Abstract
Sayeok-tang (SYT) is a traditional herbal formula comprising three medicinal herbs: Glycyrrhiza uralensis, Zingiber officinale, and Aconitum carmichaeli. Several studies have employed liquid chromatography-mass spectrometry (LC-MS) to qualitatively analyze the components and metabolites of SYT in vitro and in vivo; however, studies on quantitative analysis of SYT, which is important for quality control, are absent or limited to only a few components. In this study, ultrahigh-performance liquid chromatography coupled with quadrupole (UPLC-Q)-Orbitrap-MS was used to screen the phytochemicals of SYT, revealing a total of 42 compounds. Among them, 24 compounds were simultaneously quantified within 20 min via UPLC-TQ-MS/MS in the multiple reaction monitoring mode. The developed analytical method was validated for its linearity (r2 ≥ 0.9992), precision (0.36–2.96%), accuracy (−6.52–4.64%), and recovery (94.39–119.07%) for all analytes, exhibiting acceptable results. The validated method was applied in the analysis of SYT extracts, and the 24 compounds were quantified in the range of 0.004–6.882 mg/g (CV ≤ 3.746%). Among them, liquiritin apioside (6.870–6.933 mg/g), glycyrrhizic acid (5.418–5.540 mg/g), and liquiritin (1.303–1.331 mg/g) from G. uralensis were identified as the relatively abundant compounds. The presented validated analytical method is highly promising for the comprehensive quality control of SYT, offering fast, highly sensitive, and reliable analysis.
1. Introduction
Sayeok-tang (SYT), known as Shigyaku-to in Japan and Sini-tang in China, is a traditional herbal formula of Shang Han Lun, comprising three medicinal herbs: Glycyrrhiza uralensis, Zingiber officinale, and Aconitum carmichaeli [1]. Previous studies have shown that SYT is effective in treating cardiovascular diseases, including the improvement of early ventricular remodeling and cardiac function in heart failure following myocardial infarction [2,3,4,5]. Clinical studies on the therapeutic effects of SYT on ischemia/reperfusion injury in patients with acute myocardial infarction and on angina pectoris in coronary artery disease have also been reported [6,7]. SYT has also been applied to improve lung injury caused by sepsis through various mechanisms. SYT ameliorates the symptoms and pathology associated with sepsis, such as pulmonary histopathological lesions in cecal ligation and puncture mice models by modulating gut microbiota [8] and improves sepsis-induced acute lung injury by regulating the ACE2-Ang (1–7)-Mas axis and inhibiting the mitogen-activated protein kinase signaling pathway [9]. Additionally, SYT has been shown to possess anti-inflammatory and antioxidant properties that attenuate acute lung injury induced by E. coli in mice [10]. A previous study predicted the association between SYT and ulcerative colitis (UC) through network pharmacology analysis and revealed the pharmacological effects of SYT on UC using rats with UC [11]. Although the various experimental and clinical efficacies of SYT are known, few studies report analytical methods for quality control of SYT.
The quality of herbal medicines contained in herbal formulas varies depending on various environmental factors; therefore, quality control is important to ensure their safety and efficacy. In recent years, ultrahigh-performance liquid chromatography coupled with high-resolution mass spectrometry (UPLC-HRMS) has become a powerful tool for chemical profiling of natural products [12]. In particular, UPLC coupled with quadrupole Orbitrap mass spectrometry (UPLC-Q-Orbitrap-MS) has been widely used to screen and identify phytochemicals in complex herbal samples owing to its excellent analytical sensitivity and specificity compared to other techniques, being ideal for identifying compounds by obtaining accurate molecular mass and multistage MSn fragment ions of analytes [13,14,15]. Currently, UPLC coupled with triple quadrupole mass spectrometry (UPLC-TQ-MS/MS) has become a promising tool for simultaneous analysis of multiple target compounds in complex mixtures at low concentrations due to its high sensitivity and fast resolution [16,17]. The multiple reaction monitoring (MRM) mode of TQ-MS/MS is a rapid and highly sensitive analytical method that can selectively identify and quantify target compounds in complex mixtures by rapidly screening the transitions from specific precursor ions to product ions [17,18]. In addition, it is frequently applied to quantitative analysis in various research fields because it provides very low detection and quantitation limits without considering peak overlap interference [19,20,21]. Even though several studies have reported the qualitative analysis of the components and metabolites of SYT in vitro and in vivo using liquid chromatography-mass spectrometry (LC-MS), studies on quantitative analysis of SYT, which is important for quality control, are absent or limited to only a few components [22,23,24,25].
Therefore, in this study, a UPLC-Q-Orbitrap-MS method was applied to screen and characterize 42 phytochemicals of SYT by comparing retention times and MS information with reference standards. In addition, simultaneous quantification of 24 phytochemicals in SYT was performed using a validated UPLC-TQ-MS/MS method in the MRM mode, enabling rapid, sensitive, and high-throughput analysis. This study offers an efficient and reliable analytical method being a valuable tool for the comprehensive quality control of SYT.
