Abstract
The treatment of hypertension has improved in the last century; attention has been directed to restoring several altered pathophysiological mechanisms. However, regardless of the current treatments, it is difficult to control blood pressure. Uncontrolled hypertension is responsible for several cardiovascular complications, such as chronic renal failure, which is frequently observed in hypertensive patients. Therefore, new approaches that may improve the control of arterial blood pressure should be considered to prevent serious cardiovascular disorders. The contribution of purinergic receptors has been acknowledged in the pathophysiology of hypertension; this review describes the participation of these receptors in the alteration of kidney function in hypertension. Elevated interstitial ATP concentrations are essential for the activation of renal purinergic receptors; this becomes a fundamental pathway that leads to the development and maintenance of hypertension. High ATP levels modify essential mechanisms implicated in the long-term control of blood pressure, such as pressure natriuresis, the autoregulation of the glomerular filtration rate and renal blood flow, and tubuloglomerular feedback responses. Any alteration in these mechanisms decreases sodium excretion. ATP stimulates the release of vasoactive substances, causes renal function to decline, and induces tubulointerstitial damage. At the same time, a deleterious interaction involving angiotensin II and purinergic receptors leads to the deterioration of renal function.
1. Introduction
During the last century, the treatment of hypertension has been focused on restoring several mechanisms involved in the development and persistence of hypertension. However, despite the advanced therapies presently offered for blood pressure control, their associated complications are far from being eliminated; consequently, cardiovascular disorders, including chronic renal disease, are often observed in patients with hypertension [1,2].
Most antihypertensive treatments have been mainly directed to block the renin–angiotensin system at its pathways, control hyperactivity of the sympathetic nervous system, restore cellular calcium alterations, and modify sodium reabsorption by the kidneys. In addition, a new treatment has been developed that blocks sodium–glucose transport with specific inhibitors. Such a variety of antihypertensive compounds should be enough to control blood pressure; however, factors associated with the control of hypertension are being ignored. Thus, insights into other pathophysiological mechanisms should be developed to preclude critical cardiovascular disorders [3].
Recent findings have improved knowledge of hypertension, which may result in a more rational treatment for this disease. The participation of the immune system, inflammation, and the development of oxidative stress are recognized as early responses to the elevation of blood pressure and the negative consequences of uncontrolled hypertension [4].
This review emphasizes the potential contributions of P2 purinergic receptors as well as the association between the tubulointerstitial inflammatory cells and ATP in the development of salt-sensitive hypertension as possible targets in the treatment of hypertension. Interested readers are directed to several recent, excellent, and comprehensive reviews covering the participation of the immune system, inflammation, and oxidative stress in hypertension [5,6,7].
The kidneys are fundamental to the regulation of blood pressure through intrinsic mechanisms such as pressure natriuresis, autoregulation of the glomerular filtration rate and plasma flow, tubuloglomerular feedback, and sodium excretion [8,9,10]; thus, they are essential organs to the initiation and continuance of hypertension. When renal function is normal, the mechanism of pressure natriuresis is intact. In this context, hypertension never occurs [10]. Conversely, a renal abnormality, such as a subtle renal lesion, may cause sustained hypertension [11]. The mechanisms involved in the development of renal injury in hypertension are currently better understood. Initially, it was thought that the systemic pressure was transmitted to the glomerulus and damaged it; this was due to an incapacity of the afferent arteriole to contract and regulate the pressure that reached the glomeruli [12]. Despite the development of hyperplasia and hypertrophy of the afferent arteriole, these are not sufficient to prevent microvascular damage in the capillary network, leading to the leakage of plasma and cells into the renal interstitium, which stimulates the development of tubulointerstitial injury [13,14]. However, inflammatory infiltration has been observed to occur since the initial stages of hypertension, and it is essential for the development of structural damage to the glomeruli, which results in end-stage renal disease [11]. Mild tubulointerstitial infiltration may induce renal dysfunction with sodium and water retention, leading to salt-sensitive hypertension [11,15]. In this regard, the experimental evidence suggests that the activation of purinergic receptors participates essentially in the pathophysiology of salt-sensitive hypertension through the constant release of vasoactive mediators [16], which promotes tubulointerstitial inflammation [11] and decreases pressure natriuresis [17].
2. Purinergic Receptors
ATP, the fundamental molecule providing an energy source within cells, possesses an extracellular membrane receptor system unrelated to energy production. In 1972, George Burnstock proposed ATP as a molecule whose extracellular effects fulfill the criteria to be mediated by membrane receptors [18]. Afterwards, it was confirmed that ATP membrane receptors exist [19,20]. Purinergic receptors are widely distributed in the human body and participate in the regulation of physiological and pathophysiological processes, since they are involved in inflammatory mechanisms as well as biochemical pathways that lead to apoptosis [20]. ATP breaks down into ADP, AMP, and adenosine. The first receptors described were P1 receptors, which are activated by adenosine; subsequently, P2 receptors activated by ATP were defined. There are two families of purinergic receptors, defined according to their pharmacological profiles: P2X receptors, which are ligand-gated membrane receptors designated 1 to 7, and P2Y receptors, a family of G-protein-coupled receptors that have seven transmembrane domains and are called 1 to 9, 12, 13, and 14 [21].
