Abstract
Background: Varicose veins and chronic venous insufficiency are chronic venous disorders involving abnormalities in the venous system. Inflammation, an increase in proteolytic enzymes, and free radicals are important factors that play a role in the varicose vein pathology. Methods: In this study, the antioxidant properties and inhibitor activities of 17 plant extracts used to treat varicose veins in traditional medicine were evaluated against varicose veins-related enzymes (hyaluronidase, elastase, collagenase, lipooxygenase, prolylendopeptidase, and xanthine oxidase). The most effective compounds responsible for the activity of the Helichrysum plicatum subsp. plicatum extract were isolated by open column chromatography techniques. The active compounds were determined to be naringenin, apigenin, and luteolin by spectroscopic methods. In the activity-guided isolation study, the xanthine oxidase enzyme inhibition method was used. Results: The fractions containing naringenin and apigenin (IC50 = 0.269 ± 0.009 µg/mL) and apigenin and luteolin (IC50 = 0.285 ± 0.019 µg/mL) compounds showed synergistic and strong effects against xanthine oxidase and were found to be as active as the positive control allopurinol (IC50 = 0.250 ± 0.006 µg/mL). In the LC-MS/MS analysis of the Helichrysum plicatum extract, quinic acid (22.649 mg compound/g extract), chlorogenic acid (14.573 mg/g extract), isoquercitrin (14.371 mg/g extract), cosmosin (9.885 mg/g extract), and astragalin (11.506 mg/g extract) were detected as the major components. Naringenin, apigenin, and luteolin were detected at concentrations of 1.457, 2.518, and 1.368 mg/g in the extract, respectively. Conclusions: In conclusion, it is predicted that the combination of naringenin, apigenin, and luteolin has a promising use as a conservative treatment option for diseases associated with varicose veins due to their synergistic effects with each other.
1. Introduction
People have long used plant-based medicines to treat a wide range of physical and mental ailments. Various tribes around the world utilise them as part of their traditional medicine. Medicinal and Aromatic Plants (MAPs) are becoming increasingly important, as they can be applied in the food, pharmaceutical, and beauty industries. The use of MAPs in everyday life highlights the necessity for future multidisciplinary research studies to provide scientific evidence of their benefits [1,2].
Chronic venous insufficiency (CVI) is often an underdiagnosed condition that diminishes patients’ quality of life and places a financial strain on healthcare resources [3]. Although CVI in the lower extremities can sometimes be asymptomatic, it frequently presents a wide clinical spectrum, ranging from cosmetic issues to serious symptoms [4]. Healthy valves and muscle pumps are essential to ensure that the blood in the venous system returns to the heart against gravity. When this system is insufficient, it leads to backward blood flow, blood stasis, and venous hypertension. Venous hypertension can trigger various events, including inflammation and venous valve insufficiency [5,6]. Inflammation and oxidative stress are contributing factors to both the development and progression of CVI. It is believed that abnormal venous flow triggers the inflammatory process in varicose veins. Lipoxygenase (LOX) enzyme inhibitors may be effective in treating varicose veins by suppressing the inflammatory response and preventing the formation of free radicals [7]. Previous research has indicated that prolylendopeptidase (POP) inhibitors enhance histological abnormalities in the venous wall while reducing the inflammatory process associated with CVI. Consequently, POP inhibitors are regarded as a new therapeutic target in the treatment of CVI [8]. Collagenase, hyaluronidase, and elastase enzymes damage venous structures when proteoglycan synthesis is low. It has been noted that the elastic lamina [9], collagen [10], and hyaluronic acid [11] content in the walls of varicose veins decreases [12]. Xanthine oxidase (XO) can catalyse the transfer of monovalent and divalent electrons to O2, producing two reactive oxygen species (ROS), superoxide anion (O2•−) and hydrogen peroxide (H2O2) [13]. Therefore, increased XO levels result in the release of ROS, exacerbating the pathology of varicose veins [14].
There are various techniques for treating superficial venous insufficiency. The treatment options for varicose veins include conservative methods (compression, lifestyle changes, and venoactive drugs) and surgical interventions (surgical ligation, stripping, microphlebectomy, sclerotherapy, and laser ablation) [6,15]. The selection of treatment methods depends on the nature of the varicose veins and the underlying physiological causes [16]. Medicinal plants and natural compounds represent one of the treatment strategies for CVI and varicose veins due to their biological activities, including anti-inflammatory effects, antioxidant properties, ability to increase vascular tone, ability to inhibit lysosomal enzymes, and anti-elastase properties [17,18]. Notably, coumarins, micronised purified flavonoid fractions, rutin derivatives (troxerutin and oxerutin), pycnogenol, anthocyanidins (delphinidin, cyanidin, and malvidin), proanthocyanidin from Vitis vinifera L. seed extracts, Ruscus aculeatus L., asiaticoside (Centella asiatica L.), Mammea africana Sabine extracts, fraxin, ginkgolide B (Ginkgo biloba L.), and saponosides (escin) play a significant role in the treatment of CVI [8,19].
In previous studies, various plants were used both externally and internally in the treatment of varicose veins in many different countries [19]. Berberis vulgaris L. [20], Brassica oleracea var. acephala L. [21], Capsella bursa-pastoris (L.) Medik. [22], Capsicum annuum L. [23], Crataegus monogyna Jacq. subsp. Monogyna [24], Ecbalium elaterium (L.) A.Rich. [25], Helichrysum plicatum DC. subsp. plicatum [26], Lamium album L. [27], Lythrum salicaria L. [28], Plantago lanceolata L. [29], Plantago major L. subsp. major [29], Sambucus ebulus L. [21], Sesamum indicum L. [30], and Urtica dioica L. [31] are utilised in the treatment of varicose veins in traditional medicine in Türkiye.
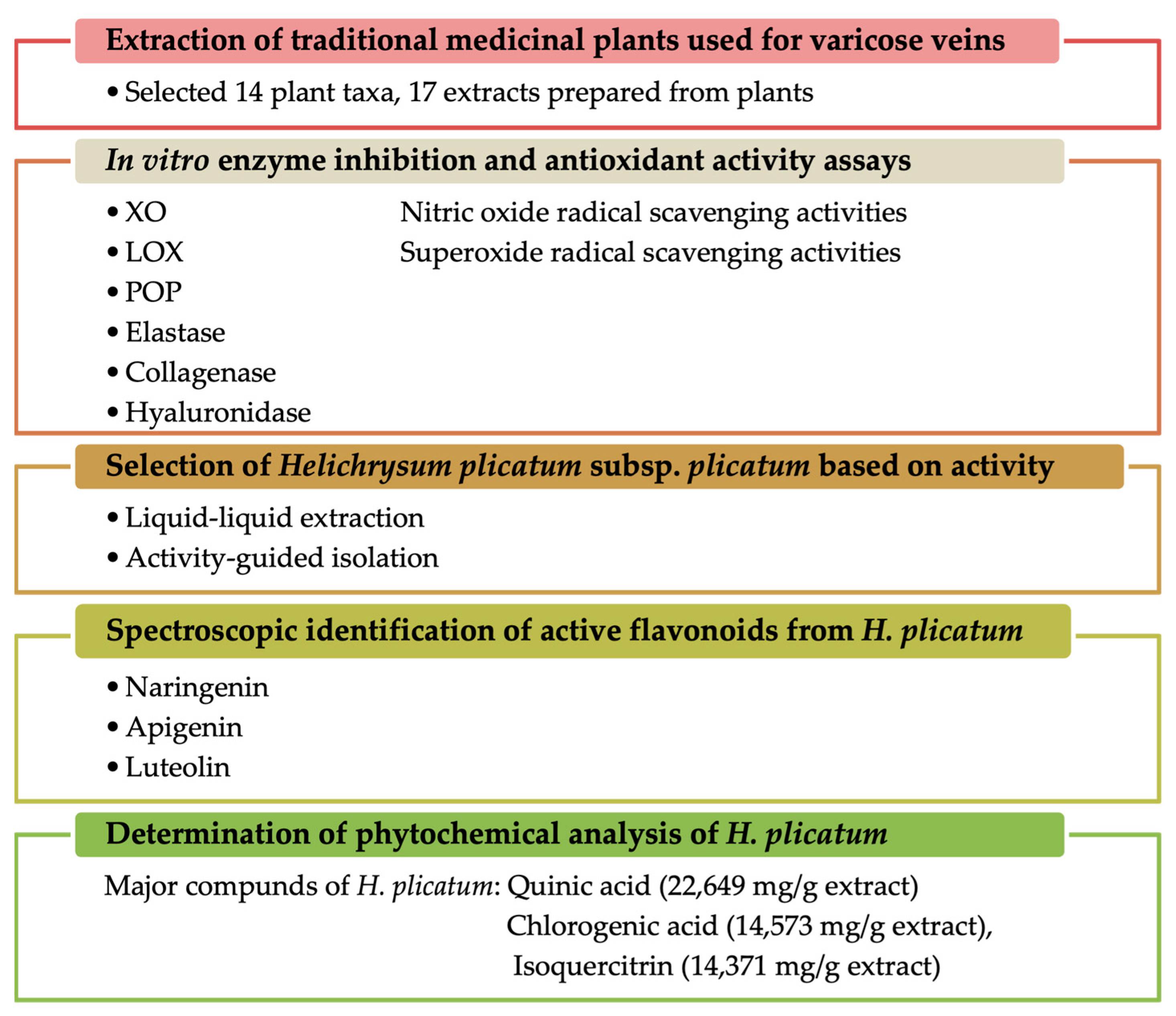
In this study, we aimed to elucidate the mechanism of action of plants that have been ethnopharmacologically used in the treatment of varicose veins, utilising various in vitro methods associated with varicose veins. The plant materials employed against varicose veins in traditional medicine were evaluated in terms of in vitro inhibition of the XO, elastase, collagenase, hyaluronidase, LOX, and POP enzymes, as well as antioxidant activities (including nitric oxide and superoxide anion radical-scavenging activities). The study also aimed to identify the compounds responsible for these activities through activity-guided fractionation. Furthermore, the phytochemical content of H. plicatum was quantitatively elucidated using LC-MS/MS. Thus, the effects of traditionally used plants were investigated through various mechanisms of action, and active compounds were isolated from the most effective plant, Helichrysum plicatum DC. subsp. plicatum (Figure 1).
