Abstract
Background: Oral squamous cell carcinoma (OSCC) is one of the most serious forms of cancer in the world. The opportunities to decrease the mortality rate would lie in the possibility of earlier identification of this pathology, and at the same time, the immediate approach of anticancer therapy. Furthermore, new treatment strategies for OSCC are needed to improve existing therapeutic options. Bioactive compounds found in medicinal plants could be used to support these strategies. It is already known that they have an increased potential for action and a safety profile; therefore, they could improve the therapeutic effect of classical chemotherapeutic agents in combination therapies. Methodology: This research was based on an extensive review of recently published studies in scientific databases (PubMed, Scopus, and Web of Science). The selection criteria were based on experimental protocols investigating molecular mechanisms, synergistic actions with conventional anticancer agents, and novel formulation possibilities (e.g., nanoemulsions and mucoadhesive films) for the targeted delivery of bioactive compounds in OSCC. Particular attention was given to in vitro, in vivo, translational, and clinical studies that have proven therapeutic relevance. Results: Recent discoveries regarding the effect of bioactive compounds in the treatment of oral cancer were analyzed, with a view to integrating them into oncological practice for increasing therapeutic efficacy and reducing the occurrence of adverse reactions and treatment resistance. Conclusions: Significant progress has been achieved in this review, allowing us to appreciate that the valorization of these bioactive compounds is emerging.
1. Introduction
Oral cancer represents one of the oncologic pathologies with increased incidence and mortality worldwide, being influenced by various factors such as chronic inflammation, smoking, alcohol consumption, low-antioxidant diet, human papilloma virus (HPV) infections, and genetic predisposition [1,2,3,4]. Over the years, the medical field has been characterized by significant advances made in the approach of conventional anticancer treatments (chemotherapy, radiotherapy, and surgery), and therefore, they are still the first treatment option for different stages of malignancy [5,6].
Since side effects and recurrence rates represent major concerns in clinical practice, numerous complementary and adjuvant therapeutic strategies have been explored and are on the increasing trend, including the use of natural compounds derived from medicinal plants [7,8,9,10], either in purified, synthesized, or nanoencapsulated forms, or as crude extracts [11,12,13]. These compounds, due to their involvement in the production of various mechanisms, such as regulation of signaling pathways involved in oncogenesis (PI3K/AKT, MAPK, NF-κB, etc.), induction of apoptosis and/or autophagy, and inhibition of proliferation by blocking the cell cycle, may exhibit significant antioxidant and anti-inflammatory effects [14,15,16].
There is a complex chemical profile of bioactive compounds that may offer promising insights into both therapeutics and prevention options for cancer, including oral cancer [17,18]. It is important to have a comprehensive knowledge of their chemical structure, to understand their biological mechanisms, and thus therapeutic targeting directions to focus on the development of synergies with conventional treatments that support and help in the validation of their contribution for clinical studies [19,20].
Thus, phenolic compounds and flavonoids are recognized for their antioxidant effects and modulation of molecular pathways related to inflammation and cell survival [18,21,22].
Other important classes are represented by that of stilbenes, with resveratrol as a representative example, known for its anticarcinogenic, antioxidant, and cardioprotective role [23,24], and quinones, and carotenoids that play a key role in protection against oxidative stress [25,26]. In parallel, numerous alkaloids possess potent cytotoxic and anti-proliferative effects and are frequently used as bases for antineoplastic drugs [21,27].
Terpenoids, present in essential oils, have gained significant attention due to their diverse pharmacological properties (antimicrobial, anti-inflammatory, and anticarcinogenic) and their alignment with the growing global interest in natural and sustainable products [28,29,30].
Understanding synergistic effects of plant extracts delves into this fascinating area of study, exploring how multiple bioactive compounds within medicinal plants interact to enhance or modulate their overall therapeutic efficacy [31,32,33].
To increase the potential therapeutic effect and reduce systemic toxicity, combinations of natural compounds with classical chemotherapeutic agents have been studied [34,35,36].
Also, research in the medical and pharmaceutical fields, due to continuous evolution, may support the development of therapies that can lead to increased efficacy through controlled delivery processes that can optimize the absorption of active principles from plants [37,38]. Polymeric drug delivery systems allow the introduction of an active substance with therapeutic properties into the body using devices or a newly designed formulation. Recent drug delivery systems emphasize biodegradable and bioreducible polymers with significant therapeutic advantages [39,40].
Thus, modern therapeutic approaches aim both to identify unique molecules with anticancer potential and to develop innovative formulations (nanoemulsions, mucoadhesive films, and liposomes) that improve bioavailability and thus lead to selective targeting of malignant cells [41,42,43]. Therefore, some compounds, such as quercetin, for example, have been incorporated into nanoparticles or orodispersible films, thus increasing local bioavailability and also increasing therapeutic efficacy in the oral cavity [44,45,46].
The purpose of this review is to present a detailed analysis of the different medicinal plants, natural extracts, and active principles that have been studied as treatment strategies for oral cancer. We identified relevant clinical and preclinical studies in the articles that reflected the mechanism by which these compounds act at the cellular and oral tumor level. Database research also highlighted the need to integrate complementary and innovative approaches in the treatment of these malignant diseases, with the aim of improving efficiency and safety in the patient’s treatment.
These studies suggested that plant-based therapies may offer a safer and potentially less invasive alternative to conventional treatments [16,47,48].
In this context, research in the field of plants and pharmaceutical treatments is becoming essential for the development of innovative therapeutic solutions to the current needs of patients with oral cancer [49,50,51,52].
2. Investigating the Therapeutic Potential of Bioactive Compounds in Oral Cancer
Compounds extracted from plants are promising directions in oncology research, aiming to develop innovative treatments for oral cancer [48,53,54,55]. Plant secondary metabolites are known for their essential roles in sustaining plant physiology, but their promising anticarcinogenic properties are now also being proven [56,57,58,59,60]. Through their ability to modulate cell signaling pathways and exert anti-inflammatory effects, bioactive compounds are emerging as potential therapeutics in oncology [58,61,62,63].
2.1. Categories, Compounds, and Biological Effects
2.1.1. Phenolic Compounds
Phenolic compounds, recognized as bioactive secondary metabolites of medicinal plants, have attracted the attention of researchers due to their multiple biological effects, with an essential role in anti-inflammatory, antioxidant, and antimicrobial therapies [64,65,66] being characterized by the presence of polyphenols. Structurally, they can be described as compounds containing at least one phenol group, the phenol itself presenting a benzene ring substituted with a hydroxyl group, systematically known as hydroxybenzene [67,68].
Polyphenols are a subcategory of phenolic compounds, represented by polyhydroxylated bioactive compounds that encompass a wide variety of compounds with similar structures. They can be divided into several main subclasses such as phenolic acids, flavonoids, lignans, stilbenes, and tannins [69,70]. Polyphenols contribute to the intake of a multitude of nutritional micronutrients in our diet, with evidence of their beneficial role being supported by studies carried out to demonstrate their direct counteracting of oxidative stress and inflammation, two major key factors in oral carcinogenesis [71,72,73]. Phenols that contain carboxylic acid are called phenolic acids; among them are ferulic acid, caffeic acid, and transferulic acid [74,75].
2.1.2. Flavonoids and Their Subclasses
Flavonoids are a class of naturally occurring secondary metabolites characterized by a polyphenolic structure and wide distribution in vegetables, fruits, as well as in various types of beverages [76,77,78]. Flavonoids are an important part of fields such as pharmacy and medicine, as well as cosmetics and nutraceuticals, due to their proven effectiveness in promoting and maintaining human health [79,80].
The beneficial effects of these compounds are attributed to their antioxidant, anti-inflammatory, and anticancer activities, along with their ability to influence the functioning of key cellular enzymes, recognized for their potential role in the management of oral cancer [76,81].
Flavonoids can be subdivided into different structural subclasses: anthocyanins, flavanones, catechins, flavonols, chalcones, flavanonols, isoflavones, and flavones that may further influence cancer cell behavior [82,83,84]. Flavones (apigenin and vitexin) are widely present in leaves, flowers, and fruits as glucosides [83,85,86].
Isoflavonoids are metabolites characteristic of leguminous plants and play essential roles in nodule induction and microbial signaling [87,88]. Isoflavones are grouped into three groups: genistein, daidzein, and glycytidine [89,90]. The molecular structure of isoflavones is like that of animal estrogens. In addition, isoflavones possess potent antioxidant activity, which may decrease the risk of cancer by inhibiting free radical-induced DNA damage [83,90].
2.1.3. Stilbenes and Their Derivatives
Stilbenes and their derivatives, known as stilbenoids (pinosylvin and pterostilbene), represent a multidisciplinary research field that combines several important key branches of chemistry, biology, physics, and medicine [91,92,93]. They exhibit pronounced antimicrobial, estrogenic, and anticarcinogenic activities. Resveratrol, one of the best-known stilbene derivatives, is valued for its potent antioxidant activity, as well as for the moderate antimicrobial, fungistatic, and fungicidal properties of its derivatives [94,95,96]. These bioactive plant compounds demonstrate therapeutic value against oral cancer through their proven mechanisms of inducing apoptosis and inhibiting tumor growth [97,98,99].
2.1.4. Flavonolignans
These compounds, flavonolignans, result from the combination of two phenylpropanoid units and present a distinctive structure that places them in the flavonoid class [100,101]. Silybum marianum flavonolignans, known as silymarin, are a complex mixture of structural constituents isolated from the fruits and seeds of the plant. They are recognized for their antihypertensive, hypolipidemic, antiatherosclerotic, and antidiabetic properties. Silymarin extracts have been used for centuries as traditional remedies for liver and gallbladder diseases [102,103,104,105]. Silymarin, also recognized for its anti-inflammatory and antioxidant effects, is a potential therapeutic candidate in reducing inflammation and oxidative stress associated with oral cancer [106,107,108,109]. Silymarin is a complex consisting of silibin, isosilibin, silidianin, and silicristin, widely used in the pharmaceutical industry and in dietary supplements [110,111,112].
2.1.5. Quinone and Carotenoids
Quinone compounds, due to their unique chemical properties and their ability to participate in redox processes, have been recognized as promising and attractive alternatives in the field of anticancer drug development [113,114].
By targeting various cellular components in biochemical pathways, quinone compounds exhibit multiple mechanisms of action, inducing cytotoxicity and apoptosis in cancer cells, making them a promising option in the treatment of oral cancer [115,116,117].
Ubiquinol-10, the reduced form of coenzyme Q10 (ubiquinone-10), acts as a potent lipophilic antioxidant in various cell membranes and LDL and is also a well-known proton-electron transporter in the inner membrane of mitochondria [118,119,120].
Carotenoids are a group of secondary metabolites produced by the terpenoid biosynthetic pathway. These natural pigments, widely distributed in plants, fungi, algae, and bacteria, are responsible for their red, orange, and yellow coloration [121,122]. Carotenoids are recognized for their antioxidant role in a variety of diseases, including cancer [118,123]. Also, carotenoids produced by microorganisms contribute to stabilizing the cell membrane, thus strengthening its integrity [121,124].
2.1.6. Alkaloids
Plants produce alkaloids, nitrogenous organic compounds characterized by their cyclic structure, the presence of an integrated nitrogen atom within the ring, and their alkaline properties [125,126].
Some alkaloids, such as amide alkaloids, can be found in free form in plants, being very weak alkaline, while others may have glycosidic forms with N-oxide groups. Some of the main classes of alkaloids found in plants are pyridine, pyrrolidine, quinoline, isoquinoline, quinazoline, steroids, and indole [127,128,129]. The bis-benzylisoquinoline alkaloids (e.g., tetrandrine) belong to the broader family of isoquinoline alkaloids, found predominantly in tropical and subtropical regions in plant families such as Menispermaceae, Berberidaceae, Lauraceae, and Ranunculaceae [130]. These alkaloids have shown significant research interest due to their anti-inflammatory, antitumor, and antiviral biological activities and increased potential in their pharmacological applications in conventional cancer therapies, including oral cancer [130,131,132,133,134,135,136].
2.1.7. Essential Oils
Essential oils (EOs) are volatile liquids obtained by extraction from different plant parts. The bioactive compounds predominantly present are terpenes (monoterpenes, diterpenes) and terpenoids (monoterpenoids, diterpenoids, and sesquiterpenoids), which are recognized for diverse biological activities, including antimicrobial, anti-inflammatory, antioxidant, antiallergic, and anticarcinogenic effects [137,138,139]. Structurally, the sesquiterpene lactones (e.g., santamarine) are terpenes that share a common base structure of 15 carbon atoms being organized into several subclasses: eudesmanolides, guaianolides and pseudoguaianolides, germacranolides and xanthanolides [140,141].
2.1.8. Phenylpropene
Simple phenylpropanoids serve as fundamental precursors in the biosynthesis of many complex natural compounds, such as flavonoids, lignans, and polyphenols. They are often associated with characteristic odors and may exhibit antimicrobial, anti-inflammatory, and anticancer effects [142,143]. These effects have also been associated with the phenylpropene t-anethole, one of these compounds, which provides the characteristic sweet flavor of anise seeds and leaves (Pimpinella anisum, family Apiaceae) [144,145,146]. These activities, through the reduction of inflammation and oxidative stress, may contribute to the modulation of the tumor micro-environment in oral cancer [147].
2.1.9. Phthalides and Xanthones
Phthalides are found in some plant families and mushroom genera and are a small group of natural compounds that can be monomeric or dimeric in structure. They are known in Asia, Europe, and North America for their anti-inflammatory, antispasmodic, and sedative properties [148,149], also being very useful in reducing chronic inflammation associated with oral pathology [150,151].
Xanthones represent secondary metabolites with remarkable structural diversity, exhibiting a wide range of antitumor, antidiabetic, and antimicrobial pharmacological properties. These aromatic compounds, mainly found in higher plants such as Clusiaceae, Hypericaceae, and Gentianaceae [152,153,154], exert influence on cancer cell proliferation and apoptosis, thereby contributing to their therapeutic relevance in oral cancer treatment [155,156,157,158].
