Abstract
The life expectancy of patients with psychotic disorders is significantly shorter than that of the general population; antipsychotic-induced metabolic disorders play a significant role in reducing life expectancy. Both metabolic syndrome (MetS) and schizophrenia are multifactorial conditions. One area where the two conditions overlap is oxidative stress, which is present in both diseases. The glutathione-S-transferase (GST) system is a major line of defense against exogenous toxicants and oxidative damage to cells. The aim of our study was to perform an association analysis of gene polymorphisms with metabolic disorders in patients with schizophrenia treated with antipsychotic therapy. Methods: A total of 639 white patients with schizophrenia (ICD-10) from Siberia (Russia) were included in the study. Genotyping was carried out using real-time polymerase chain reaction for two single-nucleotide polymorphisms (SNPs) in the GSTP1 (rs614080 and rs1695) and one SNP in the GSTO1 (rs49252). Results: We found that rs1695*GG genotype of GSTP1 is a risk factor for the development of overweight (OR 2.36; 95% CI: 1.3–4.29; p = 0.0054). In the subgroup of patients receiving first-generation antipsychotics as basic therapy, the risk of overweight was associated with carriage of the rs1695*GG (OR 5.43; 95% CI: 2.24–13.16; p < 0.001) genotype of GSTP1 in a recessive model of inheritance. In contrast, an association of rs1695*G GSTP1 with obesity (OR: 0.42; 95% CI: 0.20–0.87; p = 0.018) was shown in the dominant model of inheritance in patients receiving second-generation antipsychotics. Conclusions: The pilot results obtained confirm the hypothesis of a violation of the antioxidant status, in particular the involvement of GSTP1, in the development of antipsychotic-induced metabolic disorders in schizophrenia. Further studies with larger samples and different ethnic groups are needed to confirm the obtained results.
1. Introduction
Schizophrenia is a complex mental disorder that affects not only the cognitive and emotional aspects of patients but also their physical health. In recent years, there have been increasing findings regarding a high prevalence of metabolic disorders, such as obesity, type 2 diabetes, and dyslipidemia, in people with schizophrenia [1,2,3,4]. According to various data, the frequency of metabolic syndrome (MetS) varies widely, and, in the population of patients with schizophrenia, it is 10.1–42.7% versus 6–33.9% in the general population [5,6,7,8,9,10,11,12]. The wide range of data on the frequency of MetS occurrence may be due to the presence of various diagnostic criteria and differences in incidence among ethnic and age groups. A recent meta-analysis including the results of 12 studies involving 1953 participants found that the prevalence of MetS among patients with schizophrenia was 41.3% [13]. Obesity prevalence in schizophrenia patients is also 1.42 to 3.5 times higher than in the general population [14,15]. Cameron et al. (2017), in a large-scale study including 4658 schizophrenia cases and 19,686 healthy individuals, report that 31.6–36% of schizophrenia patients are obese by body mass index (BMI) criteria, compared with 19.5–25.4% in matched controls [15]. The increased prevalence of metabolic dysfunction in people with schizophrenia can be attributed, but is not limited to, to a number of factors, including poor diet, cigarette smoking, lack of exercise, stress, dysfunction of the hypothalamic–pituitary–adrenal axis, and use of antipsychotic medication [16]. Antipsychotic-induced metabolic disorders can significantly reduce the quality of life of patients [17], be a reason for noncompliance [18,19], and increase the risk of developing concomitant cardiometabolic diseases [20]. Risk factors for MetS in patients with schizophrenia include antipsychotic therapy and illness duration, and, in some studies, gender; however, metabolic abnormalities have also been reported in drug-naïve patients with first-episode psychosis [5,6,7,8,11]. Therefore, genes may play a role in metabolic dysregulation in patients with schizophrenia. According to case-control association studies, a number of genetic variants are potentially responsible for the high comorbidity between metabolic abnormalities and schizophrenia [21]. Majority studies focused on target genes involved in antipsychotic drug action and weight regulation, but genes responsible for other biological pathways are being identified [22,23,24].
A growing body of research has recently focused on the role of oxidative stress in the pathogenesis of obesity and metabolic syndrome in the general population. Excess adipose tissue, especially visceral, promotes the formation of proinflammatory cytokines, which leads to the activation of macrophages and increased production of free radicals [25]. This creates a vicious cycle; inflammation causes oxidative stress, and oxidative stress worsens inflammation, which ultimately contributes to the development of MetS and its associated complications [25,26]. Oxidative stress causes mitochondrial dysfunction, protein damage, lipid peroxidation, and impairing antioxidant function [26]. This also leads to dysregulation of adipocytokine production, which contributes to the development of obesity-associated vasculopathy and cardiovascular risk due to endothelial dysfunction [27]. Metabolic disorders are accompanied by a decrease in glutathione levels and changes in the activity of glutathione metabolism enzymes, the first line of defense against oxidative stress [28,29,30].
Conversely, a large body of research suggests that oxidative stress may be one of the mechanisms underlying the pathophysiology of schizophrenia. While it is not considered the primary cause of the disease, it can be incorporated into most current schizophrenia hypotheses and may play an important role in unifying these in the future [31]. It is suggested that oxidative stress may contribute to the progression of the disease and poor outcomes [32]. The association between schizophrenia and oxidative stress is hypothesized to be influenced by inflammatory and autoimmune regulation processes, neurotransmitters, such as dopamine, glutamate, and nitric oxide, mitochondrial and metal metabolism, and genetic and epigenetic factors [33,34]. A systematic review revealed that people with schizophrenia have a deficiency of glutathione and disturbances in the glutathione redox cycle [35]. Thus, oxidative stress may be the point of contact between MetS and schizophrenia.
