due to physiological changes in the gastrointestinal (GI) tract associated with aging [3].
Among medications associated with UGI AEs, direct oral anticoagulants (DOACs) have recently garnered attention for their potential to cause these AEs. DOACs—including dabigatran, rivaroxaban, apixaban, and edoxaban—were introduced as alternatives to warfarin, aiming to overcome several of its key limitations, such as a narrow therapeutic range, extensive food and drug interactions, and the need for regular monitoring [4]. In current clinical practice, DOACs are the preferred options for several conditions requiring anticoagulation therapy, including atrial fibrillation and venous thromboembolism, due to their predictable pharmacokinetics and simplified management compared to warfarin [5,6]. Despite their benefits over warfarin, UGI safety has emerged as a growing concern based on findings from both clinical trials and post-marketing surveillance. This has prompted the US FDA to include the UGI risks in the official labeling of each DOAC. Among the DOACs, dabigatran has shown a relatively high frequency of UGI AEs. In a clinical trial (RE-LY), approximately 35% of patients who received 150 mg of dabigatran reported UGI symptoms such as abdominal pain, gastroesophageal reflux disease (GERD), esophagitis, and ulcers [7]. Post-marketing surveillance has suggested a potential association between dabigatran and the development of esophageal ulcers [8]. Rivaroxaban has similarly demonstrated GI events in clinical trials. In the UNIVERSE study, approximately 12.5% of patients experienced gastroenteritis [9], and in the EINSTEIN-DVT study, 2.7% of patients complained of abdominal pain [10]. For edoxaban, abdominal pain has been identified as an additional AE in post-marketing surveillance [8]. Although apixaban is not labeled for GI AEs other than nausea, approximately 10% of patients initiating apixaban were prescribed proton pump inhibitors (PPIs) within 6 months, possibly indicating underlying UGI tolerance [11]. Given these safety concerns regarding UGI AEs associated with DOACs, clinicians often prescribe acid-suppressive agents or switch to alternative OACs [11,12]. In a previously registered study, dyspepsia accounted for 6.6% of all causes of dabigatran discontinuation, including socioeconomic factors, and 18.9% when considering only adverse events. Additionally, PPI use was identified as a strong risk factor for discontinuation. These findings support the notion that UGI tolerability influences patient adherence and impacts clinicians’ prescribing behavior and monitoring strategies [13].
In real-world settings, patients prescribed DOACs are often concurrently treated with a variety of cardiovascular (CV) drugs, such as beta-adrenergic receptor blockers (BBs), angiotensin-converting enzyme (ACE) inhibitors, diuretics, and hydroxymethylglutaryl-CoA reductase inhibitors (statins) [14]. In a study conducted by Honda et al., more than 80% of patients with atrial fibrillation on DOAC therapy had comorbid CV conditions, and over 75% were concomitantly taking five or more medications [15]. Although drug–drug interactions involving DOACs have been extensively studied, they have primarily focused on bleeding risk and interactions with cytochrome P450 enzyme (CYP) or P-glycoprotein (P-gp) inhibitors [16,17,18]. In contrast, the potential impact of such interactions on UGI AEs remains largely unexplored. Therefore, this study aims to investigate the risk of UGI AEs associated with DOAC use and to assess the potential for drug–drug interactions that contribute to these events, using data from the FDA Adverse Event Reporting System (FAERS).
2. Results
2.1. Dataset Construction
From the FAERS database, covering 1 July 2014 to 31 December 2023, a total of 12,957,061 individual case safety reports (ICSRs) were identified after removing duplicates and retaining only the latest report for each case. Among these ICSRs, 12,905,290 ICSRs reported primary suspected drugs (dataset I). Subsequently, the other dataset encompassing 558,046 ICSRs was identified as involving oral anticoagulants (OACs). After excluding 12,029 ICSRs related to two or more OACs and 181,973 ICSRs with missing information on sex or age, 364,044 ICSRs were included as eligible for analysis (dataset II). Following the categorization of dataset II according to the OAC, it was determined that a warfarin sub-dataset was present in a total of 86,406 ICSRs, an apixaban sub-dataset in 126,824 ICSRs, a dabigatran sub-dataset in 30,157 ICSRs, an edoxaban sub-dataset in 7248 ICSRs, and a rivaroxaban sub-dataset in 113,409 ICSRs. The process of constructing these datasets is illustrated in Figure 1.
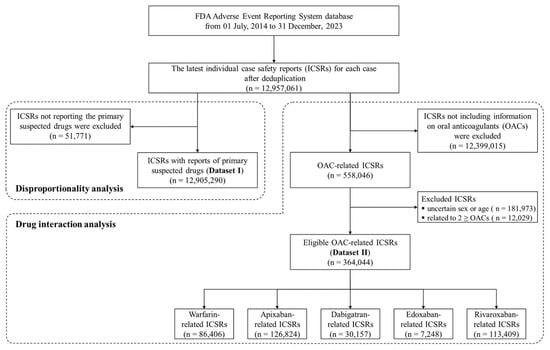
Figure 1.
Flowchart for constructing datasets in the study.
2.2. Disproportionality Analysis for OAC-Related UGI AEs
In dataset Ⅰ, there were 24,201, 122,471, 34,854, 6121, and 95,734 ICSRs reporting warfarin, apixaban, dabigatran, edoxaban, and rivaroxaban as primary suspected drugs, respectively. Only 3.2% of OAC-related ICSRs reported UGI AEs, with dabigatran showing the highest frequency at 4.7%. The results of the four metrics—the reporting odds ratio (ROR), proportional reporting ratio (PRR), information component (IC), and empirical Bayesian geometric mean (EBGM)—obtained from the disproportionality analysis are shown in Table 1. Only dabigatran showed statistically significant results for the ROR, PRR, and IC025.

Table 1.
Disproportionality analysis for OAC-related UGI AEs.
2.3. Descriptive Analysis for OAC-Related ICSRs
The demographic characteristics and co-medication information of the ICSRs in the five sub-datasets derived from dataset II are summarized in Table 2. The utilization of OACs was prevalent among individuals aged 40 years and older; however, there were notable variations in the age distribution of each OAC. Whereas warfarin and rivaroxaban demonstrated extensive utilization across all age groups, with a relatively high proportion of usage observed in the 40–64 age group, apixaban, dabigatran, and edoxaban accounted for more than half of those aged 75 years or older.

