Abstract
Sirtuin 3 (sirt3), a mitochondrial NAD+-dependent deacetylase, is an important enzyme in the maintenance of kidney functions, with critical roles in renal homeostasis, attenuation of oxidative stress, and preservation of mitochondrial homeostasis. This review aims to summarize the current literature on the mechanisms by which sirt3 impacts kidney health and disease, as well as highlight the therapeutic implications of sirt3 targeting. We conducted a PubMed search using the title word “sirt3” and the keyword “kidney” to generate our literature review sources. The animal studies that are explored in this review include cisplatin-induced acute kidney injury, cadmium-induced kidney injury, cecal ligation and puncture (CLP) and lipopolysaccharide-induced sepsis, diabetic kidney fibrosis, high-fat induced kidney disease, and ischemic kidney injury. Increasing evidence points towards a deficiency in sirt3 being an aggravator of mitochondrial dysfunction, promoting abnormal glycolysis, and contributing to the progression of diabetic kidney disease, renal fibrosis, and acute kidney injury. In contrast, pharmacological and dietary activation of sirt3 has been observed to enhance mitochondrial biogenesis, mitigate production of reactive oxygen species (ROS), and preserve the integrity of renal tubular cells under stressful conditions. Collectively, studies point towards sirt3 as a central metabolic and antioxidant regulator within the kidney, and link chronic kidney disease, as well as age-related decline in kidney function, to this enzyme. The conclusion of this review identifies future directions for translational research regarding sirt3 and NAD+-dependent regulation of mitochondrial homeostasis in renal medicine.
1. Introduction
The kidneys play an essential role in the maintenance of the body’s homeostasis through a diverse set of functions, including excretion of metabolic waste, regulation of fluid balance and blood pressure, acid–base equilibrium, and endocrine roles such as erythropoietin production and vitamin D activation [,,,]. In addition to the liver, the kidneys also contribute to systemic glucagon regulation of gluconeogenesis, especially under fasting conditions [,,]. These functions occur through the kidneys’ complex filtration system, a highly ATP-dependent process that sees renal tubular cells relying on active transport processes to reabsorb solutes along the nephron. To meet the high energy demand, nephrons contain a high density of mitochondria and consume substantial amounts of oxygen []. This metabolic profile enables efficient solute handling and allows the kidney to fulfill its functions, but leaves renal tissue highly vulnerable to mitochondrial damage, oxidative stress, and metabolic reprogramming during injury [,].
Mitochondrial dysfunction has been recognized as a central mechanism in the pathogenesis of acute kidney injury (AKI) and chronic kidney disease (CKD) [,,]. The literature surrounding these two conditions has shown that impaired oxidative phosphorylation, decreased fatty acid oxidation (FAO), and excess reactive oxygen species (ROS) production are all indicative of renal injury across models of ischemia-reperfusion, sepsis, toxin or cancer drug exposure, overnutrition, and diabetes [,,,,]. One such toxin exposure study utilized the cancer drug cisplatin to demonstrate that mitochondrial fragmentation and impaired mitochondrial homeostasis could ensue due to exposure to this toxin []. Similarly, metabolic reprogramming is caused by abnormal glycolysis and suppression of FAO, both of which can drive fibrosis in diabetic kidney disease (DKD) [,]. Such discoveries further confirm that mitochondria can be both a sensor and amplifier for renal injury [,,,].
Sirtuins are a group of proteins that evolved to manage cellular stress responses []. They are NAD-dependent enzymes using NAD as their substrate to function as deacetylases or mono(ADP-ribosyl)transferases, thereby coordinating cellular response to a variety of stressors []. For example, both caloric restriction and exercise can activate sirtuins by elevating cellular NAD levels [,]. Certain sirtuins can also act as class III histone deacetylases and play an important role in epigenetics involving gene silencing, DNA recombination, and repair [,]. Sirt3, a mitochondrial NAD+-dependent deacetylase and a known regulator of mitochondrial metabolism [,,], has been proven to act as a determinant of renal health [,,,]. Sirt3 expression has been observed in high quantities in metabolic tissues such as the kidney, where its role is to modulate the acetylation of mitochondrial proteins involved in the Krebs cycle, electron transport chain, and antioxidant defense []. It has been established that one of the mechanisms by which sirt3 is able to contribute to antioxidant defense is through the deacetylation and activation of manganese superoxide dismutase (SOD2), an important antioxidant enzyme whose expression levels are synergistically linked to sirt3 []. This example, along with others, shows sirt3’s ability to prevent oxidative injury and preserve mitochondrial integrity, thereby sustaining ATP generation.
Deficiencies or downregulation of sirt3 have been shown to contribute to the exacerbation of renal diseases [,,,]. In sepsis-induced AKI, it was found that knocking out the sirt3 gene would result in an increase in inflammasome activation, mitochondrial dysfunction, and tubular apoptosis []. Similarly, in a toxin exposure model using cisplatin to induce AKI, it was found that the presence of sirt3 resulted in amelioration of mitochondrial dysfunction, improved ATP production, and a mitigated ROS accumulation []. In ischemia-reperfusion models, sirt3 loss worsened kidney oxidative damage and renal fibrosis, while its restoration resulted in enhanced mitochondrial biogenesis and fusion [].
Further evidence of sirt3’s important role in renal function can be observed in metabolic kidney disease, where it has been demonstrated that a deficiency in sirt3 can lead to abnormal glycolysis in diabetic kidneys with fibrosis. This highlights the shift away from FAO to maladaptive energy metabolism []. High-fat diet (HFD) models also demonstrate that an absence of sirt3 can accelerate lipotoxic mitochondrial injury and real fibrosis []. These studies establish sirt3’s role in the metabolic process as a protein capable of balancing oxidative metabolism against glycolysis and modulating cellular responses to stress.
On the other hand, therapeutic activation of sirt3 has been a growing area of interest [,,], specifically into natural compounds such as silybin, resveratrol, honokiol, and melatonin. These compounds have shown promise in preclinical models in the enhancement of sirt3 expression and possible restoration of mitochondrial homeostasis [,,,]. These findings suggest that pharmacological modulation of sirt3 could provide a novel therapeutic approach for AKI and CKD. However, further mechanistic studies on these approaches must occur to optimize sirt3 targeting renal disease, before their real clinical applications can be explored.
This review aims to summarize current knowledge of sirt3 in kidney health and disease, with emphasis on its mechanistic roles in regulating metabolism, oxidative stress, apoptosis, and fibrosis. While many review articles exist in the literature regarding sirt3 and kidney disease [,,,,,], the novelty of our review is that we cover nearly all animal models of kidney injuries in the literature whereby sirt3 plays a clear role in preventing or mitigating renal dysfunction. Our review will also highlight therapeutic interventions aimed at activating sirt3 as a utility to mitigate the harm caused by AKI and CKD, with the latter including DKD. We then integrated findings across multiple experimental models of AKI, DKD, and CKD to provide a framework for understanding how modulation of sirt3 may offer new strategies for renal protection. It should be noted that this review will be limited to sirt3, as our laboratory is interested in studying the role of mitochondrial NAD-dependent redox enzymes in kidney disease. Nonetheless, this review is not meant to discount the roles of the other six sirtuin proteins in kidney disorders. For example, it has been well-established that sirt1, a cytosolic protein, also plays renoprotective roles in kidney injuries via delaying renal fibrogenesis, decreasing diabetic albuminuria, attenuating oxidative stress, and mitigating renal inflammation via NF-kB activation [,,,,,].
2. Sirt3 Structure and Function
In this section, we would like to have a brief discussion on sirt3’s structure and function. Sirt3 is one of the seven sirtuins [] and is a family member of the class 3 histone deacetylase and is thus highly involved in epigenetic regulation []. It is a mitochondrial protein whereby its major function is to remove the acetyl functional group from numerous proteins that have undergone prior acetylation due to a variety of stress challenges []. Sirt3 uses NAD+ as its substrate when it catalyzes deacetylation and restores protein function. Given the role of NAD+ in energy metabolism, redox signaling, and cell death, sirt3 can exert its function via NAD+ utilization [,]. In addition to its NAD+ binding domain, sirt3 also has a zinc-binding domain and a peptide substrate binding pocket. It should be noted that sirt3 can readily undergo posttranslational oxidative modifications such as carbonylation [,], leading to loss of its ability to deacetylate and regulate other enzymes’ functions, such as SOD2 []. Such impairment can be counteracted by polyphenols and other natural antioxidants []. Figure 1 shows a three-dimensional structure of human sirt3, and Figure 2 shows sirt3’s catalyzed reaction of protein deacetylation whereby NAD+ is used to accept the acetyl group that is usually linked to a lysine residue [,].
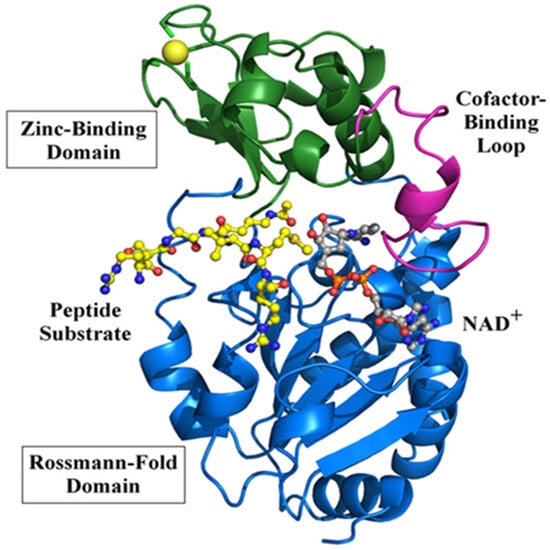
Figure 1.
Three-dimensional structure of human sirt3 showing bound peptide (acetyl-CoA synthetase 2). NAD-binding Rossmann fold domain and a zinc-binding domain are also shown. Adapted from [], Frontiers.org, 2012.
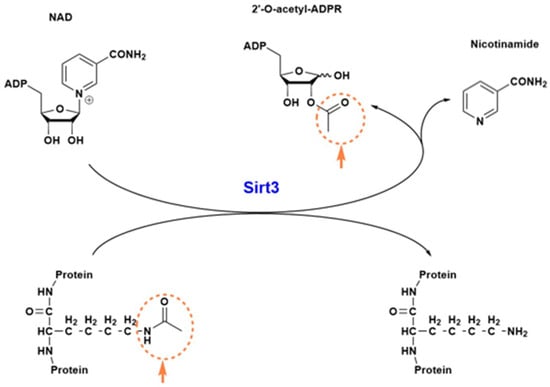
Figure 2.
Sirt3 catalyzed deacetylation reaction. The acetyl group linked to a lysine residue (circled) is transferred to NAD+, which then splits into two molecules: nicotinamide and 2′-O-acetyl-ADPR. The deacetylated protein now restores its function.
As can be seen from this reaction, nicotinamide and 2′-O-acetyl-ADP-ribose (ADPR) are the co-products of the sirt3-catalyzed reaction. Removal of the lysine-linked acetyl group restores the protein function. Therefore, sirt3 is a regulatory enzyme that has been well-recognized for its role in redox signaling, anti-oxidative stress, anti-fibrosis, and anti-apoptosis [].
3. Renoprotective Roles of Sirt3
3.1. Sirt3 Protects Against Cisplatin/Glycerol-Induced Acute Kidney Injury
Acute kidney injury is closely linked to mitochondrial dysfunction in renal tubular epithelial cells, where oxidative stress and mitochondrial damage can drive pathologies associated with this condition. Mitochondrial dysfunction that occurs because of AKI causes renal epithelial cells to lose their bioenergetic capacity and undergo apoptosis in response to injurious stimuli, such as toxins [,,]. In widely used cisplatin and glycerol-induced models of AKI [,], sirt3 was demonstrated to be a central regulator of mitochondrial integrity. In these settings, sirt3 saw a marked reduction in its expression []. Treatment of mice with cisplatin resulted in mitochondrial fragmentation in the proximal tubules of the kidney, as well as a drop in the number of renal mitochondria in AKI mice when compared to controls. This was associated with increased accumulation of nitrotyrosine, a marker of protein oxidative damage [], and decreased expression of PGC-1α, the main regulator of mitochondrial biogenesis []. In this model, both mRNA and protein expression of sirt3 dropped by nearly 80%, paralleled by decreased nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme for NAD+ biosynthesis.
In contrast, increasing sirt3 expression provided a protective feature against AKI. Treatment with AMPK agonist AICAR decreased blood urea nitrogen (BUN), reduced tubular necrosis, and preserved mitochondrial structure. This reversal was also associated with upregulation of NAMPT, PGC-1α, and, consequently, sirt3 in renal tissue []. Immunogold labeling revealed that cisplatin caused diffusion of mitochondrial proteins outside their predetermined areas. This diffusion could be reversed by AICAR treatment, which reinstated protein compartmentalization within mitochondria, suggesting that sirt3 aids in preserving organelle integrity. At the molecular level, sirt3’s protective effects extended to regulation of mitochondrial fission and fusion. In cisplatin-treated mice, dynamin-related protein 1 (DRP1) translocated to mitochondria, consistent with activation of fission pathways described previously in tubular injury []. AICAR treatment resulted in attenuated mitochondrial DRP1 accumulation, highlighting sirt3 as a restrainer of excessive fission and fragmentation [].
