Abstract
Background/Objectives: The consumption of food rich in anthocyanins, a natural pigment found in plants, has been associated with improved joint health. However, systematic efforts to summarise the effects of anthocyanins and their deglycosylated forms, anthocyanidins, in managing osteoarthritis (OA) are lacking. This scoping review aims to comprehensively summarise the current evidence regarding the role of anthocyanins and anthocyanidins in OA management and highlights potential research areas. Methods: A comprehensive literature search was performed using PubMed, Scopus, and Web of Science in January 2025 to look for primary studies published in English, with the main objective of investigating the chondroprotective effects of anthocyanins and anthocyanidins, regardless of their study designs. Results: The seven included studies showed that anthocyanins and anthocyanidins suppressed the activation of inflammatory signalling, upregulated sirtuin-6 (cyanidin only), and autophagy (delphinidin only) in chondrocytes challenged with various stimuli (interleukin-1β, oxidative stress, or advanced glycation products). Anthocyanins also preserved cartilage integrity and increased the pain threshold in animal models of OA. No clinical trial was found in this field, suggesting a translation gap. Conclusions: In conclusion, anthocyanins and anthocyanidins are potential chondroprotective agents, but more investigations are required to overcome the gap in clinical translation.
1. Introduction
Osteoarthritis (OA), a disease characterised by cartilage degeneration, subchondral osteophyte formation, and synovitis that manifests as joint pain, swelling, and stiffness [1], poises to impact global health significantly due to the increase in the ageing population. The global age-standardised prevalence rate of knee OA stood at 6967.29 per 100,000 [95% uncertainty interval (UI): 6180.7–7686.06] in 2021 [2]. The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) in 2021 assigned a disability-adjusted life-year value of 213 million (95% UI: 101.8–429.3 million) to OA, making it the 18th leading cause of disability [2].
Despite its prevalence, effective treatment for OA remains lacking, with pain relief using non-steroidal anti-inflammatory agents being the primary option. The effectiveness of symptomatic slow-acting drugs, such as glucosamine, chondroitin, and diacerein, is contentious so far. Viscosupplementation using hyaluronic acid offers merely temporary relief. Intra-articular glucocorticoid injections can relieve pain and inflammation, but their long-term safety remains a concern [3,4]. When all pharmacological agents fail, total knee replacement surgery is considered, but it comes with significant risks and potential side effects, such as thrombosis, infections, and periprosthetic fracture, which can complicate the recovery of the patients [5].
As a result, the exploration of potential compounds to mitigate OA progression, especially from nature, is gaining momentum [6]. Since inflammation and oxidative stress play key roles in the vicious cycle of cartilage destruction in OA [7,8], a compound with dual anti-inflammatory and antioxidant properties might have a role in mitigating the progression of OA. Many natural polyphenols, including flavones, stilbenes, and phenolic acids, have been investigated for their anti-OA effects, and the evidence has been extensively reviewed [8,9,10]. In contrast, reports on the anti-OA effects of anthocyanins, a class of flavonoid, are relatively scarce.
Anthocyanins, a group of hydrophilic pigments in the flavonoid class, are responsible for the red, purple, and blue colours found in various plants, such as berries, grapes, red cabbage, and eggplants. Anthocyanins are the glycosylated form of anthocyanidins (aglycones). The molecular structures of common anthocyanidins are depicted in Figure 1. Due to their attractive colours, anthocyanins help pollinate plants. They also protect plants from UV damage, drought, and pathogens [11,12]. Anthocyanins have been associated with many health benefits, such as protection from metabolic, cardiovascular, and neurodegenerative disorders as well as cancers [12,13].
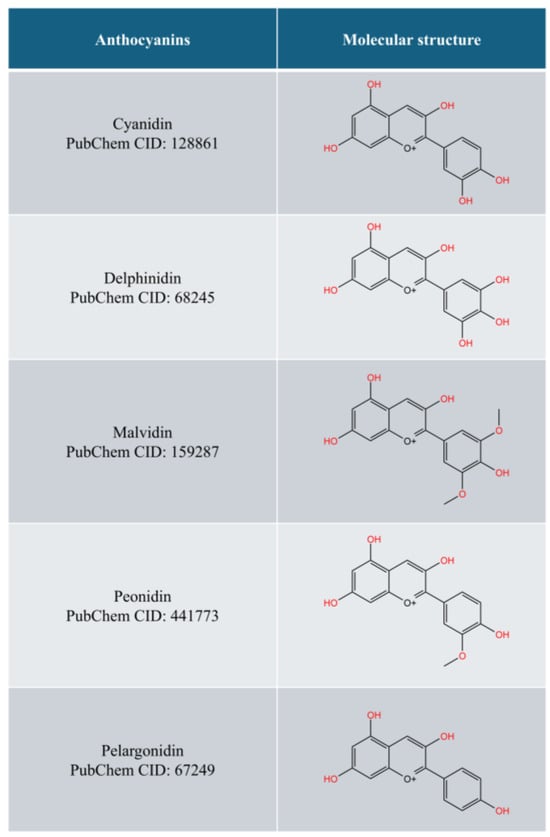
Figure 1.
The common forms of anthocyanidins (drawn with KingDraw).