3. Purinergic Receptors in the Regulation of Blood Pressure
Purinergic signaling influences blood pressure at several levels and increases the sympathetic activity of peripheral nerves either directly or by acting on neurons in the brain stem. ATP is released by endothelial cells in response to shear stress and is involved in the release of nitric oxide. The activation of P2X3 receptors in the carotid body also influences sympathetic tone. In the kidneys, purinergic receptors possess tubular and vascular effects (Table 1) [22,23].
Kidney function involves intrinsic mechanisms such as the glomerular filtration rate, tubular transport, pressure natriuresis, and urinary sodium excretion, which are involved in the long-term control of blood pressure through adjustments to the extracellular volume. The extracellular fluid balance is fundamental for the regulation of blood pressure [8,9,10]. Among several renal mediators, ATP regulates the mechanisms mentioned above. P2Y2 receptor activation increases sodium excretion and decreases blood pressure by inhibiting the epithelial sodium channel (ENaC), thereby reducing sodium reabsorption and inducing an appropriate pressure-natriuresis response when the sodium concentration in the distal nephron is elevated [24,25]. ATP’s vasoactive effects through P2X receptors participate in adjustments to the renal vascular resistances, which are essential to maintaining glomerular capillary pressure and autoregulation. When ATP increases in the extracellular fluid, it is also augmented in the renal interstitial fluid and elevates the renal perfusion pressure [26,27,28]. In this regard, the stimulation of endothelial cells by shear stress releases ATP, and the continuous liberation of the nucleotide modifies the distribution of purinergic receptors, which are preferentially expressed in areas under hypoxic conditions [19,29,30].

Table 1.
Distribution of purinergic receptors in the nephron.
Table 1.
Distribution of purinergic receptors in the nephron.
| Nephron Segments | P2X Purinergic Receptors | P2Y Purinergic Receptors |
|---|---|---|
| Proximal tubule | P2X1, P2X4, P2X5, P2X6 | P2Y1, P2Y2, P2Y4, P2Y6 |
| Loop of Henle, thick ascending limb | P2X4, P2X5 | P2Y2, P2Y4 |
| Loop of Henle, medullary thick ascending limb | P2X1, P2X4, P2X5, P2X6 | P2Y1, P2Y2, P2Y4, P2Y6 |
| Loop of Henle, thin descending limb | P2X4, P2X6 | P2Y1, P2Y2 |
| Loop of Henle, thin ascending limb | P2X4, P2X6 | P2Y2, P2Y4 |
| Distal tubule | P2X4, P2X5, P2X6 | |
| Collecting duct | P2X1, P2X2, P2X3, P2X4, P2X5, P2X6 | P2Y1, P2Y2, P2Y4, P2Y6 |
| Afferent arteriole | P2X1, P2X7 | P2Y1, P2Y2, P2Y6 |
| Efferent arteriole | P2Y1 | |
| Glomeruli | P2X2, P2X4, P2X7 | P2Y1, P2Y2, P2Y4, P2Y6 |
| Perivascular capillaries and descending vasa recta | P2X7 | P2Y1 |
| Smooth muscle cells | P2X1, P2X2, P2X3, P2X7 | P2Y1, P2Y2, P2Y4 |
| Endothelium | P2X7, P2X4 | P2Y1, P2Y2, P2Y6 |
Modified from Menzies et al. 2017 and Inscho et al. 2015 [21,26].
4. Purinergic Receptors in Hypertension
The activation of purinergic receptors may induce hypertension when extracellular ATP concentrations are elevated. Changes in the sympathetic tone and the stimulation of the renin–angiotensin system modify sodium excretion and may induce the contraction of pre- and post-glomerular arterioles. Furthermore, in Dahl salt-sensitive rats with stable hypertension [31] and angiotensin II-induced hypertensive rats, P2X1 receptors were overexpressed in the renal cortex, with no changes in P2Y1 receptor abundance [32]. P2X7 receptors have been observed in podocytes, endothelial cells, and mesangial cells in the glomeruli of hypertensive transgenic (mRen2) rats [33] and Dahl salt-sensitive hypertensive rats [31].
In angiotensin II-induced hypertensive rats, overexpression of the P2X1, P2X4, P2X7, and PY1 receptors has been observed in the intrarenal vessels, afferent arteriole, and macula densa [34,35]. It should be pointed out that purinergic receptors regulate several mechanisms engaged in the control of blood pressure, as mentioned above: pressure natriuresis [31], autoregulation of the glomerular filtration rate and blood flow [8,32,35], the tubuloglomerular feedback mechanism, and urinary sodium excretion [36,37,38]. In addition, purinergic receptors are important mediators of the progression of renal injury, at least in angiotensin II-mediated hypertension, since they stimulate vasoconstriction in the glomerular microcirculation. The afferent and efferent resistances are elevated, as well as the intraglomerular pressure, which results in a fall in the single-nephron and overall glomerular filtration rates [32,34].
Hypertensive kidneys are characterized by hypertrophy and hyperplasia of the renal vessels [39,40]. Since ATP is a powerful stimulus for the proliferation of smooth muscle cells in the renal vessels, these changes are mediated by the activation of purinergic P2X and angiotensin II AT1 receptors and are associated with interstitial injury: infiltration by lymphocytes and macrophages, the proliferation of mesangial cells and myofibroblasts around glomerular capillaries, and capillary rarefaction [41,42].