Moreover, sirt3 deacetylase activity was assessed directly, using cisplatin-treated mice. In these mice, global mitochondrial protein acetylation increased, along with the loss of sirt3 function. AICAR restored deacetylase activity, reducing protein acetylation and reestablishing mitochondrial homeostasis [,]. Together, these findings show a mechanistic synergy between AMPK, NAMPT, PGC-1α, and sirt3 that preserves mitochondrial integrity and function when exposed to agents injurious to renal tissue. By counteracting oxidative damage, preventing uncontrolled fission, and sustaining mitochondrial integrity, sirt3 acts as a defender against AKI.
3.2. Sirt3 Protects Against Cadmium-Induced Kidney Injury
Cadmium is an environmentally heavy metal that can cause kidney injury upon acute or chronic exposure []. It can induce oxidative stress and mitochondrial dysfunction by promoting the acetylation of many proteins, including forkhead O3, SOD2, and glutathione peroxidase 4 [,], which is associated with a decreased expression of Sirt3 []. When resveratrol, a natural antioxidant, was administered under experimental conditions, sirt3 expression was enhanced and protein deacetylation was facilitated, leading to improved mitochondrial biogenesis and attenuated mitochondrial ROS metabolism []. Such studies also demonstrate that natural products such as resveratrol can activate the sirt3 signaling pathway to counteract heavy metal-induced renal toxicity [,].
3.3. Sirt3 Protects Against Sepsis-Induced AKI
Sepsis is one of the most common causes of acute kidney injury (AKI), with pathogenesis driven by inflammation, oxidative stress, and mitochondrial dysfunction in renal tubular epithelial cells (RTECs) [,]. Using a cecal ligation and puncture (CLP) model of sepsis, it has been demonstrated that sirt3 plays a protective role by attenuating changes in the mitochondria, limiting ROS production, and suppressing activation of NLRP3 inflammasome []. In wild-type (WT) mice subjected to CLP, BUN and serum creatine levels increased, along with tubular vacuolar degeneration, neutrophil infiltration, and mitochondrial damage. These changes were exacerbated in sirt3 knockout (KO) mice, where sirt3 deletion resulted in higher ROS levels, decreased mitochondrial density and volume, and worsened renal dysfunction [].
Mechanistically, sirt3 protects against inflammasome-mediated injury. Mice that underwent CLP experienced upregulated NLRP3, ASC, and caspase-1, which drive expression of the proinflammatory cytokines IL-1β and IL-18. This response was enhanced in KO mice, confirming a protective anti-inflammatory role of sirt3 []. The results are backed up by reports that mark ROS as upstream activators of the NLRP3 inflammasomes, and that sirt3 mitigates this pathway by deacetylating and activating mitochondrial antioxidant enzymes such as SOD2 [,,].
Sirt3’s role as a suppressor of ROS-driven oxidative injury was highlighted by Zhao et al. through a comparison to the known antioxidant N-acetylcysteine (NAC). Treatment with NAC partially restored BUN and serum creatinine levels in both WT and KO mice. NAC treatment also reduced tubular apoptosis and mitigated inflammatory interleukins and ROS production. This overlap between an antioxidant with known AKI-protective features of sirt3 suggests that sirt3 exerts its protective effect largely through suppression of the ROS-driven oxidative injury. In vitro experimentation confirmed this relationship []. Treatment of HK-2 cells with H2O2 increased ROS production, downregulated sirt3, and upregulated NLRP3. KO mice treated with H2O2 experienced significantly enhanced apoptosis, but their treatment with NAC partially restored cells to their normal function. Additionally, sirt3 overexpression inhibited H2O2-induced apoptosis, which suggests that sirt3 protects against sepsis-induced AKI via the ROS/Caspase pathway []. In a widely used lipopolysaccharide (LPS)-induced septic kidney injury model [,], sirt3 shows similar renoprotective effects [,] as observed in the CLP model.
Together, these findings highlight sirt3’s ability to act as a regulator of the mitochondrial redox balance and inflammatory signaling in septic AKI. Sirt3’s role in suppressing ROS, inhibiting NLRP3 activation, and limiting cytokine production allows it to preserve tubular integrity and renal function under septic stress.
3.4. Sirt3 Deficiency in Diabetic Kidney Disease
A decreased expression of sirt3 has been repeatedly observed in diabetic tissues [,,,]. In the kidney, renal fibrosis is the result of diabetic kidney disease (DKD) and stands as the leading cause of end-stage renal failure worldwide []. Kidney fibrosis is defined by features such as refraction of peritubular capillaries, accumulation of extracellular matrix (ECM) proteins, like collagen, expansion of activated myofibroblasts, and infiltration of inflammatory cells [,,]. Although fibroblasts play a key role in the pathology of kidney fibrosis, their origin is not clearly defined. Researchers have suggested that the activation of myofibroblasts occurs because of the activation of resident fibroblasts and activation of mesenchymal transition programs in neighboring cells [,,,,,,].
Sirt3 deficiency was proven to play a role in the development of kidney fibrosis in a streptozotocin (STZ)-induced diabetic CD-1 mouse model. Upon injection of these mice with STZ, sirt3’s expression was found to be markedly decreased [,,]. The role of sirt3 in diabetic kidney disease was further explored by Srivastava et al., who demonstrated that sirt3 deficiency drives metabolic reprogramming in the diabetic kidney, pushing proximal tubular epithelial cells toward aerobic glycolysis as opposed to FAO []. In sirt3 KO mice with STZ-induced diabetes, renal cortical tissues experienced downregulation of PGC-1α, PPARα, CPT1a, and other FAO-related genes. This downregulation of FAO genes was coupled with the upregulation of the glycolytic enzymes hexokinase-2, pyruvate kinase M2 (PKM2), and pyruvate dehydrogenase kinase-1. Such changes in metabolic processes impaired mitochondrial respiration, elevated lactate production, and promoted ROS accumulation [].
Notably, diabetic mice displayed an induction of PKM2 dimers, whereby dimerized PKM2 regulates HIF1α and IL-1β production and induces the activation of pro-glycolytic enzymes during inflammation and tumorigenesis, linking glycolysis directly to fibrosis [,,,,]. These findings corroborate prior studies that defective FAO in renal tubular cells is a sufficient trigger to fibrogenesis and that HIF-1α-driven glycolysis promotes myofibroblast activation and ECM accumulation [,]. Mechanistically, sirt3 maintains mitochondrial integrity and redox balance through deacetylation of mitochondrial enzymes, which sustains FAO and represses HIF-1α-mediated transcription of glycolytic enzymes [,,,]. When sirt3 is absent, mitochondrial hyperacetylation, NAD+ depletion, and oxidative stress all occur, reinforcing glycolysis and fibroblast activation. Such evidence helps confirm that sirt3 is an important metabolic factor whose loss amplifies profibrotic metabolic signaling, making it a promising therapeutic target for diabetic kidney disease. It should be noted that the contents of sirt3 are robustly decreased in diabetic kidney injury, regardless of what animal models of DKD are used. Moreover, in terms of metformin and sirt3, it has been shown that metformin can activate AMPK, which in turn can decrease sirt3 SUMOylation via activating the cellular de-SUMOylation system, thereby leading to potential attenuation of kidney injury [].
Table 1 shows the widely used rodent models of DKD for studying the role of sirt3 in diabetic nephropathy.


Table 1.
Animal models that have been used for studying the role of sirt3 in DKD.
Table 1.
Animal models that have been used for studying the role of sirt3 in DKD.
| Diabetes Induction Method | References |
|---|---|
| High-fat diet (HFD) | [,,] |
| Streptozotocin (STZ) | [,,,,,,] |
| db/db mouse | [,,] |
| STZ/HFD | [,] |
| STZ/High sugar/HFD | [] |
| Zucker diabetic fatty rats | [,,] |
3.5. Sirt3 Protects Against High-Fat Diet-Induced Renal Injury
Metabolic syndrome, which includes clinical conditions such as obesity and hyperglycemia, is an established risk factor for chronic kidney disease [,,]. Metabolic diseases have been shown to be associated with mitochondrial dysfunction that can be caused by post-translational lysine acetylation of mitochondrial proteins, a process that is in part controlled by sirt3 [,]. For example, sirt3 content can be downregulated by overnutrition, leading to diminished sirt3 capabilities of maintaining cellular homeostasis [,]. Likewise, in type 2 diabetic mouse models, it was found that mice that develop nephropathy because of hyperglycemia had decreased renal sirt3 mRNA expression and activity. This decrease in sirt3 function was associated with an increase in ROS levels and mitochondrial abnormalities [].
Locatelli et al. demonstrated that sirt3-deficient mice exposed to high-fat diets developed more severe renal injury than wild-type controls []. Specifically, sirt3 deficiency led to earlier and more severe albuminuria than WT controls, beginning at four months of feeding compared to six months in WT mice. The progression of albuminuria was associated with podocyte dysfunction and glomerular capillary rarefaction []. Histological analysis showed increased mesangial matrix expansion, tubular vacuolization, and lipid accumulation in proximal tubules of sirt3-/- mice when compared to WT mice that were treated by the same diet. Inflammatory cell infiltration, specifically that of the Mac-2-positive macrophages, saw an increase in the sirt3-deficient mice.
The increasingly severe renal injuries observed in sirt3-/- mice were a result of elevated oxidative stress, mitochondrial abnormalities, and structural aberrations in tubular cells that occurred because of sirt3 being absent. This highlights sirt3’s enzymatic role in maintaining mitochondrial integrity during nutrient overload []. Sirt3 deficiency impaired the glomerular filtration barrier by causing podocyte cytoskeletal dysfunction, which was proven by decreased nestin expression, and by reducing glomerular endothelial density (CD31 expression). Taken together, these findings indicate that sirt3 can limit lipotoxicity-induced mitochondrial damage, inflammation, and podocyte injury in the setting of high-fat diets [].
3.6. Sirt3 Protects Against Ischemia-Reperfusion-Induced Kidney Injury
Acute kidney injury is often incurred by ischemia-reperfusion injury [,,,], which can be induced by surgery, hemorrhage, and trauma []. It has been found that ischemic kidney injury is accompanied by increased acetylation of SOD2 and p53 [] as well as early-stage renal fibrosis []. When sirt3 was inhibited by its selective inhibitors, such as 3-(1H-1,2,3-triazol-4-yl) pyridine (3-TYP), ischemic kidney injury was accentuated [], indicating the involvement of sirt3 in ischemic injury. In contrast, when sirt3 was overexpressed, ischemic kidney injury was significantly mitigated. It was further found that the underlying mechanism was due to sirt3’s enhancement of mitochondrial fusion and activation of the ERK-OPA1 signaling pathway []. Sirt3 can also exert its renoprotective effects on ischemic kidney injury by modulating the DRP1 pathway involved in mitophagy []. Such studies collectively highlight the nephroprotective nature of sirt3 in ischemic kidney injury.
3.7. Dietary Restriction and Pharmacological Activation of Sirt3 in Renal Protection
Sirt3’s central role in protecting the kidneys from various deleterious conditions via maintenance of mitochondrial homeostasis and defending against oxidative stress leaves it as an ideal target of therapeutic strategies. In this section, we would like to discuss sirt3 activation by dietary restriction or caloric restriction and the pharmacological agents involved in renoprotection against kidney injuries.
3.7.1. Dietary Restriction
Dietary restriction (DR), also known as caloric restriction (CR), is an established strategy for the retardation of aging and aging-associated diseases [,,,]. In the kidney, it has been demonstrated by Andrianova et al. that CR can upregulate sirt3 and ameliorate age-related kidney dysfunction []. The underlying mechanism is that sirt3 upregulation by CR mitigates oxidative stress as reflected by decreased levels of protein oxidation and lipid peroxidation. Moreover, mitochondrial membrane potential and mitochondrial integrity are also enhanced by CR []. The same research group also found that CR can confer ischemic tolerance to the kidney via sirt3 activation in senescence-accelerated rats []. CR achieves this mainly by sirt3-mediated enhancement of autophagy and mitophagy, leading to improvement in mitochondrial homeostasis and function []. It should be noted that fasting can also activate sirt3 for beneficial purposes [,]. It should also be noted that exercise can confer renoprotection against kidney injury by promoting mitochondrial dynamics via elevating the ATP/ADP ratio and NAD/NADH ratio, leading to AMPK/sirt3 activation and metabolic reprogramming and NF-kB inflammation suppression [,]. On the contrary, these mechanisms could not be activated in a sedentary lifestyle.
