Abstract
2,3,4-trisubstituted thiazoles 3a–i, having a methyl group in position four, were synthesized by the reaction of 1,4-disubstituted thiosemicarbazides with chloroacetone in ethyl acetate/Et3N at room temperature or in ethanol under reflux. The structures of new compounds were determined using NMR spectroscopy, mass spectrometry, and elemental analyses. Moreover, the structure of compound 3a was unambiguously confirmed with X-ray analysis. The cell viability assay of 3a–i at 50 µM was greater than 87%, and none of the tested substances were cytotoxic. Compounds 3a–i demonstrated good antiproliferative activity, with GI50 values ranging from 37 to 86 nM against the four tested human cancer cell lines, compared to the reference erlotinib, which had a GI50 value of 33 nM. The most potent derivatives were found to be compounds 3a, 3c, 3d, and 3f, with GI50 values ranging from 37 nM to 54 nM. The EGFR-TK and BRAFV600E inhibitory assays’ results matched the antiproliferative assay’s results, with the most potent derivatives, as antiproliferative agents, also being the most potent EGFR and BRAFV600E inhibitors. The docking computations were employed to investigate the docking modes and scores of compounds 3a, 3c, 3d, and 3f toward BRAFV600E and EGFR. Docking computations demonstrated the good affinity of compound 3f against BRAFV600E and EGFR, with values of −8.7 and −8.5 kcal/mol, respectively.
1. Introduction
Kinases control many essential cancer processes, including tumor growth, metastasis, neovascularization, and treatment resistance. Hence, the development of kinase inhibitors has become a top priority, with several of them receiving FDA approval for a variety of cancer purposes [1,2,3,4,5].
One approach for simultaneously inhibiting two or more targets is combination chemotherapy. However, two or more drugs’ pharmacokinetic profiles and metabolic stabilities frequently differ. Furthermore, drug–drug interactions may occur during combination chemotherapy [6,7,8]. An alternative method for addressing these issues is to use a single drug to suppress two or more targets [9,10,11,12]. This approach may even make patients’ treatment easier. The FDA has approved many dual-target or multi-target cancer treatments. Dasatinib is a multi-targeted kinase inhibitor that can potentially be a highly effective anticancer medication [13,14,15,16,17].
The acquired BRAFV600E mutation was suggested as a resistance mechanism after therapy with an EGFR inhibitor [18,19]. The development of resistance in colorectal cancer was also linked to the feedback stimulation of EGFR signaling [20,21,22]. Additionally, BRAF inhibition can cause EGFR to become active, promoting tumor growth [23,24]. A BRAF/EGFR combination was used to adopt these issues. In a number of studies, the BRAF/EGFR combination was found to have a significant therapeutic effect in patients with metastatic colorectal cancer that had BRAFV600E mutations [18,25,26]. As a result, sequentially inhibiting the two kinases may provide a solution to the EGFR activation problem.
Thiazole and its derivatives are also among the most active chemicals, ranking first in anticancer activity [27,28,29]. As well, thiazole-containing molecules were identified in a number of therapeutically available anticancer medicines (Figure 1), including tiazofurin (I) [30], dasatinib (II) [31,32], and dabrafenib (III) [33,34].
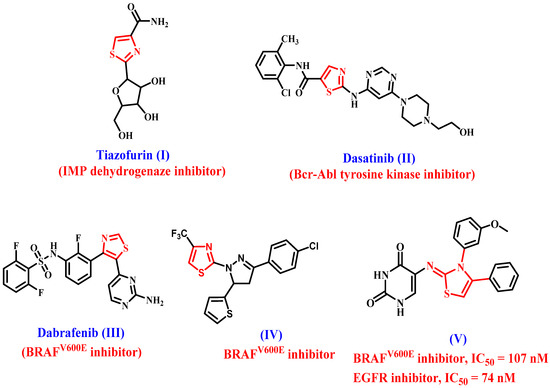
Figure 1.
Structures of thiazole-based anticancer drugs I–III and compounds IV and V.
Abdel-Maksoud et al. [35] investigated several thiazole-based compounds as potential BRAFV600E inhibitors. Compound IV (Figure 1) had the most potent antiproliferative activity, with a competitive BRAFV600E inhibitory action (IC50 = 0.05 µM). Furthermore, compound IV significantly affected dose-dependent apoptosis.
We recently reported on the design and synthesis of two series of thiazole-based compounds as potent antiproliferative agents targeting EGFR and BRAFV600E [36]. Compound V (Figure 1) was shown to be the most potent derivative of all synthesized compounds, with a GI50 value of 0.90 µM against the four evaluated cancer cell lines when compared to the reference doxorubicin (GI50 = 1.10 µM). Compound V inhibited EGFR and BRAFV600E with IC50 values of 74 ± 7 and 107 ± 10 nM, respectively, and was more effective than erlotinib against EGFR (IC50 = 80 nM).
Moreover, the sulfonamide moiety is commonly employed in medicinal chemistry as efficient bioisosteres of the carboxylic group [37,38]. The sulfonamide motif could build a network of hydrogen bonds similar to the carboxylic group. As the carboxylic group’s bioisosteres, it could avoid some of the carboxylic group’s limitations, such as metabolic instability, toxicity, and limited passive diffusion across biological membranes [37]. As a result, the sulfonamide moiety gained popularity in medicinal chemistry, and a wide range of sulfonamide derivatives with diverse biological properties, such as anticancer activity [39,40,41], were produced.
In light of the aforementioned information, and as part of our enduring effort to develop potent antiproliferative agents that are dual inhibitors of EGFR and BRAFV600E [42,43,44,45], we describe the synthesis of a new set of thiazole-based compounds 3a–i (Figure 2) in this article as antiproliferative agents that target EGFR and/or mutant BRAF. Scaffold A and B molecules had a methyl group in position 4, a hydrazo group in position 2, a physiologically active tosyl group for the scaffold B compounds, and a 2,4-dinitrophenyl group for the scaffold A compounds (Figure 2). The cell viability of the novel derivatives was tested against a normal human mammary gland epithelial (MCF-10A) cell line. The antiproliferative action of 3a–i was tested on a panel of four human cancer cell lines. The ability to inhibit EGFR and mutant BRAF was further assessed for the most active antiproliferative derivatives. Finally, the most potent compounds’ binding modes and docking scores toward BRAFV600E and EGFR targets were investigated.
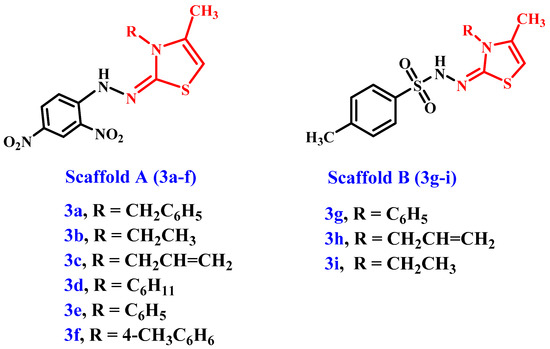
Figure 2.
The new target compounds’ 3a–i structures.
2. Results and Discussion
2.1. Chemistry
This study aimed to develop new thiazole derivatives in a straightforward manner. Indeed, a novel series of (Z)-3-substituted-2-(2-substituted)-hydrazinylidene)-4-methyl-2,3-dihydrothiazoles 3a–i were synthesized in an excellent yield of 78–99% via a mixture of substituted hydrazine-carbothioamides 1a–i [46,47,48,49] and chloroacetone (2) in ethyl acetate as a solvent. Et3N catalyzed the reaction by stirring overnight at room temperature or refluxing in ethanol for 6–10 hrs (Scheme 1). For example, compound 3a was obtained in (AcOEt/Et3N: 98%) and (EtOH: 85%) yield after recrystallization (Table 1). The structure assignment for all obtained products 3a–i was confirmed by IR, NMR analysis (Supplementary File Figures S2–S32) of the expected chemical shifts, mass spectrometry, elemental analysis, and X-ray crystallography.
