Complexes of Ruthenium(II) as Promising Dual-Active Agents against Cancer and Viral Infections
Abstract
:1. Introduction
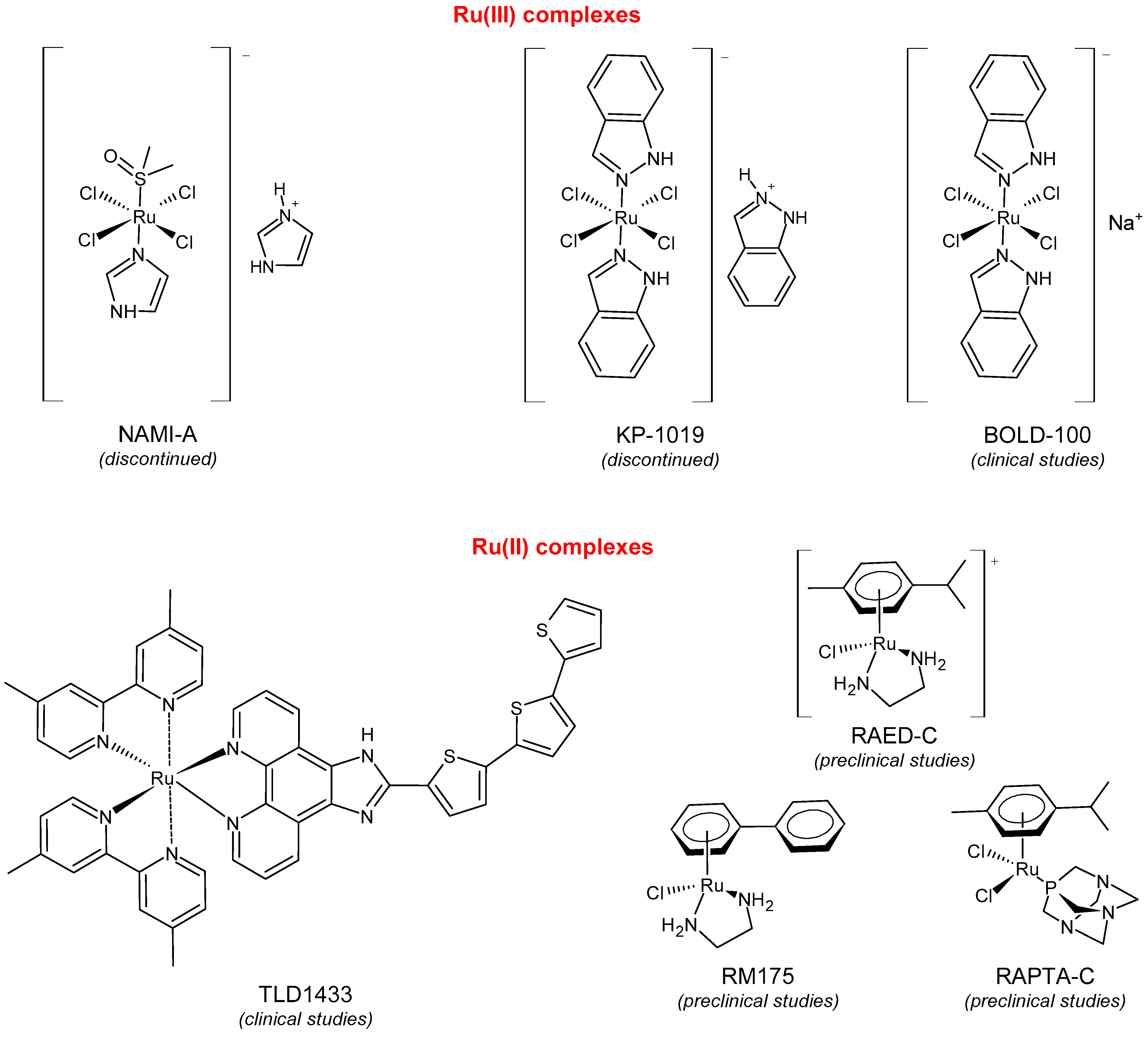
2. Ruthenium(II/III) Complexes in Clinic Trials and Advanced Preclinical Studies as Anticancer Agents
2.1. BOLD-100
2.2. TLD1433
2.3. RAPTA-C
3. Ruthenium Complexes Acting against Viruses
4. Preclinical Studies on Ru(II) Complexes
4.1. Preclinical In Vitro and In Vivo Studies on Ru(II) Complexes as Anticancer Agents
| Structure | Compound | Cytotoxicity Studies | Ref. |
|---|---|---|---|
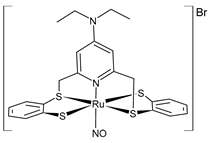 | [Ru(NO)(Et2NpyS4)]Br (1) | IC50 = 53 ± 1.3 µg/mL (HepG2) | Shereef et al. 2022 [136] |
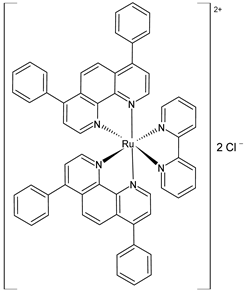 | [Ru(dip)2(bpy)]Cl2, (2) | IC50 = 9.7 ± 0.4 µM (A375) IC50 = 4.9 ± 0.9 µM (A2058) IC50 = 3.9 ± 0.6 µM (MCF7) IC50 = 0.8 ± 0.6 µM (MDA-MB-231) | Gurgul et al. (2022) [137] |
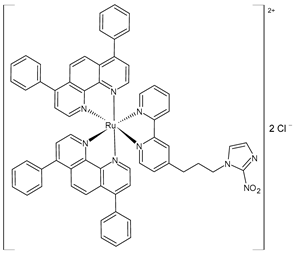 | [Ru(dip)2(bpy-NitroIm)]Cl2, (3) | IC50 = 11.2 ± 0.9 µM (A375) IC50 = 10.8 ± 0.8 µM (A2058) IC50 = 13 ± 2 µM (MCF7) IC50 = 3.8 ± 0.2 µM (MDA-MB-231) | Gurgul et al. (2022) [137] |
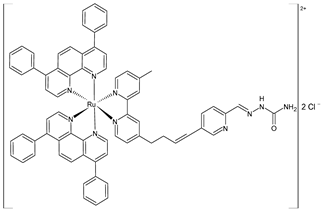 | [Ru(dip)2(bpy-NitroIm)]Cl2 (4) | IC50 = 15.0 ± 0.6 µM (A375) IC50 = 4.7 ± 0.5 µM (A2058) IC50 = 13.1 ± 0.3 µM (MCF7) IC50 = 1.8 ± 0.3 µM (MDA-MB-231) | Gurgul et al. (2022) [137] |
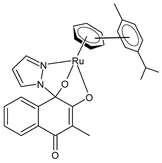 | [3-Methyl-4-oxo-(1H-κN2-pyrazol-1-yl)-1,4-dihydronaphtalene-1,2-bis(olato)-κO1-κO2)(η6-p-cymenyl)ruthenium(II)] (5) | IC50 = 1.2 ± 0.2 µM (CH1/PA-1, after 96 h) IC50 = 0.094 ± 0.031 µM (SW480, after 96 h) IC50 = >50 µM (A549, after 96 h) | Cseh et al. (2022) [138] |
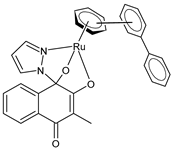 | [3-Methyl-4-oxo-(1H-κN2-pyrazol-1-yl)-1,4-dihydronaphtalene-1,2-bis(olato)-κO1-κO2)(η6-biphenyl)ruthenium(II)] (6) | IC50 = 1.2 ± 0.2 µM (CH1/PA-1, after 96 h) IC50 = 0.072 ± 0.019 µM (SW480, after 96 h) IC50 = 30 ± 3 µM (A549, after 96 h) | Cseh et al. (2022) [138] |
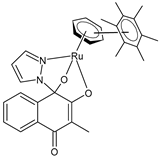 | [3-Methyl-4-oxo-(1H-κN2-pyrazol-1-yl)-1,4-dihydronaphtalene-1,2-bis(olato)-κO1-κO2)(η6-hexamethylbenzene)ruthenium(II)] (7) | IC50 = 3.4 ± 0.6 µM (CH1/PA-1, after 96 h) IC50 = 0.27 ± 0.06 µM (SW480, after 96 h) IC50 = 35 ± 4 µM (A549, after 96 h) | Cseh et al. (2022) [138] |
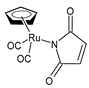 | η5-cyclopentadienyl)Ru (CO)2(η1-N-maleimidato (8) | IC50 = 5.62 µM (HL-60) | Juszczak et al. (2022) [140] |
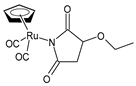 | (η5-cyclopentadienyl)Ru(CO)2-N-ethoxysuccinimidato (9) | IC50 > 250 µM (HL-60) | Juszczak et al. (2022) [140] |
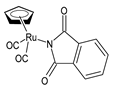 | η5-cyclopentadienyl)Ru(CO)2-N-phthalimidato (10) | IC50 > 250 µM (HL-60) | Juszczak et al. (2022) [140] |
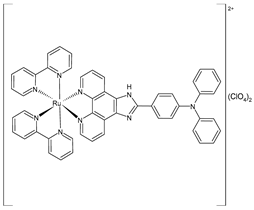 | [Ru(2,2′-bipyridine)2(IPP)](ClO4)2 (11) | IC50 = 15.1 ± 0.2 µM (B16) IC50 = 19.7 ± 1.4 µM (HepG2) IC50 = 16.9 ± 0.7 µM (A549) | Liang et al. (2022) [141] |
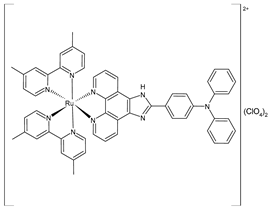 | [Ru(4,4′-dimethyl-2,2′-bipyridine)2(IPP)](ClO4)2 (12) | IC50 = 14.3 ± 0.1 µM (B16) IC50 = 19.1 ± 1.7 µM (HepG2) IC50 = 13.0 ± 0.5 µM (A549) | Liang et al. (2022) [141] |
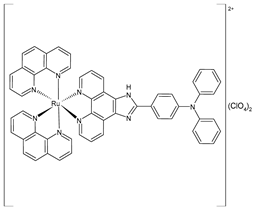 | [Ru(1,10-phenanthroline)2(IPP)](ClO4)2 (13) | IC50 = 26.0 ± 2.1 µM (B16) IC50 = 36.8 ± 1.7 µM (HepG2) IC50 = 32.3 ± 0.4 µM (A549) | Liang et al. (2022) [141] |
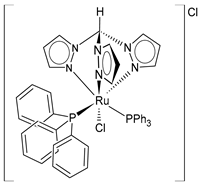 | [RuCl(κ3-tris(1-pyrazolyl)methane)(PPh3)2]Cl (14) | IC50 = 2.4 ± 0.6 µM (MCF-7) IC50 = 4.0 ± 0.4 µM (HeLa) IC50 = 2.6 ± 0.4 µM (518A2) IC50 = 1.5 ± 0.1 µM (HCT-116) IC50 = 2.2 ± 0.2 µM (RD) | Cervinka et al. (2022) [142] |
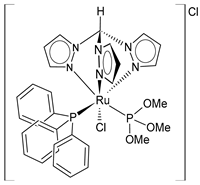 | [RuCl(κ3-tris(1-pyrazolyl)methane) (PPh3){P(OMe)3}]Cl (15) | IC50 = 6 ± 1 µM (MCF-7) IC50 = 10 ± 2 µM (HeLa) IC50 = 6.8 ± 0.8 µM (518A2) IC50 = 6.7 ± 0.4 µM (HCT-116) IC50 = 6 ± 1 µM (RD) | Cervinka et al. (2022) [142] |
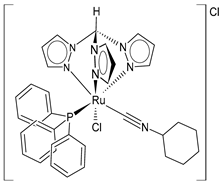 | [RuCl(κ3-tris(1-pyrazolyl)methane)(PPh3)(CNCy)]Cl (16) | IC50 = 10 ± 2 µM (MCF-7) IC50 = 15 ± 1 µM (HeLa) IC50 = 10 ± 2 µM (518A2) IC50 = 8 ± 2 µM (HCT-116) IC50 = 6.6 ± 0.7 µM (RD) | Cervinka et al. (2022) [142] |
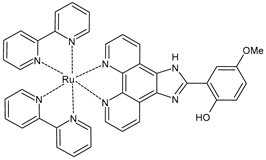 | [Ru(bpy)2L](ClO4)2 (17) | IC50 = 99.80 ± 1.9 (HeLa) µM (after 24 h) | Priya et al. (2023) [143] |
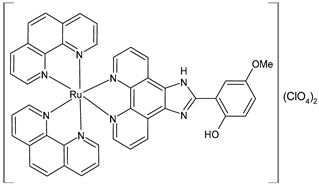 | [Ru(phenyl)2L](ClO4)2 (18) | IC50 = 24.5 ± 1.45 µM (HeLa) (after 24 h) | Priya et al. (2023) [143] |
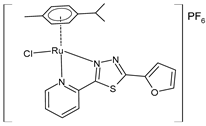 | [Ru(η6-pcym)(L1)Cl]PF6 (19) | IC50 = 8.69 ± 1.75 µM (A2780, 48 h) IC50 = 12.48 ± 4.83 µM (A2780cis) (after 48 h) | Křikavová et al. (2023) [144] |
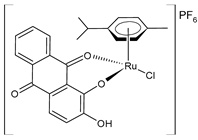 | [Ru(L)Cl(η6-p-cymene)] (20) | IC50 = 42.2 ± 3.6 µM (MDA-MB-231) IC50 = 32.8 ± 1.2 µM (MCF-7) IC50 > 100 µM (A549) | de Araujo-Neto et al. (2023) [145] |
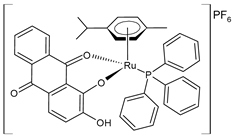 | [Ru(L)(η6-p-cymene)(PPh3)]PF6 (21) | IC50 = 6.5 ± 0.1 µM (MDA-MB-231) IC50 = 9.0 ± 0.1 µM (MCF-7) IC50 = 17.8 ± 0.8 µM (A549) | de Araujo-Neto et al. (2023) [145] |
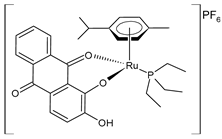 | [Ru(L)(η6-p-cymene)(PEt3)]PF6 (22) | IC50 = 45.4 ± 1.4 µM (MDA-MB-231) IC50 > 100 µM (MCF-7) IC50 = 52.6 ± 1.2 µM (A549) | de Araujo-Neto et al. (2023) [145] |
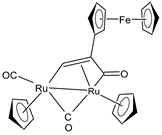 | [Ru2Cp2(CO)(μ-CO){μ-η1:η3-CH=C(Fc)C(=O)}] (23) | IC50 > 100 µM (A549) IC50 > 100 µM (SW480) IC50 = 4.1 ± 0.9 µM (A2780) IC50 = 4.1 ± 0.9 µM (A2780cis) | Bresciani et al. (2023) [146] |
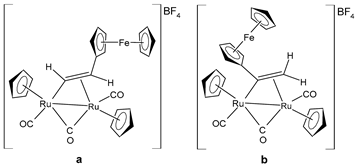 | [Ru2Cp2(CO)2(μ-CO){μ-η1:η2-CH=CH(Fc)}]BF4 (24a) [Ru2Cp2(CO)2(μ-CO){μ-η1:η2-C(Fc)CH2}]BF4 (24b) | IC50 = 41 ± 5 µM (A549) IC50 = 38 ± 2 µM (SW480) IC50 = 8 ± 4 µM (A2780) IC50 = 11.0 ± 0.2 µM (A2780cis) | Bresciani et al. (2023) [146] |
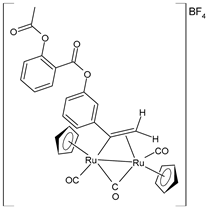 | [Ru2Cp2(CO)2(μ-CO){μ-η1:η2-C(3-C6H4-Asp)=CH2}]BF4 (25) | IC50 = 19 ± 3 µM (A549) IC50 = 22 ± 2 µM (SW480) IC50 = 7.9 ± 1.3 µM (A2780) IC50 = 9.0 ± 1.3 µM (A2780cis) | Bresciani et al. (2023) [146] |
 | [Ru2Cp2(CO)2(μ-CO){μ-η1:η2-C(H)CPh2}]BF4 (26a) [Ru2Cp2(CO)2(μ-CO){μ-η1:η2-C(Ph)CH(Ph)}]BF4 (26b) | IC50 = 34 ± 2 µM (A549) IC50 = 34 ± 2 µM (SW480) IC50 = 8.5 ± 6 µM (A2780) IC50 = 10.6 ± 0.8 µM (A2780cis) | Bresciani et al. (2023) [146] |
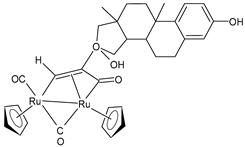 | [Ru2Cp2(CO)2{µ-η1:η3-CH=C(17α-estradiol)C(=O)}] (27) | IC50 = 6.2 ± 1.2 µM (A2780) IC50 = 7.3 ± 2.4 µM (A2780cisR) IC50 = 19.0 ± 4.5 µM (MCF-7) IC50 = 24.0 ± 3.8 µM (HOS) IC50 > 50 µM (A549) IC50 > 50 µM (PANC-1) IC50 >50 µM (Caco-2) IC50 = 36.0 ± 4.1 µM (PC-3) IC50 = 5.5 ± 0.9 µM (HeLa) | Bresciani (2023) [147] |
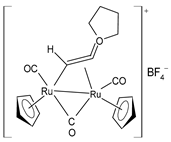 | [Ru2Cp2(CO)3{µ-η1:η3-CH=C= (cyclopentylidene)}]BF4 (28) | IC50 = 4.2 ± 0.9 µM (A2780) IC50 = 6.4 ± 1.9 µM (A2780cisR) IC50 = 16.2 ± 1.7 µM (MCF-7) IC50 = 14.6 ± 0.5 µM (HOS) IC50 = 25.3 ± 1.9 µM (A549) IC50 = 28.4 ± 3.9 µM (PANC-1) IC50 > 50 µM (Caco-2) IC50 = 22.2 ± 2.4 µM (PC-3) IC50 = 17.5 ± 2.9 µM (HeLa) | Bresciani (2023) [147] |
