Possible Association Between Concomitant Use of SSRIs with NSAIDs and an Increased Risk of Adverse Events Among People with Depressive Disorders: Data Mining of FDA Adverse Event Reporting System
Abstract
1. Introduction
2. Results
2.1. Results of Research Subject Selection
2.2. Basic Characteristics of the Research Subjects
2.3. Frequency of Use of SSRI and NSAIDs in AE Reports in Patients with Depression
2.4. Signal Detection for Various SSRIs and NSAIDs and AEs of Interest
2.5. Signal Detection for Various SSRI-NSAID Combinations and AEs of Interest
3. Discussion
4. Materials and Methods
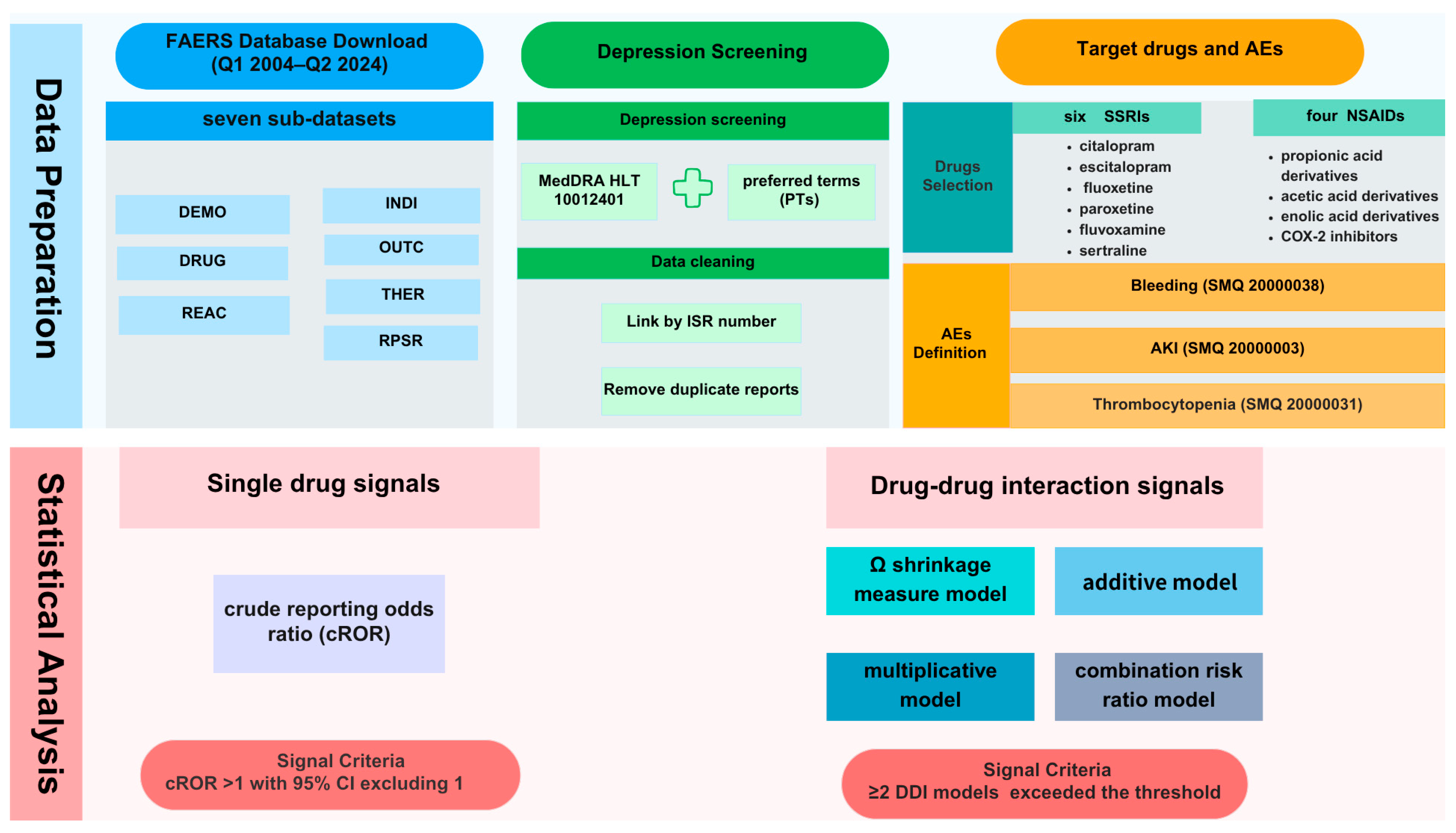
4.1. Data Source
4.2. Depression Screening and Data Cleaning
4.3. Selection of Target Adverse Events
4.4. Target Drugs and Definition of AEs
4.5. Statistical Analysis
- (1)
- Ω shrinkage measure model:
- (2)
- Additive model:
- (3)
- Multiplicative model:
- (4)
- CRR model:
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 7 December 2024).