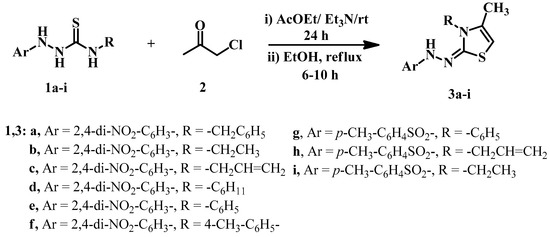
Scheme 1.
Syntheses of substituted thiazoles 3a–i.

Table 1.
Yield percentage of 3a–i via method A and method B.
Compound 3a was chosen as a representative example, which was assigned as (Z)-3-benzyl-2-(2-(2,4-dinitrophenyl)hydrazinylidene)-4-methyl-2,3-dihydrothiazole and exhibited a molecular formula C17H15N5O4S with mass m/z (385). Elemental analysis and mass spectrometry confirmed that 3a was formed by an interaction between one mole of N-benzyl-2-(2,4-dinitrophenyl)hydrazinecarbothioamide (1a) and one mole of chloroacetone (2), with the loss of a molecule of HCl and another molecule of H2O. FTIR analysis of thiazole compound 3a showed different peaks at 3286 cm−1 due to hydrazo-NH stretching, 3085 cm−1 for aromatic stretching-CH, and 2978 cm−1 for aliphatic-CH as well as two peaks at 1609 and 1548 cm−1 for C=N and C=C, respectively. IR showed a peak at 1399 and 1117 cm−1 for the NO2 group. Further, in the 1H NMR spectrum of 3a, five singlet signals were distinguished at δH = 1.98, 5, 6.42, 8.68, and 10.38 ppm, which were assigned as CH3, benzyl-CH2, thiazole-H, 2,4-dinitrophenyl-H-3, and hydrazono-NH, respectively (Figure 3). The 13C NMR spectrum for compound 3a revealed signals at δC = 13.78, 47.16, and 94.49 ppm, which were assigned as CH3, benzyl-CH2, and thiazole-C5, respectively. Moreover, C4 and C2 gave signals at δC = 135.13 and 164.14 ppm, respectively.
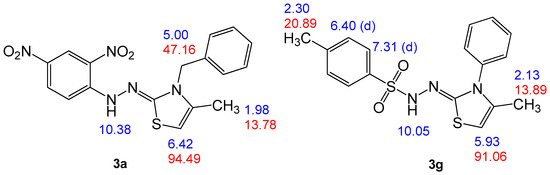
Figure 3.
Structure elucidation of compounds 3a and 3g.
To make comparable results, we chose another compound, 3g, which was assigned as (Z)-4-methyl-N′-(4-methyl-3-phenylthiazol-2(3H)-ylidene) benzenesulfonohydrazide, with a molecular formula C17H17N3O2S2 (m/z = 359). Compound 3g is analogous for the above compound by replacing the benzyl group and 2,4-dinitrophenyl with phenyl and 4-methyl-benzensulfonoyl groups, respectively. The 1H NMR for this compound is similar for compound 3a unless the 1,4-disubstituted benzene gives characteristic signals as a doublet at δH = 6.40 (d, 2H) and 7.31 ppm (d, 2H), which were assigned as H-m and H-o, respectively. Moreover, by comparing the data for the two compounds, as shown in Figure 3, it is clear that the reaction behaves the same with the difference in the substitutes and that the difference is a slight difference in the chemical shift’s results for the difference only in the nature of the substituted groups.
Another example is compound 3b, which was assigned as (Z)-2-(2-(2,4-dinitrophenyl)-hydrazinylidene)-3-ethyl-4-methyl-2,3-dihydrothiazole and has the same spectral data as compound 3a except that the benzyl group was replaced with ethyl, which gives two characteristic signals as triplet–quartet and appears in its 1H NMR spectrum at δH = 1.28–1.38 (t, J = 3 Hz; 3H, ethyl-CH3) and 3.88–3.98 ppm (q, J = 3 Hz; 2H, ethyl-CH3) and was confirmed from its 13C NMR spectrum, with two signals at δH = 12.98 (ethyl-CH3) and 30.67 ppm (ethyl-CH2).
Furthermore, the structures for the obtained products were confirmed via X-ray crystallography. Moreover, the X-ray measurements of compound 3b showed that the molecule (except the C-atom of the ethyl substituent, C21) is virtual planar. The aromatic ring is coplanar with the thiazole ring, and the ethyl group has the hours conformation structure. The angle between the thiazole and the aromatic ring is 6.37(7)°, between the thiazole and the hydrazinylidene moiety is 2.98(14)°, and between the aromatic ring and the hydrazinylidene moiety is 6.56(9)° (angle between the L.S. planes of the moieties). In addition, the geometric structure around the exocyclic C=N has cissoid geometry concerning the thiazole S-atom and the hydrazo-group (Figure 4). The geometrical parameters (selected bond distance, bond angles, and dihedral angles; see Table 2) are in good correlation with the theoretical values.
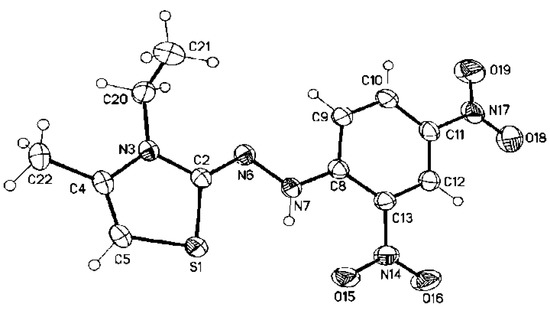
Figure 4.
The crystal structure of (Z)-2-(2-(2,4-dinitrophenyl)hydrazinylidene)-3-ethyl-4-methyl-2,3-dihydrothiazole 3b.

Table 2.
Selected geometric parameters (Å, °) for 3b.
Based on the above results and the X-ray confirmation of our obtained products, the proposed mechanism is as follows. First, the nucleophilic attack of sulfur on the primary carbon atom results in the formation of the intermediate, B (S-alkylation), via the transition state, A. Another nucleophilic attack on the nitrogen atom on the carbonyl carbon gives the intermediate, C, followed by water molecule (dehydration) loss to give the target product. The reaction mechanism proceeds via the SN2 reaction type (Scheme 2).
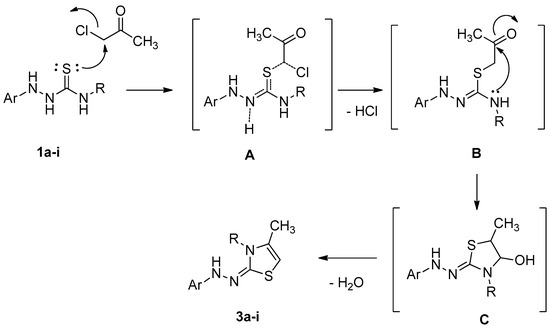
Scheme 2.
The hypothesized mechanism for the synthesis of thiazole compounds 3a–i.
2.2. Biology
2.2.1. Cell Viability Assay
The human mammary gland epithelial (MCF-10A) cell line was used to test the viability of the novel compounds [50,51]. After four days of incubation on MCF-10A cells, the vitality of compounds 3a–i was determined using the MTT method. According to Table 3, the cell viability at 50 µM was greater than 87% for all tested agents, and none of the tested substances were cytotoxic.

Table 3.
IC50 values of compounds 3a–i against four cancer cell lines.
2.2.2. Antiproliferative Assay
The MTT assay was used to investigate the antiproliferative activity of 3a–i against four human cancer cell lines: the colon cancer (HT-29) cell line, pancreatic cancer (Panc-1) cell line, lung cancer (A-549) cell line, and breast cancer (MCF-7) cell line, using erlotinib as the reference [52,53]. Table 3 shows the median inhibitory concentration (IC50).