Genetic variations in antioxidant defense genes and reactive oxygen species producing enzymes may influence the risk of obesity and related metabolic complications [36]. The glutathione S-transferase (GST) supergene family plays a pivotal role in antioxidant defense mechanisms, detoxifying electrophilic xenobiotics, and inactivating a variety of endogenous products with reduced glutathione [37,38]. The human cytosolic GST superfamily currently encompasses a minimum of 16 genes, which are divided into eight distinct classes, designated as follows: Alpha, Mu, Pi, Theta, Zeta, Sigma, and Omega [39]. A substantial body of research has characterized human GSTs, which have been found to exhibit polymorphism at varying frequencies based on ethnicity. The GSTP1 gene has several polymorphic variants, of which the most functionally significant is rs1695, which results in the substitution of isoleucine for valine at codon 105 (Ile105Val). Functional genomic studies in COS-1 cells have revealed that rs1695 is one of several polymorphisms that can significantly alter GSTP1 enzyme activity, protein levels, and substrate affinity; this may affect GSTP1′s role in drug metabolism and, potentially, disease pathogenesis and/or drug response [40]. The rs4925 polymorphism in GSTO1 results in a substitution of alanine for aspartate at codon 140 (Ala140Asp). This polymorphism influences the kinetics of deglutathionylation, thioltransferase, and glutathionylation reactions [41,42].
The present study investigated the possible associations between three polymorphisms in GSTP1 and GSTO1 genes and the risk of overweight and MetS in patients with schizophrenia.
2. Results
2.1. Association of Studied SNPs with MetS, Anthropometric and Laboratory Parameters in the Overall Group of Patients
We studied two single nucleotide polymorphisms (SNPs) in the GSTP1 (rs614080 and rs1695) and one SNP in the GSTO1 (rs49252). All SNPs studied were in Hardy–Weinberg equilibrium. Characteristics of studied SNPs are presented in Table S1. We found no associations between the studied SNPs and MetS (Table S2). We found statistically significant differences in the frequencies of rs1695 genotypes in the GSTP1 between normal and overweight schizophrenia patients (χ2 = 6.749, p = 0.034; Table 1).

Table 1.
Frequencies of alleles and genotypes of polymorphic variants of the GST in patients with schizophrenia depending on BMI.
Two models, codominant and recessive, were statistically significant. However, the information criteria (Akaike and Bayesian) are the lowest for the recessive model, which defines it as the best model. Consequently, the GG genotype carriage was identified as a risk factor for overweight in patients with schizophrenia (OR = 2.36; 95% CI: 1.30–4.29; p = 0.0054) (Table 2). There was no interaction between the SNP and the covariates (gender and smoking) (Table S3).

Table 2.
Models of predisposing effect inheritance of rs1695 in the GSTP1 gene in patients with schizophrenia.
We found that the carrying of GSTP1 rs614080*GG is associated with higher total cholesterol (p = 0.046) and low-density lipoprotein (p = 0.028) levels. No other differences in lipid and glucose levels were found in carriers of different genotypes of the genes studied (Table 3).

Table 3.
Association between GST gene polymorphisms and blood lipids and glucose in patients with schizophrenia.

Table 4.
Association between GST gene polymorphisms and anthropometric parameters in patients with schizophrenia.
2.2. Association of Studied SNPs with MetS, Anthropometric and Laboratory Parameters in the Group of Patients Receiving First-Generation Antipsychotics
First-generation antipsychotics (FGAs) and second-generation antipsychotics (SGAs) have different effects on the development of MS; metabolic disorders more often develop after taking atypical antipsychotics [8,38,43]. Although the data are inconclusive, FGAs have been shown to predominantly have prooxidant properties. In contrast, SGAs do not significantly affect redox processes or exhibit antioxidant activity [34,44]. Because the sample consisted of patients with chronic schizophrenia who had received long-term antipsychotic therapy, we analyzed the associations of the selected SNPs with metabolic parameters depending on whether the patients received FGAs or SGAs as part of their basic therapy.
In the group of patients who received FGAs, we also found no associations between the studied SNPs and MetS (Table S4). We found statistically significant differences in the frequencies of rs1695 genotypes (χ2 = 13.793; p = 0.001) and alleles (χ2 = 4.976; p = 0.026) in the GSTP1 between normal and overweight schizophrenia patients (Table 5).

Table 5.
The frequencies of alleles and genotypes of polymorphic variants of the GST in patients with schizophrenia receiving FGAs depending on BMI.
The risk of overweight was associated with carriage of the rs1695*GG (OR 5.43; 95% CI: 2.24–13.16; p < 0.001) genotype in a recessive model of inheritance (Table 6). There was no interaction between the SNP and the covariates (gender and smoking) (Table S3).

Table 6.
Models of predisposing effect inheritance of rs1695 in the GSTP1 gene in patients with schizophrenia receiving FGAs.
We did not find significant associations of the studied SNPs with blood lipids and glucose (Table S5). The carrying of GSTP1 rs1695*GG is associated with higher abdominal fat fold (p = 0.037) and visceral fat level (p = 0.047; Table 7).