Table 2.
Demographic characteristics and co-medication information of the OAC-related ICSRs.
Co-medication was identified at a high rate among OAC users, with more than 70% of all users taking additional medications. Polypharmacy was particularly prevalent among warfarin and edoxaban users, at approximately 92% and 90%, respectively. The most frequently reported co-administered CV drug class was BB, used by between 24.2% and 41.4% of each OAC group. This was followed by diuretics (18.7–37.4%), statins (19.1–27.3%), anti-platelets (12.7–27.9%), angiotensin receptor blockers (ARBs; 13.2–22.6%), and ACE inhibitors (10.7–16.6%). Additionally, PPIs were commonly used for acid suppression and prevention of UGI AEs, with a prevalence ranging from 17.0% to 34.5%. The proportion of ICSRs receiving acid-suppressive therapy, including PPI use, was nearly twice as high with edoxaban at 39.7% compared to with other OACs.
Approximately 5% of OAC-related ICSRs involved UGI AEs: 5569 ICSRs (6.4%) in the warfarin group, 5735 (4.5%) in the apixaban group, 1698 (5.6%) in the dabigatran group, 456 (6.3%) in the edoxaban group, and 6231 (5.5%) in the rivaroxaban group.
2.4. Drug Interaction Analysis
For each OAC sub-dataset, exact matching was independently performed 14 times based on exposure to either a positive control or drugs of interest, resulting in 70 separate matched datasets. The matched datasets comprised approximately 79,000 ICSRs for warfarin (min, 77,636; max, 79,898), 84,000 for apixaban (min, 82,522; max, 84,170), 20,000 for dabigatran (min, 19,706; max, 20,446), 6000 for edoxaban (min, 5799; max, 6377), and 81,000 for rivaroxaban (min, 79,988; max, 81,634).
2.4.1. Drug Interaction of OACs and Positive Control
Within the matched OAC-related ICSRs, the concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs) was associated with a significantly increased risk of UGI AEs across all OAC types in the univariate analysis (Table 3). The crude reporting odds ratios (cRORs) indicated a 26% to 148% elevation in UGI AE risk. However, after adjusting for age, sex, and co-medication, dabigatran showed no statistically significant increase in risk (adjusted reporting odds ratio [aROR], 1.21; 95% CI, 0.97–1.49), suggesting a negligible drug interaction effect. In contrast, the increased risk remained significant for the other OACs: the aROR was 1.39 (95% CI, 1.25–1.54) for warfarin, 1.95 (95% CI, 1.78–2.14) for apixaban, 1.77 (95% CI, 1.29–2.40) for edoxaban, and 1.86 (95% CI, 1.71–2.03) for rivaroxaban.

Table 3.
Results of drug interaction analysis with NSAIDs.
2.4.2. Drug Interaction of OACs and Pharmacokinetic Modulators
In the matched dataset comparing CYP inhibitor users and non-users, concomitant use of CYP inhibitors ranged from 24.3% to 31.5% depending on the type of OAC. A total of 57 CYP inhibitors were evaluated, with amiodarone (24.7%), clopidogrel (23.7%), and diltiazem (18.5%) being the most frequently reported. In a separately matched dataset for P-gp inhibitors, approximately 16.8% of ICSRs involved P-gp use, with the highest proportion observed in the dabigatran group (25.6%) and the lowest in the rivaroxaban group (14.0%). The most commonly reported P-gp inhibitors were amiodarone (39.7%), digoxin (34.0%), and diltiazem (29.7%).
As shown in Figure 2a, the pooled analysis, which evaluated the use of the CYP inhibitor class in relation to UGI AE risk across each OAC, identified a significant signal only for warfarin (aROR, 1.19; 95% CI, 1.11–1.27). In individual drug analyses, six CYP inhibitors—clarithromycin, dasabuvir, duloxetine, ombitasvir, paritaprevir, and ticlopidine—were positively associated with warfarin. Additionally, one CYP inhibitor was associated with apixaban, two with dabigatran, and four each with edoxaban and rivaroxaban. On the other hand, with regard to P-gp inhibitors, the use of the P-gp inhibitor group was not significantly associated with UGI AEs in any OAC group (Figure 2b). Nine OAC–P-gp inhibitor pairs showed positive signals. The strongest association was observed between dabigatran and itraconazole (aROR, 9.07; 95% CI, 3.09–25.60), although this finding was based on only 16 cases. The results for individual drugs are listed in Supplementary Tables S1–S5.
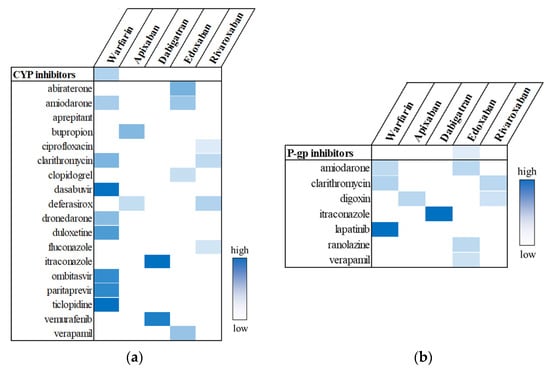
Figure 2.
Potential drug interactions between OACs and CYP inhibitors (a) or P-gp inhibitors (b). Statistically significant signals are highlighted in color, with greater intensity indicating higher aROR values.
2.4.3. Drug Interaction of OACs and Cardiovascular Drugs
To evaluate potential UGI AE risks associated with concomitant use of OACs and CV drugs, 11 separate matched datasets were constructed for each CV drug class. These datasets were developed independently, ensuring that each analysis was conducted with a mutually exclusive and statistically independent dataset.
A total of 139 CV drugs were included, encompassing BBs, ACE inhibitors, ARBs, dihydropyridine calcium channel blockers (DHP-CCBs), non-dihydropyridine calcium channel blockers (NDHP-CCBs), diuretics, statins, other lipid-lowering agents, amiodarone analogs, digitalis glycosides, and anti-platelets. The most frequently reported medications in each drug class were as follows: metoprolol (41.2%) for BBs, lisinopril (43.3%) for ACE inhibitors, losartan (36.0%) for ARBs, amlodipine (80.2%) for DHP-CCBs, diltiazem (79.9%) for NDHP-CCBs, furosemide (63.0%) for diuretics, atorvastatin (50.0%) for statins, ezetimibe (46.3%) for other lipid-lowering agents, amiodarone (91.8%) for amiodarone analogs, digoxin (93.4%) for digitalis glycosides, and aspirin (85.5%) for anti-platelets.