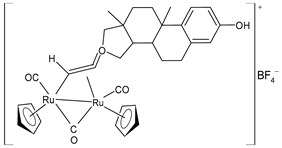 | [Ru2Cp2(CO)3{µ-η1:η2-CH=C= (estradiolylidene)}]BF4 (29) | IC50 = 3.4 ± 0.6 µM (A2780) IC50 = 4.6 ± 1.3 µM (A2780cisR) IC50 = 11.6 ± 1.5 µM (MCF-7) IC50 = 12.6 ± 0.5 µM (HOS) IC50 = 16.1 ± 1.3 µM (A549) IC50 = 19.8 ± 2.3 µM (PANC-1) IC50 = 36.0 ± 2.7 µM (Caco-2) IC50 = 42.8 ± 0.8 µM (PC-3) IC50 = 5.5 ± 0.9 µM (HeLa) | Bresciani (2023) [147] |
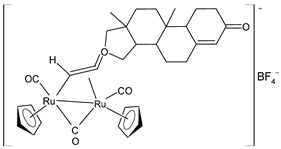 | [Ru2Cp2(CO)3{µ-η1:η2-CH=C= (testosteronylidene)}]BF4 (30) | IC50 = 6.3 ± 1.3 µM (A2780) IC50 = 11.7 ± 2.4 µM (A2780cisR) IC50 = 22.0 ± 4.0 µM (MCF-7) IC50 = 17.7 ± 2.8 µM (HOS) IC50 = 20.7 ± 1.4 µM (A549) IC50 = 30.0 ± 0.6 µM (PANC-1) IC50 = 42.8 ± 0.8 µM (Caco-2) IC50 = 19.6 ± 3.7 µM (PC-3) IC50 = 16.3 ± 1.3 µM (HeLa) | Bresciani (2023) [147] |
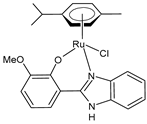 | [Ru(η6-p-cym)(L)Cl] (31) | IC50 = 11.84 ± 0.42 µM (HeLa) IC50 = 25.67 ± 0.56 µM (MCF-7) | Nayek et al. (2023) [148] |
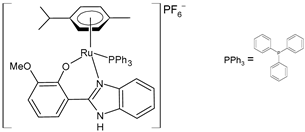 | [Ru(η6-p-cym)(L)PPh3]PF6 (32) | IC50 = 7.29 ± 0.38 µM (HeLa) IC50 = 19.97 ± 0.39 µM (MCF-7) | Nayek et al. (2023) [148] |
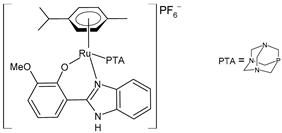 | [Ru(η6-p-cym)(L)(PTA)]PF6 (33) | IC50 = 13.25 ± 0.35 µM (HeLa) IC50 = 28.70 ± 0.48 µM (MCF-7) | Nayek et al. (2023) [148] |
 | [Ru(bipy)2(4-F-Sal)] (34) | IC50 = 5.76 × 10−6 M; 4.75 × 10−6 M (MCF-7, after 24 h and 48 h, respectively) IC50 = > 10 × 10−6 M (U-118MG, after 24 h and 48 h) | Schoeller et al. (2023) [149] |
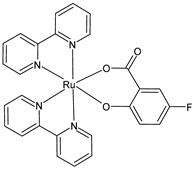 | [Ru(bipy)2(5-F-Sal)] (35) | IC50 = < 2 × 10−6 M (MCF-7, after 24 h and 48 h) IC50 = 3.56 × 10−6 M; 4.72 × 10−6 M (U-118MG, after 24 h and 48 h, respectively) | Schoeller et al. (2023) [149] |
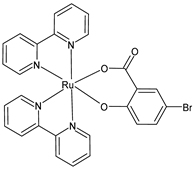 | [Ru(bipy)2(5-Br-Sal)] (36) | IC50 = 4.23 × 10−6 M; 4.92 × 10−6 M (MCF-7, after 24 h and 48 h, respectively) IC50 = 5.35 × 10−6 M; 3.95 × 10−6 M (U-118MG, after 24 h and 48 h, respectively) | Schoeller et al. (2023) [149] |
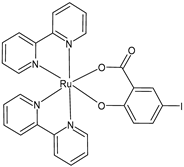 | [Ru(bipy)2(5-I-Sal)] (37) | IC50 < 2 × 10−6 M (MCF-7, after 24 h and 48 h) IC50 = 4.08 × 10−6 M; 2.65 × 10−6 M (U-118MG, after 24 h and 48 h, respectively) | Schoeller et al. (2023) [149] |
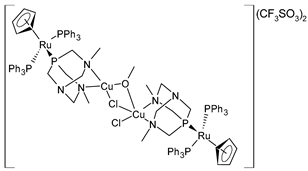 | [{RuCp(PPh3)2-μ-dmoPTA-1κP:2κ2-N,N′-CuCl}2-μ-Cl-μ-OCH3](CF3SO3)2·(CH3OH)4 (38) | GI50 = 28 ± 3.3 nM (A549, after 48 h) GI50 = 32 ± 0.2 nM (HBL-100, after 48 h) GI50 = 21 ± 1.7 nM (HeLa, after 48 h) GI50 = 27 ± 13 nM (SW1573, after 48 h) GI50 = 20 ± 7.8 nM (T-47D, after 48 h) GI50 = 21 ± 9.2 nM (WiDr, after 48 h) | Alguacil et al. (2023) [150] |
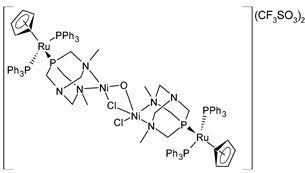 | [{RuCp(PPh3)2-μ-dmoPTA-1κP:2κ2-N,N′-NiCl}2-μ-Cl-μ-OH](CF3SO3)2 (39) | GI50 = 34 ± 8.4 nM (A549, after 48 h) GI50 = 31 ± 11 nM (HBL-100, after 48 h) GI50 = 28 ± 2.5 nM (HeLa, after 48 h) GI50 = 41 ± 6.8 nM (SW1573, after 48 h) GI50 = 23 ± 1.6 nM (T-47D, after 48 h) GI50 = 34 ± 8.7 nM (WiDr, after 48 h) | Alguacil et al. (2023) [150] |
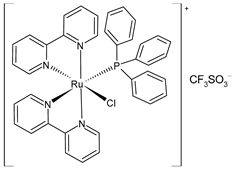 | cis-[Ru(bpy)2(PPh3)Cl] CF3SO3 (40) | IC50 = 73.31 ± 0.10 µM and 1.16 ± 0.10 µM (HL-60, after 24 h and 72 h, respectively) IC50 = 3.45 ± 0.99 µM (A549, after 24 h) IC50 = 1.62 ± 0.33 µM (DU145, after 24 h). IC50 = 13.58 ± 2.11 µM (HeLa, after 24 h) | Mitchell et al. (2023) [151] |
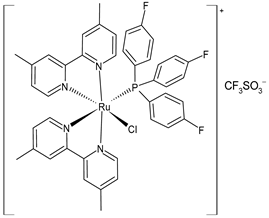 | cis-[Ru4.4′-Me2bpy)2{P(C6H4F)3}Cl] CF3SO3 (41) | IC50 = 2.74 ± 0.56 µM and 0.98 ± 0.24 µM (HL-60, after 24 h and 72 h) IC50 = 2.78 ± 0.34 µM (A549, after 24 h) IC50 = 1.42 ± 0.20 µM (DU145, after 24 h). IC50 = 5.67 ± 2.19 µM (HeLa, after 24 h) | Mitchell et al. (2023) [151] |
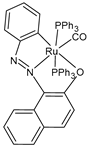 | [Ru(L1)(CO)(PPh3)2] (42) | IC50 = 5.1 ± 1.2 µM (MCF-7) IC50 = 36.2 ± 1.5 µM (A549) IC50 = 65.3 ± 1.2 µM (MDA-MB-231) IC50 = 42.1 ± 3.1 µM (AGS) | Das et al. (2023) [152] |
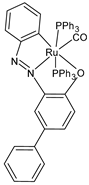 | [Ru(L2)(CO)(PPh3)2] (43) | IC50 = 6.3 ± 3.1 µM (MCF-7) IC50 = 21.3 ± 3.2 µM (A549) IC50 = 53.2 ± 1.3 µM (MDA-MB-231) IC50 = 51.1 ± 1.4 µM (AGS) | Das et al. (2023) [152] |
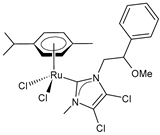 | 1-(2-methoxy-2-phenylethyl)-3-methyl) (4,5-dichloroimidazol-2-ylidene) (p-cymene) ruthenium(II) chloride (44) | IC50 = 24.14 ± 0.07 µM (MDA-MB-231) IC50 = 26.05 ± 0.9 µM (MCF-7) IC50 = 48.43 ± 0.8 µM (SH-SY5Y) | Ceramella et al. (2023) [153] |
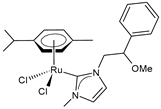 | 1-(2-methoxy-2-phenylethyl)-3-methyl-imidazol-2-ylidene) (p-cymene) ruthenium(II) chloride (45) | IC50 = 40.57 ± 1.1 µM (MDA-MB-231) IC50 = 54.75 ± 1.1 µM (MCF-7) IC50 = 66.86 ± 0.8 µM (SH-SY5Y) | Ceramella et al. (2023) [153] |
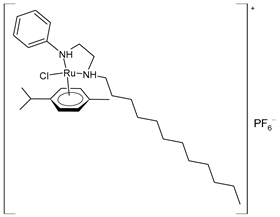 | (46) | Cell viability reduction = ~50% at 10 nM (HepG2) | Kavukcu et al. (2023) [154] |
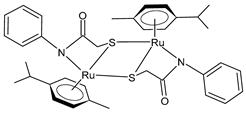 | (47) | Cell viability reduction = ~50% at 10 nM (HepG2) | Kavukcu et al. (2023) [154] |
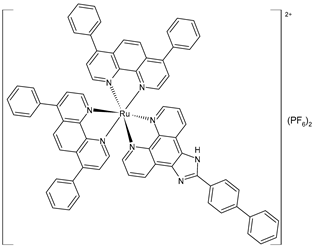 liposome | [Ru(4,7-diphenyl-1,10-phenanthroline)2(2-(1,1′-biphenyl-4-yl)-1H-imidazo [4,5-f][1,10]phenanthroline)](PF6)2 (48)lipo | IC50 = 9.3 ± 0.3 µM (A549) IC50 = 17.4 ± 0.3 µM (HepG2) IC50 = 3.4 ± 0.1 µM (SGC-7901) IC50 = 14.8 ± 0.4 µM (HeLa) IC50 = 5.9 ± 0.2 µM (Bel-7402) IC50 = 7.2 ± 0.2 µM (B16) | Chen et al. (2023) [155] |
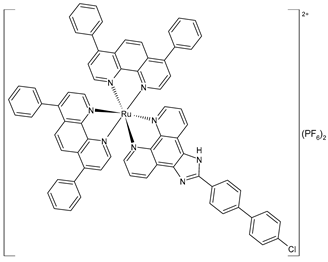 liposome | [Ru(4,7-diphenyl-1,10-phenanthroline)2(2-(4′-chloro-1,1′-biphenyl-4-yl)-1H-imidazo [4,5-f][1,10]phenanthroline)](PF6)2 (49)lipo | IC50 = 7.7 ± 0.2 µM (A549) IC50 = 15.0 ± 0.2 µM (HepG2) IC50 = 3.5 ± 0.1 µM (SGC-7901) IC50 = 14.7 ± 0.6 µM (HeLa) IC50 = 5.8 ± 0.1 µM (Bel-7402) IC50 = 5.1 ± 0.1 µM (B16) | Chen et al. (2023) [155] |
4.2. Preclinical In Vitro Studies and In Silico Studies on Ru(II) Complexes as Promising Dual-Active Agents against Cancer and Viruses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Cancer Cells Mentioned in the Text
| 518A2 | melanoma cell lines |
| A375 | malignant melanoma cell lines |
| A2780 | ovarian cancer cell lines |
| A2780cis | ovarian cancer cell lines |
| A2780cisR | cisplatin-resistant human ovarian carcinoma |
| A549 | lung cancer cells |
| AGS | gastric adenocarcinoma cell line |
| B16 | mouse melanoma cells |
| BALB/3T3 | non-tumoral cells |
| BEL-7402 | hepatocellular carcinoma |
| Caco-2 | human colorectal adenocarcinoma |
| CCD-18Co | colon healthy cell lines |
| CCD-1072Sk | foreskin fibroblasts healthy cell lines |
| CH1/PA-1 | teratocarcinoma cells |
| DU145 | prostate adenocarcinoma cells |
| HCT-116 | human colon cancer cells |
| HeLa | human cervix adenocarcinoma cancer cells |
| HBL-100 | breast cancer cells |
| HepG2 | human liver cancer cells |
| HEK293 | human embryonic kidney nontumoral cell lines |
| HIV-1 | type 1 human immunodeficiency virus |
| HNSCCs | head and neck squamous carcinoma cells |
| IC50 | half-maximal (50%) inhibitory concentration |
| HOS | human osteosarcoma |
| HPV | human papillomavirus |
| K562 | chronic myelogenous leukemia cells |
| LS174 | colon adenocarcinoma cells |
| MCF-7 | breast cancer cells |
| MCF-10A | nontumor breast cell lines |
| MDA-MB-231 | triple negative breast cancer cells |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MRC-5 | non-tumor lung cell lines |
| MRC5pd30 | normal human fibroblasts |
| NAs | nano-architectures |
