Abstract
To combat the problem of the increasing drug resistance of microorganisms, it is necessary to constantly search for new medicinal substances that will demonstrate more effective mechanisms of action with a limited number of side effects. Naphthyridines are N-heterocyclic compounds containing a fused system of two pyridine rings, occurring in the form of six structural isomers with different positions of nitrogen atoms, which exhibit a wide spectrum of pharmacological activity, in particular antimicrobial properties. This review presents most of the literature data about the synthetic and natural naphthyridine derivatives that have been reported to possess antimicrobial activity.
1. Introduction
Infectious diseases are an increasing problem in modern medicine. The growing drug resistance of bacterial and pathogenic fungi strains is one of the greatest threats to human health and life. Antimicrobial resistance may appear within a few years after the introduction of a new therapeutic agent to treatment. In order to combat growing drug resistance and increase the effectiveness and safety of therapy, it is necessary to constantly search for new medicinal substances that demonstrate more effective mechanisms of action with a limited number of adverse effects. Researchers are investigating new antimicrobial compounds using traditional techniques such as broth dilution, well diffusion, and disc diffusion, and as well as various antimicrobial assays, such as cross-streaking, poisoned food, time–kill kinetics, co-cultures, and resazurin assays. More advanced techniques such as impedance analysis, flow cytometry, and bioluminescence techniques provide rapid and accurate results and can show the effect of antimicrobials on cellular integrity [1]. Drugs can be obtained by isolating the desired compounds from natural products, developing new methods of organic synthesis, or modifying existing chemical structures. Naphthyridines are N-heterocyclic compounds, which are also called “diazanaphthalenes” or “benzodiazines”, due to the presence of a fused system of two pyridine rings, occurring in the form of six isomers with different positions of nitrogen atoms (Figure 1).
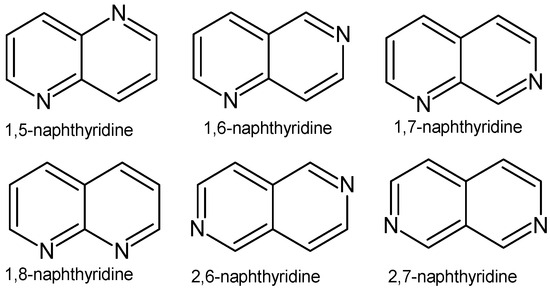
Figure 1.
Naphthyridine isomers.
According to a previous report, naphthyridine derivatives exhibit a broad spectrum of pharmacological activity, including anti-infective, anti-cancer, neurological, cardiovascular, and immunological effects [2]. Their wide range of effects makes them a promising subject of scientific research for their therapeutic use. The first naphthyridine derivative introduced into clinical practice in 1967 as an antibacterial agent was nalidixic acid 1 (Figure 2). This review presents naphthyridine derivatives with antimicrobial activity.
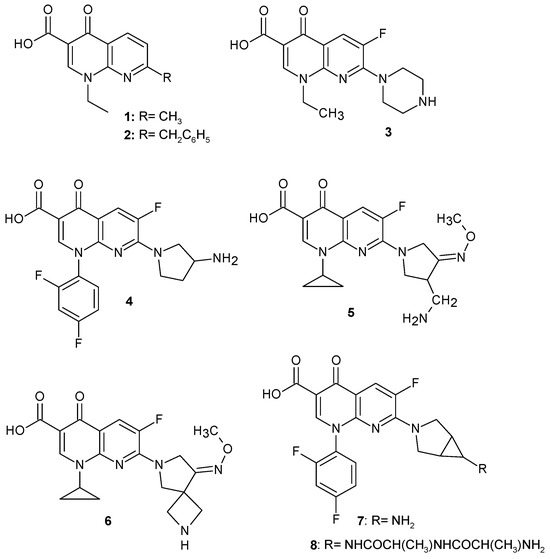
Figure 2.
Nalidixic acid and its derivatives used in medicine.
2. 1,8-Naphthyridine Derivatives
2.1. Antimicrobial Activity of Nalidixic Acid Derivatives
4-Oxo-1,8-naphthyridine-3-carboxylic acid is the basic structure of several commercially available antibacterial drugs. The first drug from this group of compounds was obtained and reported by G. Lesher et al. [3]—1-ethyl-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylic acid 1, called nalidixic acid (Figure 2). This compound was introduced into medicine in 1967 for the treatment of urinary tract infections caused by Gram-negative bacteria. Nalidixic acid 1 selectively and reversibly blocks DNA replication in bacteria by inhibiting the A subunit of bacterial DNA gyrase [4]. The marketing authorization for nalidixic acid has been suspended in the EU and other countries due to side effects. However, in the following years, as a result of structural modification, many nalidixic acid derivatives with antibacterial activity were obtained, and some of them were introduced into medicine (Figure 2).
Amfonelic acid 2 is 7-benzyl derivative of nalidixic acid, but despite its antibiotic properties, it is used as a dopaminergic stimulant [5].
In the search for new structural analogues of fluoroquinolone antibiotics, a fluorine atom was introduced into the 1,8-naphthyridine ring, and a number of 6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid derivatives with antimicrobial activity were obtained, including several medicinal substances, 3–8 (Figure 2).
Another analogue containing a piperazine ring was enoxacin: 1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid 3, whose mechanism of action is to block bacterial DNA replication by binding to DNA gyrase and which inhibits microRNA expression in many Gram-positive and Gram-negative bacteria [6]. Enoxacin 3 is a structural analogue of norfloxacin (an antibiotic from the fluoroquinolone group) and can be used to treat a wide range of infections, in particular gastroenteritis, respiratory tract infections, urinary tract infections, and gonorrhoea under the brand name Penetrex@ [7].
The introduction of a pyrrolidine ring into the structure resulted in obtaining a number of compounds with antibacterial activity. Some of them, 4–8 (Figure 2), were introduced in medicine.
Tosufloxacin: 7-(3-aminopyrrolidin-1-yl)-1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid 4 was developed by Toyama Chemical and introduced to the market in Japan under the trade name Ozex@. The use of this drug has been limited due to side effects: severe thrombocytopenia, nephritis, and hepatotoxicity [8].
Gemifloxacin mesylate: (R,S)-7-(3-aminomethyl-4-syn-methoxyimino-1-pyrrolidinyl)-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid 5 is indicated for the treatment of acute bacterial exacerbation of chronic bronchitis and mild to moderate community-acquired pneumonia caused by Staphylococcus aureus, Staphylococcus pyogenes, Haemophilus influenza, Klebsiella pneumoniae, Legionella pneumophila, Moraxella catarrhalis, Chlamydia pneumoniae, and Mycoplasma pneumoniae, as well as the multi-drug resistant strain S. pneumoniae, under the brand name Factive@ [9,10]. Gemifloxacin acts by inhibiting DNA replication by binding itself to DNA gyrase and topoisomerase IV [11].
Zabofloxacin: 1-cyclopropyl-6-fluoro-1,4-dihydro-7-[8-(methoxyimino)-2,6-diazaspiro [3.4]oct-6-yl]-4-oxo-1,8-naphthyridine-3-carboxylic acid 6 is an investigational antibiotic for treating multidrug-resistant infections caused by Gram-positive bacteria and Neisseria gonorrhoeae [12,13,14,15].
Trovafloxacin: 7-[(1S,5R)-6-amino-3-azabicyclo[3.1.0]hexan-3-yl]-1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid 7 is an inhibitor of topoisomerase IV and DNA synthesis. This drug has been marketed under the trade name Trovan@ by Pfizer since 1997 as a broad-spectrum antibacterial drug used against Gram-positive streptococci and Gram-negative pathogens [16], including a resistant strain of Neisseria gonorrhoeae [17].
Alatrofloxacin is the alaninamide of trovofloxacin: 7-[(1R,5S)-6-{[(2S)-1-{[(2S)-2-aminopropanoyl]amino}-1-oxopropan-2-yl]amino}-3-azabicyclo[3.1.0]hexan-3-yl]-1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid 8, which was also developed by Pfizer and marketed under the brand name Trovan@ IV as a prodrug quickly hydrolyzed to trovofloxacin. Trovafloxacin 7 and alatrofloxacin 8 were both withdrawn from the market due to the risk of hepatotoxicity [18].
In the Abbott laboratory [19], new nalidixic acid derivatives with pyrrolidine and azetidine moieties were obtained, which turned out to be novel ribosome inhibitors (NRIs) that selectively inhibit bacterial protein synthesis by structurally disrupting the tRNA/30S complex at the decoding site [20]. Pyrrolidine derivatives 9a and 9c (Figure 3) exhibit selective and broad-spectrum antibacterial activity, including drug-resistant respiratory pathogens, and are nontoxic to human cell lines [21]. Among the fluorinated 1,8-naphthyridines with an azetidine moiety, the most potent compounds were derivatives 10a and 10b (Figure 3) [20].
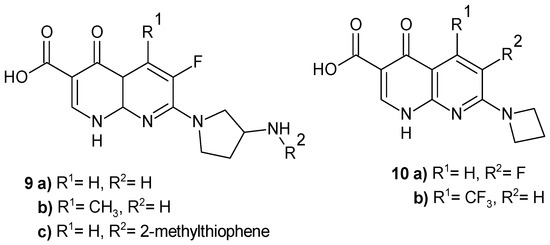
Figure 3.
Nalidixic acid derivatives 9–10.
By introducing a cyclopropyl substituent into the structure of fluorinated 1,8-naphthyridine derivatives, some active compounds were obtained (Figure 4). The highest antimicrobial activity was shown by compounds 11a–c. 7-[3-(R)-amino-2-(S)-methyl-1-azetidinyl]-1-cyclopropyl-1,4-dihydro-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid 11a, synthesized at Laboratorios Dr. Esteve S.A., Barcelona, Spain [22], turned out to be more active than the comparative drugs ciprofloxacin, fleroxacin, lomefloxacin, norfloxacin, enoxacin, and ofloxacin against Staphylococcus, Actinetobacter, Pseudomonas aeruginosa, Xanthomonas maltophilia, Neisseria gonorrhoeae, and Enterococcus, including also some ciprofloxacin-resistant strains [23]. 7-(3-Amino-1-pyrrolidinyl)-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid 11b was developed at the Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. [24] and used in clinical trials [25,26]. Derivative 11b is active against various resistant bacteria, comparable to the activity of ciprofloxacin or ofloxacin against E. coli, but much more potent against Staphylococci [27]. Clinical trials showed that compound 11b and its analogue 7-[(3R)-3-(2-aminopropan-2-yl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid 11c showed high activity against antibiotic-resistant Enterococcus strains [26].
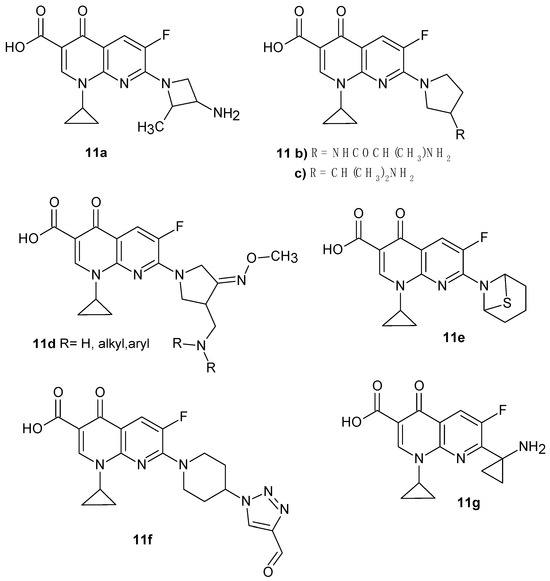
Figure 4.
1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid derivatives.
Hong et al. [28] synthesized new pyrrolidine derivatives of nalidixic acid 11d (Figure 4), which showed very strong antibacterial activity against Gram-negative and Gram-positive bacteria, including methicillin-resistant S. aureus. Obtained by the same scientist, (R,S)-7-(3-aminomethyl-4-syn-methoxyimino-1-pyrrolidinyl)-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid 5 (Figure 2) was introduced into medicine as gemifloxacin.
7-(2-Thia-5-azabicyclo[2.2.1]heptan-5-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid 11e, obtained by X. Huang et al. [29], showed very good antibacterial activity against multidrug-resistant strains of Streptococcus pneumonia in comparison with ciprofloxacin and vancomycin. 1-Cyclopropyl-6-fluoro-7-(4-(4-formyl-1H-1,2,3-triazol-1-yl)piperidin-1-yl)-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid 11f exhibited a comparable activity against multidrug-resistant strains, especially against S. aureus and Staphylococcus epidermidis, compared to ciprofloxacin and vancomycin [30].
Y. Todo et al. [31] synthesized 7-(1-aminocyclopropyl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8- naphthyridine-3-carboxylic acid 11g (Figure 4) and 7-(1-aminocyclopropyl)-1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid 12c (Figure 5), which exhibited a comparable antibacterial activity against Gram-positive and Gram-negative bacteria strains compared to ciprofloxacin and ofloxacin.
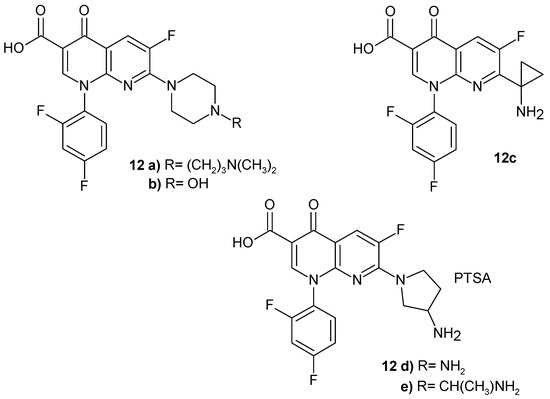
Figure 5.
1-(2,4-Difluorophenyl)-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid derivatives 12a–e.
The study results showed that the combination of a cyclopropyl group or a substituted phenyl group at N-1 and a 3-amino-2-methyl-1-azetidinyl group at C-7 provided the best overall antibacterial, pharmacokinetic, and physicochemical properties of the naphthyridine derivatives tested [32].
H.K. Gencer et al. [33] synthesized new derivatives of 1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid 12a–e (Figure 5) with antibacterial activity. Compound 12a with a piperazine moiety is a strong inhibitor of DNA gyrase and is non-genotoxic and less cytotoxic compared to the reference drugs: trovafloxacin, moxifloxacin, and ciprofloxacin.
J. Domagala et al. [34] also synthesized new analogues of 2,4-difluorophenyl derivatives 12, the most active of which was 7-[3-(1-aminoethyl)pyrrolidin-1-yl]-1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid 12e (Figure 5), demonstrating the best overall combination of safety and effectiveness.
(T-3262) p-toluenesulfonic acid salt of DL-7-(3-amino-1-pyrrolidinyl)-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid monohydrate 12d (Figure 5) showed excellent activity against Gram-positive bacteria strains, particularly Streptococci [35]. It was found to be comparable to ciprofloxacin and ofloxacin [36].
Cooper et al. [37] synthesized a series of 1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid derivatives with a piperazine moiety that displayed in vivo activity against S. aureus. The most active was compound 12b (Figure 5).
Synthesized by Egawa et al. [38] 1-alkyl-7-(3-amino-1-pyrrolidinyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acids 13a–b and 1-vinyl-7-[3-(methylamino)-1-pyrrolidinyl] analogue 13c (Figure 6) were found to be more active than enoxacin.
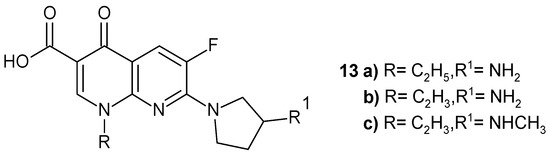
Figure 6.
Structures of derivatives 13a–c.
Biological studies aimed at assessing the antimicrobial activity of nalidixic acid-D-(+)-glucosamine conjugate 14 (Figure 7) indicated an improvement in the activity of the synthesized derivatives against various strains of resistant bacteria, while demonstrating a lower cytotoxic effect [39].
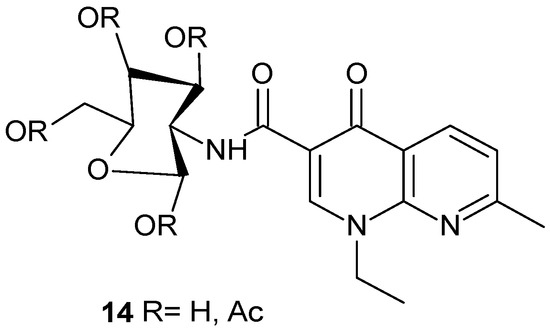
Figure 7.
Nalidixic acid-D-(+)-glucosamine conjugate 14.
N. Aggarwal et al. [40] synthesized new nalidixic acid derivatives with 1,2,4-triazole moiety 15–18 (Figure 8). Many of these compounds possessed good antibacterial and slightly less antifungal activity, but the most potent were 3-{4-(3-bromobenzylideneamino)-5-mercapto-4H-1,2,4-triazol-3-yl}-1-ethyl-7-methyl-1,8-naphthyridin-4(1H)-one 15a and 3-{6-(2-chlorophenyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazol-3-yl}-1-ethyl-7-methyl-1,8-naphthyridin-4(1H)-one 16a.
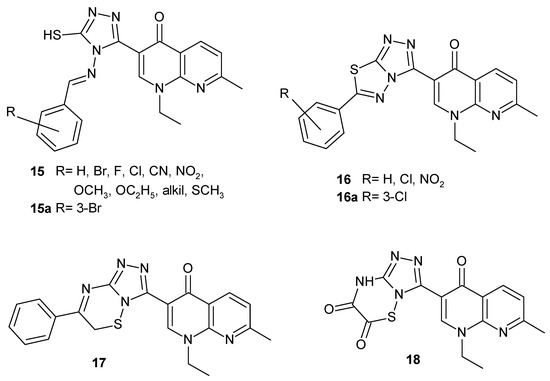
Figure 8.
Nalidixic acid-based 1,2,4-triazole derivatives 15–18.
V. K. Gurjar et al. [41] synthesized a series of 1,8-naphthyridine-3-carboxylic acid amides, 19 (Figure 9), and assessed their antibacterial potential. These derivatives showed very good bactericidal action toward E. coli and a weaker one toward S. aureus strains.
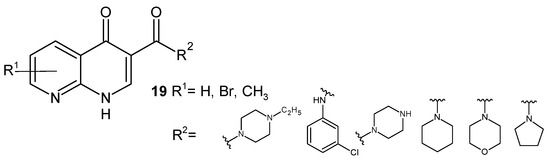
Figure 9.
Structures of 1,8-naphthyridine-3-carboxylic acid amides 19.
Synthesized by Sriram et al. [42], 1-tert-butyl-1,4-dihydro-7-(4,4-dimethyloxazolidin-3-yl)-6-nitro-4-oxo-1,8-naphthyridine-3-carboxylic acid 20a (Figure 10) showed an antitubercular activity against MDR-TB more potent than isoniazid. Compound 20a also exhibited high in vivo activity in an animal model, reducing the bacterial load in lung and spleen tissues [42].
