Supercritical Extraction and Identification of Bioactive Compounds in Dryopteris fragrans (L.) Schott
Abstract
1. Introduction
2. Results
Supercritical CO2 Extraction from the Aerial Parts of D. fragrans Plant
- − Pressure of 200 bar, extraction temperature of 55 °C, extraction time of 1 h. The yield of biologically active substances was 0.0000062 kg/0.0001 kg of plant sample; the proportion of the EtOH modifier was 2%.
- − Pressure of 250 bar, extraction temperature of 55 °C, extraction time of 1 h. The yield of biologically active substances was 0.0000059 kg/0.0001 kg of plant sample; the proportion of the EtOH modifier was 2%.
3. Discussion
3.1. Global Metabolome Profile of D. fragrans
3.2. Flavones
3.3. Flavonols
3.4. Anthocyanins
3.5. Diterpenoids
3.6. Phlorodlucinol Derivatives
3.7. Newly Identified Chemical Compounds in D. Fragrans
4. Materials and Methods
4.1. Materials
4.2. Chemicals and Reagents
4.3. Extraction
4.4. Liquid Chromatography
4.5. Mass Spectrometry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Class of Compounds | Identification | Formula | Calculated Mass | Observed Mass [M−H]− | Observed Mass [M+H]+ | MS/MS Stage 1 Fragmentation | MS/MS Stage 2 Fragmentation | MS/MS Stage 3 Fragmentation | References | |
|---|---|---|---|---|---|---|---|---|---|---|
| POLYPHENOLS | ||||||||||
| 1 | Flavone | Dihydroxyflavone * | C15H10O4 | 254.237 | 255 | 237; 185; 113 | 167 | 125 | Chinese herbal formula Jian-Pi-Yi-Shen pill [39]; Ribes pauciflorum [34] | |
| 2 | Flavone | Formononetin [Biochanin B; Formononetol] * | C16H12O4 | 268.264 | 269 | 241; 223 | 223 | D. jacutense [47]; M. varia [36]; M. amurensis [43], R. fragrans [42]; Chinese herbal formula Jian-Pi-Yi-Shen pill [39] | ||
| 3 | Flavone | Apigenin [5,7-Dixydroxy-2-(4-Hydroxyphenyl)-4H-Chromen-4-One] | C15H10O5 | 270.236 | 271 | 225 | 179 | R. meyeri [29]; L. japonica [26]; Andean blueberry [32] | ||
| 4 | Flavone | Trihydroxy(iso)flavone | C15H10O5 | 270.236 | 271 | 196 | 168 | 113 | Propolis [48] | |
| 5 | Flavone | Luteolin * | C15H10O6 | 286.236 | 287 | 187; 277 | Inula gaveolens [49]; A. absinthium [50] | |||
| 6 | Flavone | Herbacetin [3,5,7,8-Tetrahydroxy-2-(4-hydro-xyphenyl)-4H-chromen-4-one] * | C15H10O7 | 302.235 | 303 | 275; 203 | 245; 175 | 233; 175 | L. caerulea [51] | |
| 7 | Flavone | Trimethoxy flavone | C18H16O5 | 312.316 | 313 | 267 | 239 | 197; 113 | A. cordifolia [52] | |
| 8 | Flavone | Pentahydroxy dimethoxyflavone | C17H14O9 | 362.287 | 363 | 344; 300; 256 | 238; 146 | G. linguiforme [52] | ||
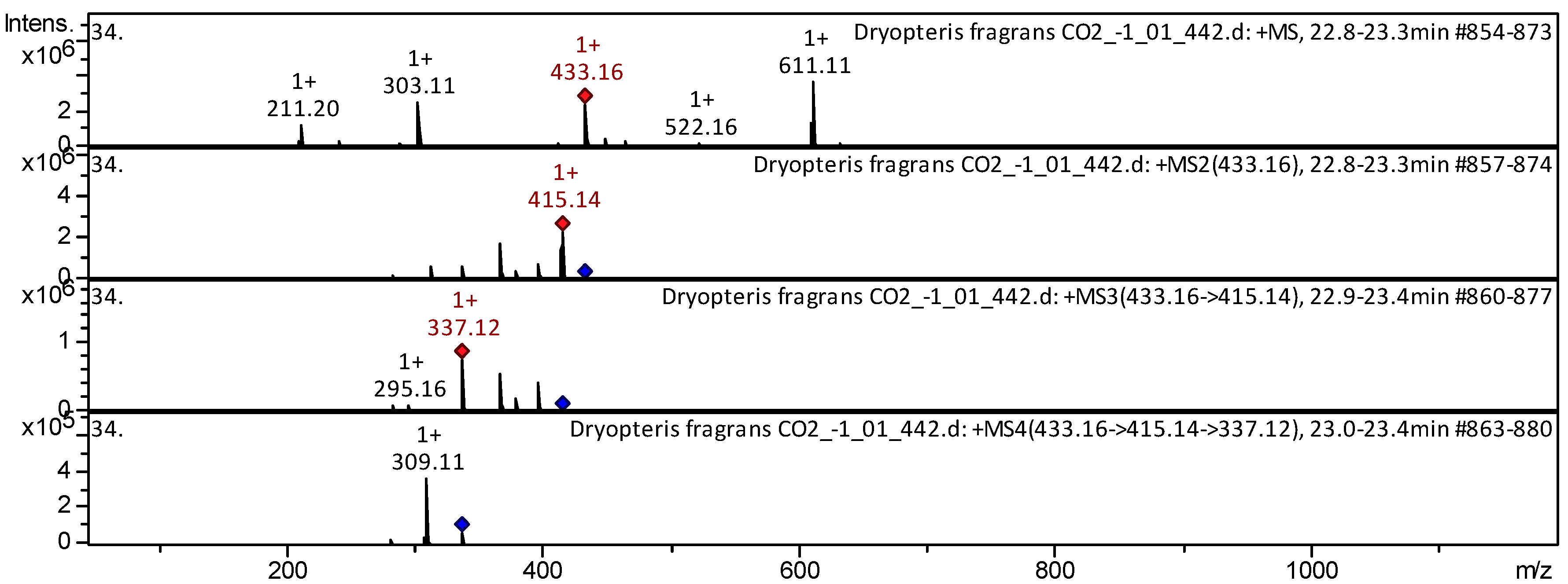
| 9 | Flavone | Vitexin [Apigenin 8-C-Glucoside] | C21H20O10 | 432.377 | 433 | 415; 367; 313 | 337; 283 | 309 | T. aestivum [23]; D. ramosa [24] | |
| 10 | Flavone | Isovitexin [Saponaretin; Homovitexin] | C21H20O10 | 432.377 | 433 | 415; 367; 313 | 337; 283 | 309 | Passiflora incarnata [27]; Chilean currants [30] | |
| 11 | Flavone | Genistein 8-C-glucoside * | C21H20O10 | 432.377 | 433 | 415 | 337 | 309 | Mexican lupine species [28] | |
| 12 | Flavone | Genistein 6-C-glucoside * | C21H20O10 | 432.377 | 433 | 415 | 337 | 309 | Mexican lupine species [28] | |
| 13 | Flavone | Acacetin 8-C-glucoside * | C22H22O10 | 446.404 | 447 | 429; 377 | 410; 358; 301; 251 | 377; 340; 251 | Mexican lupine species [28] | |
| 14 | Flavone | Luteolin 7-O-glucoside [Cynaroside; Luteoloside] | C21H20O11 | 448.376 | 449 | 431; 287 | 213 | T. aestivum [23]; L. japonicum [26]; Passiflora incarnata [27]; Mexican lupine species [28] | ||
| 15 | Flavone | Luteolin 8-C-Glucoside [Orientin; Lutexin] | C21H20O11 | 448.376 | 449 | 431 | 353; 299 | 325 | T. aestivum [23]; Aspalathus linearis [25] | |
| 16 | Flavone | Luteolin 6-C-glucoside [Isoorientin; Homoorientin] | C21H20O11 | 448.376 | 449 | 431 | 353; 299 | 325 | T. aestivum [23]; D. ramosa [24]; Aspalathus linearis [25] | |
| 17 | Flavone | Luteolin C-hexoside * | C21H20O11 | 448.376 | 449 | 431 | 353; 299 | 325 | T. aestivum [23] | |
| 18 | Flavone | Eriodictyol-O-hexoside | C21H22O11 | 450.392 | 449 | 287 | 151 | Andean blueberry [32]; F. glaucescens; F. pottsii [52] | ||
| 19 | Flavone | (S)-eriodictyol-6-C-β-D-glucopyranoside | C21H22O11 | 450.392 | 451 | 433 | 414; 363; 299; 233 | 344; 258; 213 | Aspalathus linearis [25] | |
| 20 | Flavone | (R)-eriodictyol-6-C-β-D-glucopyranoside | C21H22O11 | 450.392 | 451 | 433 | 414; 363; 299; 233 | 344; 258; 213 | Aspalathus linearis [25] | |
| 21 | Flavone | 6,4′-Dimethoxyisoflavone-7-O-glucoside * | C23H24O10 | 460.430 | 461 | 443; 419; 306 | 425; 373; 186 | 407; 257 | Astragali radix [53] | |
| 22 | Flavone | Chrysoeriol 6-C-glucoside | C22H22O11 | 462.403 | 463 | 445; 403; 375; 329; 237 | 426; 401; 347 | 385; 357; 269 | T. aestivum [54] | |
| 23 | Flavone | Chrysoeriol 8-C-glucoside [Scoparin] | C22H22O11 | 462.403 | 463 | 445; 403; 375; 329; 237 | 426; 401; 347 | 385; 357; 269 | Mexican lupine species [28]; Citrus sinensis [33] | |
| 24 | Flavone | Chrysoeriol C-hexoside | C22H22O11 | 462.403 | 463 | 445; 403; 375; 329; 237 | 426; 401; 347 | 385; 357; 269 | T. aestivum [54] | |
| 25 | Flavone | Eriodictyol-7-O-glucuronide * | C21H20O12 | 464.376 | 465 | 289 | 271; 163 | 145 | Mentha [55] | |
| 26 | Flavone | Luteolin 8-C-pentoside-6-C-hexoside | C26H28O15 | 580.491 | 581 | 563; 397; 325 | 251 | 223 | T. aestivum [56] | |
| 27 | Flavone | Luteolin 8-C-hexoside-6-C-pentoside | C26H28O15 | 580.491 | 581 | 563; 397; 325 | 251 | 223 | T. aestivum [56] | |
| 28 | Flavonol | Kaempferol | C15H10O6 | 286.236 | 287 | 269; 149 | 239; 181 | L. japonica [26]; R. meyeri [29]; Andean blueberry [32] | ||
| 29 | Flavonol | Quercetin | C15H10O7 | 302.235 | 303 | 284 | 240 | R. meyeri [29]; Propolis [48] | ||