2.1.10. Phytosterols
Phytosterols are known due to their chemical diversity, structural complexity, inherent biological activity, as well as their easy availability, accessibility, and lack of toxic effects. Besides their ability to inhibit intestinal absorption of cholesterol, which thus leads to a decrease in its level in plasma [159,160], phytosterols can be used as auxiliary agents in oral anticancer therapy [161,162,163]. Among the best-known forms of free plant sterols, found in significant amounts in vegetable oils and nuts, are campesterol, brassicasterol, β-sitosterol, and stigmasterol [164,165].
2.1.11. Other Compounds
Cyclic peptides are polypeptide chains with a cyclic structure, possessing clinical importance for various biological activities, such as antibacterial or bactericidal (gramicidin, tirocidin, vancomycin) and immunosuppressive (cyclosporin A) [166,167,168].
Proteases are proteolytic enzymes that likely arose in the early stages of protein evolution, initially as simple destructive enzymes required for protein catabolism and amino acid generation in primitive organisms. Over time, these proteases led to complex functions influencing the regulation of gene expression, diverse physiological processes (ovulation, fertilization, hemostasis, blood clotting, stem cell mobilization), and regulation of cell homeostasis (inflammation, immunity, autophagy, necrosis, and apoptosis) [169,170,171].
The diverse biological activities and complex structure of these compounds (polyphenols, flavonoids, stilbenes, quinones, alkaloids, phytosterols) provide a rich source for future novel anticancer strategies in oral cancer pathology [18,172,173,174].
2.2. Phytotherapeutic Perspectives in Oral Cancer Pathology
The summary of the main characteristics of the included studies can be found in Table 1 (plant extracts), Table 2 (phytochemical compounds), and Table 3 (formulations/combinations of phytochemical compounds).

Table 1.
Overview of plant-derived extracts and their importance in oral cancer treatment.

Table 2.
Overview of phytochemical compounds and their importance in oral cancer treatment.

Table 3.
Overview of phytochemical compounds formulation/combination and their mechanisms in oral cancer treatment.
2.2.1. Effectiveness of Plants and Natural Compounds Based on the Included Studies
In vitro tests have highlighted that most of the analyzed natural compounds, such as piperlongumine, α-mangostin, quercetin, and transferic acid, exert their action through similar molecular pathways, as described below [69,70,71,72].
A recent in vitro study investigating the anticarcinogenic effects of piperlongumine (PL) on the oral cell lines MC-3 and HSC-4 demonstrated that PL simultaneously activated apoptosis and autophagy, the two mechanisms involved in tumor cell death. Therefore, PL represents an effective therapeutic agent for the treatment of oral cancer. Further investigations to optimize dose and evaluate efficacy in combination with other autophagy inhibitors are needed to confirm previous findings [196].
In another study regarding α-mangosteen, found in Garcinia mangostana by using OSCC lines, the authors highlighted that this bioactive compound significantly inhibited the process of cell proliferation and participated in the induction of apoptosis. One of the valuable aspects in the oncology science observed in this study is that α-mangosteane presented a lower cytotoxicity compared to normal cells. Cell cycle phase arrest and inhibitory effects on mitochondrial apoptosis signaling pathways were also identified [206].
In addition to the previous research, a study was performed on quercetin, known for its antioxidant activity, which revealed that quercetin not only blocks proliferation but can also trigger cell death by endoplasmic reticulum stress (ER stress). Experiments conducted on the SAS cell line demonstrated that apoptosis is induced by the activation of proapoptotic proteins (CHOP/ATF) as well as by the release of cytochrome C from mitochondria [210].
In another study, the beneficial role of transferrin acid was highlighted by the activation of the caspase cascade, as a result of the reduction in the expression of antiapoptotic genes (Mcl-1) and the stimulation of the expression of proapoptotic genes (Bax). The conclusion of the study highlighted that this compound, in the early stages, can prevent tumor spreading through the essential mechanisms of blocking proliferation by mitochondrial mechano-mechanisms, and can be very valuable [202].
The flavonoid quercetin from propolis has been shown to potentiate tumor growth inhibitory activity. The beneficial effects of propolis in the treatment of oral cancer are the activation of immune effector cells such as cytotoxic T lymphocytes and macrophages, acceleration of cancer cell apoptosis, prevention of metastasis, anti-angiogenesis effect, mitosis-suppressing effect, immunomodulatory and antioxidant effect [230].
Another study has concluded that isoflavones from Trifolium pratense (red clover) could inhibit the proliferation of OSCC cells, with a relatively low toxicity profile [231].
In one research study on extracts from cranberry and grape seed, a selective apoptosis was induced, particularly in CAL27 and SCC25 cells, by activating the cell cycle inhibition process and increasing caspase activity by proanthocyanidins [182].
According to the authors, in a study that analyzed the performance of coenzyme Q10 (ubiquinone) and β-carotene in oral cancer patients, it was reported that T3 and T4 stages are directly proportional to antioxidant deficiencies, associated with both faster tumor progression and an altered metabolic status. Also, the same research suggested that antioxidant supplementation could partially improve the metabolic profile, with further studies needed to achieve a better understanding of the therapeutic benefits and optimize dosages [193].
Various bioactive compounds, such as silymarin, coenzyme Q10, and resveratrol, have begun to demonstrate clinical relevance. For example, silymarin (420 mg/day) reduced the severity and delayed the onset of radiotherapy-induced oral mucositis in both head and neck cancer patients [232], and systemic administration (140 mg three times per day) indicated a possible attenuation of chemotherapy-induced hepatotoxicity [233] although it remains a supplement without FDA approval [232]. Even though clinical studies on CoQ10, especially in OSCC, are limited, recent research has confirmed its safety and efficacy in preventing cardiotoxicity and decreasing cancer treatment-induced fatigue [234]. In a 2024 study, resveratrol induced feroptosis via the p53/SLC7A11 pathway in OSCC cell lines [191,229], and a March 2025 review supported its potent antiproliferative and antimetastatic actions in oral cancer models [235]; however, it does not hold drug approval [229].
Although the concrete results of the selected studies are presented in detail in this review, their limitations should also be mentioned. First, a major current limitation is the frequent use of non-standardized in vitro and in vivo models, which makes it difficult to directly compare the results. For example, nanoparticle penetration varies significantly between two-dimensional (2D) and three-dimensional (3D) models in OSCC [235,236]. Non-uniformity of dose, extract purity, and delivery systems in studies may lead to inconsistent results [237]. In addition, some information is conflicting, e.g., the dual role of antioxidants, as protectants but also as pro-oxidants, a role that may lead to cancer progression or inhibition depending on the context [238,239,240]. Due to the scarcity of high-quality clinical trials, future research should prioritize clinically relevant experiments.
2.2.2. Synergism Between Natural Compounds and Conventional Medicines
Various studies show that co-treatment of thymoquinone with cisplatin enhances the selectivity and efficacy of the chemotherapeutic agent on oral malignant cells, also protecting some normal cells. The mechanism is based on the induction of apoptosis in tumor cells by altering the expression levels of pro/antiapoptosis-related genes (e.g., p53, Bcl-2, caspase) and even reducing the dose of cisplatin, which could help to decrease the associated systemic toxicity [225].
As a separate study, the synergy of anethole (a phenylpropene derived from essential oils) with the standard chemotherapeutic agent cisplatin was investigated in oral cancer cell lines. Studies have shown the apoptogenic potential of anethole in combination with cisplatin by inhibiting important molecular pathways involved in tumor progression (MAPK, NF-κB, and β-catenin). This combined effect may allow a lower dose of cisplatin to potentially allow a lower dose of cisplatin to have an efficient therapeutic response with reduced side effects and a more tolerable treatment overall [226].
2.2.3. Advanced Topical Delivery Systems Used in This Specific Pathology
Many plant-derived compounds, such as flavonoids, stilbenes, and terpenoids, exhibit poor aqueous solubility, rapid metabolism, and low permeability, which limit their therapeutic potential in oral cancer treatment [241].
Recently, advances have been made showing that nanoparticles can significantly increase the bioavailability of natural products both in vitro and in vivo [40,242,243,244].
The stability, solubility, cellular uptake/internalization efficacy, specificity, tolerability, therapeutic index, and therapeutic efficacy of examples of natural compounds such as resveratrol and curcumin have been improved by integration into nanoemulsion-based drug delivery systems [245,246,247,248].
A new modern area of high interest for researchers is the discovery of new formulation modalities (mucoadhesive gels, nano-encapsulated formulations, orodispersible films), which allow for direct topical application to damaged surfaces in order to increase absorption, therapeutic efficacy, and reduce systemic effects, as can be seen in Figure 1 [109,249,250].
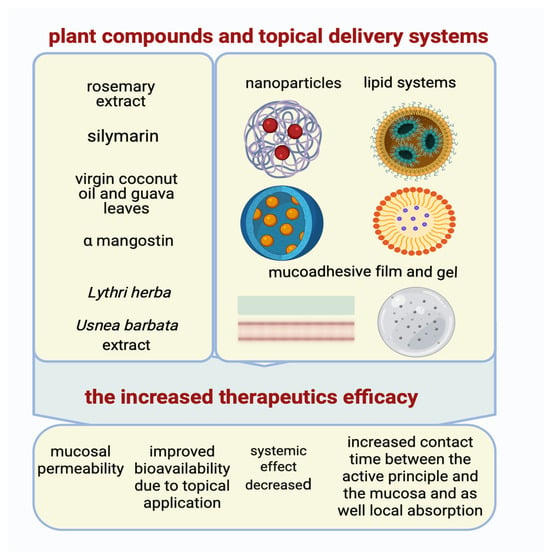
Figure 1.
The advanced topical delivery systems and the enhanced anticancer activity of compounds (Created in BioRender. Schroder, V. (2025) https://BioRender.com/tz17glc, accessed on 29 June 2025).
Oral mucoadhesive films containing Usnea barbata (L.) F.H. Wigg extract, dispersed in canola oil, has established an increase in potential therapeutic effects in the complementary therapy of oral squamous cell carcinoma [249,250].
In our previously published articles, results showed a correlation between Lythri herba and chitosan concentrations and membranes’ swelling and stability, showing it could be a promising material for biomedical applications [251].
Our studies have also focused on incorporating cannabidiol oil as an active component in chitosan-based films, with the aim of identifying a new pharmaceutical application [252].
In one study, researchers encapsulated polydatin (e.g., PLGA), a stilbene derivative known for its chemopreventive actions, in nanoparticles to protect this bioactive compound from premature degradation. This procedure was used in experimental models of oral carcinoma, and the result showed a facile release of the compound in the oral area as well as an increased anticarcinogenic efficacy [221].
Other researchers have studied the increased stability of silymarin as a result of loading it into nanostructured lipid carriers (NLCs), which are subsequently incorporated into a mucoadhesive gel formed in situ. Optimization of the therapeutic effect and reduction of the occurrence of potential systemic adverse effects were sustained by increasing oral mucosal permeability and providing a targeted release [109].
In another research, the possibility of increasing the therapeutic efficacy of α-mangostin against oral cancer was studied. An orodispersible mucoadhesive film was formulated with direct application to the oral lesion, resulting in an increased contact time between the active principle and the mucosa as well as local absorption, in direct accordance with the occurrence of a reduced number of systemic adverse effects [222].
Another investigation focused on the incorporation of rosemary extract into chitosan nanoparticles, which showed enhanced diffusion to the oral tumor and limited diffusion to other tissues due to the increased stability of the bioactive substances [223].
In another example, a nanoemulsion, based on a combination of guava leaves and virgin coconut oils, was formulated and later integrated into an orodispersible film. The incidence of systemic effects was reduced, and the anticarcinogenic effect was potentiated as a result of improved bioavailability due to topical application [224].
These advanced delivery formulation technologies are imperative for solving bioavailability barriers and optimizing the therapeutic efficacy of natural compounds in oral cancer therapy [250].
3. Advanced Methods Used for Bioproducts Cellular Activity Assay
Natural products have been used for centuries in a diverse spectrum of healing systems due to their powerful immunomodulatory, anti-inflammatory, and antiviral properties [253]. In our previous studies, the antioxidant effect of aqueous extract obtained from Prunus spinosa’s dried fruits was tested by using it to reduce the level of the biomarker IL-6 in various forms of periodontitis [254].
To evaluate the anticarcinogenic activity of phytochemical compounds on oral squamous cell carcinoma, multiple experimental methods have emerged over the last few decades (Figure 2) and have been applied in recent studies [187,188] (Figure 2).
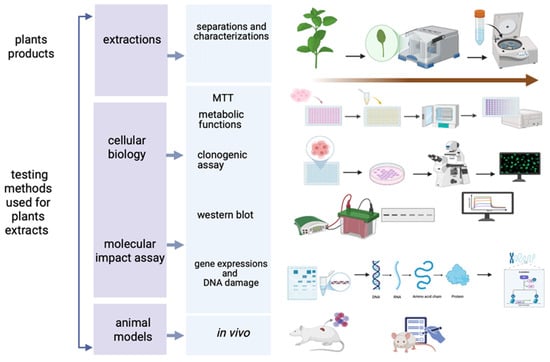
Figure 2.
The testing methods used for the plant bioproducts cellular activity assay (Created in BioRender. Schroder, V. (2025) https://BioRender.com/4j8a7i6, accessed on 29 June 2025).
Among the most versatile and popular assays used techniques are the MTT [255] assay for cellular metabolic activity, applied to oroselol [187], yohimbine [194], prenylflavones [195], piperlongumine (PL) [196], semilicoisoflavone B (SFB) [197], transferic acid [202], blumeatin [207], quercetin [209,210], sulforaphane [211], anethole [212], destruxin B [213], tetrandrine [136], β-sitosterol [217], burmanic acid (BURA) [220] and silymarin [106].
The clonogenic (or colony forming) assay, used for evaluating the radiation sensitivity of different cell lines [256], has been applied in studies on oroselol [187], prenylflavones [195] and quercetin [209,210], while Transwell assess cell migratory and invasive capacities [257] in relevant studies with oroselol [187], prenylflavones [195], quercetin [209,210], sulforaphane [211], blumeatin [207], kaempferol [208,216] and pinosylvin [216].