In general, the examined compounds 3a–i displayed good antiproliferative activity, with average IC50 (GI50) values ranging from 37 to 86 nM against the four tested human cancer cell lines, compared to the reference erlotinib (GI50 = 33 nM).
The most potent derivatives were compounds 3a, 3c, 3d, and 3f, with GI50 values ranging from 37 nM to 54 nM. Compound 3f (Ar = 2,4-di-NO2-C6H3, R = 4-CH3-C6H5) was the most potent derivative of all synthesized compounds, with a GI50 value of 37 nM against the four tested human cancer cell lines, comparable to the reference erlotinib (GI50 = 33 nM). By replacing the p-tolyl group in compound 3f with a cyclohexyl moiety, compound 3d (Ar = 2,4-di-NO2-C6H3, R = C6H11) was found to be the second-most potent compound, with a GI50 value of 46 nM, being 1.3-fold less potent than compound 3f, demonstrating the importance of the p-tolyl moiety in antiproliferative activity.
The benzyl derivative, 3a (Ar = 2,4-di-NO2-C6H3, R = CH2-C6H5), was less potent than 3f and 3d, with a GI50 value of 48 nM against the tested four cancer cell lines, while the allyl derivatives, 3c (Ar = 2,4-di-NO2-C6H3, R = CH2CH = CH2), showed moderate antiproliferative activity, with a GI50 value more than 50 nM. These findings show that allyl and benzyl groups are not preferred for the antiproliferative activity of scaffold A compounds 3a–f.
The remaining scaffold A compounds, 3b (Ar = 2,4-di-NO2-C6H3, R = CH2CH3) and 3e (Ar = 2,4-di-NO2-C6H3, R = C6H5), had GI50 values of 80 nM and 72 nM, respectively. Compounds 3b and 3e were 2.2- and 2-fold less potent than 3f, respectively, indicating weak antiproliferative activity (Table 3).
With GI50 values of 60 nM, 65 nM, and 86 nM, scaffold B compounds 3g, 3h, and 3i demonstrated moderate-to-weak antiproliferative activity. Compound 3i (Ar = p-CH3-C6H4-SO2, R = CH2CH3) was the least potent derivative of any of the synthesized compounds, with a GI50 value of 86 nM, which is less potent than its congeners, 3b (scaffold A), which has the same structure, but the aryl moiety was p-CH3-C6H4-SO2, while in 3b it was 2,4-di-NO2-C6H3. These findings demonstrated that 2,4-di-NO2-C6H3 significantly affects the antiproliferative action of the newly synthesized compounds.
2.2.3. Assay for EGFR Inhibition
The most promising antiproliferative compounds, 3a, 3c, 3d, and 3f, were further evaluated for their suppressive impact on EGFR as a probable target for their mechanism of action [50,54,55]. Table 4 compares the IC50 values to erlotinib, which worked as a control.

Table 4.
IC50 of compounds 3a, 3c, 3d, and 3f against EGFR and BRAFV600E.
The EGFR-TK inhibitory assay results matched the antiproliferative assay results, with the most potent derivatives, as antiproliferative agents, also being the most potent EGFR inhibitors. Compounds 3a, 3c, 3d, and 3f inhibited EGFR, with IC50 values ranging from 89 to 98 nM, but the tested compounds were less potent than erlotinib (IC50 = 80 nM). The most potent antiproliferative agent, compound 3f (Ar = 2,4-di-NO2-C6H3, R = 4-CH3-C6H5), was also the most potent EGFR inhibitor, with an IC50 value of 89 ± 7, being 1.1-fold less potent than standard erlotinib.
Compounds 3a (Ar = 2,4-di-NO2-C6H3, R = CH2-C6H5) and 3d (Ar = 2,4-di-NO2-C6H3, R = C6H11) ranked third and second in EGFR suppression, with IC50 values of 93 ± 8 and 91 ± 7 nM, respectively, being 1.15-fold less potent than erlotinib (IC50 = 80 ± 5 nM). These findings suggest that EGFR-TK could be a molecular target for the tested compound’s antiproliferative action.
2.2.4. BRAFV600E Inhibitory Assay
Derivatives 3a, 3c, 3d, and 3f were further investigated as possible BRAFV600E inhibitors [56]. Table 4 displays the IC50 values compared to erlotinib, which was used as a control. According to Table 4, the evaluated derivatives had a promising BRAFV600E suppressive action, with IC50 values ranging from 93 to 126 nM, making them approximately 1.5-fold less effective than erlotinib (IC50 = 60 nM). Compound 3f, the most potent derivative in the antiproliferative and EGFR suppressive assays, was also the most effective derivative as anti-BRAFV600E (IC50 = 93 ± 8 nM). These findings show that compound 3a has potent antiproliferative activity as a dual EGFR/BRAFV600E inhibitor, implying that further structural modifications may be required to obtain a more potent lead compound for future development.
2.3. In Silico Study
AutoDock4.2.6 software was used to investigate the binding scores and poses of compounds 3a, 3c, 3d, and 3f against BRAFV600E and EGFR. The estimated docking features and scores are listed in Table 5. As tabulated in Table 5, all inspected compounds revealed good docking scores against BRAFV600E and EGFR targets, ranging from −7.8 to −8.7 kcal/mol and from −7.9 to −8.5 kcal/mol, respectively. The good docking scores of the inspected compounds toward BRAFV600E and EGFR may be imputed to their capability of forming H-bonds and vdW, pi-based, and hydrophobic interactions with the proximal residues within the active sites of the investigated targets (Table 5).

Table 5.
Predicted binding features and docking scores for the top investigated compounds toward EGFR and BRAFV600E.
Compound 3f demonstrated superior docking scores of −8.7 and −8.5 kcal/mol against BRAFV600E and EGFR, respectively. Inspecting the docking mode of compound 3f with the EGFR active site unveiled that this compound formed one H-bond with CYS773 (2.18 Å). Moreover, compound 3f exhibited two carbon–hydrogen bonds with LEU768 and pi–anion interaction with ASP831. On the other hand, compound 3f, complexed with BRAFV600E, demonstrated three H-bonds with LYS483 (2.15 Å), THR529 (2.38 Å), and ASP594 (2.12 Å). Additionally, compound 3f established pi–cation interaction with LYS483 and pi–pi stacking interaction with PHE583 and TRP531 residues (Figure 5).
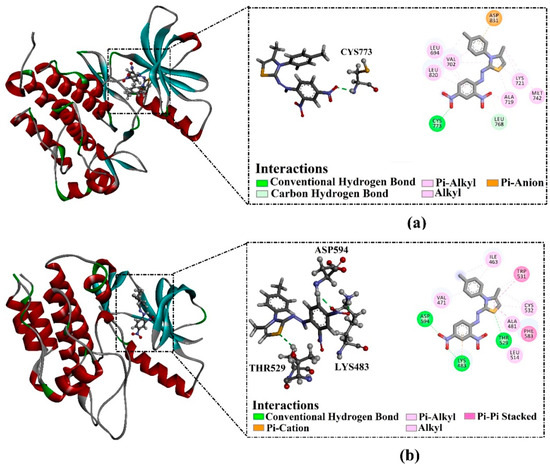
Figure 5.
Molecular interactions of compound 3f within (a) EGFR and (b) BRAFV600E active sites.
Compound 3d showed the second-lowest docking score, with values of −8.5 and −8.1 kcal/mol against BRAFV600E and EGFR, respectively. Compound 3d displayed one hydrogen bond with the CYS773 (2.17 Å) within the active site of EGFR. However, compound 3d demonstrated four H-bonds with the LYS483 (1.93Å), GLY596 (2.32 Å), THR529 (2.66 Å), and ASP594 (2.14 Å) of BRAFV600E.
