Abstract
Acute lymphoblastic leukemia (ALL) is a malignant tumor caused by abnormal proliferation of B-line or T-line lymphocytes in the bone marrow. Traditional treatments have limitations. Because of their unique advantages, nanodrug delivery systems (NDDSs) show great potential in the treatment of ALL. In this paper, the pathological features of ALL, the limitations of current therapeutic methods, and the definition and composition of NDDSs were reviewed. Research strategies for the use of NDDSs in the treatment of ALL were discussed. In addition, challenges and future development directions of NDDSs in the treatment of ALL were also discussed, aiming to provide reference for the application of NDDSs in the diagnosis and treatment of ALL.
1. Introduction
Acute lymphoblastic leukemia (ALL) is a malignant tumor of the blood system, with an incidence of 20% in adults with acute leukemia and 80% in children with acute leukemia [1]. It is characterized by the abnormal proliferation and aggregation of juvenile lymphocytes in bone marrow and lymphoid tissue. The incidence of ALL peaks between the ages of 1 and 4, and then drops sharply, reaching its lowest point between the ages of 25 and 45, and rising slightly after the age of 50, with a higher incidence in men than in women [2]. At present, chemotherapy is still the first-line treatment for ALL, but traditional chemotherapy drugs have some problems, such as poor targeting and severe toxicity and side effects, which limit their clinical application. Another conventional treatment is chimeric antigen receptor T cell (CAR T cell) therapy, which has shown great promise in the treatment of childhood ALL [2]. However, there is currently only one FDA-approved CD-19-targeted CAR-T cell product (tisagenlecleucel) available for use in patients under 25 years of age [3]. Moreover, relapse remains a major obstacle to the treatment of ALL. As a new therapeutic strategy, nanodrug delivery systems (NDDSs) have been widely investigated for use in the treatment of ALL, due to their advantages of improving drug targeting, reducing toxic side effects, and enhancing therapeutic effects.
This paper aims to review the latest research strategies regarding the use of NDDSs in the treatment of ALL, discusses their application advantages, challenges, and future development directions, and provides new ideas and a theoretical basis for the precision treatment of ALL.
2. ALL
ALL is a common malignant disease of the blood system. It is mainly characterized by abnormal clonal proliferation of immature T and B lymphocytes, infiltrating the bone marrow, blood, or other tissues and organs, and causing abnormal hematopoietic function of the bone marrow. The clinical manifestations of ALL are diverse and involve multiple systems. The main manifestations are anemia, fever, bleeding, lymph node, liver and spleen enlargement, and bone and joint pain. ALL is the most common tumor in patients under 15 years of age, and its incidence in childhood is much higher than that of acute myeloid leukemia. ALL accounts for 20% of acute leukemia in adults and 80% of acute leukemia in children [1]. The etiology and pathogenesis of ALL are not clear, may be caused by genetic factors, environmental factors, viral infection, radiation factors, and chemical factors.
The main pathophysiological features of ALL include the following aspects: it involves extensive and uncontrolled abnormal proliferation of leukemia cells, which can infiltrate tissues and organs of the whole body, in addition to involving the hematopoietic system; some leukemia stem cells (LSCs) in ALL are in a relatively resting state, and can escape the killing of most cell cycle-specific cytotoxic drugs and lurk in the body for a long time, becoming the root of relapse; ALL has a complex bone marrow microenvironment, where ALL cells interact with the bone marrow hematopoietic microenvironment, and the proliferation of ALL cells and the progression of the disease depend on the bone marrow microenvironment; and there is a blood–bone marrow barrier (BMB) between the blood circulation and bone marrow, which is very important for allowing blood cells to enter the circulation regularly. In addition, it can selectively “trap” nutrients, drugs, and other substances, resulting in unsatisfactory clearance effect of residual LSCs in bone marrow.
Combination chemotherapy is the foundational treatment for ALL. The current chemotherapy regimen for ALL patients mainly consists of induced remission, consolidation therapy, and maintenance therapy phases. Although chemotherapy can cure 80% of ALL patients, the systemic toxic side effects related to chemotherapy are still a difficult problem affecting the survival rate and quality of life of ALL patients. Hematopoietic stem cell transplantation (HSCT) is another important treatment method for ALL patients, and is of great value in the treatment of refractory and recurrent ALL [4]. HSCT is usually decided on the basis of the patient’s condition after the combination chemotherapy has achieved a complete response. But relapse is still the leading cause of transplant failure, and analysis of data from patients with relapsed T-ALL found that patients who had not received high doses of chemotherapy had little chance of a cure after autologous or allogeneic HSCT [5]. For refractory/recurrent ALL, with rechemotherapy and retransplantation, the overall survival rate is only 10% to 20%, and the prognosis is poor. With the advent of immunotherapy, there is hope for patients with refractory/recurrent ALL. At present, the immunotherapy used to treat refractory and relapsing ALL is CAR-T, and the re-complete response rate of refractory/relapsing B-ALL can reach 90% [6,7]. However, there are still some problems with CAR-T cell therapy, such as adverse reactions encephalopathy, cytokine release syndrome (CRS), and graft-versus-host disease (GVHD) [8]. Moreover, CAR-T cell therapy currently has a rather high recurrence rate [9], so it cannot be used as a radical treatment. Therefore, more novel and effective treatment strategies for leukemia still need to be developed.
3. Nanodrug Delivery Systems
An NDDS refers to the use of nanotechnology to encapsulate or adsorb drugs in nanoscale vehicles to achieve targeted delivery and controlled release of drugs. The complete NDDS consists of three main parts, including the nanovehicle type, drug loading, and surface modification (Figure 1).
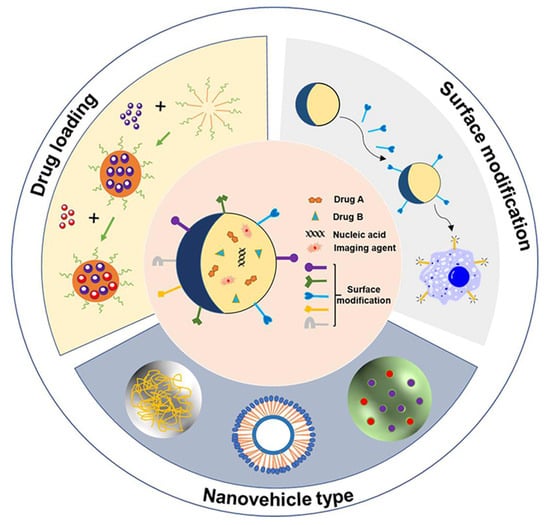
Figure 1.