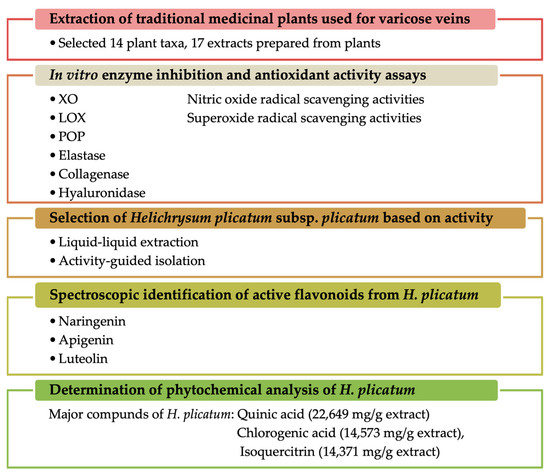
Figure 1.
Summary of the experimental design and the main findings.
2. Results
2.1. Results of Activity-Guided Isolation
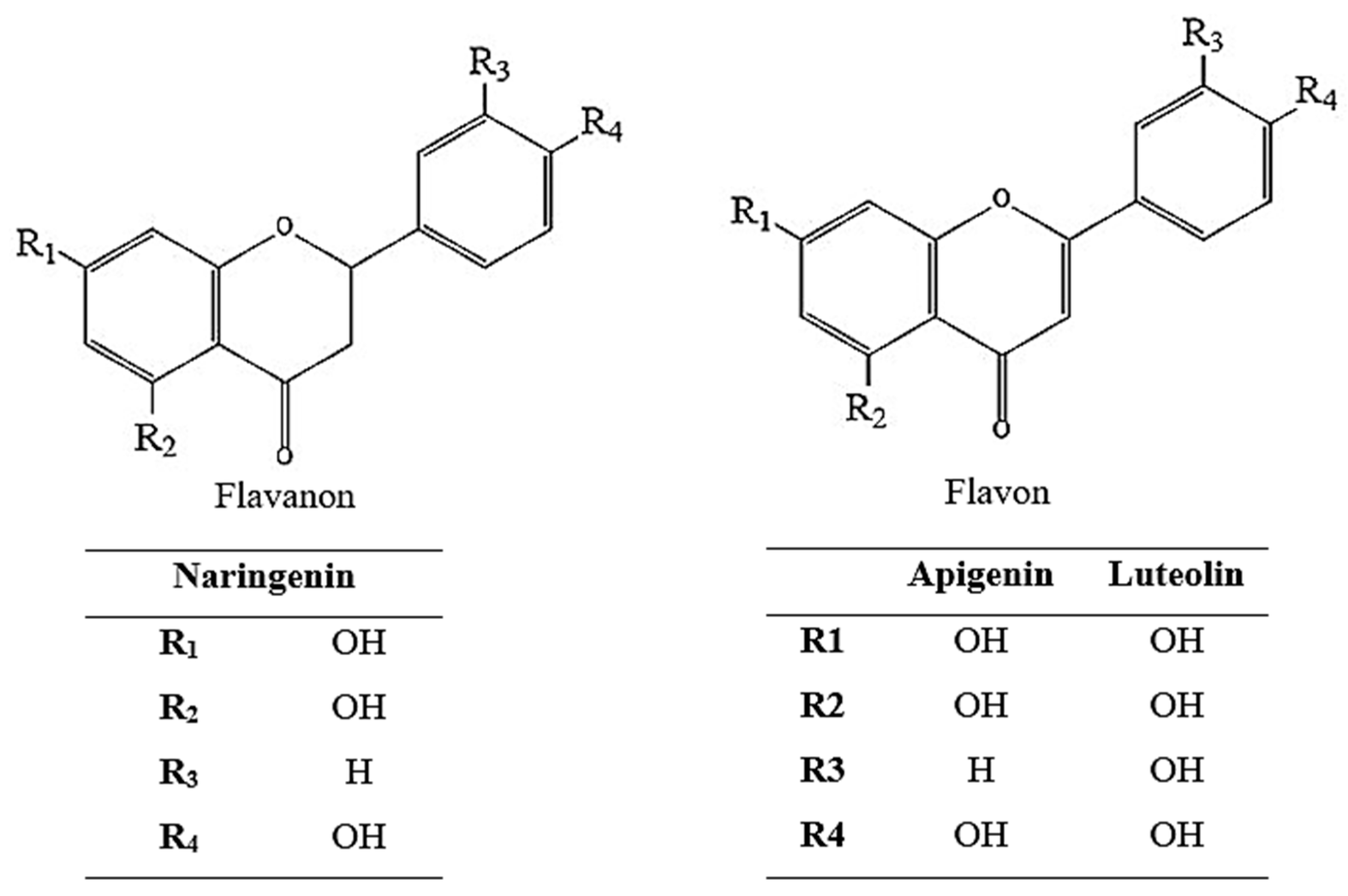
As a result of the activity-guided isolation studies, compounds 1, 2, and 3 were isolated from the EtOAc sub-extract. The isolated compounds were identified (Figure 2 and Table 1) using 1H-NMR, 13C-NMR, HMBC, COSY, and HMQC spectroscopic methods (Supplementary Material File S1). Furthermore, comparison of the spectral data of the compounds with spectra in the literature identified them as naringenin [32], apigenin [33], and luteolin [34].
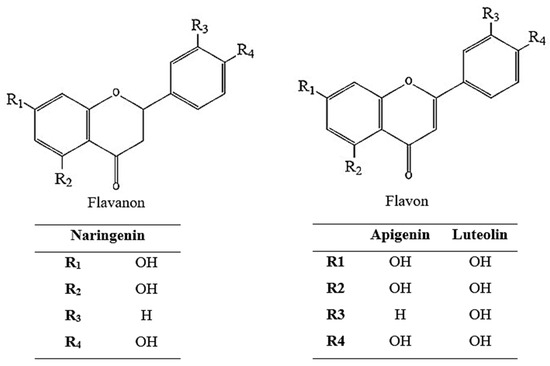
Figure 2.
Chemical structure of naringenin, apigenin, and luteolin.

Table 1.
1H and 13C NMR spectrum results for naringenin, apigenin, and luteolin.
2.2. LC-MS/MS Analysis Results
In the phytochemical analysis conducted on the ethanolic extract of H. plicatum aerial parts, 53 phenolic compounds were analysed, and a total of 20 phenolic compounds were identified (Figure 3 and Table 2). Quinic acid (22.649 mg/g extract), chlorogenic acid (14.573 mg/g extract), isoquercitrin (14.371 mg/g extract), cosmosiin (9.885 mg/g extract), and astragalin (11.506 mg/g extract) were identified as the major components. Naringenin, apigenin, and luteolin, which were isolated through activity-guided isolation from the H. plicatum extract, were found at concentrations of 1.457 mg/g extract, 2.518 mg/g extract, and 1.368 mg/g extract, respectively. Additionally, benzoic acid, fumaric acid, gallic acid, protocatechuic acid, caffeic acid, protocatechuic aldehyde, salicylic acid, cynaroside, quercetin, kaempferol, amentoflavone, 1,5-dicaffeoylquinic acid, caffeic acid, and acacetin compounds were found in low amounts in the H. plicatum extract.
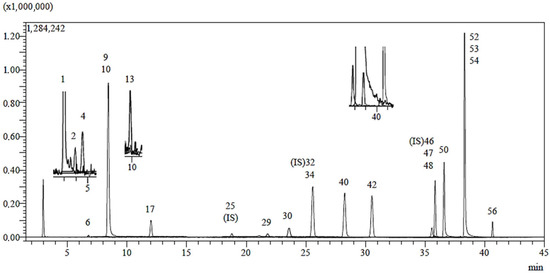
Figure 3.
LC-MS/MS chromatogram of H. plicatum ethanolic extract.

Table 2.
Identification and quantification of phenolic compounds in H. plicatum ethanolic extract.
2.3. Results of Enzyme Inhibition Assays
The findings regarding the collagenase, hyaluronidase, XO, and LOX enzyme inhibition effects of the extracts are given in Table 3. None of the extracts were found to have elastase enzyme inhibitory activity.

Table 3.
Enzyme inhibitory activities of plants used in the treatment of varicose veins.
