Abstract
Objectives: Early and noninvasive detection of fibrotic interstitial lung disease (fILD) is a critical but unmet clinical necessity. This study aimed to evaluate the feasibility of using 99mTc-HYNIC-Glu(PEG4-oncoFAPi)2 (denoted as 99mTc-H-PoFP2), a novel 99mTc-labeled radiopharmaceutical that targets fibroblast activation protein (FAP), for single-photon emission computed tomography (SPECT) imaging of pulmonary fibrosis in a mouse model and preliminary clinical studies. Methods: 99mTc-H-PoFP2 could be conveniently afforded using a kit formula with high radiochemical purity and stability. The binding specificity and affinity of 99mTc-H-PoFP2 for FAP were validated by an in vitro binding assay. The in vivo characteristics of 99mTc-H-PoFP2 were also determined. Results: 99mTc-H-PoFP2 was eliminated quickly via the urinary system, leading to low normal tissue uptake and a high target/background ratio. SPECT imaging demonstrated significantly enhanced uptake of the 99mTc-H-PoFP2 in bleomycin-induced fibrotic lung tissues, with visual effects superior to those of normal mice. Thus, a pilot clinical study of 99mTc-H-PoFP2 SPECT/CT imaging was conducted in 12 patients diagnosed with fILD. The physiological biodistribution of 99mTc-H-PoFP2 in patients was predominantly observed in the kidneys, bladder, liver, and pancreas, with relatively minor accumulation in the thyroid, salivary glands, and spleen. fILD patients exhibited elevated pulmonary 99mTc-H-PoFP2 uptake in the affected lung regions. Furthermore, the uptake of 99mTc-HPoFP2 demonstrated moderate correlations with the results of pulmonary function tests (PFTs). A higher gender–age–physiology (GAP) index was associated with elevated standardized uptake value maximum (SUVmax) and target-to-background ratio (TBR) values. Conclusions: Collectively, this study demonstrates the potential of 99mTc-HPoFP2 for SPECT imaging and assessing fILD by targeting FAP overexpressed in fibrotic lung tissues. This strategy offers new possibilities for noninvasive and precise assessment of pulmonary fibrosis.
1. Introduction
Fibrotic interstitial lung disease (fILD) is a chronic lung disease characterized by abnormal collagen accumulation in the lung parenchyma, leading to dyspnoea, progressive pulmonary dysfunction, and even death []. Once fibrosis is established, anti-fibrotic treatment merely delays the decline of lung function, leading to a median survival of 3–4 years []. Currently, clinical diagnosis of pulmonary fibrosis mainly involves pulmonary function tests (PFTs) and high-resolution computed tomography (HRCT) []. Although CT is capable of detecting morphologic changes in the diseased lung, its effectiveness is limited to relatively late stages of the disease when scar tissue formation is evident, and it cannot provide disease activity information in pulmonary fibrosis. Lung tissue biopsy and histopathological examination are the gold standards for diagnosis. However, this method is invasive and exhibits high sampling variability because of obtaining only a few lung tissues. In addition, these examinations cannot predict precisely the disease course in individual patients, which is often highly variable []. Therefore, there is an urgent need to develop a noninvasive method to monitor ongoing fibrosis and accurately identify progressors, which might significantly benefit the clinical management of fILD and their prognosis.
Nuclear medicine imaging can visualize biological processes at the molecular level in a noninvasive manner. Functional tissue imaging of fibrosis or fibrotic activity is able to complement the structural information afforded by HRCT. A promising marker for monitoring fibrogenesis is the fibroblast activation protein (FAP), a serine protease predominantly expressed on the membranes of activated fibroblasts under various pathological conditions, including cancer, fibrosis, inflammation, or wound healing. Its over-expression is confined to these pathologic sites and not typically found in most healthy tissue, making it an ideal target for radiotracers development []. Several fibroblast activation protein inhibitor (FAPI) based radiotracers have been developed for non-invasive diagnosis and quantitative evaluation of FAP expression in pulmonary fibrosis []. Preclinical and clinical studies using FAPI tracer imaging have yielded promising outcomes. Most of these radiotracers are labeled with either gallium-68 (68Ga) or fluorine-18 (18F) for positron emission tomography (PET) imaging. Despite the growing availability of PET imaging over the past decades, it remains inaccessible in some developing regions. In contrast, SPECT imaging offers a more cost-effective and convenient option with superior time-cost efficiency, possibly making it better suited for the current conditions in developing regions. Additionally, SPECT imaging involves a relatively lower radiation dose, which is advantageous for patients requiring serial follow-up examinations. The widespread use and longer half-life (6 h) of 99mTc enable more flexible imaging windows than PET. However, limited investigations on 99mTc-FAPI SPECT imaging in fILD were reported, with only one study conducted to date [].
In this study, we developed a novel 99mTc-labeled FAPI-based radiotracer, 99mTc-HYNIC-Glu(PEG4-oncoFAPi)2 (denoted as 99mTc-H-PoFP2), for SPECT imaging of pulmonary fibrosis. To obtain optimal diagnostic performance, we strategically introduced PEG4 linkers and constructed a homodimer structure. To our knowledge, the introduction of PEG4 linkers could improve the hydrophilicity and prolong the circulation time of radiopharmaceuticals [], while the homodimer structure could strengthen the binding affinity through multivalent interactions with FAP overexpressed in fibrotic tissues []. Therefore, the designed structures were expected to obtain enhanced diagnostic effectiveness for lesion detection, with higher accumulation in the lesions and faster clearance of unbound tracers. Furthermore, we investigated the safety, biodistribution, and clinical feasibility of this optimized radiopharmaceutical in 12 patients diagnosed with fILD, with the aim of advancing noninvasive diagnostic methodologies for this debilitating condition.
2. Results
2.1. 99mTc-Radiolabeling and In Vitro Evaluation of 99mTc-H-PoFP2
The labeling precursor (H-PoFP2) and reference compounds were prepared successfully with high chemical purity (>95%) and identified with mass spectrometry (Figures S1 and S2). H-PoFP2 can be easily formulated as a kit for 99mTc radiolabeling conveniently in routine clinical application, and the 99mTc-labeling procedure could be completed within 25 min (Figure 1A). The in vivo stability of 99mTc-H-PoFP2 was determined by testing the murine urine samples at different time intervals post-injection (p.i.). Negligible decomposition was observed, and the intact 99mTc-H-PoFP2 was greater than 95% within the tested time (Figure 1B). 99mTc-H-PoFP2 exhibited significant binding values (presented as percentage of the total added dose (%AD) per 0.2 μg hFAP with the number of 25.65 ± 1.88 for the binding group and 0.03 ± 0.01 for the block group, where p < 0.0001) (Figure 1C). The dissociation constant Kd of 99mTc-H-PoFP2 to hFAP was 5.35 ± 0.42 nM (Figure 1D). Although the assay setup was different, the binding affinity of 99mTc-H-PoFP2 was comparable to that of other FAPI-based radiotracers [,,,]. In addition, the partition coefficients (LogD) of 99mTc-H-PoFP2 demonstrated high hydrophilic properties with the value of −3.56 ± 0.09, which aligned with the rapid clearance and short circulation half-life (T1/2α = 3.02 min; T1/2β = 166.10 min) (Figure S5).