Composition of NDDS, including nanovehicle type, drug loading, and surface modification. NDDS includes various nanovehicle types that carry drugs such as chemotherapy (Drug A, Drug B), nucleic acid and imaging agents, and surface modification with peptides, antibodies, ligands, biotin and polysaccharide.
Nanovehicles are the skeleton of the NDDS, and are mainly divided into organic nanomaterials and inorganic nanomaterials, according to the different properties of materials (Figure 2). The most common organic nanovehicles include polymer nanoparticles, liposomes, proteins, and peptide-based nanoparticles. Commonly used inorganic nanovehicles are metals, mesoporous silicon oxide, carbon tubes, and quantum dots [10,11,12]. Compared with organic nanomaterials, inorganic nanomaterials have a variety of physicochemical properties that are closely related to their size and composition. However, inorganic nanovehicles are non-biodegradable and cannot deliver highly effective drugs, so in most cases, they must be combined with organic materials [10,13,14].
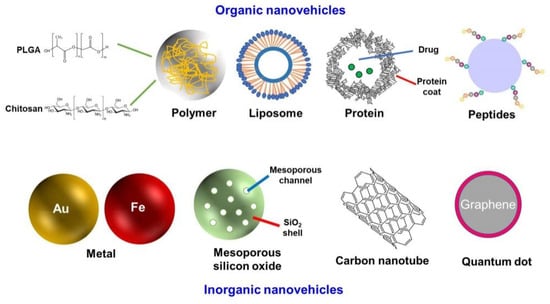
Figure 2.
Different nanovehicles for NDDSs.
The core element of the NDDS is the loaded drug, mainly including chemotherapy, protein, peptide, genes, or the combination of two or more drugs, which can achieve better efficacy through synergistic action. In addition, diagnostic imaging agents are integrated into nanovehicles for co-delivery with chemotherapy drugs, which can be used in tumor diagnosis and cancer cell metastasis monitoring. In order to obtain better delivery results, suitable nanovehicles should be selected to encapsulate different drugs, according to the nature of the drugs and the site of action. For example, proteins and peptides generally have short half-lives in circulation, and are quickly eliminated or degraded after intravenous administration. Polymer nanoparticles are ideal drug delivery nanovehicles for protein and peptide drugs [15]. Nucleic acid drugs have poor stability in vivo and poor ability to penetrate cell membranes, due to their negative charge, high molecular weight, and strong hydrophilicity [16]. Liposomes [17], polymer nanoparticles [18], and cationic vehicle-based inorganic nanoparticles [19] are the three most common types of non-viral nanovehicles used for nucleic acid drug delivery.
Although some drug delivery systems have advantages of low autoimmune stimulation, stability, low toxicity, and low cost, their targeted delivery may remain suboptimal. Therefore, in order to achieve better therapeutic effects and reduce toxic effects of drugs, targeted molecules such as peptides, antibodies, and ligands are often modified on the surface of nanovehicles [20].
4. Research Strategies for the Use of NDDSs in the Treatment of ALL
4.1. Targeted Delivery Strategy: Enhancing Drug Specificity
4.1.1. Active Targeting Modification
ALL cell surface markers (such as CD71, CD3, CD19) are specifically recognized by targeted ligands (such as antibodies, peptides, and aptamers) which are modified on the surface of nanovehicles. Krishnan et al. used a CD19-targeting ligand to modify hydrophilic poly(ethylene glycol) (PEG) and hydrophobic poly(ε-caprolactone) (PCL) polymer nanoparticles (81 nm) containing doxorubicin (in vitro experiment: 100 nM and 1 μM; in vivo experiment: 2.5 mg/kg), which could be specifically delivered to leukemia cells. CD 19 modification enabled nanoparticles to be absorbed through clathrin-dependent endocytosis, enhanced the accumulation of doxorubicin in cells, and induced apoptosis [21]. The CD19/CD3 bispecific antibody Blinatumomab has been used to treat ALL by binding T cells and leukemia cell surface antigens [22]. Durfee et al. prepared 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC)/chol/DSPE-PEG2000-NH2 lipid bilayer structures (137 nm) supported by mesoporous silica nanoparticles. They wrapped the anticancer drug gemcitabine (0–30 μM) in it, and then modified the nanocapsules with anti-epidermal growth factor antibody as the targeting agent. The results showed that the nanocapsules had high specificity and a better therapeutic effect on leukemia cell lines compared with the nanocapsules without anti-epidermal growth factor antibody as the targeting agent [23].
ALL cells originate in the bone marrow and spread throughout the blood. Bone marrow plays an important role in the progression of ALL, so it is considered a target for ALL treatment. Developing a bone marrow targeting system could enable more drugs to be brought to the lesion site for a better antitumor response. Some abnormal signals in pathologic bone marrow have been extensively studied, such as overexpression of the chemokine CXCR4, tissue hypoxia, and increased reactive oxygen species (ROS) levels. These could be used as sensitive release conditions for the design of bone marrow-targeted drug nanovehicles. Xu et al. used polymer DSPE-mPEG2000 micelles to deliver chemically synthesized CXCR4, a chemokine receptor highly expressed in a variety of leukemia cells and strongly associated with drug resistance and relapse, to antagonize polypeptides and doxorubicin (0–20 μM). The micelles (20 nm) could effectively bind to leukemia cells expressing CXCR4, downregulating the phosphorylation of Akt-, Erk-, and Mcl-1-signaling proteins mediated by the CXCR4/CXCL12 axis, and significantly reducing leukemia cells in the peripheral blood and bone marrow, as well as spleen and liver infiltration of drug-resistant and refractory leukemia mice, and thus significantly prolonging survival [24].
LSCs, which exist in bone marrow niches, are an important factor in the relapse of leukemia resistance. Targeting these niches could activate LSCs, make them sensitive to treatment, and enhance drug clearance. It has been found that the TIM-3 protein is highly expressed on the surface of LSCs. Chen et al. synthesized a 244 nm protein nanogel loaded with the immune checkpoint blocker aCD47 and the TLR7/8 agonist Resiquimod, and modified TIM-3 targeting antibodies on its surface. The nanoparticle could actively target LSCs in the blood circulation, and achieve efficient drug enrichment in bone marrow niches, significantly inducing immunosuppression by activating the “eat me” signal of macrophages, and enhancing the anti-leukemia immune response to overcome the problem of relapse of leukemia resistance [25].
The low-hemoperfusion nature of bone (also known as the BMB) prevents the passage of drugs, resulting in unsatisfactory eradication of residual LSCs in bone marrow. Xue et al. constructed a bisphosphonate (BP) lipid nanomaterial (about 100 nm) with high affinity for bone minerals as a means of overcoming biological barriers to efficiently deliver mRNA therapeutics to the bone marrow microenvironment in vivo [26]. In addition, methods used in other biological barriers, such as modifying the penetrating TAT peptide (HIV transcription activator-derived peptide) on the surface of nanovehicles to promote transmembrane transportation, also holds promise for future applications across the BMB.
