Abstract
Breast and gynecologic cancers are significant global threats to women’s health and those living with the disease require lifelong physical, financial, and social support from their families, healthcare providers, and society as a whole. Cancer vaccines offer a promising means of inducing long-lasting immune response against the disease. Among various types of cancer vaccines available, peptide vaccines offer an effective strategy to elicit specific anti-tumor immune responses. Peptide vaccines have been developed based on tumor associated antigens (TAAs) and tumor specific neoantigens which can also be of viral origin. Molecular alterations in HER2 and non-HER2 genes are established to be involved in the pathogenesis of female-specific cancers and hence were exploited for the development of peptide vaccines against these diseases, most of which are in the latter stages of clinical trials. However, prophylactic vaccines for viral induced cancers, especially those against Human Papillomavirus (HPV) infection are well established. This review discusses therapeutic and prophylactic approaches for various types of female-specific cancers such as breast cancer and gynecologic cancers with special emphasis on peptide vaccines. We also present a pipeline for the design and evaluation of a multiepitope peptide vaccine that can be active against female-specific cancers.
1. Introduction
For many years, the gold standard in cancer treatment is conventional methods like radiation therapy, chemotherapy, and surgery [1,2]. Consistent scientific effort has resulted in the development of a number of alternative potential treatment strategies to circumvent the therapeutic limitations of the current conventional methods [2,3,4,5]. Cancer cells are unique in their ability to bypass the immune system for their survival [6]. Activating the immune system to recognize and tackle tumors is a potentially effective therapeutic strategy against cancer. Several immunotherapeutic modalities for cancer include monoclonal antibody therapy [7,8,9] immune checkpoint blockade (ICB) [10], chimeric antigen receptor (CAR) T cell therapy [11], oncolytic viral therapy [12,13] natural killer (NK) cell therapy [14] and cancer vaccines [13,15]. Even though various immunotherapeutic approaches are available, cancer vaccines offer a promising method of inducing long-term immune response against cancer [16]. Among the various cancer vaccines available, peptide vaccines offer a promising strategy to elicit specific anti-tumor immune responses. This review focuses on immunotherapy, with special reference to peptide vaccines as therapeutic and prophylactic agents for treating cancers in women.
2. Female-Specific Cancers
The influence of cancer and its outcome on women needs special attention since it impacts the economic, emotional and social well-being of an individual extending to the society. Moreover, the enormous global discrepancies in female cancer survival make female-specific cancers a major public health concern [17]. A brief summary of the various aspects of female- specific cancers is depicted in Table 1.

Table 1.
Various aspects of female-specific cancers.
3. Cancer Vaccines
Advances in bioengineering and material science have helped the development of different types of cancer vaccines which can arrest tumor progression and prevent recurrence [32]. An efficient cancer vaccine should ideally be able to reinforce the body’s natural defenses against cancer by eliciting potent CD4+ and CD8+ T effector and memory response [12]. Cancer vaccines come in a variety of forms which include cell-based vaccines/whole tumor cells [33,34], viral/bacterial-based vaccines [34,35,36], gene-based vaccines [13,34,37], and protein/peptide vaccines [34,38].
Cancer vaccines stimulate both cellular and humoral immune response by utilizing tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs), thus preventing tumor growth and killing tumor cells [39]. Traditional vaccine preparations that target TAAs are having low immunogenic capacity and risk of toxicity to normal cells [40]. TSAs or neoantigens, on the other hand, are only expressed by cancer cells and elicit strong immune responses because of the lack of immunological tolerance [41]. TSAs are highly specific and are used in the production and design of personalized vaccines [40]. The possibilities of various approaches in cancer vaccines with potential against female-specific cancers are summarized in Figure 1.
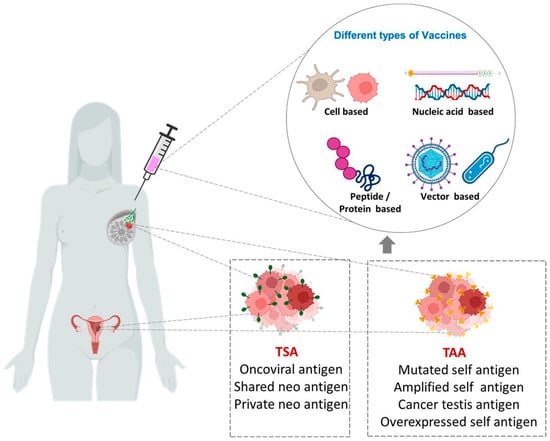
Figure 1.
Different types of vaccines against female-specific cancers. TSAs (Tumor specific antigens) and TAAs (Tumor associated antigens) are exploited for the development of various types of vaccines such as Cell-based, Nucleic acid-based, Vector-based and Peptide/Protein-based vaccines.
Vaccines based on peptides have an advantage over other forms of therapy. Metastatic cancers that have spread to various parts of the body can be treated with peptide vaccines, and they are nontoxic compared to other treatment strategies [42]. Unlike other immunotherapeutic techniques like CAR T cell therapy which targets a cell surface antigen, peptide vaccines are using multiple epitopes positioned outside or inside of tumor cells [43]. By developing a peptide vaccine devoid of B cell epitopes, the risk of hypersensitivity could be avoided, and highly heterogeneous tumors could be effectively targeted by peptide-based vaccines [44]. Though the role of B cell epitopes in cancer vaccine design is underappreciated, recent investigations reveal the significance of multiepitope vaccines encompassing B cell epitopes along with T helper and cytotoxic T lymphocyte (CTL) epitopes in prophylaxis and therapy of cancer [45].
4. Peptide Vaccines
Peptide vaccines are composed of synthetic peptides which are highly immunogenic and elicit desired and specific adaptive immune response [46]. They come in a variety of forms, including multivalent long peptide vaccines, multi-peptide vaccines containing CTL and T helper-epitopes, peptide cocktail vaccines, hybrid peptide vaccines, personalized peptide vaccines, and peptide-pulsed dendritic cell vaccines [47]. The efficacy of peptide vaccines are widely studied against neurodegenerative diseases [48,49], infectious diseases [50] like human immunodeficiency virus (HIV) [51,52], hepatitis C virus [53], tuberculosis [54], foot and mouth disease [55], cancer [6], etc. The general aspects of peptide vaccines in the context of cancer therapy are summarized in Figure 2.
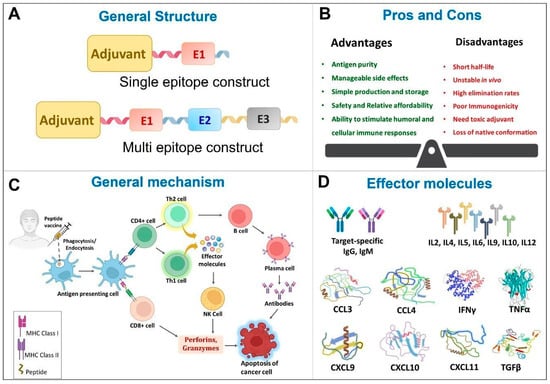
Figure 2.
General features of Peptide vaccines. CCL—Chemokine (C-C motif) ligand; CXCL—Chemokine (C-X-C motif) ligand; E1—Epitope 1, E2—Epitope 2, E3—Epitope 3, IL—Interleukin; IFN—Interferon; NK—Natural killer; TGF—Tumor growth factor; Th—helper T cells; TNF—Tumor necrosis factor.
The differential expression of TAAs and TSAs on normal cells and cancer cells are made use of in designing peptide-based cancer vaccines [6,42,56]. Synthetic long peptides (SLPs) consist of 25–35 amino acids that are derived from TAAs or TSAs from a major type of peptide-based cancer vaccines [57,58]. Cancer vaccination trials with SLPs demonstrated inhibition of growth of transplanted tumors in mice [59]. Survivin-based vaccine, composed of a pool of three SLPs with eight CD4+ epitopes and six CD8+ epitopes, has shown to activate both CD4+ and CD8+ immune responses in mouse models for colorectal cancer. Fusion proteins made by combining Xcl1 with Ovalbumin SLP antigen and IgG1 Fc fragment were shown to elicit specific T cell response and sustained tumor control against the poorly immunogenic B16-OVA melanoma tumor [60].
Short peptides composed of 8–10 amino acids utilize Class I major histocompatibility complex (MHC) receptors and initiate CD8+ T cell response. Cancer neoepitope vaccine, based on MHC1 restricted short peptide, Nes2LR was reported to induce functional CD8+ T cell responses and prevent tumor growth in murine renal carcinoma model [61]
Recombinant overlapping peptides (ROPs) developed as a design strategy for peptide vaccines consist of a single-chain polypeptide with multiple epitopes. They can produce strong immunogenic responses in CD4+ and CD8+ T cells [62]. Immunoinformatics approaches were utilized to construct a multi-epitope peptide vaccine against breast cancer using immunogenic regions of the BORIS cancer-testis antigen containing multiple CTL epitopes. The selected regions were linked together by GPGPG linker followed by incorporation of T helper epitopes and the toll-like receptor (TLR)-4/MD-2 agonist. The resulting vaccine was reverse translated and then inserted into pcDNA3.1 to form the DNA vaccine [63]. Further investigations were carried out which revealed that co immunization of the multiepitope peptide vaccine and the resultant DNA vaccine significantly inhibited the growth of breast tumors, decreased tumor weight, inhibited metastasis, and enhanced survival time in murine mammary carcinoma [64].
5. Peptide Vaccines Developed for Female-Specific Cancers
5.1. Based on Genes Involved in Pathogenesis of Cancer
Accumulation of genetic and epigenetic alterations is well established to be involved in the process of carcinogenesis. The molecular alterations involved in pathogenesis of cancer include gene amplification, gene fusion, mutation and overexpression. Multiple studies have investigated the association of breast and gynecologic malignancies with overexpression and/or amplification of HER2 [65,66,67,68,69,70,71,72,73], as well as non-HER2 genes such as BRCA1/BRCA2, CHEK2, PTEN, MUCI, Tp53, MAGE, etc. [23,74,75,76,77,78,79,80]. The alterations identified in these genes are used successfully to design peptide vaccines with therapeutic efficiency against female-specific cancers.
5.1.1. HER2 Based Peptide Vaccines for Female-Specific Cancers
HER2 is a receptor tyrosine kinase which is found to be involved in cell proliferation and survival [72]. Several HER2 derived peptide vaccines have been designed and are in the latter stages of clinical trials. B cell or T cell peptide-based vaccines, liposome-based vaccines with B cell peptides, and mature dendritic cells (DCs) loaded with TAA/TSA are a few of the diverse approaches employed [81].
HER2, a well-studied TAA is validated as a therapeutic target in breast cancer for the development of therapeutic vaccines. The present status of development of vaccines for breast cancer based on HER2 is summarized in Table 2.

Table 2.
HER2 based peptide vaccines for breast cancer HER2/neu-derived GP2 and GP2–P4.
Systematic review and meta-analysis were carried out to investigate the outcomes of HER2 based peptide vaccines in breast cancer. Both Chamani et al. [91] and You et al. [92] have reported that E75 vaccine is effective and safe in breast cancer while You et al. reported that GP2 vaccine elicited a strong immune response [92].
Overexpression, gene amplification and gene mutation of HER2 has been found to occur in patients with gynecologic malignancies with possible therapeutic implications [93]. In one of the earlier studies, intradermal immunization with a peptide vaccine based on HER2/neu combined with granulocyte macrophage colony stimulating factor (GM-CSF) as an adjuvant induced CD4+ T helper-specific immunological response in patients with breast and ovarian cancer. Patients produced HER2 specific T cell responses which could migrate out of the peripheral circulation [94].
Autologous DCs pulsed with HER2/neu or MUC1-derived peptides can effectively induce antigen-specific T cells in patients with advanced breast and ovarian cancer. The immunizations were shown to be well tolerated with no side effects in a pilot study involving 10 participants. MUC1 peptide specific T cells were found in patients vaccinated with HER2/new-derived peptides [95]. The uptake of tumor cells by DCs that are involved in cross-priming [96] and induction of other tumor antigen-specific CTLs could be the possible mechanism for this observation.
HER2/neu-specific antibody immunity was assessed in 35 patients with breast and ovarian cancer after immunization with HER2 based-peptides and successful immune response was recorded in majority of the patients. Moreover, epitope spreading to p53 was observed in 20% of the vaccinated patients [82].
Monthly vaccination of 6 breast/ovarian cancer patients having HER2/neu-overexpressing tumors with HER2/neu-derived HLA-A2-peptide and GM-CSF as adjuvant, for six months was found to induce interferon-gamma (IFN-γ) secreting CD8+ T lymphocytes targeting HER2/neu. The minimal and transient nature of immune responses necessitated the need for CD4+ T cell support to maintain immunization [97].
In a Phase 1 study, 9 participants with epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were administered with 5 class I MHC-restricted synthetic peptides derived from multiple ovarian cancer-associated proteins, as well as a class II MHC-restricted synthetic helper peptide derived from tetanus toxoid protein. All of the peptides used were immunogenic, including HER2/neu 754–762 peptide which stimulated CD8+ T cell responses. Authors suggested that the low potency of immunogenicity in ovarian cancers requires additional immunomodulation [98].
5.1.2. Non-HER2 Based Peptide Vaccines for Female-Specific Cancers
Genes other than HER2 are also established to have a role in the pathogenesis of breast and gynecologic cancers. Mutations in MSH6, CHEK2, BRCA1, BRCA2, ATM, PMS2, PALB2, and MSH2 were found to occur more frequently than in any other gene in the analysis of breast and uterine cancer patients. The frequency of BRCA1, MLH1, MSH2, MSH6, PMS2, and PTEN mutations was higher in breast and uterine cancer than in breast cancer, whereas the frequency of ATM mutations was higher in breast and uterine cancer than in uterine cancer alone [99]. In 90% of mucinous ovarian carcinomas, KRAS, BRAF, and/or ERRB2 gene amplifications are present, demonstrating the therapeutic potency of RAS/MEK pathway in this subtype [100]. Table 3 describes the state-of-the-art progress in the development of peptide vaccines designed against non-HER2 genes for female-specific cancers.

