Abstract
Trastuzumab emtansine (T-DM1) is a mainstay therapy for HER2-positive metastatic breast cancer (mBC). However, identifying patients who will benefit most remains a challenge due to the lack of reliable biomarkers. The recently developed pan-immune-inflammation value (PIV), a novel immune-inflammation marker, could aid in this regard, considering the immunomodulatory effects of T-DM1. Therefore, we aimed to evaluate the association between the PIV and the efficacy of T-DM1 in patients with HER2-positive mBC. A total of 122 HER2-positive mBC patients treated with T-DM1 were included. Receiver operating characteristic (ROC) curve analyses were conducted to determine the optimal PIV threshold value for survival prediction. Kaplan–Meier survival curves and Cox regression analyses were used for univariable and multivariable survival analyses, respectively. The median age was 51 years, and 95.1% of the patients had ECOG PS 0-1. The optimal PIV cutoff value was identified as 338 in ROC analyses (AUC: 0.667, 95% CI: 0.569–0.765, p = 0.002). The multivariate analysis revealed that patients in the high-PIV group had significantly shorter OS (HR: 2.332; 95% CI: 1.408–3.861; p = 0.001) and PFS (HR: 2.423; 95% CI: 1.585–3.702; p < 0.001) than patients in the low-PIV group. Additionally, both ORR and DCR were significantly lower in the high-PIV group (36.6% vs. 61.3%, p = 0.011; 56.1% vs. 76.0%, p = 0.027). Our findings suggest that pre-treatment PIV may be a novel prognostic biomarker for HER2-positive mBC patients receiving T-DM1. A low PIV level is associated with more favorable outcomes. Future prospective studies are warranted to validate these findings and explore the potential utility of PIV in aiding treatment decisions.
1. Introduction
Human epidermal growth factor receptor-2 (HER2) gene amplification occurs in approximately 20% of invasive breast cancers and portends a poorer prognosis with higher rates of recurrence and shorter progression-free survival (PFS) and overall survival (OS) [1]. However, the emergence of HER2-targeted therapies, including tyrosine kinase inhibitors, monoclonal antibodies, and antibody–drug conjugates (ADCs) has significantly altered the therapeutic landscape for HER2-positive metastatic breast cancer (mBC) [2].
T-DM1 is an antibody–drug conjugate (ADC) that combines the HER2-targeting activity of the monoclonal antibody trastuzumab with the cytotoxic effect of the microtubule-disrupting agent emtansine [3]. In the phase III EMILIA trial, T-DM1 demonstrated a significant improvement in PFS (9.6 vs. 6.4 months; HR: 0.65; 95% CI: 0.55–0.77; p < 0.001 and OS 30.9 vs. 25.1 months; HR: 0.68; 95% CI: 0.55–0.85; p < 0.001) compared with capecitabine plus lapatinib for patients with HER2-positive mBC who were previously treated with trastuzumab and a taxane-based treatment [4]. Another phase III trial, TH3RESA, confirmed improvements in both PFS and OS with T-DM1 in HER2-positive mBC patients who had progressed on at least two prior HER2-targeted therapies [5].
While T-DM1 improved outcomes in the second or later lines of treatment of HER2+ mBC, the progression is inevitable for most patients, and reliable biomarkers for long-term efficacy and early progression are absent. A study by Müller et al. suggests that the benefit of T-DM1 in HER2-positive breast cancer might partly be due to immune system activation, as shown by an increase in tumor-infiltrating lymphocytes (TILs) following neoadjuvant treatment [6]. A recent study illustrates that immunogenic cell death is a key mechanism of action of T-DM1 [7]. These findings suggest that the benefit of T-DM1 might be partially facilitated through an immune response against breast cancer, and the efficacy of T-DM1 could be predicted by the reflectors of the immune-inflammatory system. The pan-immune-inflammation value (PIV), a recently established marker of tumor immunity in early and advanced breast cancer, could be a useful biomarker in this regard [8,9]. The rationale for establishing the PIV as a prognostic marker lies in the understanding of the pivotal role of inflammation in cancer progression [9]. Elevated PIV values have been associated with poorer outcomes across various cancer types, as they often indicate an enhanced inflammatory milieu [10,11,12]. However, the association between the PIV levels and survival with T-DM1 was not investigated previously. Given the absence of established prognostic or predictive biomarkers for T-DM1 therapy in HER2-positive mBC, we aimed to assess PIV’s utility as a potential indicator of treatment efficacy.