Table 7.
Association between GST gene polymorphisms and anthropometric parameters in patients with schizophrenia receiving FGAs.
2.3. Association of Studied SNPs with MetS, Anthropometric and Laboratory Parameters in the Group of Patients Receiving Second-Generation Antipsychotics
In the group of patients who received SGAs, we also found no associations between the studied SNPs and MetS (Table S6). We found statistically significant differences in the frequencies of rs1695 alleles (χ2 = 5.87; p = 0.015) in the GSTP1 between normal and obese schizophrenia patients (Table 8).

Table 8.
The frequencies of alleles and genotypes of polymorphic variants of the GST gene in patients with schizophrenia receiving SGAs depending on BMI.
The dominant model of inheritance showed an association between rs1695 in GSTP1 and obesity (OR: 0.42, 95% CI: 0.20–0.87; p = 0.018; Table 9). There was no interaction between the SNP and the covariates (gender and smoking) (Table S3).

Table 9.
Models of predisposing effect inheritance of rs1695 in the GSTP1 gene in patients with schizophrenia receiving SGAs.
The carrying of GSTP1 rs614080*GG is associated with higher total cholesterol (p = 0.015) and low-density lipoprotein (p = 0.036) levels (Table 10). We did not find significant associations of the studied SNPs with anthropometric parameters (Table S7).

Table 10.
Association between GST gene polymorphisms and blood lipids and glucose in patients with schizophrenia receiving SGAs.
3. Discussion
We found associations of polymorphic variants rs1695 and rs614080 of the GSTP1 gene with metabolic buildups in patients with schizophrenia. No associations were found for the rs4925 polymorphic variant GSTO1. GSTP1 is widely expressed in various tissues of the human body, including adipose tissue [45]. According to the GeneOntology database, in addition to the implementation of biological processes associated with glutathione and xenobiotic metabolism, GSTP1 is involved in long-chain fatty acid biosynthesis processes [46]. According to the results of the association analysis, only the GSTP1 rs1695 polymorphic variant showed statistically significant results. We used the GTEx Portal database (https://gtexportal.org; accessed on 10 May 2025) [47] to evaluate the tissue-specific effects of the G minor allele of the studied SNP. The results of tissue-specific eQTL analysis for GSTP1 rs1695 polymorphism are presented in Figure 1.
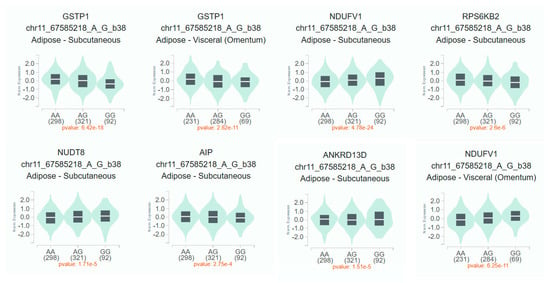
Figure 1.
Tissue-specific eQTL analysis for GSTP1 rs1695 polymorphism. Notes. The square boundaries are the 1st and 3rd quartiles, the line inside the square is the median.
The rs1695-G allele was associated with decreased GSTP1 gene expression in subcutaneous (p = 6.42 × e−18) and visceral (p = 2.82 × e−11) adipose tissue. Meanwhile, this allele was associated with increased mRNA levels of NDUFV1 genes in subcutaneous (p = 4.78 × e−24) and visceral (p = 6.25 × e−11) adipose tissue and NUDT8 in subcutaneous adipose tissue (p = 1.71 × e−5). Carriage of the rs1695-G allele was also associated with decreased expression of RPS6KB2 (p = 2.6 × e−6), AIP (p = 2.75 × e−4), and ANKRD13D (p = 1.5 × e−5) genes in subcutaneous adipose tissue. Thus, there is sufficient convincing evidence regarding the probable involvement of the rs1695 GSTP1 polymorphism in the development of metabolic disorders.
To our knowledge, our study was the first to show the contribution of rs1695 and rs614080 of the GSTP1 gene to the development of metabolic disorders in patients with schizophrenia. Previous genetic studies have focused on mentally healthy individuals. The role of the rs1695 polymorphism in the development of type 2 diabetes mellitus has been widely studied, but data on its contribution remain ambiguous. The results of a meta-analysis (18 studies, 2595 patients with type 2 diabetes mellitus and 2888 healthy individuals) did not reveal associations between the studied polymorphic variant and the risk of type 2 diabetes mellitus, although the authors point out the heterogeneity of the data and the need for further research [48]. GSTP1 rs1695 is associated with increased risk of obesity and cardiometabolic abnormalities in young adults from Brazil; individuals carrying at least one G allele have a 2.4 times higher chance of being obese compared to those with the A/A genotype [49]. In contrast, a study conducted in a Mexican population found no associations of this SNP with obesity [50]. However, the authors conclude that rs614080 was significantly associated with BMI and GSTP1 expression levels in adipose tissue [50].
A number of studies have examined associations of polymorphisms of other GST classes and metabolic disorders in schizophrenia. The GSTM1 null genotype, particularly when combined with smoking or the GSTT1 present genotype, may increase the risk of metabolic abnormalities, such as being overweight and having decreased high-density lipoprotein cholesterol levels [51]. The genotype rs1917760*T/T GSTK1 was linked to a higher BMI score in male schizophrenia patients, while in female patients this genotype was associated with a lower BMI score [52]. Schizophrenia patients with the GSTK1 rs1917760*T allele or GSTM1 deletion had a higher risk of being overweight. This was confirmed for male patients with schizophrenia and/o patients who currently smoke [53].