Table 3.
Non-HER2 based peptide vaccines for female-specific cancers.
5.2. Based on Viruses Involved in Pathogenesis of Female-Specific Cancers
Viruses are known to interact with host factors creating a tumor microenvironment (TME) that facilitates tumorigenesis [121]. Viruses are a possible cause of 15% of all human cancers, which is a sizable proportion of the worldwide cancer burden [122]. Human papilloma virus (HPV), Epstein-Barr virus (EBV), Mouse mammary tumor virus (MMTV) and Bovine leukemia virus (BLV) are known to be involved in the pathogenesis of female-specific cancers including breast and gynecologic cancers [123].
5.2.1. Human Papilloma Virus (HPV)
HPV is a double-stranded, circular DNA [124] and the most prevalent sexually transmitted virus. High-risk HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59) are the leading causes of genital tract cancers, including cervical, vulvar, vaginal, penile, and anal cancers, and a subset of head and neck cancers [125]. Subtypes 16, 18, and 33 are associated with 29% of breast cancer. [126] Two viral proteins, E6 and E7, are critical in initiating oncogenesis in infected cells, resulting in unregulated proliferation, unrestrained telomerase activity, and ultimately, cervical cancer progression [127]. HPV oncoproteins E6 can inactivate tumor suppressor protein p53 and E7 can inactivate pRb [128] which leads to the development of cancer.
The discovery of the etiologic involvement of HPV in the development and progression of cancers, mainly cervical cancer, has led to intensive research on prophylactic strategies. Currently available prophylactic vaccines exploit the ability of HPV capsid protein L1 to form virus-like particles (VLP) which are similar to native virions [129]. They induce the production of neutralizing antibodies that bind to viral particles and block their entry into host cells and effectively prevent HPV infections [130]. VLPs lack a viral genome and are neither infectious nor carcinogenic. In addition, they can provoke a robust humoral immune response with high and persistent neutralizing antibodies [131].
Three prophylactic peptide vaccines are commercially available against HPV infection, all of them based on L1 VLP. They are Gardasil®4, a quadrivalent vaccine [132], Cervarix™, a bivalent vaccine [133] and Gardasil®9, a nonavalent vaccine [133]. These prophylactic vaccines induce strong immune responses and produce high titer of antibodies [134]. HPV prophylactic vaccine is an ineffective treatment for an already infected person [135], but they can protect up to 100% of females between the ages of 9 and 26 from cervical cancer caused by HPV [136]. Table 4 enlists the currently available prophylactic peptide vaccines that could offer protection against HPV infection.

Table 4.
HPV prophylactic peptide vaccines.
The clinical success of therapeutic cancer vaccines is very low due to the immunosuppressive nature of the TME. So, administration of cancer vaccine as a prophylactic measure in individuals at high risk of cancer or premalignant conditions will enhance the clinical efficacy [152].
Therapeutic vaccines for HPV are of four major categories such as live vector-based vaccines, peptide and protein-based vaccines, nucleic acid-based vaccines and whole cell vaccines [153]. peptide-based antigens are being extensively explored in the design of therapeutic vaccines against HPV infection.
In one of the initial studies, the impact of HPV16 E6 and E7 SLP vaccination on antigen-specific T cell response in cervical cancer patients was studied by Welters M J P et al. Patients were vaccinated with overlapping long peptides emulsified in Montanide ISA-51. Both CD4+ and CD8+ T cell responses to HPV16 E6 and E7 were observed [154].
In another study, women with HPV16 positive grade 3 vulvar intraepithelial neoplasia vaccinated with long peptides from the HPV16 viral oncoproteins E6 and E7 in incomplete Freund’s adjuvant showed strong interferon-γ–associated proliferative CD4+ T cell response and a broad response of CD8+ interferon-γ T cells. Positive outcomes appear to be linked to the activation of HPV16 specific immunity [155]. Studies indicated that mHSP110, a chaperone immunoadjuvant, enhanced the immune response to peptide vaccine based on HPV16 oncoprotein E7 derived CTL epitope E7 (49–57), inhibited tumor growth and prolonged survival time in mouse models for cervical cancer [156].
Cornelis et al. have reported that 20 women with high-grade vulvar intraepithelial neoplasia on receiving a synthetic peptide vaccine composed of 13 overlapping peptides with incomplete Freund adjuvant (mineral oil-based, Montanide ISA-51). In 9 individuals, the long-peptide vaccine completely regressed all lesions and eradicated HPV16. Clinical response was strongly linked with vaccine-induced T cell response [157,158].
A single administration of HPV vaccine having CpG oligodeoxynucleotides as an adjuvant and HPV16 E7 43–77 peptide as antigen was reported to elicit prophylactic and therapeutic effects on cervical cancer in mice models. Injection of vaccine increased cellular immunity mediated by CD4+ IFN-γ+ T cells and CD8+ IFN-γ+ T cells. Vaccine administration decreased numbers of immunosuppressive cells including regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) [159]. Further studies were carried out with modified formulation of the vaccine in TC-1 grafted tumor. Subcutaneous injection of mannose-modified DCs-targeting liposomes loaded with HPV16 E7 peptide and CpG ODN vaccine stimulated powerful E7 specific CTL response and elevated the percentage of CD4+ T cells, CD8+ T cells and IFN-γ producing cells. Expression of IL-12, IFN-γ, TNF-α, and IL-2 were significantly increased, while those of IL-4 and TGF-β significantly decreased [160].
HPV16 E6/E7 synthetic overlapping long peptide vaccine was investigated for its therapeutic effect in high-grade cervical squamous intraepithelial lesions and was found to increase HPV16 specific T cell immunity which lasted up to one year [161] HPV16 SLP vaccination combined with Carboplatin and Paclitaxel chemotherapy was found to induce robust T cell response in a mouse tumor model and in patients with advanced cervical cancer [162]. Tri-therapy where HPV16 E7 SLP was administered, combined with Carboplatin/Paclitaxel followed by TLR9 agonist CpG resulted in regression of genital HPV16 tumors [163]. In 77 patients with advanced, recurrent, or metastatic cervical cancer undergoing Carboplatin/Paclitaxel chemotherapy, administration of ISA101, an SLP vaccine containing HPV E6/E7 showed type 1 T cell response and prolonged survival [164]. In a Phase I/II clinical study, immune responses of ISA101 vaccine with or without Polyethylene Glycol (PEG)ylated IFN-α as combination therapy with Carboplatin and Paclitaxel were evaluated. Enhanced tumor-specific immunity was observed and addition of PEGylated IFN-α enhanced the immune response [165].
A therapeutic vaccine for HPV called Pepcan (HPV16 E6 peptides combined with Candida skin testing reagent called Candin) was administered intradermally in 31 patients with high-grade squamous intraepithelial lesions and was reported to decrease the viral load in nine of the patients [166]. The Phase II clinical trial of Pepcan to the two treatment arms- Pepcan and Candin, in 99 patients with cervical high grade squamous intra-epitheial lesions was recently completed [167].
A bivalent therapeutic vaccine against HPV16/18 genotypes composed of a fusion protein containing the extra domain A of human fibronectin and HPV16/18 E7 viral antigens was developed by Arribillaga L et al. The vaccine induced E7-specific CTL response and eradicated pre-existing tumors [168]. Promising results have arrived from a Phase I clinical trial using the novel therapeutic vaccine, Hespecta (HPV E6 Peptide conjugated to Amplivant®) which showed a T cell specific immune response [169].
HPV16 E5 has also been proven to be a promising target for cervical cancer therapy. Administration of E5 peptide-based on epitopes predicted by immunoinformatics in combination with CpG has induced strong cell-mediated immunity, decreased tumor volume and increased survival time in mice models [170].
Administration of different immunodominant epitopes of HPV in combination was found to elicit increased immune response. Studies report induction of Th1 immune response and high Granzyme B secretion which indicates CTL activity in mice receiving E7 and E5 peptides together when compared to those receiving the peptides individually [171].
A multiepitope vaccine consisting of linked segments of E5, E6 and E7 peptides (E765m) was developed and inserted into the major immune dominant region (MIR) of hepatitis B virus core antigen (HBc) to form HBc-E765m chimeric virus-like particles (cVLPs). E5-TC-1 tumor-bearing mice immunized with cVLPs elicited high E5-, E6- and E7- specific CTLs, IgG antibody responses and increased levels of IFN-γ, IL-4 and IL-5. Tumor growth was also suppressed, which indicated that the novel vaccine provides a promising platform for immunotherapy in HPV16-associated cervical intraepithelial neoplasia [172]. An SLP vaccine containing HPV16 E7 antigen in combination with TLR9 agonist CpG formulated in an oil-in-water emulsion was found to inhibit tumor growth and induce robust CD8+ T cell response in TC-1 murine model [173].
Strategies using nanoparticles have been employed for the design of therapeutic peptide vaccines against HPV infection. Tat-E7/pGM-CSF nanoparticles are a promising new strategy for boosting the efficacy of peptide-based cervical cancer vaccinations. The HIV-1 Tat cell-penetrating peptide was fused with the HPV16 E7 CTL epitope and GM-CSF. In prophylactic and therapeutic mouse models, the vaccination resulted in lower tumor growth and improved long-term survival and higher frequency of CD8+ memory T cells [174]. In another study, Rahimian et al. used a double emulsion solvent evaporation technique to create polymeric nanoparticles (NPs) based on hydrophilic polyester loaded with an SLP derived from HPV16 E7 oncoprotein and a TLR3 ligand. There was a substantial increase in HPV specific CD8+ T cells when the HPV SLP antigen encapsulated in nanoparticles was administered. These biodegradable polymeric nanoparticles are an efficient alternative for adjuvant in cancer vaccinations since they cause no adverse reactions on administration [175].
A therapeutic HPV nanovaccine candidate was created by Zhang et al. using poly [d, l-lactic-co-glycolic acid] (PLGA), to encapsulate HPV-16 E744–62. Adenosine triphosphate (ATP) was added to the design as a novel adjuvant element. The PLGA encapsulation improved antigen presentation to antigen presenting cells (APCs), triggered the immunological response, ATP induced DC maturation, and improved antigen recognition and uptake by DCs [176]. Another novel liposomal nanoparticle based therapeutic peptide vaccine PDS0101 composed of cationic lipid R-DOTAP and 6 HPV16 E6/E7 peptides was developed. The ongoing clinical trial is evaluating the efficacy of this multipeptide vaccine when used in conjunction with chemotherapy and radiation therapy in 35 patients with stage IB3-IVA cervical cancer [177]. Future directions for HPV therapeutic vaccine development include the production of new potent adjuvants, novel antigen targets, and an enrichment of preclinical models.
5.2.2. Mouse Mammary Tumor Virus (MMTV)
MMTV is a beta retrovirus that causes mammary cancers in both wild and laboratory mice [178] and has also been identified in 40% of human breast cancers [179]. It has been proposed earlier that the zoonotic transmission of MMTV from the mice, Mus musculus domesticus could account for the geographic differences in breast cancer incidence [180]. Stewart et al. has recently reported evidence for correlation of spikes in breast cancer incidence in Australia and New Zealand with mouse population outbreaks [181].
Ever since the involvement of MMTV with breast cancer was identified, investigations have been carried out to recognize TAA that can serve as potential vaccine targets. In one study using TgMMTV-neu mouse, three early-stage tumor antigens (PDHX, STK39, and OTUD6B) were identified by serological analysis of cDNA expression libraries (SEREX) screen that could serve as superior antigen targets for the inhibition of tumor growth [182]. MMTV-p14, the signal peptide of the MMTV envelope precursor, was found to be expressed on breast cancer cells. Protective vaccination using p14 with alum as an adjuvant revealed enhanced immune response which demonstrates p14 as a target for prophylactic vaccination in MMTV associated cancers [183]. Earlier, the feasibility of using MMTV-p14 for vaccination was demonstrated in Balb/c mice that harbor MMTV [184].
Proteins gp36 and gp52 which are part of the MMTV envelope were reported to be present in primary cultures of human breast cancer [185]. Protective efficacies of vaccines consisting of synthetic peptides based upon the primary sequence of gp52 were studied in mice models. Vaccinating Balb/c mice with surface accessible peptide region EP-3 of major viral envelope glycoprotein (gp52) of C3H-MuMTV was found to result in significant decrease in frequency of early onset tumors [186].
5.2.3. Epstein-Barr Virus (EBV)
EBV is a DNA virus that belongs to the gamma Herpesviridae family [187]. The presence of the EBV genome was identified in a large subset of breast cancers by polymerase chain reaction (PCR), Southern blot analysis and immunohistochemical detection of Epstein-Barr nuclear antigen 2 (EBNA-2) [188]. Activation of HER2/HER3 signaling cascade is known to be involved in the malignant transformation induced by EBV [189].
Statistical association of EBV infection with increased breast carcinoma was demonstrated by meta-analysis [190]. Breast tumors showed viral products like EBNA-1, BZLF1, BARF-1, BARF-0, BXLF-2 and BFRF-3 [187]. Epidemiological studies suggest that EBV increases the risk for breast cancer and this association is stronger in Asian countries than in European countries, though EBV infection is not involved in the progression of breast cancer. Also, there is an association between EBV and breast cancer in areas where nasopharyngeal carcinoma is endemic [191].
Studies conducted by Li W et al. on the immune response of mice to EBV latent membrane protein 2 (LMP2) multi-epitope antigen demonstrated that priming with DNA vaccine and boosting with peptide vaccine elicited a robust humoral immune response and efficient CTL activity [192]. In vivo studies have reported that LMP-1 vaccines suppress LMP-1 expressing tumor growth and metastasis in nasopharyngeal carcinoma animal models [193]. Studies also suggest a correlation between expression of EBV LMP-1 and aggressive ER-negative breast cancer [194]. This opens a possible avenue for the development of LMP-1-based peptide vaccine as a therapeutic strategy against breast cancer.
