A Comparative Study of the Rapid (IKr) and Slow (IKs) Delayed Rectifier Potassium Currents in Undiseased Human, Dog, Rabbit, and Guinea Pig Cardiac Ventricular Preparations
Abstract
1. Introduction
2. Results
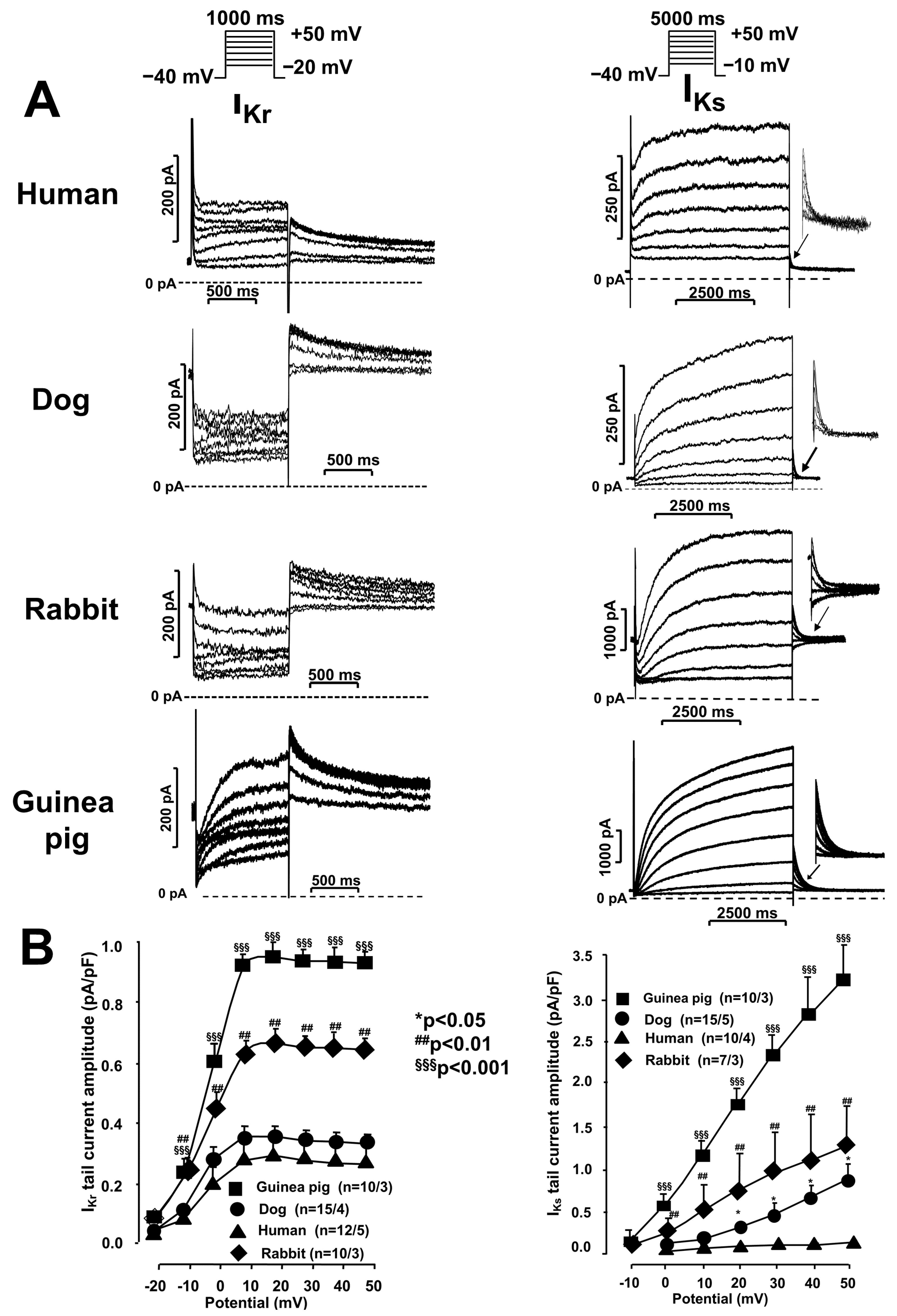
2.1. Ion Current Measurements Using the Patch Clamp Technique
IKr and IKs Currents in Undiseased Human, Canine, Rabbit, and Guinea Pig Cardiomyocytes
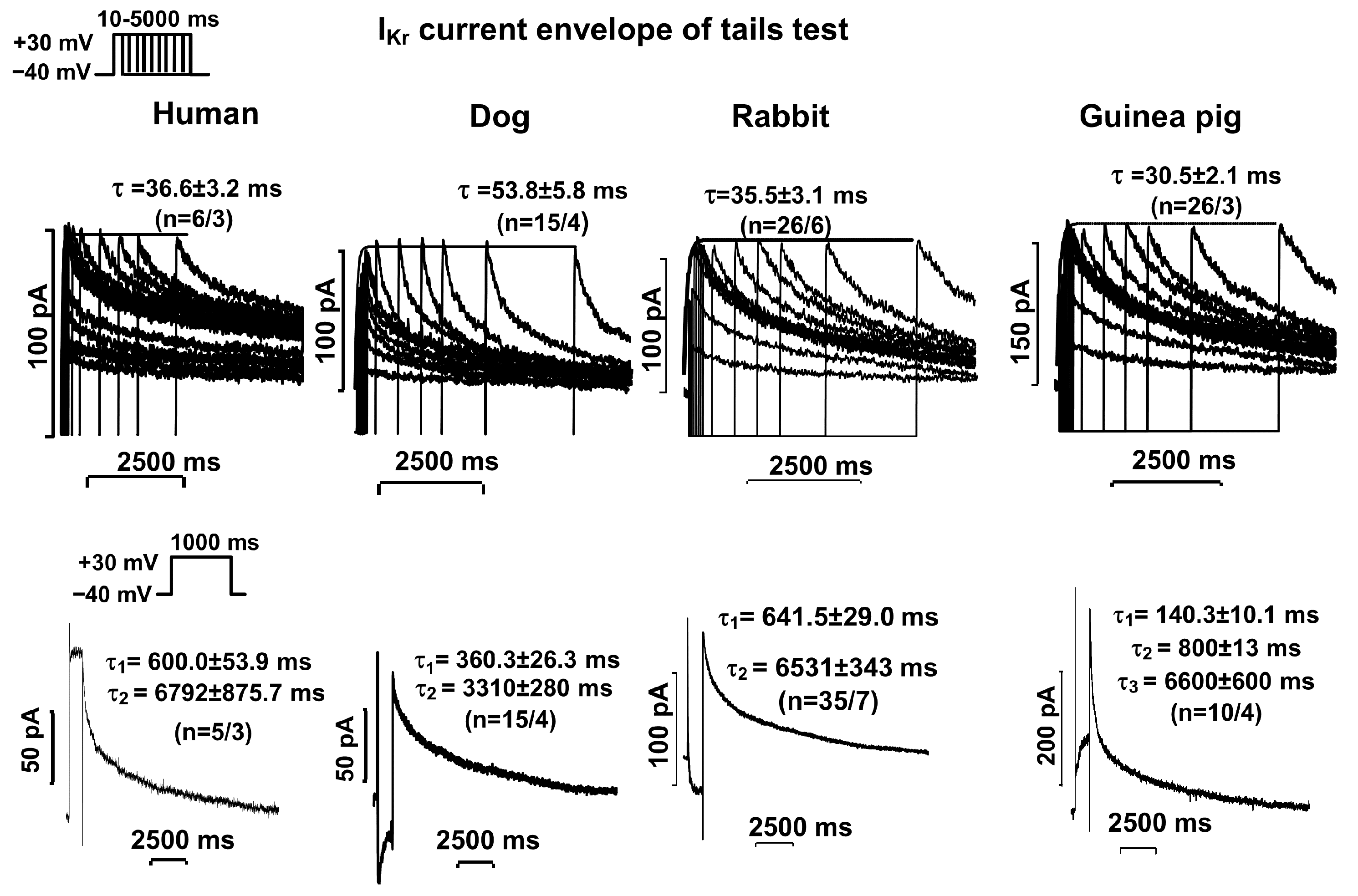
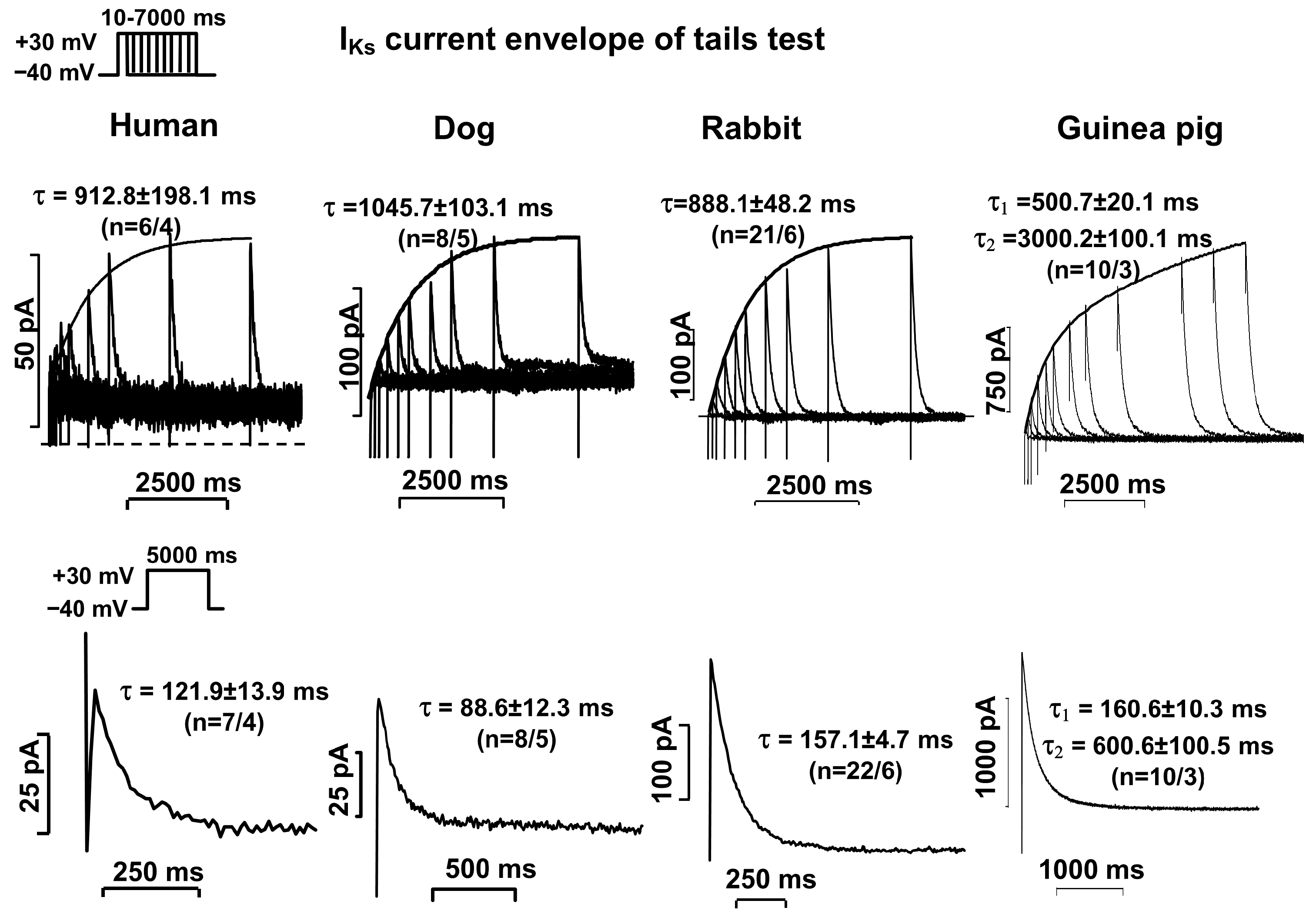
2.2. Activation and Deactivation Kinetics of IKr and IKs Currents
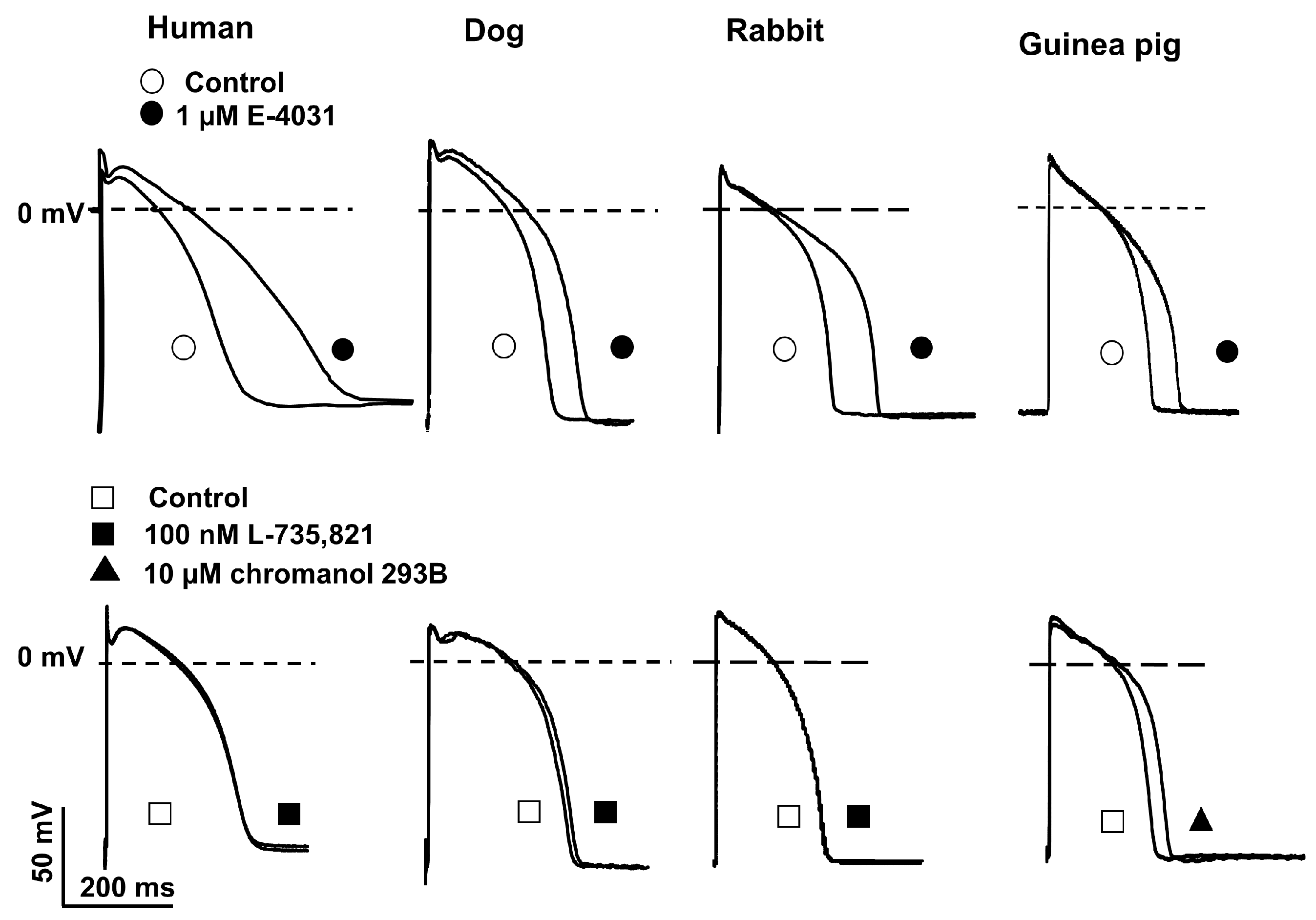
2.3. Action Potential Measurements Using Conventional Microelectrode Techniques
3. Discussion
3.1. Summary of Results of Previous Publications Describing IKr and IKs Current Measurements in Various Mammalian Species
3.2. Comparing Our Results with the Literature
4. Conclusions
5. Materials and Methods
5.1. Animals
5.2. Healthy Human Heart Muscle Preparations
5.3. Ion Current Measurement Using the Patch Clamp Technique
5.4. Action Potential Measurement Using Conventional Microelectrode Techniques
5.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.N.; Vaughan Williams, E.M. A third class of anti-arrhythmic action. Effects on atrial and ventricular intracellular potentials, and other pharmacological actions on cardiac muscle, of MJ 1999 and AH 3474. Br. J. Pharmacol. 1970, 39, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N. Comparative mechanisms of action of antiarrhythmic agents: Significance of lengthening repolarization. In Control of Cardiac Arrhythmias by Lengthening Repolarization; Singh, B.N., Ed.; Futura Publishing Co.: New York, NY, USA, 1988; pp. 53–127. [Google Scholar]