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Yang, J.; Huangfu, X.; Tong, D.; He, A. Regional Gray Matter Volume Mediates the Relationship between Neuroticism and Depressed Emotion. Front. Psychol. 2022, 13, 993694. [Google Scholar] [CrossRef]
- Barth, J.; Munder, T.; Gerger, H.; Nüesch, E.; Trelle, S.; Znoj, H.; Jüni, P.; Cuijpers, P. Comparative Efficacy of Seven Psychotherapeutic Interventions for Patients with Depression: A Network Meta-Analysis. PLoS Med. 2013, 10, e1001454. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Salian, H.H.; Raghav, M.V.; Rawat, V.S.; Divakar, A. Cost-Minimization Analysis of Escitalopram, Fluoxetine, and Amitriptyline in the Treatment of Depression. Indian J. Pharmacol. 2023, 55, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huan, J.; Lin, L.; Hu, Y. Association of Systemic Inflammatory Biomarkers with Depression Risk: Results from National Health and Nutrition Examination Survey 2005–2018 Analyses. Front. Psychiatry 2023, 14, 1097196. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Irwin, M.R. Novel Neuroimmunologic Therapeutics in Depression: A Clinical Perspective on What We Know so Far. Brain Behav. Immun. 2020, 83, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Guo, W.; Feng, Y.; Deng, H.; Li, G.; Nie, H.; Guo, G.; Yu, H.; Ma, Y.; Wang, J.; et al. Efficacy and Safety of Anti-Inflammatory Agents for the Treatment of Major Depressive Disorder: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.; Zhang, Y.; Li, J.; Li, Z.; Zhang, Y.; Ye, X.; Tang, Q.; Sun, W. Comparative Efficacy and Acceptability of Anti-Inflammatory Agents on Major Depressive Disorder: A Network Meta-Analysis. Front. Pharmacol. 2021, 12, 691200. [Google Scholar] [CrossRef] [PubMed]
- Köhler-Forsberg, O.; Lydholm, C.N.; Hjorthøj, C.; Nordentoft, M.; Mors, O.; Benros, M.E. Efficacy of Anti-Inflammatory Treatment on Major Depressive Disorder or Depressive Symptoms: Meta-Analysis of Clinical Trials. Acta Psychiatr. Scand. 2019, 139, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Anglin, R.; Yuan, Y.; Moayyedi, P.; Tse, F.; Armstrong, D.; Leontiadis, G.I. Risk of Upper Gastrointestinal Bleeding with Selective Serotonin Reuptake Inhibitors with or without Concurrent Nonsteroidal Anti-Inflammatory Use: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2014, 109, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-Y.; Park, M.-J.; Lee, S.H.; Choi, S.-H.; Kim, M.-H.; Choi, N.-K.; Lee, J.; Park, B.-J. Risk of Intracranial Haemorrhage in Antidepressant Users with Concurrent Use of Non-Steroidal Anti-Inflammatory Drugs: Nationwide Propensity Score Matched Study. BMJ 2015, 351, h3517. [Google Scholar] [CrossRef] [PubMed]
- Sakaeda, T.; Tamon, A.; Kadoyama, K.; Okuno, Y. Data Mining of the Public Version of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 2013, 10, 796–803. [Google Scholar] [CrossRef] [PubMed]
- de Abajo, F.J.; Rodríguez, L.A.G.; Montero, D. Association between Selective Serotonin Reuptake Inhibitors and Upper Gastrointestinal Bleeding: Population Based Case-Control Study. BMJ 1999, 319, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, J.; Wang, D.; Cen, B.; Lang, J.D.; DuBois, R.N. The COX-2-PGE2 Pathway Promotes Tumor Evasion in Colorectal Adenomas. Cancer Prev. Res. 2022, 15, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Seo, H.