2. Results
2.1. Baseline Characteristics
A total of 122 patients were included. The median age was 51 years (IQR 43–61) at T-DM1 initiation. Most patients (76.2%) exhibited HER2 IHC 3+, and 54.9% (n = 67) were hormone-receptor-positive (ER+ or PR+). The majority of patients had only one organ with metastasis at T-DM1 treatment initiation (65.6%); the most common were bone, brain, and lung (33.6%, 27.0%, and 24.6%, respectively). Prior to T-DM1, the percentages of patients receiving pertuzumab, lapatinib, and anthracycline therapy were 36.9%, 21.3%, and 55.7%, respectively. T-DM1 administration varied across treatment lines as a second-line (43.5%), third-line (29.5%), or later-line (27.0%) treatment (Table 1).

Table 1.
Baseline patient characteristics of study cohort (n = 122).
The ROC analysis with OS as the endpoint indicated that the optimal PIV cutoff value was 338 (AUC: 0.667, 95% CI: 0.569–0.765, p = 0.002) (Figure 1). Patients were categorized into the low-PIV and high-PIV groups based on this PIV value. Demographics and baseline characteristics were balanced for both groups, except for a significantly higher proportion of patients in the high-PIV group receiving pertuzumab prior to T-DM1 treatment compared to the low-PIV group (p = 0.044) (Table 2).
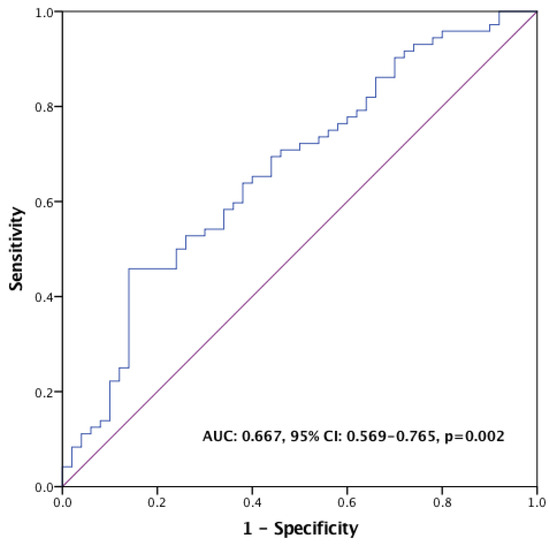
Figure 1.
ROC curve for PIV in the prediction of overall survival.

Table 2.
Comparisons of patient characteristics in the low-PIV and high-PIV groups (n = 122).
2.2. Survival Outcomes
After a median of 23.4 months of follow-up, 72 (59%) patients died, and 100 (82%) patients had a PFS event. The median OS was 33.5 months (95% CI, 26.1–40.9), and the median PFS was 9.7 months (95% CI, 7.7–11.8). Notably, the median OS was significantly lower in the high-PIV group (17.6 months) compared to the low-PIV group (38.1 months) (HR = 2.21; 95% CI: 1.39–3.54; p = 0.001) (Figure 2). Similarly, patients with high PIV levels who were treated with T-DM1 exhibited a shorter median PFS (6.47 months) compared to those with low PIV levels (10.84 months) (HR = 2.34; 95% CI: 1.54–3.55; p < 0.001) (Figure 3).