3.7.2. Pharmacological Activation
Similar to CR, numerous pharmacological agents and natural products have been found to be able to activate sirt3, leading to amelioration of renal damage in a variety of animal models of kidney injury. For example, Yi et al. and Yang et al. have demonstrated that green tea polyphenols can attenuate kidney injury by a high-fat diet via activation of the ketogenic pathways and sirt3 [,]. Sun et al. reported that beta-nicotinamide mononucleotide alleviates septic acute kidney injury by activating the sirt3 signaling pathway []. Shi et al. reported that butyrate can attenuate high-fat diet-induced glomerulopathy via the sirt3 pathway []. Drugs such as dapagliflozin and roxadustat can also exert renoprotective effects via sirt3 activation []. Table 2 lists certain pharmacological agents and natural products that can activate the sirt3 signaling pathway and mitigate kidney injury in a variety of animal models of kidney disease.


Table 2.
Pharmacological agents and natural products that can activate the sirt3 signaling pathway involved in renoprotection in a variety of animal models of kidney injury.
Table 2.
Pharmacological agents and natural products that can activate the sirt3 signaling pathway involved in renoprotection in a variety of animal models of kidney injury.
| Pharmacological Agents/Natural Products | Kidney Injury Model | Main Mechanisms | References |
|---|---|---|---|
| Apelin | Cisplatin-AKI | Mitochondrial homeostasis | [] |
| Kaempferol | High glucose-tubular cell damage | Decreased oxidative stress and apoptosis | [] |
| Calycosin | A variety of models | Anti-oxidative stress | [] |
| S-nitrosoglutathione | Septic kidney injury | Inhibition of pyroptosis | [] |
| Sodium thiosulfate | Cisplatin-induced kidney injury | Increased H2S content | [] |
| β-nicotiamide mononucleotide | Septic AKI | Increased NAD+ content | [,] |
| Artemisinin | Diclofenac-induced kidney injury | Mitochondrial homeostasis | [] |
| Butyrate | High fat-induced glomerulopathy | Decreased oxidative stress | [] |
| Luseogliflozin (SGLT2 inhibitor) | Ischemia-reperfusion injury | Suppression of ferroptosis | [] |
| Ligustilide | Ischemia-reperfusion injury | Mitochondrial homeostasis | [] |
| Tanshinone IIA | Doxorubicin-induced kidney injury | Decreased oxidative stress | [] |
| Linagliptin | Cisplatin-induced kidney injury | Enhanced mitophagy | [] |
| Dapagliflozin | Streptozotocin-DKD | Metabolic reprogramming | [] |
| Dexmedetomidine | IFNα-induced glomerulopathy | Decreased inflammation and oxidative stress | [] |
| Diosmin | UUO-mouse model | Decreased renal fibrosis | [] |
| Dihydromyricetin | Gentamicin-induced nephropathy | Anti-oxidative stress | [] |
| Salvianolic acid B | Diabetic nephropathy | Inhibiting oxidative stress | [] |
| Dahuang-Gancao decoction | LPS-induced kidney injury | Decreased apoptosis/inflammation | [] |
| Eprosartan | Ischemic-AKI | Decreased oxidative stress | [] |
| Shilajit | 5-fluorouracil kidney injury | Decreased oxidative stress | [] |
| Dexpanthenol | Glycerol-induced kidney injury | Decreased oxidative stress | [] |
| Nicotinamide riboside | db/db mice | Decreased inflammation | [] |
| Canagliflozin | High salt renal injury | Decreased oxidative stress | [] |
| Metrnl | DKD | Mitochondrial homeostasis | [] |
| Maresin-1 | Cecal ligation and puncture | Anti-inflammation | [] |
| Notoginsenoside Fc | Acetaminophen-induced nephropathy | Decreased mitochondrial damage | [] |
| Poricoic acid A | UUO rats | Suppressing renal fibrosis | [] |
| TUG891/Fatty acid receptor 4 | Ischemic/cisplatin/cecal ligation | Mitigating cell senescence | [] |
| Magnesium isoglycyrrhizinate | Cisplatin-AKI | Decreased mito-DNA damage | [] |
| Mitoquinone | Ischemia-reperfusion injury | Decreased oxidative damage | [] |
| Molineria recurvata | Streptozotocin-DKD | Antioxidation and anti-inflammation | [] |
| Eplerenone | Ischemia-reperfusion injury | Preserving mitochondrial function | [] |
| Liquiritigenin | Cisplatin-induced AKI | Improved mitochondrial function | [] |
| Matrine | Cisplatin-induced AKI | Enhanced mitochondrial function | [] |
| Fluorofenidone | UUO/ischemic injury | Reduced mitochondrial damage | [] |
| Honokiol | Cisplatin-AKI | Inhibiting mitochondrial fission | [] |
| Purple rice husk | DKD | Maintaining redox balance | [] |
| Melatonin | Contrast-induced AKI | Anti-oxidative stress | [] |
| Melatonin | Cecal ligation & puncture | Decreased oxidative stress | [] |
| Melatonin | Ischemic-AKI | Mitochondrial homeostasis | [] |
| Tenovin-1 | HFD-induced DKD | Antioxidant, anti-inflammation | [] |
| N-acetylcysteine | Bisphenol A-induced renal injury | Restoring mitochondrial integrity | [] |
| Mega-3 fatty acids | Rat 5/6 nephrectomy | Nrf2 activation | [] |
| Apigenin | DKD | Restoring redox balance | [] |
| Honokiol | DKD | Anti-oxidative stress | [] |
| Rhein | 5/6 nephrectomy-CKD | Anti-fibrosis & anti-oxidation | [] |
| Empagliflozin | Streptozotocin-DKD | Inhibiting epithelial-mesenchymal transition | [] |
| Jian-Pi-Yi-Shen formula | Adenine-induced CKD | Maintaining mitochondrial dynamics | [] |
| Croton hookeri | Streptozotocin-DKD | Inhibiting oxidative stress | [] |
| β-lapachone | Cisplatin-AKI | NQO1 activation | [,] |
| Spironolactone | 5/6 nephrectomy | Increased p-eNOS production | [] |
| Renalase | Cisplatin-AKI | Attenuating mitochondrial fission | [] |
| Stanniocalcin-1 | DKD | Inhibiting BNIP3 | [] |
| Metformin | Folic acid-AKI, ischemic AKI | Maintaining mitochondrial integrity | [] |
| Intermedin | 5/6 nephrectomy | Inhibiting oxidative stress | [] |
| Pyrroloquinoline quinine | High glucose/HK-2 cells | Inhibiting ROS production | [] |
| Mito-TEMPO | 5/6 nephrectomy | Mitigating renal fibrosis | [] |
| Fenofibrate | Salt-induced hypertension | Mitochondrial homeostasis | [] |
| Dioscin | Fructose-induced renal injury | Decreased oxidative stress | [] |
| Green tea polyphenols | High fat-induced renal damage | Decreased oxidative stress | [] |
| Lactobacillus rhamnosus GG | Deoxynivalenol-induced kidney injury | Decreased oxidative damage | [] |
| Annexin A1 | Ischemic-AKI | Increased mitochondrial function | [] |
| Jinshuiqing | IgA nephropathy | Anti-inflammation | [] |
| Tert-butylhydroquinone | Contrast-induced nephropathy | Increased antioxidant capacity | [] |
| Curcumin | Cisplatin-AKI | Mitochondrial homeostasis | [,] |
| Silybin | Cisplatin-AKI | Attenuated mitochondrial dysfunction | [] |
| Resveratrol | Cadmium-induced renal damage | Maintaining redox balance | [] |
| Resveratrol/exercise | Salt-induced hypertension | Enhanced mitophagy | [] |
| ACE inhibitor/nicorandil | CKD | Decreased oxidative stress | [] |
| Coumarin derivative SZC-6 | CKD | Restoring mitochondrial function | [] |
Please note that this table is not meant to be exhaustive. Abbreviations: AKI, acute kidney injury; DKD, diabetic kidney disease; CKD, chronic kidney disease; HFD, high-fat diet; LPS, lipopolysaccharide; UUO, unilateral ureteral obstruction.
The studies shown in Table 2 underline the ability of sirt3 to be a suitable therapeutic target for renal disease and demonstrate various dietary supplements and natural compounds as effective sirt3 activators. Through upregulation of sirt3, these activators have shown the ability to restore mitochondrial homeostasis and enhance the kidney’s antioxidant defense. Through these actions, they may provide an avenue for preventing kidney injury in clinical settings. It should be noted that those plant compounds shown in Table 2 may only play a modulating role involving sirt3 activation. It should also be noted that many of the agents/mechanisms that activate sirt3 could also activate other sirtuins. Therefore, sirt3 activation shown in Table 1 may lack specificity, which needs to be addressed in future studies. Finally, given that sirt3 plays its renoprotective roles through protein deacetylation, as shown in Figure 2, Table 3 lists some of the well-studied sirt3 substrates involved in kidney function and dysfunction.


Table 3.
Substrates that can be deacetylated by sirt3 in kidney disease.
Table 3.
Substrates that can be deacetylated by sirt3 in kidney disease.
| Substrate Protein | Disease Model | Reference |
|---|---|---|
| Mitochondrial pyruvate carrier 2 | DKD | [] |
| Glycogen synthase kinase-3β | UUO | [] |
| Carnitine palmitoyltransferase 1α | DKD | [] |
| P53 | Adenine-induced CKD | [] |
| Krüppel-like factor 15 | DKD | [] |
| Transcription factor A | LPS-induced sepsis | [] |
| Mitochondrial SOD2 | Folic acid/ischemia model | [] |
| Beta-catenin (lysine 49) | UUO | [] |
| ATP-dependent metalloprotease (YME1L1) | LPS-AKI | [] |
| Optic atrophy 1 | Cisplatin-AKI | [] |
| Pyruvate dehydrogenase E1α | UUO | [] |
| Peroxisome proliferator-activated receptor gamma coactivator-1 alpha | 5/6 Nephrectomy | [] |
| ATP synthase β | Cisplatin-AKI | [] |
| Forkhead box protein O3a | Hypertensive renal injury | [] |
4. Conclusions
Sirt3 is a pivotal enzyme in renal mitochondrial regulation, conferring protective mechanisms by maintaining oxidative metabolism, suppressing ROS production, and preserving mitochondrial integrity [,,]. Across models of acute kidney injury, ischemic kidney injury, diabetic kidney disease, and nephropathy induced by drugs, heavy metals, and high-fat diet, sirt3 deficiency has been noted to aggravate mitochondrial damage, promote abnormal glycolysis, and accelerate fibrosis [,,]. Conversely, sirt3 upregulation or pharmacological stimulation reinstates mitochondrial homeostasis, preserves fatty acid oxidation, and inhibits inflammatory and apoptotic pathways in the kidney [,,,,]. It should be noted that sirt3 could also be activated to enhance cancer cell adaptation and survival. Therefore, pharmacological inhibition of sirt3 activity could promote cancer cell death, thereby benefiting kidney health []. Figure 3 summarizes animal models of kidney injury and sirt3’s renal protective mechanisms discussed in this review and Figure 4 shows the signaling pathways that can be activated by sirt3 discussed in the review.
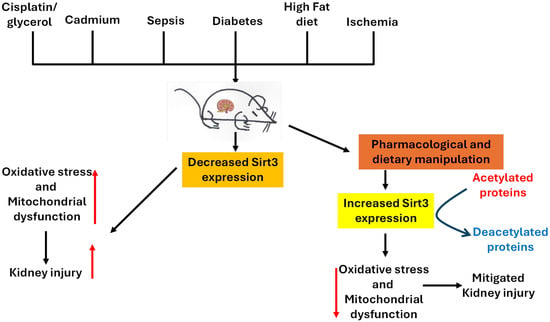
Figure 3.
A schematic diagram showing animal models and sirt3’s renal protective mechanisms discussed in the article. Sirt3 can be activated by diet manipulation and pharmacological agents or natural products to enhance mitochondrial function by mitigating oxidative stress, renal fibrosis, and inflammation in a variety of kidney injuries.
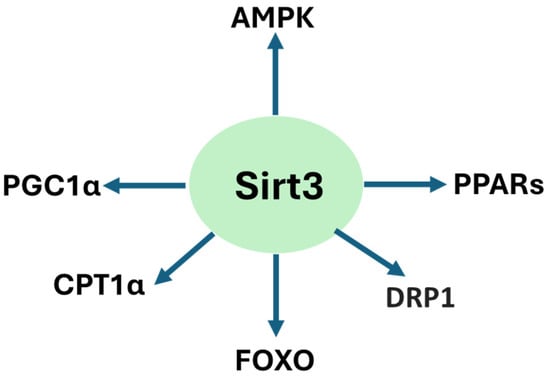
Figure 4.
Sirt3-activated signaling pathways involved in renoprotection discussed in the review.