2. Results and Discussion
2.1. Qualitative Analysis of SYT
SYT extracts were analyzed via UPLC-Q-Orbitrap-MS to identify the phytochemicals attributed to the three herbal medicines: G. uralensis, Z. officinale, and A. carmichaeli [26]. The different compounds were separated within 20 min using an Acquity BEH C18 column (100 × 2.1 mm, 1.7 µm, Waters, Milford, MA, USA) with gradient elution of 0.1% (v/v) aqueous formic acid and acetonitrile. Both the positive and negative ESI modes were used to acquire MS spectra. A total of 42 compounds, including vicenin-2, schaftoside, daidzin, neoliquiritin, liquiritin apioside, liquiritin, ferulic acid, genistin, isoliquiritin apioside, isoliquiritin, ononin, licochalcone B, liquiritigenin, licochalcone A, genistein, naringenin, echinatin, isoliquiritigenin, formononetin, glycyrrhizic acid, glabridin, and glycyrrhetinic acid from G. uralensis [27], 6-gingerol, 8-gingerol, 6-shogaol, diacetoxy-6-gingerdiol, 10-gingerol, and 8-shogaol from Z. officinale [28], and karacolidine, mesaconine, senbusine A, karacoline, aconine, napellonine, hypaconine, fuziline, bullatine B, talatisamine, benzoylmesaconine, benzoylaconine, benzoylhypacoitine, and hypaconitine from A. carmichaeli [29,30,31], were identified by comparing their retention times, precursor ions, and MS/MS fragments to those of reference standards. The characteristics of all the identified compounds in SYT based on MS data are summarized in Table 1. Alkaloids from A. carmichaeli and phenols from Z. officinale were clearly detected in the positive ion mode, whereas the compounds from G. uralensis were ionized in similar proportions in the positive and negative ion modes. The LC chromatogram at 250 nm and base peak chromatograms in the positive and negative ion modes of SYT extracts are presented in Figure 1.

Table 1.
Phytochemicals identified in SYT via UPLC-Q-Orbitrap-MS analysis.
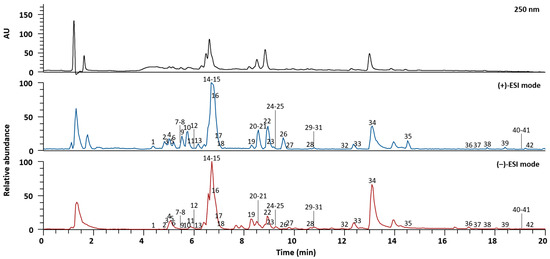
Figure 1.
LC chromatogram and base peak chromatograms in the positive and negative ion modes of SYT extracts confirmed by UPLC-Q-Orbitrap-MS. Information on each compound corresponding to each number is presented in Table 1.
2.2. Quantitative Analysis
To quantify the 24 phytochemicals identified in the SYT extracts, UPLC-TQ-MS/MS analysis was performed in dynamic MRM mode optimized for each analyte, and all analytes were detected within 20 min under 0.1% (v/v) aqueous formic acid-acetonitrile gradient conditions. The MRM mode of TQ-MS/MS is an ideal method for selectively identifying and quantifying compounds in complex mixtures by rapidly screening for transitions from specific precursor ions to product ions [18]. The optimized MRM parameters for each of the 24 compounds and internal standards (IS), including ionization mode, MRM transitions, and collision energy, are summarized in Table 2. The retention times, precursor ions, and product ions of each analyte were compared to those of reference standards. Most analytes were detected in the positive ion mode, while five analytes, liquiritin apioside, liquiritin, isoliquiritin apioside, isoliquiritin, and glycyrrhizic acid, were more suitably ionized in the negative ion mode. The MRM chromatograms of the analytes in the positive or negative ion modes are shown in Figure 2.

Table 2.
Optimized MRM parameters for the 24 compounds in SYT extracts.
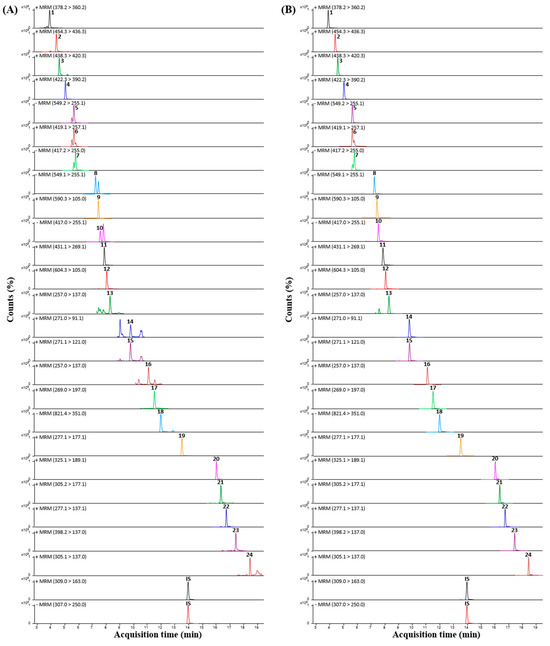
Figure 2.
Multiple reaction monitoring (MRM) chromatograms of the 24 compounds in the (A) SYT extracts and (B) standard mixture.