We found that the type of antipsychotic medication a person takes affected the associations obtained between GSTP1 rs1695 and BMI. We showed that in patients taking FGAs long-term, carriage of the mutant rs1695*G allele was associated with an increased risk of being overweight. Although SGAs are traditionally associated with an increased incidence of metabolic disorders, FGAs can also lead to weight gain and lipid and glucose imbalances [54,55,56]. One of the possible mechanisms of weight gain against the background of taking FGAs may be oxidative stress, which occurs, among other things, due to decreased GSTP1 activity in individuals with the rs1695*G allele. Interestingly, patients who have taken SGAs for an extended period have shown associations between rs1695 in GSTP1 and obesity. It has been shown that SGAs do not have a substantial effect on redox processes or demonstrate antioxidant properties [34,44]. It is possible that metabolic disorders caused by SGAs develop in ways that are not related to oxidative stress. SGAs profoundly disrupt glucose and lipid homeostasis in the liver, pancreas, adipose tissue, and skeletal muscle, as well as by acting on hypothalamic centers [43]. Further accumulation of glucose and/or lipids, as well as an increase in the volume of adipose tissue, leads to imbalance in the pro-antioxidant system and mitochondrial dysfunction [57,58]. However, we cannot yet explain this finding with complete certainty, so we are treating it with caution. Further genetic studies in larger patient cohorts are needed to more accurately understand the identified associations.
The present study has some limitations. First, the crucial limitations of our study include the cross-sectional study design and the small sample size. Based on this, the studied groups differed in terms of gender and age composition, duration of the disease, and frequency of smoking. However, these results are typical of a clinical situation where metabolic abnormalities are found in patients with schizophrenia. To reduce the significance of these parameters in the association analysis, we adjusted for gender, age, and smoking. A second limitation is that the study was conducted on a group of chronic patients with schizophrenia who had received long-term antipsychotic treatment; therefore, it is not possible to ascertain the extent to which the patients adhered to the treatment regimen over the prolonged period. Despite the limitations stated above, the results of the present study suggest evidence that GSTP1 variants can influence metabolic abnormalities in patients with schizophrenia.
4. Materials and Methods
4.1. Patients
This cross-sectional, case-control study included 639 patients with schizophrenia from the Siberian Federal District (Russia). Patients were recruited from the clinics of the Mental Health Research Institute Tomsk National Research Medical Center, the Tomsk Clinical Psychiatric Hospital, the Hospital of the Siberian State Medical University, the Kemerovo Regional Clinical Psychiatric Hospital, and the N.N. Solodnikova Clinical Psychiatric Hospital of Omsk.
The inclusion criteria in the study were verified diagnosis of schizophrenia according to ICD-10 (International Classification of Diseases 10th revision) criteria, assessed via a structured clinical interview (Structured Clinical Interview for the DSM [SCID]), aged 18 to 55 years, provided informed consent, apparent Caucasian descent, absence of severe organic diseases or somatic disorders in a state of decompensation, and those receiving continuous antipsychotic treatment. The severity of psychopathological symptoms was assessed using the Positive and Negative Syndrome Scale (PANSS). Data were gathered regarding baseline antipsychotic therapy and any concomitant treatments at the time of the examination and over the preceding six months (medicines and doses administered and duration of current medication use). For dose standardization, the daily dose of a chlorpromazine equivalent (CPZeq) was used. MetS was diagnosed according to the International Diabetes Federation (IDF, 2005) criteria [59]. Measurements of the fatty components in the body composition of the participants were conducted using a measuring tape, the non-invasive bioimpedance analysis medical device “Omron BF508”, and an electronic caliper.
Clinical and demographic data of the study sample are presented in Table 11.

Table 11.
Clinical and demographic parameters of the patients.
4.2. Laboratory Methods
After a 12 h fast, blood samples were taken through antecubital venipuncture into vacutainer tubes with EDTA (for DNA) or a clot activator (for serum). DNA from venous peripheral blood was isolated using the standard phenol–chloroform method. Genotyping was performed using real-time polymerase chain reaction (RT-PCR) with the BioMaster UDG HS-qPCR Lo-ROX (2×) PCR kit (BioLabMix, Novosibirsk, Russia) and region-specific primers (DNA-Synthesis, Moscow, Russia) on the amplifier QuantStudio™ 5 Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). The equipment is located at the core facility of Medical Genomics, Tomsk National Research Medical Center, Russian Academy of Sciences.
The polymorphic variants of the GSTP1 (rs614080 and rs1695) and GSTO1 (rs49252) genes were selected for genotyping. These gene polymorphisms were chosen for the following reasons:
- Minor allele frequency of at least 5%.
- Availability of information on previous studies of this polymorphism.
- Marker localization.
None of these criteria were decisive, but the presence of at least one item was sufficient for inclusion in the study.
Measurement of the concentration of glucose, total cholesterol, triglycerides, low-density lipoproteins, and high-density lipoproteins in blood serum was performed using standard biochemical methods using commercial kits (Cormay, Łomianki, Poland) on a semi-automatic biochemical analyzer (Cormay Multi Plus, Cormay, Łomianki, Poland).