-J.; Abdi, S.; Huh, B. All about Pain Pharmacology: What Pain Physicians Should Know. Korean J. Pain. 2020, 33, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Hyttel, J. Pharmacological Characterization of Selective Serotonin Reuptake Inhibitors (SSRIs). Int. Clin. Psychopharmacol. 1994, 9 (Suppl. S1), 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv. Pharmacol. Sci. 2019, 2019, 3418975. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute Kidney Injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Pannu, N.; Nadim, M.K. An Overview of Drug-Induced Acute Kidney Injury. Crit. Care Med. 2008, 36, S216–S223. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, S.; Guirguis, A.; Schifano, N.; Corkery, J.M.; Semeraro, F.; Mosca, A.; D’Andrea, G.; Duccio Papanti, G.; Arillotta, D.; Floresta, G.; et al. Comparative Safety of Prescribed Esketamine and Ketamine in Relation to Renal and Urinary Disorders: A Pharmacovigilance Perspective. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2025, 136, 111213. [Google Scholar] [CrossRef] [PubMed]
- Lecardeur, L.; Lefebvre, A.; Meunier-Cussac, S. Rhabdomyolysis after Escitalopram Treatment in a Young Adult with Melancholic Depression. J. Clin. Psychopharmacol. 2015, 35, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Gong, W.; Cui, Z.; Li, J.; Lu, Y. Rhabdomyolysis in a Male Adolescent Associated with Monotherapy of Fluvoxamine. Eur. J. Hosp. Pharm. 2023, 30, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Gaddameedi, S.R.; Cherukuri, P.B.; Khan, M.A.; Atreya, S.; Bandari, V.; Ashok, M.; Shah, S. Alcoholism and Immobility Induced Rhabdomyolysis Culminating in Hemodialysis. Cureus 2024, 16, e59316. [Google Scholar] [CrossRef] [PubMed]
- Abdissa, D. Purposeful Review to Identify Risk Factors, Epidemiology, Clinical Features, Treatment and Prevention of Chronic Kidney Disease of Unknown Etiology. Int. J. Nephrol. Renovasc. Dis. 2020, 13, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Sangam, S.; Malik, S.; Hodo, F.; Patel, B.; Bengualid, V.; Sharma, S. A Case of Legionella Pneumonia with Rhabdomyolysis, with Extremely High Creatinine Kinase without Acute Kidney Injury in an Adult. Eur. J. Case Rep. Intern. Med. 2024, 11, 004539. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Tachi, T.; Teramachi, H. Review of Statistical Methodologies for Detecting Drug–Drug Interactions Using Spontaneous Reporting Systems. Front. Pharmacol. 2019, 10, 1319. [Google Scholar] [CrossRef] [PubMed]
- Kontsioti, E.; Maskell, S.; Pirmohamed, M. Exploring the Impact of Design Criteria for Reference Sets on Performance Evaluation of Signal Detection Algorithms: The Case of Drug–Drug Interactions. Pharmacoepidemiol. Drug Saf. 2023, 32, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, S.; Vickers-Smith, R.; Harris, D.; Papanti Pelletier, G.D.; Corkery, J.M.; Guirguis, A.; Martinotti, G.; Sensi, S.L.; Schifano, F. Is There a Risk for Semaglutide Misuse? Focus on the Food and Drug Administration’s FDA Adverse Events Reporting System (FAERS) Pharmacovigilance Dataset. Pharmaceuticals 2023, 16, 994. [Google Scholar] [CrossRef] [PubMed]
- Alomar, M.; Tawfiq, A.M.; Hassan, N.; Palaian, S. Post Marketing Surveillance of Suspected Adverse Drug Reactions through Spontaneous Reporting: Current Status, Challenges and the Future. Ther. Adv. Drug Saf. 2020, 11, 2042098620938595. [Google Scholar] [CrossRef] [PubMed]
- FAERS Public Dashboard—Frequently Asked Questions (FAQs). Available online: https://fis.fda.gov/extensions/FPD-FAQ/FPD-FAQ.html#_Toc514144622 (accessed on 20 April 2025).