The MS fragmentation patterns from the precursor ions to the dominant product ions were confirmed through UPLC-TQ-MS/MS analysis in the dynamic MRM mode. The six Aconitum alkaloids, karacoline, fuziline, bullatine B, talatisamine, benzoylmesaconine, and benzoylaconine, exhibited protonated molecular ions [M + H]+ at m/z 378.2, 454.3, 438.3, 422.3, 590.3, and 604.3, respectively. Karacoline, fuziline, and bullatine B lost a water molecule (18 Da) from their precursor ions to form [M + H − H2O]+ ions at m/z 360.2, 436.3, and 420.3, respectively [30,31,32]. Talatisamine generated a fragment ion [M + H − CH3OH]+ at m/z 390.2 by losing a methanol molecule (32 Da) from the precursor ion. Benzoylmesaconine and benzoylaconine generated a product ion at m/z 105.0, corresponding to the benzoyl group [33]. Among the 13 constituents of G. uralensis, five compounds, liquiritin apioside, liquiritin, isoliquiritin apioside, isoliquiritin, and glycyrrhizic acid, exhibited [M − H]− ions at m/z 549.2, 417.2, 549.1, 417.0, and 821.4, respectively. Liquiritin and isoliquiritin generated [M − H − Glc]− ions at m/z 255.0 and 255.1, respectively, which resulted from the loss of glucose (162 Da). In the case of liquiritin apioside and isoliquiritin apioside, a fragment ion [M − H − Api − Glc]− was produced at m/z 255.1 by losing an apiosyl glucoside from the precursor ion. Glycyrrhizic acid produced a fragment ion [2GluA − H]− at m/z 351.0, indicating the loss of two glucuronic acids [34]. In the positive ion mode, protonated molecular ions [M + H]+ of the remaining eight compounds from G. uralensis were observed. For neoliquiritin and ononin, the precursor ions at m/z 419.1 and 431.1 eliminated a glucose molecule (162 Da) to generate fragment ions [M + H − Glc]+ at m/z 257.1 and 269.1, respectively. Liquiritigenin and isoliquiritigenin exhibited [M + H]+ ions at m/z 257.0 and had the same fragment ions [M + H − C8H8O]+ at m/z 137.0 [35,36]. The precursor ion [M + H]+ of formononetin observed at m/z 269.0 subsequently underwent several fragmentations, including loss of CH4 (16 Da) and 2CO (56 Da), to generate a specific fragment ion [M + H − C3H4O2]+ at m/z 197.0 [37]. The fragment ions of echinatin at m/z 121.0 and genistein at m/z 91.1 were generated from the precursor ions [M + H]+ at m/z 271.1 and 271.0, respectively [38,39]. Regarding glabridin, a characteristic fragment ion [M + H − C8H8O2]+ was identified at m/z 189, generated by a Retro-Diels-Alder reaction from the precursor ion at m/z 325.1 [M + H]+ [40,41]. The precursor ions of 6-gingerol and 8-gingerol in the form [M + H − H2O]+ were identified at m/z 277.1 and 305.2, respectively, while the [M + H − H2O − C6H12O]+ and [M + H − H2O − C8H16O]+ fragment ions were generated at m/z 177.1, respectively, by the loss of the neutral alkyl moiety and rearrangement [42]. Diacetoxy-6-gingerdiol exhibited an m/z 398.2 [M + NH4]+ and fragment ion at m/z 137.0. Regarding 6-shogaol and 8-shogaol, the precursor ions [M + H]+ were observed at m/z 277.1 and 305.1, respectively, and the fragment ions [M + H − C9H16O]+ and [M + H − C11H20O]+ were produced at m/z 137.1 and 137.0, respectively [28].
2.3. Method Validation for Quantitative Analysis
The linearity, limits of detection (LOD) and quantification (LOQ), precision, accuracy, and recovery were evaluated to validate the developed analytical method. The calibration curves for each analyte were linear over a wide concentration range and observed appropriate results without weighting compared to using weighting factors such as 1/x, 1/x2, 1/y, or 1/y2. The correlation coefficients are within the acceptable limits (r2 ≥ 0.9992). The LODs and LOQs of the 24 analytes ranged from 0.007–5.165 ng/mL and 0.020–15.651 ng/mL, respectively. The linear ranges, regression equations, correlation coefficient values, LODs, and LOQs of the 24 compounds are listed in Table 3. Precision was expressed as the coefficient of variation (CV) (%) of the observed concentration values for six replicates of the reference standards at three concentration levels (low, medium, and high). The intra- and inter-day precisions of the 24 compounds were less than 2.54% and 2.96%, respectively, and the accuracies, expressed as the relative error (RE) (%), ranged from −6.52 to 4.37% and −5.41 to 4.64%, respectively (Table 4). Recovery tests were performed by adding the standard solutions of the 24 compounds at three different concentrations (low, medium, and high) to the original sample of known concentration (Table 5). The recovery (%) of all analytes ranged from 94.39 to 119.07% (CV ≤ 4.75%). These verified results demonstrate that the established UPLC-TQ-MS/MS method exhibits acceptable linearity, sensitivity, precision, accuracy, and recovery and is suitable for the quantitative analysis of 24 phytochemicals in SYT.