4.3. Statistics
The statistical analysis was conducted using R package version 4.0.4 and SPSS Statistics version 26. A power analysis was conducted with “pwr” package for R. The power of the sample was 0.95 (with a significance level of 0.05 and Cohen’s w = 0.16). The Hardy–Weinberg equilibrium of genotypic frequencies was examined by the chi-squared test. Association analysis was performed using the chi-squared (χ2) test. SNPStats online web tool (https://www.snpstats.net/start.htm?q=snpstats/start.htm; accessed on 2 May 2025) was used to examine the inheritance model (data were adjusted for age, smoking, and sex). Comparisons of quantitative data were performed using the Kruskal–Wallis and Mann–Whitney tests. Bonferronni correction was used for multiple comparisons. Differences between the compared groups were considered statistically significant at p < 0.05.
5. Conclusions
The rs1695 variant of the GSTP1 is significantly associated with the BMI of schizophrenia patients receiving antipsychotic therapy. In the subgroup of patients receiving FGAs, we found an association of overweight with the rs1695*GG genotype of GSTP1 (OR: 5.43; 95% CI: 2.24–13.16; p < 0.001) in a recessive model of inheritance. Conversely, we demonstrated an association between rs1695*G GSTP1 and obesity (OR: 0.42; 95% CI: 0.20–0.87; p = 0.018) in a dominant inheritance model in patients receiving SGAs. The rs1695*GG variant of GSTP1 is also associated with higher abdominal fat fold (p = 0.037) and visceral fat levels (p = 0.047) in patients treated with FGAs. Among patients who received SGAs, we found that carrying the GSTP1 rs614080*GG variant was associated with higher total cholesterol (p = 0.015) and low-density lipoprotein (p = 0.036) levels. Our results support the hypothesis that antioxidant status is impaired, particularly with regard to the role of the GSTP1 gene in antipsychotic-induced metabolic disorders in schizophrenia. These results are preliminary and require further research with larger sample sizes and different ethnic groups.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph18070941/s1, Table S1. SNPs’ characteristics and results of the Hardy–Weinberg equilibrium test; Table S2. The frequencies of alleles and genotypes of GST polymorphic variants gene in the patients with and without MetS; Table S3. The frequencies of alleles and genotypes of GST polymorphic variants in the patients who received FGAs; Table S4. Association between GST gene polymorphisms and blood lipids and glucose in patients who received FGAs; Table S5. The frequencies of alleles and genotypes of GST polymorphic variants in the patients who received SGAs; Table S6. Association between GST gene polymorphisms and anthropometric parameters in patients who received SGAs. Table S7. Association between GST gene polymorphisms and anthropometric parameters in the groups of patients received second-generation antipsychotics.
Author Contributions
Conceptualization, S.A.I. and N.A.B.; methodology, E.G.K.; software, V.V.T.; formal analysis, V.V.T. and I.A.M.; investigation, D.A.P., E.V.M., N.M.V., D.Z.P., and I.A.M.; resources, N.M.V.; writing—original draft preparation, I.A.M., N.M.V., V.V.T., and E.G.K.; writing—review and editing, E.V.M., D.Z.P., S.A.I., and N.A.B.; supervision, I.A.M., S.A.I., and N.A.B.; project administration, I.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, Russia, grant number 23-75-10088. https://rscf.ru/en/project/23-75-10088/ (accessed on 15 May 2025).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethical Committee of the Mental Health Research Institute of the Tomsk National Research Medical Center of the Russian Academy of Sciences (Protocol 187, approval on 24 April 2018 and Protocol 165, approval on 18 September 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated for this study will not be made publicly available, but they are available upon reasonable request from Svetlana A. Ivanova (ivanovaniipz@gmail.com) following approval of the Board of Directors of the MHRI, in line with local guidelines and regulations.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI | Body mass index |
| CI | Confidence interval |
| FGA | First-generation antipsychotic |
| GST | Glutathione-S-transferase |
| MetS | Metabolic syndrome |
| OR | Odds ratio |
| SGA | Second-generation antipsychotic |
| SNP | Single-nucleotide polymorphism |
References
- Goldfarb, M.; De, H.M.; Detraux, J.; Di, P.K.; Munir, H.; Music, S.; Piña, I.; Ringen, P.A. Severe Mental Illness and Cardiovascular Disease. JACC 2022, 80, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Wang, S.; Qu, C.; Zheng, K.; Sun, P. Schizophrenia and Type 2 Diabetes Risk: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2024, 15, 1395771. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Liu, Q.; Fang, H.; Zhou, Y.; Forster, M.T.; Li, Z.; Zhang, X. The Prevalence and Clinical Correlates of Metabolic Syndrome and Cardiometabolic Alterations in 430 Drug-Naive Patients in Their First Episode of Schizophrenia. Psychopharmacology 2021, 238, 3643–3652. [Google Scholar] [CrossRef]