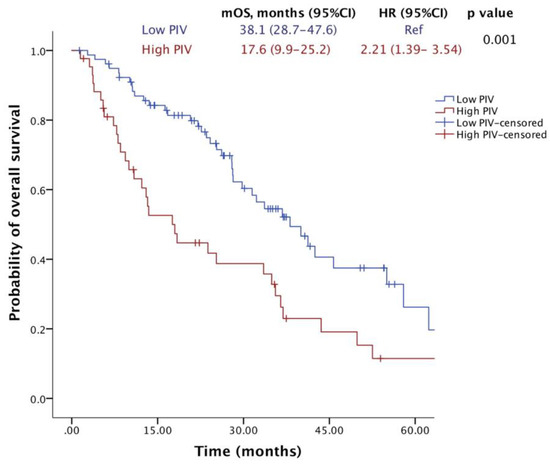
Figure 2.
Overall survival of patients receiving T-DM1 according to PIV levels.
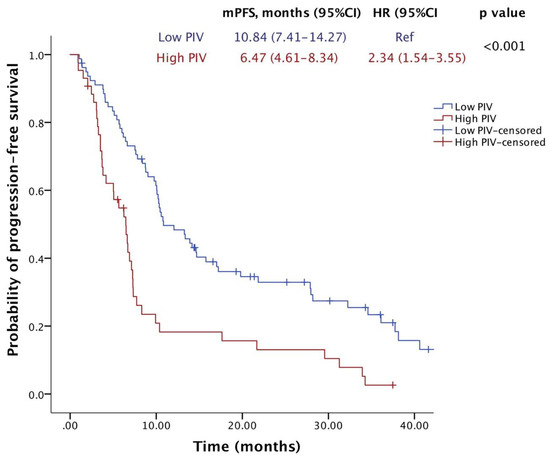
Figure 3.
Progression-free survival of patients receiving T-DM1 according to PIV levels.
In addition to higher PIV levels, higher NLR levels (high vs. low, p = 0.023), higher ECOG score (0 vs. 1–2, p = 0.005), receiving T-DM1 as a later line of treatment (≥2 vs. <2, p = 0.017), and prior use of pertuzumab (p = 0.047) were significantly associated with a worse OS in univariate analyses (Table 3). Similarly, a higher number of metastatic sites (≥2 vs. <2, p = 0.001), receiving T-DM1 as a later line of treatment (≥2 vs. <2, p = 0.010), and higher PIV levels (p < 0.001) were significant factors for PFS in univariate analyses. In multivariate analyses, patients with higher PIV levels (HR: 2.332, 95% CI: 1.408–3.861, p = 0.001), a higher number of metastatic sites (HR: 1.603, 95% CI 1.026–2.685, p = 0.046), and higher ECOG status scores (HR 2.726, 95% CI: 1.628–4.564, p < 0.001) had decreased OS. As for PFS, higher PIV levels (HR 2.423, 95% CI 1.585–3.702, p < 0.001) and receiving T-DM1 in a later line (HR 1.735, 95% CI 1.141–2.637, p = 0.010) were significant independent factors in multivariable analysis (Table 4).

Table 3.
Univariable and multivariable cox regression analyses for OS.

Table 4.
Univariable and multivariable cox regression analyses for PFS.
2.3. Radiological Responses
The ORR was 52.6% in the overall population; 18 patients (15.5%) experienced CR, and 43 patients (36%) experienced PR. Another 16.4% of patients achieved SD, which corresponds to a DCR of 69%; 31% had progressive disease (PD) as their best response. The high-PIV group exhibited significantly lower ORR and DCR compared to the low-PIV group (36.6% vs. 61.3%, p = 0.011; 56.1% vs. 76.0%, p = 0.027) (Figure 4).
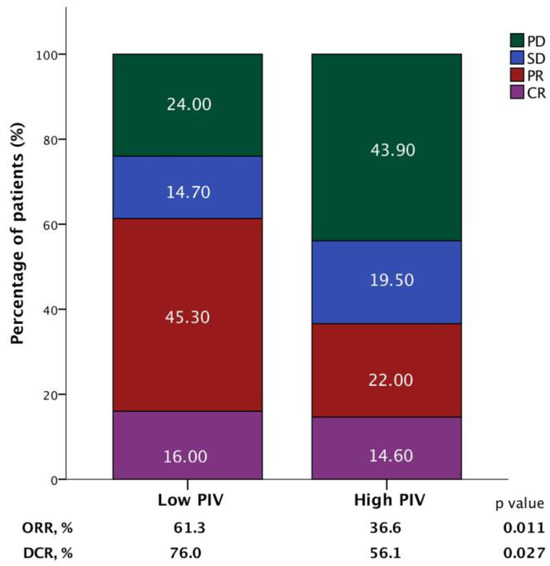
Figure 4.