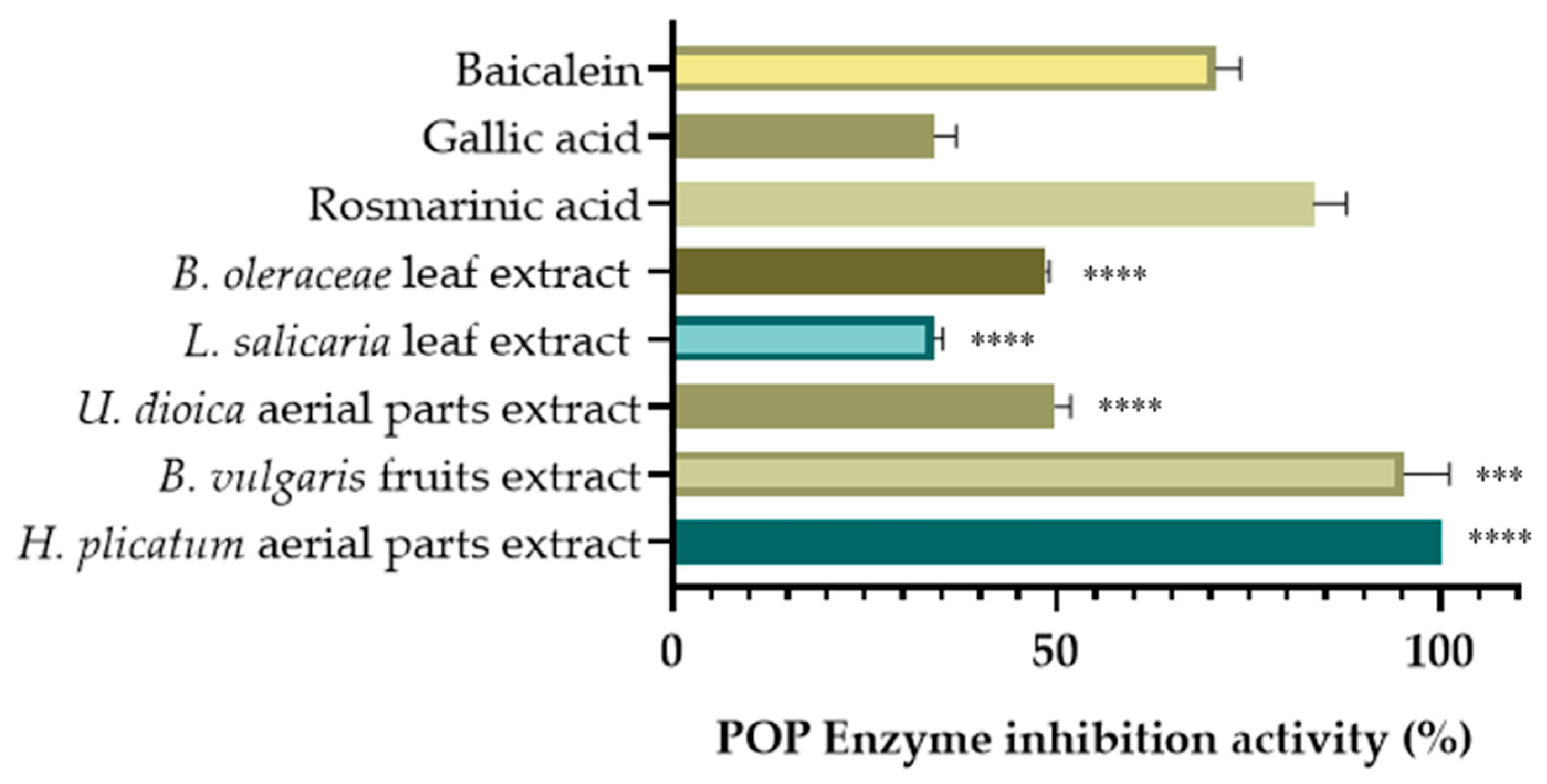
Among the screened extracts, the extract of the H. plicatum aerial part (IC50 = 85.85 ± 0.91 µg/mL) exhibited high XO inhibitory activity, while the L. salicaria leaf (IC50 = 70.76 ± 3.34 µg/mL), B. vulgaris fruit (IC50 = 114.63 ± 5.86 µg/mL), and B. oleraceae leaf (IC50 = 124.85 ± 0.64 µg/mL) extracts demonstrated significant hyaluronidase inhibitory activity. The extract of the U. dioica aerial parts (45.82% ± 2.48) also displayed considerable LOX enzyme inhibitory activity and was evaluated for POP enzyme inhibitory activity at a final concentration of 100 µg/mL (Figure 4).
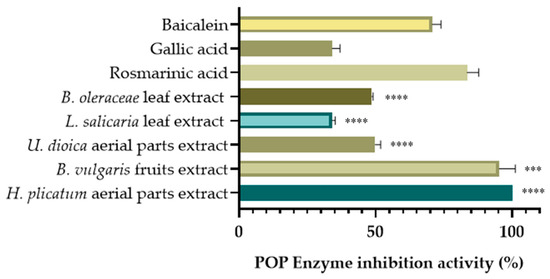
Figure 4.
POP inhibitory activities of selected extracts. (*** p < 0.001, **** p < 0.0001).
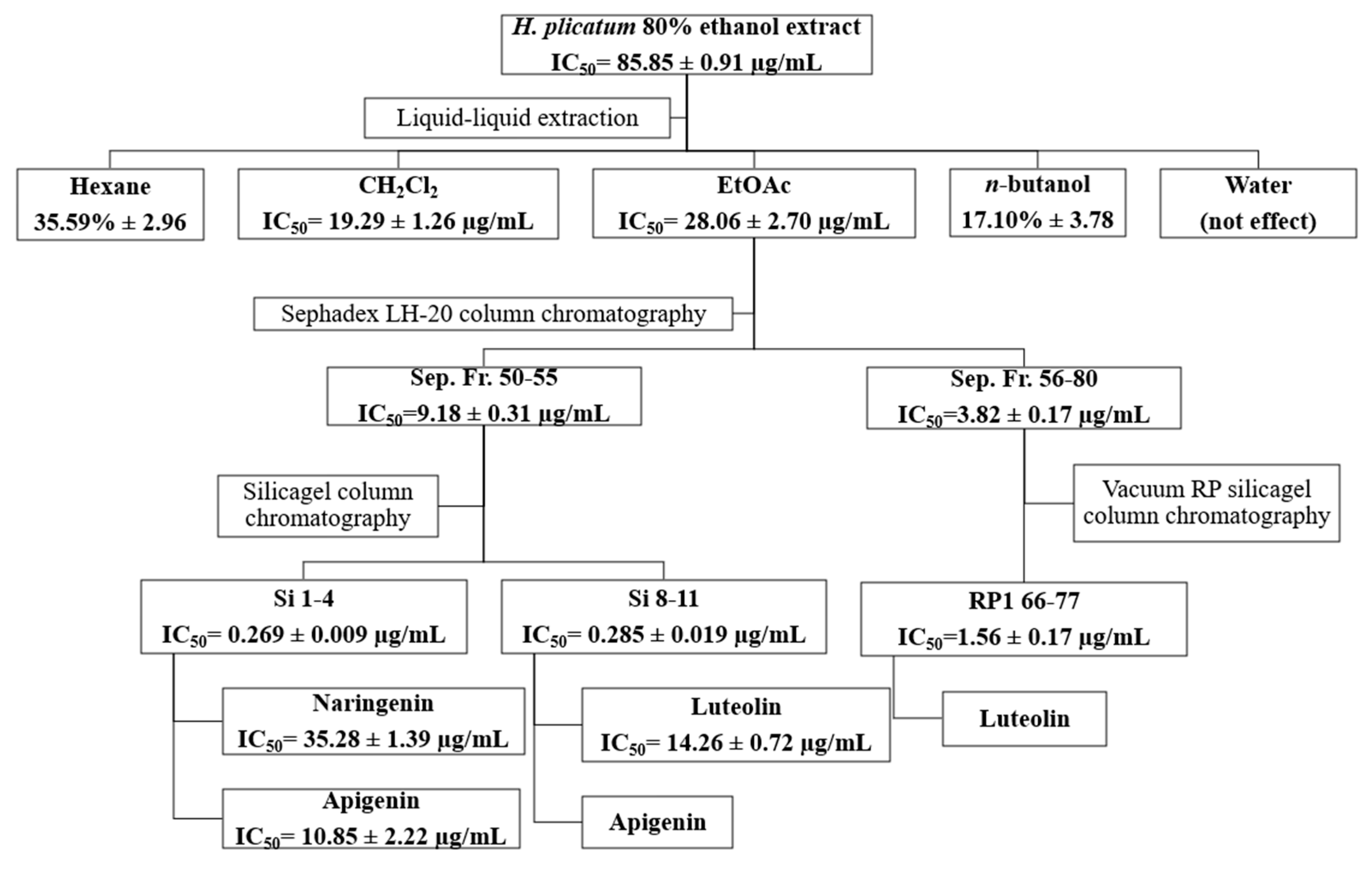
For the activity-guided isolation, the H. plicatum extract with the highest XO (IC50 = 85.85 ± 0.91 µg/mL) and POP inhibitory activities (99.99% ± 0.01) was selected. Based on the enzyme inhibitory activities of the sub-extracts (Table 4), the ethyl acetate (EtOAc) extract of H. plicatum was chosen for further isolation studies as it was effective against both hyaluronidase (76.28 ± 4.10%) and XO (IC50 = 28.06 ± 2.70 µg/mL). None of the sub-extracts exhibited anti-elastase activity. Due to the high XO inhibitory activity of H. plicatum and its EtOAc sub-extract, the activity of the fractions and pure compounds obtained from the activity-guided isolation study against the XO enzyme was assessed.

Table 4.
Enzyme inhibitory activities of sub-extracts obtained from H. plicatum extract.