Table 3.
Regression equations, linear ranges, correlation coefficients, LODs, and LOQs of the 24 compounds present in SYT.

Table 4.
Precision and accuracy data for the 24 compounds in SYT.

Table 5.
Recovery data for the 24 compounds in SYT.
2.4. Quantification of 24 Phytochemicals in SYT
The validated UPLC-TQ-MS/MS method in MRM mode was subsequently applied to the quantitative analysis of 24 phytochemicals in three batches of SYT samples. The contents of the 24 compounds were measured in the range of 0.004 to 6.882 mg/g (CV ≤ 3.746%) based on the calibration curve, and the average contents of each batch for all analytes are presented in Table 6. Among these compounds, liquiritin apioside (6.870–6.933 mg/g), glycyrrhizic acid (5.418–5.540 mg/g), and liquiritin (1.303–1.331 mg/g) from G. uralensis were relatively abundant in all three batches of SYT samples.

Table 6.
Contents of the 24 compounds in SYT extracts.
Several researchers have reported in previous studies that the contents of the components in the three herbal medicines of SYT vary depending on seasonal and geographical factors [43,44,45,46,47]. The content and composition of SYT ingredients may be influenced by environmental changes, geographical location, soil conditions, and harvest time. These influence factors can affect the overall quality and efficacy of herbal medicines [48,49]. Although we have developed and validated fast and sensitive UPLC-MS-based methods for the quality control in SYT, the evaluation of its phytochemical diversity and complexity considering various influence factors were not included in this study. In this regard, further studies are required to investigate various seasonality or to compare with other blends coming from geographical locations with different characteristics. Therefore, our precise and sensitive analytical methods can provide sufficiently valuable and helpful information for investigating various subsequent studies of SYT quality control.
3. Materials and Methods
3.1. Materials and Reagents
The three herbal medicines included in SYT, Glycyrrhiza uralensis, Zingiber officinale, and Aconitum carmichaeli, were purchased from the herbal medicine market Kwangmyungdang Pharmaceutical (Ulsan, Republic of Korea), and the voucher specimens were deposited at the KM Convergence Research Division of the Korea Institute of Oriental Medicine (Daejeon, Republic of Korea). The 42 reference standards (purity ≥ 95%) used in the qualitative analysis of SYT were purchased from TargetMol (Boston, MA, USA). The 24 reference standards (purity ≥ 98%), karacoline, fuziline, bullatine B, talatisamine, liquiritin apioside, neoliquiritin, liquiritin, isoliquiritin apioside, benzoylmesaconine, isoliquiritin, ononin, benzoylaconine, liquiritigenin, echinatin, genistein, isoliquiritigenin, formononetin, glycyrrhizic acid, 6-gingerol, glabridin, 8-gingerol, 6-shogaol, diacetoxy-6-gingerdiol, and 8-shogaol were purchased from ChemFaces Biochemical (Wuhan, China) and used for quantitative analysis. Warfarin was used as IS and was obtained from Sigma-Aldrich (St. Louis, MO, USA). Methanol, water, acetonitrile, and formic acid (LC-MS grade) were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
3.2. Preparation of Standard Solutions
The 24 reference standards and warfarin (IS) were each prepared at a concentration of 1.0 mg/mL in methanol. These stock solutions were then further diluted with methanol to obtain a series of standard solutions for the calibration curves and method validation. The concentration of IS was consistently fixed at 5.0 ng/mL in all standard solutions.
3.3. Extraction of SYT
SYT (228 g), containing a mixture of the three herbal medicines Glycyrrhiza uralensis, Zingiber officinale, and Aconitum carmichaeli in a ratio of 1:1.5:0.75, was extracted via refluxing with distilled water at 100 °C for 3 h. The extract solution was filtered, concentrated using a rotary evaporator system under vacuum, and freeze-dried to obtain a powdered extract (57.72 g, 25.32%). The powdered SYT extract was dissolved in methanol at a concentration of 50 μg/mL, filtered through a syringe filter (0.2 μm pore size), and used as a sample solution for analysis.
3.4. UPLC-Q-Orbitrap-MS Conditions
Qualitative analysis of SYT was performed using a Dionex UltiMate 3000 system connected to a Thermo Q-Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with an electrospray ionization (ESI) source according to the previously reported methods [50]. The phytochemicals in SYT were identified by gradient elution of 0.1% (v/v) aqueous formic acid and acetonitrile on an Acquity BEH C18 column (100 × 2.1 mm, 1.7 µm, Waters, Milford, MA, USA) maintained at 40 °C. MS analysis was conducted with an ESI source in both the positive and negative modes and MS spectra were acquired at a normalized collision energy of 25 eV in full MS-ddMS2 mode over a scan range of 100–1500 m/z. The source parameters were set as follows: ion spray voltage, 3.8 kV; capillary temperature, 320 °C; sheath gas pressure, 40 arbitrary units (au); auxiliary gas pressure, 10 au; Slens RF level, 60; and resolution, 70,000 (full MS) and 17,500 (ddMS2). All data were processed using Thermo Xcalibur v.3.0 and Tracefinder v.3.2 (Thermo Fisher Scientific, Bremen, Germany).