In the pooled analyses, several classes of CV drugs demonstrated statistically significant associations with an increased risk of UGI AEs when used concomitantly with specific OACs (Figure 3). A significant signal was observed for warfarin when co-administered with amiodarone analogs (aROR, 1.22; 95% CI, 1.04–1.43), though no other CV drug classes showed associations with warfarin. Apixaban showed several significant signals across a range of CV drug classes (Figure 3b). Increased UGI AE risks were observed in apixaban-related ICSRs in combination with ACE inhibitors (aROR, 1.34; 95% CI, 1.24–1.45), ARBs (aROR, 1.23; 95% CI, 1.14–1.33), DHP-CCBs (aROR, 1.30; 95% CI, 1.21–1.41), or digitalis glycosides (aROR, 1.72; 95% CI, 1.49–2.00). Additionally, as shown in Figure 3d, edoxaban was significantly associated with elevated UGI AE risk when co-administered with ARBs (aROR, 1.88; 95% CI, 1.48–2.37), amiodarone analogs (aROR, 1.73; 95% CI, 1.06–2.85), and anti-platelets (aROR, 1.56; 95% CI, 1.20–2.03). These findings suggested potential vulnerabilities when edoxaban is administered concurrently with these drug classes.
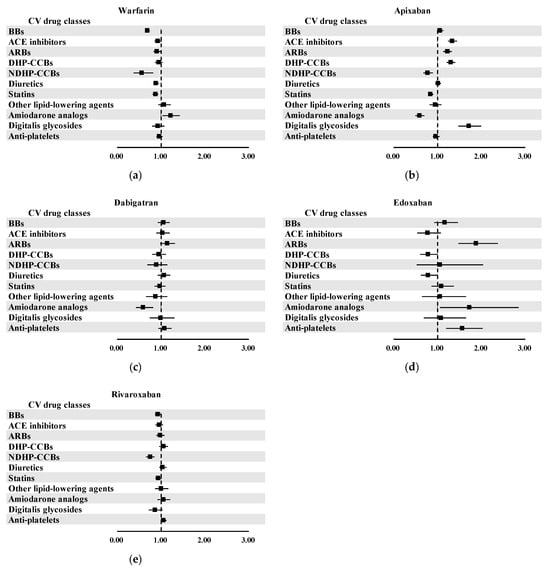
Figure 3.
Results of pooled analysis for potential drug interactions between OACs and CV drugs: (a) warfarin; (b) apixaban; (c) dabigatran; (d) edoxaban; and (e) rivaroxaban.
In the individual drug-level analyses, a total of 62 OAC-CV drug pairs showed statistically significant associations with UGI AEs: 8 for warfarin, 20 for apixaban, 10 for dabigatran, 12 for edoxaban, and 12 for rivaroxaban (Figure 4). Among these, the strongest signals were observed for apixaban–digitalis (aROR, 16.05; 95% CI, 3.94–61.20), apixaban–bendroflumethiazide (aROR, 11.63; 95% CI, 8.97–15.08), and apixaban–felodipine (aROR, 11.10; 95% CI, 8.91–13.81), suggesting a markedly elevated UGI AE risk for these specific combinations. A complete set of results, including both significant and non-significant OAC-CV drug pairs, is provided in Supplementary Tables S1–S5.
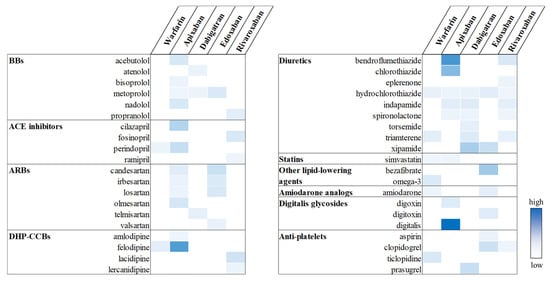
Figure 4.
Potential drug interactions between OACs and CV drugs. Statistically significant signals are highlighted in color, with greater intensity indicating higher aROR values.
3. Discussion
This exploratory pharmacovigilance study aimed to update the safety profile of OACs by evaluating their association with UGI AEs, using ICSRs from the FAERS database. Although only dabigatran exhibited a positive signal, suggesting an increased risk of UGI AEs in the disproportionality analysis, subsequent analysis of potential drug interactions with co-medications suggested that not only dabigatran but also other OACs require caution in clinical practice due to possible UGI complications. In particular, apixaban was identified as the OAC most frequently co-reported with CV drugs that have potential drug interactions, suggesting the need for careful consideration when prescribing apixaban and other CV drugs concomitantly.
Approximately 280,000 ICSRs reported OACs as the primary suspected drug in dataset Ⅰ, among which only 3.2% involved UGI AEs. This low reporting rate was attributed to reporting bias, whereby the reporting rate of AEs varies depending on their clinical significance, severity, and awareness [19]. As UGI AEs are often not considered clinically serious or life-threatening, they are often underreported compared to more severe AEs, such as major bleeding [20]. Indeed, despite the low reporting rate of UGI AEs, disproportionality analysis found a statistically significant signal for dabigatran, indicating a potential association with increased risk of UGI AEs. This finding is consistent with a previous study that suggested dabigatran is associated with a higher incidence of UGI AEs compared to warfarin [7].
Although no positive signals were observed for other OACs, investigating the incidence of UGI AEs remains essential for improving patient adherence and preventing potential complications [11]. UGI symptoms, including ulcer-like symptoms, acid reflux, and dyspepsia, are recognized risk factors for UGI bleeding [21]. This emphasizes the necessity of rigorously evaluating GI tolerability in patients prescribed OACs. Furthermore, even when the incidence of UGI AEs associated with a specific OAC is low, the risk could be amplified by concomitant use of interacting drugs [22,23]. This highlights the need for a comprehensive understanding of OAC-related UGI AEs in light of the potential drug interactions.
