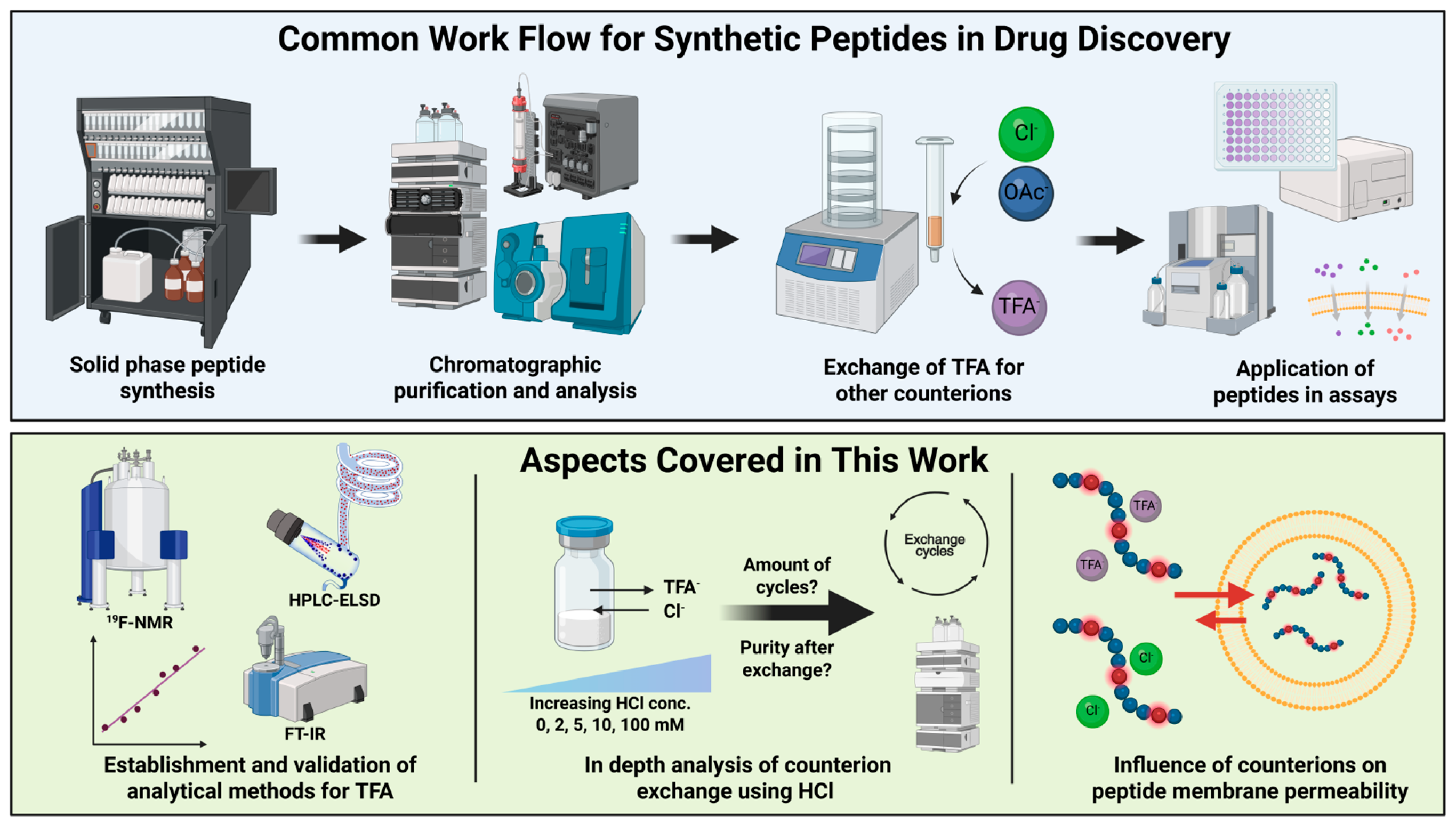
Towards a Consensus for the Analysis and Exchange of TFA as a Counterion in Synthetic Peptides and Its Influence on Membrane Permeation
Abstract
1. Introduction
2. Results and Discussion
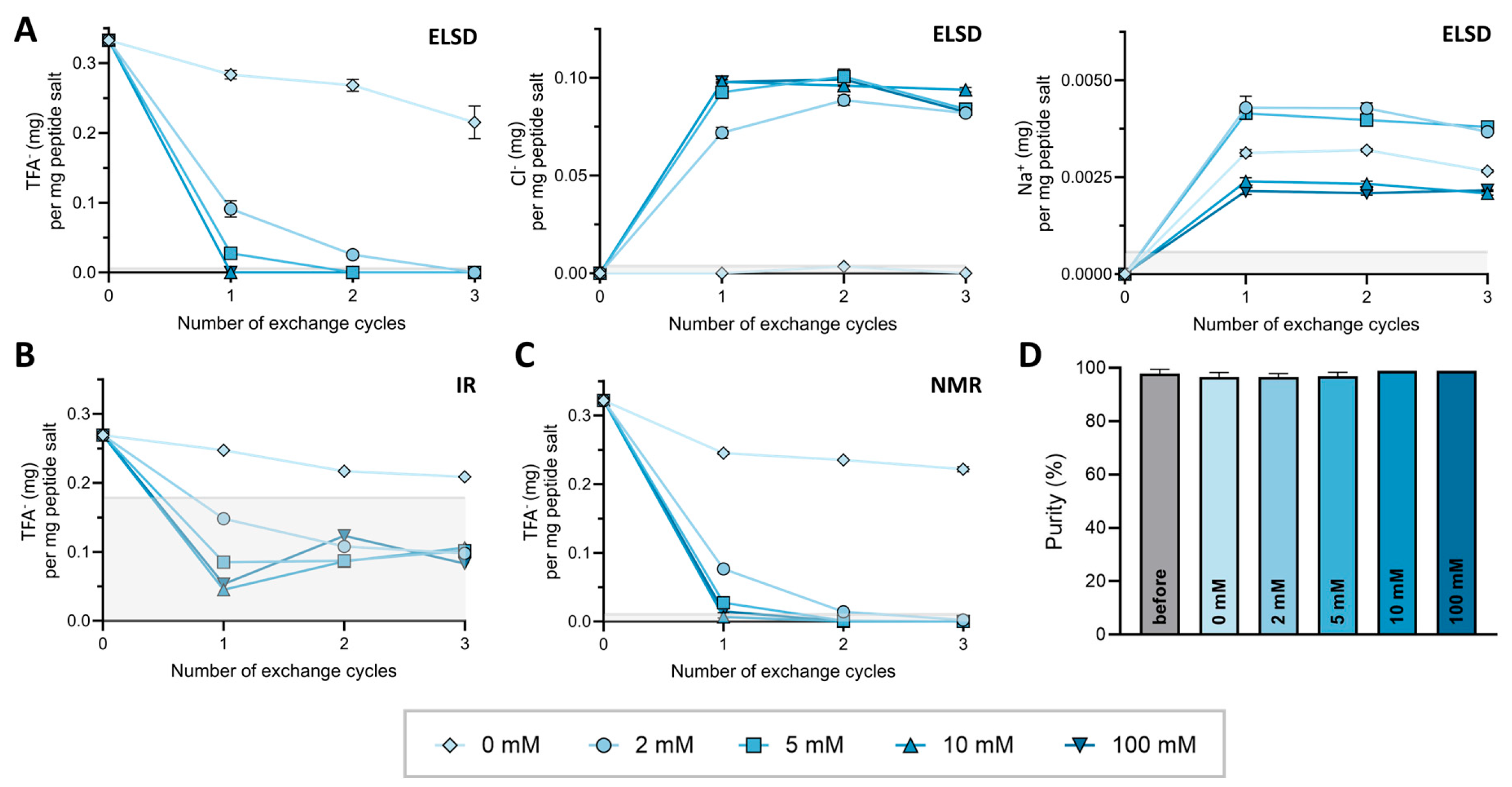
2.1. Method Development, Validation, and Comparison for TFA Quantification
2.2. Testing Different Counterion Exchange Protocols
2.3. Effect of Peptide Sequence on Counterion Exchange
2.4. Influence of Counterions on Passive Membrane Permeation
3. Material and Methods
3.1. Method Development and Validation for TFA− Detection
3.2. Peptide Synthesis, Purification, and Characterization
3.3. Monitoring of Counterion Exchange
3.4. Liposomal Fluorescence Assay for Permeation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.J.; de Campos, L.J.; Xing, H.; Conda-Sheridan, M. Peptide-based therapeutics: Challenges and solutions. Med. Chem. Res. 2024, 33, 1275–1280. [Google Scholar] [CrossRef]
- Erckes, V.; Steuer, C. A story of peptides, lipophilicity and chromatography—Back and forth in time. RSC Med. Chem. 2022, 13, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, R.B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Chan, W.; White, P. Fmoc Solid Phase Peptide Synthesis: A Practical Approach; Oxford University Press: Oxford, UK, 1999. [Google Scholar] [CrossRef]
- Carpino, L.A.; Han, G.Y. 9-Fluorenylmethoxycarbonyl function, a new base-sensitive amino-protecting group. J. Am. Chem. Soc. 1970, 92, 5748–5749. [Google Scholar] [CrossRef]
- Shibue, M.; Mant, C.T.; Hodges, R.S. Effect of anionic ion-pairing reagent hydrophobicity on selectivity of peptide separations by reversed-phase liquid chromatography. J. Chromatogr. A 2005, 1080, 68–75. [Google Scholar] [CrossRef]
- Sikora, K.; Jaskiewicz, M.; Neubauer, D.; Migon, D.; Kamysz, W. The Role of Counter-Ions in Peptides—An Overview. Pharmaceuticals 2020, 13, 442. [Google Scholar] [CrossRef]
- Streuli, A.; Coxon, C.R.; Steuer, C. Simultaneous Quantification of Commonly Used Counter Ions in Peptides and Active Pharmaceutical Ingredients by Mixed Mode Chromatography and Evaporative Light Scattering Detection. J. Pharm. Sci. 2021, 110, 2997–3003. [Google Scholar] [CrossRef]