Western blotting has been one of the key methods in apoptosis and tumor progression for identifying specific proteins from a complex mixture of proteins extracted from cells by molecular weight [258]. This method was used for analysis PARP cleavage, LC3/BECN1 expression and caspases and has been used to investigate oroselol [187], resveratrol [189,190,191], prenylflavones [195], semilicoisoflavone B (SFB) [197], vitexin [199], blumeatin [207], quercetin [209,210], sulforaphane [211], anethole [212], destruxin B [213], cathepsin S [209,210], and tetrandrine [136] in the relevant articles in oral squamous cell lines. Corroborated with this method, similarly, α-mangostin [206], pinosylvin [216], CAPE [217,218], β-sitosterol [217], santamarin [219], burmannic acid (BURA) [220], and silymarin [106] demonstrated antitumor activity. In the case of lycopene, scrape-loading assays and electron microscopy demonstrated its superior ability to enhance gap-junction intercellular communication compared to β-carotene [215].
Flow cytometry has been used for analysis for the qualitative and quantitative assessment of cells in studies [259] for cell cycle and apoptosis involving yohimbine [194], prenylflavones [195], SFB [197], fisetin [203], blumeatin [207], quercetin [209,210], anethole [212], destruxin B [213], tetrandrine [136] and BURA [220].
For gene expression analysis, qRT-PCR was used for resveratrol [189,190,191], transferic acid [202], vitexin [199], and dehydroandrographolide (DA) [201]. Confocal microscopy allows for high-resolution imaging in thick tissues [260], and it was used to assess the effects of fisetin [203], quercetin [209,210], and sulforaphane [211].
Assessment of DNA damage was relevant in studies involving demethoxyymurrapanine (DEMU) [200], santamarin [219], and BURA [220], indicating a significant pro-apoptotic potential. In vivo studies have been tested for resveratrol [189,190,191], carnosic acid (CA) [204], DA [201], and silymarin [106], confirming antitumor activity in animal models.
4. Cell Signaling Mechanisms and the Trigger in Therapeutics Approaches
The results of studies indicate a wide range of compounds whose general effects are aimed at preventing cell damage and, respectively, activating the anti-inflammatory defense mechanisms, triggering cellular mechanisms such as autophagy, apoptosis, or cell death necrosis (Figure 3).
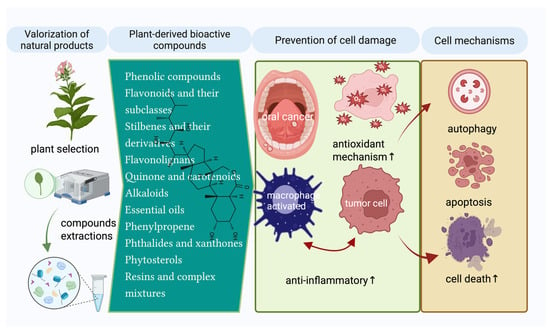
Figure 3.
The main plant-derived bioactive compounds and cell modulating mechanisms are assigned (Created in BioRender. Schroder, V. (2025) https://BioRender.com/y5n3o3a, accessed on 29 June 2025).
This review article also addresses the cell signaling mechanisms by which plant extracts and bioactive compounds exert antiproliferative effects in oral cancer, especially in oral squamous cell carcinoma (OSCC), highlighted in multiple studies presented below. In addition, in the context of OSCC, compounds such as anthocyanins [188] and cannabinoids [198] have been analyzed in review articles for their role in molecular mechanisms and therapeutic benefits. Also, Z-Ligustilide has been evaluated in TW2.6 hypoxic oral cancer cells [205]. Ubiquinones and β-carotene have been explored in a clinical study, demonstrating therapeutic or preventive implications in OSCC [193].
Several relevant studies highlight the crucial role of signaling pathways in mediating the antiproliferative effects of plant extracts [179,180,181,182,186] and bioactive compounds such as demethoxymurrapanin, fisetin, carnosic acid, Z-ligustilides, etc. on OSCC carcinoma, highlighting its role in induction of mitochondrial apoptosis, activation of caspases-3, -8, -9, induction of BAX and/or inhibition of BCL2, DNA fragmentation and oxidative stress [200,203,204,205,207]. In addition, other compounds that follow the same principle of induction of programmed cell death by altering mitochondrial membrane potential, cytochrome c release, and activation of apoptotic cascades, such as α-mangostin, quercetin, β-sitosterol, burmannic acid, silymarin, etc., are mentioned in this review [106,206,209,210,213,217,218,219,220].
In a clinical study based on the use of silymarin, tumor growth inhibition, activation of DR5 receptor, and extrinsic apoptosis signaling pathway, a decrease in Bcl-2 levels followed by activation of caspases in succession was identified, which reinforced the pro-apoptotic characteristic of this natural compound. Another aspect highlighted the absence of significant hepatic and renal side effects [106].
In several current review articles, the authors present comprehensive results on the prevention and treatment of oral cancer using plant extracts, such as Pinus densiflora and Trifolium pratens, which act by inhibition of oncogenic transcription (e.g., STAT3), activation of MAPK/ERK, PARP, PUMA, and p53 pathways [176,181,231].
Also, other studies have observed that the methanolic extract of Potentilla discolor (MEPD) has a predominant effect by increasing pro-apoptotic PUMA expression and inhibiting STAT3 activation. In this regard, studies on STAT3 overexpression have highlighted the reduced therapeutic response for MEPD and the complementary apoptotic effect when combined with a STAT3 inhibitor such as crypto-tanshinone [176].
A major challenge in treating oral squamous cell carcinoma (OSCC) is drug resistance. Plant-derived bioactive compounds such as curcumin, resveratrol, and quercetin have been able to demonstrate their efficacy in this regard by inhibiting P-glycoprotein (P-gp), an essential efflux transporter encoded by MDR1. These compounds can improve drug sensitivity in resistant cancer cells by reducing drug efflux and increasing intracellular chemotherapeutic accumulation by down-regulating MDR1 and interfering with the ATP-binding domain of P-glycoprotein [261].
The taxonomy used for plants has been extracted from the reference https://www.ncbi.nlm.nih.gov/datasets/taxonomy/tree/ [175] (accessed on 24 April 2025).
Current researchers are focusing on the main signaling pathways based on stimulation of cellular and humoral immunity, including direct action on tumor cells that may be regulated by Saraca indica and Momordica charantia [183,184].
Extracts such as those of Imperata cylindrica, Camellia sinensis, and Cardiospermum halicacabum show antiproliferative effect on oral cancer cells, accompanied by apoptosis, cell cycle blockage, and DNA damage [177,178,185].
Underlining the potential of seaweed extracts as natural anticarcinogenic agents, another in vitro study with seaweed extracts showed that Padina gymnospora selectively targets oral tumor cells by upregulating pro-apoptotic signals and regulating the expression of many proteins involved in apoptotic pathways [180].
Recent systematic reviews have explored the potential of compounds such as oroselol, resveratrol, piperlongumine, semilicoisoflavone B, etc. in cell lines and some in vivo models, as therapeutics for oral cancer, highlighting their ability to induce apoptosis through various signaling pathways related to the induction of autophagy, apoptosis, and modulation of signaling pathways (PI3K/AKT, MAPK, NF-κB) [191,192,195,196,197].
This review focuses on highlighting several representative natural plant compounds—ubiquinones and β-carotene, transferic acid, propolis, etc.—that may lead to cancer cell death, for the regulation of pathways involved in antioxidant and anti-inflammatory effects, with action on oxidative stress and metabolism [193,194,200,202,230]. Extensive studies have further demonstrated the ability of some alkaloids, e.g., yohimbine, or flavonoids, such as prenylflavones from Artocarpus altilis, to induce programmed cell death in oral tumor cells that are resistant to conventional treatment [194,195].
The importance of natural products such as dehydroandrographolides, vitexin, resveratrol, and transferic acid in blocking numerous signaling pathways that favor carcinogenesis, such as inhibition of migration and invasion by suppressing metalloproteinase activity (MMP-2, MMP-9), and ERK1/2, NF-κB, AP-1 signaling pathways, and VEGF, u-PA expression is explored in reviewed articles [190,191,192,195,210].
Findings have indicated that cannabinoids can be used as compounds with immunomodulatory and CB1/CB2-specific receptor-regulating activity, inducing apoptosis and inhibiting proliferation of oral cancer cells [198].
Other mechanisms may support the prevention of tumor development and maintenance of tissue homeostasis through mechanisms underlying antiproliferation and increased intercellular communication, as can be demonstrated for lycopene [215].
While numerous plant-derived compounds show anticarcinogenic potential in oral squamous cell carcinoma (OSCC), their therapeutic use should be undertaken with caution due to reported toxicities. For example, silymarin, known for its antioxidant and anticancer effects in OSCC, may cause mild liver damage when administered in high doses or over extended periods [262]. Kavalactones in Kava extract, which have exhibited antiproliferative activity, are associated with hepatotoxic effects such as necrosis and drug-induced hepatitis [263]. Ginkgolic acids, known for their pro-apoptotic effects in cancer cells, carry significant risks including neurotoxicity, genotoxicity, and mutagenicity [264,265]. Moreover, resveratrol, long studied for its anti-inflammatory and anticarcinogenic properties in OSCC, can produce o-quinone metabolites that contribute to liver and kidney toxicity as well as skin disorders [266]. Such examples highlight the importance of extensive toxicological evaluations, standardization, and clinical validation to ensure the safety and efficacy of using plant-derived compounds in OSCC therapy.
These findings strongly support the argument for further studies, especially large-scale comparative studies and rigorous clinical trials, to test the natural compound’s role in oral carcinoma for their efficacy and safety. Integrating these compounds into contemporary treatment modalities, either as a single agent or in combination with established agents such as cisplatin, may provide less toxic and more precisely targeted adjuvant therapy options [37,225,226].
5. Materials and Methods
Comprehensive research literature published in prestigious international scientific journals, was performed through worldwide databases (PubMed, Scopus, SpringerLink, Google Scholar, Embase, Web of Science), using keywords such as: “oral squamous cell carcinoma”, “oral cancer”, “bioactive natural compounds”, “signaling pathways”, “adjuvant treatment”, “nanotechnology”. Selected studies were limited to publications within the last 5 years, i.e., between 2020 and 2025. This time limit was established to reflect the latest developments in the innovative herbal and pharmaceutical therapies for oral cancer. Priorities were given to in vivo and in vitro studies with clear evidence of the efficacy of these treatments.
6. Conclusions
In the literature, there is extensive evidence for many natural agents acting as supplements in the management of oral carcinoma. These mechanisms are predominantly cell cycle arrest, induction of apoptosis via multiple pathways, inhibition of migration/invasion, and local reduction of inflammation and oxidative stress under certain conditions. In addition, they are also very effective in combination with standard chemotherapeutic agents in enhancing their antitumor effect and decreasing their systemic toxicity.
However, most investigations are limited to preclinical studies (from in vitro to animal models), but there is a growing clinical interest in testing the efficacy and safety of these agents.
Future developments could involve nanoparticles and orodispersible forms, as well as controlled clinical trials to find out whether these phytocompounds can be commonly used as adjuvants to oral oncology treatments. Thus, natural agents could soon become real options for patients’ prognosis and quality of life.
These results highlight the need to further explore the optimization of new plant-based compounds for optimal delivery, bioavailability, and synergy with existing therapies.
In addition, one of its most important aspects was the possibility of synergism with conventional drugs that enhance the efficacy of a chemotherapeutic agent while minimizing side effects on normal cells.