4.1.2. Passive Targeting
The passive targeting strategy of NDDSs makes use of the microenvironment characteristics of tumor tissues or lesion sites (such as increased vascular permeability and loss of lymphatic drainage), so that the nanoparticles can naturally accumulate in specific areas, thereby increasing the concentration of drugs at the lesion site and reducing damage to normal tissues [27,28]. Bone marrow infiltration is an important pathological feature in ALL, and vascular permeability in the bone marrow microenvironment is significantly higher than that in normal tissues. Nanoparticles (typically 10–200 nm) passively accumulate around leukemia cells in bone marrow or lymphoid tissues through enhanced permeability and retention (EPR) effects. For example, liposomes or polymer nanocarriers (such as PLGA) enter the bone marrow through the blood circulation and accumulate around leukemia cells, extending the duration of drug action [29,30]. The sustained release function of nanocarriers can maintain an effective concentration of drugs in the bone marrow and reduce the need for frequent administration.
4.2. Intelligent Responsive Delivery System: Precise Control of Drug Release
Combined with the physical and chemical properties of the ALL microenvironment (such as pH, enzyme activity, and oxidative stress level), stimulus-responsive nanovehicles are designed to achieve spatiotemporal controlled release of drugs. There are two types of stimulation-responsive drug release. One involves the nanovehicles themselves being stimulation-responsive, such as pH-responsive polymer nanoparticles. When stimulated by external signals, they produce physical or chemical changes, such as swelling, solubility, dissociation, and other behaviors, so as to release drugs [31,32]. The other involves modifying stimulation-responsive groups or molecules on the internal and external surfaces of nanoparticles, and using reversible binding between the modification groups and drugs, or setting up “gated” molecules on the particles, to achieve controlled release of drugs under different types of stimulation. The stimulus signal may be exogenous, such as temperature and light, or endogenous, such as redox [33,34,35]. Premature release of drugs is effectively avoided by constructing stimulation-responsive intelligent nanodrug carriers, which significantly reduce the toxic side effects caused by drug leakage during transportation. Wei et al. prepared a folate receptor-targeted and glutathione (GSH)-responsive polymer prodrug nanoparticle (170~220 nm) by coupling 6-MP (0.1–50 μg/mL) with carboxymethyl chitosan via GSH-sensitive carbonyl vinyl sulfide. In the high-GSH environment of leukemia, the drug release in tumor cells was significantly improved, and the inhibition rate of leukemia cells was higher, while their cytotoxicity was lower in normal cells [36]. Wang et al. synthesized an amphiphilic pH-sensitive poly (ethylene glycol) methylether-B-(polylactic acid copolymer (B-aminoester)) with a size of 158 nm to support paclitaxel (PTX, 0–10 μg/mL) to improve the therapeutic effect of leukemia. The release of PTX from the micelles was significantly accelerated by decreasing the pH from 7.4 to 5.0, and the plasma circulation time was significantly prolonged. Compared with free PTX, the cytotoxic effects of PTX micelles on leukemia cells at all time points were significantly improved [37].
4.3. Overcoming Drug Resistance
Drug resistance of leukemia cells is one of the main causes of leukemia treatment failure [38,39]. Chemotherapeutic drugs are co-loaded with resistance reversal agents (such as P-glycoprotein inhibitors) or small-molecule inhibitors on the same nanovehicles to reduce drug efflux efficiency. For example, polymer nanoparticles can be loaded with both paclitaxel and P-gp modulator tariquidar, enhancing intracellular drug accumulation [40]. MRX-2843, a small-molecule MERTK and FLT3 kinase inhibitor currently involved in clinical trials for the treatment of relapsed/refractory leukemia and solid tumors, has been found to have a strong synergistic effect with vincristine. Kelvin et al., who developed a two-drug lipid (composed of DSPC, distearyl phosphatidyl glycerol (DSPG) and cholesterol lipids) nanoparticle (about 100 nm), found that MRX-2843 (0–800 nM) increased the sensitivity of early T cell precursor acute lymphoblastic leukemia (ETP-ALL) cells to vincristine in vivo [41]. The delivery of histone deacetylase inhibitors (HDACis) [42] or DNA methyltransferase inhibitors [43] through epigenetic regulation has also been studied to reverse epigenetic abnormalities in leukemia cells and restore chemotherapy sensitivity. Studies have shown that chemotherapy resistance and recurrence of leukemia are associated with impaired bone marrow immunosurveillance [44,45,46]. Li et al. developed an L-phenylalanine-based metabolic reprogramming immune surveillance activation nanomedical drug (MRIAN, 80 nm) to disrupt the immunosuppressive function of myeloid-derived suppressor cells in bone marrow, which effectively targeted bone marrow and activated immune surveillance in ALL. In ALL mouse models, MRIAN loaded with doxorubicin (Dox, in vitro experiment: 0.5 μM; in vivo experiment: 3 mg/kg) specifically targeted leukemia cells, but did not affect normal hematopoietic cells, thus significantly enhancing antitumor efficacy and limiting bone marrow damage and cardiotoxic side effects of doxorubicin therapy [47].
4.4. Multi-Mechanism Synergistic Therapy
4.4.1. Synergistic Gene Therapy
Synergistic gene therapy is a promising anti-leukemia strategy. For example, nanovehicles have been used to deliver siRNA to target oncogenes (such as BCL11B and STAT5A) in order to inhibit the proliferation of ALL cells and induce apoptosis [48,49]. In recent years, with the rise of gene editing technology, the use of nanovehicles to deliver gene editing systems has attracted more and more attention. The gene editing delivery system based on DNA nanomachines developed by Tang Wantao et al. showed excellent tumor targeting and biocompatibility, and produced significant gene editing and therapeutic effects on living tumors [50]. A novel pegylated phospholipid-based cationic lipid nanoparticle (composed of ionizable lipid, DSPC, cholesterol, dimyristoyl-rac-glycero-3-methoxypolyethylene glycol (DMG-PEG), and DSPE-PEG) delivery system (71–80 nm) that compresses and encapsulates Cas9/sgRNA plasmids has been constructed for efficient and safe delivery of CRISPR/Cas9 systems in vitro and in vivo [51].