| PANC-1 | human pancreatic carcinoma cells |
| PC-3 | human prostate carcinoma cells |
| PLpro | papain-like protease |
| RD | rhabdomyo-sarcoma cells |
| SCC-25 | human squamous cell carcinoma (HPV-negative) |
| SW480 | colon adenocarcinoma cell lines |
| SW1573 | lung cancer cells |
| ROS | reactive oxygen species |
| SGC-7901 | gastric adenocarcinoma |
| SiHa | human cervical cancer cells |
| TCID50 | median tissue culture infective dose |
| U-118MG | glioma cell lines |
| UPCI-SCC-154 | human squamous cell carcinoma (HPV-positive) |
| WiDr | colon cancer cells |
References
- Singh, V.K.; Singh, V.K.; Mishra, A.; Singh, A.A.; Prasad, G.; Singh, A.K. Recent advancements in coordination compounds and their potential clinical application in the management of diseases: An up-to-date review. Polyhedron 2023, 241, 116485. [Google Scholar] [CrossRef]
- De, S.; Kazi, S.; Banerjee, S.; Banerjee, S.; Sarkar, N.; Shah, S.K.; Kuo, Y.-C.; Kumar, S.A. Metallotherapeutic complexes with high selective properties for anti-neoplastic therapy. Coord. Chem. Rev. 2024, 498, 215462. [Google Scholar] [CrossRef]
- Gamberi, T.; Hanif, M. Metal-based complexes in cancer treatment. Biomedicines 2022, 10, 2573. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, R.; Wiese-Szadkowska, M.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Helmin-Basa, A. Latest developments in metal complexes as anticancer agents. Coord. Chem. Rev. 2022, 452, 214307. [Google Scholar] [CrossRef]
- Ceramella, J.; Mariconda, A.; Sirignano, M.; Iacopetta, D.; Rosano, C.; Catalano, A.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Novel Au carbene complexes as promising multi-target agents in breast cancer treatment. Pharmaceuticals 2022, 15, 507. [Google Scholar] [CrossRef]
- Prathima, T.S.; Choudhury, B.; Ahmad, M.G.; Chanda, K.; Balamurali, M.M. Recent developments on other platinum metal complexes as target-specific anticancer therapeutics. Coord. Chem. Rev. 2023, 490, 215231. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef]
- Todorov, L.; Kostova, I. Recent Trends in the development of novel metal-based antineoplastic drugs. Molecules 2023, 28, 1959. [Google Scholar] [CrossRef]
- Esquezaro, P.G.; Manzano, C.M.; Nakahata, D.H.; ISantos, I.A.; Ruiz, U.E.; Santiago, M.B.; Silva, N.B.; Martins, C.H.; Pereira, D.H.; Bergamini, F.R.G.; et al. Synthesis, spectroscopic characterization and in vitro antibacterial and antiviral activities of novel silver(I) complexes with mafenide and ethyl-mafenide. J. Mol. Struct. 2021, 1246, 131261. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Recent overview of potent antioxidant activity of coordination compounds. Antioxidants 2023, 12, 213. [Google Scholar] [CrossRef]
- Abate, C.; Carnamucio, F.; Giuffrè, O.; Foti, C. Metal-Based Compounds in Antiviral Therapy. Biomolecules 2022, 12, 933. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Pathak, P.; Grishina, M.; Yadav, J.P.; Verma, A.; Kumar, P. Metal Complexes in cancer treatment: Journey so far. Chem. Biodivers. 2023, 20, e202300061. [Google Scholar] [CrossRef]
- Anthony, E.A.; Bolitho, E.M.; Bridgewater, R.J.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S.; Aquaro, S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses 2022, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Musib, D.; Roy, M. Transition metal complexes as potential tools against SARS-CoV-2: An in silico approach. New J. Chem. 2021, 45, 1924. [Google Scholar] [CrossRef]
- Cirri, D.; Pratesi, A.; Marzo, T.; Messori, L. Metallo therapeutics for COVID-19. Exploiting metal-based compounds for the discovery of new antiviral drugs. Expert Opin. Drug Discov. 2021, 16, 39–46. [Google Scholar] [CrossRef]
- Karges, J.; Cohen, S.M. Metal complexes as antiviral agents for SARS-CoV-2. ChemBioChem 2021, 22, 2600–2607. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Sivanesan, I. A Comprehensive survey on the expediated anti-COVID-19 options enabled by metal complexes—Tasks and trials. Molecules 2023, 28, 3354. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J. Ruthenium in medicine: Current clinical uses and future prospects. Platin. Met. Rev. 2001, 45, 62–69. [Google Scholar]
- D’Amato, A.; Mariconda, A.; Longo, P. New insights into the catalytic activity of second generation Hoveyda–Grubbs complexes having phenyl substituents on the backbone. Inorganics 2023, 11, 244. [Google Scholar] [CrossRef]
- Rajabi, S.; Rüttger, F.; Lücken, J.; Dechert, S.; John, M.; Meyer, F. Ruthenium Complexes of Rigid, Dianionic, Tetradentate N-Donor Ligands and their Potential as Catalysts for Water Oxidation. Eur. J. Inorg. Chem. 2023, 26, e202200597. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, P.; Huang, Z.; Liao, J.; Huang, G.; Liang, T.; Zhang, Z. Ruthenium(II)-catalyzed remote C–H sulfonylation of 2-pyridones. Org. Lett. 2023, 25, 5779–5783. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, A.; Ma, X.; Ciancaleoni, G.; Zacchini, S.; Biancalana, L.; Guelfi, M.; Pampaloni, G.; Nolan, S.P.; Marchetti, F. Ruthenium(II) tris-pyrazolylmethane complexes in transfer hydrogenation reactions. Eur. J. Inorg. Chem. 2023, 26, e202300078. [Google Scholar] [CrossRef]
- Hafeez, J.; Bilal, M.; Rasool, N.; Hafeez, U.; Adnan Ali Shah, S.; Imran, S.; Amiruddin Zakaria, Z. Synthesis of ruthenium complexes and their catalytic applications: A review. Arab. J. Chem. 2022, 15, 104165. [Google Scholar] [CrossRef]
- Donnici, C.L.; Araujo, M.H.; Stoianoff, M.A.R. Ruthenium complexes as antifungal agents. In Ruthenium Complexes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 293–318. [Google Scholar]
- Munteanu, A.C.; Uivarosi, V. Ruthenium complexes in the fight against pathogenic microorganisms. An extensive review. Pharmaceutics 2021, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Ruthenium complexes as anticancer agents. Curr. Med. Chem. 2006, 13, 1085–1107. [Google Scholar] [CrossRef] [PubMed]
- Shutkov, I.A.; Okulova, Y.N.; Mazur, D.M.; Melnichuk, N.A.; Babkov, D.A.; Sokolova, E.V.; Spasov, A.A.; Milaeva, E.R.; Nazarov, A.A. New organometallic Ru(II) compounds with lonidamine motif as antitumor agents. Pharmaceutics 2023, 15, 1366. [Google Scholar] [CrossRef]
- Pete, S.; Roy, N.; Kar, B.; Paira, P. Construction of homo and heteronuclear Ru(II), Ir(III) and Re(I) complexes for target specific cancer therapy. Coord. Chem. Rev. 2022, 460, 214462. [Google Scholar] [CrossRef]
- Ribeiro, G.H.; Costa, A.R.; de Souza, A.R.; da Silva, F.V.; Martins, F.T.; Plutin, A.M.; Batista, A.A. An overview on the anticancer activity of Ru(II)/acylthiourea complexes. Coord. Chem. Rev. 2023, 488, 215161. [Google Scholar] [CrossRef]
- Rafols, L.; Josa, D.; Aguila, D.; Barrios, L.A.; Roubeau, O.; Cirera, J.; Soto-Cerrato, V.; Pérez-Tomás, R.; Martinez, M.; Grabulosa, A. Piano-stool ruthenium(II) complexes with delayed cytotoxic activity: Origin of the lag time. Inorg. Chem. 2021, 60, 7974–7990. [Google Scholar] [CrossRef]