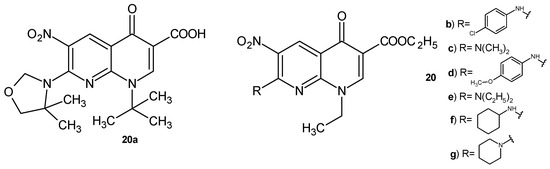
Figure 10.
Nalidixic acid derivatives with a 6-nitro group, 20a–g.
N. Suzuki et al. [43] prepared other nalidixic acid derivatives containing a 6-nitro substituent. 7-Amino substituted 1-etyl-1,4-dihydro-6-nitro-4-oxo-1,8-naphthyridine-3-carboxylates were evaluated for their activity against Trichomonas vaginalis. Compounds 20b–g (Figure 10) showed the same activity as metronidazole.
K.P. Chennam et al. [44] obtained N-(2-hydroxybenzylidene)-1-ethyl-1,4-dihydro-7-methyl-4-oxo-1,8 naphthyridine-3-carbohydrazide 21a and its complexes, with Cu (II) 21b, Co (II) 21c, and Zn (II) 21d (Figure 11). In vitro antibacterial studies against two Gram-positive (Bacillus subtilis and S. aureus) and two Gram-negative (Escherichia coli and K. pneumoniae) strains showed that the activities of metal complexes 21b–d and hydrazide 21a were comparable to ampicillin, but the most potent was the complex of Cu (II) 21b.
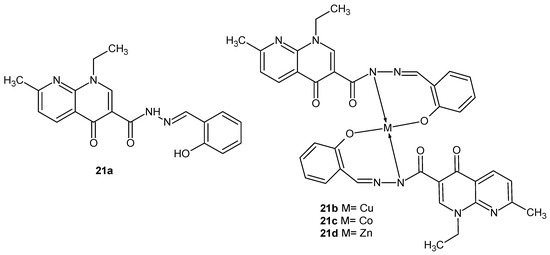
Figure 11.
Nalidixic acid hydrazide derivative and its metal complexes, 21a–d.
S. Massari et al. [45] obtained 7-[4-(1,3-benzothiazol-2-yl)piperazin-1-yl]-1-methyl-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid 22 (Figure 12), which showed a very potent and selective anti-HIV activity due to its ability to inhibit Tat-mediated transcription.
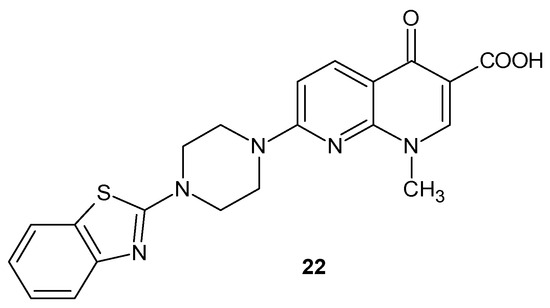
Figure 12.
7-[4-(1,3-benzothiazol-2-yl)piperazin-1-yl]-1-methyl-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid 22.
2.2. 1,8-Naphthyridine Derivatives with Antimicrobial Activity
E. Laxminarayana et al. [46] evaluated many 1,8-naphthyridine derivatives for their in vitro antibacterial activity against S. aureus, Bacillus cereus, B. subtilis, Micrococcus luteus, K. pneumoniae, Salmonella paratyphi A, E. coli, Bacillus magaterium, Proteus vulgaris, and Enterobacter aerogenes. Some derivatives (Figure 13) exhibited good activity, especially against P. vulgaris (23a–d, 24a–b) and S. aureus (25a–b), but all the compounds were less active than the standard tetracycline.
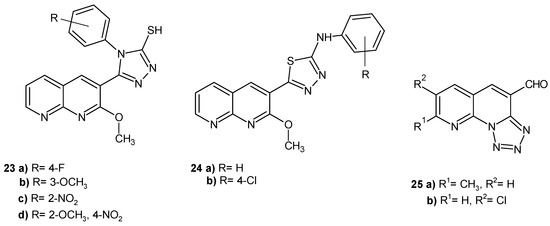
Figure 13.
Antibacterial 1,8-naphthyridine derivatives 23–25.
S. Raja et al. [47] evaluated hydrazono, 26–27, and azo, 28–29, derivatives of 1,8-naphthyridine (Figure 14) for their antibacterial and antifungal activity. The activity was due to the presence of a 4-chloro substituent on the phenyl ring of the pyrazolinone and pyrazole nucleus. Compounds with a 4-chlorophenyl ring, 26–29, were found to be most active against B. subtilis, S. aureus, E. coli, P. aeruginosa, Aspergillus niger, and Candida albicans, comparable to ampicillin and griseofulvin.
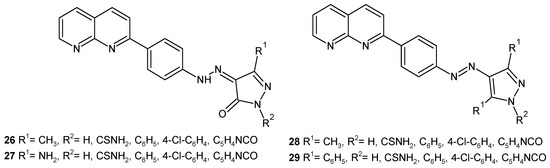
Figure 14.
Hydrazono and azo derivatives of 1,8-naphthyridine 26–29.
Synthesized by K. Md and R. Domala [48], 5-(2-phenyl-1,8-naphthyridine-3-yl)-1,3,4-oxadiazole-2-amine 30a and its amide derivatives 30b–d (Figure 15) were tested against S. aureus, E. coli, and C. albicans. Propionamide 30c, pentanamide 30b, and benzamide 30d exhibited high antifungal activity. The pentanamide 30b derivative also showed good antibacterial properties against Staphylococcus aureus, comparable to ampicillin.
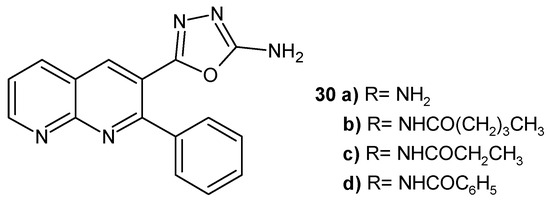
Figure 15.
1,8-Naphthyridines with oxadiazole and phenyl ring 30a–d.
N.G. Mohamed et al. [49] obtained 7-methyl-1,8-naphthyridinone derivatives substituted with a 1,2,4-triazole ring, 31a–m (Figure 16), as potential DNA-gyrase inhibitors. Most of the compounds exhibited selective antibacterial activity against B. subtilis resistant strains, and some of the obtained compounds were active against A. actinomycetemcomitans. The introduction of bromine at C-6 of the naphthyridine scaffold enhanced the antibacterial activity. The tested compounds showed a moderate to high inhibitory effect, having an IC50 range 1.7–13.2 µg/mL against DNA gyrase. The most potent were brominated derivatives 31b and 31f.
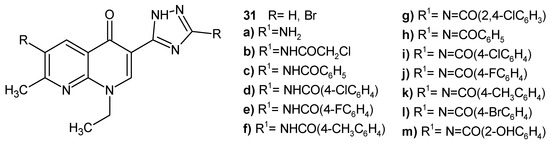
Figure 16.
1,8-Naphthyridinones 31a–m.
2-(β-D-galactopyranosylmethyl)-1,8-naphthyridine 32 (Figure 17), obtained by Nagarajan et al. [50], turned out to be more effective than tetracycline against Gram-negative bacteria such as K. pneumoniae, P. aeruginosa, and P. vulgaris, as well as against S. aureus.
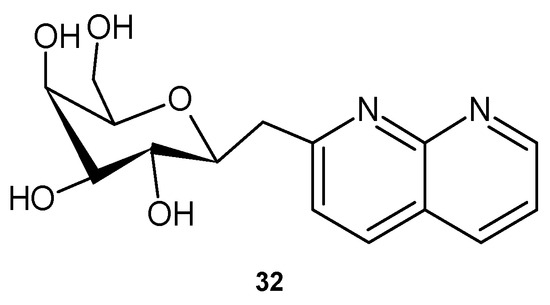
Figure 17.
2-(β-D-galactopyranosylmethyl)-1,8-naphthyridine 32.
M. A. Hussein et al. [51] synthesized a series of heteroaromatic polymers containing 4-ethoxy-2,7-dicarboxyaldehyde-1,8-naphthyridine moieties (Figure 18). Polymers 33a–b showed significant activity against Gram-negative bacteria (P. aeruginosa, Serratia marcescens, and E. coli) compared to ampicillin, and compound 33b also exhibited a strong antifungal influence against A. niger and Fusarium oxysporum.
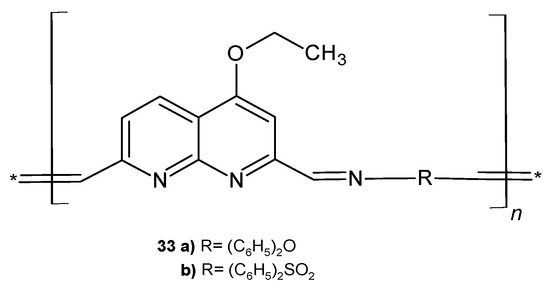
Figure 18.
Polymers 33a–b.
Gordon B. Bavlin and Weng-Lai Tan [52] obtained 5-amino derivatives of 1,8-naphthyridine 34–35 (Figure 19), which showed minimal antimalarial activity in vivo against Plasmodium vinckei vinckei.
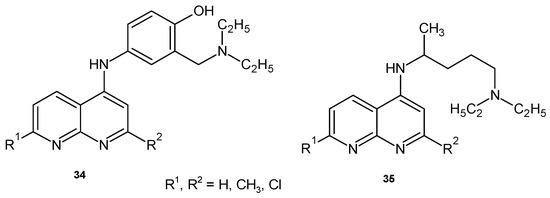
Figure 19.
1,8-Naphthyridine derivatives 34–35.
S. Olepu et al. [53] developed a new class of PFT (protein farnesyltransferase) inhibitors. 6-Cyano-2-oxo-tetrahydro-1,8-naphthyridine derivatives 36–37 (Figure 20) selectively inhibited malaria PFT with activities in the low nanomolar range. The resulting compounds were more potent in malaria PFT than in the mammalian enzyme. These derivatives were also more metabolically stable than tetrahydroquinoline.
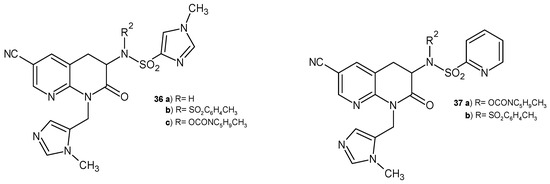
Figure 20.
Antimalarial 2-oxo-tetrahydro-1,8-naphthyridine derivatives 36–37.
J. M. Quintela et al. [54] obtained 3-cyano-2-ethoxy-4-phenyl-7-substituted-1,8-naphthyridines and submitted them to in vitro tests for antiparasitic activity against the ciliates Philasterides dicentrarchi. The presence of the piperazine ring was found to be essential for strong antiprotozoal activity. The most active compounds were 38a–b (Figure 21), whose activity was comparable to that of norfloxacin.
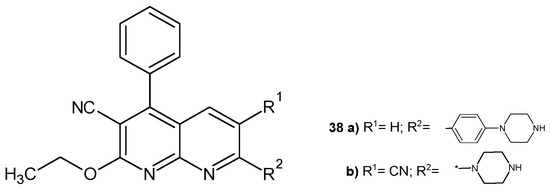
Figure 21.
3-Cyano-2-ethoxy-4-phenyl-7-substituted-1,8-naphthyridines 38a–b.
K.K. Priya et al. [55] prepared 7-(2-(4-bromobenzylidene)hydrazinyl)-6-aryl-1,8-naphthyridine-2-carbonitrile 39 and 6-aryl-[1,2,4]triazolo [4,3-a][1,8]naphthyridine 40 derivatives (Figure 22), which were evaluated for their antimicrobial activity against bacterial strains (B. megaterium, M. luteus, S. typhi, and E. coli) and fungal strains (A. niger, Aspergillus flavus). All compounds showed good levels of activity, comparable to reference drugs (streptomycin and nystatin).
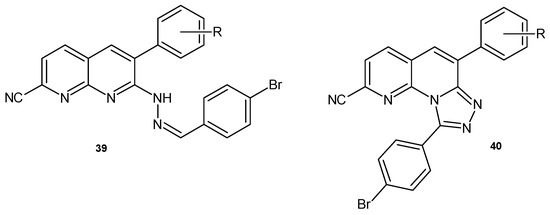
Figure 22.
6-Aryl-1,8-naphthyridine-2-carbonitrile derivatives 39–40.
A. Narender et al. [56] prepared 2-cyclopeopyl-1,8-naphthyridine derivatives, which were tested in vitro against S. aureus, E. coli, K. pneumoniae, S. paratyphi A, S. paratyphi B, and M. luteus. The most active derivatives (growth inhibition zones were 6–16 mm at a concentration of 1 mg/mL) were compounds 41a–b, 42a–b, and 43a–b (Figure 23).
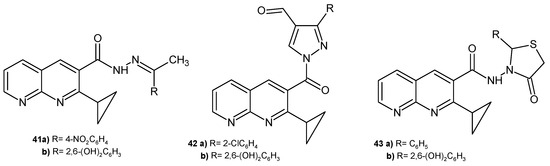
Figure 23.
2-Cyclopropyl-1,8-naphthyridine derivatives with antibacterial activity.
F.A. Omar et al. [57] synthesized a series of 1,8-naphthyridine-3-thiosemicarbazides, 44, and 1,8-naphthyridine-3-(1,3,4-oxadiazoles), 45 (Figure 24), as potential DNA gyrase inhibitors. These derivatives 44–45 were screened for antibacterial activity against S. aureus, B. cereus, E. coli, K. pneumoniae, P. aeruginosa, and Mycobacterium smegmatis. The tested compounds were not active against P. aeruginosa. The highest activity against S. aureus was shown by compounds 44a–b, 45a–b (MIC values in the range of 6–7 mM), while the most active against M. smegmatis were derivatives 44b–d, 45c–e (MIC values in the range of 5.4–7.1 mM). The bromination of the naphthyridine skeleton resulted in better antibacterial activity against B. cereus. Molecular docking and a DNA gyrase inhibition assay showed that derivatives 44b, 44e, 45a, and 45d were the most active, comparable to nalidixic acid.
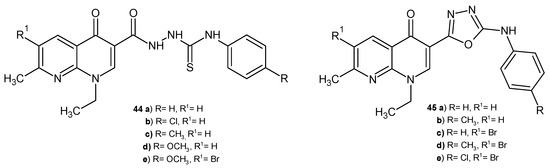
Figure 24.
Thiosemicarbazides and 1,3,4-oxadiazoles of 1,8-naphthyridine 44–45.
G. Turan-Zitouni et al. [58] evaluated the obtained N-benzylidene-N′-(5-methyl-2-acetamido [1,8]naphthyridin-7-yl)hydrazine derivatives 46 (Figure 25) for antimicrobial activity against M. luteus, B. subtilis, Salmonella typhimirium, S. aureus, E. coli, Listeria monocytogenes, and C. albicans. Tested compounds 46 showed an antibacterial activity comparable to streptomycin only against S. typhimirium. All of these derivatives were also effective against C. albicans compared to ketoconazole.
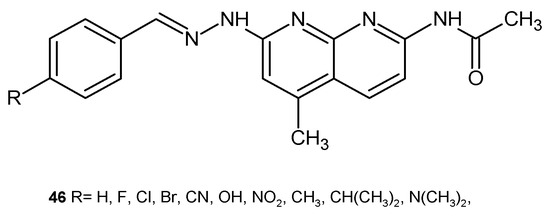
Figure 25.
N-benzylidene-N′-(5-methyl-2-acetamido[1,8]naphthyridin-7-yl)hydrazine derivatives 46.
D. Ramesh et al. [59] synthesized a series of 3-(2-methyl-1,8-naphthyridin-3-yl) ureas, 47 (Figure 26), as potential antifungal agents. The obtained compounds were found to be active against the following fungal strains: Alternaria alternata 47a–d, F. oxysporum 47a,b,e, and Curvularia lunata 47a,b,f.
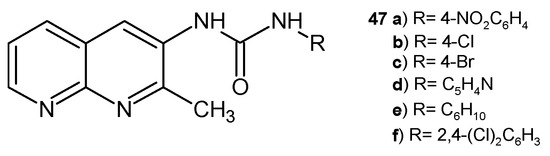
Figure 26.
3-(2-Methyl-1,8-naphthyridin-3-yl)ureas 47.
V.K. Gurjar et al. [60] obtained 1-benzyl-N-cyclohexyl-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxamide 48 (Figure 27) as an effective antimicrobial agent similar to ciprofloxacine.
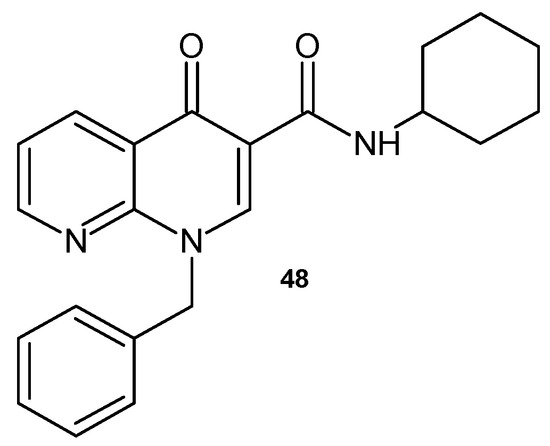
Figure 27.
1-Benzyl-N-cyclohexyl-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxamide 48.
D. Bhambi et al. [61] obtained tetrahydrobenzo[b][1,8]naphthyridine derivatives substituted with a phthalimidoxy group, 49a–j (Figure 28). All compounds were evaluated for antimicrobial activity against Proteus mirabilis, B. subtilis, K. pneumonia, E. coli, S. albicans, and Aspergillus fumigatus and showed moderate antibacterial activity but strong antifungal efficacy.

Figure 28.
Benzo[b][1,8]naphthyridine derivatives 49a–j.
4-Amino-2-phenyl-5,7-di(thien-2-yl)-1,8-naphthyridine-3-carbonitrile 50 (Figure 29), obtained by E. Mohamed et al. [62], was evaluated for its antimicrobial activity against E. coli, B. subtilis, A. flavus, and C. albicans and showed no high antibacterial activity but better antifungal activity against A. flavus.
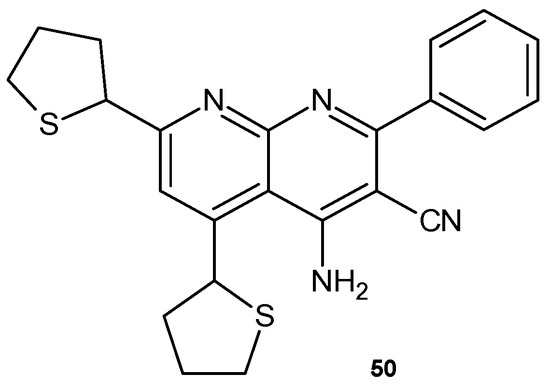
Figure 29.
