Abstract
Cutaneous alternariosis is a rare condition, more frequently presented in immunocompromised patients, which usually requires long courses of systemic antifungals that may interact with other medications. The presented series shows three cases of cutaneous alternariosis in immunocompromised patients and organ transplant recipients that were successfully treated with photodynamic therapy and oral antifungals, allowing a reduction in the systemic treatment duration and therefore decreasing the risk of side effects and drug interactions.
1. Introduction
Alternaria is a dematiaceous fungus, with widespread presence in the natural environment, that causes phaeohyphomycosis, a relatively infrequent human infection. This melanized mold can lead to a diverse range of clinical conditions, varying from localized manifestations in specific organs to more widespread systemic infections [1]. Alternaria infects mainly immunocompromised hosts; although there have been occasional instances of infections in individuals with healthy immune systems [2,3], these cases seldom progress to invasive diseases [4].
The skin and the subcutaneous tissues are the most frequently infected sites by this fungus, although other types of infections such as oculomycosis, sinus infections, onychomycosis, and invasive diseases have been described [1].
The treatment of cutaneous alternariosis includes, when feasible, the reduction of immunosuppression, in addition to long courses of antifungals, which often interact with the immunosuppressants taken by organ transplant recipients (OTRs). Excision of the lesions can also be an option in certain cases, often combined with systemic treatment [5].
Recently, some authors have shown the use of antimicrobial photodynamic therapy (PDT) as an effective treatment of phaeohyphomycosis [6,7], and also chromoblastomycosis [4,8]. PDT is based on the use of photosensitizing molecules that end up generating reactive oxygen species (ROS) that destroy the target cells when being irradiated with light of a suitable wavelength and a proper dose [9]. Some reports have shown that PDT is effective in the treatment of cutaneous infections due to pathogens such as multi-resistant mycobacteria, in combination with systemic treatments [10]. Moreover, it also offers the possibility of a reduction in the duration of systemic treatment of fungal infections such as onychomycosis [11]. In relation with cutaneous alternariosis, Liu et al. [4] reported a case of an infection due to Alternaria alternata treated with oral itraconazole followed by PDT with complete healing. Moreover, the combination of systemic antifungal and PDT has also been described in other cutaneous fungal infections with promising results [6,7,8].