The chondroprotective effects of anthocyanin are suggested by the benefits of red orange [14], purple corn [15], and purple rice extracts [16], which are rich in anthocyanins, in suppressing the progression of OA in preclinical models. Anthocyanins have been demonstrated to activate nuclear factor erythroid 2-related factor 2 (Nrf2) [17], the key regulator of the antioxidant response, which protects cells, including chondrocytes, from oxidative damage [18]. Anthocyanins also block the activation of nuclear factor kappa-B (NFκB) [19], the critical inflammatory pathway associated with various diseases, including OA [20]. More importantly, some studies have suggested that they stimulate the differentiation of mesenchymal stem cells into chondrocytes, which could enhance the regeneration of cartilage [21]. However, the anti-OA effects of anthocyanins have not been systematically reviewed.
Therefore, this scoping review aims to provide a comprehensive overview of the current evidence regarding the role of anthocyanins in OA management, highlighting their potential therapeutic benefits and identifying gaps in the existing research that warrant further investigation.
2. Methods
This scoping review was designed according to the steps outlined by Arksey and O’Malley [22], and adhered to the checklist of PRISMA for Scoping Reviews (Table S1) [23]. The steps involved were (1) identifying the research question, (2) identifying relevant studies, (3) study selection, (4) charting the data, and (5) collating, summarising, and reporting the results. The protocol of this scoping review is available at Open Science Framework (Url: https://osf.io/8xjrk/?view_only=181e036019cf46b5ad6d58825a7381b0, assessed on 20 January 2025).
2.1. Identifying the Research Question
The current scoping review addressed the question, “What are the effects of anthocyanin and anthocyanidin supplementation on OA?” The research question of this review was designed based on the Population, Concept, and Context (PCC) outlined in Table 1. The research question was addressed with evidence derived from studies using preclinical models of OA (in vitro or in vivo) and patients with OA (clinical trials) treated with anthocyanins or anthocyanidins.

Table 1.
The Population, Concept and Context of the current scoping review.
2.2. Identifying Relevant Studies
In January 2025, a systematic literature search was performed on three major scholarly databases, i.e., PubMed, Scopus, and Web of Science, using the search string (Anthocyanin* OR Anthocyanidin* OR Aurantinidin OR Capensinidin OR Cyanidin OR Delphinidin OR Europinidin OR Hirsutidin OR Malvidin OR Pelargonidin OR Peonidin OR Petunidin OR Pulchellidin OR Rosinidin) AND (Osteoarthritis OR Osteoarthrosis OR Cartilage OR Chondrocyte*). The search string was applied to titles and abstracts to avoid unspecific results. All items between the inception of databases and the date of search were included. No additional filter was applied during the search.
Primary studies published in English with the main objective of studying the effects of anthocyanin or anthocyanidin supplementation on joint health parameters in OA models or patients were included. Items without primary data, such as editorials, letters, reviews (including systematic reviews and meta-analyses), and perspectives, were excluded. Conference abstracts and proceedings were excluded due to incomplete data and duplication with full articles. Studies investigating the effects of anthocyanin OR anthocyanidin exposure on normal chondrocytes or animals without OA induction were excluded. Studies using crude extracts or mixed formulations were excluded because the effects of anthocyanins OR anthocyanidins could not be delineated.
2.3. Study Selection
Reference management was performed using Endnote version 20.6 (Clarivate, Philadelphia, PA, USA). The search results from three databases were merged and deduplicated using Endnote. A manual examination was performed to ensure the deduplication was successful. The titles and abstracts were screened by two researchers (X.M., K.-Y.C.) independently based on the inclusion and exclusion criteria. Next, the full texts were obtained for the eligible items and screened by the same researchers. Disagreements were resolved by discussion and the opinions of the third researcher (S.O.E.). The reference list of the included articles and relevant reviews was screened to identify potential articles left out during the literature search.
2.4. Charting the Data
Data from the articles included was extracted by two researchers (X.M., K.-Y.C.) using a standardised Google Form (Google, Mountain View, CA, USA). The data extracted included authors, year of publication, study designs, OA models adopted, treatment details (compounds, dosages, and treatment periods), major findings, limitations, and conclusions.
2.5. Collating, Summarising, and Reporting the Results
The data were summarised and reported qualitatively based on important pathological OA features, such as chondrocyte viability, anabolic and catabolic balance of cartilage, cartilage integrity, and pain and behaviour changes. Data synthesis based on statistical methods was not conducted because the study designs, OA models, and reported outcomes were heterogeneous and difficult to combine meaningfully. The role of anthocyanins and anthocyanidins in mitigating OA in each cascade of the pathogenesis of the disease, current research gaps in the field, and limitations of the review are covered in Section 4.
3. Results
The literature search uncovered 44 unique articles from three scholarly databases, of which 37 were excluded because they were not within the scope (n = 12), did not contain primary data (n = 14), did not use OA models (n = 6), used crude extracts (n = 4), or were conference abstracts (n = 1). Seven articles were included in the current scoping review. The results of the article screening and selection are summarised in Figure 2.