- Allenspach, M.D.; Fuchs, J.A.; Doriot, N.; Hiss, J.A.; Schneider, G.; Steuer, C. Quantification of hydrolyzed peptides and proteins by amino acid fluorescence. J. Pept. Sci. 2018, 24, e3113. [Google Scholar] [CrossRef]
- Gaussier, H.; Morency, H.; Lavoie, M.C.; Subirade, M. Replacement of trifluoroacetic acid with HCl in the hydrophobic purification steps of pediocin PA-1: A structural effect. Appl. Environ. Microbiol. 2002, 68, 4803–4808. [Google Scholar] [CrossRef]
- Andrushchenko, V.V.; Vogel, H.J.; Prenner, E.J. Optimization of the hydrochloric acid concentration used for trifluoroacetate removal from synthetic peptides. J. Pept. Sci. 2007, 13, 37–43. [Google Scholar] [CrossRef]
- Roux, S.; Zekri, E.; Rousseau, B.; Paternostre, M.; Cintrat, J.C.; Fay, N. Elimination and exchange of trifluoroacetate counter-ion from cationic peptides: A critical evaluation of different approaches. J. Pept. Sci. 2008, 14, 354–359. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.; Lin, C.-X.; Xiao, C.; Mulvey, T.; Cooper, G.; Reid, I. Trifluoroacetate, a contaminant in purified proteins, inhibits proliferation of osteoblasts and chondrocytes. Am. J. Physiol.Endocrinol. Metab. 1999, 277, E779–E783. [Google Scholar] [CrossRef]
- Sikora, K.; Jaśkiewicz, M.; Neubauer, D.; Bauer, M.; Bartoszewska, S.; Barańska-Rybak, W.; Kamysz, W. Counter-ion effect on antistaphylococcal activity and cytotoxicity of selected antimicrobial peptides. Amino Acids 2018, 50, 609–619. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, A.G.; Rodríguez-Mejía, K.G.; Cuero-Amu, K.J.; Ardila-Chantré, N.; Reyes-Calderón, J.E.; González-López, N.M.; Huertas-Ortiz, K.A.; Fierro-Medina, R.; Rivera-Monroy, Z.J.; García-Castañeda, J.E. A New Methodology for Synthetic Peptides Purification and Counterion Exchange in One Step Using Solid-Phase Extraction Chromatography. Processes 2024, 13, 27. [Google Scholar] [CrossRef]
