LC/MS-MS Analysis of Phenolic Compounds in Hyoscyamus albus L. Extract: In Vitro Antidiabetic Activity, In Silico Molecular Docking, and In Vivo Investigation against STZ-Induced Diabetic Mice
Abstract
1. Introduction
2. Results
2.1. Total Phenolic, Flavonoid, and Tannin Contents
2.2. Screening and Measurement of Phenolic Compounds
2.3. In Vitro Test Methods for Inhibiting Enzymes
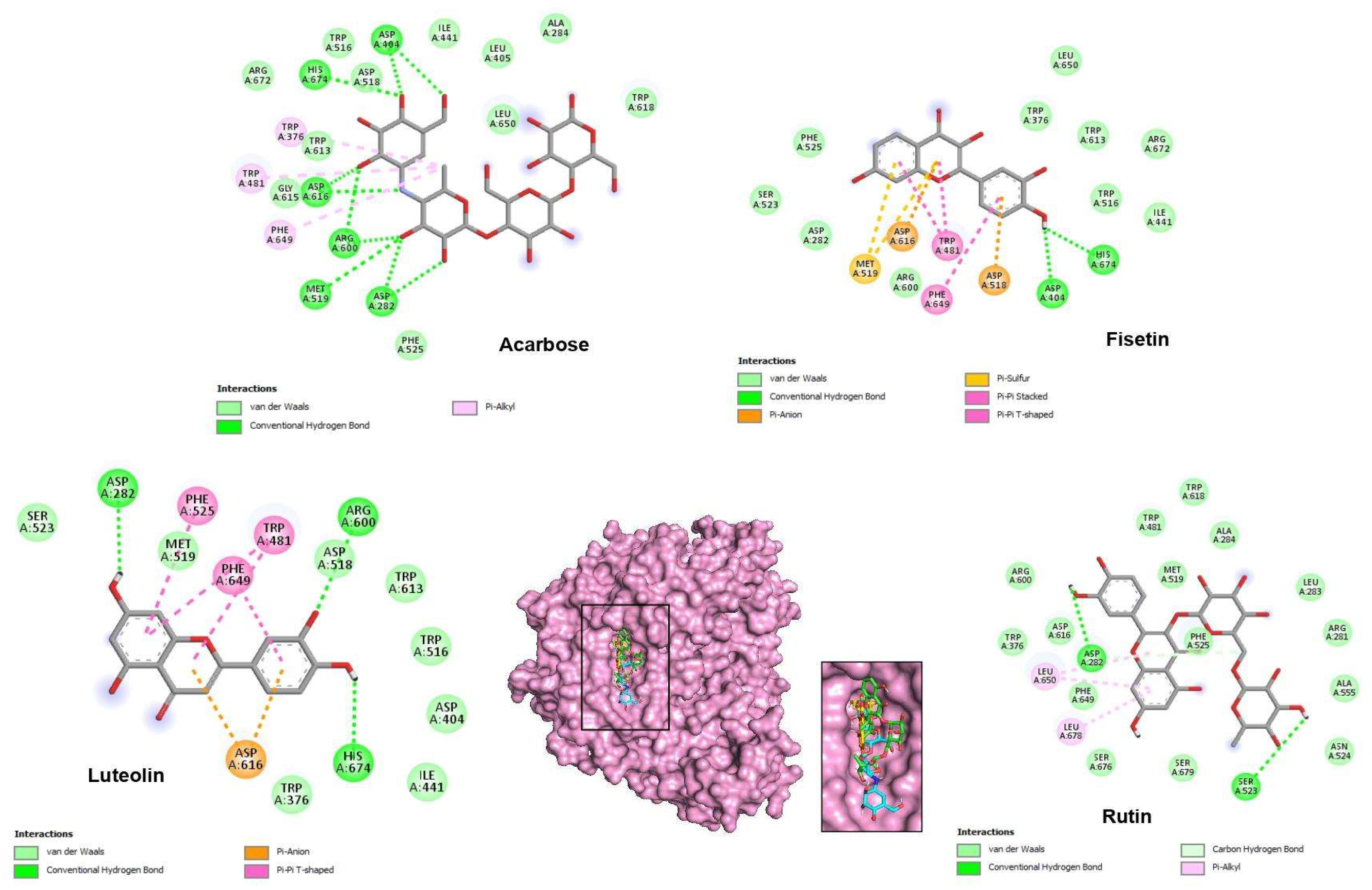
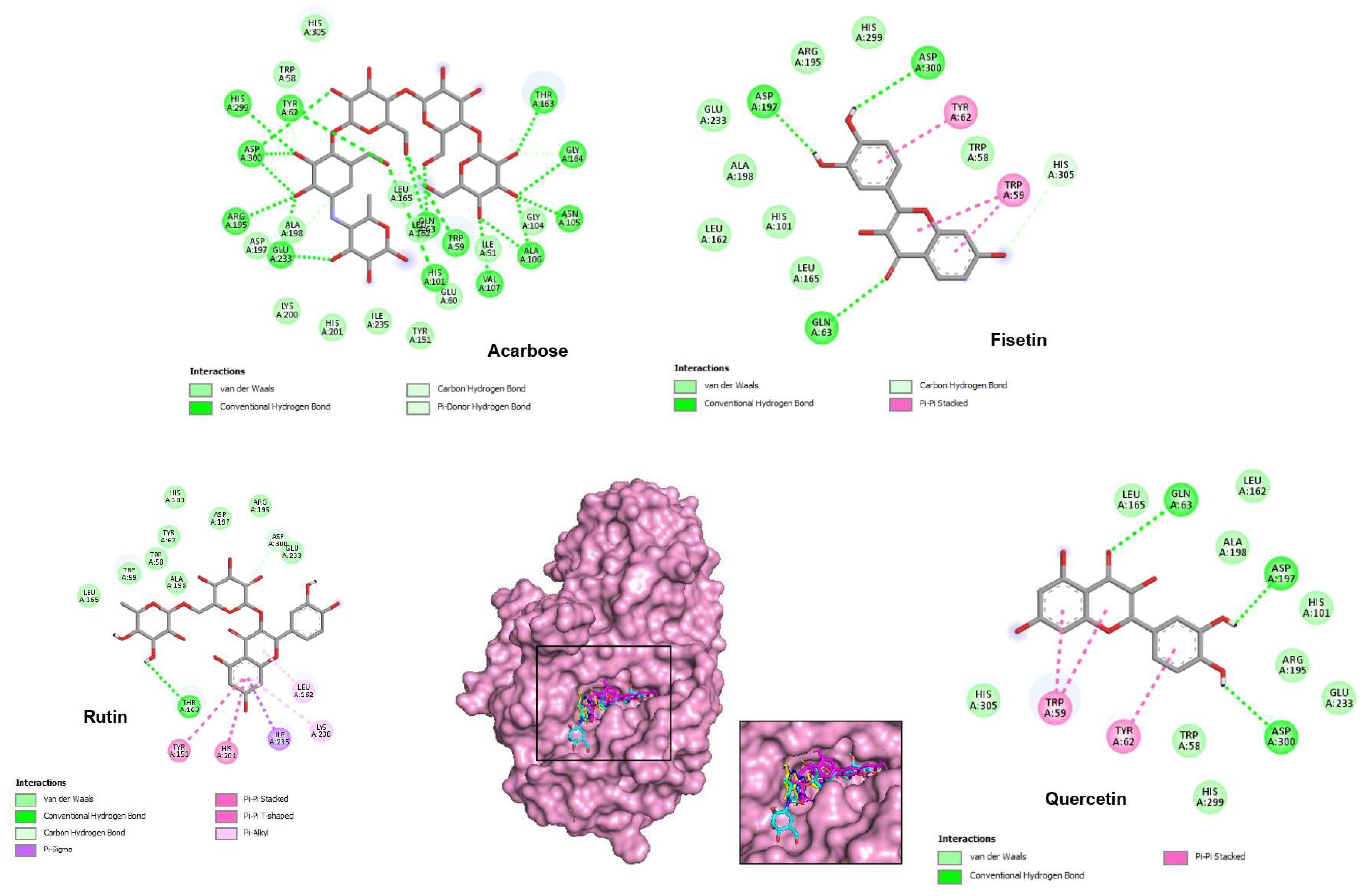
2.4. Molecular Docking Studies
2.5. Drug Similarity and the ADMET Profile
2.6. In Vivo Antidiabetic
2.6.1. Acute Oral Toxicity
2.6.2. Antidiabetic Activity in Streptozotocin-Induced Hyperglycemia Model
3. Discussion
4. Materials and Methods
4.1. Instruments and Chemicals
4.2. Plant Material
4.3. Total Bioactive Compound Determination (TPC, TFC, and TTC)
4.4. Plant Extract Preparation for LC-MS/MS and an Enzyme Inhibitory Test
4.5. Polyphenolic Detection and Quantitation
4.6. Enzymatic Inhibitory Assay
4.7. Computational Docking Methodology
4.8. Pharmacokinetic and ADMET Profile
4.9. In Vivo Antidiabetic Activity
4.9.1. Animals
4.9.2. Acute Toxicity
4.9.3. Hypoglycemic Activity
4.9.4. Antihyperglycemic Activity in Streptozotocin-Induced Hyperglycemia Model
- Group 1: normal control mice;
- Group 2: diabetic control mice;
- Group 3: diabetic mice treated with H. albus at a concentration of 10 mg;
- Group 4: diabetic mice treated with H. albus at a concentration of 20 mg;
- Group 5: diabetic mice administered glibenclamide at a dose of 20 mg/kg.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour. Technol. 2008, 99, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Asgar, M.d.A. Antidiabetic Potential of Phenolic Compounds: A Review. Int. J. Food Prop. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini-Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Magalhães, P.O.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef]
- Singh, S.K.; Rai, P.K.; Jaiswal, D.; Watal, G. Evidence-based critical evaluation of glycemic potential of Cynodon dactylon. Evid. Based Complement. Alternat. Med. 2008, 5, 415–420. [Google Scholar] [CrossRef]
- Mata, R.; Cristians, S.; Escandón-Rivera, S.; Juárez-Reyes, K.; Rivero-Cruz, I. Mexican antidiabetic herbs: Valuable source of inhibitors of α-glucosidase. J. Nat. Prod. 2013, 76, 468–483. [Google Scholar] [CrossRef]