Author Contributions
Conceptualization, G.M., V.S. and I.M.I.; methodology, G.M., I.M.I. and V.S.; formal analysis and investigation, G.M.; resources, I.M.I.; data curation, G.M., I.M.I. and V.S.; writing—original draft preparation, G.M., I.M.I. and V.S.; writing—review and editing, G.M., V.S. and I.M.I.; visualization, G.M.; supervision, V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Coletta, R.D.; Yeudall, W.A.; Salo, T. Current Trends on Prevalence, Risk Factors and Prevention of Oral Cancer. Front. Oral Health 2024, 5, 1505833. [Google Scholar] [CrossRef]
- Tenore, G.; Nuvoli, A.; Mohsen, A.; Cassoni, A.; Battisti, A.; Terenzi, V.; Della Monaca, M.; Raponi, I.; Brauner, E.; De Felice, F.; et al. Tobacco, Alcohol and Family History of Cancer as Risk Factors of Oral Squamous Cell Carcinoma: Case-Control Retrospective Study. Appl. Sci. 2020, 10, 3896. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Bakhshi, A.; Norouzi, N.; Fallah, A.; Gharib, S. Environmental and Genetic Risk Factors of Oral Cancer: An Updated Review. Clin. Cancer Investig. J. 2022, 11, 1–8. [Google Scholar]
- Aghiorghiesei, O.; Zanoaga, O.; Nutu, A.; Braicu, C.; Campian, R.S.; Lucaciu, O.; Berindan Neagoe, I. The World of Oral Cancer and Its Risk Factors Viewed from the Aspect of MicroRNA Expression Patterns. Genes 2022, 13, 594. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Prager, P. Rapid Communication The Role of Chemotherapy in Modern Cancer Treatment: Advancements, Challenges, and Future Directions. J. Mol. Oncol. Res. 2024, 8, 244. [Google Scholar]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative Approaches for Cancer Treatment: Current Perspectives and New Challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Mitea, G.; Iancu, I.M.; Schröder, V.; Roșca, A.C.; Iancu, V.; Crețu, R.-M.; Mireșan, H. Therapeutic Potential of Prunus Species in Gastrointestinal Oncology. Cancers 2025, 17, 938. [Google Scholar] [CrossRef]
- Boța, M.; Vlaia, L.; Jîjie, A.-R.; Marcovici, I.; Crişan, F.; Oancea, C.; Dehelean, C.A.; Mateescu, T.; Moacă, E.-A. Exploring Synergistic Interactions between Natural Compounds and Conventional Chemotherapeutic Drugs in Preclinical Models of Lung Cancer. Pharmaceuticals 2024, 17, 598. [Google Scholar] [CrossRef]
- Moody, R.; Wilson, K.; Jaworowski, A.; Plebanski, M. Natural Compounds with Potential to Modulate Cancer Therapies and Self-Reactive Immune Cells. Cancers 2020, 12, 673. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef]
- Chowdhury, S.; Kar, K.; Mazumder, R. Exploration of Different Strategies of Nanoencapsulation of Bioactive Compounds and Their Ensuing Approaches. Future J. Pharm. Sci. 2024, 10, 72. [Google Scholar] [CrossRef]
- Habib, M.; Jan, K.; Bashir, K. Extraction and Characterization of Bioactive Compounds from Different Sources. In Bioactive Components; Springer Nature: Singapore, 2023; pp. 121–141. [Google Scholar]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural Products Targeting the PI3K-Akt-MTOR Signaling Pathway in Cancer: A Novel Therapeutic Strategy. Semin. Cancer Biol. 2022, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Iqbal, M.O.; Khan, H.; Ahmed, M.M.; Farooq, M.; Aadil, M.M.; Jamaludin, M.I.; Hazafa, A.; Tsai, W.-C. A Review of Twenty Years of Research on the Regulation of Signaling Pathways by Natural Products in Breast Cancer. Molecules 2022, 27, 3412. [Google Scholar] [CrossRef] [PubMed]
- Mitea, G.; Schröder, V.; Iancu, I.M.; Mireșan, H.; Iancu, V.; Bucur, L.A.; Badea, F.C. Molecular Targets of Plant-Derived Bioactive Compounds in Oral Squamous Cell Carcinoma. Cancers 2024, 16, 3612. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, W.; Zhang, H.; Guo, Q.; Yang, W.; Li, B.; Sun, Z.; Gao, S.; Cui, R. Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front. Oncol. 2019, 9, 1153. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting Cancer Signaling Pathways by Natural Products: Exploring Promising Anti-Cancer Agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Ali Abdalla, Y.O.; Subramaniam, B.; Nyamathulla, S.; Shamsuddin, N.; Arshad, N.M.; Mun, K.S.; Awang, K.; Nagoor, N.H. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. J. Trop. Med. 2022, 2022, 5794350. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Brisdelli, F.; D’Andrea, G.; Bozzi, A. Resveratrol: A Natural Polyphenol with Multiple Chemopreventive Properties (Review). Curr. Drug Metab. 2009, 10, 530–546. [Google Scholar] [CrossRef]
- Kushwaha, S.P.; Hasan, S.M.; Ahmad, U.; Singh, K.; Kumar, A.; Arif, M.; Kushwaha, P.; Hafeez, A.; Shoaib, A. Quinones as Antioxidants. In Quinone-Based Compounds in Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 83–101. [Google Scholar]
- Bufka, J.; Vaňková, L.; Sýkora, J.; Křížková, V. Exploring Carotenoids: Metabolism, Antioxidants, and Impacts on Human Health. J. Funct. Foods 2024, 118, 106284. [Google Scholar] [CrossRef]
- Patil, M.A.; Sarkate, A.P.; Nirmal, N.P.; Sakhale, B.K. Alkaloids as Potential Anticancer Agent. In Recent Frontiers of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 203–224. [Google Scholar]
- Siddiqui, T.; Khan, M.U.; Sharma, V.; Gupta, K. Terpenoids in Essential Oils: Chemistry, Classification, and Potential Impact on Human Health and Industry. Phytomed. Plus 2024, 4, 100549. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Mohamed, J.; Ainane, T. Exploring the Potent Anticancer Activity of Essential Oils and Their Bioactive Compounds: Mechanisms and Prospects for Future Cancer Therapy. Pharmaceuticals 2023, 16, 1086. [Google Scholar] [CrossRef]
- Carlos, D. Synergistic Effects of Plant Extracts: Understanding Complex Interactions in Medicinal Plants. J. Pharmacogn. Nat Prod. 2024, 10, 313. [Google Scholar]
- Arora, S.; Saquib, S.A.; Algarni, Y.A.; Kader, M.A.; Ahmad, I.; Alshahrani, M.Y.; Saluja, P.; Baba, S.M.; Abdulla, A.M.; Bavabeedu, S.S. Synergistic Effect of Plant Extracts on Endodontic Pathogens Isolated from Teeth with Root Canal Treatment Failure: An In Vitro Study. Antibiotics 2021, 10, 552. [Google Scholar] [CrossRef]
- Cheon, C.; Ko, S.-G. Synergistic Effects of Natural Products in Combination with Anticancer Agents in Prostate Cancer: A Scoping Review. Front. Pharmacol. 2022, 13, 963317. [Google Scholar] [CrossRef]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic Effects of Plant Derivatives and Conventional Chemotherapeutic Agents: An Update on the Cancer Perspective. Medicina 2019, 55, 110. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, P.; Li, J.; Zhou, Y.; Wang, B.; Lu, Z. The Effect of Doxorubicin Curcumin Co-Loaded Lipid Nanoparticles and Doxorubicin on Osteosarcoma before Surgery. Cancer Nanotechnol. 2024, 15, 11. [Google Scholar] [CrossRef]
- Kciuk, M.; Alam, M.; Ali, N.; Rashid, S.; Głowacka, P.; Sundaraj, R.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E.; et al. Epigallocatechin-3-Gallate Therapeutic Potential in Cancer: Mechanism of Action and Clinical Implications. Molecules 2023, 28, 5246. [Google Scholar] [CrossRef] [PubMed]
- Iancu, V.; Iancu, I.M.; Roncea, F.N.; Mireșan, H.; Cazacincu, R.G.; Schroder, V.; Blebea, N.M.; Mitea, G. Pharmaco-technical profile of Lythri herba freeze-dried extract based on SeDeM methodology. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021; IEEE: New York, NY, USA, 2021; pp. 1–4. [Google Scholar]
- Topor, G.; Nechita, A.; Strambu, I.R.; Bratu, I.C.; Stefan, S.; Campanu, M.; Dumitriu-Buzia, O.; Agop, D.; Forna, S.P. Analysis of the Effects of Nonsteroidal Anti-Inflammatory Drugs (Nsaids) in the Oral Cavity. Rom. J. Oral Rehabil. 2024, 16, 468–478. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Geszke-Moritz, M.; Moritz, M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers 2024, 16, 2536. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef]
- Kumar, R.; Islam, T.; Nurunnabi, M. Mucoadhesive Carriers for Oral Drug Delivery. J. Control. Release 2022, 351, 504–559. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, B.; Wan, Y.; Sun, Y.; Wang, L.; Sun, J.; Li, C. Drug Delivery Based Pharmacological Enhancement and Current Insights of Quercetin with Therapeutic Potential against Oral Diseases. Biomed. Pharmacother. 2020, 128, 110372. [Google Scholar] [CrossRef]
- Attar, E.S.; Chaudhari, V.H.; Deokar, C.G.; Dyawanapelly, S.; Devarajan, P.V. Nano Drug Delivery Strategies for an Oral Bioenhanced Quercetin Formulation. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 495–514. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.G.R.; Pinheiro, M.; Neves, A.R. Nanotechnology Innovations to Enhance the Therapeutic Efficacy of Quercetin. Nanomaterials 2021, 11, 2658. [Google Scholar] [CrossRef]
- Kumar, M.; Jha, A.K. Exploring the Potential of Dietary Factors and Plant Extracts as Chemopreventive Agents in Oral Squamous Cell Carcinoma Treatment. Front. Oral Health 2023, 4, 1246873. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, M.; Quispe, C.; Sharifi-Rad, J.; Pons-Fuster, E.; Lopez-Jornet, P.; Zam, W.; Das, T.; Dey, A.; Kumar, M.; Pentea, M.; et al. Naturally-Occurring Bioactives in Oral Cancer: Preclinical and Clinical Studies, Bottlenecks and Future Directions. Front. Biosci. Sch. 2022, 14, 1403024. [Google Scholar] [CrossRef]
- Burcher, J.T.; DeLiberto, L.K.; Allen, A.M.; Kilpatrick, K.L.; Bishayee, A. Bioactive Phytocompounds for Oral Cancer Prevention and Treatment: A Comprehensive and Critical Evaluation. Med. Res. Rev. 2023, 43, 2025–2085. [Google Scholar] [CrossRef]
- Issa, H.; Loubaki, L.; Al Amri, A.; Zibara, K.; Almutairi, M.H.; Rouabhia, M.; Semlali, A. Eugenol as a Potential Adjuvant Therapy for Gingival Squamous Cell Carcinoma. Sci. Rep. 2024, 14, 10958. [Google Scholar] [CrossRef]
- Esquivel-Chirino, C.; Bolaños-Carrillo, M.A.; Carmona-Ruiz, D.; Lopéz-Macay, A.; Hernández-Sánchez, F.; Montés-Sánchez, D.; Escuadra-Landeros, M.; Gaitán-Cepeda, L.A.; Maldonado-Frías, S.; Yáñez-Ocampo, B.R.; et al. The Protective Role of Cranberries and Blueberries in Oral Cancer. Plants 2023, 12, 2330. [Google Scholar] [CrossRef]
- Shukla, N.P.; Senapathya, G.J. Current Review on Nanophytomedicines in the Treatment of Oral Cancer: Recent Trends and Treatment Prospects. Crit. Rev. Ther. Drug Carr. Syst. 2025, 42, 89–118. [Google Scholar] [CrossRef]
- Adeola, H.A.; Bano, A.; Vats, R.; Vashishtha, A.; Verma, D.; Kaushik, D.; Mittal, V.; Rahman, M.d.H.; Najda, A.; Albadrani, G.M.; et al. Bioactive Compounds and Their Libraries: An Insight into Prospective Phytotherapeutics Approach for Oral Mucocutaneous Cancers. Biomed. Pharmacother. 2021, 141, 111809. [Google Scholar] [CrossRef]
- Sachdeva, A.; Dhawan, D.; Jain, G.K.; Yerer, M.B.; Collignon, T.E.; Tewari, D.; Bishayee, A. Novel Strategies for the Bioavailability Augmentation and Efficacy Improvement of Natural Products in Oral Cancer. Cancers 2022, 15, 268. [Google Scholar] [CrossRef]
- Salehi, B.; Lopez-Jornet, P.; Pons-Fuster López, E.; Calina, D.; Sharifi-Rad, M.; Ramírez-Alarcón, K.; Forman, K.; Fernández, M.; Martorell, M.; Setzer, W.; et al. Plant-Derived Bioactives in Oral Mucosal Lesions: A Key Emphasis to Curcumin, Lycopene, Chamomile, Aloe Vera, Green Tea and Coffee Properties. Biomolecules 2019, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Anjali; Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of Plant Secondary Metabolites in Defence and Transcriptional Regulation in Response to Biotic Stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Selwal, N.; Rahayu, F.; Herwati, A.; Latifah, E.; Supriyono; Suhara, C.; Kade Suastika, I.B.; Mahayu, W.M.; Wani, A.K. Enhancing Secondary Metabolite Production in Plants: Exploring Traditional and Modern Strategies. J. Agric. Food Res. 2023, 14, 100702. [Google Scholar] [CrossRef]
- Katyal, P. Plant Secondary Metabolites: Functions in Plants and Pharmacological Importance. In Plant Secondary Metabolites; Springer Nature: Singapore, 2022; pp. 437–457. [Google Scholar]
- Kumar, S.; Saini, R.; Suthar, P.; Kumar, V.; Sharma, R. Plant Secondary Metabolites: Their Food and Therapeutic Importance. In Plant Secondary Metabolites; Springer Nature: Singapore, 2022; pp. 371–413. [Google Scholar]