- Varró, A.; Papp, J.G. The impact of single cell voltage clamp on the understanding of the cardiac ventricular action potential. Cardioscience 1992, 3, 131–144. [Google Scholar] [PubMed]
- Whalley, D.W.; Wendt, D.J.; Grant, A.O. Basic concepts in cellular cardiac electrophysiology: Part I: Ion channels, membrane currents and action potential. Pacing Clin. Electrophysiol. 1955, 18, 1556–1574. [Google Scholar] [CrossRef] [PubMed]
- Noble, D.; Tsien, R.W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J. Physiol. 1969, 200, 205–231. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, E. Voltage-and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J. Pharmacol. Exp. Ther. 1992, 262, 809–817. [Google Scholar] [PubMed]
- Gintant, G.A. Two components of delayed rectifier current in canine atrium and ventricle. Does IKs play a role in the reverse rate dependence of Class III agents ? Circ. Res. 1996, 78, 26–37. [Google Scholar] [CrossRef]
- Liu, D.W.; Antzelevitch, C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell. Circ. Res. 1995, 76, 351–365. [Google Scholar] [CrossRef]
- Apkon, J.; Nerbonne, J.M. Characterization of two distinct depolarization-activated K+-currents in isolated adult rat ventricular myocytes. J. Gen. Physiol. 1991, 97, 973–1011. [Google Scholar] [CrossRef]
- Salata, J.J.; Jurkiewicz, N.K.; Jow, B.; Folander, K.; Guinosso, P.J.; Raynor, B.; Swanson, R.; Fermini, B. IK of rabbit ventricle is composed of two currents: Evidence for IKs. Am. J. Physiol. 1996, 271, H2477–H2489. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.C.; Jurkiewicz, N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990, 96, 195–215. [Google Scholar] [CrossRef]