3.5. UPLC-TQ-MS/MS Conditions
Quantitative analysis of the 24 compounds in SYT was performed with an Agilent 1290 Infinity II UPLC system equipped with a 6495C triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) with a jet-stream ESI source. The 24 compounds were separated on an Acquity BEH C18 column (100 × 2.1 mm, 1.7 µm, Waters, Milford, MA, USA) maintained at 40 °C by gradient elution of 0.1% (v/v) aqueous formic acid (A) and acetonitrile (B) using the following method: 3% B for 0–1 min, 3–15% B for 1–2 min, 15–50% B for 2–13 min, 50–100% B for 13–20 min, and 100% B for 20–23 min at a flow rate of 0.25 mL/min. The mass spectrometer was operated in the dynamic MRM mode, and the MRM data were collected in the positive or negative ion mode depending on the optimal ionization conditions for each compound. The ESI source conditions involved a drying gas temperature of 130 °C, drying gas flow of 11 L/min, nebulizer pressure of 25 psi, sheath gas temperature of 400 °C, sheath gas flow of 12 L/min, capillary voltage of 3500 V (positive) and 3000 V (negative), and nozzle voltage of 500 V (positive) and 1500 V (negative). Agilent MassHunter Workstation v.10.1 software (Agilent Technologies, Santa Clara, CA, USA) was used for all data acquisition and processing.
3.6. Validation of the UPLC-TQ-MS/MS Method
Calibration curves of the 24 reference standards were established from the peak areas of standard solutions at nine different concentration levels, and the linear relationships between the peak area (y) and corresponding concentration (x, ng/mL) of each standard were expressed via the regression equation (y = ax + b). Standard solutions were measured five times repeatedly to obtain the calibration curves. The LOD and LOQ for the 24 compounds were calculated using the slope of the calibration curve and the standard deviation (SD) of the intercept as follows: LOD = 3.3 × (SD of the response/slope of the calibration curve) and LOQ = 10 × (SD of the response/slope of the calibration curve). To assess precision, three standard solutions containing low, medium, and high concentrations of each standard were analyzed repeatedly (n = 6) in one day and three consecutive days to measure the intra- and inter-day variation. Precision was expressed as CV (%) of the measured concentration values and calculated using the following formula: CV (%) = (SD/Mean) × 100. Accuracy was represented by RE (%) and calculated as follows: RE (%) = (observed concentration − expected concentration)/expected concentration × 100. Recovery tests were performed by spiking standard solutions of three different concentrations (low, medium, and high) into samples of known concentration. The recovery (%) was calculated according to the following equation: recovery (%) = (found concentration − original concentration)/spiked concentration × 100.
4. Conclusions
The phytochemicals of SYT were studied via UPLC-Q-Orbitrap-MS analyses, and a total of 42 compounds were identified in the positive and negative ESI modes. The qualitative analysis results, including retention time and MS data, were compared with those of reference standards. Within 20 min, 24 compounds were simultaneously quantified in the MRM mode using the optimized UPLC-TQ-MS/MS method. The method was validated for its linearity, precision, accuracy, and recovery, exhibiting acceptable results and confirming that the established analytical method is suitable for quantifying the components of SYT. Our study offers a valuable tool for the comprehensive quality control of SYT.
Author Contributions
Conceptualization, Y.-H.H.; investigation, Y.J.K. and S.J.; writing—original draft preparation, Y.J.K.; writing—review and editing, Y.J.K. and Y.-H.H.; supervision, Y.-H.H.; funding acquisition, Y.-H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Institute of Oriental Medicine, grant number KSN2213020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, H.; Liu, M.; Zhang, W.; Chen, J.; Zhu, Z.; Cao, H.; Chai, Y. Comparative pharmacokinetics of three monoester-diterpenoid alkaloids after oral administration of Acontium carmichaeli extract and its compatibility with other herbal medicines in Sini Decoction to rats. Biomed. Chromatogr. 2015, 29, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Liao, W.; Dong, X.; Yang, G.; Zhu, Z.; Li, W.; Chai, Y.; Lou, Z. Metabonomic profiles delineate the effect of traditional Chinese medicine sini decoction on myocardial infarction in rats. PLoS ONE 2012, 7, e34157. [Google Scholar] [CrossRef]