- Wang, Z.F.; Huang, X.Q.; Wu, R.C.; Xiao, Y.; Zhang, S.H. Antitumor studies evaluation of triphenylphosphine ruthenium complexes with 5, 7-dihalo-substituted-8-quinolinoline targeting mitophagy pathways. J. Inorg. Biochem. 2023, 248, 112361. [Google Scholar] [CrossRef] [PubMed]
- Florio, D.; La Manna, S.; Annunziata, A.; Iacobucci, I.; Monaco, V.; Di Natale, C.; Mollo, V.; Ruffo, F.; Monti, M.; Marasco, D. Ruthenium complexes bearing glucosyl ligands are able to inhibit the amyloid aggregation of short histidine-peptides. Dalton Trans. 2023, 52, 8549. [Google Scholar] [CrossRef] [PubMed]
- Honorato, J.; Oliveira, K.M.; Leite, C.M.; Colina-Vegas, L.; Nóbrega, J.A.; Castellano, E.E.; Ellena, J.; Correa, R.S.; Batista, A.A. “Half-sandwich”/Ru II anticancer complexes containing triphenylphosphine and p-substituted benzoic acids. J. Brazil. Chem. Soc. 2020, 31, 2237–2249. [Google Scholar] [CrossRef]
- Srivastava, P.; Shukla, M.; Kaul, G.; Chopra, S.; Patra, A.K. Rationally designed curcumin based Ruthenium(II) antimicrobials effective against drug-resistant: Staphylococcus aureus. Dalton Trans. 2019, 48, 11822–11828. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Mariconda, A.; Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Longo, P. Biological activities of ruthenium NHC complexes: An update. Antibiotics 2023, 12, 365. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, H.; Zhong, R.; Yang, Y.; Huang, C.; Chen, J.; Liang, L.; Chen, Y.; Liu, Y. Synthesis, RNA-sequence and evaluation of anticancer efficacy of ruthenium(II) polypyridyl complexes toward HepG2 cells. J. Inorg. Biochem. 2023, 244, 112230. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, H.; Yang, Y.; Liu, H.; Chen, J.; Wang, Y.; Liang, L.; Hu, H.; Liu, Y. Synthesis, characterization, molecular docking, RNA-sequence and anticancer efficacy evaluation in vitro of ruthenium(II) complexes on B16 cells. J. Inorg. Biochem. 2023, 247, 112329. [Google Scholar] [CrossRef]
- Khan, R.A.; Alterary, S.S.; BinSharfan, I.I.; Alsaeedi, H.; AlFawaz, A.; Khan, M.S.; Jaafar, M.H.; Shi, Y.; Arman, H.D.; Alsalme, A. Piano-stool type (η6-p-cymene) ruthenium(II) thiazole-derived motifs complexes: Synthesis, crystal structures, DFT studies, molecular docking and in-vitro binding studies with HSA and cytotoxicity. Inorg. Chim. Acta 2022, 537, 120925. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Mariconda, A.; Giuzio, F.; Saturnino, C.; Longo, P.; Sinicropi, M.S. Metal Complexes with Schiff Bases as Antimicrobials and Catalysts. Inorganics 2023, 11, 320. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Catalano, A.; Mariconda, A.; Rosano, C.; Saturnino, C.; El-Kashef, H.; Longo, P. Metal complexes with Schiff bases: Data collection and recent studies on biological activities. Int. J. Mol. Sci. 2022, 23, 14840. [Google Scholar] [CrossRef]
- Parveen, S. Recent advances in anticancer ruthenium Schiff base complexes. Appl. Organometal. Chem. 2020, 34, e5687. [Google Scholar] [CrossRef]
- Međedović, M.; Mijatović, A.; Baošić, R.; Lazić, D.; Milanović, Ž.; Marković, Z.; Milovanović, J.; Arsenijević, D.; Stojanović, B.; Arsenijević, M. Synthesis, characterization, biomolecular interactions, molecular docking, and in vitro and in vivo anticancer activities of novel ruthenium(III) Schiff base complexes. J. Inorg. Biochem. 2023, 248, 112363. [Google Scholar] [CrossRef] [PubMed]
- Sumithaa, C.; Ganeshpandian, M. Half-sandwich ruthenium arene complexes bearing clinically approved drugs as ligands: The importance of metal–drug synergism in metallodrug design. Mol. Pharm. 2023, 20, 1453–1479. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, K.M.; Niloy, M.S.; Shakil, M.S.; Islam, M.A. Ruthenium complexes: An alternative to platinum drugs in colorectal cancer treatment. Pharmaceutics 2021, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Popolin, C.P.; Cominetti, M.R. A review of ruthenium complexes activities on breast cancer cells. Mini-Rev. Med. Chem. 2017, 17, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, Y.; Shi, H.; Wang, Y.; Zhang, Q. Ruthenium complexes as promising candidates against lung cancer. Molecules 2021, 26, 4389. [Google Scholar] [CrossRef] [PubMed]
- Paulus, L.; Gallardo-Villagrán, M.; Carrion, C.; Ouk, C.; Martin, F.; Therrien, B.; Léger, D.Y.; Liagre, B. The effect of photosensitizer metalation incorporated into arene–ruthenium assemblies on prostate cancer. Int. J. Mol. Sci. 2023, 24, 13614. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) compounds: Next-generation anticancer metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef]
- Dyson, P.J.; Sava, G. Metal-Based Antitumour Drugs in the Post Genomic Era. Dalton Trans. 2006, 16, 1929–1933. [Google Scholar] [CrossRef]
- Hong, W.X.; Huang, F.; Huan, T.; Xu, X.; Han, Q.; Wang, G.; Xu, H.; Duan, S.; Duan, Y.; Long, X.; et al. Comparative studies on DNA-binding and in vitro antitumor activity of enantiomeric ruthenium(II) complexes. J. Inorg. Biochem. 2018, 180, 54–60. [Google Scholar] [CrossRef]
- Sonkar, C.; Sarkar, S.; Mukhopadhyay, S. Ruthenium (II)–arene complexes as anti-metastatic agents, and related techniques. RSC Med. Chem. 2022, 13, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Shamsi, F.; Azam, A. Ruthenium complexes: An emerging ground to the development of metallopharmaceuticals for cancer therapy. Mini Rev. Med. Chem. 2016, 16, 772–786. [Google Scholar] [CrossRef]
- Kanaoujiya, R.; Singh, M.; Singh, J.; Srivastava, S. Ruthenium based anticancer compounds and their importance. J. Sci. Res. 2020, 64, 264–268. [Google Scholar] [CrossRef]
- Silva, M.J.S.A.; Vinck, R.; Wang, Y.; Saubaméa, B.; Tharaud, M.; Dominguez-Jurado, E.; Karges, J.; Gois, P.M.P.; Gasser, G. Towards selective delivery of a ruthenium(II) polypyridyl complex-containing bombesin conjugate into cancer cells. ChemBioChem 2023, 24, e202200647. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.K.; Mukhopadhyay, S. Target based chemotherapeutic advancement of ruthenium complexes. Coord. Chem. Rev. 2021, 448, 214169. [Google Scholar] [CrossRef]
- Yang, G.G.; Su, X.X.; Liang, B.B.; Pan, Z.Y.; Cao, Q.; Mao, Z.W. A platinum–ruthenium hybrid prodrug with multi-enzymatic activities for chemo-catalytic therapy of hypoxic tumors. Chem. Sci. 2022, 13, 11360–11367. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, M.; Kluska, M.; Kosińska, A.; Rudolf, B.; Woźniak, K. Antioxidant activity of ruthenium cyclopentadienyl complexes bearing succinimidato and phthalimidato ligands. Molecules 2022, 27, 2803. [Google Scholar] [CrossRef]
- Małecka, M.; Skoczyńska, A.; Goodman, D.M.; Hartinger, C.G.; Budzisz, E. Biological properties of ruthenium (II)/(III) complexes with flavonoids as ligands. Coord. Chem. Rev. 2021, 436, 213849. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J.; Ellis, D.J.; Salter, P.A.; Scopelliti, R. Synthesis and characterisation of some water soluble ruthenium(II)–arene complexes and an investigation of their antibiotic and antiviral properties. J. Organomet. Chem. 2003, 668, 35–42. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; Santos, I.D.A.; Martins, D.O.S.; Gonçalves, Y.G.; Cardoso-Sousa, L.; Sabino-Silva, R.; Von Poelhsitz, G.; Franca, E.D.F.; Nicolau-Junior, N.; Pacca, C.C.; et al. Organometallic complex strongly impairs Chikungunya virus entry to the host cells. Front. Microbiol. 2020, 11, 608924. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chen, H.J.; Wu, Y.C.; Tsai, S.W.; Liu, Y.H.; Bhattacharya, U.; Lin, D.; Tai, H.C.; Kong, K.V. Highly efficient singlet oxygen generation by BODIPY–ruthenium(II) complexes for promoting neurite outgrowth and suppressing Tau Protein aggregation. Inorg. Chem. 2023, 62, 1102–1112. [Google Scholar] [CrossRef]