- Mobin, M.; Khan, M.N.; Abbas, Z.K. Ecotype difference in bioactive constituents and In vitro antioxidant activities of some Saudi medicinal plants. Eur. J. Med. Plants 2015, 7, 125–136. [Google Scholar] [CrossRef]
- Nejadhabibvash, F.; Rahmani, F.; Heidari, R.; Jamei, R. Assessment of genetic diversity among Hyoscyamus genotypes based on ISSR markers. Int. J. Agric. Crop Sci. 2012, 4, 1300–1306. [Google Scholar]
- Mansouri, N.; Benslama, O.; Arhab, R. Homology modeling, docking, and molecular dynamics studies of some secondary metabolites of actinomycetes as biocontrol agents against the 3HNR enzyme of the phytopathogenic fungus Alternaria alternata. J. Biomol. Struct. Dyn. 2021, 41, 871–883. [Google Scholar] [CrossRef]
- Dufour, D.R.; Lott, J.A.; Nolte, F.S.; Gretch, D.R.; Koff, R.S.; Seeff, L.B. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin. Chem. 2007, 46, 2027–2049. [Google Scholar] [CrossRef]
- Dhuley, J.N. Effect of some Indian herbs on macrophage functions in ochratoxin A treated mice. J. Ethnopharmacol. 1997, 58, 15–20. [Google Scholar] [CrossRef]
- Gulcin, I.; Elias, R.; Gepdiremen, A.; Chea, A.; Topal, F. Antioxidant activity of bis benzylisoquinoline alkaloids from Stephania rotunda: Cepharanthine and fangchinoline. J. Enzyme Inhib. Med. Chem. 2010, 25, 44–53. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Thakur, K.; Wei, C.K.; Wang, H.; Zhang, J.G.; Wei, Z.J. Evaluation of inhibitory activity of natural plant polyphenols on Soybean lipoxygenase by UFLCmass spectrometry. S. Afr. J. Bot. 2019, 120, 179–185. [Google Scholar] [CrossRef]
- Sung, B.; Chung, H.Y.; Kim, N.D. Role of Apigenin in Cancer Prevention via the Induction of Apoptosis and Autophagy. J. Cancer Prev. 2016, 21, 216–226. [Google Scholar] [CrossRef]
- Perk, A.A.; Shatynska-mytsyk, I.; Gerçek, Y.C.; Boztas, K.; Yazgan, M.; Fayyaz, S.; Farooqi, A.A. Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell Int. 2014, 14, 124. [Google Scholar] [CrossRef]
- Tlili, H.; Hanen, N.; Ben Arfa, A.; Neffati, M.; Boubakri, A.; Buonocore, D.; Dossena, M.; Verri, M.; Doria, E. Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLoS ONE 2019, 14, e0213049. [Google Scholar] [CrossRef]
