Abstract
The route of administration of implanted cells may affect the outcome of cell therapy by directing cell migration to the damaged site. However, the question of the relationship between the route of administration, the efficacy of colonisation of a given organ, and the efficacy of cell therapy has not been resolved. The aim of the study was to localise transplanted intravenously and intraperitoneally human amniotic epithelial cells (hAECs) in the tissues of mice, both healthy and injured, in an animal experimental model of acute liver failure (ALF). Mice intoxicated with D-Galactosamine (D-GalN) at a dose of 150 mg/100 g body weight received D-GalN alone or with a single dose of hAECs administered by different routes. Subsequently, at 6, 24, and 72 h after D-GaIN administration and at 3, 21, and 69 h after hAEC administration, lungs, spleen, liver, and blood were collected from recipient mice. The degree of liver damage and regeneration was assessed based on biochemical blood parameters, histopathological evaluation (H&E staining), and immunodetection of proliferating (Ki67+) and apoptotic (Casp+) cells. The biodistribution of the administered cells was based on immunohistochemistry and the identification of human DNA. It has been shown that after intravenous administration, in both healthy and intoxicated mice, most of the transplanted hAECs were found in the lungs, while after intraperitoneal administration, they were found in the liver. We concluded that a large number of hAECs implanted in the lungs following intravenous administration can exert a therapeutic effect on the damaged liver, while the regenerative effect of intraperitoneally injected hAECs on the liver was very limited due to the relatively lower efficiency of cell engraftment.
1. Introduction
Acute liver failure (ALF) is caused by many factors, such as chronic use of medication (e.g., amiodarone, paracetamol), viral infections (e.g., hepatitis B virus), or diseases (e.g., Wilson’s disease) [1,2]. Despite the liver’s ability to self-regenerate from injuries induced by damaging factors, once the capacity for self-repair is exceeded, permanent and irreversible damage to the organ begins. The resulting functional disorders of the liver can lead to the sudden death of patients [3].
The significance of research on the mechanisms of hepatotoxicity and new therapies for the treatment of liver damaged by chemical agents or viruses results from the ineffectiveness of the currently used therapeutic methods, the large number of people requiring organ transplants in the absence of available donors, high treatment costs, and the lifelong need to take immunosuppressive drugs. Hepatotoxicity studies conducted on animals make it possible to analyse the mechanisms of damage, verify the efficacy of the used drugs or cells, and determine the degree of safety of their use [4,5]. However, some human liver diseases cannot be fully replicated in vivo. One example is ALF caused by the hepatitis virus, the effect of which is specific only to humans [6]. Therefore, experimental models are used in which intoxication results in symptoms that mimic human diseases. The compounds that are used to induce ALF include D-galactosamine (D-GaIN) and carbon tetrachloride (CCl4) [5]. Their effects vary depending on the dose administered and the frequency of administration. D-GaIN is an amino acid derivative and a highly hepatospecific toxin. The action of D-GaIN is to reduce the number of unbound uridine molecules, which prevents the synthesis of macromolecules, including RNA and protein, and causes massive necrosis of hepatocytes [7]. Just one dose of D-GaIN administered intraperitoneally can cause focal hepatocyte necrosis and a range of effects very similar to those observed in human viral hepatitis [8]. D-GaIN causes inflammatory infiltrates of histiocytes, nuclear polymorphism, dilatation of sinusoidal vessels, and the appearance of Councilman bodies [9].
In cell therapies for many diseases, attempts are made to use stem cells obtained from both adult and perinatal tissues. However, Clinicaltrials.com has only six registered projects using cell therapies to treat ALF. These studies aim to check the safety of the administered cells and determine their therapeutic properties in the treatment of damaged livers. These experiments use hepatocytes administered into the portal vein, mesenchymal stem cells isolated from bone marrow, and a commercial line isolated from adipose tissue, administered intravenously. Due to the advantages of human amniotic epithelial cells (hAECs), which have immunomodulatory properties and do not form tumours after transplantation, they are receiving increasing attention in cell therapies [10,11]. hAECs may be an efficient tool in cell therapy in the absence of an effective method of treating liver diseases. Their isolation raises no ethical concerns, and between 20 and 100 million cells can be obtained from a single amnion [12,13]. The first trials of clinical applications of hAECs showed that their administration does not cause side effects, even in newborns and adults [14,15]. Therefore, they also started to be administered to adults with liver fibrosis to establish a safe intravenous cell dose [16].
So far, it has not been possible to determine which route of hAEC administration for therapeutic purposes in ALF is safest and most efficient in the treatment of damage to the liver. It is fundamental to establish the biodistribution of transplanted cells in the recipient’s body as well as the number of implanted cells in the target organ necessary for its faster and more effective regeneration. The answer to the question of whether the implantation of transplanted cells in the damaged organ is necessary for their therapeutic effect is of great practical importance, especially since such implantation may be difficult if the degree of tissue destruction is advanced. Studies conducted on animal models as well as clinical trials show that intravenous injection is an efficient and convenient injection route for the mesenchymal stem cells (MSCs) and hAECs tested to date [16,17,18]. The direct introduction of cells into the bloodstream promotes their colonisation in the damaged organ. Compared to the above, intraperitoneal administration is faster and less invasive, but it promotes cell dispersion in the body, which may have a different impact on their therapeutic effect [19]. In addition, it is not known whether the inflammation occurring in the recipient significantly affects the biodistribution of the administered cells, promoting or not faster and more efficient cell migration to the damaged organ, and how to use this relationship to better design amniotic cell therapies.
It was therefore hypothesised that transplanted amniotic epithelial cells may colonise the recipient’s organs in the order and time related to the route of their administration and the degree of liver damage, and the biodistribution of the administered cells in the intoxicated body may be closely related to the therapeutic effect.
The aim of this study was to compare the effectiveness of intraperitoneal and intravenous administration of isolated cells of human amniotic epithelium in colonising organs in a healthy body and one intoxicated with a hepatospecific toxin, and to assess the therapeutic effect of hAECs in an experimental animal model of ALF.
2. Results
2.1. Characteristics of Isolated hAECs
In primary culture, the obtained cells were mostly adherent and had typical epithelial cell morphology (Figure 1) and marker expression (Figure 2).
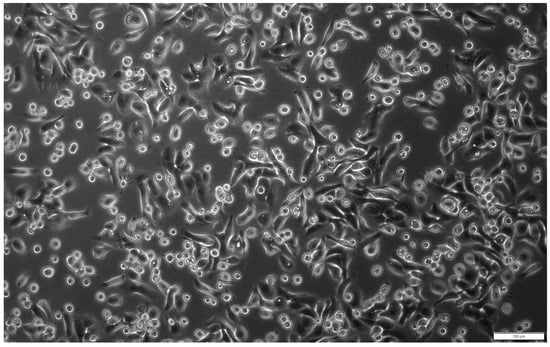
Figure 1.
Morphology of hAECs in 24 h primary culture. The isolated cells showed adherence to the culture plate and were characterised by the typical cobblestone-like shape of epithelial cells. Magn. ×100; scale bar = 100 µm.
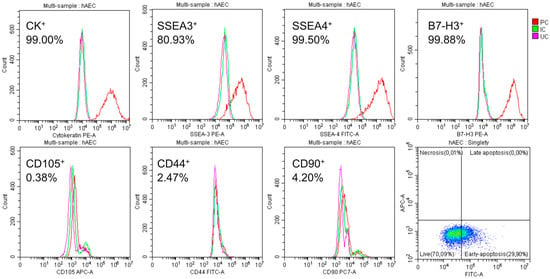
Figure 2.
Cytometric characterization of isolated hAECs. After 48 h of culturing, large numbers of SSEA-4+ and SSEA-3+; CK14, 15, 16, and 19+; and B7H3+ were detected; and only a few CD105+, CD44+, and CD90+ were detected. The percentage given is the average of the six populations tested. Only a few cells showed characteristics of necrotic cells: PC—cells labelled with the corresponding antibody, IC—isotype control, and UC—cells not labelled with antibodies.
On average, 63% of the isolated amniotic cells were alive and did not show apoptotic features, 10% were in early apoptosis, and 27% were in late apoptosis. There was significant expression of the pluripotency surface markers SSEA-3 (over 80%) and SSEA-4 (almost 100%) and the immunomodulatory protein B7-H3 (almost 100%). The epithelial nature of the cells was confirmed by demonstrating the presence of the cytokeratins 14, 15, 16, and 19 and the absence of mesenchymal markers, i.e., CD105, CD44, and CD90 (Figure 2).