5. Beneficial Effects of Purinergic Receptor Blockade in Renal Microcirculation in Hypertension
In the context of P2X1 and P2X7 activation, vasoactive compounds such as inflammatory cytokines are released and are responsible for detrimental effects in renal microcirculation [43,44]. P2X7 receptor activation promotes a pro-inflammatory condition, since it induces the release of the cytokines Il-1β, Il-18, TNF-α, and MCP1, which are responsible for the stimulation of pathways that induce vasoconstriction [31,34,45,46]. Since P2X7 receptors are expressed in the smooth muscle cells of intrarenal vessels [36] and P2X1 receptors are expressed in the endothelial and smooth muscle cells, blockade with a purinergic antagonist was found to induce vasodilation in the renal microcirculation in hypertension [45,46]. When renal hemodynamics were evaluated after 14 days of infusion of angiotensin II in hypertensive rats, an acute blockade with broad (PPADS, pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid) or specific P2X1 (NF 449, 4,4′,4,4-(carbonilbis(imino-5,1,3-benzenetriybis(carbonylimino)))tetrakis-benzene-l,3-disulfonic acid) and P2X7 (A438079, 6-difluoro-4-isopropyloxybenzyl alcohol) antagonists induced a vasodilatory response [32,34]. This was characterized by a fall in the resistances, an increase in the glomerular plasma flow, an increase in the ultrafiltration coefficient, and a return of the single-nephron glomerular filtration rate to nearly normal values (Figure 1) [34]. In the same model, when angiotensin II was administered at the same time as a P2X and P2Y purinergic antagonist (PPADS) for 14 days, the trophic effects of angiotensin II were prevented in the tubulointerstitium; indeed, inflammatory infiltration and afferent arteriole hypertrophy were significantly inhibited without changes in blood pressure neither of the angiotensin II concentrations [47].
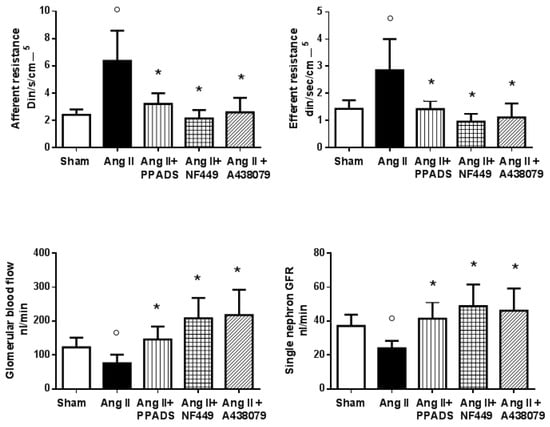
Figure 1.
Renal hemodynamics in rats with Ang II-mediated hypertension (14 days, Ang II, 435 ng/kg/min) during an acute infusion of a broad purinergic receptor blocker (PPADS, 30 mg⋅kg−1) and specific P2X1 (NF 449, 30 nM⋅kg−1⋅h−1) and P2X7 (A 438079, 80 μm⋅kg−1) receptor antagonists. The purinergic antagonists induced a decrease in the afferent and efferent resistances (* < 0.05 to 0.019; ο < 0.05 vs. Sham) that produced a significant elevation in the glomerular blood flow; as a result of these changes, the single-nephron glomerular filtration rate returned to near-normal levels. These results show that, in Ang II-induced hypertension, the renal vasoconstriction induced by Ang II is associated with important actions of the P2X1 and P2X7 receptors (Modified by Franco et al. [32,34]).
6. Purinergic Receptors in Inflammation and Immunity
Inflammation is associated with ischemia and hypoxia, free radical oxygen species, and necrosis and apoptosis pathways [48,49,50]. Extracellular ATP is a potent stimulus for inflammation and may explain the immune activation that has been observed in hypertension. This issue is not currently understood. In this regard, to increase the ATP concentration in the interstitial space, the release of intracellular ATP is needed. This can be accomplished through cellular membrane channels called pannexins and connexins during the inflammatory process [51,52]. In the acute inflammatory reaction, ATP is metabolized to ADP and adenosine, then ectoenzymes such as ATPase, apyrase, alkaline phosphatase, and ectonucleotidases, decrease the concentration of extracellular ATP [50]. In contrast, in angiotensin II-induced hypertension, renal ecto-adenosine deaminase (which metabolizes adenosine) is decreased and induces the elevation of interstitial adenosine [53]. This fact should be pointed out, since the equilibrium between the vasodilator and vasoconstrictor effects of adenosine may be lost due to adenosine’s vasoactive properties [54]. It is important to mention that inflammatory cells possess P2X and P2Y receptors; this is the reason why interstitial ATP has chemoattractant effects [55]. In addition, inflammatory cells have the ability to enable the non-specific release of ATP when exposed to a deleterious stimulus, which induces the release of cytokines and chemoattractant factors [56,57,58]. The elevation of interstitial ATP modifies the expression and distribution of purinergic receptors when associated with the inflammatory reaction and induces the assembly of the NLRP3 (nucleotide-binding domain-like receptor pyrin domain containing 3) inflammasome [59,60,61], which is an essential step for the initiation of a proliferative reaction and the development of fibrosis in persistent hypertension [59].
The P2X7 receptor has been linked to the ensemble of the NRLP3 inflammasome, but the mechanism of the coupling is only partially known. In this regard, when ATP is elevated in the interstitial space, it induces protein phosphorylation, which, in turn, modulates protein ubiquitination and activates the NLRP3 inflammasome and the release of IL-1β and caspase-1 in macrophages and dendritic cells [60,61,62]; however, not all the effects of ATP are deleterious, since phagocytes and dendritic cells are also involved in tissue repair [63].