- Bourebaba, L.; Saci, S.; Touguit, D.; Gali, L.; Terkmane, S.; Oukil, N.; Bedjou, F. Evaluation of antidiabetic effect of total calystegines extracted from Hyoscyamus albus. Biomed. Pharmacother. 2016, 82, 337–344. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Rehman, N.U.; Rafiq, K.; Khan, A.; Halim, S.A.; Ali, L.; Al-Saady, N.; Al-Balushi, A.B.; Al-Busaidi, H.K.; Al-Harrasi, A. α-glucosidase Inhibition and Molecular Docking Studies of Natural Brominated Metabolites from Marine Macro Brown Alga Dictyopteris hoytii. Mar. Drugs 2019, 17, 666. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.M.; Mayer, D.A.; Siebert, D.A.; Micke, G.A.; Alberton, M.D.; Tavares, L.B.B.; Oliveira, D. Kinetics analysis of the inhibitory effects of alpha-glucosidase and identification of compounds from Ganoderma lipsiense mycelium. Appl. Biochem. Biotechnol. 2020, 191, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Vaya, J.; Mahmood, S.; Goldblum, A.; Aviram, M.; Volkova, N.; Shaalan, A.; Musa, R.; Tamir, S. Inhibition of LDL oxidation by flavonoids in relation to their structure and calculated enthalpy. Phytochemistry 2003, 62, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Ferruzzi, M.G.; Hamaker, B.R. Structural requirements of flavonoids for the selective inhibition of α-amylase versus α-glucosidase. Food Chem. 2021, 370, 130981. [Google Scholar] [CrossRef] [PubMed]
- Maurus, R.; Begum, A.; Williams, L.K.; Fredriksen, J.R.; Zhang, R.; Withers, S.G.; Brayer, G.D. Alternative Catalytic Anions Differentially Modulate Human R-Amylase Activity and Specificity. Biochemistry 2008, 47, 3332–3344. [Google Scholar] [CrossRef]
- Uitdehaag, J.C.; Mosi, R.; Kalk, K.H.; van der Veen, B.A.; Dijkhuizen, L.; Withers, S.G.; Dijkstra, B.W. X-ray structures along the reaction pathway of cyclodextrin glycosyltransferase elucidate catalysis in the R-amylase family. Nat. Struct. Biol. 1999, 6, 432–436. [Google Scholar] [CrossRef]
- Akshatha, J.V.; Kumar, H.S.S.; Prakash, S.H.; Nalini, M.S. In silico docking studies of α-amylase inhibitors from the antidiabetic plant Leucas ciliate Benth. and an endophyte, Streptomyces longisporoflavus. 3 Biotech 2021, 11, 51. [Google Scholar] [CrossRef]
- Baker, E.N.; Hubbard, R.E. Hydrogen bonding in globular proteins. Prog. Biophys. Mol. Biol. 1984, 44, 97–179. [Google Scholar] [CrossRef]
- Jia, W.; Gao, W.; Tang, L. Antidiabetic herbal drugs officially approved in China. Phytother. Res. 2003, 17, 1127–1134. [Google Scholar] [CrossRef]