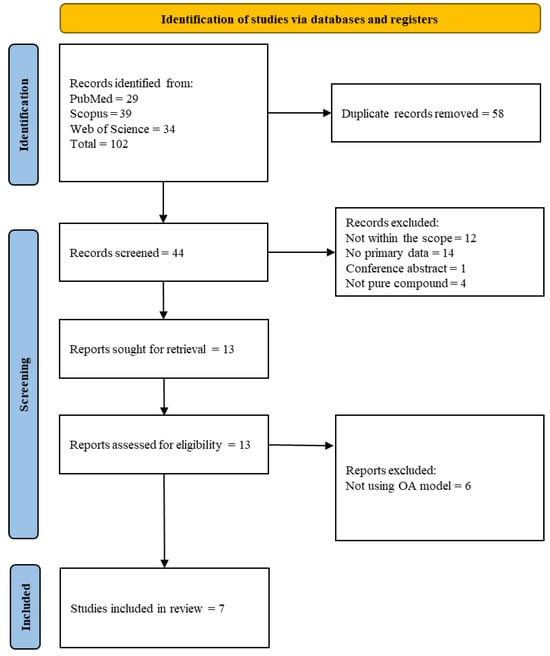
Figure 2.
PRISMA flow chart (adapted from https://www.prisma-statement.org/prisma-2020-flow-diagram, assessed on 20 January 2025).
3.1. Study Characteristics
Four studies used in vitro models, and three studies used a combination of in vitro and in vivo models to examine the effects of anthocyanins and anthocyanidins in OA models. No clinical studies have been conducted on this topic. For in vitro studies, primary chondrocytes of humans (normal [15,16] and OA patients [24,25]) and mice [26,27], as well as human chondrocyte cell line C28/I2 [28], have been used. Most studies used interleukin-1β (IL-1β) to mimic OA changes in in vitro studies [16,24,25,26,27], whereas a study used advanced glycation products to mimic OA changes in diabetic conditions [15], and one study used hydrogen peroxide to emulate oxidative stress in OA. For in vivo studies, C57B/L male mice [25,26] and Wistar rats (sex not specified) [27] were used. The OA induction methods were medial meniscus destabilisation (MMD) in mice [25,26] and monosodium iodoacetate (MIA) in Wistar rats [27].
The compounds investigated included cyanidin, delphinidin, malvidin, peonidin, pelargonidin, and their corresponding aglycone forms. In in vitro studies, various dosages (1.25–50 µM) and treatment periods were attempted depending on the objectives, while in vivo studies used oral dosages ranging from 5 to 20 mg/kg/day for 14 days to 8 weeks.
3.2. Effects on Chondrocyte Viability
Chondrocytes undergo apoptosis when exposed to stressors, such as IL-1β and oxidative stress [27,29]. Since chondrocytes are the only cell type in cartilage to synthesise the extracellular matrix (ECM), the capacity for cartilage to regenerate decreases, leading to OA [30].
Treatment with anthocyanins prevented the apoptosis of chondrocytes [27,28]. For instance, malvidin reduced the number of chondrocytes in rats expressing senescence-associated B-galactosidase after IL-1β exposure. This effect was attributed to the suppression of nuclear-factor kappa-B activation [27]. Delphinidin reduced the terminal deoxynucleotidyl transferase dUTP nick-end labelled C28/I2 chondrocytes after hydrogen peroxide exposure. This reduction was accompanied by a suppression of pro-apoptotic markers [cleaved caspase-3 and cleaved poly (ADP-ribose) polymerase] and an increase in anti-apoptotic markers (B-cell lymphoma-extra large) [28]. This effect was associated with the activation of autophagy, marked by increased microtubule-associated protein 1A/1B-light chain 3 expression and autophagic vacuoles [28].
3.3. Effects on Anabolic and Catabolic Processes of Chondrocytes
The anabolic process in chondrocytes is reflected by the expression of ECM components, such as aggrecan and type II collagen. Meanwhile, the catabolic process in chondrocytes is reflected by the expression of metalloproteinases (MMPs) responsible for breaking down the ECM [31]. In OA, inflammation skewed the balance between anabolic and catabolic processes to the latter, contributing to cartilage degradation [32].
Various in vitro and in vivo studies have showcased the ability of anthocyanins and anthocyanidins to promote the anabolic process and prevent the catabolic process of chondrocytes. Notably, pelargonidin-treated primary mouse chondrocytes stained strongly for its proteoglycan content, and showed reduced disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMSTS5) and MMP-13 expressions [26]. Similarly, in mice with MMD-induced OA, pelargonidin treatment (10–20 mg/kg/d for 8 weeks) increased aggrecan expression while decreasing MMP-13 expression at the cartilage [26].
In another study, peonidin-3-glycoside and its deglycosylated form, as well as cyanidin-3-glycoside but not its deglycosylated form, reduced MMP-1, -3, and -13 mRNA expression [16]. The regulatory effects of cyanidin were further validated in another study, whereby cyanidin-treated chondrocytes from OA patients exposed to IL-1β maintained the protein expression of aggrecan, type II collagen, and SRY-box transcription factor 9, while reducing the expression of MMP-13 and ADAMTS4. These effects are mediated by sirtuin-6 [25].
3.4. Effects of Cartilage Integrity
Cartilage integrity is the ultimate measure of joint health because it represents the net effects of treatment. In animal studies, the joint can be harvested and sectioned, and the cartilage morphology can be evaluated under a microscope and scored [33,34]. Surprisingly, only one included study used the Osteoarthritis Research Society International (OARSI) Score to assess cartilage integrity in rats with MMD-induced OA. The OARSI Score reduced after pelargonidin supplementations (20–30 mg/kg/day for 8 weeks) [26].
3.5. Effects on Pain Threshold
OA is manifested as joint pain in animals and humans with OA [35]. MIA-induced OA is an excellent model for evaluating pain response [36]. Malvidin (10 and 20 mg/kg/day for 14 days) increased the paw pressure and joint compression threshold of rats with MIA-induced OA, indicating greater pain tolerance [27].