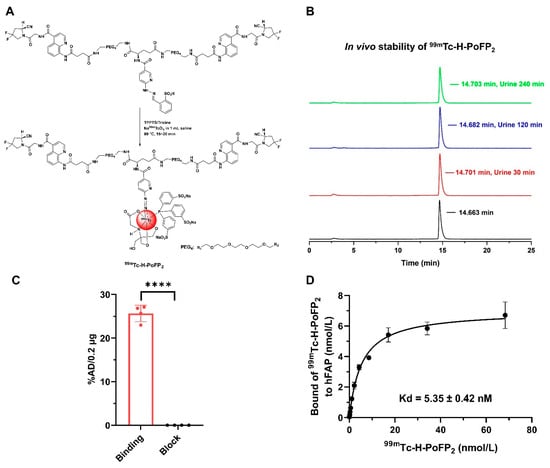
Figure 1.
Radiolabeling and characteristics of 99mTc-H-PoFP2. (A) H-PoFP2 was labeled with 99mTc via a kit formula. (B) 99mTc-H-PoFP2 was afforded without purification, and the in vivo stability of 99mTc-H-PoFP2 was determined. The radiolabeling yield and radiochemical purity of 99mTc-H-PoFP2 were determined by radio-HPLC. (C) The binding specificity to human FAP of 99mTc-H-PoF2 was determined by protein binding assay. In the block study, 99mTc-H-PoFP2 was incubated with unlabeled precursor H-PoFP2. **** indicates that p < 0.0001. (D) The binding affinity of 99mTc-H-PoF2 to human FAP was determined by protein saturation binding assay. TPPTS: trisodium triphenylphosphine-3,3′,3″-trisulfonate.
2.2. 99mTc-H-PoFP2 Showed Promising Detection Ability in Bleomycin-Induced Lung Fibrosis Model
We explored the ability of 99mTc-H-PoFP2 in the detection of the bleomycin-induced lung fibrosis model. In the mice with pulmonary fibrosis, obvious parenchymal regions in the lungs could be seen from the CT signals, which proved to be fibrotic lesions by hematoxylin and eosin staining (HE) and Masson staining (Figure 2A). Meanwhile, in the corresponding SPECT/CT images of these regions, accumulation of radioactive signals can also be visualized, suggesting that the regions where the probe accumulated were the pulmonary fibrosis regions. These regions also showed strong FAP expression by immunohistochemistry staining (IHC) (Figure 2B). On the contrary, in the CT and SPECT images of normal mice, no obvious fibrotic CT or radioactive signals could be seen. Additionally, whole-body planar SPECT/CT images showed the quick renal excretion and specific accumulation in fibrotic lung tissues with high target-background contrast (Figure S6). These results indicated that 99mTc-H-PoFP2 SPECT/CT imaging had promising application values in the diagnosis of pulmonary fibrosis. Therefore, we also conducted a preliminary study in patients diagnosed with pulmonary fibrosis.
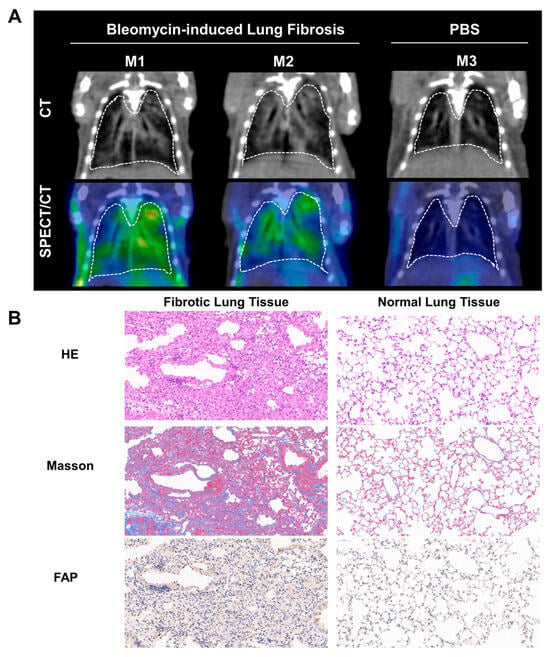
Figure 2.
99mTc-H-PoFP2 could detect fibrotic regions in bleomycin-induced lung fibrotic mice. (A) SPECT/CT imaging of 99mTc-H-PoFP2 in bleomycin-induced lung fibrotic mice and normal mice (n = 3). (B) HE, Masson staining, and FAP immunostaining of murine fibrotic and normal lung tissue. (Left panel: 10×, right panel: 20×).
2.3. Dynamic Biodistribution in Organs and Dosimetry Analysis
A summary of the demographic characteristics of 12 fILD patients is presented in Table 1. No adverse events associated with the administration of 99mTc-H-PoFP2 (723.9 ± 52.4 MBq) were reported. 99mTc-H-PoFP2 uptake was predominantly observed in the kidneys, urinary bladder, pancreas, thyroid, salivary glands, liver, and spleen. There was significant uptake in the chest regions of patients at the early time points, which remained visible even at 6 h p.i. A representative illustration of 99mTc-H-PoFP2 distribution from whole-body scintigraphy was depicted in Figure 3A. Dosimetry calculation analysis was conducted in six representative patients using HERMES Hybrid Viewer 4.0 Dosimetry according to OLINDA/EXM (version 2.0) methodology in the guideline []. The regions of interest and geometric mean count of target organs were derived from sequential background-corrected anterior and posterior images. Time–activity curves of target organs were shown in Figure 3B. As shown in Table 2, organ-specific absorbed doses and effective doses for 99mTc-H-PoFP2 were calculated with the OLINDA/EAM software (version 2.0), and the International Commission on Radiological Protection (ICPR) 103 tissue weighting factors were used for effective dose estimation. The average total-body effective dose was determined to be 0.00724 mSv/MBq.

Table 1.
Baseline demographic data of patients enrolled in the study.