The inhibitory activities of the fractions obtained using Sephadex LH-20 (Sep. Fr.), silica gel (Si) and vacuum reverse-phase (RP1) silica gel column chromatography techniques against XO are presented in Table 5. The most active fractions isolated from the EtOAc sub-extract via Sephadex LH-20 column chromatography were Sep. Fr. 50–55 (IC50 = 9.18 ± 0.31 µg/mL) and Sep. Fr. 56–80 (IC50 = 3.82 ± 0.17 µg/mL). Among the fractions obtained from silica gel column chromatography for Sep. 50–55, the most active were Si. 1–4 and Si. 8–11. For the fractions acquired from reverse-phase silica gel column chromatography for Sep. 56–80, the most active fraction was RP 66–77. The Si. 1–4, Si. 8–11, and RP 66–77 fractions exhibited similar TLC profiles; consequently, the subsequent activity-guided isolation studies used these fractions.

Table 5.
XO enzyme inhibitory activities of fractions obtained by various column chromatography methods from H. plicatum EtOAc sub-extract.
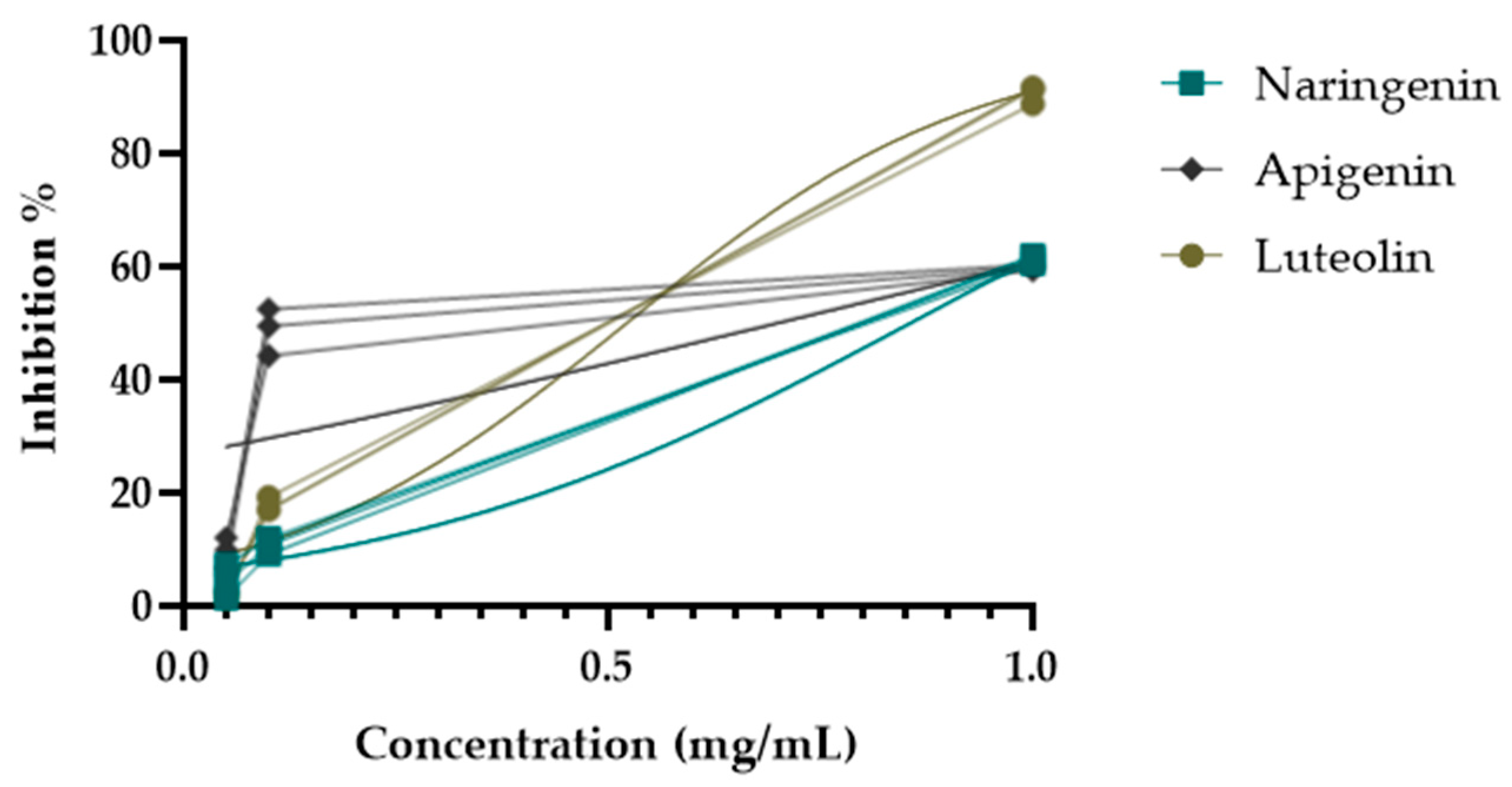
During the activity-guided isolation study of the H. plicatum extract, the inhibitory activity against XO increased. Apigenin (IC50 = 10.85 ± 2.22 µg/mL****) was identified as having a higher activity than luteolin (IC50 = 14.26 ± 0.72 µg/mL****) and naringenin (IC50 = 35.28 ± 1.39 µg/mL****) (Figure 5). Figure 6 provides a summary of the activity-guided isolation study results.
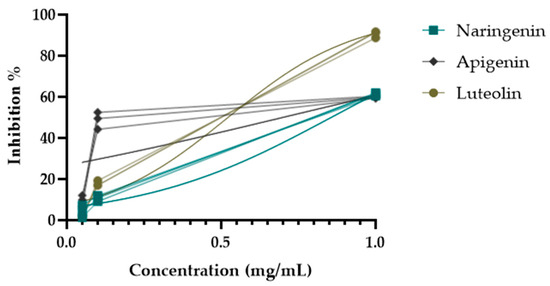
Figure 5.
XO enzyme inhibitory activities of isolated compounds.
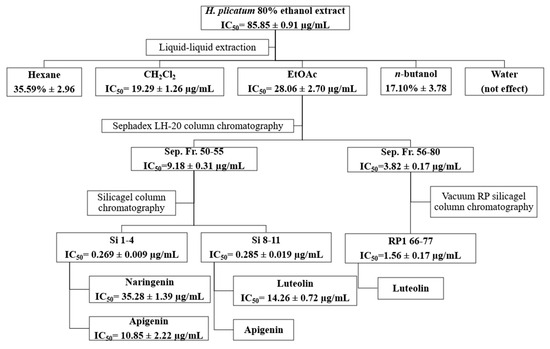
Figure 6.
Isolation of the active compounds by activity-guided fractionation.
2.4. Results of Antioxidant Activity Assays
The nitric oxide and superoxide radical-scavenging activities of the 80% ethanol extracts of the plant materials are presented in Table 6. In terms of nitric oxide radical-scavenging activity, H. plicatum (IC50 = 152.70 ± 3.68 µg/mL) and L. salicaria (IC50 = 133.00 ± 6.79 µg/mL) extracts exhibited high antioxidant activity. For the determination of superoxide radical-scavenging activity, the experimental results were obtained using vitamin C as the standard. L. salicaria demonstrated the strongest effect (274.77 mg vit C equivalent/g extract).

Table 6.
Antioxidant activities of plants employed in the treatment of varicose veins in traditional medicine.
3. Discussion
Approximately 150,000 new patients are diagnosed with CVI each year, and about USD 500 million is allocated to care for these patients [35]. The treatment of varicose veins and CVI is challenging because the cause remains unclear, and various factors contribute to the pathophysiology. Interventional treatment aimed at improving venous function is considered the first-line approach before other symptomatic treatment options are employed. If interventional treatment is not applicable or desired, or if symptoms persist following a therapeutic intervention, compression and pharmacological therapy may be used alone or in combination as symptom-based treatment options [36]. The most important group of pharmacological treatments is venoactive drugs [37,38]. The mechanism of action of venoactives is based on reducing capillary permeability, decreasing the release of inflammatory mediators, and enhancing venous tone. Generally, the application of flavonoids or flavonoid-rich fractions is beneficial in achieving therapeutic outcomes. Other secondary metabolites that positively affect vascular walls include triterpenic saponins. The mechanism of action of triterpenic saponins differs somewhat and includes the inhibition of certain enzymes responsible for degrading the structure of the vascular wall [5,39]. In venous pathophysiology, elevated venous pressure and changes in the flow force create an abnormal biomechanical environment in the vascular walls and valves. These biomechanical abnormalities lead to the premature release and activation of enzymes that induce endothelial dysfunction, degrade the extracellular matrix, and trigger a cascade of leukocyte infiltration and inflammation [40]. XO, hyaluronidase, elastase, collagenase, LOX, and POP are among the enzymes that create the abnormal conditions, such as inflammation, decreased resistance to venous pressure, increased vascular permeability, and oxidative stress, observed in CVI. Therefore, it is crucial to identify medicinal plants that can inhibit these enzymes for the treatment of varicose veins and CVI.