- Ardino, C.; Sannio, F.; Pasero, C.; Botta, L.; Dreassi, E.; Docquier, J.-D.; D’Agostino, I. The impact of counterions in biological activity: Case study of antibacterial alkylguanidino ureas. Mol. Divers. 2023, 27, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Pini, A.; Lozzi, L.; Bernini, A.; Brunetti, J.; Falciani, C.; Scali, S.; Bindi, S.; Di Maggio, T.; Rossolini, G.M.; Niccolai, N.; et al. Efficacy and toxicity of the antimicrobial peptide M33 produced with different counter-ions. Amino Acids 2012, 43, 467–473. [Google Scholar] [CrossRef]
- Ma, T.G.; Ling, Y.H.; McClure, G.D.; Tseng, M.T. Effects of trifluoroacetic acid, a halothane metabolite, on C6 glioma cells. J. Toxicol. Environ. Health 1990, 31, 147–158. [Google Scholar] [CrossRef]
- Dekant, W.; Dekant, R. Mammalian toxicity of trifluoroacetate and assessment of human health risks due to environmental exposures. Arch. Toxicol. 2023, 97, 1069–1077. [Google Scholar] [CrossRef]
- Barlow, N.; Chalmers, D.K.; Williams-Noonan, B.J.; Thompson, P.E.; Norton, R.S. Improving Membrane Permeation in the Beyond Rule-of-Five Space by Using Prodrugs to Mask Hydrogen Bond Donors. ACS Chem. Biol. 2020, 15, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, L.O.; Yang, J.; Raines, R.T. N(α) -Methylation of arginine: Implications for cell-penetrating peptides. J. Pept. Sci. 2023, 29, e3468. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.; Millan, D.S.; Perez, M.; Wakenhut, F.; Whitlock, G.A. Intramolecular hydrogen bonding to improve membrane permeability and absorption in beyond rule of five chemical space. MedChemComm 2011, 2, 669–674. [Google Scholar] [CrossRef]
- Kramer, S.D.; Aschmann, H.E.; Hatibovic, M.; Hermann, K.F.; Neuhaus, C.S.; Brunner, C.; Belli, S. When barriers ignore the “rule-of-five”. Adv. Drug Deliv. Rev. 2016, 101, 62–74. [Google Scholar] [CrossRef]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef]
- Sikora, K.; Neubauer, D.; Jaskiewicz, M.; Kamysz, W. Citropin 1.1 Trifluoroacetate to Chloride Counter-Ion Exchange in HCl-Saturated Organic Solutions: An Alternative Approach. Int. J. Pept. Res. Ther. 2018, 24, 265–270. [Google Scholar] [CrossRef]
- Kaiser, E.; Rohrer, J. Determination of residual trifluoroacetate in protein purification buffers and peptide preparations by ion chromatography. J. Chromatogr. A 2004, 1039, 113–117. [Google Scholar] [CrossRef]