2.2. Assessment of the Degree of Liver Damage
2.2.1. Assessment of Biochemical Parameters of Collected Blood
In order to assess the degree of liver damage, blood was collected at 6, 24, and 72 h after the administration of 150 mg/g bw D-GaIN and at 3, 21, and 69 h after the administration of 2 × 106 hAECs. The collected blood was used to determine the activities of alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), alkaline phosphatase (ALP), and the content of total protein (TP) and glucose (Table 1).

Table 1.
Changes in blood parameters after administration of D-GaIN, and/or hAECs depending on the route of cell injection. Statistically significant (p < 0.05) as compared to the: a—control; b—D-GaIN.
The toxic effect of D-GalN was manifested by a statistically significant increase in ALAT and ASAT activities by 81% with a maximum at 24 h and in ALP activity from 41% to 58% between 6 and 72 h, as well as a decrease in TP content at 6 h and a significant decrease in glucose concentration at all-time points, exceeding 300% at 24 h.
The administration of hAECs to non-intoxicated mice had visible effects on the values of certain liver parameters in the blood. The effect of intraperitoneal administration was less marked than that of intravenous administration and was particularly visible in a statistically significant, approximately 60% increase in ALP activity and a significant (24–85%) decrease in glucose concentration at all-time points. For comparison, already 3 h after intravenous administration of hAECs, there was a statistically significant increase in ALAT and ASAT activities, and at 21 h also in ALP activity. These values then decreased over time. By contrast, there was a statistically insignificant (30–40%) decrease in glucose concentration, comparable at subsequent time points.
The vast majority of changes in ALAT, ASAT, and ALP activities observed after the combined administration of D-GalN and hAECs were statistically insignificant, as were changes in glucose concentration. Nevertheless, differences should be noted in the values of some parameters, especially ASAT activity and glucose concentration, between intraperitoneal and intravenous hAEC administration, amounting to several dozen percent. These changes were more severe after intravenous administration.
2.2.2. Evaluation of Histopathological Changes in the Liver
Control mice showed single lymphocytes and apoptotic cells and no parenchymal damage in the liver.
Administration of D-GaIN induced liver damage, manifested primarily by ballooning degeneration and the presence of apoptotic bodies and leukocyte clusters within hepatic acini (Figure 3). The greatest damage was recorded 24 h after intoxication, which was reflected by statistically significant differences in histopathological scores of the assessed parameters of liver damage (Figure 4). These changes decreased at 72 h after D-GaIN administration.
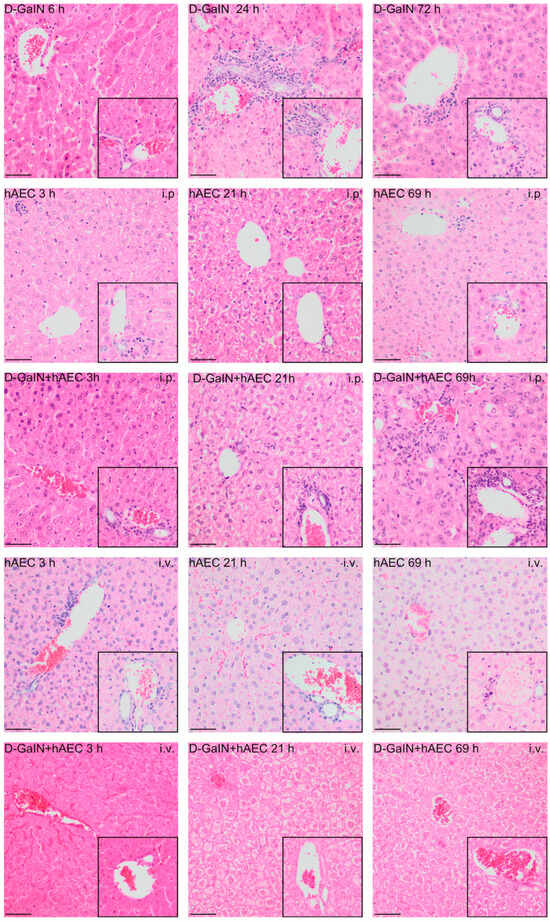
Figure 3.
Histopathological changes in mouse livers after administration of D-GaIN and/or hAECs intraperitoneally or intravenously. The larger image shows the pericentral zone 3 and zone 2 of the hepatic acini. The inset in the lower right corner shows the area around periportal zone 1. 6/24/72 h after D-GaIN administration; 3/21/69 h after hAEC administration; i.v.—intravenous administration, i.p.—intraperitoneal administration. Control mice were injected with NaCl and showed no histopathological changes in the liver. H&E staining; Magn. ×200, scale bar = 40 µm.
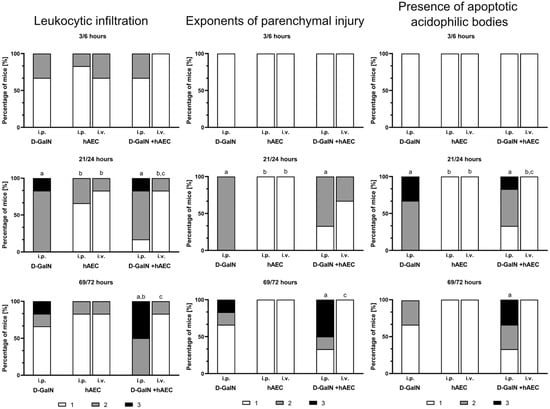
Figure 4.
Histopathological assessment of liver damage in mice scored 1, 2, or 3 according to a three-grade histopathological classification. First number (3, 21, 69) at a given time point—time since hAEC administration; second number (6, 24, 72) at a given time point—time since D-GaIN or NaCl administration. i.p.—intraperitoneal administration; i.v.—intravenous administration; p < 0.05 as compared to a—the control, b—D-GaIN, c—intraperitoneal injection of hAECs. Control mice were injected with NaCl and showed no histopathological changes in the liver.
After both intravenous and intraperitoneal administration of hAECs, only a small number of leukocyte clusters and single apoptotic bodies were observed throughout the experiment. No signs of parenchymal damage, such as necrosis or degenerative changes in hepatocytes, were observed at any of the examined time points.
Intraperitoneal administration of hAECs after intoxication of mice increased the effects of damage observed after administration of toxin alone, manifested by a rise over time in the number of animals in which the area infiltrated by leukocytes, including degenerative changes, and the number of apoptotic cells increased. Intravenous injection of hAECs after administration of D-GaIN contributed to reducing liver damage because examination revealed only single foci of leukocytic infiltration and histopathological changes in the liver parenchyma and only very few apoptotic cells compared to the administration of D-GaIN alone and intraperitoneal administration of hAECs to intoxicated mice.
2.2.3. Assessment of Liver Cell Proliferation and Apoptosis
There was a variable relationship between, on the one hand, an increase in the number of proliferating cells and, on the other hand, the degree of liver damage and route of hAEC administration (Figure 5).

Figure 5.
Immunodetection of proliferating cells in the liver based on the presence of Ki67 protein (upper images) and the number of proliferating Ki67+ cells in the liver expressed per cross-sectional area of 0.3779 mm2 (lower graphs) after administration of D-GaIN or NaCl (6/24/72 h) or after administration of hAECs (3/21/69 h). p < 0.05 as compared to a—the control, b—D-GaIN, and c—intraperitoneal injection of hAECs. Magn. ×200. Scale bar = 40 µm.
After the administration of D-GalN, the number of Ki67+ increased over time compared to the control group, but this increase was not statistically significant.
The number of proliferating cells did not increase after intraperitoneal administration of hAECs but increased statistically significantly after intravenous administration of hAECs (4-fold at 3 h and 2-fold at 21 and 69 h compared to both the control group and the group receiving hAECs intraperitoneally).
Comparing the intoxicated study groups, it was noted that just 3 h after intraperitoneal administration of hAECs, the number of proliferating cells was statistically significantly (9-fold) higher in the group receiving both hAECs and D-GaIN than in the group receiving only D-GaIN. In addition, a statistically significantly higher number of Ki67+ cells was observed in the groups receiving hAECs intravenously and D-GaIN compared to the group receiving only the toxin at 3 and 21 h after intoxication. These differences disappeared with increasing observation time (up to 72 h).
After administration of D-GaIN, the number of apoptotic cells in the examined livers increased at 24 h, when it was significantly higher compared to the control group, and then decreased significantly (Figure 6).
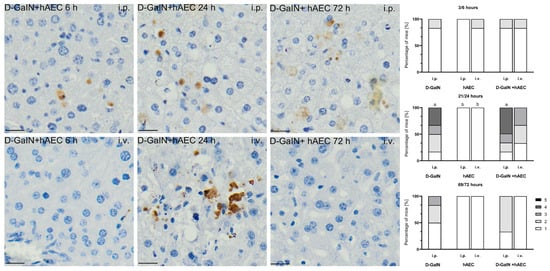
Figure 6.