We present three additional cases of cutaneous alternariosis in immunocompromised patients, two of them OTRs, treated with systemic antifungals and PDT (Table 1), and a review of the literature about the role of PDT in the treatment of phaeohyphomycosis and chromoblastomycosis. In our series, the combination of both treatment modalities allowed shortening the antifungal treatment duration, diminishing the possibility of interactions with other drugs, especially in the ORTs, and side effects.

Table 1.
Summary of the cases of cutaneous Alternaria infection treated with photodynamic therapy and systemic antifungals. COPD: Chronic Obstructive Pulmonary Disease. PDT: photodynamic therapy. 5-ALA: 5-aminolevulinic acid (Ameluz®). MAL: methyl-aminolevulinate (Metvix®).
2. Case 1
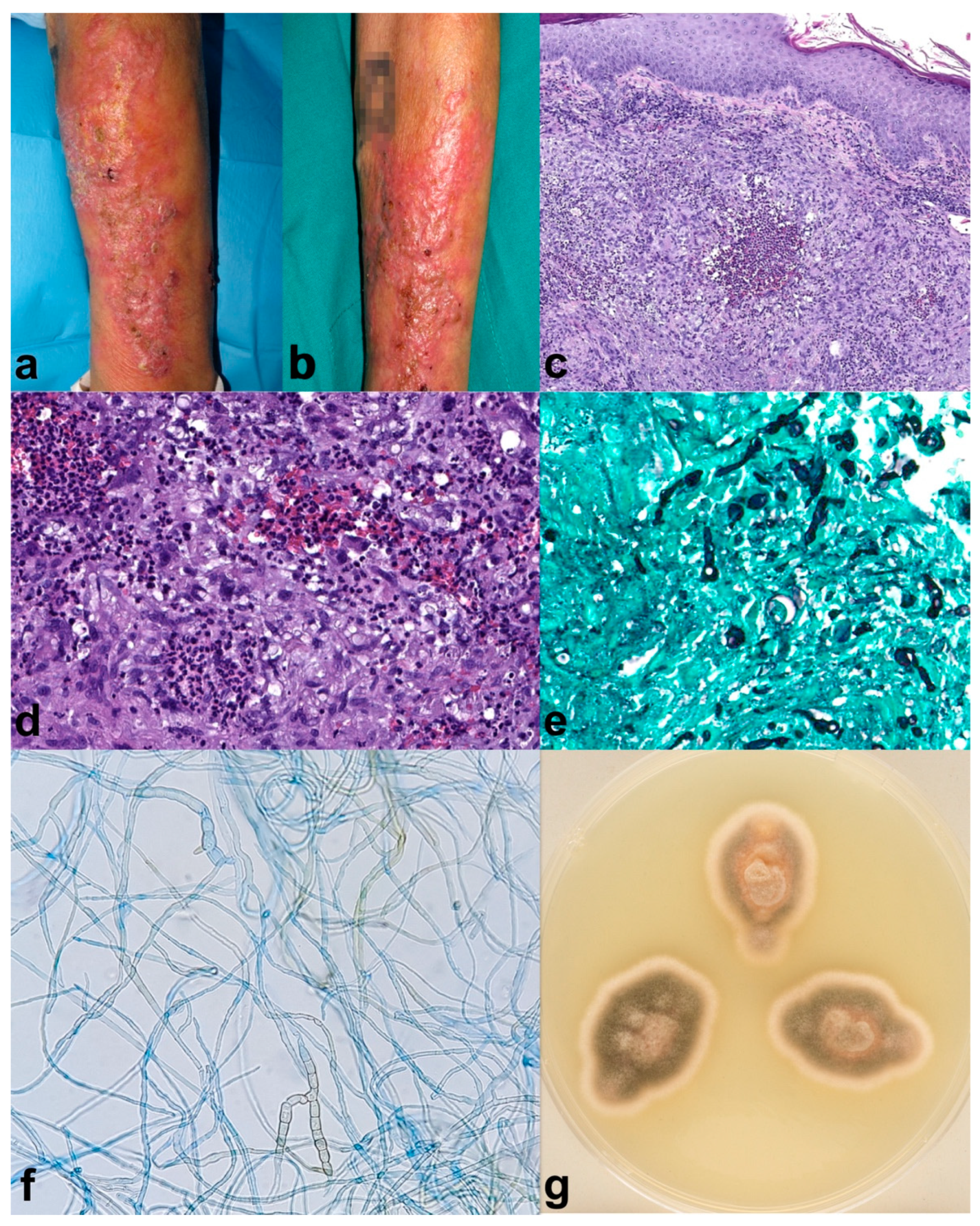
A 70-year-old male with a personal history of Chronic Obstructive Pulmonary Disease (COPD), requiring frequent courses of oral steroids (30–40 mg of prednisone for 10 to 14 days every month), diabetes mellitus type 2 with poor control, and larynx squamous cell carcinoma. The patient developed verrucous plaques on the left forearm for four months before consulting (Figure 1a,b). Moreover, he mentioned gardening often and outdoor farming activities. The histopathological analysis showed light acanthosis, with mild hyperkeratosis and foci of parakeratosis; at the dermal layer, there was granulomatous perifollicular inflammation with multinuclear, surrounding broken follicles (Figure 1c,d). Grocott (Figure 1e) and PAS staining revealed structures compatible with hyphae, and Ziehl–Nielsen was negative. Tissue culture showed a grey-olive green color and woolly texture colonies (Figure 1g). Microscopic study showed septate, brown hyphae compatible with those of Alternaria spp. (Figure 1f). Alternaria infectoriae was identified by PCR sequencing. The patient received one month of itraconazole 100 mg twice daily, and fivefour months of 50 mg twice daily achieving partial improvement, followed by two sessions of daylight photodynamic therapy (DL-PDT) with 5-aminolevulinic acid (5-ALA, Ameluz®) consistent of 30 min of incubation followed by 2 hours of exposure to sunlight, once every 10 days. Complete healing was achieved after de DL-PDT, with no remission relapse after two months. The patient died shortly after due to COPD worsening.
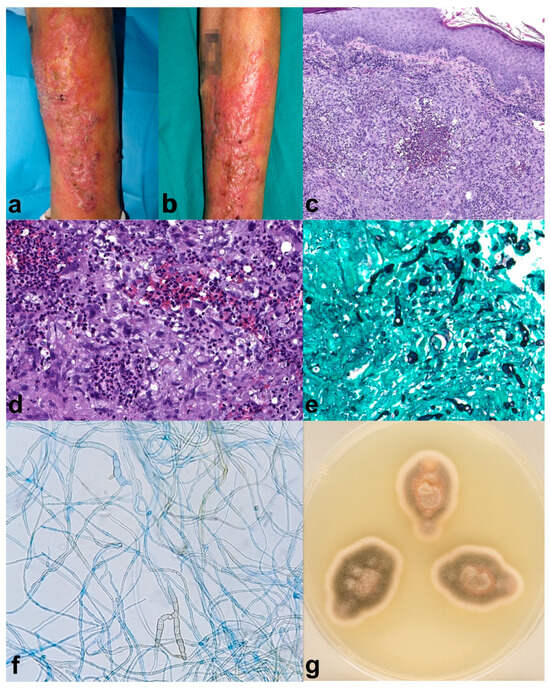
Figure 1.