Compound 3a exposed the third-lowest docking score, with values of −8.4 and −8.0 kcal/mol against BRAFV600E and EGFR, respectively (Table 5). Compound 3a made one H-bond with the CYS773 (2.21 Å) of EGFR, while compound 3a exhibited three H-bonds with the LYS483 (2.15 Å), THR529 (2.38 Å), and ASP594 (2.12 Å) within the BRAFV600E binding pocket.
Compound 3c also unveiled good docking scores, with values of −7.8 and −7.9 kcal/mol against BRAFV600E and EGFR, respectively (Table 5). Observably, compound 3c established one H-bond with the CYS773 (2.15 Å) of EGFR and two H-bonds with the LYS483 (2.13 Å) and ASP593 (1.93 Å) of BRAFV600E.
Erlotinib, a reference drug, showed docking scores of −8.4 and −8.6 kcal/mol toward BRAFV600E and EGFR, respectively (Table 5). From Table 5, erlotinib demonstrated two H-bonds with the CYS773 (1.91 Å) and MET769 (1.62 Å) within the EGFR binding pocket. In addition, erlotinib also displayed two H-bonds with CYS532 (2.02 Å) and THR529 (2.07 Å).
3. Material and Methods
3.1. Chemistry
General information: refer to Supplementary Information.
The starting materials, 1a–i, were synthesized in accordance with the documented methods [46,47,48]
3.1.1. General Procedure of Synthesis of Trisubstituted Thiazoles 3a–i
Method A
In a conical flask containing 10 mL ethyl acetate and two drops of Et3N as a catalyst, 0.092 gm of chloroacetone were dissolved (2). To this mixture, 1 mmol of thiosemicarbazides 1a–i in 10 mL ethyl acetate was added drop by drop while stirring. After addition was complete, the reaction mixture was stirred for 24 h. The reaction mixture was monitored with TLC. After the reaction was completed, the formed precipitate was filtered off and recrystallized from ethanol to afford products 3a–i as fine crystals.
Method B
In a 50 mL round-bottom flask containing 20 mL absolute ethanol, a molar ratio (1:1) mixture of chloroacetone and substituted thiosemicarbazides 1a–i was added. The flask was fitted with a condenser and was refluxed for 6–10 h. The reaction was monitored with TLC to assure the reaction completion. Then, the reaction mixture was cooled to room temperature, and the formed precipitate was filtered off and recrystallized from ethanol to afford products 3a–i.
(Z)-3-Benzyl-2-(2-(2,4-Dinitrophenyl)Hydrazineylidene)-4-Methyl-2,3-Dihydrothiazole (3a)
This compound was found as red crystals from methanol in (98% and 85%) yield, with m.p., 215–217 °C; 1H NMR (DMSO-d6): δ 1.98 (s, 3H, CH3), 5.00 (s, 2H, benzyl-CH2), 6.42 (s, 1H, H-5), 7.10–8.28 (m, 6H, Ar-H), 7.96–8.01 (d, 1H, Ar-H), 8.68 (s, 1H, Ar-H), 10.38 (s, 1H, NH) ppm; 13C NMR (DMSO-d6): δ 13.7 (CH3), 47.1 (benzyl-CH2), 94.5 (C5), 115.5, 123.4, 126.6, 127.4, 127.7, 128.7 (Ar-CH), 129.5, 136.7, 136.7, 144.3 (Ar-C), 135.1 (C4), 164.1 (C2) ppm; IR: ν = 3286 (NH),3085 (Ar-CH), 2978 (ali-CH), 1609 (C=N), 1548 (Ar-C=C), 1399, 1117 (NO2) cm−1; MS (70 eV): m/z (%) = 385 (M+, 30). Anal. Calcd. For C17H15N5O4S (385.40): C, 52.98; H, 3.92; N, 18.17; S, 8.32. Found: C, 52.89; H, 3.81; N, 18.06; S, 8.23.
(Z)-2-(2-(2,4-Dinitrophenyl)Hydrazineylidene)-3-Ethyl-4-Methyl-2,3-Dihydrothiazole (3b)
This compound was found as red crystals from methanol in (99% and 84%) yield, with m.p., 176–177 °C; 1H NMR (DMSO-d6): δ 1.28–1.38 (t, 3H, J = 3, CH3), 2.1 (s, 3H, CH3), 3.88–3.98 (q, 2H, J = 3, CH2), 6.14 (s, 1H, H-5), 7.48–7.54 (d, 1H, Ar-H), 8.22–8.28 (d, 1H, Ar-H), 8.82 (s, 1H, Ar-H), 10.46 (s, 1H, NH) ppm; 13C NMR (DMSO-d6): δ 12.9 (ethyl-CH3), 13.4 (CH3), 30.6 (ethyl-CH2), 94.0 (C5), 115.5, 123.4, 127.6 (Ar-CH), 129.7, 136.8, 144.3 (Ar-C), 135.0 (C4), 163.6 (C2) ppm. IR: ν = 3115 (NH), 3078 (Ar-CH), 2988 (ali-CH), 1612 (C=N), 1557 (Ar-C=C), 1373, 1132 (NO2). MS (70 eV): m/z (%) = 323 (M+, 54). Anal. Calcd. For C12H13N5O4S (323.33): C, 44.58; H, 4.05; N, 21.66; S, 9.92. Found: C, 44.46; H, 3.98; N, 21.57; S, 9.87.
(Z)-3-Allyl-2-(2-(2,4-Dinitrophenyl)Hydrazineylidene)-4-Methyl-2,3-Dihydrothiazole (3c)
This compound was found as red crystals from methanol in (96% and 83%) yield, with m.p. 197–198 °C; 1H NMR (DMSO-d6): δ 2.16 (s, 3H, CH3), 4.52–4.58 (m, 2H, allyl-CH2), 5.10–5.28 (m, 2H, allyl-CH2=), 5.96–6.06 (m, 1H, allyl-CH=), 6.16 (s, 1H, H-5), 7.46–7.52 (d, 1H, Ar-H), 8.22–8.28 (d, 1H, Ar-H), 8.85 (s, 1H, Ar-H), 10.48 (s, 1H, NH) ppm; 13C NMR (DMSO-d6): δ 13.4 (CH3), 46.1 (allyl-CH2), 94.17 (C5), 116.6 (allyl-CH2=), 115.6, 123.4, 128.3 (Ar-CH), 129.7, 136.9, 144.3 (Ar-C), 135.2 (C4), 136.9 (allyl-CH=), 164.4 (C2) ppm. IR: ν = 3105 (NH),3093 (Ar-CH), 2978 (ali-CH), 1606 (C=N), 1562 (Ar-C=C), 1374, 1206 (NO2). MS (70 eV): m/z (%) = 335 (M+, 93). Anal. Calcd. For C13H13N5O4S (335.34): C, 46.56; H, 3.91; N, 20.88; S, 9.56. Found: C, 46.48; H, 3.85; N, 20.79; S, 9.47.
(Z)-3-Cyclohexyl-2-(2-(2,4-Dinitrophenyl)Hydrazineylidene)-4-Methyl-2,3-Dihydrothi-Azole (3d)
This compound was found as red crystals from methanol in (94% and 78%) yield, with m.p., 204–206 °C; 1H NMR (DMSO-d6): δ 1.34–1.46 (m, 10H, cyclohexyl-CH2), 1.69–184 (m, 1H, cyclohexyl-CH), 2.21 (s, 3H, CH3), 6.10 (s, 1H, H-5), 7.36–7.39 (d, 1H, Ar-H), 8.29–8.32 (dd, 1H, Ar-H), 8.84–8.85 (d, 1H, Ar-H), 10.48 (s, 1H, NH) ppm. IR: ν = 3110 (NH),3015 (Ar-CH), 2925 (ali-CH), 1608 (C=N), 1544 (Ar-C=C), 1323, 1034 (NO2). MS (70 eV): m/z (%) = 377 (M+, 80) Anal. Calcd. For C16H19N5O4S (377.42): C, 50.92; H, 5.07; N, 18.56; S, 8.49. Found: C, 50.82; H, 4.93; N, 18.48; S, 8.43.