- Yuandani; Jantan, I.; Salim, E.; Septama, A.W.; Rullah, K.; Nainu, F.; Fasihi Mohd Aluwi, M.F.; Emran, T.B.; Roney, M.; Khairunnisa, N.A.; et al. Mechanistic Insights into Anti-inflammatory and Immunosuppressive Effects of Plant Secondary Metabolites and Their Therapeutic Potential for Rheumatoid Arthritis. Phytother. Res. 2024, 38, 2931–2961. [Google Scholar] [CrossRef]
- Rao, M.R.P.; Ghadge, I.; Kulkarni, S.; Madgulkar, A.R. Importance of Plant Secondary Metabolites in Modern Therapy. In Plant Specialized Metabolites: Phytochemistry, Ecology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 25–55. [Google Scholar]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- de Souza Silva, A.P.; de Camargo, A.C.; Lazarini, J.G.; Franchin, M.; Sardi, J.d.C.O.; Rosalen, P.L.; de Alencar, S.M. Phenolic Profile and the Antioxidant, Anti-Inflammatory, and Antimicrobial Properties of Açaí (Euterpe oleracea) Meal: A Prospective Study. Foods 2022, 12, 86. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef]
- Al Mamari, H.H. Phenolic Compounds: Classification, Chemistry, and Updated Techniques of Analysis and Synthesis; IntechOpen: London, UK, 2022. [Google Scholar]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Oluwole, O.; Fernando, W.B.; Lumanlan, J.; Ademuyiwa, O.; Jayasena, V. Role of Phenolic Acid, Tannins, Stilbenes, Lignans and Flavonoids in Human Health—A Review. Int. J. Food Sci. Technol. 2022, 57, 6326–6335. [Google Scholar] [CrossRef]
- Anand, S.; Sowbhagya, R.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Almalik, A.I.; Ahmad, W.; Tripathi, T.; Elderdery, A.Y. Polyphenols and Their Nanoformulations: Protective Effects against Human Diseases. Life 2022, 12, 1639. [Google Scholar] [CrossRef]
- Khan, K.R.; Dawda, H.; Mukundan, U. Polyphenols: Methods of Extraction. Sci. Rev. Chem. Commun. 2014, 51, 1–6. [Google Scholar]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as Natural Phenolic Compounds and Their Role in Therapeutics: An Overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.C.; Fortes, G.A.C.; Camargo, L.T.F.M.; Camargo, A.J.; Ferri, P.H. Antioxidant Effects of Polyphenolic Compounds and Structure-Activity Relationship Predicted by Multivariate Regression Tree. LWT 2021, 137, 110366. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Zhuang, W.-B.; Li, Y.-H.; Shu, X.-C.; Pu, Y.-T.; Wang, X.-J.; Wang, T.; Wang, Z. The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses. Molecules 2023, 28, 3599. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Pentu, N.; M, S.B.; Tadikonda, R.R. Flavanoids: An era of nutraceuticals turning in to medicinal agents. Asian J. Pharm. Clin. Res. 2024, 17, 9–17. [Google Scholar] [CrossRef]
- Čižmárová, B.; Hubková, B.; Tomečková, V.; Birková, A. Flavonoids as Promising Natural Compounds in the Prevention and Treatment of Selected Skin Diseases. Int. J. Mol. Sci. 2023, 24, 6324. [Google Scholar] [CrossRef]
- Kim, K.; Vance, T.M.; Chun, O.K. Greater Flavonoid Intake Is Associated with Improved CVD Risk Factors in US Adults. Br. J. Nutr. 2016, 115, 1481–1488. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef]
- de Oliveira, N.K.S.; Almeida, M.R.S.; Pontes, F.M.M.; Barcelos, M.P.; de Paula da Silva, C.H.T.; Rosa, J.M.C.; Cruz, R.A.S.; da Silva Hage-Melim, L.I. Antioxidant Effect of Flavonoids Present in Euterpe oleracea Martius and Neurodegenerative Diseases: A Literature Review. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- do Nascimento, R.P.; dos Santos, B.L.; Amparo, J.A.O.; Soares, J.R.P.; da Silva, K.C.; Santana, M.R.; Almeida, Á.M.A.N.; da Silva, V.D.A.; Costa, M.d.F.D.; Ulrich, H.; et al. Neuroimmunomodulatory Properties of Flavonoids and Derivates: A Potential Action as Adjuvants for the Treatment of Glioblastoma. Pharmaceutics 2022, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Pejčić, T.; Zeković, M.; Bumbaširević, U.; Kalaba, M.; Vovk, I.; Bensa, M.; Popović, L.; Tešić, Ž. The Role of Isoflavones in the Prevention of Breast Cancer and Prostate Cancer. Antioxidants 2023, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Luo, K. Biosynthesis and Metabolic Engineering of Isoflavonoids in Model Plants and Crops: A Review. Front. Plant Sci. 2024, 15, 1384091. [Google Scholar] [CrossRef]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A Review on Its Anti-Inflammatory Properties. Front. Pharmacol. 2022, 13, 820969. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxid. Med. Cell Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Ding, G.; Gong, Y.; Jing, C.; Peng, G.; Liu, S.; Niu, L.; Zhang, S.; Luo, Z.; Li, H.; et al. Stilbene-Benzophenone Dyads for Free Radical Initiating Polymerization of Methyl Methacrylate under Visible Light Irradiation. Dye. Pigment. 2016, 132, 27–40. [Google Scholar] [CrossRef]
- Begh, M.Z.A.; Khan, J.; Zehravi, M.; Sweilam, S.H.; Raja, A.D.; Muthukumar, A.; Haque, M.A.; Kar, N.R.; Singh, L.P.; Priya, B.D.; et al. Targeting Neurological Disorders with Stilbenes: Bridging the Preclinical-Clinical Gap. Int. J. Biol. Sci. 2024, 20, 5474–5494. [Google Scholar] [CrossRef]
- Mendonça, E.L.S.S.; Xavier, J.A.; Fragoso, M.B.T.; Silva, M.O.; Escodro, P.B.; Oliveira, A.C.M.; Tucci, P.; Saso, L.; Goulart, M.O.F. E-Stilbenes: General Chemical and Biological Aspects, Potential Pharmacological Activity Based on the Nrf2 Pathway. Pharmaceuticals 2024, 17, 232. [Google Scholar] [CrossRef]
- Kluska, M.; Jabłońska, J.; Prukała, W. Analytics, Properties and Applications of Biologically Active Stilbene Derivatives. Molecules 2023, 28, 4482. [Google Scholar] [CrossRef]
- Singh, D.; Chauhan, N.; Koli, M.; Nayak, S.K.; Subramanian, M. Dimer Stilbene, a Resveratrol Analogue Exhibits Synergy with Antibiotics That Target Protein Synthesis in Eradicating Staphylococcus aureus Infection. Biochimie 2022, 201, 128–138. [Google Scholar] [CrossRef]
- Kobylka, P.; Kucinska, M.; Kujawski, J.; Lazewski, D.; Wierzchowski, M.; Murias, M. Resveratrol Analogues as Selective Estrogen Signaling Pathway Modulators: Structure–Activity Relationship. Molecules 2022, 27, 6973. [Google Scholar] [CrossRef]
- Shan, Z.; Yang, G.; Xiang, W.; Pei-jun, W.; Bin, Z. Effects of Resveratrol on Oral Squamous Cell Carcinoma (OSCC) Cells in Vitro. J. Cancer Res. Clin. Oncol. 2014, 140, 371–374. [Google Scholar] [CrossRef]
- Borys, F.; Tobiasz, P.; Poterała, M.; Fabczak, H.; Krawczyk, H.; Joachimiak, E. Systematic Studies on Anti-Cancer Evaluation of Stilbene and Dibenzo[b,f]Oxepine Derivatives. Molecules 2023, 28, 3558. [Google Scholar] [CrossRef]
- Yu, X.-D.; Yang, J.; Zhang, W.-L.; Liu, D.-X. Resveratrol Inhibits Oral Squamous Cell Carcinoma through Induction of Apoptosis and G2/M Phase Cell Cycle Arrest. Tumor Biol. 2016, 37, 2871–2877. [Google Scholar] [CrossRef]
- Valentová, K.; Havlík, J.; Kosina, P.; Papoušková, B.; Jaimes, J.D.; Káňová, K.; Petrásková, L.; Ulrichová, J.; Křen, V. Biotransformation of Silymarin Flavonolignans by Human Fecal Microbiota. Metabolites 2020, 10, 29. [Google Scholar] [CrossRef]
- Song, K.; Li, M.; Yang, Y.; Zhang, Z.; Zhu, Q.; Liu, J.; Wang, A. Natural Flavonolignans as Potential Therapeutic Agents against Common Diseases. J. Pharm. Pharmacol. 2022, 74, 337–350. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, X.; Huang, Z.; Luo, S.; Zhang, X.; Sun, W.; Lan, T.; He, R. Determination of Flavonolignan Compositional Ratios in Silybum marianum (Milk Thistle) Extracts Using High-Performance Liquid Chromatography. Molecules 2024, 29, 2949. [Google Scholar] [CrossRef]
- Mohammadi, S.; Asbaghi, O.; Afrisham, R.; Farrokhi, V.; Jadidi, Y.; Mofidi, F.; Ashtary-Larky, D. Impacts of Supplementation with Silymarin on Cardiovascular Risk Factors: A Systematic Review and Dose–Response Meta-Analysis. Antioxidants 2024, 13, 390. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Panayiotou, C.; Vardas, M.; Balaskas, N.; Kostomitsopoulos, N.G.; Tsaroucha, A.K.; Valsami, G. A Comprehensive Review of the Cardiovascular Protective Properties of Silibinin/Silymarin: A New Kid on the Block. Pharmaceuticals 2022, 15, 538. [Google Scholar] [CrossRef]
- Tajmohammadi, A.; Razavi, B.M.; Hosseinzadeh, H. Silybum marianum (Milk Thistle) and Its Main Constituent, Silymarin, as a Potential Therapeutic Plant in Metabolic Syndrome: A Review. Phytother. Res. 2018, 32, 1933–1949. [Google Scholar] [CrossRef] [PubMed]
- Won, D.-H.; Kim, L.-H.; Jang, B.; Yang, I.-H.; Kwon, H.-J.; Jin, B.; Oh, S.H.; Kang, J.-H.; Hong, S.-D.; Shin, J.-A.; et al. In Vitro and In Vivo Anti-Cancer Activity of Silymarin on Oral Cancer. Tumor Biol. 2018, 40, 101042831877617. [Google Scholar] [CrossRef]
- Ahmadian, E. Silymarin and Oral Health. Adv. Dent. Oral Health 2018, 9, 555772. [Google Scholar] [CrossRef]
- Sharma, U.; Sahni, P.K.; Sharma, B.; Gupta, M.; Kaur, D.; Mathkor, D.M.; Haque, S.; Khatoon, S.; Tuli, H.S.; Mishra, A.; et al. Silymarin: A Promising Modulator of Apoptosis and Survival Signaling in Cancer. Discov. Oncol. 2025, 16, 66. [Google Scholar] [CrossRef]
- Shete, M.B.; Deshpande, A.S.; Shende, P. Enhancement of In-Vitro Anti-Oral Cancer Activities of Silymarin Using Dispersion of Nanostructured Lipid Carrier in Mucoadhesive in-Situ Gel. Int. J. Pharm. 2023, 636, 122860. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, M.C.; D’Antoni, C.L.; Domínguez Rubio, A.P.; Alaimo, A.; Pérez, O.E. Chitosan-Tripolyphosphate Nanoparticles Designed to Encapsulate Polyphenolic Compounds for Biomedical and Pharmaceutical Applications—A Review. Biomed. Pharmacother. 2021, 142, 111970. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, V.; Srećković, N.; Popović-Djordjević, J.B. Silybin and Silymarin: Phytochemistry, Bioactivity, and Pharmacology. In Handbook of Dietary Flavonoids; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–45. [Google Scholar]
- Muchiri, R.N.; van Breemen, R.B. Chemical Standardization of Milk Thistle (Silybum marianum L.) Extract Using UHPLC-MS/MS and the Method of Standard Addition. J. Am. Soc. Mass. Spectrom. 2024, 35, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Hassan, A.A.; Mohamed, N.K.; Ramadan, M.; Abd El-Aal, A.S.; Bräse, S.; Nieger, M. Synthesis of Quinone-Based Heterocycles of Broad-Spectrum Anticancer Activity. J. Chem. Res. 2021, 45, 562–571. [Google Scholar] [CrossRef]
- Singh, R.; Zubair, R.K.; Suresh, S.; Lonari, S.B.; Phatake, R.S. Naturally Occurring and Structural Analogues of Quinones Offering New Research Directions for the Discovery of Anticancer Drugs. In Quinone-Based Compounds in Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 29–53. [Google Scholar]
- Faizan, S.; Mohammed Abdo Mohsen, M.; Amarakanth, C.; Justin, A.; Ravishankar Rahangdale, R.; Raghu Chandrashekar, H.; Prashantha Kumar, B.R. Quinone Scaffolds as Potential Therapeutic Anticancer Agents: Chemistry, Mechanism of Actions, Structure-Activity Relationships and Future Perspectives. Results Chem. 2024, 7, 101432. [Google Scholar] [CrossRef]
- Ciftci, H.; Sever, B.; Kaya, N.; Bayrak, N.; Yıldız, M.; Yıldırım, H.; Tateishi, H.; Otsuka, M.; Fujita, M.; TuYuN, A.F. Studies on 1,4-Quinone Derivatives Exhibiting Anti-Leukemic Activity along with Anti-Colorectal and Anti-Breast Cancer Effects. Molecules 2022, 28, 77. [Google Scholar] [CrossRef]
- Córdova-Delgado, M.; Fuentes-Retamal, S.; Palominos, C.; López-Torres, C.; Guzmán-Rivera, D.; Ramírez-Rodríguez, O.; Araya-Maturana, R.; Urra, F.A. FRI-1 Is an Anti-Cancer Isoquinolinequinone That Inhibits the Mitochondrial Bioenergetics and Blocks Metabolic Shifts by Redox Disruption in Breast Cancer Cells. Antioxidants 2021, 10, 1618. [Google Scholar] [CrossRef]
- Safari, M.R.; Rrezaei, M.; Taherkhani, H. Effects of Ubiquinol-10 and Beta-Carotene on the in Vitro Susceptibility of Low-Density Lipoprotein to Copper-Induced Oxidation. Med. J. Islam. Repub. Iran. 2005, 2, 169–174. [Google Scholar]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10 Analogues: Benefits and Challenges for Therapeutics. Antioxidants 2021, 10, 236. [Google Scholar] [CrossRef]
- Zaki, N.M. Strategies for Oral Delivery and Mitochondrial Targeting of CoQ10. Drug Deliv. 2014, 1868–1881, 993747. [Google Scholar] [CrossRef]