| 30 | Flavonol | Myricetin | C15H10O8 | 318.235 | 319 | 273; 219 | 191 | 209 | Andean blueberry [32] | |
| 31 | Flavonol | Kaempferol 3-O-pentoside | C20H18O10 | 418.350 | 417 | 195 | 151 | 136 | Andean blueberry [32]; R. dikuscha [34] | |
| 32 | Flavonol | Quercetin 3-O-pentoside | C20H18O11 | 434.350 | 433 | 387 | 301; 231 | 283 | R. meyeri [29] | |
| 33 | Flavonol | Quercetin 3-D-xyloside [Reynoutrin] | C20H18O11 | 434.350 | 433 | 387 | 301 | 284 | Cranberry [31] | |
| 34 | Flavonol | Quercetin-3-O-arabinoside | C20H18O11 | 434.350 | 433 | 387 | 301; 233 | 285 | Cranberry [31] | |
| 35 | Flavonol | Astragalin [Kaempferol 3-O-glucoside] * | C21H20O11 | 448.376 | 447 | 429; 225 | 183 | 181 | L. japonica [26]; R. meyeri [29] | |
| 36 | Flavonol | Kaempferol-3-O-hexoside | C21H20O11 | 448.376 | 447 | 327; 285; 151 | 255 | Andean blueberry [32] | ||
| 37 | Flavonol | Quercitrin [Quercetin 3-O-rhamnoside] * | C21H20O11 | 448.376 | 447 | 327 | 299 | Cranberry [31] | ||
| 38 | Flavonol | Quercetin-3-O-hexoside | C21H20O12 | 464.376 | 463 | 301 | 271; 179 | 151 | A. absinthium [50] | |
| 39 | Flavonol | Hyperoside [Quercetin 3-O-galactoside; Hyperin] | C21H20O12 | 464.376 | 463 | 301 | 271; 179 | 151 | T. aestivum [23]; L. japonica [26]; Cranberry [31]; Andean blueberry [32] | |
| 40 | Flavonol | Quercetin 3-O-glucoside [Isoquercitrin; Hirsutrin] | C21H20O12 | 464.376 | 465 | 303 | 229; 165 | 201; 161 | T. aestivum [23]; R. meyeri [29]; L. japonica [26]; Cranberry [31] | |
| 41 | Flavonol | Myricitrin * | C21H20O12 | 464.376 | 465 | 447 | 428; 235 | 383 | Chilean currants [30] | |
| 42 | Flavonol | Rutin [Quercetin 3-O-rutinoside] | C27H30O16 | 610.517 | 611 | 303 | 257; 165 | 229 | R. meyeri [29]; R. magellanicum [30]; L. japonica [26] | |
| 43 | Flavonol | Quercetin-O-rhamnosyl-hexoside | C27H30O16 | 610.517 | 611 | 303; 465 | 257; 165 | 229; 201 | Papaya [57] | |
| 44 | Flavan-3-ol | (Epi)-catechin derivative | 379 | 379 | 261 | 233 | 151 | PubChem | ||
| 45 | Flavan-3-ol | (Epi)-afzelechin derivative | C18H16O10 | 392.313 | 393 | 275; 245; 215 | 245; 175 | 175; 127 | Z. marina [35] | |
| 46 | Flavan-3-ol | (Epi)-catechin derivative | 424 | 425 | 291 | 261; 191 | 191 | PubChem | ||
| 47 | Gallotannin | Strictinin [galloyl-HHDP-hexose] | C27H22O18 | 634.453 | 635 | 561; 461 | 433 | Juglans regia [58] | ||
| 48 | Anthocyanin | Apigenidin | C15H11O4 | 255.245 | 256 | 182 | 154 | 113 | T. aestivum [59] | |
| 49 | Anthocyanin | Delphinidin | C15H11O7 | 303.243 | 303 | 257; 165 | 229 | A. cordifolia [52] | ||
| 50 | Anthocyanin | Cyanidin-3-O-glucoside | C21H21O11+ | 449.384 | 449 | 287 | 213 | 185 | R. magellanicum [30] | |
| 51 | Anthocyanin | Cyanidin-3-O-hexoside | C21H21O11+ | 449.384 | 449 | 287 | 213; 165 | Andean blueberry [32] | ||
| 52 | Anthocyanin | Cyanidin-3-O-β-galactoside | C21H21O11 | 449.384 | 449 | 287 | 287; 213 | 185 | T. aestivum [23,59] | |
| 53 | Anthocyanin | Delphinidin 3-O-glucoside | C21H21O12+ | 465.390 | 465 | 303 | 257; 165 | 229; 201 | R. magellanicum [30] | |
| 54 | Anthocyanin | Delphinidin 3-O-hexoside | C21H21O12+ | 465.390 | 465 | 303 | 257; 165 | 229; 173 | Andean blueberry [32]; R. dikuscha [34] | |
| 55 | Anthocyanin | Delphinidin 3-O-β-galactoside | C21H21O12+ | 465.390 | 465 | 303 | 257; 165 | 229; 173 | R. dikuscha [34] | |
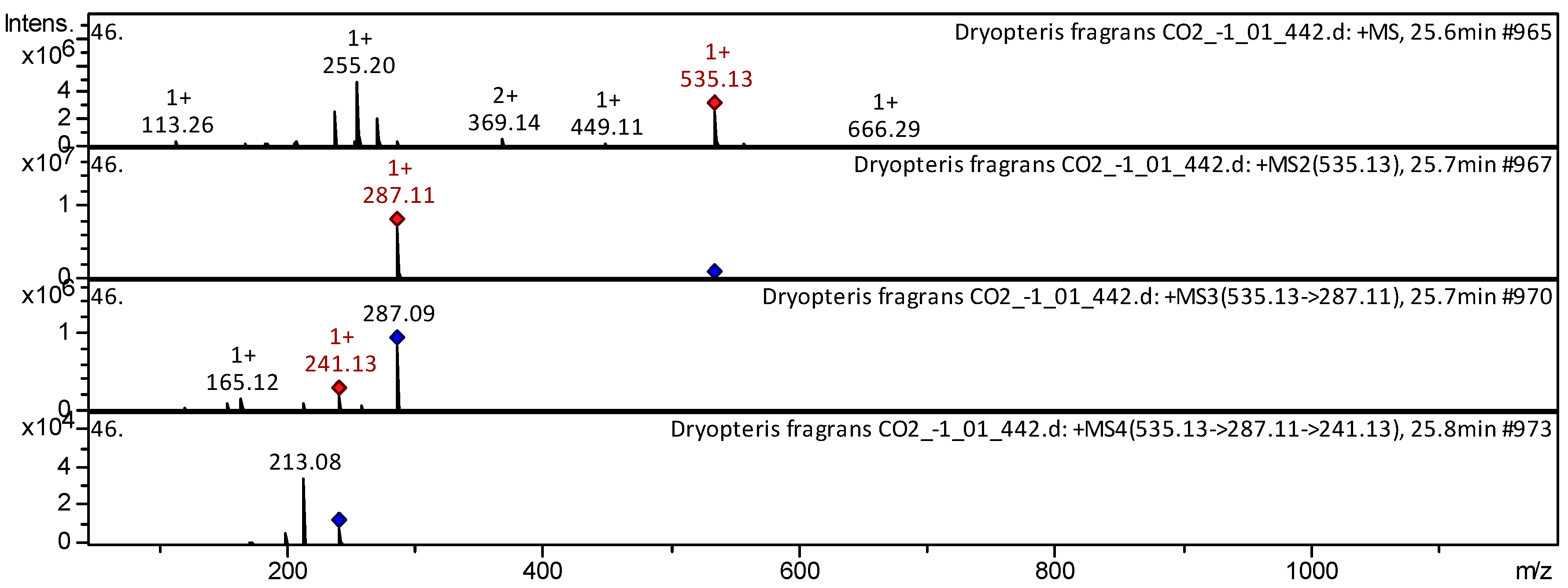
| 56 | Anthocyanin | Cyanidin 3-(6”-malonylglucoside) * | C24H23O14 | 535.431 | 535 | 287 | 241; 165 | 213 | Medicago varia [36]; T. aestivum [37] | |
| 57 | Anthocyanin | Cyanidin malonyl hexoside * | C24H23O14 | 535.431 | 535 | 287 | 241; 165 | 213 | T. aestivum [23]; R. magellanicum [30] | |
| 58 | Anthocyanin | Delphinidin-3-O-(6”-O-malonyl)-β-D-glucoside * | C24H23O15 | 551.430 | 551 | 303 | 257; 165 | 229 | T. aestivum [37] | |
| 59 | Anthocyanin | Delphinidin 3-O-rutinoside [Tulipanin] | C27H31O16 | 611.525 | 611 | 303; 465 | 257; 165 | L. caerulea [51]; R. aureum [34] | ||
| 60 | Anthocyanin | Delphinidin 3-O-(6-O-p-coumaroyl) glucoside * | C30H27O14 | 611.527 | 611 | 303; 465 | 257; 165 | R. pauciflorum [34] | ||
| 61 | Hydroxycinnamic acid | Caffeic acid [(2E)-3-(3,4-Dihydroxyphenyl)acrylic acid] | C9H8O4 | 180.157 | 181 | 135 | 119 | R. meyeri [29]; L. japonica [26] | ||
| 62 | Hydroxycinnamic acid | 3,4-Dihydroxyhydrocinnamic acid [Dihydrocaffeic acid] | C9H10O4 | 182.173 | 183 | 155 | 127 | 145 | Chilean currants [30] | |
| 63 | Ethyl protocatechuate [3,4-Dihydroxybenzoic Acid Ethyl Ester] | C9H10O4 | 182.173 | 183 | 155 | 126 | Medicago varia [36] | |||
| 64 | Trans-cinnamic acid | Ferulic acid | C10H10O4 | 194.184 | 193 | 176 | 132 | L. japonica [26]; Andean blueberry [32] | ||
| 65 | Hydroxycinnamic acid | Hydroxyferulic acid | C10H10O5 | 210.183 | 211 | 183; 113 | 165; 113 | Andean blueberry [32]; R. davurica [60] | ||
| 66 | Hydroxycinnamic acid | Sinapic acid [trans-Sinapic acid] * | C11H12O5 | 224.21 | 225 | 197 | 152 | 151 | Andean blueberry [32] | |
| 67 | Hydroxybenzoic acid | Ellagic acid [Benzoaric acid; Elagostasine] | C14H6O8 | 302.192 | 301 | 257; 165 | 229 | 201; 173 | R. meyeri [29] | |
| 68 | Hydroxycinnamic acid; | Chlorogenic acid [3-O-Caffeoylquinic acid] | C16H18O9 | 354.308 | 353 | 163 | 145 | 117 | Artemisia annua [50]; R. magellanicum [30] | |
| 69 | Hydroxycinnamic acid | Cryptochlorogenic acid [4-O-Caffeoylquinic acid; Quinic acid 4-O-Caffeate] | C16H18O9 | 354.308 | 353 | 163 | 145 | 117 | Juglans regia [58]; Artemisia annua [50]; R. magellanicum [30]; | |
| 70 | Neochlorogenic acid [5-O-Caffeoylquinic acid] | C16H18O9 | 354.308 | 355 | 163 | 145 | 117 | Artemisia annua [50]; R. magellanicum [30]; L. japonica [26] | ||
| 71 | Caffeic acid derivative | C16H18O9Na | 377.298 | 377 | 341 | 179 | Embelia [61] | |||