Case 1. (a,b) Erythematous verrucous plaques on the left forearm. (c) (H-E 8×) Histopathological analysis showed light acanthosis, with mild hyperkeratosis and foci of parakeratosis; at the dermal layer, there was granulomatous perifollicular inflammation with multinuclear, surrounding broken follicles. (d) (H-E 20×) Some yeast forms can also be identified. Grocott (e) (20×) and PAS staining revealed structures compatible with hyphae, and Ziehl–Nielsen was negative. (g) Macroscopic appearance of the culture showed a grey-olive green color and woolly texture colonies. (f) (40×) The septate, brown hyphae seen microscopically were compatible with Alternaria spp. Alternaria infectoriae was identified by PCR sequencing. Partial improvement was seen five months after Itraconazole (100 mg/12 h for 1 month and Itraconazole 50 mg/12 h for 4 months), which was followed by two sessions of 5-aminolevulinic acid (5-ALA) daylight photodynamic therapy, achieving resolution of the lesions (no photography available). The patient died shortly after due to COPD worsening.
3. Case 2
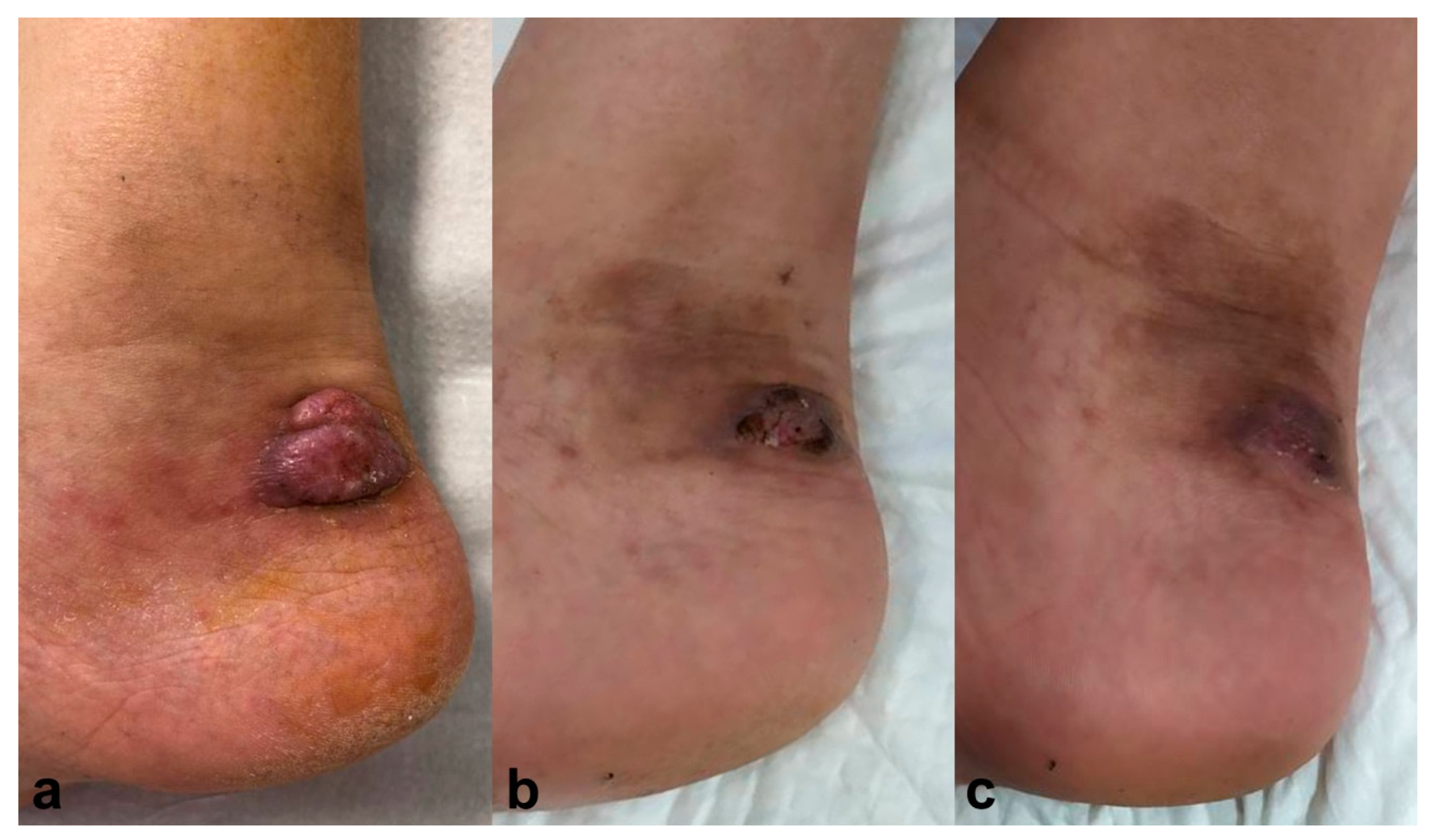
A 62-year-old male, treated with tacrolimus (0.1 mg/kg/day) and 10 mg/day of prednisone due to a kidney transplant, presented with a tumoral lesion on the lateral side of his right ankle two months ago (Figure 2a). Histopathology analysis showed hyphae compatible with phaeohyphomycotic infection, and culture confirmed Alternaria spp. Simultaneous treatment with voriconazole 400 mg/day for three months and PDT with methyl-aminolevulinate (MAL-PDT), Metvix®, was started with 1 hour incubation, progressively increasing the time to 3 hours; and subsequent illumination with Aktilite® increasing fluences from 37 to 74 J/cm2. MAL-PDT was performed once a week, with a total of 12 sessions, achieving complete resolution after six months, with no relapse after twelve months follow-up (Figure 2b).