In the clinical setting, patients prescribed OACs are commonly exposed to polypharmacy rather than exclusive treatment with OACs. This is largely attributable to the high prevalence of comorbid CV disorders among OAC users, which often require concomitant use of CV drugs [15,16]. Given this clinical practice, the present study incorporated 11 CV drug classes as frequently co-administered medications in patients receiving OAC therapy, as well as CYP and P-gp inhibitors, which are the most important drugs evaluated for drug interaction. Previous drug interaction studies using ICSRs have employed a metric approach, wherein two drugs of interest are categorized into four classes of exposure and then evaluated for the occurrence of AEs [24,25,26]. While the metric approach is effective for assessing whether the interaction of two drugs is additive, synergistic, or antagonistic, it has limitations when it comes to considering the effects of other drugs. Therefore, in this study, a multivariate logistic regression analysis was employed to provide a more comprehensive reflection of the effects of CV drugs frequently used by patients taking OACs [27,28].
Given that NSAIDs are well documented to cause UGI AEs such as gastric ulcers and heartburn, they were used as a positive control in the drug interaction analysis for OACs [29]. A potential increase in the UGI AE risk was observed for four of the five OACs, excluding dabigatran. The absence of a signal for dabigatran is explained by the heightened clinical awareness of its UGI risk profile, which has been extensively documented since the RE-LY trial in 2009 [7]. These risks were recognized early after the approval of dabigatran, and clinical guidelines have since recommended the concomitant use of acid suppressants and the avoidance of NSAIDs to mitigate GI bleeding risk [30,31]. As our study included ICSRs from July 2014, it is plausible that these precautions were already being implemented, resulting in more selective NSAID use in patients treated with dabigatran—possibly those at lower risk of UGI AEs. Conversely, the UGI risk profiles of other OACs were not well established at the time of approval. Even now, these risks remain insufficiently characterized or have recently been identified through post-marketing surveillance [8]. The differences in the available GI risk profiles across OACs have influenced prescribing behaviors, particularly the more conservative administration of NSAIDs in dabigatran users. It may have resulted in selection bias and attenuated the observed signal. Additionally, underreporting in spontaneous reporting systems may have contributed to the absence of a detected interaction.
Drug-induced UGI symptoms, including abdominal pain, gastric discomfort, and dyspepsia, are commonly associated with many drugs used in clinical practice. While a variety of medications are implicated in UGI AEs, the underlying mechanisms have been well characterized only for a few drug classes, notably NSAIDs and anticholinergics. For most other drugs, the mechanisms remain largely unexplored [32,33]. Among the OACs, only dabigatran was studied for its association with UGI AEs, which are attributed to the tartaric acid in its formulation rather than dabigatran itself [34,35]. Although UGI AEs are generally less severe than other AEs such as bleeding, they can affect patient adherence. Hwang et al. reported that UGI AEs were the most common reason for discontinuation of dabigatran [36]. Current clinical guidelines from the American College of Cardiology/American Heart Association, the European Society of Cardiology, the American Society of Hematology, and the American College of Chest Physicians recommend OAC therapy ranging from several months to lifelong use. Given this, managing UGI tolerability issues is essential for achieving long-term treatment success. However, these guidelines address drug interactions and polypharmacy primarily from the perspective of reducing bleeding risk and do not provide specific recommendations regarding UGI AEs [5,6,37,38].
To address this gap, the present study focused on UGI tolerability under polypharmacy conditions involving OAC-CV drug combinations and identified several potential interactions. In particular, a high frequency of positive signals was observed for apixaban when co-administered with CV drugs. Apixaban has been perceived as safer than other OACs in vulnerable patients with an elevated bleeding risk, such as those with reduced renal function, advanced age, and low body weight, compared with other OACs [39,40,41]. This perception has led to its higher frequency of use in clinical practice [42]. The positive signals observed for UGI AEs may have been attributable to the intensive use of apixaban in these vulnerable patients. Given this pattern of use, particular attention should be paid to the potential risk of UGI AEs in these high-risk populations. In this context, caution is warranted when interpreting the strong signal observed with the concomitant use of apixaban and digitalis glycosides. Digitalis glycosides are well established to be associated with gastrointestinal toxicity [43], supporting the possibility of a true pharmacologic interaction. At the same time, the absence of a similar signal with other DOACs indicates that residual confounding related to patient characteristics and prescribing preferences may also have contributed. Moreover, combinations involving diuretics–OACs and ARBs–OACs were frequently associated with positive signals, suggesting a broader scope of potential drug-related UGI risks. These findings emphasize the need to evaluate UGI AEs at both the individual OAC and co-administered CV drug levels, thereby providing a complementary perspective to current guidance regarding UGI tolerability.
Several limitations are present in this study. Firstly, the FAERS data have inherent limitations such as underreporting, reporting bias, and the absence of denominator data, which could influence the results and warrant caution in their interpretation. Although these factors affect both disproportionality and drug interaction analyses, certain biases—such as those arising from market share differences—are less likely to affect the drug interaction analysis because comparisons were restricted within each OAC user group, using those without the specific CV drug as the reference. Secondly, the ICSR data from the FAERS is incomplete. The omission of non-suspect drugs frequently occurred, resulting in the underreporting of co-medications. In addition, the incorporation of other important clinical information, such as indications and dosage, often rendered the data inadequate for reliable assessment. To mitigate such incompleteness, ICSRs with missing data on age and sex were excluded, and the number of co-medications was incorporated as a matching variable to adjust for the overall medication burden. Thirdly, this study evaluated signals through a comparison of OAC monotherapy and OACs co-administered with CV drugs; however, there was no identification of the UGI AE risk associated with CV drugs alone. The potential interactions between OACs and non-CV drugs have been insufficiently accounted for in this analysis. An additional important limitation of the present study is that it included only a limited number of CV drugs. In real-world clinical settings, patients are frequently prescribed a broader range of pharmacotherapies.
Despite these limitations, the present study has several methodological strengths that enhance the validity and applicability of the findings. First, we established a clinically relevant definition of UGI AEs by screening approximately 2400 lowest-level terms (LLTs), as the identification of UGI AEs was not standardized in the FAERS database. This rigorous selection process ensured a comprehensive and systematic approach to defining UGI AEs and improved the consistency and clinical interpretability of the definition. Second, a total of 195 drugs, spanning 11 CV drug classes and pharmacokinetic modulators, were included in the present study. This extensive inclusion reflects real-world prescribing patterns, where patients on OAC therapy are commonly exposed to these drugs. By encompassing a broad and clinically significant range of co-medications, the study improves the generalizability and external applicability of its results to clinical practice. Third, independently matched datasets were constructed for each drug of interest to minimize the confounding effects of co-medications and to allow for more precise attribution of the UGI AE risk to specific OAC-CV drug pairs. Such class-specific matching enhances the internal validity of the analysis, facilitating a more focused interpretation of drug interactions in clinical decision making. Several combinations of OACs with commonly used CV drugs, such as ACE inhibitors, ARBs, DHP-CCBs, digitalis glycosides, and amiodarone analogs, were associated with statistically significant signals for UGI AEs. These findings highlight the need for caution when prescribing OACs alongside such medications and support the development of tailored OAC strategies based on co-medication profiles to ensure UGI safety.