(Z)-2-(2-(2,4-Dinitrophenyl)Hydrazineylidene)-4-Methyl-3-Phenyl-2,3-Dihydrothiazole (3e)
This compound was found as red crystals from methanol in (94% and 83%) yield, with m.p., 231–233 °C; 1H NMR (DMSO-d6): δ 1.80 (s, 3H, CH3), 6.34 (s, 1H, H-5), 7.14–7.17 (d, 1H, Ar-H), 7.49–7.36 (m, 5H, Ar-H), 8.26–8.30 (dd, 1H, Ar-H), 8.85–8.86 (d, 1H, Ar-H), 10.47 (s, 1H, NH) ppm; 13C NMR (DMSO-d6): δ 14.5 (CH3), 95.3 (C5), 115.5, 123.3, 127.8, 128.5, 128.9, 129.6 (Ar-CH), 129.7, 136.2, 136.5, 144.5 (Ar-C), 135.2 (C4), 165.5 (C2) ppm; IR: ν = 3226 (NH), 3118 (Ar-CH), 2975 (ali-CH), 1603 (C=N),1555 (Ar-C=C), 1355, 1133 (NO2) cm−1; MS (70 eV): m/z (%) = 371 (M+, 8). Anal. Calcd. For C16H13N5O4S (371.37): C, 51.75; H, 3.53; N, 18.86; S, 8.63. Found: C, 51.68; H, 3.49; N, 18.79; S, 8.52.
(Z)-2-(2-(2,4-Dinitrophenyl)Hydrazineylidene)-4-Methyl-3-(p-Tolyl)-2,3-Dihydrothi- Azole (3f)
This compound was found as red crystals from methanol in (92% and 86%) yield, with m.p., 198–199 °C; 1H NMR (DMSO-d6): δ 1.87 (s, 3H, CH3), 2.40 (s, 3H, tolyl-CH3), 6.32 (s, 1H, H-5), 7.14–7.17 (d, 1H, Ar-H), 7.35–7.39 (m, 4H, Ar-H), 8.19–8.24 (dd, 1H, Ar-H), 8.81–8.83 (d, 1H, Ar-H), 10.47 (s, 1H, NH) ppm; 13C NMR (DMSO-d6): δ 14.5 (CH3), 20.7 (tolyl-CH3), 95.1 (C5), 115.5, 123.6, 127.8, 128.2, 129.7 (Ar-CH), 130.1, 134.1, 137.5, 138.4, 144.4 (Ar-C), 136.1 (C4), 165.4 (C2) ppm. IR: ν = 3226 (NH),3088 (Ar-CH), 2945 (ali-CH), 1607 (C=N), 1559 (Ar-C=C), 1383, 1172 (NO2). MS (70 eV): m/z (%) = 385 (M+, 13). Anal. Calcd. For C17H15N5O4S (385.40): C, 52.98; H, 3.92; N, 18.17; S, 8.32. Found: C, 52.89; H, 3.87; N, 18.11; S, 8.25.
(Z)-4-Methyl-N′-(4-Methyl-3-Phenylthiazol-2(3H)-Ylidene) Benzenesulfonohydrazide (3g)
This compound was found as pale-yellow crystals from methanol in (90% and 83%) yield, with m.p., 193–194 °C; 1H NMR (DMSO-d6): δ 2.13 (s, 3H, CH3), 2.30 (s, 3H, tolyl-CH3), 5.93 (s, 1H, H-5), 6.40 (d, 2H, tolyl-H-m), 6.93 (m, 1H, Ar-H), 7.19 (m, 2H, Ar-H), 7.31 (d, 2H, tolyl-H-o), 7.69 (m, 2H, Ar-H), 10.92 (s, 1H, NH) ppm; 13C NMR (DMSO-d6): δ 13.8 (CH3), 20.9 (tolyl-CH3), 91.0 (C5), 120.5, 122.9, 127.7, 128.9, 129.2 (Ar-CH), 137.6, 143.5, 148.5 (Ar-C), 136.0 (C4), 155.2 (C2) ppm. IR: ν = 3130 (NH),3062 (Ar-CH), 2920 (ali-CH), 1595 (C=N), 1561 (Ar-C=C). MS (70 eV): m/z (%) = 359 (M+, 100). Anal. Calcd. For C17H17N3O2S2 (359.46): C, 56.80; H, 4.77; N, 11.69; S, 17.84. Found: C, 56.73; H, 4.69; N, 11.63; S, 17.73.
(Z)-N′-(3-Allyl-4-Methylthiazol-2(3H)-Ylidene)-4-Methyl Benzenesulfonohydrazide (3h)
This compound was found as pale-yellow crystals from methanol in (89% and 83%) yield, with m.p., 236–238 °C. IR: ν = 3115 (NH),3078 (Ar-CH), 2988 (ali-CH), 1612 (C=N), 1557 (Ar-C=C), 1373, 1132 (NO2) cm−1. MS (70 eV): m/z (%) = 323 (M+, 100). Anal. Calcd. For C14H17N3O2S2 (323.43): C, 51.99; H, 5.30; N, 12.99; S, 19.83. Found: C, 51.91; H, 5.23; N, 12.92; S, 19.78.
(Z)-N′-(3-Ethyl-4-Methylthiazol-2(3H)-Ylidene)-4-Methyl Benzenesulfonohydrazide (3i)
This compound was found as pale-yellow crystals from methanol in (87% and 78%) yield, with m.p., 236–238 °C. 1H NMR (DMSO-d6): δ 1.26–1.39 (t, 3H, CH3), 2.12 (s, 3H, CH3), 3.80–3.96 (q, 2H, CH2), 5.91 (s, 1H, H-5), 6.42 (d, 2H, Ar-H), 7.13 (d, 2H, Ar-H), 10.89 ppm (s, 1H, NH). IR: ν = 3112 (NH), 3044 (Ar-CH), 2946 (ali-CH), 1613 (C=N), 1545 (Ar-C=C), cm−1;. MS (70 eV): m/z (%) = 311 (M+, 65). Anal. Calcd. For C13H17N3O2S2 (311.42): C, 50.14; H, 5.50; N, 13.49; O, 10.27; S, 20.59. Found: C, 50.09; H, 5.41; N, 13.39; S, 20.53.
3.1.2. Crystal X-ray Structure Determination of 3b
Compound 3b was obtained as single crystals by recrystallization from methanol. Bruker D8 Venture diffractometer with Photon II detector at 298(2) K using Cu-Kα radiation (λ = 1.54178 Å) was used to study the single-crystal X-ray diffraction. Moreover, we used dual space methods (SHELXT for 5a) [57,58] for the structure solution, and refinement was carried out using SHELXL-2014 (full-matrix least-squares on F2) [59]. Hydrogen atoms were localized by difference electron density determination and refined using a riding model. Semi-empirical absorption corrections and a general RIGU restraint were applied.
3b: red crystals, C12H13N5O4S, Mr = 323.33, crystal size 0.20 × 0.04 × 0.02 mm, triclinic, space group P-1 (No. 2), a = 7.0981(2) Å, b = 8.2929(2) Å, c = 13.0081(4) Å, α = 101.598(1)°, β = 103.030(1)°, γ = 92.366(1)°, V = 727.74(4) Å3, Z = 2, ρ = 1.476 Mg/m−3, µ(Cu-Kα) = 2.24 mm−1, F(000) = 336, T = 298 K, 2θmax = 144.4°, 13,774 reflections, of which 2874 were independent (Rint = 0.058), 200 parameters, 165 restraints (see cif-file for details), R1 = 0.063 (for 2692 I > 2σ(I)), wR2 = 0.170 (all data), S = 1.07, largest diff. peak/hole = 0.78/−0.40 e Å−3.