- Zhang, C.; Wang, H.; Chen, X.; Liu, Y.; Jiang, P. Safety of Lifitegrast: A Real-World Pharmacovigilance Study Based on FAERS. PLoS ONE 2025, 20, e0321307. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Huang, P.; Zhang, J.; Liu, J.; Yan, Z.; Ma, X. Drug-Induced Cardiac Arrest: A Pharmacovigilance Study from 2004–2024 Based on FAERS Database. Front. Cardiovasc. Med. 2025, 12, 1498700. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Ueno, A.; Otsubo, M.; Katsuno, H.; Sugita, I.; Kanematsu, Y.; Yoshida, A.; Esaki, H.; Tachi, T.; Teramachi, H. A New Search Method Using Association Rule Mining for Drug-Drug Interaction Based on Spontaneous Report System. Front. Pharmacol. 2018, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Kayanuma, G.; Nagashima, T.; Toda, C.; Nagayasu, K.; Kaneko, S. Early Detection of Adverse Drug Reaction Signals by Association Rule Mining Using Large-Scale Administrative Claims Data. Drug Saf. 2023, 46, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.; Prada Jardim, A.; Albera, C. Artificial Intelligence: Applications in Pharmacovigilance Signal Management. Pharmaceut. Med. 2025, 39, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Fusaroli, M.; Salvo, F.; Khouri, C.; Raschi, E. The Reporting of Disproportionality Analysis in Pharmacovigilance: Spotlight on the READUS-PV Guideline. Front. Pharmacol. 2024, 15, 1488725. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Sakai, T.; Tanabe, K.; Umemura, T.; Goto, N.; Ohtsu, F. Visualization of Kinase Inhibition-Related Adverse Events Using the Japanese Adverse Drug Event Report Database. Drugs Real World Outcomes 2021, 8, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lee-Kelland, R.; Zehra, S.; Mappa, P. Fluoxetine Overdose in a Teenager Resulting in Serotonin Syndrome, Seizure and Delayed Onset Rhabdomyolysis. BMJ Case Rep. 2018, 2018, bcr2018225529. [Google Scholar] [CrossRef] [PubMed]