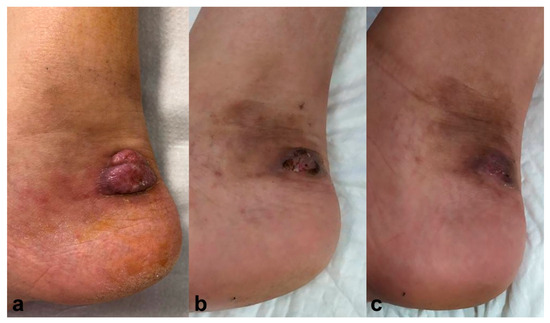
Figure 2.
Case 2. (a) Erythematous-violaceus tumoral lesion on the lateral side of the right ankle. (b) Resolution six months after simultaneous treatment with voriconazole 400 mg/day for three months and photodynamic therapy with methyl-aminolevulinate (Metvix®). Resolution after six months and no recurrence after twelve months follow-up (c).
4. Case 3
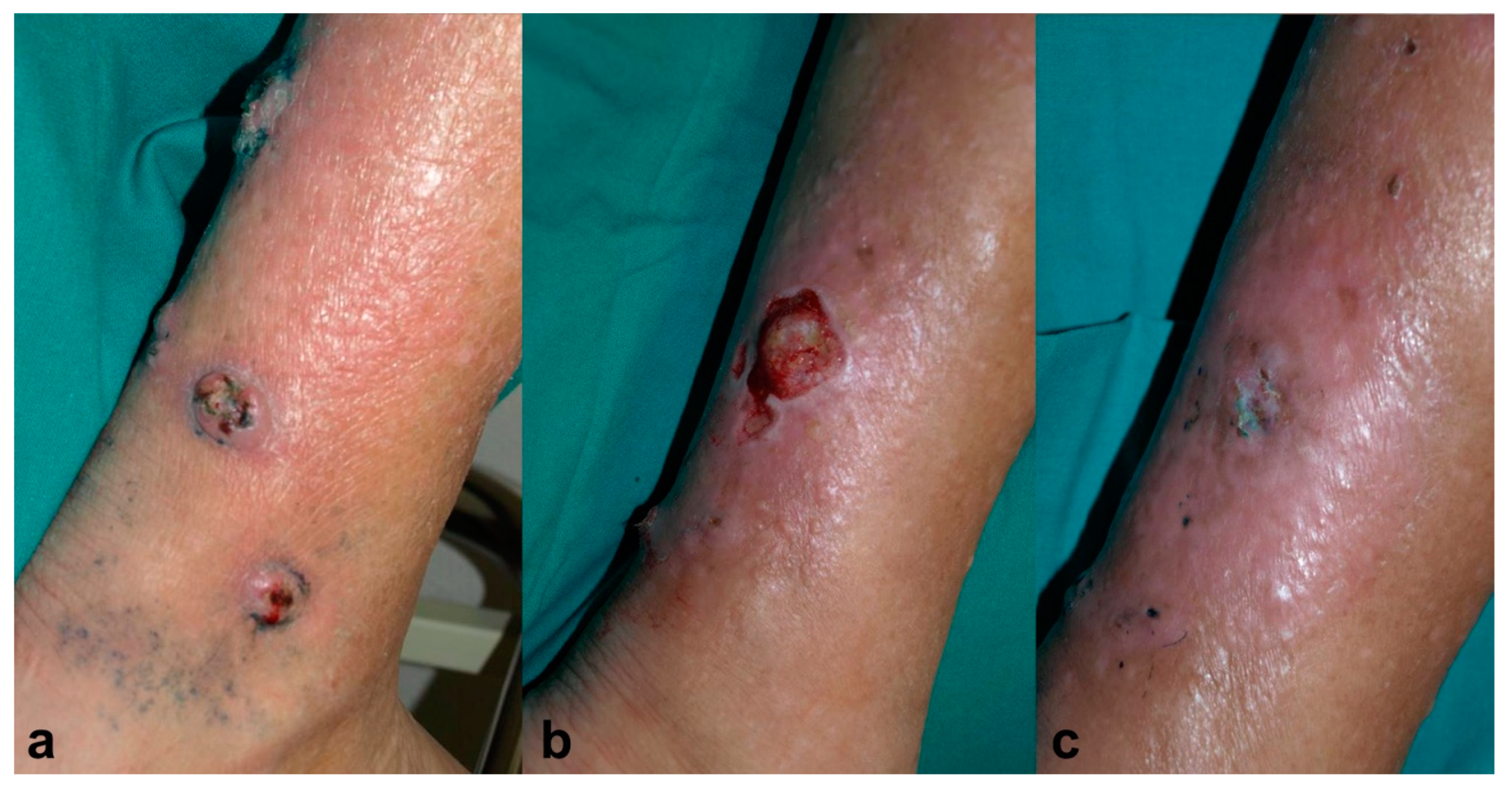
An 81-year-old male, recipient of a kidney transplant, under treatment with tacrolimus (0.2 mg/kg/day) and 5 mg/day of prednisone, was referred to the dermatology clinic for four ulcerated lesions surrounded by grey-blue macules on his right leg for the past month (Figure 3a). A skin biopsy showed findings consistent with deep fungal infection and Alternaria spp. grew in the tissue culture. It was decided to treat the patient with MAL-PDT, Metvix®, with three hours of incubation followed by illumination with Aktilite® 37 J/cm2 (twice weekly). After 11 sessions, he showed partial improvement, but the culture was still positive; therefore, the photosensitizer was switched to methylene blue 1% solution (MB-PDT) with 30 minutes of incubation under occlusion and illuminated with Aktilite® (fluence 74 J/cm2) once a week, and exposure to daylight for half an hour every day until the disappearance of the blue color. After 10 sessions of MB-PDT, since the biggest lesion persisted, voriconazole 400mg per day was added to MB-PDT (Figure 3b). After 1.5 months, the lesion was clinical and microbiologically cured, and voriconazole also had to be discontinued due to acute renal failure. No recurrence had been presented after three years of follow-up (Figure 3c).
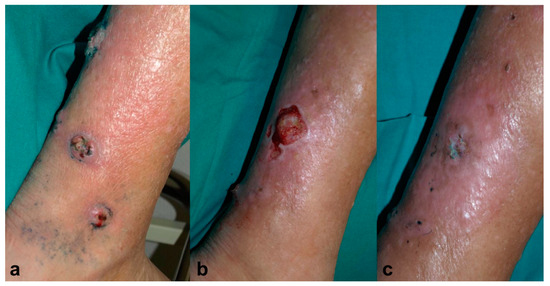