In our study, we first prepared 80% ethanolic extracts of plants used in traditional medicine for treating varicose veins to obtain various secondary metabolites from different plant parts, and conducted in vitro activity tests. When the enzyme inhibition results of the extracts were evaluated, the extract of H. plicatum, which demonstrated the highest inhibitory activity against POP (99.99% ± 0.01) and XO (IC50 = 85.85 ± 0.91 µg/mL), was selected for activity-guided isolation studies. H. plicatum has not previously been studied for the inhibition of enzymes associated with varicose veins. When the activity of the other plant extracts was assessed, it was found that the B. vulgaris fruit extract (95.24% ± 5.84) exhibited significant inhibitory activity against the POP enzyme. It is predicted that this effect may be due to berberine [41], which is known to be the major component of B. vulgaris. Studies have confirmed that berberine (IC50 = 142.0 ± 21.5 µM) is a potent POP inhibitor [42,43]. The extract from U. dioica (45.82% ± 2.48), which had the highest inhibitory activity against the LOX enzyme, has been extensively studied for its inflammation-reducing properties. In one study, after U. dioica was administered orally to experimental animals, it inhibited rat paw oedema similarly to indomethacin. The anti-inflammatory activity of U. dioica is attributed to the inhibition of cyclooxygenase, LOX, and cytokine production [44]. The extract from S. indicum seeds exhibited moderate inhibitory activity against the LOX enzyme (IC50 = 41.07 ± 3.77). Research has shown that sesamol, a lignan structure found in S. indicum, possesses strong LOX inhibitory properties that act in a dose-dependent manner (IC50 = 51.84 μM) [45]. Our results indicate that the L. salicaria leaf extract (IC50 = 70.76 ± 3.34 μg/mL) displayed the highest inhibitory activity against the hyaluronidase enzyme. In another study, it was demonstrated that the aerial parts of L. salicaria inhibited hyaluronidase activity in a dose-dependent manner with an IC50 of 10.1 ± 1.2 μg/mL and induced 94.4 ± 0.6% inhibition at a concentration of 20 μg/mL [46]. Further investigations revealed that L. salicaria achieved 64.9% (IC50 = 8.1 ± 0.8 μg/mL) inhibition of the hyaluronidase enzyme at a concentration of 10 μg/mL [47]. However, the NO radical- (IC50 = 133.00 ± 6.79 μg/mL) and superoxide anion-scavenging (274.77 vit C mg/extract g) activities of L. salicaria were the highest among our extracts. In one study, the IC50 values of the L. salicaria leaf extracts prepared using various methods showed an NO radical-scavenging activity between 0.73 ± 0.03 mg/mL and 1.41 ± 0.07 mg/mL. This activity was found to be weaker than that of quercetin (IC50 = 0.16 ± 0.01 mg/mL), which was used as the positive control [48]. In our study, it was observed that the L. salicaria extract demonstrated a weaker effect than vitamin C (IC50 = 25.28 ± 2.98 µg/mL), which was also used as a positive control. It can be concluded that this study provided results similar to those of previous studies.
The sub-extracts were prepared with different polarities using the H. plicatum ethanolic extract. The activity-guided isolation study used the EtOAc sub-extract, which was the most active against XO (IC50 = 28.06 ± 2.70 µg/mL) and hyaluronidase (76.28% ± 4.10). Due to the inhibitory activity of the H. plicatum extract against XO, this enzyme was selected as the determinant in the activity-guided isolation study. Eight fractions were obtained through Sephadex LH-20 column chromatography, with the active fractions being Sep. Fr. 50–55 (IC50 = 9.18 ± 0.31 µg/mL) and Sep. Fr. 56–80 (IC50 =3.82 ± 0.17 µg/mL). An evaluation of the TLC profiles of the Sep. Fr. 50–55 and Sep. Fr. 56–80 fractions showed that they contained similar compounds, which were targeted for the next step of the isolation process. Sep. Fr. 50–55 was separated using the silica gel column chromatography technique, while Sep. Fr. 56–80 was separated using vacuum reverse-phase silica gel column chromatography. In the isolation studies, the preparative TLC method was also employed, resulting in the isolation of compound 1, compound 2, and compound 3. The isolated compounds were identified by NMR to be naringenin, apigenin, and luteolin, respectively. Although the phytochemical content of H. plicatum has been elucidated through advanced chromatographic techniques, no isolation studies focused on biological activity have been conducted. This study marks the first time that three compounds predicted to be active were isolated from H. plicatum. Phytochemical studies of H. plicatum have confirmed the presence of compounds such as apigenin, naringenin, luteolin, kaempferol, and their glycosides, quercetin, dicaffeoylquinic acid, isoquercitrin, β-sitosterol, nonacosanoic acid, astragalin, β-sitosterol-3-O-β-D-glucoiranoside, helicrhysin A, helicrhysin B, isosalipurposide, chlorogenic acid, and chalcone derivatives [49,50,51]. In our study, we detected quinic acid, benzoic acid, fumaric acid, gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, protocatechuic aldehyde, salicylic acid, cynaroside, isoquercitrin, cosmosiin, astragalin, quercetin, naringenin, luteolin, kaempferol, apigenin, amentoflavone, and acacetin compounds in the H. plicatum extracts. These results are similar to those of other phytochemical studies.
During the activity-guided isolation study process starting from H. plicatum, the inhibitory activity against XO increased until the extract was reduced to the pure compounds. Si. 1–4 fractions (IC50 = 0.269 ± 0.009 µg/mL), naringenin, and apigenin were identified as the major constituents of the TLC profile. Si. 8–11 (IC50 = 0.285 ± 0.019 µg/mL), where apigenin and luteolin were the primary components in the TLC profile and exhibited a synergistic effect with one another, was found to be as effective as the positive control allopurinol (IC50 = 0.250 ± 0.006 µg/mL). When the inhibitory activities of the isolated compounds naringenin (IC50 = 35.28 ± 1.39 µg/mL), apigenin (IC50 = 10.85 ± 2.22 µg/mL), and luteolin (IC50 = 14.26 ± 0.72 µg/mL) against the XO enzyme were evaluated individually, the effect was weaker (Figure 6).
In other studies, naringenin demonstrated moderate inhibitory activity against XO [52,53]. Additionally, another study found that naringenin (IC50 = 4.57 μmol/L) was as active as the positive control allopurinol (IC50 = 4.05 μmol/L) [54]. One study revealed that apigenin was a strong competitive inhibitor of XO with an IC50 of 3.57 μM. The phenolic group of apigenin extends to the vicinity of the hydrophobic cavity, the active site of XO, through hydrogen bonds and electrostatic interactions [55]. Among the flavonoids isolated from Blumea balsamifera L., luteolin (IC50 = 2.38 ± 0.01 µM) exhibited the highest XO inhibitory activity [56]. Furthermore, another study found that luteolin (IC50 = 0.96 µM) displayed the highest activity after kaempferol, quercetin, and isorhamnetin [57].
Regarding the structure–activity relationship of flavonoids, it has been reported that the double bond at C-2 and C-3, along with the OH groups at C-5 and C-7, play an important role in inhibiting XO [58,59]. Our study observed that naringenin, which has lower XO-inhibiting activity compared to other flavonoids, may have a reduced effect due to the saturation of the double bond at C-2 and C-3. Previous studies have shown that the inhibitory activity against and affinity for XO decrease in the presence of the OH group at the C-3′ position in the B ring [59]. In this study, luteolin may exhibit lower XO-inhibiting activity than apigenin due to the OH group at C-3′.
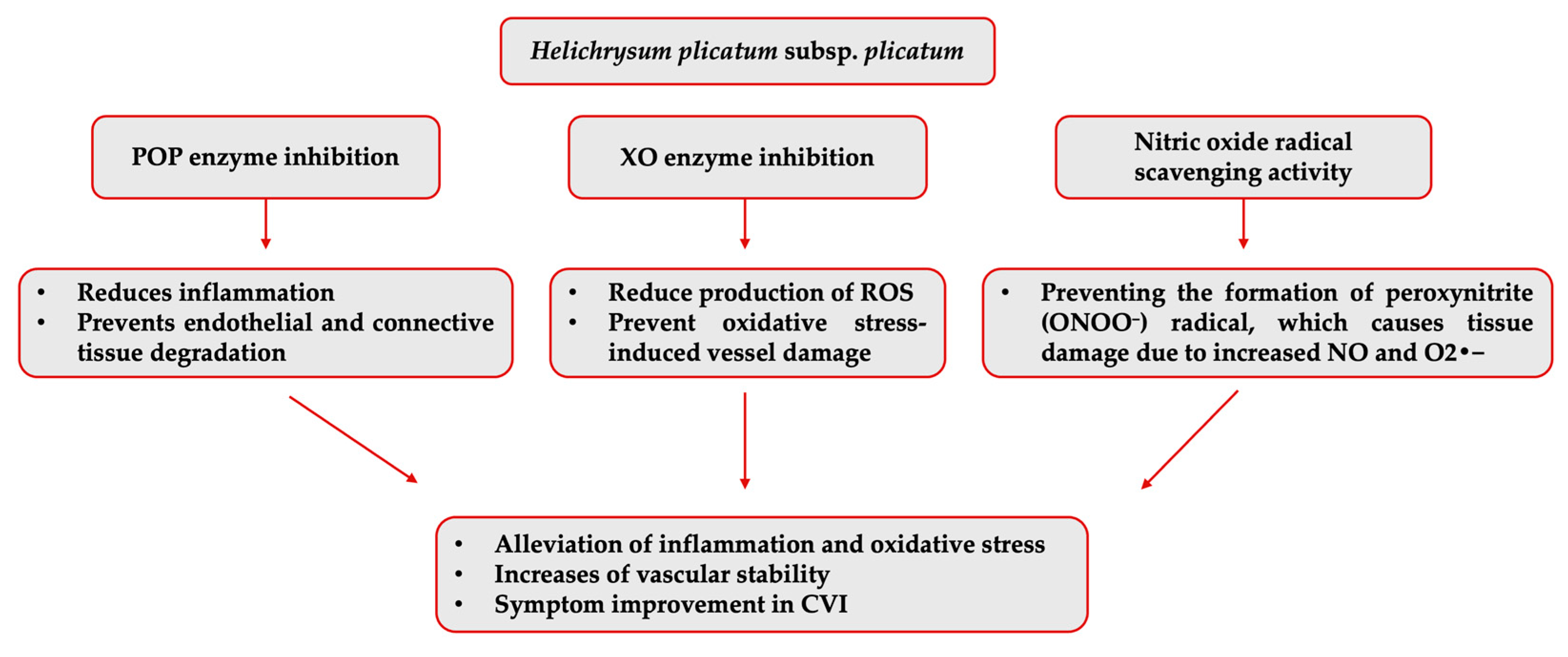
The isolated compounds were not individually evaluated regarding their inhibitory activities against the other varicose vein-related enzymes. However, another enzyme that H. plicatum (99.99% ± 0.01) has displayed high inhibitory activity against is POP. Activity studies have found that naringenin [60] did not exhibit inhibitory activity against the POP enzyme, and luteolin showed high inhibitory activity (IC50 = 0.17 ppm); there was no data for apigenin [61]. Luteolin may contribute to the high activity of H. plicatum. However, previous structure–activity studies suggested that quinic acid and chlorogenic acid, which are the predominant components in H. plicatum extracts, may be responsible for the POP inhibitory activity due to the presence of the pyrogallol group [62]. This study determined that naringenin, apigenin, and luteolin, as the potential active compounds in the H. plicatum extract that can treat varicose veins, may help reduce oxidative stress by supporting antioxidant mechanisms in CVI due to their strong ability to inhibit the XO enzyme when combined (Figure 7).