- Liu, J.; Peter, K.; Shi, D.; Zhang, L.; Dong, G.; Zhang, D.; Breiteneder, H.; Bauer, R.; Jakowitsch, J.; Ma, Y. Anti-inflammatory effects of the chinese herbal formula sini tang in myocardial infarction rats. Evid. Based Complement. Altern. Med. 2014, 2014, 309378. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, J.; Han, Q.; Wang, Z.; Wang, Z.; Dong, X.; Li, J.; Liu, L.; Shen, X. The Effect and Mechanism of Chinese Herbal Formula Sini Tang in Heart Failure after Myocardial Infarction in Rats. Evid. Based Complement. Altern. Med. 2018, 2018, 5629342. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peter, K.; Shi, D.; Zhang, L.; Dong, G.; Zhang, D.; Breiteneder, H.; Jakowitsch, J.; Ma, Y. Traditional formula, modern application: Chinese medicine formula sini tang improves early ventricular remodeling and cardiac function after myocardial infarction in rats. Evid. Based Complement. Altern. Med. 2014, 2014, 141938. [Google Scholar] [CrossRef]
- Wu, W.K.; Su, J.W.; Lin, S.G. Clinical study on effect of sini decoction on ischemia/reperfusion injury by Holter monitoring in patients with acute myocardial infarction treated with thrombolytic therapy. Zhongguo Zhong Xi Yi Jie He Za Zhi 2001, 21, 744–746. [Google Scholar]
- Liang, Y. Clinical study on “sini” decoction on treating stenocardia for coronary heart disease. Zhong Yao Cai 2005, 28, 737–739. [Google Scholar]
- Wang, W.; Chen, Q.; Yang, X.; Wu, J.; Huang, F. Sini decoction ameliorates interrelated lung injury in septic mice by modulating the composition of gut microbiota. Microb. Pathog. 2020, 140, 103956. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, J.; Wang, W.; Liu, S.; Yang, X.; Chen, M.; Cheng, L.; Lu, J.; Guo, T.; Huang, F. Sini decoction ameliorates sepsis-induced acute lung injury via regulating ACE2-Ang (1-7)-Mas axis and inhibiting the MAPK signaling pathway. Biomed. Pharmacother. 2019, 115, 108971. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Q.; Liu, S.; Yang, X.; Zhang, Y.; Huang, F. Sini decoction alleviates E. coli induced acute lung injury in mice via equilibrating ACE-AngII-AT1R and ACE2-Ang-(1-7)-Mas axis. Life Sci. 2018, 208, 139–148. [Google Scholar] [CrossRef]
- Ji, E.; Wang, T.; Xu, J.; Fan, J.; Zhang, Y.; Guan, Y.; Yang, H.; Wei, J.; Zhang, G.; Huang, L. Systematic Investigation of the Efficacy of Sinitang Decoction Against Ulcerative Colitis. Front. Pharmacol. 2020, 11, 1337. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, X.J.; Zhou, N.; Cai, N.; Xu, J.H.; Wang, Q.B.; Li, J.J.; Liu, Q.; Lin, P.C.; Shang, X.Y. Rapid Characterizaiton of Chemical Constituents of the Tubers of Gymnadenia conopsea by UPLC-Orbitrap-MS/MS Analysis. Molecules 2020, 25, 898. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Zhong, X.; Li, J.; Wang, X.; Ji, L.; Shang, X. Rapid Characterization of Chemical Components in Edible Mushroom Sparassis crispa by UPLC-Orbitrap MS Analysis and Potential Inhibitory Effects on Allergic Rhinitis. Molecules 2019, 24, 3014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, S.; Zhou, W.; Meng, J.; Deng, K.; Zhou, H.; Hu, N.; Suo, Y. Rapid qualitative and quantitative analyses of eighteen phenolic compounds from Lycium ruthenicum Murray by UPLC-Q-Orbitrap MS and their antioxidant activity. Food Chem. 2018, 269, 150–156. [Google Scholar] [CrossRef]
- Zhong, J.; Ren, D.; Shang, Y.; Huang, S.; Li, Y.; Hu, Y.; Yi, L. Targeted identification of glycosylated flavones and isomers in green tea through integrated ion-filtering strategy and mass-fragmentation characteristics based on the UPLC-Q-Orbitrap-MS/MS platform. Food Chem. 2022, 377, 131901. [Google Scholar] [CrossRef]
- Liu, G.D.; Zhao, Y.W.; Li, Y.J.; Wang, X.J.; Si, H.H.; Huang, W.Z.; Wang, Z.Z.; Ma, S.P.; Xiao, W. Qualitative and quantitative analysis of major constituents from Dazhu Hongjingtian capsule by UPLC/Q-TOF-MS/MS combined with UPLC/QQQ-MS/MS. Biomed. Chromatogr. 2017, 31, e3887. [Google Scholar] [CrossRef]
- Li, X.Y.; Fu, Y.J.; Fu, Y.F.; Wei, W.; Xu, C.; Yuan, X.H.; Gu, C.B. Simultaneous quantification of fourteen characteristic active compounds in Eucommia ulmoides Oliver and its tea product by ultra-high performance liquid chromatography coupled with triple quadrupole mass spectrometry (UPLC-QqQ-MS/MS). Food Chem. 2022, 389, 133106. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, D.; Fan, M.; Young Quek, S. UPLC-QqQ-MS/MS-based phenolic quantification and antioxidant activity assessment for thinned young kiwifruits. Food Chem. 2019, 281, 97–105. [Google Scholar] [CrossRef]
- Cheng, X.; Li, B.P.; Han, Z.X.; Zhang, F.L.; Jiang, Z.R.; Yang, J.S.; Luo, Q.Z.; Tang, L. Qualitative and quantitative analysis of the major components in Qinghao Biejia decoction by UPLC-Orbitrap Fusion-MS/MS and UPLC-QQQ-MS/MS and evaluation of their antibacterial activities. Phytochem. Anal. 2022, 33, 809–825. [Google Scholar] [CrossRef]