As a highlight, natural compounds such as silybin, resveratrol, honokiol, and melatonin have demonstrated their abilities to enhance sirt3 activity and elicit renoprotection in preclinical models [,,]. While these findings establish sirt3 as an appealing therapeutic target, more studies will be needed to clarify their tissue-specific mechanisms, evaluate long-term modulation strategies, and translate these results into safe, effective therapies for use in clinics [,].
Taken together, current evidence positions sirt3 both as a biomarker of renal metabolic health and as a primary target for the development of new medications. The future of research integrating mechanistic insight with translational intent will be decisive for realizing sirt3’s potential to prevent or reverse kidney injury in human disease. Given that sirt3 competes with other NAD+-utilizing enzymes such as NAD kinase [,], CD38 [,], and dihydrolipoamide dehydrogenase [,], as well as other alpha-keto acid dehydrogenase complexes [,], functional characterization of these other NAD+-dependent enzymes and their relationship to sirt3 would also need to be investigated in a variety of kidney diseases.
Author Contributions
Original draft preparation, R.S.A.; review and editing, R.S.A. and L.-J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
L.J. Yan was supported in part by a grant from the Diabetes Action Research and Education Foundation and by a Bridge grant (Grant number 2400071) from UNT Health Fort Worth.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Diep, T.N.; Liu, H.; Yan, L.J. Beneficial Effects of Butyrate on Kidney Disease. Nutrients 2025, 17, 722. [Google Scholar] [CrossRef]
- Kamt, S.F.; Liu, J.; Yan, L.J. Renal-Protective Roles of Lipoic Acid in Kidney Disease. Nutrients 2023, 15, 1732. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, J.; Qin, Y.; Zhang, Y.; Wang, A.; Ma, R.; Han, M.; Hui, Y.; Guo, S.; Ning, X.; et al. The protective mechanism of SIRT3 and potential therapy in acute kidney injury. QJM Int. J. Med. 2024, 117, 247–255. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490. [Google Scholar] [CrossRef]
- Bankir, L.; Bouby, N.; Speth, R.C.; Velho, G.; Crambert, G. Glucagon revisited: Coordinated actions on the liver and kidney. Diabetes Res. Clin. Pract. 2018, 146, 119–129. [Google Scholar] [CrossRef]
- Sharma, R.; Sahoo, B.; Srivastava, A.; Tiwari, S. Reduced insulin signaling and high glucagon in early insulin resistance impaired fast-fed regulation of renal gluconeogenesis via insulin receptor substrate. J. Cell Biochem. 2022, 123, 1327–1339. [Google Scholar] [CrossRef]
- Meyer, C.; Dostou, J.M.; Gerich, J.E. Role of the human kidney in glucose counterregulation. Diabetes 1999, 48, 943–948. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Liu, H.; Yan, L.-J. The Role of Ketone Bodies in Various Animal Models of Kidney Disease. Endocrines 2023, 4, 236–249. [Google Scholar] [CrossRef]
- Forbes, J.M.; Thorburn, D.R. Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 291–312. [Google Scholar] [CrossRef]
- Mapuskar, K.A.; Vasquez-Martinez, G.; Mayoral-Andrade, G.; Tomanek-Chalkley, A.; Zepeda-Orozco, D.; Allen, B.G. Mitochondrial Oxidative Metabolism: An Emerging Therapeutic Target to Improve CKD Outcomes. Biomedicines 2023, 11, 1573. [Google Scholar] [CrossRef]
- Chang, L.Y.; Chao, Y.L.; Chiu, C.C.; Chen, P.L.; Lin, H.Y. Mitochondrial Signaling, the Mechanisms of AKI-to-CKD Transition and Potential Treatment Targets. Int. J. Mol. Sci. 2024, 25, 1518. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, X.; Xia, F.; Shi, M.; Zhao, D.; Li, L.; Jiang, H. Mitochondrial dysfunction and metabolic reprogramming in acute kidney injury: Mechanisms, therapeutic advances, and clinical challenges. Front Physiol. 2025, 16, 1623500. [Google Scholar] [CrossRef]
- Yan, L.J. Folic acid-induced animal model of kidney disease. Anim. Models Exp. Med. 2021, 4, 329–342. [Google Scholar] [CrossRef]
- Haoxin, L.; Yucheng, W.; Ying, W.; Liang-Jun, Y. Rodent Models of Streptozotocin-Induced Diabetes as Suitable Paradigms for Studying Diabetic Kidney Disease. Free Radic. Antioxid. 2024, 14, 32–33. [Google Scholar]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Emma, F.; Montini, G.; Parikh, S.M.; Salviati, L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat. Rev. Nephrol. 2016, 12, 267–280. [Google Scholar] [CrossRef]
- Morigi, M.; Perico, L.; Rota, C.; Longaretti, L.; Conti, S.; Rottoli, D.; Novelli, R.; Remuzzi, G.; Benigni, A. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Investig. 2015, 125, 715–726. [Google Scholar] [CrossRef]
- Takasu, M.; Kishi, S.; Nagasu, H.; Kidokoro, K.; Brooks, C.R.; Kashihara, N. The Role of Mitochondria in Diabetic Kidney Disease and Potential Therapeutic Targets. Kidney Int. Rep. 2025, 10, 328–342. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Li, J.; Kitada, M.; Fujita, H.; Yamada, Y.; Goodwin, J.E.; Kanasaki, K.; Koya, D. SIRT3 deficiency leads to induction of abnormal glycolysis in diabetic kidney with fibrosis. Cell Death Dis. 2018, 9, 997. [Google Scholar] [CrossRef]
- Perico, L.; Remuzzi, G.; Benigni, A. Sirtuins in kidney health and disease. Nat. Rev. Nephrol. 2024, 20, 313–329. [Google Scholar] [CrossRef]
- Wang, X.; Luo, T.; Yang, Y.; Yang, L.; Liu, M.; Zou, Q.; Wang, D.; Yang, C.; Xue, Q.; Liu, S.; et al. TRPA1 protects against contrast-induced renal tubular injury by preserving mitochondrial dynamics via the AMPK/DRP1 pathway. Free Radic. Biol. Med. 2024, 224, 521–539. [Google Scholar] [CrossRef]
- Wang, Z.; Do Carmo, J.M.; Da Silva, A.A.; Fu, Y.; Hall, J.E. Mechanisms of Synergistic Interactions of Diabetes and Hypertension in Chronic Kidney Disease: Role of Mitochondrial Dysfunction and ER Stress. Curr. Hypertens. Rep. 2020, 22, 15. [Google Scholar] [CrossRef]
- Pavlovic, N.; Krizanac, M.; Kumric, M.; Vukojevic, K.; Bozic, J. Mitochondrial Dysfunction: The Silent Catalyst of Kidney Disease Progression. Cells 2025, 14, 794. [Google Scholar] [CrossRef]
- Bosch-Presegue, L.; Vaquero, A. Sirtuins in stress response: Guardians of the genome. Oncogene 2014, 33, 3764–3775. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, B.; Wang, H.; Lv, S.; Chen, H.; Liu, D. Caloric restriction, Sirtuins, and cardiovascular diseases. Chin. Med. J. 2024, 137, 921–935. [Google Scholar] [CrossRef]
- Chong, M.C.; Silva, A.; James, P.F.; Wu, S.S.X.; Howitt, J. Exercise increases the release of NAMPT in extracellular vesicles and alters NAD+ activity in recipient cells. Aging Cell 2022, 21, e13647. [Google Scholar] [CrossRef]
- Veena, K.V.; Siddamalla, S.; Deenadayal, M.; Sisinthy, S.; Bhanoori, M. Histone deacetylase 1, Sirtuin 1, and Sirtuin 3 single-nucleotide polymorphisms and the risk of endometriosis in South Indian women. J. Obstet. Gynaecol. 2022, 42, 3230–3235. [Google Scholar] [CrossRef]
- Mahlknecht, U.; Voelter-Mahlknecht, S. Chromosomal characterization and localization of the NAD+-dependent histone deacetylase gene sirtuin 1 in the mouse. Int. J. Mol. Med. 2009, 23, 245–252. [Google Scholar] [CrossRef]
- Trinh, D.; Al Halabi, L.; Brar, H.; Kametani, M.; Nash, J.E. The role of SIRT3 in homeostasis and cellular health. Front. Cell. Neurosci. 2024, 18, 1434459. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Zhao, Y.; Wang, X. Mechanisms of SIRT3 regulation of aging and aging-related diseases and advances in drug therapy. Gerontology 2025, 1–22. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, Z. Roles of SIRT3 in aging and aging-related diseases. Int. J. Biol. Sci. 2025, 21, 5135–5163. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Zhang, T.; Liu, M.; Chi, Y. Role of Sirtuin3 in high glucose-induced apoptosis in renal tubular epithelial cells. Biochem. Biophys. Res. Commun. 2016, 480, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ni, H.; Kuang, B.; Wang, Z.; Hou, S.; Gu, S.; Gong, N. Sirtuin 3 in renal diseases and aging: From mechanisms to potential therapies. Pharmacol. Res. 2024, 206, 107261. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, T.; Chen, J.; Fan, Z.; Gao, F.; Yu, Z.; Jiang, Y. SIRT3/6: An amazing challenge and opportunity in the fight against fibrosis and aging. Cell. Mol. Life Sci. 2024, 81, 69. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, F.; Arnould, T.; Decleves, A.E. The Role of Mitochondrial Sirtuins (SIRT3, SIRT4 and SIRT5) in Renal Cell Metabolism: Implication for Kidney Diseases. Int. J. Mol. Sci. 2024, 25, 6936. [Google Scholar] [CrossRef]
- Lombard, D.B.; Tishkoff, D.X.; Bao, J. Mitochondrial sirtuins in the regulation of mitochondrial activity and metabolic adaptation. Handb. Exp. Pharmacol. 2011, 206, 163–188. [Google Scholar]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, D.; Huang, S.; Liu, S.; Zhang, B.; Wang, F.; Lu, J.; Chen, J.; Li, S. Impaired Nicotinamide Adenine Dinucleotide Biosynthesis in the Kidney of Chronic Kidney Disease. Front. Physiol. 2021, 12, 723690. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Le, J.W.; Sun, M.; Zhu, J.H. Sirtuin 3 deficiency promotes acute kidney injury induced by sepsis via mitochondrial dysfunction and apoptosis. Iran. J. Basic Med. Sci. 2021, 24, 675–681. [Google Scholar] [PubMed]
- Pezzotta, A.; Perico, L.; Corna, D.; Morigi, M.; Remuzzi, G.; Benigni, A.; Imberti, B. Sirt3 deficiency promotes endothelial dysfunction and aggravates renal injury. PLoS ONE 2023, 18, e0291909. [Google Scholar] [CrossRef]
- Wang, J.; Ren, X.; Lu, H.; Guo, Z.; Li, X.; Tian, Y.; Yin, Y.; Qin, Z.; Yun, K.; Wu, M.; et al. Restoration of SIRT3 Expression in Aged Mice Alleviates UUO-Induced Renal Fibrosis by Reducing GSK-3beta Hyperacetylation. Adv. Sci. 2025. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhang, L.; Sui, M.X.; Zhu, Y.H.; Zeng, L. Protective effects of sirtuin 3 in a murine model of sepsis-induced acute kidney injury. Sci. Rep. 2016, 6, 33201. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhang, L.; Sui, M.X.; Zhu, Y.H.; Zeng, L. Activation of Sirtuin 3 by Silybin Attenuates Mitochondrial Dysfunction in Cisplatin-induced Acute Kidney Injury. Front. Pharmacol. 2017, 8, 178. [Google Scholar] [CrossRef]
- Locatelli, M.; Macconi, D.; Corna, D.; Cerullo, D.; Rottoli, D.; Remuzzi, G.; Benigni, A.; Zoja, C. Sirtuin 3 Deficiency Aggravates Kidney Disease in Response to High-Fat Diet through Lipotoxicity-Induced Mitochondrial Damage. Int. J. Mol. Sci. 2022, 23, 8345. [Google Scholar] [CrossRef] [PubMed]
- Kobroob, A.; Kumfu, S.; Chattipakorn, N.; Wongmekiat, O. Modulation of Sirtuin 3 by N-Acetylcysteine Preserves Mitochondrial Oxidative Phosphorylation and Restores Bisphenol A-Induced Kidney Damage in High-Fat-Diet-Fed Rats. Curr. Issues Mol. Biol. 2024, 46, 4935–4950. [Google Scholar] [CrossRef]