4-Amino-2-phenyl-5,7-di(thien-2-yl)-1,8-naphthyridine-3-carbonitrile 50.
C. Oliveira-Tintino et al. [63] investigated the antibacterial activity of 1,8-naphthyridinesulphonamides. The tested compounds did not show direct antibacterial activity but effectively reduced the MIC of multidrug-resistant bacteria by associating with ethidium bromide and norfloxacin. Additionally, molecular docking studies showed that 1,8-naphthyridinesulfonamides 51a–d (Figure 30) can attenuate the resistance of S. aureus via molecular mechanisms related to the inhibition of the NorA efflux pump [63].
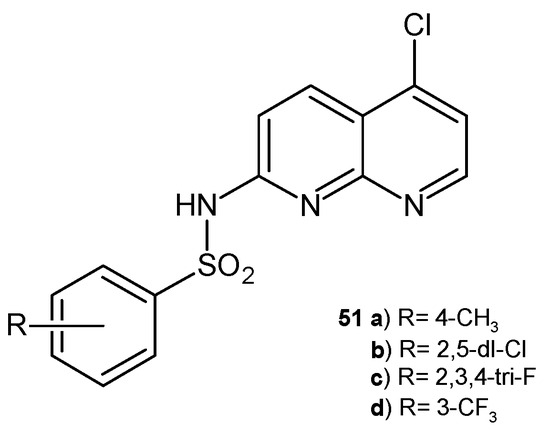
Figure 30.
1,8-Naphthyridine sulphonamides 51a–d.
K. M. Fayyadh et al. [64] evaluated 2-chloro-1,8-naphthyridine derivatives for their antibacterial activity. Among the tested compounds, 2-chloro-1,8-naphthyridine-3-carbaldyhyde 52 (Figure 31) showed moderate activity against E. coli and high activity against S. pyogenes.
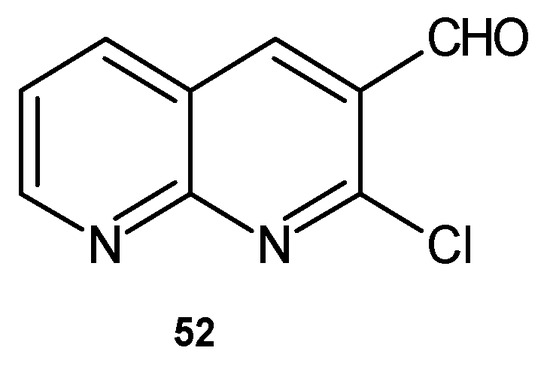
Figure 31.
2-Chloro-1,8-naphthyridine-3-carbaldyhyde 52.
M. A. Seefeld et al. [65] obtained a series of naphthyridin-2-one derivatives with an indole moiety as inhibitors of bacterial enoyl-ACP reductases FabI and FabK. The most active derivatives, 53a–b (Figure 32), were found to be potent FabI and FabK inhibitors. Additionally, compound 53b showed good in vivo efficacy after oral administration in a rat model of infection with a multidrug-resistant strain of S. aureus.
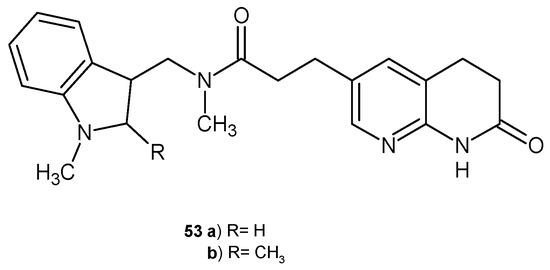
Figure 32.
Naphthyridin-2-one derivatives 53 as FabI and FabK inhibitors.
4-Amino-6-benzotriazol-1-yl-1,2-dihydro-5-methyl-2-oxo-1,8-naphthyridine-3-carbonitrile 54 (Figure 33), obtained by F. Al-Omran et al. [66], showed strong bactericidal and fungicidal activity against S. aureus and A. niger but was inactive against E. coli, B. subtilis, and F. oxysporium.
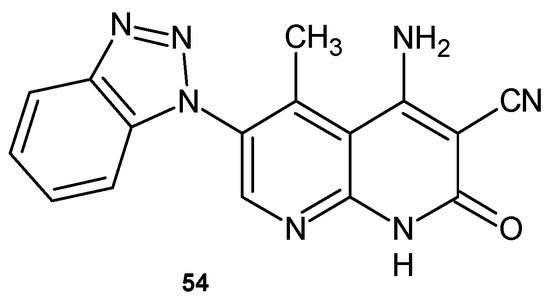
Figure 33.
4-Amino-6-benzotriazol-1-yl-1,2-dihydro-5-methyl-2-oxo-1,8-naphthyridine-3-carbonitrile 54.
4,7-Diamino-2-oxo-1,2-dihydro-1,8-naphthyridine-3,6-dicarbonitrile and 4,7-diamino-2-thioxo-1,2-dihydro-1,8-naphthyridine-3,6-dicarbonitrile, 55 (Figure 34), synthesized by El-Remaily et al. [67], showed moderate activity against Staphylococcus albus. Compounds 55 were found to be highly toxic to Artemia salina larvae.
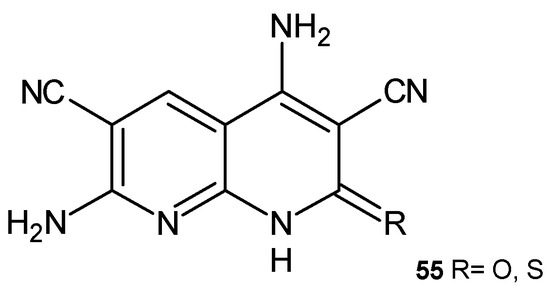
Figure 34.
4,7-Diamino-2-oxo/thioxo-1,2-dihydro[1,8]naphthyridine-3,6-dicarbonitrile, 55.
S. Banoth et al. [68] prepared imidazo[1,2-a][1,8]naphthyridine derivatives 56–57 (Figure 35). The obtained compounds were evaluated for their antibacterial and antifungal activity. Compounds 56a–b and 57 demonstrated high activity against S. aureus, E. coli, Candida metapsilosis, and A. niger, comparable to that of penicillin and griseofulvin.
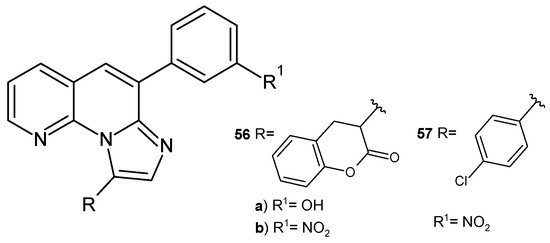
Figure 35.
Imidazo [1,2-a][1,8]naphthyridine derivatives 56–57.
A series of N-3-diaryl-1,8-naphthyridin-2-amines were synthesized by D. Ravi et al. [69] and evaluated for their antibacterial activity against B. subtilis and E. coli using the filter paper disc technique. The most active, similar to streptomycin, were derivatives 58a–c (Figure 36).
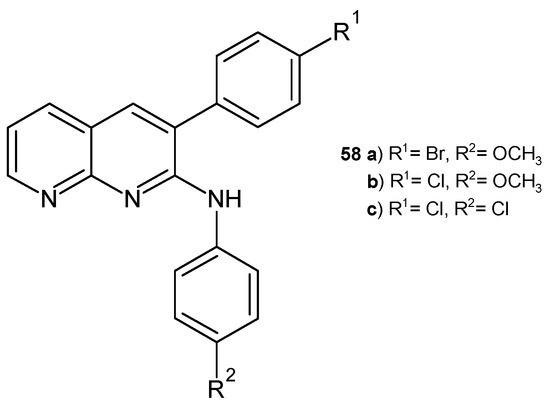
Figure 36.
N-3-diaryl-1,8-naphthyridin-2-amine derivatives 58a–c.
B. Sakram et al. [70] synthesized 9-(3-fluoro-4-methoxyphenyl)-6-aryl-[1,2,4]triazolo [4,3-a][1,8]naphthyridine derivatives. These compounds were screened in vitro for their antibacterial activity against S. aureus B. subtilis, E. coli, and K. pneumoniae and for antifungal activity against A. flavus and F. oxysporum. Derivatives 59a–c (Figure 37) showed maximal inhibition zones against all tested microorganisms compared to ciprofloxacin and amphotericin B. Molecular docking studies demonstrated the maximum interaction of the tested compounds 59a–c with His228.
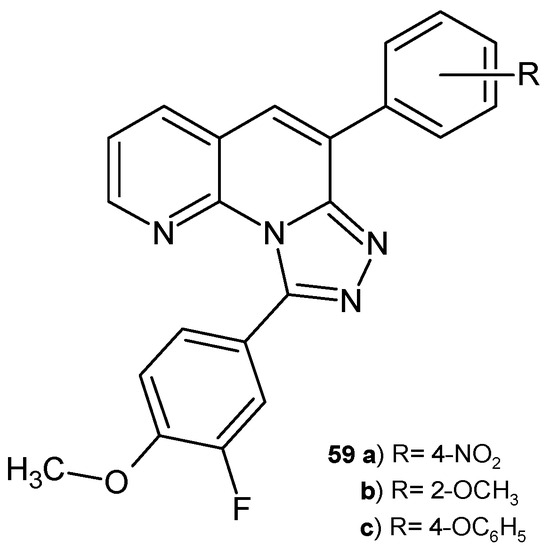
Figure 37.
9-(3-Fluoro-4-methoxyphenyl)-6-aryl-[1,2,4]triazolo [4,3-a][1,8]naphthyridine derivatives 59a–c.
B. Sakram et al. [71] obtained 4-aryl-2-(3-(2-(trifluoromethyl)phenyl)-1,8-naphthyridin-2-yl)phthalazin-1(2H)-ones 60a–h (Figure 38). Some of the compounds 60a–d showed good antibacterial and antifungal activity. Derivatives containing 4-OCH3 and 4-OH substitution in the benzene ring exhibited better antimicrobial activity (the growth inhibition zones were 15–19 mm at a concentration of 250 ppm).
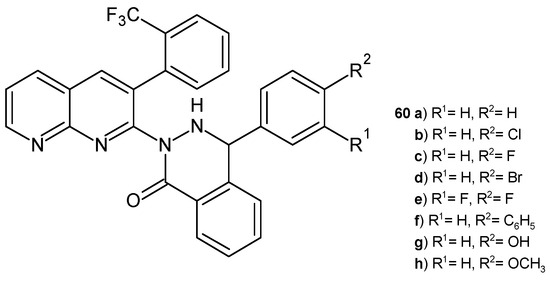
Figure 38.
4-Aryl-2-(3-(2-(trifuoromethyl)phenyl)-1,8-naphthyridin-2-yl)phthalazin-1(2H)-ones 60a–h.
2-(2-(3-Nitrophenyl)-1,8-naphthyridin-3-yl)-5-phenyl-1,3,4-oxadiazoles derivatives 61a–h (Figure 39) were synthesized by B. Sakram et al. [72]. These compounds were evaluated for their antibacterial and antifungal activity in vitro. The most active against S. aureus, E. coli, A. niger, and C. metapsilosis were the 4-hydroxy 61c and 4-fluoro 61f derivatives.
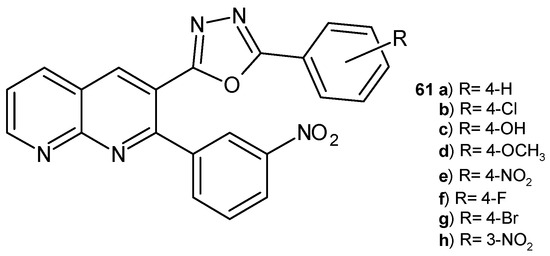
Figure 39.
2-(2-(3-Nitrophenyl)-1,8-naphthyridin-3-yl)-5-phenyl-1,3,4-oxadiazoles derivatives 61a–h.
2-Bromo-N-(3-aryl-1,8-naphthyridin-2-yl)thiazole-4-carboxamide derivatives 62a–f (Figure 40), synthesized by B. Sonyanaik et al. [73], were screened for their in vitro antibacterial activity against S. aureus and E. coli, as well as for their antifungal activity against pathogenic fungal strains A. niger and A. flavus. Compounds 62a–b showed high antimicrobial efficacy. Zones of inhibition (in mm) were comparable to those of griseofulvin and ampicillin.
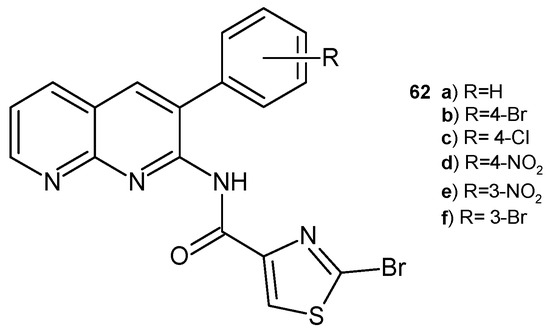
Figure 40.
2-Bromo-N-(3-aryl-1,8-naphthyridin-2-yl)thiazole-4-carboxamide derivatives 62a–f.
N-(3-aryl-1,8-naphthyridin-2-yl)-5-(2-methyl-1,8-naphthyridin-3-yl)thiazol-2-amine derivatives 63a–e (Figure 41), synthesized by A. Ashok et al. [74], were evaluated for their in vitro antibacterial and antifungal activity against S. aureus, E. coli, A. niger, and C. albicans. Compounds containing a chloro substituent, 63b and 63d, displayed the highest activity when compared with penicillin or griseofulvin (MIC values in the range of 35.5–75.5 μg/mL).
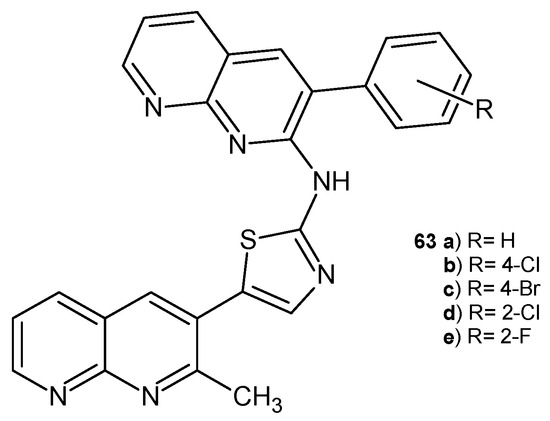
Figure 41.
N-(3-aryl-1,8-naphthyridin-2-yl)-5-(2-methyl-1,8-naphthyridin-3-yl)thiazol-2-amine derivatives 63a–e.
Kumar Parangi et al. [75] synthesized 5-(2-methyl-1,8-naphthyridin-3-yl)-1,3,4-oxadiazol-2-amine derivatives 64a–k (Figure 42), which were screened for their in vitro antibacterial and antifungal activity against strains of S. aureus, E. coli, and C. albicans. Compound 64b showed high activity against S. aureus (the 1 mm zone at the concentration of 100 µg/mL), similar to ampicillin. Derivatives 64b and 64i demonstrated moderate properties against E. coli. Compounds 64a, 64f, and 64i showed significant antifungal activity compared to ketoconazole. Molecular docking studies showed that compounds 64b, 64f, 64g, 64i, 64j, and 64k interacted with the target protein more efficiently [75].
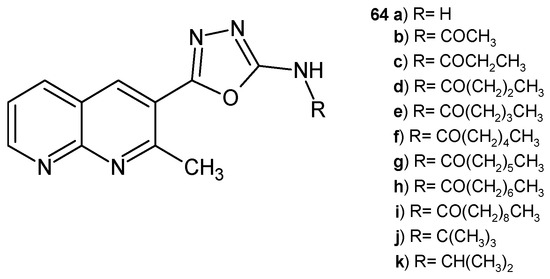
Figure 42.
5-(2-methyl-1,8-naphthyridin-3-yl)-1,3,4-oxadiazol-2-amines 64a–k.
D. Ravi et al. [76] obtained 2-methoxy-3-aryl-1,8-naphthyridines 65a–j (Figure 43), which were evaluated for their antimicrobial activity against bacterial strains P. vulgaris, S. typhimurium, E. aerogenes, and S. aureus and fungi strains Malassezia pachydermatis and C. albicans. The compound with fluorine substitution at the para position of the phenyl ring, 65e, and the derivative with a trifluoromethyl group, 65g, exhibited very good antimicrobial activity against the tested strains (MIC = 35–125 µg/mL). Based on the molecular docking results, compounds 65e and 65g showed the highest docking and hydrogen-bonding score and a good affinity towards the DNA gyrase receptor.
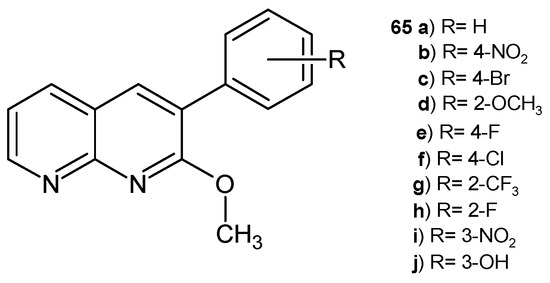
Figure 43.
2-Methoxy-3-aryl-1,8-naphthyridine derivatives 65a–j.
B. Sakram et al. [77] obtained 2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxylates 66a–k (Figure 44), which were screened for their antimicrobial activity against S. pyogenes, E. coli, Saccharomyces cerevisiae, and Aspergillus terreus by the agar well diffusion method, using ciprofloxacin and nystatin as standards. Compounds 66b, 66i, and 66j showed the highest antibacterial and antifungal activity, similar to the reference drugs.
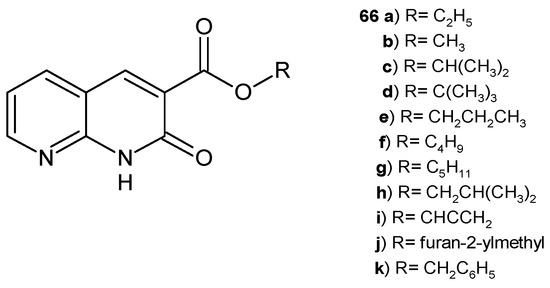
Figure 44.
2-Oxo-1,2-dihydro-1,8-naphthyridine-3-carboxylates 66a–k.
The same researchers [78] synthesized 3-iodo-1,8-naphthyridines 67a–g (Figure 45), which were evaluated for their antimicrobial activity against S. aureus, B. subtilis, E. coli, K. pneumoniae, A. flavus, and F. oxysporum. All the tested compounds showed good antibacterial and antifungal properties, but the most potent derivatives, 67d and 67g, exhibited maximum zones of inhibition against the tested strains as compared to ciprofloxacin and amphotericin B.
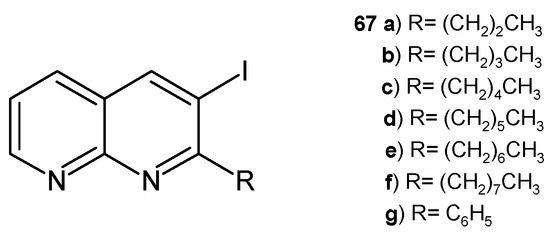
Figure 45.