Bar graphs demonstrating the association between the objective response rate (ORR) and disease control rate (DCR) according to the pan-immune-inflammation value (PIV) in patients receiving T-DM1.
3. Discussion
In the present study, we demonstrated that lower baseline PIV levels were significantly associated with improved OS and PFS compared to higher PIV levels. This association was observed independently of age, hormone receptor status, or HER2 status. We also demonstrated a statistically significant association with the treatment response, as evidenced by higher ORR and DCR in the low-PIV group compared to the high-PIV group. To the best of our knowledge, this study represents the first study to evaluate the association between PIV level and survival outcomes in patients with HER2-positive mBC receiving T-DM1.
Following the pivotal phase III trials EMILIA [4] and TH3RESA [5], which established T-DM1 as a standard therapeutic option for HER2-positive breast cancer, this single-center retrospective analysis offers valuable real-world data on treatment effectiveness. Our study population demonstrated a median PFS of 9.7 months, which is comparable to the 9.6 months reported in the EMILIA study and longer than the 6.2 months reported in the TH3RESA study. Similarly, the observed OS of 33.5 months surpassed the 30.9 months and 22.7 months reported in EMILIA and TH3RESA, respectively. This disparity in OS might be attributable to potential differences in the prior lines of therapy received by patients enrolled in these trials. Although T-DM1 has become a promising treatment for HER2-positive breast cancer, the factors affecting its effectiveness are still unclear.
Trastuzumab deruxtecan (T-DXd) is a recently developed ADC incorporating a humanized anti-HER2 antibody, a peptide-based cleavable linker, and a novel potent topoisomerase I inhibitor payload [13]. The DESTINY-Breast03 trial demonstrated a significant improvement in both PFS and OS with T-DXd compared to T-DM1 in patients with HER2-positive mBC who had previously been treated with trastuzumab and a taxane [14]. These findings led to FDA approval of T-DXd as a second-line treatment of adult patients with HER2-positive mBC. Despite demonstrating superior efficacy in trials, limited national health insurance coverage restricts access to T-DXd, making T-DM1 the more frequently utilized second-line therapy in clinical practice. It is also important to consider the toxicity profiles of these treatments; T-DXd has been associated with significant adverse effects, including interstitial lung disease (ILD) and pneumonitis, which require vigilant monitoring due to their potential severity. In contrast, T-DM1 generally exhibits a more favorable toxicity profile, with most adverse events being manageable and less severe, such as thrombocytopenia and elevated liver enzymes. This difference in toxicity profiles is crucial when evaluating the overall risk–benefit ratio of these therapies for individual patients.
The significant correlation between a high HER2 expression level and improved T-DM1 efficacy is well documented and appears likely to be mediated by increased HER2-mediated endocytosis and subsequent lysosomal degradation of the antibody–drug conjugate [15,16]. This mechanism potentially leads to higher intracellular concentrations of the cytotoxic payload, DM1, within HER2-overexpressing cancer cells [16]. High HER2 mRNA levels were found to be associated with better tumor responses and PFS in the metastatic setting treated with T-DM1 [17]. Interestingly, Baselga et al. revealed a more pronounced effect of T-DM1 on OS compared to PFS in patients with high HER2 mRNA levels [16]. This observation suggests that T-DM1 may exert its anti-tumor effects beyond direct HER2 targeting.
