Abstract
Background/Objectives: Bcr-Abl inhibitors such as imatinib have been used to treat chronic myeloid leukemia (CML). However, the efficacy of these drugs has diminished due to mutations in the kinase domain, notably the T315I mutation. Therefore, in this study, new purine derivatives were designed as Bcr-Abl inhibitors based on 3D-QSAR studies. Methods: A database of 58 purines that inhibit Bcr-Abl was used to construct 3D-QSAR models. Using chemical information from these models, a small group of new purines was designed, synthesized, and evaluated in Bcr-Abl. Viability assays were conducted on imatinib-sensitive CML cells (K562 and KCL22) and imatinib-resistant cells (KCL22-B8). In silico analyses were performed to confirm the results. Results: Seven purines were easily synthesized (7a–g). Compounds 7a and 7c demonstrated the highest inhibition activity on Bcr-Abl (IC50 = 0.13 and 0.19 μM), surpassing the potency of imatinib (IC50 = 0.33 μM). 7c exhibited the highest potency, with GI50 = 0.30 μM on K562 cells and 1.54 μM on KCL22 cells. The GI50 values obtained for non-neoplastic HEK293T cells indicated that 7c was less toxic than imatinib. Interestingly, KCL22-B8 cells (expressing Bcr-AblT315I) showed greater sensitivity to 7e and 7f than to imatinib (GI50 = 13.80 and 15.43 vs. >20 μM, respectively). In silico analyses, including docking and molecular dynamics studies of Bcr-AblT315I, were conducted to elucidate the enhanced potency of 7e and 7f. Thus, this study provides in silico models to identify novel inhibitors that target a kinase of significance in CML.
1. Introduction
Leukaemia is a type of blood cancer that is characterized by the uncontrolled proliferation of white blood cells that exhibit an altered morphology and functionality. This phenomenon arises because of a series of mutations that are expressed during the differentiation of these cells into leukocytes [1,2]. In chronic myeloid leukaemia (CML), approximately 95% of patients exhibit a genetic abnormality referred to as the Philadelphia chromosome (Ph) [3]. This abnormality arises from a translocation event between chromosomes 9 and 22 which results in the creation of the Bcr-Abl fusion oncogene. Bcr-Abl is a protein tyrosine kinase that is aberrantly activated and plays a role in the signalling pathway that regulates cell division [4]. Consequently, Bcr-Abl has emerged as a significant therapeutic target for CML, with imatinib being the first-line treatment [5]. Approximately one-third of patients encounter treatment failure with this medication, largely because of point mutations in Bcr-Abl. These mutations can modify the binding of the inhibitor or lead to conformational changes in the enzyme [6]. Over 80 mutations that encode amino acid substitutions that confer varying levels of imatinib resistance have been identified [7]. These mutations can affect residues that directly interact with imatinib. One of the most common mutations is the substitution of isoleucine for threonine at position T315 (T315I), which is found in 2–20% of cases of CML. This mutation causes the enzyme to remain in the active conformation rather than the inactive conformation required for imatinib binding [7]. These findings have led to the development of more potent second-generation inhibitors such as dasatinib and nilotinib. However, like imatinib, they exhibit limited efficacy against certain Bcr-Abl mutations and present other adverse side effects [4,8]. Currently, the only approved third-generation inhibitor is ponatinib, which is specifically designed to overcome the T315I mutation [9].
Owing to the limitations of the Bcr-Abl inhibitors that are used clinically, numerous studies have focused on developing novel inhibitors that exhibit reduced side effects or maintain their efficacy in mutated proteins [8,10]. The most common Bcr-Abl inhibitors are those that bind directly to the ATP-binding site, which has resulted in the identification of the purine scaffold as a potential new inhibitor [10]. In this context, our research group has recently designed, synthesized, and assessed a small library of purine derivatives that are modified at the 2, 6, and 9 positions and which can inhibit Bcr-Abl with varying degrees of potency [11,12,13]. Compounds I–III (as shown in Figure 1) exhibited notable inhibition of Bcr-Abl, with IC50 values between 0.040 and 0.090 μM in the Abl kinase inhibition assay [13]. Moreover, compound III displayed a higher selectivity for Bcr-Abl compared to other tyrosine kinases, such as BTK, and a serine/threonine kinase, CDK-2. It also demonstrated low micromolar cytotoxicity across various leukaemia cell lines and effectively diminished the phosphorylation of downstream proteins within the Bcr-Abl signalling pathway [13]. Additionally, the optimal substituent at N-9 was identified as the cyclopropylmethyl group found in compounds I–III, which was evidenced by the elevated IC50 values of compounds IV–VI, which possess longer hydrophobic substituents. This conclusion was supported by molecular docking studies which correlated with the dimensions of the hydrophobic pocket in Bcr-Abl [12].
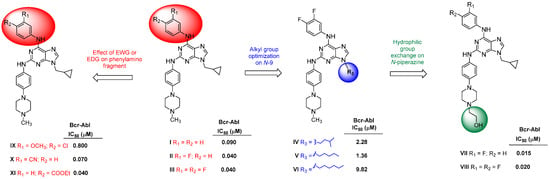
Figure 1.
The above chemical structures of purine derivatives with Bcr-Abl activity were developed by our group [11,12,13].
Following this, we synthesized new 2,6,9-trisubstituted purines that were derived from our earlier Bcr-Abl inhibitors, with compound VII (see Figure 1) being particularly significant [11]. Compound VII demonstrated enhanced potency against Bcr-Abl (IC50 = 0.015 μM) in comparison to imatinib and nilotinib and exhibited robust antiproliferative effects on three CML cell lines with Bcr-Abl rearrangement (GI50 = 0.7–1.3 μM). Additionally, compound VII inhibited the proliferation of KCL22 cells harbouring the Bcr-Abl T315I, E255K, and Y253H point mutations at micromolar concentrations. In contrast, imatinib and nilotinib were ineffective against KCL22 cells with Bcr-AblT315I (GI50 > 20 μM), while VII–VIII (Figure 1) displayed a GI50 ranging from 6.4 to 11.5 μM. Molecular docking studies clarified the structure–activity relationship of these purines concerning Bcr-AblWT and Bcr-AblT315I. Lastly, cell cycle flow cytometry assays and immunodetection indicated that compound VII induced a G1 phase arrest and downregulated downstream Bcr-Abl protein levels in these cells [11]. The influence of certain electron-donating and electron-withdrawing groups (EDG and EWG) in the para- and meta-positions of the phenylamino fragment at C-6 (compounds IX–XI, Figure 1) on Bcr-Abl activity has been explored, although definitive conclusions were not reached [12].
Several in silico approaches have been considered for the design of new compounds that can be used as tyrosine kinase inhibitors (TKIs) [14,15,16,17,18,19,20]. QSAR methods have become significant in the development of anticancer drugs due to allowing the prediction of biological activity from molecular structures and thereby aiding in the prioritization of compounds prior to their synthesis. In the context of TKIs, QSAR models allow for a deeper understanding of the structural features that are responsible for their potency and selectivity, guiding the design of more effective analogues with improved pharmacological profiles, as shown by Gond et al. [18]. Likewise, Gagic et al. conducted a review in which they discussed significant studies on the design of novel kinase inhibitors with anticancer applications using methods such as 3D-QSAR, structure-based design, and virtual screening [14]. Urich et al. conducted a de novo study, starting from 84,000 fragments, which were recombined and evaluated through docking to identify new kinase inhibitors [15]. Additionally, mathematical studies have been conducted that used 2D-QSAR with multiple linear regression to identify new PIM1 kinase inhibitors, and their results were validated through molecular dynamics simulations [16]. Moreover, VX-680, an Aurora A kinase inhibitor developed through the use of CoMFA/CoMSIA-based 3D-QSAR models that were combined with molecular docking, exhibited significant potency and in vivo efficacy [21]. Other noteworthy instances include the creation of SYK kinase inhibitors through a blend of pharmacophore modelling, 3D-QSAR, and molecular dynamics, which produced hits with affinities comparable to fostamatinib [22], and the enhancement of p38 MAP kinase inhibitors using CoMFA and CoMSIA [23], which aided in the elucidation of structure–activity relationships within a series of active derivatives. These successful applications underscore the versatility and impact of 3D-QSAR methods in the rational design of selective kinase inhibitors.
Recognizing the potential of in silico molecular modelling techniques to substantially reduce time costs and expenses by efficiently narrowing synthetic optimization options, in this research we utilized our purine derivatives as a basis to develop structure-based 3D-QSAR models. These models were pivotal in designing and synthesizing a small set of new molecules in order to evaluate their inhibition on Bcr-Abl. This study focuses on two main approaches, CoMFA and CoMSIA, which quantitatively evaluate the connections between the molecular polarity/electrostatic characteristics and hydrophobicity of compounds. Furthermore, docking studies were performed on Bcr-AblWT to interpret the experimental results. The newly synthesized purine derivatives were also tested on imatinib-sensitive and -resistant CML cell lines. Docking and molecular dynamics studies were also conducted for the KCL22-B8 line, which is resistant to imatinib due to its Bcr-AblT315I mutation.
2. Results and Discussion
2.1. 3D-QSAR
An in silico study was carried out to organize previously observed structure–activity relationship data and to propose a systematic series of structural modifications that lead to a new family of purines. First, the biological activity (pIC50 = −logIC50) of a database of 58 purines (Table S1) was correlated with the steric and electrostatic potentials of these purines. The results can be visualized through coloured polyhedra that surround specific fragments of these compounds, which suggests that alterations in the volume and electronic properties of these compounds are necessary to enhance their biological activity. We present our findings in the next section.
2.1.1. Statistical Results
Table 1 summarizes the statistical outcomes of the CoMFA and CoMSIA models. We searched for optimal models using successive field combinations (refer to Table S2). A key parameter for assessing the statistical reliability of a QSAR model is the q2 value, which should exceed 0.5. This cross-validation coefficient indicates the internal predictive capability of the QSAR model. For CoMFA, the model that incorporated both field contributions (CoMFA-SE) achieved a value of 0.576. The CoMSIA model that considered steric, electrostatic, and hydrophobic features (CoMSIA-SEH) presented a q2 of 0.637. The r2test value, which was used to evaluate the external predictive capability of the model displayed in the summary of accepted models in Table 1, was 0.863 for CoMFA-SE, while the CoMSIA-SEH model presented a value of 0.842. The best models also presented a low standard error of the estimate (SEE) and a high non-cross-validation coefficient (r2) (0.939 for CoMFA and 0.934 for CoMSIA). The optimal number of components (N) was consistently low across all models (N = 6 for CoMFA and N = 6 for CoMSIA). Although some CoMSIA models reported higher q2 values, we chose a model with a greater number of fields to gain more insight into its structure–activity relationship.

Table 1.
Summary of accepted models.
Table 2 presents the external validation results for the CoMFA-SE and CoMSIA-SEH models (hereafter referred to as the ‘CoMFA’ and ‘CoMSIA’ models). Both models exhibited high r2 test values (0.863 and 0.842, respectively), which indicated satisfactory external predictive capacity. However, as noted by Golbraikh and Tropsha [24,25], while high q2 and r2 test values (conditions 1 and 2) are essential, they alone do not suffice for model validation. A reliable QSAR model should have a close alignment between predicted and the experimental activity, ideally approximating the line y = x. This was confirmed by meeting conditions [3a or 3b], [4a or 4b], [5a or 5b], and 6, as detailed in Table 2. Condition 7, known as the r2m metric, quantitatively assesses the closeness between the observed and predicted activity for the test set. In general, the CoMSIA model displayed better statistical parameters than the CoMFA model.

Table 2.
Overview of external validation parameters for CoMFA and CoMSIA.
The experimental and predicted activity values along with residuals for the most effective CoMFA and CoMSIA models are displayed in Table S3. All compounds exhibited low residuals with a balanced distribution of positive and negative values. Figure 2A,B depict graphs that compare the experimental and predicted activity, while Figure 2C,D illustrate the residuals and Figure 2E,F show the predictive graphics. The CoMFA and CoMSIA models demonstrated satisfactory predictive capabilities across the entire dataset (training and test sets) and exhibited robust predictive power.