- McFarland, D.; Merchant, D.; Khandai, A.; Mojtahedzadeh, M.; Ghosn, O.; Hirst, J.; Amonoo, H.; Chopra, D.; Niazi, S.; Brandstetter, J.; et al. Selective Serotonin Reuptake Inhibitor (SSRI) Bleeding Risk: Considerations for the Consult-Liaison Psychiatrist. Curr. Psychiatry Rep. 2023, 25, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Ide, J.; Shoaibi, A.; Wagner, K.; Weinstein, R.; Boyle, K.E.; Myers, A. Patterns of Comorbidities and Prescribing and Dispensing of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Among Patients with Osteoarthritis in the USA: Real-World Study. Drugs Aging 2024, 41, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.M.; Qasswal, M.; Ahsan, M.J.; Walters, R.W.; Chandra, S. Selective Serotonin Reuptake Inhibitors Increase Risk of Upper Gastrointestinal Bleeding When Used with NSAIDs: A Systemic Review and Meta-Analysis. Sci. Rep. 2022, 12, 14452. [Google Scholar] [CrossRef]
- Andersohn, F.; Konzen, C.; Bronder, E.; Klimpel, A.; Garbe, E. Citalopram-Induced Bleeding Due to Severe Thrombocytopenia. Psychosomatics 2009, 50, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Gkika, D.A.; Siadimas, I.; Christodoulopoulos, I.; Efthymiopoulos, P.; Kyzas, G.Z. The Multifaceted Effects of Non-Steroidal and Non-Opioid Anti-Inflammatory and Analgesic Drugs on Platelets: Current Knowledge, Limitations, and Future Perspectives. Pharmaceuticals 2024, 17, 627. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, A.A.; Gunawan, V.A.; Iragama, F.R.; Alfiansyah, R.; Hertanto, D.M.; Tjempakasari, A.; Thaha, M. Risk Factors and Clinical Characteristics of Acute Kidney Injury in Patients with COVID-19: A Systematic Review and Meta-Analysis. Pathophysiology 2023, 30, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, S.; Vickers-Smith, R.; Guirguis, A.; Corkery, J.M.; Martinotti, G.; Schifano, F. A Focus on Abuse/Misuse and Withdrawal Issues with Selective Serotonin Reuptake Inhibitors (SSRIs): Analysis of Both the European EMA and the US FAERS Pharmacovigilance Databases. Pharmaceuticals 2022, 15, 565. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-J.; Hu, T.; Jiang, C.-L. Cool the Inflamed Brain: A Novel Anti-Inflammatory Strategy for the Treatment of Major Depressive Disorder. Curr. Neuropharmacol. 2024, 22, 810–842. [Google Scholar] [CrossRef] [PubMed]
