Abstract
Multiple myeloma (MM) is a challenging hematological cancer which typically grows in bone marrow. MM accounts for 10% of hematological malignancies and 1.8% of cancers. The recent treatment strategies have significantly improved progression-free survival for MM patients in the last decade; however, a relapse for most MM patients is inevitable. In this review we discuss current treatment, important pathways for proliferation, survival, immune suppression, and resistance that could be targeted for future treatments.
1. The Pathogenesis of Multiple Myeloma
Multiple myeloma (MM) is a mature B-cell neoplasm that is characterized by uncontrolled growth of plasma cells (PCs) in bone marrow (BM) which leads to excessive secretion of antibodies. The progression of MM is a multistep process that starts with an asymptomatic premalignant condition known as monoclonal gammopathy of undetermined significance (MGUS), in which BM produces abnormal PCs and secretes M protein instead of normal antibodies [1]. With the increase in oncogenic mutations, MGUS evolves into smoldering MM (SMM), which is characterized by a higher serum level of M protein and a higher percentage of clonal PCs. About 50% of patients with SMM show a constant increase of M protein and develop MM [2]. While both MGUS and SMM are asymptomatic, complications of accumulated proteins may start to affect the kidneys [3]. Almost 20–40% of MM patients have renal disease by the time of diagnosis [4].
The complexity of MM is attributed to the clinical and biological heterogeneity of the disease that further genetically evolves during its progression [5]. MM cells have a wide range of genetic changes including point mutations, insertions, deletions, multiploidy, and chromosomal translocations [6]. For example, trisomic MM and patients with t(11;14) are considered standard-risk patients. On the other hand, MM patients with t(4;14), t(14;16), t(14;20), p53 mutation, gain 1q, or del(17p) are considered to be high-risk [7,8]. Moreover, the bone marrow microenvironment (BMM) plays an important role in disease development, progression, and resistance [9]. All these factors enhance different signaling pathways that contribute to proliferation, survival, invasion, angiogenesis, and osteoclastogenesis [10]. There are many signaling pathways that protect against apoptosis and support MM growth which become activated through the adhesion of MM to the BMM. These activated pathways include phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR), and nuclear factor kappa B (NF-κB), janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3), which support MM growth and protect against apoptosis (Figure 1). The activation of these pathways leads to upregulation and secretion of several cytokines and factors from both MM and BMM cells such as interleukin-6 (IL-6), insulin-like growth factor-1, VEGF, tumor necrosis factor alpha (TNF-α), and transforming growth factor-β [11,12].
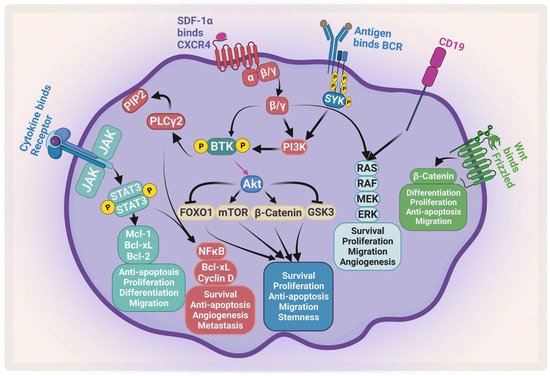
Figure 1.
Major signaling pathways in MM. MM receives several survival and proliferation signals: JAK2/STAT3 pathway activates antiapoptotic proteins and activates NF-κB, the PI3K/AKT/mTOR pathways become activated when stromal-derived factor 1 α (SDF-1α) binds to CXCR4 and/or antigen binds to B-cell receptor (BCR). The RAS/MEK/ERK pathway becomes activated by BCR and CD19. Wnt/β-catenin pathway activation enhances differentiation, survival, migration, and antiapoptotic signals. Ras: rat sarcoma; Raf: rapidly accelerated fibrosarcoma; MEK: mitogen-activated protein kinase kinase. Created by Biorender.com.
The BMM contains several specialized cells that are responsible for skeletal integrity, immunity, and blood formation [13,14] (Figure 2). Its compartments are classified into niches: the immune niche, the vascular niche and the endosteal niche [15]. Each niche contains various cell types such as B-cells, T-cells, myeloid-derived suppressor cells, osteoclasts, natural killer (NK) cells, mesenchymal stem cells, BM stromal cells (BMSCs), osteoblasts, and endothelial progenitor cells [16].
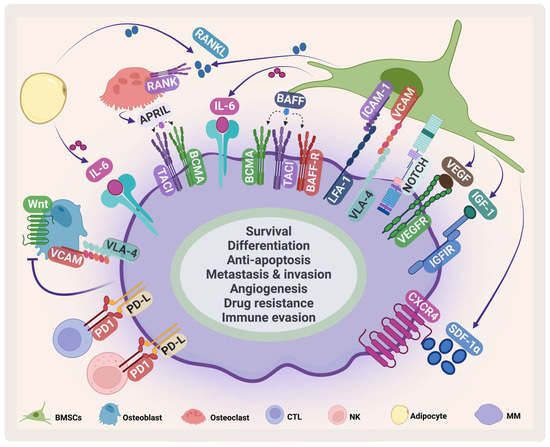
Figure 2.
MM microenvironment. MM cells interact with several cells in the BMM such as BMSCs, osteoclasts, osteoblasts, cytotoxic T-cell (CTLs), natural killer (NK) cells, adipocytes. This interaction enhances MM survival and inhibits immune cells. VCAM: vascular cell adhesion protein; LFA-1: lymphocyte function-associated antigen-1; ICAM-1: intercellular adhesion molecule 1; IGF-1: insulin-like growth factor-1. Created by Biorender.com.
BMSCs are a heterogeneous cell population that supports hematopoiesis in normal conditions. They play an important role in supporting the survival, proliferation, and drug resistance of MM [17]. They communicate with MM in several ways. The direct cell adhesion contact through adhesion molecules such as very late antigen 4 (VLA-4), vascular cell adhesion protein, lymphocyte function-associated antigen-1, and intercellular adhesion molecule 1 stimulates IL-6 secretion by BMSCs [18] and mediates drug resistance through cellular-adhesion-mediated drug resistance (CAM-DR) [19]. Stromal cell-derived factor-1α (SDF-1α), a chemokine produced by BMSCs, plays an important role in embryogenesis, angiogenesis, hematopoiesis, and inflammation [20,21]. It stimulates homing and migration of cells through G protein-coupled receptor C-X-C chemokine receptor type 4 (CXCR4) [22]. The SDF-1α/CXCR4 axis plays an important role in survival, angiogenesis, metastasis, invasion, and adhesion in MM (Figure 2) [23]. It has been shown that the SDF-1α level in MM is elevated and this elevation contributes to activating several signaling pathways and induces mitogen-activated protein kinase kinase1/2, AKT phosphorylation, mitogen-activated protein kinase (MAPK), and NF-κB in MM cell lines and patient samples [24,25]. The SDF-1α/CXCR4 axis mediates drug resistance through different pathways that are involved in CAM-DR, affecting adhesion molecules, enhancing IL-6 mediated drug resistance, and stimulating pathways including MAP/extracellular signal-regulated kinase (ERK), wingless/integrated3 (Wnt3)/Ras homolog family member A/Ras homologous -associated protein kinase, and Ras homologous/Ras homologous -kinase [26,27,28,29,30].
Other cytokines and growth factors secreted from BMSCs suppress the immune response and facilitate MM immune evasion. One of the most important cytokines in MM is IL-6, which is secreted by different BM cells including BMSCs, osteoclasts, and macrophages [31]. IL-6 secretion stimulates the JAK/STAT3 pathway, which leads to increased survival and proliferation through the upregulation of Mcl-1, Bcl-xL, Bcl-2, c-Myc, and cyclin D1 [32,33,34,35]. As MM progresses, osteoclast activity increases, which in turn causes bone lesions. During disease progression, an imbalance occurs in receptor activator of NF-κB ligand (RANKL), and osteoprotegerin [36].
In autologous hematopoietic progenitor cell (HPC) transplantation, plerixafor, a specific antagonist of SDF-1α binding to CXCR4, was approved in 2008 to induce hematopoietic stem cells (HSCs) and progenitor cells (HPCs) trafficking. It has been shown that it augments granulocyte colony-stimulating factor (G-CSF)-induced mobilization of HSCs and HPCs [37,38].
2. The Existing Therapies for MM
In the 1960s, oral melphalan, an alkylating agent, in combination with prednisone was considered the frontline treatment for MM [39,40]. Then, FDA-approved thalidomide, an immunomodulatory agent (IMiDs), was introduced in MM therapy. Thalidomide enhanced the overall survival (OS) and showed longer progression-free survival (PFS) regardless of patient age when used in combination with melphalan and prednisolone (clinical trial # NCT00232934, and ISRCTN90692740) [41,42]. Additionally in the 1980s, autologous stem cell transplantation (ASCT) followed by a high dose of therapy was introduced and became the standard of care among younger patients with normal renal function [43,44].
The discovery and the introduction of proteasome inhibitors (PIs) in 2003 has tremendously improved PFS in patients [45]. Bortezomib became the first line of treatment for MM in newly diagnosed MM (NDMM) patients. For relapsed and refractory MM (RRMM) patients, it was used in combination with melphalan and prednisone (Table 1) [46]. After the success of bortezomib, other PIs such as carfilzomib and ixazomib were approved for the MM treatment. In the TOURMALINE-MM1 trial (NCT01564537), oral ixazomib was tested in combination with lenalidomide and dexamethasone (IRd) on RRMM patients and it has significantly improved the PFS (20.6 months in the IRd group vs. 14.7 months in the Rd group (lenalidomide and dexamethasone) at a median follow-up of 14.7 months). The overall response rate (ORR) was 78% in the IRd group and 72% in the Rd group. The median OS was not reached at a median follow-up of approximately 23 months. The additional adverse effects between the two groups were limited and there was a similar quality of life between the IRd and the Rd groups [47].

Table 1.
FDA-approved medications for MM from 2006 to 2013.
The introduction of a new generation of IMiDs such as lenalidomide in 2005, in combination with PIs, increased survival from 14.8 to 30.9 months [56]. Currently, a triple therapy (PI, IMiD, and corticosteroids) is the first line of treatment for MM followed by autologous stem cell transplant (ASCT). Lenalidomide is usually recommended as a maintenance therapy for MM patients [57]. A combination of bortezomib, lenalidomide, and dexamethasone (VRd) was tested on NDMM in the SWOG-S077 phase III clinical trial (NCT00644228) versus Rd. There was a significant improvement in median PFS (43 months in VRd group vs. 30 months in Rd group) and in median OS (75 months in VRd vs. 64 months in the control group). The ORR was 82% in the VRd group vs. 72% in the Rd group [58]. The next generation IMiD, pomalidomide is shown to be effective and is one of the treatment options that is usually considered in combination after the first relapse for patients who are refractory to lenalidomide [59]. It was first approved in 2013 in combination with dexamethasone for RRMM patients [60]. Then, it was approved in combination with anti-CD38 monoclonal antibody and a steroid for RRMM patients who have previously received two therapies including lenalidomide and bortezomib [60,61,62,63].
Introducing daratumumab, an anti-CD38, in clinical trials (MAIA, ALCYONE, CASTOR, and POLLUX) with different combinations improved minimal residual disease negativity (MRD), and PFS [64] (Table 2). In 2019, daratumumab, lenalidomide, and dexamethasone (DRd) treatment was approved in NDMM patients who are ineligible for transplant after phase III MAIA trial (NCT02252172). In this study, DRd showed significant improvement in PFS (not reached) compared with lenalidomide and dexamethasone (Rd) (31.9 months). The median OS was not reached at a median follow-up of 56.2 months. The common adverse effects of this treatment are neutropenia, pneumonia, anemia, and lymphopenia. Treatment-related-death was 4% in the DRd group compared to 3% in the Rd control group [65,66]. In addition, daratumumab was tested in combination with bortezomib and melphalan-prednisone (D-VMP) in a phase III ALCYONE trial (NCT02195479) in NDMM patients. The 18-month PFS was 71.6%. At a median follow-up of 16.5 months, 22.3% of the patients were negative for MRD. The common adverse effects were neutropenia, thrombocytopenia, and anemia [67]. After the CASSIOPEIA phase III trial (NCT02541383) on ND transplant-eligible MM patients, daratumumab was approved to be used in combination with bortezomib, thalidomide, and dexamethasone (D-VTd) in 2019. At a median follow-up of 35.4 months, PFS was not reached versus 46.7 months with the control group. The most common adverse effects were lymphopenia, hypertension, and neutropenia [68].

Table 2.
FDA-approved medications for MM from 2014 to 2019.
Treatment choices differ according to age, cytogenic abnormalities, and eligibility for transplantation. Maintenance therapy for standard-risk MM patients is lenalidomide. However, bortezomib is used as a maintenance therapy for high-risk ND patients who are determined to be eligible for ASCT. ND high-risk patients who are eligible for ASCT start with three to four cycles of VRd or three to four cycles of quadruplet regimen of daratumumab, bortezomib, lenalidomide, and dexamethasone (DVRd) [59].