The use of immunoinformatics approaches has resulted in the prediction of potential T cell and B cell epitopes for nine antigenic EBV proteins. The integrative meta-analytical approach could model these epitopes as effective candidates for peptide vaccine development towards the treatment of EBV associated cancers [195]. A computational meta-analysis integrated with dynamics could predict a panel of epitopes including B cell epitopes and cytotoxic T cell epitopes. These peptides were then docked against the MHC molecules and the selected peptides were subjected to molecular dynamics simulation and stability analysis. The validated peptides are suggested to aid in the development of vaccines that could be effective against multiple diseases caused by EBV [196]. A multiepitope based polyvalent vaccine against EBV associated tumors was developed using immunoinformatics approach. Molecular docking of the vaccine construct against TLRs revealed that it could elicit humoral and cellular immune responses [197].
5.2.4. Bovine Leukemia Virus (BLV)
BLV is a delta retrovirus that most closely resembles human T cell lymphotropic virus 1 (HTLV-1) [198]. Association of breast cancer with exposure to BLV has been reported by Buehring G C et al. [199]. Literature search reveals a few investigations that have been carried out on development of peptide vaccines that could be effective against BLV associated tumors.
In one of the initial studies, Kabeya H et al. had reported that recombinant baculovirus (rgp51) and synthetic multiple antigenic peptides (MAP) of T helper, T cytotoxic, and B cell epitopes of BLV gp51 protected sheep from BLV [200].
Inoculation of mannan-coated liposome encapsulating 20-mer synthetic peptide of BLV envelope glycoprotein gp51 in BALB/c mice induced specific delayed-type hypersensitivity, lymphocyte proliferative responses, and weak cytotoxic lymphocyte response [201]. Glycoprotein gp51-peptide epitope covalently linked to a mutant bacteriophage carrier (mQβ) using two different linker strategies, isothiocyanate and dinitrophenyl adipate were reported to elicit long-lasting neutralizing antibodies in mice [202].
A prophylactic multi-epitope vaccine against BLV was computationally developed for breast cancer. The vaccine construct consisted of five antigenic CTL and four helper T lymphocyte (HTL) epitopes linked by AAY and GPGPG, respectively. β-defensin (TLR3 agonist) was added as an adjuvant using EAAAK linker. Immune simulation study confirmed that the designed vaccine could produce a higher response exhibited by helper T and cytotoxic T cell during vaccination. Also, NK and DCs demonstrated elevated macrophage activity [203].
An in silico approach was used to predict the reliable B and T helper cell epitopes of BLV that can be used for vaccine design. Immunogenic regions of linear and conformational epitopes were selected and the tertiary structure of the final epitope was modeled. The structures of both conformational epitopes were the same as that of the whole extracellular part of gp60 SU (surface glycoprotein of BLV, the major target for the host immunity against the virus) [204].
6. A Pipeline for the Design and Evaluation of Peptide Vaccine in Female-Specific Cancers
One of the initial steps in the design of a peptide vaccine is the identification of appropriate epitopes with immunogenicity. Immunoinformatics approaches can be effectively used for the prediction of epitopes in vaccine research. The multi-epitope vaccine construct is an acceptable choice for future research [205]. Epitopes can also be designed based on the sequence of TAA or TSA that are encoded by mutated cancer genes [42,206,207]. In the case of viral induced cancers, the viral antigens can serve as a guide for epitope-based vaccine design [208,209,210]. After an epitope is predicted, a multi-epitope vaccine can be designed. Then the allergenicity, antigenicity and physicochemical properties of the vaccine construct is analyzed [211]. It is followed by preclinical or in vitro studies in cell lines and/or in animal models/humanized animal models and various phases of clinical trials before the successful development of a commercial vaccine. A flow chart illustrating a model pipeline for the design and evaluation of peptide vaccine in female-specific cancers is shown in Figure 3.
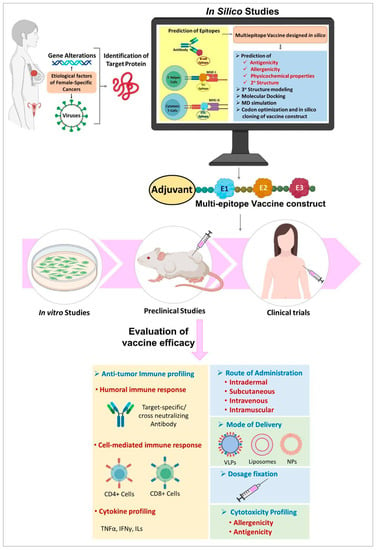
Figure 3.
Study design for development of peptide vaccine. MD—Molecular dynamics, NP—Nanoparticle, VLP—Virus like particles.
7. Delivery Systems for Peptide Vaccines
When used alone as vaccines, peptides do not elicit robust in vivo immune responses due to their rapid degradation at the injection site, lack of costimulatory effects and immune signals essential for APC activation [42]. In early phase clinical trials of peptide-based vaccines, overlapping long peptides were used along with adjuvants to boost immune response. For example, a vaccine made by combining HPV E6 and E7 peptides in Montanide ISA 51 adjuvant was well tolerated and induced the development of IFN-related T cell response in advanced cervical cancer patients [212]. So adjuvants and/or delivery systems are required to induce a satisfactory immune response and also protect the antigen from degradation and deliver it to the targeted cells. Delivery systems are self-adjuvating and they aid in the delivery of peptides to APCs to generate optimum T cell responses. Poly lactic-co-glycolic acid (PLGA) [213] and liposomes [214] are two drug delivery methods that have been studied for many years and have proven safety and efficacy for the treatment of cancer. Nanoparticles are an effective antigen presentation and delivery system for stimulating an optimal immune response [215]. The physical features of nanoparticles, such as size, shape, and surface characteristics can be easily modified to induce immunological responses against the associated antigen. Vaccines have been delivered via polymeric nanoparticles such as nanogels [216], dendrimers [216], hydrogels [217], and micelles [218] that have been conjugated with immune stimulants. Conjugation of peptides to inorganic nanoparticles like gold nanoparticles increases the stability and reproducibility of the conjugate [219]. A recent study by Firdaus et al. shows that a completely specified, natural, hydrophobic amino-acid-based polymer (Polyleucine) conjugated to peptide antigen works well for vaccine delivery mechanism [220]. VLPs can efficiently act as vehicles of antigens to APCs, where they are cross-presented in association with both MHC class I and class II molecules, eliciting both humoral and cellular immune responses [221]. Recently, adenovirus-inspired non-infectious VLP was shown to stimulate anti-tumor immune response in mouse modes of melanoma [222] present shortcomings of peptide vaccines necessitate additional research on more effective adjuvants, routes of administration, and novel delivery systems.
8. Limitations and Adverse Effects of Peptide Vaccines
Peptide vaccines provide a number of benefits, including simplicity of synthesis, low production costs, adaptability to antigens, and high specificity. However, they also have a number of drawbacks, including MHC constraints, poor immunogenic potency, and the necessity for an adjuvant. Even though stability and immunogenicity of peptide vaccines can be improved by conjugating them with adjuvants, the unwanted immune response elicited by the adjuvant is a challenge. The extent and diversity of MHC alleles in various populations and races also pose a barrier that a specific peptide may not induce much cell-mediated immunity in individuals with diverse MHC class I molecules. Since the vaccine must match the HLA in patients, a peptide vaccine for the entire human population cannot be designed due to the presence of HLA polymorphisms [223]. Even though peptides present on MHC-II molecules that are recognized by helper T cells could considerably improve efficacy, it is extremely difficult to predict the immunogenicity of MHC-II-restricted peptides due to their greater diversity and complexity than MHC-I-restricted peptides [224]. Short peptides may induce T cell tolerance since they can directly bind to MHC on non-professional APCs [225]. Also, the constrained conformation of short peptides will prevent them from folding into the three-dimensional structure that is required for proper immunogenicity [226,227]. Although algorithms for predicting T cell epitopes are widely employed, their accuracy and sensitivity are somewhat limited due to the fact that the spatial configuration of T cell epitopes changes when antigens bind to cell surface receptors. Consequently, false-positive and false-negative immune responses are possible [228]. Tumor-specific CD4+ T cell responses often target self-derived epitopes. This will hinder the immune system from realizing its entire potential in fighting against cancer, presenting another important challenge in peptide vaccine development [229].
9. Future Prospects
The increasing incidence of female-specific cancers across the globe remains a great challenge that needs to be addressed with therapeutic and prophylactic approaches. In the case of viral-induced female-specific cancers, the development of novel prophylactic vaccines is all the more important. Even though vaccinations against HPV are widely used, prophylactic strategies against retroviruses causing female-specific cancers, such as MMTV and BLV are not even in the nascent stages of development. The major concern regarding retroviruses is that they stably integrate into the host genome, enter long-term latency in some cells, and evade immune response making vaccination difficult [230].
Peptide-based vaccination approaches have several advantages over other forms of therapies in eliciting appropriate anti-tumor immune responses. This should be addressed in conjunction with the fact that the anticancer immune response of peptide vaccines can be attenuated by factors such as the complexity, continuous evolution of the TME and the influence of neoantigen-specific T cell immunity [231]. Also, due to their rapid degradation at the injection site, lack of costimulation, and lack of signals needed for APC activation, peptide vaccines may not induce robust immune reactions in vivo [232]. Thus more studies are warranted on the development of potent adjuvants or immunostimulators and efficient delivery systems that are capable of producing effective T cell responses.
The identification of optimal antigen targets, streamlining immunization regimens and exploring novel biomarkers that could predict the efficacy of vaccine response are all major domains that deserve additional attention in the near future [42]. Empirical research on the design and effectiveness of combining peptide-based cancer vaccination with other forms of existing therapy is also the need of the hour.
The concept of multi-epitope-based peptide vaccines is quite interesting for both therapeutic and prophylactic purposes. Advances in artificial intelligence should be effectively exploited for the development of new algorithms for the prediction of peptide-binding epitopes which would aid in the design of neoantigen-based cancer vaccines. Personalized peptide-based cancer vaccines, emerging as a promising strategy for eliciting a diversified antitumor immune response that is appropriate and useful to individual cancer patients, which is currently an expensive and time-consuming affair, also need more attention.