3-Iodo-1,8-naphthyridine derivatives 67a–g.
A series of 2-{4-[(3-aryl-1,8-naphthyridin-2-yl)amino]-phenyl}-1H-benzo[de]isoquinoline-1,3(2H)-dione derivatives, 68a–h (Figure 46), was obtained by B. Sakram et al. [79] and screened for their antimicrobial activity against E. coli, B. subtilis, K. pneumoniae, and S. aureus. The tested compounds showed moderate activity, and only derivatives with the trifluoro group, 68b, exhibited high activity against B. subtilis, exhibiting a maximum zone of inhibition close to that of chloramphenicol.
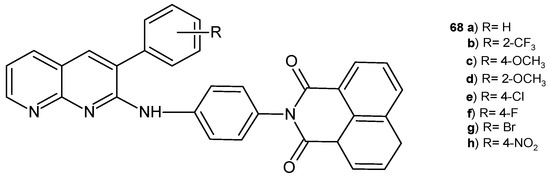
Figure 46.
2-{4-[(3-Aryl-1,8-naphthyridin-2-yl)amino]-phenyl}-1H-benzo[de]isoquinoline-1,3(2H)-diones 68a–h.
B. Sonyanaik et al. [80] synthesized 6-(2-chloro-4-fluorophenyl)-9-phenylimidazo [1,2-a][1,8]naphthyridine derivatives 69a–h (Figure 47), which were evaluated for their in vitro antibacterial activity against S. aureus and E. coli and antifungal activity against A. niger and C. metapsilosis using the agar diffusion method. All the tested compounds showed good antimicrobial activity, but the most potent was compound 69d, with properties similar to that of the reference drugs (penicillin and clotrimazole). Molecular docking studies confirmed the data on antimicrobial activity.
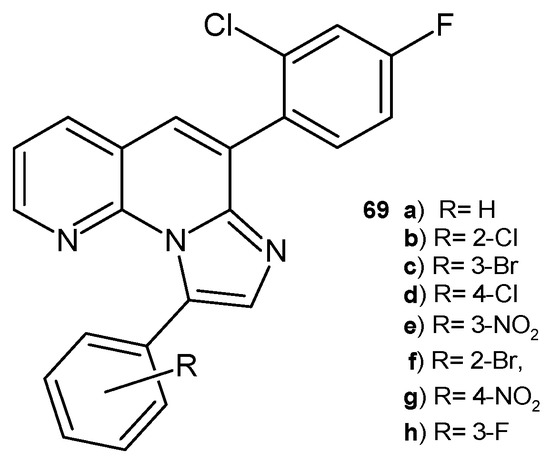
Figure 47.
6-(2-Chloro-4-fluorophenyl)-9-phenylimidazo[1,2-a][1,8]naphthyridines 69a–h.
Silver(I) complexes with 1,8-naphthyridine, 70a–b (Figure 48), were prepared by D. P. Aśanin et al. [81] and biologically evaluated as potential antimicrobial agents. The tested complexes showed significant in vitro activity against S. aureus, P. aeruginosa, and Candida species (with MIC values in the range of 1.56–7.81 µg/mL) and low in vivo toxicity in the C. elegans nematode model.
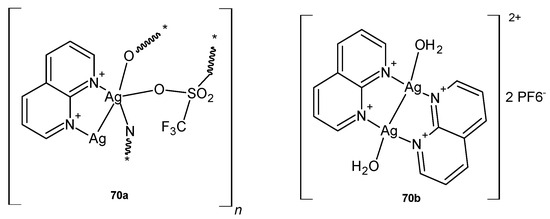
Figure 48.
Silver(I) complexes with 1,8 naphthyridine 70a–b.
K. M. Pandaya et al. [82] obtained β-lactam derivatives of 1,8-naphthyridines, 71a–j (Figure 49), and assessed their antimicrobial activity. 3-Chloro-1-((3-(2-(trifluoromethyl)phenyl)-1,8-naphthyridin-2-yl)amino)azetidin-2-one derivatives 71a, 71d, 71g, 71i, and 71j were found to have a good efficacy against S. pneumoniae, Clostridium tetani, S. typhi, and Vibrio cholera comparable to ampicillin and ciprofloxacin. Derivatives 71a, 71b, 71d, and 71g showed promising antitubercular activity, with MIC values of 6.45 µg/mL, 6.14 µg/mL, 4.19 µg/mL, and 3.11 µg/mL, respectively [82].
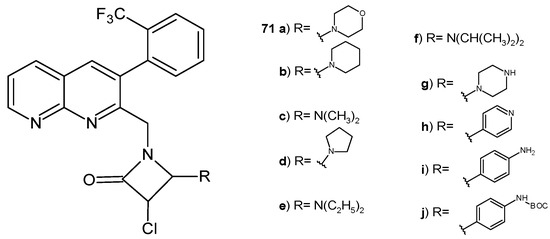
Figure 49.
3-Chloro-1-((3-(2-(trifluoromethyl)phenyl)-1,8-naphthyridin-2-yl)amino)azetidin-2-ones 71a–j.
N-methyl-N-(2-methyl-1H-indol-3-ylmethyl)-3-(7-oxo-5,6,7,8-tetrahydro-1,8-naphthyridin-3-yl)acrylamide 72 (Figure 50) showed excellent activity against multidrug-resistant strains of S. aureus and S. epidermidis and excellent in vivo efficacy in a rat model of S. aureus infection [83]. Compound 72 exhibited good FabI inhibitory activity and weak FabK inhibitory activity. Derivative 72 also showed activity against a broader range of Gram-negative bacteria. Studies indicate that this compound is a substrate for the H. influenzae efflux pump [83].
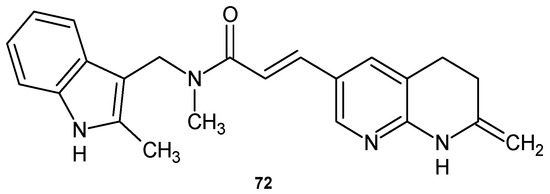
Figure 50.
N-methyl-N-(2-methyl-1H-indol-3-ylmethyl)-3-(7-oxo-5,6,7,8-tetrahydro-1,8-naphthyridin-3-yl)acrylamide 72.
P. M. Sivakumar et al. [84] investigated the influence of substituents in the 3- or 4-phenyl-1,8-naphthyridine scaffold, 73 (Figure 51), on the antitubercular and antibacterial activity of these derivatives. The piperidinyl moiety at position 2 or 7 enhanced the antitubercular activity. Morpholinyl substitution at these positions resulted in weaker activity. 7-Amino, 7-chloro, or 7-methoxy derivatives showed moderate to good antimycobacterial activity, but the 6-amino or 6-nitro derivatives were found to be inactive. However, the 6-nitro substituent resulted in the highest activity against S. aureus, and 7-amino derivatives exhibited very good activity against E. coli. QSAR results showed that compounds with a large number of hydrogen bond donors and a low heat of formation and solvent-accessible surface area are effective antibacterial agents. Most of the active compounds showed a positive correlation, while the least active compounds showed a negative correlation between antitubercular activity and logP [84].
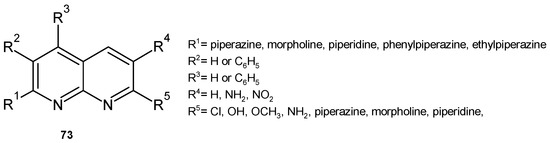
Figure 51.
1,8-Naphthyridine derivatives 73.
2-Aryl-1,8-naphthyridine derivatives 74 (Figure 52), synthesized by G. V. Bhasker et al. [85], were evaluated for their antibacterial activity against E. coli, S. aureus, K. pneumonia, and B. subtilis. All the tested compounds were inactive against B. subtilis. In vitro screening results showed that some compounds, 74a–c, exhibited antibacterial activity against E. coli and S. aureus with zones of inhibition of 4–6 mm.
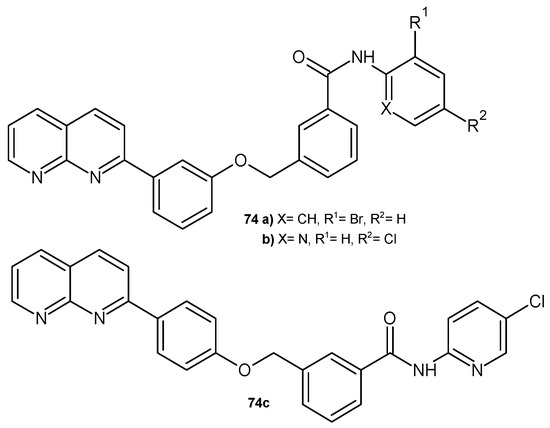
Figure 52.
2-Aryl-1,8-naphthyridine derivatives 74a–c.
2.3. Polycyclic Derivatives of 1,8-Naphthyridine
E. Melcón-Fernandez et al. [86] synthesized and evaluated the antileishmanial activity of 1,8-substituted fused naphthyridines 75–78 (Figure 53) in in vitro and ex vivo tests against Leishmania infantum. The presence of a nitrogen atom in the naphthyridine-fused ring (compounds 76 and 78) is important for the increased biological activity. Compounds 75 and 77 showed significant activity against leishmaniasis but also high toxicity.
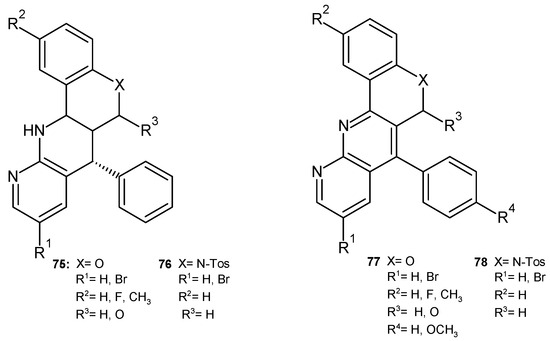
Figure 53.
Fused naphthyridines 75–78.
A series of naphtho[2,3-b][1,8]naphthyridine derivatives 79–81 (Figure 54) synthesized by Elkanzi [87] were tested in vitro for their antibacterial and antifungal activity. Compounds 79a, 79e, 80a, 82b displayed the highest activity against S. aureus, and compounds 79c, 80b, 81b exhibited very good activity against B. subtilis and E. coli compared to ampicillin. Most of these compounds displayed good activity, but the presence of a chloro or nitro group at the para position of the phenyl ring improved their efficiency. Compounds 79a, 79b–c, 79d–e, 80b–c, and 81a–c displayed potent antifungal activity against A. flavus and C. albicans similar to that of amphotericin B. Molecular docking studies showed that the most active antimicrobial compounds have good energy-binding properties in the active site of topoisomerase II [87].
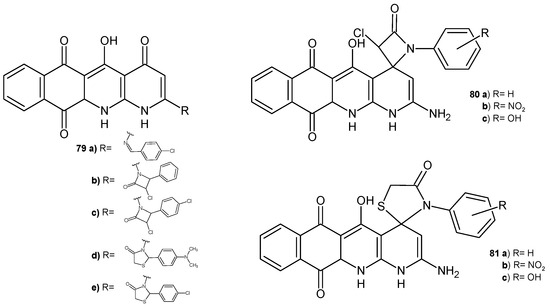
Figure 54.
Naphtho[2,3-b][1,8]naphthyridine derivatives 79–81.
A series of chromeno-1,8-naphthyridine derivatives, 82 (Figure 55), prepared by J. D. Gohil et al. [88], was evaluated for their antimicrobial activity against bacterial strains S. pneumonia, B. subtilis, C. tetani, E. coli, S. typhi, and V. cholera and fungal strains C. albicans and A. fumigatus using the broth microdilution method. The obtained compounds showed moderate antifungal properties. The presence of fluorine increased the antibacterial activity of the tested compounds. The most potent were derivatives 82b–c and 82h–i (MIC = 62.5–200 µg/mL). Compounds with a pyridine moiety also showed better antibacterial activity. Compound 82l exhibited excellent activity against all Gram-positive bacteria strains tested (MIC = 62.5–100 µg/mL), and compound 82f was strongly active against C. tetani (MIC = 62.5 µg/mL).
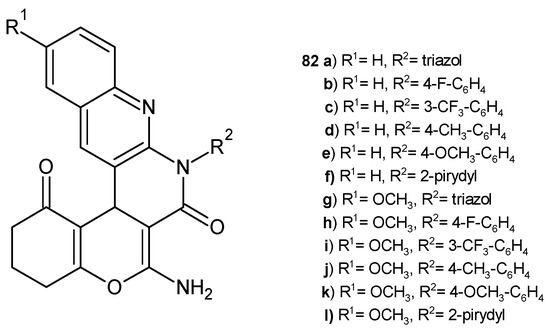
Figure 55.
Chromeno-1,8-naphthyridine derivatives 82a–l.
2.4. 1,8-Naphthyridine Conjugated with Ketolide
D. Abbanat et al. [89] prepared a ketolide combined with 1,8-naphthyridine, 83a (Figure 56). Compound 83a exhibited good activity (MIC = 0.25 µg/mL) against a macrolide-resistant strain of S. pneumoniae.
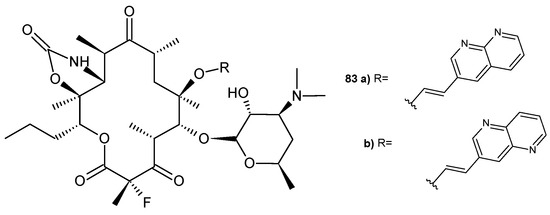
Figure 56.
Ketolides containing naphthyridine scaffold, 83a–b.
3. 1,5-Naphthyridine Derivatives
3.1. 1,5-Naphthyridines Conjugated with Antibiotics
The same researchers [89] also tested the ketolide conjugated with a 1,5-naphthyridine scaffold, 83b (Figure 56), against the same macrolide-resistant strain of S. pneumoniae. Ketolides 83a–b inhibited protein synthesis in vitro. The MIC90 values of the tested ketolides were several times lower than those of telithromycin and erythromycin A [89].
Modifications of the penicillin molecule have led to progress in antibiotic therapy. 6{D(-)-α(4-hydroxyl-1,5-naphthyridine-3-carboxamido)phenylacetamido} sodium penicillinate, 84 (Figure 57), is a semisynthetic penicillin with a broad spectrum of activity against Gram-positive cocci and Gram-negative bacilli. Its activity against P. aeruginosa is similar to that of aminoglycoside antibiotics [90].
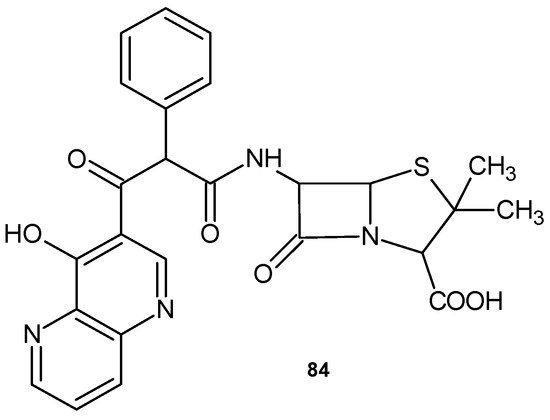
Figure 57.
6{D(-)-a(4-hydroxyl-1,5-naphthyridine-3-carboxamido)phenylacetamido} sodium penicillinate 84.
3.2. Synthetic 1,5-Naphthyridine Derivatives
A.K. Parhi et al. [91] prepared N-{[8-(4-tert-butylphenyl)-1,5-naphthyridin-4-yl]methyl}guanidine 85 (Figure 58), which showed antibacterial activity against methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA), with MIC = 8.0 μg/mL.
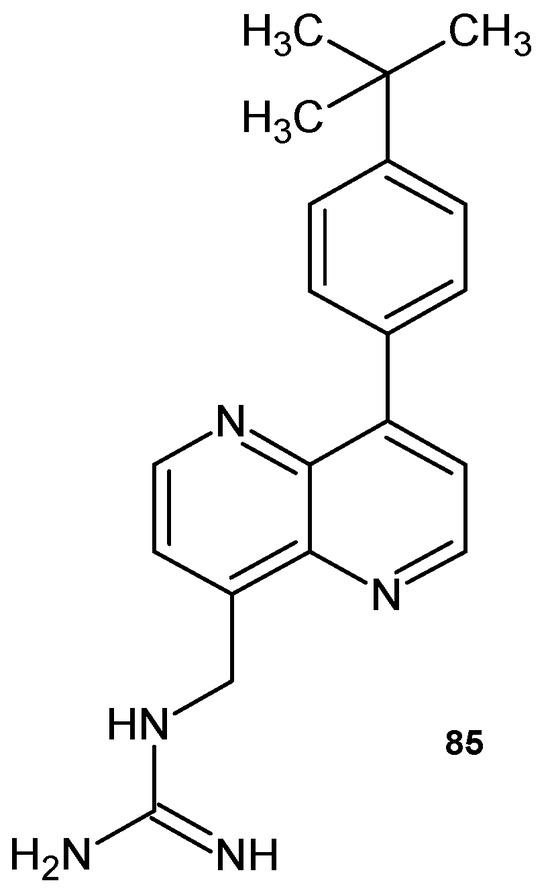
Figure 58.
N-{[8-(4-tert-butylphenyl)-1,5-naphthyridin-4-yl]methyl}guanidine 85.
J-P. Surivet et al. [92] obtained 3-fluoro-6-methoxy-1,5-naphthyridine derivatives as inhibitors of bacterial type II topoisomerases (topoisomerase IV and DNA gyrase). The most potent compounds, 86a–b (Figure 59), were found to be dual inhibitors of DNA gyrase and topoisomerase IV, with broad antibacterial activity and a low spontaneous development of resistance. (1S,2R)-1-((2S,5R)-5-(((2,3-dihydro-[1,4]oxathiino [2,3-c]pyridin-7-yl)methyl)amino)tetrahydro-2H-pyran-2-yl)-2-(3-fluoro-6-methoxy-1,5-naphthyridin-4-yl) ethane-1,2-diol 86b also showed moderate clearance in rats and satisfactory in vivo efficacy against S. aureus in a murine model of infection.
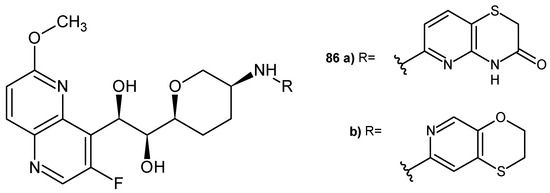
Figure 59.
2-(3-Fluoro-6-methoxy-1,5-naphthyridin-4-yl)ethane-1,2-diol derivatives 86a–b.