- Yawson, G.K.; Will, M.F.; Huffman, S.E.; Strandquist, E.T.; Bothwell, P.J.; Oliver, E.B.; Apuzzo, C.F.; Platt, D.C.; Weitzel, C.S.; Jones, M.A.; et al. A dual-pronged approach: A ruthenium(III) complex that modulates amyloid-β aggregation and disrupts its formed aggregates. Inorg. Chem. 2022, 61, 2733–2744. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, P.; Li, J.; Gong, Y.; Li, X.; Liu, Y.; Yu, K.; Liu, Z. Half-sandwich iridium(III), rhodium(III), and ruthenium(II) complexes chelating hybrid sp2-N/sp3-N donor ligands to achieve improved anticancer selectivity. Inorg. Chem. 2023, 62, 15118–15137. [Google Scholar] [CrossRef] [PubMed]
- Sadique, S.; Baqer, A.A.; Salman, A.W.; Iqbal, M.A.; Kadim, M.M.; Jamil, F.; Majeed, A.; Manahil, S.; Altaf, A. Ruthenium complexes for breast cancer therapy. Rev. Inorg. Chem. 2023, in press. [Google Scholar] [CrossRef]
- Skoczynska, A.; Lewinski, A.; Pokora, M.; Paneth, P.; Budzisz, E. An overview of the potential medicinal and pharmaceutical properties of Ru (II)/(III) complexes. Int. J. Mol. Sci. 2023, 24, 9512. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, S.; Xu, G.; Man, X.; Yang, T.; Zhang, Z.; Liang, H.; Yang, F. Developing a ruthenium(III) complex to trigger gasdermin E-mediated pyroptosis and an immune response based on decitabine and liposomes: Targeting inhibition of gastric tumor growth and metastasis. J. Med. Chem. 2023, 66, 13072–13085. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, C.Y.; Nam, T.G. Ruthenium complexes as anticancer agents: A brief history and perspectives. Drug. Des. Dev. Ther. 2020, 14, 5375–5392. [Google Scholar] [CrossRef] [PubMed]
- Kenny, R.G.; Marmion, C.J. Toward multi-targeted platinum and ruthenium drugs—A new paradigm in cancer drug treatment regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Swaminathan, S.; Deepak, R.J.; Karvembu, R. Interweaving catalysis and cancer using Ru-and Os-arene complexes to alter cellular redox state: A structure-activity relationship (SAR) review. Coord. Chem. Rev. 2023, 491, 215230. [Google Scholar] [CrossRef]
- Borutzki, Y.; Skos, L.; Gerner, C.; Meier-Menches, S.M. Exploring the potential of metal-based candidate drugs as modulators of the cytoskeleton. ChemBioChem 2023, 24, e202300178. [Google Scholar] [CrossRef]
- Toupin, N.; Herroon, M.K.; Thummel, R.P.; Turro, C.; Podgorski, I.; Gibson, H.; Kodanko, J.J. Metalloimmunotherapy with rhodium and ruthenium complexes: Targeting tumor-associated macrophages. Chem. Eur. J. 2022, 28, e202104430. [Google Scholar] [CrossRef] [PubMed]
- Kanaoujiya, R.; Srivastava, S.; Singh, R.; Mustafa, G. Recent advances and application of ruthenium complexes in tumor malignancy. Mater. Today Proc. 2023, 72, 2822–2827. [Google Scholar] [CrossRef]
- Bijelic, A.; Theiner, S.; Keppler, B.K.; Rompel, A. X-ray structure analysis of indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019) bound to human serum albumin reveals two ruthenium binding sites and provides insights into the drug binding mechanism. J. Med. Chem. 2016, 59, 5894–5903. [Google Scholar] [CrossRef] [PubMed]
- Neuditschko, B.; Legin, A.A.; Baier, D.; Schintlmeister, A.; Reipert, S.; Wagner, M.; Keppler, B.K.; Berger, W.; Meier-Menches, S.M.; Gerner, C. Interaction with ribosomal proteins accompanies stress induction of the anticancer metallodrug BOLD-100/KP1339 in the endoplasmic reticulum. Angew. Chem. Int. Ed. Engl. 2021, 60, 5063–5068. [Google Scholar] [CrossRef] [PubMed]
- Alessio, E.; Messori, L. NAMI-A and KP1019/1339, two iconic ruthenium anticancer drug candidates face-to-face: A case story in medicinal inorganic chemistry. Molecules 2019, 24, 1995. [Google Scholar] [CrossRef] [PubMed]
- Hinton, S.R.; Corpuz, E.L.; Holman, K.L.M.; Meyer, S.C. A split β-lactamase sensor for the detection of DNA modification by cisplatin and ruthenium-based chemotherapeutic drugs. J. Inorg. Biochem. 2022, 236, 111986. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M.M.; Giram, P.; Foster, B.A.; You, Y. Photodynamic therapy for bladder cancers, a focused review. Photochem. Photobiol. 2023, 99, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.S.; Babak, M.V.; Hartinger, C.G.; Dyson, P.J. The Development of RAPTA Compounds for the Treatment of Tumors. Coord. Chem. Rev. 2016, 306, 86–114. [Google Scholar] [CrossRef]
- Casini, A.; Gabbiani, C.; Sorrentino, F.; Rigobello, M.P.; Bindoli, A.; Geldbach, T.J.; Marrone, A.; Re, N.; Hartinger, C.G.; Dyson, P.J.; et al. Emerging Protein Targets For Anticancer Metallodrugs: Inhibition of thioredoxin reductase and cathepsin B by antitumor ruthenium(II)−arene compounds. J. Med. Chem. 2008, 51, 6773–6781. [Google Scholar] [CrossRef]
- Aird, R.E.; Cummings, J.; Ritchie, A.A.; Muir, M.; Morris, R.E.; Chen, H.; Sadler, P.J.; Jodrell, D.I. In vitro and in vivo activity and cross resistance profiles of novel ruthenium (II) organometallic arene complexes in human ovarian cancer. Br. J. Cancer 2002, 86, 1652–1657. [Google Scholar] [CrossRef]
- Romero-Canelon, I.; Sadler, P.J. Next-generation metal anticancer complexes: Multitargeting via redox modulation. Inorg. Chem. 2013, 52, 12276–12291. [Google Scholar] [CrossRef] [PubMed]
- Scolaro, C.; Bergamo, A.; Brescacin, L.; Delfino, R.; Cocchietto, M.; Laurenczy, G.; Geldbach, T.J.; Sava, G.; Dyson, P.J. In vitro and in vivo evaluation of ruthenium(II)-arene PTA complexes. J. Med. Chem. 2005, 48, 4161–4171. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; Aird, R.E.; Murdoch, P.D.; Chen, H.M.; Cummings, J.; Hughes, N.D.; Parsons, S.; Parkin, A.; Boyd, G.; Jodrell, D.I.; et al. Inhibition of cancer cell growth by ruthenium(II) arene complexes. J. Med. Chem. 2001, 44, 3616–3621. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, A.; Melchart, M.; Fernandez, R.; Parsons, S.; Oswald, I.D.; Parkin, A.; Fabbiani, F.P.; Davidson, J.E.; Dawson, A.; Aird, R.E.; et al. Structure-activity relationships for cytotoxic ruthenium(II) arene complexes containing N,N-, N,O-, and O,O-chelating ligands. J. Med. Chem. 2006, 49, 6858–6868. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Haribabu, J.; Balakrishnan, N.; Vasanthakumar, P.; Karvembu, R. Piano stool Ru(II)-arene complexes having three monodentate legs: A comprehensive review on their development as anticancer therapeutics over the past decade. Coord. Chem. Rev. 2022, 459, 214403. [Google Scholar] [CrossRef]