Author Contributions
Conceptualization and design of manuscript, M.M., Writing-Original draft preparation, M.M., M.L. and C.R.D., Preparation of Figures, C.R.D., Preparation of Tables, M.L. and J.S.S., Project Administration, J.A.S., Validation and formal analysis, J.J.V., Data curation, J.T.V., Visualization and supervision, L.A., Writing-Review and Editing, V.S.L., Resources and literature survey, A.M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| AAHS | Amorphous aluminum hydroxyphosphate sulfate |
| AE37, AE36, E75 and GP2 | Immunogenic peptides from the HER2/neu |
| APC | Antigen presenting cells |
| B16-OVA | B16 Ovalbumin |
| BLV | Bovine leukemia virus |
| BORIS | Brother of Regulator of Imprinted Sites |
| BRCA1/BRCA2 | Breast Cancer gene |
| CA-125 | Cancer antigen 125 |
| CAR T cell | Chimeric antigen receptor T cell |
| CCL-Chemokine | (C-C motif) ligand |
| CEA | Carcinoembryonic antigen |
| CHEK2 | Checkpoint kinase 2 |
| CIN | Cervical squamous intraepithelial neoplasia |
| CpG-ODN | Oligodeoxynucleotides of cytosine and guanine |
| CRP | C-reactive protein |
| CTL | Cytotoxic T lymphocyte |
| CTLA-4 | Cytotoxic T lymphocyte-associated antigen-4 |
| cVLPs | Chimeric virus-like particles |
| CXCL | Chemokine (C-X-C motif) ligand |
| DCs | Dendritic cells |
| dLOS (CIA06) | A novel proprietary immune adjuvant |
| DTH | Delayed type hypersensitivity |
| E1, E2, E3 | Epitope 1, 2, 3 |
| EBNA-2 | Epstein-Barr nuclear antigen 2 |
| EBV | Epstein-Barr virus. |
| ECD | Extracellular domain |
| EGFR | Epidermal growth factor receptor |
| ER | Estrogen receptor; |
| ErbB2 | Erythroblastic oncogene B |
| FR | Folate receptor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HBc | Hepatitis B virus core antigen |
| HER 2 | Human epidermal growth factor receptor 2 |
| HIV | Human immunodeficiency virus |
| HLA | Human leukocyte antigens |
| HPV | Human Papillomavirus |
| HSP | Heat shock protein; |
| HTL | Helper T lymphocyte |
| HTLV-1 | Human T cell lymphotropic virus 1 |
| ICB | Immune checkpoint blockade |
| ICD | Intracellular domain |
| IFN | Interferon |
| IgG | Immunoglobulin G |
| IL | Interleukin |
| KLH- | Keyhole limpet hemocyanin |
| LMP2 | Latent membrane protein 2 |
| MAGE | Melanoma Antigen Gene |
| MAP | Multiple antigenic peptides |
| MD | Molecular dynamics |
| MDSCs | Myeloid-derived suppressor cells |
| MHC | Major histocompatibility complex |
| MIR | Major immune dominant region |
| MMTV | Mouse Mammary Tumour Virus |
| MUC1 | Mucin1 |
| NK | Natural killer cell |
| NP | Nanoparticle |
| NPBC | Non-palpable breast cancer |
| NPS | Nelipepimut-S |
| OLP | Overlapping peptide |
| PCR | Polymerase chain reaction |
| PEG | Polyethylene Glycolylated |
| PEP-DC | DCs pulsed with up to ten peptides |
| Poly-ICLC | Polyinosinic-polycytidylic acid |
| PLGA | Poly lactic-co-glycolic acid |
| PTEN | Phosphatase and TENsin homolog |
| RNF | Ring finger protein; ROC-recurrent ovarian cancer |
| ROPs | Recombinant overlapping peptides |
| SEREX | Serological analysis of cDNA expression libraries |
| SLP | Synthetic long peptide |
| SSA | Serum amyloid A |
| STING | Stimulator of interferon genes |
| TAAs | Tumor associated antigens |
| TCR | T-cell receptor |
| TGF | Tumor growth factor |
| Th | helper T cells |
| TLR | Toll-like receptor |
| TNBC | Triple-negative breast cancer |
| TNF | Tumor necrosis factor |
| TME | Tumor microenvironment |
| Tp53 | Tumor protein 53 |
| TSAs | Tumor-specific antigens |
| TUBO | Cell lines cloned from a BALB/c mouse mammary carcinoma |
| VLP | Virus like particles. |
| wt p53 | Wild type p53 |
| WT1 | Wilms’ tumor 1 |
References
- Qiao, J.; Liu, Z.; Fu, Y.-X. Adapting conventional cancer treatment for immunotherapy. J. Mol. Med. 2016, 94, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Hoda, M. Potential Alternatives to Conventional Cancer Therapeutic Approaches: The Way Forward. Curr. Pharm. Biotechnol. 2021, 22, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, C.L.; Kapoor, E.; Sood, R.; Thielen, J.M.; Jatoi, A.; Faubion, S.S. Menopausal hormone therapy in cancer survivors: A narrative review of the literature. Maturitas 2016, 92, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.L.; Silva-de-Oliveira, A.F.S.; Andraus, R.A.C.; Maia, L.P. Effects of low level laser therapy in cancer cells—A systematic review of the literature. Lasers Med. Sci. 2020, 35, 523–529. [Google Scholar] [CrossRef]
- Ross, J.S.; Schenkein, D.P.; Pietrusko, R.; Rolfe, M.; Linette, G.P.; Stec, J.; Stagliano, N.E.; Ginsburg, G.S.; Symmans, W.F.; Pusztai, L.; et al. Targeted Therapies for Cancer 2004. Am. J. Clin. Pathol. 2004, 122, 598–609. [Google Scholar] [CrossRef]
- Stephens, A.J.; Burgess-Brown, N.A.; Jiang, S. Beyond Just Peptide Antigens: The Complex World of Peptide-Based Cancer Vaccines. Front. Immunol. 2021, 12, 2629. [Google Scholar] [CrossRef]
- Takei, J.; Suzuki, H.; Asano, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-Mouse CCR4 Monoclonal Antibody (C4Mab-1) by N-Terminal Peptide Immunization. Monoclon. Antibodies Immunodiagn. Immunother. 2022, 41, 87–93. [Google Scholar] [CrossRef]
- Nezafat, N.; Ghasemi, Y.; Javadi, G.; Khoshnoud, M.J.; Omidinia, E. A novel multi-epitope peptide vaccine against cancer: An in silico approach. J. Theor. Biol. 2014, 349, 121–134. [Google Scholar] [CrossRef]
- De Campos, N.S.P.; Souza, B.S.; da Silva, G.C.P.; Porto, V.A.; Chalbatani, G.M.; Lagreca, G.; Janji, B.; Suarez, E.R. Carbonic Anhydrase IX: A Renewed Target for Cancer Immunotherapy. Cancers 2022, 14, 1392. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Fry, T.J.; Mackall, C.L. T-cell adoptive immunotherapy for acute lymphoblastic leukemia. Hematology Am. Soc. Hematol. Educ. Program 2013, 2013, 348–353. [Google Scholar] [CrossRef]
- Schlom, J. Therapeutic Cancer Vaccines: Current Status and Moving Forward. JNCI J. Natl. Cancer Inst. 2012, 104, 599–613. [Google Scholar] [CrossRef]
- Gatti-Mays, M.E.; Redman, J.M.; Collins, J.M.; Bilusic, M. Cancer vaccines: Enhanced immunogenic modulation through therapeutic combinations. Hum. Vaccines Immunother. 2017, 13, 2561–2574. [Google Scholar] [CrossRef]
- Michel, T.; Ollert, M.; Zimmer, J. A Hot Topic: Cancer Immunotherapy and Natural Killer Cells. Int. J. Mol. Sci. 2022, 23, 797. [Google Scholar] [CrossRef]
- Cebon, J. Perspective: Cancer vaccines in the era of immune checkpoint blockade. Mamm. Genome 2018, 29, 703–713. [Google Scholar] [CrossRef]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 1–24. [Google Scholar] [CrossRef]
- Ginsburg, O.; Bray, F.; Coleman, M.P.; Vanderpuye, V.; Eniu, A.; Kotha, S.R.; Sarker, M.; Huong, T.T.; Allemani, C.; Dvaladze, A.; et al. The global burden of women’s cancers: A grand challenge in global health. Lancet 2017, 389, 847–860. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Diseases and Conditions-Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions?customer_id=856-310-7743&mc_id=google&campaign=19807197113&geo=1007828&kw=mayoclinic&ad=650933999493&network=g&sitetarget=&adgroup=147643045435&extension=&target=kwd-98051161&matchtype=e&device=c&account=8563107743&invsrc=india&placementsite=enterprise&gclid=Cj0KCQjw_r6hBhDdARIsAMIDhV80obPMuAybSmabKG5o7bfYGuiMqecyuCzOrgx4PUm32MPsx2TZtYMaAuPCEALw_wcB (accessed on 7 April 2023).
- Cancer Types|Cancer Resources|American Cancer Society. Available online: https://www.cancer.org/cancer.html (accessed on 7 April 2023).
- Alibek, K.; Kakpenova, A.; Mussabekova, A.; Sypabekova, M.; Karatayeva, N. Role of viruses in the development of breast cancer. Infect. Agents Cancer 2013, 8, 32. [Google Scholar] [CrossRef]
- Peng, L.; Xu, T.; Long, T.; Zuo, H. Association Between BRCA Status and P53 Status in Breast Cancer: A Meta-Analysis. Med. Sci. Monit. 2016, 22, 1939–1945. [Google Scholar] [CrossRef]
- Walsh, T.; Casadei, S.; Coats, K.H.; Swisher, E.; Stray, S.M.; Higgins, J.; Roach, K.C.; Mandell, J.; Lee, M.K.; Ciernikova, S.; et al. Spectrum of Mutations in BRCA1, BRCA2, CHEK2, and TP53 in Families at High Risk of Breast Cancer. JAMA 2006, 295, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.W.; O’Hara, A.J. The genomics and genetics of endometrial cancer. Adv. Genom. Genet. 2012, 2012, 33. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, S.; Zohura, F.T.; Islam, S.; Shimonty, A.; Khan, A.-A.; Parveen, R.; Sharmin, F.; Ahsan, C.R.; Islam, A.B.M.M.K.; Yasmin, M. Mutational profiles of marker genes of cervical carcinoma in Bangladeshi patients. BMC Cancer 2021, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tamandani, D.M.K.; Sobti, R.C.; Shekari, M.; Huria, A. CPG Island methylation of TMS1/ASC and CASP8 genes in cervical cancer. Eur. J. Med. Res. 2009, 14, 71–75. [Google Scholar] [CrossRef][Green Version]
- Du, J.; Zhou, S.; Wang, L.; Yu, M.; Mei, L. Downregulation of ERBB3 decreases the proliferation, migration and invasion of cervical cancer cells though the interaction with MTK 1. Oncol Lett. 2018, 16, 3453–3458. [Google Scholar] [CrossRef]
- Toss, A.; Tomasello, C.; Razzaboni, E.; Contu, G.; Grandi, G.; Cagnacci, A.; Schilder, R.J.; Cortesi, L. Hereditary Ovarian Cancer: Not OnlyBRCA1 and 2 Genes. BioMed Res. Int. 2015, 2015, 341723. [Google Scholar] [CrossRef]
- The AACR Project GENIE Consortium; André, F.; Arnedos, M.; Baras, A.S.; Baselga, J.; Bedard, P.L.; Berger, M.F.; Bierkens, M.; Calvo, F.; Cerami, E.; et al. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef]
- Capria, A.; Tahir, N.; Fatehi, M. Vulva Cancer. In Decision Making in Radiation Oncology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 703–724. [Google Scholar] [CrossRef]
- Zięba, S.; Kowalik, A.; Zalewski, K.; Rusetska, N.; Goryca, K.; Piaścik, A.; Misiek, M.; Bakuła-Zalewska, E.; Kopczyński, J.; Kowalski, K.; et al. Somatic mutation profiling of vulvar cancer: Exploring therapeutic targets. Gynecol. Oncol. 2018, 150, 552–561. [Google Scholar] [CrossRef]
- Viswanath, D.I.; Liu, H.-C.; Huston, D.P.; Chua, C.Y.X.; Grattoni, A. Emerging biomaterial-based strategies for personalized therapeutic in situ cancer vaccines. Biomaterials 2022, 280, 121297. [Google Scholar] [CrossRef]
- Martin-Liberal, J.; de Olza, M.O.; Hierro, C.; Gros, A.; Rodon, J.; Tabernero, J. The expanding role of immunotherapy. Cancer Treat. Rev. 2017, 54, 74–86. [Google Scholar] [CrossRef]
- Shibata, H.; Zhou, L.; Xu, N.; Egloff, A.M.; Uppaluri, R. Personalized cancer vaccination in head and neck cancer. Cancer Sci. 2021, 112, 978–988. [Google Scholar] [CrossRef]
- Toussaint, B.; Chauchet, X.; Wang, Y.; Polack, B.; Le Gouëllec, A. Live-attenuated bacteria as a cancer vaccine vector. Expert Rev. Vaccines 2014, 12, 1139–1154. [Google Scholar] [CrossRef]
- Russell, S.J.; Barber, G.N. Oncolytic Viruses as Antigen-Agnostic Cancer Vaccines. Cancer Cell 2018, 33, 599–605. [Google Scholar] [CrossRef]
- McNamara, M.A.; Nair, S.K.; Holl, E.K. RNA-Based Vaccines in Cancer Immunotherapy. J. Immunol. Res. 2015, 2015, 794528. [Google Scholar] [CrossRef]
- Obara, W.; Kanehira, M.; Katagiri, T.; Kato, R.; Kato, Y.; Takata, R. Present status and future perspective of peptide-based vaccine therapy for urological cancer. Cancer Sci. 2018, 109, 550–559. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 1–23. [Google Scholar] [CrossRef]
- Nwonu, E.J. Approaches to cancer vaccination. In Vaccinology and Methods in Vaccine Research; ACM: New York, NY, USA, 2022; pp. 177–199. [Google Scholar] [CrossRef]
- Lee, C.-H.; Yelensky, R.; Jooss, K.; Chan, T.A. Update on Tumor Neoantigens and Their Utility: Why It Is Good to Be Different. Trends Immunol. 2018, 39, 536–548. [Google Scholar] [CrossRef]
- Abd-Aziz, N.; Poh, C.L. Development of Peptide-Based Vaccines for Cancer. J. Oncol. 2022, 2022, 9749363. [Google Scholar] [CrossRef]
- Sotillo, E.; Barrett, D.M.; Black, K.L.; Bagashev, A.; Oldridge, D.; Wu, G.; Sussman, R.; LaNauze, C.; Ruella, M.; Gazzara, M.R.; et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015, 5, 1282–1295. [Google Scholar] [CrossRef]
- Quakkelaar, E.D.; Fransen, M.F.; van Maren, W.W.C.; Vaneman, J.; Loof, N.M.; van Heiningen, S.H.; Verbeek, J.S.; Ossendorp, F.; Melief, C.J.M. IgG-Mediated Anaphylaxis to a Synthetic Long Peptide Vaccine Containing a B Cell Epitope Can Be Avoided by Slow-Release Formulation. J. Immunol. 2014, 192, 5813–5820. [Google Scholar] [CrossRef]