CCDC 2265616 (3b) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif (accessed on 24 June 2023).
3.2. Biology
3.2.1. Cell Viability Assay
The human mammary gland epithelial (MCF-10A) cell line was used to test the viability of compounds 3a–i [50,60]. See Supplementary Information.
3.2.2. Antiproliferative Assay
The MTT assay was used to investigate 3a–i’s antiproliferative activity versus four human cancer cell lines: colon cancer (HT-29) cell line, pancreatic cancer (Panc-1) cell line, lung cancer (A-549) cell line, and breast cancer (MCF-7) cell line, using erlotinib as the reference [52,53]. See Supplementary Information.
3.2.3. EGFR Inhibitory Assay
Compounds 3a, 3c, 3d, and 3f were further evaluated for their suppressive effect versus EGFR as a probable molecular target for their mechanism of action [50,54]. See Supplementary Information.
3.2.4. BRAFV600E Inhibitory Assay
Derivatives 3a, 3c, 3d, and 3f were further investigated as possible BRAFV600E inhibitors [61]. See Supplementary Information.
3.3. In Silico Study
The crystal structures of BRAFV600E and EGFR, with PDB codes 3OG7 [62] and 1M17 [63], respectively, were prepared for all docking computations. All heteroatoms, water molecules, ligands, and ions were removed to prepare the PDB files. Modeler software was applied to construct all missing amino acids [64,65]. The protonation state of titratable residues of the investigated targets was estimated using PropKa software at pH 7.0 [66]. The 3D structure of the investigated compounds was energetically minimized using the MMFF94S force field within SZYBKI software [67,68].
For docking computations, AutoDock4.2.6 software was utilized [69]. All docking parameters were set to default values, except GA run and energy evaluation, which were 250 and 25,000,000, respectively. The active site of the investigated targets was inspected by a grid box with a size of 50 Å × 50 Å × 50 Å. The grid maps were generated using the AutoGrid program with a spacing of 0.375 Å. Gasteiger–Marsili method was employed to assign the atomic charges of the chemical compounds [70]. Discovery Studio module of Biovia software 17.1.0.115 was utilized to visualize all drug–protein interactions [71].
4. Conclusions
Using simple interactions between thiosemicarbazides and chloroacetone, a novel set of heterocycles with thiazole rings was developed. All obtained derivatives were validated using various spectral data such as IR, NMR, mass spectrometry, elemental analysis, and X-ray crystallography. The newly synthesized compounds, 3a–i, were evaluated against a panel of four human cancer cell lines, with compounds 3a, 3c, 3d, and 3f being the most potent variants. The in vitro assay results demonstrated that compound 3f possesses potent antiproliferative activity as a dual EGFR/BRAFV600E inhibitor, signaling that further structural modifications may be needed to establish a more potent lead molecule for future development. Finally, the docking analysis results showed that all inspected compounds revealed good docking scores toward BRAFV600E and EGFR.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph16071014/s1. Figure S1: The crystal structure of (Z)-2-(2-(2,4-dinitrophenyl)hydrazineylidene)-3-ethyl-4-methyl-2,3-dihydrothiazole 3b; Figures S2–S32: IR and NMR analysis of the structure assignment for all obtained products 3a–i.
Author Contributions
B.G.M.Y., E.M.E.-S., A.A.H. and H.N.T.: conceptualization, methodology, writing, editing, and revision. L.H.A.-W.: writing, editing, and revision. S.B.: writing and editing. M.N.: X-ray analysis. M.A.A.I.: docking study. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Princess Nourah Bint Abdulrahman University Researchers Supporting Project Number PNURSP2023R3, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Acknowledgments
The authors extend their appreciation to Princess Nourah Bint Abdulrahman University Researchers Supporting Project Number PNURSP2023R3, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia. The authors also acknowledge support by the KIT-Publication Fund of the Karlsruhe Institute of Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Bin Emran, T.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.-R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 2581. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef]
- Nishal, S.; Jhawat, V.; Gupta, S.; Phaugat, P. Utilization of kinase inhibitors as novel therapeutic drug targets: A review. Oncol. Res. 2023, 30, 221–230. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Gouda, A.M.; Abou-Ghadir, O.F.; Salem, O.I.; Ali, A.T.; Farghaly, H.S.H.; Abdelrahman, M.H.; Trembleau, L.; Abdu-Allah, H.H.; Youssif, B.G. Design and synthesis of novel 2,3-dihydropyrazino[1,2-a]indole-1,4-dione derivatives as antiproliferative EGFR and BRAFV600E dual inhibitors. Bioorg. Chem. 2020, 104, 104260. [Google Scholar] [CrossRef]
- Jarvinen, T.A.; Liu, E.T. Simultaneous amplification of HER-2 (ERBB2) and topoisomerase IIα (TOP2A) genes-molecular basis for combination chemotherapy in cancer. Curr. Cancer Drug Targets 2006, 6, 579–602. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Lehár, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Iii, G.F.S.; Staunton, J.E.; Jin, X.; et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef]
- Kendall, J.M. Designing a research project: Randomised controlled trials and their principles. Emerg. Med. J. 2003, 20, 164–168. [Google Scholar] [CrossRef]
- Lim, Z.-F.; Ma, P.C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J. Hematol. Oncol. 2019, 12, 134. [Google Scholar] [CrossRef]
- Hoelder, S.; Clarke, P.A.; Workman, P. Discovery of small molecule cancer drugs: Successes, challenges and opportunities. Mol. Oncol. 2012, 6, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.N.; Bhatt, R.; Rotow, J.; Rohrberg, J.; Olivas, V.; Wang, V.E.; Hemmati, G.; Martins, M.M.; Maynard, A.; Kuhn, J.; et al. Aurora kinase A drives the evolution of resistance to third-generation EGFR inhibitors in lung cancer. Nat. Med. 2019, 25, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-P.; Murakami, M.; Wu, Y.-S.; Lin, C.-L.; Li, Y.-Q.; Huang, Y.-H.; Hung, T.-H.; Ambudkar, S.V. The multitargeted tyrosine kinase inhibitor SKLB610 resensitizes ABCG2-overexpressing multidrug-resistant cancer cells to chemotherapeutic drugs. Biomed. Pharmacother. 2022, 149, 112922. [Google Scholar] [CrossRef]
- Kanojia, D.; Garg, M.; Martinez, J.; MT, A.; Luty, S.B.; Doan, N.B.; Said, J.W.; Forscher, C.; Tyner, J.W.; Koeffler, H.P. Kinase profiling of liposarcomas using RNAi and drug screening assays identified druggable targets. J. Hematol. Oncol. 2017, 10, 173. [Google Scholar] [CrossRef]
- Sun, D.; Zhao, Y.; Zhang, S.; Zhang, L.; Liu, B.; Ouyang, L. Dual-target kinase drug design: Current strategies and future directions in cancer therapy. Eur. J. Med. Chem. 2020, 188, 112025. [Google Scholar] [CrossRef]