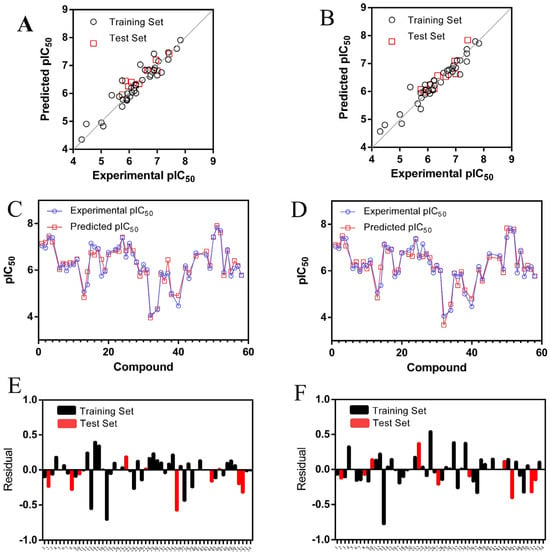
Figure 2.
Graphs comparing the experimental and predicted pIC50 values for molecules in the training and test sets are shown for the (A) CoMFA and (B) CoMSIA models. Residual plots are presented for (C) CoMFA and (D) CoMSIA. Predictions for each molecule in the entire dataset are illustrated for (E) CoMFA and (F) CoMSIA.
2.1.2. Outliers
In the CoMFA and CoMSIA models, molecules 5, 21, 44, 46, and 47 were identified as outliers. These compounds produced predictive values with substantial residuals (greater than one logarithmic unit). To evaluate the model’s robustness, a Y-randomization test was performed (Table S4) [26]. The dependent variable (biological activity) was randomly shuffled, and a new QSAR model was generated using the original independent variable matrix. If numerous randomizations yield negative or subpar q2 and r2ncv values (q2 < 0.5, r2ncv < 0.6), it confirms that the results from the final model formulation are not coincidental. In our analysis, the new QSAR models (after several iterations) exhibited low q2 and r2ncv values (see Table S4).
2.1.3. Applicability Domain
The applicability domain (AD) represents a theoretical region in chemical space that encompasses both the model descriptors and the modelled response. This allows for estimating the uncertainty in predicting a compound based on its similarity to the training compounds used in model development. In this study, we employed the method established by Roy et al. for AD determination [27]. This approach relies on the fundamental principles of standardization. The calculations were conducted using a freely available application on the author’s website, and indicated that all compounds fell within the applicability domain.
2.1.4. Contour Map Analysis
Contour maps represented by coloured polyhedra around the molecules were generated in our study to illustrate the steric, electrostatic, and hydrophobic properties of the molecules. Differently coloured polyhedra indicate regions with favourable or unfavourable molecular characteristics. Figure 3 and Figure 4 showcase the distinct maps for the most active compound (VII or 51 according to Table S3, pIC50 = 7.833, on the left) and the least active compound (32, pIC50 = 4.063, on the right).
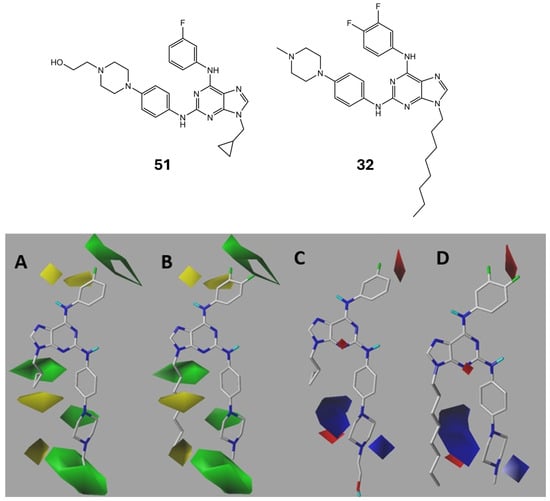
Figure 3.
CoMFA steric (A for 51 and B for 32) and electrostatic (C for 51 and D for 32) contour maps. Green: sterically favoured; yellow sterically disfavoured. Blue: electropositively favoured; red: electronegatively favoured.
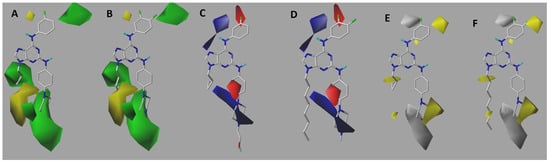
Figure 4.
CoMSIA steric (A = 51 and B = 32), electrostatic (C = 51 and D = 32), and hydrophobic (E = 51 and F = 32) contour maps. Green: sterically favoured; yellow: sterically disfavoured (A,B). Blue: electropositively favoured; red: electronegatively favoured (C,D). Yellow: hydrophobically favoured; grey: hydrophobically unfavourable (E,F).
According to the CoMFA-SE analysis, green and yellow polyhedra are depicted in the steric contour maps. The green polyhedra signify the favourability of high-volume substituents, whereas the yellow polyhedra indicate that bulky compounds are unfavourable. In Figure 3A, the most active molecule is presented, while Figure 3B depicts the least active molecule. The meta-position in the aniline fragment was favourable for the presence of bulky groups, whereas the ortho-position did not favour such bulky substitutions. Alkylation with a small group at the N-9 position is favourable. However, when the carbon chain becomes excessively large (7–8 atoms of carbon), it becomes disavowable. In the electrostatic contour map, red polyhedra signify the favourability of electronegative or electron-rich groups, whereas blue indicates the favourability of electron-deficient groups. A red polyhedron is visible at the meta- and para-positions of the aminophenyl fragment. This indicates that the electronegative functional groups on the ring are the foundation of its activity. The presence of the piperazine ring is favourable for both steric and electronic activities.
With respect to the contour maps obtained by the CoMSIA method, additional information can be extracted by considering not only the same fields seen in the CoMFA model, but also the hydrophobic fields. Steric contour maps are shown in Figure 4A,B. Overall, the conclusions drawn were similar to those obtained in the CoMFA model, with one difference. This map suggests that bulky substitutions must be present at the para-positions of the aniline fragment. Finally, Figure 4E,F show hydrophobic contour maps. The yellow colour indicates favourable hydrophobic substituent activity, whereas the silver-coloured polyhedron suggests favourability for the presence of hydrophilic groups. A yellow polyhedron was observed in piperazine, suggesting that, if this group was to be modified, it should be replaced by a ring with higher lipophilicity or hydrophobicity. Furthermore, a silver polyhedron was apparent in the vicinity of this ring. Therefore, in conjunction with the results of the steric map, the addition of hydrophilic and bulky groups to the ring was suggested. Alkylation at N-9 contributes to hydrophobicity. Therefore, cyclopropane was considered favourable. One alternative suggestion is to explore other groups that contribute to hydrophobicity, such as cyclopentane rings. This directly aligns with the conclusions derived from the steric contour maps.
In general, the results obtained from the CoMFA and CoMSIA methods are related to each other. This is conducive to the rational design of new structures.
2.2. Design and Synthesis
Based on our previous experimental results and those of the 3D-QSAR study, we used this information to design a small series of seven trisubstituted purine derivatives with different potencies in Bcr-Abl. The proposed structures for the synthesis conducted in this study are shown in Figure 5. To design new purine derivatives, the following is postulated:
- (i)
- Considering the CoMFA and CoMSIA contour maps (green and yellow polyhedra in Figure 3A,B and Figure 4A,B) and by comparing the results of both models for purine 51 (or VII, the most active compound of the series, IC50 = 0.015 μM), it can be determined that the methylcyclopropyl substituent is much more favourable than the n-hexyl substituent (less active compound, 32, IC50 = 86.46 μM). Therefore, it is important to maintain the substitution at N-9 with a methylcyclopropyl group (blue region in Figure 5);
- (ii)
- It is important to maintain the hydroxymethyl group attached to piperazine, because it is the most active compound (VII and VIII, Figure 1), and according to CoMSIA (Figure 4E), the presence of a hydrophilic group favours activity on Bcr-Abl. Likewise, it is interesting to explore the increase in the size of this fragment (green region in Figure 5) because we reported that this fragment is in the solvent-exposed region and is present in dasatinib;
- (iii)
- Based on the CoMSIA analysis, it is proposed that the presence of an electronegative atom in the phenylamino fragment at C-2 of purine would favour activity. That is, the isosteric replacement of benzene by pyridine was considered for these new purine derivatives (orange region in Figure 5);
- (iv)
- In Figure 4C,D, it is observed that the inhibitory activity of Bcr-Abl is stronger in the presence of electron-rich groups or electronegative atoms in the red polyhedra, as well as in the presence of electron-deficient substituents in the blue polyhedra. Thus, the substitution of the fluorine atoms in the meta- and/or para-positions of the C-6 phenylamino ring by atoms or groups with different electronic and steric properties will be considered (red region in Figure 5). These modifications will allow us to obtain more conclusive information regarding the optimal subtraction patterns of this moiety because this issue is still unclear.
In summary, this design incorporates some chemical modifications at the C-2, N-9, and C-6 positions in the purine ring that have not been previously explored by our group, nor have they been considered into other purine derivatives reported as Bcr-Abl inhibitors by other researchers.
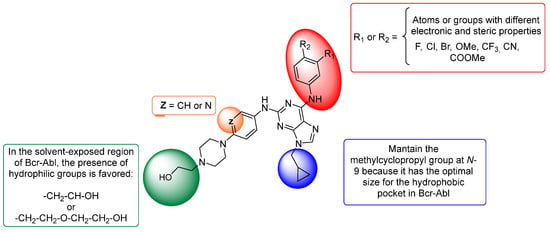
Figure 5.
Proposal of novel purine derivatives as potential inhibitors of Bcr-Abl based on 3D-QSAR models.
Subsequently, seven designed 2,6,9-trisubstituted purines were synthesized (7a–g) using the straightforward and efficient synthetic techniques outlined in Scheme 1 and previously reported by our group [11,12,13]. The synthesis began with 2,6-dichloropurine (1) as the starting material. Initially, compound 1 was alkylated with methylcyclopropyl bromide under basic conditions, which resulted in compound 2 (excluding its N-7 regioisomer) [11,12,13,28,29,30]. To yield 3a–f, regioselective nucleophilic substitution (SNAr) at the C-6 position was performed with the corresponding aniline using n-butanol as the solvent and N,N-diisopropylethylamine as the base. The reaction was carried out under reflux for 12 h to yield the desired intermediates. Following this, a Buchwald–Hartwig C-N coupling reaction at the C-2 of 3a–f with 6a–c was catalysed by palladium (II) and enhanced via microwave irradiation, which yielded the desired compounds 7a–g with yields ranging from 10% to 52%. Aniline derivatives 6a–c were synthesized beforehand from 4-nitro-1-fluorobenzene (4a) or 2-fluor-5-nitropyridine (4b) through two synthetic steps, as depicted in Scheme 1. Compounds 4a and 4b were substituted with piperazine derivatives to form 5a–c (93–98%), which were then quantitatively converted to the necessary aniline derivatives 6a–c. All compounds underwent purification through column chromatography, and their structures were confirmed via spectral analysis (1H NMR, 13C NMR, and HRMS; see Section 3 and Supplementary Materials).
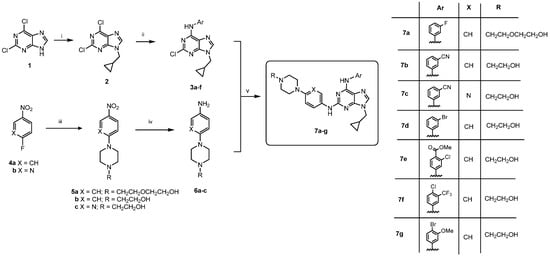
Scheme 1.
Reagents and conditions: (i) Methylcyclopropyl bromide, K2CO3, DMF, 12 h, r.t., 60%. (ii) Anilines, DIPEA, n-butanol, reflux, 12 h, 50–85%. (iii) 2-(2-(Piperazin-1-yl)ethoxy)ethan-1-ol or N-(2-hydroxyethyl)piperazine, K2CO3, DMF, r.t., 6 h, 93–98%. (iv) H2, Pd-C, ethanol, r.t., 4–6 h, quant. (v) K2CO3 2M, Pd(OAc)2, XantPhos, dioxane, MW, 170 °C, 10 min, 10–52%.