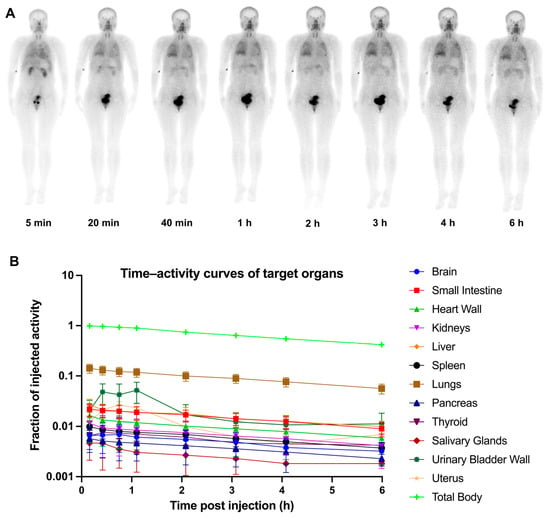
Figure 3.
Serial whole-body anterior projection images (A) and time–activity curves of target organs (B) of a 34-year-old female patient at different time points (5 min, 20 min, 40 min, 1 h, 2 h, 3 h, 4 h, and 6 h p.i.) following intravenous administration of 99mTc-H-PoFP2.

Table 2.
Organ absorbed and effective doses (mSv/MBq) of 99mTc-H-PoFP2. (ICRP-103).
2.4. Uptake of 99mTc-H-PoFP2 in fILD Patients and Relationship with PFT and GAP Index
In fILD patients, SPECT/CT images revealed elevated 99mTc-H-PoFP2 uptake predominantly in subpleural and peripheral areas, corresponding to pathologic regions of pulmonary fibrosis, including reticular abnormalities, traction bronchiectasis, and honeycombing as seen on HRCT. Exemplary cases are illustrated in Figure 4. In one instance (Figure 4A), massive honeycombing opacities were noted in the subpleural and peripheral regions, particularly in the basal parts, while mild elevation of 99mTc-H-PoFP2 uptake was observed. With an intense 99mTc-H-PoFP2 uptake with a standardized uptake value maximum (SUVmax) of 3.7, a target-to-background ratio (TBR) of 1.5 was observed throughout the entire lung, corresponding to reticular abnormalities and honeycombing opacities shown on HRCT. Additionally, a solid lesion (2.8 cm × 2.6 cm with SUVmax of 4.2, TBR of 1.7) was identified in the basal parts of the right lung, exhibiting higher uptake than the surrounding fibrosis region. In another case (Figure 4B), markedly increased 99mTc-H-PoFP2 uptake (SUVmax 8.2, TBR 3.9) was observed throughout the whole lung, with predominant reticular abnormalities and honeycombing on HRCT. In addition, increased uptake of 99mTc-H-PoFP2 around the right knee joint was also observed.
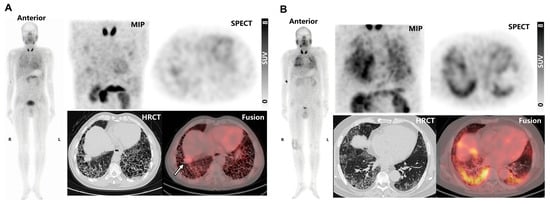
Figure 4.
(A) 99mTc-H-PoFP2 SPECT/CT and HRCT images of a 65-year-old male patient. The solid lesion in the right basal part was noted by a white arrow. (B) 99mTc-H-PoFP2 SPECT/CT and HRCT images of a 63-year-old male patient. MIP, maximum intensity projection.
Correlations between SUVmax and TBR with corresponding FVC and DLCO were assessed (Figure 5A,B). SUVmax demonstrated a moderate negative correlation with both FVC and DLCO (R = −0.57, p = 0.055 for FVC; R = −0.40, p = 0.199 for DLCO). Similarly, TBR also showed moderated negative correlations with FVC and DLCO as well. Patients were further stratified according to GAP index scores. Stage II patients (n = 5/12; 41.7%) exhibited significantly higher SUVmax and TBR values compared to those in Stage I (n = 7/12; 58.3%) (SUVmax: 5.00 ± 1.02 vs. 8.34 ± 1.54, p = 0.005; TBR: 1.85 ± 0.35 vs. 3.50 ± 1.30, p = 0.045) (Figure 5C).
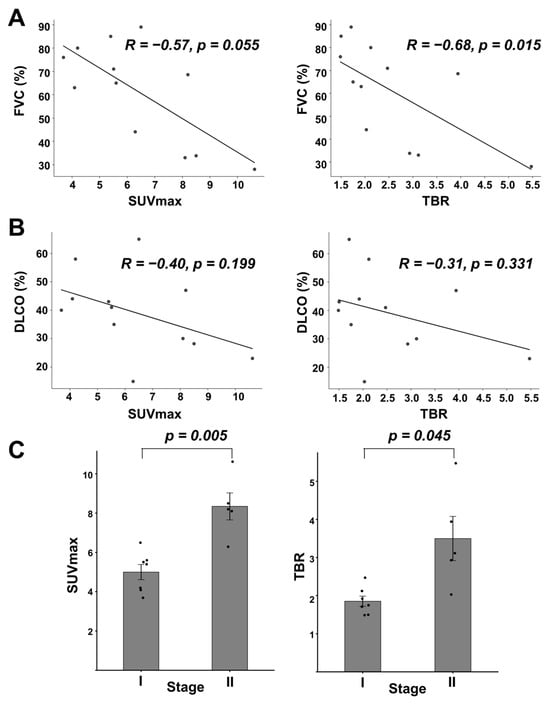
Figure 5.
Relationship between 99mTc-H-PoFP2 uptake with PFT and GAP index. (A) Linear correlation between SUVmax or TBR with FVC (% predicted). For SUVmax, R = −0.57, p = 0.055; for TBR, R = −0.68, p = 0.015. (B) Linear correlation between SUVmax or TBR with DLCO (% predicted). For SUVmax, R = −0.40, p = 0.199; for TBR, R = −0.31, p = 0.331. (C) SUVmax or TBR stratified by GAP index. SUVmax was 5.00 ± 1.02 vs. 8.34 ± 1.54 for Stages I and II, respectively (p = 0.005). TBRs were 1.85 ± 0.35 vs. 3.50 ± 1.30 for Stages I and II, respectively (p = 0.045).