- Hou, M.; Lin, C.; Ma, Y.; Shi, J.; Liu, J.; Zhu, L.; Bian, Z. One-step enrichment of phenolics from Chaenomeles speciosa (Sweet) Nakai fruit using macroporous resin: Adsorption/desorption characteristics, process optimization and UPLC-QqQ-MS/MS-based quantification. Food Chem. 2024, 439, 138085. [Google Scholar] [CrossRef]
- Li, Y.; Su, H.; Yin, Z.P.; Li, J.E.; Yuan, E.; Zhang, Q.F. Metabolism, tissue distribution and excretion of taxifolin in rat. Biomed. Pharmacother. 2022, 150, 112959. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xiao, S.; Li, Z.; Ai, N.; Fan, X. Chemical and Metabolic Profiling of Si-Ni Decoction Analogous Formulae by High performance Liquid Chromatography-Mass Spectrometry. Sci. Rep. 2015, 5, 11638. [Google Scholar] [CrossRef]
- He, L.P.; Di, B.; Du, Y.X.; Yan, F.; Su, M.X.; Liu, H.Q.; You, L.J. Development and validation of a high-performance liquid chromatography-tandem mass spectrometry method for the rapid simultaneous quantification of aconitine, mesaconitine, and hypaconitine in rat plasma after oral administration of Sini decoction. J. Anal. Toxicol. 2009, 33, 588–594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tan, G.; Liu, M.; Dong, X.; Wu, S.; Fan, L.; Qiao, Y.; Chai, Y.; Wu, H. A strategy for rapid analysis of xenobiotic metabolome of Sini decoction in vivo using ultra-performance liquid chromatography-electrospray ionization quadrupole-time-of-flight mass spectrometry combined with pattern recognition approach. J. Pharm. Biomed. Anal. 2014, 96, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Zhu, Z.; Jing, J.; Lv, L.; Lou, Z.; Zhang, G.; Chai, Y. Characterization of constituents in Sini decoction and rat plasma by high-performance liquid chromatography with diode array detection coupled to time-of-flight mass spectrometry. Biomed. Chromatogr. 2011, 25, 913–924. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Hu, Y.; Zhang, X.; Cao, L.; Dong, Z.; Li, L.; Hu, Z. Bacterial diversity in the intestinal mucosa of heart failure rats treated with Sini Decoction. BMC Complement. Med. Ther. 2022, 22, 93. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Braun, M.; Wetterauer, P.; Wetterauer, B.; Wink, M. Antioxidant, Cytotoxic, and Antimicrobial Activities of Glycyrrhiza glabra L., Paeonia lactiflora Pall., and Eriobotrya japonica (Thunb.) Lindl. Extracts. Medicines 2019, 6, 43. [Google Scholar] [CrossRef]
- Jiang, H.; Sólyom, A.M.; Timmermann, B.N.; Gang, D.R. Characterization of gingerol-related compounds in ginger rhizome (Zingiber officinale Rosc.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2957–2964. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, M.; Wei, X.; Shi, J.; Geng, Z.; Yang, S.; Fu, C.; Guo, L. Rapid discovery of chemical constituents and absorbed components in rat serum after oral administration of Fuzi-Lizhong pill based on high-throughput HPLC-Q-TOF/MS analysis. Chin. Med. 2019, 14, 6. [Google Scholar] [CrossRef]
- Chen, R.; Ning, Z.; Zheng, C.; Yang, Y.; Zhang, C.; Ou, X.; Chen, K.; Yu, H.; Wei, X.; Zhao, Q.; et al. Simultaneous determination of 16 alkaloids in blood by ultrahigh-performance liquid chromatography-tandem mass spectrometry coupled with supported liquid extraction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1128, 121789. [Google Scholar] [CrossRef]
- Sun, L.; You, G.; Cao, X.; Wang, M.; Ren, X. Comparative investigation for raw and processed Aconiti Lateralis Radix using chemical UPLC-MS profiling and multivariate classification techniques. J. Food Drug Anal. 2019, 27, 365–372. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Z.; Luo, L.; He, Y.; Ma, Y.; Wen, C.; Wang, X.; Shen, X. Development of an ultra-high-performance liquid chromatography-tandem mass spectrometry method for the simultaneous determination of crassicauline A, fuziline, karacoline, and songorine in rat plasma and application in their pharmacokinetics. Biomed. Chromatogr. 2024, 38, e5821. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, X.G.; Liu, L.; Li, P.; Yang, H. Targeted profiling and relative quantification of benzoyl diterpene alkaloids in Aconitum roots by using LC-MS/MS with precursor ion scan. J. Sep. Sci. 2018, 41, 3515–3526. [Google Scholar] [CrossRef]