Regarding the inflammatory effect of ATP, it has been observed that immunosuppressor compounds such as mycophenolate mofetil (MMF), non-steroidal anti-inflammatory drugs (pentosan polysulfate), and genetic manipulations are associated with a reduction in tubulointerstitial infiltration and decreased renal injury [64,65,66]. The infiltration of macrophages is reduced along with NFkB, IL-1β, and TNFα [62], and treatment with the drugs mentioned above prevents the elevation of blood pressure [62]. The angiotensin II-induced hypertension model can be distinguished by producing severe hypertension associated with remarkable vasoconstriction; angiotensin II was administered for 14 days, followed by 5 weeks of a high-salt diet (NaCl: 4%) [67]. When angiotensin II and MMF were given simultaneously, barely borderline hypertension developed, a mild elevation of renal resistances was observed, and the glomerular blood flow and glomerular filtration rate were near normal levels; these changes were associated with a significant reduction in tubulointerstitial infiltration [64,65,67].
The development of hypertension and salt sensitivity and the activation of purinergic receptors can be summarized as follows: in situations of hyperactivity of the sympathetic nervous system, overstimulation of the renin–angiotensin system, or a genetic predisposition, some stress situations may induce a temporary elevation in blood pressure (11, 65, 66). When this elevation exceeds the limits of renal autoregulation (>140 mmHg), an increase in interstitial ATP occurs, as well as mild interstitial injury. The transmission of blood pressure damages the peritubular capillary walls, allowing the leakage of plasma and leukocytes to the tubulointerstitium; leukocytes induce local inflammation and enhance the severity of the microvascular and tubulointerstitial injury [65,67]. These alterations induce focal ischemia, cytokine release, upregulation of adhesion molecules, and capillary rarefaction, maintaining the inflammatory reaction [11]. In this context, the angiotensin II effects, the elevation of ATP, and tubulointerstitial inflammation become critical factors for the progression of renal injury [41,64,68,69,70]. These factors enhance the sensitivity of the renal mechanisms involved in the regulation of blood pressure and sodium excretion, leading to sodium retention (Figure 2) [71,72].
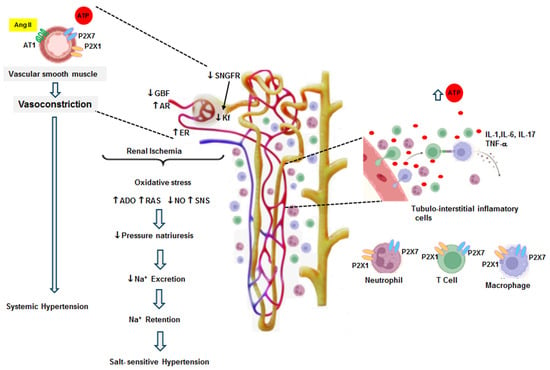
Figure 2.
The vasoconstrictor effects of Ang II (14 days) and both P2X1 and P2X7 receptors are shown in this figure. Ang II infusion induced systemic hypertension and an elevation of interstitial fluid concentrations of ATP as well as local Ang II. The direct effect of Ang II associated with its regulatory response to hypertension produced renal vasoconstriction. The glomerular hemodynamics were characterized by an increase in the afferent resistance (AR) and efferent resistance (ER), which resulted in a decrease in the glomerular blood flow (GBF) and a diminished ultrafiltration coefficient (Kf). These alterations produced a reduction in the single-nephron glomerular filtration rate. These changes induced renal ischemia, leading to an overexpression of P2X receptors in the smooth muscle of intrarenal arterioles. Simultaneously, tubulointerstitial inflammatory cell infiltration contributed to a further elevation in interstitial ATP and the overexpression of P2X receptors in the intrarenal arterioles and on the surface of inflammatory cells. These changes induced the release of cytokines, growth factors, and chemoattractant factors; these factors exacerbated inflammatory cell infiltration and the intensity of renal vasoconstriction. Under these conditions, oxidative stress, the augmentation of adenosine (ADO), a decrease in nitric oxide (NO), an increase in the local production of Ang II (RAS), and the stimulation of sympathetic tone (SNS) developed. These alterations adjusted the sodium excretion and reduced pressure natriuresis, leading to decreased sodium excretion compared to the expected level for the elevation of blood pressure. This resulted in sodium retention, and salt-sensitive hypertension developed (modified from Graciano et al. 2008 [47] and Franco et al. 2019 [43]).
While the blood pressure increases, glomerular perfusion improves, since hypoxia and tubular ischemia decrease, resulting in the recovery of the oxygenation and perfusion of the kidneys [8,9]. At the same time, the elevation of glomerular blood flow stimulates nitric oxide release, leading to an increase in sodium excretion [8,9]. The blood pressure remains elevated as a result of the tubulointerstitial changes mentioned above, but the preservation of hypertension is required to maintain normal sodium excretion [45]. Then, salt sensitivity develops and sodium homeostasis is restored, but at the cost of hypertension [8,9]. Therefore, tubulointerstitial injury without glomerular damage is a condition frequently observed in the early stages of hypertension [67]. The vascular resistance increases initially in response to hypertension, inducing afferent arteriole hypertrophy. Despite these adaptative adjustments, after some time, hyperperfusion and glomerular hypertension develop, as well as glomerular capillary injury, resulting in a decrease in sodium excretion [73,74,75].