Immunodetection of apoptotic cells based on the presence of caspase-3 protein (left images). Percentage of mice assigned a score of 1–5 on a histopathological scale (1–5) assessing the number of apoptotic Casp+ cells in the liver (right graphs). First number (3, 21, 69) at a given time point—time since hAEC administration; second number (6, 24, 72) at a given time point—time since D-GaIN administration. p < 0.05 as compared to—a—the control, b—D-GaIN; i.p.—intraperitoneal administration; i.v.—intravenous administration. Magn. ×200. Scale bar = 40 µm.
In the groups receiving only hAECs intraperitoneally or intravenously, no apoptotic cells were detected at any of the tested time points, or very few apoptotic cells were visible.
In the groups of D-GaIN-intoxicated mice receiving hAECs, a statistically significantly higher number (by 15%) of apoptotic cells in the liver was observed at 24 h after intraperitoneal administration of cells compared to the group receiving only the toxin. In the case of intravenous administration of hAECs after previous intoxication, the number of apoptotic cells in the examined livers was lower compared to the intoxicated-only groups.
2.3. Identification of Transplanted Human Amniotic Cells in the Organs of Recipient Mice
The immunohistochemical method of identifying transplanted hAECs in the liver, spleen, lungs, and blood of recipient mice involved detection of human nuclear mitotic apparatus protein (NuMA), epithelial cell marker cytokeratin 14, and human immunomodulatory protein B7-H3 (Figure 7).
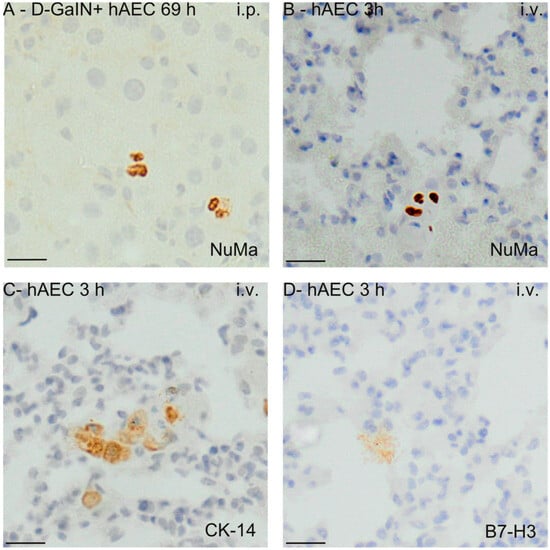
Figure 7.
Visualisation of hAECs delivered intraperitoneally or intravenously into the mouse liver (A) and lungs (B–D) at the time points corresponding to the largest number of identified hAEC cells. The examples shown in the images concern immunodetection of NuMa (A,B), CK14 (C), and B7H3 (D) markers in the liver and lungs. 3/69 h—hours after hAEC administration; i.v.—intravenous administration; i.p.—intraperitoneal administration. Magn. ×200. Scale = 40 µm.
The number of implanted cells was estimated by detecting the gene encoding human cytochrome b in mice. After intraperitoneal administration to healthy mice, human DNA was present in the liver and spleen in the greatest amount 3 h after hAEC injection, and then the concentration of DNA in these organs decreased over time—in the spleen statistically significantly. In intoxicated mice, after hAEC administration, the concentration of human DNA increased significantly in the spleen and liver at 21 and 69 h. In both healthy and intoxicated mice, only small amounts of human DNA were detected in the lungs (Figure 8).
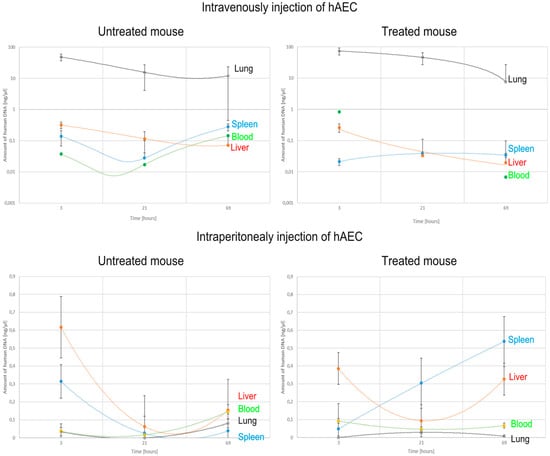
Figure 8.
Changes in the concentration of human DNA detected in mouse organs and blood after intravenous or intraperitoneal administration of hAECs depending on the time after cell injection.
In contrast, after intravenous administration of hAECs, the largest amounts of human DNA were detected in the lungs of both intoxicated and non-intoxicated mice, but this amount decreased statistically significantly over time. Little or no human DNA was detected in the liver, spleen, and blood of these mice.
Summarising the obtained results, it can be concluded that the administration of D-galactosamine is sufficient to induce liver damage and affect the biodistribution of the administered cells. When liver damage was present, the cells administered intraperitoneally became implanted mainly in the spleen. However, when administered intravenously, they accumulated in the lungs (Table 2, Figure 8).

Table 2.
Number of hAECs estimated from the concentration of human DNA in intoxicated and untreated mouse lungs, spleen, liver, and blood, depending on the route of cell administration. Values are medians of hAECs. p < 0.05 as compared to—a—intraperitoneal injection of hAEC, b—3 h in untreated mice; c—3 h in treated mice; d—21 h in untreated mice; e—21 h in treated mice; f—69 h in untreated mice (n = 6).
3. Discussion
Despite the many transplants performed, both from living and dead donors, the liver is still the second most-awaited organ transplant, right after kidneys [20]. The number of people requiring transplantation of damaged livers is increasing, while the number of potential donors is still insufficient. The significance of research on new alternative therapies, including stem cell therapy, for the treatment of liver damaged by chemical agents or viruses results from the ineffectiveness of the currently used therapeutic methods based on liver or hepatocyte transplantation [21,22].
The present experiment assessed the therapeutic effect of transplanted hAECs on the liver in an animal model of ALF induced by hepatospecific D-GaIN toxin. Depending on the dose and the number of injections, D-GaIN can cause liver symptoms similar to those caused by the human hepatitis virus [23,24]. Despite the well-known mechanisms of D-GaIN action on the liver, no studies have yet been conducted to test the therapeutic effect of stem cells in this model in connection with their organ biodistribution.
In the present experiment, a dose of 150 mg of D-GaIN per 100 g of body weight was used, which is believed to cause ALF but does not lead to liver necrosis [25,26]. We confirmed the efficacy of this dose by observing an increase in the values of representative blood parameters and histopathological features of hepatitis at 6 h after intoxication. The full-blown phase of ALF after D-GaIN intoxication, expressed through blood parameter values and the microscopic image of liver tissue, was observed 24 h after intoxication. At this time point, the number of apoptotic cells was the highest, which could be due to metabolization of D-GaIN and inhibition of mRNA synthesis, followed by activation of TNF-α-dependent signalling pathways [24,27]. Additionally, there was a statistically significant reduction in blood glucose levels, which may indicate that due to the competitive effect of D-GaIN against D-galactose, there was a reduction in the ability of the liver to synthesize glycogen and convert it to glucose in subsequent stages [4,5]. This would reflect the hypoglycaemic state that occurs in many ALF patients, often caused by depletion of hepatic glycogen stores and impaired gluconeogenesis [28,29]. The last phase of induced damage observed at 72 h was the regeneration phase, in which, although the features of hepatitis and degenerative changes in the parenchyma were still visible, the severity of these changes as well as the values of liver markers in the blood slowly returned to the physiological state. This was related to the inhibition of apoptotic changes and an increase in the number of proliferating cells. In total, the obtained effects of D-GalN intoxication reflect the three-stage course of action of the toxin, i.e., the initial phase visible already at 6 h after intoxication, the greatest effects of ALF at 24 h, and the self-repair processes noticeable at 72 h after intoxication [30,31].
Amniotic cells isolated from human placentas and used in the experimental model constituted a relatively homogeneous population of hAECs [10,32] characterised by the presence of epithelial cell-specific cytokeratins and the absence of markers typical of mesenchymal cells. They had some characteristics of pluripotent cells, such as the presence of the surface marker proteins SSEA-3 and SSEA-4 [33]. On average, 98% of them had the B7-H3 protein on their surface, which has a significant effect on the immune response by stimulating Th1 and Th2 lymphocytes and reducing the secretion of IL-2 and IFN-γ and the proliferation of T cells [34].