- van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.M.; Lindquist, M.; Orre, R.; Egberts, A.C.G. A Comparison of Measures of Disproportionality for Signal Detection in Spontaneous Reporting Systems for Adverse Drug Reactions. Pharmacoepidemiol. Drug Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Tachi, T.; Teramachi, H. Comparison of Signal Detection Algorithms Based on Frequency Statistical Model for Drug-Drug Interaction Using Spontaneous Reporting Systems. Pharm. Res. 2020, 37, 86. [Google Scholar] [CrossRef] [PubMed]
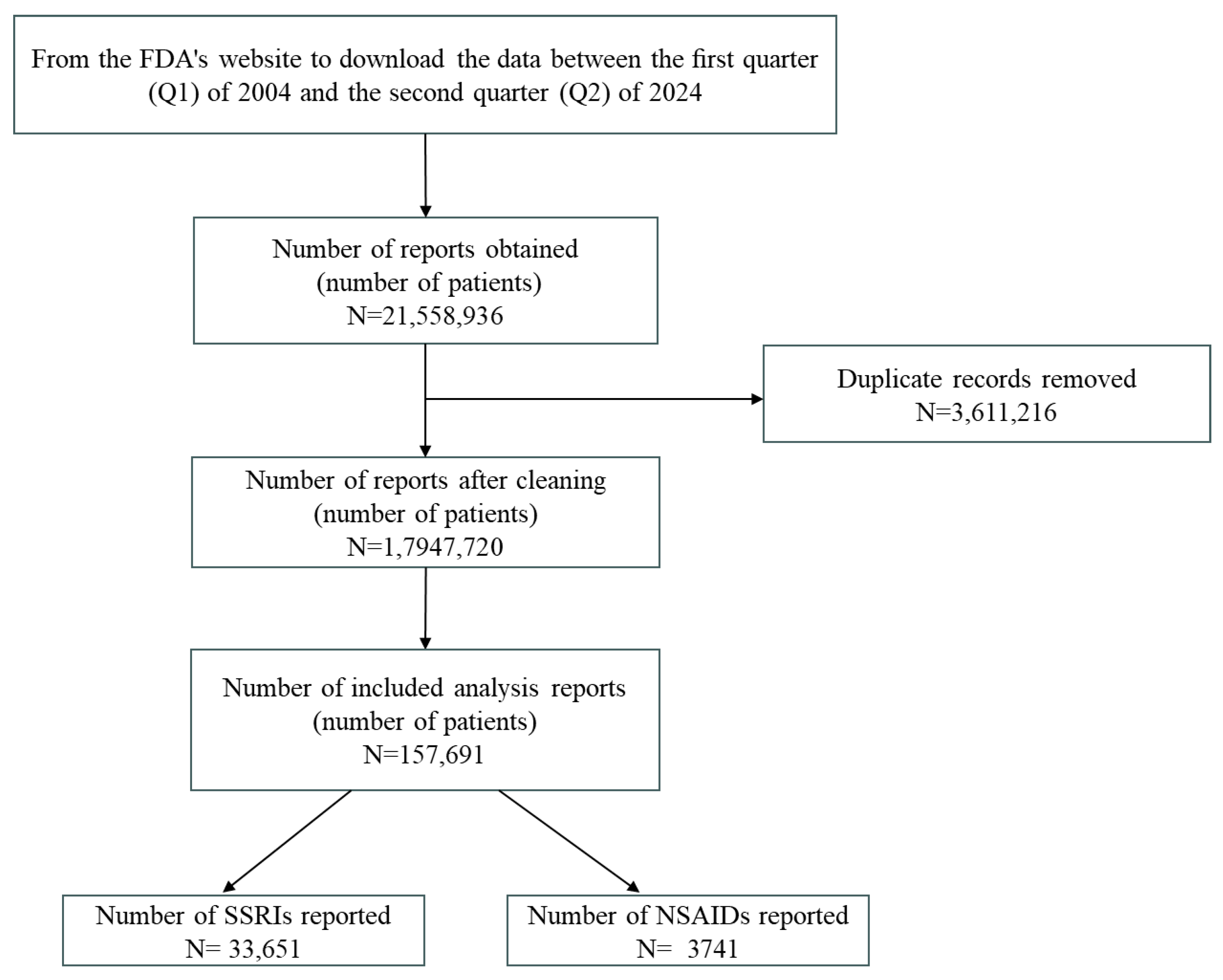

| Control | Only SSRIs | Only NSAIDs | SSRIs + NSAIDs | ||
|---|---|---|---|---|---|
| n | 121,449 | 32,501 | 2591 | 1150 | |
| Age, (mean (SD)) | 47.91 (19.77) | 47.01 (23.02) | 49.85 (17.46) | 47.57 (20.53) | |
| Age, n (%) | <18 | 6014 (5.0) | 2502 (7.7) | 77 (3.0) | 67 (5.8) |
| 18–45 | 29,284 (24.1) | 9784 (30.1) | 626 (24.2) | 349 (30.3) | |
| 46–64 | 32,700 (26.9) | 6981 (21.5) | 864 (33.3) | 344 (29.9) | |
| >65 | 17,711 (14.6) | 6625 (20.4) | 376 (14.5) | 204 (17.7) | |
| Unknown | 35,740 (29.4) | 6609 (20.3) | 648 (25.0) | 186 (16.2) | |
| Sex, n (%) | Female | 78,358 (68.1) | 19,323 (65.2) | 1711 (68.5) | 738 (70.6) |
| Male | 36,418 (31.6) | 10,193 (34.4) | 780 (31.2) | 302 (28.9) | |
| Unknown | 303 (0.3) | 107 (0.4) | 6 (0.2) | 5 (0.5) | |
| Year, n (%) | 2004–2008 | 25,546 (21.0) | 3821 (11.8) | 443 (17.1) | 137 (11.9) |
| 2009–2013 | 28,356 (23.3) | 5916 (18.2) | 698 (26.9) | 232 (20.2) | |
| 2014–2018 | 36,173 (29.8) | 9877 (30.4) | 822 (31.7) | 351 (30.5) | |
| 2019–2024 | 31,374 (25.8) | 12,887 (39.7) | 628 (24.2) | 430 (37.4) | |
| Country, n (%) | United States | 76,135 (62.7) | 6132 (18.9) | 1677 (64.7) | 158 (13.7) |
| United Kingdom | 5719 (4.7) | 9692 (29.8) | 201 (7.8) | 522 (45.4) | |
| France | 5631 (4.6) | 3494 (10.8) | 46 (1.8) | 67 (5.8) | |
| Germany | 3927 (3.2) | 2309 (7.1) | 105 (4.1) | 78 (6.8) | |
| Others | 20,895 (17.2) | 9573 (29.5) | 392 (15.1) | 271 (23.6) | |
| Not Specified | 9142 (7.5) | 1301 (4.0) | 170 (6.6) | 54 (4.7) |
| Drugs | Bleeding | Thrombocytopenia | Acute Kidney Injury | |
|---|---|---|---|---|
| SSRI | ||||
| citalopram | 2.81 [2.30, 3.44] | 1.12 [0.87, 1.43] | 1.39 [1.20, 1.60] | |
| escitalopram | 2.27 [1.67, 3.06] | 1.09 [0.75, 1.58] | 1.36 [1.10, 1.67] | |
| fluoxetine | 1.36 [0.93, 1.97] | 2.11 [1.60, 2.78] | 1.16 [0.93, 1.44] | |