7. Perspectives
The treatment of hypertension with P2X antagonists has not yet been explored, but these antagonists are an attractive possibility for the control of blood pressure and the prevention of cardiovascular complications [76]. P2X7 antagonists are under development by several drug companies, and they have been tested in patients with inflammatory diseases, such as rheumatoid arthritis, neuroinflammation, pain, and cancer [77]. However, the implications of purinergic receptors in the pathophysiology of hypertension should be considered. Despite the favorable effects obtained in experimental hypertension models, the main limitations for the use of this treatment in patients will be identifying the correct moment to initiate the therapy during hypertension and selecting markers to evaluate the effects of the therapy, since inflammatory processes are chronic. Patients with uncontrolled hypertension or indicators of possible cardiovascular complications should benefit.
Alterations in purinoceptor function lead to various diseases [76], including neurological, rheumatic, cardiovascular, and renal diseases as well as cancer, among others [77]. To date, only P2Y12 antagonists have been used for their effect in antiplatelet therapy (suramin, pentoxifylline, clopidogrel, prasugrel, cangrelor, and ticagrelor). Newly developed compounds such as the P2Y2 agonist denufusol (cystic fibrosis) and the P2X7 antagonist AZD 9056 (Crohn’s disease) demonstrate the therapeutic potential of agonists and antagonists of purinergic receptors in various human diseases [78].
8. Conclusions
Uncontrolled hypertension is associated with renal vasoconstriction. It stimulates hypoxia, oxidative stress, autoimmunity, and inflammation, which are involved in the pathophysiological mechanisms that induce salt sensitivity. The particular combination of factors such as elevated sheer stress, a high interstitial ATP concentration, the activation of P2 receptors, and elevated renal interstitial Ang II collectively controls the release of interleukins and growth factors, which contributes to the development of hypertensive renal injury. In addition, the evidence presented in this review suggests that purinergic antagonists may help to prevent the progression of renal damage to chronic kidney disease in hypertensive patients.
Author Contributions
Conceptualization, M.F. and R.B.-P.; writing—review and editing, M.F. and R.B.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chobufo, M.D.; Gayam, V.; Soluny, J.; Rahman, E.U.; Enoru, S.; Foryoung, J.B.; Agbor, V.N.; Dufresne, A.; Nfor, T. Prevalence and control rates of hypertension in the USA: 2017–2018. Int. J. Cardiol. Hypertens. 2020, 6, 100044. [Google Scholar] [CrossRef]
- Ostchega, Y.; Hughes, J.P.; Zhang, G.; Nwankwo, T.; Graber, J.; Nguyen, D.T. Differences in Hypertension Prevalence and Hypertension Control by Urbanization Among Adults in the United States, 2013–2018. Am. J. Hypertens. 2022, 35, 31–41. [Google Scholar] [CrossRef]
- Muselli, M.; Bocale, R.; Necozione, S.; Desideri, G. Is the response to antihypertensive drugs heterogeneous? Rationale for personalized approach. Eur. Heart J. 2024, 26 (Suppl. S1), i60–i63. [Google Scholar] [CrossRef]
- Shokoples, B.G.; Paradis, P.; Schiffrin, E.L. Immunological insights into hypertension: Unraveling triggers and potential therapeutic avenues. Hypertens. Res. 2024, 47, 2115–2125. [Google Scholar] [CrossRef]
- Camargo, L.L.; Rios, F.J.; Montesano, A.C.; Touyz, R.M. Reactive oxygen species in hypertension. Nat. Rev. Cardiol. 2024; ahead of print. [Google Scholar] [CrossRef]
- Guzic, T.J.; Nosalki, T.; Maffia, P.; Drummond, G.R. Immune and inflammatory mechanisms in hypertension. Nat. Rev. Cardiol. 2024, 21, 396–416. [Google Scholar] [CrossRef]
- Hao, X.M.; Liu, Y.; Hailati, D.; Gong, Y.; Zhang, X.D.; Yue, B.N.; Liu, J.P.; Wu, X.L.; Yang, K.Z.; Wang, J.; et al. Mechanisms of inflammation modulation by different immune cells in hypertensive nephrophaty. Front. Immunol. 2024, 13, 1333170. [Google Scholar] [CrossRef]
- Guyton, A.C. The surprising kidney-fluid mechanism for pressure control--its infinite gain. Hypertension 1990, 16, 725–730. [Google Scholar] [CrossRef]
- Guyton, A.C. Blood pressure control: Special role of the kidneys and body fluids. Science 1991, 252, 1813–1816. [Google Scholar] [CrossRef]
- Ivy, J.R.; Bailey, M.A. Pressure natriuresis and the renal control of arterial blood pressure. J. Physiol. 2014, 592, 3955–3967. [Google Scholar] [CrossRef]
- Johnson, R.J.; Herrera-Acosta, J.; Schreiner, G.F.; Rodríguez-Iturbe, B. Subtle acquires renal injury as a mechanism of salt-sensitive hypertension. N. Engl. J. Med. 2002, 346, 913–923. [Google Scholar] [CrossRef]
- Brenner, B.M.; Lawler, E.V.; Mackenzie, H.S. The hyperfiltration theory: A paradigm shifts in nephrology. Kidney Int. 1996, 49, 1774. [Google Scholar] [CrossRef]