Figure 3.
Case 3. (a) Ulcerated lesions on the right leg surrounded by grey-blue macules. (b) Partial resolution after 11 sessions of photodynamic therapy with methyl-aminolevulinate (Metvix®) (MAL-PDT) and 10 sessions of photodynamic therapy with methylene blue (MB-PDT). (c) Complete healing after 1.5 months of simultaneous treatment with voriconazole 400 mg/day and MB-PDT (6 sessions), leaving mild erythema and residual hyperpigmentation.
5. Discussion
Cutaneous alternariosis is caused by the dematiaceous fungus Alternaria, which is frequently isolated in opportunistic deep cutaneous mycoses [5]. Alternaria is widely distributed and can be found in plants, water, soil water, or objects, among others [2]. The most frequent transmission mechanism in cutaneous infections is direct inoculation during or after a traumatism, commonly causing phaeohyphomycosis. However, cases of chromoblastomycosis due to Alternaria, characterized by the histopathologic finding of sclerotic or muriform cells, have also been reported [4,12,13]. The clinical presentation is usually non-specific and asymptomatic and may present as small papules or nodules, multinodular plaques, ulcers, cellulitis, or even blisters, usually in exposed areas of outdoor workers, as they are more prone to trauma [5,6].
Histopathological study often shows a dense dermal suppurative granulomatous infiltrate and fungal structures, which are more evident with different stains such as Grocott, Periodic acid–Schiff (PAS), or Masson-Fontana. These findings are enough to guide the management, which commonly involves empirical treatment with high doses of antifungals. Confirmation of the fungal species requires culture or direct sequencing [5].