- Nordin, M.L.; Norpi, A.S.M.; Ng, P.Y.; Yusoff, K.; Abu, N.; Lim, K.P.; Azmi, F. HER2/neu-Based Peptide Vaccination-Pulsed with B-Cell Epitope Induced Efficient Prophylactic and Therapeutic Antitumor Activities in TUBO Breast Cancer Mice Model. Cancers 2021, 13, 4958. [Google Scholar] [CrossRef] [PubMed]
- Marintcheva, B. Viruses as Tools for Vaccine Development. In Harnessing the Power of Viruses; ACM: New York, NY, USA, 2018; pp. 217–242. [Google Scholar] [CrossRef]
- Yamada, A.; Sasada, T.; Noguchi, M.; Itoh, K. Next-generation peptide vaccines for advanced cancer. Cancer Sci. 2013, 104, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Vassilakopoulou, V.; Karachaliou, C.-E.; Evangelou, A.; Zikos, C.; Livaniou, E. Peptide-Based Vaccines for Neurodegenerative Diseases: Recent Endeavors and Future Perspectives. Vaccines 2021, 9, 1278. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, M.; Fiorini, E.; Siegert, S.; Carpintero, R.; Rincon-Restrepo, M.; Lopez-Deber, P.; Piot, N.; Ayer, M.; Rentero, I.; Babolin, C.; et al. An amyloid beta vaccine that safely drives immunity to a key pathological species in Alzheimer’s disease: Pyroglutamate amyloid beta. Brain Commun. 2022, 4, fcac022. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, X.; Liu, Y.; Li, D.; Cun, Y.; Zhang, Y.; Wang, R.; Hao, Y.; Wang, S.; Chen, C.; et al. A D-peptide-based HIV gelatinous combination vaccine improves therapy in ART-delayed macaques of chronic infection. Nano Today 2022, 42, 101353. [Google Scholar] [CrossRef]
- Combadière, B.; Beaujean, M.; Chaudesaigues, C.; Vieillard, V. Peptide-Based Vaccination for Antibody Responses Against HIV. Vaccines 2019, 7, 105. [Google Scholar] [CrossRef]
- Sumadi, F.A.; Jamil, A.S.; Auliyana, N.; H, M.N.; K, R.N. Developing an Epitope-Based Peptide Vaccine for the Hepatitis C Virus Using an in Silico Approach. KnE Med. 2022, 212–222. [Google Scholar] [CrossRef]
- Gong, W.; Pan, C.; Cheng, P.; Wang, J.; Zhao, G.; Wu, X. Peptide-Based Vaccines for Tuberculosis. Front. Immunol. 2022, 13, 104. [Google Scholar] [CrossRef]
- Forner, M.; Cañas-Arranz, R.; Defaus, S.; de León, P.; Rodríguez-Pulido, M.; Ganges, L.; Blanco, E.; Sobrino, F.; Andreu, D. Peptide-Based Vaccines: Foot-and-Mouth Disease Virus, a Paradigm in Animal Health. Vaccines 2021, 9, 477. [Google Scholar] [CrossRef]
- Liu, W.; Tang, H.; Li, L.; Wang, X.; Yu, Z.; Li, J. Peptide-based therapeutic cancer vaccine: Current trends in clinical application. Cell Prolif. 2021, 54, e13025. [Google Scholar] [CrossRef]
- Speetjens, F.M.; Kuppen, P.J.; Welters, M.J.; Essahsah, F.; Brink, A.M.E.V.v.D.; Lantrua, M.G.K.; Valentijn, A.R.P.; Oostendorp, J.; Fathers, L.M.; Nijman, H.W.; et al. Induction of p53-Specific Immunity by a p53 Synthetic Long Peptide Vaccine in Patients Treated for Metastatic Colorectal Cancer. Clin. Cancer Res. 2009, 15, 1086–1095. [Google Scholar] [CrossRef]
- Van Poelgeest, M.I.; Welters, M.J.; van Esch, E.M.; Stynenbosch, L.F.; Kerpershoek, G.; van Persijn van Meerten, E.L.; van den Hende, M.; Löwik, M.J.; Berends-van der Meer, D.M.; Fathers, L.M.; et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J. Transl. Med. 2013, 11, 88. [Google Scholar] [CrossRef]
- Rabu, C.; Rangan, L.; Florenceau, L.; Fortun, A.; Charpentier, M.; Dupré, E.; Paolini, L.; Beauvillain, C.; Dupel, E.; Latouche, J.-B.; et al. Cancer vaccines: Designing artificial synthetic long peptides to improve presentation of class I and class II T cell epitopes by dendritic cells. Oncoimmunology 2019, 8, e1560919-10. [Google Scholar] [CrossRef]
- Botelho, N.K.; Tschumi, B.O.; Hubbell, J.A.; Swartz, M.A.; Donda, A.; Romero, P. Combination of Synthetic Long Peptides and XCL1 Fusion Proteins Results in Superior Tumor Control. Front. Immunol. 2019, 10, 294. [Google Scholar] [CrossRef]
- He, X.; Zhou, S.; Dolan, M.; Shi, Y.; Wang, J.; Quinn, B.; Jahagirdar, D.; Huang, W.-C.; Tsuji, M.; Pili, R.; et al. Immunization with short peptide particles reveals a functional CD8+ T-cell neoepitope in a murine renal carcinoma model. J. Immunother. Cancer 2021, 9, e003101. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, J.; Zhu, R.; Shi, W.; Xia, X.; Edwards, M.; Finch, W.; Coombs, A.; Gao, J.; Chen, K.; et al. Protective cellular immunity generated by cross-presenting recombinant overlapping peptide proteins. Oncotarget 2017, 8, 76516–76524. [Google Scholar] [CrossRef]
- Mahdevar, E.; Safavi, A.; Abiri, A.; Kefayat, A.; Hejazi, S.H.; Miresmaeili, S.M.; Mobarakeh, V.I. Exploring the cancer-testis antigen BORIS to design a novel multi-epitope vaccine against breast cancer based on immunoinformatics approaches. J. Biomol. Struct. Dyn. 2021, 40, 6363–6380. [Google Scholar] [CrossRef]
- Mahdevar, E.; Kefayat, A.; Safavi, A.; Behnia, A.; Hejazi, S.H.; Javid, A.; Ghahremani, F. Immunoprotective effect of an in silico designed multiepitope cancer vaccine with BORIS cancer-testis antigen target in a murine mammary carcinoma model. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 182–191. [Google Scholar] [CrossRef]
- Burstein, H.J. The Distinctive Nature of HER2-Positive Breast Cancers. N. Engl. J. Med. 2005, 353, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.G.; Modi, S. HER2 breast cancer therapies: A review. Biologics 2009, 3, 289. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Hausdorf, D.E.; Schalper, K.A.; Bai, Y.; Black, J.; Santin, A.D.; Rimm, D.L. Objective, domain-specific HER2 measurement in uterine and ovarian serous carcinomas and its clinical significance. Gynecol. Oncol. 2017, 145, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Buza, N.; English, D.P.; Santin, A.D.; Hui, P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod. Pathol. 2013, 26, 1605–1612. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Krishnamurti, U.; Silverman, J.F. HER2 in Breast Cancer: A review and update. Adv. Anat. Pathol. 2014, 21, 100–107. [Google Scholar] [CrossRef]
- Ben-Baruch, N.E.; Bose, R.; Kavuri, S.M.; Ma, C.X.; Ellis, M.J. HER2-Mutated Breast Cancer Responds to Treatment With Single-Agent Neratinib, a Second-Generation HER2/EGFR Tyrosine Kinase Inhibitor. J. Natl. Compr. Cancer Netw. 2015, 13, 1061–1064. [Google Scholar] [CrossRef]
- King, M.-C.; Marks, J.H.; Mandell, J.B. Breast and Ovarian Cancer Risks Due to Inherited Mutations in BRCA1 and BRCA2. Science 2003, 302, 643–646. [Google Scholar] [CrossRef]
- Nielsen, F.C.; van Overeem Hansen, T.; Sørensen, C.S. Hereditary breast and ovarian cancer: New genes in confined pathways. Nat. Rev. Cancer 2016, 16, 599–612. [Google Scholar] [CrossRef]
- Kuusisto, K.M.; Bebel, A.; Vihinen, M.; Schleutker, J.; Sallinen, S.L. Screening for BRCA1, BRCA2, CHEK2, PALB2, BRIP1, RAD50, and CDH1 mutations in high-risk Finnish BRCA1/2-founder mutation-negative breast and/or ovarian cancer individuals. Breast Cancer Res. 2011, 13, 1–13. [Google Scholar] [CrossRef]
- Mills, G. The role of genetic abnormalities of PTEN and the phosphatidylinositol 3-kinase pathway in breast and ovarian tumorigenesis, prognosis, and therapy. Semin. Oncol. 2001, 28, 125–141. [Google Scholar] [CrossRef]
- Matsukita, S.; Nomoto, M.; Kitajima, S.; Tanaka, S.; Goto, M.; Irimura, T.; Kim, Y.S.; Sato, E.; Yonezawa, S. Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in mucinous carcinoma of the breast: Comparison with invasive ductal carcinoma. Histopathology 2003, 42, 26–36. [Google Scholar] [CrossRef]
- Kwon, S.; Kang, S.H.; Ro, J.; Jeon, C.-H.; Park, J.-W.; Lee, E.S. The melanoma antigen gene as a surveillance marker for the detection of circulating tumor cells in patients with breast carcinoma. Cancer 2005, 104, 251–256. [Google Scholar] [CrossRef][Green Version]
- Ayyoub, M.; Scarlata, C.-M.; Hamaï, A.; Pignon, P.; Valmori, D. Expression of MAGE-A3/6 in Primary Breast Cancer is Associated With Hormone Receptor Negative Status, High Histologic Grade, and Poor Survival. J. Immunother. 2014, 37, 73–76. [Google Scholar] [CrossRef]
- Tobias, J.; Garner-Spitzer, E.; Drinić, M.; Wiedermann, U. Vaccination against Her-2/neu, with focus on peptide-based vaccines. ESMO Open 2022, 7, 100361. [Google Scholar] [CrossRef]
- Disis, M.L.; Goodell, V.; Schiffman, K.; Knutson, K.L. Humoral Epitope-Spreading Following Immunization with a HER-2/neu Peptide Based Vaccine in Cancer Patients. J Clin Immunol. 2004, 24, 571–578. [Google Scholar] [CrossRef]
- Peoples, G.E.; Gurney, J.M.; Hueman, M.T.; Woll, M.M.; Ryan, G.B.; Storrer, C.E.; Fisher, C.; Shriver, C.D.; Ioannides, C.G.; Ponniah, S. Clinical Trial Results of a HER2/neu (E75) Vaccine to Prevent Recurrence in High-Risk Breast Cancer Patients. J. Clin. Oncol. 2005, 23, 7536–7545. [Google Scholar] [CrossRef]
- Carmichael, M.G.; Benavides, L.C.; Holmes, J.P.; Gates, J.D.; Mittendorf, E.A.; Ponniah, S.; Peoples, G.E. Results of the first phase 1 clinical trial of the HER-2/neupeptide (GP2) vaccine in disease-free breast cancer patients: United States Military Cancer Institute Clinical Trials Group Study I-04. Cancer 2010, 116, 292–301. [Google Scholar] [CrossRef]
- Takahashi, R.; Toh, U.; Iwakuma, N.; Takenaka, M.; Otsuka, H.; Furukawa, M.; Fujii, T.; Seki, N.; Kawahara, A.; Kage, M.; et al. Feasibility study of personalized peptide vaccination for metastatic recurrent triple-negative breast cancer patients. Breast Cancer Res. 2014, 16, R70. [Google Scholar] [CrossRef]
- Antonilli, M.; Rahimi, H.; Visconti, V.; Napoletano, C.; Ruscito, I.; Zizzari, I.G.; Caponnetto, S.; Barchiesi, G.; Iadarola, R.; Pierelli, L.; et al. Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: Clinical and immunological data of a phase I/II clinical trial. Int. J. Oncol. 2016, 48, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Dillon, P.M.; Petroni, G.R.; Smolkin, M.E.; Brenin, D.R.; Chianese-Bullock, K.A.; Smith, K.T.; Olson, W.C.; Fanous, I.S.; Nail, C.J.; Brenin, C.M.; et al. A pilot study of the immunogenicity of a 9-peptide breast cancer vaccine plus poly-ICLC in early stage breast cancer. J. Immunother. Cancer 2017, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Mittendorf, E.A.; Hale, D.F.; Myers, J.W.; Peace, K.M.; Jackson, D.O.; Greene, J.M.; Vreeland, T.J.; Clifton, G.T.; Ardavanis, A.; et al. Prospective, randomized, single-blinded, multi-center phase II trial of two HER2 peptide vaccines, GP2 and AE37, in breast cancer patients to prevent recurrence. Breast Cancer Res. Treat. 2020, 181, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Toh, U.; Sakurai, S.; Saku, S.; Takao, Y.; Okabe, M.; Iwakuma, N.; Shichijo, S.; Yamada, A.; Itoh, K.; Akagi, Y. Early phase II study of mixed 19-peptide vaccine monotherapy for refractory triple-negative breast cancer. Cancer Sci. 2020, 111, 2760–2769. [Google Scholar] [CrossRef] [PubMed]
- Barati, N.; Nikpoor, A.R.; Mosaffa, F.; Razazan, A.; Badiee, A.; Motavallihaghi, S.S.; Behravan, J.; Jaafari, M.R. AE36 HER2/neu-derived peptide linked to positively charged liposomes with CpG-ODN as an effective therapeutic and prophylactic vaccine for breast cancer. J. Drug Deliv. Sci. Technol. 2021, 67, 102904. [Google Scholar] [CrossRef]
- Chamani, R.; Ranji, P.; Hadji, M.; Nahvijou, A.; Esmati, E.; Alizadeh, A.M. Application of E75 peptide vaccine in breast cancer patients: A systematic review and meta-analysis. Eur. J. Pharmacol. 2018, 831, 87–93. [Google Scholar] [CrossRef]
- You, Z.; Zhou, W.; Weng, J.; Feng, H.; Liang, P.; Li, Y.; Shi, F. Application of HER2 peptide vaccines in patients with breast cancer: A systematic review and meta-analysis. Cancer Cell Int. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Erickson, B.K.; Zeybek, B.; Santin, A.D.; Fader, A.N. Targeting human epidermal growth factor receptor 2 (HER2) in gynecologic malignancies. Curr. Opin. Obstet. Gynecol. 2020, 32, 57–64. [Google Scholar] [CrossRef]
- Generation of Immunity to the HER-2/neu Oncogenic Protein in Patients with Breast and Ovarian Cancer Using a Peptide-Based Vaccine-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/10389911/ (accessed on 7 April 2023).
- Brossart, P.; Wirths, S.; Stuhler, G.; Reichardt, V.L.; Kanz, L.; Brugger, W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood 2000, 96, 3102–3108. [Google Scholar] [CrossRef]
- Albert, M.L.; Sauter, B.; Bhardwaj, N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998, 392, 86–89. [Google Scholar] [CrossRef]
- Immunization of Cancer Patients with a HER-2/neu, HLA-A2 Peptide, p369-377, Results in Short-Lived Peptide-Specific Immunity-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12006513/ (accessed on 7 April 2023).