- Hildebrandt, J.; Häfner, N.; Kritsch, D.; Görls, H.; Dürst, M.; Runnebaum, I.B.; Weigand, W. Highly cytotoxic osmium(II) compounds and their ruthenium(II) analogues targeting ovarian carcinoma cell lines and evading cisplatin resistance mechanisms. Int. J. Mol. Sci. 2022, 23, 4976. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, D.; Le, Q.; Wang, Y.; Wang, W. Ruthenium-based antitumor drugs and delivery systems from monotherapy to combination therapy. Nanoscale 2022, 14, 16339–16375. [Google Scholar] [CrossRef] [PubMed]
- Milović, E.; Janković, N.; Petronijević, J.; Joksimović, N.; Kosanić, M.; Stanojković, T.; Matić, I.; Grozdanić, N.; Klisurić, O.; Stefanović, S. Synthesis, characterization, and biological evaluation of tetrahydropyrimidines: Dual-activity and mechanism of action. Pharmaceutics 2022, 14, 2254. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, F.; Guo, H.; Wang, S.; Ni, S.; Zhou, Y.; Wang, Z.; Bao, H.; Wang, Y. Antitussive and anti-inflammatory dual-active agents developed from natural product lead compound 1-methylhydantoin. Molecules 2019, 24, 2355. [Google Scholar] [CrossRef]
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R.R. In vitro dual (anticancer and antiviral) activity of the carotenoids produced by haloalkaliphilic archaeon Natrialba sp. M6. Sci. Rep. 2020, 10, 5986. [Google Scholar] [CrossRef]
- Aldea, M.; Michot, J.-M.; Danlos, F.-X.; Ribas, A.; Soria, J.-C. Repurposing of anticancer drugs expands possibilities for antiviral and anti-inflammatory discovery in COVID-19. Cancer Discov. 2021, 11, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Trondl, R.; Heffeter, P.; Kowol, C.R.; Jakupec, M.A.; Berger, W.; Keppler, B.K. NKP-1339, the first ruthenium-based anticancer drug on the edge to clinical application. Chem. Sci. 2014, 5, 2925–2932. [Google Scholar] [CrossRef]
- Meier-Menches, S.M.; Gerner, C.; Berger, W.; Hartinger, C.G.; Keppler, B.K. Structure-activity relationships for ruthenium and osmium anticancer agents towards clinical development. Chem. Soc. Rev. 2018, 47, 909–928. [Google Scholar] [CrossRef] [PubMed]
- Pötsch, I.; Baier, D.; Keppler, B.K.; Berger, W. Challenges and chances in the preclinical to clinical translation of anticancer metallodrugs. RSC Metallobiol. 2019, 14, 308–347. [Google Scholar] [CrossRef]
- Burris, H.A.; Bakewell, S.; Bendell, J.C.; Infante, J.; Jones, S.F.; Spigel, D.R.; Weiss, G.J.; Ramanathan, R.K.; Ogden, A.; Von Hoff, D.; et al. Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: A First-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open 2016, 1, e000154. [Google Scholar] [CrossRef]
- Farkas, E.; Marmion, C.J. (Eds.) Targeted Metallo-Drugs: Design, Development, and Modes of Action; CRC Press: Boca Raton, FL, USA, 2023; ISBN 9781032223308. [Google Scholar]
- Spratlin, J.L.; O’Kane, G.; Goodwin, R.A.; McWhirter, E.; Thompson, D.; Halani, K.; Jones, M.; Snow, M.; McAllister, E.R.; Machado, A.; et al. BOLD-100-001 (TRIO039): A phase 1b dose-escalation study of BOLD-100 in combination with FOLFOX chemotherapy in patients with advanced gastrointestinal solid cancers: Interim safety, tolerability, and efficacy. J. Clin. Oncol. 2022, 40 (Suppl. S16), 3031. [Google Scholar] [CrossRef]
- Spratlin, J.; O’Kane, G.; Oh, D.Y.; Rha, S.Y.; McWhirter, E.; Elimova, E.; Kavan, P.; Choi, M.K.; Kim, D.W.; Goodwin, R.; et al. Abstract CT149: BOLD-100-001 (TRIO039): A phase 1b/2a dose-escalation study of BOLD-100 in combination with FOLFOX chemotherapy in patients with pre-treated advanced colorectal cancer: Interim efficacy, safety and tolerability analysis. Cancer Res. 2023, 83 (Suppl. S8), CT149. [Google Scholar] [CrossRef]
- Park, B.J.; Raha, P.; Pankovich, J.; Bazett, M. Utilization of cancer cell line screening to elucidate the anticancer activity and biological pathways related to the ruthenium-based therapeutic BOLD-100. Cancers 2022, 15, 28. [Google Scholar] [CrossRef]
- Labach, D.S.; Kohio, H.P.; Tse, E.A.; Paparisto, E.; Friesen, N.J.; Pankovich, J.; Bazett, M.; Barr, S.D. The metallodrug BOLD-100 is a potent inhibitor of SARS-CoV-2 replication and has broad-acting antiviral activity. Biomolecules 2023, 13, 1095. [Google Scholar] [CrossRef]
- Bakewell, S.; Conde, I.; Fallah, Y.; McCoy, M.; Jin, L.; Shajahan-Haq, A.N. Inhibition of DNA repair pathways and induction of ROS are potential mechanisms of action of the small molecule inhibitor BOLD-100 in breast cancer. Cancers 2020, 12, 2647. [Google Scholar] [CrossRef]
- Flocke, L.S.; Trondl, R.; Jakupec, M.A.; Keppler, B.K. Molecular mode of action of NKP-1339—A clinically investigated ruthenium-based drug—Involves ER- and ROS-related effects in colon carcinoma cell lines. Investig. New Drugs 2016, 34, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Schoenhacker-Alte, B.; Mohr, T.; Pirker, C.; Kryeziu, K.; Kuhn, P.S.; Buck, A.; Hofmann, T.; Gerner, C.; Hermann, G.; Koellensperger, G.; et al. Sensitivity towards the GRP78 inhibitor KP1339/IT-139 is characterized by apoptosis induction via caspase 8 upon disruption of ER homeostasis. Cancer Lett. 2017, 404, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Carlos, A.J.; Ha, D.P.; Yeh, D.W.; Van Krieken, R.; Tseng, C.C.; Zhang, P.; Gill, P.; Machida, K.; Lee, A.S. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. J. Biol. Chem. 2021, 296, 100759. [Google Scholar] [CrossRef] [PubMed]
- Wernitznig, D.; Kiakos, K.; Del Favero, G.; Harrer, N.; Machat, H.; Osswald, A.; Jakupec, M.A.; Wernitznig, A.; Sommergruber, W.; Keppler, B.K. First-in-class ruthenium anticancer drug (KP1339/IT-139) induces an immunogenic cell death signature in colorectal spheroids in vitro. Metallomics 2019, 11, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Mucke, H.A. Patent highlights October–November 2021. Pharm. Pat. Anal. 2022, 11, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Iacopetta, D.; Sinicropi, M.S.; Andreu, I.; Mariconda, A.; Saturnino, C.; Giuzio, F.; Longo, P.; Aquaro, S.; Catalano, A. Drugs for COVID-19: An update. Molecules 2022, 27, 8562. [Google Scholar] [CrossRef] [PubMed]
- Lohitesh, K.; Chowdhury, R.; Mukherjee, S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: An insight. Cancer Cell Int. 2018, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Baier, D.; Schoenhacker-Alte, B.; Rusz, M.; Pirker, C.; Mohr, T.; Mendrina, T.; Kirchhofer, D.; Meier-Menches, S.M.; Hohenwallner, K.; Schaier, M.; et al. The anticancer ruthenium compound BOLD-100 targets glycolysis and generates a metabolic vulnerability towards glucose deprivation. Pharmaceutics 2022, 14, 238. [Google Scholar] [CrossRef]
- Jang, M.; Kim, S.S.; Lee, J. Cancer cell metabolism: Implications for therapeutic targets. Exp. Mol. Med. 2013, 45, e45. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Baier, D.; Mendrina, T.; Schoenhacker-Alte, B.; Pirker, C.; Mohr, T.; Rusz, M.; Regner, B.; Schaier, M.; Sgarioto, N.; Raynal, N.J.M.; et al. The lipid metabolism as target and modulator of BOLD-100 anticancer activity: Crosstalk with histone acetylation. Adv. Sci. 2023, 10, 2301939. [Google Scholar] [CrossRef] [PubMed]
- Intravesical Photodynamic Therapy (PDT) in BCG Refractory/Intolerant Non-Muscle Invasive Bladder Cancer (NMIBC) Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT03945162 (accessed on 11 October 2023).