Surgery can be an effective treatment option; however, it might not be enough or possible in some cases such as in large and multifocal cases that require a combination with systemic antifungals [5], or certain locations in which the surgery would be too aggressive, or in which healing would be difficult.
High doses of antifungals for several months are recommended to treat cutaneous alternariosis. Itraconazole 400 mg/day or voriconazole 400 mg/day are two of the most common options [5]. Nevertheless, the use of these treatments in OTRs requires close monitoring due to their interaction with calcineurin inhibitors, as well as other adverse reactions such as hepatotoxicity. A combination of surgery plus different antifungals is commonly needed to achieve cure rates around 90% due to adverse events to the antifungal drugs or lack of response to them [5], which is emerging as a concern even in immunocompetent patients [2].
PDT has been shown to be effective in different types of infections including bacterial infections [9], fungal infections [11,14], multi-resistant mycobacteria, or other pathogens [10,15]. The antimicrobial efficacy of PDT has been extensively explored through numerous in vitro studies since the 1990s, and unlike traditional antibiotics, PDT’s diverse cellular targets make resistance development less likely [16].
Recently, there have been reports of encouraging outcomes achieved through the utilization of PDT (using methylene blue as a photosensitizer rather than ALA) in the management of chromoblastomycosis [17,18]. Moreover, several authors have also shown the utility of PDT with ALA as an adjuvant of antifungal therapy for the treatment of different phaeohyphomycosis and chromoblastomycosis (Table 2).
Yang and colleagues [19] documented a challenging case of chromoblastomycosis caused by F. monophora that showed improvement following treatment with ALA-PDT. Moreover, Liu et al. [4] described a 50-year-old man with a chromoblastomycosis because of Alternaria alternata, successfully treated with a short course of systemic itraconazole and subsequent 5-aminolevulinic acid-photodynamic therapy.
The presented case series shows similar results of PDT in phaeohyphomycosis due to Alternaria and highlights the challenges of diagnosing and managing opportunistic co-infection in an immunocompromised host. As previously described in fungal infections such as onychomycosis [11], PDT with MAL or 5-ALA potentiates the effects of the systemic antifungals, both sequentially or simultaneously, permitting a reduction in the duration of the systemic treatment, and therefore, diminishing the possibility of drug adverse events and interactions. In addition, the beneficial effects of PDT on host tissues, such as growth factor stimulation and immune response enhancement, potentially foster improved wound healing, particularly in common presentations of this condition such as chronic ulcers [20].

Table 2.
Summary of previous reports of chromoblastomycosis and phaeohyphomycosis treated with PDT. CBM: chromoblastomycosis. PHM: Phaeohyphomycosis. ALA: 5-aminolevulinic acid. N/S: not specified. The sign “+” indicates simultaneously.
Table 2.
Summary of previous reports of chromoblastomycosis and phaeohyphomycosis treated with PDT. CBM: chromoblastomycosis. PHM: Phaeohyphomycosis. ALA: 5-aminolevulinic acid. N/S: not specified. The sign “+” indicates simultaneously.
| Study | Pathogen | Oral Treatment | PDT Photosensitizer (Incubation) | Source of Light | Total Number of Sessions | Outcome |
|---|---|---|---|---|---|---|
| Wang et al. 2023 [21] | C. lunata (PHM) | None (surgery + PDT) | 20% ALA (4 h) | LED 635 nm (80 J/cm2) | 3 (every 9 days) | Healing |
| Yang et al. 2020 [22] | F. monophora (CBM) | Itraconazole 400 mg/d (2 months) + PDT | 20% ALA (2 h) | LED 630 nm (90 J/cm2) | 3 (every 10 days) | Healing |
| Lan et al. 2021 [23] | F. monophora (CBM) | Terbinafine 250 mg/d, Itraconazole 400 mg/d (4 months) and isotretinoin 20 mg/d (1 month), before ALA-PDT | 20% ALA (3 h) + previous vaporization of hyperkeratosis with CO2 laser | LED 633 nm (96 J/cm2) | 4 (weekly) | Improvement |
| Liu et al. 2019 [7] | E. spinifera (PHM) | Itraconazole 200 mg/d + terbinafine 250 mg/d (5 months) + PDT | 20% ALA (4 h) | LED 633 nm (120 mW/cm2, 25 min) | 3 (weekly) | Healing |
| Hu et al. 2019 [8] | F. nubica | Itraconazole 200 mg/d (1 year) + PDT | 20% ALA (4 h) | LED 635 nm (36.8 mW/cm2) | 4 (weekly) | Improvement |