3.6. Mechanism of Actions
Most of the studies attributed the joint benefits of anthocyanins and anthocyanidins to their anti-inflammatory effects, particularly through the suppression of NFκB activation [15,16,24,25,27]. The nuclear translocation of NFκB or p65 is required for the translation of pro-inflammatory genes [37]. Treatment with anthocyanins reduced the phosphorylation and nuclear translation of p65 in chondrocytes. However, the mechanisms of different anthocyanins and anthocyanidins could be different. A study illustrated that cyanidin, peonidin, and their deglycosylated forms suppressed the activation of the IκB kinase complex and I-kappa-B related to the canonical NFκB pathway in human primary chondrocytes exposed to IL-1β, but only the glycosylated forms prevent the activation of p-65 [16]. Only peonidin, among the four compounds tested, can activate JNK pathway. None are effective in activating p-38 [16]. JNK and p38, along with ERK, form MAPK signalling, which is also vital to the inflammation process in OA [38]. However, in AGE-exposed human primary chondrocytes, cyanidin-, peonidin-, and malvidin-3-glycoside could suppress the activation of IKK, IκB, p65, ERK, p-38, and JNK altogether [39]. The model difference could be responsible for the discrepancy in the molecular effects of anthocyanins.
Autophagy is another cellular mechanism critical for maintaining chondrocytes’ health [40]. Chloroquine, an autophagy inhibitor, was shown to ameliorate the anti-apoptotic effects of delphinidin in human chondrocyte C28/I2 exposed to hydrogen peroxide [28]. Sirtuin-6, an anti-senescence molecule, also plays a role in mediating the chondroprotective effects of anthocyanins. Sirtuin-positive cells increased in mice with MMD-induced OA treated with 5 mg/kg/day cyanidins for 8 weeks [25]. Cyanidin also improved the expression of sirtuin-6 in primary human chondrocytes exposed to IL-1β, but its silencing abolished the chondroprotective effects of cyanidins [25].
The study designs and major findings of the included studies are summarised in Table 2.

Table 2.
Summary of published studies on the effects of anthocyanins and anthocyanidins on OA.
4. Discussion
The pathogenesis of OA is multifaceted, involving not only mechanical wear and tear but also biochemical signalling that exacerbates synovial inflammation and cartilage degradation [41]. The mechanical wear and tear of cartilage generates particles known as damage-associated molecular patterns, which bind with Toll-like receptors (TLRs) on the surface of invading macrophages, chondrocytes, and synoviocytes. This binding recruits adapters with TLR-IL1 domains to initiate downstream signalling cascades, which include kinases such as MAPK and IKKs, both of which regulate the phosphorylation of transcription factors important in inflammation [42]. Pro-inflammatory cytokines stimulate chondrocytes and synoviotes to produce MMPs such as MMP-1, MMP-3, and MMP-13, degrading collagen and proteoglycans, leading to cartilage destruction [43]. As evidenced by previous studies, anthocyanins are able to suppress inflammation via the regulation of MAPK and NFκB signalling and reduce the cartilage degradation process by downregulating MMP expression [15,16].
In OA, chondrocytes, the sole cell type in cartilage, increase ECM production in an attempt to repair the damaged cartilage. The increased workload drives the hypertrophy of chondrocytes. They eventually undergo premature senescence, loss of functions, and apoptosis [44]. Sirtuin-6 is known to play a role in DNA repair and preserving mitochondrial functions in chondrocytes [45]. Malvidin was shown to downregulate β-galactosidase, a cellular senescence marker, in the cartilage of mice with OA [27]. In addition, as an activator of sirtuin-6, cyanidin preserved anabolic processes while reducing catabolic processes in chondrocytes in OA conditions [25]. Interestingly, cyanidin also prevents chondrogenic and hypertrophic differentiation of mesenchymal stem cells by blocking autophagy [46]. This observation warrants the examination of the effects of cyanidins on hypertrophic chondrocytes in OA conditions.
Autophagy is an important process of chondrocyte homeostasis, as it prevents the accumulation of damaged organelles that might negatively impact the functions of chondrocytes, eventually leading to apoptosis [47]. Autophagy is activated during early OA as a protective mechanism but is downregulated as the disease progresses. Thus, the delicate stimulation of the autophagy process could mitigate the progression of OA [48]. Delphinidin could enhance autophagy in chondrocytes assaulted with oxidative damage and prevent their apoptosis. Blocking autophagy could nullify the benefits of delphinidin, signifying the central role of this process in chondroprotection [28].
The enhanced anabolism and reduced catabolism of cartilage could explain the preservation of cartilage integrity in mice with OA treated with pelargonidin. On the other hand, the increased pain threshold, as observed in malvidin-treated rats with OA, could be a result of improvement in joint structure (although premature to be concluded in a short-term study as in [27]), but is more likely a result of the suppression of the inflammation important in exacerbating pain transduction. Inflammatory mediators may activate peripheral nociceptors by stimulating the further release of inflammatory mediators and sensitising primary afferent neurons to other stimuli [49].
The mechanism of anthocyanins and anthocyanidins in managing OA is summarised in Figure 3.