| 72 | Phenolic acid | Ellagic acid pentoside [Ellagic acid 4-O-xylopyranoside] | C19H14O12 | 434.307 | 433 | 387 | 301; 271 | 283; 257; 231 | R. pauciflorum; R. aureum [34] | |
| 73 | Phenolic acid | Dicaffeoylferuoylquinic acid * | 692.345 | 693 | 352; 261 | 261; 123 | 149 | PubChem | ||
| 74 | Phenylpropanoid | 1-O-Caffeoylquininc acid methyl ether | C17H20O9 | 368.335 | 369 | 207 | 192 | 153 | Pear [62] | |
| 75 | Dihydrochalcone | Phloretin [Dihydronaringenin; Phloretol] * | C15H14O5 | 274.268 | 275 | 257 | 230 | 229 | G. linguiforme [51,52] | |
| 76 | Stilbene | Pinosylvin [ 3,5-Stilbenediol] * | C14H12O2 | 212.243 | 213 | 167; 139 | 139 | L. caerulea [51]; R. triste [34] | ||
| 77 | Stilbene | Resveratrol [trans-Resveratrol; 3,4′,5-Trihydroxystilbene] * | C14H12O3 | 228.243 | 229 | 211 | 183; 127 | 138 | A. cordifolia; F. glaucescens; F. herrerae [52]; R. pauciflorum [34] | |
| 78 | Hydroxycoumarin | Umbelliferone [Skimmetin; Hydragin] * | C9H6O3 | 162.142 | 163 | 145 | 117 | Z. marina [35]; L. caerulea [51] | ||
| 79 | Coumarin | Fraxetin * | C10H8O5 | 208.167 | 209 | 191 | 117 | Embelia [61] | ||
| 80 | Coumarin | 3,4/6,8-Dihydro-5,7-dihydroxy-2-oxo-2H-1-benzopyran-3-acetic acid * | C11H10O6 | 238.193 | 239 | 221 | 203 | 185 | PubChem | |
| 81 | Coumarin | Umbelliferone hexoside * | C15H16O8 | 324.282 | 325 | 289; 127 | 271; 127 | 253; 146 | F. glaucescens [52]; R. triste [34] | |
| 82 | Coumarin glucoside | Tomenin * | C17H20O10 | 384.334 | 385 | 223 | 177 | 149 | Pubchem | |
| 83 | Coumarin | Fraxin (Fraxetin-8-O-glucoside) * | C16H18O10 | 370.308 | 371 | 209 | 163; 111 | 119 | R. rugosa [60] | |
| 85 | Lignan | Dimethyl-secoisolariciresinol * | C22H30O6 | 390.470 | 391 | 373; 211 | 345; 239 | 299; 247 | Lignans [63] | |
| 86 | Lignan | Podophyllotoxin [Podofilox; Condylox; Condyline] * | C22H22O8 | 414.405 | 415 | 396; 344; 284; 209 | 378; 326 | 350 | Lignans [63] | |
| OTHERS | ||||||||||
| 87 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one [DDMP] | C6H8O4 | 144.125 | 145 | 127 | Radix polygoni multiflori [64] | ||||
| 88 | Methoxyeugenol | C11H14O3 | 194.227 | 195 | 163 | 145 | 117 | Ocimum [65] | ||
| 89 | 1,3-Dimethyluric acid [Oxytheophylline; 1,3-Dimethylurate] | C7H8N4O3 | 196.163 | 197 | 169 | 113; 151 | Coffee [66] | |||
| 90 | Benzofuran | Loliolide | C11H16O3 | 196.242 | 197 | 179 | 161 | 133 | D. ramosa [24]; Jatropha [67]; A. martjanovii [68] | |
| 91 | Naphthoquinone | 5,8-Dihydroxy-6-methyl-2,3-dihydro-1,4-naphthalenedione/8-Hydroxy-2-methoxy-2,3-dihydro-1,4-naphthalenedione * | C11H10O4 | 206.194 | 207 | 189 | 161 | 133 | J. mandshurica [69] | |
| 92 | Galactaric acid [Mucic acid;] * | C6H10O8 | 210.138 | 211 | 193; 165 | 165 | Soybean [70]; Stevia rebaudiana [71] | |||
| 93 | Polysaccharides | Glucaric acid [D-Glucaric acid; Saccharic acid; D-Glutarate] * | C6H10O8 | 210.138 | 211 | 193 | 165 | 147 | Soybean [70]; Cherimoya, Papaya [57] | |
| 94 | Aminoalkylindole | 5-Methoxydimethyltryptamine * | C13H18N2O | 218.294 | 219 | 201; 161 | 159 | Camellia kucha [72] | ||
| 95 | Sesquiterpenoid | Caryophyllene oxide [Caryophyllene-alpha-oxide] * | C15H24O | 220.350 | 221 | 203; 163 | 135 | R. davurica [60] | ||
| 96 | Naphthoquinone | 1,8-Dihydroxy-anthraquinone [Chrysazin] * | C14H8O4 | 240.210 | 241 | 213 | 195 | J. mandshurica [69] | ||
| 97 | Peptide | 5-oxo-L-propyl-L-isoleucine | C11H18N2O4 | 242.271 | 243 | 197 | 169 | 113 | Solanum tuberosum [73] | |
| 98 | Propionic acid | Ketoprofen [Orudis; 2-(3-Benzoylphenyl) propionic acid; Profenid] * | C16H14O3 | 254.280 | 253 | 209; 191; 165; 141 | 194; 167; 124 | 165; 125 | Ginkgo biloba [74] | |
| 99 | Naphthoquinone | Chrysophanol [Chrysophanolic acid] * | C15H10O4 | 254.237 | 255 | 237; 112 | 112 | Chinese herbal formula Jian-Pi-Yi-Shen pill [39] | ||
| 100 | Naphthoquinone | 1,4,8-Trihydroxyanthraquinone * | C14H8O5 | 256.210 | 257 | 183; 113 | 155 | 113 | J. mandshurica [69] | |
| 101 | Naphthoquinone | 1,2,5-Trihydroxyanthraquinone * | C14H8O5 | 256.210 | 257 | 183; 113 | 155 | 113 | J. sigillata [75] | |
| 102 | Omega-3-fatty acid | Stearidonic acid [6,9,12,15-Octadecatetraenoic acid] | C18H28O2 | 276.413 | 277 | 257 | 229 | 187 | G. linguiforme [52]; R. triste [34] | |
| 103 | Dihydrotanshinone I * | C18H20O3 | 278.302 | 279 | 261; 123 | 177 | 135 | Salviae Miltiorrhizae [40] | ||
| 104 | Omega-3-fatty acid | Linolenic acid (Alpha-Linolenic acid; Linolenate) | C18H30O2 | 278.429 | 279 | 261; 219; 177 | 177; 219 | 135 | Salviae Miltiorrhizae [41]; M. amurensis [43] | |
| 105 | Sesquiterpenoid | Artemisinin [Arteannuin; Artemisinine] * | C15H22O5 | 282.332 | 281 | 237 | 235 | 191 | A. absinthium [50]; A. martjanovii [68] | |
| 106 | Omega-9 unsaturated fatty acid | Oleic acid (Cis-9-Octadecenoic acid; Cis-Oleic acid) | C18H34O2 | 282.461 | 281 | 237 | 235 | 191 | Huolisu Oral Liquid [38] | |
| 107 | Dihydroxy anthraquinone | 6-methyl-aloe-emodin | C16H12O5 | 284.263 | 285 | 211; 141 | 183; 113 | 165 | Chinese herbal formula Jian-Pi-Yi-Shen pill [39] | |
| 108 | Isocoumarin | Brevifolincarboxylic acid * | C13H8O8 | 292.197 | 291 | 248 | 205 | 204; 146 | P. granatum [76]; Carpinus betulus [77] | |
| 109 | 1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)-2-propanol | C15H16O6 | 292.283 | 291 | 248 | 205; 178 | 148 | Ribes magellanicum [30] | ||
| 110 | Diterpenoid | Tanshinone IIA * [Tanshinone II; Tanshinone B] | C19H18O3 | 294.344 | 295 | 277 | 259; 175 | 199 | Huolisu Oral Liquid [38]; Radix Salviae [40] | |
| 111 | Diterpenoid | Cryptotanshinone * | C19H20O3 | 296.360 | 297 | 279 | 261; 177 | 177 | Huolisu Oral Liquid [38]; Radix Salviae [40] | |
| 112 | Diterpenoid | Isocryptotanshinone II * | C19H20O3 | 296.360 | 297 | 279 | 261; 177 | 177 | Salviae Miltiorrhizae [41] | |