- Li, G.R.; Feng, J.; Yue, L.; Carrier, M.; Nattel, S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ. Res. 1996, 78, 689–696. [Google Scholar] [CrossRef]
- Heath, B.M.; Terrar, D.A. The deactivation kinetics of the delayed rectifier components IKr and IKs in guinea pig isolated ventricular myocytes. Exp. Physiol. 1996, 81, 605–621. [Google Scholar] [CrossRef]
- Singh, B.N. Antiarrhythmic drugs: A reorientation in light of recent developments in the control of disorders of rhythm. Am. J. Cardiol. 1998, 81, 3D–13D. [Google Scholar] [CrossRef]
- Jurkiewicz, N.K.; Sanguinetti, M.C. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ. Res. 1993, 71, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, D.A. Electromechanical characterization of the effects of racemic sotalol and its optical isomers on isolated canine ventricular trabecular muscles and Purkinje strands. Can. J. Physiol. Pharm. 1985, 63, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Woosley, R.L. Sotalol. N. Engl. J. Med. 1994, 331, 31–38. [Google Scholar]
- Hondeghem, L.M.; Snyders, D.J. Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation 1990, 81, 686–690. [Google Scholar] [PubMed]
- Busch, A.E.; Suessbrich, H.; Waldegger, S.; Sailer, E.; Greger, R.; Lang, H.; Lang, F.; Gibson, K.J.; Maylie, J.G. Inhibition of IKs in guinea pig cardiac myocytes and guinea pig IsK channels by the chromanol 293B. Pflügers Arch. Eur. J. Physiol. 1996, 432, 1094–1096. [Google Scholar] [CrossRef]