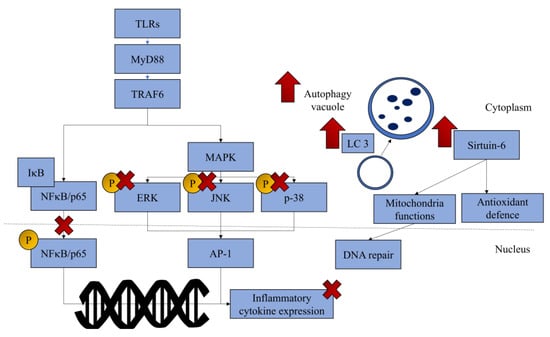
Figure 3.
The regulatory mechanism of anthocyanins and anthocyanidins in mitigating the progression of OA. Most anthocyanins and anthocyanidins can prevent inflammation and specific anthocyanins, such as cyanidin, which has been reported to upregulate sirtuin-6, whereas delphinidin has been reported to upregulate autophagy. (Drawn with Microsoft PowerPoint.) Abbreviations: AP-1, AP-1 transcription factor; ERK, extracellular signal-regulated kinase; IκB, I-kappa-B; JNK, c-Jun N-terminal kinase; LC3, microtubule-associated protein 1A/1B-light chain 3; MAPK, mitogen-activated protein kinase; NFκB, nuclear factor kappa-B; TRAF6, tumour necrosis factor receptor-associated factor 6; up arrow (↑), upregulation; cross symbol (×), inhibition.
Though not explored in the current review, anthocyanins and anthocyanidins could manage OA by indirect mechanisms. Firstly, anthocyanins have been demonstrated to mitigate metabolic syndromes and obesity in many studies [50]. Since obesity is a significant risk factor for OA due to increased mechanical loading on the joint, systemic inflammation and oxidative stress [51], anthocyanins and anthocyanidins could relieve the effects of obesity on the joint. Secondly, previous studies have demonstrated that anti-osteoporosis medications can help improve joint health in patients with OA [52]. This benefit may be a consequence of enhanced subchondral structures, which transfer less loading stress to the cartilage [53]. The intake of anthocyanin-rich food has been associated with improved bone health in middle-aged and older adults at risk of osteoporosis, according to a meta-analysis [54]. Preclinical studies have demonstrated the potential pro-osteogenic and anti-resorptive effects of anthocyanins [55]. Thus, anthocyanins could preserve subchondral bone and cartilage integrity at the same time. Thirdly, the bioavailability of anthocyanins is low after oral consumption [56]. They are metabolised to other phenolic compounds like protocatechuic acid (PCA) [57], with potent chondroprotective effects. A study found that PCA deterred the loss of ECM components from cartilage explants in culture and showed shared anti-inflammatory mechanisms with anthocyanins [16]. It has also been incorporated into biomaterials for OA treatment [58]. Further studies should be conducted to examine whether these metabolites are responsible for the chondroprotective effects of anthocyanins. Lastly, there is a surge of interest in the gut–joint axis, whereby the relationship between gut microbiota and OA is mediated by inflammation, oxidative stress, and metabolic dysfunction [59,60]. Anthocyanins have been shown to regulate gut microbiota composition in achieving its therapeutic potentials [61,62,63]; therefore, their chondroprotective effects might be mediated by the gut–joint axis.
Several limitations were noted from the studies included in the current review. For the in vitro studies, the replication of findings in more than one chondrocyte source should provide stronger evidence on the effects of anthocyanins and anthocyanidins. The upstream mechanism leading to the activation of MAPKs and IKK, such as tumour necrosis factor receptor-associated factor 6 (TRAF6) and myeloid differentiation primary response 88 (MyD88), which are central to various inflammatory pathways [64], have yet to be explored. Similarly, the regulatory mechanisms of sirtuin-6, autophagy, and Nrf2 by anthocyanins and anthocyanidins are not precise as of current and warrant further investigation. Cartilage explants provide a simple three-dimensional culture system to observe the effects of xenobiotics on cartilage metabolism, such as ECM component release and retention. However, this type of experiment has only been attempted for PCA, as well as anthocyanin-rich food extracts [15,16], but not anthocyanins or anthocyanidins. For the in vivo studies, only one explored the effects of anthocyanins on cartilage integrity, and one examined the effects of anthocyanins on pain response. For clinical translation, the preservation of cartilage tissue and the amelioration of symptoms are important and should be emphasised in the future. Most importantly, none of the vivo studies answered whether anthocyanins, anthocyanidins, or their metabolites could enter the circulation and subsequently deposit in the joint space to exert their effects. The apparent lack of clinical trials in the field should be of concern because the effects of anthocyanins or anthocyanidins have not been verified in humans. Our research in clinicaltrial.gov dated January 2025 revealed no ongoing or completed trials on this topic. This gap in clinical translation should be bridged in the future.
The current scoping review is not without its limitations. We only searched for items published in the English language in three major scholarly databases. Thereby, selection bias could not be avoided due to exclusion of studies published in other languages and not indexed by these databases. We did not search for grey literature, so there might be a bias in including studies reporting favourable outcomes. We also did not conduct quality assessments of the studies included. However, an appraisal of possible limitations of the studies is mentioned in Table 2. Nevertheless, this scoping review provides the current landscape of the research on the chondroprotective effects of anthocyanins or anthocyanidins and suggests possible future studies for this field.