S. B. Singh et al. [93] also synthesized 3-fluoro-6-methoxy-1,5-naphthyridine derivatives containing an oxabicyclooctane linker, but with a pyridoxazinone moiety, 87a–b (Figure 60), as inhibitors of bacterial type II topoisomerases (topoisomerase IV and DNA gyrase). These compounds showed broad-spectrum antibacterial activity against MRSA and Gram-negative pathogens Acinetobacter baumannii and E. coli. They showed activity against quinolone-resistant S. aureus and S. pneumoniae strains, but exhibited weaker activity against P. aeruginosa. The S enantiomer (α-hydroxy) of 87b was slightly more potent than its R enantiomer. Both derivatives showed potent activity against S. aureus and E. coli gyrase and better activity against E. coli topoisomerase IV compared to S. aureus topoisomerase IV. Compounds 87a–b were stable in human, dog, and mouse liver microsomal incubations.
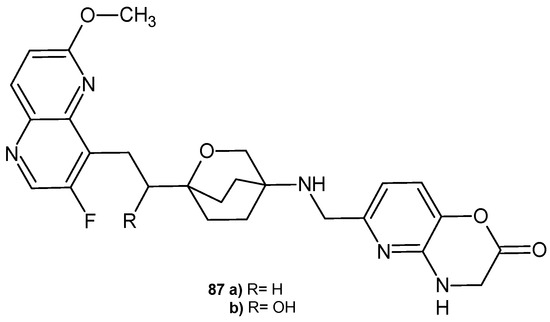
Figure 60.
3-Fluoro-6-methoxy-1,5-naphthyridine derivatives 87a–b.
The 2-hydroxy derivative 87b showed improved in vivo efficacy in a murine S. aureus bacteremia survival model. The influence of other substituents instead of the hydroxyl group on the activity of the derivatives was investigated [94]. Single and double substitutions with OH, NH2, COOH, F, and CH3 groups indicated that a single hydroxyl substitution at position 2 is preferred for potency. Furthermore, the monohydroxy compound showed better efficacy after intravenous administration in the murine model of S. aureus infections. The influence of substituents in the 1,5-naphthyridine system on the activity of the derivatives was also tested [95]. The attachment of methoxy and CN groups at C-2 and halogen and hydroxy at C-7 seems to be acceptable, while substitutions at the remaining three carbons generally have a detrimental effect on the antibacterial activity. Substitution of the fluorine atom with a chlorine or CN group did not result in loss of in vitro activity but only weaker properties in the in vivo S. aureus infection model [95].
L. Li et al. [96] also prepared DNA gyrase and topoisomerase IV inhibitors. A series of dioxane-linked 3-fluoro-6-methoxy-1,5-naphthyridine derivatives 88 (Figure 61), showed improved activity against methicillin-resistant S. aureus, penicillin-resistant S. pneumoniae, vancomycin-resistant Enterococcus faecium, and S. pyogenes. These compounds also reduced hERG inhibition.
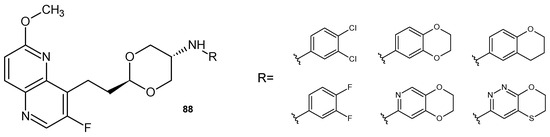
Figure 61.
2-[2-(3-Fluoro-6-methoxy-1,5-naphthyridin-4-yl)ethyl]-1,3-dioxan-5-amine derivatives 88.
Pyronaridine 89 (Figure 62) is a Mannich base derivative of 1,5-naphthyridine. 2-Methoxy-7-chloro-10-[3,5-bis(pyrrolidinyl-1-methyl-)4-hydroxyphenyl]aminobenzyl-1,5-naphthyridine 89 has been clinically tested as an antimalarial drug effective in treating malaria-infected patients in chloroquine resistance regions [97]. It has been used as an antimalarial agent against Plasmodium falciparum and Plasmodium vivax since the 1970s. The combination of pyronaridine 89 and artesunate (Pyramax@) has been found to provide more potent antiviral activity with less toxicity and is therefore being investigated as a possible treatment for SARS-CoV-2 [98].
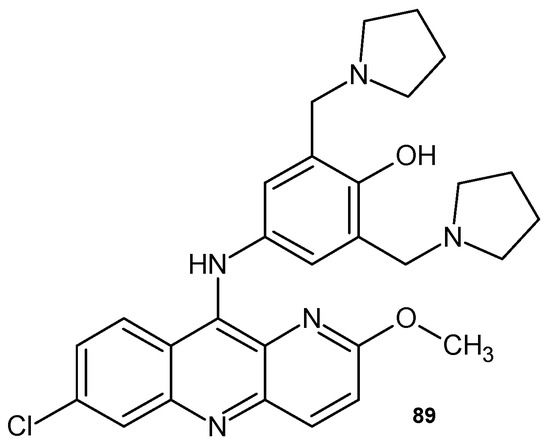
Figure 62.
Pyronaridine 89.
E. Melcón-Fernandez et al. [86] prepared a series of fused 1,5-naphthyridines, 90–91 (Figure 63), and 1,8-naphthyridines, 75–78 (Figure 53), and evaluated their antileishmanial activity using in vitro and ex vivo assays. 1,8-Naphthyridines 75–78 showed better activity against leishmaniasis than 1,5-naphthyridine derivatives 90–91. The presence of a nitrogen atom in the ring fused to the naphthyridine system was important for the increased activity against L. infantum amastigotes. Naphthyridines fused to quinoline were more active than those fused to the chromene ring. Quinoline derivatives exhibited EC50 values in the range of 4.93–5.53 µM and were also less toxic.
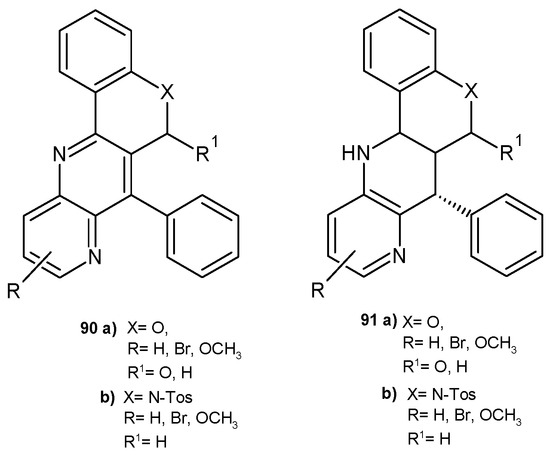
Figure 63.
Chromeno [4,3-b]naphthyridines and quinolino [4,3-b]naphthyridines 90–91.
S. Durić et al. [99] prepared silver(I) complexes with 1,5-naphthyridine: [Ag(NO3)(1,5-naph)]n 92a, [Ag(CF3COO)(1,5-naph)]n 92b, and [Ag(CF3SO3)(1,5-naph)]n 92c (Figure 64). The obtained complexes showed good antibacterial activity, with MIC values in the range of 2.5–100 μg/mL, and better antifungal activity against Candida spp., with MIC values in the range of 0.78–6.25 μg/mL. Compound 92c showed the best therapeutic potential due to the lowest MIC values against the tested Candida strains, as well as a non-toxic in vivo response in zebrafish embryos. Complexes 92a and 92b effectively inhibited C. albicans biofilms, while complex 92a also inhibited the formation of mixed C. albicans/P. aeruginosa biofilms [99].
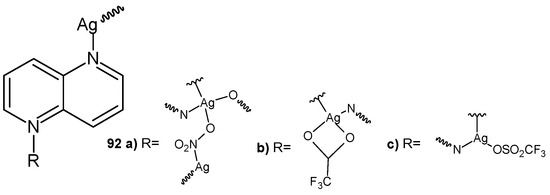
Figure 64.
Silver(I) complexes with 1,5-naphthyridines 92a–c.
3.3. Alkaloids Containing a 1,5-Naphthyridine Scaffold
Canthin-6-one 93a and 10-methoxycanthin-6-one 93b (Figure 65) were isolated from Zanthoxylum paracanthum as promising antimicrobial substances [100]. Alkaloids 93a–b showed strong activity against methicillin-resistant S. aureus strains, with MIC values of 0.98 µg/mL and 3.91 µg/mL, respectively. Antifungal activity against C. albicans was presented, with MIC values of 3.91 and 7.81 µg/mL for canthin-6-one 93a and 10-methoxycanthin-6-one 93b, respectively. Moreover, 10-hydroxycanthin-6-one 93b showed antifungal effects against Fusarium graminearum and Fusarium solani (growth inhibition rate 74.5% and 57.9%), and antibacterial activity against B. cereus, with MIC = 15.62 µg/mL [101]. Alkaloids 93a–b also showed antimycobacterial effects against M. smegmatis, M. phlei, and M. fortuitum, with MIC values in the range of 2–8 µg/mL [102]. Canthin-6-one 93a exhibited antiparasitic activity in mice infected with Trypanosoma cruzi. Due to its low toxicity, alkaloid 93a is a promising candidate for the treatment of Chagas disease [103].
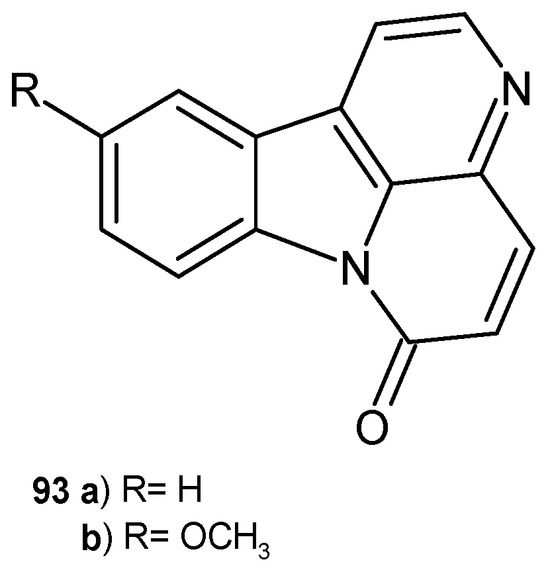
Figure 65.
Canthin-6-one 93a and 10-methoxycanthin-6-one 93b.
4. 1,6-Naphthyridine Derivatives
4.1. Alkaloids Containing a 1,6-Naphthyridine Scaffold
Aaptamine (8,9-dimethoxy-1H-benzo[de][1,6]naphthyridine 94a (Figure 66) isolated from Aaptos aaptos displayed antiviral activity against HIV-1 [104]. Alkaloid 94a and its dibenzyl derivative 94b showed anti-amoebic effect towards Acanthamoeba castellanii, with IC50 = 45 µg/mL and IC50 = 8 µg/mL, respectively [105].
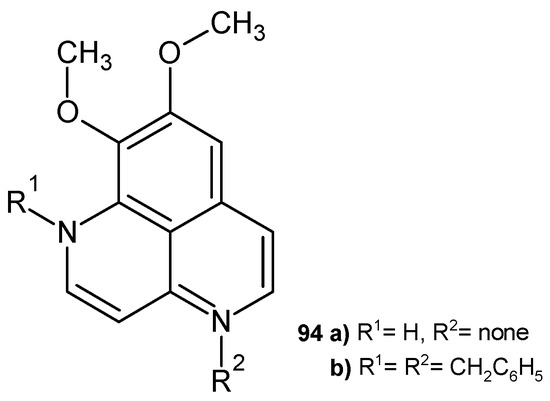
Figure 66.
Aaptamine 94a and 1,4-dibenzylaaptamine 94b.
4.2. Synthetic 1,6-Naphthyridine Derivatives
D.P. Aśanin et al. [106] prepared silver(I) and gold(III) coordination compounds with 1,6-naphthyridine scaffolds. {[Ag(1,6-naph)(H2O)](BF4)}n 95 (Figure 67) showed good antifungal activity against C. albicans and Candida parapsilosis, with MIC values of 0.49 and 3.9 µg/mL, respectively, while no significant antibacterial activity was observed against S. aureus, L. monocytogenes, P. aeruginosa, E. coli, and K. pneumoniae. [AuCl3(1,6-naph)] 96 (Figure 67) exhibited moderate activity only against S. aureus, L. monocytogenes, P. aeruginosa, and E. coli, with MIC = 62.5 µg/mL. Both polymers 95–96 were less cytotoxic to human health fibroblast cell lines than the silver-based antimicrobial drug.
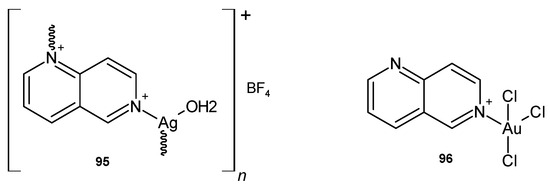
Figure 67.
Silver(I) and gold(III) coordination compounds with 1,6-naphthyridine scaffold.
Ibrahim and El-Gohary [107] obtained 7-amino-3-[(6-hydroxy-4,7-dimethoxy-1-benzofuran-5-yl)carbonyl]-5-oxo-5,6-dihydro-1,6-naphthyridine-8-carbonitrile 97 (Figure 68), with high antibacterial activity against Gram-positive strains S. aureus and B. subtilis and moderate activity against Gram-negative bacteria strains E. coli and S. typhimurium. This compound 97 also showed moderate antifungal activity against C. albicans and A. fumigatus.
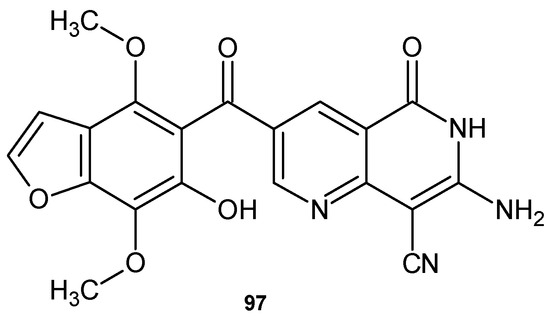
Figure 68.
7-Amino-3-[(6-hydroxy-4,7-dimethoxy-1-benzofuran-5-yl)carbonyl]-5-oxo-5,6-dihydro-1,6-naphthyridine-8-carbonitrile 97.
8-Hydroxy-1,6-naphthyridine derivatives 98–99 (Figure 69) were synthesized by R. J. Wall et al. [108] and showed high activity against Trypanosoma brucei and Leishmania donovani. The influence of divalent cations Ca2+, Cu2+, Fe2+, Mg2+, and Mn2+ on the potency of derivatives 98–99 against T. brucei was also assessed. The addition of FeCl2 led to a significant increase in the EC50 of the tested compounds. In contrast, the addition of tolerated levels of other divalent cations had little effect on the potency of these compounds. The addition of 100 M FeCl2 reduced the potency of the tested compounds in the case of L. donovani.
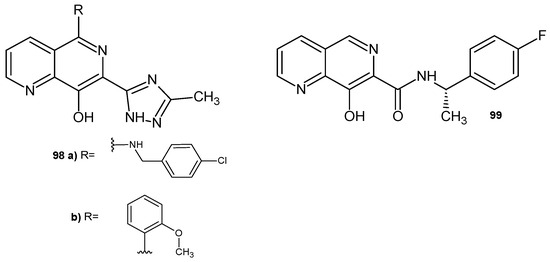
Figure 69.
8-Hydroxy-1,6-naphthyridine derivatives 98–99.
5. 2,6-Naphthyridine Derivatives
5.1. Synthetic 2,6-Naphthyridine Derivatives
A. Bishnoi et al. [109] synthesized benzo[h][1,2,4]triazolo [3,4-a][2,6]naphthyridine derivatives as potential antibacterial agents. The activity of the obtained compounds, 100a–d (Figure 70), was tested against Gram-positive and Gram-negative bacterial strains S. aureus, B. subtilis, P. aeruginosa, and E. coli, as well as on fungal strains C. albicans, A. niger, and A. fumigatus using the disc diffusion method. No activity was observed against A. niger. Compounds 100b–c exhibited good activity against C. albicans, with MIC values of 12.5 mg/mL and 25 mg/mL, respectively. Compound 100a showed activity against A. fumigatus, with MIC = 25 mg/mL. Compounds 100d and 100c were the most active antibacterial agents, with MIC values of 12.5 mg/mL against B. subtilis and S. aureus [109].
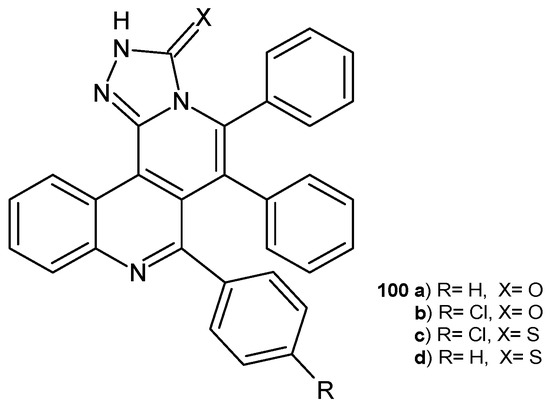
Figure 70.
Benzo[h][1,2,4]triazolo[3,4-a][2,6]naphthyridine derivatives 100a–d.
5.2. An Alkaloid Containing a 2,6-Naphthyridine Scaffold
Calycanthine 101 (Figure 71)—an alkaloid containing a 2,6-naphthyridine scaffold, which was isolated from Chimonanthus praecox seeds—exhibited significant activity against two plant pathogenic fungi, Exserohilum turcicum and Bipolaris maydis, with EC50 values of 103.1 and 29.3 mg/mL, respectively [110].
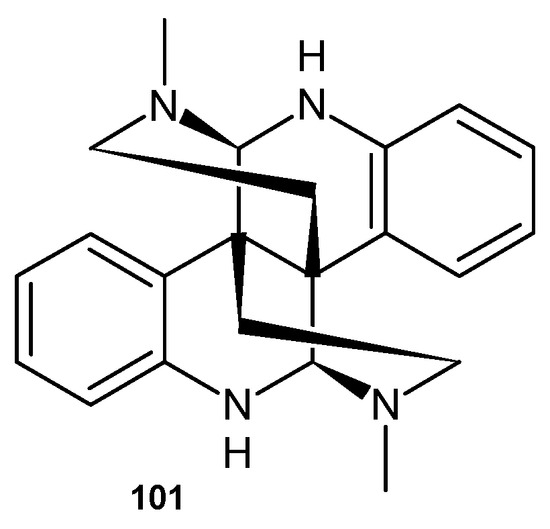
Figure 71.
Calycanthine 101.
6. 2,7-Naphthyridine Derivatives
6.1. Synthetic 2,7-Naphthyridine Derivatives
Z. Hossaini et al. [111] investigated the antibacterial activity of synthesized isoquino [1,2-a][2,7]-naphthyridine derivatives 102a–g (Figure 72). The evaluation displayed that compounds 102a–d exhibit very good activity against S. aureus, B. cereus, E. coli, and K. pneumoniae strains, comparable to streptomycin and gentamicin.
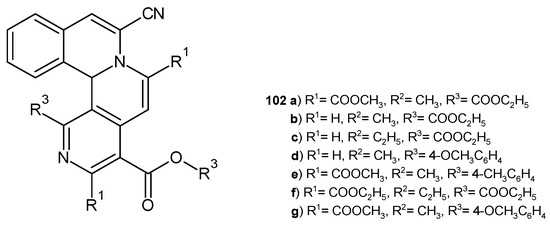
Figure 72.