The therapeutic landscape for cancer is shifting towards a deeper understanding of the interplay between the anti-tumor immune response and direct cytotoxic effects of anti-cancer therapies [18]. Tumor-infiltrating lymphocytes (TILs) are a readily obtainable biomarker of the intra-tumoral immune response and are gaining interest as promising predictors of treatment response [19]. The KRISTINE phase III study demonstrated a positive association between TIL infiltration and the response to T-DM1 treatment [20]. Moreover, the KRISTINE study revealed that higher expressions of both HER2 and immune markers within the tumor microenvironment correlated with higher pCR rates, suggesting that T-DM1 sensitivity might be linked to the activation of the anti-tumor immune response [20]. Additionally, a recent study demonstrated that immunogenic cell death induction is one of the mechanisms of T-DM1 sensitivity in vitro and in vivo [7]. Collectively, this growing body of evidence underscores the possibility that T-DM1’s efficacy may be partially mediated by immunity.
Although pre-clinical data suggest that the T-DM1’s anti-tumor mechanism involves immune system activation, the clinical utility of peripheral blood immune cell subsets as prognostic markers in HER2-positive mBC patients receiving T-DM1 therapy remains to be elucidated. The evaluation of the neutrophil–lymphocyte ratio (NLR) or platelet–lymphocyte ratio (PLR) in the HER2-positive breast cancer subgroup did not identify a statistically significant correlation with the clinical outcomes [21]. Similarly, Ulas et al. reported no such significant association between NLR or PLR and survival outcomes in HER2-positive BC patients treated with adjuvant trastuzumab [22]. Conversely, Imamura et al. observed a significant correlation between elevated baseline NLR and worse PFS and OS in HER2-positive mBC patients treated with second line T-DM1 [23]. This finding is intriguing, especially considering the potential of T-DM1 to induce immune activation, as evidenced by a significant increase in lymphocytes following T-DM1 initiation in patients with low NLR. These conflicting findings underscore the need for further research to definitively establish the prognostic utility of the blood cell parameters HER2+ mBC, and these findings may ultimately contribute to unraveling the mechanism and potentially aid in patient selection for optimal treatment benefit for T-DM1.
Emerging evidence suggests the PIV as a novel promising predictor of clinical outcomes in cancer patients [24]. Fuca et al. demonstrated that PIV showed a robust association with both OS and PFS in advanced colorectal cancer, and PIV’s prognostic capacity appears to surpass that of other well-established inflammatory-immune markers, such as NLR and PLR, possibly due to the inclusion of four different peripheral blood cell indices. A recent meta-analysis encompassing 15 studies and approximately 5000 patients found a significant association between elevated PIV and worse OS (HR = 2.00, 95% CI: 1.51–2.64) and PFS (HR = 1.80, 95% CI: 1.39–2.32) in cancer patients [10]. Compared to individual blood cell parameters, PIV can provide a more comprehensive reflection of the complexity of the immune landscape and various cellular components, each potentially reflecting and regulating distinct aspects of anti-tumor immunity [25].
Peripheral blood lymphocyte levels may reflect an immune reaction or potential immunity against tumor-associated antigens [26]. In contrast, neutrophils impede anti-tumor immunity through the release of different pro-tumorigenic cytokines, growth factors, and chemokines, fostering tumor progression via mechanisms such as neo-angiogenesis, metastasis, and the creation of an immunosuppressive tumor microenvironment [27,28]. Intriguingly, macrophages potentially suppress the anti-tumor immune response of T lymphocytes by releasing chemokines, contributing to the immunosuppressive state [29]. Collectively, neutrophils, monocytes, and platelets exhibit a pro-inflammatory signature in the peripheral blood of cancer patients, potentially reflecting the host’s inflammatory response to the tumor. In contrast, lymphocyte levels may serve as a biomarker for the immune system’s modulatory potential during cancer therapy. Since PIV integrates both pro-tumor and anti-tumor factors within the TME, it has the potential to serve as a surrogate marker for the degree of immunosuppression. A growing body of evidence implicates that a high PIV may represent an immune-suppressive state in the TME. We therefore postulated that T-DM1 efficacy may be higher in patients with a low PIV, which reflects lower immune suppression, and T-DM1-associated immune induction may be expected. Consistent with this hypothesis, we found a robust and independent impact of baseline PIV on survival outcomes, with higher PIV levels predicting worse PFS and OS outcomes in patients receiving T-DM1 therapy.