3. Discussion
Fibrotic interstitial lung disease (fILD) represents a spectrum of chronic lung disorders that share the common feature of progressive fibrosis of the pulmonary parenchyma, leading to significant functional impairment and early mortality [,]. The diagnosis of fILD necessitates a multidisciplinary approach, integrating clinical assessments, radiological findings, and sometimes histopathological results. For early diagnosis, HRCT and histology/biopsy exhibit limitations. In contrast, nuclear medicine imaging has been routinely utilized for clinical diagnoses of cancer, cardiovascular, and neuroimaging due to its molecular-level insights and functional assessment []. Preclinical studies have demonstrated that activated fibroblasts play a pivotal role in the pathogenesis and progression of the fibrotic process in fILD [,,,]. Several clinical investigations into FAPI PET imaging in fILD patients have yielded robust and promising results regarding the performance of FAPI PET in disease evaluation, stratification of risk, and response to anti-fibrosis therapy [,,,]. Investigations into 99mTc-labeled FAPI for SPECT imaging remain limited []. There are especially few 99mTc-labeled FAPI tracers for imaging lung fibrosis.
Previously, we reported 99mTc-HFAPI for imaging idiopathic pulmonary fibrosis []. However, 99mTc-HFAPI would be decomposed in the body and was mainly excreted through the hepatobiliary system, resulting in a relatively high uptake in the gastrointestinal tract []. In this study, we designed a novel 99mTc-labeled FAPI-based radiopharmaceutical with optimized pharmacokinetics, guided by rational structural modifications. Specifically, we adopted the dimerization strategy to enhance the binding affinity to FAP and improve the in vivo stability of the radiopharmaceutical. Meanwhile, we introduced two PEG4 linkers to increase the hydrophilicity of the probe, which may shift the primary excretion pathway from hepatobiliary to renal (LogD = −3.56, indicating strong hydrophilicity), reduce gastrointestinal uptake, and lower the background signal. Collectively, these structural modifications endowed 99mTc-H-PoFP2 with superior in vivo stability compared to 99mTc-HFAPI. Regarding binding affinity, 99mTc-H-PoFP2 showed comparable Kd values to 99mTc-HFAPI (5.35 nM and 4.49 nM) []. The dimeric structures theoretically confer higher affinity for FAP than the monomer []. The lack of a significant decrease in Kd may be attributed to modifications in the targeting motif between 99mTc-H-PoFP2 and 99mTc-HFAPI. Nevertheless, 99mTc-H-PoFP2 demonstrated excellent in vivo FAP-targeting specificity in tumor-bearing mice (Figures S3 and S4) and bleomycin-induced lung fibrosis animal models (Figure 2). Critically, the regions with high radioactive signals in SPECT images were consistent with fibrotic foci identified by CT imaging and pathological staining results (HE, Masson, and FAP IHC). Notably, a discrepancy between SPECT and CT signals was observed. Mouse 2 showed stronger CT intensity but lower SPECT signal than Mouse 1 (Figure 2A). This inconsistency may arise from the distinct biological information captured by the imaging modality between CT and SPECT. CT reflects anatomical structural changes associated with fibrosis severity, while 99mTc-H-PoFP2 SPECT imaging specially indicates FAP expression. FAP is upregulated in activated fibroblasts during the active phase of fibrosis and its expression decreases as fibrosis progresses to a chronic stage whilst structural fibrosis persists in the bleomycin-induced pulmonary fibrosis mouse model [,]. The SPECT/CT imaging was performed on day 14 post-intratracheal injection, and the mouse model should be at the fibrotic stage with high FAP expression. However, the individual mice may exhibit inherent variability during the establishment of the bleomycin-induced fibrosis model []. M1 likely remained in the active fibrotic phase with high FAP expression and a strong SPECT signal, while M2 may have transitioned to an early chronic phase with reduced FAP activity, a weak SPECT signal, and ongoing structural fibrosis, which are strong CT signals. Further studies should be conducted to determine the relationship between 99mTc-H-PoFP2 SPECT imaging and CT imaging at different stages of pulmonary fibrosis. Furthermore, in another study regarding 99mTc-HFAPI SEPCT/CT imaging for pulmonary fibrosis, the inconsistency was also observed with lower 99mTc-HFAPI uptake in areas of severe lung fibrosis defined by HRCT []. This may be attributed to the decreased blood flow in end-stage fibrosis, which affects the 99mTc-FAPI uptake. Finally, several technical factors may also affect 99mTc-H-PoFP2 uptake. Given that pulmonary fibrosis lesions exhibit a diffuse rather than focal distribution, and that SPECT has lower spatial resolution, the uptake in diffuse lesions may be filtered out during SPECT data reconstruction, ultimately compromising FAPI uptake and resulting in the inconsistency between CT and SPECT signals.
Based on the excellent performance in preclinical studies, we validated 99mTc-H-PoFP2 in 12 fILD patients. No adverse effects were reported during the tracer administration or examination. The average total-body effective dose (0.00724 mSv/MBq) was comparable to other known single photon tracers and lower than that of 68Ga or 18F-labled FAPI (0.0164 mSv/MBq for 68Ga-FAPI-04; 0.00780 mSv/MBq for 68Ga-FAPI-46; 0.0124 mSv/MBq for 18F-NOTA-FAPI-04) [,]. We found accumulated uptake of 99mTc-H-PoFP2 in fibrotic lung regions. The markedly increased 99mTc-H-PoFP2 regions corresponded well with HRCT patterns of pulmonary fibrosis. Interestingly, 99mTc-H-PoFP2 exhibited high uptake around the right knee joint in the patient (Figure 4B) who had experienced right knee pain for several months, potentially indicating arthritis. The high uptake of 99mTc-H-PoFP2 suggests the presence of active fibrosis in the joint. Several studies have reported that FAPI PET may assist in diagnosing and monitoring disease activity in rheumatoid arthritis and psoriatic arthritis [,]. Interestingly, despite observing extensive honeycombing on HRCT, only mild 99mTc-H-PoFP2 uptake was noted in one case (Figure 4A). Experimental studies have found that FAP expression is induced during the early phase of lung fibroblast activation []. Pathologic sections of explanted lungs of fILD patients confirmed lower FAP expression in the late-stage fibrotic regions, like honeycombing, where matrix deposition and tissue remodeling predominate rather than being overtaken by ongoing active fibrosis []. In addition, the lower resolution of SPECT could reduce its sensitivity as smaller foci of fibroblastic activity could be obscured by larger inactive areas, resulting in reduced or undetectable uptake signals []. Thus, the uptake of 99mTc-H-PoFP2 might be lower. Additionally, a solid lesion with high 99mTc-H-PoFP2 uptake was identified in this patient. Given that lung cancer is a frequent complication of fILD, this lesion should be further clarified. However, due to the deterioration of lung function in this patient, the lesion had not been managed. Different time–activity curves have been observed in the dynamic FAPI PET imaging for fILD and lung cancer lesions []. Whether 99mTc-H-PoFP2 uptake differs in the fILD and lung cancer lesion could be further investigated. Importantly, 99mTc-H-PoFP2 uptake showed moderate negative correlations with PFT parameters, FVC, and DLCO, which were consistent with other FAPI PET studies. Moreover, our study revealed that patients at a higher GAP index stage exhibited significantly higher SUVmax and TBR values compared to those at a lower stage. Studies have demonstrated that the GAP index stratifies patients into different stages (I, II, and III) based on their risk of mortality, with higher scores indicating worse prognosis and predicting mortality in ILD patients [,]. Our findings suggest that the 99mTc-H-PoFP2 SPECT imaging is not only useful for diagnosing the presence of fILD but also may reflect the severity of lung fibrosis and predict disease progression.