- Little, M.J.; Aubry, N.; Beaudoin, M.-E.; Goudreau, N.; LaPlante, S.R. Quantifying trifluoroacetic acid as a counterion in drug discovery by 19F NMR and capillary electrophoresis. J. Pharm. Biomed. Anal. 2007, 43, 1324–1330. [Google Scholar] [CrossRef]
- Peters, F.T.; Drummer, O.H.; Musshoff, F. Validation of new methods. Forensic Sci. Int. 2007, 165, 216–224. [Google Scholar] [CrossRef]
- ICH Harmonised Guideline. Validation of Analytical Procedures Q2 (R2); ICH: Geneva, Switzerland, 2022. [Google Scholar]
- Chaloin, L.; Vidal, P.; Lory, P.; Mery, J.; Lautredou, N.; Divita, G.; Heitz, F. Design of carrier peptide-oligonucleotide conjugates with rapid membrane translocation and nuclear localization properties. Biochem. Biophys. Res. Commun. 1998, 243, 601–608. [Google Scholar] [CrossRef]
- Morris, M.C.; Depollier, J.; Mery, J.; Heitz, F.; Divita, G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 2001, 19, 1173–1176. [Google Scholar] [CrossRef]
- Elmquist, A.; Lindgren, M.; Bartfai, T.; Langel, U. VE-cadherin-derived cell-penetrating peptide, pVEC, with carrier functions. Exp. Cell Res. 2001, 269, 237–244. [Google Scholar] [CrossRef]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Copolovici, D.M.; Langel, K.; Eriste, E.; Langel, U. Cell-penetrating peptides: Design, synthesis, and applications. ACS Nano 2014, 8, 1972–1994. [Google Scholar] [CrossRef] [PubMed]
- Hermann, K.F.; Neuhaus, C.S.; Micallef, V.; Wagner, B.; Hatibovic, M.; Aschmann, H.E.; Paech, F.; Alvarez-Sanchez, R.; Kramer, S.D.; Belli, S. Kinetics of lipid bilayer permeation of a series of ionisable drugs and their correlation with human transporter-independent intestinal permeability. Eur. J. Pharm. Sci. 2017, 104, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Eyer, K.; Paech, F.; Schuler, F.; Kuhn, P.; Kissner, R.; Belli, S.; Dittrich, P.S.; Kramer, S.D. A liposomal fluorescence assay to study permeation kinetics of drug-like weak bases across the lipid bilayer. J. Control. Release 2014, 173, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.E.; Paci, M.B.; De Pauli, C.P.; Giacomelli, C.E. Infrared study of trifluoroacetic acid unpurified synthetic peptides in aqueous solution: Trifluoroacetic acid removal and band assignment. Anal. Biochem. 2011, 410, 118–123. [Google Scholar] [CrossRef]