5. Conclusions
Anthocyanins and anthocyanidins exert chondroprotective effects via the modulation of inflammation signalling pathways (MAPK and NFκB) in chondrocytes. Notably, cyanidin also upregulates sirtuin-6, and delphinidin upregulates autophagy in chondrocytes. Specific types of anthocyanins, like malvidin, have been found to be effective in abolishing inflammatory pain, and pelargonidin has been found to preserve cartilage integrity. A translation gap exists to date as no clinical trials on this topic can be found. Future research efforts should be geared towards this direction to verify the effectiveness of anthocyanins and anthocyanidins in managing OA.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph18030301/s1. Table S1: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist.
Author Contributions
Conceptualisation, S.O.E. and K.-Y.C.; methodology, X.M., S.O.E. and K.-Y.C.; validation, K.-Y.C.; formal analysis, X.M., S.O.E. and K.-Y.C.; investigation, X.M., S.O.E. and K.-Y.C.; resources, K.-Y.C.; writing—original draft preparation, X.M. and S.O.E.; writing—review and editing, K.-Y.C.; visualisation, K.-Y.C.; supervision, K.-Y.C.; project administration, K.-Y.C.; funding acquisition, K.-Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universiti Kebangsaan Malaysia through the Research University Grant (GUP-2024-026).
Acknowledgments
We thank Universiti Kebangsaan Malaysia for funding this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wenjian Zhao, Y.L.; Wang, Y.; Chin, K.-Y. Osteoarthritis: An integrative overview from pathogenesis to management. Malays. J. Pathol. 2024, 46, 369–378. [Google Scholar]
- Li, H.Z.; Liang, X.Z.; Sun, Y.Q.; Jia, H.F.; Li, J.C.; Li, G. Global, regional, and national burdens of osteoarthritis from 1990 to 2021: Findings from the 2021 global burden of disease study. Front. Med. 2024, 11, 1476853. [Google Scholar] [CrossRef]
- Nicholas, E.; Cheng, J.; Moley, P.J. Non-operative Treatment Options for Osteoarthritis in the Hip. HSS J. 2023, 19, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Cherniavskyi, V.V.; Baylo, A.E.; Onyshuk, L.O.; Tishchenko, V.V. Critical evaluation of the current role of SYSADOA in the management of osteoarthritis (literature review). Pain Jt. Spine 2024, 14, 96–105. [Google Scholar] [CrossRef]
- Atthakorn, J.; Chaturong, P. Complications after Total Knee Arthroplasty: Stiffness, Periprosthetic Joint Infection, and Periprosthetic Fracture. In Arthroplasty; Alessandro, Z., Hechmi, T., Eric, L., Eds.; IntechOpen: Rijeka, Croation, 2022; p. Ch. 8. [Google Scholar]
- Fang, S.; Zhang, B.; Xiang, W.; Zheng, L.; Wang, X.; Li, S.; Zhang, T.; Feng, D.; Gong, Y.; Wu, J.; et al. Natural products in osteoarthritis treatment: Bridging basic research to clinical applications. Chin. Med. 2024, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediators Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.Q.; Nie, L.Y.; Qian, L.N.; Zhao, K. Efficacy and Safety of Polyphenols for Osteoarthritis Treatment: A Meta-Analysis. Clin. Ther. 2021, 43, e241–e253.e242. [Google Scholar] [CrossRef]
- Arias, C.; Salazar, L.A. Autophagy and Polyphenols in Osteoarthritis: A Focus on Epigenetic Regulation. Int. J. Mol. Sci. 2021, 23, 421. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, X.; Lin, X.; Mostafa, S.; Zou, H.; Wang, L.; Jin, B. Plant anthocyanins: Classification, biosynthesis, regulation, bioactivity, and health benefits. Plant Physiol. Biochem. 2024, 217, 109268. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Leporini, M.; Tenuta, M.C.; Tundis, R. The Role of Anthocyanins in Drug Discovery: Recent Developments. Curr. Drug Discov. Technol. 2020, 17, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Frasca, G.; Panico, A.M.; Bonina, F.; Messina, R.; Rizza, L.; Musumeci, G.; Rapisarda, P.; Cardile, V. Involvement of inducible nitric oxide synthase and cyclooxygenase-2 in the anti-inflammatory effects of a red orange extract in human chondrocytes. Nat. Prod. Res. 2010, 24, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Chuntakaruk, H.; Kongtawelert, P.; Pothacharoen, P. Chondroprotective effects of purple corn anthocyanins on advanced glycation end products induction through suppression of NF-κB and MAPK signaling. Sci. Rep. 2021, 11, 1895. [Google Scholar] [CrossRef]
- Wongwichai, T.; Teeyakasem, P.; Pruksakorn, D.; Kongtawelert, P.; Pothacharoen, P. Anthocyanins and metabolites from purple rice inhibit IL-1β-induced matrix metalloproteinases expression in human articular chondrocytes through the NF-κB and ERK/MAPK pathway. Biomed. Pharmacother. 2019, 112, 108610. [Google Scholar] [CrossRef]