No signs of parenchymal damage, such as necrosis, were observed after either intravenous or intraperitoneal administration of hAECs to native mice. Also, no degenerative changes, signs of inflammation, or increased apoptosis were observed. In our experiment, we found no significant changes in the blood total protein concentration, although other observations indicate that after intrasplenic administration, hAECs engrafted in the mouse liver contributed to increased hepatic gene expression and secretion of normal liver proteins, including α1-antitrypsin and albumin [35,36]. Nevertheless, we recorded a significant increase in the number of proliferating cells in the liver already at 3 h after intravenous injection of hAECs, associated with a statistically significant increase in ALAT and ASAT activities, which was not observed after intraperitoneal administration of hAECs. Some substances secreted by hAECs, such as EFG and IGF, are known to have a pro-proliferative effect [37,38,39]. In addition, we noted a significant and route-independent effect of hAECs on ALP activity and blood glucose concentration. Based on preclinical studies, it is known that changes in ALP activity may be a marker of liver damage, but especially in rodents, the assessment of this activity should take into account the presence of extrahepatic forms of the enzyme, such as the intestinal isoform of ALP, showing low activity that increases, e.g., after a meal or transiently during fasting [40]. Elevated ALP activity, however, may be the result of obstruction of bile ducts caused by their blockage by the administered cells [41] and may be a manifestation of functional disorders in the liver along with changes in aminotransferase activity and increased proliferative activity of hepatocytes. Previous studies performed after administration of bone marrow cells to mice indicate that such elevated ALP activity may also reflect the role of this enzyme as a ‘signal regulator’ determining the fate of the administered cells, which was associated with the differentiation of the administered cells into osteoblasts [42]. On the other hand, the decrease in blood glucose levels observed by us in this study, particularly severe after intraperitoneal administration of hAECs, may be related to decreased appetite in injected rodents or to the passage of hAECs into the intestines and impaired glucose absorption [19,43].
The dynamics of hAEC biodistribution in healthy and intoxicated mice depended on the route of cell administration. Following intraperitoneal administration of hAECs to healthy mice, we found the presence of these cells in the blood only after 21 h, which is due to the longer time required for them to get from the peritoneum into the bloodstream and can occur in two ways. The main migration route of the administered cells is through mesenteric vessels, which carry the implanted cells to the portal vein and then to the liver. This justifies the biodistribution of the cells administered this way in the liver even before reaching the systemic circulation [44]. In addition, hAECs can enter from the peritoneal cavity into the peritoneal lymphatic vessels and only then into the bloodstream [19,45], while they can also cause clogging of small blood vessels in the liver [46,47]. The opposite situation was observed in intoxicated mice, i.e., the number of hAECs administered intraperitoneally and present in the blood increased up to 24 h and then decreased to zero at 72 h after intoxication, which coincided with increased colonisation in the spleen and, somewhat more slowly, the liver. At the same time, a small number of engrafted cells were detected in mouse lungs, both in the group receiving hAECs alone and after administration of D-GaIN and hAECs.
In general, intraperitoneal administration of hAECs was much more efficient in colonising both healthy and damaged livers. Intoxication with D-GalN, inducing histopathological changes in the liver characteristic of ALF, significantly contributed to the penetration of intraperitoneally administered hAECs into the spleen and liver. Presumably, an important role was played here by increased chemotaxis, caused mainly by the secretory activity of cells in the damaged organ [46,48,49,50]. Nevertheless, the number of cells populating the liver after intraperitoneal administration was not high enough to reveal the repair processes in the organ when assessing inflammation, histopathological changes, and the apoptotic activity of the cells. On the contrary, after the administration of hAECs, there was an increase in leukocyte infiltration, indicators of liver parenchymal damage, and the number of apoptotic cells with a simultaneous inhibition of their proliferative activity, which was hardly observed after hAEC administration to native mice.
Regarding intravenous administration of cells, it was previously shown that in healthy recipient mice, hAECs administered caudally colonise the liver as early as 1 h after implantation, but they also localise in the thoracic cavity, abdominal cavity, and hind legs [18]. We observed that after intravenous administration, the number of hAECs in the blood obviously increased at 3 h after administration, both in the group of mice receiving only hAECs and, to a greater extent, in the D-GaIN/hAEC group. In both cases, hAECs quickly disappeared from the blood, which, however, was not accompanied by an increase in their number in the liver, while there was a slight increase in their number in the spleen of intoxicated animals. The obtained results are consistent with data on MSCs, which suggested a change in the biodistribution of implanted cells when organ damage occurs [51]. However, it is known that, due to the nature of vascular connections, the lungs are potentially the most important target for migration of the cells transplanted via the intravenous route [52]. As expected, we identified hAECs in the lungs already 3 h after intravenous administration, but after 69 h, their number, although decreasing, was still significant. Similar quantitative changes in hAECs in the lungs of healthy mice were demonstrated in another study at 1, 3, and 24 h [18]. However, at 3 h after intravenous administration of hAECs to intoxicated mice, we found that the number of hAECs in the blood was higher and in the lungs was lower compared to healthy mice. This indicates that during the first hours after cell injection into intoxicated mice, only part of the administered hAECs engrafted in the lungs. Some of these cells, probably due to active attraction by chemoattractants, migrated through the circulatory system towards the site of the damage [53] but did not colonise the liver in proportionally high numbers, which can possibly be related to the toxic effect of D-GalN. Initially, the lower effectiveness of hAEC engraftment in the lungs of intoxicated mice increased at 21 h after hAEC administration, which is the time when the greatest liver damage was observed. The number of hAECs in the lungs decreased with time after intoxication, which is consistent with the results of a study on engraftment and migration of labelled MSCs administered intravenously to mice with liver necrosis, which showed that the number of cells present in the lungs decreased over time, while the number of cells engrafted in the liver and spleen remained constant and low [54]. It also appears that the cells engrafted in the lungs after intravenous administration, even if they are unable to migrate and effectively localise in the damaged organ, can produce anti-inflammatory substances into the bloodstream and have a therapeutic effect on cells of the liver damaged in the D-GaIN model already a short time after intoxication. This can take place through the secretion of anti-inflammatory substances, such as TGF-β and IL-10 [55], and substances stimulating regeneration and/or inhibiting apoptosis of hepatocytes, such as TNF-α, IL-1b, IL-2, and IL-6 [56,57], as well as through the stimulation of the proliferative activity of liver cells. The activated Kupffer cells produce substances such as TNF-α and IL-1β, which induce increased expression of E- and P-selectins on vascular endothelial cells, enabling the inflow of not only neutrophils and monocytes but also hAECs [50,58]. A paracrine therapeutic effect was also obtained in the ALF mouse model after administration of MSCs derived both from bone marrow and adipose tissue, which, despite not being engrafted in the liver, reduced the hepatotoxic effects [59,60]. Compared to intravenous administration, intraperitoneal administration of the cells should potentially favour their direct effect on the damaged liver due to their close proximity to this organ and the possibility of more intense attraction to the damage site by chemoattractants such as SDF-1. However, it was shown that in order to improve the functional efficiency of a damaged liver, the number of cells needed for its proper regeneration would have to correspond to approximately 10–15% of the organ’s weight, which enabled, among others, a reduction of blood bilirubin levels in a rat model of Crigler-Najjar type I deficiency [61,62].
The most visible effect of administering hAECs to mice previously intoxicated with D-GaIN was a significant reduction in the increases in ALAT, ASAT, and ALP activities and the decreases in glucose levels observed in mice intoxicated with D-GaIN alone. The vast majority of changes in ALAT, ASAT, and ALP activities observed after the combined administration of D-GalN and hAECs were statistically insignificant, as were changes in glucose levels. Nevertheless, differences should be noted in the values of some parameters, especially ASAT activity and glucose concentration, between intraperitoneal and intravenous hAEC administration, amounting to several dozen percent and increasing over time after the administration of hAECs. These differences indicate a decreasing toxic effect after 21 h, especially quickly after intravenous administration.
Summarising the data obtained on liver damage caused by D-GaIN administration alone and/or after hAECs administration by two routes, intravenous and intraperitoneal, we found that regardless of whether intoxication occurred or not, hAECs administered intravenously engrafted in large numbers in the lungs, while those administered intraperitoneally engrafted in the liver. Paradoxically, however, the therapeutic effect is different: it is pronounced after intravenous administration, most likely due to the large number of engrafted cells and increased concentration of factors secreted by the administered hAECs, while after intraperitoneal administration, it does not occur and the inflammatory and degenerative changes intensify, which may be due to the insufficient effectiveness of the engraftment of hAECs in the damaged organ.