4.4.2. Immunosynergistic Therapy
Immunotherapy has become a powerful clinical strategy for the treatment of leukemia. Checkpoint inhibitors are the most studied class of immunotherapy to date. Li Q et al. designed a nanoplatform (110 nm) based on glycinic acid (0–100 μM) to enhance the blocking of PD-1/PD-L1 and improve the immune response of T cells to leukemia [52]. Han X et al. designed and constructed a combined delivery platform based on platelet-hematopoietic stem cell-anti-programmed death-1 antibody (aPD1) conjugate. Due to the homing ability of hematopoietic stem cells to bone marrow, the assembly of a hematopoietic stem cell–platelet–aPD1 system effectively delivered aPD1 in a mouse model of leukemia [53]. CAR-T therapy is highly effective in relapsed/refractory ALL, but there is a risk of CRS and off-targeting. Nanoparticles optimized the delivery of CAR-T cells, such as the delivery of CAR genes via mRNA-LNP (80 nm), achieving high CAR expression and significant cytotoxicity to leukemia cells in vitro [54]. In addition, the delivery of leukemia-associated antigen WT1 and adjuvant CpG oligonucleotide via a nanodelivery system (610 nm) activated antigen presenting cells and T cell responses, enhancing the DC-mediated anti-leukemia immune response [55].
4.4.3. Integrated Diagnosis and Treatment Platform
The core of the integrated diagnosis and treatment with NDDSs involves integrating the diagnosis and treatment functions into the same nanoplatform to achieve the synergistic effect of accurate disease detection and targeted therapy. For example, surface-enhanced Raman scattering (SERS)-based nanoparticles (60 nm) were used to detect early leukemia cell lesions and accurately distinguish between live, apoptotic, and necrotic leukemia cells [56]. Intraoperative real-time navigation was realized by labeling tumor cells with near-infrared fluorescence (NIR) quantum dots (10–12 nm) [57]. Magnetic iron oxide nanoparticles combined with chemotherapy drugs were used to achieve simultaneous treatment and efficacy monitoring [58]. The research strategies for the use of NDDSs in the treatment of ALL are summarized in Figure 3.
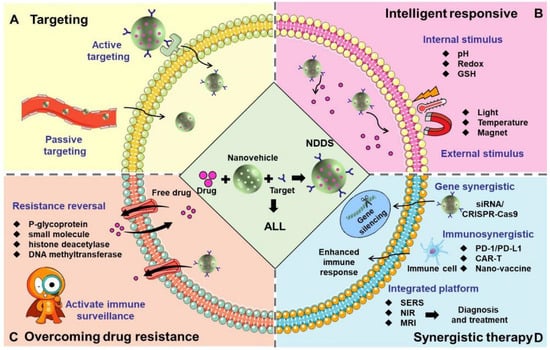
Figure 3.
The research strategies for the use of NDDSs in the treatment of ALL. (A) Targeting: NDDS precisely delivers drugs to ALL cells, leukemia stem cells, the bone marrow microenvironment and blood-bone marrow barrier through active or passive targeting. (B) Intelligent responsive: NDDS precisely controls drug release through internal stimulus (pH, redox, GSH) or external stimulus (light, temperature and magnet). (C) Overcoming drug resistance: Chemotherapeutic drugs are co-loaded with resistance reversal agents (such as P-glycoprotein inhibitors), small-molecule inhibitors and histone deacetylase or DNA methyltransferase inhibitors on the same nanovehicles to reduce drug efflux efficiency. In addition, activating the immune surveillance function could also reverse drug resistance and recurrence. (D) Synergistic therapy: NDDS combines with gene therapy (delivering siRNA and CRISPR/Cas9 to achieve gene silencing), immunotherapy (delivering PD1/PD-L1, CAR-T, and nano-vaccines to enhance the immune response), and a comprehensive diagnosis and treatment platform (combining SERS, NIR, and MRI) to achieve multi-mechanism synergistic therapy.
5. Statistics on the Application of NDDSs in ALL
Using “Acute lymphoblastic leukemia” and “Drug delivery” as key words, we searched in PUBMED, and a total of 316 articles were retrieved. The number of papers published in this field has increased significantly over the past two decades (Figure 4). However, in general, compared with other fields, there are relatively few research articles, with more attention paid by hospitals and hematology institutes. The representative applications of NDDSs used in ALL are summarized in Table 1, which shows that the nanovehicle types are mainly polymer, liposome, protein, and inorganic nanoparticles, and most studies are still mainly focused on the cellular level, with a lack of more in-depth studies at the animal and patient levels. The specific research progress is summarized below.
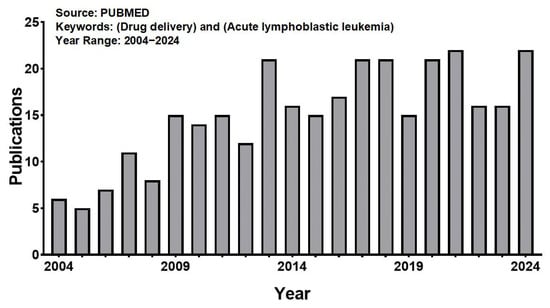
Figure 4.
The number of publications obtained using the keywords “drug delivery” and “acute lymphoblastic leukemia” when searching the PUBMED for the period 2004–2024.

Table 1.
Representative applications of NDDSs used in ALL.
6. Clinical Translation and Challenges of NDDSs
According to reports in the literature, as of now, 16 antitumor nanomaterials have been approved for marketing, including 8 liposomes, 3 polymer micelles, and 5 nanoparticles (2 inorganic nanocarriers) [68]. Due to the variety of blood tumors and complex subtypes, there are a few nanodrugs applicable to blood tumors so far. The dual drug liposome (composed of DSPC, DSPG, and cholesterol lipids) preparation of cytarabine and daunorubicin in a 5:1 fixed drug ratio (trade name Vyxeos, also known as CPX-351) was approved by the FDA in 2017 for the treatment of clinical AML patients. The vincristine sulfate sphingomyelin liposome (Marqibo) nanodrug was approved by the FDA in 2012 for the treatment of Philadelphia chromosome-negative (Ph) adults with acute lymphoblastic leukemia (ALL) who have relapsed or progressed after two or more anti-leukemia treatments. In addition, there is a large number of nanomedicines in the clinical research stage, involving nearly 200 clinical trials [68,69,70]. Among these preclinical nanomedicines, relatively simple liposomes and micelles still dominate, and most of these drugs are still in the early stages of clinical trials, Phase I or II. Nanomedicines that have been approved clinically, and some examples of nanomedicines currently under clinical investigation for the treatment of leukemia, are shown in Table 2.

Table 2.
Nanomedicines approved or in clinical trials for leukemia.