2.1. Mechanism of Action of Proteasome Inhibitors
PIs kill myeloma cells through different pathways (Figure 3). Inhibition of proteosomes leads to the accumulation of ubiquitinated proteins that would otherwise be degraded in the proteosome. This leads to the accumulation of these proteins in the endoplasmic reticulum (ER), which in turn causes ER stress, which leads to ER stress-dependent apoptosis and activation of the Jun amino-terminal kinases (JNKs) pathway, increasing the Fas ligand, caspase 8, and caspase 3 [82,83]. Furthermore, mitochondrial injury occurs due to the direct effect of ubiquitinated proteins and the indirect effect of the ER stress that releases reactive oxygen species (ROS) [84]. The direct apoptosis effect of PIs can also occur through accumulation and phosphorylation of P53, which stimulates pro-apoptotic proteins such as Bcl-2-associated X protein (Bax), NADPH oxidase activator (NOXA), cytochrome-c release, and inhibition of the antiapoptotic protein Mcl-1 [85,86].
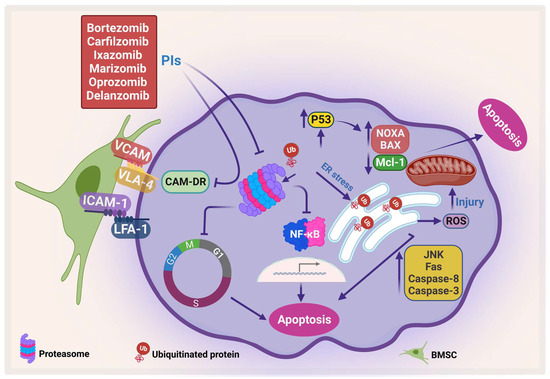
Figure 3.
Proteasome inhibitors (PIs) induce apoptosis and inhibit CAM-DR. PIs induce protein accumulation, which leads to ER stress and activates JNK, which in turn activates caspase-8 and caspase-3, increases Fas, and generates ROS. In addition, PI enhances pro-apoptotic proteins, NOXA and BAX, and inhibits Mcl-1, which leads to apoptosis. Moreover, PI inhibits CAM-DR through inhibition of adhesion molecules (adapted from [86]). Created by Biorender.com.
Bortezomib is the first-generation FDA-approved PI that reversibly inhibits the chymotrypsin-like activity of the proteasomes [87]. Bortezomib has been shown to inhibit NF-κB, which in turn inhibits its downstream pathways and their products, including IL-6, vascular endothelial growth factor (VEGF), c-Myc, and cyclin D1 [88]. On the other hand, bortezomib has been shown to induce constitutive NF-κB activity which could be due to the difference in response among different cell clones [89,90] (reviewed in [86]). In addition, bortezomib ameliorates CAM-DR by inhibiting the expression of adhesion molecules such as VLA-4. Therefore, it resensitizes MM cells to treatment [91]. Even though introducing bortezomib has numerous benefits for patients, several side effects such as peripheral neuropathy may occur. The second-generation PI, carfilzomib, which does not cross the blood–brain barrier may have lower incidence of neuropathy. However, caution should be taken in using carfilzomib in elderly patients as it has shown cardiovascular side effects (reviewed at [92]). Similarly, ixazomib showed lower neurotoxicity than bortezomib as well as more efficacy in clinical trials [93].
2.2. Mechanism of Action of Immunomodulatory Drugs
IMiDs target some proteins for ubiquitination and proteasomal degradation through binding with cereblon ubiquitin ligase, forming an E3 ubiquitin ligase complex with DNA damage-binding protein 1, Cullin-4A, and RING box protein-1 (Figure 4). They target IKAROS family zinc finger 1 and 3 (IKZF1 and IKZF3), which are transcription factors that play an important role in lymphocyte biology. IKZF3 is an essential transcription factor in plasma cell development and therefore its degradation affects MM progression [94].
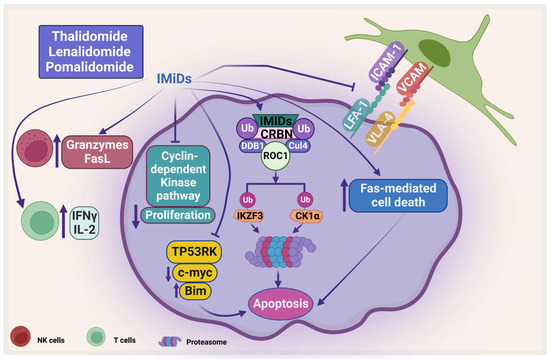
Figure 4.
The direct and indirect effect of IMiDs on MM. IMiDs enhances apoptosis and inhibits MM proliferation through the cyclin-dependent pathway. IMiDs works indirectly through activating the immune cells. CRBN: Cereblon, DDB1: DNA damage-binding protein 1, CUL4: Cullin-4A, and ROC1: RING box protein-1. Created by Biorender.com.
Another protein that has been shown to be degraded by IMiDs is casein kinase 1 alpha (CK1α), which plays an important role in the pathogenesis of MM. CK is one of the serine/threonine kinases that is important in cell survival and has been shown to be important in different types of cancer including MM [95]. Manni et al. have shown that CK1α is overexpressed in most patients’ samples and its inhibition leads to apoptosis, a decrease in β-catenin and AKT expression, and an increase in p53 and p21 expression. In addition, the same group showed that CK1α inhibition enhances the cytotoxicity of bortezomib and lenalidomide on MM [96]. Interestingly, both CK1α and CK2 have been shown to sustain activation of important signaling pathways such as JAK/STAT, NF-κB, and PI3K/AKT [95,97,98]. IMiDs also inhibit the proliferation of PCs by inhibiting the cyclin-dependent kinase pathway through inducing P21 [99]. Moreover, IMiDs induce direct apoptosis in PCs by activation of Fas-mediated cell death [100,101]. Moreover, Hideshima et al. showed that IMiDs inhibit the kinase activity of p53-related protein kinase (TP53RK), which correlate negatively with MM patients’ survival. TP53RK phosphorylate serine 15 of p53, which in turn affects MM growth. The binding of IMiDs to TP53RK triggers apoptosis by inducing pro-apoptotic protein Bim. It also affects the metastasis of MM cells by inhibiting c-Myc protein [102].
The treatment of IMiDs helps in restoring the immune homeostasis. Several mechanisms have been proposed for IMiDs-mediated immune restoration, for example thalidomide stimulates the proliferation of T-cells and increases the secretion of interferon-gamma (IFN-γ) and interleukin-2 (IL-2) [103,104]. Along these lines, lenalidomide has been shown to stimulate T-cell-mediated cytotoxicity, induction of T-cell proliferative responses to allogeneic dendritic cells, and suppresses expression of programmed cell death protein-1 (PD-1) [105,106]. In addition, lenalidomide and pomalidomide suppress forkhead box P3 transcription factor and T regulatory cell expansion [107]. Moreover, lenalidomide enhances the expression of Fas ligand on NK cells and increases granzyme secretion which correlates to an increase in Ab-dependent cellular cytotoxicity (ADCC) [100]. Lenalidomide and pomalidomide inhibit the expression of adhesion molecules and inhibit the RANKL/osteoprotegerin ratio, which leads to inhibition of osteoclast formation [108].
2.3. Mechanism of Action of Histone Deacetylase Inhibitors
Dysregulation in epigenetics including histone acetylation has been shown in different types of cancer including MM [109,110]. Mithraprabhu et al. have shown that class I histone deacetylase (HDAC) is significantly upregulated in MM patients’ samples compared with normal PCs [111]. Moreover, upregulation of HDAC1 was correlated with poor prognosis and shorter OS [111].
Removal of the acetyl group from the lysine residue on histone by HDACs causes transcription repression (Figure 5). HDACs affect different proteins via deacetylation either directly or indirectly by affecting the function of the chaperone protein that is needed for their stabilization. HDACs cause hyperacetylation and therefore destabilization for the chaperone protein heat shock protein 90 (HSP90), which inhibits its association with CXCR4 leading to proteasomal degradation of CXCR4 in acute myeloid leukemia (AML) cells [112,113]. HDACs also cause degradation of protein phosphatase 3 catalytic subunit alpha (PPP3CA), which is overexpressed in MM, and patients show poor prognosis when it is overexpressed. As HDAC6 plays an important role in the aggresomal protein degradation, its inhibition significantly synergizes with proteasomal inhibition in MM [114]. Hideshima et al. have shown that a selective HDAC6 inhibitor increased the cytotoxicity of bortezomib in vitro and overcame its resistance through JNK activation and ER stress [115]. In 2015, panobinostat was approved for treatment of RRMM patients in combination with dexamethasone. HDAC inhibitors (HDACis) have been shown to affect the acetylation of histone and non-histone proteins; they therefore affect different cell processes including apoptosis, survival, angiogenesis, and the cell cycle [116,117].
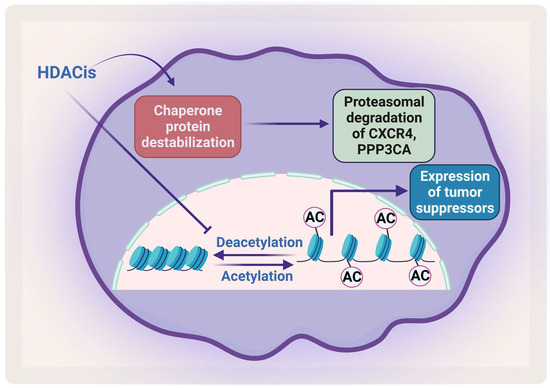
Figure 5.
The effect of HDACis on MM. HDACis cause proteasomal degradation of CXCR4 and PPP3CA and enhance the expression of tumor suppressors. Created by Biorender.com.
3. The Development of Immunotherapies in MM
Currently, there are rapid advances in immunotherapy to treat different types of cancer including MM. Several approaches for immunotherapies have been developed such as inhibiting immune check points, targeting antigens, development of antibody–drug conjugates, chimeric antigen receptor (CAR)-T cells, CAR-NK cell therapy, or using bispecific antibodies or bispecific T-cell engagers antibodies (BiTEs) to attach to more than one target [118]. It is important in immunotherapy to have a specific target, which ideally are surface proteins that are only expressed on target cells to minimize the side effects. MM cells express B-cell maturation antigen (BCMA), CD56, CD117, CD150 (or SLAMF1, signaling lymphocytic activation molecule1), CD48 (SLAMF2), CD229 (SLAMF3), CD352 (SLAMF6), CD319 (SLAMF7 or CS1), CD86, CD184, CD200, and CD272 [119,120,121,122]. BCMA, a tumor necrosis factor receptor superfamily 17 (TNFRSF17) member, is one of the most studied antigens for development of immunotherapies [123].
3.1. Monoclonal Antibodies
Monoclonal antibodies can be used to target surface markers on cancer cells or block immune checkpoints between immune and cancer cells. Daratumumab (anti-CD38) is the first monoclonal antibody that was approved to treat MM in 2015. CD38 is a type II transmembrane glycoprotein that is highly expressed in MM and other hematological malignancies, and it is expressed on myeloid and lymphoid cells [124]. It has been shown that daratumumab induces ADCC, complement-dependent cytotoxicity [125], and Ab-dependent cellular phagocytosis (ADCP) [126]. Moreover, immune profiling of daratumumab in patients’ samples from two clinical trials (NCT00574288 [GEN501] and NCT01985126 [SIRIUS]) showed that daratumumab exerts immunomodulatory effects via suppression of the immunosuppressive cells such as myeloid-derived suppressor, regulatory T-, and regulatory B-cells [127]. In addition, it enhances the expansion of T-helper cells and cytotoxic T-cells [127]. Another anti-CD38 antibody, isatuximab, has been approved for RRMM and NDMM patients, and shown a significant increase in ORR in different clinical trials (Table 3). It is being tested in quadruple therapy regimen with lenalidomide, bortezomib, and dexamethasone in a phase III clinical trial (NCT03617731) [128]. There was a significant improvement in minimal residual disease negativity (50% vs. 36% in the control group). Moreover, addition of isatuximab has significantly improved the very good partial response (VGPR): 77% vs. 61% in the control group [128].

Table 3.
FDA-approved medications for MM from 2017 to 2022.
Elotuzumab (anti-SLAMF7) was also approved in 2015 to be used in combination with lenalidomide and dexamethasone for MM patients who have received at least three prior therapies. SLAMF7 or CS1 is a type I transmembrane glycoprotein which belongs to the Ig superfamily, and it is highly expressed in MM cells (≥95% of cases of MM), normal PCs, and other immune cells such as dendritic, NK, and some T-cell subsets [142]. Elotuzumab induces ADCC by specific binding to SLAMF7 on MM through its Fab portion, and engages NK cells through binding of its FC portion with CD16 on NK cells. Therefore, NK cells become activated and release cytotoxic granules to kill MM cells and release IFN-γ to stimulate other immune cells [142]. Elotuzumab has been shown to induce ADCP through binding of its FC portion with Fc-gamma receptors on macrophages [143]. Moreover, elotuzumab inhibited soluble SLAMF7-induced growth of MM in vitro and in vivo [144]. Additionally, treatment using elotuzumab inhibits the adhesion of myeloma cells to BMSCs [142].