- Herrera-Bravo, J.; Beltrán, J.F.; Huard, N.; Saavedra, K.; Saavedra, N.; Alvear, M.; Lanas, F.; Salazar, L.A. Anthocyanins Found in Pinot Noir Waste Induce Target Genes Related to the Nrf2 Signalling in Endothelial Cells. Antioxidants 2022, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Ahmad, I.; Haqqi, T.M. Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radic. Biol. Med. 2018, 116, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Semmarath, W.; Mapoung, S.; Umsumarng, S.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Cyanidin-3-O-glucoside and Peonidin-3-O-glucoside-Rich Fraction of Black Rice Germ and Bran Suppresses Inflammatory Responses from SARS-CoV-2 Spike Glycoprotein S1-Induction In Vitro in A549 Lung Cells and THP-1 Macrophages via Inhibition of the NLRP3 Inflammasome Pathway. Nutrients 2022, 14, 2738. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- Saulite, L.; Jekabsons, K.; Klavins, M.; Muceniece, R.; Riekstina, U. Effects of malvidin, cyanidin and delphinidin on human adipose mesenchymal stem cell differentiation into adipocytes, chondrocytes and osteocytes. Phytomedicine 2019, 53, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Social. Res. Methodol. Theory Pract. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Chen, D.; Haqqi, T.M. Delphinidin inhibits IL-1β-induced activation of NF-κB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes. Rheumatology 2013, 52, 998–1008. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, Z.M.; Hu, J.N.; Jin, Y.; Guo, Q.; Xu, J.J.; Chen, Z.X.; Jiang, R.H.; Wu, Y.S. Cyanidin ameliorates the progression of osteoarthritis via the Sirt6/NF-κB axis in vitro and in vivo. Food Funct. 2019, 10, 5873–5885. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Li, H.; Luo, C.; Hu, W.; Weng, T.J.; Shuang, F. Pelargonidin ameliorates inflammatory response and cartilage degeneration in osteoarthritis via suppressing the NF-κB pathway. Arch. Biochem. Biophys. 2023, 743, 109668. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Shi, K.; Chen, G.; Shen, Y.; Pan, T. Malvidin attenuates pain and inflammation in rats with osteoarthritis by suppressing NF-κB signaling pathway. Inflamm. Res. 2017, 66, 1075–1084. [Google Scholar] [CrossRef]
- Lee, D.Y.; Park, Y.J.; Song, M.G.; Kim, D.R.; Zada, S.; Kim, D.H. Cytoprotective Effects of Delphinidin for Human Chondrocytes against Oxidative Stress through Activation of Autophagy. Antioxidants 2020, 9, 83. [Google Scholar] [CrossRef]
- Wang, B.W.; Jiang, Y.; Yao, Z.L.; Chen, P.S.; Yu, B.; Wang, S.N. Aucubin Protects Chondrocytes Against IL-1β-Induced Apoptosis In Vitro and Inhibits Osteoarthritis in Mice Model. Drug Des. Devel Ther. 2019, 13, 3529–3538. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef] [PubMed]
- Sumsuzzman, D.M.; Choi, J.; Khan, Z.A.; Kamenos, G.; Hong, Y. Melatonin Maintains Anabolic-Catabolic Equilibrium and Regulates Circadian Rhythm During Osteoarthritis Development in Animal Models: A Systematic Review and Meta-analysis. Front. Pharmacol. 2021, 12, 714974. [Google Scholar] [CrossRef]
- Arra, M.; Abu-Amer, Y. Cross-talk of inflammation and chondrocyte intracellular metabolism in osteoarthritis. Osteoarthritis Cartilage 2023, 31, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Ekeuku, S.O.; Ahmad, F.; Chin, K.-Y. Changes of Grip Strength, Articular Cartilage and Subchondral Bone in Monoiodoacetate-Induced Osteoarthritis in Rats. Sains Malays. 2022, 51, 3741–3754. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Nor Muhamad, M.L.; Aminuddin, A.A.; Ahmad, F.; Wong, S.K.; Mark-Lee, W.F.; Chin, K.-Y. Effects of emulsified and non-emulsified palm tocotrienol on bone and joint health in ovariectomised rats with monosodium iodoacetate-induced osteoarthritis. Biomed. Pharmacother. 2024, 170, 115998. [Google Scholar] [CrossRef]
- Yu, H.; Huang, T.; Lu, W.W.; Tong, L.; Chen, D. Osteoarthritis Pain. Int. J. Mol. Sci. 2022, 23, 4642. [Google Scholar] [CrossRef] [PubMed]
- Han, F.Y.; Brockman, D.A.; Nicholson, J.R.; Corradini, L.; Smith, M.T. Pharmacological characterization of the chronic phase of the monoiodoacetate-induced rat model of osteoarthritis pain in the knee joint. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1515–1522. [Google Scholar] [CrossRef]
- Bagaev, A.V.; Garaeva, A.Y.; Lebedeva, E.S.; Pichugin, A.V.; Ataullakhanov, R.I.; Ataullakhanov, F.I. Elevated pre-activation basal level of nuclear NF-kappaB in native macrophages accelerates LPS-induced translocation of cytosolic NF-kappaB into the cell nucleus. Sci. Rep. 2019, 9, 4563. [Google Scholar] [CrossRef] [PubMed]
- Saklatvala, J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr. Drug Targets 2007, 8, 305–313. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ahmad, F.; Ima-Nirwana, S. Regulation of inflammatory response and oxidative stress by tocotrienol in a rat model of non-alcoholic fatty liver disease. J. Funct. Foods 2020, 74, 104209. [Google Scholar] [CrossRef]