- Kulkarni, G.; Richards, K.; Black, P.C.; Rendon, R.; Chin, J.; Shore, N.; Jayram, G.; Kramolowsky, E.; Saltzstein, D.; Agarwal, A.; et al. MP63-01 an interim analysis of a phase ii clinical study of intravesical photodynamic therapy in patients with bcg-unresponsive non-muscle invasive bladder cancer (NMIBC) carcinoma in-situ (CIS). J. Urol. 2023, 209 (Suppl. S4), e871. [Google Scholar] [CrossRef]
- Chen, Q.; Ramu, V.; Aydar, Y.; Groenewoud, A.; Zhou, X.-Q.; Jager, M.J.; Cole, H.; Cameron, C.G.; McFarland, S.A.; Bonnet, S.; et al. TLD1433 photosensitizer inhibits conjunctival melanoma cells in zebrafish ectopic and orthotopic tumour models. Cancers 2020, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Murayama, Y.; Takamatsu, T.; Otsuji, E.; Tanaka, H. 5-Aminolevulinic acid-induced protoporphyrin ix fluorescence imaging for tumor detection: Recent advances and challenges. Int. J. Mol. Sci. 2022, 23, 6478. [Google Scholar] [CrossRef] [PubMed]
- Karges, J. Clinical development of metal complexes as photosensitizers for photodynamic therapy of cancer. Angew. Chem. Int. Ed. 2022, 61, e202112236. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Karvembu, R. Dichloro Ru(II)-p-cymene-1,3,5-triaza-7-phosphaadamantane (RAPTA-C): A case study. ACS Pharm. Translat. Sci. 2023, 6, 982–996. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.; Mantoo, I.A.; Arjmand, F.; Tabassum, S.; Yousuf, I. An overview of advancement of organoruthenium(II) complexes as prospective anticancer agents. Coord. Chem. Rev. 2023, 487, 215169. [Google Scholar] [CrossRef]
- Rausch, M.; Dyson, P.J.; Nowak-Sliwinska, P. Recent considerations in the application of RAPTA-C for cancer treatment and perspectives for its combination with immunotherapies. Adv. Ther. 2019, 2, 1900042. [Google Scholar] [CrossRef]
- Weiss, A.; Ding, X.; van Beijnum, J.R.; Wong, I.; Wong, T.J.; Berndsen, R.H.; Dormond, O.; Dallinga, M.; Shen, L.; Schlingemann, R.O.; et al. Rapid optimization of drug combinations for the optimal angiostatic treatment of cancer. Angiogenesis 2015, 18, 233–244. [Google Scholar] [CrossRef]
- Coverdale, J.P.C.; Laroiya-McCarron, T.; Isolda Romero-Canelón, I. Designing ruthenium anticancer drugs: What have we learnt from the key drug candidates? Inorganics 2019, 7, 31. [Google Scholar] [CrossRef]
- Weiss, A.; Berndsen, R.H.; Ding, X.; Ho, C.M.; Dyson, P.J.; Van Den Bergh, H.; Griffioen, A.W.; Nowak-Sliwinska, P. A streamlined search technology for identification of synergistic drug combinations. Sci. Rep. 2015, 5, 14508. [Google Scholar] [CrossRef] [PubMed]
- Berndsen, R.H.; Weiss, A.; Abdul, U.K.; Wong, T.J.; Meraldi, P.; Griffioen, A.W.; Dyson, P.J.; Nowak-Sliwinska, P. Combination of ruthenium(II)-arene complex [Ru(ƞ6-p-cymene)Cl2(pta)] (RAPTA-C) and the epidermal growth factor receptor inhibitor erlotinib results in efficient angiostatic and antitumor activity. Sci. Rep. 2017, 7, 43005. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Berndsen, R.H.; Dubois, M.; Müller, C.; Schibli, R.; Griffioen, A.W.; Dyson, P.J.; Nowak-Sliwinska, P. In vivo anti-tumor activity of the organometallic ruthenium(II)-arene complex [Ru(ƞ6-p-cymene)Cl2(Pta)] (RAPTA-C) in human ovarian and colorectal carcinomas. Chem. Sci. 2014, 5, 4742–4748. [Google Scholar] [CrossRef]
- Lu, M.; Wang, S.; Khine, Y.Y.; Hong, Y.; Zheng, J.; Lu, H.; Stenzel, M.H. Dual drug delivery system of RAPTA-C and paclitaxel based on fructose coated nanoparticles for metastatic cancer treatment. Biochem. Biophys. Res. Commun. 2023, 640, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Marzo, T.; Messori, L. A Role for metal-based drugs in fighting COVID-19 infection? The Case of Auranofin. ACS Med. Chem. Lett. 2020, 11, 1067–1068. [Google Scholar] [CrossRef]
- De Paiva, R.E.F.; Marçal Neto, A.; Santos, I.A.; Jardim, A.C.G.; Corbi, P.P.; Bergamini, F.R.G. What is holding back the development of antiviral metallodrugs? A literature overview and implications for SARS-CoV-2 therapeutics and future viral outbreaks. Dalton Trans. 2020, 49, 16004–16033. [Google Scholar] [CrossRef] [PubMed]
- Chuong, C.; DuChane, C.M.; Webb, E.M.; Rai, P.; Marano, J.M.; Bernier, C.M.; Merola, J.S.; Weger-Lucarelli, J. Noble metal organometallic complexes display antiviral activity against SARS-CoV-2. Viruses 2021, 13, 980. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Development of metal complexes for treatment of coronaviruses. Int. J. Mol. Sci. 2022, 23, 6418. [Google Scholar] [CrossRef]
- Kojima, S.; Hasegawa, T.; Yonemura, T.; Sasaki, K.; Yamamoto, K.; Makimura, Y.; Takahashi, T.; Suzuki, T.; Suzuki, Y.; Kobayashi, K. Ruthenium complexes carrying a disialo complex-type oligosaccharide: Enzymatic synthesis and its application to a luminescent probe to detect influenza viruses. Chem. Commun. 2003, 11, 1250–1251. [Google Scholar] [CrossRef]
- Wong, E.L.-M.; Sun, R.W.-Y.; Chung, N.P.-Y.; Lin, C.-L.S.; Zhu, N.; Che, C.-M. A mixed-valent ruthenium−oxo oxalato cluster Na7[Ru4(μ3-O)4(C2O4)6] with potent anti-HIV activities. J. Am. Chem. Soc. 2006, 128, 4938–4939. [Google Scholar] [CrossRef]
- Gil-Moles, M.; Türck, S.; Basu, U.; Pettenuzzo, A.; Bhattacharya, S.; Rajan, A.; Ma, X.; Büssing, R.; Wölker, J.; Burmeister, H.; et al. Metallodrug profiling against SARS-CoV-2 target proteins identifies highly potent inhibitors of the S/ACE2 interaction and the Papain-like Protease PLpro. Chem. Eur. J. 2021, 27, 17928–17940. [Google Scholar] [CrossRef] [PubMed]
- Janković, N.; Milović, E.; Jovanović, J.Đ.; Marković, Z.; Vraneš, M.; Stanojković, T.; Matić, I.; Crnogorac, M.Đ.; Klisurić, O.; Cvetinov, M. A new class of half-sandwich ruthenium complexes containing Biginelli hybrids: Anticancer and anti-SARS-CoV-2 activities. Chem. Biol. Interact. 2022, 363, 110025. [Google Scholar] [CrossRef] [PubMed]
- Shereef, H.A.; Shaban, S.Y.; Moemen, Y.S.; El-Khouly, M.E.; El-Nahas, A.M. Biophysicochemical studies of a ruthenium(II) nitrosyl thioether-thiolate complex binding to BSA: Mechanistic information, molecular docking, and relationship to antibacterial and cytotoxic activities. Appl. Organometal. Chem. 2022, 36, e6583. [Google Scholar] [CrossRef]
- Gurgul, I.; Janczy-Cempa, E.; Mazuryk, O.; Lekka, M.; Łomzik, M.; Suzenet, F.; Gros, P.C.; Brindell, M. Inhibition of metastasis by polypyridyl Ru(II) complexes through modification of cancer cell adhesion—In Vitro functional and molecular studies. J. Med. Chem. 2022, 65, 10459–10470. [Google Scholar] [CrossRef]
- Cseh, K.; Geisler, H.; Stanojkovska, K.; Westermayr, J.; Brunmayr, P.; Wenisch, D.; Gajic, N.; Hejl, M.; Schaier, M.; Koellensperger, G.; et al. Arene variation of highly cytotoxic tridentate naphthoquinone-based ruthenium(II) complexes and in-depth in vitro studies. Pharmaceutics 2022, 14, 2466. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Talon, S.; de Bellis, M.; Desaphy, J.-F.; Franchini, C.; Lentini, G.; Catalano, A.; Corbo, F.; Tortorella, V.; Conte-Camerino, D. Inhibition of skeletal muscle sodium currents by mexiletine analogues: Specific hydrophobic interactions rather than lipophilia per se account for drug therapeutic profile. Naunyn Schmiedeberg’s Arch. Pharmacol. 2003, 367, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, M.; Kluska, M.; Kosińska, A.; Palusiak, M.; Rybarczyk-Pirek, A.J.; Wzgarda-Raj, K.; Rudolf, B.; Woźniak, K. Cytotoxicity of piano-stool ruthenium cyclopentadienyl complexes bearing different imidato ligands. Appl. Organomet. Chem. 2022, 36, e6595. [Google Scholar] [CrossRef]
- Liang, L.; Wu, X.; Shi, C.; Wen, H.; Wu, S.; Chen, J.; Huang, C.; Wang, Y.; Liu, Y. Synthesis and characterization of polypyridine ruthenium(II) complexes and anticancer efficacy studies in vivo and in vitro. J. Inorg. Biochem. 2022, 236, 111963. [Google Scholar] [CrossRef]
- Cervinka, J.; Gobbo, A.; Biancalana, L.; Markova, L.; Novohradsky, V.; Guelfi, M.; Zacchini, S.; Kasparkova, J.; Brabec, V.; Marchetti, F. Ruthenium (II)–tris-pyrazolylmethane complexes inhibit cancer cell growth by disrupting mitochondrial calcium homeostasis. J. Med. Chem. 2022, 65, 10567–10587. [Google Scholar] [CrossRef]
- Priya, F.C.; Kumar, D.S. pH dependent spectrophotometric study, cytotoxicity and antimicrobial activity of mononuclear ruthenium(II) polypyridine complexes. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Křikavová, R.; Romanovová, M.; Jendželovská, Z.; Majerník, M.; Masaryk, L.; Zoufalý, P.; Milde, D.; Moncol, J.; Herchel, R.; Jendželovský, R.; et al. Impact of the central atom and halido ligand on the structure, antiproliferative activity and selectivity of half-sandwich Ru (II) and Ir (III) complexes with a 1,3,4-thiadiazole-based ligand. Dalton Transact. 2023, 52, 12717–12732. [Google Scholar] [CrossRef] [PubMed]