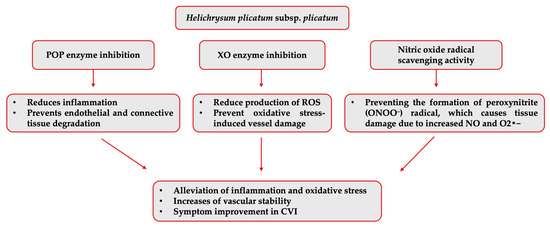
Figure 7.
Potential mechanism of action of H. plicatum extract against varicose veins.
4. Materials and Methods
4.1. Plant Materials and Extraction Methods
Information on the plant species screened in this study is presented in Table 7. H. plicatum was collected from Osmaniye province, while the other plant taxa were sourced from Konya province. The collected plant samples were identified by Prof. Dr. Osman Tugay. Herbarium samples have been preserved in the herbarium of the Faculty of Science, Selçuk University.

Table 7.
The extraction yields of plant materials and their applications in the treatment of varicose veins in traditional medicine.
All plant samples were macerated using 80% ethanol at room temperature for 5 days. Subsequently, filter paper was used to filter the samples, and the remaining filtrate was evaporated to dryness in a rotary evaporator (Büchi, Flawil, Switzerland). The extracts were weighed and dissolved in dimethyl sulfoxide (DMSO) to reach a concentration of 2 mg/mL, which was used in the in vitro experiments.
4.2. Activity-Guided Isolation Study
The ethanol extracts of H. plicatum (30 g) were dissolved in 90% methanol (MeOH) and subjected to liquid–liquid fractionation using hexane, dichloromethane (CH2Cl2), EtOAc, n-butanol, and water. The organic phases were evaporated to dryness, yielding five fractions along with the remaining aqueous phase. Finally, the aqueous phase was lyophilised.
4.2.1. Fractionation of EtOAc Extract
These fractions were subsequently screened for their inhibitory activities against varicose veins-related enzymes. The most active EtOAc (4.9966 g) fraction was fractionated using Sephadex LH-20 column chromatography (3 cm × 60 cm). MeOH served as the mobile phase for the separation. The profiles that exhibited similarities in their TLC profiles were combined, yielding a total of eight fractions (Sep. Fr.). The XO enzyme inhibitory activities of these fractions (Sep. Fr.) were tested.
4.2.2. Fractionation of Sep. Fr. 50–55
The active fraction Sep. Fr. 50–55 was separated using the silica gel column chromatography technique [mobile phase: chloroform CHCl3/MeOH/water at ratios of 90:10:1, 80:20:2, 70:30:3, 61:32:7, and 50:5:5]. Compounds 1 and 2 were isolated from the Si 1–4 fraction using the preparative (prep) TLC method [stationary phase: silica gel; mobile phase: CHCl3/MeOH/water (90:10:1)].
4.2.3. Fractionation of Sep. Fr. 56–80
Sep. Fr. 56–80 was separated into four fractions using the vacuum reverse-phase silica gel column chromatography technique [mobile phase: water/MeOH at ratios of 100:0, 98:2, 93:7, 80:20, 75:25, 70:30, 50:50, 25:75, and 0:100]. The most active RP1 66–77 fraction was isolated from compound 3 by employing the vacuum reverse-phase silica gel column chromatography technique [mobile phase: water/MeOH at ratios of 85:15, 82:18, 79:21, 75:25, 72:28, 70:30, 68:32, 65:35, 60:40, 57:43, 55:45, and 50:50].
4.3. LC-MS/MS Analysis Methods
The LC-MS/MS analysis was performed using a Shimadzu Nexera model ultrahigh-performance liquid chromatography (UHPLC) system, coupled with a tandem mass spectrometer, to quantitatively evaluate 53 phytochemicals. The conditions of the chromatographic analysis were the same as those used in previous studies [63,64].
4.4. Microtiter Assays for Enzyme Inhibition
The enzyme inhibition assays conducted in this study included elastase, collagenase, hyaluronidase, XO, and prolyl POP inhibition and were performed using an ELISA microplate reader (Spectramax i3x microplate reader with Softmax® Pro Software for Windows 10; Molecular Devices, San Jose, USA). Elastase inhibition by the extracts was assessed using a modified spectrophotometric method based on Kraunsoe et al. (1996) [65] and Lee et al. (2009) [66]. Collagenase inhibition was evaluated using the modified spectrophotometric method by Barrantes and Guinea 2003 [67], which was originally developed by Wart and Steinbrink (1981) [68]. The LOX inhibitory activities of the extracts were determined following the method of Chung et al. (2009), with minor modifications [69]. XO inhibition was measured spectrophotometrically by monitoring the absorbance at 295 nm from uric acid formed during an enzymatic reaction [70]. Inhibition of the POP enzyme was conducted on the five extracts that exhibited the highest activity for the other enzymes. To determine POP enzyme inhibitory activity, a Fluorogenic Prolyl OligoPeptidase (POP) test kit (catalogue number: 80106) from BPS Bioscience Inc. (San Diego, CA, USA) was utilised.
4.5. Antioxidant Activity Assays
The determination of the superoxide radical (O2•−)-scavenging effect was conducted using the method described by Duh et al. [71,72]. To assess the nitric oxide (NO)-scavenging effect, the method of Sreejayan and Rao was utilised with some modifications [73].
4.6. Statistical Analysis
For the activity assays, the test groups were compared with the positive control using one-way ANOVA followed by Dunnett’s multiple comparison test and the results were statistically analysed (GraphPad Prism 8.0). p values ≤ 0.05 were considered statistically significant.
5. Conclusions
In conclusion, an activity-guided isolation study was conducted and the H. plicatum extract demonstrated the highest activity in in vitro enzyme inhibition assays. Naringenin, apigenin, and luteolin were identified as the most active compounds. The fact that these isolated compounds belong to the flavonoid class, which includes venoactive drugs used to treat CVI disease, underscores the importance of flavonoids in this condition. This study determined that naringenin, apigenin, and luteolin may help reduce oxidative stress by bolstering the antioxidant mechanisms in CVI due to their ability to inhibit the XO enzyme. Furthermore, the observation that the naringenin–apigenin and apigenin–luteolin fractions displayed XO inhibitory activity as robust as that of allopurinol suggests that these compounds might have synergistic effects, and their combined application may yield more effective results for CVI. Our research into active extracts and isolated compounds using the human endothelial cell line (HUVEC) is ongoing. We are currently conducting a cell culture test using HUVECs and chemically inducing hypoxia to mimic the conditions associated with varicose veins. We plan to perform in vivo experiments based on the results obtained from cell culture and in silico drug-likeness parameters, as well as ADME profile analysis of active and major compounds in future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph18060926/s1, Figure S1: 1H-NMR spectrum of Naringenin (Compound 1); Figure S2: 13C-NMR spectrum of Naringenin (Compound 1); Figure S3: COSY spectrum of Naringenin (Compound 1); Figure S4: HMBC spectrum of Naringenin (Compound 1); Figure S5: HMQC spectrum of Naringenin (Compound 1); Figure S6: ESI-MS spectrum of Naringenin (Compound 1) -ESI MS spectrum and +ESI MS spectrum, respectively; Figure S7: 1H-NMR spectrum of Apigenin (Compound 2); Figure S8: 13C-NMR spectrum of Apigenin (Compound 2); Figure S9: COSY spectrum of Apigenin (Compound 2); Figure S10: HMBC spectrum of Apigenin (Compound 2); Figure S11: HMQC spectrum of Apigenin (Compound 2); Figure S12: ESI-MS spectrum of Apigenin (Compound 2) -ESI MS spectrum and +ESI MS spectrum, respectively; Figure S13: 1H spectrum of Luteolin (Compound 3); Figure S14: 13C spectrum of Luteolin (Compound 3); Figure S15: COSY spectrum of Luteolin (Compound 3); Figure S16: HMBC spectrum of Luteolin (Compound 3); Figure S17: HMQC spectrum of Luteolin (Compound 3); Figure S18: ESI-MS spectrum of Luteolin (Compound 3) -ESI MS spectrum and +ESI MS spectrum, respectively.