- Timalsina, D.; Bhusal, D.; Devkota, H.P.; Pokhrel, K.P.; Sharma, K.P. α-Amylase Inhibitory Activity of Catunaregam spinosa (Thunb.) Tirveng.: In Vitro and In Silico Studies. Biomed Res. Int. 2021, 2021, 4133876. [Google Scholar] [CrossRef]
- McCue, P.; Vattem, D.; Shetty, K. Inhibitory effect of clonal oregano extracts against porcine pancreatic amylase in vitro. Asia Pac. J. Clin. Nutr 2004, 13, 401–408. [Google Scholar]
- Kim, J.S.; Kwon, C.S.; Son, K.H. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci. Biotechnol. Biochem. 2000, 64, 2458–2461. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Duffy, E.M. prediction of drug solubility from Monte Carlo simulations. Bioorg. Med. Chem. Lett. 2000, 10, 1155–1158. [Google Scholar] [CrossRef]
- Van de Waterbeemd, H.; Gifford, E. ADMET in silico modeling: Towards prediction paradise? Nat. Rev. Drug Discov. 2003, 2, 192–204. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Khanna, V.; Shoba Ranganathan, S. Physiochemical property space distribution among human metabolites, drugs and toxins. BMC Bioinform. 2009, 10 (Suppl. 15), S10. [Google Scholar] [CrossRef]
- Nebert, D.W.; Russell, D.W. Clinical Importance of the Cytochromes P450. Lancet 2002, 360, 1155–1162. [Google Scholar] [CrossRef]
- Guttman, Y.; Kerem, Z. Computer-Aided (In Silico) Modeling of Cytochrome P450-Mediated Food–Drug Interactions (FDI). Int. J. Mol. Sci. 2022, 23, 8498. [Google Scholar] [CrossRef]
- Sayyad, M.; Tiang, N.; Kumari, Y.; Goh, B.H.; Jaiswal, Y.; Rosli, R.; Williams, L.; Shaikh, M.F. Acute Acute toxicity profiling of the ethyl acetate fraction of Swietenia macrophylla seeds and in- vitro neuroprotection studies. Saudi Pharm. J. 2017, 25, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Umale, S.; Deck, C.; Bourdet, N.; Dhumane, P.; Soler, L.; Marescaux, J.; Willinger, R. Experimental mechanical characterization of abdominal organs: Liver, kidney & spleen. J. Mech. Behav. Biomed. Mater. 2012, 17, 22–33. [Google Scholar] [PubMed]
- Manickam, M.; Ramanathan, M.; Jahromi, M.A.; Chansouria, J.P.; Ray, A.B. Antihyperglycemic activity of phenolics from Pterocarpus marsupium. J. Nat. Prod. 1997, 60, 609–610. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005, 579, 1653–1657. [Google Scholar] [CrossRef]