Immune checkpoints are major contributors of cancer evasion [145]. Targeting inhibitory immune checkpoints have revolutionized cancer therapy. There are several immune checkpoint inhibitors that have been shown to inhibit immune function such as cytotoxic T-lymphocyte antigen 4, T-cell immunoglobulin mucin-3, programmed cell death 1 (PD-1) or its ligand, programmed cell death ligand 1 (PD-L1, also referred to as B7-H1 or CD274) [146]. There are many mAbs targeting immune checkpoints approved by the FDA to treat different kinds of cancer as they prolong OS [147]. Tamura et al. have shown that PD-L1 is upregulated in MM and its upregulation is induced by IL-6. Moreover, PD-L1+ RPMI8226 cells have higher Bcl-2 and FasL expression compared with PD-L1− RPMI8226 cells and PDL-1 upregulation is associated with drug resistance, higher MM cell percentages in BM, and higher serum lactate dehydrogenase levels [148]. Stromal-cell-induced MM growth was abrogated by blocking PD1/PD-L1 and enhanced in combination with lenalidomide ex vivo [149]. Durvalumab and nivolumab, PD-1 inhibitors, are being tested in combination with other compounds such as IMiDs, daratumumab, and venetoclax (Bcl-2 inhibitor) [118,150]. A phase Ib KEYNOTE-013 trial (NCT01953692) for pembrolizumab, a PD-1 inhibitor, in RRMM patients showed 2.7 months PFS and 20.2 months OS after a median follow-up of 19.9 months [151]. In 2017, the FDA terminated two phase III trials, KEYNOTE-183 (NCT02576977), and KEYNOTE-185 (NCT02579863), in which pembrolizumab was tested in RRMM and NDMM patients with pomalidomide or lenalidomide, respectively, in addition to a low dose of dexamethasone. In the trials, there was an increase of progression risk and death as the PFS in the pembrolizumab-Pd arm was shorter than in the Pd arm [151].
Siltuximab, an anti-IL-6 monoclonal antibody, was not effective when tested alone or in combination with dexamethasone in a in a phase II clinical trial, NCT00911859 [152]. Similarly, another phase II clinical study showed that the combination of siltuximab with bortezomib did not improve PFS or OS of RRMM patients [153]. However, when testing siltuximab on patients with SMM, there was a delay in the progression of high-risk SMM [154].
3.2. Antibody–Drug Conjugate
To enhance specificity of cytocidal compounds and reduce their off-target effects, an engineered monoclonal antibody is used as a carrier directed toward a tumor-associated antigen. Antibody–drug conjugate (ADC) is one of the exciting and fast evolving approaches in immunotherapy that enhances specificity. This engineered antibody is conjugated with the cytotoxic agent (the payload) through a linker, which should be stable in circulation to guarantee the attachment of the toxic payload until the antibody gets internalized inside the cell (Figure 6E) [155].
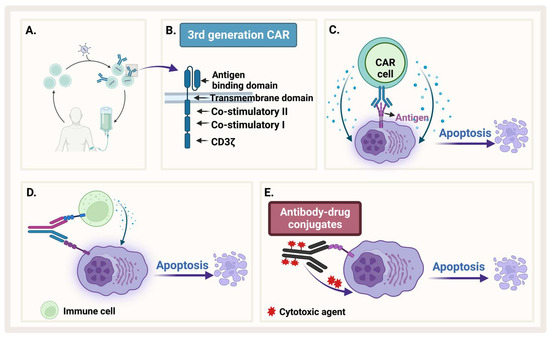
Figure 6.
Immunotherapy. (A) Generation of CAR-T. (B) The different generations of CAR-T. (C) CAR-T recognizes an antigen on MM and causes apoptosis. (D) Bispecific antibody binds both MM and an immune cell. (E) Antibody–drug conjugate recognizes MM and releases cytotoxic agent. Created by Biorender.com.
Belantamab mafodotin was the first-in-class monoclonal antibody that was approved to treat MM [156]. It is an anti-BCMA that is covalently linked to monomethyl auristatin F, a microtubule inhibitor [157]. There are some other ADCs that have been developed as anti-BCMA drug conjugates; for example, HDP-101, an anti-BCMA drug conjugate, has been tested in preclinical trials. It is conjugated to alpha-amanitin, a eukaryotic RNA polymerase II inhibitor, to inhibit transcription and therefore translation [158,159,160].
3.3. Bispecific Antibodies
Bispecific antibodies are monoclonal antibodies that have two binding sites for different antigens or two different epitopes on the same antigen (Figure 6D). They work through four different mechanisms: (1) bind target cell and an immune cell to assist immune response, (2) block two signaling pathways to prevent immune escape of cancerous cells, (3) block two immune checkpoints, and (4) drive connection of protein complexes [161]. The most common mechanism known for bispecific antibodies is to simultaneously bind two different antigens on two different cells such as a cancer cell and a T-cell to bring them into proximity and facilitate antitumor immune response [162].
In 2022, the FDA approved Teclistamab as the first bispecific antibody to treat MM patients who had received at least four prior treatments. It targets both CD3 on T-cells and BCMA, which is overexpressed on MM cells. Bringing T-cell and MM cells in proximity creates an immunological synapse and initiates cytolytic cascades and stimulates proinflammatory cytokines. MM cell death and lysis occur after T-cell activation. Teclistamab was approved after a phase I/II MajesTEC-1 trial (NCT04557098) in triple-class RRMM with five previous therapy lines. The ORR was 63.0% at a median follow-up of 14.1 months with 39.4% of patients showing complete response; PFS was 11.3 months. The common adverse effects are cytokine release syndrome, thrombocytopenia, neutropenia, and anemia. Additionally, 14% of patients had neurotoxic events. Currently, a phase III trial (NCT05083169), which is still recruiting, will be testing the combination of teclistamab with daratumumab (Tec-Dara) versus DPd (daratumumab, pomalidomide, and dexamethasone) or DVd (daratumumab, bortezomib, dexamethasone) in RRMM [140,141].
Elranatamab, another bispecific antibody, also targets CD3 and BCMA and is now in a phase III trial for RRMM. It has been tested as monotherapy as well as in combination in Magnetis MM clinical trials. It showed a good ORR (around 60%) and it was well tolerated in patients [163]. Talquetamab, is a bispecific antibody that targets both CD3 and the orphan G protein coupled receptor, class C group 5 member D (GPRC5D) that has been shown to be expressed suggested as tumor load in MM patients [164,165].
3.4. Chimeric Antigen Receptor-Modified T-Cells and NK Cells
CARs are genetically engineered transmembrane receptors designed to recognize and target specific antigens on cell surfaces. CAR-T cells are generally engineered in vitro after collecting autologous patients’ T-cells and transducing them with engineered lentivirus (Figure 6A). With CAR therapy, the immune system of patients can be reprogrammed and directed to target cancer cells [151]. CARs in general are composed of an extracellular domain that recognizes antigens such as single-chain variable fragment (scFv), and an intracellular activation domain (Figure 6B). The first generation of CAR, which was engineered in 1992, contains only CD3ζ. Then, one costimulatory domain, CD28 or 4-1BB (second generation), or two costimulatory domains (third generation), were added to enhance CAR function [123]. After in vitro engineering, CAR cells are reinfused in patients’ bodies so that they would recognize and attach to specific antigens on tumor cells (Figure 6C). This engagement stimulates the signaling cascade in engineered cells. In the case of engineered T-cells, for example, engagement of CAR-T with its target can stimulate different signaling pathways such as the PI3K and MAPK pathways, which activate T-cells to release pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-6, and IL-2 [123,166]. This activation of CAR-T cells is required to exert cytotoxic function and lysis of tumor cells. However, immune-mediated adverse reactions such as cytokine release syndrome (CRS) is one of the issues that researchers try to ameliorate by using different methods such as introducing “suicide” genes [167]. The field of CAR engineering to design next generation therapies is rapidly moving forward. The fourth generation of CAR is called T-cells redirected for antigen-unrestricted cytokine-initiated killing, in which a constitutively expressed chemokine is added to the second generation of CARs. When the CAR is activated, the cytokine is released to induce tumor killing [168]. To add a third synergistic signal to CD3ζ CD28, a truncated IL-2 receptor is added with a binding site for STAT3 and integrated into the second generation of CARs to produce the fifth generation of CARs [169]. Researchers are improving the CAR-T design by adding other targets in the same CAR-T construct and testing other costimulatory domains as well as reprogramming several types of immune cells.
The first clinical trial of an anti-BCMA CAR-T (Construct: anti-BCMA scFv, CD8α hinge and transmembrane regions, the cytoplasmic portion of the CD28 costimulatory moiety, and the CD3ζ T-cell activation domain) started in 2014 (NCT02215967) after conditioning with cyclophosphamide and fludarabine. Antimyeloma activity and remissions of poor prognosis RRMM patients have been reported; however, side effects of CRS were seen in two patients out of eleven. Moreover, antigen escape has been seen in patients treated with anti-BCMA CAR-T [170,171].
Various anti-BCMA CAR-T therapies have been developed and investigated in clinical trials (Table 4). One of the challenges facing immunotherapy targeting antigens is the antigen escape that has been reported in many targeted antigens, including BCMA, which leads to relapse of MM patients and shorter durations of remission [172]. For this reason, researchers are attempting to find a ligand that can bind with more than one antigen. The BAFF ligand binds with three receptors on mature B cells: BAFF-R, BCMA, and transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI). Therefore, Wong et al. constructed a BAAF ligand-CAR-T cell (Construct: extracellular BAFF ligand, short spacer, hinge from human IgG1, CD28 transmembrane and signaling domains, OX40, and CD3ζ), which can interact with all three proteins (BAFF-R, BCMA, and TACI). BAAF L-CAR-T was significantly activated when co-cultured with U266, RPMI8226, or MM.1S MM cells. Furthermore, BAAF L-CAR-T showed a significant cytotoxic effects in vivo using xenograft models injected with MM1.S [173]. A phase I clinical trial is ongoing, which will study the efficacy of BAAF ligand-CAR-T in MM patients (NCT05546723).

Table 4.
Some of the ongoing CAR-T and CAR-NK trials.
Currently, idecabtagene (BCMA CAR-T) and ciltacabtagene autoleucel are the only CAR-Ts approved to treat RRMM. Idecabtagene vicleucel (ide-cel; bb2121) was approved in March 2021 after the phase II KarMMa trial (NCT03361748) on RRMM patients who received ≥3 prior regimens. The ORR was 73.0% at a median follow-up of 11.3 months with PFS around 8.6 months. The common adverse effects are CRS, cytopenias, and neurotoxicity [135]. Likewise, ciltacabtagene autoleucel (cilta-cel), which contains two anti-BCMA-targeting single domains was approved in 2022 after a phase Ib/II CARTITUDE-1 trial (NCT03548207) on double refractory MM patients who received four or more prior lines of therapy, including a PI, an IMiD, and an anti-CD38 monoclonal antibody. The ORR was 100% at a median follow-up of 9 months. PFS was 6 months with 76% CR and 21% had VGPR. The common adverse effects are CRS, neutropenia, thrombocytopenia, and leukopenia [139].
Even though anti-BCMA CAR-T showed promising results in MM clinical trials, there are concerns about toxicity, CRS, and aplasia [174,175]. Therefore, NK cells could be better candidates [176]. CAR-NK trafficking of CAR-NK cells to the BM is challenging. To solve this issue, Yu et al. have an exciting in vivo study with a xenograft mouse model in which they modified anti-BMCA CAR-NK cells to express CXCR4 to enable the CAR-NK infiltration in the BM. For the CAR-NK construct, they used BCMA-specific scFv binding domain and compared two intracellular activation domains: CD3ζ and DAP12. There was no statistically significant difference in the performance of CAR-NK between the two activation domains. Expressing CXCR4 in NK cells significantly increased migration towards SDF-1α in vitro and promoted their migration toward BM in vivo. Moreover, expressing CXCR4 in anti-BCMA CAR-NK significantly inhibited tumor burden after 42 days [177]. An early phase I trial started in June 2021 and the final data was expected to be collected in December 2022 (NCT04727008). Other clinical trials for anti-BMCA CAR-NK are still recruiting patients (Table 4).
4. Glance into the Future
Even though the MM survival rate has improved with current treatments, generally 19–25% patients do not respond to PIs during the first-time treatment and almost 50% of RRMM patients do not respond to PIs, which leads to a serious problem [118]. The OS of RRMM patients is about 8 months [46,118]. Therefore, it is necessary to find new treatments and combination strategies to overcome drug resistance and improve survival for MM patients. Several new targets have been identified, and new molecules have been developed. Some of these molecules have been introduced in the clinical trial and some are still in the pre-clinical phase.
4.1. Targeting the Apoptotic Pathway in MM
Apoptosis, a regulated cell death, is an essential mechanism in regulating development and maintaining homeostasis, plays a crucial role in preventing oncogenesis. The unrestrained growth of MM can occur due to the loss of control of apoptosis that happens through upregulation of antiapoptotic proteins such as Mcl-1, Bcl-2, and Bcl-xL all of which protect against genomic instability. Activation of different signaling cascades such as JAK2/STAT3, NF-κB, PI3K/AKT/mTOR, and Wnt/β-catenin pathways through the tumor microenvironment leads to upregulation of antiapoptotic proteins.
The efforts of targeting different molecules in apoptotic and survival pathways has shown promising results. Many small molecules that can inhibit survival and/or stimulate apoptosis have been developed, some of which are still in clinical trials.
Bcl-2 Family Inhibitors
The Bcl-2 family is one of the most important targets, and their inhibitors are either in clinical or in preclinical stages. For example, venetoclax, a Bcl-2 inhibitor, is approved for the treatment of hematological malignancies including chronic lymphocytic leukemia, AML, and small lymphocytic lymphoma [178,179]. There are around 25 clinical trials for venetoclax with different drug combinations ongoing in MM [180]. In a phase III BELLINI clinical trial (NCT02755597) for RRMM patients, a significant improvement in PFS could not be achieved when venetoclax was used in combination with bortezomib and dexamethasone [181]. However, the study showed that venetoclax is more effective in patients with t (11;14) and high Bcl-2 expression compared to the other patients [181].