2.3. Kinase Inhibition and Structure–Activity Relationship
To align with this study’s objective of identifying novel Bcr-Abl inhibitors, the next step involved evaluating all synthesized compounds for their kinase inhibition capability. As shown in Table 3, compounds such as 7a, 7c, and 7d displayed enhanced activity relative to imatinib, as evidenced by their lower IC50 values (0.13, 0.19, and 0.21 μM compared to 0.33 μM, respectively). From a chemical standpoint, a structure–activity relationship (SAR) can be discerned for this target.

Table 3.
The IC50 values for the in vitro inhibition of Abl1 kinase by purines, along with the predicted pIC50 values derived from CoMFA and CoMSIA models and their corresponding residuals.
As indicated in Table 3, compound 7a exhibited the greatest inhibition among all the purine derivatives, achieving an IC50 of 0.13 μM on Bcr-Abl. However, it was less effective than its counterpart VII, which had an IC50 of 0.015 μM. This reduction in activity might be attributed to the elongation of the piperazine-bound 2-hydroxyethyl chain in the C-2-linked aminophenyl segment of the purine. This outcome was anticipated because this alteration was not considered in the purine base utilized in the 3D-QSAR study but was intentionally included in the design to explore this variable for potential future conjugations.
Likewise, from Table 3, it can also be observed that compound 7c presented an IC50 of 0.19 μM in Bcr-Abl, being more potent than its counterpart 7b, which had an IC50 of 0.46 μM. This increase in activity may be due to the isosteric replacement of the benzene ring by the pyridine of the phenylamino fragment attached to the C-2 of the purine. This result indicates that the modification suggested by the 3D-QSAR was accurate.
On the other hand, in general terms, it can be appreciated that, of these seven new purine derivatives, except for 7b, the most effective were typically those with a single substitution at the meta-position of the phenylamino group attached to the C-6 position of the purine. Likewise, to evaluate the effect of the proposed modifications in detail, it is necessary to compare each new purine with its respective counterpart in Figure 1. In the case of 7b, replacement of the methyl group by the hydroxyethyl group in the piperazine moiety reduced its potency against Bcr-Abl (IC50 = 0.46 μM) compared to its analogue X (IC50 = 0.070 μM). In addition, the change in F by CN in C-6 linked to the phenylamino fragment of the purine was not beneficial either, as evidenced by the comparison between 7b and VII. In the case of VIII and the new compounds 7e, 7f, and 7g, it was observed that it was not better to change the disubstitutions of the two fluorine atoms to other substituents, which would indicate that a significant increase in the size of the substituents was not favourable for the activity in Bcr-Abl. Therefore, it is necessary to revise the depth proposed by the 3D-QSAR in this region. This was confirmed by comparing VII with 7d, where it was evident that the exchange of a fluorine atom for a bromine atom decreased the compound’s inhibition.
Therefore, as can be seen in the above analysis, compounds 7a–g presented a varied inhibitory activity and, in some cases, were adjusted in accordance with what was predicted by the 3D-QSAR models, according to the low value of the residual, as was done for 7a (Table 3). This could be explained by the fact that the substitutions did not favour the interactions of these ligands in the Bcr-Abl binding pocket. This point will be addressed in subsequent molecular docking studies.
2.4. In Silico Studies for Validation Design of New Purines
To explain the different potencies of the designed purines as Bcr-Abl inhibitors and the different values of the experimental and predicted pIC50 (Table 3), docking studies were performed. The molecular docking protocol was initially validated through self-docking of the co-crystallized ligand for purvalanol B-Bcr-Abl (PDB ID: 6BL8) [31]. These purines were localised to the ATP-binding site (Figure S1), and their interaction patterns and binding energies were analysed systematically. As shown in Table 4, these purines exhibited distinct scoring values due to their structural diversity. In general, a clear trend can be observed among the pIC50 values and IFD scores, as shown in Figure 6. A linear relationship was observed for most of the designed compounds: 7a, 7b, 7c, 7d, and 7f. Only compounds 7e and 7 g deviated from this correlation, which is an interesting result because both compounds showed the highest predicted pIC50 values (Table 4) and the lowest potency on Bcr-Abl.

Table 4.
Comparison of the inducing fit docking score in Bcr-Abl and experimental pIC50 values.
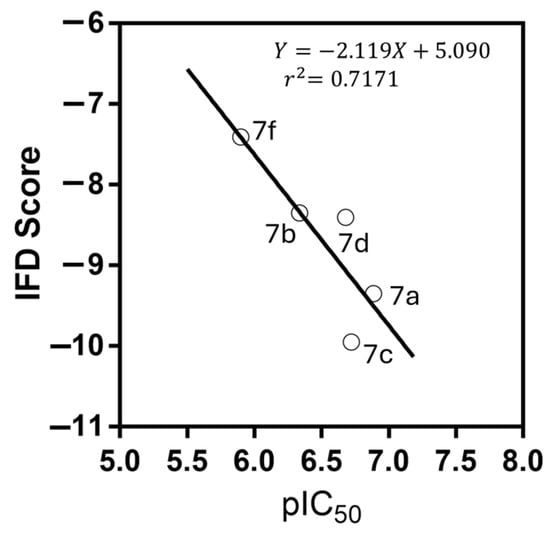
Figure 6.
Correlation between experimental pIC50 and IFD score (XP GScore, kcal/mol). The equation for the line is shown along with r2 for the linear regression. Compounds 7e and 7g did not exhibit this trend.
Moreover, the binding interactions and orientation patterns of purine derivatives are critical to their Bcr-Abl inhibition. As previously reported [12], multiple interaction networks were observed between 7a and 7g within the ATP-binding pocket of this kinase (Figure 7A–G). 7b and 7c exhibited similar docking behaviour due to the only structural difference between them being the aminophenyl or aminopyridine fragment linked at C-2 of the purine. In contrast, 7a displays a slightly different interaction profile than the other purines because the presence of the phenyl-piperazine-hydroxyethyl fragment is spatially displaced, which promotes the formation of a new hydrogen bond with GLU329. In 7g, the para-bromo atom in the phenylamino fragment at C-6 is exposed to the solvent, as is the case with the para-chlorine atom in 7f. This suggests that steric factors may limit the coupling values, despite retention of the double interaction with MET318. In addition, 7d formed more hydrogen bonds than 7e, which agrees with the experimental differences in the IC50 values for Bcr-Abl. This behaviour was attributed to the solvent exposure of the aniline fragment. The groups in 7e were more exposed to the solvent than those in 7d, as the bromine group in 7d is non-polar while the groups in 7e are polar. This caused the aromatic ring in 7d to shift away from the binding site, hindering the potential formation of a π-stacking interaction with PHE317. Although 7d could form more favourable interactions than 7e, the polar solvent affinity effect leads the docking score to favour 7e.
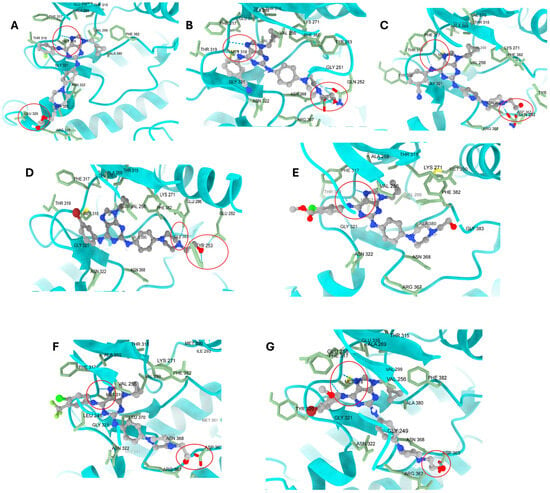
Figure 7.
Visualization of all poses obtained from molecular docking between Bcr-Abl and 7a–g. (A) for 7a; (B) for 7b; (C) for 7c; (D) for 7d; (E) for 7e; (F) for 7f; and (G) for 7g. All compounds exhibited double interactions with the MET318 backbone, except for 7d. In the hydroxyethyl fragment, it was observed that, in nearly all cases, it formed a hydrogen bond with ASP363, GLN252, and GLU329, generating an anchoring effect. The exception was 7e, where this fragment did not interact with any other amino acid. The most important interactions are enclosed in a red circle.
A comprehensive structural analysis corroborates the enhanced activity of our lead compounds, specifically 7a and 7c, in comparison to imatinib. The CoMFA and CoMSIA contour maps indicate that the incorporation of a cyclopropylmethyl group at N-9 in compound 7a corresponds with sterically favorable regions, thereby augmenting the compound’s binding affinity. Additionally, the isosteric substitution of the phenyl ring with a pyridine ring at the C-2 position in compound 7c enhances its electrostatic complementarity within the Bcr-Abl active site. Molecular docking analysis reveals that these compounds sustain critical interactions with MET318 and establish additional hydrogen bonds—such as with GLU329 in 7a and ASP363 in 7c—and thus stabilize their binding conformations. The increased potency of 7a and 7c can be attributed to their improved steric compatibility and electronic interactions, as predicted by the 3D-QSAR models and validated by docking. These findings provide a mechanistic rationale for their superior activity.
2.5. Cytotoxic Studies on CML Cell Lines Sensitive and Resistant to Imatinib
Initially, all compounds under investigation were evaluated for their cytotoxic effects on CML cell lines with Bcr-AblWT rearrangement and their sensitivity to imatinib, including K562 and KCL22 (CML in blast crisis), as well as on non-cancerous HEK-293T cells (Table 5). The cytotoxic effects of the 2,6,9-trisubstituted purines demonstrated variability that was contingent upon the cell type and the specific compounds being assessed. Among the cell lines, K562 was the most responsive, with all compounds showing GI50 values ranging from 0.30 to 4.04 μM, whereas KCL22 was the least responsive, with only five compounds exhibiting GI50 values below 10 μM. Compound 7c emerged as the most effective derivative, with GI50 values of 0.30 and 1.54 μM for the K562 and KCL22 cell lines, respectively. Interestingly, 7c demonstrated comparable potency to imatinib in K562 cells but was six times more potent than imatinib in KCL22 cells. It should also be noted that 7c is more potent in K562 cells than any of the 58 previously studied purines [11,12,13]. In addition, by comparing 7c and 7b, it was possible to establish that the substitution of the pyridylamino fragment by the phenylamino fragment at the C-2 position of purine increased the cytotoxicity in both cells, which was consistent with enhanced Abl inhibition. Compound 7c was 13-fold more potent than 7b on K562 cells and approximately 8.5-fold more potent on KCL22 cells.

Table 5.
In vitro cytotoxicity is expressed as GI50 values of purines on CML cell lines and non-neoplastic cells (HEK-293T) along with corresponding selectivity index (SI) values.
According to what has been previously analysed, it should be considered that, although it is important that these purines inhibit the growth of CML cell lines containing Bcr-AblWT, it is equally interesting to study the response of these compounds in a cell line that has the Bcr-AblT315I mutation, KCL22-B8. This is important because this mutation is responsible for resistance to imatinib [32] and nilotinib [33]. Therefore, the KCL22-B8 cell line provides further evidence regarding the future of these purines. Looking at the results in Table 5, there was a lower sensitivity to the synthesized compounds compared to the cell lines without the mutation. However, compounds 7f, 7e, and 7g presented GI50 values of 13.8, 16.56, and 15.43 μM, respectively, which are lower values than that presented by imatinib. These results suggested that 7f, 7e, and 7g could also inhibit Bcr-AblT315I.
Conversely, it is important to examine the results obtained from the HEK293T cell line, a non-neoplastic cell line, as it serves as a control in cell cytotoxicity studies, facilitating the comparison of its response with that of cancer cells. In this context, it is expected that the GI50 values in the HEK-293T cells will be elevated, which would indicate a lower sensitivity to the compound and, therefore, lower toxicity in healthy cells. In this study, compound 7c showed the highest value (GI50 > 25 μM), followed by 7b with a GI50 of 14.75 μM and 7a with a GI50 of 6.85 μM. These results suggest that, among the compounds tested, 7c was the least cytotoxic to non-tumour cells. In cytotoxicity assays, the selectivity index (SI) is an important measure that helps to evaluate the specificity of a compound to preferentially affect tumour cells versus non-tumour cells. The SI was calculated as the ratio of the GI50 value in a non-tumour cell line (such as HEK-293T) to the value in a cancer cell line (K562, KCL22, or KCL22-B8). The SI values of the studied purines are listed in Table 6. Based on these values, compound 7c stands out for its high SI towards the K562 cell line (SI value = 83), which is higher than that of imatinib (SI value = 43). Other compounds showed moderately lower SI values than imatinib (5.65–1.1). For KCL22, compound 7c again stood out, with an SI value of 16, which was higher than that of imatinib (SI value = 1.1). For 7a and 7d, the SI values were 2.9 and 2.7. On the other hand, in the KCL22-B8 cell line, the SI values were lower than those of other cell lines and, for almost all derivatives, those for purine were lower than those for imatinib, except for 7c, which had an SI value = 0.8.