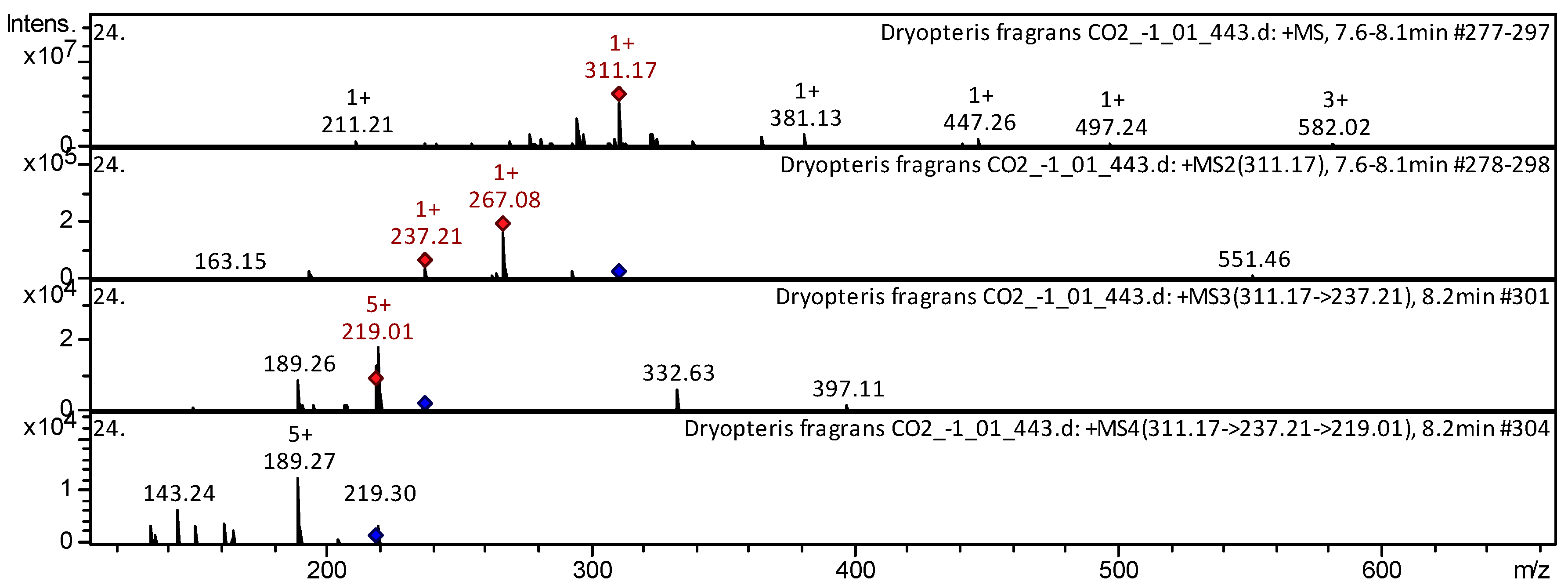
| 113 | Diterpenoid | Tanshinone IIB [(S)-6-(Hydroxymethyl)-1,6-Dimethyl-6,7,8,9-Tetrahydrophenanthro[1,2-B]Furan-10,11-Dione] * | C19H18O4 | 310.343 | 309 | 237 | 222 | 180 | Salviae Miltiorrhizae [41]; Huolisu Oral Liquid [38] | |
| 114 | Diterpenoid | 7-Dehydroabietic acid | C20H26O3 | 314.418 | 315 | 287 | 187; 259 | D. ramosa [24] | ||
| 115 | Long-Chain Polyunsaturated Fatty Acid | Docosahexaenoic acid [Doconexent; Cervonic acid] | C22H32O2 | 328.488 | 329 | 311; 283; 255 | 283 | PubChem | ||
| 116 | Cyclohexenecarboxylic acid | Caffeoyl shikimic acid | C16H16O8 | 336.293 | 337 | 301; 193 | 255; 227; 173 | 199; 170 | R. aureum, R. pauciflorum [34]; Carpinus betulus [77] | |
| 117 | Cyclohexenecarboxylic acid | 4-O-caffeoyl shikimic acid | C16H16O8 | 336.293 | 335 | 317 | 273; 205 | 189; 121 | Inula viscosa [77,78] | |
| 118 | Naphthoquinone | 4,8-Dihydroxy-1-naphthyl-beta-D-glucopyranoside | C16H18O8 | 338.309 | 339 | 147 | 119 | J. mandshurica [68,69] | ||
| 119 | Disaccharide | Trehalose | C13H24O13 | 388.321 | 387 | 341 | 178 | Pubchem | ||
| 120 | Sterol | Fucosterol [Fucostein; Trans-24-Ethylidenecholesterol] | C29H48O | 412.690 | 413 | 395; 268; 209 | 378; 254 | 350; 208 | F. pottsii [52]; L. caerulea [51] | |
| 121 | Sterol | Beta-Sitostenone | C29H48O | 412.690 | 413 | 209; 149 | 191; 149 | 149 | F. herrerae [52]; | |
| 122 | Steroidal alkaloid | Solasodine | C27H43NO2 | 413.635 | 414 | 396; 209 | 378; 326 | 350 | Jatropha [67] | |
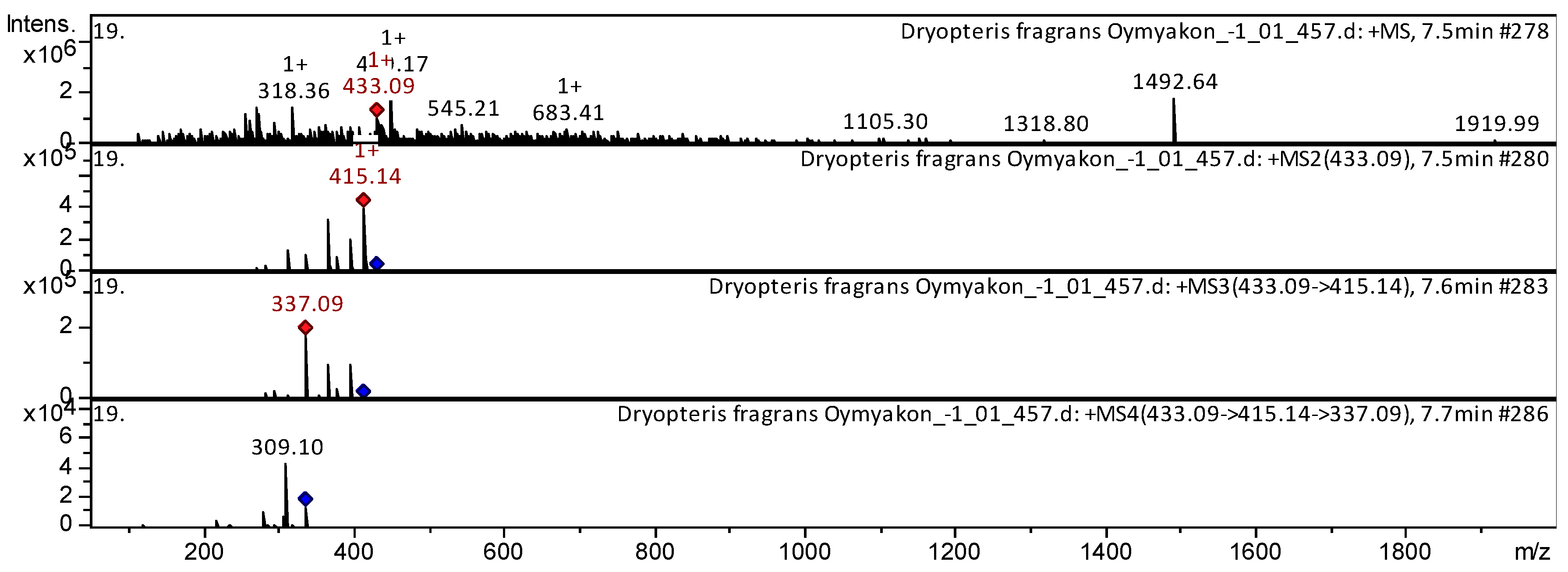
| 123 | Phlorodlucinol derivative | Disflavaspidic acid PB | C23H28O8 | 432.463 | 433 | 415 | 337 | 309 | D. fragrans [20] | |
| 124 | Phlorodlucinol derivative | Aspidin AB | C23H28O8 | 432.463 | 433 | 415 | 337 | 309 | D. fragrans [20] | |
| 125 | Phlorodlucinol derivative | Albaspidin PP | C23H28O8 | 432.463 | 433 | 415 | 337 | 309 | D. fragrans [20] | |
| 126 | Amorphygenin | Dalbinol [12A-Hydroxyamorphygenin] | C23H22O8 | 426.416 | 427 | 409; 291; 233 | 339 | 241; 173 | D. ramosa [24] | |
| 127 | Pentacyclic triterpene | Lupeol [Fagarasterol; Clerodol; Lupenol] * | C30H50O | 426.717 | 427 | 409; 291; 233 | 339 | 241; 173 | J. mandshurica [69] | |
| 128 | Triterpenoid | Uvaol | C30H50O2 | 442.716 | 443 | 425; 219 | 206; 151 | F. herrerae [52] | ||
| 129 | Phlorodlucinol derivative | Albaspidin PB | C24H30O8 | 446.490 | 445 | 427; 401; 347; 247 | 385; 341; 297; 247 | 368; 343; 398; 283 | D. fragrans [20] | |
| 130 | Phlorodlucinol derivative | Flavaspidic acid BB | C24H30O8 | 446.490 | 445 | 401; 223 | 179; 153 | 150 | D. fragrans [20] | |
| 131 | Phlorodlucinol dimer | Saroaspidin A | C24H30O8 | 446.490 | 445 | 427; 401; 347; 247 | 385; 341; 297; 247 | 368; 343; 398; 283 | D. fragrans [20] | |
| 132 | Anabolic steroid | Vebonol * | C30H44O3 | 452.668 | 453 | 435; 210 | 226; 336 | 210 | H. polyrhizus [79] | |
| 133 | Triterpenoid | Betulonic acid * | C30H46O3 | 454.684 | 455 | 438; 237 | 420; 321; 248; 159 | 375 | R. rugosa [60] | |
| 134 | Triterpenic acid | Ursolic acid * | C30H48O3 | 456.700 | 457 | 439; 223 | 421; 209 | 379; 268 | J. mandshurica [69]; Ocimum [65]; Pear [62] | |
| 135 | Triterpenoid | 1-Hydroxy-3-oxours-12-en-28-oic acid | C30H46O4 | 470.683 | 471 | 453; 237 | 435; 383 | 417; 365 | Pear [62] | |
| 136 | Cyclohexenecarboxylic acid | Caffeoylquinate shikimate derivative | 510.618 | 511 | 493; 317 | 475; 282 | 457; 405 | Phoenix dactylifera [80] | ||
| 137 | Indole sesquiterpene alkaloid | Sespendole * | C33H45NO4 | 519.714 | 520 | 184 | 125 | H. polyrhizus [79] | ||
| 138 | Eucaglobulin B * | C25H34O12 | 526.530 | 527 | 509; 351; 182 | 464; 291 | 418; 139 | Eucalyptus genus [81] | ||
| 139 | Carotenoid | Antheraxanthin [All-Trans-Antheraxanthin] | C40H56O3 | 584.870 | 585 | 566; 377; 237 | 548; 475; 381 | 485; 389; 259 | Carotenoids [82] | |
| 140 | (all-E)-violaxanthin butyrate | 670.123 | 671 | 653; 431 | 575; 353 | 353 | Carotenoids [82] | |||
| 141 | Steroidal alkaloid | Alpha-solanine | C45H73NO15 | 868.958 | 868 | 706; 560; 398 | 327; 157 | S. tuberosum [83] |
References
- Malyshev, L.I. Fam. Dryopteridaceae. Summary of the Flora of Asiatic Russia: Vascular Plants; Publishing House of the Siberian Branch of the Russian Academy of Sciences: Novosibirsk, Russia, 2012. (In Russian) [Google Scholar]
- Makarov, A.A. Medicinal Plants of Yakutia and Prospects for Their Development; Publishing House of the Siberian Branch of the Russian Academy of Sciences: Novosibirsk, Russia, 2002; 264p. (In Russian) [Google Scholar]
- Nikolin, E.G. Local floras of the Verkhneindigirsky resource reserve (North-Eastern Yakutia). Bot. J. 2020, 105, 627–645. (In Russian) [Google Scholar] [CrossRef]
- Lebedev, V.V. Medicinal Plants of Yakutia Used for Gastrointestinal Diseases; Publishing House of the Siberian Branch of the Russian Academy of Sciences: Omsk, Russia, 1969; 190p. (In Russian) [Google Scholar]
- Lebedev, V.V. Dryopteris fragrans in dyspepsia of calves. Zemlya Sib. Far East 1984, 8, 35. (In Russian) [Google Scholar]
- Makarov, A.A. Herbal Remedies of the Yakut Folk Medicine; Book Publishing House: Yakutsk, Russia, 1974; 64p. (In Russian) [Google Scholar]
- Ito, H.; Muranaka, T.; Mori, K.; Jin, Z.X.; Yoshida, T. Dryofragin and aspidin PB, piscicidal components from Dryopteris fragrans. Chem. Pharm. Bull. 1997, 45, 1720–1722. [Google Scholar] [CrossRef]
- Kuang, H.; Zhang, Y.; Li, G.; Zeng, W.; Wang, H.; Song, Q. A new phenolic glycoside from the aerial parts of Dryopteris fragrans. Fitoterapia 2008, 79, 319–320. [Google Scholar] [CrossRef]
- Kuang, H.; Sun, C.; Zhang, Y.L.; Zhang, Y.L.; Chen, D.; Yang, B.Y.; Xia, Y.G. Three drimane sesquiterpene glucoside from the aerial parts of Dryopteris fragrans (L.) Schot. Fitoterapia 2009, 80, 134–137. [Google Scholar] [CrossRef]