The overexpression of another Bcl-2 family member, Mcl-1, is reported in MM patients. The expression of Mcl-1 is associated with 1q21 [182] amplification, where approximately 40% of ND and 70% of RR patients show gain (3 copies) or amplification (≥4 copies) in 1q21 [183,184]. The overexpression of Mcl-1 is associated with relapse and poor prognosis [185]. Moreover, Mcl-1 dependent cancers are resistant to pan Bcl-2/Bcl-xL inhibitors (ABT-737), and venetoclax [186]. Therefore, Mcl-1 is an attractive target for MM [187,188,189]. There are several clinical trials ongoing for Mcl-1 inhibitors, for example MIK665 (also named S64315) is tested in phase I trials for refractory or relapsed lymphoma or MM patients [190]. One of these trials (NCT02992483) is completed, but results are not yet released [191]. The other trial (NCT04702425) is still recruiting with the goal of testing the combination of MIK665 with a Bcl-2 inhibitor, VOB560 [191]. Both in vitro and in vivo studies have shown that the combination of Mcl-1 inhibitor, MIK665, with Bcl-2 inhibitors showed strong and durable antitumor responses [192,193]. Other potential Mcl-1 inhibitors, PRT14 and AMG176 (Tapotoclax), are currently in phase I (NCT04543305, NCT02675452).
Marinopyrrole A (also named maritoclax), a natural product from marine-derived streptomycetes was discovered by the Wang group as a selective Mcl-1 antagonist [194]. They found that Maritoclax induces caspase-3 activation by directly binding to Mcl-1 and targeting it for proteasomal degradation and sensitizes cancer cells to ABT-737. The researchers showed that maritoclax disrupts the interaction between Bim and Mcl-1 [194]. Along these lines, we showed that maritoclax potentiates the apoptotic effect of ABT-737 in human melanoma cells [195].
4.2. Targeting MM Cancer Stem Cells
It has been demonstrated that cancer stem cells (CSCs) are critical in relapse [196]. The mechanism of how CSCs are involved in relapse is not well understood; however, it has been demonstrated that intracellular drug detoxification and drug efflux which retribute to the overexpression of aldehyde dehydrogenase (ALDH) and ATP-binding cassette transporter G2 (ABCG2) are key players [197]. Several studies have been made to investigate phenotypic characteristics and identify surface markers for CSCs. However, the molecular profile of CSCs is still controversial. The studies have demonstrated that CD138− PCs exhibit tumor-initiating potential, self-renewal capability, and drug resistance, which suggests that CD138− is a CSC [198]. Furthermore, it is likely that because of the heterogenous characteristic of MM, there are different stem cell subsets [199]. For example, the markers CD19+CD38−CD27+, CD19+CD34−Lchain(λ)+ALDH+, and CD19−CD45−CD38+CD138+ have also been identified as CSCs in MM [200].
Some natural compounds have been shown to be effective in targeting CSCs. The ethanolic extract of scutellaria, a traditional Chinese herbal remedy, and its derivatives baicalein, wogonin, and baicalin have been shown to decrease the expression level of ABCG2 protein in RPMI-8226 [201].
Salinomycin, a monocarboxylic polyether antibiotic derived from Streptomyces albus [202], is shown to inhibit stemness in cancer cells. It overcomes ABC transporter-mediated multidrug resistance and induces apoptosis in human leukemia stem-cell-like cells [203]. Moreover, Kastritis et al. showed that salinomycin decreases the side population fraction of MM, which is known to efflux Hoechst stain and represent a stem-cell-like population [204].
ALDHs are detoxifying enzymes that have been shown to be highly expressed in HSCs and CSCs [205]. The overexpression of ALDH in cancer has been associated with drug resistance, relapse, and poor prognosis [206]. Zhou et al. showed that ALDH is associated with chromosomal instability in MM and that an ALDH1+ subset from two MM cell lines had a higher clonogenic potential than an ALDH1− cell subset [207]. Moreover, Yang et al. found that overexpression of ALDH1 increased MM drug resistance in vivo [208]. Even though ALDH inhibitors are not in the clinical trials for MM, it is a compelling target to explore, and researchers are developing and modifying different ALDH inhibitors (reviewed in [209]).
4.3. Targeting the Bone Marrow Microenvironment
The interaction of myeloma cells to BMM is a hallmark of MM. This interaction supports myeloma cell survival and plays critical role in pathogenesis, thus targeting of BMM has been one of the areas of active research. Along these lines, SDF-1α/CXCR4 axis, Bruton’s tyrosine kinase (BTK), JAK/STAT, NF-κB, and RANK/RANK-L have been identified as prime targets.
Plerixafor, a specific antagonist of SDF-1α binding to CXCR4, was approved in 2008 to induce HSCs and human progenitor cells (HPCs) trafficking. It has been shown that it augments granulocyte colony-stimulating factor (G-CSF)-induced mobilization of HSCs and HPCs [38,39]. Our lab has shown that gambogic acid, a xanthonoid derived from Garcinia hanburyi, blocks RANKL-induced osteoclastogenesis, suppressing the SDF-1α- induced chemotaxis of MM cells [210].
Bruton’s Tyrosine Kinase Inhibitors
Kinases play essential roles in survival pathways; therefore, a wide range of kinase inhibitors have been developed and investigated regarding MM. There are numerous potential targets for kinase inhibitors, including but not limited to non-receptor tyrosine kinases, receptor tyrosine kinases (RTKs), PI3K/AKT/mTOR pathway kinases, protein kinase C, mitogen-activated protein kinase, cell cycle control kinases, casein kinase, and glycogen synthase kinase. At this writing, there are about 100 clinical trials testing different kinase inhibitors in the targeting of MM survival pathways.
One of the essential kinases that is important during B-cell development is BTK, which belongs to the Tec family of kinases. BTK is shown to be overexpressed and activated in MM stem-cell-like cells [211]. BTK is activated by different pathways including B-cell receptor (BCR), toll-like receptor, chemokine, and Fc receptor signaling pathways [212]. When activated, it translocates from the cytosol to the cell membrane and gets phosphorylated by one of the members of the spleen tyrosine kinase (SYK) or Src family kinases on the tyrosine residue at position 551 (Y551) [213]. Then, an autophosphorylation occurs on tyrosine at position 223 (Y223). The activation of BTK stimulates several downstream signals that are associated with survival, proliferation, drug resistance, and migration.
Ibrutinib and acalabrutinib are FDA-approved BTK inhibitors to treat B-cell malignancies other than MM. Ibrutinib inhibits BTK by covalently binding cysteine at position 481 (C481). BTK inhibitors are not yet approved for MM; however, clinical trials are ongoing (Table 2). After the excitement of the effective results of ibrutinib and acalabrutinib in treating B-cell malignancies, resistance occurred due to acquired mutations in the kinase domain such as the Cys481 to Ser (C481S) mutation, which disrupts the covalent binding of these drugs. Therefore, we developed a new BTK inhibitor, KS151, that avoids binding with the Cys481 residue. Importantly, this new molecule was effective in a stem-cell-like population and kills MM cells [214].
5. Conclusions
Most MM patients experience a relapse after treatment which can occur from drug resistance and antigen escape. The genomic instability that is associated with the disease progression adds to the complexity of disease progression. Moreover, there are multiple mutational drivers that can lead to MM disease and contribute to its heterogeneity [109,215]. Misund et al. showed that MM progression is associated with phenotypic transformation and several changes in the transcriptomic levels in patients’ sample which can be targeted in future [216]. There are various pathways that are disrupted in MM that can affect other pathways by cross talk activation or repression. Therefore, it is essential to combine different treatments to overcome different feedback loops that can counteract the effect of some inhibitors, overcome resistance, and lower the side effects of given medications.
The treatment of MM continues to progress, and the future of immunotherapy in combination with other treatments will focus on understanding the MM stem-cell-like cells and finding putative tumor-associated antigens that can be targeted with immunotherapy as well as small molecules, improving the efficacy and the specificity of immunotherapy, and targeting the BMM compartments such as BMSCs to overcome the BMM’s protective niche. Over the past decade, immunotherapy has been introduced and has shown promising results. There is a need to have more clinical studies regarding treatment sequences to know the effect of immunotherapy on NDMM patients. Personalized treatments should also be considered as MM has a heterogeneous phenotype. New targets should be investigated with novel compounds to minimize toxicity and side effects and increase patients’ OS and quality of life.
Author Contributions
Conceptualization, W.O.E. and M.K.P.; writing—original draft preparation, W.O.E.; writing—review and editing, W.O.E., K.B.C., T.B.-A., S.C.J. and M.K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Camden Research Initiative fund (M.K.P., T.B.-A. and S.C.J.), New Jersey Health Foundation (S.C.J. and M.K.P.), and an inter-department fund from Cooper Medical School of Rowan University, Camden, NJ, USA (M.K.P.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors are thankful to Rachel King, Cooper Medical School of Rowan University Library for careful proofreading and editing the manuscript.
Conflicts of Interest
The authors do not report any conflicts of interest.
References
- Kyle, R.A.; Durie, B.G.; Rajkumar, S.V.; Landgren, O.; Blade, J.; Merlini, G.; Kröger, N.; Einsele, H.; Vesole, D.H.; Dimopoulos, M.; et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010, 24, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Atrash, S.; Robinson, M.; Slaughter, D.; Aneralla, A.; Brown, T.; Robinson, J.; Ndiaye, A.; Sprouse, C.; Zhang, Q.; Symanowski, J.T.; et al. Evolving changes in M-protein and hemoglobin as predictors for progression of smoldering multiple myeloma. Blood Cancer J. 2018, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Landgren, O.; Mateos, M.V. Smoldering multiple myeloma. Blood 2015, 125, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Terpos, E.; Chanan-Khan, A.; Leung, N.; Ludwig, H.; Jagannath, S.; Niesvizky, R.; Giralt, S.; Fermand, J.P.; Bladé, J.; et al. Renal impairment in patients with multiple myeloma: A consensus statement on behalf of the International Myeloma Working Group. J. Clin. Oncol. 2010, 28, 4976–4984. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Khong, T.; Ramachandran, M.; Chow, A.; Klarica, D.; Mai, L.; Walsh, S.; Broemeling, D.; Marziali, A.; Wiggin, M.; et al. Circulating tumour DNA analysis demonstrates spatial mutational heterogeneity that coincides with disease relapse in myeloma. Leukemia 2017, 31, 1695–1705. [Google Scholar] [CrossRef]
- Bergsagel, P.L.; Kuehl, W.M. Promiscuous Structural Variants Drive Myeloma Initiation and Progression. Blood Cancer Discov. 2020, 1, 221–223. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rajkumar, S.V. The multiple myelomas-current concepts in cytogenetic classification and therapy. Nat. Rev. Clin. Oncol. 2018, 15, 409–421. [Google Scholar] [CrossRef]