- Xiong, Z.; Hu, X.; Wang, R.; Li, C.; Cheng, H.; Zhao, W.; Shen, Y.; Wang, L.; Li, W.; Zhu, X.; et al. Jingtian granule alleviates adenine-induced renal fibrosis in mice through SIRT3-Mediated deacetylation of P53. Front. Pharmacol. 2025, 16, 1526414. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.; Li, J. A review on SIRT3 and its natural small molecule activators as a potential Preventive and therapeutic target. Eur. J. Pharmacol. 2024, 963, 176155. [Google Scholar] [CrossRef]
- Pillai, V.B.; Sundaresan, N.R.; Jeevanandam, V.; Gupta, M.P. Mitochondrial SIRT3 and heart disease. Cardiovasc. Res. 2010, 88, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, Y.; Ding, N.; Wei, J.; Li, C. The role of Sirt1 in kidney disease. Int. Urol. Nephrol. 2025, 57, 147–158. [Google Scholar] [CrossRef]
- Kitada, M.; Kume, S.; Takeda-Watanabe, A.; Kanasaki, K.; Koya, D. Sirtuins and renal diseases: Relationship with aging and diabetic nephropathy. Clin. Sci. 2013, 124, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Morigi, M.; Benigni, A. Mitochondrial Sirtuin 3 and Renal Diseases. Nephron 2016, 134, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Yu, D.; Zhao, H.; Li, P. Sirtuin 3 in diabetic kidney disease: Mechanisms and pharmacotherapy. Ren. Fail. 2025, 47, 2543927. [Google Scholar] [CrossRef]
- Ye, K.; Zhao, Y.; Huang, W.; Zhu, Y. Sodium butyrate improves renal injury in diabetic nephropathy through AMPK/SIRT1/PGC-1alpha signaling pathway. Sci. Rep. 2024, 14, 17867. [Google Scholar]
- Chu, W.; Sun, X.; Yan, Y. Study on the regulation of renal tubular cell apoptosis by SIRT1/NF-kappaB signaling pathway in septic acute kidney injury. Ren. Fail. 2025, 47, 2499904. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Ma, F.; Fu, T.; Yang, L.; Mao, H.; Wang, Y.; Peng, L.; Li, P.; Zhan, Y. Roles of Sirt1 and its modulators in diabetic microangiopathy: A review. Int. J. Biol. Macromol. 2024, 264 Pt 2, 130761. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Lee, K.; He, J.C. SIRT1 Is a Potential Drug Target for Treatment of Diabetic Kidney Disease. Front. Endocrinol. 2018, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, R.; Lee, K.; He, J.C. The Role of SIRT1 in Diabetic Kidney Disease. Front. Endocrinol. 2014, 5, 166. [Google Scholar] [CrossRef]
- Guan, Y.; Hao, C.M. SIRT1 and Kidney Function. Kidney Dis. 2016, 1, 258–265. [Google Scholar] [CrossRef]
- Moniot, S.; Weyand, M.; Steegborn, C. Structures, substrates, and regulators of Mammalian sirtuins—Opportunities and challenges for drug development. Front. Pharmacol. 2012, 3, 16. [Google Scholar] [CrossRef]
- Morris, B.J. Seven sirtuins for seven deadly diseases of aging. Free Radic. Biol. Med. 2013, 56, 133–171. [Google Scholar] [CrossRef] [PubMed]
- Yu, D. Sirtuin 3 as a promising target in disease therapy: Model action and drug discovery. Eur. J. Med. Chem. 2025, 297, 117929. [Google Scholar] [CrossRef]
- Osborne, B.; Bentley, N.L.; Montgomery, M.K.; Turner, N. The role of mitochondrial sirtuins in health and disease. Free Radic. Biol. Med. 2016, 100, 164–174. [Google Scholar] [CrossRef]
- Benigni, A.; Perico, L.; Macconi, D. Mitochondrial Dynamics Is Linked to Longevity and Protects from End-Organ Injury: The Emerging Role of Sirtuin 3. Antioxid. Redox Signal. 2016, 25, 185–199. [Google Scholar] [CrossRef]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Dagostino, C.; Gliozzi, M.; Malafoglia, V.; Sansone, L.; Palma, E.; Tafani, M.; Russo, M.A.; et al. Antioxidant modulation of sirtuin 3 during acute inflammatory pain: The ROS control. Pharmacol. Res. 2020, 157, 104851. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, X.; Huang, C.; Gao, J.; Shen, W. Frataxin Loss Promotes Angiotensin II-Induced Endothelial-to-Mesenchymal Transition. J. Am. Heart Assoc. 2024, 13, e034316. [Google Scholar] [CrossRef]
- Oppedisano, F.; Nesci, S.; Spagnoletta, A. Mitochondrial sirtuin 3 and role of natural compounds: The effect of post-translational modifications on cellular metabolism. Crit. Rev. Biochem. Mol. Biol. 2024, 59, 199–220. [Google Scholar] [CrossRef]
- Sharma, A.; Mahur, P.; Muthukumaran, J.; Singh, A.K.; Jain, M. Shedding light on structure, function and regulation of human sirtuins: A comprehensive review. 3 Biotech 2023, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Feng, L.; Yan, Y.; Ye, H.; Tang, K.; Guo, X.; Ma, Y. SIRT3 deficiency aggravates mitochondrial metabolic disorder and podocyte injury in DKD via MPC2 acetylation. Cell. Signal. 2025, 135, 112029. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Luo, R.; Xu, Z.; Zhang, C.; Zhang, Z.; Ren, P.; He, X.; Zhang, J.; Liu, Y. Fe-flavonoid nanozyme as dual modulator of oxidative stress and autophagy for acute kidney injury repair. Theranostics 2025, 15, 8658–8674. [Google Scholar] [CrossRef] [PubMed]
- Iskander, A.; Yan, L.J. Cisplatin-Induced Kidney Toxicity: Potential Roles of Major NAD+-Dependent Enzymes and Plant-Derived Natural Products. Biomolecules 2022, 12, 1078. [Google Scholar] [CrossRef]
- Yan, L.J. Analysis of oxidative modification of proteins. Curr. Protoc. Protein Sci. 2009, 14, 14–24. [Google Scholar]
- Ye, S.; Zhang, M.; Tang, S.C.W.; Li, B.; Chen, W. PGC1-alpha in diabetic kidney disease: Unraveling renoprotection and molecular mechanisms. Mol. Biol. Rep. 2024, 51, 304. [Google Scholar] [CrossRef]
- Brooks, C.; Wei, Q.; Cho, S.G.; Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Investig. 2009, 119, 1275–1285. [Google Scholar] [CrossRef]
- Canto, C.; Auwerx, J. NAD+ as a signaling molecule modulating metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Fu, B.; Zhao, J.; Peng, W.; Wu, H.; Zhang, Y. Resveratrol rescues cadmium-induced mitochondrial injury by enhancing transcriptional regulation of PGC-1alpha and SOD2 via the Sirt3/FoxO3a pathway in TCMK-1 cells. Biochem. Biophys. Res. Commun. 2017, 486, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Liang, N.N.; Zhang, X.Y.; Ren, Y.H.; Wu, W.Z.; Liu, Z.B.; He, Y.Z.; Zhang, Y.H.; Huang, Y.C.; Zhang, T.; et al. Mitochondrial GPX4 acetylation is involved in cadmium-induced renal cell ferroptosis. Redox Biol. 2024, 73, 103179. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Ge, J.; Lv, M.W.; Talukder, M.; Guo, K.; Li, Y.H.; Li, J.L. Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy. Food Funct. 2020, 11, 1856–1868. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Lv, W.; Xue, L.; Liang, L.; Liu, D.; Li, C.; Liao, J.; Jin, Y. Endotoxin induced acute kidney injury modulates expression of AQP1, P53 and P21 in rat kidney, heart, lung and small intestine. PLoS ONE 2023, 18, e0288507. [Google Scholar] [CrossRef]
- Won, J.P.; Lee, H.G.; Yoon, H.J.; Seo, H.G. Biochanin A-mediated anti-ferroptosis is associated with reduction of septic kidney injury. Life Sci. 2024, 358, 123124. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Gu, J.; Li, T.; Chen, H.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X. Inhibition of aerobic glycolysis alleviates sepsis-induced acute kidney injury by promoting lactate/Sirtuin 3/AMPK-regulated autophagy. Int. J. Mol. Med. 2021, 47, 19. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Yang, Y.; Cheng, L.; Yang, X.; Liu, H.; Li, W.; Wan, Y.; Yang, D. Sirt3 mitigates LPS-induced mitochondrial damage in renal tubular epithelial cells by deacetylating YME1L1. Cell Prolif. 2023, 56, e13362. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Z.; Zhang, H.; Wang, L.; Sun, F.; Li, Q.; Cao, T.; Wang, B.; Ma, H.; You, M.; et al. Sodium-glucose exchanger 2 inhibitor canagliflozin promotes mitochondrial metabolism and alleviates salt-induced cardiac hypertrophy via preserving SIRT3 expression. J. Adv. Res. 2025, 70, 255–269. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, Y.; Song, J.; Wang, S.; Lu, J.; Wei, F.; Li, X. Urinary exosomes exacerbate diabetic kidney disease by promoting NLRP3 inflammasome activation via the microRNA-516b-5p/SIRT3/AMPK pathway. Am. J. Physiol. Endocrinol. Metab. 2025, 328, E911–E923. [Google Scholar] [CrossRef]
- Wu, J.; Luo, X.; Thangthaeng, N.; Sumien, N.; Chen, Z.; Rutledge, M.A.; Jing, S.; Forster, M.J.; Yan, L.J. Pancreatic mitochondrial complex I exhibits aberrant hyperactivity in diabetes. Biochem. Biophys. Rep. 2017, 11, 119–129. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Z.; Yan, L.J. Redox imbalance and mitochondrial abnormalities in the diabetic lung. Redox Biol. 2017, 11, 51–59. [Google Scholar] [CrossRef]
- Roxburgh, S.A.; Kattla, J.J.; Curran, S.P.; O’Meara, Y.M.; Pollock, C.A.; Goldschmeding, R.; Godson, C.; Martin, F.; Brazil, D.P. Allelic depletion of grem1 attenuates diabetic kidney disease. Diabetes 2009, 58, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Hanai, J.; Sugimoto, H.; Mammoto, T.; Charytan, D.; Strutz, F.; Kalluri, R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003, 9, 964–968. [Google Scholar] [CrossRef]
- Kanasaki, K.; Taduri, G.; Koya, D. Diabetic nephropathy: The role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol 2013, 4, 7. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Taduri, G.; O’Connell, J.; Teng, Y.; Cooke, V.G.; Woda, C.; Sugimoto, H.; Kalluri, R. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 2013, 19, 1047–1053. [Google Scholar] [CrossRef]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1819–1834. [Google Scholar] [CrossRef]
- Grande, M.T.; Lopez-Novoa, J.M. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat. Rev. Nephrol. 2009, 5, 319–328. [Google Scholar] [CrossRef]
- Grgic, I.; Duffield, J.S.; Humphreys, B.D. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr. Nephrol. 2012, 27, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.P.; Koya, D.; Kanasaki, K. MicroRNAs in kidney fibrosis and diabetic nephropathy: Roles on EMT and EndMT. BioMed Res. Int. 2013, 2013, 125469. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Grahovac, G.; Zeisberg, M.; Kalluri, R. Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes 2007, 56, 1825–1833. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Shi, S.; Kanasaki, M.; Nagai, T.; Kitada, M.; He, J.; Nakamura, Y.; Ishigaki, Y.; Kanasaki, K.; Koya, D. Effect of Antifibrotic MicroRNAs Crosstalk on the Action of N-acetyl-seryl-aspartyl-lysyl-proline in Diabetes-related Kidney Fibrosis. Sci. Rep. 2016, 6, 29884. [Google Scholar] [CrossRef]