- Chianese-Bullock, K.A.; Irvin, W.P.J.; Petroni, G.R.; Murphy, C.; Smolkin, M.; Olson, W.C.; Coleman, E.; Boerner, S.A.; Nail, C.J.; Neese, P.Y.; et al. A Multipeptide Vaccine is Safe and Elicits T-cell Responses in Participants With Advanced Stage Ovarian Cancer. J. Immunother. 2008, 31, 420–430. [Google Scholar] [CrossRef]
- Fulk, K.; Milam, M.R.; Li, S.; Yussuf, A.; Black, M.H.; Chao, E.C.; LaDuca, H.; Stany, M.P. Women with breast and uterine cancer are more likely to harbor germline mutations than women with breast or uterine cancer alone: A case for expanded gene testing. Gynecol. Oncol. 2019, 152, 612–617. [Google Scholar] [CrossRef]
- Mackenzie, R.; Kommoss, S.; Winterhoff, B.J.; Kipp, B.R.; Garcia, J.J.; Voss, J.; Halling, K.; Karnezis, A.; Senz, J.; Yang, W.; et al. Targeted deep sequencing of mucinous ovarian tumors reveals multiple overlapping RAS-pathway activating mutations in borderline and cancerous neoplasms. BMC Cancer 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Mukherjee, P.; Madsen, C.S.; Ginardi, A.R.; Tinder, T.L.; Jacobs, F.; Parker, J.; Agrawal, B.; Longenecker, B.M.; Gendler, S.J. Mucin 1-Specific Immunotherapy in a Mouse Model of Spontaneous Breast Cancer. J. Immunother. 2003, 26, 47–62. Available online: https://journals.lww.com/immunotherapy-journal/fulltext/2003/01000/mucin_1_specific_immunotherapy_in_a_mouse_model_of.6.aspx (accessed on 7 April 2023). [CrossRef]
- Leffers, N.; Lambeck, A.J.; Gooden, M.J.; Hoogeboom, B.; Wolf, R.; Hamming, I.E.; Hepkema, B.G.; Willemse, P.H.; Molmans, B.H.; Hollema, H.; et al. Immunization with a P53 synthetic long peptide vaccine induces P53-specific immune responses in ovarian cancer patients, a phase II trial. Int. J. Cancer 2009, 125, 2104–2113. [Google Scholar] [CrossRef]
- Vermeij, R.; Leffers, N.; Hoogeboom, B.-N.; Hamming, I.L.; Wolf, R.; Reyners, A.K.; Molmans, B.H.; Hollema, H.; Bart, J.; Drijfhout, J.W.; et al. Potentiation of a p53-SLP vaccine by cyclophosphamide in ovarian cancer: A single-arm phase II study. Int. J. Cancer 2012, 131, E670–E680. [Google Scholar] [CrossRef]
- Sabbatini, P.; Tsuji, T.; Ferran, L.; Ritter, E.; Sedrak, C.; Tuballes, K.; Jungbluth, A.A.; Ritter, G.; Aghajanian, C.; Bell-McGuinn, K.; et al. Phase I Trial of Overlapping Long Peptides from a Tumor Self-Antigen and Poly-ICLC Shows Rapid Induction of Integrated Immune Response in Ovarian Cancer Patients. Clin. Cancer Res. 2012, 18, 6497–6508. [Google Scholar] [CrossRef]
- Leffers, N.; Vermeij, R.; Hoogeboom, B.-N.; Schulze, U.R.; Wolf, R.; Hamming, I.E.; van der Zee, A.G.; Melief, K.J.; van der Burg, S.H.; Daemen, T.; et al. Long-term clinical and immunological effects of p53-SLP® vaccine in patients with ovarian cancer. Int. J. Cancer 2012, 130, 105–112. [Google Scholar] [CrossRef]
- Rahma, O.E.; Ashtar, E.; Czystowska, M.; Szajnik, M.E.; Wieckowski, E.; Bernstein, S.; Herrin, V.E.; Shams, M.A.; Steinberg, S.M.; Merino, M.; et al. A gynecologic oncology group phase II trial of two p53 peptide vaccine approaches: Subcutaneous injection and intravenous pulsed dendritic cells in high recurrence risk ovarian cancer patients. Cancer Immunol. Immunother. 2012, 61, 373–384. [Google Scholar] [CrossRef]
- Miyatake, T.; Ueda, Y.; Morimoto, A.; Enomoto, T.; Nishida, S.; Shirakata, T.; Oka, Y.; Tsuboi, A.; Oji, Y.; Hosen, N.; et al. WT1 peptide immunotherapy for gynecologic malignancies resistant to conventional therapies: A phase II trial. J. Cancer Res. Clin. Oncol. 2013, 139, 457–463. [Google Scholar] [CrossRef]
- Fujita, T.; Yoshida, A.; Nishimura, H.; Koshikawa, K.; Nagura, N.; Yoshida, K.; Miyoshi, Y.; Yamauchi, H.; Nakamura, S.; Nakamura, Y.; et al. Phase I clinical trial of multi-antigen peptide vaccines therapy using cancer-testis antigens for patients with advanced or recurrent breast cancer. J. Clin. Oncol. 2012, 30 (Suppl. 15), e13037. [Google Scholar] [CrossRef]
- Kawano, K.; Tsuda, N.; Matsueda, S.; Sasada, T.; Watanabe, N.; Ushijima, K.; Yamaguchi, T.; Yokomine, M.; Itoh, K.; Yamada, A.; et al. Feasibility study of personalized peptide vaccination for recurrent ovarian cancer patients. Immunopharmacol. Immunotoxicol. 2014, 36, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Chiba, A.; Izawa, H.; Yanagida, E.; Okamoto, M.; Shimodaira, S.; Yonemitsu, Y.; Shibamoto, Y.; Suzuki, N.; Nagaya, M.; et al. The feasibility and clinical effects of dendritic cell-based immunotherapy targeting synthesized peptides for recurrent ovarian cancer. J. Ovarian Res. 2014, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, M.; Hijikata, Y.; Yamada, K.; Tanaka, Y.; Kishimoto, J.; Inoue, H.; Marumoto, T.; Takahashi, A.; Okazaki, T.; Takeda, K.; et al. Phase I clinical trial of a five-peptide cancer vaccine combined with cyclophosphamide in advanced solid tumors. Clin. Immunol. 2016, 166–167, 48–58. [Google Scholar] [CrossRef]
- Kalli, K.R.; Block, M.S.; Kasi, P.M.; Erskine, C.L.; Hobday, T.J.; Dietz, A.; Padley, D.; Gustafson, M.P.; Shreeder, B.; Puglisi-Knutson, D.; et al. Folate Receptor Alpha Peptide Vaccine Generates Immunity in Breast and Ovarian Cancer Patients. Clin. Cancer Res. 2018, 24, 3014–3025. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yin, Z.; McKay, C.; Pett, C.; Yu, J.; Schorlemer, M.; Gohl, T.; Sungsuwan, S.; Ramadan, S.; Baniel, C.; et al. Protective Epitope Discovery and Design of MUC1-based Vaccine for Effective Tumor Protections in Immunotolerant Mice. J. Am. Chem. Soc. 2018, 140, 16596–16609. [Google Scholar] [CrossRef]
- Hijikata, Y.; Okazaki, T.; Tanaka, Y.; Murahashi, M.; Yamada, Y.; Yamada, K.; Takahashi, A.; Inoue, H.; Kishimoto, J.; Nakanishi, Y.; et al. A phase I clinical trial of RNF43 peptide-related immune cell therapy combined with low-dose cyclophosphamide in patients with advanced solid tumors. PLoS ONE 2018, 13, e0187878. [Google Scholar] [CrossRef]
- Hasegawa, K.; Ikeda, Y.; Kunugi, Y.; Kurosaki, A.; Imai, Y.; Kohyama, S.; Nagao, S.; Kozawa, E.; Yoshida, K.; Tsunoda, T.; et al. Phase I Study of Multiple Epitope Peptide Vaccination in Patients With Recurrent or Persistent Cervical Cancer. J. Immunother. 2018, 41, 201–207. [Google Scholar] [CrossRef]
- Sarivalasis, A.; Boudousquié, C.; Balint, K.; Stevenson, B.J.; Gannon, P.O.; Iancu, E.M.; Rossier, L.; Lluesma, S.M.; Mathevet, P.; Sempoux, C.; et al. A Phase I/II trial comparing autologous dendritic cell vaccine pulsed either with personalized peptides (PEP-DC) or with tumor lysate (OC-DC) in patients with advanced high-grade ovarian serous carcinoma. J. Transl. Med. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Brown, T.A.; Byrd, K.; Vreeland, T.J.; Clifton, G.T.; Jackson, D.O.; Hale, D.F.; Herbert, G.S.; Myers, J.W.; Greene, J.M.; Berry, J.S.; et al. Final analysis of a phase I/IIa trial of the folate-binding protein-derived E39 peptide vaccine to prevent recurrence in ovarian and endometrial cancer patients. Cancer Med. 2019, 8, 4678–4687. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, L.; Gao, N.; Diao, Y.; Zhong, J.; Deng, Y.; Wang, Z.; Jin, G.; Wang, X. Synthetic MUC1 breast cancer vaccine containing a Toll-like receptor 7 agonist exerts antitumor effects. Oncol. Lett. 2020, 20, 2369–2377. [Google Scholar] [CrossRef]
- Morisaki, T.; Hikichi, T.; Onishi, H.; Morisaki, T.; Kubo, M.; Hirano, T.; Yoshimura, S.; Kiyotani, K.; Nakamura, Y. Intranodal Administration of Neoantigen Peptide-loaded Dendritic Cell Vaccine Elicits Epitope-specific T Cell Responses and Clinical Effects in a Patient with Chemorefractory Ovarian Cancer with Malignant Ascites. Immunol. Investig. 2020, 50, 562–579. [Google Scholar] [CrossRef]
- Kang, J.; Lee, H.-J.; Lee, J.; Hong, J.; Kim, Y.H.; Disis, M.L.; Gim, J.-A.; Park, K.H. Novel peptide-based vaccine targeting heat shock protein 90 induces effective antitumor immunity in a HER2+ breast cancer murine model. J. Immunother. Cancer 2022, 10, e004702. [Google Scholar] [CrossRef]
- Dzobo, K. The Role of Viruses in Carcinogenesis and Molecular Targeting: From Infection to Being a Component of the Tumor Microenvironment. OMICS A J. Integr. Biol. 2021, 25, 358–371. [Google Scholar] [CrossRef]
- Hausen, H.Z. Viruses in Human Cancers. Science 1991, 254, 1167–1173. [Google Scholar] [CrossRef]
- Lawson, J.S.; Heng, B. Viruses and Breast Cancer. Cancers 2010, 2, 752–772. [Google Scholar] [CrossRef]
- Burk, R.D.; Harari, A.; Chen, Z. Human papillomavirus genome variants. Virology 2013, 445, 232–243. [Google Scholar] [CrossRef]
- Garbuglia, A.R.; Lapa, D.; Sias, C.; Capobianchi, M.R.; Del Porto, P. The Use of Both Therapeutic and Prophylactic Vaccines in the Therapy of Papillomavirus Disease. Front. Immunol. 2020, 11, 188. [Google Scholar] [CrossRef]
- Lazzeroni, M.; Serrano, D. Potential Use of Vaccines in the Primary Prevention of Breast Cancer in High-Risk Patients. Breast Care 2012, 7, 281–287. [Google Scholar] [CrossRef]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2020, 10, 3116. [Google Scholar] [CrossRef]
- Enokida, T.; Moreira, A.; Bhardwaj, N. Vaccines for immunoprevention of cancer. J. Clin. Investig. 2021, 131, 6956. [Google Scholar] [CrossRef] [PubMed]
- Kirnbauer, R.; Booy, F.; Cheng, N.; Lowy, D.R.; Schiller, J.T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 1992, 89, 12180–12184. [Google Scholar] [CrossRef] [PubMed]
- Chabeda, A.; Yanez, R.J.R.; Lamprecht, R.; Meyers, A.E.; Rybicki, E.P.; Hitzeroth, I.I. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018, 5, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, Y.; Du, J. Human Papillomavirus Vaccines: An Updated Review. Vaccines 2020, 8, 391. [Google Scholar] [CrossRef]
- Hanna, E.; Bachmann, G. HPV vaccination with Gardasil®: A breakthrough in women’s health. Expert Opin. Biol. Ther. 2006, 6, 1223–1227. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Kitchener, H.C. Cervarix™—A bivalent L1 virus-like particle vaccine for prevention of human papillomavirus type 16- and 18-associated cervical cancer. Expert Opin. Biol. Ther. 2007, 7, 391–396. [Google Scholar] [CrossRef]
- Stanley, M.; Joura, E.; Yen, G.P.; Kothari, S.; Luxembourg, A.; Saah, A.; Walia, A.; Perez, G.; Khoury, H.; Badgley, D.; et al. Systematic literature review of neutralizing antibody immune responses to non-vaccine targeted high-risk HPV types induced by the bivalent and the quadrivalent vaccines. Vaccine 2021, 39, 2214–2223. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Luckett, R.; Feldman, S. Impact of 2-, 4- and 9-valent HPV vaccines on morbidity and mortality from cervical cancer. Hum. Vaccines Immunother. 2015, 12, 1332–1342. [Google Scholar] [CrossRef]
- Highlights of Prescribing Information. Available online: www.vaers.hhs.gov (accessed on 7 April 2023).
- Monie, A.; Hung, C.F.; Roden, R.; Wu, T.-C. Cervarix™: A vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics 2008, 2, 107. [Google Scholar] [CrossRef]
- Asif, M.; Siddiqui, A.; Perry, C.M. ADIS DRUG PROFILE Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18) Recombinant Vaccine (Gardasil ®). Drugs 2006, 66, 1263–1271; discussion 1272–1273. [Google Scholar]
- Roden, R.B.S.; Stern, P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 2018, 18, 240–254. [Google Scholar] [CrossRef]
- Illah, O.; Olaitan, A. Updates on HPV Vaccination. Diagnostics 2023, 13, 243. [Google Scholar] [CrossRef]
- Glaxosmithkline. Cervarix, Suspension for Intramuscular Injection. Available online: www.vaers.hhs.gov (accessed on 7 April 2023).
- Fda, C. Package Insert-Gardasil 9. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil-9 (accessed on 7 April 2023).