- Meads, M.B.; Hazlehurst, L.A.; Dalton, W.S. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin. Cancer Res. 2008, 14, 2519–2526. [Google Scholar] [CrossRef]
- Bommert, K.; Bargou, R.C.; Stühmer, T. Signalling and survival pathways in multiple myeloma. Eur. J. Cancer 2006, 42, 1574–1580. [Google Scholar] [CrossRef]
- Yosifov, D.Y.; Reufsteck, C.; Konstantinov, S.M.; Berger, M.R. Interleukin-6, osteopontin and Raf/MEK/ERK signaling modulate the sensitivity of human myeloma cells to alkylphosphocholines. Leuk. Res. 2012, 36, 764–772. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.; Lokhorst, H.M.; Bloem, A.C. Growth factors and antiapoptotic signaling pathways in multiple myeloma. Leukemia 2005, 19, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, I.M.; Detappe, A.; Anderson, K.C.; Steensma, D.P. The bone-marrow niche in MDS and MGUS: Implications for AML and MM. Nat. Rev. Clin. Oncol. 2018, 15, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Pawlyn, C.; Morgan, G.J. Evolutionary biology of high-risk multiple myeloma. Nat. Rev. Cancer 2017, 17, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Neumeister, P.; Schulz, E.; Pansy, K.; Szmyra, M.; Deutsch, A.J. Targeting the Microenvironment for Treating Multiple Myeloma. Int. J. Mol. Sci. 2022, 23, 7627. [Google Scholar] [CrossRef]
- Di Marzo, L.; Desantis, V.; Solimando, A.G.; Ruggieri, S.; Annese, T.; Nico, B.; Fumarulo, R.; Vacca, A.; Frassanito, M.A. Microenvironment drug resistance in multiple myeloma: Emerging new players. Oncotarget 2016, 7, 60698–60711. [Google Scholar] [CrossRef]
- Mitsiades, C.S.; Mitsiades, N.S.; Munshi, N.C.; Richardson, P.G.; Anderson, K.C. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: Interplay of growth factors, their receptors and stromal interactions. Eur. J. Cancer 2006, 42, 1564–1573. [Google Scholar] [CrossRef]
- Uchiyama, H.; Barut, B.A.; Mohrbacher, A.F.; Chauhan, D.; Anderson, K.C. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood 1993, 82, 3712–3720. [Google Scholar] [CrossRef]
- Morgan, G.J.; Walker, B.A.; Davies, F.E. The genetic architecture of multiple myeloma. Nat. Rev. Cancer 2012, 12, 335–348. [Google Scholar] [CrossRef]
- Bleul, C.C.; Fuhlbrigge, R.C.; Casasnovas, J.M.; Aiuti, A.; Springer, T.A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J. Exp. Med. 1996, 184, 1101–1109. [Google Scholar] [CrossRef]
- Janssens, R.; Struyf, S.; Proost, P. The unique structural and functional features of CXCL12. Cell Mol. Immunol. 2018, 15, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Jones, D.; Borghesani, P.R.; Segal, R.A.; Nagasawa, T.; Kishimoto, T.; Bronson, R.T.; Springer, T.A. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 9448–9453. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, P.; Haier, J.; Schlüter, K.; Domikowsky, B.; Wendel, C.; Wiesner, U.; Kubitza, R.; Engers, R.; Schneider, S.W.; Homey, B.; et al. CXCR4 regulates the early extravasation of metastatic tumor cells in vivo. Neoplasia 2009, 11, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Chauhan, D.; Hayashi, T.; Podar, K.; Akiyama, M.; Gupta, D.; Richardson, P.; Munshi, N.; Anderson, K.C. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol. Cancer Ther. 2002, 1, 539–544. [Google Scholar]
- Hideshima, T.; Chauhan, D.; Schlossman, R.; Richardson, P.; Anderson, K.C. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: Therapeutic applications. Oncogene 2001, 20, 4519–4527. [Google Scholar] [CrossRef]
- Waldschmidt, J.M.; Simon, A.; Wider, D.; Müller, S.J.; Follo, M.; Ihorst, G.; Decker, S.; Lorenz, J.; Chatterjee, M.; Azab, A.K.; et al. CXCL12 and CXCR7 are relevant targets to reverse cell adhesion-mediated drug resistance in multiple myeloma. Br. J. Haematol. 2017, 179, 36–49. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, H.M.; Lv, Y.Q.; Tang, S.M.; Cheng, P. Blockade of SDF-1/CXCR4 reduces adhesion-mediated chemoresistance of multiple myeloma cells via interacting with interleukin-6. J. Cell Physiol. 2019, 234, 19702–19714. [Google Scholar] [CrossRef]
- Sun, L.; Liu, L.; Liu, X.; Wang, Y.; Li, M.; Yao, L.; Yang, J.; Ji, G.; Guo, C.; Pan, Y.; et al. MGr1-Ag/37LRP induces cell adhesion-mediated drug resistance through FAK/PI3K and MAPK pathway in gastric cancer. Cancer Sci. 2014, 105, 651–659. [Google Scholar] [CrossRef]
- Kobune, M.; Chiba, H.; Kato, J.; Kato, K.; Nakamura, K.; Kawano, Y.; Takada, K.; Takimoto, R.; Takayama, T.; Hamada, H.; et al. Wnt3/RhoA/ROCK signaling pathway is involved in adhesion-mediated drug resistance of multiple myeloma in an autocrine mechanism. Mol. Cancer Ther. 2007, 6, 1774–1784. [Google Scholar] [CrossRef]
- Schmidmaier, R.; Baumann, P.; Simsek, M.; Dayyani, F.; Emmerich, B.; Meinhardt, G. The HMG-CoA reductase inhibitor simvastatin overcomes cell adhesion-mediated drug resistance in multiple myeloma by geranylgeranylation of Rho protein and activation of Rho kinase. Blood 2004, 104, 1825–1832. [Google Scholar] [CrossRef]
- Shay, G.; Hazlehurst, L.; Lynch, C.C. Dissecting the multiple myeloma-bone microenvironment reveals new therapeutic opportunities. J. Mol. Med. 2016, 94, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.S.Y.; Chng, W.J.; de Mel, S. STAT3: A Promising Therapeutic Target in Multiple Myeloma. Cancers 2019, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A.; Kortylewski, M.; Kujawski, M.; Zhang, C.; Reckamp, K.; Armstrong, B.; Wang, L.; Kowolik, C.; Deng, J.; Figlin, R.; et al. Targeting Stat3 in the myeloid compartment drastically improves the in vivo antitumor functions of adoptively transferred T cells. Cancer Res. 2010, 70, 7455–7464. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, M.; Zhang, C.; Herrmann, A.; Reckamp, K.; Scuto, A.; Jensen, M.; Deng, J.; Forman, S.; Figlin, R.; Yu, H. Targeting STAT3 in adoptively transferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res. 2010, 70, 9599–9610. [Google Scholar] [CrossRef]
- Raje, N.S.; Bhatta, S.; Terpos, E. Role of the RANK/RANKL Pathway in Multiple Myeloma. Clin. Cancer Res. 2019, 25, 12–20. [Google Scholar] [CrossRef]
- Liles, W.C.; Rodger, E.; Broxmeyer, H.E.; Dehner, C.; Badel, K.; Calandra, G.; Christensen, J.; Wood, B.; Price, T.H.; Dale, D.C. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion 2005, 45, 295–300. [Google Scholar] [CrossRef]
- Flomenberg, N.; Devine, S.M.; Dipersio, J.F.; Liesveld, J.L.; McCarty, J.M.; Rowley, S.D.; Vesole, D.H.; Badel, K.; Calandra, G. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood 2005, 106, 1867–1874. [Google Scholar] [CrossRef]
- Bergsagel, D.E.; Sprague, C.C.; Austin, C.; Griffith, K.M. Evaluation of new chemotherapeutic agents in the treatment of multiple myeloma. IV. L-Phenylalanine mustard (NSC-8806). Cancer Chemother Rep. 1962, 21, 87–99. [Google Scholar]
- Hoogstraten, B.; Sheehe, P.R.; Cuttner, J.; Cooper, T.; Kyle, R.A.; Oberfield, R.A.; Townsend, S.R.; Harley, J.B.; Hayes, D.M.; Costa, G.; et al. Melphalan in multiple myeloma. Blood 1967, 30, 74–83. [Google Scholar] [CrossRef]
- Gay, F.; Larocca, A.; Wijermans, P.; Cavallo, F.; Rossi, D.; Schaafsma, R.; Genuardi, M.; Romano, A.; Liberati, A.M.; Siniscalchi, A.; et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: Analysis of 1175 patients. Blood 2011, 117, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Bringhen, S.; Caravita, T.; Merla, E.; Capparella, V.; Callea, V.; Cangialosi, C.; Grasso, M.; Rossini, F.; Galli, M.; et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: Randomised controlled trial. Lancet 2006, 367, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Osserman, E.F.; DiRe, L.B.; DiRe, J.; Sherman, W.H.; Hersman, J.A.; Storb, R. Identical twin marrow transplantation in multiple myeloma. Acta Haematol. 1982, 68, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Harousseau, J.L.; Stoppa, A.M.; Sotto, J.J.; Fuzibet, J.G.; Rossi, J.F.; Casassus, P.; Maisonneuve, H.; Facon, T.; Ifrah, N.; et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N. Engl. J. Med. 1996, 335, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Twombly, R. First proteasome inhibitor approved for multiple myeloma. J. Natl. Cancer Inst. 2003, 95, 845. [Google Scholar] [CrossRef]
- San Miguel, J.F.; Schlag, R.; Khuageva, N.K.; Dimopoulos, M.A.; Shpilberg, O.; Kropff, M.; Spicka, I.; Petrucci, M.T.; Palumbo, A.; Samoilova, O.S.; et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N. Engl. J. Med. 2008, 359, 906–917. [Google Scholar] [CrossRef]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Blood, E.; Vesole, D.; Fonseca, R.; Greipp, P.R. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: A clinical trial coordinated by the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2006, 24, 431–436. [Google Scholar] [CrossRef]
- Services USDoHaH. Hematology/Oncology (Cancer) Approvals & Safety Notifications: Previous News Items; U.S. Food and Drug Administration. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications (accessed on 1 March 2023).
- Dimopoulos, M.; Spencer, A.; Attal, M.; Prince, H.M.; Harousseau, J.-L.; Dmoszynska, A.; Miguel, J.S.; Hellmann, A.; Facon, T.; Foà, R.; et al. Lenalidomide plus Dexamethasone for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2007, 357, 2123–2132. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Nagler, A.; Sonneveld, P.; Bladé, J.; Hajek, R.; Spencer, A.; San Miguel, J.; Robak, T.; Dmoszynska, A.; Horvath, N.; et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: Combination therapy improves time to progression. J. Clin. Oncol. 2007, 25, 3892–3901. [Google Scholar] [CrossRef]
- Richardson, P.G.; Xie, W.; Mitsiades, C.; Chanan-Khan, A.A.; Lonial, S.; Hassoun, H.; Avigan, D.E.; Oaklander, A.L.; Kuter, D.J.; Wen, P.Y.; et al. Single-agent bortezomib in previously untreated multiple myeloma: Efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J. Clin. Oncol. 2009, 27, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortega, I.; Querol, S.; Encuentra, M.; Ortega, S.; Serra, A.; Sanchez-Villegas, J.M.; Grifols, J.R.; Pujol-Balaguer, M.M.; Pujol-Bosch, M.; Martí, J.M.; et al. Plerixafor in patients with lymphoma and multiple myeloma: Effectiveness in cases with very low circulating CD34+ cell levels and preemptive intervention vs remobilization. Bone Marrow Transpl. 2015, 50, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Administration USFaD. Carfilzomib. Available online: http://wayback.archive-it.org/7993/20170113081106/http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm312945.htm (accessed on 1 March 2023).
- Richardson, P.G.; Siegel, D.S.; Vij, R.; Hofmeister, C.C.; Baz, R.; Jagannath, S.; Chen, C.; Lonial, S.; Jakubowiak, A.; Bahlis, N.; et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: A randomized phase 2 study. Blood 2014, 123, 1826–1832. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Lauwers-Cances, V.; Marit, G.; Caillot, D.; Moreau, P.; Facon, T.; Stoppa, A.M.; Hulin, C.; Benboubker, L.; Garderet, L.; et al. Lenalidomide Maintenance after Stem-Cell Transplantation for Multiple Myeloma. N. Engl. J. Med. 2012, 366, 1782–1791. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kumar, S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020, 10, 94. [Google Scholar] [CrossRef]
- Miguel, J.S.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 1055–1066. [Google Scholar] [CrossRef]
- Sanofi. Sanofi: FDA Approves Sarclisa® (isatuximab-irfc) for Patients with Relapsed Refractory Multiple Myeloma. Available online: https://www.sanofi.com/en/media-room/press-releases/2020/2020-03-02-18-51-16-1993727 (accessed on 1 March 2023).