Isoquino[1,2-a][2,7]naphthyridine derivatives 102a–g.
Pyrimido[4,5-c][2,7]naphthyridine derivatives obtained by A. Gangjee et al. [112] were evaluated as inhibitors of dihydrofolate reductase (DHFR) from Pneumocystis carinii and Toxoplasma gondii. The most potent analogue was the trimethoxyphenyl derivative 103 (Figure 73), with IC50 = 86 nM.
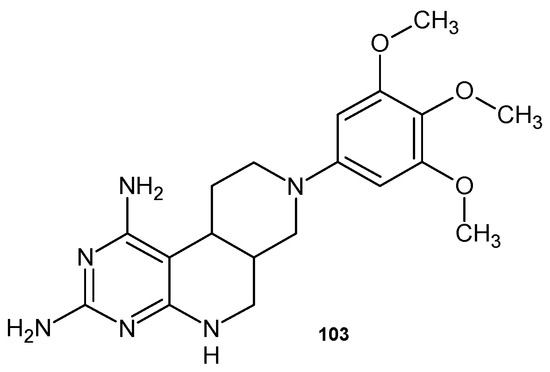
Figure 73.
8-(3,4,5-Trimethoxyphenyl)-5,6,6a,7,8,9,10,10a–octahydropyrimido[4,5-c][2,7]naphthyridine -1,3-diamine 103.
2,7-Naphthyridine-4-carbonitrile derivatives 104 and thieno[2,3-c][2,7]naphthyridine derivatives 105 (Figure 74) were evaluated for their antimicrobial activity against four bacterial strains (E. coli, S. aureus, P. aeruginosa, and B. cereus) and four fungal strains (Geotrichum candidium, C. albicans, Trichophyton rubrum, and A. flavus) by measuring the inhibition zones [113]. The acetyl group in this structure enhanced the strong antibacterial effect. Compounds 104a–b and 105a,d were the most potent against B. cereus and E. coli, with MIC values in the range of 7.0–8.0 μg/mL. Derivatives 105a,b,e showed significant activity against S. aureus, with MIC values in the range of 6.0–8.0 μg/mL. The P. aeruginosa strain turned out to be sensitive only to compound 104a (MIC = 6.0 μg/mL). Substitution of the acetyl group with a benzoyl group (compound 105c) resulted in strong antifungal activity against G. candidium, T. rubrum, and A. flavus, with MIC values in the range of 7.0–9.0 μg/mL. Derivatives 105c and 105f showed very good antifungal activity against A. flavus and T. rubrum, with MIC values in the range of 7.0–8.0 μg/mL. C. albicans was sensitive to the 4-chlorophenyl derivatives 104d and 105d (MIC= 8.0 μg/mL) [113].
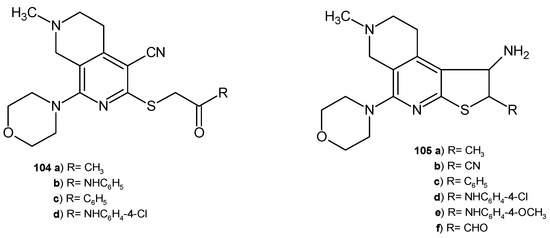
Figure 74.
2,7-Naphthyridine-4-carbonitrile derivatives 104 and thieno [2,3-c][2,7]naphthyridine derivatives 105.
6.2. Alkaloids Containing a 2,7-Naphthyridine Scaffold
Eupolauridine-indeno[1,2,3-ij][2,7]naphthyridine 106a (Figure 75), extracted from the root bark of Cleistopholis patens by Hufford et al. [114], exhibited in vitro efficacy against fungal pathogens C. albicans, Cryptococcus neoformans, and A. fumigatus and selective inhibition of fungal topoisomerase I [115]. This alkaloid 106a showed significant activity against C. albicans, with MIC = 5.25 μg/mL [115]. S. Taghavi-Moghadam et al. [116] prepared eupolauridine derivatives 106–107 (Figure 75), which were evaluated for their antimicrobial activity. Compounds 106b, 106g, and 107a–b exhibited antifungal activity toward C. albicans, with MIC values in the range of 0.098–0.78 µg/mL, and compounds 106b–g and 107a–b acted similarly toward C. neoformans, with MIC values in the range of 0.39–0.78 µg/mL. However, none of the synthesized analogues 106–107 were effective against A. fumigatus. Only compounds 107a–b showed very good antibacterial activity against S. aureus, MRS, and P. aeruginosa, with IC50 values in the range of 2–5 µg/mL. Derivatives 106c and 106e showed significant antimalarial activity.
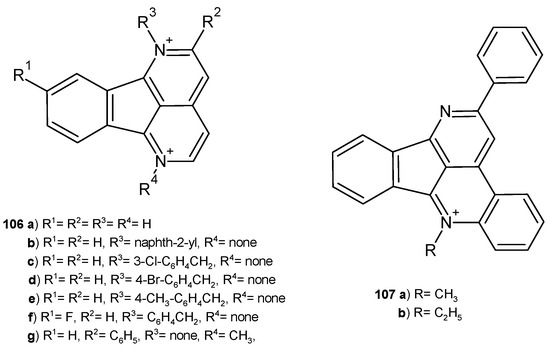
Figure 75.
Eupolauridine and its derivatives, 106–107.
Ascididemin 108 (Figure 76) exhibited antimicrobial activity against Cladosporium resinae, E. coli, and B. subtilis and also very good potency (MIC = 0.35 mM) against Mycobacterium tuberculosis [117]. Pyrido[2,3,4-kl]acridin-6-one derivatives prepared by D.R. Appleton et al. [118] were evaluated for their ability to inhibit the growth of M. tuberculosis in vitro. The most potent were 4-ethylthiopyrido[2,3,4-kl]acridin-6-one 109, with an MIC = 0.34 mM, and 110a–b, with MIC values of 0.58.5 mM and 0.61 mM, respectively.
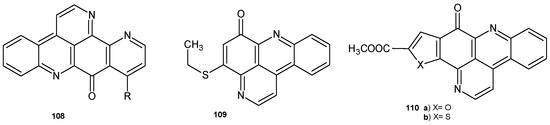
Figure 76.
Pyrido[2,3,4-kl]acridin-6-one derivatives 108–110.
Kitahara et al. [119] obtained eupomatidines 111a–c (Figure 77), which were evaluated for their antifungal activity against C. albicans, Paecilomyces variotii, and Trichophyton mentagrophytes. Eupomatidine-1 111a showed activity against all strains, with EC50 values of 50 μg/mL, 6.25 μg/mL, and 0.4 μg/mL, respectively. Eupomatidines 111b–c were active only toward T. mentagrophytes, with EC50 values of 3.1 μg/mL and 6.25 μg/mL, respectively.
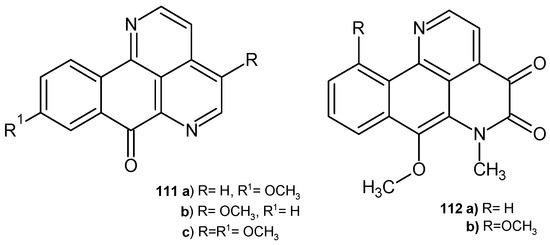
Figure 77.
Eupomatidines 111a–c, imbiline 112a, and hadranthine A 112b.
7-Methoxy-6-methyl-4,5-dihydronaphthol[1,2,3-ij][2,7]naphthyridine-4,5-(6H)-dione derivatives imbiline 112a and hadranthine A 112b (Figure 77), isolated from Duguetia hadrantha, were evaluated for their antimalarial and antimicrobial activities. These alkaloids showed weak antimalarial potency against chloroquine-resistant Plasmodium falciparum. Imbiline 112a was found to be inactive against S. aureus, as well as against fungal strains C. albicans and C. neoformans. Hadranthine A 112b exhibited activity against C. albicans, with MIC = 20 μg/mL [120].
Sampangine 113a and 3-methoxysampangine 113b (Figure 78) were also isolated from D. hadrantha and then evaluated for their antimalarial, antifungal, and cytotoxic potential [121]. Both alkaloids 113a–b showed activity against P. falciparum without cytotoxicity toward VERO cells. These alkaloids 113a–b also exhibited activity against Mycobacterium intracellulare, comparable to rifampin [122]. Sampangine 113a was also found to be a potent antifungal agent against Paecilomyces variotii and Trichophyton mentagrophytes, with EC50 = 0.2 μg/mL [120]. 3-Methoxysampangine 113b showed significant antifungal activity against C. albicans, C. neoformans, and A. fumigatus, better than amphotericin B [122].
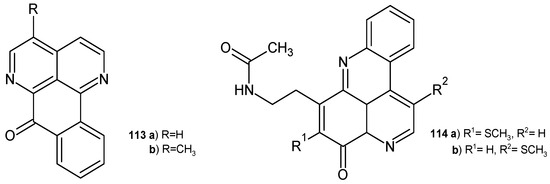
Figure 78.
Sampangines 113a–b and diplamines 114a–b.
Diplamines 114a–b (Figure 78), isolated from the tunicate Diplosoma sp. [123], exhibited moderate antimicrobial activity towards B. subtilis, E. coli, C. albicans, and T. mentagrophytes [124].
Amphimedine analogues 115–117 (Figure 79) isolated from marine sponges were evaluated for their antimicrobial activities. Neoamphimedine 116 showed antitrypanosomal activity against T. brucei, with IC50 = 0.21 μM. Demethyldeoxyamphimedine 116 exhibited antibacterial activity against Listonella anguillarum and M. luteus [125]. Petrosamine B 117 inhibited Helicobacter pylori aspartyl semialdehyde dehydrogenase (IC50 = 306 μM) [126].
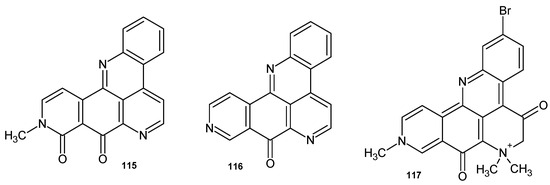
Figure 79.
Amphimedine analogues 115–117.
Ascididemin 118 and 12-deoxyascididemin 119 (Figure 80) showed significant activity against T. brucei, with IC50 values of 0.077 and 0.032 μM, respectively [127]. Ascididemin 118 exhibited antimicrobial activity against C. resinae, E. coli, and B. subtilis [117] and very good efficacy against M. tuberculosis, with MIC = 0.35 μM [118].
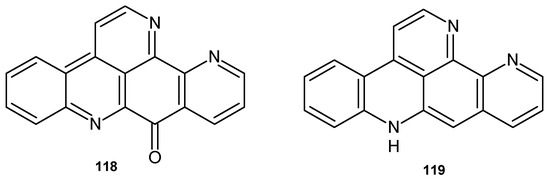
Figure 80.
Ascididemines 118–119.
7. Conclusions
Naphthyridine derivatives have been studied for their various pharmacological activities. This review presents the literature data on synthetic and natural naphthyridine derivatives that have been reported to possess antimicrobial activity. Most publications refer to the 1,8-naphthyridine isomer, since the first naphthyridine derivative introduced into therapy in 1967 was the antibacterial nalidixic acid. Since then, several other 1,8-naphthyridine derivatives (mainly fluoro derivatives) have been approved for therapy, as well as one derivative of the 1,5-naphthyridine isomer as the antimalarial drug pyronaridine. Although the vast majority of naphthyridines with antimicrobial properties are derivatives of the 1,8-isomer, many scientific reports also refer to the 1,5-naphthyridine derivatives. So far, no 1,7-naphthyridine derivative has been obtained that would exhibit antimicrobial activity. Alkaloids containing in their structure the 1,5-, 1,6-, 2,6- or 2,7-naphthyridine system have also been isolated [128]. The antibacterial, antifungal, antimalarial, or antiparasitic properties of these alkaloids have been studied. Some of them were also active against drug-resistant strains. Canthin-6-one, 10-methoxycanthin-6-one, and eupolauridine derivatives were active against methicillin-resistant S. aureus, while imbiline and hadranthine A were active against chloroquine-resistant P. falciparum. Despite the broad antimicrobial activity of naphthyridine derivatives, the molecular mechanisms of their action are still poorly understood, which makes them an interesting target for deeper research. We believe that this review will contribute to further research on the properties of naphthyridine derivatives, which will allow the use of these compounds in the fight against the increasing drug resistance of microorganisms.
Author Contributions
Conceptualization, A.W.; methodology, A.W.; formal analysis, A.W. and M.M.; investigation, A.W. and M.M.; resources, A.W.; data curation, A.W.; writing—original draft preparation, A.W.; writing—review and editing, A.W. and M.M.; visualization, A.W.; supervision, A.W. and M.M.; funding acquisition, A.W. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Wroclaw Medical University, grant number SUBK.D090.24.037.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hossain, T.J. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Madaan, A.; Verma, R.; Kumar, V.; Singh, A.T.; Jain, S.K.; Jaggi, M. 1,8-Naphthyridine Derivatives: A Review of Multiple Biological Activities. Arch. Pharm. 2015, 348, 837–860. [Google Scholar] [CrossRef]
- Lesher, G.Y.; Froelich, E.J.; Gruett, M.D.; Bailey, J.H.; Brundage, R.P. 1,8-naphthyridine derivatives. A new class of chemotherapeutic agents. J. Med. Chem. 1962, 5, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, A.M.; Geroldi, D.; Siccardi, A.; Falaschi, A. Studies on the Mode of Action of Nalidixic Acid. Eur. J. Biochem. 1972, 25, 359–365. [Google Scholar] [CrossRef]
- Wang, K.; Gong, C.; Xiao, W.; Abdukader, A.; Wang, D. Accessing 1,8-Naphthyridone-3-carboxylic Acid Derivatives and Application to the Synthesis of Amfonelic Acid. J. Org. Chem. 2024, 89, 5811–5824. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.; Andrews, J.M.; Danks, G. In-vitro activity of enoxacin (CI-919), a new quinoline derivative, compared with that of other antimicrobial agents. J. Antimicrob. Chemother. 1984, 13, 237–244. [Google Scholar] [CrossRef]
- Jałbrzykowska, K.; Chrzanowska, A.; Roszkowski, P.; Struga, M. The New Face of a Well-Known Antibiotic: A Review of the Anticancer Activity of Enoxacin and Its Derivatives. Cancers 2022, 14, 3056. [Google Scholar] [CrossRef]
- Ethan Rubinstein. History of Quinolones and Their Side Effects. Chemotherapy 2001, 47 (Suppl. S3), 3–8. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.; Giménez, M.J.; Alou, L.; Gómez-Lus, M.L.; Aguilar, L.; Prieto, J. Ex vivo serum activity (killing rates) after gemifloxacin 320 mg versus trovafloxacin 200 mg single doses against ciprofloxacin-susceptible and -resistant Streptococcus pneumoniae. Int. J. Antimicrob. Agents 2002, 20, 144–146. [Google Scholar] [CrossRef]
- Goldstein, E.J.C.; Citron, D.M.; Warren, Y.; Tyrrell, K.; Merriam, C.V. In Vitro Activity of Gemifloxacin (SB 265805) against Anaerobes. Antimicrob. Agents Chemother. 1999, 43, 2231–2235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yague, G.; Morris, J.E.; Pan, X.; Gould, K.A.; Fisher, L.M. Cleavable-Complex Formation by Wild-Type and Quinolone-Resistant Streptococcus pneumoniae Type II Topoisomerases Mediated by Gemifloxacin and Other Fluoroquinolones. Antimicrob. Agents Chemother. 2002, 46, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, H.; Seol, M.; Choi, D.; Choi, E.; Kwak, J. In Vitro and In Vivo Antibacterial Activities of DW-224a, a New Fluoronaphthyridone. Antimicrob. Agents Chemother. 2006, 50, 2261–2264. [Google Scholar] [CrossRef]
- Kwon, A.-R.; Min, Y.-H.; Ryu, J.-M.; Choi, D.-R.; Shim, M.-J.; Choi, E.-C. In vitro and in vivo activities of DW-224a, a novel fluoroquinolone antibiotic agent. J. Antimicrob. Chemother. 2006, 58, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Biedenbach, D.J.; Ambrose, P.G.; Wikler, M.A. Zabofloxacin (DW-224a) activity against Neisseria gonorrhoeae including quinolone-resistant strains. Diagn. Microbiol. Infect. Dis. 2008, 62, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Oh, S.-H.; Kim, H.-S.; Choi, D.-R.; Kwak, J.-H. Antimicrobial Activity of Zabofloxacin against Clinically Isolated Streptococcus pneumoniae. Molecules 2016, 21, 1562. [Google Scholar] [CrossRef]
- Babich, J.W.; Rubin, R.H.; Graham, W.A.; Wilkinson, R.A.; Vincent, J.; Fischman, A.J. 18F-Labeling and Biodistribution of the Novel Fluoro-Quinolone Antimicrobial Agent, Trovafloxacin (CP 99,219). Nucl. Med. Biol. 1996, 23, 995–998. [Google Scholar] [CrossRef]
- Jones, R.N.; Barrett, M.S.; Deguchi, T. Antimicrobial Activity of Trovafloxacin Tested against Ciprofloxacin-Susceptible and-Resistant Neisseria gonomhoeae Interpretive Criteria and Comparisons with Etest Results. Diagn. Microbiol. Infect. Dis. 1997, 28, 193–200. [Google Scholar] [CrossRef]
- Qureshi, Z.P.; Seoane-Vazquez, E.; Rodriguez-Monguio, R.; Stevenson, K.B.; Szeinbach, S.L. Market withdrawal of new molecular entities approved in the United States from 1980 to 2009. Pharmacoepidemiol. Drug Saf. 2011, 20, 772–777. [Google Scholar] [CrossRef]
- Clark, R.F.; Wang, S.; Ma, Z.; Weitzberg, M.; Motter, C.; Tufano, M.; Wagner, R.; Gu, Y.G.; Dandliker, P.J.; Lerner, C.G.; et al. Novel inhibitors of bacterial protein synthesis: Structure-activity relationships for 1,8-naphthyridine derivatives incorporating position 3 and 4 variants. Bioorg. Med. Chem. Lett. 2004, 14, 3299–3302. [Google Scholar] [CrossRef]
- Shen, L.L.; Black-Schaefer, C.; Cai, Y.; Dandliker, P.J.; Beutel, B.A. Mechanism of action of a novel series of naphthyridine-type ribosome inhibitors: Enhancement of tRNA footprinting at the decoding site of 16S rRNA. Antimicrob. Agents Chemother. 2005, 49, 1890–1897. [Google Scholar] [CrossRef]
- Dandliker, P.J.; Pratt, S.D.; Nilius, A.M.; Black-Schaefer, C.; Ruan, X.; Towne, D.L.; Clark, R.F.; Englund, E.E.; Wagner, R.; Weitzberg, M.; et al. Novel Antibacterial Class. Antimicrob. Agents Chemother. 2003, 47, 3831–3839. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N. In vitro evaluation of E-4695, a new fluoro-naphthyridine. Eur. J. Clin. Microbiol. Infect. Dis. 1992, 11, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J.; Gargallo-Viola, D.; Robert, M.; Tudela, E.; Xicota, M.A.; Garcia, J.; Esteve, M.; Coll, R.; Pares, M.; Roser, R. E-4695, a new C-7 azetidinyl fluoronaphthyridine with enhanced activity against gram-positive and anaerobic pathogens. Antimicrob. Agents Chemother. 1995, 39, 413–421. [Google Scholar] [CrossRef]