Although the clinical transformation of nanomedicines has made great progress, the biosafety of nanomedicines has attracted much attention, due to their different pharmacological characteristics, tissue distribution, and safety, resulting from the reassembly of various components of nanomedicines. The size, shape, specific surface area, surface charge, physical and chemical structure and aggregation state of nanomaterials will affect the absorption and metabolism of the drug and the drug itself, and consequently produce different levels of human cytotoxicity [71]. Although a lot of research has been conducted on the toxicity of nanoparticles, due to the wide range of types of nanoparticles, the toxicities of different types of nanomedicines are not the same, and the toxicity of the same nanomedicine to different tissues or cells and different sizes of nanomedicine to the same tissues or cells will be different. The behavior and fate of nanomedicines in vivo is one of the core issues in toxicology research. Although a large number of studies have revealed some mechanisms, many key scientific problems remain unknown or controversial, especially dynamic interactions in complex physiological environments, cumulative effects after long-term administration, degradation kinetics of degradable nanomaterials, and the secondary toxicity of metabolites. Therefore, it is necessary to establish a more systematic and standard toxicological evaluation method to study the safety of nanomedicine more comprehensively and deeply. At present, the safety evaluation methods of nanodrugs are similar to those of traditional drugs. In addition to acute toxicity evaluation, based on factors such as complement activation, hemolysis, inflammation, and oxidative stress, it is more necessary to strengthen long-term toxicity analysis. Artificial intelligence and molecular simulation are also needed to construct quantitative nanostructure–toxicity relationships (physicochemical properties) and clarify the molecular mechanisms related to toxicity [72]. In recent years, some governments around the world have attached great importance to the safety of nanomedicines and established new methods and technologies for safety evaluation of nanomedicines.
In addition, due to the complex microstructure and composition of nanomedicines, their construction process involves multi-step or complex technology, and there is a lack of controllable and reproducible nanomedicine synthesis methods, resulting in their large-scale production and quality control being restricted [73,74]. Physicochemical parameters are crucial to the biological effects of nanomedicines, so the production of industrial-grade nanomedicines requires strict control of physicochemical properties between batches, and involves higher requirements for chemistry, manufacturing, and control. Advances in microfluidic technology [75], 3D printing [76], and other technologies may help to open up possibilities for large-scale production of nanomedicines in the future.
7. Conclusions and Prospects
Nanodrug delivery systems provide a new strategy and hope for the treatment of ALL. By improving drug targeting, reducing toxic side effects, and enhancing therapeutic effects, NDDSs are expected to overcome the limitations of traditional therapeutic methods. However, translating nanomedicines from research to clinical practice faces significant challenges. The size, shape, and surface chemistry of nanomaterials affect their distribution in vivo, cellular absorption, and overall efficacy, so, looking ahead, the prospects for programmable nanomedicine in clinical applications are bright. Continued research and development is essential to optimize nanoparticle design and enhance targeting and delivery mechanisms. New methods for safety evaluation of nanomedicines, new technologies, and policies for the safe and effective use of nanomedicine must be established through close collaboration between researchers, clinicians, and regulatory authorities. Future research should focus on improving the biosafety, therapeutic effects, and clinical transformation of nanomedicines, as well as providing more accurate and effective treatment for ALL patients. Although there are still some challenges, with the continuous progress of nanotechnology and in-depth understanding of the pathological mechanisms underlying ALL, the application of NDDSs in clinical environments has broad prospects.
Author Contributions
Conceptualization, A.Y. and J.W.; writing—original draft, A.Y. and Z.Z.; writing—review and editing, A.Y., Y.L. and J.W.; funding acquisition, A.Y. and J.W.; supervision, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Beijing Natural Science Foundation (7244291), the National Natural Science Foundation of China (U23A20420), and the Research Foundation of Capital Institute of Pediatrics (JCYJ-2023-15, JHYJ-2023-03).
Data Availability Statement
No data are associated with this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zazuli, Z.; Irham, L.M.; Adikusuma, W.; Sari, N.M. Identification of Potential Treatments for Acute Lymphoblastic Leukemia through Integrated Genomic Network Analysis. Pharmaceuticals 2022, 15, 1562. [Google Scholar] [CrossRef]
- Aureli, A.; Marziani, B.; Venditti, A.; Sconocchia, T.; Sconocchia, G. Acute Lymphoblastic Leukemia Immunotherapy Treatment: Now, Next, and Beyond. Cancers 2023, 15, 3346. [Google Scholar] [CrossRef]
- Hayashino, K.; Terao, T.; Nishimori, H.; Kitamura, W.; Kobayashi, H.; Kamoi, C.; Seike, K.; Fujiwara, H.; Asada, N.; Ennishi, D.; et al. Outcomes of allogeneic SCT versus tisagenlecleucel in patients with R/R LBCL and poor prognostic factors. Int. J. Hematol. 2025, 121, 232–243. [Google Scholar] [CrossRef]