| paroxetine | 2.17 [1.52, 3.10] | 2.68 [2.01, 3.59] | 1.26 [0.98, 1.61] | |
| fluvoxamine | 3.58 [0.88, 14.47] | 2.84 [0.70,11.49] | 3.24 [1.43, 7.34] | |
| sertraline | 1.69 [1.31, 2.17] | 1.08 [0.83, 1.41] | 0.80 [0.66, 0.97] | |
| NSAID | ||||
| propionic acid derivatives | 3.17 [2.18, 4.61] | 1.34 [0.82, 2.21] | 1.32 [0.97, 1.79] | |
| acetic acid derivatives | 1.69 [0.70, 4.08] | 0.53 [0.13, 2.13] | 1.30 [0.75, 2.25] | |
| enolic acid derivatives | 1.99 [0.64, 6.21] | 0.52 [0.07, 3.72] | 0.78 [0.29, 2.09] | |
| selective cyclooxygenase (COX)-2 inhibitors | 1.24 [0.17, 8.87] | 2.99 [0.96, 9.38] | 2.24 [1.00, 5.06] |
| DDI Combination | n111 | n11+ | Ω Shrinkage Model | Additive Model | Multiplicative Model | CRR Model |
|---|---|---|---|---|---|---|
| DDI for Bleeding | ||||||
| SSRI + NSAID | 19 | 1150 | 0.49 (−0.16, 1.34) | −0.001 | 0.87 | 2.25 |
| Citalopram + NSAID | 9 | 415 | 0.56 (−0.39, 1.50) | 0 | 0.9 | 2.43 |
| Fluoxetine + NSAID | 3 | 229 | 0.20 (−1.43, 1.83) | −0.005 | 0.99 | 1.31 |
| Sertraline + NSAID | 5 | 417 | −0.03 (−1.30, 1.23) | −0.001 | 0.71 | 1.21 |
| Citalopram + NSAID1 | 8 | 271 | 0.89 (−0.11, 1.89) | 0.01 | 1.14 | 3.13 |
| Sertraline + NSAID1 | 4 | 322 | −0.16 (−1.58, 1.25) | −0.01 | 0.63 | 1.06 |
| DDI for Thrombocytopenia | ||||||
| SSRI+NSAID | 12 | 1150 | 0.68 (−0.13, 1.50) | −0.004 | 1.71 | 1.55 |
| Citalopram + NSAID | 3 | 415 | 0.18 (−1.45, 1.82) | −0.008 | 1.14 | 1.26 |
| Fluoxetine + NSAID | 3 | 229 | 0.28 (−1.35, 1.92) | −0.006 | 1.17 | 1.37 |
| Paroxetine + NSAID | 3 | 85 | 1.17 (−0.46, 2.81) | 0.014 | 2.65 | 2.99 |
| Sertraline + NSAID | 3 | 417 | 0.21 (−1.42, 1.85) | −0.008 | 1.18 | 1.26 |
| Citalopram + NSAID1 | 3 | 271 | 0.67 (−0.96, 2.30) | −0.001 | 1.73 | 1.89 |
| Fluoxetine + NSAID1 | 3 | 160 | 0.69 (−0.94, 2.33) | −0.001 | 1.64 | 1.98 |
| Sertraline + NSAID1 | 3 | 326 | 0.46 (−1.17, 2.09) | −0.01 | 1.45 | 1.54 |
| DDI for Acute Kidney Injury | ||||||
| SSRI+NSAID | 27 | 1150 | 0.50 (−0.05, 1.04) | −0.02 | 1.39 | 1.6 |
| Citalopram + NSAID | 13 | 415 | 0.62 (−0.16, 1.40) | −0.11 | 13.35 | 1.86 |
| Escitalopram + NSAID | 4 | 150 | 0.29 (−1.13, 1.70) | −0.02 | 1.17 | 1.57 |
| Sertraline + NSAID | 11 | 417 | 0.94 (0.09, 1.80) | −0.01 | 2.14 | 1.62 |