4. Materials and Methods
4.1. Experimental Model of Hepatotoxicity
The study was conducted on adult, eight-week-old female BALB/c mice obtained from the Centre for Experimental Medicine at the Medical University of Silesia in Katowice. During the experiment, the animals were kept in separate cages under standard conditions: temperature of 22 °C ± 2 °C, humidity of 50–60%, light/dark cycle of 12 h/12 h, and light intensity of 60–400 lux. Water and food (Labofeed) were available ad libitum.
Healthy mice were divided into four main groups of six mice each, receiving single injections of placebo (group I—intraperitoneally, group II—intravenously), hAECs administered intraperitoneally (group III), and hAECs administered intravenously (group IV), respectively.
Mice subjected to intoxication received D-GalN by intraperitoneal injection at a single dose of 150 mg/100 g bw. The injection contained galactosamine hydrochloride solution with pH = 6.8 equilibrated with NaOH solution, administered using disposable sterile insulin syringes.
Mice injected with D-GalN were divided into three subgroups: one received D-GaIN alone (group V), the second also received hAECs intraperitoneally (group VI), and the third also received hAECs intravenously (group VII) (Table 3).

Table 3.
Description of the study groups.
Subsequently, at 6, 24, and 72 h after D-GaIN administration and at 3, 21, and 69 h after hAEC administration, lungs, spleen, liver, and blood were collected from recipient mice (Figure 9). hAECs were visualised in the collected tissues and blood by immunohistochemistry and identified by the detection of human DNA. In addition, biochemical and morphological testing of mouse blood was performed to assess recipient health and liver damage, and histological staining with haematoxylin and eosin was used to assess histopathological changes induced in the liver.
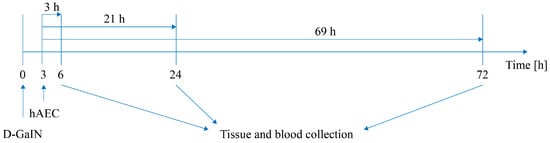
Figure 9.
Scheme of the experiment. Intoxication time precedes hAEC administration by 3 h.
4.2. Isolation of hAECs
Human placentas were collected from women aged 18–35 who were patients of the Department of Gynaecology and Obstetrics with the Division of Gynaecologic Oncology at the Brothers Hospitallers Hospital in Katowice after obtaining their informed consent. The placentas came from normal pregnancies terminated at full term by caesarean section performed for obstetric reasons (transverse or longitudinal pelvic position of the foetus, foetopelvic disproportion) or for non-obstetric indications in the absence of symptoms of placental insufficiency. The study was performed on cells isolated from six placentas with the approval of the Bioethics Committee at the Medical University of Silesia in Katowice (permission no. KNW/0022/KB/29C/19).
Inclusion criteria: good health, uncomplicated pregnancy, and full-term delivery.
Exclusion criteria: smoking, drinking alcohol or use of other stimulants during pregnancy, positive test for group B streptococcus, and chronic non-obstetric diseases prior to or during pregnancy.
The placentas were collected in a sterile container containing phosphate buffer (PBS) supplemented with an anticoagulant agent (5 mM EDTA) and an antibiotic–antimycotic solution (penicillin 0.1 U/mL, streptomycin sulphate 0.1 mg/mL, and amphotericin B 0.25 μg/mL). The amniotic membrane was mechanically separated from the chorion, after which fragments measuring approximately 1 cm × 1 cm were cut off from the membrane and decellularized by enzymatic digestion with trypsin (0.05%, 40 min, 37 °C). The cell suspension obtained after digestion was centrifuged (Eppendorf Centrifuge 5810R, at 500× g, 5 min, 4 °C) and suspended in culture medium (DMEM, 10% FBS, 1% AA, 10 ng/μL EGF). The hAECs were cultured in an incubator under standard conditions, i.e., 37 °C and 5% CO2. The cells were seeded at a density of 160,000/cm2 in a 25 cm2 culture flask. After 48 h of culture, cell viability was assessed, and the phenotype was characterised. The remaining isolated cells were banked.
4.3. Assessment of Viability and Phenotype of Isolated Amniotic Cells
After 48 h of culturing isolated cells, the phenotype was analysed by detection of the following markers: pluripotency markers SSEA-3 (PE Rat anti-SSEA3 cat. No. 560237; BD Biosciences, Franklin Lakes, NJ, USA) and SSEA-4 (FITC Mouse anti-SSEA-4, cat. no. 560126; BD Biosciences, Franklin Lakes, NJ, USA); the immunomodulatory protein B7H3 (PE anti-B7-H3 (CD276), cat. no. FAB1027P (R&D Systems, Minneapolis, MN, USA); cytokeratins CK14, CK15, CK16, and CK19 (Kit PE Mouse Anti-Human Cytokeratin 14/15/16/19, cat. no. 550953; BD Biosciences, Franklin Lakes, NJ, USA); and mesenchymal markers CD105, CD44, and CD90 (FITC Mouse Anti-Human CD44, cat. no. 555478; Alexa Fluor 647 Mouse anti-Human CD105, cat. no. 561439; PE-Cy7 Mouse Anti-Human CD90, cat. no. 561558, BD Biosciences, Franklin Lakes, NJ, USA). To evaluate the phenotype of the obtained cells, after digestion from the culture flask with TrypLE Express enzyme solution (Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA), hAECs in the amount of 1 × 106 were suspended in culture medium, centrifuged (500× g, 5 min, 4 °C), resuspended in a staining solution (PBS supplemented with 10% FBS and 5 mM EDTA) containing fluorochrome-conjugated antibodies or appropriate isotype controls, and incubated at room temperature for 30 min. After washing the cell suspension in the staining solution and centrifuging (500× g, 5 min, 4 °C), the cells were resuspended in the staining solution and analysed in a flow cytometer (CytoFlex; Beckman Coulter, Brea, CA, USA). Cytometric analysis was performed after 10,000 cells were counted.
To assess cell viability and apoptotic cell content in the culture, after digestion from the culture flask, 100,000 cells were resuspended in 1 x concentrated binding solution from the manufacturer’s kit (FITC Annexin V Apoptosis Detection Kit I, cat. no. 556547, BD Biosciences, Franklin Lakes, NJ, USA) and labelled with propidium iodide and Annexin V solution. The cells were then incubated with the antibody for 15 min at room temperature in the dark. In the final step, the labelled cell suspension was diluted fivefold to inhibit the reaction and analysed in a flow cytometer (CytoFlex; Beckman Coulter, Brea, CA, USA). Cytometric analysis was performed after 10,000 cells had been counted.
4.4. Delivery of Isolated hAECs
Forty-eight hours before the planned administration, hAECs were thawed, seeded in culture flasks, and cultured in an incubator (Sanyo MCO-19M, Osaka, Japan) under standard conditions, i.e., 37 °C and 5% CO2 concentration. After a predetermined culture time, the cells were digested from the surface of the culture flask with TrypLE Express enzyme solution (Thermo Fisher Scientific, Waltham, MA, USA), and the obtained 2 × 106 hAECs were suspended in 0.9% NaCl and administered to mice intraperitoneally or into the caudal vein. The cells were administered in a single dose using a 27 G 1/2-inch (0.4 × 14) insulin needle syringe (BD). The mice in the control group received 250 µL of saline.
4.5. Evaluation of the Liver Damage
For histopathological and immunohistochemical evaluation, the liver, spleen, lungs, and blood were harvested from mice euthanized 6, 24, and 72 h after D-GaIN administration and 3, 21, and 69 h after hAEC administration.
4.5.1. Blood Parameters in the Recipient Mice
Blood tests were performed at Labo-Vet (Czechowice-Dziedzice, Poland) 6, 24, and 72 h after administration of D-GaIN and 3, 21, and 69 h after administration of hAECs or 72 h after administration of NaCl. Haematological tests were performed on an ABX Micros ESV60 haematology analyser using a combination of two measurement methods: impedance and photometry. Biochemical blood tests (ALAT, ASAT, ALP, total protein, and glucose) were performed using an Accent-200 analyser, which measures the absorbance of the reaction mixture.
4.5.2. Histopathological Evaluation
In order to standardise the assessment, a three-grade classification of liver damage markers was introduced (Table 4).

Table 4.
Histopathological scale to assess the degree of liver damage.