- Gerlach, U. IKs channel blockers: Potential antiarrhythmic agents. Drug Future 2001, 26, 473–484. [Google Scholar] [CrossRef]
- Beuckelmann, D.J.; Näbauer, M.; Erdmann, E. Alteration of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ. Res. 1993, 73, 379–385. [Google Scholar] [CrossRef]
- Veldkamp, M.W.; Van Gineken, A.C.G.; Opthof, T.; Bouman, L.N. Delayed rectifier channels in human ventricular myocytes. Circulation 1995, 92, 3497–3504. [Google Scholar] [CrossRef]
- Follmer, C.H.; Lodge, N.J.; Cullinan, C.A.; Colatsky, T.J. Modulation of the delayed rectifier IK by cadmium in cat ventricular myocytes. Am. J. Physiol. 1992, 262, C75–C83. [Google Scholar] [CrossRef]
- Hirano, Y.; Hiraoka, M. Changes in K+ currents induced by Ba2+ in guinea pig ventricular muscles. Am. J. Physiol. 1986, 251, H24–H33. [Google Scholar] [CrossRef] [PubMed]
- Varró, A.; Lathrop, D.A.; Hester, S.B.; Nánási, P.P.; Papp, J.G. Ionic currents and action potentials in rabbit, rat and guinea pig ventricular myocytes. Basic. Res. Cardiol. 1993, 88, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Yang, S.R.; Huang, M.H.; Liu, Y.C.; Wu, S.N. Characterization of chromanol 293B-induced block of the delayed-rectifier K+ current in heart-derived H9c2 cells. Life Sci. 2005, 76, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Q.; Thomas, G.; Antzelevitch, C. Role of the delayed rectifier component IKs in cardiac repolarization [reply]. J. Cardiovasc. Electrophysiol. 2001, 12, 1205–1206. [Google Scholar]
- Varró, A.; Lathrop, D.A.; Papp, J.G. Role of the delayed rectifier component IKs in cardiac repolarization. J. Cardiovasc. Electrophysiol. 2001, 12, 1204–1205. [Google Scholar]