Despite these promising results, there are several limitations of our study. Firstly, we only investigated 99mTc-H-PoFP2 SPECT imaging in pulmonary fibrosis models at a single time point, but did not explore whether 99mTc-H-PoFP2 SPECT imaging could monitor the progression of pulmonary fibrosis and response to anti-fibrotic treatment. We also did not conduct a biodistribution study in the pulmonary fibrosis mouse model, and thus we lack quantitative distribution data of 99mTc-H-PoFP2 in this model. In a subsequent experimental design, we should optimize the study protocol to ensure the comprehensiveness and rigor of the research. Secondly, while we preliminarily verified the feasibility of 99mTc-H-PoFP2 SPECT imaging in fILD, our present study is a single-time-point analysis. Further well-designed studies with a larger cohort are warranted to see whether 99mTc-H-PoFP2 SPECT imaging could monitor the response to anti-fibrotic therapy. Additionally, although the preclinical data validated the correlation between immunohistochemical expression of FAP and 99mTc-H-PoFP2 uptake, the patient study could not confirm this result due to the lack of patients’ tissue biopsy.
4. Materials and Methods
4.1. Reagents and Materials
All the reagents and solvents were purchased commercially and were of analytical grade. Na99mTcO4 was purchased from Beijing Atom High-Tech Co., Ltd. (Beijing, China). The radio high-performance liquid chromatography (radio-HPLC) was conducted with an Agilent 1260 HPLC system (Santa Clara, CA, USA). A semi-preparative column (YMC-Pack ODS-AC-18, 250 × 10 mm, 5 μm) and a C18 column (YMC-Pack ODS-A, 250 × 4.6 mml, 5 μm) (YMC Co., Ltd., Kyoto, Japan) were used for chemical synthesis and radiochemical analysis, respectively. FAP was purchased from Sino Biological, Inc. (Beijing, China).
4.2. Chemical Synthesis and 99mTc Radiolabeling
The detailed synthesis route and chemical characterization of HYNIC-(PEG4-oncoFAPi)2 (denoted as H-PoFP2) are described in the Supporting Information. The 99mTc-H-PoFP2 radiolabeling procedure was performed as follows. In total, 1 mL of Na99mTcO4 solution was added to a lyophilized kit containing tricine (6.5 mg), trisodium triphenylphosphine-3,3′,3″-trisulfonate (TPPTS) (5 mg), succinic acid (59 mg), sodium hydroxide (20 mg), and H-PoFP2 (25 μg). Next, the kit was heated to 100 °C for 20~25 min. After cooling to room temperature, 99mTc-H-PoFP2 was analyzed with radio-HPLC. Phase A was phosphate-buffered saline (PBS), and phase B was ACN with 0.05% TFA. The flow rate was 1 mL/min. The gradient mobile phase started from 15% phase B at 0~5 min and progressed to 85% phase B at 20 min and 15% phase B at 25 min. The product was then formulated in PBS and passed through a 0.22 μm Millipore filter (Burlington, MA, USA) into a sterile vial for further use.
4.3. Determination of the Partition Coefficient (LogD)
An aliquot of 99mTc-H-PoFP2 (74 kBq) was added to a centrifuge tube with 5.0 mL PBS (pH 7.4) and 5.0 mL n-octanol and vortexed for 10 min. After centrifugation (20,000 rpm, 10 min), three samples (500 μL × 3) were taken from each phase and measured in a γ-counter. The octanol/PBS partition coefficient (LogP) was determined as the logarithm of the octanol/PBS ratio. The determination of LogD was carried out twice with triplicate samples. The values of logD are given as mean values ± standard deviation.
4.4. In Vivo Stability Determination
For the in vivo stability studies, normal BALB/c mice were injected with 99mTc-H-PoFP2 (18 MBq) through the tail vein. Murine urine samples at 30, 120, and 240 min post-injection (p.i.) were collected and mixed with acetonitrile, and then passed through a 0.22 μm Millipore filter. The percentage of intact 99mTc-H-PoFP2 was then analyzed by radio-HPLC.
4.5. In Vitro Binding Specificity and Affinity Determination
For the protein binding assay, 100 μL of human FAP (2 μg/mL) was coated onto a detachable 96-well plate at 4 °C for 24 h. The coating solution was then removed, the plates were washed three times with cold PBS, and then blocked with 4% BSA for 30 min. For binding specificity determination, 99mTc-H-PoFP2 was diluted with PBS and added to the 96-well plate, and incubated at 37 °C for 1 h with or without excess unlabeled precursor H-PoFP2 (binding group and block group). After incubation, the plates were washed five times with ice-cold PBS solution containing 1% BSA. These wells were then put into corresponding radioimmunoassay tubes, and the cpm of each well was measured. For binding affinity determination, 99mTc-H-PoFP2 was diluted with PBS to prepare solutions of increasing concentration (0−200 nmol/L), which were added to the 96-well plate and incubated at 37 °C for 1 h. After incubation, the plates were washed five times with ice-cold PBS solution containing 1% BSA. The wells were then put into corresponding radioimmunoassay tubes, the cpm of each well was measured, and nonlinear fitting calculations were carried out using GraphPad 9.0 software to obtain the apparent dissociation constant (Kd) of 99mTc-H-PoFP2 to human FAP. Four parallel samples were set at each test point, and the experiment was repeated twice.
4.6. Establishment of Bleomycin-Induced Lung Fibrosis Model
All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Peking Union Medical College Hospital (approval number XHDW-2024-97). C57BL6 mice (female, 6 weeks of age) were purchased from the Department of Animal Experiment, Peking University Health Science Center, and were housed under a 12 h light/12 h dark cycle, with free access to food and water. To establish bleomycin-induced lung fibrotic mouse models, C57/BL6 mice received a single intratracheal injection of bleomycin (2.5~3.5 U/kg, Santa Cruz Biotechnology, Dallas, TX, USA) or saline under anesthesia (3% isoflurane). These mice were imaged at day 14 after intratracheal injection (n = 3 for each group).