- Zhao, D.D.; Zhao, Q.S.; Liu, L.; Chen, Z.Q.; Zeng, W.M.; Lei, H.; Zhang, Y.L. Compounds from Dryopteris Fragrans (L.) Schott with Cytotoxic Activity. Molecules 2014, 19, 3345–3355. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zeng, W.M.; Li, G.Y.; Liu, G.Q.; Zhao, D.D.; Wang, J.; Zhang, Y.L. Characterization of a new sesquiterpene and antifungal activities of chemical constituents from Dryopteris fragrans (L.) Schott. Molecules 2014, 19, 507–513. [Google Scholar] [CrossRef]
- Han, X.; Li, Z.; Li, C.Y.; Jia, W.N.; Wang, H.T.; Wang, C.H. Phytochemical constituents and biological activities of plants from the Genus Dryopteris. Chem. Biodivers. 2015, 12, 1131–1162. [Google Scholar] [CrossRef]
- Peng, B.; Bai, R.F.; Li, P.; Han, X.Y.; Wang, H.; Zhu, C.C.; Zeng, Z.P.; Chai, X.Y. Two new glycosides from Dryopteris fragrans with anti-inflammatory activities. J. Asian Nat. Prod. Res. 2022, 24, 1064–1070. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Duan, D.H.; Zhang, Y.H.; Huang, S.X.; Chang, Y. Cytotoxicity-guided isolation of two new phenolic derivatives from Dryopteris fragrans (L.) Schott. Molecules 2018, 23, 1652. [Google Scholar] [CrossRef]
- Yan, P.; Lai, Q.F.; Li, M.; Jin, X.B.; Wie, G.; Chen, W.Q.; Ye, L.B. New anticancer agents: Design, synthesis, biological activity, and molecular docking of bicyclic phloroglucinol derivatives. ChemistrySelect 2021, 6, 453–1457. [Google Scholar] [CrossRef]
- Peng, B.; Han, X.Y.; Wang, H.; Zhang, W.J.; Zeng, Z.P. Two new cadinane-type sesquiterpenoid glycosides from Dryopteris fragrans with anti-inflammatory activities. J. Asian Nat. Prod. Res. 2022, 24, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.L.; Lai, Q.F.; Lai, W.H.; Li, M.; Jin, X.B.; Ye, L.B. Phloroglucinol derivatives as anti-tumor agents: Synthesis, biological activity evaluation and molecular docking studies. Med. Chem. Res. 2022, 31, 65–176. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, D.J.; Song, J.-S.; Kim, J.-A.; Lee, B.-H.; Son, Y.K. Identification and Comparison of Bioactive Components of Two Dryopteris Sp. Extract Using LC-QTOF-MS. Plants 2022, 11, 3233. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Jiang, T.; Zhang, L.; Huang, Y.; Wan, J.; Song, G.; Lin, H.; Shen, Z.; Tang, C. Analysis of Chemical Composition and In Vitro Antidermatophyte Activity of Ethanol Extracts of Dryopteris fragrans (L.) Schott. J. Ethnopharmacol. 2018, 226, 36–43. [Google Scholar] [CrossRef]
- Chung, N.C.; Miasojedow, B.; Startek, M.; Gambin, A. Jaccard/Tanimoto similarity test and estimation methods for biological presence-absence data. BMC Bioinform. 2019, 20, 644. [Google Scholar] [CrossRef]
- Lipkus, A.H. A proof of the triangle inequality for the Tanimoto distance. J. Math. Chem. 1999, 26, 263–265. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Hou, H.; Ma, X.; Sun, S.; Wang, H.; Kong, L. Metabolomics and gene expression analysis reveal the accumulation patterns of phenylpropanoids and flavonoids in different colored-grain wheats (Triticum aestivum L.). Food Res. Int. 2020, 138, 109711. [Google Scholar] [CrossRef]
- Zia-ur-Rehman; Rasheed, H.M.; Bashir, K.; Gurgul, A.; Wahid, F.; Che, C.-T.; Shahzadi, I.; Khan, T. UHPLC-MS/MS-GNPS based phytochemical investigation of Dryopteris ramosa (Hope) C. Chr. and evaluation of cytotoxicity against liver and prostate cancer cell lines. Heliyon 2022, 8, e11286. [Google Scholar] [CrossRef]
- Fantoukh, O.I.; Wang, Y.-H.; Parveen, A.; Hawwal, M.F.; Ali, Z.; Al-Hamoud, G.A.; Chittiboyina, A.G.; Joubert, E.; Viljoen, A.; Khan, I.A. Chemical Fingerprinting Profile and Targeted Quantitative Analysis of Phenolic Compounds from Rooibos Tea (Aspalathus linearis) and Dietary Supplements Using UHPLC-PDA-MS. Separations 2022, 9, 159. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Zou, L.; Liu, X.; Chen, J.; Tan, M.; Mei, Y.; Wei, L. Comparison of Multiple Bioactive Constituents in the Flower and the Caulis of Lonicera japonica Based on UFLC-QTRAP-MS/MS Combined with Multivariate Statistical Analysis. Molecules 2019, 24, 1936. [Google Scholar] [CrossRef] [PubMed]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; De Chaves, D.S.A.; Romaniuk, A.; Rybczynska, M.; Gryszczynska, A.; Sawikowska, A.; Kachlicki, P.; Mikolajczak, P.L.; et al. Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Braz. J. Pharmacol. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- Wojakowska, A.; Piasecka, A.; Garcia-Lopez, P.M.; Zamora-Natera, F.; Krajewski, P.; Marczak, L.; Kachlicki, P.; Stobiecki, M. Structural analysis and profiling of phenolic secondary metabolites of Mexican lupine species using LC–MS techniques. Phytochemistry 2013, 92, 71–86. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, H.; Wang, Q.; Liu, H.; Shen, H.; Xu, W.; Ge, J.; He, D. Rapid qualitative profiling and quantitative analysis of phenolics in Ribes meyeri leaves and their antioxidant and antidiabetic activities by HPLC-QTOF MS/MS and UHPLC-MS/MS. Sep. Sci. 2021, 44, 1404–1420. [Google Scholar] [CrossRef]
- Burgos-Edwards, A.; Jimenez-Aspee, F.; Theoduloz, C.; Schmeda-Hirschmann, G. Colonic fermentation of polyphenols from Chilean currants (Ribes spp.) and its effect on antioxidant capacity and metabolic syndrome-associated enzymes. Food Chem. 2018, 258, 144–155. [Google Scholar] [CrossRef]
- Wang, Y.; Vorsa, N.; Harrington, P.; Chen, P. Nontargeted Metabolomic Study on Variation of Phenolics in Different Cranberry cultivars Using UPLC-IM−HRMS. J. Agric. Food Chem. 2018, 66, 12206–12216. [Google Scholar] [CrossRef]