- Varró, A.; Baláti, B.; Iost, N.; Takács, J.; Virág, L.; Lathrop, D.A.; Lengyel, C.; Tálosi, L.; Papp, J.G. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J. Physiol. 2000, 523, 67–81. [Google Scholar] [CrossRef]
- Jost, N.; Virag, L.; Bitay, M.; Takács, J.; Lengyel, C.; Biliczki, P.; Nagy, Z.; Bogáts, G.; Lathrop, D.A.; Papp, J.G.; et al. Restricting excessive cardiac action potential and QT prolongation: A vital role for IKs in human ventricular muscle. Circulation 2005, 112, 1392–1399. [Google Scholar] [CrossRef]
- Lengyel, C.; Iost, N.; Virág, L.; Varró, A.; Lathrop, D.A.; Papp, J.G. Pharmacological block of the slow component of the outward delayed rectifier current (I(Ks)) fails to lengthen rabbit ventricular muscle QT(c) and action potential duration. Br. J. Pharmacol. 2001, 132, 101–110. [Google Scholar] [CrossRef]
- Jost, N.; Virág, L.; Hála, O.; Varró, A.; Thormählen, D.; Papp, J.G. Effect of the antifibrillatory compound tedisamil (KC-8857) on transmembrane currents in mammalian ventricular myocytes. Curr. Med. Chem. 2004, 11, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Jost, N.; Papp, J.G.; Varró, A. Slow delayed rectifier potassium current (IKs) and the repolarization reserve. Ann. Noninvasive Electrocardiol. 2007, 12, 64–78. [Google Scholar] [CrossRef]
- Jost, N.; Virág, L.; Comtois, P.; Ördög, B.; Szuts, V.; Seprényi, G.; Bitay, M.; Kohajda, Z.; Koncz, I.; Nagy, N.; et al. Ionic mechanisms limiting cardiac repolarization reserve in humans compared to dogs. J. Physiol. 2013, 591, 4189–4206. [Google Scholar] [CrossRef] [PubMed]