4.5.3. Assessment of Liver Damage Based on Histological Staining with Haematoxylin and Eosin
The tissues were fixed in 10% buffered formalin and then embedded in paraffin. Paraffin blocks were cut on a Microm HM 350 S microtome (Thermo Fisher Scientific, Waltham, MA, USA) into 4-μm-thick sections, which were deparaffinised in xylene and decreasing concentrations of alcohols and then stained with haematoxylin and eosin (H&E) or stained immunohistochemically. The degree of liver damage was assessed after H&E staining by the presence of the following markers: interface hepatitis, parenchymal liver damage, and the presence of acidophilic bodies.
4.5.4. Evaluation of Proliferative and Apoptotic Activity of Hepatic Cells
Paraffin-embedded sections obtained from mouse livers, after deparaffinisation and rehydration, were immunohistochemically stained to detect Ki-67, a marker of proliferation, and caspase-3, a marker of apoptosis. For this purpose, specific antibodies and isotype controls were used, as well as a positive control, which was a section prepared from a human tonsil. The unmasking of Ki-67 antigen and caspase-3 was performed using a citric acid solution (Vector, Newark, CA, USA) by incubation for 60 min. To eliminate non-specific binding, the prepared liver sections were incubated for 60 min in a 2.5% horse serum solution (Vector, Newark, CA, USA). The sections were then incubated with either rabbit anti-Ki67 antibody (ab16667; dilution 1:400, Abcam, Cambridge, UK) or rabbit anti-caspase 3 antibody (#9661; dilution 1:500; Cell Signalling, Danvers, MA, USA) for 20 h at 4 °C, and with a peroxidase-conjugated secondary anti-rabbit antibody (ImPreSS Vector Laboratories, Newark, CA, USA) for 30 min at room temperature. The staining reaction was carried out using 0.05% diaminobenzidine (Vector, Newark, CA, USA). The sections were then stained with haematoxylin to visualise cell nuclei, dehydrated in a series of increasing concentrations of alcohols and xylene, and sealed with a coverslip in DPX medium. To assess the number of positive cells on each section, fifteen random fields of 0.3779 mm2 each were photographed at 100× magnification. Ki67 immunodetection results were analysed using ImageJ 1.52e software and expressed as the average number of positive cells present per area. A semi-quantitative ordinal scale was used to assess the abundance of Casp+ apoptotic cells expressing caspase-3, namely: (1) no Casp+ cells in section; (2) <10 Casp+ cells; (3) 10–25 Casp+ cells; (4) 26–50 Casp+ cells; and (5) >50 Casp+ cells per area (0.3779 mm2) (Table 5).

Table 5.
Scale for assessing the number of Casp+ apoptotic cells in recipient mouse liver sections per surface area (0.3779 mm2).
4.6. Identification of Transplanted Human Amniotic Cells in Recipient Mouse Tissues
4.6.1. Immunohistochemical Detection of hAECs
hAECs were identified in paraffin sections obtained from mouse lungs, liver, and spleen collected at 6, 24, and 72 h after intoxication or at 3, 21, and 69 h after hAEC administration. Detection of hAECs in mouse organs was carried out by identifying human proteins, i.e., the nuclear protein of the mitotic apparatus NuMa, the immunomodulatory protein B7-H3, and the intermediate filament protein cytokeratin 14. For this purpose, deparaffinised sections were incubated with a 2.5% goat serum solution to eliminate non-specific binding of primary antibodies. The sections were incubated with the appropriate primary antibody (Rabbit Anti-NuMA antibody, cat. no. ab84680; Rabbit Anti-B7H3 antibody, cat. no. ab105922; Rabbit Recombinant Anti-Cytokeratin 14 antibody, cat. no. ab51054; all from Abcam, Cambridge, UK) or isotypic control for 20 h at 4 °C. This was followed by a 30 min incubation with a peroxidase-conjugated anti-rabbit secondary antibody (ImmPress Vector, Newark, CA, USA). Visualisation was achieved with 0.05% diaminobenzidine (Vector Laboratories), after which the sections were stained with haematoxylin to visualise cell nuclei, dehydrated in a series of increasing concentrations of alcohols and xylene, and sealed with a coverslip in DPX medium.
4.6.2. Identification of Human DNA from Transplanted hAECs
The presence of human amniotic cells was assessed in recipient mouse tissues and blood by identifying the human mitochondrial cytochrome b gene. Total DNA was extracted from mouse tissues using the DNA/RNA Extracol Kit (EurX, Gdańsk, Poland) and from blood using the Quick Blood DNA Purification Kit according to the manufacturer’s instructions. The amplification reaction was carried out on a Roche LightCycler 480, and the specificity of the resulting product was confirmed by agarose gel electrophoresis and the determination of the melting temperature (Tm) curve. For the detection of cytochrome b, the FastStart Essential DNA Green Master reagent kit (Roche, Indianapolis, USA) and oligonucleotide primers (Thermo Fisher Scientific, Waltham, MA, USA) specific for the tested gene, were used (Table 6).

Table 6.
The primers used for the qPCR reaction.
The concentration of human DNA, as detected by cytochrome b sequencing, was determined using a standard DNA concentration curve (TaqMan DNA Template Reagents, Thermo Fisher Scientific, Waltham, MA, USA) and compared with the concentrations obtained from a known number of isolated hAECs, i.e., 1000, 5000, 10,000, 100,000, 250,000, 500,000, 1 million, and 1.5 million. Using the equation defining the relationship between the concentration of human DNA and the CT value obtained from 100 ng of the DNA of the tested mouse tissue, the concentration obtained per 1 mg of DNA isolated from the tested tissues was calculated so that it was possible to compare the content of human DNA in samples with different concentrations of starting DNA. A comparison of the obtained concentration of human DNA from a given number of hAECs to the data from the standard curve allowed a preliminary estimate of the number of implanted cells in mouse organs.
4.7. Statistical Analysis
Statistical analysis was performed using Statistica 13.3 software (TIBCO, USA). Different groups were compared using Kruskal–Wallis test for non-normally distributed data and an ANOVA with a post hoc Tukey test for normally distributed data. The level of significance was set at p < 0.05 for all statistical tests. Values were expressed as median (Me) with the 25th and 75th quartiles and minimum and maximum for non-normally distributed data, and for normally distributed variables, they were presented as mean and standard deviation.
5. Conclusions
- The biodistribution of transplanted hAECs varies depending on the route of administration and the degree of liver damage.
- After intravenous administration, most of the cells engraft in the lungs, while after intraperitoneal administration, they engraft primarily in the liver in both intoxicated and native mice.
- The increased presence of hAECs in the spleen is associated with progressive liver toxicity regardless of the route of administration—the greater the liver damage, the greater the number of hAECs in the spleen.
- After intravenous administration, a large number of the grafted hAECs in the lungs can have a therapeutic effect on the damaged liver.
- The therapeutic effect of intraperitoneally administered hAECs on histopathological and regenerative changes in the liver is very limited due to the low efficiency of cell engraftment in the damaged organ.
Author Contributions
Conceptualization, funding acquisition and data curation, P.W. and P.C.; methodology, P.W., P.C., M.K., E.B., M.H., E.K., A.S.-S. and R.S.; laboratory investigation, P.W., M.K., E.B., M.H., E.K., A.G. and A.S.-S.; clinical tasks, J.T. and R.S.; writing—original draft preparation, review and editing, P.W. and P.C.; supervision, P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the Medical University of Silesia in Katowice (Poland), grant no: PCN-2-003/K/1/I, PCN-2-005/N/0/O and PCN-2-048/N/2/I and in part by National Science Center (Poland), grant no: 2021/41/N/NZ7/00355. For the purpose of Open Access, the author has applied a CC-BY public copyright license to any Author Accepted Manuscript (AAM) version arising from this submission.
Institutional Review Board Statement
The studies involving human participants were reviewed and approved by the Bioethics Committee of the Medical University of Silesia (SUM) in Katowice (decision no KNW/0022/KB/29C/19; 4 March 2019). The animal study was reviewed and approved by the Local Ethical Committee on Animal Experiments (SUM in Katowice), decision no: 69/2019 (24 June 2019).