- de Araujo-Neto, J.H.; Guedes, A.P.; Leite, C.M.; Moraes, C.A.F.; Santos, A.L.; Brito, R.D.S.; Rocha, T.L.; Mello-Andrade, F.R.; Ellena, J.; Batista, A.A. “Half-sandwich” ruthenium complexes with alizarin as anticancer agents: In vitro and in vivo studies. Inorg. Chem. 2023, 62, 6955–6969. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, G.; Boni, S.; Funaioli, T.; Zacchini, S.; Pampaloni, G.; Busto, N.; Biver, T.; Marchetti, F. Adding diversity to a diruthenium biscyclopentadienyl scaffold via alkyne incorporation: Synthesis and biological studies. Inorg. Chem. 2023, 62, 12453–12467. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, G.; Vančo, J.; Funaioli, T.; Zacchini, S.; Malina, T.; Pampaloni, G.; Dvořák, Z.; Trávníček, Z.; Marchetti, F. Anticancer potential of diruthenium complexes with bridging hydrocarbyl ligands from bioactive alkynols. Inorg. Chem. 2023, 62, 15875–15890. [Google Scholar] [CrossRef] [PubMed]
- Nayek, S.; Singh, S.; Sonawane, A.; Grabchev, I.; Ganguly, R.; Mukhopadhyay, S. Studies on anticancer properties with varying co-ligands in a Ru(II) arene benzimidazole system. Dalton Transact. 2023, 52, 7104–7118. [Google Scholar] [CrossRef]
- Schoeller, M.; Piroš, M.; Litecká, M.; Koňariková, K.; Jozefíková, F.; Šagátová, A.; Zahradníková, E.; Valentová, J.; Moncol, J. Bipyridine ruthenium(II) complexes with halogen-substituted salicylates: Synthesis, crystal structure, and biological activity. Molecules 2023, 28, 4609. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, A.; Scalambra, F.; Lorenzo-Luis, P.; Puerta, A.; González-Bakker, A.; Mendoza, Z.; Padrón, J.M.; Romerosa, A. Tetranuclear Ru2Cu2 and Ru2Ni2 complexes with nanomolar anticancer activity. Dalton Transact. 2023, 52, 9541–9545. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Gowda, A.S.; Olivelli, A.G.; Huckaba, A.J.; Parkin, S.; Unrine, J.M.; Oza, V.; Blackburn, J.S.; Ladipo, F.; Heidary, D.K.; et al. Triarylphosphine-coordinated bipyridyl Ru(II) complexes induce mitochondrial dysfunction. Inorg. Chem. 2023, 62, 10940–10954. [Google Scholar] [CrossRef]
- Das, A.; Mandal, S.; Mukherjee, R.; Naskar, R.; Murmu, N.; Mondal, T.K. Synthesis of Ru(II) cyclometallated complexes via C (aryl)–S bond activation: X-ray structure, DNA/BSA protein binding and antiproliferative activity. New J. Chem. 2023, 47, 17359–17372. [Google Scholar] [CrossRef]
- Ceramella, J.; Troiano, R.; Iacopetta, D.; Mariconda, A.; Pellegrino, M.; Catalano, A.; Saturnino, C.; Aquaro, S.; Sinicropi, M.S.; Longo, P. Synthesis of novel N-Heterocyclic carbene-ruthenium(II) complexes, “precious” tools with antibacterial, anticancer and antioxidant properties. Antibiotics 2023, 12, 693. [Google Scholar] [CrossRef]
- Kavukcu, S.B.; Ensarioğlu, H.K.; Karabıyık, H.; Vatansever, H.S.; Türkmen, H. Cell death mechanism of organometallic ruthenium(II) and iridium(III) arene complexes on HepG2 and Vero cells. ACS Omega 2023, 8, 37549–37563. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, W.; Yang, Y.; Zhong, R.; Hu, H.; Huang, C.; Chen, J.; Liang, L.; Liu, Y. Significant increase of anticancer efficacy in vitro and in vivo of liposome entrapped ruthenium(II) polypyridyl complexes. Eur. J. Med. Chem. 2023, 115541. [Google Scholar] [CrossRef] [PubMed]
- Santi, M.; Mapanao, A.K.; Biancalana, L.; Marchetti, F.; Voliani, V. Ruthenium arene complexes in the treatment of 3D models of head and neck squamous cell carcinomas. Eur. J. Med. Chem. 2021, 212, 113143–113157. [Google Scholar] [CrossRef] [PubMed]
- Santi, M.; Frusca, V.; Ermini, M.L.; Mapanao, A.K.; Sarogni, P.; Gonnelli, A.; Giannini, M.; Zamborlin, A.; Biancalana, L.; Marchetti, F.; et al. Hybrid nano-architectures loaded with metal complexes for the co-chemotherapy of head and neck carcinomas. J. Mater. Chem. B 2023, 11, 325–334. [Google Scholar] [CrossRef]
- Wang, M.-F.; Li, Y.; Bi, X.-D.; Guo, Y.-X.; Liu, M.; Zhang, H.; Gao, F. Polypyridyl ruthenium complexes as bifunctional TAR RNA binders and HIV-1 reverse transcriptase inhibitors. J. Inorg. Biochem. 2022, 234, 111880. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Su, J.; Chen, M.; Chen, T.; Jia, W.; Zhu, B. Selenium-ruthenium complex blocks H1N1 influenza virus-induced cell damage by activating GPx1/TrxR1. Theranostics 2023, 13, 1843–1859. [Google Scholar] [CrossRef]
| Structure | Compound | Cytotoxicity Studies | Antiviral Studies | Ref. |
|---|---|---|---|---|
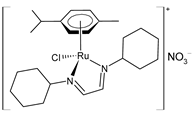 | [RuCl{κ2N-(HC=N-cyclohexyl)2}(η6-p-cymene)]NO3 (50) | IC50 = 78.5 µM (SCC-25, after 72 h) IC50 = 91.8 µM (UPCI-SCC-154, after 72 h) | Santi et al. (2021) [156] | |
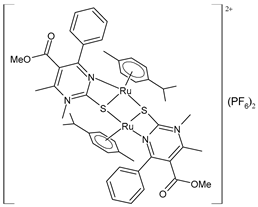 | [(p-cymene)Ru(4a)]2 (PF6)2 (51) | IC50 = 34.70 ± 1.23 µM (HeLa) IC50 = 61.99 ± 0.36 µM (A549) IC50 = 67.43 ± 1.24 µM (LS174) IC50 = 14.14 ± 1.11 µM (A375) IC50 = 11.44 ± 1.19 µM (K652) IC50 = 59.96 ± 11.50 μM (EA.hy926) | ΔGbind = −6.40 kcal/mol Ki = 20.25 μM | Janković et al. (2022) [135] |
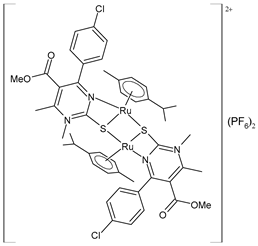 | [(p-cymene)Ru(4b)]2 (PF6)2 (52) | IC50 = 16.39 ± 0.43 µM (HeLa) IC50 = 24.87 ± 1.14 µM (A549) IC50 = 32.78 ± 3.38 µM (LS174) IC50 = 14.00 ± 0.10 µM (A375) IC50 = 11.45 ± 0.15 µM (K652) IC50 = 35.24 ± 1.08 μM (EA.hy926) | ΔGbind = −6.24 kcal/mol Ki = 26.84 μM | Janković et al. (2022) [135] |
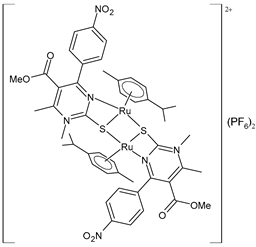 | [(p-cymene)Ru(4c)]2 (PF6)2 (53) | IC50 = 17.89 ± 0.7 µM (HeLa) IC50 = 33.85 ± 2.74 µM (A549) IC50 = 34.00 ± 1.39 µM (LS174) IC50 = 13.94 ± 0.25 µM (A375) IC50 = 8.63 ± 0.24 µM (K652) IC50 = 33.85 ± 1.68 μM (EA.hy926) | ΔGbind = −5.53 kcal/mol Ki = 88.62 μM | Janković et al. (2022) [135] |
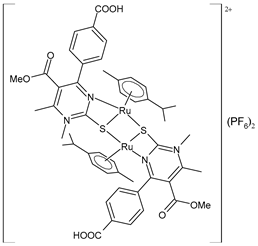 | [(p-cymene)Ru(4d)]2 (PF6)2 (54) | IC50 = 69.66 ± 4.33 µM (HeLa) IC50 = not active (A549) IC50 = 81.79 ± 4.28 µM (LS174) IC50 = 199.53 ± 0.67 µM (A375) IC50 = 198.09 ± 1.58 µM (K652) IC50 = not active (EA.hy926) | ΔGbind = −5.32 kcal/mol Ki = 124.98 μM | Janković et al. (2022) [135] |
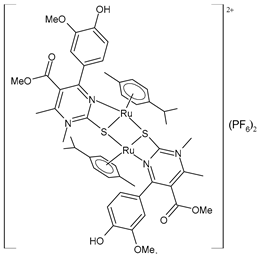 | [(p-cymene)Ru(4e)]2 (PF6)2 (55) | IC50 = 78.28 ± 3.26 µM (HeLa) IC50 = not active (A549) IC50 = 97.77 ± 1.43 µM (LS174) IC50 = 116.66 ± 5.72 µM (A375) IC50 = 130-48 ± 3.13 µM (K652) IC50 = not active (EA.hy926) | ΔGbind = −7.34 kcal/mol Ki = 4.18 μM | Janković et al. (2022) [135] |
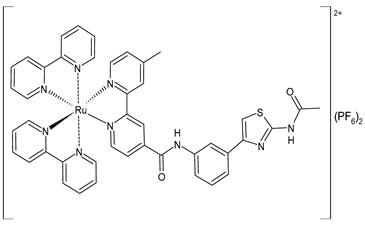 | [Ru(bpy)2(L1)] (PF6)2 (56) | CC50 = 226 ± 12 µM (HL-7702 normal cells) | IC50 = 1.85 ± 0.09 µM (M-MuLV RT) EC50 = 0.168 ± 0.009 µM (HIV-RT) | Wang et al. (2022) [158] |
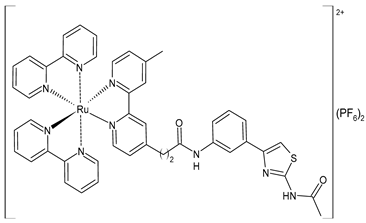 | [Ru(bpy)2(L2)] (PF6)2 (57) | CC50 = 247 ± 11 µM (HL-7702 normal cells) | IC50 = 3.62 ± 0.10 µM (M-MuLV RT) EC50 = 0.357 ± 0.023 µM (HIV-RT) | Wang et al. (2022) [158] |
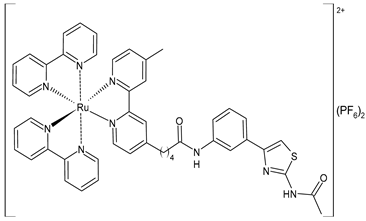 | [Ru(bpy)2(L3)] (PF6)2 (58) | CC50 = 239 ± 16 µM (HL-7702 normal cells) | IC50 = 4.74 ± 0.11 µM (M-MuLV RT) EC50 = 0.446 ± 0.032 µM (HIV-RT) | Wang et al. (2022) [158] |
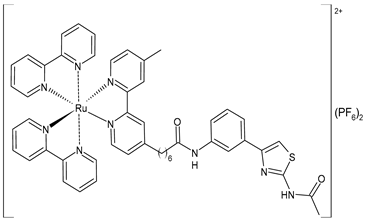 | [Ru(bpy)2(L4)] (PF6)2 (59) | CC50 = 231 ± 18 µM (HL-7702 normal cells) | IC50 = 5.49 ± 0.26 µM (M-MuLV RT) EC50 = 0.522 ± 0.032 µM (HIV-RT) | Wang et al. (2022) [158] |
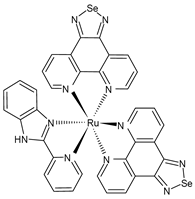 | Ru(biim) (PhenSe)2 (60) | TCID50 = 1.04·102/0.1 mL (H1N1+ RuSe group) | Li et al. (2023) [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amato, A.; Mariconda, A.; Iacopetta, D.; Ceramella, J.; Catalano, A.; Sinicropi, M.S.; Longo, P. Complexes of Ruthenium(II) as Promising Dual-Active Agents against Cancer and Viral Infections. Pharmaceuticals 2023, 16, 1729. https://doi.org/10.3390/ph16121729
D’Amato A, Mariconda A, Iacopetta D, Ceramella J, Catalano A, Sinicropi MS, Longo P. Complexes of Ruthenium(II) as Promising Dual-Active Agents against Cancer and Viral Infections. Pharmaceuticals. 2023; 16(12):1729. https://doi.org/10.3390/ph16121729
Chicago/Turabian StyleD’Amato, Assunta, Annaluisa Mariconda, Domenico Iacopetta, Jessica Ceramella, Alessia Catalano, Maria Stefania Sinicropi, and Pasquale Longo. 2023. "Complexes of Ruthenium(II) as Promising Dual-Active Agents against Cancer and Viral Infections" Pharmaceuticals 16, no. 12: 1729. https://doi.org/10.3390/ph16121729
APA StyleD’Amato, A., Mariconda, A., Iacopetta, D., Ceramella, J., Catalano, A., Sinicropi, M. S., & Longo, P. (2023). Complexes of Ruthenium(II) as Promising Dual-Active Agents against Cancer and Viral Infections. Pharmaceuticals, 16(12), 1729. https://doi.org/10.3390/ph16121729