| Citalopram + NSAID1 | 11 | 271 | 1.08 (0.23, 1.93) | −0.003 | 2.17 | 2.42 |
| Sertraline + NSAID1 | 6 | 326 | 0.37 (−0.78, 1.53) | −0.021 | 1.41 | 1.11 |
| With an Adverse Event of Interest | Without an Adverse Event of Interest | |
|---|---|---|
| With a drug of interest | a | b |
| Without a drug of interest | c | d |
| AEs of Interest | Other AEs | Total | |
|---|---|---|---|
| Concomitant use of SSRIs and NSAIDs | n111 | n110 | n11+ |
| SSRIs without NSAIDs | n101 | n100 | n10+ |
| NSAIDs without SSRIs | n011 | n010 | n01+ |
| Neither SSRIs nor NSAIDs | n001 | n000 | n00+ |
| Total | n++1 | n++0 | n+++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, X.; Wu, J.; Zhang, X.; Wei, F.; Li, L.; Li, H.; Wang, X.; Wang, B.; Wu, W.; et al. Possible Association Between Concomitant Use of SSRIs with NSAIDs and an Increased Risk of Adverse Events Among People with Depressive Disorders: Data Mining of FDA Adverse Event Reporting System. Pharmaceuticals 2025, 18, 1062. https://doi.org/10.3390/ph18071062
Zhang Y, Liu X, Wu J, Zhang X, Wei F, Li L, Li H, Wang X, Wang B, Wu W, et al. Possible Association Between Concomitant Use of SSRIs with NSAIDs and an Increased Risk of Adverse Events Among People with Depressive Disorders: Data Mining of FDA Adverse Event Reporting System. Pharmaceuticals. 2025; 18(7):1062. https://doi.org/10.3390/ph18071062
Chicago/Turabian StyleZhang, Yi, Xiaoyu Liu, Jianru Wu, Xuening Zhang, Fenfang Wei, Limin Li, Hongqiao Li, Xinru Wang, Bei Wang, Wenyu Wu, and et al. 2025. "Possible Association Between Concomitant Use of SSRIs with NSAIDs and an Increased Risk of Adverse Events Among People with Depressive Disorders: Data Mining of FDA Adverse Event Reporting System" Pharmaceuticals 18, no. 7: 1062. https://doi.org/10.3390/ph18071062
APA StyleZhang, Y., Liu, X., Wu, J., Zhang, X., Wei, F., Li, L., Li, H., Wang, X., Wang, B., Wu, W., & Hong, X. (2025). Possible Association Between Concomitant Use of SSRIs with NSAIDs and an Increased Risk of Adverse Events Among People with Depressive Disorders: Data Mining of FDA Adverse Event Reporting System. Pharmaceuticals, 18(7), 1062. https://doi.org/10.3390/ph18071062