- Markowitz, L.E.; Schiller, J.T. Human Papillomavirus Vaccines. J. Infect. Dis. 2021, 224 (Suppl. 5), S367–S378. [Google Scholar] [CrossRef]
- Akhatova, A.; Azizan, A.; Atageldiyeva, K.; Ashimkhanova, A.; Marat, A.; Iztleuov, Y.; Suleimenova, A.; Shamkeeva, S.; Aimagambetova, G. Prophylactic Human Papillomavirus Vaccination: From the Origin to the Current State. Vaccines 2022, 10, 1912. [Google Scholar] [CrossRef]
- Zou, Z.; Fairley, C.K.; Ong, J.J.; Hocking, J.; Canfell, K.; Ma, X.; Chow, E.P.F.; Xu, X.; Zhang, L.; Zhuang, G. Domestic HPV vaccine price and economic returns for cervical cancer prevention in China: A cost-effectiveness analysis. Lancet Glob. Health 2020, 8, e1335–e1344. [Google Scholar] [CrossRef]
- Cecolin®|WHO-Prequalification of Medical Products (IVDs, Medicines, Vaccines and Immunization Devices, Vector Control). Available online: https://extranet.who.int/pqweb/content/cecolin%C2%AE (accessed on 26 March 2023).
- Inc, E. EG-HPV: Human Papillomavirus Vaccine Based on Virus-Like Particle (VLP) Technology (HPV 16 & 18 L1 VLP) and a Novel Proprietary Immune Adjuvant EGvac (Adjuvant System CIA05+Alum) that Enhances the Immune Response against HPV through Both B Cell (Humoral) and T Cell (Cellular) Immune Stimulation. Available online: http://www.vaers.hhs.gov/ (accessed on 7 April 2023).
- Kim, K.S.; Park, S.A.; Ko, K.-N.; Yi, S.; Cho, Y.J. Current status of human papillomavirus vaccines. Clin. Exp. Vaccine Res. 2014, 13, 271–273. [Google Scholar] [CrossRef]
- Negahdaripour, M.; Eslami, M.; Nezafat, N.; Hajighahramani, N.; Ghoshoon, M.B.; Shoolian, E.; Dehshahri, A.; Erfani, N.; Morowvat, M.H.; Ghasemi, Y. A novel HPV prophylactic peptide vaccine, designed by immunoinformatics and structural vaccinology approaches. Infect. Genet. Evol. 2017, 54, 402–416. [Google Scholar] [CrossRef]
- Negahdaripour, M.; Nezafat, N.; Heidari, R.; Erfani, N.; Hajighahramani, N.; Ghoshoon, M.B.; Shoolian, E.; Rahbar, M.R.; Najafipour, S.; Dehshahri, A.; et al. Production and Preliminary In Vivo Evaluations of a Novel in silico-designed L2-based Potential HPV Vaccine. Curr. Pharm. Biotechnol. 2020, 21, 316–324. [Google Scholar] [CrossRef]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 4, 7. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Zhang, H.; Shen, J.; Chen, J.; Hong, J.; Xu, Y.; Qian, C. Prophylactic and Therapeutic HPV Vaccines: Current Scenario and Perspectives. Front. Cell. Infect. Microbiol. 2022, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Welters, M.J.; Kenter, G.G.; Piersma, S.J.; Vloon, A.P.; Lowik, M.J.; der Meer, D.M.B.-V.; Drijfhout, J.W.; Valentijn, A.R.P.; Wafelman, A.R.; Oostendorp, J.; et al. Induction of Tumor-Specific CD4+ and CD8+ T-Cell Immunity in Cervical Cancer Patients by a Human Papillomavirus Type 16 E6 and E7 Long Peptides Vaccine. Clin. Cancer Res. 2008, 14, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Kenter, G.G.; Welters, M.J.P.; Valentijn, A.R.P.M.; Lowik, M.J.G.; Berends-van der Meer, D.M.A.; Vloon, A.P.G.; Essahsah, F.; Fathers, L.M.; Offringa, R.; Drijfhout, J.W.; et al. Vaccination against HPV-16 Oncoproteins for Vulvar Intraepithelial Neoplasia. N. Engl. J. Med. 2009, 361, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Xu, Y.; Mao, L.; Ou, R.; Ding, Z.; Zhang, X.; Tang, J.; Li, B.; Jia, Z.; Tian, Z.; et al. Heat shock protein 110 improves the anti-tumor effects of the cytotoxic T lymphocyte epitope E7 in mice. Cancer Biol. Ther. 2010, 9, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Melief, C.J. Synthetic Vaccine for the Treatment of Lesions Caused by High Risk Human Papilloma Virus. Cancer J. 2011, 17, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Melief, C.J.M. Treatment of Established Lesions Caused by High-risk Human Papilloma Virus Using a Synthetic Vaccine. J. Immunother. 2012, 35, 215–216. [Google Scholar] [CrossRef]
- Yang, Y.; Che, Y.; Zhao, Y.; Wang, X. Prevention and treatment of cervical cancer by a single administration of human papillomavirus peptide vaccine with CpG oligodeoxynucleotides as an adjuvant in vivo. Int. Immunopharmacol. 2019, 69, 279–288. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Yang, Y.; Jia, W.; Su, T.; Che, Y.; Feng, Y.; Yuan, X.; Wang, X. Mannose-Modified Liposome Co-Delivery of Human Papillomavirus Type 16 E7 Peptide and CpG Oligodeoxynucleotide Adjuvant Enhances Antitumor Activity Against Established Large TC-1 Grafted Tumors in Mice. Int. J. Nanomed. 2020, 15, 9571–9586. [Google Scholar] [CrossRef]
- Berzofsky, J.; de Vos van Steenwijk, P.J.; Ramwadhdoebe, T.H.; Löwik, M.J.G.; van der Minne, C.E.; Berends-van der Meer, D.M.A.; Fathers, L.M.; Valentijn, R.P.M.; Oostendorp, J.; Fleuren, G.J.; et al. Faculty Opinions recommendation of A placebo-controlled randomized HPV16 synthetic long-peptide vaccination study in women with high-grade cervical squamous intraepithelial lesions. Cancer Immunol. Immunother. 2012, 61, 1485–1492. [Google Scholar] [CrossRef]
- Welters, M.J.; van der Sluis, T.C.; van Meir, H.; Loof, N.M.; van Ham, V.J.; van Duikeren, S.; Santegoets, S.J.; Arens, R.; de Kam, M.L.; Cohen, A.F.; et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci. Transl. Med. 2016, 8, 334ra52. [Google Scholar] [CrossRef]
- Domingos-Pereira, S.; Galliverti, G.; Hanahan, D.; Nardelli-Haefliger, D. Carboplatin/paclitaxel, E7-vaccination and intravaginal CpG as tri-therapy towards efficient regression of genital HPV16 tumors. J. Immunother. Cancer 2019, 7, 122. [Google Scholar] [CrossRef]
- Melief, C.J.M.; Welters, M.J.P.; Vergote, I.; Kroep, J.R.; Kenter, G.G.; Ottevanger, P.B.; Tjalma, W.A.A.; Denys, H.; van Poelgeest, M.I.E.; Nijman, H.W.; et al. Strong vaccine responses during chemotherapy are associated with prolonged cancer survival. Sci. Transl. Med. 2020, 12, aaz8235. [Google Scholar] [CrossRef]
- Study of the Therapeutic Vaccine (ISA101/ISA101b) to Treat Advanced or Recurrent Cervical Cancer-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02128126 (accessed on 7 April 2023).
- Coleman, H.N.; Greenfield, W.W.; Stratton, S.L.; Vaughn, R.; Kieber, A.; Moerman-Herzog, A.M.; Spencer, H.J.; Hitt, W.C.; Quick, C.M.; Hutchins, L.F.; et al. Human papillomavirus type 16 viral load is decreased following a therapeutic vaccination. Cancer Immunol. Immunother. 2016, 65, 563–573. [Google Scholar] [CrossRef]
- A Clinical Trial of PepCan to Two Therapy Arms for Treating Cervical High-Grade Squamous Intraepithelial Lesions-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02481414 (accessed on 7 April 2023).
- Arribillaga, L.; Echeverria, I.; Belsue, V.; Gomez, T.; Lozano, T.; Casares, N.; Villanueva, L.; Domingos-Pereira, S.; Romero, P.J.; Nardelli-Haefliger, D.; et al. Bivalent therapeutic vaccine against HPV16/18 genotypes consisting of a fusion protein between the extra domain A from human fibronectin and HPV16/18 E7 viral antigens. J. Immunother. Cancer 2020, 8, e000704. [Google Scholar] [CrossRef]
- Speetjens, F.M.; Welters, M.J.P.; Slingerland, M.; van Poelgeest, M.I.; van Steenwijk, P.J.d.V.; Roozen, I.; Boekestijn, S.; Loof, N.M.; Zom, G.G.; Valentijn, A.R.P.M.; et al. Intradermal vaccination of HPV-16 E6 synthetic peptides conjugated to an optimized Toll-like receptor 2 ligand shows safety and potent T cell immunogenicity in patients with HPV-16 positive (pre-)malignant lesions. J. Immunother. Cancer 2022, 10, e005016. [Google Scholar] [CrossRef]
- Liao, S.-J.; Deng, D.-R.; Zeng, D.; Zhang, L.; Hu, X.-J.; Zhang, W.-N.; Li, L.; Jiang, X.-F.; Wang, C.-Y.; Zhou, J.-F.; et al. HPV16 E5 peptide vaccine in treatment of cervical cancer in vitro and in vivo. J. Huazhong Univ. Sci. Technol. 2013, 33, 735–742. [Google Scholar] [CrossRef]
- Namvar, A.; Panahi, H.A.; Agi, E.; Bolhassani, A. Development of HPV16,18,31,45 E5 and E7 peptides-based vaccines predicted by immunoinformatics tools. Biotechnol. Lett. 2020, 42, 403–418. [Google Scholar] [CrossRef]
- Qi, W.; Qingfeng, L.; Jing, Z.; Maolin, Z.; Zhihui, Z.; Shanli, Z.; Jun, C.; Pengfei, J.; Lifang, Z. A novel multi-epitope vaccine of HPV16 E5E6E7 oncoprotein delivered by HBc VLPs induced efficient prophylactic and therapeutic antitumor immunity in tumor mice model. Vaccine 2022, 40, 7693–7702. [Google Scholar] [CrossRef]
- Maynard, S.K.; Marshall, J.D.; MacGill, R.S.; Yu, L.; Cann, J.A.; Cheng, L.I.; McCarthy, M.P.; Cayatte, C.; Robbins, S.H. Vaccination with synthetic long peptide formulated with CpG in an oil-in-water emulsion induces robust E7-specific CD8 T cell responses and TC-1 tumor eradication. BMC Cancer 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Tang, J.; Yin, R.; Tian, Y.; Huang, Z.; Shi, J.; Fu, X.; Wang, L.; Wu, Y.; Hao, F.; Ni, B. A novel self-assembled nanoparticle vaccine with HIV-1 Tat49-57/HPV16 E749-57 fusion peptide and GM-CSF DNA elicits potent and prolonged CD8+ T cell-dependent anti-tumor immunity in mice. Vaccine 2012, 30, 1071–1082. [Google Scholar] [CrossRef]
- Rahimian, S.; Fransen, M.F.; Kleinovink, J.W.; Christensen, J.R.; Amidi, M.; Hennink, W.E.; Ossendorp, F. Polymeric nanoparticles for co-delivery of synthetic long peptide antigen and poly IC as therapeutic cancer vaccine formulation. J. Control. Release 2015, 203, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, W.; Yuan, M.; Li, W.; Hua, L.; Yang, Z.; Gao, F.; Li, S.; Ye, C.; Chen, Y.; et al. Employing ATP as a New Adjuvant Promotes the Induction of Robust Antitumor Cellular Immunity by a PLGA Nanoparticle Vaccine. ACS Appl. Mater. Interfaces 2020, 12, 54399–54414. [Google Scholar] [CrossRef] [PubMed]
- A Vaccine (PDS0101) and Chemoradiation for the Treatment of Stage IB3-IVA Cervical Cancer, the IMMUNOCERV Trial-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04580771 (accessed on 10 June 2023).
- Dudley, J.P.; Golovkina, T.V.; Ross, S.R. Lessons Learned from Mouse Mammary Tumor Virus in Animal Models. ILAR J. 2016, 57, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.S.; Glenn, W.K. Mouse Mammary Tumour Virus (MMTV) in Human Breast Cancer—The Value of Bradford Hill Criteria. Viruses 2022, 14, 721. [Google Scholar] [CrossRef]
- Stewart, T.H.M.; Sage, R.D.; Stewart, A.F.R.; Cameron, D.W. Breast cancer incidence highest in the range of one species of house mouse, Mus domesticus. Br. J. Cancer 2000, 82, 446–451. [Google Scholar] [CrossRef]
- Stewart, A.F.R.; Chen, H.-H. Revisiting the MMTV Zoonotic Hypothesis to Account for Geographic Variation in Breast Cancer Incidence. Viruses 2022, 14, 559. [Google Scholar] [CrossRef]
- Stanton, S.E.; Gad, E.; Corulli, L.R.; Lu, H.; Disis, M.L. Tumor-associated antigens identified early in mouse mammary tumor development can be effective vaccine targets. Vaccine 2019, 37, 3552–3561. [Google Scholar] [CrossRef]
- Braitbard, O.; Roniger, M.; Bar-Sinai, A.; Rajchman, D.; Gross, T.; Abramovitch, H.; La Ferla, M.; Franceschi, S.; Lessi, F.; Naccarato, A.G.; et al. A new immunization and treatment strategy for mouse mammary tumor virus (MMTV) associated cancers. Oncotarget 2016, 7, 21168–21180. [Google Scholar] [CrossRef]
- Abstract #367: Use of MMTV-p14 as a vaccine against MMTV-Bearing Tumors|Cancer Research|American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/69/9_Supplement/367/556331/Abstract-367-Use-of-MMTV-p14-as-a-vaccine-against (accessed on 7 April 2023).