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Richardson, P.G.; Laubach, J.P.; Munshi, N.C.; Anderson, K.C. Early or delayed transplantation for multiple myeloma in the era of novel therapy: Does one size fit all? Hematol. Am. Soc. Hematol. Educ. Program. 2014, 2014, 255–261. [Google Scholar] [CrossRef]
- Cavo, M.; San-Miguel, J.; Usmani, S.Z.; Weisel, K.; Dimopoulos, M.A.; Avet-Loiseau, H.; Paiva, B.; Bahlis, N.J.; Plesner, T.; Hungria, V.; et al. Prognostic value of minimal residual disease negativity in myeloma: Combined analysis of POLLUX, CASTOR, ALCYONE, and MAIA. Blood 2022, 139, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.K.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Rajkumar, S.V. MAIA under the microscope-bringing trial design into focus. Nat. Rev. Clin. Oncol. 2019, 16, 339–340. [Google Scholar] [CrossRef]
- Mateos, M.V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef]
- Moreau, P.; Dimopoulos, M.A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Špička, I.; Baker, R.; Kim, K.; et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): A multicentre, open-label, randomised phase 3 trial. Lancet 2021, 397, 2361–2371. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Hungria, V.T.; Yoon, S.S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Günther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Overall survival of patients with relapsed multiple myeloma treated with panobinostat or placebo plus bortezomib and dexamethasone (the PANORAMA 1 trial): A randomised, placebo-controlled, phase 3 trial. Lancet Haematol. 2016, 3, e506–e515. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Stewart, A.K.; Masszi, T.; Špička, I.; Oriol, A.; Hájek, R.; Rosiñol, L.; Siegel, D.; Mihaylov, G.G.; Goranova-Marinova, V.; et al. Carfilzomib, lenalidomide, and dexamethasone in patients with relapsed multiple myeloma categorised by age: Secondary analysis from the phase 3 ASPIRE study. Br. J. Haematol. 2017, 177, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Weiss, B.M.; Usmani, S.Z.; Singhal, S.; Chari, A.; Bahlis, N.J.; Belch, A.; Krishnan, A.; Vescio, R.A.; Mateos, M.V.; et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 2016, 387, 1551–1560. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Weisel, K.; San-Miguel, J.; Shpilberg, O.; Grosicki, S.; Špička, I.; Walter-Croneck, A.; et al. Elotuzumab, lenalidomide, and dexamethasone in RRMM: Final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J. 2020, 10, 91. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Palumbo, A.; San-Miguel, J.; Shpilberg, O.; Anderson, K.; Grosicki, S.; Spicka, I.; et al. Elotuzumab plus lenalidomide/dexamethasone for relapsed or refractory multiple myeloma: ELOQUENT-2 follow-up and post-hoc analyses on progression-free survival and tumour growth. Br. J. Haematol. 2017, 178, 896–905. [Google Scholar] [CrossRef]
- Bahlis, N.J.; Dimopoulos, M.A.; White, D.J.; Benboubker, L.; Cook, G.; Leiba, M.; Ho, P.J.; Kim, K.; Takezako, N.; Moreau, P.; et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 2020, 34, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Lentzsch, S.; Weisel, K.; Avet-Loiseau, H.; Mark, T.M.; Spicka, I.; Masszi, T.; Lauri, B.; Levin, M.D.; Bosi, A.; et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of CASTOR. Haematologica 2018, 103, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- Auner, H.W.; Gavriatopoulou, M.; Delimpasi, S.; Simonova, M.; Spicka, I.; Pour, L.; Dimopoulos, M.A.; Kriachok, I.; Pylypenko, H.; Leleu, X.; et al. Effect of age and frailty on the efficacy and tolerability of once-weekly selinexor, bortezomib, and dexamethasone in previously treated multiple myeloma. Am. J. Hematol. 2021, 96, 708–718. [Google Scholar] [CrossRef]
- Vogl, D.T.; Dingli, D.; Cornell, R.F.; Huff, C.A.; Jagannath, S.; Bhutani, D.; Zonder, J.; Baz, R.; Nooka, A.; Richter, J.; et al. Selective Inhibition of Nuclear Export With Oral Selinexor for Treatment of Relapsed or Refractory Multiple Myeloma. J. Clin. Oncol. 2018, 36, 859–866. [Google Scholar] [CrossRef]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Meriin, A.B.; Gabai, V.L.; Yaglom, J.; Shifrin, V.I.; Sherman, M.Y. Proteasome inhibitors activate stress kinases and induce Hsp72. Diverse effects on apoptosis. J. Biol. Chem. 1998, 273, 6373–6379. [Google Scholar] [CrossRef]
- Tani, E.; Kitagawa, H.; Ikemoto, H.; Matsumoto, T. Proteasome inhibitors induce Fas-mediated apoptosis by c-Myc accumulation and subsequent induction of FasL message in human glioma cells. FEBS Lett. 2001, 504, 53–58. [Google Scholar] [CrossRef]
- Ling, Y.H.; Liebes, L.; Zou, Y.; Perez-Soler, R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J. Biol. Chem. 2003, 278, 33714–33723. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Mitsiades, C.; Akiyama, M.; Hayashi, T.; Chauhan, D.; Richardson, P.; Schlossman, R.; Podar, K.; Munshi, N.C.; Mitsiades, N.; et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood 2003, 101, 1530–1534. [Google Scholar] [CrossRef]
- Ito, S. Proteasome Inhibitors for the Treatment of Multiple Myeloma. Cancers 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Thibaudeau, T.A.; Smith, D.M. A Practical Review of Proteasome Pharmacology. Pharmacol. Rev. 2019, 71, 170–197. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Richardson, P.; Chauhan, D.; Palombella, V.J.; Elliott, P.J.; Adams, J.; Anderson, K.C. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001, 61, 3071–3076. [Google Scholar]
- Hideshima, T.; Ikeda, H.; Chauhan, D.; Okawa, Y.; Raje, N.; Podar, K.; Mitsiades, C.; Munshi, N.C.; Richardson, P.G.; Carrasco, R.D.; et al. Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood 2009, 114, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Keats, J.J.; Fonseca, R.; Chesi, M.; Schop, R.; Baker, A.; Chng, W.J.; Van Wier, S.; Tiedemann, R.; Shi, C.X.; Sebag, M.; et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell 2007, 12, 131–144. [Google Scholar] [CrossRef]
- Noborio-Hatano, K.; Kikuchi, J.; Takatoku, M.; Shimizu, R.; Wada, T.; Ueda, M.; Nobuyoshi, M.; Oh, I.; Sato, K.; Suzuki, T.; et al. Bortezomib overcomes cell-adhesion-mediated drug resistance through downregulation of VLA-4 expression in multiple myeloma. Oncogene 2009, 28, 231–242. [Google Scholar] [CrossRef]
- Groen, K.; van de Donk, N.; Stege, C.; Zweegman, S.; Nijhof, I.S. Carfilzomib for relapsed and refractory multiple myeloma. Cancer Manag. Res. 2019, 11, 2663–2675. [Google Scholar] [CrossRef]
- Sana, M.K.; Abdullah, S.M.; Javed, S.; Ehsan, H.; Faizan, U.; Khalid, F.; Jaan, A.; Tayyeb, M.; Abdullah, S.; Anwer, F. Efficacy of Ixazomib and Bortezomib with Lenalidomide Combination Regimens for Multiple Myeloma: A Systematic Review. Blood 2020, 136, 40–41. [Google Scholar] [CrossRef]
- Krönke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Manni, S.; Carrino, M.; Piazza, F. Role of protein kinases CK1α and CK2 in multiple myeloma: Regulation of pivotal survival and stress-managing pathways. J. Hematol. Oncol. 2017, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Manni, S.; Carrino, M.; Manzoni, M.; Gianesin, K.; Nunes, S.C.; Costacurta, M.; Tubi, L.Q.; Macaccaro, P.; Taiana, E.; Cabrelle, A.; et al. Inactivation of CK1α in multiple myeloma empowers drug cytotoxicity by affecting AKT and β-catenin survival signaling pathways. Oncotarget 2017, 8, 14604–14619. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Qin, H.; Frank, S.J.; Deng, L.; Litchfield, D.W.; Tefferi, A.; Pardanani, A.; Lin, F.T.; Li, J.; Sha, B.; et al. A CK2-dependent mechanism for activation of the JAK-STAT signaling pathway. Blood 2011, 118, 156–166. [Google Scholar] [CrossRef]
- Yu, M.; Yeh, J.; Van Waes, C. Protein kinase casein kinase 2 mediates inhibitor-kappaB kinase and aberrant nuclear factor-kappaB activation by serum factor(s) in head and neck squamous carcinoma cells. Cancer Res. 2006, 66, 6722–6731. [Google Scholar] [CrossRef]
- Schafer, P.H.; Gandhi, A.K.; Zhang, L.-H.; Kang, J.; Capone, L.; Parton, S.; Wu, L.; Bartlett, B. Opposing Effects of Dexamethasone on Lenalidomide Activity in Multiple Myeloma: Additive/Synergistic Effects on Anti-Proliferative Activity on Myeloma Cells and Antagonistic Effects on Immune Function. Blood 2008, 112, 2761. [Google Scholar] [CrossRef]
- Wu, L.; Adams, M.; Carter, T.; Chen, R.; Muller, G.; Stirling, D.; Schafer, P.; Bartlett, J.B. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin. Cancer Res. 2008, 14, 4650–4657. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.; Ritchie, D.; Stewart, A.K.; Neeson, P.; Harrison, S.; Smyth, M.J.; Prince, H.M. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010, 24, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Cottini, F.; Nozawa, Y.; Seo, H.S.; Ohguchi, H.; Samur, M.K.; Cirstea, D.; Mimura, N.; Iwasawa, Y.; Richardson, P.G.; et al. p53-related protein kinase confers poor prognosis and represents a novel therapeutic target in multiple myeloma. Blood 2017, 129, 1308–1319. [Google Scholar] [CrossRef]
- Haslett, P.A.; Corral, L.G.; Albert, M.; Kaplan, G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J. Exp. Med. 1998, 187, 1885–1892. [Google Scholar] [CrossRef]
- Davies, F.E.; Raje, N.; Hideshima, T.; Lentzsch, S.; Young, G.; Tai, Y.T.; Lin, B.; Podar, K.; Gupta, D.; Chauhan, D.; et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 2001, 98, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Luptakova, K.; Glotzbecker, B.; Mills, H.; Stroopinsky, D.; Vasir, B.; Rosenblatt, J.; Kufe, D.; Avigan, D. Lenalidomide Decreases PD-1 Expression, Depletes Regulatory T-Cells and Improves Cellular Response to a Multiple Myeloma/Dendritic Cell Fusion Vaccine In Vitro. Blood 2010, 116, 492. [Google Scholar] [CrossRef]
- Luptakova, K.; Rosenblatt, J.; Glotzbecker, B.; Mills, H.; Stroopinsky, D.; Kufe, T.; Vasir, B.; Arnason, J.; Tzachanis, D.; Zwicker, J.I.; et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol. Immunother. 2013, 62, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Galustian, C.; Meyer, B.; Labarthe, M.-C.; Dredge, K.; Klaschka, D.; Henry, J.; Todryk, S.; Chen, R.; Muller, G.; Stirling, D.; et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol. Immunother. 2009, 58, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Bolzoni, M.; Storti, P.; Bonomini, S.; Todoerti, K.; Guasco, D.; Toscani, D.; Agnelli, L.; Neri, A.; Rizzoli, V.; Giuliani, N. Immunomodulatory drugs lenalidomide and pomalidomide inhibit multiple myeloma-induced osteoclast formation and the RANKL/OPG ratio in the myeloma microenvironment targeting the expression of adhesion molecules. Exp. Hematol. 2013, 41, 387–397.e381. [Google Scholar] [CrossRef]
- Walker, B.A.; Mavrommatis, K.; Wardell, C.P.; Ashby, T.C.; Bauer, M.; Davies, F.E.; Rosenthal, A.; Wang, H.; Qu, P.; Hoering, A.; et al. Identification of novel mutational drivers reveals oncogene dependencies in multiple myeloma. Blood 2018, 132, 587–597. [Google Scholar] [CrossRef]
- Dutta, R.; Tiu, B.; Sakamoto, K.M. CBP/p300 acetyltransferase activity in hematologic malignancies. Mol. Genet. Metab. 2016, 119, 37–43. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Kalff, A.; Chow, A.; Khong, T.; Spencer, A. Dysregulated Class I histone deacetylases are indicators of poor prognosis in multiple myeloma. Epigenetics 2014, 9, 1511–1520. [Google Scholar] [CrossRef]
- West, A.C.; Johnstone, R.W. New and emerging HDAC inhibitors for cancer treatment. J. Clin. Investig. 2014, 124, 30–39. [Google Scholar] [CrossRef]
- Mandawat, A.; Fiskus, W.; Buckley, K.M.; Robbins, K.; Rao, R.; Balusu, R.; Navenot, J.M.; Wang, Z.X.; Ustun, C.; Chong, D.G.; et al. Pan-histone deacetylase inhibitor panobinostat depletes CXCR4 levels and signaling and exerts synergistic antimyeloid activity in combination with CXCR4 antagonists. Blood 2010, 116, 5306–5315. [Google Scholar] [CrossRef]
- Hideshima, T.; Bradner, J.E.; Wong, J.; Chauhan, D.; Richardson, P.; Schreiber, S.L.; Anderson, K.C. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc. Natl. Acad. Sci. USA 2005, 102, 8567–8572. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Qi, J.; Paranal, R.M.; Tang, W.; Greenberg, E.; West, N.; Colling, M.E.; Estiu, G.; Mazitschek, R.; Perry, J.A.; et al. Discovery of selective small-molecule HDAC6 inhibitor for overcoming proteasome inhibitor resistance in multiple myeloma. Proc. Natl. Acad. Sci. USA 2016, 113, 13162–13167. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.L.; Fabre, C.; Lonial, S.; Richardson, P.G. Histone deacetylase inhibitors in multiple myeloma: Rationale and evidence for their use in combination therapy. Clin. Lymphoma Myeloma Leuk. 2013, 13, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Hirano, M.; Kobayashi, M.; Futami, M.; Tojo, A. HDAC Inhibitors Exert Anti-Myeloma Effects through Multiple Modes of Action. Cancers 2019, 11, 475. [Google Scholar] [CrossRef]
- Castella, M.; Fernández de Larrea, C.; Martín-Antonio, B. Immunotherapy: A Novel Era of Promising Treatments for Multiple Myeloma. Int. J. Mol. Sci. 2018, 19, 3613. [Google Scholar] [CrossRef]
- O’Connor, B.P.; Raman, V.S.; Erickson, L.D.; Cook, W.J.; Weaver, L.K.; Ahonen, C.; Lin, L.L.; Mantchev, G.T.; Bram, R.J.; Noelle, R.J. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 2004, 199, 91–98. [Google Scholar] [CrossRef]
- Tembhare, P.R.; Yuan, C.M.; Venzon, D.; Braylan, R.; Korde, N.; Manasanch, E.; Zuchlinsky, D.; Calvo, K.; Kurlander, R.; Bhutani, M.; et al. Flow cytometric differentiation of abnormal and normal plasma cells in the bone marrow in patients with multiple myeloma and its precursor diseases. Leuk. Res. 2014, 38, 371–376. [Google Scholar] [CrossRef]
- Raja, K.R.; Kovarova, L.; Hajek, R. Review of phenotypic markers used in flow cytometric analysis of MGUS and MM, and applicability of flow cytometry in other plasma cell disorders. Br. J. Haematol. 2010, 149, 334–351. [Google Scholar] [CrossRef]