- Kanasaki, K.; Shi, S.; Kanasaki, M.; He, J.; Nagai, T.; Nakamura, Y.; Ishigaki, Y.; Kitada, M.; Srivastava, S.P.; Koya, D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes 2014, 63, 2120–2131. [Google Scholar] [CrossRef] [PubMed]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.R.; Sheedy, F.J.; Gleeson, L.E.; Van den Bosch, M.W.M.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G.W.; et al. Pyruvate Kinase M2 Regulates Hif-1alpha Activity and IL-1beta Induction and Is a Critical Determinant of the Warburg Effect in LPS-Activated Macrophages. Cell Metab. 2015, 21, 65–80. [Google Scholar] [CrossRef]
- Hamabe, A.; Konno, M.; Tanuma, N.; Shima, H.; Tsunekuni, K.; Kawamoto, K.; Nishida, N.; Koseki, J.; Mimori, K.; Gotoh, N.; et al. Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 2014, 111, 15526–15531. [Google Scholar] [CrossRef]
- Liang, J.; Cao, R.; Zhang, Y.; Xia, Y.; Zheng, Y.; Li, X.; Wang, L.; Yang, Y.; Lu, Z. PKM2 dephosphorylation by Cdc25A promotes the Warburg effect and tumorigenesis. Nat. Commun. 2016, 7, 12431. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef]
- Morita, M.; Kanasaki, K. Sodium-glucose cotransporter-2 inhibitors for diabetic kidney disease: Targeting Warburg effects in proximal tubular cells. Diabetes Metab. 2020, 46, 353–361. [Google Scholar] [CrossRef]
- Zhu, M.; He, J.; Xu, Y.; Zuo, Y.; Zhou, W.; Yue, Z.; Shao, X.; Cheng, J.; Wang, T.; Mou, S. AMPK activation coupling SENP1-Sirt3 axis protects against acute kidney injury. Mol. Ther. 2023, 31, 3052–3066. [Google Scholar] [CrossRef]
- Yi, W.; Xie, X.; Du, M.; Bu, Y.; Wu, N.; Yang, H.; Tian, C.; Xu, F.; Xiang, S.; Zhang, P.; et al. Green Tea Polyphenols Ameliorate the Early Renal Damage Induced by a High-Fat Diet via Ketogenesis/SIRT3 Pathway. Oxid. Med. Cell. Longev. 2017, 2017, 9032792. [Google Scholar] [CrossRef]
- Kundu, A.; Gali, S.; Sharma, S.; Park, J.H.; Kyung, S.Y.; Kacew, S.; Kim, I.S.; Lee, K.Y.; Kim, H.S. Tenovin-1 Ameliorates Renal Fibrosis in High-Fat-Diet-Induced Diabetic Nephropathy via Antioxidant and Anti-Inflammatory Pathways. Antioxidants 2022, 11, 1812. [Google Scholar] [CrossRef]
- Wang, L.F.; Li, Q.; Le Zhao, J.; Wen, K.; Zhang, Y.T.; Zhao, Q.H.; Ding, Q.; Li, J.H.; Guan, X.H.; Xiao, Y.F.; et al. CD38 deficiency prevents diabetic nephropathy by inhibiting lipid accumulation and oxidative stress through activation of the SIRT3 pathway. Biochem. Cell Biol. 2025, 103, 1–12. [Google Scholar] [PubMed]
- Wang, X.X.; Wang, D.; Luo, Y.; Myakala, K.; Dobrinskikh, E.; Rosenberg, A.Z.; Levi, J.; Kopp, J.B.; Field, A.; Hill, A.; et al. FXR/TGR5 Dual Agonist Prevents Progression of Nephropathy in Diabetes and Obesity. J. Am. Soc. Nephrol. 2018, 29, 118–137. [Google Scholar] [CrossRef]
- Kundu, A.; Dey, P.; Sarkar, P.; Karmakar, S.; Tae, I.H.; Kim, K.S.; Park, J.H.; Lee, S.H.; Lee, B.M.; Renthlei, L.; et al. Protective effects of Croton hookeri on streptozotocin-induced diabetic nephropathy. Food Chem. Toxicol. 2020, 135, 110873. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Lee, H.E.; Kar, B.; Vishal, V.; Dash, S.; Kim, I.S.; Bhakta, T.; Kim, H.S. Molineria recurvata Ameliorates Streptozotocin-Induced Diabetic Nephropathy through Antioxidant and Anti-Inflammatory Pathways. Molecules 2022, 27, 4985. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.Y.; Wei, Z.T.; Liu, S.K.; Sun, J.L.; Mao, Y.H.; Xu, Y.D.; Yang, Y. Therapeutic effect and mechanism of combination therapy with ursolic acid and insulin on diabetic nephropathy in a type I diabetic rat model. Front. Pharmacol. 2022, 13, 969207. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Zha, H.; Zhang, Y.; Shi, L.; Wang, J.; Huang, H.; Yue, R.; Hu, B.; Zhu, J.; et al. Dapagliflozin attenuates AKI to CKD transition in diabetes by activating SIRT3/PGC1-alpha signaling and alleviating aberrant metabolic reprogramming. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167433. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Song, S.; Liu, S.; Li, Q.; Zou, W.; Ke, J.; Wang, C. (Pro)renin receptor mediates tubular epithelial cell pyroptosis in diabetic kidney disease via DPP4-JNK pathway. J. Transl. Med. 2024, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Zhang, S.; Sun, L.; Qian, M.; Xu, H. Salvianolic Acid B Inhibits Oxidative Stress in Glomerular Mesangial Cells Alleviating Diabetic Nephropathy by Regulating SIRT3/FOXO1 Signaling. Kidney Blood Press. Res. 2023, 48, 738–751. [Google Scholar] [CrossRef]
- Ogura, Y.; Kitada, M.; Xu, J.; Monno, I.; Koya, D. CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging 2020, 12, 11325–11336. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Richa, S.; Dey, P.; Kim, K.S.; Son, J.Y.; Kim, H.R.; Lee, S.Y.; Lee, B.H.; Lee, K.Y.; Kacew, S.; et al. Protective effect of EX-527 against high-fat diet-induced diabetic nephropathy in Zucker rats. Toxicol. Appl. Pharmacol. 2020, 390, 114899. [Google Scholar] [CrossRef]
- Ogura, Y.; Kitada, M.; Monno, I.; Kanasaki, K.; Watanabe, A.; Koya, D. Renal mitochondrial oxidative stress is enhanced by the reduction of Sirt3 activity, in Zucker diabetic fatty rats. Redox Rep. 2018, 23, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Singh, A.K.; Kari, J.A. Metabolic syndrome and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013, 22, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, W.; Jiang, W.; Yu, W.; Lin, Y.; Zhang, T.; Yao, J.; Zhou, L.; Zeng, Y.; Li, H.; et al. Regulation of cellular metabolism by protein lysine acetylation. Science 2010, 327, 1000–1004. [Google Scholar] [CrossRef]
- Hallows, W.C.; Yu, W.; Smith, B.C.; Devries, M.K.; Ellinger, J.J.; Someya, S.; Shortreed, M.R.; Prolla, T.; Markley, J.L.; Smith, L.M.; et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol. Cell 2011, 41, 139–149. [Google Scholar] [CrossRef]
- Locatelli, M.; Zoja, C.; Zanchi, C.; Corna, D.; Villa, S.; Bolognini, S.; Novelli, R.; Perico, L.; Remuzzi, G.; Benigni, A.; et al. Manipulating Sirtuin 3 pathway ameliorates renal damage in experimental diabetes. Sci. Rep. 2020, 10, 8418. [Google Scholar] [CrossRef]
- Fu, Y.; Xiang, Y.; Wei, Q.; Ilatovskaya, D.; Dong, Z. Rodent models of AKI and AKI-CKD transition: An update in 2024. Am. J. Physiol. Ren. Physiol. 2024, 326, F563–F583. [Google Scholar] [CrossRef]
- Smith, T.; Zaidi, A.; Brown, C.V.M.; Pino-Chavez, G.; Bowen, T.; Meran, S.; Fraser, D.; Chavez, R.; Khalid, U. Robust Rat and Mouse Models of Bilateral Renal Ischemia Reperfusion Injury. In Vivo 2024, 38, 1049–1057. [Google Scholar] [CrossRef]
- Yan, Y.; Bai, J.; Zhou, X.; Tang, J.; Jiang, C.; Tolbert, E.; Bayliss, G.; Gong, R.; Zhao, T.C.; Zhuang, S. P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. Am. J. Physiol.-Cell Physiol. 2015, 308, C463–C472. [Google Scholar] [CrossRef]
- Chang-Panesso, M.; Kadyrov, F.F.; Lalli, M.; Wu, H.; Ikeda, S.; Kefaloyianni, E.; Abdelmageed, M.M.; Herrlich, A.; Kobayashi, A.; Humphreys, B.D. FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J. Clin. Investig. 2019, 129, 5501–5517. [Google Scholar] [CrossRef]
- Ouyang, J.; Zeng, Z.; Fang, H.; Li, F.; Zhang, X.; Tan, W. SIRT3 Inactivation Promotes Acute Kidney Injury Through Elevated Acetylation of SOD2 and p53. J. Surg. Res. 2019, 233, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, X.; Jian, Y.; Liu, J.; Ke, X.; Chen, S.; Yang, D.; Yang, D. SIRT3 deficiency exacerbates early-stage fibrosis after ischaemia-reperfusion-induced AKI. Cell. Signal. 2022, 93, 110284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, J.; Li, X.; Liu, Z.; Han, Y.; Xu, X.; Li, X.; Tang, Y.; Liu, Y.; Yu, T.; et al. Sirt3 modulate renal ischemia-reperfusion injury through enhancing mitochondrial fusion and activating the ERK-OPA1 signaling pathway. J. Cell. Physiol. 2019, 234, 23495–23506. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, X.; Xu, Y.; Han, C.; Cai, X.; Wu, Y.; Geng, Y.; Liu, C. Low-protein Calorie-restriction Mitigates Diabetic Mice Kidney Injury via the Gut-Kidney Axis. Int. J. Vitam. Nutr. Res. 2025, 95, 37311. [Google Scholar] [CrossRef]
- Taufani, I.P.; Laila, S.R.; Tasminatun, S.; Simaremare, S.R.S.; Mardiana, M.; Situmorang, J.H. Calorie and time-restricted feeding improves liver and kidney histopathology in streptozotocin-induced type 1 diabetic rats. Front. Physiol. 2025, 16, 1629751. [Google Scholar] [CrossRef]
- Sohal, R.S.; Forster, M.J. Caloric restriction and the aging process: A critique. Free Radic. Biol. Med. 2014, 73, 366–382. [Google Scholar] [CrossRef] [PubMed]
- Andrianova, N.V.; Zorova, L.D.; Pevzner, I.B.; Kolosova, N.G.; Plotnikov, E.Y.; Zorov, D.B. Calorie Restriction Provides Kidney Ischemic Tolerance in Senescence-Accelerated OXYS Rats. Int. J. Mol. Sci. 2022, 23, 15224. [Google Scholar] [CrossRef] [PubMed]
- Andrianova, N.V.; Zorova, L.D.; Pevzner, I.B.; Popkov, V.A.; Chernikov, V.P.; Silachev, D.N.; Plotnikov, E.Y.; Zorov, D.B. Resemblance and differences in dietary restriction nephroprotective mechanisms in young and old rats. Aging 2020, 12, 18693–18715. [Google Scholar] [CrossRef]
- Dittenhafer-Reed, K.E.; Richards, A.L.; Fan, J.; Smallegan, M.J.; Fotuhi Siahpirani, A.; Kemmerer, Z.A.; Prolla, T.A.; Roy, S.; Coon, J.J.; Denu, J.M. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab. 2015, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.M.; Carmona, J.J.; Michan, S.; Chen, K.Y.; Manabe, Y.; Ward, J.L., 3rd; Goodyear, L.J.; Tong, Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging 2009, 1, 771–783. [Google Scholar] [CrossRef]
- Zhang, B.; Cui, S.; Bai, X.; Zhuo, L.; Sun, X.; Hong, Q.; Fu, B.; Wang, J.; Chen, X.; Cai, G. SIRT3 overexpression antagonizes high glucose accelerated cellular senescence in human diploid fibroblasts via the SIRT3-FOXO1 signaling pathway. Age 2013, 35, 2237–2253. [Google Scholar] [CrossRef]
- Yang, H.; Zuo, X.Z.; Tian, C.; He, D.L.; Yi, W.J.; Chen, Z.; Zhang, P.W.; Ding, S.B.; Ying, C.J. Green Tea Polyphenols Attenuate High-Fat Diet-Induced Renal Oxidative Stress through SIRT3-Dependent Deacetylation. Biomed. Environ. Sci. 2015, 28, 455–459. [Google Scholar]
- Sun, C.; Xiong, H.; Guo, T. beta-Nicotinamide Mononucleotide Alleviates Sepsis-associated Acute Kidney Injury by Activating NAD+/SIRT3 Signaling. Cell Biochem. Biophys. 2025, 83, 2089–2099. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, L.; Zheng, R.; Luo, X.; Yue, F.; Xiang, X.; Qiu, A.; Xie, J.; Russell, R.; Zhang, D. Butyrate attenuates high-fat diet-induced glomerulopathy through GPR43-Sirt3 pathway. Br. J. Nutr. 2025, 133, 1–10. [Google Scholar] [CrossRef]
- Sung, P.H.; Yue, Y.; Chen, Y.L.; Chiang, J.Y.; Cheng, B.C.; Yang, C.C.; Chai, H.T.; Yip, H.K. Combined dapagliflozin and roxadustat effectively protected heart and kidney against cardiorenal syndrome-induced damage in rodent through activation of cell stress-Nfr2/ARE signalings and stabilizing HIF-1alpha. Biomed. Pharmacother. 2024, 180, 117567. [Google Scholar] [CrossRef]