- Chang, S.-C.; Goh, K.K.; Lu, M.-L. Metabolic Disturbances Associated with Antipsychotic Drug Treatment in Patients with Schizophrenia: State-of-the-Art and Future Perspectives. World J. Psychiatry 2021, 11, 696–710. [Google Scholar] [CrossRef]
- Saddichha, S.; Manjunatha, N.; Ameen, S.; Akhtar, S. Metabolic Syndrome in First Episode Schizophrenia—A Randomized Double-Blind Controlled, Short-Term Prospective Study. Schizophr. Res. 2008, 101, 266–272. [Google Scholar] [CrossRef]
- Bajaj, S.; Varma, A.; Srivastava, A.; Verma, A.K. Association of Metabolic Syndrome with Schizophrenia. Indian J. Endocrinol. Metab. 2013, 17, 890. [Google Scholar] [CrossRef]
- Sugawara, N.; Yasui-Furukori, N.; Sato, Y.; Umeda, T.; Kishida, I.; Yamashita, H.; Saito, M.; Furukori, H.; Nakagami, T.; Hatakeyama, M.; et al. Prevalence of Metabolic Syndrome among Patients with Schizophrenia in Japan. Schizophr. Res. 2010, 123, 244–250. [Google Scholar] [CrossRef]
- De Hert, M.A.; van Winkel, R.; Van Eyck, D.; Hanssens, L.; Wampers, M.; Scheen, A.; Peuskens, J. Prevalence of the Metabolic Syndrome in Patients with Schizophrenia Treated with Antipsychotic Medication. Schizophr. Res. 2006, 83, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Saari, K.M.; Lindeman, S.M.; Viilo, K.M.; Isohanni, M.K.; Jarvelin, M.-R.; Lauren, L.H.; Savolainen, M.J.; Koponen, H.J. A 4-Fold Risk of Metabolic Syndrome in Patients with Schizophrenia: The Northern Finland 1966 Birth Cohort Study. J. Clin. Psychiatry 2005, 66, 559–563. [Google Scholar] [CrossRef]
- Suvisaari, J.M.; Saarni, S.I.; Perala, J.; Suvisaari, J.V.; Harkanen, T.; Lonnqvist, J.; Reunanen, A. Metabolic Syndrome among Persons with Schizophrenia and Other Psychotic Disorders in a General Population Survey. J. Clin. Psychiatry 2007, 68, 1045–1055. [Google Scholar] [CrossRef]
- McEvoy, J.P.; Meyer, J.M.; Goff, D.C.; Nasrallah, H.A.; Davis, S.M.; Sullivan, L.; Meltzer, H.Y.; Hsiao, J.; Scott Stroup, T.; Lieberman, J.A. Prevalence of the Metabolic Syndrome in Patients with Schizophrenia: Baseline Results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia Trial and Comparison with National Estimates from NHANES III. Schizophr. Res. 2005, 80, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Challa, F.; Getahun, T.; Sileshi, M.; Geto, Z.; Kelkile, T.S.; Gurmessa, S.; Medhin, G.; Mesfin, M.; Alemayehu, M.; Shumet, T.; et al. Prevalence of Metabolic Syndrome among Patients with Schizophrenia in Ethiopia. BMC Psychiatry 2021, 21, 620. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Maghami, N.; Ammari, T.; Mosafer, H.; Abdullahi, R.; Rasoulpoor, S.; Babajani, F.; Mahmodzadeh, B.; Mohammadi, M. Global Prevalence of Metabolic Syndrome in Schizophrenia Patients: A Systematic Review and Meta-Analysis. J. Prev. 2024, 45, 973–986. [Google Scholar] [CrossRef]
- Coodin, S. Body Mass Index in Persons with Schizophrenia. Can. J. Psychiatry 2001, 46, 549–555. [Google Scholar] [CrossRef]
- Cameron, I.M.; Hamilton, R.J.; Fernie, G.; MacGillivray, S.A. Obesity in Individuals with Schizophrenia: A Case Controlled Study in Scotland. BJPsych Open 2017, 3, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Sneller, M.H.; De Boer, N.; Everaars, S.; Schuurmans, M.; Guloksuz, S.; Cahn, W.; Luykx, J.J. Clinical, Biochemical and Genetic Variables Associated with Metabolic Syndrome in Patients with Schizophrenia Spectrum Disorders Using Second-Generation Antipsychotics: A Systematic Review. Front. Psychiatry 2021, 12, 625935. [Google Scholar] [CrossRef]
- Moreno, T.S.-A.; Ruiz-Doblado, S.; Hernández-Fleta, J.L.; Touriño-Gonzalez, R.; León-Pérez, P. Quality of Life in a Sample of Schizophrenic Patients with and without Metabolic Syndrome. J. Psychiatr. Intensive Care 2010, 6, 101–108. [Google Scholar] [CrossRef]
- Chandra, I.S.; Kumar, K.L.; Reddy, M.P.; Reddy, C.M.P.K. Attitudes toward Medication and Reasons for Non-Compliance in Patients with Schizophrenia. Indian J. Psychol. Med. 2014, 36, 294–298. [Google Scholar] [CrossRef]
- Perkins, D.O. Predictors of Noncompliance in Patients with Schizophrenia. J. Clin. Psychiatry 2002, 63, 1121–1128. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Lange, S.M.M. Metabolic Syndrome in Psychiatric Patients: Overview, Mechanisms, and Implications. Dialogues Clin. Neurosci. 2018, 20, 63–73. [Google Scholar] [CrossRef]