- Cohen, M.A.; Huband, M.D.; Mailloux, G.B.; Yoder, S.L.; Roland, G.E.; Domagala, J.M.; Heifetz, C.L. In Vitro Antibacterial Activities of PD 131628, a New 1,8-Naphthyridine Anti-Infective Agent. Antimicrob. Agents Chemother. 1991, 35, 141–146. [Google Scholar] [CrossRef]
- Hussain Qadri, S.M.; Ueno, Y.; Saldin, H.; Burdette, J.M.; Lee, G.C. CI-990 (PD 131112): A New Quinolone Prodrug. Ann. Saudi Med. 1993, 13, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.A.; Yoder, S.L.; Huband, M.D.; Roland, G.E.; Courtney, C.L. In vitro and in vivo activities of clinafloxacin, CI-990 (PD 131112), and PD 138312 versus enterococci. Antimicrob. Agents Chemother. 1995, 39, 2123–2127. [Google Scholar] [CrossRef]
- Lewin, C.S. Antibacterial activity of a 18-Naphthyridine quinolone. J. Med. Microbiol. 1992, 36, 353–357. [Google Scholar] [CrossRef]
- Hong, C.Y.; Kim, Y.K.; Chang, J.H.; Kim, S.H.; Choi, H.; Nam, D.H.; Kim, Y.Z.; Kwak, J.K. Novel Fluoroquinolone Antibacterial Agents Containing Oxime-Substituted (Aminomethyl)pyrrolidines: Synthesis and Antibacterial Activity of 7-(4-(Aminomethyl)-3-(methoxyimino)pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro[1,8]naphthyridine-3-carboxylic Acid (LB20304). J. Med. Chem. 1997, 40, 3584–3593. [Google Scholar]
- Huang, X.; Chen, D.; Wu, N.; Zhang, A.; Jia, Z.; Li, X. The synthesis and biological evaluation of a novel series of C7 non-basic substituted fluoroquinolones as antibacterial agents. Bioorg. Med. Chem. Lett. 2009, 19, 4130–4133. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, A.; Chen, D.; Jia, Z.; Li, X. 4-Substituted 4-(1H-1,2,3-triazol-1-yl)piperidine: Novel C7 moieties of fluoroquinolones as antibacterial agents. Bioorg. Med. Chem. Lett. 2010, 20, 2859–2863. [Google Scholar] [CrossRef]
- Todo, Y.; Takagi, H.; Iino, F.; Fukuoka, Y.; Takahata, M.; Okamoto, S.; Saikawa, I.; Narita, H. Pyridonecarboxylic acids as antibacterial agents. IX. Synthesis and structure-activity relationship of 3-substituted 10-(1-aminocyclopropyl)-9-fluoro-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de]- 1,4-benzoxazine-6-carboxylic acids and their 1-thio and 1-aza analogues. Chem. Pharm. Bull. 1994, 42, 2569–2574. [Google Scholar] [CrossRef][Green Version]
- Frigola, J.; Torrens, A.; Castrillo, J.A.; Mas, J.; Vañó, D.; Berrocal, J.M.; Calvet, C.; Salgado, L.; Redondo, J.; Garcia-Granda, S.; et al. 7-Azetidinylquinolones as Antibacterial Agents. 2.1 Synthesis and Biological Activity of 7-(2,3-Disubstituted-l-azetidinyl)-4-oxoquinoline-and-1,8-naphthyridine-3-carboxylic Acids. Properties and Structure-Activity Relationships of Quinolones with an Azetidine Moiety2 Chart 1. J. Med. Chem 1994, 37, 4195–4210. [Google Scholar]
- Gençer, H.K.; Levent, S.; Acar Çevik, U.; Özkay, Y.; Ilgın, S. New 1,4-dihydro[1,8]naphthyridine derivatives as DNA gyrase inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 1162–1168. [Google Scholar] [CrossRef]
- Domagala, J.M.; Hagen, S.E.; Joannides, T.; Kiely, J.S.; Laborde, E.; Schroeder, M.C.; Sesnie, J.A.; Shapiro, M.A.; Suto, M.J.; Vanderroest, S. Quinolone Antibacterials Containing the New 7-[3-(l-Aminoethyl)-l-pyrrolidinyl] Side Chain: The Effects of the 1-Aminoethyl Moiety and Its Stereochemical Configurations on Potency and in Vivo Efficacy H R = Et. J. Med. Chem. 1993, 36, 871–882. [Google Scholar] [CrossRef]
- Takahata, M.; Otsuki, M.; Nishino, T. In-vitro and in-vivo activities of T-3262, a new pyridone carboxylic acid. J. Antimicrob. Chemother. 1988, 22, 143–154. [Google Scholar] [CrossRef]
- Espinoza, A.M.; Chin, N.-X.; Novelli, A.; Neu, H.C. Comparative In Vitro Activity of a New Fluorinated 4-Quinolone, T-3262 (A-60969). Antimicrob. Agents Chemother. 1988, 32, 663–670. [Google Scholar] [CrossRef]
- Cooper, C.S.; Klock, P.L.; Chu, D.T.W.; Hardy, D.J.; Swanson, R.N.; Plattner, J.J. Preparation and in Vitro and in Vivo Evaluation of Quinolones with Selective Activity against Gram-Positive Organisms. J. Med. Chem. 1992, 35, 1392–1398. [Google Scholar] [CrossRef]
- Egawa, H.; Miyamoto, T.; Minamida, A.; Nishimura, Y.; Okada, H.; Uno, H.; Matsumoto, J. Pyridonecarboxylic Acids as Antibacterial Agents. 4.1 Synthesis and Antibacterial Activity of 7-(3-Amino-l-pyrrolidinyl)-l-ethyl-6-fluoro-l,4-dihydro-4-oxol,8-naphthyridine-3-carboxylic Acid and Its Analogues. J. Med. Chem. 1984, 27, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.M.; Suaifan, G.A.R.Y.; Shehadeh, M.B.; Okechukwu, P.N. Design, synthesis, and biological evaluation of 1,8-naphthyridine glucosamine conjugates as antimicrobial agents. Drug. Dev. Res. 2019, 80, 179–186. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kumar, R.; Dureja, P.; Khurana, J.M. Synthesis, antimicrobial evaluation and QSAR analysis of novel nalidixic acid based 1,2,4-triazole derivatives. Eur. J. Med. Chem. 2011, 46, 4089–4099. [Google Scholar] [CrossRef]
- Gurjar, V.K.; Shukla, S.; Gondaliya, N.; Puwar, N. Design, Synthesis, in Silico Study and Biological Evaluation of 1,8-Naphthyridine Derivatives as Potential Antibacterial Agents. Orient. J. Chem. 2023, 39, 320–334. [Google Scholar] [CrossRef]
- Sriram, D.; Senthilkumar, P.; Dinakaran, M.; Yogeeswari, P.; China, A.; Nagaraja, V. Antimycobacterial activities of novel 1-(cyclopropyl/tert-butyl/4- fluorophenyl)-1,4-dihydro-6-nitro-4-oxo-7-(substituted secondary amino)-1,8-naphthyridine-3-carboxylic acid. J. Med. Chem. 2007, 50, 6232–6239. [Google Scholar] [CrossRef]
- Suzuki, N.; Kato, M.; Dohmori, R. Synthesis of 1,8-naphthyridine derivatives and their activities against Trichomonas vaginalis. Yakugaku Zasshi 1979, 99, 155–164. [Google Scholar] [CrossRef]
- Chennam, K.P.; Ravi, M.; Ushaiah, B.; Srinu, V.; Eslavath, R.K.; Devi, C.S. Synthesis, characterization, DNA interactions, DNA cleavage, radical scavenging activity, antibacterial, anti-proliferative and docking studies of new transition metal complexes. J. Fluoresc. 2016, 26, 189–205. [Google Scholar] [CrossRef]
- Massari, S.; Daelemans, D.; Barreca, M.L.; Knezevich, A.; Sabatini, S.; Cecchetti, V.; Marcello, A.; Pannecouque, C.; Tabarrini, O. A 1,8-naphthyridone derivative targets the HIV-1 Tat-mediated transcription and potently inhibits the HIV-1 replication. J. Med. Chem. 2010, 53, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayana, E.; Karunakar, T.; Shankar, S.S.; Chary, M.T. A study on antibacterial activity of substituted 1, 8-naphthyridines containing carbaldehydes, methylene hydrazines, thiadiazol amines and triazole thiols. J. Adv. Drug Res. 2012, 2, 6–11. [Google Scholar]
- Raja, S.; Jayaveera, K.N.; Subramanyam, S.; Sunil, A.; Reddy, K.; Prakash, C.R. Synthesis and biological evaluation of novel 1,8-naphthyridines containing pyrazolinone, pyrazole, isoxazolinone, isoxazole and pyrimidine-2-ones. Int. J. Pharm. Sci. Res. 2016, 7, 2573–2585. [Google Scholar] [CrossRef]
- Md, K.; Domala, R. Synthesis, Antimicrobial Activities and Molecular Docking Studies of New N-Acylated Derivatives of 5-(2-Phenyl-1,8-naphthyridin-3-yl)-1,3,4-oxadiazol-2-amine. Asian J. Chem. 2024, 36, 628–634. [Google Scholar] [CrossRef]
- Mohamed, N.G.; Sheha, M.M.; Hassan, H.Y.; Abdel-Hafez, L.J.M.; Omar, F.A. Synthesis, antimicrobial activity and molecular modeling study of 3-(5-amino-(2H)-1,2,4-triazol-3-yl]-naphthyridinones as potential DNA-gyrase inhibitors. Bioorg. Chem. 2018, 81, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Arjun, P.; Raaman, N.; Das, T.M. Regioselective facile one-pot Friedländer synthesis of sugar-based heterocyclic biomolecules. Carbohydr. Res. 2010, 345, 1988–1997. [Google Scholar] [CrossRef]
- Hussein, M.A.; Abdel-Rahman, M.A.; Geies, A.A. New heteroaromatic polyazomethines containing naphthyridine moieties: Synthesis, characterization, and biological screening. J. Appl. Polym. Sci. 2012, 126, 2–12. [Google Scholar] [CrossRef]
- Bavlin, G.B.; Tan, W.-L. Potential Antimalarials. I 1,s-Naphthyridines. Aust. J. Chem 1984, 37, 1065–1073. [Google Scholar]
- Olepu, S.; Suryadevara, P.K.; Rivas, K.; Yokoyama, K.; Verlinde, C.L.M.J.; Chakrabarti, D.; van Voorhis, W.C.; Gelb, M.H. 2-Oxo-tetrahydro-1,8-naphthyridines as selective inhibitors of malarial protein farnesyltransferase and as anti-malarials. Bioorg. Med. Chem. Lett. 2008, 18, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Quintela, J.M.; Peinador, C.; González, L.; Iglesias, R.; Paramá, A.; Álvarez, F.; Sanmartín, M.L.; Riguera, R. Piperazine N-substituted naphthyridines, pyridothienopyrimidines and pyridothienotriazines: New antiprotozoals active against Philasterides dicentrarchi. Eur. J. Med. Chem. 2003, 38, 265–275. [Google Scholar] [CrossRef]
- Priya, K.K.; Jella, K.S. Synthesis and Biological Evaluation of 1,2,4-Triazolo[4,3-a][1,8] Naphthyridines under Microwave Condition. Chem. Biol. Lett. 2024, 11, 664–667. [Google Scholar] [CrossRef]
- Narender, A.; Kumar, M.R.; Laxminarayana, E.; Chary, M.T. Synthesis and antimicrobial activity of pyrazole derivatives of 2-cyclopropyl-1,8-naphthyridines. Asian J. Chem. 2009, 21, 6885–6903. [Google Scholar]
- Omar, F.A.; Abelrasoul, M.; Sheha, M.M.; Hassan, H.Y.; Ibrahiem, Y.M. Synthesis, Antibacterial Activity and Molecular Docking of Substituted Naphthyridines as Potential DNA Gyrase Inhibitors. Chem. Select 2018, 3, 2604–2612. [Google Scholar] [CrossRef]
- Turan-Zitouni, G.; Dilek Altintop, M.; Özdemir, A.; Demirci, F.; Abu Mohsen, U.; Asım Kaplancıklı, Z. Cukurova Medical Journal Novel Naphthyridine-Hydrazone Derivatives and their Antimicrobial Activity Yeni Naftiridin Hidrazon Türevleri ve Anti-Mikrobiyal Aktiviteleri. Cukurova Med. J. 2014, 39, 234–239. [Google Scholar] [CrossRef]
- Ramesh, D.; Chary, M.T.; Laxminarayana, E.; Sreenivasulu, B. Synthesis and antimicrobial activity of 1-alkyl and aryl-3-(2-methyl-1,8-naphthyridin-3-yl)ureas. Indian J. Chem. 2010, 49B, 1271–1273. [Google Scholar] [CrossRef]
- Gurjar, V.K.; Pal, D.; Mazumder, A.; Mazumder, A.R. Research Paper Synthesis, Biological Evaluation and Molecular Docking Studies of Novel 1,8-Naphthyridine-3-carboxylic Acid Derivatives as Potential Antimicrobial Agents (Part-1). Indian J. Pharm. Sci. 2021, 82, 41–53. [Google Scholar] [CrossRef]
- Bhambi, D.; Salvi, V.K.; Bapna, A.; Pemawat, G.; Talesara, G.L. Synthesis and antimicrobial evaluation of some alkoxyphthalimide derivatives of naphthyridine. Indian J. Chem. 2009, 48, 607–704. [Google Scholar]
- Mohamed, E.A.; Abdelmajeid, A.; Behalo, M.S.; Aly, A.A.; Hebash, K.A. Green Synthesis, Cytotoxicity and Antimicrobial Activities of Some New Pyrazolines, Pyrimidines and Naphthyridines Based on 1,3-Di(thien-2-yl)prop-2-en-1-one Using Choline Chloride-Urea Mixture As A Deep Eutectic Solvent. Egypt. J. Chem. 2022, 65, 651–663. [Google Scholar] [CrossRef]
- De Morais Oliveira-Tintino, C.D.; Muniz, D.F.; Dos Santos Barbosa, C.R.; Pereira, R.L.S.; Begnini, I.M.; Rebelo, R.A.; da Silva, L.E.; Mireski, S.L.; Nasato, M.C.; Krautler, M.I.L.; et al. The 1,8-naphthyridines sulfonamides are NorA efflux pump inhibitors. J. Glob. Antimicrob. Resist. 2021, 24, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Fayyadh, K.M.; Mayeed, H.S. Synthesis and Spectroscopic Studies of Some Heterocyclic Compounds (Oxazepane -4, 7-Dione, Azetidin-2-one) Derived from 2-Chloro -1, 8-Naphthyridine-3-Carbaldyhyd and Studying their Bacterial Activity. J. Glob. Pharma Technol. 2018, 10, 353–359. [Google Scholar]
- Seefeld, M.A.; Miller, W.H.; Newlander, K.A.; Burgess, W.J.; DeWolf, W.E., Jr.; Elkins, P.A.; Head, M.S.; Jakas, D.R.; Janson, C.A.; Keller, P.M.; et al. Indole naphthyridinones as inhibitors of bacterial enoyl-ACP reductases FabI and FabK. J. Med. Chem. 2003, 46, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Al-Omran, F.; Mohareb, R.M.; El-Khair, A.A. Synthesis and biological effects of new derivatives of benzotriazole as antimicrobial and antifungal agents. J. Heterocycl. Chem. 2002, 39, 877–883. [Google Scholar] [CrossRef]
- El-Remaily, M.A.A.; Elhady, O.M.; Abdel-Raheem, E.M.M. Synthesis and Antimicrobial Screening of Fused Heterocyclic Pyridines. J. Heterocycl. Chem. 2017, 54, 871–878. [Google Scholar] [CrossRef]
- Banoth, S.; Perugu, S.; Boda, S. Green Synthesis of Fused Imidazo[1,2-a][1,8]naphthyridine Derivatives Catalyzed by DABCO under Solvent-Free Solid-State Conditions and Their Biological Evaluation. J. Heterocycl. Chem. 2018, 55, 709–715. [Google Scholar] [CrossRef]
- Ravi, D.; Rambabu, S.; Ashok, K.; Madhu, P.; Sakram, B. An Exceedingly Mild, Green Synthesis of Substituted N-3-diaryl-1,8-naphthyridin-2-amine Derivatives and Their Antimicrobial Activity. J. Heterocycl. Chem. 2018, 55, 957–963. [Google Scholar] [CrossRef]
- Sakram, B.; Ashok, K.; Rambabu, S.; Ravi, D.; Kurumanna, A.; Abbas, N.K. Molecular Modeling, Ionic Liquid Cu(II)-Catalyzed Synthesis of 9-(3-Fluoro-4-methoxyphenyl)-6-aryl-[1,2,4]triazolo[4,3-a][1,8] Naphthyridines under Microwave Irradiation and Their Antimicrobial Activity. J. Heterocycl. Chem. 2018, 55, 2392–2399. [Google Scholar] [CrossRef]
- Sakram, B.; Ravi, D.; Raghupathi, M.; Kumar, B.S.; Anantha Lakshmi, P. A facile greener synthesis, antimicrobial evaluation and molecular modelling of new 4-aryl-2-(3-(2-(trifluoromethyl)phenyl)-1,8-naphthyridin-2-yl)phthalazin-1(2H)-one derivatives. Res. Chem. Intermed. 2019, 45, 2007–2022. [Google Scholar] [CrossRef]
- Sakram, B.; Sonyanaik, B.; Ashok, K.; Rambabu, S.; Ravi, D.; Kurumanna, A.; Shyam, P. Eco-friendly synthesis of 1,8-naphthyridine 5-aryl-1,3,4-oxadiazole derivatives under solvent-free solid-state conditions and their antimicrobial activity. Res. Chem. Intermed. 2017, 43, 1881–1892. [Google Scholar] [CrossRef]
- Sonyanaik, B.; Ravi, D.; Kishore, K.; Shyam, P.; Sakram, B. Green pathway for the construction of aryl-1,8-naphthyridine-thiazole scaffolds and in vitro-antimicrobial evaluation, DNA binding interactions, viscosity measurements, molecular modeling studies and ADME properties. Res. Chem. Intermed. 2024, 50, 265–279. [Google Scholar] [CrossRef]
- Ashok, A.; Sonyanaik, B.; Sakram, B. Green synthesis of substituted 1,8-naphthyridin-thiazole scaffolds, molecular docking studies and biological evaluation. Res. Chem. Intermed. 2023, 49, 1029–1041. [Google Scholar] [CrossRef]