| Hu et al. 2019 [8] | F. pedrosoi | Terbinafine 250 mg/d (8 months) + PDT | 20% ALA (4 h) | LED 635 nm (36.8 mW/cm2) | 4 (weekly) | Healing |
| Hu et al. 2019 [8] | F. pedrosoi | Itraconazole 200 mg/d + terbinafine 250 mg/d (2 years) + PDT | 20% ALA (4 h) | LED 635 nm (36.8 mW/cm2) | 3 (weekly or every 2 weeks) | Improvement |
| Hu et al. 2019 [8] | F. monophora | Terbinafine 250 mg/d | 20% ALA (4 h) | LED 635 nm (36.8 mW/cm2) | 18 (weekly) | Healing |
| Hu et al. 2019 [8] | F. monophora | Terbinafine 250 mg/d | 20% ALA (4 h) | LED 635 nm (36.8 mW/cm2) | 10 (weekly) | Improvement |
| Huang et al. 2019 [24] | F. pedrosoi (CBM) | Itraconazole 400 mg/d (2 moths) before PDT | 10% ALA (4 h) | LED 633 nm (80–100 mW/cm2, 25 min) | 6 (every 2 weeks) | Healing |
| Liu et al. 2014 [4] | Alternaria alternata (CBM) | Itraconazole 400 mg/d (15 weeks) before PDT | 20% ALA (3 h) | LED 633 nm (80 mW/cm2) | 3 | Healing |
| Yang et al. 2012 [19] | F. monophora (CBM) | Terbinafine 250 mg/d (5 months) and voriconazole 200 mg/d (2 months) + PDT | ALA | N/S | 10 (weekly) | Improvement |
| Pereira Lyon et al. 2011 [17] | 10 cases of CBM | Itraconazole after PDT | 20% methylene blue preparation in Eucerin cream (4 h) | LED 660 nm (28 J/cm2) | 6 (weekly) | Improvement with PDT and healing after itraconazole |
6. Conclusions
PDT has been shown to be effective for the treatment of various types of infections including cutaneous fungal infections. The presented case series, as well as previous reports, supports its role as a simple and effective treatment for cutaneous alternariosis and other cutaneous fungal infections, which has a synergistic effect with oral antifungals. Combination of PDT with systemic antifungals allows an important reduction in the treatment duration and the healing time. By shortening the systemic antifungal treatments, the possible interactions with other drugs are also reduced, increasing the safety of the treatment. This is particularly important in certain populations, such as organ transplant recipients, who need immunosuppressive treatments that require monitoring when taking certain systemic antifungals and are more susceptible to these types of opportunistic infections.
Author Contributions
Conceptualization, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G.; methodology, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G.; software, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G.; formal analysis, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G.; investigation, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G.; resources, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G.; data curation, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G.; writing—original draft preparation, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G.; writing—review and editing, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G.; visualization, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G.; supervision, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G.; project administration, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G.; funding acquisition, P.G.-P., T.G.-C., M.Á.-S., M.A.G., M.G.-G., A.B.-R., M.A.-B., A.N.-B. and Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
All patients signed informed consent for the publication of clinical images and case details.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pastor, F.J.; Guarro, J. Alternaria infections: Laboratory diagnosis and relevant clinical features. Clin. Microbiol. Infect. 2008, 14, 734–746. [Google Scholar] [CrossRef]
- Colosi, I.A.; Crișan, M.; Țoc, D.A.; Colosi, H.A.; Georgiu, C.; Sabou, M.; Costache, C. First Reported Case of a Clinically Nonresponsive-to-Itraconazole Alternaria alternata Isolated from a Skin Infection of a Nonimmunocompromised Patient from Romania. J. Fungi 2023, 9, 839. [Google Scholar] [CrossRef]
- Jebari, M.; Mtibaa, L.; Abid, R.; Hannechi, S.; Souid, H.; Battikh, R.; Louzir, B.; Jemli, B. Unusual location of cutaneous alternariosis in an immunocompetent patient. IDCases 2022, 27, e01356. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Xia, X.-J. Successful sequential treatment with itraconazole and ALA-PDT for chromoblastomycosis because of Alternaria alternata: Itraconazole and ALA-PDT for chromoblastomycosis. Dermatol. Ther. 2014, 27, 357–360. [Google Scholar] [CrossRef]