4.7. SPECT/CT Imaging in Bleomycin-Induced Lung Fibrosis Model
99mTc-H-PoFP2 (37 MBq) was injected into the bleomycin-induced lung fibrotic mice and normal mice via the tail vein. At 1 h post-injection, the mice were anesthetized via inhalation of 2% isoflurane and imaged using SPECT/CT. SPECT imaging parameters were as listed: 140 keV photopeak and a 20% window width, with a 30 s frame duration. The parameters of helical CT scans were 50 kVp, 0.67 mA, a 210 rotation, and a 300 ms exposure time. SPECT and CT images were fused using the NanoScan SPECT/CT system by Mediso Ltd. (Budapest, Hungary). Representative CT images and SPECT/CT fused images were shown.
4.8. HE and Immunohistochemical Analysis of Fibrotic and Normal Lung Tissues
HE and Masson staining of bleomycin-induced fibrotic and normal lung tissues were performed following standard procedures. For FAP staining, lung tissues were incubated with a rabbit anti-FAP antibody (1:200; ab28244; Abcam, Waltham, MA, USA) overnight at 4 °C. Slide sections were then incubated with an HRP-conjugated goat anti-rabbit IgG antibody (1:200 dilution, GB23303, Servicebio, Wuhan, China) for 1 h and visualized following incubation with diaminobenzidine substrate.
4.9. Patients Enrolling and Characteristics
The clinical study was granted approval by the Institutional Review Board of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College (Ethics committee approval No. I-22YJ129). Prior to participation, all patients provided written informed consent. The calculation of radiation dosimetry, evaluation of biodistribution, and feasibility of 99mTc-H-PoFP2 imaging were conducted in 12 patients diagnosed with fILD. The fILD diagnoses were established by multidisciplinary team discussions based on clinical manifestations and radiologic patterns on HRCT. PFTs were executed in accordance with standardized protocols []. Key parameters, including forced vital capacity (FVC) (% predicted) and diffusing capacity of the lungs for carbon monoxide (DLCO) (% predicted), were documented. Additionally, the gender–age–physiology (GAP) index score, which has been used to evaluate the mortality risk in ILD patients, was calculated as well []. In brief, the score is derived from four variables, including gender, age, FVC, and DLCO. The cumulative score categorizes patients into Stage I (0–3 points), Stage II (4–5 points), or Stage III (6–8 points) [].
4.10. Whole-Body Scintigraphy and SPECT/CT Imaging Protocol
Sequential whole-body scintigraphy was performed at 5 min, 20 min, 40 min, 1 h, 2 h, 3 h, 4 h, and 6 h post-injection of 99mTc-H-PoFP2 (723.9 ± 52.4 MBq) on a dual-head Insight NM/CT Pro SPECT/CT scanner (Novel Medical, Beijing, China). Chest SPECT/CT was conducted approximately 200 ± 20 min post-injection. The SPECT data were normalized and corrected for attenuation, decay, and scatter utilizing an iterative reconstruction algorithm provided by the manufacturer-supplied technique. Patients were asked to report any abnormalities during the examination.
4.11. Distribution and Dosimetry Calculation
The overall biodistribution of 99mTc-H-PoFP2 was assessed visually. Biodistribution and dosimetry calculation analysis were conducted in six representative patients using HERMES Hybrid Viewer 4.0 Dosimetry (HERMES Medical Solutions) according to OLINDA/EXM (version 2.0) methodology as previously described []. In brief, regions of interest (ROIs) were manually delineated over the target organs, including the lungs, heart, liver, kidneys, pancreas, spleen, thyroid, salivary glands, urinary bladder, and residual body. The geometric mean counts were derived from background-corrected anterior and posterior counts. The number of disintegrations and the time–activity curve were generated for each target organ. Absorbed dose of target organs and effective dose were calculated for dosimetry estimation.
4.12. Image Analysis and Quantification
99mTc-H-PoFP2 SPECT images were visually compared with HRCT findings regarding the sites and extent of tracer uptake. For quantitative or semi-quantitative analysis, a cylindrical radioactive source with well-determined 99mTc activity was used to determine the conversion factors in the volumes of interest to standardized uptake values (SUVs). SUVs for ROIs were computed based on the corresponding patient weight, injected activity, and 99mTc camera calibration factor with HERMES HybridRecon standardized uptake value (SUV) SPECT. SUVmax was recorded based on the highest voxel value within the fibrotic region for each patient. The target-to-background ratio (TBR) was then calculated by normalizing the SUVmax relative to the blood pool as the reference background.
4.13. Statistical Analysis
All statistical analysis was executed using GraphPad Prism software, version 9.0.0. (GraphPad Software Inc., San Diego, CA, USA). Data were presented as mean ± standard deviation (SD). Differences between group means were compared using Student’s t-test. Correlation analyses were performed using the Spearman correlation test. A p-value < 0.05 was considered statistically significant.
5. Conclusions
In conclusion, our findings underscore the promising potential of 99mTc-H-PoFP2 SPECT imaging in pulmonary fibrosis, which offers a viable noninvasive assessment method for the diagnosis, staging, and prognosis of fILD. Further research is needed to validate these findings and explore the broader clinical applications of 99mTc-H-PoFP2 imaging in the management of fILD.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph18121779/s1. Figure S1: Synthesis route of HYNIC-(PEG4-oncoFAP)2 (denoted as H-PoFP2); Figure S2: Representative HPLC chromatogram images and TOF MS results of HYNIC-(PEG4-oncoFAP)2 and related compounds; Figure S3: SPECT/CT imaging of 99mTc-H-PoFP2 in U87MG tumor-bearing mice; Figure S4: Ex vivo biodistribution of 99mTc-H-PoFP2 in U87MG tumor-bearing mice; Figure S5. Blood clearance rate curve of 99mTc-H-PoFP2 in ICR mice; Figure S6. Whole-body planar SPECT/CT images of bleomycin-induced pulmonary fibrosis mice (M1, M2, and M4) and normal mice (M3, M5, and M6); Table S1: Detailed biodistribution data 99mTc-H-PoFP2 in U87MG tumor-bearing mice.