- Iacoboni, G.; Dietrich, S.; Liebers, N. CAR T-cell therapy versus allogeneic HSCT for relapsed or refractory mantle cell lymphoma. Lancet Haematol. 2023, 10, e951–e952. [Google Scholar] [CrossRef]
- Gross, T.G.; Hale, G.A.; He, W.; Camitta, B.M.; Sanders, J.E.; Cairo, M.S.; Hayashi, R.J.; Termuhlen, A.M.; Zhang, M.J.; Davies, S.M.; et al. Hematopoietic stem cell transplantation for refractory or recurrent non-Hodgkin lymphoma in children and adolescents. Biol. Blood Marrow. Transplant. 2010, 16, 223–230. [Google Scholar] [CrossRef]
- Cappell, K.M.; Kochenderfer, J.N. Long-term outcomes following CAR T cell therapy: What we know so far. Nat. Rev. Clin. Oncol. 2023, 20, 359–371. [Google Scholar] [CrossRef]
- Myers, R.M.; Shah, N.N.; Pulsipher, M.A. How I use risk factors for success or failure of CD19 CAR T cells to guide management of children and AYA with B-cell ALL. Blood 2023, 141, 1251–1264. [Google Scholar] [CrossRef]
- Li, Y.; Ming, Y.; Fu, R.; Li, C.; Wu, Y.; Jiang, T.; Li, Z.; Ni, R.; Li, L.; Su, H.; et al. The pathogenesis, diagnosis, prevention, and treatment of CAR-T cell therapy-related adverse reactions. Front. Pharmacol. 2022, 13, 950923. [Google Scholar] [CrossRef]
- Haradhvala, N.J.; Leick, M.B.; Maurer, K.; Gohil, S.H.; Larson, R.C.; Yao, N.; Gallagher, K.M.E.; Katsis, K.; Frigault, M.J.; Southard, J.; et al. Distinct cellular dynamics associated with response to CAR-T therapy for refractory B cell lymphoma. Nat. Med. 2022, 28, 1848–1859. [Google Scholar] [CrossRef]
- Pavelić, K.; Kraljević Pavelić, S.; Bulog, A.; Agaj, A.; Rojnić, B.; Čolić, M.; Trivanović, D. Nanoparticles in Medicine: Current Status in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 12827. [Google Scholar] [CrossRef]
- Gallo, E.; Diaferia, C.; Smaldone, G.; Rosa, E.; Pecoraro, G.; Morelli, G.; Accardo, A. Fmoc-FF hydrogels and nanogels for improved and selective delivery of dexamethasone in leukemic cells and diagnostic applications. Sci. Rep. 2024, 14, 9940. [Google Scholar] [CrossRef]
- Jiang, X.; Bugno, J.; Hu, C.; Yang, Y.; Herold, T.; Qi, J.; Chen, P.; Gurbuxani, S.; Arnovitz, S.; Ulrich, B.; et al. Targeted Treatment of FLT3 -Overexpressing Acute Myeloid Leukemia with MiR-150 Nanoparticles Guided By Conjugated FLT3 Ligand Peptides. Blood 2015, 126, 3784. [Google Scholar] [CrossRef]
- Alshammari, B.H.; Lashin, M.M.A.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and inorganic nanomaterials: Fabrication, properties and applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef]
- Yanar, F.; Carugo, D.; Zhang, X. Hybrid Nanoplatforms Comprising Organic Nanocompartments Encapsulating Inorganic Nanoparticles for Enhanced Drug Delivery and Bioimaging Applications. Molecules 2023, 28, 5694. [Google Scholar] [CrossRef]
- Butreddy, A.; Gaddam, R.P.; Kommineni, N.; Dudhipala, N.; Voshavar, C. PLGA/PLA-Based Long-Acting Injectable Depot Microspheres in Clinical Use: Production and Characterization Overview for Protein/Peptide Delivery. Int. J. Mol. Sci. 2021, 22, 8884. [Google Scholar] [CrossRef]
- Thomas, O.S.; Weber, W. Overcoming Physiological Barriers to Nanoparticle Delivery-Are We There Yet? Front. Bioeng. Biotechnol. 2019, 7, 415. [Google Scholar] [CrossRef]
- El Moukhtari, S.H.; Garbayo, E.; Amundarain, A.; Pascual-Gil, S.; Carrasco-León, A.; Prosper, F.; Agirre, X.; Blanco-Prieto, M.J. Lipid nanoparticles for siRNA delivery in cancer treatment. J. Control. Release 2023, 361, 130–146. [Google Scholar] [CrossRef]
- Dilliard, S.A.; Siegwart, D.J. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat. Rev. Mater. 2023, 8, 282–300. [Google Scholar] [CrossRef]
- Wang, L.; Yan, Y. A Review of pH-Responsive Organic-Inorganic Hybrid Nanoparticles for RNAi-Based Therapeutics. Macromol. Biosci. 2021, 21, e2100183. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Krishnan, V.; Xu, X.; Kelly, D.; Snook, A.; Waldman, S.A.; Mason, R.W.; Jia, X.; Rajasekaran, A.K. CD19-Targeted Nanodelivery of Doxorubicin Enhances Therapeutic Efficacy in B-Cell Acute Lymphoblastic Leukemia. Mol. Pharm. 2015, 12, 2101–2111. [Google Scholar] [CrossRef]
- Paul, S.; Jabbour, E.; Nichols, E.D.; Short, N.J.; Kantarjian, H. Blinatumomab for the treatment of acute lymphoblastic leukemia in a real-world setting: Clinical vignettes. Leuk. Lymphoma 2025, 66, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Durfee, P.N.; Lin, Y.S.; Dunphy, D.R.; Muñiz, A.J.; Butler, K.S.; Humphrey, K.R.; Lokke, A.J.; Agola, J.O.; Chou, S.S.; Chen, I.M.; et al. Mesoporous Silica Nanoparticle-Supported Lipid Bilayers (Protocells) for Active Targeting and Delivery to Individual Leukemia Cells. ACS Nano 2016, 10, 8325–8345. [Google Scholar] [CrossRef]
- Zhang, M.; Ge, Y.; Xu, S.; Fang, X.; Meng, J.; Yu, L.; Wang, C.; Liu, J.; Wen, T.; Yang, Y.; et al. Nanomicelles co-loading CXCR4 antagonist and doxorubicin combat the refractory acute myeloid leukemia. Pharmacol. Res. 2022, 185, 106503. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, D.; Yao, Y.; Xiao, Y.; Zhao, Z.; Zhang, Z.; Liu, S.; Wang, X.; Yang, S.; Huang, P. Leukemia Cell Hitchhiking Hypoxia Responsive Nanogel for Improved Immunotherapy of Acute Myeloid Leukemia. Adv. Funct. Mater. 2024, 34, 2411439. [Google Scholar] [CrossRef]
- Xue, L.; Gong, N.; Shepherd, S.J.; Xiong, X.; Liao, X.; Han, X.; Zhao, G.; Song, C.; Huang, X.; Zhang, H.; et al. Rational Design of Bisphosphonate Lipid-like Materials for mRNA Delivery to the Bone Microenvironment. J. Am. Chem. Soc. 2022, 144, 9926–9937. [Google Scholar] [CrossRef]
- Arandhara, A.; Bhuyan, P.; Das, B.K. Exploring lung cancer microenvironment: Pathways and nanoparticle-based therapies. Discov. Oncol. 2025, 16, 159. [Google Scholar] [CrossRef] [PubMed]