- Aita, S.E.; Capriotti, A.L.; Cavaliere, C.; Cerrato, A.; Giannelli Moneta, B.; Montone, C.M.; Piovesana, S.; Lagana, A. Andean Blueberry of the Genus Disterigma: A High-Resolution Mass Spectrometric Approach for the Comprehensive Characterization of Phenolic Compounds. Separations 2021, 8, 58. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. First evidence of C- and O-glycosyl flavone in blood orange (Citrus sinensis (L.) Osbeck) juice and their influence on antioxidant properties. Food Chem. 2014, 149, 244–252. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Nawaz, M.A.; Sabitov, A.S.; Golokhvast, K.S. Genus Ribes: Ribes aureum, Ribes pauciflorum, Ribes triste, Ribes dikuscha, comparative mass spectrometric study of polyphenolic composition and other bioactive components. Int. J. Mol. Sci. 2024, 25, 10085. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Tekutyeva, L.A.; Podvolotskaya, A.B.; Stepochkina, V.D.; Zakharenko, A.M.; Golokhvast, K.S. Zostera marina L. Supercritical CO2-Extraction and Mass Spectrometric Characterization of Chemical Constituents Recovered from Seagrass. Separations 2022, 9, 182. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Nawaz, M.A.; Ivanova, E.P.; Cherevach, E.I.; Golokhvast, K.S. Supercritical CO2-based Extraction and Detection of Phenolic Compounds and Saponins from the Leaves of Three Medicago varia Mart. Varieties by Tandem Mass Spectrometry. Processes 2024, 12, 1041. [Google Scholar] [CrossRef]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; Saini, M.K. Transfer of grain colors to elite wheat cultivars and their characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, K.; Wei, L.; Chen, D.; Chen, Q.; Jiao, M.; Li, X.; Huang, J.; Gong, Z.; Kang, N.; et al. The Molecular Mechanism of Antioxidation of Huolisu Oral Liquid Based on Serum Analysis and Network Analysis. Front. Pharmacol. 2021, 12, 710976. [Google Scholar] [CrossRef]
- Wang, F.; Huang, S.; Chen, Q.; Hu, Z.; Li, Z.; Zheng, P.; Liu, X.; Li, S.; Zhang, S.; Chen, J. Chemical characterisation and quantification of the major constituents in the Chinese herbal formula Jian-Pi-Yi-Shen pill by UPLC-Q-TOF-MS/MS and HPLC-QQQ-MS/MS. Phytochem. Anal. 2020, 31, 915–929. [Google Scholar] [CrossRef]
- Hu, P.; Liang, Q.-L.; Luo, G.-A.; Zhao, Z.-Z.; Jiang, Z.-H. Multi-component HPLC Fingerprinting of Radix Salviae miltiorrhizae and Its LC-MS-MS Identification. Chem. Pharm. Bull. 2005, 53, 677–683. [Google Scholar] [CrossRef]
- Yang, S.T.; Wu, X.; Rui, W.; Guo, J.; Feng, Y.F. UPLC/Q-TOF-MS Analysis for Identification of Hydrophilic Phenolics and Lipophilic Diterpenoids from Radix salviae miltiorrhizae. Acta Chromatogr. 2015, 27, 711–728. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Sabitov, A.S.; Senotrusova, T.A.; Lee, N.G.; Vitomskova, E.A.; Golokhvast, K.S. Ribes fragrans Pall.: Supercritical CO2-extraction and Complete Plant Metabolome. Bot. Pacifica 2024, 13, 61–72. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Cherevach, E.I.; Tekutyeva, L.A.; Fedoreev, S.A.; Mishchenko, N.P.; Tarbeeva, D.V.; Demidova, E.N.; Kirilenko, N.S.; Golokhvast, K.S. Maackia amurensis Rupr. et Maxim.: Supercritical CO2-extraction and Mass Spectrometric Characterization of Chemical Constituents. Molecules 2023, 28, 2026. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Tai-Sun, S.; Chung, G.; Golokhvast, K. Supercritical CO2 Extraction and Identification of Ginsenosides in Russian and North Korean Ginseng by HPLC with Tandem Mass Spectrometry. Molecules 2020, 25, 1407. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Ercisli, S.; Grudev, V.; Golokhvast, K. Comparative Analysis of Far East Sikhotinsky Rhododendron (Rh. sichotense) and East Siberian Rhododendron (Rh. adamsii) Using Supercritical CO2-Extraction and HPLC-ESI-MS/MS Spectrometry. Molecules 2020, 25, 3774. [Google Scholar] [CrossRef]
- Pharmacopoeia of the Eurasian Economic Union, Approved by Decision of the Board of Eurasian Economic Commission No. 100. Available online: https://eec.eaeunion.org/upload/medialibrary/37c/PHARMACOPOEIA-of-the-Eurasian-Economic-Union.pdf (accessed on 11 August 2020).
- Okhlopkova, Z.M.; Razgonova, M.P.; Rozhina, Z.R.; Egorova, P.S.; Golokhvast, K.S. Dracocephalum jacutense Peschkova from Yakutia: Extraction and Mass Spectrometric Characterization of 128 Chemical Compounds. Molecules 2023, 28, 4402. [Google Scholar] [CrossRef] [PubMed]
- Belmehdi, O.; Bouyahya, A.; Jeko, J.; Cziaky, Z.; Zengin, G.; Sotkó, G.; El Baaboua, A.; Skali-Senhaji, N.; Abrini, J. Synergistic interaction between propolis extract, essential oils, and antibiotics against Staphylococcus epidermidis and methicillin resistant Staphylococcus aureus. Int. J. Second Metab. 2021, 8, 195–213. [Google Scholar] [CrossRef]
- Silinsin, M.; Bursal, E. UHPLC-MS/MS phenolic profiling and in vitro antioxidant activities of Inula graveolens (L.) Desf. Nat. Prod. Res. 2018, 31, 1467–1471. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Wozniak, K.; Luca, S.V. Unveiling the Phytochemical Profile and Biological Potential of Five Artemisia Species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Navaz, M.A.; Sabitov, A.S.; Zinchenko, Y.N.; Rusakova, E.A.; Petrusha, E.N.; Golokhvast, K.S.; Tikhonova, N.G. The Global metabolome profiles of four varieties of Lonicera caerulea, established via tandem mass spectrometry. Horticulturae 2023, 9, 1188. [Google Scholar] [CrossRef]
- Hamed, A.R.; El-Hawary, S.S.; Ibrahim, R.M.; Abdelmohsen, U.R.; El-Halawany, A.M. Identification of Chemopreventive Components from Halophytes Belonging to Aizoaceae and Cactaceae Through LC/MS–Bioassay Guided Approach. J. Chrom. Sci. 2021, 59, 618–626. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.J.; Xu, W.; Huang, J.; Zhu, D.; Qiu, X.H. Rapid Characterization and Identification of Flavonoids in Radix Astragali by Ultra-High-Pressure Liquid Chromatography Coupled with Linear Ion Trap Orbitrap Mass Spectrometry. J. Chromatogr. Sci. 2015, 53, 945–952. [Google Scholar] [CrossRef]