- Bao, J.; Chen, Z.; Xu, L.; Wu, L.; Xiong, Y. Rapamycin protects chondrocytes against IL-18-induced apoptosis and ameliorates rat osteoarthritis. Aging 2020, 12, 5152–5167. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Mandraju, R.; Troutman, T.D.; Pasare, C. Toll-Like Receptor Function and Signaling. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Malemud, C.J. Chapter Seven—Matrix Metalloproteinases and Synovial Joint Pathology. In Progress in Molecular Biology and Translational Science; Matrix Metalloproteinases and Tissue Remodeling in Health and Disease: Target Tissues and Therapy; Khalil, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 148, pp. 305–325. [Google Scholar]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef]
- Sun, K.; Wu, Y.; Zeng, Y.; Xu, J.; Wu, L.; Li, M.; Shen, B. The role of the sirtuin family in cartilage and osteoarthritis: Molecular mechanisms and therapeutic targets. Arthritis Res. Ther. 2022, 24, 286. [Google Scholar] [CrossRef]
- Cao, Z.; Huang, S.; Dou, C.; Xiang, Q.; Dong, S. Cyanidin suppresses autophagic activity regulating chondrocyte hypertrophic differentiation. J. Cell Physiol. 2018, 233, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Li, J.J.; Zhou, H.; Zhu, X.W.; Zhang, P.H.; Huang, B.; Zhao, W.T.; Zhao, X.F.; Chen, E.S. Chondrocyte autophagy mechanism and therapeutic prospects in osteoarthritis. Front. Cell Dev. Biol. 2024, 12, 1472613. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Bahar, M.E.; Kim, C.-W.; Seo, M.-S.; Song, M.-G.; Song, S.-Y.; Kim, S.-Y.; Kim, D.-R.; Kim, D.-H. Autophagy in Osteoarthritis: A Double-Edged Sword in Cartilage Aging and Mechanical Stress Response: A Systematic Review. J. Clin. Med. 2024, 13, 3005. [Google Scholar] [CrossRef] [PubMed]
- Mironidou-Tzouveleki, M. Inflammation and pain. Ann. Gen. Psychiatry 2010, 9, S2. [Google Scholar] [CrossRef]
- Godyla-Jabłoński, M.; Raczkowska, E.; Jodkowska, A.; Kucharska, A.Z.; Sozański, T.; Bronkowska, M. Effects of Anthocyanins on Components of Metabolic Syndrome—A Review. Nutrients 2024, 16, 1103. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezhiyan, U.; Varughese, I.; Sun, A.R.; Wu, X.; Crawford, R.; Prasadam, I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front. Immunol. 2022, 13, 907750. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.C.; Chang, C.C.; Wu, W.T.; Lee, R.P.; Yao, T.K.; Peng, C.H.; Yeh, K.T. Effect of Osteoporosis Treatments on Osteoarthritis Progression in Postmenopausal Women: A Review of the Literature. Curr. Rheumatol. Rep. 2024, 26, 188–195. [Google Scholar] [CrossRef]
- Zhu, X.; Chan, Y.T.; Yung, P.S.H.; Tuan, R.S.; Jiang, Y. Subchondral Bone Remodeling: A Therapeutic Target for Osteoarthritis. Front. Cell Dev. Biol. 2020, 8, 607764. [Google Scholar] [CrossRef]
- Quek, Y.Y.; Cheng, L.J.; Ng, Y.X.; Hey, H.W.D.; Wu, X.V. Effectiveness of anthocyanin-rich foods on bone remodeling biomarkers of middle-aged and older adults at risk of osteoporosis: A systematic review, meta-analysis, and meta-regression. Nutr. Rev. 2023, 82, 1187–1207. [Google Scholar] [CrossRef]
- Mao, W.; Huang, G.; Chen, H.; Xu, L.; Qin, S.; Li, A. Research Progress of the Role of Anthocyanins on Bone Regeneration. Front. Pharmacol. 2021, 12, 773660. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic Acid Is the Major Human Metabolite of Cyanidin-Glucosides123. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Hsu, Y.H.; Lin, Y.C.; Nguyen, T.T.; Chen, H.W.; Nabilla, S.C.; Hou, S.Y.; Chang, F.C.; Chung, R.J. 3d printing of collagen/oligomeric proanthocyanidin/oxidized hyaluronic acid composite scaffolds for articular cartilage repair. Polymers 2021, 13, 3123. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Ram, P.R.; Jeyaraman, N.; Yadav, S. The Gut-Joint Axis in Osteoarthritis. Cureus 2023, 15, e48951. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Hao, L.; Huang, G. Exploring the Interconnection between Metabolic Dysfunction and Gut Microbiome Dysbiosis in Osteoarthritis: A Narrative Review. Biomedicines 2024, 12, 2182. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Wang, F.; Li, Y.; Zheng, X. Black Current Anthocyanins Improve Lipid Metabolism and Modulate Gut Microbiota in High-Fat Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2021, 65, e2001090. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Zhou, Y.; Zheng, X. Black rice anthocyanins alleviate hyperlipidemia, liver steatosis and insulin resistance by regulating lipid metabolism and gut microbiota in obese mice. Food Funct. 2021, 12, 10160–10170. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Deng, R.; Chu, Q.; Zheng, X. Pomegranate peel anthocyanins prevent diet-induced obesity and insulin resistance in association with modulation of the gut microbiota in mice. Eur. J. Nutr. 2022, 61, 1837–1847. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Yang, M.; Liu, M. Rutin prevents inflammation induced by lipopolysaccharide in RAW 264.7 cells via conquering the TLR4-MyD88-TRAF6-NF-κB signalling pathway. J. Pharm. Pharmacol. 2021, 73, 110–117. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).