- Muccio, V.E.; Saraci, E.; Gilestro, M.; Gattei, V.; Zucchetto, A.; Astolfi, M.; Ruggeri, M.; Marzanati, E.; Passera, R.; Palumbo, A.; et al. Multiple myeloma: New surface antigens for the characterization of plasma cells in the era of novel agents. Cytom. B Clin. Cytom. 2016, 90, 81–90. [Google Scholar] [CrossRef]
- Teoh, P.J.; Chng, W.J. CAR T-cell therapy in multiple myeloma: More room for improvement. Blood Cancer J. 2021, 11, 84. [Google Scholar] [CrossRef]
- Deaglio, S.; Mehta, K.; Malavasi, F. Human CD38: A (r)evolutionary story of enzymes and receptors. Leuk. Res. 2001, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- de Weers, M.; Tai, Y.T.; van der Veer, M.S.; Bakker, J.M.; Vink, T.; Jacobs, D.C.; Oomen, L.A.; Peipp, M.; Valerius, T.; Slootstra, J.W.; et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol. 2011, 186, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Overdijk, M.B.; Verploegen, S.; Bögels, M.; van Egmond, M.; Lammerts van Bueren, J.J.; Mutis, T.; Groen, R.W.; Breij, E.; Martens, A.C.; Bleeker, W.K.; et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 2015, 7, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; van de Donk, N.W.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, H.; Mai, E.K.; Bertsch, U.; Fenk, R.; Nievergall, E.; Tichy, D.; Besemer, B.; Dürig, J.; Schroers, R.; von Metzler, I.; et al. Addition of isatuximab to lenalidomide, bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible patients with multiple myeloma (GMMG-HD7): Part 1 of an open-label, multicentre, randomised, active-controlled, phase 3 trial. Lancet Haematol. 2022, 9, e810–e821. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.Z.; Nahi, H.; Legiec, W.; Grosicki, S.; Vorobyev, V.; Spicka, I.; Hungria, V.; Korenkova, S.; Bahlis, N.J.; Flogegard, M.; et al. Final analysis of the phase III non-inferiority COLUMBA study of subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma. Haematologica 2022, 107, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Prawitz, T.; Popat, R.; Suvannasankha, A.; Sarri, G.; Hughes, R.; Wang, F.; Hogea, C.; Ferrante, S.A.; Gorsh, B.; Willson, J.; et al. DREAMM-2: Indirect Comparisons of Belantamab Mafodotin vs. Selinexor + Dexamethasone and Standard of Care Treatments in Relapsed/Refractory Multiple Myeloma. Adv. Ther. 2021, 38, 5501–5518. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Quach, H.; Mateos, M.-V.; Landgren, O.; Leleu, X.; Siegel, D.S.; Weisel, K.; Yang, H.; Klippel, Z.K.; Zahlten-Kumeli, A.; et al. Carfilzomib, Dexamethasone, and Daratumumab Versus Carfilzomib and Dexamethasone for the Treatment of Patients with Relapsed or Refractory Multiple Myeloma (RRMM): Primary Analysis Results from the Randomized, Open-Label, Phase 3 Study Candor (NCT03158688). Blood 2019, 134, LBA-6. [Google Scholar] [CrossRef]
- Delimpasi, S.; Mateos, M.V.; Auner, H.W.; Gavriatopoulou, M.; Dimopoulos, M.A.; Quach, H.; Pylypenko, H.; Hájek, R.; Leleu, X.; Dolai, T.K.; et al. Efficacy and tolerability of once-weekly selinexor, bortezomib, and dexamethasone in comparison with standard twice-weekly bortezomib and dexamethasone in previously treated multiple myeloma with renal impairment: Subgroup analysis from the BOSTON study. Am. J. Hematol. 2022, 97, E83–E86. [Google Scholar] [CrossRef]
- Richardson, P.G.; Oriol, A.; Larocca, A.; Bladé, J.; Cavo, M.; Rodriguez-Otero, P.; Leleu, X.; Nadeem, O.; Hiemenz, J.W.; Hassoun, H.; et al. Melflufen and Dexamethasone in Heavily Pretreated Relapsed and Refractory Multiple Myeloma. J. Clin. Oncol. 2021, 39, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kanapuru, B.; George, B.; Lin, X.; Xu, Z.; Bryan, W.W.; Pazdur, R.; Theoret, M.R. FDA Approval Summary: Idecabtagene Vicleucel for Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2022, 28, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Dimopoulos, M.A.; Yong, K.; Mikhael, J.; Risse, M.L.; Asset, G.; Martin, T. Isatuximab plus carfilzomib/dexamethasone versus carfilzomib/dexamethasone in patients with relapsed/refractory multiple myeloma: IKEMA Phase III study design. Future Oncol. 2020, 16, 4347–4358. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Terpos, E.; Boccadoro, M.; Delimpasi, S.; Beksac, M.; Katodritou, E.; Moreau, P.; Baldini, L.; Symeonidis, A.; Bila, J.; et al. Apollo: Phase 3 Randomized Study of Subcutaneous Daratumumab Plus Pomalidomide and Dexamethasone (D-Pd) Versus Pomalidomide and Dexamethasone (Pd) Alone in Patients (Pts) with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 5–6. [Google Scholar] [CrossRef]
- Drug USFa. FDA Approves Darzalex Faspro, Kyprolis, and Dexamethasone for Multiple Myeloma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-darzalex-faspro-kyprolis-and-dexamethasone-multiple-myeloma (accessed on 1 March 2023).
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Girgis, S.; Lin, S.X.W.; Pillarisetti, K.; Banerjee, A.; Stephenson, T.; Ma, X.; Shetty, S.; Yang, T.Y.; Hilder, B.W.; Jiao, Q.; et al. Translational Modeling Predicts Efficacious Therapeutic Dosing Range of Teclistamab for Multiple Myeloma. Target Oncol. 2022, 17, 433–439. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Veillette, A.; Guo, H. CS1, a SLAM family receptor involved in immune regulation, is a therapeutic target in multiple myeloma. Crit. Rev. Oncol. Hematol. 2013, 88, 168–177. [Google Scholar] [CrossRef]
- Kurdi, A.T.; Glavey, S.V.; Bezman, N.A.; Jhatakia, A.; Guerriero, J.L.; Manier, S.; Moschetta, M.; Mishima, Y.; Roccaro, A.; Detappe, A.; et al. Antibody-Dependent Cellular Phagocytosis by Macrophages is a Novel Mechanism of Action of Elotuzumab. Mol. Cancer Ther. 2018, 17, 1454–1463. [Google Scholar] [CrossRef]
- Kikuchi, J.; Hori, M.; Iha, H.; Toyama-Sorimachi, N.; Hagiwara, S.; Kuroda, Y.; Koyama, D.; Izumi, T.; Yasui, H.; Suzuki, A.; et al. Soluble SLAMF7 promotes the growth of myeloma cells via homophilic interaction with surface SLAMF7. Leukemia 2020, 34, 180–195. [Google Scholar] [CrossRef]
- Muenst, S.; Läubli, H.; Soysal, S.D.; Zippelius, A.; Tzankov, A.; Hoeller, S. The immune system and cancer evasion strategies: Therapeutic concepts. J. Intern. Med. 2016, 279, 541–562. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Le Calvez, B.; Moreau, P.; Touzeau, C. Immune checkpoint inhibitors for the treatment of myeloma: Novel investigational options. Expert Opin. Investig. Drugs 2021, 30, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Ishibashi, M.; Yamashita, T.; Tanosaki, S.; Okuyama, N.; Kondo, A.; Hyodo, H.; Shinya, E.; Takahashi, H.; Dong, H.; et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia 2013, 27, 464–472. [Google Scholar] [CrossRef]
- Görgün, G.; Samur, M.K.; Cowens, K.B.; Paula, S.; Bianchi, G.; Anderson, J.E.; White, R.E.; Singh, A.; Ohguchi, H.; Suzuki, R.; et al. Lenalidomide Enhances Immune Checkpoint Blockade-Induced Immune Response in Multiple Myeloma. Clin. Cancer Res. 2015, 21, 4607–4618. [Google Scholar] [CrossRef]
- Boussi, L.S.; Avigan, Z.M.; Rosenblatt, J. Immunotherapy for the treatment of multiple myeloma. Front. Immunol. 2022, 13, 1027385. [Google Scholar] [CrossRef]
- Ribrag, V.; Avigan, D.E.; Green, D.J.; Wise-Draper, T.; Posada, J.G.; Vij, R.; Zhu, Y.; Farooqui, M.Z.H.; Marinello, P.; Siegel, D.S. Phase 1b trial of pembrolizumab monotherapy for relapsed/refractory multiple myeloma: KEYNOTE-013. Br. J. Haematol. 2019, 186, e41–e44. [Google Scholar] [CrossRef]
- San-Miguel, J.; Bladé, J.; Shpilberg, O.; Grosicki, S.; Maloisel, F.; Min, C.K.; Polo Zarzuela, M.; Robak, T.; Prasad, S.V.; Tee Goh, Y.; et al. Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood 2014, 123, 4136–4142. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Gercheva, L.; Williams, C.; Sutherland, H.; Robak, T.; Masszi, T.; Goranova-Marinova, V.; Dimopoulos, M.A.; Cavenagh, J.D.; Špička, I.; et al. A phase 2, randomized, double-blind, placebo-controlled study of siltuximab (anti-IL-6 mAb) and bortezomib versus bortezomib alone in patients with relapsed or refractory multiple myeloma. Am. J. Hematol. 2015, 90, 42–49. [Google Scholar] [CrossRef]
- Brighton, T.A.; Khot, A.; Harrison, S.J.; Ghez, D.; Weiss, B.M.; Kirsch, A.; Magen, H.; Gironella, M.; Oriol, A.; Streetly, M.; et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Study of Siltuximab in High-Risk Smoldering Multiple Myeloma. Clin. Cancer Res. 2019, 25, 3772–3775. [Google Scholar] [CrossRef]
- Birrer, M.J.; Moore, K.N.; Betella, I.; Bates, R.C. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J. Natl. Cancer Inst. 2019, 111, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Ketchum, E.B.; Clarke, A.; Clemmons, A.B. Belantamab Mafodotin-blmf: A Novel Antibody-Drug Conjugate for Treatment of Patients With Relapsed/Refractory Multiple Myeloma. J. Adv. Pract. Oncol. 2022, 13, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Offidani, M.; Corvatta, L.; Morè, S.; Olivieri, A. Belantamab Mafodotin for the Treatment of Multiple Myeloma: An Overview of the Clinical Efficacy and Safety. Drug Des. Devel. Ther. 2021, 15, 2401–2415. [Google Scholar] [CrossRef] [PubMed]
- Lindell, T.J.; Weinberg, F.; Morris, P.W.; Roeder, R.G.; Rutter, W.J. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science 1970, 170, 447–449. [Google Scholar] [CrossRef]
- Pahl, A.; Lutz, C.; Hechler, T. Amanitins and their development as a payload for antibody-drug conjugates. Drug Discov. Today Technol. 2018, 30, 85–89. [Google Scholar] [CrossRef]
- Kaplan, C.D.; Larsson, K.M.; Kornberg, R.D. The RNA polymerase II trigger loop functions in substrate selection and is directly targeted by alpha-amanitin. Mol. Cell 2008, 30, 547–556. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, A.; Cassidy, S.; Webster, R.M. Bispecific antibodies in oncology. Nat. Rev. Drug Discov. 2022, 21, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Grosicki, S.; Mellqvist, U.-H.; Pruchniewski, Ł.; Crafoord, J.; Trudel, S.; Min, C.-K.; White, D.; Alegre, A.; Hansson, M.; Ikeda, T.; et al. Elranatamab in Combination with Daratumumab for Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Results from the Phase 3 Magnetismm-5 Study Safety Lead-in Cohort. Blood 2022, 140, 4407–4408. [Google Scholar] [CrossRef]
- Atamaniuk, J.; Gleiss, A.; Porpaczy, E.; Kainz, B.; Grunt, T.W.; Raderer, M.; Hilgarth, B.; Drach, J.; Ludwig, H.; Gisslinger, H.; et al. Overexpression of G protein-coupled receptor 5D in the bone marrow is associated with poor prognosis in patients with multiple myeloma. Eur. J. Clin. Investig. 2012, 42, 953–960. [Google Scholar] [CrossRef]
- Cohen, Y.; Gutwein, O.; Garach-Jehoshua, O.; Bar-Haim, A.; Kornberg, A. GPRC5D is a promising marker for monitoring the tumor load and to target multiple myeloma cells. Hematology 2013, 18, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, P.; Huang, H. Engineering better chimeric antigen receptor T cells. Exp. Hematol. Oncol. 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Bonifant, C.L.; Jackson, H.J.; Brentjens, R.J.; Curran, K.J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics 2016, 3, 16011. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Oertle, J.; Warren, D.; Prato, D. Chimeric antigen receptor (CAR) T cell therapy for malignant cancers: Summary and perspective. J. Cell. Immunother. 2016, 2, 59–68. [Google Scholar] [CrossRef]
- Kagoya, Y.; Tanaka, S.; Guo, T.; Anczurowski, M.; Wang, C.H.; Saso, K.; Butler, M.O.; Minden, M.D.; Hirano, N. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat. Med. 2018, 24, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018, 36, 2267–2280. [Google Scholar] [CrossRef]
- Ali, S.A.; Shi, V.; Maric, I.; Wang, M.; Stroncek, D.F.; Rose, J.J.; Brudno, J.N.; Stetler-Stevenson, M.; Feldman, S.A.; Hansen, B.G.; et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016, 128, 1688–1700. [Google Scholar] [CrossRef]
- Fernández de Larrea, C.; Staehr, M.; Lopez, A.V.; Ng, K.Y.; Chen, Y.; Godfrey, W.D.; Purdon, T.J.; Ponomarev, V.; Wendel, H.G.; Brentjens, R.J.; et al. Defining an Optimal Dual-Targeted CAR T-cell Therapy Approach Simultaneously Targeting BCMA and GPRC5D to Prevent BCMA Escape-Driven Relapse in Multiple Myeloma. Blood Cancer Discov. 2020, 1, 146–154. [Google Scholar] [CrossRef]
- Wong, D.P.; Roy, N.K.; Zhang, K.; Anukanth, A.; Asthana, A.; Shirkey-Son, N.J.; Dunmire, S.; Jones, B.J.; Lahr, W.S.; Webber, B.R.; et al. A BAFF ligand-based CAR-T cell targeting three receptors and multiple B cell cancers. Nat. Commun. 2022, 13, 217. [Google Scholar] [CrossRef]
- Fan, F.X.-H.; Zhao, W.-h.; Liu, J.; He, A.; Chen, Y.-X.; Cao, X.-m.; Yang, N.; Wang, B.; Zhang, P.; Zhang, Y.; et al. Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma. J. Clin. Oncol. 2017, 35. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Lin, Y.; Raje, N.S.; Siegel, D.S.D.; Munshi, N.C.; Liedtke, M.; Jagannath, S.; Maus, M.V.; Turka, A.; Lam, L.P.; et al. First-in-human multicenter study of bb2121 anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: Updated results. J. Clin. Oncol. 2017, 35, 3010. [Google Scholar] [CrossRef]
- Pittari, G.; Vago, L.; Festuccia, M.; Bonini, C.; Mudawi, D.; Giaccone, L.; Bruno, B. Restoring Natural Killer Cell Immunity against Multiple Myeloma in the Era of New Drugs. Front. Immunol. 2017, 8, 1444. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.Y.; Du, Z.; Zhang, X.; Chng, W.J.; Wang, S. CXCR4 and anti-BCMA CAR co-modified natural killer cells suppress multiple myeloma progression in a xenograft mouse model. Cancer Gene Ther. 2022, 29, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Shimony, S.; Stone, R.M.; Stahl, M. Venetoclax combination therapy in acute myeloid leukemia and myelodysplastic syndromes. Curr. Opin. Hematol. 2022, 29, 63–73. [Google Scholar] [CrossRef]
- FDA.gov. FDA Approves Venetoclax for CLL and SLL. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-venetoclax-cll-and-sll (accessed on 1 March 2023).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=Myeloma&term=Venetoclax&cntry=&state=&city=&dist= (accessed on 1 March 2023).