- Roden, D.M. Taking the “idio” out of “idiosyncratic”: Predicting torsades de pointes. Pacing Clin. Electrophysiol. 1998, 21, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Roden, D.M. Long QT syndrome: Reduced repolarization reserve and the genetic link. J. Intern. Med. 2006, 259, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.F.; Gaspo, R.; Busch, A.E.; Lang, H.J.; Li, G.R.; Nattel, S. Effects of the chromanol 293B, a selective blocker of the slow component of the delayed rectifier K+ current on repolarisation in human and guinea pig ventricular myocytes. Cardiovasc. Res. 1998, 38, 441–450. [Google Scholar] [CrossRef]
- Biliczki, P.; Virág, L.; Iost, N.; Papp, J.G.; Varró, A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: Role of repolarization reserve. Br. J. Pharmacol. 2001, 137, 361–368. [Google Scholar] [CrossRef]
| Species | IKr Current | IKs Current | ||
|---|---|---|---|---|
| Activation Time Constant (ms) | Deactivation Time Constant (ms) | Activation Time Constant (ms) | Deactivation Time Constant (ms) | |
| Human | τ = 36.6 ± 3.2 (n = 6/3) | τ1 = 600.0 ± 53.9 τ2 = 6792 ± 876 (n = 5/3) | τ = 912.8 ± 198.1 (n = 6/4) | τ = 121.9 ± 13.9 (n = 7/4) |
| Dog | τ = 53.8 ± 5.8 (n = 15/4) | τ1 = 360.3 ± 26.3 τ2 = 3310 ± 280 (n = 15/4) | τ = 1046 ± 103 (n = 8/5) | Τ = 88.6 ± 12.3 (n = 8/5) |
| Rabbit | τ = 35.5 ± 3.1 (n = 26/6) | τ1 = 641.5 ± 29.0 τ2 = 6531 ± 343 (n = 35/7) | τ = 888.1 ± 48.2 (n = 21/6) | τ = 157.1 ± 4.7 (n = 22/6) |