Author Contributions
Study conception and design: G.Y., J.W., J.S., L.H., Q.W., and F.W.; Experiment construction and implementation: G.Y., J.W., J.S.; Data acquisition and processing: G.Y., Y.L., X.Z., and Y.Z.; Writing of Manuscript: G.Y. and J.W.; Supervision and coordination of the study: J.S., L.H., Q.W., and F.W. All authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (Grant Nos. 2024YFC2419400 and 2022YFA1206100); the National Natural Science Foundation of China (Grant Nos. U24A20758, 92159201, and 82472023); the Beijing Natural Science Foundation (Grant No. L242062); the National High Level Hospital Clinical Research Funding (Grant Nos. 2022-PUMCH-D-001, 2022-PUMCH-D-002, 2022-PUMCH-C-020, and 2022-PUMCH-A-107); the Nuclear Energy R&D project No. HNKF202223(36); and the CAMS Innovation Fund for Medical Sciences (CIFMS) (2023-I2M-2-005, 2021-I2M-1-005). No other potential conflict of interest relevant to this article was reported.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Ethics committee approval No. I-22YJ129; date 23 August 2022). All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Peking Union Medical College Hospital (approval number XHDW-2024-97, date 21 June 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors appreciate Xiaoyu Zhao and Hannan Gao of Medical Isotopes Research Center and Department of Radiation Medicine of Peking University (Beijing, China) for their help with bleomycin-induced lung fibrosis model establishment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maher, T.M. Interstitial Lung Disease: A Review. JAMA 2024, 331, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Wijsenbeek, M.; Cottin, V. Spectrum of Fibrotic Lung Diseases. N. Engl. J. Med. 2020, 383, 958–968. [Google Scholar] [CrossRef]
- Muri, J.; Durcová, B.; Slivka, R.; Vrbenská, A.; Makovická, M.; Makovický, P.; Škarda, J.; Delongová, P.; Kamarád, V.; Vecanová, J. Idiopathic Pulmonary Fibrosis: Review of Current Knowledge. Physiol. Res. 2024, 73, 487–497. [Google Scholar] [CrossRef]
- Allison, M.B.; Catana, C.; Zhou, I.Y.; Caravan, P.; Montesi, S.B. Molecular Imaging of Pulmonary Fibrosis. J. Nucl. Med. 2025, 66, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Huang, Y.; Simms, A.E.; Mazur, A. Fibroblast Activation Protein-α: A Key Modulator of the Microenvironment in Multiple Pathologies. Int. Rev. Cell Mol. Biol. 2012, 297, 83–116. [Google Scholar] [CrossRef]
- Bundgaard-Nielsen, M.; Johnsen, R.H.; Mortensen, J.; Shaker, S.B.; Nielsen, C.T.H. Radio-Labelled Fibroblast Activation Protein Inhibitors in Interstitial Lung Diseases—A Systematic Review. Autoimmun. Rev. 2025, 24, 103856. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Zhang, Y.; Wang, J.; Wu, Y.; Yang, G.; Shi, J.; Wang, F.; Xu, Z.; Jing, H. 99mTc-Labeled FAPI SPECT Imaging in Idiopathic Pulmonary Fibrosis: Preliminary Results. Pharmaceuticals 2023, 16, 1434. [Google Scholar] [CrossRef]
- Yang, G.; Gao, H.; Luo, C.; Zhao, X.; Luo, Q.; Shi, J.; Wang, F. Palmitic Acid-Conjugated Radiopharmaceutical for Integrin Avβ3-Targeted Radionuclide Therapy. Pharmaceutics 2022, 14, 1327. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, B.; Fang, J.; Pang, Y.; Li, S.; Xie, C.; Sun, L.; Zhang, X.; Guo, Z.; Lin, Q.; et al. Synthesis, Preclinical Evaluation, and a Pilot Clinical PET Imaging Study of 68Ga-Labeled FAPI Dimer. J. Nucl. Med. 2022, 63, 862–868. [Google Scholar] [CrossRef]
- Ma, M.; Yang, G.; Zhao, M.; Liu, Y.; Ge, X.; Jia, B.; Gao, S. Synthesis and Preliminary Study of 99mTc-Labeled HYNIC-FAPi for Imaging of Fibroblast Activation Proteins in Tumors. Mol. Pharm. 2024, 21, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Diao, L.; Li, Z.; Ding, D.; Han, P.; Jiang, Y.; Yin, G.; Feng, J.; Wang, Q.; Jiang, J.; et al. Design and Preclinical Evaluation of 99mTc-Labeled Dimer FAPI-46 Derivatives as Potential Tumor Radiotracers. Eur. J. Med. Chem. 2025, 287, 117343. [Google Scholar] [CrossRef]
- Meng, L.; Fang, J.; Zhang, J.; Li, H.; Xia, D.; Zhuang, R.; Chen, H.; Huang, J.; Li, Y.; Zhang, X.; et al. Rational Design and Comparison of Novel 99mTc-Labeled FAPI Dimers for Visualization of Multiple Tumor Types. J. Med. Chem. 2024, 67, 8460–8472. [Google Scholar] [CrossRef] [PubMed]
- Stokke, C.; Gnesin, S.; Tran-Gia, J.; Cicone, F.; Holm, S.; Cremonesi, M.; Blakkisrud, J.; Wendler, T.; Gillings, N.; Herrmann, K.; et al. EANM Guidance Document: Dosimetry for First-in-Human Studies and Early Phase Clinical Trials. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1268–1286. [Google Scholar] [CrossRef] [PubMed]
- Goobie, G.C.; Marinescu, D.-C.; Adegunsoye, A.; Bourbeau, J.; Carlsten, C.; Clifford, R.L.; Doiron, D.; Duan, Q.; Gibson, K.F.; Grant-Orser, A.; et al. Accelerated Epigenetic Aging Worsens Survival and Mediates Environmental Stressors in Fibrotic Interstitial Lung Disease. Eur. Respir. J. 2025, 65, 2401618. [Google Scholar] [CrossRef] [PubMed]
- Wijsenbeek, M.; Suzuki, A.; Maher, T.M. Interstitial Lung Diseases. Lancet 2022, 400, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singh, S.S.; Gayana, S. Fibroblast Activation Protein Inhibitor PET/CT: A Promising Molecular Imaging Tool. Clin. Nucl. Med. 2021, 46, e141–e150. [Google Scholar] [CrossRef]
- Walsh, S.L.F.; Wells, A.U.; Sverzellati, N.; Devaraj, A.; von der Thüsen, J.; Yousem, S.A.; Colby, T.V.; Nicholson, A.G.; Hansell, D.M. Relationship between Fibroblastic Foci Profusion and High Resolution CT Morphology in Fibrotic Lung Disease. BMC Med. 2015, 13, 241. [Google Scholar] [CrossRef]
- Harada, T.; Watanabe, K.; Nabeshima, K.; Hamasaki, M.; Iwasaki, H. Prognostic Significance of Fibroblastic Foci in Usual Interstitial Pneumonia and Non-Specific Interstitial Pneumonia. Respirology 2013, 18, 278–283. [Google Scholar] [CrossRef]