- Guan, Y.M.; Diao, Z.L.; Huang, H.D.; Zheng, J.F.; Zhang, Q.D.; Wang, L.Y.; Liu, W.H. Bioactive peptide apelin rescues acute kidney injury by protecting the function of renal tubular mitochondria. Amino Acids 2021, 53, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.C.; Li, Y.; Wu, D.; Zhang, X.G.; Hou, F. The protective effect of kaempferol on high glucose-stimulated renal tubular epithelial cells. BMC Nephrol. 2025, 26, 477. [Google Scholar] [CrossRef]
- Dalal, D.; Singh, L.; Singh, A. Calycosin and kidney health: A molecular perspective on its protective mechanisms. Pharmacol. Rep. 2025, 77, 658–669. [Google Scholar] [CrossRef]
- Fan, H.; Sun, M.; Zhu, J.H. S-nitrosoglutathione inhibits pyroptosis of kidney tubular epithelial cells in sepsis via the SIRT3/SOD2/mtROS signaling pathway. Ren. Fail. 2025, 47, 2472987. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Alornyo, K.K.; Adams, I.; Adjei, S.; Amoah, D.; Obeng-Kyeremeh, R. Chemoprotective Mechanism of Sodium Thiosulfate Against Cisplatin-Induced Nephrotoxicity Is via Renal Hydrogen Sulfide, Arginine/cAMP and NO/cGMP Signaling Pathways. Int. J. Mol. Sci. 2025, 26, 384. [Google Scholar] [CrossRef]
- Cao, T.; Ni, R.; Ding, W.; Ji, X.; Fan, G.C.; Zhang, Z.; Peng, T. Nicotinamide mononucleotide as a therapeutic agent to alleviate multi-organ failure in sepsis. J. Transl. Med. 2023, 21, 883. [Google Scholar] [CrossRef] [PubMed]
- Hellal, D.; El-Khalik, S.R.A.; Arakeep, H.M.; Radwan, D.A.; Abo Safia, H.S.; Farrag, E.A.E. Activation of sirtuin 3 and maintenance of mitochondrial homeostasis by artemisinin protect against diclofenac-induced kidney injury in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 5593–5609. [Google Scholar] [CrossRef]
- Hirashima, Y.; Nakano, T.; Torisu, K.; Aihara, S.; Wakisaka, M.; Kitazono, T. SGLT2 inhibition mitigates transition from acute kidney injury to chronic kidney disease by suppressing ferroptosis. Sci. Rep. 2024, 14, 20386. [Google Scholar] [CrossRef]
- Xia, K.; Jin, Z.; Qiu, Q.; Zhou, Y.; Lu, Y.; Qiu, T.; Zhou, J.; Chen, Z. Ligustilide alleviates oxidative stress during renal ischemia-reperfusion injury through maintaining Sirt3-dependent mitochondrial homeostasis. Phytomedicine 2024, 134, 155975. [Google Scholar] [CrossRef]
- Fan, Y.; Kang, S.; Shao, T.; Xu, L.; Chen, J. Activation of SIRT3 by Tanshinone IIA ameliorates renal fibrosis by suppressing the TGF-beta/TSP-1 pathway and attenuating oxidative stress. Cell. Signal. 2024, 122, 111348. [Google Scholar] [CrossRef] [PubMed]
- Ewees, M.G.E.; Mostafa-Hadeab, G.; Saber, S.; El-Meguid, E.A.A.; Sree, H.T.A.; Abdel Rahman, F.E.S.; Mahmoud, N.I. Linagliptin mitigates cisplatin-induced kidney impairment via mitophagy regulation in rats, with emphasis on SIRT-3/PGC-1alpha, PINK-1 and Parkin-2. Toxicol. Appl. Pharmacol. 2024, 491, 117048. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Li, X.; Wu, J. Sirtuin 3 is required for the dexmedetomidine-mediated alleviation of inflammation and oxidative stress in nephritis. Immun. Inflamm. Dis. 2024, 12, e1135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.M.; Li, X.L.; Zhu, Y.; Shi, R.; Wang, Z.J.; Xiao, J.P.; Wang, D.G. Diosmin ameliorates renal fibrosis through inhibition of inflammation by regulating SIRT3-mediated NF-kappaB p65 nuclear translocation. BMC Complement. Med. Ther. 2024, 24, 29. [Google Scholar] [CrossRef]
- Matouk, A.I.; Awad, E.M.; Mousa, A.A.K.; Abdelhafez, S.M.N.; Fahmy, U.A.; El-Moselhy, M.A.; Abdel-Naim, A.B.; Anter, A. Dihydromyricetin protects against gentamicin-induced nephrotoxicity via upregulation of renal SIRT3 and PAX2. Life Sci. 2024, 336, 122318. [Google Scholar] [CrossRef]
- Lotfi, B.; Bagheri, Y.; Abdollahpour, A.; Ahmadian, E.; Matin, S.; Firouzfar, A.; Zununi Vahed, S.; Khajepour, F. Protective effect of Eprosartan against ischemic acute renal injury: Acting on NF-kappaB, caspase 3, and Sirtuin 1. Int. Immunopharmacol. 2023, 115, 109690. [Google Scholar] [CrossRef]
- Ezer, M.; Ozturkler, M.; Yildiz-Dalginli, K.; Atakisi, E.; Beseren-Havadar, H.; Atakisi, O. Investigation of the molecular and cellular effects of Shilajit on 5-fluorouracil (5-FU)-induced nephrotoxicity in rats. Iran. J. Basic Med. Sci. 2025, 28, 565–574. [Google Scholar]
- Koyuncu, F.; Solmaz, F.A.; Gulle, K.; Ilhan, I.; Tepebasi, M.Y.; Ozden, E.S.; Kirdemir, P. Effect of dexpanthenol on glycerol-induced acute kidney injury by targeting the PGC-1alpha/SIRT3 pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 12299–12306. [Google Scholar]
- Myakala, K.; Wang, X.X.; Shults, N.V.; Krawczyk, E.; Jones, B.A.; Yang, X.; Rosenberg, A.Z.; Ginley, B.; Sarder, P.; Brodsky, L.; et al. NAD metabolism modulates inflammation and mitochondria function in diabetic kidney disease. J. Biol. Chem. 2023, 299, 104975. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, J.; Zhang, T.; He, L.; Ma, S.; Zuo, Q.; Zhang, G.; Wang, Y.; Guo, Y. Canagliflozin ameliorates epithelial-mesenchymal transition in high-salt diet-induced hypertensive renal injury through restoration of sirtuin 3 expression and the reduction of oxidative stress. Biochem. Biophys. Res. Commun. 2023, 653, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, L.; Jin, B.; Wu, Y.; Xu, L.; Chang, X.; Hu, L.; Wang, G.; Huang, Y.; Song, L.; et al. Metrnl Alleviates Lipid Accumulation by Modulating Mitochondrial Homeostasis in Diabetic Nephropathy. Diabetes 2023, 72, 611–626. [Google Scholar] [CrossRef]
- Sun, M.; Wang, F.; Li, H.; Li, M.; Wang, Y.; Wang, C.; Zhang, Y.; Zhang, D.; Li, J.; Yao, S. Maresin-1 Attenuates Sepsis-Associated Acute Kidney Injury via Suppressing Inflammation, Endoplasmic Reticulum Stress and Pyroptosis by Activating the AMPK/SIRT3 Pathway. J. Inflamm. Res. 2024, 17, 1349–1364. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Cheng, D.; Zhang, H.; Zhang, W.; Shen, Y.; Huang, Q.; An, X.; Wang, B.; Yu, Z.; et al. Notoginsenoside Fc ameliorates renal tubular injury and mitochondrial damage in acetaminophen-induced acute kidney injury partly by regulating SIRT3/SOD2 pathway. Front. Med. 2022, 9, 1055252. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Q.; Chen, L.; Guo, Y.; Wu, X.Q.; Zhao, T.T.; Zhao, H.L.; Zhang, H.J.; Yan, M.H.; Zhang, G.Q.; Li, P. Poricoic acid A suppresses renal fibroblast activation and interstitial fibrosis in UUO rats via upregulating Sirt3 and promoting beta-catenin K49 deacetylation. Acta Pharmacol. Sin. 2023, 44, 1038–1050. [Google Scholar] [CrossRef]
- Yang, L.; Wang, B.; Guo, F.; Huang, R.; Liang, Y.; Li, L.; Tao, S.; Yin, T.; Fu, P.; Ma, L. FFAR4 improves the senescence of tubular epithelial cells by AMPK/SirT3 signaling in acute kidney injury. Signal Transduct. Target. Ther. 2022, 7, 384. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Hu, J.; Li, H.; Guo, S.; Chen, B.; Liu, C.; Wang, G.; Zhou, F. Magnesium Isoglycyrrhizinate Reduces the Target-Binding Amount of Cisplatin to Mitochondrial DNA and Renal Injury through SIRT3. Int. J. Mol. Sci. 2022, 23, 13093. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhang, Y.; Xiong, Y.; Zhu, Z.; Wang, L.; Liu, X. Mitochondria-Targeted Antioxidant Mitoquinone Maintains Mitochondrial Homeostasis through the Sirt3-Dependent Pathway to Mitigate Oxidative Damage Caused by Renal Ischemia/Reperfusion. Oxid. Med. Cell. Longev. 2022, 2022, 2213503. [Google Scholar] [CrossRef] [PubMed]
- Barati, A.; Rahbar Saadat, Y.; Meybodi, S.M.; Nouraei, S.; Moradi, K.; Kamrani Moghaddam, F.; Malekinejad, Z.; Hosseiniyan Khatibi, S.M.; Zununi Vahed, S.; Bagheri, Y. Eplerenone reduces renal ischaemia/reperfusion injury by modulating Klotho, NF-kappaB and SIRT1/SIRT3/PGC-1alpha signalling pathways. J. Pharm. Pharmacol. 2023, 75, 819–827. [Google Scholar] [CrossRef]
- Zhou, M.; Dai, Y.; Ma, Y.; Yan, Y.; Hua, M.; Gao, Q.; Geng, X.; Zhou, Q. Protective Effects of Liquiritigenin against Cisplatin-Induced Nephrotoxicity via NRF2/SIRT3-Mediated Improvement of Mitochondrial Function. Molecules 2022, 27, 3823. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, J.; Li, Y.; Yuan, L.; Liu, F.; Yuan, Y.; Tang, X. Matrine alleviates cisplatin-induced acute kidney injury by inhibiting mitochondrial dysfunction and inflammation via SIRT3/OPA1 pathway. J. Cell. Mol. Med. 2022, 26, 3702–3715. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lv, X.; Zhang, Y.; Han, Y.; Li, J.; Zeng, J.; Tang, D.; Meng, J.; Yuan, X.; Peng, Z.; et al. Fluorofenidone Inhibits UUO/IRI-Induced Renal Fibrosis by Reducing Mitochondrial Damage. Oxid. Med. Cell. Longev. 2022, 2022, 2453617. [Google Scholar] [CrossRef]
- Mao, R.W.; He, S.P.; Lan, J.G.; Zhu, W.Z. Honokiol ameliorates cisplatin-induced acute kidney injury via inhibition of mitochondrial fission. Br. J. Pharmacol. 2022, 179, 3886–3904. [Google Scholar] [CrossRef]
- Wongmekiat, O.; Lailerd, N.; Kobroob, A.; Peerapanyasut, W. Protective Effects of Purple Rice Husk against Diabetic Nephropathy by Modulating PGC-1alpha/SIRT3/SOD2 Signaling and Maintaining Mitochondrial Redox Equilibrium in Rats. Biomolecules 2021, 11, 1224. [Google Scholar] [CrossRef]
- Zhang, C.; Suo, M.; Liu, L.; Qi, Y.; Zhang, C.; Xie, L.; Zheng, X.; Ma, C.; Li, J.; Yang, J.; et al. Melatonin Alleviates Contrast-Induced Acute Kidney Injury by Activation of Sirt3. Oxid. Med. Cell. Longev. 2021, 2021, 6668887. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, L.; Wu, J.; An, S.; Fang, H.; Han, Y.; Huang, Q.; Chen, Z.; Zeng, Z. Melatonin Attenuates Sepsis-Induced Small-Intestine Injury by Upregulating SIRT3-Mediated Oxidative-Stress Inhibition, Mitochondrial Protection, and Autophagy Induction. Front. Immunol. 2021, 12, 625627. [Google Scholar] [CrossRef]
- Kobroob, A.; Kongkaew, A.; Wongmekiat, O. Melatonin Reduces Aggravation of Renal Ischemia-Reperfusion Injury in Obese Rats by Maintaining Mitochondrial Homeostasis and Integrity through AMPK/PGC-1alpha/SIRT3/SOD2 Activation. Curr. Issues Mol. Biol. 2023, 45, 8239–8254. [Google Scholar] [CrossRef]
- Kobroob, A.; Peerapanyasut, W.; Kumfu, S.; Chattipakorn, N.; Wongmekiat, O. Effectiveness of N-Acetylcysteine in the Treatment of Renal Deterioration Caused by Long-Term Exposure to Bisphenol A. Biomolecules 2021, 11, 655. [Google Scholar] [CrossRef]
- Son, S.H.; Lee, S.M.; Lee, M.H.; Son, Y.K.; Kim, S.E.; Won, S.A. Omega-3 Fatty Acids Upregulate SIRT1/3, Activate PGC-1alpha via Deacetylation, and Induce Nrf1 Production in 5/6 Nephrectomy Rat Model. Mar. Drugs 2021, 19, 182. [Google Scholar] [CrossRef]