Table 6.
Binding free energies (ΔG) from MMGBSA calculations for Bcr-AblWT and Bcr-AblT315I complexes.
Finally, it should be noted that, although these newly designed purines did not show higher potency on Bcr-Abl than previous compounds, it is interesting to consider that some purines are capable of inhibiting growth in CML cells that are sensitive and resistant to imatinib and have better SI values than imatinib, such as 7c and 7a (for K562 and KCL22) and 7b (for KCL22-B8).
2.6. In Silico Studies for Bcr-AblT15I
To elucidate the antiproliferative effects of these purines on KCL22 and KCL22-B8 cells, we posited that they were connected to the presence of Bcr-Abl in either the wild-type or T315I mutant states, and then determined the degree of inhibition by these purines. To verify this hypothesis, we first conducted molecular docking studies on Bcr-AblT315I (PDB ID: 4TWP [34]) for 7e and 7f, the most active compounds in KCL22-B8 cells. The binding affinity energy for 7e was −10.186 kcal/mol and for 7f it was −10.457 kcal/mol; these energies were consistent with the GI50 values (15.43 for 7e and 13.80 for 7f μM). This result is interesting because both compounds were less active in Bcr-AblWT inhibition, and it could be hypothesized that they are more active in Bcr-AblT15I.
Figure 8A,C depict 7f and 7e in Bcr-AblWT, while Figure 8B,D show their docking in Bcr-AblT315I. In both cases, interactions with MET318 were retained, despite the mutation. Both ligands also establish new interactions with hydroxyethyl fragments. The pose of 7e shifts compared to its position in Bcr-AblWT due to the rotational flexibility of the ligand fragment and its polar end, which anchors with polar residues such as ASP325.
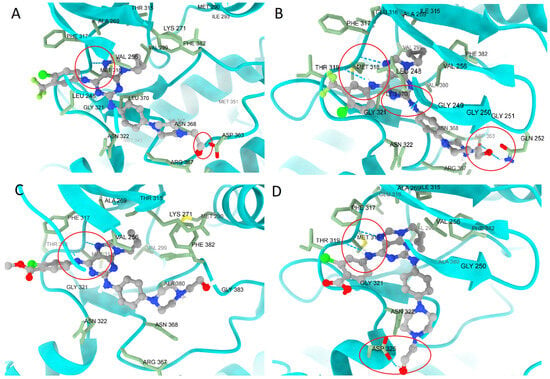
Figure 8.
Comparison of the poses of (A) 7f in Bcr-AblWT, (B) Bcr-AblT315I, (C) 7e in Bcr-AblWT, and (D) Bcr-AblT315I. In both cases, the ligands remained largely unaffected by the T315I mutation, which suggests stable docking in the presence of this mutation. Additionally, each ligand gains a new hydrogen bond: 7f interacts with GLN252, LEU248, and ASP353, while 7e interacts with ASP325. The most important interactions are enclosed in a red circle.
Molecular dynamics (MD) studies were conducted to investigate, in depth, the fact that 7e and 7f are better inhibitors of Bcr-AblT315I than Bcr-AblWT. With this goal, the stability of both ligands in complexes with WT and mutated kinases were evaluated, and imatinib was also included using NAMD 3.0.1 under the conditions described in the Methods section [35]. To assess the structural stability of each system, the root-mean-square deviation (RMSD) values for the ligand atoms were calculated over 200 ns production trajectories. These analyses provide insights into global conformational changes and the extent of equilibration over time.
Figure S2 shows the RMSD profiles of the ligands in their respective complexes over the 200 ns MD simulations. The results indicated that the ligand stability varies depending on the protein state, with distinct RMSD trends being observed for imatinib, 7f, and 7e in both Bcr-AblWT and Bcr-AblT315I. The RMSD fluctuations ranged from 1.5 to 3 Å for these compounds. The RMSD values of the proteins in their respective complexes are shown in Figure S3. Fluctuations occurred in the range of 2–5 Å, with the largest fluctuation being observed for the protein in the imatinib–Bcr-AblWT complex. The smallest fluctuation was found in the 7e complex, both in the WT and mutated forms.
Additionally, MMGBSA calculations were performed throughout the production trajectory to estimate the ligand-binding free energies using the MolAICal v.1.3 software [36]. This approach enabled a comparative assessment of how the T315I mutation affects ligand affinity, revealing differences in the overall binding energy contributions and interaction patterns within the active site. From Table 6, we can observe that imatinib exhibits a better free energy in the WT form (−38.08 kcal/mol) than in the mutated form (−28.18 kcal/mol), which is in agreement with its IC50 values. In contrast, the 7e and 7f showed higher binding energies in the mutant form (−33.52 and −30.06 kcal/mol, respectively), which is related to their GI50 values on KCLL22-B8 cells.
2.7. Calculated Physicochemical Properties and ADME Parameters
In the drug discovery and development process, it is essential to consider both pharmacological characteristics and pharmacokinetic profiles. Thus, predicting or determining these pharmacokinetic properties—encompassing administration, distribution, metabolism, and excretion (ADME)—is crucial for optimizing a bioactive compound until it qualifies for preclinical studies [37]. The SwissADME online platform (http://www.swissadme.ch/index.php, accessed on 1 March 2025) was utilized to evaluate the physicochemical properties of purine derivatives 7a–g in accordance with Lipinski’s rules. As indicated in Table 7, among the seven purine derivatives that we analysed, only 7b closely meets all criteria for favourable permeability and bioavailability, based on its molecular weight (MW), hydrogen bond donor (HBD), hydrogen bond acceptor (HBA), and cLogP values [38]. Furthermore, following Veber’s rules, compounds 7b–d and 7f–g possess a topological polar surface area (TPSA) and a number of rotatable bonds (NRB) that are below the thresholds of 140 Å2 and ≤10 NRB, respectively (Table 7) [39]. These metrics suggest that 7b and 7c are likely to penetrate cell membranes effectively and exhibit good oral absorption, in line with the Lipinski and Veber rules. Moreover, all purine derivatives exhibit high oral gastrointestinal (GI) absorption and do not penetrate the blood–brain barrier (BBB), which is favourable for minimizing potential central nervous system side effects. All compounds are substrates of P-glycoprotein, and only two of them—compounds 7b and 7c—show a high risk of hERG inhibition. Additionally, the SwissADME platform provided a bioavailability radar plot that allowed us to assess parameters such as flexibility (FLEX), lipophilicity (LIPO), solubility (INSOLU), size (SIZE), polarity (POLAR), and saturation (INSATU). If all parameters fall within the desired range (pink region), the compound is expected to demonstrate good oral absorption. Figure 9 illustrates that 7b and 7c fulfil these criteria.

Table 7.
Calculated physicochemical properties, ADME parameters, and toxicity predicted for 7a–g.
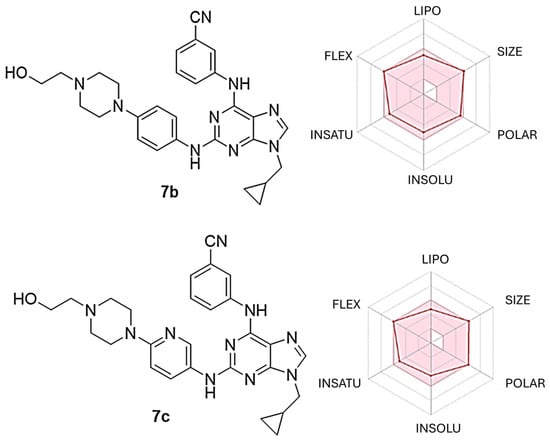
Figure 9.
Radar plot illustrating the predicted bioavailability of compounds 7b and 7c. The pink region represents the optimal value range for each property associated with oral bioavailability, while the predicted properties are shown by red lines.
3. Materials and Methods
3.1. 3D-QSAR Studies
3.1.1. Selection of Conformers and Molecular Alignment
CoMFA and CoMSIA studies were conducted utilizing Sybyl X v.1.2 software (Tripos International, St. Louis, MS, USA). To identify the most suitable conformers for each molecule, every compound was drafted in ChemDraw v. 15.1.0 (PerkinElmer, Waltham, MA, USA) and subjected to initial geometry optimization via MM2 molecular mechanics, which was implemented in ChemBio3D v.15.1.0 software. The mol2 structures were imported into Sybyl, where MMFF94 charges were assigned to each atom. Superimposed molecules were minimized using Powell’s method [40].
3.1.2. CoMFA and CoMSIA Field Calculation
To derive the CoMFA and CoMSIA descriptor fields, the aligned training set molecules were positioned within a three-dimensional cubic lattice, with a grid spacing of 2 Å in the x, y, and z dimensions to encompass the entire set. The CoMFA steric and electrostatic field energies were calculated utilizing a sp3 carbon probe atom with a Van der Waals radius of 1.52 Å and a charge of +1.0. The cutoff values for both the steric and electrostatic fields were set at 30.0 kcal/mol. For CoMSIA analysis, standard settings (probe with charge +1.0, radius 1 Å, hydrophobicity +1.0, H-bond donating +1.0, and H-bond accepting +1.0 [41]) were employed to compute five distinct fields: steric, electrostatic, hydrophobic, donor, and acceptor. The Gaussian-type distance dependence was utilized to evaluate the relative attenuation of the field position of each atom in the lattice, which resulted in a smoother sampling of the fields surrounding the molecules compared to CoMFA. An attenuation factor α of 0.3 was established as the default value.
3.1.3. Internal Validation and Partial Least Squares (PLS) Analysis
PLS analysis was implemented to establish a linear correlation between the CoMFA and CoMSIA descriptors (independent variables) and the activity values (dependent variables) [42]. To identify the most effective model, cross-validation analysis was executed using the leave-one-out (LOO) method (and sample distance PLS [SAMPLS]) to generate the square of the cross-validation coefficient (q2) and optimal number of components (N). Non-cross validation was performed with a column filter value of 2.0 to expedite the analysis and diminish noise. q2, which is a measure of the internal quality of the models, was obtained using Equation (1):
where , , and are observed, mean, and predicted activity in the training set, respectively.
3.1.4. External Validation
The models were subjected to external validation criteria based on the test proposed by Golbraikh and Tropsha [24,25], which considers a QSAR model predictive if the following conditions are met:
It has been demonstrated [32] that all aforementioned criteria are essential for a comprehensive evaluation of a QSAR model’s predictive capability. Furthermore, the external predictive power of the developed 3D-QSAR models employing the test set was analysed by accounting for metrics, as shown below [27]:
where and are the squared correlation coefficients between the observed and predicted activities of the test set with and without the (0,0) intercept, respectively. For a significant external model validation, the value of should be greater than 0.5.
3.1.5. Applicability Domain Calculation
The AD was evaluated using a straightforward standardization method reported by Roy et al. [43]. First, each descriptor “i” for each compound “k” was standardized (Sik). Each compound must have a maximum value of [Si]max(k) ≤ 3. In the case that [Si]max(k) > 3 and its minimum value [Si]min(k) < 3, then the Snew(k) parameter must be calculated and has to fulfil the condition Snew(k) = , where is the mean of Sik values for compound k and is the standard deviation for such values. The software is freely accessible on the authors’ website: https://teqip.jdvu.ac.in/QSAR_Tools/ (accessed on 17 May 2025).
3.2. Chemistry
All reagents and chemicals utilized in the chemical synthesis of intermediates and final compounds were procured from Sigma-Aldrich (St. Louis, MO, USA). Intermediates previously published were synthesized according to established procedures, as referenced in the relevant literature. HPLC-grade solvents were acquired from Merck (Darmstadt, Germany) and Fisher Scientific (Waltham, MA, USA).