- Egger, C.; Cannet, C.; Gérard, C.; Suply, T.; Ksiazek, I.; Jarman, E.; Beckmann, N. Effects of the Fibroblast Activation Protein Inhibitor, PT100, in a Murine Model of Pulmonary Fibrosis. Eur. J. Pharmacol. 2017, 809, 64–72. [Google Scholar] [CrossRef]
- Rosenkrans, Z.T.; Massey, C.F.; Bernau, K.; Ferreira, C.A.; Jeffery, J.J.; Schulte, J.J.; Moore, M.; Valla, F.; Batterton, J.M.; Drake, C.R.; et al. 68Ga-FAPI-46 PET for Non-Invasive Detection of Pulmonary Fibrosis Disease Activity. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3705–3716. [Google Scholar] [CrossRef]
- Albu, M.T.; Matei, A.-E.; Distler, J.H.W.; Giesel, F.L.; Mori, Y. Fibroblast Activation Protein Inhibitor PET/CT as an Emerging Diagnostic Modality in Interstitial Lung Disease and Other Fibrotic Conditions. Rheumatol. Immunol. Res. 2024, 5, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Distler, J.H.W.; Treutlein, C.; Tascilar, K.; Müller, A.-T.; Atzinger, A.; Matei, A.-E.; Knitza, J.; Györfi, A.-H.; Lück, A.; et al. 68Ga-FAPI-04 PET-CT for Molecular Assessment of Fibroblast Activation and Risk Evaluation in Systemic Sclerosis-Associated Interstitial Lung Disease: A Single-Centre, Pilot Study. Lancet Rheumatol. 2021, 3, e185–e194. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, K.; Nakuz, T.S.; Kulterer, O.C.; Geßl, I.; Simader, E.; Mrak, D.; Bonelli, M.; Kiener, H.P.; Prayer, F.; Prosch, H.; et al. FAPi PET/CT for Assessment and Visualisation of Active Myositis-Related Interstitial Lung Disease: A Prospective Observational Pilot Study. EClinicalMedicine 2024, 72, 102598. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Luo, Q.; Wang, X.; Fang, Q.; Fu, Z.; Li, J.; Lai, Y.; Chen, X.; Xu, X.; Peng, X.; et al. Comprehensive Analysis of Fibroblast Activation Protein Expression in Interstitial Lung Diseases. Am. J. Respir. Crit. Care Med. 2023, 207, 160–172. [Google Scholar] [CrossRef]
- Boschi, A.; Urso, L.; Uccelli, L.; Martini, P.; Filippi, L. 99mTc-Labeled FAPI Compounds for Cancer and Inflammation: From Radiochemistry to the First Clinical Applications. EJNMMI Radiopharm. Chem. 2024, 9, 36. [Google Scholar] [CrossRef]
- Younis, M.H.; Lan, X.; Cai, W. PET with a 68Ga-Labeled FAPI Dimer: Moving towards Theranostics. J. Nucl. Med. 2022, 63, 860–861. [Google Scholar] [CrossRef]
- Moeller, A.; Ask, K.; Warburton, D.; Gauldie, J.; Kolb, M. The Bleomycin Animal Model: A Useful Tool to Investigate Treatment Options for Idiopathic Pulmonary Fibrosis? Int. J. Biochem. Cell Biol. 2008, 40, 362–382. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, X.; Xu, X.; Ding, J.; Liu, S.; Hou, X.; Li, N.; Zhu, H.; Yang, Z. Clinical Translational Evaluation of Al18F-NOTA-FAPI for Fibroblast Activation Protein-Targeted Tumour Imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4259–4271. [Google Scholar] [CrossRef]
- Bentestuen, M.; Nalliah, S.; Stolberg, M.M.K.; Zacho, H.D. How to Perform FAPI PET? An Expedited Systematic Review Providing a Recommendation for FAPI PET Imaging with Different FAPI Tracers. Semin. Nucl. Med. 2024, 54, 345–355. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Jiang, L.; Tian, R.; Li, Q. 68Ga-FAPI-04 PET/CT Imaging in a Patient With Psoriatic Arthritis. Clin. Nucl. Med. 2025, 50, 83–84. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, Q.; Zhou, Z.; Li, M.; Wei, Y.; Jiang, X.; Yang, H.; Li, F. 68Ga-FAPI PET/CT for Rheumatoid Arthritis: A Prospective Study. Radiology 2023, 307, e222052. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Kim, G.H.J.; Rerkpichaisuth, V.; Teng, P.Y.; Armstrong, W.R.; Carlucci, G.; Dahlbom, M.; Abtin, F.; Lari, S.M.; Fishbein, G.A.; et al. Correlation of FAPI PET Uptake with Immunohistochemistry in Explanted Lungs from Patients with Advanced Interstitial Lung Disease. J. Nucl. Med. 2024, 65, 1789–1794. [Google Scholar] [CrossRef]
- Alqahtani, F.F. SPECT/CT and PET/CT, Related Radiopharmaceuticals, and Areas of Application and Comparison. Saudi Pharm. J. 2023, 31, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Röhrich, M.; Leitz, D.; Glatting, F.M.; Wefers, A.K.; Weinheimer, O.; Flechsig, P.; Kahn, N.; Mall, M.A.; Giesel, F.L.; Kratochwil, C.; et al. Fibroblast Activation Protein–Specific PET/CT Imaging in Fibrotic Interstitial Lung Diseases and Lung Cancer: A Translational Exploratory Study. J. Nucl. Med. 2022, 63, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, C.J.; Vittinghoff, E.; Ley, B.; Lee, J.S.; Mooney, J.J.; Jones, K.D.; Elicker, B.M.; Wolters, P.J.; Koth, L.L.; King, T.E.; et al. Predicting Survival across Chronic Interstitial Lung Disease: The ILD-GAP Model. Chest 2014, 145, 723–728. [Google Scholar] [CrossRef]
- Ley, B.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.H.; Tomassetti, S.; Lee, J.S.; Poletti, V.; Buccioli, M.; Elicker, B.M.; Jones, K.D.; et al. A Multidimensional Index and Staging System for Idiopathic Pulmonary Fibrosis. Ann. Intern. Med. 2012, 156, 684–691. [Google Scholar] [CrossRef]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS Standards for Single-Breath Carbon Monoxide Uptake in the Lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef]
- Fujii, H.; Hara, Y.; Saigusa, Y.; Tagami, Y.; Murohashi, K.; Nagasawa, R.; Aoki, A.; Izawa, A.; Seki, K.; Watanabe, K.; et al. ILD-GAP Combined with the Charlson Comorbidity Index Score (ILD-GAPC) as a Prognostic Prediction Model in Patients with Interstitial Lung Disease. Can. Respir. J. 2023, 2023, 5088207. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, F.; Gao, L.; Sun, L.; Hou, Y.; Liu, Y. An Integrated Framework of Projection and Attenuation Correction for Quantitative SPECT/CT Reconstruction. In Proceedings of the 2021 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Piscataway, NJ, USA, 16–23 October 2021; pp. 1–3. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).