- Melana, S.M.; Nepomnaschy, I.; Hasa, J.; Djougarian, A.; Djougarian, A.; Holland, J.F.; Pogo, B.G. Detection of human mammary tumor virus proteins in human breast cancer cells. J. Virol. Methods 2010, 163, 157–161. [Google Scholar] [CrossRef]
- Dion, A.S.; Knittel, J.J.; Morneweck, S.T. Virus envelope-based peptide vaccines against virus-induced mammary tumors. Virology 1990, 179, 474–477. [Google Scholar] [CrossRef]
- Arias-Calvachi, C.; Blanco, R.; Calaf, G.M.; Aguayo, F. Epstein–Barr Virus Association with Breast Cancer: Evidence and Perspectives. Biology 2022, 11, 799. [Google Scholar] [CrossRef]
- Bonnet, M.; Guinebretiere, J.-M.; Kremmer, E.; Grunewald, V.; Benhamou, E.; Contesso, G.; Joab, I. Detection of Epstein-Barr Virus in Invasive Breast Cancers. Gynecol. Oncol. 1999, 91, 1376–1381. [Google Scholar] [CrossRef]
- Lin, J.-H.; Tsai, C.-H.; Chu, J.-S.; Chen, J.-Y.; Takada, K.; Shew, J.-Y. Dysregulation of HER2/HER3 Signaling Axis in Epstein-Barr Virus-Infected Breast Carcinoma Cells. J. Virol. 2007, 81, 5705–5713. [Google Scholar] [CrossRef]
- Huo, Q.; Zhang, N.; Yang, Q. Epstein-Barr Virus Infection and Sporadic Breast Cancer Risk: A Meta-Analysis. PLoS ONE 2012, 7, e31656. [Google Scholar] [CrossRef][Green Version]
- He, J.-R.; Tang, L.-Y.; Yu, D.-D.; Su, F.-X.; Song, E.-W.; Lin, Y.; Wang, S.-M.; Lai, G.-C.; Chen, W.-Q.; Ren, Z.-F. Epstein-Barr virus and breast cancer: Serological study in a high-incidence area of nasopharyngeal carcinoma. Cancer Lett. 2011, 309, 128–136. [Google Scholar] [CrossRef]
- Li, W.; Chen, Q.; Chen, H.; Rao, P.; Xue, X.; Chen, S.; Zhu, S.; Zhang, L. Immune response of mice to a latency membrane protein 2 multiepitope antigen of Epstein-Barr virus applied as DNA vaccine and/or peptide vaccine. Acta Virol. 2013, 57, 51–58. [Google Scholar] [CrossRef]
- Lin, M.-C.; Lin, Y.-C.; Chen, S.-T.; Young, T.-H.; Lou, P.-J. Therapeutic vaccine targeting Epstein-Barr virus latent protein, LMP1, suppresses LMP1-expressing tumor growth and metastasis in vivo. BMC Cancer 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Salih, M.M.; Higgo, A.A.; Khalifa, A.S.; Eed, E.M. Incidence of Epstein-Barr Virus Among Women With Breast Cancer Using Monoclonal Antibodies for Latent Membrane Protein 1 (LMP1). Vivo 2022, 36, 1513–1518. [Google Scholar] [CrossRef]
- Olotu, F.A.; Soliman, M.E. Immunoinformatics prediction of potential B-cell and T-cell epitopes as effective vaccine candidates for eliciting immunogenic responses against Epstein–Barr virus. Biomed. J. 2021, 44, 317–337. [Google Scholar] [CrossRef]
- Ali, A.; Khan, A.; Kaushik, A.C.; Wang, Y.; Ali, S.S.; Junaid, M.; Saleem, S.; Cho, W.C.S.; Mao, X.; Wei, D.-Q. Immunoinformatic and systems biology approaches to predict and validate peptide vaccines against Epstein–Barr virus (EBV). Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Omoboyede, V.; Ibrahim, O.; Umar, H.I.; Bello, T.; Adedeji, A.A.; Khalid, A.; Fayojegbe, E.S.; Ayomide, A.B.; Chukwuemeka, P.O. Designing a vaccine-based therapy against Epstein-Barr virus-associated tumors using immunoinformatics approach. Comput. Biol. Med. 2022, 150, 106128. [Google Scholar] [CrossRef] [PubMed]
- Aida, Y.; Murakami, H.; Takahashi, M.; Takeshima, S.-N. Mechanisms of pathogenesis induced by bovine leukemia virus as a model for human T-cell leukemia virus. Front. Microbiol. 2013, 4, 328. [Google Scholar] [CrossRef] [PubMed]
- Buehring, G.C.; Shen, H.M.; Jensen, H.M.; Jin, D.L.; Hudes, M.; Block, G. Exposure to Bovine Leukemia Virus Is Associated with Breast Cancer: A Case-Control Study. PLoS ONE 2015, 10, e0134304. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, H.; Ohashi, K.; Ohishi, K.; Sugimoto, C.; Amanuma, H.; Onuma, M. An effective peptide vaccine to eliminate bovine leukaemia virus (BLV) infected cells in carrier sheep. Vaccine 1996, 14, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Peptide-Based Bovine Leukemia Virus (BLV) Vaccine that Induces BLV-Env Specific Th-1 Type Immunity-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/9209348/ (accessed on 7 April 2023).
- Chugh, S.; Swenson, C.; Yuzbasiyan-Gurkan, V.; Huang, X. Design and Synthesis of Bovine Leukemia Virus-Associated Peptide-Based Qβ Conjugate Eliciting Long-Lasting Neutralizing Antibodies in Mice. ACS Infect. Dis. 2022, 8, 1031–1040. [Google Scholar] [CrossRef]
- Samad, A.; Meghla, N.S.; Nain, Z.; Karpiński, T.M.; Rahman, S. Immune epitopes identification and designing of a multi-epitope vaccine against bovine leukemia virus: A molecular dynamics and immune simulation approaches. Cancer Immunol. Immunother. 2022, 71, 2535–2548. [Google Scholar] [CrossRef]
- Hooshmand, N.; Fayazi, J.; Tabatabaei, S.; Behbahan, N.G.G. Prediction of B cell and T-helper cell epitopes candidates of bovine leukaemia virus (BLV) by in silico approach. Veter.- Med. Sci. 2020, 6, 730–739. [Google Scholar] [CrossRef]
- Fadilah, F.; Linda, E.; Indah, P.R.; Anbar, I.K. Immunoinformatics studies and design of breast cancer multiepitope peptide vaccines: Diversity analysis approach. J. Appl. Pharm. Sci. 2021, 11, 35–45. [Google Scholar] [CrossRef]
- Prawiningrum, A.F.; Paramita, R.I.; Panigoro, S.S. Immunoinformatics Approach for Epitope-Based Vaccine Design: Key Steps for Breast Cancer Vaccine. Diagnostics 2022, 12, 2981. [Google Scholar] [CrossRef]
- Curigliano, G.; Bagnardi, V.; Ghioni, M.; Louahed, J.; Brichard, V.; Lehmann, F.F.; Marra, A.; Trapani, D.; Criscitiello, C.; Viale, G. Expression of tumor-associated antigens in breast cancer subtypes. Breast 2020, 49, 202–209. [Google Scholar] [CrossRef]
- Sanami, S.; Rafieian-Kopaei, M.; Dehkordi, K.A.; Pazoki-Toroudi, H.; Azadegan-Dehkordi, F.; Mobini, G.R.; Alizadeh, M.; Nezhad, M. In silico design of a multi-epitope vaccine against HPV16/18. BMC Bioinform. 2022, 23, 1–24. [Google Scholar] [CrossRef]
- Jabbar, B.; Rafique, S.; Salo-Ahen, O.M.H.; Ali, A.; Munir, M.; Idrees, M.; Mirza, M.U.; Vanmeert, M.; Shah, S.Z.; Jabbar, I.; et al. Antigenic Peptide Prediction From E6 and E7 Oncoproteins of HPV Types 16 and 18 for Therapeutic Vaccine Design Using Immunoinformatics and MD Simulation Analysis. Front. Immunol. 2018, 9, 3000. [Google Scholar] [CrossRef]
- Robles-Oteiza, C.; Wu, C.J. Reinvigorating therapeutic cancer vaccines. Curr. Opin. Immunol. 2022, 76, 102176. [Google Scholar] [CrossRef]
- Sanami, S.; Azadegan-Dehkordi, F.; Rafieian-Kopaei, M.; Salehi, M.; Ghasemi-Dehnoo, M.; Mahooti, M.; Alizadeh, M.; Bagheri, N. Design of a multi-epitope vaccine against cervical cancer using immunoinformatics approaches. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Hussein, W.M.; Toth, I.; Skwarczynski, M. Advances in Peptide-based Human Papillomavirus Therapeutic Vaccines. Curr. Top. Med. Chem. 2012, 12, 1581–1592. [Google Scholar] [CrossRef]
- Dölen, Y.; Gileadi, U.; Chen, J.-L.; Valente, M.; Creemers, J.H.A.; Van Dinther, E.A.W.; van Riessen, N.K.; Jäger, E.; Hruby, M.; Cerundolo, V.; et al. PLGA Nanoparticles Co-encapsulating NY-ESO-1 Peptides and IMM60 Induce Robust CD8 and CD4 T Cell and B Cell Responses. Front. Immunol. 2021, 12, 641703. [Google Scholar] [CrossRef]
- Wallis, J.; Katti, P.; Martin, A.M.; Hills, T.; Seymour, L.W.; Shenton, D.P.; Carlisle, R.C. A liposome-based cancer vaccine for a rapid and high-titre anti-ErbB-2 antibody response. Eur. J. Pharm. Sci. 2020, 152, 105456. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Nanoparticles to Improve the Efficacy of Peptide-Based Cancer Vaccines. Cancers 2020, 12, 1049. [Google Scholar] [CrossRef]
- Duinkerken, S.; Horrevorts, S.K.; Kalay, H.; Ambrosini, M.; Rutte, L.; De Gruijl, T.D.; Garcia-Vallejo, J.J.; Van Kooyk, Y. Glyco-Dendrimers as Intradermal Anti-Tumor Vaccine Targeting Multiple Skin DC Subsets. Theranostics 2019, 9, 5797–5809. [Google Scholar] [CrossRef]
- Petrizzo, A.; Conte, C.; Tagliamonte, M.; Napolitano, M.; Bifulco, K.; Carriero, V.; De Stradis, A.; Tornesello, M.L.; Buonaguro, F.M.; Quaglia, F.; et al. Functional characterization of biodegradable nanoparticles as antigen delivery system. J. Exp. Clin. Cancer Res. 2015, 34, 114. [Google Scholar] [CrossRef]
- Kang, S.; Ahn, S.; Lee, J.; Kim, J.Y.; Choi, M.; Gujrati, V.; Kim, H.; Kim, J.; Shin, E.-C.; Jon, S. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J. Control. Release 2017, 256, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Taguchi, H. Nanoparticle-Based Peptide Vaccines. Micro Nanotechnol. Vaccine Dev. 2017, 149–170. [Google Scholar] [CrossRef]
- Firdaus, F.Z.; Bartlett, S.; Hussein, W.M.; Lu, L.; Wright, Q.; Huang, W.; Nahar, U.J.; Yang, J.; Khongkow, M.; Veitch, M.; et al. Liposomal Formulations of a Polyleucine–Antigen Conjugate as Therapeutic Vaccines against Cervical Cancer. Pharmaceutics 2023, 15, 602. [Google Scholar] [CrossRef] [PubMed]
- Buonaguro, L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M. Developments in virus-like particle-based vaccines for infectious diseases and cancer. Expert Rev. Vaccines 2014, 10, 1569–1583. [Google Scholar] [CrossRef]
- Besson, S.; Boucher, E.; Laurin, D.; Manches, O.; Aspord, C.; Hannani, D.; Fender, P. Stimulation of the immune system by a tumor antigen-bearing adenovirus-inspired VLP allows control of melanoma growth. Mol. Ther.-Methods Clin. Dev. 2022, 28, 76–89. [Google Scholar] [CrossRef]
- Hartmaier, R.J.; Charo, J.; Fabrizio, D.; Goldberg, M.E.; Albacker, L.A.; Pao, W.; Chmielecki, J. Genomic analysis of 63,220 tumors reveals insights into tumor uniqueness and targeted cancer immunotherapy strategies. Genome Med. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Desrichard, A.; Snyder, A.; Chan, T.A. Cancer Neoantigens and Applications for Immunotherapy. Clin. Cancer Res. 2016, 22, 807–812. [Google Scholar] [CrossRef]
- Slingluff, C.L.; Slingluff, C.L., Jr. The Present and Future of Peptide Vaccines for Cancer. Cancer J. 2011, 17, 343–350. [Google Scholar] [CrossRef]
- Bijker, M.S.; Eeden, S.J.F.v.D.; Franken, K.L.; Melief, C.J.M.; van der Burg, S.H.; Offringa, R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur. J. Immunol. 2008, 38, 1033–1042. [Google Scholar] [CrossRef]
- Dalvi, H.; Bhat, A.; Iyer, A.; Jyothi, V.G.S.S.; Jain, H.; Srivastava, S.; Madan, J. Armamentarium of Cryoprotectants in Peptide Vaccines: Mechanistic Insight, Challenges, Opportunities and Future Prospects. Int. J. Pept. Res. Ther. 2021, 27, 2965–2982. [Google Scholar] [CrossRef]
- Moldovan, I.; Targoni, O.; Zhang, W.; Sundararaman, S.; Lehmann, P.V. How frequently are predicted peptides actually recognized by CD8 cells? Cancer Immunol. Immunother. 2016, 65, 847–855. [Google Scholar] [CrossRef]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy—New insights into old paradigms. Cancer Gene Ther. 2020, 28, 5–17. [Google Scholar] [CrossRef]
- Abdala, A.; Alvarez, I.; Brossel, H.; Calvinho, L.; Carignano, H.; Franco, L.; Gazon, H.; Gillissen, C.; Hamaidia, M.; Hoyos, C.; et al. BLV: Lessons on vaccine development. Retrovirology 2019, 16, 1–6. [Google Scholar] [CrossRef]
- Tang, M.; Cai, J.-H.; Diao, H.-Y.; Guo, W.-M.; Yang, X.; Xing, S. The progress of peptide vaccine clinical trials in gynecologic oncology. Hum. Vaccines Immunother. 2022, 18, 2062982. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).