- Kumar, S.K.; Harrison, S.J.; Cavo, M.; de la Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.; Salwender, H.; Suzuki, K.; Kim, I.; et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1630–1642. [Google Scholar] [CrossRef]
- Slomp, A.; Moesbergen, L.M.; Gong, J.N.; Cuenca, M.; von dem Borne, P.A.; Sonneveld, P.; Huang, D.C.S.; Minnema, M.C.; Peperzak, V. Multiple myeloma with 1q21 amplification is highly sensitive to MCL-1 targeting. Blood Adv. 2019, 3, 4202–4214. [Google Scholar] [CrossRef]
- Wu, K.L.; Beverloo, B.; Lokhorst, H.M.; Segeren, C.M.; van der Holt, B.; Steijaert, M.M.; Westveer, P.H.; Poddighe, P.J.; Verhoef, G.E.; Sonneveld, P.; et al. Abnormalities of chromosome 1p/q are highly associated with chromosome 13/13q deletions and are an adverse prognostic factor for the outcome of high-dose chemotherapy in patients with multiple myeloma. Br. J. Haematol. 2007, 136, 615–623. [Google Scholar] [CrossRef]
- Hanamura, I.; Stewart, J.P.; Huang, Y.; Zhan, F.; Santra, M.; Sawyer, J.R.; Hollmig, K.; Zangarri, M.; Pineda-Roman, M.; van Rhee, F.; et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: Incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood 2006, 108, 1724–1732. [Google Scholar] [CrossRef]
- Wuilleme-Toumi, S.; Robillard, N.; Gomez, P.; Moreau, P.; Le Gouill, S.; Avet-Loiseau, H.; Harousseau, J.L.; Amiot, M.; Bataille, R. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia 2005, 19, 1248–1252. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.G. Anti-cancer drug discovery and development: Bcl-2 family small molecule inhibitors. Commun. Integr. Biol. 2012, 5, 557–565. [Google Scholar] [CrossRef]
- Al-Odat, O.; von Suskil, M.; Chitren, R.; Elbezanti, W.; Srivastava, S.; Budak-Alpddogan, T.; Jonnalagadda, S.; Aggarwal, B.; Pandey, M. Mcl-1 Inhibition: Managing Malignancy in Multiple Myeloma. Front. Pharmacol. 2021, 12, 699629. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Contractor, R.; Tsao, T.; Samudio, I.; Ruvolo, P.P.; Kitada, S.; Deng, X.; Zhai, D.; Shi, Y.X.; Sneed, T.; et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 2006, 10, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Winter, P.S.; Xie, A.; Roth, C.; Martz, C.A.; Stein, E.M.; Anderson, G.R.; Tingley, J.P.; Wood, K.C. Targeting MCL-1/BCL-XL Forestalls the Acquisition of Resistance to ABT-199 in Acute Myeloid Leukemia. Sci. Rep. 2016, 6, 27696. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Phase I Study of MIK665, a Mcl-1 Inhibitor, in Patients With Refractory or Relapsed Lymphoma or Multiple Myeloma. Available online: https://clinicaltrials.gov/ct2/show/NCT02992483?term=MIK665&cond=myeloma&draw=2&rank=1 (accessed on 1 March 2023).
- Maragno, A.L.; Mistry, P.; Kotschy, A.; Szlavik, Z.; Murray, J.; Davidson, J.; Toumelin-Braizat, G.L.; Chanrion, M.; Bruno, A.; Claperon, A.; et al. Abstract 4482: S64315 (MIK665) is a potent and selective Mcl1 inhibitor with strong antitumor activity across a diverse range of hematologic tumor models. Cancer Res. 2019, 79, 4482. [Google Scholar] [CrossRef]
- Halilovic, E.; Chanrion, M.; Mistry, P.; Wartmann, M.; Qiu, S.; Sanghavi, S.; Chen, Y.; Lysiak, G.; Maragno, A.L.; Pfaar, U.; et al. Abstract 4477: MIK665/S64315, a novel Mcl-1 inhibitor, in combination with Bcl-2 inhibitors exhibits strong synergistic antitumor activity in a range of hematologic malignancies. Cancer Res. 2019, 79, 4477. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. VOB560-MIK665 Combination First in Human Trial in Patients With Hematological Malignancies (Relapsed/Refractory Non-Hodgkin Lymphoma, Relapsed/Refractory Acute Myeloid Leukemia, or Relapsed/Refractory Multiple Myeloma). Available online: https://clinicaltrials.gov/ct2/show/NCT04702425 (accessed on 1 March 2023).
- Doi, K.; Li, R.; Sung, S.S.; Wu, H.; Liu, Y.; Manieri, W.; Krishnegowda, G.; Awwad, A.; Dewey, A.; Liu, X.; et al. Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. J. Biol. Chem. 2012, 287, 10224–10235. [Google Scholar] [CrossRef]
- Pandey, M.K.; Gowda, K.; Doi, K.; Sharma, A.K.; Wang, H.G.; Amin, S. Proteasomal degradation of Mcl-1 by maritoclax induces apoptosis and enhances the efficacy of ABT-737 in melanoma cells. PLoS ONE 2013, 8, e78570. [Google Scholar] [CrossRef]
- Matsui, W.; Wang, Q.; Barber, J.P.; Brennan, S.; Smith, B.D.; Borrello, I.; McNiece, I.; Lin, L.; Ambinder, R.F.; Peacock, C.; et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008, 68, 190–197. [Google Scholar] [CrossRef]
- Gao, M.; Kong, Y.; Yang, G.; Gao, L.; Shi, J. Multiple myeloma cancer stem cells. Oncotarget 2016, 7, 35466–35477. [Google Scholar] [CrossRef]
- Reghunathan, R.; Bi, C.; Liu, S.C.; Loong, K.T.; Chung, T.H.; Huang, G.; Chng, W.J. Clonogenic multiple myeloma cells have shared stemness signature associated with patient survival. Oncotarget 2013, 4, 1230–1240. [Google Scholar] [CrossRef]
- Johnsen, H.E.; Bogsted, M.; Schmitz, A.; Bodker, J.S.; El-Galaly, T.C.; Johansen, P.; Valent, P.; Zojer, N.; Van Valckenborgh, E.; Vanderkerken, K.; et al. The myeloma stem cell concept, revisited: From phenomenology to operational terms. Haematologica 2016, 101, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Kellner, J.; Liu, B.; Kang, Y.; Li, Z. Fact or fiction--identifying the elusive multiple myeloma stem cell. J. Hematol. Oncol. 2013, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.G.; Liu, L.P.; Li, C.Y.; Zhang, M.; Chen, Y.; Qin, J.; Gu, Y.Y.; Li, Z.; Wu, X.L.; Mo, S.L. Scutellaria extract decreases the proportion of side population cells in a myeloma cell line by down-regulating the expression of ABCG2 protein. Asian Pac. J. Cancer Prev. 2013, 14, 7179–7186. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Shibuya, M.; Sugawara, H.; Kawaguchi, O.; Hirsoe, C. Salinomycin, a new polyether antibiotic. J. Antibiot. 1974, 27, 814–821. [Google Scholar] [CrossRef]
- Fuchs, D.; Daniel, V.; Sadeghi, M.; Opelz, G.; Naujokat, C. Salinomycin overcomes ABC transporter-mediated multidrug and apoptosis resistance in human leukemia stem cell-like KG-1a cells. Biochem. Biophys Res. Commun. 2010, 394, 1098–1104. [Google Scholar] [CrossRef]
- Kastritis, E.; Jakubikova, J.; Delmore, J.; Klippel, S.; McMillin, D.W.; Ooi, M.; Jacobs, H.M.; Laubach, J.P.; Richardson, P.G.; Anderson, K.C.; et al. Preclinical Studies of Salinomycin In Multiple Myeloma (MM) Models: Targeting of Side Population (SP) Cells In the Context of Tumor–Microenvironment Interactions. Blood 2010, 116, 1574. [Google Scholar] [CrossRef]
- Ruiu, R.; Tarone, L.; Rolih, V.; Barutello, G.; Bolli, E.; Riccardo, F.; Cavallo, F.; Conti, L. Cancer stem cell immunology and immunotherapy: Harnessing the immune system against cancer’s source. Prog. Mol. Biol. Transl. Sci. 2019, 164, 119–188. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem. Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, Y.; Gu, Z.; Wang, H.; Xia, J.; Wu, X.; Zhan, X.; Levasseur, D.; Zhou, Y.; Janz, S.; et al. ALDH1 activity identifies tumor-initiating cells and links to chromosomal instability signatures in multiple myeloma. Leukemia 2014, 28, 1155–1158. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, W.; Xia, J.; Gu, Z.; Wendlandt, E.; Zhan, X.; Janz, S.; Tricot, G.; Zhan, F. NEK2 mediates ALDH1A1-dependent drug resistance in multiple myeloma. Oncotarget 2014, 5, 11986–11997. [Google Scholar] [CrossRef]
- Dinavahi, S.S.; Bazewicz, C.G.; Gowda, R.; Robertson, G.P. Aldehyde Dehydrogenase Inhibitors for Cancer Therapeutics. Trends Pharmacol. Sci. 2019, 40, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Kale, V.P.; Song, C.; Sung, S.S.; Sharma, A.K.; Talamo, G.; Dovat, S.; Amin, S.G. Gambogic acid inhibits multiple myeloma mediated osteoclastogenesis through suppression of chemokine receptor CXCR4 signaling pathways. Exp. Hematol. 2014, 42, 883–896. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, J.; Gu, Z.; Salama, M.E.; Das, S.; Wendlandt, E.; Xu, H.; Huang, J.; Tao, Y.; Hao, M.; et al. Bruton tyrosine kinase is a therapeutic target in stem-like cells from multiple myeloma. Cancer Res. 2015, 75, 594–604. [Google Scholar] [CrossRef]
- Von Suskil, M.; Sultana, K.N.; Elbezanti, W.O.; Al-Odat, O.S.; Chitren, R.; Tiwari, A.K.; Challagundla, K.B.; Srivastava, S.K.; Jonnalagadda, S.C.; Budak-Alpdogan, T.; et al. Bruton’s Tyrosine Kinase Targeting in Multiple Myeloma. Int. J. Mol. Sci. 2021, 22, 5707. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, D.J.; Scharenberg, A.M.; Park, H.; Wahl, M.I.; Lin, S.; Kato, R.M.; Fluckiger, A.C.; Witte, O.N.; Kinet, J.P. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science 1996, 271, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Elbezanti, W.O.; Al-Odat, O.S.; Chitren, R.; Singh, J.K.; Srivastava, S.K.; Gowda, K.; Amin, S.; Robertson, G.P.; Nemmara, V.V.; Jonnalagadda, S.C.; et al. Development of a novel Bruton’s tyrosine kinase inhibitor that exerts anti-cancer activities potentiates response of chemotherapeutic agents in multiple myeloma stem cell-like cells. Front. Pharmacol. 2022, 13, 894535. [Google Scholar] [CrossRef] [PubMed]
- Maura, F.; Bolli, N.; Angelopoulos, N.; Dawson, K.J.; Leongamornlert, D.; Martincorena, I.; Mitchell, T.J.; Fullam, A.; Gonzalez, S.; Szalat, R.; et al. Genomic landscape and chronological reconstruction of driver events in multiple myeloma. Nat. Commun. 2019, 10, 3835. [Google Scholar] [CrossRef]
- Misund, K.; Hofste Op Bruinink, D.; Coward, E.; Hoogenboezem, R.M.; Rustad, E.H.; Sanders, M.A.; Rye, M.; Sponaas, A.M.; van der Holt, B.; Zweegman, S.; et al. Clonal evolution after treatment pressure in multiple myeloma: Heterogenous genomic aberrations and transcriptomic convergence. Leukemia 2022, 36, 1887–1897. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).