The melting points (m.p.) of all synthesized compounds were measured uncorrected using a Kofler Thermogerate apparatus (Reichert, Werke A.G., Wien, Vienna, Austria). The 1H and 13C nuclear magnetic resonance (NMR) spectra of the synthesis intermediates and final compounds were recorded on BRUKER AVANCE III HD-400 (400 MHz (1H) and 100 MHz (13C)) and 200 MHz [200 MHz (1H) and 50 MHz (13C)] spectrometers. The compounds were dissolved in CDCl3 or DMSO-d6, which tetramethylsilane (TMS) being utilized as an internal standard. The chemical shifts in the NMR spectra are expressed in parts per million (ppm) and coupling constants (J) are given in hertz (Hz) where applicable. The multiplicity observed in the 1H NMR spectra is indicated as s (singlet), d (doublet), t (triplet), and dd (doublet doublet). High-resolution mass spectra (HRMS) or mass spectra (MS) were obtained using a Q-TOF mass spectrometer (Synapt G2-Si, Waters, Milford, MA, USA) equipped with an electrospray ionization (ESI) source. Briefly, the measurement process involved injecting an acetonitrile solution of the sample directly into the ESI source using a syringe pump at a flow rate of 10 µL/min. Positive-mode molecular ions were detected on the Q-TOF mass spectrometer. Reaction monitoring and verification of the purity of the synthesized products post-column chromatography were conducted through thin-layer chromatography (TLC) using Merck GF-254 type 60 silica gel. The purity of the final compounds for biological assays was established via TLC, HRMS, and HPLC.
3.2.1. General Procedure for the Synthesis of Intermediates 2a–2a′
A solution of 2,6-dichloro-9H-purine (1, 1.0 g, 5.29 mmol), methylcyclopropyl bromide (0.79 g, 5.82 mmol), and K2CO3 (2.19 g, 15.87 mmol) in DMF (10 mL) was prepared, and the mixture was stirred at room temperature for 12 h. The mixture was then filtered and evaporated. The residue was purified by silica gel chromatography using a polar mobile phase (EtOAc/hexane, 1:1) to yield products 2a and 2a′.
2,6-Dichloro-9-(cyclopropylmethyl)-9H-purine (2a). White solid, yield 60%. The analytical data corresponded to the literature [13].
2,6-Dichloro-9-(cyclopropylmethyl)-7H-purine (2a′). White solid, yield 18%, and analytical data corresponded to the literature [13].
3.2.2. General Procedure for the Synthesis of Intermediates 3a–f
A solution of 2a (200 mg, 0.83 mmol), the corresponding aniline (0.90 mmol), and DIPEA (0.3 mL, 1.65 mmol) in n-butanol (5 mL) was prepared and stirred at 110 °C for 12 h. To halt the reaction, ice water was added, and the mixture was washed twice with water, which resulted in solid formation. If two phases were noted, extraction was performed with EtOAc (3 × 15 mL), and the organic layer was dried with anhydrous Na2SO4 before concentration. The residue was purified via silica gel chromatography using a polar mobile phase (AcOEt/hexane 60:40) to acquire products 3a–f.
2-Chloro-9-(cyclopropylmethyl)-N-(3-fluorophenyl)-9H-purin-6-amine (3a): White solid, yield 85%. The analytical data corresponded to the literature [13].
3-((2-Chloro-9-(cyclopropylmethyl)-9H-purin-6-yl)amino)benzonitrile (3b): White solid, yield 76%. The analytical data corresponded to the literature [12].
N-(3-bromophenyl)-2-chloro-9-(cyclopropylmethyl)-9H-purin-6-amine (3c): Brown solid, yield 54%, m.p. 127.3–129.5 °C. 1H NMR (400 MHz, chloroform-d) δ 8.17 (s, 1H, NH), 7.96 (s, 1H, CH), 7.90 (s, 1H, CH), 7.73 (m, 1H, CH), 7.22 (d, J = 4.7 Hz, 2H, 2 CH), 4.02 (d, J = 7.3 Hz, 2H, CH2), 1.30 (m, 1H, CH), 0.69 (t, J = 6.9 Hz, 2H, CH2), 0.45 (t, J = 5.2 Hz, 2H, CH2). 13C NMR (101 MHz, chloroform-d) δ 153.91, 152.02, 150.95, 140.86, 139.51, 130.37, 126.79, 123.03, 122.60, 119.22, 118.75, 48.74, 11.03, 4.40 (2C).
Methyl-2-chloro-4-((2-chloro-9-(cyclopropylmethyl)-9H-purin-6-yl)amino)benzoate (3d): white solid, yield 30%, m.p. 127.3–131.5 °C. 1H NMR (200 MHz, DMSO-d6) δ 10.74 (s, 1H, NH), 8.46 (s, 1H, CH), 8.25 (d, J = 2.0 Hz, 1H, CH), 7.97 (dd, J = 8.7, 2.1 Hz, 1H, CH), 7.86 (d, J = 8.7 Hz, 1H, CH), 4.04 (d, J = 7.2 Hz, 2H, CH2), 3.83 (s, 3H, CH3), 1.46–1.14 (m, 1H, CH), 0.64–0.36 (m, 4H, 2 CH2). 13C NMR (101 MHz, DMSO-d6) δ 165.28, 152.29, 152.11, 151.79, 143.86, 143.48, 133.38, 132.54, 123.18, 121.99, 119.76, 118.82, 52.68, 48.27, 11.60, 4.30 (2C).
2-Chloro-N-(4-chloro-3-(trifluoromethyl)phenyl)-9-(cyclopropylmethyl)-9H-purin-6-amine (3e): white solid, yield 60%, m.p. 103.5–105.2 °C. 1H NMR (400 MHz, chloroform-d) δ 8.70 (s, 1H, NH), 8.10 (d, J = 2.7 Hz, 1H, CH), 8.04–7.96 (m, 1H, CH), 7.91 (s, 1H, CH), 7.45 (d, J = 8.7 Hz, 1H, CH), 4.01 (d, J = 7.4 Hz, 2H, CH2), 1.29 (dq, J = 13.8, 6.9, 5.9 Hz, 1H, CH), 0.67 (m, 2H, CH2), 0.44 (m, 2H, CH2). 13C NMR (101 MHz, chloroform-d) δ 153.89, 151.80, 151.06, 140.88, 137.39, 132.00 (2C), 128.72 (d, 2JC-F = 31.3 Hz), 126.06–119.06 (dd, 1JC-F = 216.6 Hz) 123.86, 118.93 (q, 3JC-F = 5.7 Hz) 48.82, 10.99, 4.39 (2C).
N-(4-bromo-3-methoxyphenyl)-2-chloro-9-(cyclopropylmethyl)-9H-purin-6-amine (3f): Yellow solid, yield 83%, m.p. 115.6–117.2 °C. 1H NMR (400 MHz, chloroform-d) δ 8.20 (s, 1H, NH), 7.94–7.86 (m, 2H, 2 CH), 7.45 (d, J = 8.5 Hz, 1H, CH), 7.01 (dd, J = 8.6, 2.4 Hz, 1H, CH), 4.04 (d, J = 7.2 Hz, 2H, CH2), 3.96 (s, 3H, CH3), 1.32 (tdd, J = 10.7, 9.5, 7.9, 4.8 Hz, 1H, CH), 0.74–0.65 (m, 2H, CH2), 0.45 (dd, J = 5.1 Hz, 2H, CH2). 13C NMR (101 MHz, chloroform-d) δ 156.13, 153.77, 151.94, 150.82, 140.67, 138.88, 132.99, 119.17, 112.81, 105.37, 104.51, 56.20, 48.74, 11.03, 4.39 (2C).
3.2.3. General Procedures for the Synthesis of Intermediates 5a–c
A solution of 4a or 4b (7.0 mmol), 2-(2-(Piperazin-1-yl)ethoxy)ethan-1-ol or 2-hydroxyethyl piperazine (7.1 mmol), and K2CO3 (2.9 g, 21.1 mmol) in DMF (10 mL) was prepared and stirred at room temperature for 12 h. The reaction mixture was filtered and evaporated. The solid was subsequently recrystallized in MeOH to yield products 5a–c.
2-(2-(4-(4-Nitrophenyl)piperazin-1-yl)ethoxy)ethan-1-ol (5a): Orange solid, yield 98%, m.p. 84.5 °C. 1H NMR (200 MHz, chloroform-d) 8.19–8.02 (m, 2H, 2 CH), 6.90–6.75 (m, 2H, 2 CH), 3.71 (m, 4H, 2 CH2), 3.63 (m, 2H, 2 CH2), 3.53–3.38 (m, 4H, 2 CH2), 2.67 (q, J = 5.1 Hz, 6H, 3 CH2). 13C NMR (50 MHz, chloroform-d) δ 154.77, 138.49, 125.88 (2C), 112.70 (2C), 72.40, 67.58, 61.85, 57.76, 52.74 (2C), 46.71 (2C).
2-(4-(4-Nitrophenyl)piperazin-1-yl)ethan-1-ol, (5b): Yellow solid, yield 97%. The analytical data corresponded to the literature [11].
2-(4-(5-Nitropyridin-2-yl)piperazin-1-yl)ethan-1-ol (5c): Yellow solid, yield 98%, m.p. 112.0–115.1 °C. 1H NMR (200 MHz, chloroform-d) δ 9.01 (d, J = 2.8 Hz, 1H, CH), 8.19 (dd, J = 9.5, 2.8 Hz, 1H, CH), 6.57 (d, J = 9.5 Hz, 1H, CH), 4.11 (m, 1H, OH), 3.85–3.76 (m, 4H, 2 CH2), 3.69 (t, J = 5.3 Hz, 2H, CH2), 2.63 (dt, J = 5.0, 2.5 Hz, 6H, 3 CH2). 13C NMR (50 MHz, chloroform-d) δ 160.32, 146.40, 135.05, 132.99, 104.54, 59.38, 57.91, 52.53 (2C), 44.87 (2C).
3.2.4. General Procedures for the Synthesis Compounds 6a–c
A solution of 5a–c (500 mg, 1.69 mmol) and 10% Pd/C (34 mg) in ethanol (10 mL) was prepared under a hydrogen atmosphere. After 12 h, the reaction mixture was filtered through celite and concentrated to yield 6a–c.
2-(2-(4-(4-Aminophenyl)piperazin-1-yl)ethoxy)ethan-1-ol (6a): Purple solid, yield 100%, m.p. 126.3–127.8 °C. 1H NMR (400 MHz, chloroform-d) δ 6.75 (d, J = 8.2 Hz, 2H, 2 CH), 6.58 (d, J = 8.2 Hz, 2H, 2 CH), 3.67–3.62 (m, 4H, 2 CH2), 3.58–3.54 (m, 2H, CH2), 3.06–2.99 (m, 4H, 2 CH2), 2.67–2.61 (m, 4H, 2 CH2), 2.59 (t, J = 5.5 Hz, 2H, CH2). 13C NMR (101 MHz, chloroform-d) δ 144.35, 140.24, 118.71 (2C), 116.20 (2C), 72.43, 67.62, 61.93, 57.91, 53.51 (2C), 50.66 (2C).
2-(4-(4-aminophenyl)piperazin-1-yl)ethan-1-ol (6b): Purple solid, yield 98%. The analytical data corresponded to literature [11].
2-(4-(4-aminophenyl)piperazin-1-yl)ethan-1-ol (6c): Purple solid, yield 98%, m.p. 129.3–133.5 °C. 1H NMR (200 MHz, chloroform-d) δ 7.79 (d, J = 2.9 Hz, 1H, CH), 6.98 (dd, J = 8.8, 3.0 Hz, 1H, CH), 6.57 (d, J = 8.8 Hz, 1H, CH), 3.66 (t, J = 5.4 Hz, 2H, CH2), 3.45–3.31 (m, 4H, 2 CH2), 3.08 (s, 2H, NH2 and OH), 2.61 (dt, J = 8.9, 5.2 Hz, 6H, 3 CH2). 13C NMR (50 MHz, chloroform-d) δ 154.40, 135.28, 134.72, 126.07, 108.45, 59.42, 57.79, 52.79 (2C), 46.77 (2C).