- Neuringer, J.R.; Brenner, B.M. Hemodynamic theory of progressive renal disease: A 10-year update in brief review. Am. J. Kidney Dis. 1993, 22, 98–104. [Google Scholar] [CrossRef]
- Tolins, J.P.; Shultz, P.; Raij, L. Mechanisms of hypertensive glomerular injury. Am. J. Cardiol. 1988, 62, 54G–58G. [Google Scholar] [CrossRef]
- Sealey, J.E.; Blumenfield, J.D.; Bell, G.M.; Pecker, M.S.; Sommers, S.C.; Laragh, J.H. On the renal basis for essential hypertension: Nephron heterogeneity with discordant renin secretion and sodium excretion causing hypertensive vasoconstriction-volume relationship. J. Hypertens. 1988, 6, 763–777. [Google Scholar] [CrossRef]
- Franco, M.; Martínez, F.; Rodríguez-Iturbe, B.; Johnson, R.J.; Santamaría, J.; Montoya, A.; Nepomuceno, T.; Bautista, R.; Tapia, E.; Herrera-Acosta, J. Angiotensin II, interstitial inflammation, and the pathogenesis of salt-sensitive hypertension. Am. J. Physiol. Renal Physiol. 2006, 291, F1281–F1287. [Google Scholar] [CrossRef]
- Franco, M.; Tapia, E.; Bautista, R.; Pacheco, U.; Santamaria, J.; Quiroz, Y.; Johnson, R.J.; Rodriguez-Iturbe, B. Impaired pressure natriuresis resulting in salt-sensitive hypertension is caused by tubulointerstitial immune cell infiltration in the kidney. Am. J. Physiol. Renal Physiol. 2013, 304, F982–F990. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. Bioessays 2012, 34, 218–225. [Google Scholar] [CrossRef]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar]
- Schrader, J. Ectonucleotidases as bridge between the ATP and adenosine world: Reflections on Geoffrey Burnstock. Purinergic Signal. 2022, 18, 193–198. [Google Scholar] [CrossRef]
- Van Beusecum, J.; Inscho, E.W. Regulation of renal function and blood pressure control by P2 purinoceptors in the kidney. Curr. Opin. Pharmacol. 2015, 21, 82–88. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergical signaling in the cardiovascular system. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.-J.; Lv, J.; Cao, X. Purinoceptor: A novel target for hypertension. Purinergic Signal. 2023, 19, 185–197. [Google Scholar] [CrossRef]
- Soares, A.G.; Contreras, J.; Mironova, E.; Archer, C.R.; Stockand, J.D.; Abd El-Aziz, T.M. P2Y2 receptor decreases blood pressure by inhibiting ENaC. JCI Insight 2023, 24, e167704. [Google Scholar] [CrossRef]
- Toney, G.M.; Vallon, V.; Stockand, J.D. Intrinsic control of sodium excretion in the distal nephron by inhibitory purinergic regulation of the epithelial Na+ channel. Curr. Opin. Nephrol. Hypertens. 2012, 21, 52–60. [Google Scholar] [CrossRef]
- Menzies, R.I.; Frederick, T.W.T.; Unwin, R.J.; Bailey, M.A. Purinergic signaling in kidney disease. Kidney Int. 2017, 91, 315–316. [Google Scholar] [CrossRef]
- Nishiyama, A.; Majid, D.S.; Taher, K.A.; Miyatake, A.; Navar, L.G. Relation between interstitial ATP concentrations and autoregulaion-mediated changes in vascular resistance. Cir. Res. 2000, 86, 656–662. [Google Scholar] [CrossRef]
- Palygin, O.; Evans, L.C.; Cowley, A.W., Jr.; Staruschenko, A. Acute in vivo analysis of ATP release in rat kidneys in response to changes of renal perfusion pressure. J. Am. Heart Assoc. 2017, 6, e006658. [Google Scholar] [CrossRef]
- Yamamoto, K.; Korenaga, R.; Kamiya, A.; Ando, J. Fluid shear stress activates Ca2+ influx into human endothelial cells via P2X4 purinoceptors. Circ. Res. 2000, 87, 385–391. [Google Scholar] [CrossRef]
- Vallon, V.; Unwin, R.; Inscho, E.W.; Leipziger, J.; Kishore, B.K. Extracellular nucleotides and P2 receptors in renal function. Physiol. Rev. 2020, 100, 211–269. [Google Scholar] [CrossRef]
- Ji, X.; Naito, Y.; Hirokawa, G.; Weng, H.; Hiura, Y.; Takahashi, R.; Iwai, N. 2X (7) receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertens. Res. 2012, 35, 173–179. [Google Scholar] [CrossRef]
- Franco, M.; Bautista, R.; Tapia, E.; Soto, V.; Santamaría, J.; Osorio, H.; Pacheco, U.; Sánchez-Lozada, L.G.; Kobori, H.; Navar, L.G. Contribution of renal purinergic receptors to renal vasoconstriction in angiotensin II-induced hypertensive rats. Am. J. Physiol. Renal Physiol. 2011, 300, F1301–F1309. [Google Scholar] [CrossRef]
- Vonend, O.; Turner, C.M.; Chan, C.M.; Loesch, A.; DellÄnn, G.C.; Srai, K.S.; Burnstock, G.; Unwin, R.J. Glomerular exression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int. 2004, 66, 157–166. [Google Scholar] [CrossRef][Green Version]
- Franco, M.; Bautista-Pérez, R.; Cano-Martínez, A.; Pacheco, U.; Santamaría, J.; Del Valle-Mondragón, L.; Pérez-Méndez, O.; Navar, L.G. Physiopathological implications of P2X1 and P2X7 receptors in regulation of glomerular hemodynamics in angiotensin II-induced hypertension. Am. J. Physiol. Renal Physiol. 2017, 312, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Menzies, R.I.; Unwin, R.J.; Bailey, M.A. Renal P2 receptors and hypertension. Acta Physiol. 2015, 213, 232–241. [Google Scholar] [CrossRef]