- Zou, X.; Tang, X.-Y.; Qu, Z.-Y.; Sun, Z.-W.; Ji, C.-F.; Li, Y.-J.; Guo, S.-D. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: A review. Int. J. Biol. Macromol. 2022, 202, 539–557. [Google Scholar] [CrossRef]
- Ho, C.-C.; Liao, W.-Y.; Lin, C.-A.; Shih, J.-Y.; Yu, C.-J.; Yang, J.C.-H. Acquired BRAF V600E Mutation as Resistant Mechanism after Treatment with Osimertinib. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2017, 12, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Nana, F.A.; Ocak, S. Targeting BRAF Activation as Acquired Resistance Mechanism to EGFR Tyrosine Kinase Inhibitors in EGFR-Mutant Non-Small-Cell Lung Cancer. Pharmaceutics 2021, 13, 1478. [Google Scholar] [CrossRef]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.-Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef]
- Amodio, V.; Yaeger, R.; Arcella, P.; Cancelliere, C.; Lamba, S.; Lorenzato, A.; Arena, S.; Montone, M.; Mussolin, B.; Bian, Y.; et al. EGFR Blockade Reverts Resistance to KRASG12C Inhibition in Colorectal Cancer. Cancer Discov. 2020, 10, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Prahallad, A.; Sun, C.; Huang, S.; Di Nicolantonio, F.; Salazar, R.; Zecchin, D.; Beijersbergen, R.L.; Bardelli, A.; Bernards, R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012, 483, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.; Markman, B.; Ananda, S.; Tebbutt, N.C.; Michael, M.; Solomon, B.J.; McArthur, G.A.; Tie, J.; Gibbs, P.; Ritchie, D.; et al. A phase I/II trial of combined BRAF and EGFR inhibition in patients (pts) with BRAF V600E mutated (BRAFm) metastatic colorectal (mCRC): The EViCT (Erlotinib and Vemurafenib in Combination Trial) study. J. Clin. Oncol. 2017, 35, 3557. [Google Scholar] [CrossRef]
- Liu, H.; Nazmun, N.; Hassan, S.; Liu, X.; Yang, J. BRAF mutation and its inhibitors in sarcoma treatment. Cancer Med. 2020, 9, 4881–4896. [Google Scholar] [CrossRef]
- Corcoran, R.B.; André, T.; Atreya, C.E.; Schellens, J.H.; Yoshino, T.; Bendell, J.C.; Hollebecque, A.; McRee, A.J.; Siena, S.; Middleton, G. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAFV600E-Mutant Colorectal Can-cerBRAF/EGFR/MEK Inhibition in BRAFV600E Colorectal Cancer. Cancer Discov. 2018, 8, 428–443. [Google Scholar] [CrossRef]
- Bahrami, A.; Hesari, A.; Khazaei, M.; Hassanian, S.M.; Ferns, G.A.; Avan, A. The therapeutic potential of targeting the BRAF mutation in patients with colorectal cancer. J. Cell Physiol. 2017, 233, 2162–2169. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Zimenkovsky, B.; Lesyk, R. Synthesis and anticancer activity of new thiopyrano [2, 3-d] thiazoles based on cinnamic acid amides. Sci. Pharm. 2014, 82, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Janowska, S.; Khylyuk, D.; Bielawska, A.; Szymanowska, A.; Gornowicz, A.; Bielawski, K.; Noworól, J.; Mandziuk, S.; Wujec, M. New 1,3,4-Thiadiazole Derivatives with Anticancer Activity. Molecules 2022, 27, 1814. [Google Scholar] [CrossRef]
- Arshad, M.F.; Alam, A.; Alshammari, A.A.; Alhazza, M.B.; Alzimam, I.M.; Alam, A.; Mustafa, G.; Ansari, S.; Alotaibi, A.M.; Alotaibi, A.A.; et al. Thiazole: A Versatile Standalone Moiety Contributing to the Development of Various Drugs and Biologically Active Agents. Molecules 2022, 27, 3994. [Google Scholar] [CrossRef]
- Franchetti, P.; Cappellacci, L.; Grifantini, M.; Barzi, A.; Nocentini, G.; Yang, H.; O’Connor, A.; Jayaram, H.N.; Carrell, C.; Goldstein, B.M. Furanfurin and Thiophenfurin: Two Novel Tiazofurin Analogs. Synthesis, Structure, Antitumor Activity, and Interactions with Inosine Monophosphate Dehydrogenase. J. Med. Chem. 1995, 38, 3829–3837. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Ruiz, C.H.; Koenig, M.; Cameron, M.D. Characterization of Dasatinib and Its Structural Analogs as CYP3A4 Mechanism-Based Inactivators and the Proposed Bioactivation Pathways. Drug Metab. Dispos. 2009, 37, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Laneuville, P.; Rousselot, P.; Snyder, D.S.; Rea, D.; Shah, N.P.; Paar, D.; Abruzzese, E.; Hochhaus, A.; Lipton, J.H.; et al. Incidence, outcomes, and risk factors of pleural effusion in patients receiving dasatinib therapy for Philadelphia chromosome-positive leukemia. Haematologica 2018, 104, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Hu-Lieskovan, S.; Mok, S.; Moreno, B.H.; Tsoi, J.; Robert, L.; Goedert, L.; Pinheiro, E.M.; Koya, R.C.; Grae-ber, T.G.; Comin-Anduix, B. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF V600E melanoma. Sci. Transl. Med. 2015, 7, 279ra41. [Google Scholar] [CrossRef]
- Argenziano, G.; Banzi, C.; De Blasio, S.; Lallas, A.; Longo, C.; Moscarella, E.; Alfano, R. Dabrafenib: A new opportunity for the treatment of BRAF V600-positive melanoma. OncoTargets Ther. 2016, ume 9, 2725–2733. [Google Scholar] [CrossRef]
- Abdel-Maksoud, M.S.; Kim, M.-R.; El-Gamal, M.I.; El-Din, M.M.G.; Tae, J.; Choi, H.S.; Lee, K.-T.; Yoo, K.H.; Oh, C.-H. Design, synthesis, in vitro antiproliferative evaluation, and kinase inhibitory effects of a new series of imidazo [2, 1-b] thiazole derivatives. Eur. J. Med. Chem. 2015, 95, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Alshammari, M.B.; Ahmad, A.; Gomaa, H.A.M.; Youssif, B.G.M.; Bräse, S.; Ibrahim, M.A.A.; Mohamed, A.H. Design, synthesis, docking and mechanistic studies of new thiazolyl/thiazolidinylpyrimidine-2,4-dione antiproliferative agents. Arab. J. Chem. 2023, 16, 104612. [Google Scholar] [CrossRef]
- Ballatore, C.; Huryn, D.M.; Smith, A.B. Carboxylic Acid (Bio)Isosteres in Drug Design. Chemmedchem 2013, 8, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Ammazzalorso, A.; De Filippis, B.; Giampietro, L.; Amoroso, R. N-acylsulfonamides: Synthetic routes and biological potential in medicinal chemistry. Chem. Biol. Drug Des. 2017, 90, 1094–1105. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Alsaid, M.S.; El-Gaby, M.S.; Safwat, N.A.; Elaasser, M.M.; Soliman, A.M. Biological evaluation of some new N-(2,6-dimethoxypyrimidinyl) thioureido benzenesulfonamide derivatives as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2016, 124, 299–310. [Google Scholar] [CrossRef]
- Alaoui, S.; Dufies, M.; Driowya, M.; Demange, L.; Bougrin, K.; Robert, G.; Auberger, P.; Pagès, G.; Benhida, R. Synthesis and anti-cancer activities of new sulfonamides 4-substituted-triazolyl nucleosides. Bioorg. Med. Chem. Lett. 2017, 27, 1989–1992. [Google Scholar] [CrossRef]
- Wan, Y.; Fang, G.; Chen, H.; Deng, X.; Tang, Z. Sulfonamide derivatives as potential anti-cancer agents and their SARs elucidation. Eur. J. Med. Chem. 2021, 226, 113837. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Mahmoud, M.A.; Mostafa, Y.A.; Raslan, A.E.; Youssif, B.G. Novel piperine-carboximidamide hybrids: Design, synthesis, and antiproliferative activity via a multi-targeted inhibitory pathway. J. Enzym. Inhib. Med. Chem. 2023, 38, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, H.A.; Shaker, M.E.; Alzarea, S.I.; Hendawy, O.; Mohamed, F.A.; Gouda, A.M.; Ali, A.T.; Morcoss, M.M.; Abdelrahman, M.H.; Trembleau, L.; et al. Optimization and SAR investigation of novel 2,3-dihydropyrazino[1,2-a]indole-1,4-dione derivatives as EGFR and BRAFV600E dual inhibitors with potent antiproliferative and antioxidant activities. Bioorg. Chem. 2022, 120, 105616. [Google Scholar] [CrossRef]