- Wu, X.; Liu, M.; Wei, G.; Guan, Y.; Duan, J.; Xi, M.; Wang, J. Renal protection of rhein against 5/6 nephrectomied-induced chronic kidney disease: Role of SIRT3-FOXO3alpha signalling pathway. J. Pharm. Pharmacol. 2020, 72, 699–708. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Takagi, S.; Nitta, K.; Kitada, M.; Srivastava, S.P.; Takagaki, Y.; Kanasaki, K.; Koya, D. Renal protective effects of empagliflozin via inhibition of EMT and aberrant glycolysis in proximal tubules. JCI Insight 2020, 5, e129034. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Zhang, B.; Luo, D.; Huang, S.; Wang, F.; Zheng, L.; Lu, J.; Chen, J.; Li, S. Jian-Pi-Yi-Shen Formula Alleviates Chronic Kidney Disease in Two Rat Models by Modulating QPRT/NAD+/SIRT3/Mitochondrial Dynamics Pathway. Evid.-Based Complement. Altern. Nat. Med. 2021, 2021, 6625345. [Google Scholar] [CrossRef]
- Liu, H.; Diep, T.N.; Yan, L.-J. Mitigating Oxidative Stress and Inflammation: The Protective Role of β-lapachone in Kidney Disease. In Inflammation: From Chemical Mediators and Pathophysiology to Practice and Treatment; Wang, Y.-X., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2025; pp. 1–18. [Google Scholar]
- Oh, G.S.; Kim, H.J.; Choi, J.H.; Shen, A.; Choe, S.K.; Karna, A.; Lee, S.H.; Jo, H.J.; Yang, S.H.; Kwak, T.H.; et al. Pharmacological activation of NQO1 increases NAD+ levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 2014, 85, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Lee, A.S.; Liu, S.H.; Chang, K.C.; Shen, M.Y.; Chang, C.T. Spironolactone ameliorates endothelial dysfunction through inhibition of the AGE/RAGE axis in a chronic renal failure rat model. BMC Nephrol. 2019, 20, 351. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, Q.; Yuan, Y.; Zhang, C.; Wu, L.; Liu, X.; Cao, W.; Guo, H.; Duan, S.; Xu, X.; et al. Renalase attenuates mitochondrial fission in cisplatin-induced acute kidney injury via modulating sirtuin-3. Life Sci. 2019, 222, 78–87. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Xiao, L.; Liu, G.; Sun, L.; He, L. STC-1 ameliorates renal injury in diabetic nephropathy by inhibiting the expression of BNIP3 through the AMPK/SIRT3 pathway. Lab. Investig. 2019, 99, 684–697. [Google Scholar] [CrossRef]
- Liu, S.M.; Zhang, Y.R.; Chen, Y.; Ji, D.R.; Zhao, J.; Fu, S.; Jia, M.Z.; Yu, Y.R.; Tang, C.S.; Huang, W.; et al. Intermedin Alleviates Vascular Calcification in CKD through Sirtuin 3-Mediated Inhibition of Mitochondrial Oxidative Stress. Pharmaceuticals 2022, 15, 1224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Wang, Y.; Zhao, K.; Chi, Y.; Wang, B. Pyrroloquinoline quinine protects HK-2 cells against high glucose-induced oxidative stress and apoptosis through Sirt3 and PI3K/Akt/FoxO3a signaling pathway. Biochem. Biophys. Res. Commun. 2019, 508, 398–404. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ding, W.; Wang, Y. Mito-TEMPO Alleviates Renal Fibrosis by Reducing Inflammation, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress. Oxid. Med. Cell. Longev. 2018, 2018, 5828120. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, L.; Pignieri, A.; Fiasche, M.; Giudici, M.; Crestani, M.; Mitro, N.; Abbate, M.; Zoja, C.; Rottoli, D.; Foray, C.; et al. Fenofibrate attenuates cardiac and renal alterations in young salt-loaded spontaneously hypertensive stroke-prone rats through mitochondrial protection. J. Hypertens. 2018, 36, 1129–1146. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Xu, L.; Tao, X.; Yin, L.; Qi, Y.; Xu, Y.; Han, X.; Tang, Z.; Ma, X.; Liu, K.; et al. Protective effects of dioscin against fructose-induced renal damage via adjusting Sirt3-mediated oxidative stress, fibrosis, lipid metabolism and inflammation. Toxicol. Lett. 2018, 284, 37–45. [Google Scholar] [CrossRef]
- Ma, K.; Bai, Y.; Li, J.; Ren, Z.; Li, J.; Zhang, J.; Shan, A. Lactobacillus rhamnosus GG ameliorates deoxynivalenol-induced kidney oxidative damage and mitochondrial injury in weaned piglets. Food Funct. 2022, 13, 3905–3916. [Google Scholar] [CrossRef]
- Suliman, H.; Ma, Q.; Zhang, Z.; Ren, J.; Morris, B.T.; Crowley, S.D.; Ulloa, L.; Privratsky, J.R. Annexin A1 Tripeptide Mimetic Increases Sirtuin-3 and Augments Mitochondrial Function to Limit Ischemic Kidney Injury. Front. Physiol. 2021, 12, 683098. [Google Scholar] [CrossRef]
- Zha, Z.; Song, W.; Wang, R.; Zhang, X.; Liu, X.; Wang, L. Therapeutic Mechanisms of Jinshuiqing in IgA Nephropathy: A Transcriptomic Analysis. Med. Sci. Monit. Basic Res. 2025, 31, e950343. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Shao, X.; Wang, H.; Liu, X.; Ke, X.; Xiong, C.; Wei, L.; Zou, H. tert-Butylhydroquinone Treatment Alleviates Contrast-Induced Nephropathy in Rats by Activating the Nrf2/Sirt3/SOD2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 4657651. [Google Scholar] [CrossRef]
- Ugur, S.; Ulu, R.; Dogukan, A.; Gurel, A.; Yigit, I.P.; Gozel, N.; Aygen, B.; Ilhan, N. The renoprotective effect of curcumin in cisplatin-induced nephrotoxicity. Ren. Fail. 2015, 37, 332–336. [Google Scholar] [CrossRef]
- Ortega-Dominguez, B.; Aparicio-Trejo, O.E.; Garcia-Arroyo, F.E.; Leon-Contreras, J.C.; Tapia, E.; Molina-Jijon, E.; Hernandez-Pando, R.; Sanchez-Lozada, L.G.; Barrera-Oviedo, D.; Pedraza-Chaverri, J. Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem. Toxicol. 2017, 107 Pt A, 373–385. [Google Scholar] [CrossRef]
- Bal, N.B.; Sadi, G.; Bostanci, A.; Kiremitci, S.; Adanir, I.; Uludag, M.O.; Demirel-Yilmaz, E. Effect of regular exercise and resveratrol on hypertension-induced cellular stress response and senescence in renal and vascular tissues of rats. J. Cardiovasc. Pharmacol. 2025, 10, 1097. [Google Scholar] [CrossRef]
- Shiraishi, T.; Tamura, Y.; Taniguchi, K.; Higaki, M.; Ueda, S.; Shima, T.; Nagura, M.; Nakagawa, T.; Johnson, R.J.; Uchida, S. Combination of ACE inhibitor with nicorandil provides further protection in chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2014, 307, F1313–F1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xuan, A.; Zheng, L.; Li, D.; Chen, C.; Liu, H.; Lu, G.; Cheng, Z.; Zou, Y.; Zhi, S.; et al. Novel coumarin derivative SZC-6 as an allosteric activator of SIRT3 alleviates diabetic kidney disease via the SIRT3-Foxo3a signaling axis. Free Radic. Biol. Med. 2025, 240, 29–45. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Zhang, Y.; Ke, Q.; Su, W.; Zhou, Y.; Jiang, L.; Dai, C.; Wen, P. SIRT3 regulates CPT1a acetylation and fatty acid oxidation in renal tubular epithelial cells under diabetic condition. Mol. Biol. Rep. 2025, 52, 603. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Chang, J.; Liu, Y.; Yin, J.; Zeng, X. Apelin/APJ alleviates diabetic nephropathy by improving glomerular endothelial cells dysfunction via SIRT3-KLF15. Mol. Med. Rep. 2025, 31, 122. [Google Scholar] [CrossRef]
- Deng, Z.; He, M.; Hu, H.; Zhang, W.; Zhang, Y.; Ge, Y.; Ma, T.; Wu, J.; Li, L.; Sun, M.; et al. Melatonin attenuates sepsis-induced acute kidney injury by promoting mitophagy through SIRT3-mediated TFAM deacetylation. Autophagy 2024, 20, 151–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, P.; Luo, J.; Ding, H.; Cao, H.; He, W.; Zen, K.; Zhou, Y.; Yang, J.; Jiang, L. Sirtuin 3 regulates mitochondrial protein acetylation and metabolism in tubular epithelial cells during renal fibrosis. Cell Death Dis. 2021, 12, 847. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhu, L.; Li, L.; Liu, J.; Chen, Y.; Cheng, J.; Peng, T.; Lu, Y. S-Sulfhydration of SIRT3 by Hydrogen Sulfide Attenuates Mitochondrial Dysfunction in Cisplatin-Induced Acute Kidney Injury. Antioxid. Redox Signal. 2019, 31, 1302–1319. [Google Scholar] [CrossRef]
- Lin, J.R.; Zheng, Y.J.; Zhang, Z.B.; Shen, W.L.; Li, X.D.; Wei, T.; Ruan, C.C.; Chen, X.H.; Zhu, D.L.; Gao, P.J. Suppression of Endothelial-to-Mesenchymal Transition by SIRT (Sirtuin) 3 Alleviated the Development of Hypertensive Renal Injury. Hypertension 2018, 72, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jiang, S.; Gao, S.; Wang, Y.; Cai, X.; Fang, J.; Yan, T.; Craig Wan, C.; Cai, Y. Sirtuins: Research advances on the therapeutic role in acute kidney injury. Phytomedicine 2022, 101, 154122. [Google Scholar] [CrossRef]
- Lambona, C.; Zwergel, C.; Valente, S.; Mai, A. SIRT3 Activation a Promise in Drug Development? New Insights into SIRT3 Biology and Its Implications on the Drug Discovery Process. J. Med. Chem. 2024, 67, 1662–1689. [Google Scholar] [CrossRef]
- Aventaggiato, M.; Arcangeli, T.; Vernucci, E.; Barreca, F.; Sansone, L.; Pellegrini, L.; Pontemezzo, E.; Valente, S.; Fioravanti, E.; Russo, M.A.; et al. Pharmacological Activation of SIRT3 Modulates the Response of Cancer Cells to Acidic pH. Pharmaceuticals 2024, 17, 810. [Google Scholar] [CrossRef]
- Dai, H.; Sinclair, D.A.; Ellis, J.L.; Steegborn, C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol. Ther. 2018, 188, 140–154. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Zhou, L. Natural products for kidney disease treatment: Focus on targeting mitochondrial dysfunction. Front. Pharmacol. 2023, 14, 1142001. [Google Scholar] [CrossRef]
- Oka, S.I.; Titus, A.S.; Zablocki, D.; Sadoshima, J. Molecular properties and regulation of NAD+ kinase (NADK). Redox Biol. 2023, 59, 102561. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. NADH/NAD+ Redox Imbalance and Diabetic Kidney Disease. Biomolecules 2021, 11, 730. [Google Scholar] [CrossRef]
- Rabelink, T.J.; de Vries, A.P.J. CD38—A new target in renal immune disease. Nat. Rev. Nephrol. 2024, 20, 641–642. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, R.; Li, W.; Ren, M.; Thangthaeng, N.; Sumien, N.; Liu, R.; Yang, S.; Simpkins, J.W.; Forster, M.J.; et al. Administration of 5-methoxyindole-2-carboxylic acid that potentially targets mitochondrial dihydrolipoamide dehydrogenase confers cerebral preconditioning against ischemic stroke injury. Free Radic. Biol. Med. 2017, 113, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Wang, Y. Roles of Dihydrolipoamide Dehydrogenase in Health and Disease. Antioxid. Redox Signal. 2023, 39, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lu, G.; Gao, C.; Wang, Y.; Sun, H. Tissue-specific and nutrient regulation of the branched-chain alpha-keto acid dehydrogenase phosphatase, protein phosphatase 2Cm (PP2Cm). J. Biol. Chem. 2012, 287, 23397–23406. [Google Scholar] [CrossRef]
- Szabo, E.; Nagy, B.; Czajlik, A.; Komlodi, T.; Ozohanics, O.; Tretter, L.; Ambrus, A. Mitochondrial Alpha-Keto Acid Dehydrogenase Complexes: Recent Developments on Structure and Function in Health and Disease. Subcell. Biochem. 2024, 104, 295–381. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).