- Menzies, R.I.; Unwin, R.J.; Rash, R.; Beard, D.A.; Cowley, A.W., Jr.; Carlson, B.E.; Mullins, J.J.; Bailey, M.A. Effect of P2X4 and P2X7 receptor antagonism on the pressure diuresis relationship in rats. Front. Physiol. 2013, 4, 235. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.D.; Komlosi, P.; Zhang, Z.R. ATP as a mediator of macula densa cell signalling. Purinergic Signal. 2009, 5, 461–471. [Google Scholar] [CrossRef]
- Osmond, D.A.; Inscho, E.W. P2X(1) Receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. Am. J. Physiol. Renal Physiol. 2010, 298, F1360–F1368. [Google Scholar] [CrossRef]
- Wang, D.J.; Huang, N.N.; Heppel, L.A. Extracellular ATP and ADP stimulate proliferation of porcine aortic smooth muscle cells. J. Cell Physiol. 1992, 153, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Erlinge, D.; Yoo, H.; Edvinsson, L.; Reis, D.J.; Wahlestedt, C. Mitogenic effects of ATP on vascular smooth muscle cells vs. other growth factors and sympathetic cotransmitters. Am. J. Physiol. Heart Circ. Physiol. 1993, 265, H1089–H1097. [Google Scholar] [CrossRef]
- Johnson, R.J.; Alpers, C.E.; Yoshimura, A.; Lombardi, D.; Prinzl, P.; Floege, J.; Schwartz, S.M. Renal injury from angiotensin II-mediated hypertension. Hypertension 1992, 19, 464–474. [Google Scholar] [CrossRef]
- Lombardi, D.; Gordon, K.L.; Polinsky, P.; Suga, S.; Schwartz, S.M.; Johnson, R.J. Salt-sensitive hypertension develops after short term exposure to angiotensin II. Hypertension 1999, 33, 1013–1019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Franco, M.; Pérez-Méndez, O.; Kulthinee, S.; Navar, L.G. Integration of purinergic and angiotensin II receptor function in renal vascular responses and renal injury in angiotensin II-dependent hypertension. Purinergic Signal. 2019, 15, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Naito, Y.; Weng, H.; Endo, K.; Ma, X.; Iwai, N. P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am. J. Physiol. Renal Physiol. 2012, 303, F1207–F1215. [Google Scholar] [CrossRef] [PubMed]
- Menzies, R.I.; Howarth, A.R.; Unwin, R.J.; Frederick, W.K.; Mullis, J.J.; Bailey, M.A. Inhibition of the purinergic P2X7 receptor improves renal perfusion pressure in angiotensin-II-infused rats. Kidney Int. 2015, 88, 1079–1087. [Google Scholar] [CrossRef]
- Turner, C.M.; Vonend, O.; Chan, C.; Burnstock, G.; Unwin, R.J. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: An immunohistologycal study. Cells Tissues Organs 2003, 175, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Graciano, M.L.; Nishiyama, A.; Jackson, K.; Seth, D.M.; Ortiz, R.M.; Prieto-Carrasquero, M.; Kobori, H.; Navar, L.G. Purinergic Receptors Contribute to Early Mesangial Transformation and Renal Vessel Hypertrophy during Angiotensin II-Induced Hypertension. Am. J. Physiol. Renal Physiol. 2008, 294, F161–F169. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Liu, S.C.; Hu, S.Q.; Lu, J.F.; Wu, C.L.; Hu, D.X.; Zhang, W.J. ATP ion channel P2X purinergic receptors in inflammation response. Biomed. Pharmacother. 2023, 158, 114205. [Google Scholar] [CrossRef] [PubMed]
- Wihlborg, A.K.; Wang, L.; Braun, O.O.; Eyjolfsson, A.; Gustafsson, R.; Gudbjartsson, T.; Erlinge, D. ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.; Koch-Nolte, F.; Dahl, G. Purine Release, Metabolism, and signaling in the inflammatory response. Annu. Rev. Immunol. 2019, 37, 325–347. [Google Scholar] [CrossRef]
- Takenaka, T.; Inoue, T.; Kanno, Y.; Osaka, H.; Hill, C.E.; Suzuki, H. Conexin 37 and 40 transduce purinergic signals mediating renal autoregulation. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R1–R11. [Google Scholar] [CrossRef]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Bautista-Pérez, R.; Pérez-Méndez, O.; González, L.; Pacheco, U.; Sánchez-Lozada, L.G.; Tapia, E.; Martínez, F. Renal interstitial adenosine is increased in angiotensin II-induced hypertensive rats. Am. J. Physiol. Renal Physiol. 2008, 294, F84–F92. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.G.; Navar, L.G. Afferent arteriolar vasodilator effect of adenosine predominantly involves A2B receptor activation. Am. J. Physiol. Renal Physiol. 2010, 299, F310–F315. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Pittman, K.; Menezes, G.B.; Hirota, S.A.; Slaba, I.; Waterhouse, C.C.; Beck, P.L.; Muruve, D.A.; Kubes, P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010, 330, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Dosh, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP release by inflammatory cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, C.; Guo, C.; Lund, H.; Feng, D.; Mattson, D.L. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am. J. Physiol. Renal Physiol. 2011, 300, F734–F742. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.M.; Ling, Y.H.; Huuskes, B.M.; Ferens, D.M.; Saini, N.; Chan, C.T.; Diep, H.; Kett, M.M.; Samuel, C.S.; Kemp-Harper, B.K.; et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc. Res. 2019, 115, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Gomvault, A.; Baron, L.; Couillin, I. ATP reléase and purinergic signalling in NLRP3 inflammasome activation. Front. Immunol. 2012, 3, 414. [Google Scholar] [CrossRef]