The present study has certain limitations. First, the single-center and retrospective design of the study restricts the generalizability of the results to the entire HER2-positive mBC population. Additionally, the retrospective data collection approach introduces the possibility of selection bias, potentially influencing the observed correlations between PIV and clinical outcomes. Our analysis did not include TILs, which were previously found to predict the clinical outcomes in the CLEOPATRA trial [30]. Finally, the optimal cutoff value for PIV remains debated, but our study chose a more stringent methodology for cutoff selection to enhance accuracy. However, despite these limitations, a robust association between baseline PIV levels and survival outcomes was observed.
4. Materials and Methods
4.1. Patient Population
Patients diagnosed with HER2-positive mBC who received T-DM1 between January 2013 and December 2023 at Hacettepe University Oncology Hospital were enrolled in this study. HER2 positivity was defined by an immunohistochemistry (IHC) score of 3 or fluorescence in situ hybridization (FISH) positivity with an IHC score of 2, as per the ASCO CAP guidelines. Patients were followed for survival data until 21 March 2024, until either the date of the last available patient record or censored date. Patients with missing clinical data were excluded.
Baseline characteristics including demographic features, Eastern Cooperative Oncology Group (ECOG) performance status, HER2 status, hormonal status, metastatic sites at diagnosis, prior lines of therapy before T-DM1 (trastuzumab, pertuzumab, lapatinib, antracycline, and taxane), and baseline laboratory parameters were collected along with the survival data. Pre-treatment complete blood count (CBC) data used for PIV calculation were collected within the week preceding T-DM1 therapy initiation. The PIV was calculated using the established formula: [monocyte count (103/mL) × neutrophil count (103/mL) × platelet count (103/mL)]/lymphocyte count (103/mL) [31].
This study was approved by the Hacettepe University Institutional Review Board (Approval Number: SBA 24/456) and conducted in line with ethical standards established in the Helsinki Declaration and its subsequent amendments. All applicable local and national regulations were complied with in the study.
4.2. Statistical Analyses
Continuous variables were expressed as median and interquartile range (IQR), while categorical variables were summarized as percentages and frequencies. The optimal PIV cutoff value for predicting survival outcomes was determined by Youden’s J index from receiver operating characteristic (ROC) curve analysis [32]. Based on this cutoff value, patients were divided into high-PIV and low-PIV groups. The comparison of patient characteristics between the PIV groups involved using the Mann–Whitney U test for continuous data and Fisher’s exact test or chi-square test for categorical variables.
PFS was defined as the time from first T-DM1 administration to disease progression or death, whichever occurred first. OS was defined as time from the first T-DM1 administration to last follow-up and/or death. The assessment of tumor response followed the Response Evaluation Criteria in Solid Tumors (RECIST) guideline version 1.1 [33]. The overall response rate (ORR) was the proportion of patients achieving a complete response (CR) or partial response (PR) as their best overall response. The disease control rate (DCR) was the proportion of patients achieving CR, PR, or stable disease (SD) as the best overall response. Kaplan–Meier analyses were used to estimate survival analysis, and the log-rank test compared survival times between prognostic subgroups. Multivariable Cox proportional hazards models quantified the independent effects of prognostic factors on survival with hazard ratios (HRs) and 95% confidence intervals (CIs). The multivariable survival models were constructed with the inclusion of parameters with p values below 0.10 in the univariate analyses via backwards variable selection. Data were analyzed with SPSS, version 25.0 (IBM Corp., Armonk, NY, USA). A p value of less than 0.05 was considered significant.
5. Conclusions
In conclusion, our findings suggest that baseline PIV levels may serve as a predictive marker for T-DM1 efficacy. Further prospective studies are required to confirm these findings, elucidate the mechanism, and ultimately optimize patient selection for improved clinical outcomes.