- Clement, A.; Yong, D.; Brechet, C. Simultaneous identification of sugars by HPLC using evaporative light scattering detection (ELSD) and refractive index detection (RI). Application to plant tissues. J. Liq. Chromatogr. Relat. Technol. 1992, 15, 805–817. [Google Scholar] [CrossRef]
- Montesano, D.; Cossignani, L.; Giua, L.; Urbani, E.; Simonetti, M.S.; Blasi, F. A Simple HPLC-ELSD Method for Sugar Analysis in Goji Berry. J. Chem. 2016, 2016, 6271808. [Google Scholar] [CrossRef]
- Camdzic, D.; Dickman, R.A.; Joyce, A.S.; Wallace, J.S.; Ferguson, P.L.; Aga, D.S. Quantitation of total PFAS including trifluoroacetic acid with fluorine nuclear magnetic resonance spectroscopy. Anal. Chem. 2023, 95, 5484–5488. [Google Scholar] [CrossRef]
- Erckes, V.; Hilleke, M.; Isert, C.; Steuer, C. PICKAPEP: An application for parameter calculation and visualization of cyclized and modified peptidomimetics. J. Pept. Sci. 2024, 30, e3646. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.I.; Chankeshwara, S.V.; Abdulkarim, Z.; Ghiandoni, G.M. pIChemiSt—Free Tool for the Calculation of Isoelectric Points of Modified Peptides. J. Chem. Inf. Model. 2023, 63, 187–196. [Google Scholar] [CrossRef]
- Nugrahadi, P.P.; Hinrichs, W.L.J.; Frijlink, H.W.; Schöneich, C.; Avanti, C. Designing Formulation Strategies for Enhanced Stability of Therapeutic Peptides in Aqueous Solutions: A Review. Pharmaceutics 2023, 15, 935. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, S.; Ye, S.; Wei, S.; Chu, B.; Wang, R.; Li, H.; Zhang, T. The role of trifluoroacetic acid in new particle formation from methanesulfonic acid-methylamine. Atmos. Environ. 2023, 311, 120001. [Google Scholar] [CrossRef]
- Madani, F.; Lindberg, S.; Langel, Ü.; Futaki, S.; Gräslund, A. Mechanisms of Cellular Uptake of Cell-Penetrating Peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef]
- Jiao, C.-Y.; Delaroche, D.; Burlina, F.; Alves, I.D.; Chassaing, G.; Sagan, S. Translocation and Endocytosis for Cell-penetrating Peptide Internalization. J. Biol. Chem. 2009, 284, 33957–33965. [Google Scholar] [CrossRef]