4. Materials and Methods
4.1. Data Resource and Dataset Construction
We analyzed ICSRs available through the FAERS database from 1 July 2014 to 31 December 2023. The FAERS database was composed of seven structured ASCII files, which were merged for analysis. To address duplicate reporting for the same ICSR, records were deduplicated based on the unique ICSR identifier, age, sex, active pharmaceutical ingredient of the product, adverse drug event, indication, reporting date, and reporting country. Only the most recent report per ICSR was retained to ensure data consistency.
Two distinct datasets were then constructed for analysis. Dataset I was created by selecting records in which the drug was reported as the primary suspect and used for traditional disproportionality analysis. The other dataset, dataset II, was established by extracting ICSRs mentioning warfarin, apixaban, dabigatran, edoxaban, or rivaroxaban from the deduplicated latest report dataset, regardless of their role in the report. ICSRs involving more than one OAC or lacking definitive information on age or sex were subsequently excluded. Dataset II was used to evaluate potential drug interactions of OACs subsequently divided into five sub-datasets, each corresponding to one of the OACs.
4.2. Definition of Upper Gastrointestinal Adverse Events
UGI AEs of interest were defined using the LLTs from the Medical Dictionary for Regulatory Activities (MedDRA) version 26.0 (Maintenance and Support Organization, McLean, VA, USA). We reviewed 2384 LLTs included in the following two level 1 Standardized MedDRA Queries (SMQs): GI perforation, ulceration, hemorrhage, or obstruction and GI nonspecific inflammation and dysfunctional conditions. To refine the selection toward UGI-specific events, LLTs anatomically related to the oral cavity, esophagus, stomach, and duodenum were included, while those associated with the jejunum, ileum, cecum, colon, sigmoid, rectum, and anus were excluded. With respect to the clinical symptoms, the focus was on LLTs indicative of UGI discomfort, dyspepsia, reflux, ulcers, and inflammation. Consequently, 545 LLTs were identified as UGI AEs of interest (Supplementary Table S6).
4.3. Disproportionality Analysis
The conventional disproportionality analysis was conducted by creating a two-by-two contingency table: A, number of ICSRs exposed to an OAC and experiencing UGI AEs; B, number of ICSRs exposed to an OAC but not experiencing UGI AEs; C, number of ICSRs not exposed to an OAC and experiencing UGI AEs; and D, number of ICSRs not exposed to an OAC and not experiencing UGI AEs [44]. Subsequently, the association between OACs and UGI AEs was determined using four metrics: ROR, PRR, IC, and EBGM. The formulas for these metrics are shown in Table 4.

Table 4.
Formulas for disproportionality analysis metrics.
4.4. Definition of Drugs of Interest
4.4.1. Positive Control
To reinforce the methodological rigor of the analysis, NSAIDs were incorporated as positive controls, given their well-established association with UGI AEs [45]. The NSAIDs included in this study are listed in Table 5, along with other drugs of interest.

Table 5.
List of drugs of interest.
4.4.2. Pharmacokinetic Modulators
Drugs that could pharmacokinetically influence the plasma concentration of OACs were identified as drugs of interest. These included CYP and P-gp inhibitors, which increase systemic exposure to OACs and thereby elevate the risk of UGI AEs. The definition of CYP and P-gp inhibitors was based on the US FDA categorization of modulators as either moderate or strong [46]. Additionally, diltiazem and digoxin, which were not included in the FDA classification, were defined as P-gp inhibitors based on their well-known P-gp inhibiting properties [47].
CYP inducers were excluded from the evaluation of direct effects in the drug interaction analysis. This was due to the fact that risk-reducing effects could not be assessed using ICSR data, which includes only cases reporting AEs. However, we defined CYP inducers according to the US FDA’s definition of moderate or strong inducers in order to adjust for their potential effects as covariates in the drug interaction analysis. The drug list of CYP inhibitors, P-gp inhibitors, and CYP inducers is provided in Table 5.
4.4.3. Cardiovascular Drugs
CV drugs are among the most commonly prescribed co-medications in patients receiving OAC therapy. To assess potential drug interactions of clinical relevance, eleven classes of CV drugs were defined as drugs of interest: BBs, ACE inhibitors, ARBs, DHP-CCBs, NDHP-CCBs, diuretics, statins, other lipid-lowering agents, amiodarone analogs, digitalis glycosides, and anti-platelets. A list of drugs included in each class is presented in Table 5.
4.4.4. Acid-Suppressive Agents
Acid-suppressive agents, which affect the occurrence of UGI AEs, were included as covariates in this study. These agents included PPIs, histamine 2 receptor antagonists (H2RAs), and potassium-competitive acid blockers (PCABs). The included drugs in each class are listed in Table 5.
4.5. Drug Interaction Analysis
4.5.1. Classification of OAC-Related ICSRs
The drug interaction analysis was conducted using dataset Ⅱ, which included only ICSRS related to OACs. The classification of these ICSRs was based on two criteria: exposure to drugs of interest as co-medication and experience of UGI AEs. The OAC-related ICSRs were then categorized into four groups: N00, no exposure to drugs of interest and no experience of UGI AEs; N01, no exposure to drugs of interest and experience of UGI AEs; N10, exposure to drugs of interest and no experience of UGI AEs; and N11, exposure to drugs of interest and experience of UGI AEs. The initial classification was conducted based on pharmacologic drug classes (e.g., BBs, ACE inhibitors, ARBs), CYP inhibitors, and P-gp inhibitors to enable class-level comparison in the pooled analysis. For individual drug-level analysis, N10 and N11 were redefined for each specific drug (e.g., atenolol, bisoprolol, metoprolol), while N00 and N01 remained fixed as ICSRs unexposed to any drug within the corresponding drug class.