Author Contributions
Conceptualization, T.K.S., D.C.G. and S.A.; methodology, T.K.S., A.A., O.T.D. and G.K.; formal analysis, T.K.S., A.A., O.T.D. and D.C.G.; investigation, T.K.S., A.A., G.K., D.C.G. and S.A.; writing—original draft preparation, T.K.S., A.A., O.T.D., G.K., D.C.G. and S.A.; writing—review and editing, D.C.G. and S.A.; visualization, T.K.S., A.A., O.T.D., G.K., D.C.G. and S.A.; supervision, D.C.G. and S.A.; project administration, T.K.S., D.C.G. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Hacettepe University (Approval Number: SBA 24/456).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cronin, K.A.; Harlan, L.C.; Dodd, K.W.; Abrams, J.S.; Ballard-Barbash, R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Investig. 2010, 28, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, G.; Gampenrieder, S.P.; Greil, R. HER2 Directed Antibody-Drug-Conjugates beyond T-DM1 in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 1115. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Kim, S.B.; Martin, A.G.; LoRusso, P.M.; Ferrero, J.M.; Badovinac-Crnjevic, T.; Hoersch, S.; Smitt, M.; Wildiers, H. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): Final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017, 18, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Kreuzaler, M.; Khan, T.; Thommen, D.S.; Martin, K.; Glatz, K.; Savic, S.; Harbeck, N.; Nitz, U.; Gluz, O. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 2015, 7, ra188–ra315. [Google Scholar] [CrossRef] [PubMed]
- Gedik, M.E.; Saatci, O.; Oberholtzer, N.; Uner, M.; Akbulut-Caliskan, O.; Cetin, M.; Aras, M.; Ibis, K.; Caliskan, B.; Banoglu, E.; et al. Targeting TACC3 induces immunogenic cell death and enhances T-DM1 response in HER2-positive breast cancer. Cancer Res. 2024, 84, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhang, L.P.; Xie, S.Y.; Huang, H.Y.; Chen, X.Y.; Jiang, T.C.; Guo, L.; Lin, H.X. Pan-Immune-Inflammation Value: A New Prognostic Index in Operative Breast Cancer. Front. Oncol. 2022, 12, 830138. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, F.; Fucà, G.; Zattarin, E.; Lobefaro, R.; Zambelli, L.; Leporati, R.; Rea, C.; Mariani, G.; Bianchi, G.V.; Capri, G.; et al. The Pan-Immune-Inflammation-Value Predicts the Survival of Patients with Human Epidermal Growth Factor Receptor 2 (HER2)—Positive Advanced Breast Cancer Treated with First-Line Taxane-Trastuzumab-Pertuzumab. Cancers 2021, 13, 1964. [Google Scholar] [CrossRef]
- Su, Z.; Tang, J.; He, Y.; Zeng, W.H.; Yu, Q.; Cao, X.L.; Zou, G.R. Pan-immune-inflammation value as a novel prognostic biomarker in nasopharyngeal carcinoma. Oncol. Lett. 2024, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Şahin, A.B.; Cubukcu, E.; Ocak, B.; Deligonul, A.; Oyucu Orhan, S.; Tolunay, S.; Gokgoz, M.S.; Cetintas, S.; Yarbas, G.; Senol, K.; et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci. Rep. 2021, 19, 14662. [Google Scholar] [CrossRef] [PubMed]
- Efil, S.C.; Guner, G.; Guven, D.C.; Celikten, B.; Celebiyev, E.; Taban, H.; Akyol, A.; Isik, A.; Kilickap, S.; Yalcin, S.; et al. Prognostic and predictive value of tumor infiltrating lymphocytes in combination with systemic inflammatory markers in colon cancer. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102171. [Google Scholar] [CrossRef] [PubMed]