- Kumar Parangi, R.; Domala, R. Synthesis, characterization, biological evaluation and docking studies of novel 5-(2-methyl-1,8-naphthyridin-3-yl)-1,3,4-oxadiazol-2-amine and some of its derivatives using symmetrical anhydrides. Results Chem. 2023, 5, 100795. [Google Scholar] [CrossRef]
- Ravi, D.; Sonyanaik, B.; Sakram, B. Green design, synthesis of novel 2-methoxy-3-aryl-1,8-naphthyridines and their biological and molecular docking study. Results Chem. 2022, 4, 100611. [Google Scholar] [CrossRef]
- Sakram, B.; Madhu, P.; Sonyanaik, B.; Rambabu, S.; Ravi, D.; Kurumanna, A. Ferric Chloride-catalyzed Synthesis of 2-Oxo-1,2-dihydro-1,8-naphthyridine-3-carboxylate Derivatives and Their Biological Evaluation. Russ. J. Gen. Chem. 2018, 88, 1224–1227. [Google Scholar] [CrossRef]
- Sakram, B.; Ashok, K.; Rambabu, S.; Sonyanaik, B.; Ravi, D. A novel and efficient synthesis of 3-iodo substituted 1,8-naphthyridines by electrophilic cyclization of 2-amino nicotinaldehyde and their antimicrobial activity. Russ. J. Gen. Chem. 2017, 87, 1794–1799. [Google Scholar] [CrossRef]
- Sakram, B.; Ravi, D.; Ashok, K.; Rambabu, S.; Sonyanaik, B.; Kurumanna, A. An Efficient Microwave-Assisted Synthesis of Novel 2-{4-[(3-Aryl-1,8-naphthyridin-2-yl)amino]phenyl}-1H-benzo[de]isoquinoline-1,3(2H)-diones and Their Antimicrobial Activity. Russ. J. Gen. Chem. 2018, 88, 780–788. [Google Scholar] [CrossRef]
- Sonyanaik, B.; Sakram, B.; Shyam, P.; Madhu, P.; Govan, M. Green Synthesis and Biological Evaluation of Novel Fused 6-(2-Chloro-4-fluorophenyl)-9-arylimidazo[1,2-a][1,8]naphthyridine Derivatives Catalyzed by DABCO. Russ. J. Gen. Chem. 2018, 88, 1495–1501. [Google Scholar] [CrossRef]
- Ašanin, D.P.; Bogojevic, S.S.; Perdih, F.; Andrejević, T.P.; Milivojevic, D.; Aleksic, I.; Nikodinovic-Runic, J.; Glišić, B.; Turel, I.; Djuran, M.I. Structural characterization, antimicrobial activity and BSA/DNA binding affinity of new silver(I) complexes with thianthrene and 1,8-naphthyridine. Molecules 2021, 26, 1871. [Google Scholar] [CrossRef] [PubMed]
- Pandya, K.M.; Battula, S.; Kumar, K.A.A.; Patel, R.J.; Patel, N.B. Design & synthesis of hybrid pharmacophore of β-lactam, 1,8-naphthyridine, and secondary amines; Discover their in vitro antimicrobial, anticancer properties & DFT and ADMET prediction studies. Med. Chem. Res. 2023, 32, 1098–1108. [Google Scholar] [CrossRef]
- Payne, D.J.; Miller, W.H.; Berry, V.; Brosky, J.; Burgess, W.J.; Chen, E.; DeWolf, W.E.; Fosberry, A.P.; Greenwood, R.; Head, M.S.; et al. Discovery of a novel and potent class of fabI-directed antibacterial agents. Antimicrob. Agents Chemother. 2002, 46, 3118–3124. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Iyer, G.; Doble, M. QSAR studies on substituted 3- or 4-phenyl-1,8-naphthyridine derivatives as antimicrobial agents. Med. Chem. Res. 2012, 21, 788–795. [Google Scholar] [CrossRef]
- Bhasker, G.V.; Satyanarayana, G.V.; Laxminarayana, E.; Latha, A.; Chary, M.T. Synthesis, Antibacterial Activity, and Docking Studies of Some New 2-Substituted-1,8-naphthyridine Derivatives. Indian J. Chem. 2018, 28, 227–242. [Google Scholar]
- Melcón-Fernandez, E.; Martín-Encinas, E.; Palacios, F.; Galli, G.; Reguera, R.M.; Martínez-Valladares, M.; Balaña-Fouce, R.; Alonso, C.; Pérez-Pertejo, Y. Antileishmanial Effect of 1,5- and 1,8-Substituted Fused Naphthyridines. Molecules 2024, 29, 74. [Google Scholar] [CrossRef] [PubMed]
- Elkanzi, N.A.A.; Hrichi, H.; Muqbil Alsirhani, A.; Bakr, R.B. Antioxidant and Antimicrobial Potential of 1,8-Naphthyridine Based Scaffolds: Design, Synthesis and in Silico Simulation Studies within Topoisomerase II. Chem. Biodivers. 2024, 21, e202301746. [Google Scholar] [CrossRef]
- Gohil, J.D.; Patel, H.B.; Patel, M.P. Synthesis and evaluation of new chromene based [1,8]naphthyridines derivatives as potential antimicrobial agents. RSC Adv. 2016, 6, 74726–74733. [Google Scholar] [CrossRef]
- Abbanat, D.; Webb, G.; Foleno, B.; Li, Y.; Macielag, M.; Montenegro, D.; Wira, E.; Bush, K. In vitro activities of novel 2-fluoro-naphthyridine-containing ketolides. Antimicrob. Agents Chemother. 2005, 49, 309–315. [Google Scholar] [CrossRef]
- Bodey, G.P.; Weaver, S.; Pan, T. PC-904, a New Semisynthetic Penicillin. Antimicrob. Agents Chemother. 1978, 13, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Parhi, A.K.; Zhang, Y.; Saionz, K.W.; Pradhan, P.; Kaul, M.; Trivedi, K.; Pilch, D.S.; Lavoie, E.J. Antibacterial activity of quinoxalines, quinazolines, and 1,5-naphthyridines. Bioorg. Med. Chem. Lett. 2013, 23, 4968–4974. [Google Scholar] [CrossRef] [PubMed]
- Surivet, J.P.; Zumbrunn, C.; Rueedi, G.; Hubschwerlen, C.; Bur, D.; Bruyère, T.; Locher, H.; Ritz, D.; Keck, W.; Seiler, P.; et al. Design, synthesis, and characterization of novel tetrahydropyran-based bacterial topoisomerase inhibitors with potent anti-gram-positive activity. J. Med. Chem. 2013, 56, 7396–7415. [Google Scholar] [CrossRef]
- Singh, S.B.; Kaelin, D.E.; Wu, J.; Miesel, L.; Tan, C.M.; Meinke, P.T.; Olsen, D.; Lagrutta, A.; Bradley, P.; Lu, J.; et al. Oxabicyclooctane-linked novel bacterial topoisomerase inhibitors as broad spectrum antibacterial agents. ACS Med. Chem. Lett. 2014, 5, 609–614. [Google Scholar] [CrossRef]
- Singh, S.B.; Kaelin, D.E.; Wu, J.; Miesel, L.; Tan, C.M.; Meinke, P.T.; Olsen, D.; Lagrutta, A.; Wei, C.; Liao, Y.; et al. C1-C2-linker substituted 1,5-naphthyridine analogues of oxabicyclooctane-linked NBTIs as broad-spectrum antibacterial agents (part 7). MedChemComm 2015, 6, 1773–1780. [Google Scholar] [CrossRef]
- Singh, S.B.; Kaelin, D.E.; Wu, J.; Miesel, L.; Tan, C.M.; Meinke, P.T.; Olsen, D.; Lagrutta, A.; Wei, C.; Peng, X.; et al. Structure activity relationship of substituted 1,5-naphthyridine analogs of oxabicyclooctane-linked novel bacterial topoisomerase inhibitors as broad-spectrum antibacterial agents (Part-4). Bioorg. Med. Chem. Lett. 2015, 25, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Okumu, A.A.; Nolan, S.; English, A.; Vibhute, S.; Lu, Y.; Hervert-Thomas, K.; Seffernick, J.T.; Azap, L.; Cole, S.L.; et al. 1,3-Dioxane-Linked Bacterial Topoisomerase Inhibitors with Enhanced Antibacterial Activity and Reduced hERG Inhibition. ACS Infect. Dis. 2019, 5, 1115–1128. [Google Scholar] [CrossRef]
- Auparakkitanon, S.; Chapoomram, S.; Kuaha, K.; Chirachariyavej, T.; Wilairat, P. Targeting of hematin by the antimalarial pyronaridine. Antimicrob. Agents Chemother. 2006, 50, 2197–2200. [Google Scholar] [CrossRef]
- Krishna, S.; Augustin, Y.; Wang, J.; Xu, C.; Staines, H.M.; Platteeuw, H.; Kamarulzaman, A.; Sall, A.; Kremsner, P. Repurposing Antimalarials to Tackle the COVID-19 Pandemic. Trends Parasitol. 2021, 37, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Đurić, S.; Vojnovic, S.; Pavic, A.; Mojicevic, M.; Wadepohl, H.; Savić, N.D.; Popsavin, M.; Nikodinovic-Runic, J.; Djuran, M.I.; Glišić, B. New polynuclear 1,5-naphthyridine-silver(I) complexes as potential antimicrobial agents: The key role of the nature of donor coordinated to the metal center. J. Inorg. Biochem. 2020, 203, 110872. [Google Scholar] [CrossRef] [PubMed]
- Kaigongi, M.M.; Lukhoba, C.W.; Yaouba, S.; Makunga, N.P.; Githiomi, J.; Yenesew, A. In Vitro Antimicrobial and Antiproliferative Activities of the Root Bark Extract and Isolated Chemical Constituents of Zanthoxylum Paracanthum Kokwaro (Rutaceae). Plants 2020, 9, 920. [Google Scholar] [CrossRef]
- Zhao, F.; Dai, J.-K.; Liu, D.; Wang, S.-J.; Wang, J.-R. Synthesis and Evaluation of Ester Derivatives of 10-Hydroxycanthin-6-One as Potential Antimicrobial Agents. Molecules 2016, 21, 390. [Google Scholar] [CrossRef]
- O’Donnell, G.; Gibbons, S. Antibacterial Activity of Two Canthin-6-One Alkaloids FromAllium Neapolitanum. Phyther. Res. 2007, 21, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.E.; Nakayama, H.; de Arias, A.R.; Schinini, A.; de Bilbao, N.V.; Serna, E.; Lagoutte, D.; Soriano-Agatón, F.; Poupon, E.; Hocquemiller, R.; et al. Effects of Canthin-6-One Alkaloids from Zanthoxylum Chiloperone on Trypanosoma Cruzi-Infected Mice. J. Ethnopharmacol. 2007, 109, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Bowling, J.J.; Pennaka, H.K.; Ivey, K.; Wahyuono, S.; Kelly, M.; Schinazi, R.F.; Valeriote, F.A.; Graves, D.E.; Hamann, M.T. Antiviral and Anticancer Optimization Studies of the DNA-Binding Marine Natural Product Aaptamine. Chem. Biol. Drug Des. 2008, 71, 205–215. [Google Scholar] [CrossRef]
- Amin, N.M.; Hamdin, M.S.; Amir, M.; Azarnudin, T.; Asari, A.; Nur, F.; Rashid, A.A.; Mariam, S.; Nor, M. Cytotoxicity effect of Aaptamine and its derivatives on Acanthamoeba castellanii (IMR isolate). Sci. Int. 2018, 30, 309–313. [Google Scholar]
- Ašanin, D.P.; Andrejević, T.P.; Nenadovic, M.; Rodić, M.; Vojnovic, S.; Djuran, M.I.; Glišić, B. Comparative study of antimicrobial potential and DNA/BSA binding affinity of silver(I) and gold(III) coordination compounds with 1,6-naphthyridine. Polyhedron 2023, 244, 116585. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; El-Gohary, N.M. Chemical behavior of 4,9-dimethoxy-5-oxo-5H-furo[3,2-g]chromene-6-carboxaldehyde towards carbon nucleophilic reagents. J. Heterocycl. Chem. 2020, 57, 2815–2830. [Google Scholar] [CrossRef]
- Wall, R.J.; Moniz, S.; Thomas, M.G.; Norval, S.; Ko, E.-J.; Marco, M.; Miles, T.J.; Gilbert, I.H.; Horn, D.; Fairlamb, A.H.; et al. Antitrypanosomal 8-Hydroxy-Naphthyridines Are Chelators of Divalent Transition Metals. Antimicrob. Agents Chemother. 2018, 62, e00235-18. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, A.; Tiwari, A.K.; Singh, S.; Sethi, A.; Tripathi, C.M.; Banerjee, B. Synthesis, characterization, and biological evaluation of novel thiazole and pyrazole derivatives of quinoline-4-carboxylic acid as potential antimicrobial agents. Med. Chem. Res. 2013, 22, 3527–3535. [Google Scholar] [CrossRef]
- Zhang, J.W.; Gao, J.M.; Xua, T.; Zhang, X.C.; Ma, Y.T.; Jarussophon, S.; Konishi, Y. Antifungal Activity of Alkaloids from the Seeds of Chimonanthus Praecox. Chem. Biodivers. 2009, 6, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Hossaini, Z.; Tabarsaei, N.; Khandan, S.; Valipour, P.; Ghorchibeigi, M. ZnO/Ag/Fe3O4 nanoparticles supported on carbon nanotubes employing Petasites hybridus rhizome water extract: A novel organometallic nanocatalyst for the synthesis of new naphthyridines. Appl. Organomet. Chem. 2021, 35, e6114. [Google Scholar] [CrossRef]
- Gangjee, A.; Shi, J.; Queener, S.F. Synthesis and Biological Activities of Conformationally Restricted, Tricyclic Nonclassical Antifolates as Inhibitors of Dihydrofolate Reductases 1. J. Med. Chem. 1997, 40, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- El-Dean, A.M.K.; Geies, A.A.; Hassanien, R.; Abdel-Wadood, F.K.; El-Naeem, E.E.A. Novel Synthesis, Reactions, and Biological Study of New Morpholino-Thieno[2,3-c][2,7]Naphthyridines as Anti-Cancer and Anti-Microbial Agents. Russ. J. Bioorg. Chem. 2022, 48, 821–834. [Google Scholar] [CrossRef]
- Hufford, C.D.; Liu, S.; Clark, A.M.; Oguntimein, B.O. Anticandidal activity of eupolauridine and onychine, alkaloids from Cleistopholis patens. J. Nat. Prod. 1987, 50, 961–964. [Google Scholar] [CrossRef]
- Khan, S.I.; Nimrod, A.C.; Mehrpooya, M.; Nitiss, J.L.; Walker, L.A.; Clark, A.M. Antifungal activity of eupolauridine and its action on DNA topoisomerases. Antimicrob. Agents Chemother. 2002, 46, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Taghavi-Moghadam, S.; Kwong, C.D.; Secrist, J.A.; Khan, S.I.; Clark, A.M. The synthesis and biological evaluation of alkyl and benzyl naphthyridinium analogs of eupolauridine as potential antimicrobial and cytotoxic agents. Bioorg. Med. Chem. 2016, 24, 6119–6130. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, B.S.; Barrows, L.R.; Copp, B.R. Structural Requirements for Biological Activity of the Marine Alkaloid Ascididemin. Bioorg. Med. Chem. Lett. 1995, 5, 739–742. [Google Scholar] [CrossRef]
- Appleton, D.R.; Pearce, A.N.; Copp, B.R. Anti-Tuberculosis Natural Products: Synthesis and Biological Evaluation of Pyridoacridine Alkaloids Related to Ascididemin. Tetrahedron 2010, 66, 4977–4986. [Google Scholar] [CrossRef]
- Kitahara, Y.; Onikura, H.; Shibano, Y.; Watanabe, S.; Mikami, Y.; Kuboa, A. Synthesis of Eupomatidines 1,2 and 3 and Related Compounds Including Iminoquinolinequinone Structure. Yoshiyasu 1997, 53, 6001–6010. [Google Scholar]
- Muhammad, I.; Dunbar, D.C.; Takamatsu, S.; Walker, L.A.; Clark, A.M. Antimalarial, Cytotoxic, and Antifungal Alkaloids from Duguetia Hadrantha. J. Nat. Prod. 2001, 64, 559–562. [Google Scholar] [CrossRef]
- Peterson, J.R.; Zjawiony, J.K.; Liu, S.; Hufford, C.D.; Clark, A.M.; Rogers, R.D. Copyrine Alkaloids: Synthesis, Spectroscopic Characterization, and Antimycotic/Antimycobacterial Activity of A- and B-Ring-Functionalized Sampangines. J. Med. Chem. 1992, 35, 4069–4077. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Oguntimein, B.; Hufford, C.D.; Clark, A.M. 3-Methoxysampangine, a Novel Antifungal Copyrine Alkaloid from Cleistopholis Patens. Antimicrob. Agents Chemother. 1990, 34, 529–533. [Google Scholar] [CrossRef]
- Charyulu, G.A.; McKee, T.C.; Ireland, C.M. Diplamine, a Cytotoxic Polyaromatic Alkaloid from the Tunicate Diplosoma sp. Tetrahedron Lett. 1989, 30, 4201–4202. [Google Scholar] [CrossRef]
- Appleton, D.R.; Pearce, A.N.; Lambert, G.; Babcock, R.C.; Copp, B.R. Isodiplamine, Cystodytin K and Lissoclinidine: Novel Bioactive Alkaloids from the New Zealand Ascidian Lissoclinum Notti. Tetrahedron 2002, 58, 9779–9783. [Google Scholar] [CrossRef]
- Bry, D.; Banaigs, B.; Long, C.; Bontemps, N. New Pyridoacridine Alkaloids from the Purple Morph of the Ascidian Cystodytes Dellechiajei. Tetrahedron Lett. 2011, 52, 3041–3044. [Google Scholar] [CrossRef]
- Carroll, A.R.; Ngo, A.; Quinn, R.J.; Redburn, J.; Hooper, J.N.A. Petrosamine B, an Inhibitor of the Helicobacter Pylori Enzyme Aspartyl Semialdehyde Dehydrogenase from the Australian Sponge Oceanapia sp. J. Nat. Prod. 2005, 68, 804–806. [Google Scholar] [CrossRef]
- Feng, Y.; Davis, R.A.; Sykes, M.L.; Avery, V.M.; Carroll, A.R.; Camp, D.; Quinn, R.J. Antitrypanosomal Pyridoacridine Alkaloids from the Australian Ascidian Polysyncraton Echinatum. Tetrahedron Lett. 2010, 51, 2477–2479. [Google Scholar] [CrossRef]
- Chabowska, G.; Barg, E.; Wójcicka, A. Biological activity of naturally derived naphthyridines. Molecules 2021, 26, 4324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).