- Ejigah, V.; Owoseni, O.; Bataille-Backer, P.; Ogundipe, O.D.; Fisusi, F.A.; Adesina, S.K. Approaches to Improve Macromolecule and Nanoparticle Accumulation in the Tumor Microenvironment by the Enhanced Permeability and Retention Effect. Polymers 2022, 14, 2601. [Google Scholar] [CrossRef]
- Fukuta, T.; Yoshimi, S.; Tanaka, T.; Kogure, K. Leukocyte-mimetic liposomes possessing leukocyte membrane proteins pass through inflamed endothelial cell layer by regulating intercellular junctions. Int. J. Pharm. 2019, 563, 314–323. [Google Scholar] [CrossRef]
- Deshantri, A.K.; Varela Moreira, A.; Ecker, V.; Mandhane, S.N.; Schiffelers, R.M.; Buchner, M.; Fens, M. Nanomedicines for the treatment of hematological malignancies. J. Control. Release 2018, 287, 194–215. [Google Scholar] [CrossRef]
- Leng, Q.; Imtiyaz, Z.; Woodle, M.C.; Mixson, A.J. Delivery of Chemotherapy Agents and Nucleic Acids with pH-Dependent Nanoparticles. Pharmaceutics 2023, 15, 1482. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Rossi Herling, B.; Zhang, C.; Beach, M.A.; Teo, S.L.Y.; Gillies, E.R.; Johnston, A.P.R.; Such, G.K. Self-Immolative Polymer Nanoparticles with Precise and Controllable pH-Dependent Degradation. Biomacromolecules 2023, 24, 4958–4969. [Google Scholar] [CrossRef]
- Thakur, V.; Kush, P.; Pandey, R.S.; Jain, U.K.; Chandra, R.; Madan, J. Vincristine sulfate loaded dextran microspheres amalgamated with thermosensitive gel offered sustained release and enhanced cytotoxicity in THP-1, human leukemia cells: In vitro and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 113–122. [Google Scholar] [CrossRef]
- Wang, B.Y.; Lin, Y.C.; Lai, Y.T.; Ou, J.Y.; Chang, W.W.; Chu, C.C. Targeted photoresponsive carbazole-coumarin and drug conjugates for efficient combination therapy in leukemia cancer cells. Bioorg. Chem. 2020, 100, 103904. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gao, Y.; Ma, J.; Hu, M.; Xia, J.; Bao, S.; Liu, Y.; Feng, K. Cytarabine delivered by CD44 and bone targeting redox-sensitive liposomes for treatment of acute myelogenous leukemia. Regen. Biomater. 2022, 9, rbac058. [Google Scholar] [CrossRef]
- Wei, X.; Liao, J.; Davoudi, Z.; Zheng, H.; Chen, J.; Li, D.; Xiong, X.; Yin, Y.; Yu, X.; Xiong, J.; et al. Folate Receptor-Targeted and GSH-Responsive Carboxymethyl Chitosan Nanoparticles Containing Covalently Entrapped 6-Mercaptopurine for Enhanced Intracellular Drug Delivery in Leukemia. Mar. Drugs 2018, 16, 439. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, L.; Xiao, M.; Sun, Z.L.; Zhang, C.Y. Nanomedicine-based paclitaxel induced apoptotic signaling pathways in A562 leukemia cancer cells. Colloids Surf. B Biointerfaces 2017, 149, 16–22. [Google Scholar] [CrossRef]
- Calderon, A.; Han, C.; Karma, S.; Wang, E. Non-genetic mechanisms of drug resistance in acute leukemias. Trends Cancer 2024, 10, 38–51. [Google Scholar] [CrossRef]
- Sidhu, J.; Gogoi, M.P.; Krishnan, S.; Saha, V. Relapsed Acute Lymphoblastic Leukemia. Indian J. Pediatr. 2024, 91, 158–167. [Google Scholar] [CrossRef]
- Patil, Y.; Sadhukha, T.; Ma, L.; Panyam, J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J. Control. Release 2009, 136, 21–29. [Google Scholar] [CrossRef]
- Kelvin, J.M.; Chimenti, M.L.; Zhang, D.Y.; Williams, E.K.; Moore, S.G.; Humber, G.M.; Baxter, T.A.; Birnbaum, L.A.; Qui, M.; Zecca, H.; et al. Development of constitutively synergistic nanoformulations to enhance chemosensitivity in T-cell leukemia. J. Control. Release 2023, 361, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Aroonthongsawat, P.; Manocheewa, S.; Srisawat, C.; Punnakitikashem, P.; Suwanwong, Y. Enhancement of the in vitro anti-leukemic effect of the histone deacetylase inhibitor romidepsin using Poly-(D, L-lactide-co-glycolide) nanoparticles as a drug carrier. Eur. J. Pharm. Sci. 2025, 207, 107043. [Google Scholar] [CrossRef]
- Vijayaraghavalu, S.; Labhasetwar, V. Efficacy of decitabine-loaded nanogels in overcoming cancer drug resistance is mediated via sustained DNA methyltransferase 1 (DNMT1) depletion. Cancer Lett. 2013, 331, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Smyth, M.J.; Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 2013, 39, 74–88. [Google Scholar] [CrossRef]
- Andersen, M.H. The targeting of immunosuppressive mechanisms in hematological malignancies. Leukemia 2014, 28, 1784–1792. [Google Scholar] [CrossRef]
- Paczulla, A.M.; Rothfelder, K.; Raffel, S.; Konantz, M.; Steinbacher, J.; Wang, H.; Tandler, C.; Mbarga, M.; Schaefer, T.; Falcone, M.; et al. Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature 2019, 572, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; You, X.; Xu, X.; Wu, B.; Liu, Y.; Tong, T.; Chen, J.; Li, Y.; Dai, C.; Ye, Z.; et al. A Metabolic Reprogramming Amino Acid Polymer as an Immunosurveillance Activator and Leukemia Targeting Drug Carrier for T-Cell Acute Lymphoblastic Leukemia. Adv. Sci. 2022, 9, e2104134. [Google Scholar] [CrossRef]
- Zeng, X.; Nie, D.; Liu, Z.; Peng, X.; Wang, X.; Qiu, K.; Zhong, S.; Liao, Z.; Zha, X.; Li, Y.; et al. Aptamer sgc8-Modified PAMAM Nanoparticles for Targeted siRNA Delivery to Inhibit BCL11B in T-Cell Acute Lymphoblastic Leukemia. Int. J. Nanomed. 2024, 19, 12297–12309. [Google Scholar] [CrossRef]
- Nasrullah, M.; Kc, R.; Nickel, K.; Parent, K.; Kucharski, C.; Meenakshi Sundaram, D.N.; Rajendran, A.P.; Jiang, X.; Brandwein, J.; Uludağ, H. Lipopolymer/siRNA Nanoparticles Targeting the Signal Transducer and Activator of Transcription 5A Disrupts Proliferation of Acute Lymphoblastic Leukemia. ACS Pharmacol. Transl. Sci. 2024, 7, 2840–2855. [Google Scholar] [CrossRef]