4.5.2. Confounding Factors and Matching
In order to address confounding factors associated with the occurrence of UGI AEs, the following variables were included in the analysis: age, sex, acid-suppressive agents, and the number of co-medications. Co-medications were defined as all concurrently reported drugs within a single ICSR. The number of co-medications was stratified into four categories: 1 (only OAC), 2–4, 5–9, and ≥10. Utilizing these confounding factors as covariates, exact matching was conducted for each of the 14 drug classes of interest—including NSAIDs, CYP inhibitors, P-gp inhibitors, and 11 CV drug classes. This process was applied to the five sub-datasets within dataset II to minimize bias by excluding unmatched outliers while preserving the heterogeneity of real-world populations.
4.5.3. Logistic Regression
To estimate the association between co-medications and the risk of UGI AEs in patients receiving OACs, we employed logistic regression analyses. A univariate logistic regression was performed to evaluate the effect of each co-medication on the risk of UGI AEs, estimating cRORs, which were consistent with those derived from the ROR formula described in Table 4. Subsequently, a multivariate logistic regression analysis was conducted to obtain aRORs, incorporating potential confounding factors. These included age, sex, the number of co-medications, acid-suppressive agents, and the concomitant use of NSAIDs, CYP inhibitors, P-gp inhibitors, CYP inducers, and CV drugs.
The estimation of both cRORs and aRORs was conducted only when both N10 and N11 were ≥3 to ensure statistical reliability and minimize bias from sparse data. All analyses were performed with R software version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
5. Conclusions
This exploratory study evaluated potential drug interactions between OACs and commonly co-prescribed CV drugs, focusing on their association with UGI AEs. In the pooled analysis, apixaban showed positive drug interaction signals with ACE inhibitors, ARBs, DHP-CCBs, and digitalis glycosides. At the individual drug level, apixaban was associated with a broader range of CV drugs showing potential interactions compared to other OACs. Our findings highlight the importance of recognizing drug-specific interaction profiles, particularly in the context of polypharmacy with CV drugs, to ensure safe and personalized OAC therapy in clinical practice. For high-risk patients receiving OAC-CV drug combinations, clinicians should closely monitor UGI symptoms and consider the concomitant use of GI protective agents to mitigate potential risks. These considerations help minimize the risk of reduced adherence and treatment discontinuation owing to UGI AEs, ultimately improving the patient outcomes of OACs. Further large-scale cohort studies are warranted to validate these observations and inform clinical decision making.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph18091311/s1: Supplementary Table S1: Potential drug interactions between warfarin and drugs of interest. Supplementary Table S2: Potential drug interactions between apixaban and drugs of interest. Supplementary Table S3: Potential drug interactions between dabigatran and drugs of interest. Supplementary Table S4: Potential drug interactions between edoxaban and drugs of interest. Supplementary Table S5: Potential drug interactions between rivaroxaban and drugs of interest. Supplementary Table S6: Definition of upper gastrointestinal adverse events.
Author Contributions
Conceptualization, Y.S.K. and J.-E.C.; methodology, S.C. and J.-E.C.; software, S.C.; validation, S.C., Y.S.K. and J.-E.C.; formal analysis, S.C., J.P. and D.O.; investigation, S.C., J.P. and D.O.; data curation, S.C.; writing—original draft preparation, S.C.; writing—review and editing, J.-E.C.; visualization, S.C.; supervision, J.-E.C.; project administration, Y.S.K. and J.-E.C.; funding acquisition, J.-E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (RS-2023-00217123, 2022R1F1A1075439) and the Research Fund of Hanyang University (HY-2025-1103).
Institutional Review Board Statement
The requirement for ethical approval was waived due to the retrospective nature of the study.
Informed Consent Statement
The requirement for written informed consent was waived due to the retrospective nature of the study.
Data Availability Statement
The FDA Adverse Event Reporting System (FAERS) quarterly data extract files used in this study are publicly available at the following link: https://www.fda.gov/drugs/fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files (accessed on 3 April 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE | Angiotensin-converting enzyme |
| AE | Adverse event |
| ARB | Angiotensin receptor blocker |
| aROR | Adjusted reporting odds ratio |
| BB | Beta-adrenergic receptor blocker |
| CI | Confidence interval |
| cROR | Crude reporting odds ratio |
| CV | Cardiovascular |
| CYP | Cytochrome P450 enzyme |
| DHP-CCB | Dihydropyridine calcium channel blocker |
| DOAC | Direct oral anticoagulant |
| EBGM | Empirical Bayesian geometric mean |
| FAERS | Food and Drug Administration Adverse Event Reporting System |
| GERD | Gastroesophageal reflux disease |
| GI | Gastrointestinal |
| H2RA | Histamine 2 receptor antagonist |
| IC | Information component |
| ICSR | Individual case safety report |
| LCI | Lower limit of 95% confidence interval |
| LLT | Lowest-level term |
| NDHP-CCB | Non-dihydropyridine calcium channel blocker |
| NSAID | Non-steroidal anti-inflammatory drug |
| OAC | Oral anticoagulant |
| PCAB | Potassium-competitive acid blocker |
| P-gp | P-glycoprotein |
| PPI | Proton pump inhibitor |
| PRR | Proportional reporting ratio |
| ROR | Reporting odds ratio |
References
- Insani, W.N.; Wei, L.; Abdulah, R.; Alfian, S.D.; Ramadhani, N.A.; Andhika, R.; Zakiyah, N.; Adesuyan, M.; Pamela, Y.; Mustafa, R.; et al. Exploring the association of adverse drug reactions with medication adherence and quality of life among hypertensive patients: A cross-sectional study. Int. J. Clin. Pharm. 2025, 47, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Al Ramahi, R.; Tumeh, D. The prevalence and severity of upper gastrointestinal complications among patients with chronic diseases: A cross-sectional study from Palestine. BMC Gstroenterol. 2024, 24, 175. [Google Scholar] [CrossRef]
- Goriacko, P.; Veltri, K.T. Adverse Drug Effects Involving the Gastrointestinal System (Pharmacist Perspective). In Geriatric Gastroenterology, 2nd ed.; Pitchumoni, C.S., Dharmarajan, T.S., Eds.; Springer: Cham, Switzerland, 2021; pp. 297–339. [Google Scholar]
- Witt, D.M.; Clark, N.P.; Kaatz, S.; Schnurr, T.; Ansell, J.E. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 187–205. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3314–3414, Erratum in Eur. Heart J. 2025, ahead of print. [Google Scholar]
- Stevens, S.M.; Woller, S.C.; Baumann Kreuziger, L.; Doerschug, K.; Geersing, G.-J.; Klok, F.A.; King, C.S.; Murin, S.; Vintch, J.R.E.; Wells, P.S.; et al. Antithrombotic Therapy for VTE Disease: Compendium and Review of CHEST Guidelines 2012–2021. Chest 2024, 166, 388–404. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- FDALabel: Full-Text Search of Drug Product Labeling. Available online: https://nctr-crs.fda.gov/fdalabel/ui/search (accessed on 11 July 2025).