Author Contributions
Conceptualisation, F.S.S.D. and T.B.; methodology, T.B., F.S.S.D., M.Y.B., E.S., and M.A.Y.; software, M.A.Y. and T.B.; validation, M.A.Y. and T.B.; investigation, T.B., F.S.S.D., M.A.Y., E.S., and M.Y.B.; resources, O.T.; data curation, T.B., F.S.S.D., M.Y.B., E.S., and M.A.Y.; writing—original draft preparation, T.B., F.S.S.D., M.Y.B., E.S., M.A.Y., and O.T.; writing—review and editing, T.B. and F.S.S.D.; supervision, F.S.S.D.; project administration, F.S.S.D.; funding acquisition, F.S.S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Gazi University Scientific Research Projects Coordination Unit (grant number TDK-2024-9222). The current study is a part of the Ph.D. thesis of Tugsen Buyukyıldırım at the Institute of Health Sciences, Gazi University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shahrajabian, M.H.; Sun, W. Five important seeds in Traditional Medicine, and pharmacological benefits. Seeds 2023, 2, 290–308. [Google Scholar] [CrossRef]
- Salm, S.; Rutz, J.; van den Akker, M.; Blaheta, R.A.; Bachmeier, B.E. Current state of research on the clinical benefits of herbal medicines for non-life-threatening ailments. Front. Pharmacol. 2023, 14, 1234701. [Google Scholar] [CrossRef] [PubMed]
- Beebe-Dimmer, J.L.; Pfeifer, J.R.; Engle, J.S.; Schottenfeld, D. The epidemiology of chronic venous insufficiency and varicose veins. Ann. Epidemiol. 2005, 15, 175–184. [Google Scholar] [CrossRef]
- Silva, M.J.; Louzada, A.C.S.; da Silva, M.F.A.; Portugal, M.F.C.; Teivelis, M.P.; Wolosker, N. Epidemiology of 869,220 varicose vein surgeries over 12 years in Brazil: Trends, costs and mortality rate. Ann. Vasc. Surg. 2022, 82, 1–6. [Google Scholar] [CrossRef]
- Bencsik, T.; Balázs, V.L.; Farkas, Á.; Csikós, E.; Horváth, A.; Ács, K.; Kocsis, M.; Doseděl, M.; Fialová, S.B.; Czigle, S. Herbal drugs in chronic venous disease treatment: An update. Fitoterapia 2024, 179, 106256. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, R.T.; Raffetto, J.D. Chronic venous insufficiency. Circulation 2014, 130, 333–346. [Google Scholar] [CrossRef]
- Wisastra, R.; Dekker, F.J. Inflammation, cancer and oxidative lipoxygenase activity are intimately linked. Cancers 2014, 6, 1500–1521. [Google Scholar] [CrossRef]
- Casili, G.; Lanza, M.; Campolo, M.; Messina, S.; Scuderi, S.; Ardizzone, A.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Therapeutic potential of flavonoids in the treatment of chronic venous insufficiency. Vasc. Pharmacol. 2021, 137, 106825. [Google Scholar] [CrossRef]
- Buján, J.; Gimeno, M.J.; Jiménez, J.A.; Kielty, C.M.; Mecham, R.P.; Bellón, J.M. Expression of elastic components in healthy and varicose veins. World J. Surg. 2003, 27, 901–905. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Khalil, R.A. Mechanisms of lower extremity vein dysfunction in chronic venous disease and implications in management of varicose veins. Vessel Plus 2021, 5, 36. [Google Scholar] [CrossRef]
- Niebes, P. Determination of enzymes and degradation products of glycosaminoglycan metabolism in the serum of healthy and varicose subjects. Clin. Chim. Acta 1972, 42, 399–408. [Google Scholar] [CrossRef]
- Alsaigh, T.; Fukaya, E. Varicose veins and chronic venous disease. Cardiol. Clin. 2021, 39, 567–581. [Google Scholar] [CrossRef]
- Phan, S.H.; Gannon, D.E.; Ward, P.A.; Karmiol, S. Mechanism of neutrophil-induced xanthine dehydrogenase to xanthine oxidase conversion in endothelial cells: Evidence of a role for elastase. Am. J. Respir. Cell Mol. Biol. 1992, 6, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Glowinski, J.; Glowinski, S. Generation of reactive oxygen metabolites by the varicose vein wall. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Gawas, M.; Bains, A.; Janghu, S.; Kamat, P.; Chawla, P. A comprehensive review on varicose veins: Preventive measures and different treatments. J. Am. Nutr. Assoc. 2022, 41, 499–510. [Google Scholar] [CrossRef]
- Singh, A.; Gattani, R. A Narrative Review of Advancements in Understanding and Treating Varicose Veins. Cureus 2023, 15, e48093. [Google Scholar] [CrossRef]
- Okley, D. Systemic phlebotropic drugs in pharmacotherapy of chronic venous insufficiency of the lower extremities. News Pharm. 2015, 4, 74–77. [Google Scholar] [CrossRef]
- Wadworth, A.N.; Faulds, D. Hydroxyethylrutosides: A review of its pharmacology, and therapeutic efficacy in venous insufficiency and related disorders. Drugs 1992, 44, 1013–1032. [Google Scholar] [CrossRef]
- Büyükyıldırım, T.; Deniz, F.S.Ş. Natural treatment approaches for varicose veins: A brief review of the literature. Trak. Univ. J. Nat. Sci. 2024, 25, 121–132. [Google Scholar] [CrossRef]
- Akgül, G.; Yılmaz, N.; Celep, A.; Celep, F.; Çakılcıoğlu, U. Ethnobotanical purposes of plants sold by herbalists and folk bazaars in the center of Cappadocica (Nevșehir, Turkey). Indian J. Tradit. Knowl. 2016, 15, 103–108. [Google Scholar]
- Karcı, E.; Gürbüz, İ.; Akaydın, G.; Günbatan, T. Folk medicines of Bafra (samsun-Turkey). Turk. J. Biochem. 2017, 42, 381–399. [Google Scholar] [CrossRef]
- Güzel, Y.; Güzelşemme, M.; Miski, M. Ethnobotany of medicinal plants used in Antakya: A multicultural district in Hatay Province of Turkey. J. Ethnopharmacol. 2015, 174, 118–152. [Google Scholar] [CrossRef]
- Günbatan, T.; Gürbüz, İ.; Özkan, A.M.G. The current status of ethnopharmacobotanical knowledge in Çamlıdere (Ankara, Turkey). Turk. J. Bot. 2016, 40, 241–249. [Google Scholar] [CrossRef]
- Korkmaz, M.; Karakuş, S.; Selvi, S. An ethnobotanical study on medicinal plants in Erzincan, Turkey. Indian J. Tradit. Knowl. 2016, 15, 192–202. [Google Scholar]
- Sevgi, E.; Kızılarslan-Hançer, Ç.; Akkaya, M.; Altundağ-Çakır, E.; Büyükkılıç-Altınbaşak, B. An ethnobotanical survey of medicinal plants in Biga (Çanakkale-Turkey). Indian J. Tradit. Knowl. 2022, 21, 583–594. [Google Scholar]
- Korkmaz, M.; Karakurt, E. An ethnobotanical investigation to determine plants used as folk medicine in Kelkit Gümüşhane/Turkey district. Biol. Divers. Conserv. 2015, 8, 290–303. [Google Scholar]
- Akan, H. Kahta (Adıyaman) merkezi ve Narince köyünün etnobotanik açıdan araştırılması. Bitlis Eren Univ. J. Sci. 2015, 4, 219–248. [Google Scholar] [CrossRef]
- Tunalier, Z.; Koşar, M.; Küpeli, E.; Çalış, İ.; Başer, K.H.C. Antioxidant, anti-inflammatory, anti-nociceptive activities and composition of Lythrum salicaria L. extracts. J. Ethnopharmacol. 2007, 110, 539–547. [Google Scholar] [CrossRef]
- Kalankan, G.; Özkan, Z.C.; Akbulut, S. Medicinal and aromatic wild plants and traditional usage of them in Mount Ida (Balıkesir/Turkey). J. Appl. Biol. Sci. 2015, 9, 25–33. [Google Scholar]
- Sargin, S.A.; Büyükcengiz, M. Plants used in ethnomedicinal practices in Gulnar district of Mersin, Turkey. J. Herb. Med. 2019, 15, 100224. [Google Scholar] [CrossRef]
- Kültür, Ş. Medicinal plants used in Kırklareli province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Backheet, E.Y.; Farag, S.F.; Ahmed, A.S.; Sayed, H.M. Flavonoids and cyanogenic glycosides from the leaves and stem bark of Prunus persica (L.) Batsch (Meet Ghamr) peach local cultivar in Assiut region. Bull. Pharm. Sci. Assiut Univ. 2003, 26, 55–66. [Google Scholar] [CrossRef]
- Sary, H.G.; Ayoub, N.A.; Singab, A.B.; Ahmed, A.H.; Al-Azizi, M.M. Chemical constituents and molluscicidal activity of Iris pseudacorus L. cultivated in Egypt. Bull. Pharm. Sci. Assiut Univ. 2004, 27, 161–169. [Google Scholar] [CrossRef]
- Lin, L.C.; Pai, Y.F.; Tsai, T.H. Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. J. Agric. Food Chem. 2015, 63, 7700–7706. [Google Scholar] [CrossRef]
- Patel, S.K.; Surowiec, S.M. Venous insufficiency. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Stucker, M.; Debus, E.S.; Hoffmann, J.; Jünger, M.; Kröger, K.; Mumme, A.; Ramelet, A.A.; Rabe, E. Consensus statement on the symptom-based treatment of chronic venous diseases. JDDG J. Ger. Soc. Dermatol. 2016, 14, 575–583. [Google Scholar] [CrossRef]