- Tang, W.; Tong, T.; Wang, H.; Lu, X.; Yang, C.; Wu, Y.; Wang, Y.; Liu, J.; Ding, B. A DNA Origami-Based Gene Editing System for Efficient Gene Therapy In Vivo. Angew. Chem. Int. Ed. Engl. 2023, 62, e202315093. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Su, R.; Bao, X.; Cao, K.; Du, Y.; Wang, N.; Wang, J.; Xing, F.; Yan, F.; Huang, K.; et al. Glycyrrhetinic acid nanoparticles combined with ferrotherapy for improved cancer immunotherapy. Acta Biomater. 2022, 144, 109–120. [Google Scholar] [CrossRef]
- Han, X.; Li, H.; Zhou, D.; Chen, Z.; Gu, Z. Local and Targeted Delivery of Immune Checkpoint Blockade Therapeutics. Acc. Chem. Res. 2020, 53, 2521–2533. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ren, A.; Li, Y.; Shu, J.; Wu, J.; Huang, H.; Wang, J.; Hu, Y.; Mei, H. mRNA-laden lipid nanoparticle-enabled humanized CD19 CAR-T-cell engineering for the eradication of leukaemic cells. Br. J. Haematol. 2025, 206, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, S.; Duan, J.; Hu, Y.; Gu, N.; Xu, H.; Yang, X.D. Enhancement of DC-mediated anti-leukemic immunity in vitro by WT1 antigen and CpG co-encapsulated in PLGA microparticles. Protein Cell 2013, 4, 887–889. [Google Scholar] [CrossRef]
- Khetani, A.; Momenpour, A.; Alarcon, E.I.; Anis, H. Hollow core photonic crystal fiber for monitoring leukemia cells using surface enhanced Raman scattering (SERS). Biomed. Opt. Express 2015, 6, 4599–4609. [Google Scholar] [CrossRef]
- Wang, P.; Fan, Y.; Lu, L.; Liu, L.; Fan, L.; Zhao, M.; Xie, Y.; Xu, C.; Zhang, F. NIR-II nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer. Nat. Commun. 2018, 9, 2898. [Google Scholar] [CrossRef]
- Zhang, H. Thermally cross-linked superparamagnetic iron oxide nanoparticle-A10 RNA aptamer-doxorubicin conjugate. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Chamorro Rengifo, A.F.; Stefanes, N.; Toigo, J.; Mendes, C.; Santos-Silva, M.C.; Nunes, R.J.; Parize, A.L.; Minatti, E. A new and efficient carboxymethyl-hexanoyl chitosan/dodecyl sulfate nanocarrier for a pyrazoline with antileukemic activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110051. [Google Scholar] [CrossRef]
- Fan, J.; He, Q.; Jin, Z.; Chen, W.; Huang, W. A novel phosphoester-based cationic co-polymer nanocarrier delivers chimeric antigen receptor plasmid and exhibits anti-tumor effect. RSC Adv. 2018, 8, 14975–14982. [Google Scholar] [CrossRef]
- Lim, W.S.; Tardi, P.G.; Dos Santos, N.; Xie, X.; Fan, M.; Liboiron, B.D.; Huang, X.; Harasym, T.O.; Bermudes, D.; Mayer, L.D. Leukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine:daunorubicin formulation, in bone marrow xenografts. Leuk. Res. 2010, 34, 1214–1223. [Google Scholar] [CrossRef]
- de Souza Guimarães, M.; Cachumba, J.J.M.; Bueno, C.Z.; Torres-Obreque, K.M.; Lara, G.V.R.; Monteiro, G.; Barbosa, L.R.S.; Pessoa, A., Jr.; Rangel-Yagui, C.O. Peg-Grafted Liposomes for L-Asparaginase Encapsulation. Pharmaceutics 2022, 14, 1819. [Google Scholar] [CrossRef]
- Billingsley, M.M.; Singh, N.; Ravikumar, P.; Zhang, R.; June, C.H.; Mitchell, M.J. Ionizable Lipid Nanoparticle-Mediated mRNA Delivery for Human CAR T Cell Engineering. Nano Lett. 2020, 20, 1578–1589. [Google Scholar] [CrossRef]
- Zahariev, N.; Draganova, M.; Zagorchev, P.; Pilicheva, B. Casein-Based Nanoparticles: A Potential Tool for the Delivery of Daunorubicin in Acute Lymphocytic Leukemia. Pharmaceutics 2023, 15, 471. [Google Scholar] [CrossRef]
- Yang, A.; Luo, D.; Jia, Y.; Liu, Y.; Zhang, Z.; Li, S.; Liu, R.; Zhou, J.; Wang, J. Targeted delivery of AZD5363 to T-cell acute lymphocytic leukemia by mSiO2-Au nanovehicles. Colloids Surf. B Biointerfaces 2023, 230, 113505. [Google Scholar] [CrossRef]
- Ghasemi Goorbandi, R.; Mohammadi, M.R.; Malekzadeh, K. Synthesizing efficacious genistein in conjugation with superparamagnetic Fe3O4 decorated with bio-compatible carboxymethylated chitosan against acute leukemia lymphoma. Biomater. Res. 2020, 24, 9. [Google Scholar] [CrossRef]
- Zong, S.; Wang, L.; Yang, Z.; Wang, H.; Wang, Z.; Cui, Y. Black Phosphorus-Based Drug Nanocarrier for Targeted and Synergetic Chemophotothermal Therapy of Acute Lymphoblastic Leukemia. ACS Appl. Mater. Interfaces 2019, 11, 5896–5902. [Google Scholar] [CrossRef]
- de Lázaro, I.; Mooney, D.J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 2021, 20, 1469–1479. [Google Scholar] [CrossRef]
- Kumar, A.; Shahvej, S.K.; Yadav, P.; Modi, U.; Yadav, A.K.; Solanki, R.; Bhatia, D. Clinical Applications of Targeted Nanomaterials. Pharmaceutics 2025, 17, 379. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Awashra, M.; Młynarz, P. The toxicity of nanoparticles and their interaction with cells: An in vitro metabolomic perspective. Nanoscale Adv. 2023, 5, 2674–2723. [Google Scholar] [CrossRef]
- Yan, X.; Yue, T.; Winkler, D.A.; Yin, Y.; Zhu, H.; Jiang, G.; Yan, B. Converting Nanotoxicity Data to Information Using Artificial Intelligence and Simulation. Chem. Rev. 2023, 123, 8575–8637. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, W.C.; Torchilin, V.; Patri, A.K.; Hrkach, J.; Stern, S.; Lee, R.; Nel, A.; Panaro, N.J.; Grodzinski, P. Best practices in cancer nanotechnology: Perspective from NCI nanotechnology alliance. Clin. Cancer Res. 2012, 18, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, J.; Sun, R.; Han, S.; Yang, Z.; Teng, L. Microfluidics for nano-drug delivery systems: From fundamentals to industrialization. Acta Pharm. Sin. B 2023, 13, 3277–3299. [Google Scholar] [CrossRef]
- Ahmad, J.; Garg, A.; Mustafa, G.; Mohammed, A.A.; Ahmad, M.Z. 3D Printing Technology as a Promising Tool to Design Nanomedicine-Based Solid Dosage Forms: Contemporary Research and Future Scope. Pharmaceutics 2023, 15, 1448. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).