- Azar, I.; Alkassis, S.; Fukui, J.; Alsawah, F.; Fedak, K.; Al Hallak, M.N.; Sukari, A.; Nagasaka, M. Spotlight on Trastuzumab Deruxtecan (DS-8201,T-DXd) for HER2 Mutation Positive Non-Small Cell Lung Cancer. Lung Cancer (Auckl.) 2021, 12, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Leyton, J.V. Improving Receptor-Mediated Intracellular Access and Accumulation of Antibody Therapeutics-The Tale of HER2. Antibodies 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Lewis Phillips, G.D.; Verma, S.; Ro, J.; Huober, J.; Guardino, A.E.; Samant, M.K.; Olsen, S.; de Haas, S.L.; Pegram, M.D. Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-positive metastatic breast cancer. Clin. Cancer Res. 2016, 22, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Griguolo, G.; Brasó-Maristany, F.; Gonzalez-Farre, B.; Pascual, T.; Chic, N.; Saurí, T.; Kates, R.; Gluz, O.; Martínez, D.; Paré, L. ERBB2 mRNA expression and response to ado-trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Cancers 2020, 12, 1902. [Google Scholar] [CrossRef] [PubMed]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Sarwar, M.; Thorat, N.D. Targeting the tumor microenvironment: Potential strategy for cancer therapeutics. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2023, 1869, 166746. [Google Scholar] [CrossRef] [PubMed]
- Luque, M.; Sanz-Álvarez, M.; Morales-Gallego, M.; Madoz-Gúrpide, J.; Zazo, S.; Domínguez, C.; Cazorla, A.; Izarzugaza, Y.; Arranz, J.L.; Cristóbal, I.; et al. Tumor-Infiltrating Lymphocytes and Immune Response in HER2-Positive Breast Cancer. Cancers 2022, 14, 6034. [Google Scholar] [CrossRef] [PubMed]
- de Haas, S.L.; Slamon, D.J.; Martin, M.; Press, M.F.; Lewis, G.D.; Lambertini, C.; Prat, A.; Lopez-Valverde, V.; Boulet, T.; Hurvitz, S.A. Tumor biomarkers and efficacy in patients treated with trastuzumab emtansine + pertuzumab versus standard of care in HER2-positive early breast cancer: An open-label, phase III study (KRISTINE). Breast Cancer Res. 2023, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, E.Y.; Yun, J.S.; Park, Y.L.; Do, S.-I.; Chae, S.W.; Park, C.H. The prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Cancer 2018, 18, 938. [Google Scholar] [CrossRef] [PubMed]
- Ulas, A.; Avci, N.; Kos, T.; Cubukcu, E.; Olmez, O.F.; Bulut, N.; Degirmenci, M. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with HER2-positive early breast cancer receiving adjuvant trastuzumab? Lung Cancer 2015, 18, 20. [Google Scholar]
- Imamura, M.; Morimoto, T.; Egawa, C.; Fukui, R.; Bun, A.; Ozawa, H.; Miyagawa, Y.; Fujimoto, Y.; Higuchi, T.; Miyoshi, Y. Significance of baseline neutrophil-to-lymphocyte ratio for progression-free survival of patients with HER2-positive breast cancer treated with trastuzumab emtansine. Sci. Rep. 2019, 9, 1811. [Google Scholar] [CrossRef] [PubMed]
- Guven, D.C.; Yildirim, H.C.; Bilgin, E.; Aktepe, O.H.; Taban, H.; Sahin, T.K.; Cakir, I.Y.; Akin, S.; Dizdar, O.; Aksoy, S.; et al. PILE: A candidate prognostic score in cancer patients treated with immunotherapy. Clin. Transl. Oncol. 2021, 23, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Fucà, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huang, W.; Wu, Y.; Luo, Y.; Wu, B.; Cheng, J.; Chen, J.; Liu, D.; Li, C. Prognostic role of pretreatment blood lymphocyte count in patients with solid tumors: A systematic review and meta-analysis. Cancer Cell Int. 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Saxena, S.; Singh, R.K. Neutrophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim. Biophys. Acta (BBA) Rev. Cancer 2022, 1877, 188762. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Kilickap, S.; Gambichler, T.; Aksoy, S. The Association between the Pan-Immune-Inflammation Value and Cancer Prognosis: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2675. [Google Scholar] [CrossRef] [PubMed]
- Ruopp, M.D.; Perkins, N.J.; Whitcomb, B.W.; Schisterman, E.F. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom. J. 2008, 50, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1. 1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).