- Antolak, H.; Oracz, J.; Otlewska, A.; Żyżelewicz, D.; Kręgiel, D. Identification of Carotenoids and Isoprenoid Quinones from Asaia Lannensis and Asaia Bogorensis. Molecules 2017, 22, 1608. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Fu, W.; Du, M.; Chen, Z.-X.; Lei, A.-P.; Wang, J.-X. Carotenoids Biosynthesis, Accumulation, and Applications of a Model Microalga Euglenagracilis. Mar. Drugs 2022, 20, 496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, C.; Zeng, N.; Wang, D.; Zhang, N.; Li, B. Current Advances in the Biosynthesis and Sustainable Production Strategies of Carotenoids and Their Multifaceted Applications in the Food Industry: A Comprehensive Review. Food Biosci. 2025, 64, 105864. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant Carotenoids: Recent Advances and Future Perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Lichman, B.R. The Scaffold-Forming Steps of Plant Alkaloid Biosynthesis. Nat. Prod. Rep. 2021, 38, 103–129. [Google Scholar] [CrossRef]
- Bui, V.-H.; Rodríguez-López, C.E.; Dang, T.-T.T. Integration of Discovery and Engineering in Plant Alkaloid Research: Recent Developments in Elucidation, Reconstruction, and Repurposing Biosynthetic Pathways. Curr. Opin. Plant Biol. 2023, 74, 102379. [Google Scholar] [CrossRef]
- Sharma, H.; Pathak, D.; Kumar, S. Recent Progress in Isolating and Purifying Amide Alkaloids from Their Natural Habitats: A Review. Curr. Bioact. Compd. 2024, 20, E210224227226. [Google Scholar] [CrossRef]
- Pereira, A.G.; Cassani, L.; Garcia-Oliveira, P.; Otero, P.; Mansoor, S.; Echave, J.; Xiao, J.; Simal-Gándara, J.; Prieto, M.A. Plant Alkaloids: Production, Extraction, and Potential Therapeutic Properties. In Natural Secondary Metabolites; Springer International Publishing: Cham, Switzerland, 2023; pp. 157–200. [Google Scholar]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Luan, F.; He, X.; Zeng, N. Tetrandrine: A Review of Its Anticancer Potentials, Clinical Settings, Pharmacokinetics and Drug Delivery Systems. J. Pharm. Pharmacol. 2020, 72, 1491–1512. [Google Scholar] [CrossRef]
- Huang, L.; Liu, L.; Zhu, J.; Chen, N.; Chen, J.; Chan, C.-F.; Gao, F.; Yin, Y.; Sun, J.; Zhang, R.; et al. Bis-Benzylisoquinoline Alkaloids Inhibit Flavivirus Entry and Replication by Compromising Endolysosomal Trafficking and Autophagy. Virol. Sin. 2024, 39, 892–908. [Google Scholar] [CrossRef]
- Manogaran, P.; Beeraka, N.M.; Padma, V.V. The Cytoprotective and Anti-Cancer Potential of Bisbenzylisoquinoline Alkaloids from Nelumbo Nucifera. Curr. Top. Med. Chem. 2020, 19, 2940–2957. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. An Overview on the Chemistry, Pharmacology and Anticancer Properties of Tetrandrine and Fangchinoline (Alkaloids) from Stephania Tetrandra Roots. J. Integr. Med. 2021, 19, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Bhagya, N.; Chandrashekar, K.R. Autophagy and Cancer: Can Tetrandrine Be a Potent Anticancer Drug in the near Future? Biomed. Pharmacother. 2022, 148, 112727. [Google Scholar] [CrossRef] [PubMed]
- Lien, J.; Lin, M.; Chang, S.; Lai, K.; Huang, A.; Yu, F.; Chung, J. Tetrandrine Induces Programmed Cell Death in Human Oral Cancer CAL 27 Cells through the Reactive Oxygen Species Production and Caspase-dependent Pathways and Associated with Beclin-1-induced Cell Autophagy. Environ. Toxicol. 2017, 32, 329–343. [Google Scholar] [CrossRef]
- Huang, A.-C.; Lien, J.-C.; Lin, M.-W.; Yang, J.-S.; Wu, P.-P.; Chang, S.-J.; Lai, T.-Y. Tetrandrine Induces Cell Death in SAS Human Oral Cancer Cells through Caspase Activation-Dependent Apoptosis and LC3-I and LC3-II Activation-Dependent Autophagy. Int. J. Oncol. 2013, 43, 485–494. [Google Scholar] [CrossRef]
- Zuzarte, M.; Sousa, C.; Alves-Silva, J.; Salgueiro, L. Plant Monoterpenes and Essential Oils as Potential Anti-Ageing Agents: Insights from Preclinical Data. Biomedicines 2024, 12, 365. [Google Scholar] [CrossRef]
- Saha, P.; Rahman, F.I.; Hussain, F.; Rahman, S.M.A.; Rahman, M.M. Antimicrobial Diterpenes: Recent Development From Natural Sources. Front. Pharmacol. 2022, 12, 820312. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Moujir, L.; Callies, O.; Sousa, P.M.C.; Sharopov, F.; Seca, A.M.L. Applications of Sesquiterpene Lactones: A Review of Some Potential Success Cases. Appl. Sci. 2020, 10, 3001. [Google Scholar] [CrossRef]
- Page, J.E.; Nagel, J. Biosynthesis of Terpenophenolic Metabolites in Hop and Cannabis. Recent Adv. Phytochem. 2006, 40, 179–210. [Google Scholar]
- Zhu, Z.; Chen, R.; Zhang, L. Simple Phenylpropanoids: Recent Advances in Biological Activities, Biosynthetic Pathways, and Microbial Production. Nat. Prod. Rep. 2024, 41, 6–24. [Google Scholar] [CrossRef]
- Dumitrescu, E.; Muselin, F.; Tîrziu, E.; Folescu, M.; Dumitrescu, C.S.; Orboi, D.M.; Cristina, R.T. Pimpinella anisum L. Essential Oil a Valuable Antibacterial and Antifungal Alternative. Plants 2023, 12, 2428. [Google Scholar] [CrossRef]
- Koeduka, T.; Baiga, T.J.; Noel, J.P.; Pichersky, E. Biosynthesis of t-Anethole in Anise: Characterization of t-Anol/Isoeugenol Synthase and an O. -Methyltransferase Specific for a C7–C8 Propenyl Side Chain. Plant Physiol. 2009, 149, 384–394. [Google Scholar] [CrossRef]
- Evangelista, P.M.; Lima, F.L.G.d.; Cunha, L.d.S.d.; Cavalcante, M.P.; Furtado, J.A.; Silva, S.d.S.; Barros, S.L.; Silva, C.d.F.B.d.; Canuto, K.M.; Bastos, M.d.S.R.; et al. Impact of the Extraction Temperature on the Antifungal Efficiency and Valorization Potential of Anise (Pimpinella anisum L.) Essential Oil Residues: A Sustainable Strategy. Ciênc. Agrotecnologia 2024, 48, e019724. [Google Scholar] [CrossRef]
- AlBalawi, A.N.; Elmetwalli, A.; Baraka, D.M.; Alnagar, H.A.; Alamri, E.S.; Hassan, M.G. Chemical Constituents, Antioxidant Potential, and Antimicrobial Efficacy of Pimpinella anisum Extracts against Multidrug-Resistant Bacteria. Microorganisms 2023, 11, 1024. [Google Scholar] [CrossRef]
- Hammache, M.; Benchekroun, S.; Alamri, A.; Jalouli, M.; Yousry, A.M.M.; Boufahja, F.; Chahine, M.; Chandad, F.; Semlali, A. Modulation of Signature Cancer-Related Genes in Oral Cancer Cells (Ca9-22) by Anethole Treatment: Insights into Therapeutic Potential. PLoS ONE 2024, 19, e0315085. [Google Scholar] [CrossRef] [PubMed]
- León, A.; Del-Ángel, M.; Ávila, J.L.; Delgado, G. Phthalides: Distribution in Nature, Chemical Reactivity, Synthesis, and Biological Activity. In Progress in the Chemistry of Organic Natural Products; Springer: Cham, Switzerland, 2017; pp. 127–246. [Google Scholar]
- Chen, Y.; Cheng, Q.; Lv, S.; Kang, Z.; Zeng, S. Advances in the Phytochemistry and Pharmacology of Plant-Derived Phthalides. Heliyon 2023, 9, e22957. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zeng, Y.; Sun, C.; Meng, F.; Wang, Y. Recent Advances in Natural Phthalides: Distribution, Chemistry, and Biological Activities. Fitoterapia 2022, 160, 105223. [Google Scholar] [CrossRef]
- Sadıkoğulları, B.C.; Şenel, P.; Çini, N.; Faysal, A.A.; Odabaşoğlu, M.; Özdemir, A.D.; Gölcü, A. An Overview of Natural and Synthetic Phthalides Involved in Cancer Studies: Past, Present, and Future. ChemistrySelect 2022, 7, e202202004. [Google Scholar] [CrossRef]
- Remali, J.; Sahidin, I.; Aizat, W.M. Xanthone Biosynthetic Pathway in Plants: A Review. Front. Plant Sci. 2022, 13, 809497. [Google Scholar] [CrossRef]
- Oriola, A.O.; Kar, P. Naturally Occurring Xanthones and Their Biological Implications. Molecules 2024, 29, 4241. [Google Scholar] [CrossRef] [PubMed]
- Badiali, C.; Petruccelli, V.; Brasili, E.; Pasqua, G. Xanthones: Biosynthesis and Trafficking in Plants, Fungi and Lichens. Plants 2023, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Sakashita, H.; Hayashi, H.; Shiono, J.; Miyake, G.; Komine, Y.; Taira, F.; Sakashita, H. Synergism between α-Mangostin and TRAIL Induces Apoptosis in Squamous Cell Carcinoma of the Oral Cavity through the Mitochondrial Pathway. Oncol. Rep. 2017, 38, 3439–3446. [Google Scholar] [CrossRef][Green Version]
- Nauman, M.C.; Johnson, J.J. The Purple Mangosteen (Garcinia mangostana): Defining the Anticancer Potential of Selected Xanthones. Pharmacol. Res. 2022, 175, 106032. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Sun, H.; Wang, X.; Zhao, L.; Gao, Y.; Liu, X.; Zhang, S.; Wang, Y.; Yang, Y.; et al. Garcinia Xanthones as Orally Active Antitumor Agents. J. Med. Chem. 2013, 56, 276–292. [Google Scholar] [CrossRef]
- Rahayu, Y.C. Potential of Mangosteen Xanthones as Anti-Oral Cancer Agents by Induction of Apoptosis. Insisiva Dent. J. 2012, 1, 34–41. [Google Scholar]
- Yuan, L.; Zhang, F.; Jia, S.; Xie, J.; Shen, M. Differences between Phytosterols with Different Structures in Regulating Cholesterol Synthesis, Transport and Metabolism in Caco-2 Cells. J. Funct. Foods 2020, 65, 103715. [Google Scholar] [CrossRef]
- Shen, M.; Yuan, L.; Zhang, J.; Wang, X.; Zhang, M.; Li, H.; Jing, Y.; Zeng, F.; Xie, J. Phytosterols: Physiological Functions and Potential Application. Foods 2024, 13, 1754. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Zhang, Z.; Liu, J.; Hong, L. β-Sitosterol as a Promising Anticancer Agent for Chemoprevention and Chemotherapy: Mechanisms of Action and Future Prospects. Adv. Nutr. 2023, 14, 1085–1110. [Google Scholar] [CrossRef]
- Clairet, A.-L.; Boiteux-Jurain, M.; Curtit, E.; Jeannin, M.; Gérard, B.; Nerich, V.; Limat, S. Interaction between Phytotherapy and Oral Anticancer Agents: Prospective Study and Literature Review. Med. Oncol. 2019, 36, 45. [Google Scholar] [CrossRef]
- Viglianisi, G.; Polizzi, A.; Grippaudo, C.; Cocuzza, S.; Leonardi, R.; Isola, G. Chemopreventive and Biological Strategies in the Management of Oral Potentially Malignant and Malignant Disorders. Bioengineering 2024, 11, 65. [Google Scholar] [CrossRef]
- Jiménez, P.; Bustamante, A.; Echeverría, F.; Sambra, V.; Rincón-Cervera, M.Á.; Farías, C.; Valenzuela, R. Metabolic Benefits of Phytosterols: Chemical, Nutritional, and Functional Aspects. Food Rev. Int. 2024, 40, 2917–2939. [Google Scholar] [CrossRef]
- Poli, A.; Marangoni, F.; Corsini, A.; Manzato, E.; Marrocco, W.; Martini, D.; Medea, G.; Visioli, F. Phytosterols, Cholesterol Control, and Cardiovascular Disease. Nutrients 2021, 13, 2810. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.-H. Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Biomol. Ther. 2012, 20, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Martian, P.C.; Tertis, M.; Leonte, D.; Hadade, N.; Cristea, C.; Crisan, O. Cyclic Peptides: A Powerful Instrument for Advancing Biomedical Nanotechnologies and Drug Development. J. Pharm. Biomed. Anal. 2025, 252, 116488. [Google Scholar] [CrossRef]
- Ji, X.; Nielsen, A.L.; Heinis, C. Cyclic Peptides for Drug Development. Angew. Chem. Int. Ed. 2024, 63, e202308251. [Google Scholar] [CrossRef]
- López-Otín, C.; Bond, J.S. Proteases: Multifunctional Enzymes in Life and Disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef]
- Jagtap, Y.A.; Choudhary, A.; Kinger, S.; Kumar, P.; Bhattacharyya, S.; Jha, H.C.; Dhiman, R.; Sharma, V.; Suresh, A.K.; Poluri, K.M.; et al. Mitochondrial Proteostasis and Cellular Health: Insights from Chaperones and Autophagy. J. Physiol. 2025, 00, 1–28. [Google Scholar] [CrossRef]
- Bond, J.S. Proteases: History, Discovery, and Roles in Health and Disease. J. Biol. Chem. 2019, 294, 1643–1651. [Google Scholar] [CrossRef]
- Prakash, S.; Radha; Kumar, M.; Kumari, N.; Thakur, M.; Rathour, S.; Pundir, A.; Sharma, A.K.; Bangar, S.P.; Dhumal, S.; et al. Plant-Based Antioxidant Extracts and Compounds in the Management of Oral Cancer. Antioxidants 2021, 10, 1358. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Doghish, Y.A.; Doghish, A.S.; Mageed, S.S.A.; Mohammed, O.A.; Hamza, T.A.; Abdelaziz, A.A.; Moustafa, Y.M.; Abdel-Reheim, M.A.; Abbass, S.O.; Abbass, S.O.; et al. Natural Compounds Targeting MiRNAs: A Novel Approach in Oral Cancer Therapy. Funct. Integr. Genom. 2024, 24, 202. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ncbi.nlm.nih.gov/datasets/taxonomy/tree/ (accessed on 24 April 2025).