- Oniki, Y.; Kato, T.; Irie, H.; Mizuta, H.; Takagi, K. Diabetes with hyperlipidemia: A risk factor for developing joint contractures secondary to immobility in rat knee joints. J. Orthop. Sci. 2005, 10, 221–226. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Liu, X.; Zhang, W.; Li, Y. Interrelationship between diabetes and periodontitis: Role of hyperlipidemia. Arch. Oral Biol. 2015, 60, 667–674. [Google Scholar] [CrossRef]
- Howard, B.V.; Schneiderman, N.; Falkner, B.; Haffner, S.M.; Laws, A. Insulin, health behaviors, and lipid metabolism. Metabolism 1993, 42, 25–35. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Müller, L.; Gnoyke, S.; Popken, A.M.; Böhm, V. Antioxidant capacity and related parameters of different fruit formulations. LWT—Food Sci. Technol. 2010, 43, 992–999. [Google Scholar] [CrossRef]
- Topçu, G.; Ay, M.; Bilici, A.; Sarikurkcu, C.; Öztürk, M.; Ulubelen, A. A new flavone from antioxidant extracts of Pistacia terebinthus. Food Chem. 2007, 103, 816–822. [Google Scholar] [CrossRef]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Lekmine, S.; Bendjedid, S.; Benslama, O.; Martín-García, A.I.; Boussekine, S.; Kadi, K.; Akkal, S.; Nieto, G.; Sami, R.; Al-Mushhin, A.A.M.; et al. Ultrasound-Assisted Extraction, LC–MS/MS Analysis, Anticholinesterase, and Antioxidant Activities of Valuable Natural Metabolites from Astragalus armatus Willd: In Silico Molecular Docking and In Vitro Enzymatic Studies. Antioxidants 2022, 11, 2000. [Google Scholar] [CrossRef]
- Ertas, A.; Yener, I. A comprehensive study on chemical and biological profiles of three herbal teas in Anatolia; Rosmarinic and chlorogenic acids. S. Afr. J. Bot. 2020, 130, 274–281. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A comprehensive study on phytochemical characterization of Haplophyllum myrtifolium Boiss. endemic to Turkey and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind. Crop. Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Nampoothiri, S.V.; Prathapan, A.; Cherian, O.L.; Raghu, K.G.; Venugopalan, V.V.; Sundaresan, A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 2011, 49, 125–131. [Google Scholar] [CrossRef]
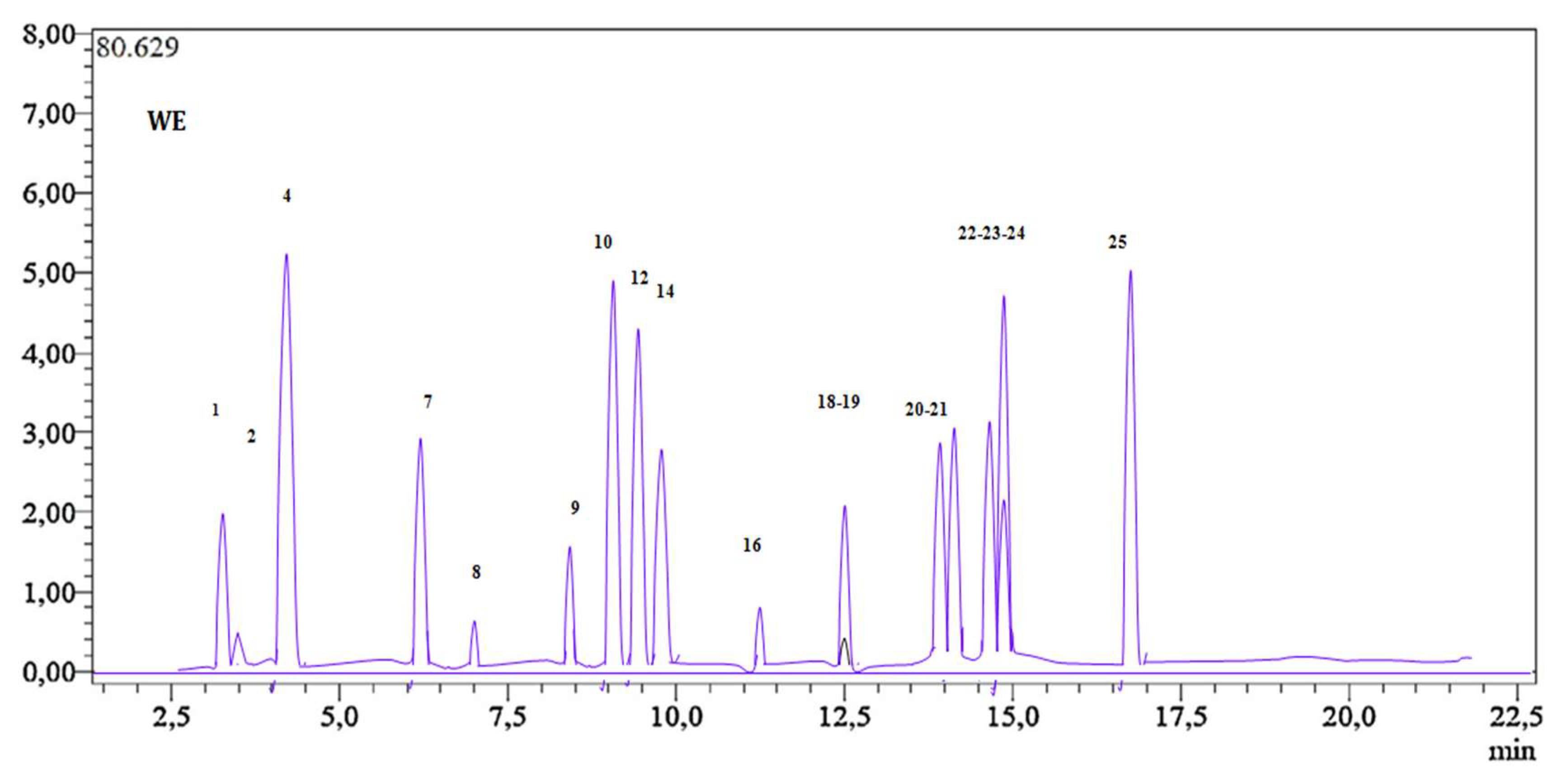
| Sample | TPC (μgEAG/mg E) | TFC (μg EQ/mgE) | TTC (μg ECT/mgE) |
|---|---|---|---|
| H. albus ethanolic extract | 245.20 ± 0.53 a | 120.55 ± 0.56 a | 60 ± 0.42 b |