- Karmakar, M.; Katsnelson, M.A.; Dubyak, G.R.; Pearlman, E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1beta secretion in response to ATP. Nat. Commun. 2016, 7, 10555. [Google Scholar] [CrossRef]
- Juliana, C.; Fernandes-Alnemri, T.; Kang, S.; Farias, A.; Qin, F.; Alnemri, E.S. Nontranscriptional priming and deubiquitylation regulate NLRP3 inflammasome activation. J. Biol. Chem. 2012, 287, 36617–36622. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.M.; Gavala, M.L.; Lenertz, L.Y.; Bertics, P.J. Extracellular ATP may contribute to tissue repair by rapidly stimulating purinergic receptor X7-dependent vascular endothelial growth factor release from primary human monocytes. J. Immunol. 2010, 185, 3028–3034. [Google Scholar] [CrossRef] [PubMed]
- Mattson, D.L.; James, L.; Berdan, E.A.; Meister, C.J. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 2006, 48, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Martínez, F.; Quiroz, Y.; Galicia, O.; Bautista, R.; Johnson, R.J.; Rodríguez-Iturbe, B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R251–R256. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Rodríguez-Iturbe, B. Mechanisms of disease: Oxidative stress and iflammation in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2006, 2, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Tapia, E.; Santamaría, J.; Zafra, I.; García-Torres, R.; Gordon, K.L.; Pons, H.; Rodríguez-Iturbe, B.; Johnson, J.R.; Herrera-Acosta, J. Renal Cortical Vasoconstriction Contributes to the Development of Salt Sensitive Hypertension after Angiotensin II Exposure. J. Am. Soc. Nephrol. 2001, 10, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.D.; Song, Y.S.; Lin, E.E.; Griffiths, R.; Kim, H.S.; Ruiz, P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1089–R1097. [Google Scholar] [CrossRef]
- Hoch, N.E.; Guzik, T.J.; Chen, W.; Deans, T.; Maalouf, S.A.; Gratze, P.; Weyand, C.; Harrison, D.G. Regulation of T-cell function by endogenously produced angiotensin II. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R208–R216. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.S.; McCormack, M.; Semprum-Prieto, L.S.; Shenouda, S.; Majid, D.S.; Kobori, H.; Navar, L.G.; Prieto, M.C. AT1 receptor-mediated augmentation of angiotensinogen, oxidative stress, and inflammation in ANG II-salt hypertension. Am. J. Physiol. Renal Physiol. 2012, 302, F85–F94. [Google Scholar] [CrossRef]
- Mironova, E.; Boiko, N.; Bugaj, V.; Kucher, V.; Stockand, J.D. Regulation of Na+ excretion and arterial blood pressure by purinergic signalling intrinsic to the distal nephron: Consequences and mechanisms. Acta Physiol. 2015, 213, 213–221. [Google Scholar] [CrossRef]
- Song, J.; Lu, Y.; Lai, E.Y.; Wei, J.; Wang, L.; Chandrashekar, K.; Wang, S.; Shen, C.; Juncos, L.A.; Liu, R. Oxidative status in the macula densa modulates tubuloglomerular feedback responsiveness in angiotensin II-induced hypertension. Acta Physiol. 2015, 213, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Matavelli, L.C.; Zhou, X.; Varagic, J.; Susic, D.; Frohlich, E.D. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H814–H819. [Google Scholar] [CrossRef] [PubMed]
- Sannai, T.; Kimur, G. Renal function reserve and sodium sensitivity in essential hypertension. J. Lab. Clin. Med. 1996, 128, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Leh, S.; Hultström, M.; Rosenberger, C.; Iversen, B.M. Afferent arteriolopathy and glomerular collapse but not segmental sclerosis induce tubular atrophy in old spontaneously hypertensive rats. Virchows Arch. 2011, 459, 99–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burnstock, G. Purinergic Signalling: Therapeutic developments. Front. Pharmacol. 2017, 8, 661. [Google Scholar] [CrossRef]
- Soni, S.; Lukhey1, M.S.; Baban, S.; Thawkar, B.S.; Chintamaneni, M.; Kaur, G.; Joshi, H.; Ramniwas, S.; Tuli, H.S. A current review on P2X7 receptor antagonist patents in the treatment of neuroinflammatory disorders: A patent review on antagonists. Naunyn-Schmiedeberg’s Arch Pharmacol. 2024, 397, 4643–4656. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.-G.; Huang, C.; et al. From purines to purinergic signalling: Molecular functions and human diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).