- Malan-Müller, S.; Kilian, S.; van den Heuvel, L.L.; Bardien, S.; Asmal, L.; Warnich, L.; Emsley, R.A.; Hemmings, S.M.J.; Seedat, S. A Systematic Review of Genetic Variants Associated with Metabolic Syndrome in Patients with Schizophrenia. Schizophr. Res. 2016, 170, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mednova, I.A.; Pozhidaev, I.V.; Tiguntsev, V.V.; Bocharova, A.V.; Paderina, D.Z.; Boiko, A.S.; Fedorenko, O.Y.; Kornetova, E.G.; Bokhan, N.A.; Stepanov, V.A. NOS1AP Gene Variants and Their Role in Metabolic Syndrome: A Study of Patients with Schizophrenia. Biomedicines 2024, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; De Hert, M.; Moons, T.; Claes, S.J.; Correll, C.U.; van Winkel, R. CNR1 Gene and Risk of the Metabolic Syndrome in Patients with Schizophrenia. J. Clin. Psychopharmacol. 2013, 33, 186–192. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, P.-Y.; Chen, C.Y.-A.; Chiu, C.-C.; Lu, M.-L.; Huang, M.-C.; Lin, Y.-K.; Chen, Y.-H. Associations of Genetic Variants of Methylenetetrahydrofolate Reductase and Serum Folate Levels with Metabolic Parameters in Patients with Schizophrenia. Int. J. Environ. Res. Public Health 2021, 18, 11333. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Otani, H. Oxidative Stress as Pathogenesis of Cardiovascular Risk Associated with Metabolic Syndrome. Antioxid. Redox Signal. 2011, 15, 1911–1926. [Google Scholar] [CrossRef]
- Baez-Duarte, B.G.; Zamora-Ginez, I.; De Jésus, K.L.; Torres-Rasgado, E.; González-Mejía, M.E.; Porchia, L.; Ruiz-Vivanco, G.; Pérez-Fuentes, R. Association of the Metabolic Syndrome with Antioxidant Defense and Outstanding Superoxide Dismutase Activity in Mexican Subjects. Metab. Syndr. Relat. Disord. 2016, 14, 154–160. [Google Scholar] [CrossRef]
- Vávrová, L.; Kodydková, J.; Zeman, M.; Dušejovská, M.; Macášek, J.; Staňková, B.; Tvrzická, E.; Žák, A. Altered Activities of Antioxidant Enzymes in Patients with Metabolic Syndrome. Obes. Facts 2013, 6, 39–47. [Google Scholar] [CrossRef]
- Cardona, F.; Tunez, I.; Tasset, I.; Murri, M.; Tinahones, F.J. Similar Increase in Oxidative Stress after Fat Overload in Persons with Baseline Hypertriglyceridemia with or without the Metabolic Syndrome. Clin. Biochem. 2008, 41, 701–705. [Google Scholar] [CrossRef]
- Murray, A.J.; Rogers, J.C.; Katshu, M.Z.U.H.; Liddle, P.F.; Upthegrove, R. Oxidative Stress and the Pathophysiology and Symptom Profile of Schizophrenia Spectrum Disorders. Front. Psychiatry 2021, 12, 703452. [Google Scholar] [CrossRef] [PubMed]
- Juchnowicz, D.; Dzikowski, M.; Rog, J.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M.; Karakuła-Juchnowicz, H. Oxidative Stress Biomarkers as a Predictor of Stage Illness and Clinical Course of Schizophrenia. Front. Psychiatry 2021, 12, 728986. [Google Scholar] [CrossRef]
- Nayok, S.B.; Shivakumar, V.; Sreeraj, V.S. Oxidative Stress in Schizophrenia. In Handbook of the Biology and Pathology of Mental Disorders; Martin, C.R., Preedy, V.R., Patel, V.B., Rajendram, R., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 1–19. ISBN 978-3-031-32035-4. [Google Scholar]
- Ermakov, E.A.; Dmitrieva, E.M.; Parshukova, D.A.; Kazantseva, D.V.; Vasilieva, A.R.; Smirnova, L.P. Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxid. Med. Cell. Longev. 2021, 2021, 8881770. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, S.; Noda, Y.; Tarumi, R.; Mimura, Y.; Yoshida, K.; Iwata, Y.; Elsalhy, M.; Kuromiya, M.; Kurose, S.; Masuda, F.; et al. Glutathione Levels and Activities of Glutathione Metabolism Enzymes in Patients with Schizophrenia: A Systematic Review and Meta-Analysis. J. Psychopharmacol. 2019, 33, 1199–1214. [Google Scholar] [CrossRef]
- Rupérez, A.; Gil, A.; Aguilera, C. Genetics of Oxidative Stress in Obesity. Int. J. Mol. Sci. 2014, 15, 3118–3144. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Lee, H. Pharmacogenetic Studies Investigating the Adverse Effects of Antipsychotics. Psychiatry Investig. 2007, 4, 66. [Google Scholar]
- Hayes, J.D.; McLellan, L.I. Glutathione and Glutathione-Dependent Enzymes Represent a Co-Ordinately Regulated Defence against Oxidative Stress. Free Radic. Res. 1999, 31, 273–300. [Google Scholar] [CrossRef]
- Moyer, A.M.; Salavaggione, O.E.; Wu, T.-Y.; Moon, I.; Eckloff, B.W.; Hildebrandt, M.A.T.; Schaid, D.J.; Wieben, E.D.; Weinshilboum, R.M. Glutathione S-Transferase P1: Gene Sequence Variation and Functional Genomic Studies. Cancer Res. 2008, 68, 4791–4801. [Google Scholar] [CrossRef]
- Menon, D.; Board, P.G. A Role for Glutathione Transferase Omega 1 (GSTO1-1) in the Glutathionylation Cycle. J. Biol. Chem. 2013, 288, 25769–25779. [Google Scholar] [CrossRef]
- Tanaka-Kagawa, T.; Jinno, H.; Hasegawa, T.; Makino, Y.; Seko, Y.; Hanioka, N.; Ando, M. Functional Characterization of Two Variant Human GSTO 1-1s (Ala140Asp and Thr217Asn). Biochem. Biophys. Res. Commun. 2003, 301, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fasciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef]
- Lepping, P.; Delieu, J.; Mellor, R.; Williams, J.H.; Hudson, P.R.; Hunter-Lavin, C. Antipsychotic Medication and Oxidative Cell Stress: A Systematic Review. J. Clin. Psychiatry 2010, 71, 1093. [Google Scholar] [CrossRef]
- BioGPS—Your Gene Portal System. Available online: http://biogps.org/#goto=welcome (accessed on 8 April 2025).