| Compound Number | RT | [M-H]− | MS2 (Collision Energy) | Cultivated H. albus (µg dry/g Extract) | |
|---|---|---|---|---|---|
| 1 | Quinic acid | 3.32 | 191.0 | 85 (22), 93 (22) | 114.7 ± 4.3 |
| 2 | Malic acid | 3.54 | 133.1 | 115 (14), 71 (17) | 3.6 ± 2.3 |
| 3 | tr-Aconiticacid | 4.13 | 172.9 | 85 (12), 129 (9) | N.D. |
| 4 | Gallic acid | 4.29 | 169.1 | 125 (14), 79 (25) | 6516 ± 1.7 |
| 5 | Chlorogenic acid | 5.43 | 353.0 | 191 (17) | N.D. |
| 6 | Protocatechuicacid | 5.63 | 153.0 | 109 (16), 108 (26) | N.D. |
| 7 | Tannic acid | 6.46 | 183.0 | 124 (22), 78 (34) | 970.0 ±1.6 |
| 8 | tr-Caffeic acid | 7.37 | 179.0 | 135 (15), 134 (24), 89 (31) | 12.71 ± 1.3 |
| 9 | Vanillin | 8.77 | 151.1 | 136 (17), 92 (21) | 76.7 ± 1.2 |
| 10 | p-Coumaric acid | 9.53 | 163.0 | 119 (15), 93 (31) | 6656.8 ± 3.4 |
| 11 | Rosmarinic acid | 9.57 | 358.9 | 161 (17), 133 (42) | N.D. |
| 12 | Rutin | 10.16 | 608.1 | 300 (37), 271 (51), 301 (38) | 5213.9 ± 1.3 |
| 13 | Hesperidin | 9.69 | 611.1 | 303, 465 | N.D. |
| 14 | Hyperoside | 10.53 | 459.1 | 300, 301 | 2123.0 ± 1.2 |
| 15 | 4-OHBenzoicacid | 11.72 | 137.0 | 93, 65 | N.D. |
| 16 | Salicylicacid | 11.72 | 137.0 | 93, 65, 75 | 43.71 ± 3.3 |
| 17 | Myricetin | 11.94 | 317.0 | 179, 151, 137 | N.D. |
| 18 | Fisetin | 12.61 | 285.0 | 135, 121 | 124.26 ± 2.1 |
| 19 | Coumarin | 12.52 | 147.0 | 103, 91, 77 | 0.8 ± 2.3 |
| 20 | Quercetin | 14.48 | 300.9 | 179, 151, 121 | 293.0 ± 6.2 |
| 21 | Naringenin | 14.66 | 271.0 | 151, 119, 107 | 923.0 ± 2.1 |
| 22 | Hesperetin | 15.29 | 301.0 | 164, 136, 108 | 883.0 ± 1.7 |
| 23 | Luteolin | 15.43 | 285.0 | 175, 151, 133 | 6251.9 ± 1.3 |
| 24 | Kaempferol | 15.43 | 285.0 | 217, 133, 151 | 212.0 ± 2.1 |
| 25 | Apigenin | 16.31 | 269.0 | 151, 117 | 6209.9 ± 1.1 |
| Sample | IC50 of Enzyme Inhibitory Assay (µg/mL) | |
|---|---|---|
| Alpha-Amylase | Alpha-Glucosidase | |
| Cultivated H. albus | 120.5 ± 1.3 | 243.2 ± 1.3 |
| Acarbose | 146.63 ± 1.1 | 270.43 ± 1.1 |
| Binding Force (Energy) (kcal/mol) | Contacts with Hydrogen (Interactions) | Hydrophobic Interactions | Van der Waals Interactions | Electrostatic Interactions | |
|---|---|---|---|---|---|
| Acarbose | −8.6 | Asp404, His674, Asp616, Arg600, Met519, Asp282 | Trp376, Trp481, Phe649 | Trp618, Ala284, Leu405, Leu650, Ile441, Trp516, Asp518, Arg672, Trp613, Gly615, Phe525 | - |