- Eggimann, G.A.; Buschor, S.; Darbre, T.; Reymond, J.-L. Convergent synthesis and cellular uptake of multivalent cell penetrating peptides derived from Tat, Antp, pVEC, TP10 and SAP. Org. Biomol. Chem. 2013, 11, 6717–6733. [Google Scholar] [CrossRef]
- Troeira Henriques, S.; Alexandre, Q.; Bagatolli, L.A.; Fabrice, H.; Castanho, M.A.R.B. Energy-independent translocation of cell-penetrating peptides occurs without formation of pores. A biophysical study with pep-1. Mol. Membr. Biol. 2007, 24, 282–293. [Google Scholar] [CrossRef]
- Elmquist, A.; Hansen, M.; Langel, Ü. Structure–activity relationship study of the cell-penetrating peptide pVEC. Biochim. Biophys. Acta BBA Biomembr. 2006, 1758, 721–729. [Google Scholar] [CrossRef]
- Guterstam, P.; Madani, F.; Hirose, H.; Takeuchi, T.; Futaki, S.; El Andaloussi, S.; Gräslund, A.; Langel, Ü. Elucidating cell-penetrating peptide mechanisms of action for membrane interaction, cellular uptake, and translocation utilizing the hydrophobic counter-anion pyrenebutyrate. Biochim. Biophys. Acta BBA Biomembr. 2009, 1788, 2509–2517. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Ristroph, K.D.; Prud’homme, R.K. Hydrophobic ion pairing: Encapsulating small molecules, peptides, and proteins into nanocarriers. Nanoscale Adv. 2019, 1, 4207–4237. [Google Scholar] [CrossRef] [PubMed]
- Nandi, R.; Amdursky, N. The Dual Use of the Pyranine (HPTS) Fluorescent Probe: A Ground-State pH Indicator and an Excited-State Proton Transfer Probe. Acc. Chem. Res. 2022, 55, 2728–2739. [Google Scholar] [CrossRef]
- Launay, M.; Tripier, M.; Guizerix, J.; Viriot, M.L.; Andre, J.C. Pyranine used as a fluorescent tracer in hydrology: pH effects in determination of its concentration. J. Hydrol. 1980, 46, 377–383. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- The Python Language Reference. Available online: https://docs.python.org/3/reference/ (accessed on 22 January 2024).
- Pandas User Guide. Available online: https://pandas.pydata.org/ (accessed on 22 January 2024).
- SciPy. Available online: https://scipy.org/ (accessed on 23 August 2024).
- Matplotlib. Available online: https://matplotlib.org/ (accessed on 23 August 2024).