3.2.5. General Procedures for the Synthesis of Final Compounds for Biological Assays 7a–g
A solution of 3a–f (200 mg, 0.49 mmol) and 6a–c (132 mg, 0.59 mmol) in dioxane (5 mL) was stirred with Pd(OAc)2 (11 mg, 0.05 mmol, 0.1 eq), Xantphos (86 mg, 0.14 mmol, 0.3 eq), and 2 M K2CO3 (aq) (0.75 mL, 0.15 mmol, and 3 eq) at 170 °C for 10 min in a microwave reactor. After cooling to room temperature, the mixture was filtered through celite, and the filtrate was diluted with dichloromethane and concentrated to dryness. The residue was purified through silica gel chromatography utilizing a polar mobile phase (methanol/dichloromethane, 5:95, 10:90) to achieve the final product. The Rf values of each compound were determined using a 5:95 mobile phase. All compounds were >98% pure as determined by HPLC analysis (refer to Supporting Information).
2-(2-(4-(4-((9-(Cyclopropylmethyl)-6-((3-fluorophenyl)amino)-9H-purin-2-yl)amino)phenyl)piperazin-1-yl)ethoxy)ethan-1-ol (7a): Red solid, yield 52%, m.p. 60.3–61.8 °C, Rf = 0.4. 1H NMR (400 MHz, chloroform-d) δ 8.11 (s, 1H, NH), 7.89 (d, J = 11.5 Hz, 1H, CH), 7.69 (s, 1H, CH), 7.53 (d, J = 8.4 Hz, 2H, 2 CH), 7.30–7.17 (m, 2H, 2 CH), 7.01 (s, 1H, NH), 6.93 (d, J = 8.4 Hz, 2H, 2 CH), 6.73 (t, J = 8.3 Hz, 1H, CH), 3.93 (d, J = 7.2 Hz, 2H, CH2), 3.77–3.67 (m, 4H, 2 CH2), 3.64 (t, J = 4.3 Hz, 2H, CH2), 3.20 (t, J = 4.8 Hz, 4H, 2 CH2), 2.71 (t, J = 5.0 Hz, 4H, 2 CH2), 2.66 (t, J = 5.6 Hz, 2H, CH2), 1.33–1.24 (m, 1H, CH), 0.67 (d, J = 7.9 Hz, 2H, CH2), 0.45 (d, J = 5.1 Hz, 2H, CH2). 13C NMR (101 MHz, chloroform-d) δ 163.06 (d, 1JC-F = 243.6 Hz), 156.82, 151.84, 151.41, 146.86, 140.85 (d, 3JC-F = 11.0 Hz), 137.92, 133.07, 129.73 (d, 4JC-F = 9.7 Hz), 121.24 (2C), 117.15 (2C), 115.15 (d, 3JC-F = 13.1 Hz), 109.28 (d, 2JC-F = 21.6 Hz), 107.31 (d, 2JC-F = 26.8 Hz), 72.48, 67.64, 61.94, 57.93, 53.42 (2C), 49.76 (2C), 48.15, 11.05, 4.30 (2C). ESI/MS for (C29H35FN8O [M + H]+): Calcd: 547.3256. Found: 547.2947.
3-((9-(Cyclopropylmethyl)-2-((4-(4-(2-hydroxyethyl)piperazin-1-yl)phenyl)amino)-9H-purin-6-yl)amino)benzonitrile (7b): Brown solid, yield 10%, m.p. 95.2–99.7 °C, Rf = 0.3. 1H NMR (400 MHz, chloroform-d) δ 8.45 (s, 1H, NH), 8.37 (s, 1H, NH), 7.73 (d, J = 6.6 Hz, 2H, 2 CH), 7.53 (d, J = 8.4 Hz, 2H, 2 CH), 7.32 (t, J = 7.8 Hz, 1H, CH), 7.28 (d, J = 3.1 Hz, 1H, CH), 7.15 (s, 1H, CH), 6.97 (d, J = 8.5 Hz, 2H, 2 CH), 3.94 (d, J = 7.2 Hz, 2H, CH2), 3.69 (t, J = 5.4 Hz, 2H, CH2), 3.19 (t, J = 4.8 Hz, 4H, 2 CH2), 3.06 (s, 1H, OH), 2.69 (t, J = 4.9 Hz, 4H, 2 CH2), 2.62 (t, J = 5.4 Hz, 2H, CH2), 1.31 (td, J = 8.1, 4.2 Hz, 1H, CH), 0.68 (d, J = 7.5 Hz, 2H, CH2), 0.46 (d, J = 5.1 Hz, 2H, CH2). 13C NMR (101 MHz, chloroform-d) δ 156.74, 151.60, 146.97, 140.21, 138.21, 132.76, 129.48, 126.02, 123.76, 122.67, 121.15 (2C), 119.06, 116.99 (3C), 112.66, 59.51, 57.90, 53.03 (2C), 49.90 (2C), 48.20, 30.92, 11.00, 4.32 (2C). ESI/MS for (C28H31N9O [M + H]+): Calcd: 510.2724. Found: 510.2725
3-((9-(Cyclopropylmethyl)-2-((6-(4-(2-hydroxyethyl)piperazin-1-yl)pyridin-3-yl)amino)-9H-purin-6-yl)amino)benzonitrile (7c): Yellow solid, yield 31%, m.p. 139.4–142.3 °C, Rf = 0.3. 1H NMR (400 MHz, DMSO-d6) δ 9.95 (s, 1H, NH), 9.03 (s, 1H, NH), 8.45 (d, J = 5.1 Hz, 2H, 2 CH), 8.28 (d, J = 8.2 Hz, 1H, CH), 8.07 (s, 1H, CH), 7.96 (dd, J = 9.0, 2.8 Hz, 1H, CH), 7.46 (dt, J = 16.4, 7.6 Hz, 2H, 2 CH), 6.83 (d, J = 9.1 Hz, 1H, CH), 4.45 (s, 1H, OH), 3.96 (d, J = 7.2 Hz, 2H, CH2), 3.55 (t, J = 6.2 Hz, 2H, CH2), 3.40 (q, J = 8.7, 7.0 Hz, 8H, 4 CH2), 2.44 (t, J = 6.2 Hz, 2H, CH2), 1.32 (ddt, J = 10.7, 7.6, 3.9 Hz, 1H, CH), 0.56 (q, J = 5.4, 4.7 Hz, 2H, CH2), 0.47 (t, J = 5.0 Hz, 2H, CH2). 13C NMR (101 MHz, DMSO-d6) δ 156.75, 155.39, 152.05, 152.01, 141.48, 139.71, 130.75, 130.13, 129.28, 125.90, 125.21, 123.07, 119.56, 115.20, 111.84, 107.28 (2C), 60.86, 59.03, 53.54 (2C), 47.59, 45.89 (2C), 11.77, 4.26 (2C). ESI/MS for (C27H30N10O [M + H]+): Calcd: 511.2677. Found: 511.2677.
2-(4-(4-((6-((3-Bromophenyl)amino)-9-(cyclopropylmethyl)-9H-purin-2-yl)amino)phenyl)piperazin-1-yl)ethan-1-ol (7d): Yellow solid, yield 30%, m.p. 109.4–114.3 °C, Rf = 0.3. 1H NMR (200 MHz, chloroform-d) δ 8.07 (s, 1H, NH), 7.76 (dd, J = 8.3, 1.2 Hz, 2H, NH, CH), 7.68 (s, 1H, CH), 7.59–7.51 (m, 2H), 7.37–7.30 (m, 1H, CH), 7.12–7.01 (m, 2H, 2 CH), 6.96–6.86 (m, 2H, 2 CH), 3.93 (d, J = 7.1 Hz, 2H, CH2), 3.71 (d, J = 4.1 Hz, 2H, CH2), 3.16 (dd, J = 6.4, 3.5 Hz, 4H, 2 CH2), 3.02 (s, 1H, OH), 2.73–2.62 (m, 4H, 2 CH2), 1.38–1.26 (m, 1H, CH), 0.75–0.57 (m, 2H, CH2), 0.44 (dt, J = 6.5, 4.8 Hz, 2H, CH2). 13C NMR (50 MHz, chloroform-d) δ 156.79, 152.17, 151.21, 146.62, 139.16, 137.67, 133.41, 128.77 (2C), 122.97, 120.87 (2C), 120.30 (2C), 116.99 (2C), 115.14, 67.07, 59.49, 57.87, 53.03 (2C), 50.13 (2C), 48.10, 11.04, 4.27 (2C). ESI/MS for (C27H31BrN8O [M − Br]−): Calcd: 483.2842 Found: 483.2596.
Methyl 2-chloro-4-((9-(cyclopropylmethyl)-2-((4-(4-(2-hydroxyethyl)piperazin-1-yl)phenyl)amino)-9H-purin-6-yl)amino)benzoate (7e): Brown solid, yield 22%, m.p. 241.4–245.3 °C, Rf = 0.3. 1H NMR (400 MHz, chloroform-d) δ 8.30 (s, 1H, NH), 8.21–8.13 (m, 1H, NH), 7.98 (d, J = 8.5 Hz, 1H, CH), 7.85 (dd, J = 8.6, 2.0 Hz, 1H, CH), 7.72 (s, 1H, CH), 7.54 (t, J = 8.6 Hz, 2H, 2 CH), 6.99–6.92 (m, 3H, 3 CH), 3.95 (dd, J = 7.3, 3.5 Hz, 2H, CH2), 3.91 (d, J = 2.3 Hz, 3H, CH3), 3.70 (t, J = 5.4 Hz, 2H, CH2), 3.20 (q, J = 5.1 Hz, 4H, 2 CH2), 2.72 (q, J = 5.3 Hz, 4H, 2 CH2), 2.64 (td, J = 5.6, 3.0 Hz, 2H, CH2), 0.86 (dt, J = 19.1, 6.9 Hz, 1H, CH), 0.72–0.62 (m, 2H, CH2), 0.50–0.43 (m, 2H, CH2). 13C NMR (101 MHz, chloroform-d) δ 166.87, 151.60, 143.61, 138.15, 130.71 (2C), 123.85, 121.43 (2C), 118.81 (2C), 117.13, 116.97 (2C), 59.41, 57.82, 52.99 (2C), 50.04 (2C), 49.96, 48.21, 29.70, 11.05, 4.32 (2C). ESI/MS for (C29H33ClN8O3 [M + H]+): Calcd: 577.2437. Found: 577.2445.
2-(4-(4-((6-((4-Chloro-3-(trifluoromethyl)phenyl)amino)-9-(cyclopropylmethyl)-9H-purin-2-yl)amino)phenyl)piperazin-1-yl)ethan-1-ol (7f): Brown solid, yield 32%, m.p. 232.4–236.4 °C, Rf = 0.3. 1H NMR (400 MHz, DMSO-d6) δ 9.81 (s, 1H, NH), 8.70 (s, 1H, NH), 8.30 (d, J = 9.1 Hz, 1H, CH), 8.07 (s, 1H, CH), 7.82 (s, 1H, CH), 7.32 (t, J = 8.4 Hz, 3H, 3 CH), 6.62 (d, J = 8.5 Hz, 2H, 2 CH), 4.19 (s, 1H, OH), 3.72 (d, J = 7.2 Hz, 2H, CH2), 3.30 (d, J = 13.5 Hz, 2H, CH2), 3.10 (s, 2H, CH2), 2.44–2.15 (m, 8H, 4 CH2), 1.09 (m, 1H, CH), 0.27 (dd, J = 6.3 Hz, 4H, 2 CH2). 13C NMR (101 MHz, DMSO-d6) δ 156.59, 152.16, 151.86, 146.38, 140.16, 139.80, 133.73, 131.89, 127.09 (t, 2JC-F = 25.5 Hz), 126.48–120.09 (dd, 1JC-F = 187.3 Hz), 125.42 (dd, 3JC-F = 6.0 Hz), 122.68 (d, 2JC-F = 15.3 Hz), 120.81 (dt, 3JC-F = 7.3 Hz), 119.55 (dd, 4JC-F = 3.6 Hz), 116.32 (2C), 115.14, 60.75, 58.99, 53.74 (2C), 49.52 (2C), 47.58, 11.79, 4.25 (2C). ESI/MS for (C28H30ClF3N8O [M + H]+): Calcd: 587.2256. Found: 587.2274.