- Gene Ontology Resource. Available online: http://geneontology.org/ (accessed on 8 April 2025).
- GTEx Portal. Available online: https://www.gtexportal.org/home/ (accessed on 8 April 2025).
- Saadat, M. Evaluation of Glutathione S-Transferase P1 (GSTP1) Ile105Val Polymorphism and Susceptibility to Type 2 Diabetes Mellitus, a Meta-Analysis. EXCLI J. 2017, 16, 1188. [Google Scholar] [CrossRef]
- Chielle, E.O.; Trott, A.; Rosa, B.d.S.; Casarin, J.N.; Fortuna, P.C.; da Cruz, I.B.M.; Moretto, M.B.; Moresco, R.N. Impact of the Ile105Val Polymorphism of the Glutathione S-Transferase P1 (GSTP1) Gene on Obesity and Markers of Cardiometabolic Risk in Young Adult Population. Exp. Clin. Endocrinol. Diabetes 2016, 125, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Villamil-Ramírez, H.; León-Mimila, P.; Macias-Kauffer, L.R.; Canizalez-Román, A.; Villalobos-Comparán, M.; León-Sicairos, N.; Vega-Badillo, J.; Sánchez-Muñoz, F.; López-Contreras, B.; Morán-Ramos, S.; et al. A Combined Linkage and Association Strategy Identifies a Variant near the GSTP1 Gene Associated with BMI in the Mexican Population. J. Hum. Genet. 2017, 62, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Saruwatari, J.; Yasui-Furukori, N.; Kamihashi, R.; Yoshimori, Y.; Oniki, K.; Tsuchimine, S.; Noai, M.; Sato, Y.; Nakagami, T.; Sugawara, N.; et al. Possible Associations between Antioxidant Enzyme Polymorphisms and Metabolic Abnormalities in Patients with Schizophrenia. NDT 2013, 9, 1683–1698. [Google Scholar] [CrossRef]
- Oniki, K.; Kamihashi, R.; Tomita, T.; Ishioka, M.; Yoshimori, Y.; Osaki, N.; Tsuchimine, S.; Sugawara, N.; Kajiwara, A.; Morita, K.; et al. Glutathione S-Transferase K1 Genotype and Overweight Status in Schizophrenia Patients: A Pilot Study. Psychiatry Res. 2016, 239, 190–195. [Google Scholar] [CrossRef]
- Oniki, K.; Ishioka, M.; Osaki, N.; Sakamoto, Y.; Yoshimori, Y.; Tomita, T.; Kamihashi, R.; Tsuchimine, S.; Sugawara, N.; Otake, K.; et al. Association between Oxidative Stress-Related Genes Polymorphisms and Metabolic Abnormalities among Schizophrenia Patients. Clin. Neuropsychopharmacol. Ther. 2017, 8, 25–37. [Google Scholar] [CrossRef]
- Chiliza, B.; Asmal, L.; Oosthuizen, P.; Van Niekerk, E.; Erasmus, R.; Kidd, M.; Malhotra, A.; Emsley, R. Changes in Body Mass and Metabolic Profiles in Patients with First-Episode Schizophrenia Treated for 12 Months with A First-Generation Antipsychotic. Eur. Psychiatr. 2015, 30, 277–283. [Google Scholar] [CrossRef]
- Pakkiyalakshmi, N.; Suriyamoorthi, M.; Ravishankar, J. A Comparative Study between First Generation and Second Generation Antipsychotics over the Development of Metabolic Syndrome in Persons with First Episode Drug Naive Schizophrenia. Int. J. Res. Med. Sci. 2018, 6, 3693. [Google Scholar]
- Panati, D.; Sudhakar, T.P.; Swetha, P.; Sayeli, V.K. A Comparative Study on Metabolic Syndrome in Patients with Schizophrenia Treated Using First-Generation and Second-Generation Antipsychotics. Arch. Ment. Health 2020, 21, 4. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Xia, N. The Interplay Between Adipose Tissue and Vasculature: Role of Oxidative Stress in Obesity. Front. Cardiovasc. Med. 2021, 8, 650214. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, K.R.H.; Katshu, M.Z.U.H.; Chakrabarti, L. Second-Generation Antipsychotics and Metabolic Syndrome: A Role for Mitochondria. Front. Psychiatry 2023, 14, 1257460. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic Syndrome—A New World-wide Definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).