Informed Consent Statement
The patients provided their written informed consent to participate in this study.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank the Labo-Vet (Veterinary Diagnostic Laboratory), Czechowice-Dziedzice, Poland, for performing the serum biochemistry analysis and the blood morphology assessment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stravitz, R.T.; Lee, W.M. Acute Liver Failure. Lancet 2019, 394, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Wajtryt, O.; Zielonka, T.M. Ostra Niewydolność Wątroby Po Podaniu Amiodaronu. Folia Cardiol. 2019, 14, 242–246. [Google Scholar] [CrossRef]
- Czechowska, G.; Celiński, K.; Wójcicka, G. Liver Fibrosis Mechanisms: The Role of Stellate Cells, Oxidative and Nitrosative Stress. Postepy Hig. Med. Dosw. 2019, 73, 421–439. [Google Scholar] [CrossRef]
- Czekaj, P.; Król, M.; Limanówka, Ł.; Skubis-Sikora, A.; Kolanko, E.; Bogunia, E.; Hermyt, M.; Michalik, M.; Sikora, B.; Prusek, A.; et al. Dynamics of Acute Liver Injury in Experimental Models of Hepatotoxicity in the Context of Their Implementation in Preclinical Studies on Stem Cell Therapy. Front. Biosci. (Landmark Ed.) 2022, 27, 237. [Google Scholar] [CrossRef] [PubMed]
- Czekaj, P.; Król, M.; Limanówka, Ł.; Michalik, M.; Lorek, K.; Gramignoli, R. Assessment of Animal Experimental Models of Toxic Liver Injury in the Context of Their Potential Application as Preclinical Models for Cell Therapy. Eur. J. Pharmacol. 2019, 861, 172597. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Z.; Jiang, J.; Wu, D.; Liu, X.; Xie, Z.; Chen, E.; Zhu, D.; Ye, C.; Zhang, X.; et al. Proteomic Signature of Acute Liver Failure: From Discovery and Verification in a Pig Model to Confirmation in Humans. Mol. Cell. Proteom. 2017, 16, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Batista Éboli, L.P.; Netto, A.A.S.; de Azevedo, R.A.; Lanzoni, V.P.; de Paula, T.S.; Goldenberg, A.; Gonzalez, A.M. Evaluating the Best Time to Intervene Acute Liver Failure in Rat Models Induced by D-Galactosamine. Acta Cir. Bras. 2016, 31, 783–792. [Google Scholar] [CrossRef]
- Decker, K.; Keppler, D. Galactosamine Hepatitis: Key Role of the Nucleotide Deficiency Period in the Pathogenesis of Cell Injury and Cell Death. Rev. Physiol. Biochem. Pharmacol. 1974, 71, 77–106. [Google Scholar] [CrossRef]
- Keppler, D.; Lesch, R.; Reutter, W.; Decker, K. Experimental Hepatitis Induced by D-Galactosamine. Exp. Mol. Pathol. 1968, 9, 279–290. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, W.; Liu, J.; Li, J.; Tan, B.; Qiu, C.; Zhu, X.; Qiu, C. Biological Characterization of Human Amniotic Epithelial Cells in a Serum-Free System and Their Safety Evaluation. Acta Pharmacol. Sin. 2018, 39, 1305–1316. [Google Scholar] [CrossRef]
- Pratama, G.; Vaghjiani, V.; Tee, J.Y.; Liu, Y.H.; Chan, J.; Tan, C.; Murthi, P.; Gargett, C.; Manuelpillai, U. Changes in Culture Expanded Human Amniotic Epithelial Cells: Implications for Potential Therapeutic Applications. PLoS ONE 2011, 6, e26136. [Google Scholar] [CrossRef] [PubMed]
- Motedayyen, H.; Esmaeil, N.; Tajik, N.; Khadem, F.; Ghotloo, S.; Khani, B.; Rezaei, A. Method and Key Points for Isolation of Human Amniotic Epithelial Cells with High Yield, Viability and Purity. BMC Res. Notes 2017, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Gramignoli, R.; Srinivasan, R.C.; Kannisto, K.; Strom, S.C. Isolation of Human Amnion Epithelial Cells According to Current Good Manufacturing Procedures. Curr. Protoc. Stem Cell Biol. 2016, 2016, 1E.10.1–1E.10.13. [Google Scholar] [CrossRef]
- Lim, R.; Malhotra, A.; Tan, J.; Chan, S.T.; Lau, S.; Zhu, D.; Mockler, J.C.; Wallace, E.M. First-In-Human Administration of Allogeneic Amnion Cells in Premature Infants with Bronchopulmonary Dysplasia: A Safety Study. Stem Cells Transl. Med. 2018, 7, 628–635. [Google Scholar] [CrossRef]
- Keung, C.; Nguyen, T.C.; Lim, R.; Gerstenmaier, A.; Sievert, W.; Moore, G.T. Local Fistula Injection of Allogeneic Human Amnion Epithelial Cells Is Safe and Well Tolerated in Patients with Refractory Complex Perianal Crohn’s Disease: A Phase I Open Label Study with Long-Term Follow Up. eBioMedicine 2023, 98, 104879. [Google Scholar] [CrossRef]
- Qiu, C.; Ge, Z.; Cui, W.; Yu, L.; Li, J. Human Amniotic Epithelial Stem Cells: A Promising Seed Cell for Clinical Applications. Int. J. Mol. Sci. 2020, 21, 7730. [Google Scholar] [CrossRef]
- Shukla, S.; Mittal, S.K.; Foulsham, W.; Elbasiony, E.; Singhania, D.; Sahu, S.K.; Chauhan, S.K. Therapeutic Efficacy of Different Routes of Mesenchymal Stem Cell Administration in Corneal Injury. Ocul. Surf. 2019, 17, 729–736. [Google Scholar] [CrossRef]
- Srinivasan, R.C.; Kannisto, K.; Strom, S.C.; Gramignoli, R. Evaluation of Different Routes of Administration and Biodistribution of Human Amnion Epithelial Cells in Mice. Cytotherapy 2018, 21, 113–124. [Google Scholar] [CrossRef]
- Packthongsuk, K.; Rathbun, T.; Troyer, D.; Davis, D.L. Porcine Wharton’s Jelly Cells Distribute throughout the Body after Intraperitoneal Injection. Stem Cell Res. Ther. 2018, 9, 38. [Google Scholar] [CrossRef]
- Nguyen, M.P.; Jain, V.; Iansante, V.; Mitry, R.R.; Filippi, C.; Dhawan, A. Clinical Application of Hepatocyte Transplantation: Current Status, Applicability, Limitations, and Future Outlook. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 185–196. [Google Scholar] [CrossRef]
- Miki, T. Clinical Hepatocyte Transplantation. Gastroenterol. Hepatol. Engl. Ed. 2019, 42, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.T.; Bolonio, M.; Cabezas, E.; Pelechá, M.; Pareja, E.; Domènech, A.; Castell, J.V.; Gómez-Lechón, M.J.; Tolosa, L. Improved In Vivo Efficacy of Clinical-Grade Cryopreserved Human Hepatocytes in Mice with Acute Liver Failure. Cytotherapy 2019, 22, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ganai, A.A.; Husain, M. Genistein Attenuates D-GalN Induced Liver Fibrosis/Chronic Liver Damage in Rats by Blocking the TGF-β/Smad Signaling Pathways. Chem. Biol. Interact. 2017, 261, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Ganai, A.A.; Ganaie, I.A.; Verma, N.; Farooqi, H. Regression of Fibrosis/Cirrhosis by Glycine Propionyl-l-Carnitine Treatment in D-Galactosamine Induced Chronic Liver Damage. Chem. Biol. Interact. 2016, 260, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, Y.; Arisaka, H.; Yoshida, S.; Mori, M.; Takahashi, M. The Protection Mechanism of Proline from D-Galactosamine Hepatitis Involves the Early Activation of ROS-Eliminating Pathway in the Liver. SpringerPlus 2015, 4, 199. [Google Scholar] [CrossRef]
- Drucker, C.; Rabe, B.; Chalaris, A.; Schulz, E.; Scheller, J.; Rose-John, S. Interleukin-6 Trans-Signaling Regulates Glycogen Consumption after d-Galactosamine-Induced Liver Damage. J. Interferon Cytokine Res. 2009, 29, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Shinohara, T. Pathological Study of Chronic D-Galactosamine Induced Hepatitis in Mice by Administration of Adjuvants an Animal Model of the Chronic Active Hepatitis. Gastroenterol. Jpn. 1981, 16, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.; Wang, L.; Xu, J.; Wen, J. Hypoglycemia on Admission in Patients with Acute on Chronic Liver Failure: A Retrospective Cohort Analyzing the Current Situation, Risk Factors, and Associations with Prognosis. Ann. Palliat. Med. 2023, 12, 163–170. [Google Scholar] [CrossRef]