2-(4-(4-((6-((4-Bromo-3-methoxyphenyl)amino)-9-(cyclopropylmethyl)-9H-purin-2-yl)amino)phenyl)piperazin-1-yl)ethan-1-ol (7g): Brown solid, yield 30%, m.p. 79.5–83.5 °C, Rf = 0.3. 1H NMR (400 MHz, chloroform-d) δ 8.07 (s, 1H, NH), 7.70 (s, 1H, CH), 7.50 (d, J = 8.6 Hz, 2H, 2 CH), 7.45–7.36 (m, 2H, CH, NH), 7.21 (dd, J = 8.7, 2.4 Hz, 1H, CH), 6.97 (s, 1H, CH), 6.90 (d, J = 8.6 Hz, 2H, 2 CH), 3.94 (d, J = 7.1 Hz, 2H, CH2), 3.72 (s, 3H, CH3), 3.69 (t, J = 5.4 Hz, 2H, CH2), 3.16 (t, J = 4.8 Hz, 4H, 2 CH2), 2.70 (t, J = 5.0 Hz, 4H, 2 CH2), 2.63 (t, J = 5.4 Hz, 2H, CH2), 1.32 (dtd, J = 12.8, 7.9, 5.2 Hz, 1H, CH), 0.66 (m, 2H, CH2), 0.45 (d, J = 5.2 Hz, 2H, CH2). 13C NMR (101 MHz, chloroform-d) δ 156.76, 155.97, 151.93, 151.42, 146.93, 139.77, 137.96, 133.07, 132.89, 121.27 (2C), 116.94 (3C), 115.26, 113.43, 104.64, 59.44, 57.87, 56.15, 53.00 (2C), 50.05 (2C), 48.15, 11.07, 4.30 (2C). ESI/MS for (C28H33BrN8O2 [M + H]+): Calcd: 593.1983. Found: 593.1987.
3.3. Biological Activity
3.3.1. Kinase Assays
The inhibitory activity of the synthesized compounds was assessed on an in-house produced recombinant Abl1 kinase. The assay utilized 500 µM peptide GGEAIYAAPFKK as a substrate in the presence of 1 µM ATP + [γ-33P]ATP (0.05 µCi per reaction) and the test compound in a final volume of 10 µL of reaction buffer (60 mM HEPES-NaOH, pH 7.5, 3 mM MgCl2, 3 mM MnCl2, 3 μM Na-orthovanadate, 1.2 mM DTT, 2.5 μg/50 μL PEG20.000). The reaction was terminated by adding 5 µL of 3% aq. H3PO4. Aliquots were spotted onto P-81 phosphocellulose (Whatman, Maidstone, UK), washed three times with 0.5% aq. H3PO4, and air-dried. Peptide phosphorylation was quantified through digital autoradiography (FLA-7000; Fujifilm, Tokyo, Japan). The concentration of test compounds required to reduce activity by 50% was determined from the dose–response curves and reported as the IC50 value.
3.3.2. Cell Cultures
The human cell line KCL22 was sourced from the German Collection of Microorganisms and Cell Cultures, while K562 and HEK-293T cells were obtained from the European Collection of Authenticated Cell Cultures. The development of the KCL22 subclone B8 (T315I, 100%) has been previously detailed [44]. All cell lines were maintained in a humidified incubator at 37 °C and 5% CO2, following the manufacturer’s instructions, in the appropriate medium supplemented with 10% foetal bovine serum, penicillin (100 IU/mL), streptomycin (100 µg/mL), and glutamine (4 mM).
3.3.3. Cytotoxicity Assay
Cytotoxicity was assessed utilizing resazurin, an indicator dyes that measures oxidation-reduction reactions in the mitochondria of living cells. Cells were seeded into 96-well plates and treated with the compounds for 72 h (six different doses of each compound, in triplicate). After treatment, a solution of resazurin (Merck, Prague, Czech Republic) was added for 4 h, and the fluorescence of resorufin corresponding to viable cell quantity was measured at 544 nm/590 nm (excitation/emission) using a Fluoroskan Ascent microplate reader (LabSystems, Helsinki, Finland). The GI50 value, representing the drug concentration lethal to 50% of the cells, was calculated from the dose–response curves using Origin 6.0.
3.4. Molecular Docking Studies
Energy minimization and protonation of the proposed compounds were conducted using the LigPrep tool in the Maestro Schrödinger suite, version 11.8 (Schrödinger, LLC, New York, NY, 2019). For induced molecular docking, the crystal structure of the Bcr-Abl receptor (PDB code: 6BL8) was employed alongside the designed structures. Protein optimization was accomplished using the Protein Preparation Wizard available in Maestro v.14.1 software. Water molecules were removed, and appropriate hydrogen bonds were added at a pH of 7.0 ± 0.5, ensuring correct ionization states for acidic and basic amino acid residues. The receptor was optimized utilizing the OPLS4 force field. The active site was defined by a grid with a 14 Å radius centred around the Met318 residue. Initial induced docking was performed using an energy grid-based ligand docking algorithm (Glide v7.0, Schrödinger v11.2). The next step combined rigid receptor docking and side-chain protein remodelling, and included employing search and minimization techniques with the Prime module. During the protein modelling phase, all residues within a 5 Å radius of each initially docked compound were refined using Prime. After this refinement, the compounds were re-docked into the optimized receptor structure using Glide. The optimal orientations for each docked compound were identified using Glide in standard precision (SP) mode and subsequently recalculated using the Glide score function (GScore) in extra-precision (XP) mode.
3.5. Molecular Dynamics
The PDB structures for wild-type Bcr-Abl (PDB ID: 6BL8) and the T315I mutant (PDB ID: 4TWP) were retrieved from the Protein Data Bank. Both structures were prepared utilizing CHARMM-GUI [45] under the CHARMM36 force field for proteins and ions, and CGenFF for ligands [46]. A simulation box measuring approximately 116 × 110 × 100 Å was established, with a cutoff of 12 Å (and switching from 10 Å), and included 0.15 M of Na+ and Cl− ions to neutralize the system and reflect physiological ionic strength. Subsequently, a 10,000-step energy minimization was executed to eliminate unfavourable contacts, followed by 2000 steps of heating to ramp from 0 K to 298 K. Next, a 50 ns equilibration was conducted to stabilize the system and to gradually release positional restraints. The ensuing 200 ns production phase was performed under NPT conditions at 298 K and 1 atm using NAMD [35]. Throughout the simulations, the Particle Mesh Ewald (PME) function was utilized for long-range electrostatics with a tolerance of 1 × 10−6, while a switching function was applied from 10 Å to the 12 Å cutoff for Van der Waals and real-space electrostatic interactions. A multiple time-step scheme was used which employed a 2 fs integration step for bonded and real-space non-bonded interactions, and all hydrogen-involving bonds were constrained by SHAKE [47]. The water model used was the TIP3P [48].
4. Conclusions
In this study, we performed a comprehensive analysis of 58 purine derivatives with inhibitory effects on Bcr-Abl that were previously synthesized by our team to develop 3D-QSAR models for the design of novel potential treatments for CML. We constructed and validated two 3D-QSAR models, CoMFA and CoMSIA, that exhibited satisfactory statistical parameters. The inhibition of Bcr-Abl was attributed to its steric, electrostatic, and hydrophobic properties. Using these data, we designed and synthesized seven new 2,6,9-trisubstituted purines. Compounds 7a and 7c demonstrated the strongest inhibitory activity against Bcr-Abl, surpassing the potency of imatinib, which indicated that the modifications proposed by the 3D-QSAR models were effective. Molecular docking studies revealed that these purines occupy the same binding site on Bcr-Abl. Notably, 7c exhibited significant cytotoxicity in two CML cell lines that are sensitive to imatinib, K562 and KCL22, indicating that it is the most potent purine developed by our group. Furthermore, based on the viability of HEK293T cells, 7c showed greater selectivity for K562 and KCL22 cells than imatinib. However, the most intriguing findings emerged when these purines were tested on imatinib-resistant KCL22-B8 cells expressing Bcr-AblT315I, in which 7e and 7f were more effective in inhibiting cell growth. Molecular dynamics studies elucidated the key interactions of these inhibitors at the binding sites of Bcr-AblWT and Bcr-AblT315I, emphasizing the differences in potency against both kinases and validating our hypothesis concerning the significance of specific fragments of the purine ring. At last, 7b and 7c were anticipated to possess favourable pharmacokinetic profiles for oral administration. Our findings will aid in the development of two validated 3D-QSAR models for designing potential CML drugs based on the purine scaffold.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph18060925/s1, Table S1. The Chemical structure of the 58 in-house purine compounds; Table S2. Statistical parameters and Field combinations for CoMFA and CoMSIA; Table S3. Experimental and predicted pIC50 and residual values for the studied compounds according to CoMFA and CoMSIA. Table S4. q2 and r2ncv values after several Y-randomization tests; 1H-, 13C-spectra, HRMS, HPLC chromatograms of selected compounds. Figure S1. Representation of the Bcr-Abl surface, providing an overview of the binding site location and the conformations adopted by the studied molecules during the docking process (PDB = 6BL8); Figure S2. Time evolution of the RMSD for Bcr-Abl complexes with different ligands. Six systems were analysed: imatinib-Bcr-AblWT (blue), imatinib-Bcr-AblT315I (red), 7f-Bcr-AblWT (green), 7f-Bcr-AblT315I (purple), 7e-Bcr-AblWT (orange), and 7e-Bcr-AblT315I (brown); Figure S3. Time evolution of the RMSD for the Bcr-Abl protein in complex with different ligands. Six systems were analysed: imatinib-Bcr-AblWT (blue), imatinib-Bcr-AblT315I (red), 7f-Bcr-AblWT (green), 7f-Bcr-AblT315I (purple), 7e-Bcr-AblWT (orange), and 7e-Bcr-AblT315I (brown).