- Mohassab, A.M.; Hassan, H.A.; Abdelhamid, D.; Gouda, A.M.; Youssif, B.G.M.; Tateishi, H.; Fujita, M.; Otsuka, M.; Abdel-Aziz, M. Design and synthesis of novel quinoline/chalcone/1,2,4-triazole hybrids as potent antiproliferative agent targeting EGFR and BRAFV600E kinases. Bioorg. Chem. 2020, 106, 104510. [Google Scholar] [CrossRef] [PubMed]
- Youssif, B.G.; Abdelrahman, M.H.; Abdelazeem, A.H.; Ibrahim, H.M.; Salem, O.I.; Mohamed, M.F.; Treambleau, L.; Bukhari, S.N.A. Design, synthesis, mechanistic and histopathological studies of small-molecules of novel in-dole-2-carboxamides and pyrazino [1, 2-a] indol-1 (2H)-ones as potential anticancer agents effecting the reactive oxygen species production. Eur. J. Med. Chem. 2018, 146, 260–273. [Google Scholar] [CrossRef]
- Noto, R.; Meo, P.L.; Gruttadauria, M.; Werber, G. A quantitative study of substituent effects on oxidative cycliza-tion of some 2-aryl-substituted aldehyde thiosemicarbazones induced by ferric chloride and cupric perchlorate. J. Heterocycl. Chem. 1999, 36, 667–674. [Google Scholar] [CrossRef]
- Krishna, P.M.; Reddy, N.G.; Harish, B.; Patil, Y.P.; Nethaji, M. Synthesis, structural studies, molecular docking and DNA binding studies of 4N-substituted hydrazinecarbothioamides. J. Mol. Struct. 2019, 1175, 97–104. [Google Scholar] [CrossRef]
- Munir, R.; Zia-ur-Rehman, M.; Murtaza, S.; Zaib, S.; Javid, N.; Awan, S.J.; Iftikhar, K.; Athar, M.M.; Khan, I. Micro-wave-Assisted Synthesis of (Piperidin-1-yl) quinolin-3-yl) methylene) hydrazinecarbothioamides as Potent Inhibitors of Cholinesterases: A Biochemical and In silico Approach. Molecules 2021, 26, 656. [Google Scholar] [CrossRef]
- Basri, R.; Ullah, S.; Halim, S.A.; Alharthy, R.D.; Rauf, U.; Khan, A.; Hussain, J.; Al-Ghafri, A.; Al-Harrasi, A.; Shafiq, Z. Synthesis, biological evaluation, and molecular docking study of chromen-linked hydrazine carbothioamides as potent α-glucosidase inhibitors. Drug Dev. Res. 2023. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Mostafa, Y.A.; Abdelrahman, M.H.; El-Bahrawy, A.H.; Trembleau, L.; Youssif, B.G.M. Synthesis and Biological Evaluation of Indole-2-Carboxamides with Potent Apoptotic Antiproliferative Activity as EGFR/CDK2 Dual Inhibitors. Pharmaceuticals 2022, 15, 1006. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays, Assay Guidance Manual [Internet]. 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK144065/ (accessed on 11 July 2023).
- Mahmoud, M.A.; Mohammed, A.F.; Salem, O.I.; Gomaa, H.A.; Youssif, B.G. New 1, 3, 4-oxadiazoles linked with the 1, 2, 3-triazole moiety as antiproliferative agents targeting the EGFR tyrosine kinase. Arch. Pharm. 2022, 355, 2200009. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.; Mohammed, A.F.; Salem, O.I.; Rabea, S.M.; Youssif, B.G. Design, synthesis, and antiproliferative properties of new 1,2,3-triazole-carboximidamide derivatives as dual EGFR/VEGFR-2 inhibitors. J. Mol. Struct. 2023, 1282, 135165. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Gomaa, H.A.; Hendawy, O.; Ali, A.T.; Farghaly, H.S.; Gouda, A.M.; Abdelazeem, A.H.; Ab-delrahman, M.H.; Trembleau, L.; Youssif, B.G. Design, synthesis, and biological evaluation of novel EGFR inhibitors con-taining 5-chloro-3-hydroxymethyl-indole-2-carboxamide scaffold with apoptotic antiproliferative activity. Bioorg. Chem. 2021, 112, 104960. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.Y.; Al-Harbi, R.A.; El-Sharief, M.A.S. Synthesis and anticancer activity of thiourea derivatives bearing a benzodioxole moiety with EGFR inhibitory activity, apoptosis assay and molecular docking study. Eur. J. Med. Chem. 2020, 198, 112363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, T.-W.; Hashizume, R.; Hariono, S.; Barkovich, K.J.; Fan, Q.-W.; Prados, M.; James, C.D.; Weiss, W.A.; Nicolaides, T. Combined BRAF V600E and MEK blockade for BRAF V600E-mutant gliomas. J. Neurooncol. 2017, 131, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, T.; Huang, Z. Structure determination of a low-crystallinity covalent organic framework by three-dimensional electron diffraction. Commun. Chem. 2023, 6, 116. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Abou-Zied, H.A.; Youssif, B.G.; Mohamed, M.F.; Hayallah, A.M.; Abdel-Aziz, M. EGFR inhibitors and apoptotic inducers: Design, synthesis, anticancer activity and docking studies of novel xanthine derivatives carrying chalcone moiety as hybrid molecules. Bioorg. Chem. 2019, 89, 102997. [Google Scholar] [CrossRef]
- Youssif, B.G.; Gouda, A.M.; Moustafa, A.H.; Abdelhamid, A.A.; Gomaa, H.A.; Kamal, I.; Marzouk, A.A. Design and synthesis of new triarylimidazole derivatives as dual inhibitors of BRAFV600E/p38α with potential antiproliferative activity. J. Mol. Struct. 2021, 1253, 132218. [Google Scholar] [CrossRef]
- Bollag, G.; Hirth, P.; Tsai, J.; Zhang, J.; Ibrahim, P.N.; Cho, H.; Spevak, W.; Zhang, C.; Zhang, Y.; Habets, G.; et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 2010, 467, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Stamos, J.; Sliwkowski, M.X.; Eigenbrot, C. Structure of the Epidermal Growth Factor Receptor Kinase Domain Alone and in Complex with a 4-Anilinoquinazoline Inhibitor. J. Biol. Chem. 2002, 277, 46265–46272. [Google Scholar] [CrossRef] [PubMed]
- Martí-Renom, M.A.; Stuart, A.C.; Fiser, A.; Sánchez, R.; Melo, F.; Šali, A. Comparative Protein Structure Modeling of Genes and Genomes. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 291–325. [Google Scholar] [CrossRef]
- Muthumanickam, S.; Boomi, P.; Subashkumar, R.; Palanisamy, S.; Sudha, A.; Anand, K.; Balakumar, C.; Saravanan, M.; Poorani, G.; Wang, Y. An Insight of Protein Structure Predictions Using Homology Modeling. In Computation in Bioinformatics: Multidisciplinary Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 265–277. [Google Scholar]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A. MMFF VI. MMFF94s option for energy minimization studies. J. Comput. Chem. 1999, 20, 720–729. [Google Scholar] [CrossRef]
- OpenEye Scientific. SZYBKI 1.9.0.3. 1.9.0.3; OpenEye Scientific Software: Santa Fe, NM, USA, 2016. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and Auto-DockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Gasteiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity—A rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Discovery Studio Visualizer (D.S.V.). Dassault Systèmes BIOVIA. Version 2019; Dassault Systèmes: San Diego, CA, USA, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).