| 19F-NMR | HPLC-ELSD | FT-IR | |
|---|---|---|---|
| Detected analytes | TFA− | Na+, Cl−, TFA− | TFA− |
| Analyte signal | Signal area at 75.4 ppm | Peak area at retention time 4.0 min (Na+), 5.4 min (TFA−), 6.6 min (Cl−) | Maximum absorbance 1190–1210 cm−1 |
| Measurement time | 4 min | 10 min | 1 min |
| Sample solvent | 10% D2O/H2O | Water | Water |
| Automation with autosampler | Yes | Yes | No |
| Sample recovery | Possible | Destructive | Possible |
| Calibration model | Linear | Quadratic | Linear |
| Weighting | 1/x | x | x |
| TFA− calibration range | 50.7–2536.5 µg/mL | 10.24–409.5 µg/mL | 503–2514 µg/mL |
| LoD TFA− | 6.82 µg/mL | 1.52 µg/mL | 253 µg/mL |
| LoQ TFA− | 20.68 µg/mL | 4.61 µg/mL | 713 µg/mL |
| Sample interference | Unlikely | Sequence dependent | Sequence dependent |
| Bias QC low/med/high | 9.5%/3.6%/0.2% | 13.2%/5.7%/0.1% | 15.9%/4.6%/3.7% |
| Intra-day deviation QC low/med/high | 1.1%/2.1%/1.7% | 3.9%/3.2%/2.5% | 16.8%/9.7%/4.5% |
| Inter-day deviation QC low/med/high | 2.8%/2.9%/2.0% | 6.6%/8.6%/6.6% | 19.5%/11.7%/10.0% |
| Peptide | Sequence * | pI | Charge at pH < 3 | MW (Da) | MW as TFA Salt (Da) | Weight Percentage TFA− (%) | MW as Cl Salt (Da) | Weight Percentage Cl− (%) |
|---|---|---|---|---|---|---|---|---|
| AT 1 | H2N-DRVYIHPFHL-COOH | 7.44 ± 0.93 | +4 | 1296.50 | 1752.58 | 26.0 | 1438.30 | 9.9 |
| AT 2 | H2N-DRVYIHPF-COOH | 7.28 ± 0.98 | +3 | 1046.20 | 1388.26 | 24.6 | 1152.55 | 9.2 |
| AT 3 | H2N-RVYIHPF-COOH | 9.05 ± 1.42 | +3 | 931.11 | 1273.17 | 26.9 | 1037.46 | 10.3 |
| AT 4 | H2N-VYIHPF-COOH | 7.26 ± 1.05 | +2 | 774.92 | 1002.96 | 22.7 | 845.82 | 8.4 |
| AntP | H2N-RQIKIWFQNRRMKWKK-CONH2 | 12.92 ± 0.82 | +8 | 2245.79 | 3157.95 | 28.9 | 2529.41 | 11.2 |
| Pep1 | H2N-KETWWETWWTEWSQPKKKRKV-COOH | 9.90 ± 2.02 | +7 | 2848.27 | 3646.41 | 21.9 | 3096.44 | 8.0 |
| pVEC | H2N-LLIILRRRIRKQAHAHSK-COOH | 12.60 ± 0.90 | +9 | 2096.57 | 3235.91 | 35.2 | 2528.81 | 17.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erckes, V.; Streuli, A.; Chamera Rendueles, L.; Krämer, S.D.; Steuer, C. Towards a Consensus for the Analysis and Exchange of TFA as a Counterion in Synthetic Peptides and Its Influence on Membrane Permeation. Pharmaceuticals 2025, 18, 1163. https://doi.org/10.3390/ph18081163
Erckes V, Streuli A, Chamera Rendueles L, Krämer SD, Steuer C. Towards a Consensus for the Analysis and Exchange of TFA as a Counterion in Synthetic Peptides and Its Influence on Membrane Permeation. Pharmaceuticals. 2025; 18(8):1163. https://doi.org/10.3390/ph18081163
Chicago/Turabian StyleErckes, Vanessa, Alessandro Streuli, Laura Chamera Rendueles, Stefanie Dorothea Krämer, and Christian Steuer. 2025. "Towards a Consensus for the Analysis and Exchange of TFA as a Counterion in Synthetic Peptides and Its Influence on Membrane Permeation" Pharmaceuticals 18, no. 8: 1163. https://doi.org/10.3390/ph18081163
APA StyleErckes, V., Streuli, A., Chamera Rendueles, L., Krämer, S. D., & Steuer, C. (2025). Towards a Consensus for the Analysis and Exchange of TFA as a Counterion in Synthetic Peptides and Its Influence on Membrane Permeation. Pharmaceuticals, 18(8), 1163. https://doi.org/10.3390/ph18081163