- Xu, L.; Li, M.; Zhou, H.; Zhang, B.; Zhang, Z.; Han, N.; Wu, T. Rapid characterization of the chemical constituents and rat metabolites of the Wen-Jing decoction by ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Sep. Sci. 2019, 42, 1174–1193. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, J.; Dai, Y.; Xia, Y. Integration of serum pharmacochemistry and metabolomics to reveal the underlying mechanism of shaoyao-gancao-fuzi decoction to ameliorate rheumatoid arthritis. J. Ethnopharmacol. 2024, 326, 117910. [Google Scholar] [CrossRef]
- Yang, S.; Chen, G.; Yuan, M.; Zou, Y.; Zhang, H.; Xu, H. UPLC-QTOF-MS with a chemical profiling approach for holistic quality evaluation between a material reference of Wen Dan decoction and its commercial preparations. Chin. Med. 2023, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Z.; Wang, T.; Chen, Q.L.; Chen, H.B.; He, Q.S.; Zhang, Y.Z. Identification of the Metabolites of Both Formononetin in Rat Hepatic S9 and Ononin in Rat Urine Samples and Preliminary Network Pharmacology Evaluation of Their Main Metabolites. Molecules 2023, 28, 7451. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhao, M.; Zhao, Y.; Jiang, X.; Wang, M.; Zhang, Y.; Zhao, C. Rapid characterization of chemical constituents of Shaoyao Gancao decoction using UHPLC coupled with Fourier transform ion cyclotron resonance mass spectrometry. RSC Adv. 2020, 10, 29528–29535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Q.; Zhang, X.; Chen, J.; Liang, X.; Kettrup, A. High-performance liquid chromatography-tandem mass spectrometry for identification of isoflavones and description of the biotransformation of kudzu root. Anal. Bioanal. Chem. 2005, 383, 787–796. [Google Scholar] [CrossRef]
- Balkrishna, A.; Verma, S.; Sharma, P.; Tomer, M.; Srivastava, J.; Varshney, A. Comprehensive and Rapid Quality Evaluation Method for the Ayurvedic Medicine Divya-Swasari-Vati Using Two Analytical Techniques: UPLC/QToF MS and HPLC-DAD. Pharmaceuticals 2021, 14, 297. [Google Scholar] [CrossRef]
- Man, S.; Guo, S.; Gao, W.; Wang, J.; Zhang, L.; Li, X. Identification of metabolic profiling of cell culture of licorice compared with its native one. Anal. Bioanal. Chem. 2013, 405, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Liu, L.; Wang, L.L.; Jiang, P.; Zhang, J.Q.; Zhang, W.D.; Liu, R.H. Screening and analysis of the multiple absorbed bioactive components and metabolites in rat plasma after oral administration of Jitai tablets by high-performance liquid chromatography/diode-array detection coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 1641–1652. [Google Scholar]
- Alsaadi, D.H.M.; Raju, A.; Kusakari, K.; Karahan, F.; Sekeroglu, N.; Watanabe, T. Phytochemical Analysis and Habitat Suitability Mapping of Glycyrrhiza glabra L. Collected in the Hatay Region of Turkey. Molecules 2020, 25, 5529. [Google Scholar] [CrossRef]
- Basar, N.; Talukdar, A.D.; Nahar, L.; Stafford, A.; Kushiev, H.; Kan, A.; Sarker, S.D. A Simple Semi-preparative Reversed-phase HPLC/PDA Method for Separation and Quantification of Glycyrrhizin in Nine Samples of Glycyrrhiza glabra Root Collected from Different Geographical Origins. Phytochem. Anal. 2014, 25, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Yudthavorasit, S.; Wongravee, K.; Leepipatpiboon, N. Characteristic fingerprint based on gingerol derivative analysis for discrimination of ginger (Zingiber officinale) according to geographical origin using HPLC-DAD combined with chemometrics. Food Chem. 2014, 158, 101–111. [Google Scholar] [CrossRef]
- Jayasundara, N.D.B.; Arampath, P. Effect of variety, location & maturity stage at harvesting, on essential oil chemical composition, and weight yield of Zingiber officinale roscoe grown in Sri Lanka. Heliyon 2021, 7, e06560. [Google Scholar] [PubMed]
- Taki, M.; Matsuba, T.; Fukuchi, M.; Aburada, M.; Okada, M. Comparison of seasonal variations on growth of Aconitum carmichaeli DEBX. and constituents of root tubers cultivated in Hokkaido and Ibaraki prefecture. Nat. Med. 2004, 58, 55–63. [Google Scholar]
- Ahmad, R.; Alqathama, A.; Aldholmi, M.; Riaz, M.; Mukhtar, M.H.; Aljishi, F.; Althomali, E.; Alamer, M.A.; Alsulaiman, M.; Ayashy, A.; et al. Biological Screening of Glycyrrhiza glabra L. from Different Origins for Antidiabetic and Anticancer Activity. Pharmaceuticals 2023, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Rupji, M.; Choudhary, I.; Osan, R.; Kapoor, S.; Zhang, H.J.; Yang, C.; Aneja, R. Efficacy based ginger fingerprinting reveals potential antiproliferative analytes for triple negative breast cancer. Sci. Rep. 2020, 10, 19182. [Google Scholar] [CrossRef]
- Jang, S.; Lee, A.; Hwang, Y.-H. Qualitative profiling and quantitative analysis of major constituents in Jinmu-tang by UHPLC-Q-Orbitrap-MS and UPLC-TQ-MS/MS. Molecules 2022, 27, 7887. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).