- Yu, H.J.; Ahn, C.H.; Yang, I.H.; Won, D.H.; Jin, B.; Cho, N.P.; Hong, S.D.; Shin, J.A.; Cho, S.D. Apoptosis Induced by Methanol Extract of Potentilla discolor in Human Mucoepidermoid Carcinoma Cells through STAT3/PUMA Signaling Axis. Mol. Med. Rep. 2018, 17, 5258–5264. [Google Scholar] [CrossRef] [PubMed]
- Keshava, R.; Muniyappa, N.; Gope, R.; Ramaswamaiah, A.S. Anti-Cancer Effects of Imperata cylindrica Leaf Extract on Human Oral Squamous Carcinoma Cell Line SCC-9 in Vitro. Asian Pac. J. Cancer Prev. 2016, 17, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Sivakali, V.; Thangavelu, L. Cytotoxic Activity of Cardiospermum halicacabum L. against Oral Cancer Cell Lines.-SCC25. Asian J. Biol. Life Sci. 2020, 9, 88–91. [Google Scholar] [CrossRef]
- Totmaj, M.A.; Dadelahi, S.; Varmazyar, S.; Abbasi, M. Scrophularia oxysepala Inhibit Oral Cancer Cell Line OECM-1 through Induction of Apoptosis. Hum. Gene 2022, 34, 201080. [Google Scholar] [CrossRef]
- Zhang, Q.; Kandasamy, K.; Alyami, N.M.; Alyami, H.M.; Natarajan, N.; Elayappan, P.K. Influence of Padina gymnospora on Apoptotic Proteins of Oral Cancer Cells—A Proteome-Wide Analysis. Appl. Biochem. Biotechnol. 2022, 194, 5945–5962. [Google Scholar] [CrossRef]
- Jo, J.R.; Park, J.S.; Park, Y.K.; Chae, Y.Z.; Lee, G.H.; Park, G.Y.; Jang, B.C. Pinus densiflora Leaf Essential Oil Induces Apoptosis via ROS Generation and Activation of Caspases in YD-8 Human Oral Cancer Cells. Int. J. Oncol. 2012, 40, 1238–1245. [Google Scholar] [CrossRef]
- Kingsley, K.; Chatelain, K.; Phippen, S.; McCabe, J.; Teeters, C.A.; O’Malley, S. Cranberry and Grape Seed Extracts Inhibit the Proliferative Phenotype of Oral Squamous Cell Carcinomas. Evid.-Based Complement. Altern. Med. 2011, 2011, 467691. [Google Scholar] [CrossRef]
- Asokan, A.; Thangavel, M. Invitro Cytotoxic Studies of Crude Methanolic Extract of Saraca indica Bark Extract. IOSR J. Pharm. Biol. Sci. 2014, 9, 26–30. [Google Scholar] [CrossRef]
- Sur, S.; Ray, R.B. Diverse Roles of Bitter Melon (Momordica charantia) in Prevention of Oral Cancer. J. Cancer Metastasis Treat. 2021, 7, 12. [Google Scholar] [CrossRef]
- Ramshankar, V.; Krishnamurthy, A. Chemoprevention of Oral Cancer: Green Tea Experience. J. Nat. Sci. Biol. Med. 2014, 5, 3–7. [Google Scholar] [CrossRef]
- Peng, S.Y.; Lin, L.C.; Chen, S.R.; Farooqi, A.A.; Cheng, Y.B.; Tang, J.Y.; Chang, H.W. Pomegranate Extract (Pomx) Induces Mitochondrial Dysfunction and Apoptosis of Oral Cancer Cells. Antioxidants 2021, 10, 1117. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R.; Meng, F.; Su, Z. Antiproliferative Activity of an Angular Furanocoumarin-Oroselol in Human Oral Cancer Cells Is Mediated via Autophagy Induction, Inhibition of Cell Migration, Invasion, and Downregulation of PI3K/AKT Signalling Pathway. Acta Biochim. Pol. 2022, 69, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Pourshahidi, S.; Davari, M. Anthocyanins: Promising Natural Compounds for Prevention and Treatment of Oral Squamous Cell Carcinoma. Middle East. J. Rehabil. Health Stud. 2020, 7, e105844. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, K.H.; Lee, H.Y. Effect of Resveratrol on Oral Cancer Cell Invasion Induced by Lysophosphatidic Acid. J. Dent. Hyg. Sci. 2018, 18, 188–193. [Google Scholar] [CrossRef]
- Fukuda, M.; Ogasawara, Y.; Hayashi, H.; Inoue, K.; Sakashita, H. Resveratrol Inhibits Proliferation and Induces Autophagy by Blocking SREBP1 Expression in Oral Cancer Cells. Molecules 2022, 27, 8250. [Google Scholar] [CrossRef]
- Alam, M.K.; Alqhtani, N.R.; Alnufaiy, B.; Alqahtani, A.S.; Elsahn, N.A.; Russo, D.; Di Blasio, M.; Cicciù, M.; Minervini, G. A Systematic Review and Meta-Analysis of the Impact of Resveratrol on Oral Cancer: Potential Therapeutic Implications. BMC Oral Health 2024, 24, 412. [Google Scholar] [CrossRef]
- Yang, H.W.; Chun-Yu Ho, D.; Liao, H.Y.; Liao, Y.W.; Fang, C.Y.; Ng, M.Y.; Yu, C.C.; Lin, F.C. Resveratrol Inhibits Arecoline-Induced Fibrotic Properties of Buccal Mucosal Fibroblasts via MiR-200a Activation. J. Dent. Sci. 2024, 19, 1028–1035. [Google Scholar] [CrossRef]
- Chan, M.Y.; Lee, B.J.; Chang, P.S.; Hsiao, H.Y.; Hsu, L.P.; Chang, C.H.; Lin, P.T. The Risks of Ubiquinone and β-Carotene Deficiency and Metabolic Disorders in Patients with Oral Cancer. BMC Cancer 2020, 20, 310. [Google Scholar] [CrossRef]
- Jabir, N.R.; Khan, M.S.; Alafaleq, N.O.; Naz, H.; Ahmed, B.A. Anticancer Potential of Yohimbine in Drug-Resistant Oral Cancer KB-ChR-8–5 Cells. Mol. Biol. Rep. 2022, 49, 9565–9573. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, M.; Parama, D.; Hegde, M.; Dr, S.; Lankalapalli, R.S.; Radhakrishnan, K.V.; Kunnumakkara, A.B. Natural Prenylflavones from the Stem Bark of Artocarpus Altilis: Promising Anticancer Agents for Oral Squamous Cell Carcinoma Targeting the Akt/MTOR/STAT-3 Signaling Pathway. ACS Omega 2024, 9, 24252–24267. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Han, E.J.; Jeon, S.J.; Lee, S.W.; Moon, J.M.; Jung, S.H.; Jung, J.Y. Piperlongumine Induces Apoptosis and Cytoprotective Autophagy via the MAPK Signaling Pathway in Human Oral Cancer Cells. Biomedicines 2023, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Ho, H.Y.; Lo, Y.S.; Lin, C.C.; Chuang, Y.C.; Abomughaid, M.M.; Hsieh, M.C.; Chen, M.K. Semilicoisoflavone B Induces Apoptosis of Oral Cancer Cells by Inducing ROS Production and Downregulating MAPK and Ras/Raf/MEK Signaling. Int. J. Mol. Sci. 2023, 24, 4505. [Google Scholar] [CrossRef]
- Cretu, B.; Zamfir, A.; Bucurica, S.; Scheau, A.E.; Savulescu Fiedler, I.; Caruntu, C.; Caruntu, A.; Scheau, C. Role of Cannabinoids in Oral Cancer. Int. J. Mol. Sci. 2024, 25, 969. [Google Scholar] [CrossRef]
- Yang, S.; Liao, P.; Pan, Y.; Chen, S.; Chou, S.; Chou, M. The Novel P53-Dependent Metastatic and Apoptotic Pathway Induced by Vitexin in Human Oral Cancer OC2 Cells. Phytother. Res. 2013, 27, 1154–1161. [Google Scholar] [CrossRef]
- Chuang, Y.; Yen, C.; Shiau, J.; Chang, F.; Duh, C.; Sung, P.; Chen, K.; Tsai, Y.; Tang, J.; Jeng, J.; et al. Demethoxymurrapanine, an Indole-naphthoquinone Alkaloid, Inhibits the Proliferation of Oral Cancer Cells without Major Side Effects on Normal Cells. Env. Toxicol. 2024, 39, 1221–1234. [Google Scholar] [CrossRef]
- Hsieh, M.-J.; Chen, J.-C.; Yang, W.-E.; Chien, S.-Y.; Chen, M.-K.; Lo, Y.-S.; Hsi, Y.-T.; Chuang, Y.-C.; Lin, C.-C.; Yang, S.-F. Dehydroandrographolide Inhibits Oral Cancer Cell Migration and Invasion through NF-ΚB-, AP-1-, and SP-1-Modulated Matrix Metalloproteinase-2 Inhibition. Biochem. Pharmacol. 2017, 130, 10–20. [Google Scholar] [CrossRef]
- Singh, A.; Tripathi, V.; Vishwas Tripathi, C. Effect of Transferulic Acid on Oral Squamous Carcinoma Cells. J. Pharmacogn. Phytochem. 2018, 7, 1955–1958. [Google Scholar]
- Shih, Y.L.; Hung, F.M.; Lee, C.H.; Yeh, M.Y.; Lee, M.H.; Lu, H.F.; Chen, Y.L.; Liu, J.Y.; Chung, J.G. Fisetin Induces Apoptosis of HSC3 Human Oral Cancer Cells through Endoplasmic Reticulum Stress and Dysfunction of Mitochondria-Mediated Signaling Pathways. Vivo 2017, 31, 1103–1114. [Google Scholar] [CrossRef][Green Version]
- Min, F.; Liu, X.; Li, Y.; Dong, M.; Qu, Y.; Liu, W. Carnosic Acid Suppresses the Development of Oral Squamous Cell Carcinoma via Mitochondrial-Mediated Apoptosis. Front. Oncol. 2021, 11, 760861. [Google Scholar] [CrossRef]
- Hsu, R.J.; Peng, K.Y.; Hsu, W.L.; Chen, Y.T.; Liu, D.W. Z-Ligustilide Induces c-Myc-Dependent Apoptosis via Activation of ER-Stress Signaling in Hypoxic Oral Cancer Cells. Front. Oncol. 2022, 12, 824043. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.H.; Kim, I.R.; Kim, H.J.; Park, B.S.; Yu, S. Bin α -Mangostin Induces Apoptosis and Cell Cycle Arrest in Oral Squamous Cell Carcinoma Cell. Evid.-Based Complement. Altern. Med. 2016, 2016, 5352412. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Su, Z. Detailed Studies on the Anticancer Action of Blumeatin Flavanone in Human Oral Carcinoma Cells: Determining Its Impact on Cellular Autophagy, DNA Damage and Cell Migration and Invasion. JBUON 2020, 25, 2011–2016. [Google Scholar] [PubMed]
- Lin, C.W.; Chen, P.N.; Chen, M.K.; Yang, W.E.; Tang, C.H.; Yang, S.F.; Hsieh, Y.S. Kaempferol Reduces Matrix Metalloproteinase-2 Expression by down-Regulating ERK1/2 and the Activator Protein-1 Signaling Pathways in Oral Cancer Cells. PLoS ONE 2013, 8, e80883. [Google Scholar] [CrossRef]
- Ma, Y.S.; Yao, C.N.; Liu, H.C.; Yu, F.S.; Lin, J.J.; Lu, K.W.; Liao, C.L.; Chueh, F.S.; Chung, J.G. Quercetin Induced Apoptosis of Human Oral Cancer SAS Cells through Mitochondria and Endoplasmic Reticulum Mediated Signaling Pathways. Oncol. Lett. 2018, 15, 9663–9672. [Google Scholar] [CrossRef]
- Manmuan, S.; Tubtimsri, S.; Chaothanaphat, N.; Manmuan, P. Quercetin for Efficient Inhibition of Cell Proliferation and Modulation Activity on Biological Behavior in KON Oral Cancer Cells. In Proceedings of the 5th International Conference and Exhibition on Pharmaceutical Sciences and Technology, Online, 23–24 June 2022. [Google Scholar]
- Chen, C.-T.; Hsieh, M.-J.; Hsieh, Y.-H.; Hsin, M.-C.; Chuang, Y.-T.; Yang, S.-F.; Yang, J.-S.; Lin, C.-W. Sulforaphane Suppresses Oral Cancer Cell Migration by Regulating Cathepsin S Expression. Oncotarget 2018, 9, 17564–17575. [Google Scholar] [CrossRef]
- Contant, C.; Rouabhia, M.; Loubaki, L.; Chandad, F.; Semlali, A. Anethole Induces Anti-Oral Cancer Activity by Triggering Apoptosis, Autophagy and Oxidative Stress and by Modulation of Multiple Signaling Pathways. Sci. Rep. 2021, 11, 13087. [Google Scholar] [CrossRef]
- Huang Liu, R.; Chen, S.P.; Lu, T.M.; Tsai, W.Y.; Tsai, C.H.; Yang, C.C.; Tzeng, Y.M. Selective Apoptotic Cell Death Effects of Oral Cancer Cells Treated with Destruxin B. BMC Complement. Altern. Med. 2014, 14, 207. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Lin, C.W.; Chen, M.K.; Chien, S.Y.; Lo, Y.S.; Chuang, Y.C.; Hsi, Y.T.; Lin, C.C.; Chen, J.C.; Yang, S.F. Inhibition of Cathepsin S Confers Sensitivity to Methyl Protodioscin in Oral Cancer Cells via Activation of P38 MAPK/JNK Signaling Pathways. Sci. Rep. 2017, 7, srep45039. [Google Scholar] [CrossRef]
- Livny, O.; Kaplan, I.; Reifen, R.; Polak-Charcon, S.; Madar, Z.; Schwartz, B. Nutrition and Cancer Lycopene Inhibits Proliferation and Enhances Gap-Junction Communication of KB-1 Human Oral Tumor Cells. J. Nutr. 2002, 132, 3754–3759. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Liu, Y.T.; Lin, J.T.; Lin, C.C.; Chuang, Y.C.; Lo, Y.S.; Hsi, Y.T.; Hsieh, M.J. Pinosylvin Reduced Migration and Invasion of Oral Cancer Carcinoma by Regulating Matrix Metalloproteinase-2 Expression and Extracellular Signal-Regulated Kinase Pathway. Biomed. Pharmacother. 2019, 117, 109160. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Raj Natarajan, S.; Ponnusamy, B.; Veeraraghavan, V.P.; Jasmine, S. Unlocking the Potential of Beta Sitosterol: Augmenting the Suppression of Oral Cancer Cells through Extrinsic and Intrinsic Signalling Mechanisms. Saudi Dent. J. 2023, 35, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-J.; Shin, J.-A.; Yang, I.-H.; Won, D.-H.; Ahn, C.H.; Kwon, H.-J.; Lee, J.-S.; Cho, N.-P.; Kim, E.-C.; Yoon, H.-J.; et al. Apoptosis Induced by Caffeic Acid Phenethyl Ester in Human Oral Cancer Cell Lines: Involvement of Puma and Bax Activation. Arch. Oral Biol. 2017, 84, 94–99. [Google Scholar] [CrossRef]