- Shah, N.J.; Royer, A.; John, S. Acute Liver Failure. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ramanathan, R.; Rupert, S.; Selvaraj, S.; Satyanesan, J.; Vennila, R.; Rajagopal, S. Role of Human Wharton’s Jelly Derived Mesenchymal Stem Cells (WJ-MSCs) for Rescue of D-Galactosamine Induced Acute Liver Injury in Mice. J. Clin. Exp. Hepatol. 2017, 7, 205–214. [Google Scholar] [CrossRef]
- Chung, H.; Kim, H.J.; Jang, K.S.; Kim, M.; Yang, J.; Kang, K.S.; Kim, H.L.; Yoon, B.I.; Lee, M.O.; Lee, B.H.; et al. Comprehensive Analysis of Differential Gene Expression Profiles on D-Galactosamine-Induced Acute Mouse Liver Injury and Regeneration. Toxicology 2006, 227, 136–144. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Mitamura, K.; Ross, M.A.; Stolz, D.B.; Strom, S.C. Identification of Stem Cell Marker-Positive Cells by Immunofluorescence in Term Human Amnion. J. Reprod. Immunol. 2007, 75, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, Q.; Jin, L. The Role of B7 Family Molecules in Maternal–Fetal Immunity. Front. Immunol. 2020, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Zhou, L.; Sagayaraj, A.; Jumat, N.H.B.; Choolani, M.; Chan, J.K.Y.; Biswas, A.; Wong, P.C.; Lim, S.G.; Dan, Y.Y. Hepatic Differentiation of Human Amniotic Epithelial Cells and In Vivo Therapeutic Effect on Animal Model of Cirrhosis. J. Gastroenterol. Hepatol. 2015, 30, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Maymó, J.L.; Riedel, R.; Pérez-Pérez, A.; Magatti, M.; Maskin, B.; Dueñas, J.L.; Parolini, O.; Sánchez-Margalet, V.; Varone, C.L. Proliferation and Survival of Human Amniotic Epithelial Cells during Their Hepatic Differentiation. PLoS ONE 2018, 13, e191489. [Google Scholar] [CrossRef] [PubMed]
- Alhomrani, M.; Correia, J.; Zavou, M.; Leaw, B.; Kuk, N.; Xu, R.; Saad, M.I.; Hodge, A.; Greening, D.W.; Lim, R.; et al. The Human Amnion Epithelial Cell Secretome Decreases Hepatic Fibrosis in Mice with Chronic Liver Fibrosis. Front. Pharmacol. 2017, 8, 748. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X.; Chen, J.; Li, X.; Wang, L.; Wang, B.; Peng, W.; Yang, C.; Li, Z.; Chen, Y.; et al. Pericentral Hepatocytes Produce Insulin-like Growth Factor-2 to Promote Liver Regeneration during Selected Injuries in Mice. Hepatology 2017, 66, 2002–2015. [Google Scholar] [CrossRef] [PubMed]
- Andrewartha, N.; Yeoh, G. Human Amnion Epithelial Cell Therapy for Chronic Liver Disease. Stem Cells Int. 2019, 2019, 8106482. [Google Scholar] [CrossRef]
- Boone, L.; Meyer, D.; Cusick, P.; Ennulat, D.; Provencher Bolliger, A.; Everds, N.; Meador, V.; Elliott, G.; Honor, D.; Bounous, D.; et al. Selection and Interpretation of Clinical Pathology Indicators of Hepatic Injury in Preclinical Studies. Vet. Clin. Pathol. 2005, 34, 182–188. [Google Scholar] [CrossRef]
- Aulbach, A.D.; Amuzie, C.J. Biomarkers in Nonclinical Drug Development, 2nd ed.; Elsevier Inc., Academic Press: Boston, MA, USA, 2017; pp. 447–471. [Google Scholar]
- Liu, W.; Zhang, L.; Xuan, K.; Hu, C.; Li, L.; Zhang, Y.; Jin, F.; Jin, Y. Alkaline Phosphatase Controls Lineage Switching of Mesenchymal Stem Cells by Regulating the Lrp6/Gsk3β Complex in Hypophosphatasia. Theranostics 2018, 8, 5575–5592. [Google Scholar] [CrossRef]
- Wang, M.; Liang, C.; Hu, H.; Zhou, L.; Xu, B.; Wang, X.; Han, Y.; Nie, Y.; Jia, S.; Liang, J.; et al. Intraperitoneal Injection (IP), Intravenous Injection (IV) or Anal Injection (AI)? Best Way for Mesenchymal Stem Cells Transplantation for Colitis. Sci. Rep. 2016, 6, 30696. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar] [PubMed]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should It Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fan, X.; Zhang, L.; Shi, G.; Aili, M.; Lu, X.; Jiang, T.; Zhang, Y. Bone Mesenchymal Stem Cell Transplantation via Four Routes for the Treatment of Acute Liver Failure in Rats. Int. J. Mol. Med. 2014, 34, 987–996. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vidal-Vanaclocha, F. The Prometastatic Microenvironment of the Liver. Cancer Microenviron. 2008, 1, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Diaz, M.; Quiñones-Vico, M.I.; de la Torre, R.S.; Montero-Vílchez, T.; Sierra-Sánchez, A.; Molina-Leyva, A.; Arias-Santiago, S. Biodistribution of Mesenchymal Stromal Cells after Administration in Animal Models and Humans: A Systematic Review. J. Clin. Med. 2021, 10, 2925. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Charron, M.; Reyes, J.; Rubinstein, W.; Strom, S.C.; Swanson, D.; Towbin, R. Use of Indium-111-Labeled Hepatocytes to Determine the Biodistribution of Transplanted Hepatocytes through Portal Vein Infusion. Clin. Nucl. Med. 2000, 25, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. Concise Review: MSC Adhesion Cascade—Insights into Homing and Transendothelial Migration. Stem Cells 2017, 35, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Park, B.N.; Lim, T.S.; Yoon, J.K.; An, Y.S. In Vivo Tracking of Intravenously Injected Mesenchymal Stem Cells in an Alzheimer’s Animal Model. Cell Transplant. 2018, 27, 1203–1209. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal Stem Cells Are Short-Lived and Do Not Migrate beyond the Lungs after Intravenous Infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Riegler, J.; Wu, J.C. Stem Cell Imaging: From Bench to Bedside. Cell Stem Cell 2014, 14, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Gholamrezanezhad, A.; Mirpour, S.; Bagheri, M.; Mohamadnejad, M.; Alimoghaddam, K.; Abdolahzadeh, L.; Saghari, M.; Malekzadeh, R. In Vivo Tracking of 111In-Oxine Labeled Mesenchymal Stem Cells Following Infusion in Patients with Advanced Cirrhosis. Nucl. Med. Biol. 2011, 38, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Gao, F.; Lin, J.; Lu, L.; Xu, H.; Xu, G.T. Conditioned Medium of Human Amniotic Epithelial Cells Alleviates Experimental Allergic Conjunctivitis Mainly by IL-1ra and IL-10. Front. Immunol. 2021, 12, 774601. [Google Scholar] [CrossRef] [PubMed]
- Gramignoli, R. Therapeutic Use of Human Amnion-Derived Products: Cell-Based Therapy for Liver Disease. Curr. Pathobiol. Rep. 2016, 4, 157–167. [Google Scholar] [CrossRef]
- Jafari, A.; Niknejad, H.; Rezaei-Tavirani, M.; Sarrami-Forooshani, R.; Gilanchi, S.; Jafari, Z. Antiproliferative and Apoptotic Effects of Conditioned Medium Released from Human Amniotic Epithelial Stem Cells on Breast and Cervical Cancer Cells. Int. J. Immunopathol. Pharmacol. 2023, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dong, V.; Nanchal, R.; Karvellas, C.J. Pathophysiology of Acute Liver Failure. Nutr. Clin. Pract. 2020, 35, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, H.; Zhou, J.; Xia, S.; Shi, X.; Ren, H. Transplantation of Mesenchymal Stem Cells Attenuates Acute Liver Failure in Mice via an Interleukin-4-Dependent Switch to the M2 Macrophage Anti-Inflammatory Phenotype. J. Clin. Transl. Hepatol. 2022, 10, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Jamshidi, S.; Dehghan, M.M.; Saheli, M.; Piryaei, A. Bone Marrow or Adipose Tissue Mesenchymal Stem Cells: Comparison of the Therapeutic Potentials in Mice Model of Acute Liver Failure. J. Cell. Biochem. 2018, 119, 5834–5842. [Google Scholar] [CrossRef]
- Iansante, V.; Mitry, R.R.; Filippi, C.; Fitzpatrick, E.; Dhawan, A. Human Hepatocyte Transplantation for Liver Disease: Current Status and Future Perspectives. Pediatr. Res. 2018, 83, 232–240. [Google Scholar] [CrossRef]
- Gilbert, J.C.; Stein, J.E.; Takeda, T. Quantitation of Transplanted Hepatic Mass Necessary Gunn Rat Model of Hyperbilirubinemia. J. Pediatr. Surg. 1991, 27, 298–301. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).