- McCrindle, B.W.; Michelson, A.D.; Van Bergen, A.H.; Suzana Horowitz, E.; Pablo Sandoval, J.; Justino, H.; Harris, K.C.; Jefferies, J.L.; Miriam Pina, L.; Peluso, C.; et al. Thromboprophylaxis for Children Post-Fontan Procedure: Insights From the UNIVERSE Study. J. Am. Heart Assoc. 2021, 10, e021765. [Google Scholar] [CrossRef]
- Prins, M.H.; Lensing, A.W.; Bauersachs, R.; van Bellen, B.; Bounameaux, H.; Brighton, T.A.; Cohen, A.T.; Davidson, B.L.; Decousus, H.; Raskob, G.E.; et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: A pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb. J. 2013, 11, 21. [Google Scholar] [CrossRef]
- Zielinski, G.D.; Teichert, M.; Klok, F.A.; Rosendaal, F.R.; Huisman, M.V.; Cannegieter, S.C.; Lijfering, W.M. Direct oral anticoagulant use and subsequent start of proton pump inhibitors as proxy for gastric complaints. Pharmacoepidemiol. Drug Saf. 2018, 27, 1371–1378. [Google Scholar] [CrossRef]
- Adelakun, A.R.; Turgeon, R.D.; De Vera, M.A.; McGrail, K.; Loewen, P.S. Oral anticoagulant switching in patients with atrial fibrillation: A scoping review. BMJ Open 2023, 13, e071907. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; Franca, L.R.; Diener, H.C.; Lu, S.; Dubner, S.J.; Ma, C.S.; Rothman, K.J.; Zint, K.; Halperin, J.L.; Olshansky, B.; et al. Dabigatran persistence and outcomes following discontinuation in atrial fibrillation patients from the GLORIA-AF registry. Am. J. Cardiol. 2020, 125, 383–391. [Google Scholar] [CrossRef]
- Proietti, M.; Vitolo, M.; Lip, G.Y.H. Integrated care and outcomes in patients with atrial fibrillation and comorbidities. Eur. J. Clin. Investig. 2021, 51, e13498. [Google Scholar] [CrossRef]
- Honda, T.; Abe, K.; Oda, M.; Harada, F.; Maruyama, K.; Aoyagi, H.; Miura, R.; Konno, K.; Arizumi, T.; Asaoka, Y.; et al. Gastrointestinal Bleeding During Direct Oral Anticoagulant Therapy in Patients With Nonvalvular Atrial Fibrillation and Risk of Polypharmacy. J. Clin. Pharmacol. 2022, 62, 1548–1556. [Google Scholar] [CrossRef]
- Foerster, K.I.; Hermann, S.; Mikus, G.; Haefeli, W.E. Drug-Drug Interactions with Direct Oral Anticoagulants. Clin. Pharmacokinet. 2020, 59, 967–980. [Google Scholar] [CrossRef]
- Hirsh Raccah, B.; Rottenstreich, A.; Zacks, N.; Muszkat, M.; Matok, I.; Perlman, A.; Kalish, Y. Drug interaction as a predictor of direct oral anticoagulant drug levels in atrial fibrillation patients. J. Thromb. Thrombolysis 2018, 46, 521–527. [Google Scholar] [CrossRef]
- Schaefer, J.K.; Errickson, J.; Kong, X.; Ali, M.A.; Chipalkatti, N.; Dorby, P.; Giuliano, C.; Haymart, B.; Kaatz, S.; Kurlander, J.E.; et al. Outcomes of Oral Anticoagulation with Concomitant NSAID Use: A Registry Based Cohort Study. Blood 2023, 142, 5129. [Google Scholar] [CrossRef]
- Peng, L.; Kui, X.; Silvia, O.; Justin, S.; Wang, Y.-J. A real-world disproportionality analysis of FDA Adverse Event Reporting System (FAERS) events for baricitinib. Expert Opin. Drug Saf. 2020, 19, 1505–1511. [Google Scholar] [CrossRef]
- Alatawi, Y.M.; Hansen, R.A. Empirical estimation of under-reporting in the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS). Expert Opin. Drug Saf. 2017, 16, 761–767. [Google Scholar] [CrossRef]
- Abrignani, M.G.; Lombardo, A.; Braschi, A.; Renda, N.; Abrignani, V. Proton pump inhibitors and gastroprotection in patients treated with antithrombotic drugs: A cardiologic point of view. World J. Cardiol. 2023, 15, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Magro, L.; Moretti, U.; Leone, R. Epidemiology and characteristics of adverse drug reactions caused by drug-drug interactions. Expert Opin. Drug. Saf. 2012, 11, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lin, Y.; Ren, W.; Fang, Z.; Liu, Y.; Tan, X.; Lv, X.; Zhang, N. Adverse drug reactions and correlations with drug-drug interactions: A retrospective study of reports from 2011 to 2020. Front. Pharmacol. 2022, 13, 923939. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jian, X.; Ting, Y.; Ling, H.; Ruwen, C.; Huimin, Y.; Jingyang, L.; Cheng, S. Analysis of hemorrhagic drug-drug interactions between P-gp inhibitors and direct oral anticoagulants from the FDA Adverse Event Reporting System. Expert Opin. Drug Saf. 2024, 23, 1453–1461. [Google Scholar] [CrossRef]
- Honma, T.; Onda, K.; Masuyama, K. Drug-drug interaction assessment based on a large-scale spontaneous reporting system for hepato- and renal-toxicity, and thrombocytopenia with concomitant low-dose methotrexate and analgesics use. BMC Pharmacol. Toxicol. 2024, 25, 13. [Google Scholar] [CrossRef]
- Sridharan, K.; Sivaramakrishnan, G. Amlodipine-Associated Angioedema: An Integrated Pharmacovigilance Assessment Using Disproportionality and Interaction Analysis and Case Reviews. J. Clin. Med. 2025, 14, 1097. [Google Scholar] [CrossRef]