- Lu, H.I.; Chen, K.L.; Yen, C.Y.; Chen, C.Y.; Chien, T.M.; Shu, C.W.; Chen, Y.H.; Jeng, J.H.; Chen, B.H.; Chang, H.W. Michelia Compressa-Derived Santamarine Inhibits Oral Cancer Cell Proliferation via Oxidative Stress-Mediated Apoptosis and DNA Damage. Pharmaceuticals 2024, 17, 230. [Google Scholar] [CrossRef]
- Liu, S.L.; Yang, K.H.; Yang, C.W.; Lee, M.Y.; Chuang, Y.T.; Chen, Y.N.; Chang, F.R.; Chen, C.Y.; Chang, H.W. Burmannic Acid Inhibits Proliferation and Induces Oxidative Stress Response of Oral Cancer Cells. Antioxidants 2021, 10, 1588. [Google Scholar] [CrossRef]
- Sankaran, V.; Murali, S.K.; Ghidan, A.Y.; Al-Antary, T.M.; David, E. Oral Cancer Preventive Potential of Polydatin: A Nanoencapsulation Approach. J. Phytol. 2020, 12, 109–116. [Google Scholar] [CrossRef]
- Tangsuksan, P.; Chuerduangphui, J.; Takahashi Yupanqui, C.; Srichana, T.; Hitakomate, E.; Pientong, C.; Ekalaksananan, T.; Nittayananta, W. Mucoadhesive Film Containing α-Mangostin Shows Potential Role in Oral Cancer Treatment. BMC Oral Health 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Ellithy, M.M.; Aly, R.M.; Tarek, H.E.-S. Nanoformulated Rosemary Extract Impact on Oral Cancer: In Vitro Study. Bull. Natl. Res. Cent. 2022, 46, 1–11. [Google Scholar] [CrossRef]
- Weerapol, Y.; Manmuan, S.; Chuenbarn, T.; Limmatvapirat, S.; Tubtimsri, S. Nanoemulsion-Based Orodispersible Film Formulation of Guava Leaf Oil for Inhibition of Oral Cancer Cells. Pharmaceutics 2023, 15, 2631. [Google Scholar] [CrossRef]
- Alaufi, O.M.; Noorwali, A.; Zahran, F.; Al-Abd, A.M.; Al-Attas, S. Cytotoxicity of Thymoquinone Alone or in Combination with Cisplatin (CDDP) against Oral Squamous Cell Carcinoma in Vitro. Sci. Rep. 2017, 7, 13131. [Google Scholar] [CrossRef]
- Semlali, A.; Ajala, I.; Beji, S.; Al-Zharani, M.M.; Rouabhia, M. Synergistic Effect of Anethole and Platinum Drug Cisplatin against Oral Cancer Cell Growth and Migration by Inhibiting MAPKase, Beta-Catenin, and NF-ΚB Pathways. Pharmaceuticals 2023, 16, 700. [Google Scholar] [CrossRef]
- Pei, C.; Zhang, J.; Li, J.; Zhou, D. Apigenin Suppresses the Low Oxaliplatin-Induced Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma Cells via LINC00857. Transl. Cancer Res. 2024, 13, 2164–2174. [Google Scholar] [CrossRef]
- Singh, A.; Chauhan, S.; Tripathi, V. Quinic Acid Attenuates Oral Cancer Cell Proliferation by Downregulating Cyclin D1 Expression and Akt Signaling. Pharmacogn. Mag. 2018, 14, S14–S19. [Google Scholar] [CrossRef]
- Angellotti, G.; Di Prima, G.; Belfiore, E.; Campisi, G.; De Caro, V. Chemopreventive and Anticancer Role of Resveratrol against Oral Squamous Cell Carcinoma. Pharmaceutics 2023, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Vagish Kumar, L.S. Propolis in Dentistry and Oral Cancer Management. N. Am. J. Med. Sci. 2014, 6, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, V.A.; Geetha, R.V. Evaluation of Anti Carcinogenic Activity of Trifolium pratense on Oral Cancer Cell—An in Vitro Study. J. Pharm. Res. Int. 2021, 33, 800–806. [Google Scholar] [CrossRef]
- Elyasi, S.; Hosseini, S.; Niazi Moghadam, M.R.; Aledavood, S.A.; Karimi, G. Effect of Oral Silymarin Administration on Prevention of Radiotherapy Induced Mucositis: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Phytother. Res. 2016, 30, 1879–1885. [Google Scholar] [CrossRef]
- Moezian, G.S.A.; Javadinia, S.A.; Sales, S.S.; Fanipakdel, A.; Elyasi, S.; Karimi, G. Oral Silymarin Formulation Efficacy in Management of AC-T Protocol Induced Hepatotoxicity in Breast Cancer Patients: A Randomized, Triple Blind, Placebo-Controlled Clinical Trial. J. Oncol. Pharm. Pract. 2022, 28, 827–835. [Google Scholar] [CrossRef]
- Mao, C.; Gong, L.; Kang, W. Effect and Mechanism of Resveratrol on Ferroptosis Mediated by P53/SLC7A11 in Oral Squamous Cell Carcinoma. BMC Oral Health 2024, 24, 773. [Google Scholar] [CrossRef]
- Li, B.; Allela, O.Q.B.; Alkhazali, W.H.; Vadia, N.; Renuka Jyothi, S.; Panigrahi, R.; Chauhan, A.S.; Singh, S.; Akhrorova, M.; Sameer, H.N.; et al. Resveratrol in Oral Cancer: A Systematic Review of Preclinical Studies on Its Anticancer Mechanisms and Therapeutic Potential. Med. Oncol. 2025, 42, 329. [Google Scholar] [CrossRef]
- Herrada Céspedes, A.; Reyes, M.; Morales, J.O. Advanced Drug Delivery Systems for Oral Squamous Cell Carcinoma: A Comprehensive Review of Nanotechnology-Based and Other Innovative Approaches. Front. Drug Deliv. 2025, 5, 1596964. [Google Scholar] [CrossRef]
- Thanikachalam, P.V.; Devaraji, M.; Jayaprakash, N.; Sivakumar, H.; Balamurugan, D.; Shanmugan, H.K. Phytochemical Innovations in Oral Cancer Therapy: Targeting Oncogenic Pathways with Natural Compounds. J. Complement. Integr. Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Bekhet, O.H.; Eid, M.E. The Interplay between Reactive Oxygen Species and Antioxidants in Cancer Progression and Therapy: A Narrative Review. Transl. Cancer Res. 2021, 10, 4196–4206. [Google Scholar] [CrossRef]
- You, A.J.; Park, J.; Shin, J.-M.; Kim, T.H. Oxidative Stress and Dietary Antioxidants in Head and Neck Cancer. Antioxidants 2025, 14, 508. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Tseng, Y.-H. The Potential of Phytochemicals in Oral Cancer Prevention and Therapy: A Review of the Evidence. Biomolecules 2020, 10, 1150. [Google Scholar] [CrossRef]
- Hu, L.; Luo, Y.; Yang, J.; Cheng, C. Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability. Molecules 2025, 30, 1184. [Google Scholar] [CrossRef]
- Kasaai, M.R. Applications of Nanoscience and Nanotechnology in Oral Cancer: A Review. In Nanomaterials in Dental Medicine; Springer: Berlin/Heidelberg, Germany, 2023; pp. 177–199. [Google Scholar]
- Saif Imam, S. Sublingual Tablets Amalgamated with Nano-Particles and Natural Products to Treat Oral Cancer. Res. J. Pharm. Technol. 2024, 17, 2056–2062. [Google Scholar] [CrossRef]
- Xu, B.; Watkins, R.; Wu, L.; Zhang, C.; Davis, R. Natural Product-Based Nanomedicine: Recent Advances and Issues. Int. J. Nanomed. 2015, 10, 6055–6074. [Google Scholar] [CrossRef]
- Kumbar, V.M.; Muddapur, U.; Bin Muhsinah, A.; Alshehri, S.A.; Alshahrani, M.M.; Almazni, I.A.; Kugaji, M.S.; Bhat, K.; Peram, M.R.; Mahnashi, M.H.; et al. Curcumin-Encapsulated Nanomicelles Improve Cellular Uptake and Cytotoxicity in Cisplatin-Resistant Human Oral Cancer Cells. J. Funct. Biomater. 2022, 13, 158. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic Applications of Curcumin Nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef]
- Semlali, A.; Beji, S.; Ajala, I.; Al-Zharani, M.; Rouabhia, M. Synergistic Effects of New Curcumin Analog (PAC) and Cisplatin on Oral Cancer Therapy. Curr. Issues Mol. Biol. 2023, 45, 5018–5035. [Google Scholar] [CrossRef] [PubMed]
- Annaji, M.; Poudel, I.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-loaded Nanomedicines for Cancer Applications. Cancer Rep. 2021, 4, 1353. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Musuc, A.M.; Mitu, M.A.; Atkinson, I.; et al. In Vitro Anticancer Activity of Mucoadhesive Oral Films Loaded with Usnea barbata (L.) F. H. Wigg Dry Acetone Extract, with Potential Applications in Oral Squamous Cell Carcinoma Complementary Therapy. Antioxidants 2022, 11, 1934. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Sarbu, I.; Musuc, A.M.; Atkinson, I.; et al. Formulation and Development of Bioadhesive Oral Films Containing Usnea barbata (L.) F.H.Wigg Dry Ethanol Extract (F-UBE-HPC) with Antimicrobial and Anticancer Properties for Potential Use in Oral Cancer Complementary Therapy. Pharmaceutics 2022, 14, 1808. [Google Scholar] [CrossRef] [PubMed]
- Iancu, I.M.; Schröder, V.; Apetroaei, M.-R.; Crețu, R.M.; Mireșan, H.; Honcea, A.; Iancu, V.; Bucur, L.A.; Mitea, G.; Atodiresei-Pavalache, G. Biocompatibility of Membranes Based on a Mixture of Chitosan and Lythri Herba Aqueous Extract. Appl. Sci. 2023, 13, 8023. [Google Scholar] [CrossRef]
- Blebea, N.M.; Schroder, V.; Mitea, G.; Iancu, V.; Apetroaei, M.M.; Apetroaei, M.R.; Negres, S. Development Membranes with Chitosan Delivery Systems for Cannabidiol. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18 November 2021; IEEE: New York, NY, USA, 2021; pp. 1–4. [Google Scholar]
- Rizvi, S.A.A.; Einstein, G.P.; Tulp, O.L.; Sainvil, F.; Branly, R. Introduction to Traditional Medicine and Their Role in Prevention and Treatment of Emerging and Re-Emerging Diseases. Biomolecules 2022, 12, 1442. [Google Scholar] [CrossRef]
- Mitea, G.; Iancu, I.M.; Blebea, N.M.; Bucur, L.A.; Schroder, V.; Badea, V.; Badea, C.F.; Nuca, C.; Radu, M.D.; Iancu, V. Cotinine and IL-6-Biomarkers for Estimating Pharmacological Processes in Various Forms of Periodontitis. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021; IEEE: NewYork, NY, USA, 2021; pp. 1–4. [Google Scholar]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 6, 469–471. [Google Scholar] [CrossRef]
- Rafehi, H.; Orlowski, C.; Georgiadis, G.T.; Ververis, K.; El-Osta, A.; Karagiannis, T.C. Clonogenic Assay: Adherent Cells. J. Vis. Exp. 2011, 13, e2573. [Google Scholar] [CrossRef]
- Justus, C.R.; Marie, M.A.; Sanderlin, E.J.; Yang, L.V. Transwell In Vitro Cell Migration and Invasion Assays. Methods Mol. Biol. 2023, 2644, 349–359. [Google Scholar]
- Ghanim, M.; Relitti, N.; McManus, G.; Butini, S.; Cappelli, A.; Campiani, G.; Mok, K.H.; Kelly, V.P. A Non-Toxic, Reversibly Released Imaging Probe for Oral Cancer That Is Derived from Natural Compounds. Sci. Rep. 2021, 11, 14069. [Google Scholar] [CrossRef]
- El-Hajjar, L.; Ali Ahmad, F.; Nasr, R. A Guide to Flow Cytometry: Components, Basic Principles, Experimental Design, and Cancer Research Applications. Curr. Protoc. 2023, 3, e721. [Google Scholar] [CrossRef]
- Elliott, A.D. Confocal Microscopy: Principles and Modern Practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef]
- Sánchez-Carranza, J.N.; Montes-Helguera, S.V.; Valladares-Méndez, A.; Salas-Vidal, E.; González-Maya, L. Natural Compounds and Derivatives as Modulators of Multidrug Resistance and Their Mechanisms of Action: Recent Studies In Vitro, In Vivo and in Silico. Discov. Appl. Sci. 2024, 6, 545. [Google Scholar] [CrossRef]
- Adam, A.B.; Titilayo, O.A.; Stedfast, A.C.; Peter, I.O. Exploring the Safety Profile of Phyllanthus Amarus: Side Effects, Contraindications, and the Importance of Dosage. Med. Med. Chem. 2024, 1, 182–192. [Google Scholar]
- Wang, Y.; Su, C.; Zhang, B.; Niu, Y.; Ren, R.; Zhao, X.; Yang, L.; Zhang, W.; Ma, X. Biological Activity, Hepatotoxicity, and Structure-Activity Relationship of Kavalactones and Flavokavins, the Two Main Bioactive Components in Kava (Piper Methysticum). Evid.-Based Complement. Altern. Med. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Boateng, I.D. Potentialities of Ginkgo Extract on Toxicants, Toxins, and Radiation: A Critical Review. Food Funct. 2022, 13, 7960–7983. [Google Scholar] [CrossRef] [PubMed]
- Jităreanu, A.; Trifan, A.; Vieriu, M.; Caba, I.-C.; Mârțu, I.; Agoroaei, L. Current Trends in Toxicity Assessment of Herbal Medicines: A Narrative Review. Processes 2022, 11, 83. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).