| Luteolin | −8.2 | Asp282, Arg600, His674 | Phe525, Phe649, Trp481 | Ser523, Met519, Trp376, Ile441, Asp404, Trp516, Trp613, Asp518 | Asp616 |
| Fisetin | −8.2 | His674, Asp404 | Trp481, Phe649 | Ile441, Trp516, Arg672, Trp613, Trp376, Leu650, Phe525, Ser523, Asp282, Arg600 | Asp616, Met519, Asp518 |
| Rutin | −8.0 | Asp282, Ser523 | Leu678, Leu650 | Asn524, Ala555, Arg281, Leu283, Ala284, Phe525, Met519, Trp481, Trp618, Arg600, Asp616, trp376, Phe649, Ser676 | - |
| Binding Force (Energy) (kcal/mol) | Contacts with Hydrogen (Interactions) | Hydrophobic Interactions | Van der Waals Interactions | |
|---|---|---|---|---|
| Acarbose | −9.2 | Gly164, Thr163, Ala106, Asn105, Trp59, Val107, His101,Gln63, Arg195, Glu233, Asp300, Tyr62, His299 | - | His305, Trp58, Ala198, Asp197, Lys200, His201, Ile235, Tyr151, Leu162, Leu165, Ile51, Gly104 |
| Fisetin | −9.3 | Asp197, Asp300, Gln63, His305 | Tyr62, Trp59 | Trp58, His299, Arg195, Glu233, Ala198, Leu162, His101, Leu165 |
| Quercetin | −9.2 | Gln63, Asp197, Asp300 | Tyr62, Trp59 | Leu162, Leu165, Ala198, His101, Arg195, Glu233, Trp58, His299, His305 |
| Rutin | −9.1 | Thr163 | Tyr151, His201, Ile235, Lys200, Leu162 | Leu165, Trp59, Trp58, Ala198, Tyr62, His101, Asp197, Arg195, Asp300, Glu233 |
| Groups | Parameters for the Renal Function | |||
|---|---|---|---|---|
| ASAT | ALAT | Urea (g/L) | Creatinine (mg/L) | |
| Group 1 (1000 mg/kg) | 156 ± 2.2 | 47.2 ± 3.5 | 0.34 ± 1.3 | <0.37 |
| Group 2(1500 mg/kg) | 159.3 ± 1.2 | 49.6 ± 3.6 | 0.43 ± 1.3 | <0.37 |
| Group 3 (2000 mg/kg) | 162.5 ± 1.4 | 48.3 ± 2.5 | 0.42 ± 1.5 | <0.37 |
| Control | 157.2 ± 5.2 | 50.0 ± 2.3 | 0.59 ± 1.4 | <0.37 |
| Amount of Blood Glucose (g/L) | |||||
|---|---|---|---|---|---|
| Groups | 0 min | 30 min | 1 h | 1 h and 30 min | 2 h |
| H. albus 10 mg/kg | 0.93 ± 0.7 | 2.4 ± 1.3 | 2.1 ± 1.7 | 1.9 ± 2.0 | 1.8 ± 1.3 |
| H. albus 20 mg/kg | 0.93 ± 1.1 | 2.01 ± 1.8 | 1.5 ± 2.2 | 0.9 ± 1.2 | 0.8 ± 0.6 |
| Glibenclamide20 mg/kg | 0.95 ± 0.8 | 0.62 ± 0.5 | 0.56 ± 0.2 | 0.47 ± 0.5 | 0.41 ± 0.3 |
| Control | 0.9 ± 1.2 | 2.3 ± 1.5 | 2.01 ± 2.1 | 1.82 ± 2.1 | 1.17 ± 1.4 |
| Groups | Amount of Blood Glucose (g/L), Body Weight (g), and Lipid Profile | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Day | 5 Day | 10 Day | 15 Day | 20 Day | |||||||||
| BGL | BW | BGL | BW | BGL | BW | BGL | BW | BGL | BW | TC | HDL | TG | |