- van Puijenbroek, E.P.; Egberts, A.C.; Heerdink, E.R.; Leufkens, H.G. Detecting drug-drug interactions using a database for spontaneous adverse drug reactions: An example with diuretics and non-steroidal anti-inflammatory drugs. Eur. J. Clin. Pharmacol. 2000, 56, 733–738. [Google Scholar] [CrossRef]
- Kobayashi, S.; Sugama, N.; Nagano, H.; Miyamori, A.; Takahashi, M.; Kushiyama, A. Analysis of Adverse Events of Cholinesterase Inhibitors and NMDA Receptor Antagonists on Arrhythmias Using the Japanese Adverse Drug Event Report Database. Drugs-Real World Outcomes 2023, 10, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Sato, K.; Kudara, N.; Shinozaki, H.; Ikeda, K.; Sato, K.; Endo, M.; Orii, S.; Suzuki, K. Upper gastrointestinal disorders induced by non-steroidal anti-inflammatory drugs. Inflammopharmacology 2008, 16, 16–20. [Google Scholar] [CrossRef]
- Nieuwlaat, R.; Healey, J.S.; Ezekowitz, M.; Reilly, P.; Formella, S.; Wallentin, L.; Yusuf, S.; Connolly, S. Management of dyspepsia symptoms on dabigatran during RELY-ABLE: Long-term follow up study after RE-LY. Eur. Heart. J. 2013, 34 (Suppl. S1), 549. [Google Scholar] [CrossRef]
- Bytzer, P.; Connolly, S.J.; Yang, S.; Ezekowitz, M.; Formella, S.; Reilly, P.A.; Aisenberg, J. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin. Gastroenterol. Hepatol. 2013, 11, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Leong, R.W.; Chan, F.K. Drug-induced side effects affecting the gastrointestinal tract. Expert Opin. Drug Saf. 2006, 5, 585–592. [Google Scholar] [CrossRef]
- Philpott, H.L.; Nandurkar, S.; Lubel, J.; Gibson, P.R. Drug-induced gastrointestinal disorders. Frontline Gastroenterol. 2014, 5, 49–57. [Google Scholar] [CrossRef]
- Toya, Y.; Nakamura, S.; Tomita, K.; Matsuda, N.; Abe, K.; Abiko, Y.; Orikasa, S.; Akasaka, R.; Chiba, T.; Uesugi, N.; et al. Dabigatran-induced esophagitis: The prevalence and endoscopic characteristics. J. Gastroenterol. Hepatol. 2016, 31, 610–614. [Google Scholar] [CrossRef]
- Yamashita, T.; Watanabe, E.; Ikeda, T.; Shiga, T.; Kusano, K.F.; Takahashi, N.; Takahashi, T.; Nozaki, A.; Kasao, M.; Fukatsu, T.; et al. Observational study of the effects of dabigatran on gastrointestinal symptoms in patients with non-valvular atrial fibrillation. J. Arrythm. 2014, 30, 478–484. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, S.R.; Park, H.S.; Lee, Y.S.; Ahn, J.H.; Choi, J.I.; Shin, D.G.; Kim, D.K.; Park, J.S.; Hwang, K.W.; et al. Adherence to dabigatran and the influence of dabigatran-induced gastrointestinal discomfort in the real-world practice. Int. J. Cardiol. 2021, 323, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar] [CrossRef]
- Oh, S.-H.; Cheon, S.; Choi, S.-Y.; Kim, Y.S.; Choi, H.-G.; Chung, J.-E. Effectiveness and Safety of Dose-Specific DOACs in Patients With Atrial Fibrillation: A Systematic Review and Network Meta-Analysis. Cardiovasc. Ther. 2025, 2025, 9923772. [Google Scholar] [CrossRef]
- Rutherford, O.W.; Jonasson, C.; Ghanima, W.; Söderdahl, F.; Halvorsen, S. Comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in atrial fibrillation: A nationwide cohort study. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.C.Y.; Torre, C.O.; Man, K.K.C.; Stewart, H.M.; Seager, S.; Van Zandt, M.; Reich, C.; Li, J.; Brewster, J.; Lip, G.Y.H.; et al. Comparative Effectiveness and Safety Between Apixaban, Dabigatran, Edoxaban, and Rivaroxaban Among Patients With Atrial Fibrillation: A Multinational Population-Based Cohort Study. Ann. Intern. Med. 2022, 175, 1515–1524, Erratum in Ann. Intern. Med. 2023, 176, 144.. [Google Scholar] [CrossRef] [PubMed]
- Mandt, S.R.; Thadathil, N.; Klem, C.; Russ, C.; McNamee, P.L.; Stigge, K.; Cheng, D. Apixaban Use in Patients with Kidney Impairment: A Review of Pharmacokinetic, Interventional, and Observational Study Data. Am. J. Cardiovasc. Drugs 2024, 24, 603–624. [Google Scholar] [CrossRef]
- Andrews, P.; Anseeuw, K.; Kotecha, D.; Lapostolle, F.; Thanacoody, R. Diagnosis and practical management of digoxin toxicity: A narrative review and consensus. Eur. J. Emerg. Med. 2023, 30, 395–401. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, R.; Zhou, Q.; Shen, Y.; Zhu, H. Analysis of post-market adverse events of istradefylline: A real-world study base on FAERS database. Sci. Rep. 2024, 14, 7659. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.M. NSAID-induced Gastrointestinal Injury: A Focused Update for Clinicians. J. Clin. Gastroenterol. 2016, 50, 5–10. [Google Scholar] [CrossRef] [PubMed]
- FDA’s Examples of Drugs that Interact with CYP Enzymes and Transporter Systems. Available online: https://www.fda.gov/drugs/drug-interactions-labeling/healthcare-professionals-fdas-examples-drugs-interact-cyp-enzymes-and-transporter-systems (accessed on 11 July 2025).
- Wessler Jeffrey, D.; Grip Laura, T.; Mendell, J.; Giugliano Robert, P. The P-Glycoprotein Transport System and Cardiovascular Drugs. J. Am. Coll. Cardiol. 2013, 61, 2495–2502, Erratum in J. Am. Coll. Cardiol. 2014, 63, 2176.. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).