Author Contributions
Conceptualization, J.M. and C.O.S.; formal analysis, C.E.-B., V.K., J.E., D.C., G.M., J.M. and C.O.S.; investigation, D.C., T.D., G.S., P.K., V.V., M.S., N.I.S., J.E., J.L., G.M. and C.E.-B.; methodology, D.C., G.S., G.S., P.K., V.V., M.S., N.I.S., G.M., J.L. and C.E.-B.; validation, D.C., T.D., G.S., G.S., P.K., V.V., M.S., N.I.S., G.M., J.L. and C.E.-B.; resources, V.K., J.M. and C.O.S.; writing—original draft preparation, D.C., T.D., J.M. and C.O.S.; writing—review and editing, D.C., T.D., V.K., J.E., C.E.-B., J.M. and C.O.S.; visualization, D.C., T.D., G.S., G.S., P.K., V.V., M.S., N.I.S., J.E., G.M., V.K., J.L., J.M. and C.O.S.; supervision, V.K., J.E., C.E.-B., J.M. and C.O.S.; project administration, V.K., M.S., J.E., J.M. and C.O.S.; funding acquisition, V.K., J.E., J.M. and C.O.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by FONDECYT (COS, Research Grant N° 1231199), the Czech Science Foundation (23-05474S), and Palacký University in Olomouc (IGA_PrF_20242025_005011). The European Union—Next Generation EU (Project National Institute for Cancer Research, Programme EXCELES, ID No. LX22NPO5102).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors thank to Kateřina Machová Poláková (Institute of Hematology and Blood Transfusion, Czech Republic) for providing the established KCL22 clone resistant to imatinib used in this study. TD is grateful to PUC for a PhD fellowship. NS is grateful to ANID, Chile, for a PhD fellowship (N° 21241377).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Westerweel, P.E.; Te Boekhorst, P.A.W.; Levin, M.-D.; Cornelissen, J.J. New Approaches and Treatment Combinations for the Management of Chronic Myeloid Leukemia. Front. Oncol. 2019, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, L.; Fidyt, K.; Patkowska, E.; Firczuk, M. Philadelphia Chromosome-Positive Leukemia in the Lymphoid Lineage-Similarities and Differences with the Myeloid Lineage and Specific Vulnerabilities. Int. J. Mol. Sci. 2020, 21, 5776. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Tiwari, A.K.; Sun, Y.; Ding, P.-R.; Ashby, C.R.; Chen, Z.-S. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: A review. Leuk. Res. 2010, 34, 1255–1268. [Google Scholar] [CrossRef]
- Baran, Y.; Saydam, G. Cumulative clinical experience from a decade of use: Imatinib as first-line treatment of chronic myeloid leukemia. J. Blood Med. 2012, 3, 139–150. [Google Scholar] [CrossRef]
- Laganà, A.; Scalzulli, E.; Bisegna, M.L.; Ielo, C.; Martelli, M.; Breccia, M. Understanding and overcoming resistance to tyrosine kinase inhibitors (TKIs) in Chronic myeloid leukemia (CML). Expert. Rev. Hematol. 2025, 18, 65–79. [Google Scholar] [CrossRef]
- Carofiglio, F.; Trisciuzzi, D.; Gambacorta, N.; Leonetti, F.; Stefanachi, A.; Nicolotti, O. Bcr-Abl Allosteric Inhibitors: Where We Are and Where We Are Going to. Molecules 2020, 25, 4210. [Google Scholar] [CrossRef]
- Rossari, F.; Minutolo, F.; Orciuolo, E. Past, present, and future of Bcr-Abl inhibitors: From chemical development to clinical efficacy. J. Hematol. Oncol. 2018, 11, 84. [Google Scholar] [CrossRef]
- Nascimento, M.; Moura, S.; Parra, L.; Vasconcellos, V.; Costa, G.; Leite, D.; Dias, M.; Fernandes, T.V.A.; Hoelz, L.; Pimentel, L.; et al. Ponatinib: A Review of the History of Medicinal Chemistry behind Its Development. Pharmaceuticals 2024, 17, 1361. [Google Scholar] [CrossRef]
- Azam, M.; Nardi, V.; Shakespeare, W.C.; Metcalf, C.A., 3rd; Bohacek, R.S.; Wang, Y.; Sundaramoorthi, R.; Sliz, P.; Veach, D.R.; Bornmann, W.G.; et al. Activity of dual SRC-ABL inhibitors highlights the role of BCR/ABL kinase dynamics in drug resistance. Proc. Natl. Acad. Sci. USA 2006, 103, 9244–9249. [Google Scholar] [CrossRef]
- Delgado, T.; Veselá, D.; Dostálová, H.; Kryštof, V.; Vojáčková, V.; Jorda, R.; Castro, A.; Bertrand, J.; Rivera, G.; Faúndez, M.; et al. New Inhibitors of Bcr-Abl Based on 2,6,9-Trisubstituted Purine Scaffold Elicit Cytotoxicity in Chronic Myeloid Leukemia-Derived Cell Lines Sensitive and Resistant to TKIs. Pharmaceutics 2024, 16, 649. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Dostálová, H.; Kryštof, V.; Jorda, R.; Delgado, T.; Castro-Alvarez, A.; Mella, J.; Cabezas, D.; Faúndez, M.; Espinosa-Bustos, C.; et al. Design, Synthesis, In Silico Studies and Inhibitory Activity towards Bcr-Abl, BTK and FLT3-ITD of New 2,6,9-Trisubstituted Purine Derivatives as Potential Agents for the Treatment of Leukaemia. Pharmaceutics 2022, 14, 1294. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Dostálová, H.; Krystof, V.; Jorda, R.; Castro, A.; Mella, J.; Espinosa-Bustos, C.; María Zarate, A.; Salas, C.O. New 2,6,9-trisubstituted purine derivatives as Bcr-Abl and Btk inhibitors and as promising agents against leukemia. Bioorg. Chem. 2020, 94, 103361. [Google Scholar] [CrossRef] [PubMed]
- Gagic, Z.; Ruzic, D.; Djokovic, N.; Djikic, T.; Nikolic, K. In silico Methods for Design of Kinase Inhibitors as Anticancer Drugs. Front. Chem. 2019, 7, 873. [Google Scholar] [CrossRef]
- Urich, R.; Wishart, G.; Kiczun, M.; Richters, A.; Tidten-Luksch, N.; Rauh, D.; Sherborne, B.; Wyatt, P.G.; Brenk, R. De Novo Design of Protein Kinase Inhibitors by in Silico Identification of Hinge Region-Binding Fragments. ACS Chem. Biol. 2013, 8, 1044–1052. [Google Scholar] [CrossRef]
- Golestanifar, F.; Garkani-Nejad, Z. In silico design and ADMET evaluation of new inhibitors for PIM1 kinase using QSAR studies, molecular docking, and molecular dynamic simulation. Heliyon 2024, 10, e38309. [Google Scholar] [CrossRef]
- Azimi, S.; Bagher, M.; Shahram, B.; Vessally, E. In Silico study and design of some new potent threonine tyrosine kinase inhibitors using molecular docking simulation. Mol. Simul. 2023, 49, 517–524. [Google Scholar] [CrossRef]
- Gond, C.; Kumar, N.; Mishra, A.; Daksh, S.; Datta, A.; Tiwari, A.K. Rational computational Design of new-Generation EGFR tyrosine kinase (EGFR-TK) inhibitors. Results Chem. 2025, 15, 102239. [Google Scholar] [CrossRef]
- Martins, D.M.; Fernandes, P.O.; Vieira, L.A.; Maltarollo, V.G.; Moraes, A.H. Structure-Guided Drug Design Targeting Abl Kinase: How Structure and Regulation Can Assist in Designing New Drugs. ChemBioChem 2024, 25, e202400296. [Google Scholar] [CrossRef]
- Koroleva, E.V.; Ermolinskaya, A.L.; Ignatovich, Z.V.; Kornoushenko, Y.V.; Panibrat, A.V.; Potkin, V.I.; Andrianov, A.M. Design, in silico Evaluation, and Determination of Antitumor Activity of Potential Inhibitors Against Protein Kinases: Application to BCR-ABL Tyrosine Kinase. Biochemistry 2024, 89, 1094–1108. [Google Scholar] [CrossRef]
- Harrington, E.A.; Bebbington, D.; Moore, J.; Rasmussen, R.K.; Ajose-Adeogun, A.O.; Nakayama, T.; Graham, J.A.; Demur, C.; Hercend, T.; Diu-Hercend, A.; et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat. Med. 2004, 10, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Parate, S.; Danishuddin; Zeb, A.; Singh, P.; Lee, G.; Jung, T.S.; Lee, K.W.; Ha, M.W. 3D-QSAR-Based Pharmacophore Modeling, Virtual Screening, and Molecular Dynamics Simulations for the Identification of Spleen Tyrosine Kinase Inhibitors. Front. Cell. Infect. Microbiol. 2022, 12, 909111. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.K.; Balupuri, A.; Cho, S.J. 3D-QSAR studies on disubstituted dibenzosuberone derivatives as p38α MAP kinase inhibitors using CoMFA and COMSIA. Med. Chem. Res. 2016, 25, 2349–2359. [Google Scholar] [CrossRef]
- Golbraikh, A.; Tropsha, A. Beware of q2! J. Mol. Graph. Model. 2002, 20, 269–276. [Google Scholar] [CrossRef]
- Tropsha, A. Best Practices for QSAR Model Development, Validation, and Exploitation. Mol. Inform. 2010, 29, 476–488. [Google Scholar] [CrossRef]
- Tropsha, A.; Gramatica, P.; Gombar, V.K. The Importance of Being Earnest: Validation is the Absolute Essential for Successful Application and Interpretation of QSPR Models. QSAR Comb. Sci. 2003, 22, 69–77. [Google Scholar] [CrossRef]
- Roy, P.P.; Roy, K. On Some Aspects of Variable Selection for Partial Least Squares Regression Models. QSAR Comb. Sci. 2008, 27, 302–313. [Google Scholar] [CrossRef]
- Espinosa-Bustos, C.; Zárate, A.M.; Castro-Álvarez, A.; Guerrero, S.; Kogan, M.J.; Salas, C.O. Synthesis, antitumoral activity, and in silico studies on Smoothened receptor of new 2,6,9-trisubstituted purine derivatives. J. Mol. Struct. 2025, 1328, 141228. [Google Scholar] [CrossRef]
- Espinosa-Bustos, C.; Bertrand, J.; Villegas-Menares, A.; Guerrero, S.; Di Marcotullio, L.; Navacci, S.; Schulte, G.; Kozielewicz, P.; Bloch, N.; Villela, V.; et al. New Smoothened ligands based on the purine scaffold as potential agents for treating pancreatic cancer. Bioorg. Chem. 2024, 151, 107681. [Google Scholar] [CrossRef]
- Villegas, A.; Satheeshkumar, R.; Ballesteros-Casallas, A.; Paulino, M.; Castro, A.; Espinosa-Bustos, C.; Salas, C.O. Convergent synthesis, drug target prediction, and docking studies of new 2,6,9-trisubstituted purine derivatives. J. Heterocycl. Chem. 2022, 59, 97–111. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, C.; Clawson, D.K.; Atwell, S.; Russell, M.; Vieth, M.; Roux, B. Predicting the Conformational Variability of Abl Tyrosine Kinase using Molecular Dynamics Simulations and Markov State Models. J. Chem. Theory Comput. 2018, 14, 2721–2732. [Google Scholar] [CrossRef]
- Willis, S.G.; Lange, T.; Demehri, S.; Otto, S.; Crossman, L.; Niederwieser, D.; Stoffregen, E.P.; McWeeney, S.; Kovacs, I.; Park, B.; et al. High-sensitivity detection of BCR-ABL kinase domain mutations in imatinib-naive patients: Correlation with clonal cytogenetic evolution but not response to therapy. Blood 2005, 106, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, E.; Manley, P.; Mestan, J.; Cowan-Jacob, S.; Ray, A.; Griffin, J.D. AMN107 (nilotinib): A novel and selective inhibitor of BCR-ABL. Br. J. Cancer 2006, 94, 1765–1769. [Google Scholar] [CrossRef] [PubMed]
- Pemovska, T.; Johnson, E.; Kontro, M.; Repasky, G.A.; Chen, J.; Wells, P.; Cronin, C.N.; McTigue, M.; Kallioniemi, O.; Porkka, K.; et al. Axitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformation. Nature 2015, 519, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Tan, S.; Xu, T.; Liu, H.; Huang, J.; Yao, X. MolAICal: A soft tool for 3D drug design of protein targets by artificial intelligence and classical algorithm. Brief. Bioinform. 2020, 22, bbaa161. [Google Scholar] [CrossRef]
- Meanwell, N.A. Improving Drug Candidates by Design: A Focus on Physicochemical Properties as a Means of Improving Compound Disposition and Safety. Chem. Res. Toxicol. 2011, 24, 1420–1456. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development. Adv. Drug Deliv. Rev. 1997, 23, 3–25.1, Erratum in Adv. Drug Deliv. Rev. 2001, 46, 3–26. https://doi.org/10.1016/S0169-409X(00)00129-0. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Powell, M.J.D. An efficient method for finding the minimum of a function of several variables without calculating derivatives. Comput. J. 1964, 7, 155–162. [Google Scholar] [CrossRef]
- Klebe, G.; Abraham, U.; Mietzner, T. Molecular Similarity Indices in a Comparative Analysis (CoMSIA) of Drug Molecules to Correlate and Predict Their Biological Activity. J. Med. Chem. 1994, 37, 4130–4146. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Cramer III, R.D.; Van Opdenbosch, N. Validation of the general purpose tripos 5.2 force field. J. Comput. Chem. 1989, 10, 982–1012. [Google Scholar] [CrossRef]
- Roy, K.; Kar, S.; Ambure, P. On a simple approach for determining applicability domain of QSAR models. Chemom. Intell. Lab. Syst. 2015, 145, 22–29. [Google Scholar] [CrossRef]
- Curik, N.; Polivkova, V.; Burda, P.; Koblihova, J.; Laznicka, A.; Kalina, T.; Kanderova, V.; Brezinova, J.; Ransdorfova, S.; Karasova, D.; et al. Somatic Mutations in Oncogenes Are in Chronic Myeloid Leukemia Acquired De Novo via Deregulated Base-Excision Repair and Alternative Non-Homologous End Joining. Front. Oncol. 2021, 11, 744373. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).