- Stallmann, J.; Schweiger, R.; Pons, C.A.; Müller, C. Wheat growth, applied water use efficiency and flag leaf metabolome under continuous and pulsed deficit irrigation. Sci. Rep. 2020, 10, 10112. [Google Scholar] [CrossRef]
- Bodalska, A.; Kowalczyk, A.; Wlodarczyk, M.; Fecka, I. Analysis of Polyphenolic Composition of a Herbal Medicinal Product—Peppermint Tincture. Molecules 2020, 25, 69. [Google Scholar] [CrossRef]
- Geng, P.; Sun, J.; Zhang, M.; Li, X.; Harnly, J.M.; Chen, P. Comprehensive characterization of C-glycosyl flavones in wheat (Triticum aestivum L.) germ using UPLC-PDA-ESI/HRMSn and mass defect filtering. J. Mass Spectrom. 2016, 51, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Spinola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Medic, A.; Jakoric, J.; Hudina, M.; Solar, A.; Veberic, R. Identification and quantification of the major phenolic constituents in Juglans regia L. peeled kernels and pellicles, using HPLC–MS/MS. Food Chem. 2021, 352, 129404. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sandhir, R.; Singh, A.; Kumar, P.; Mishra, A.; Jachak, S.; Singh, S.P.; Singh, J.; Roy, J. Comparative analysis of phenolic compound characterization and their biosynthesis genes between two diverse bread wheat (Triticum aestivum) varieties differing for chapatti (unleavened flat bread) quality. Front. Plant Sci. 2016, 7, 1870. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Bazhenova, B.B.; Zabalueva, Y.Y.; Burkhanova, A.G.; Zakharenko, A.M.; Kupriyanov, A.N.; Sabitov, A.S.; Ercisli, S.; Golokhvast, K.S. Rosa davurica Pall., Rosa rugosa Thumb., and Rosa acicularis Lindl. originating from Far Eastern Russia: Screening of 146 Chemical Constituents in Tree Species of the Genus Rosa. Appl. Sci. 2022, 12, 9401. [Google Scholar] [CrossRef]
- Vijayan, K.P.R.; Raghu, A.V. Tentative characterization of phenolic compounds in three species of the genus Embelia by liquid chromatography coupled with mass spectrometry analysis. Spectrosc. Lett. 2019, 52, 653–670. [Google Scholar] [CrossRef]
- Sun, L.; Tao, S.; Zhang, S. Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS. Molecules 2019, 24, 159. [Google Scholar] [CrossRef]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.A.; Smeds, A.I.; Sjoholm, R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2008, 43, 97–107. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Wang, C.; Guo, N.; Song, Z.; Wang, C.; Xia, L.; Lu, A. Comparative Analyses of Chromatographic Fingerprints of the Roots of Polygonum multiflorum Thunb. and Their Processed Products Using RRLC/DAD/ESI-MSn. Planta Med. 2011, 77, 1855–1860. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, B. HPLC–QTOF–MS/MS-based rapid screening of phenolics and triterpenic acids in leaf extracts of Ocimum species and their interspecies variation. J. Liq. Chromatogr. Relat. Tech. 2016, 39, 225–238. [Google Scholar] [CrossRef]
- Lang, R.; Dieminger, N.; Beusch, A.; Lee, Y.-M.; Dunkel, A.; Suess, B.; Skurk, T.; Wahl, A.; Hauner, H.; Hofmann, T. Bioappearance and pharmacokinetics of bioactives upon coffee consumption. Anal. Bioanal. Chem. 2013, 405, 8487–8503. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Mahomoodally, M.F.; Sinan, K.I.; Ak, G.; Etienne, O.K.; Sharmeen, J.B.; Brunetti, L.; Leone, S.; Di Simone, S.C.; Recinella, L.; et al. Chemical composition and biological properties of two Jatropha species: Different parts and different extraction methods. Antioxidants 2021, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Okhlopkova, Z.M.; Ercisli, S.; Razgonova, M.P.; Ivanova, N.S.; Antonova, E.E.; Egorov, Y.A.; Kucharova, E.V.; Golokhvast, K.S. Primary Determination of the Composition of Secondary Metabolites in the Wild and Introduced Artemisia martjanovii Krasch: Samples from Yakutia. Horticulturae 2023, 9, 1329. [Google Scholar] [CrossRef]
- Huo, J.-H.; Du, X.-W.; Sun, G.-D.; Dong, W.-T.; Wang, W.-M. Identification and characterization of major constituents in Juglans mandshurica using ultra performance liquid chromatography coupled with time-of-flight mass spectrometry (UPLC-ESI-Q-TOF/MS). Chin. J. Nat. Medic. 2018, 16, 0525–0545. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Wang, X.; Fu, H.; Zhao, M.; Wang, H.; Shi, L. Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress. J. Plant Physiol. 2018, 229, 132–141. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shaari, K. LC–MS metabolomics analysis of Stevia rebaudiana Bertoni leaves cultivated in Malaysia in relation to different developmental stages. Phytochem. Anal. 2021, 33, 249–261. [Google Scholar] [CrossRef]
- Qin, D.; Wang, Q.; Li, H.; Jiang, X.; Fang, K.; Wang, Q.; Li, B.; Pan, C.; Wu, H. Identification of key metabolites based on non-targeted metabolomics and chemometrics analyses provides insights into bitterness in Kucha [Camellia kucha (Chang et Wang) Chang]. Food Res. Int. 2020, 138, 109789. [Google Scholar] [CrossRef]
- Rodriguez-Perez, C.; Gomez-Caravaca, A.M.; Guerra-Hernandez, E.; Cerretani, L.; Garcia-Villanova, B.; Verardo, V. Comprehensive metabolite profiling of Solanum tuberosum L. (potato) leaves T by HPLC-ESI-QTOF-MS. Molecules 2018, 112, 390–399. [Google Scholar] [CrossRef]
- Xie, J.; Ding, C.; Ge, Q.; Zhou, Z.; Zhi, X. Simultaneous determination of ginkgolides A, B, C and bilobalide in plasma by LC–MS/MS and its application to the pharmacokinetic study of Ginkgo biloba extract in rats. J. Chromatogr. B 2008, 864, 87–94. [Google Scholar] [CrossRef]