| H. albus 10 mg/kg | 3.97 | 26.3 | 2.61 | 25.2 | 2.52 | 25.6 | 2.32 | 25.3 | 2.11 | 25.3 | 0.85 ± 1.2 | 0.45 ± 1.1 | 0.46 ± 1.3 |
| H. albus 20 mg/kg | 2.86 | 27.4 | 1.59 | 27.6 | 1.65 | 26.5 | 1.24 | 27.9 | 1.32 | 26.6 | 0.91 ± 2.2 | 0.51 ± 2.1 | 0.41 ± 2.3 |
| Glibenclamide 20 mg/kg | 3.61 | 27.5 | 3.93 | 26.3 | 3.43 | 26.1 | 2.91 | 25.9 | 2.82 | 25.3 | / | / | / |
| Untreated diabetic mice | 3.51 | 28.2 | 4.53 | 24.3 | 4.31 | 22.1 | 4.62 | 22.6 | 4.35 | 22.3 | 1.83 ± 1.6 | 0.21 ± 1.4 | 0.89 ± 1.1 |
| Normal | 1.08 | 26.6 | 1.02 | 26.5 | 0.98 | 26.1 | 1.12 | 26.8 | 1.10 | 27.4 | 0.95 ± 1.3 | 0.51 ± 2.2 | 0.34 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekmine, S.; Benslama, O.; Kadi, K.; Martín-García, A.I.; Yilmaz, M.A.; Akkal, S.; Boumegoura, A.; Alhomida, A.S.; Ola, M.S.; Ali, A. LC/MS-MS Analysis of Phenolic Compounds in Hyoscyamus albus L. Extract: In Vitro Antidiabetic Activity, In Silico Molecular Docking, and In Vivo Investigation against STZ-Induced Diabetic Mice. Pharmaceuticals 2023, 16, 1015. https://doi.org/10.3390/ph16071015
Lekmine S, Benslama O, Kadi K, Martín-García AI, Yilmaz MA, Akkal S, Boumegoura A, Alhomida AS, Ola MS, Ali A. LC/MS-MS Analysis of Phenolic Compounds in Hyoscyamus albus L. Extract: In Vitro Antidiabetic Activity, In Silico Molecular Docking, and In Vivo Investigation against STZ-Induced Diabetic Mice. Pharmaceuticals. 2023; 16(7):1015. https://doi.org/10.3390/ph16071015
Chicago/Turabian StyleLekmine, Sabrina, Ouided Benslama, Kenza Kadi, Antonio Ignacio Martín-García, Mustafa Abdullah Yilmaz, Salah Akkal, Ali Boumegoura, Abdullah S. Alhomida, Mohammad Shamsul Ola, and Ahmad Ali. 2023. "LC/MS-MS Analysis of Phenolic Compounds in Hyoscyamus albus L. Extract: In Vitro Antidiabetic Activity, In Silico Molecular Docking, and In Vivo Investigation against STZ-Induced Diabetic Mice" Pharmaceuticals 16, no. 7: 1015. https://doi.org/10.3390/ph16071015
APA StyleLekmine, S., Benslama, O., Kadi, K., Martín-García, A. I., Yilmaz, M. A., Akkal, S., Boumegoura, A., Alhomida, A. S., Ola, M. S., & Ali, A. (2023). LC/MS-MS Analysis of Phenolic Compounds in Hyoscyamus albus L. Extract: In Vitro Antidiabetic Activity, In Silico Molecular Docking, and In Vivo Investigation against STZ-Induced Diabetic Mice. Pharmaceuticals, 16(7), 1015. https://doi.org/10.3390/ph16071015