- Gloviczki, P.; Lawrence, P.F.; Wasan, S.M.; Meissner, M.H.; Almeida, J.; Brown, K.R.; Bush, R.L.; Di Iorio, M.; Fish, J.; Fukaya, E. The 2023 Society for Vascular Surgery, American Venous Forum, and American Vein and Lymphatic Society clinical practice guidelines for the management of varicose veins of the lower extremities. Part II: Endorsed by the Society of Interventional Radiology and the Society for Vascular Medicine. J. Vasc. Surg. Venous Lymphat. Disord. 2024, 12, 101670. [Google Scholar]
- Wasan, S. Chronic venous insufficiency evaluation and medical management. Curr. Cardiol. Rep. 2024, 26, 1241–1247. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef]
- Pocock, E.S.; Alsaigh, T.; Mazor, R.; Schmid-Schönbein, G.W. Cellular and molecular basis of venous insufficiency. Vasc. Cell 2014, 6, 24. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Imenshahidi, M.; Hosseinzadeh, H. A review of the effects of Berberis vulgaris and its major component, berberine, in metabolic syndrome. Iran. J. Basic Med. Sci. 2017, 20, 557. [Google Scholar]
- Cahlíková, L.; Hulová, L.; Hrabinová, M.; Chlebek, J.; Hošťálková, A.; Adamcová, M.; Šafratová, M.; Jun, D.; Opletal, L.; Ločárek, M. Isoquinoline alkaloids as prolyl oligopeptidase inhibitors. Fitoterapia 2015, 103, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Tarrago, T.; Kichik, N.; Seguí, J.; Giralt, E. The natural product berberine is a human prolyl oligopeptidase inhibitor. ChemMedChem 2007, 2, 354–359. [Google Scholar] [CrossRef]
- Dhouibi, R.; Affes, H.; Salem, M.B.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Screening of pharmacological uses of Urtica dioica and others benefits. Prog. Biophys. Mol. Biol. 2020, 150, 67–77. [Google Scholar] [CrossRef]
- Yashaswini, P.; Rao, A.A.; Singh, S.A. Inhibition of lipoxygenase by sesamol corroborates its potential anti-inflammatory activity. Int. J. Biol. Macromol. 2017, 94, 781–787. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Granica, S.; Kiss, A.K. Lythrum salicaria L.—Underestimated medicinal plant from European traditional medicine. A review. J. Ethnopharmacol. 2015, 170, 226–250. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Kiss, A.K.; Kozłowska-Wojciechowska, M. Anti-hyaluronidase and anti-elastase activity screening of tannin-rich plant materials used in traditional Polish medicine for external treatment of diseases with inflammatory background. J. Ethnopharmacol. 2011, 137, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, M.; Shabani, E.; Hashemi, Z.; Ebrahimzadeh, M. Evaluation of three methods for the extraction of antioxidants from leaf and aerial parts of Lythrum salicaria L. (Lythraceae). Int. Food Res. J. 2014, 21. [Google Scholar]
- Aydin, T. Secondary metabolites of Helichrysum plicatum DC. subsp. plicatum flowers as strong carbonic anhydrase, cholinesterase and α-glycosidase inhibitors. Z. Naturforsch. C 2020, 75, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Bigović, D.; Šavikin, K.; Janković, T.; Menković, N.; Zdunić, G.; Stanojković, T.; Djurić, Z. Antiradical and cytotoxic activity of different Helichrysum plicatum flower extracts. Nat. Prod. Commun. 2011, 6, 1934578X1100600617. [Google Scholar] [CrossRef]
- Taşkın, T.; Gezmiş, T.; Çam, M.E.; Taşkın, D.; Çelik, B.Ö.; Şenkardeş, İ.; Süzgeç-Selçuk, S. The in vitro and in vivo investigation of biological activities and phenolic analysis of Helichrysum plicatum subsp. plicatum. Braz. J. Pharm. Sci. 2020, 56, e18345. [Google Scholar] [CrossRef]
- Balázs, O.; Dombi, Á.; Zsidó, B.Z.; Hetényi, C.; Valentová, K.; Vida, R.G.; Poór, M. Inhibition of xanthine oxidase-catalyzed xanthine and 6-mercaptopurine oxidation by luteolin, naringenin, myricetin, ampelopsin and their conjugated metabolites. Biomed. Pharmacother. 2023, 167, 115548. [Google Scholar] [CrossRef] [PubMed]
- Iio, M.; Moriyama, A.; Matsumoto, Y.; Takaki, N.; Fukumoto, M. Inhibition of xanthine oxidase by flavonoids. Agric. Biol. Chem. 1985, 49, 2173–2176. [Google Scholar]
- Zhang, X.; Liu, L.; Fan, Z.; Mamadalieva, N.; Liu, C.; Guo, X.; Sun, S.; Sun, H.; Li, N.; Wang, M. Bioactive evaluation of naringenin in ameliorating hyperuricemia-induced liver injury by inhibiting xanthine oxidase. eFood 2025, 6, e70032. [Google Scholar] [CrossRef]
- Su, Z.-R.; Fan, S.-Y.; Shi, W.-G.; Zhong, B.-H. Discovery of xanthine oxidase inhibitors and/or α-glucosidase inhibitors by carboxyalkyl derivatization based on the flavonoid of apigenin. Bioorg. Med. Chem. Lett. 2015, 25, 2778–2781. [Google Scholar] [CrossRef] [PubMed]
- Nessa, F.; Ismail, Z.; Mohamed, N. Xanthine oxidase inhibitory activities of extracts and flavonoids of the leaves of Blumea balsamifera. Pharm. Biol. 2010, 48, 1405–1412. [Google Scholar] [CrossRef]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of xanthine oxidase by flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Berghe, D.V. Structure–activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary flavonoids as xanthine oxidase inhibitors: Structure–affinity and structure–activity relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef]
- Ali, H.; Ullah, K.; Siddiqui, H.; Iqbal, S.; Goren, N.; Ayatollahi, S.A.; Choudhary, M.I. Chemical constituents from Parrotia persica—Structural derivatization and their potential prolyl endopeptidase inhibition activity. Bioorg. Chem. 2020, 96, 103526. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kwak, J.H.; Lee, K.-B.; Song, K.-S. Prolyl endopeptidase inhibitors from Caryophylli Flos. Arch. Pharm. Res. 1998, 21, 207–211. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jun, M.; Choi, J.-Y.; Yang, E.-J.; Hur, J.-M.; Bae, K.; Seong, Y.-H.; Huh, T.-L.; Song, K.-S. Plant phenolics as prolyl endopeptidase inhibitors. Arch. Pharm. Res. 2007, 30, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.A. Simultaneous quantitative screening of 53 phytochemicals in 33 species of medicinal and aromatic plants: A detailed, robust and comprehensive LC–MS/MS method validation. Ind. Crops Prod. 2020, 149, 112347. [Google Scholar] [CrossRef]
- Deniz, F.S.S.; Orhan, I.E.; Filipek, P.A.; Ertas, A.; Gstir, R.; Jakschitz, T.; Bonn, G.K. Evaluation of the anti-aging properties of ethanolic extracts from selected plant species and propolis by enzyme inhibition assays and 2D/3D cell culture methods. Pharmaceuticals 2025, 18, 439. [Google Scholar] [CrossRef]
- Kraunsoe, J.A.; Claridge, T.D.; Lowe, G. Inhibition of human leukocyte and porcine pancreatic elastase by homologues of bovine pancreatic trypsin inhibitor. Biochemistry 1996, 35, 9090–9096. [Google Scholar] [CrossRef]
- Lee, S.; Sandesh, S.; Shruti, S.; Seo, S. Potent antielastase and antityrosinase activities of Astilbe chinensis. Am. J. Pharmacol. Toxicol. 2009, 4, 127–129. [Google Scholar] [CrossRef]
- Barrantes, E.; Guinea, M.a. Inhibition of collagenase and metalloproteinases by aloins and aloe gel. Life Sci. 2003, 72, 843–850. [Google Scholar] [CrossRef]
- Van Wart, H.E.; Steinbrink, D.R. A continuous spectrophotometric assay for Clostridium histolyticum collagenase. Anal. Biochem. 1981, 113, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.Y.; Soo, W.K.; Chan, K.Y.; Mustafa, M.R.; Goh, S.H.; Imiyabir, Z. Lipoxygenase inhibiting activity of some Malaysian plants. Pharm. Biol. 2009, 47, 1142–1148. [Google Scholar] [CrossRef]
- Khan, S.B.; Afza, N.; Malik, A.; Azhar-Ul-Haq; Perveen, S.; Ahmad, I.; Ejaz, A.; Iqbal Choudhary, M. Xanthine oxidase inhibiting flavonol glycoside from Amberboa ramosa. Nat. Prod. Res. 2006, 20, 335–339. [Google Scholar] [CrossRef]
- Duh, P.-D.; Tu, Y.-Y.; Yen, G.-C. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat). LWT–Food Sci. Technol. 1999, 32, 269–277. [Google Scholar] [CrossRef]
- Miser-Salihoglu, E.; Akaydin, G.; Caliskan-Can, E.; Yardim-Akaydin, S. Evaluation of antioxidant activity of various herbal folk medicines. J. Nutr. Food. Sci. 2013, 3, 222. [Google Scholar]
- Sreejayan, X.; Rao, M. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).