- Liang, J.-J.; Zhou, J.; Song, J.; Ruan, H.-L. Anthraquinone and Naphthoquinone derivatives from the pericarps of Juglans sigillata. Chem. Nat. Comp. 2019, 55, 435–439. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.G.; Glamoclija, J.; Ciric, A.; Sokovic, M.; Heropoulos, G.; Proestos, C. Antiradical-antimicrobial activity and phenolic profile of pomegranate (Punica granatum L.) juices from different cultivars: A comparative study. RSC Adv. 2015, 5, 2602–2614. [Google Scholar] [CrossRef]
- Felegyi-Toth, C.A.; Garadi, Z.; Darcsi, A.; Csernak, O.; Boldizsar, I.; Beni, S.; Alberti, A. Isolation and quantification of diarylheptanoids from European hornbeam (Carpinus betulus L.) and HPLC-ESI-MS/MS characterization of its antioxidative phenolics. J. Pharm. Biomed. Anal. 2022, 210, 114554. [Google Scholar] [CrossRef] [PubMed]
- Brahmi-Chendouh, N.; Piccolella, S.; Crescente, G.; Pacifico, F.; Boulekbache, L.; Hamri-Zeghichi, S.; Akkal, S.; Madani, K.; Pacifico, S. A nutraceutical extract from Inula viscosa leaves: UHPLC-HR-MS/MS based polyphenol profile, and antioxidant and cytotoxic activities. J. Food Drug Anal. 2019, 27, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, J.; He, Y.; Shi, M.; Han, X.; Li, W.; Zhang, X.; Wen, X. Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation. Molecules 2019, 24, 1114. [Google Scholar] [CrossRef]
- Said, R.B.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef]
- Heskes, A.M.; Goodger, J.Q.D.; Tseday, S.; Quach, T.; Williams, S.J.; Woodrow, I.E. Localization of Oleuropeyl Glucose Esters and a Flavanone to Secretory Cavities of Myrtaceae. PLoS ONE 2012, 7, e40856. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Rodrigues, D.B.; Petry, F.C.; Barros Mariutti, L.R. Carotenoid esters in foods—A review and practical directions on analysis and occurrence. Food Res. Int. 2017, 99, 830–850. [Google Scholar] [CrossRef]
- Deng, Y.; He, M.; Feng, F.; Feng, X.; Zhang, Y.; Zhang, F. The distribution and changes of glycoalkaloids in potato tubers under different storage time based on MALDI-TOF mass spectrometry imaging. Talanta 2021, 221, 121453. [Google Scholar] [CrossRef]


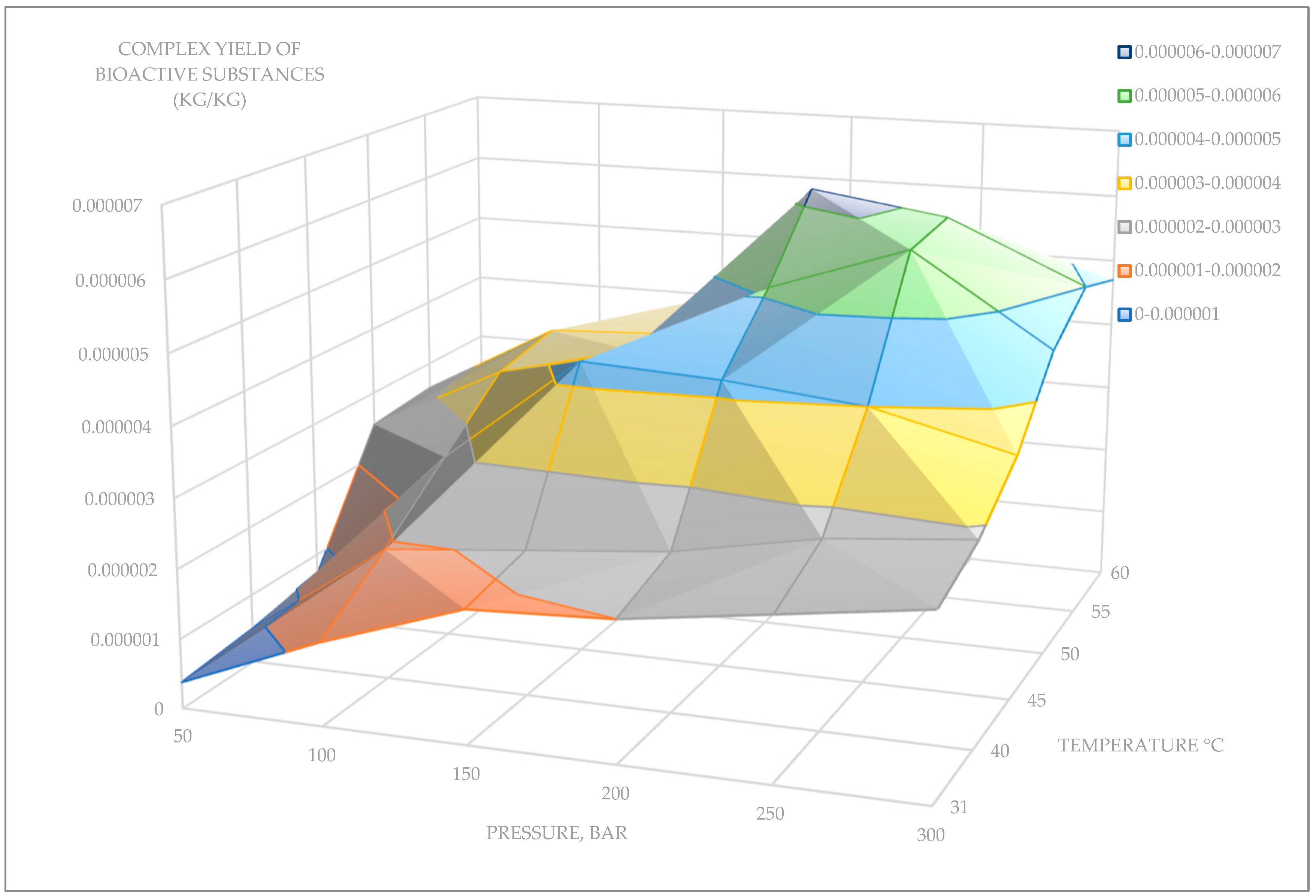
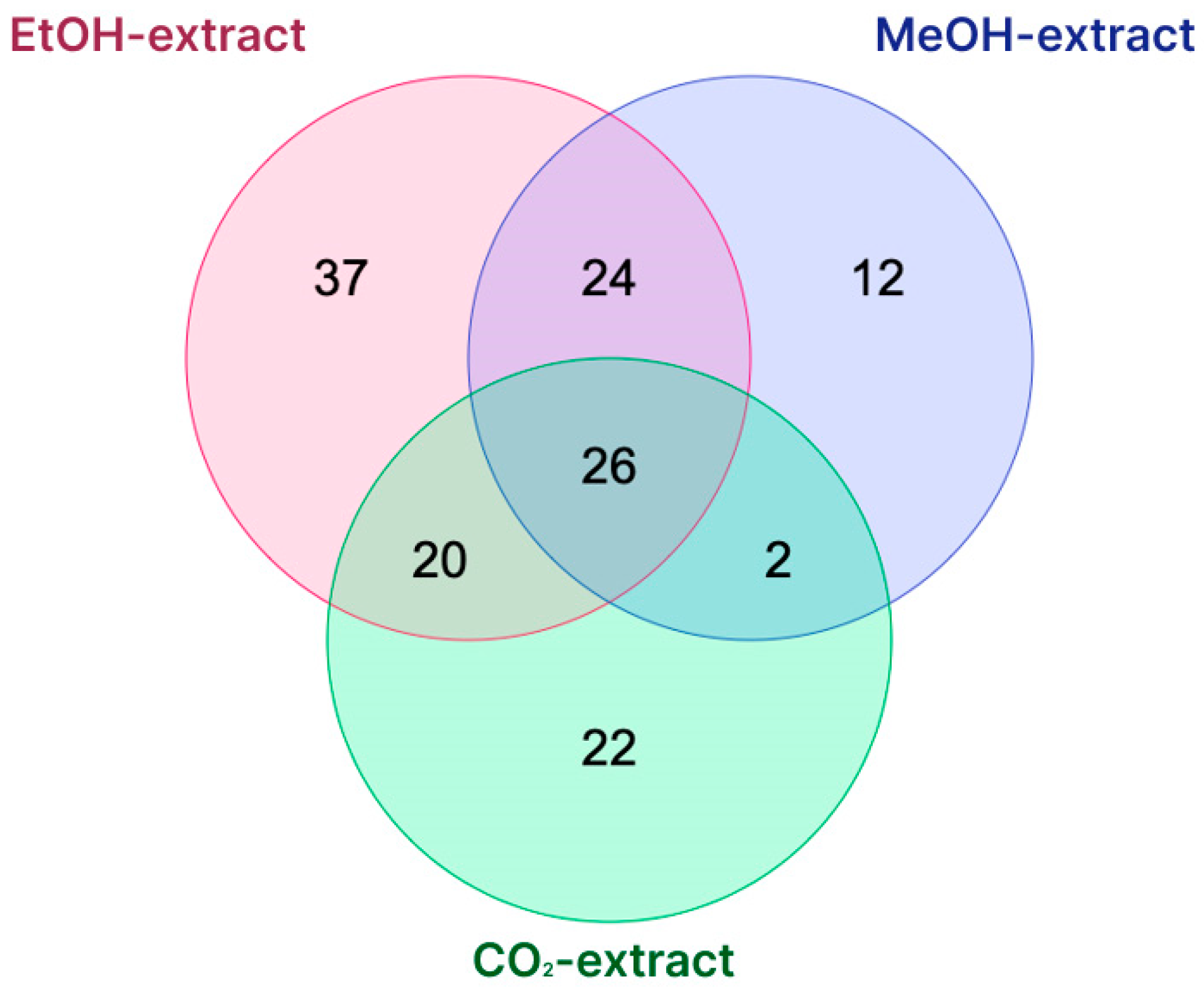

| Pressure | 50 Bar | 100 Bar | 150 Bar | 200 Bar | 250 Bar | 300 Bar |
|---|---|---|---|---|---|---|
| 31 °C | 0.00000038 | 0.0000012 | 0.0000019 | 0.000002 | 0.0000023 | 0.0000026 |
| 40 °C | 0.0000005 | 0.0000019 | 0.0000021 | 0.0000023 | 0.0000027 | 0.0000029 |
| 45 °C | 0.0000007 | 0.0000027 | 0.0000043 | 0.0000042 | 0.000004 | 0.0000035 |
| 50 °C | 0.0000025 | 0.0000035 | 0.000004 | 0.0000051 | 0.0000058 | 0.0000045 |
| 55 °C | 0.0000026 | 0.0000037 | 0.0000038 | 0.0000062 | 0.0000059 | 0.000005 |
| 60 °C | 0.0000025 | 0.0000031 | 0.000004 | 0.0000045 | 0.000004 | 0.0000047 |
| Type of Extraction | Total | Compounds |
|---|---|---|
| CO2 EtOH MeOH | 26 | Chrysoeriol C-hexoside; Albaspidin PB; Quercetin-O-rhamnosyl-hexoside; Chrysophanol; Delphinidin 3-O-hexoside; Ellagic acid; Delphinidin 3-O-glucoside; Cryptochlorogenic acid; Vitexin; Dihydroxyflavone; Chrysoeriol 6-C-glucoside; Chrysoeriol 8-C-glucoside; Flavaspidic acid BB; 1,3-Dimethyluric acid; Genistein 6-C-glucoside; Delphinidin 3-O-β-galactoside; Sinapic acid; Genistein 8-C-glucoside; Saroaspidin A; Isovitexin; Linolenic acid; Ethyl protocatechuate; 1,8-Dihydroxy-anthraquinone; Ketoprofen; Chlorogenic acid; Fraxin |
| EtOH MeOH | 24 | Kaempferol; Umbelliferone; (R)-eriodictyol-6-C-β-D-glucopyranoside; Hydroxyferulic acid; Albaspidin PP; Ferulic acid; Luteolin; Hyperoside; Apigenin; Delphinidin 3-O-rutinoside; Aspidin AB; Rutin; Eriodictyol-O-hexoside; Tomenin; Quercetin 3-O-hexoside; Quercetin 3-O-glucoside; Trimethoxy flavone; Disflavaspidic acid PB; Loliolide; Delphinidin 3-O-(6-O-p-coumaroyl) glucoside; 1-O-Caffeoylquininc acid methyl ether; Methoxyeugenol; Antheraxanthin; (S)-eriodictyol-6-C-β-D-glucopyranoside |
| CO2 EtOH | 20 | Docosahexaenoic acid; Trihydroxy(iso)flavone; Caffeic acid; Quercetin; Delphinidin; 4,8-Trihydroxyanthraquinone; 6-methyl-aloe-emodin; Luteolin 6-C-glucoside; Caffeoyl shikimic acid; Herbacetin; Vebonol; Glucaric acid; Luteolin C-hexoside; Neochlorogenic acid; (Epi)-afzelechin derivative; 2,5-Trihydroxyanthraquinone; Dihydrotanshinone I; Galactaric acid; Luteolin 8-C-Glucoside; Astragalin |
| CO2 MeOH | 2 | Eriodictyol-7-O-glucuronide; Trehalose |
| EtOH Extract (107) | MeOH Extract (64) | CO2 Extract (70) | |
|---|---|---|---|
| EtOH extract (107) | -- | 50 | 46 |
| 0.4132 | 0.3511 | ||
| MeOH extract (64) | 50 | -- | 28 |
| 0.4132 | 0.2642 | ||
| CO2 extract (70) | 46 | 28 | -- |
| 0.3511 | 0.2642 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razgonova, M.P.; Okhlopkova, Z.M.; Nawaz, M.A.; Egorova, P.S.; Golokhvast, K.S. Supercritical Extraction and Identification of Bioactive Compounds in Dryopteris fragrans (L.) Schott. Pharmaceuticals 2025, 18, 299. https://doi.org/10.3390/ph18030299
Razgonova MP, Okhlopkova ZM, Nawaz MA, Egorova PS, Golokhvast KS. Supercritical Extraction and Identification of Bioactive Compounds in Dryopteris fragrans (L.) Schott. Pharmaceuticals. 2025; 18(3):299. https://doi.org/10.3390/ph18030299
Chicago/Turabian StyleRazgonova, Mayya P., Zhanna M. Okhlopkova, Muhammad A. Nawaz, Polina S. Egorova, and Kirill S. Golokhvast. 2025. "Supercritical Extraction and Identification of Bioactive Compounds in Dryopteris fragrans (L.) Schott" Pharmaceuticals 18, no. 3: 299. https://doi.org/10.3390/ph18030299
APA StyleRazgonova, M. P., Okhlopkova, Z. M., Nawaz, M. A., Egorova, P. S., & Golokhvast, K. S. (2025). Supercritical Extraction and Identification of Bioactive Compounds in Dryopteris fragrans (L.) Schott. Pharmaceuticals, 18(3), 299. https://doi.org/10.3390/ph18030299