| Guinea pig | τ = 30.5 ± 2.1 (n = 26/3) | τ1 = 140.3 ± 10.1 τ2 = 800 ± 13 τ3 = 6600 ± 600 (n = 10/4) | τ1 = 500.7 ± 20.1 τ2 = 3000 ± 100 (n = 10/3) | τ1 = 160.6 ± 10.3 τ2 = 600.6 ± 100.5 (n = 10/3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ágoston, M.; Kohajda, Z.; Virág, L.; Baláti, B.; Nagy, N.; Lengyel, C.; Bitay, M.; Bogáts, G.; Vereckei, A.; Papp, J.G.; et al. A Comparative Study of the Rapid (IKr) and Slow (IKs) Delayed Rectifier Potassium Currents in Undiseased Human, Dog, Rabbit, and Guinea Pig Cardiac Ventricular Preparations. Pharmaceuticals 2024, 17, 1091. https://doi.org/10.3390/ph17081091
Ágoston M, Kohajda Z, Virág L, Baláti B, Nagy N, Lengyel C, Bitay M, Bogáts G, Vereckei A, Papp JG, et al. A Comparative Study of the Rapid (IKr) and Slow (IKs) Delayed Rectifier Potassium Currents in Undiseased Human, Dog, Rabbit, and Guinea Pig Cardiac Ventricular Preparations. Pharmaceuticals. 2024; 17(8):1091. https://doi.org/10.3390/ph17081091
Chicago/Turabian StyleÁgoston, Márta, Zsófia Kohajda, László Virág, Beáta Baláti, Norbert Nagy, Csaba Lengyel, Miklós Bitay, Gábor Bogáts, András Vereckei, Julius Gy. Papp, and et al. 2024. "A Comparative Study of the Rapid (IKr) and Slow (IKs) Delayed Rectifier Potassium Currents in Undiseased Human, Dog, Rabbit, and Guinea Pig Cardiac Ventricular Preparations" Pharmaceuticals 17, no. 8: 1091. https://doi.org/10.3390/ph17081091
APA StyleÁgoston, M., Kohajda, Z., Virág, L., Baláti, B., Nagy, N., Lengyel, C., Bitay, M., Bogáts, G., Vereckei, A., Papp, J. G., Varró, A., & Jost, N. (2024). A Comparative Study of the Rapid (IKr) and Slow (IKs) Delayed Rectifier Potassium Currents in Undiseased Human, Dog, Rabbit, and Guinea Pig Cardiac Ventricular Preparations. Pharmaceuticals, 17(8), 1091. https://doi.org/10.3390/ph17081091