- Ferrándiz-Pulido, C.; Martin-Gomez, M.T.; Repiso, T.; Juárez-Dobjanschi, C.; Ferrer, B.; López-Lerma, I.; Aparicio, G.; González-Cruz, C.; Moreso, F.; Roman, A.; et al. Cutaneous infections by dematiaceous opportunistic fungi: Diagnosis and management in 11 solid organ transplant recipients. Mycoses 2019, 62, 121–127. [Google Scholar] [CrossRef]
- Wang, X.; Ling, S.; Liang, G.; Sun, J. An Unusual Presentation of Cutaneous Alternariosis with Bullous Lesions in a Patient with Diabetes Mellitus. Mycopathologia 2022, 187, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Chen, Y.; Xue, R.; Zeng, W.; Xi, L.; Chen, Y. Phaeohyphomycosis due to Exophiala spinifera greatly improved by ALA-PDT: A case report. Photodiagnosis Photodyn. Ther. 2019, 28, 297–299. [Google Scholar] [CrossRef]
- Hu, Y.; Qi, X.; Sun, H.; Lu, Y.; Hu, Y.; Chen, X.; Liu, K.; Yang, Y.; Mao, Z.; Wu, Z.; et al. Photodynamic therapy combined with antifungal drugs against chromoblastomycosis and the effect of ALA-PDT on Fonsecaea in vitro. PLoS Neglected Trop. Dis. 2019, 13, e0007849. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Rezusta, A.; Gilaberte, Y. Photodynamic therapy using methylene blue, combined or not with gentamicin, against Staphylococcus aureus and Pseudomonas aeruginosa. Photodiagnosis Photodyn. Ther. 2020, 31, 101810. [Google Scholar] [CrossRef] [PubMed]
- Cerro-Muñoz, P.; Navarro-Bielsa, A.; Almenara-Blasco, M.; Algara, O.; Gracia-Cazaña, T.; Gilaberte, Y. Multiresistant Mycobacterium abscessus ulcer treated with photodynamic therapy with methyl-aminolevulinate. Dermatol. Ther. 2022, 35, e15756. [Google Scholar] [CrossRef]
- Navarro-Bielsa, A.; Gracia-Cazaña, T.; Robres, P.; Lopez, C.; Calvo-Priego, M.D.; Aspiroz, C.; Gilaberte, Y. Combination of Photodynamic Therapy and Oral Antifungals for the Treatment of Onychomycosis. Pharmaceuticals 2022, 15, 722. [Google Scholar] [CrossRef]
- Morales, A.; Charlez, L.; Remón, L.; Sanz, P.; Aspiroz, C. Cutaneous Alternariosis in a Heart Transplant Recipient. Actas Dermosifiliogr. 2010, 101, 370–372. [Google Scholar] [CrossRef]
- Thomas, E.; Bertolotti, A.; Barreau, A.; Klisnick, J.; Tournebize, P.; Borgherini, G.; Zemali, N.; Jaubert, J.; Jouvion, G.; Bretagne, S.; et al. From phaeohyphomycosis to disseminated chromoblastomycosis: A retrospective study of infections caused by dematiaceous fungi. Med. Mal. Infect. 2018, 48, 278–285. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Y. Photodynamic Therapy for the Treatment of Fungal Infections. Infect Drug Resist. 2022, 15, 3251–3266. [Google Scholar] [CrossRef]
- Leanse, L.G.; Marasini, S.; dos Anjos, C.; Dai, T. Antimicrobial Resistance: Is There a ‘Light’ at the End of the Tunnel? Antibiotics 2023, 12, 1437. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagnosis Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.P.; de Maria Pedroso e Silva Azevedo, C.; Moreira, L.M.; de Lima, C.J.; de Resende, M.A. Photodynamic Antifungal Therapy Against Chromoblastomycosis. Mycopathologia 2011, 172, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.P.; Moreira, L.M.; de Carvalho, V.S.D.; dos Santos, F.V.; de Lima, C.J.; de Resende, M.A. In vitro photodynamic therapy against Foncecaea pedrosoi and Cladophialophora carrionii. Mycoses 2013, 56, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, Y.; Zhang, J.; Li, X.; Lu, C.; Liang, Y.; Xi, L. A refractory case of chromoblastomycosis due to Fonsecaea monophora with improvement by photodynamic therapy. Med Mycol. 2012, 50, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, Y.; Yang, Z.; Zhang, T.; Feng, Y.; Li, D.; Yan, H.; Shi, D. Surgery plus photodynamic therapy for a diabetic patient with cutaneous infectious granuloma caused by Curvularia lunata. Photodiagnosis Photodyn. Ther. 2023, 41, 103253. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, W.; Luo, J.; Chen, J.; Tan, Y.; Lei, X. 5-aminolevulinic acid-based photodynamic therapy associated with Itraconazole successfully treated a case of chromoblastomycosis. Photodiagnosis Photodyn. Ther. 2020, 29, 101589. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Lu, S.; Zhang, J. Retinoid combined with photodynamic therapy against hyperkeratotic chromoblastomycosis: A case report and literature review. Mycoses 2021, 64, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Han, K.; Wang, L.; Peng, X.; Zeng, K.; Li, L. Successful treatment of chromoblastomycosis using ALA-PDT in a patient with leukopenia. Photodiagnosis Photodyn. Ther. 2019, 26, 13–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).