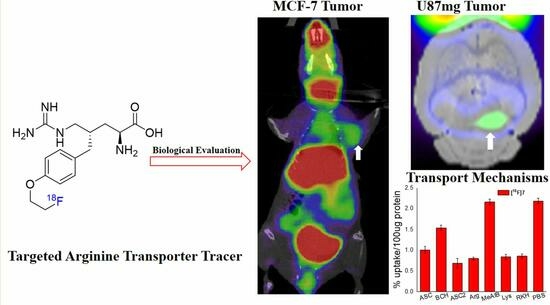
Design, Synthesis, and Biological Evaluation of a Novel [18F]-Labeled Arginine Derivative for Tumor Imaging
Abstract
:1. Introduction
2. Results
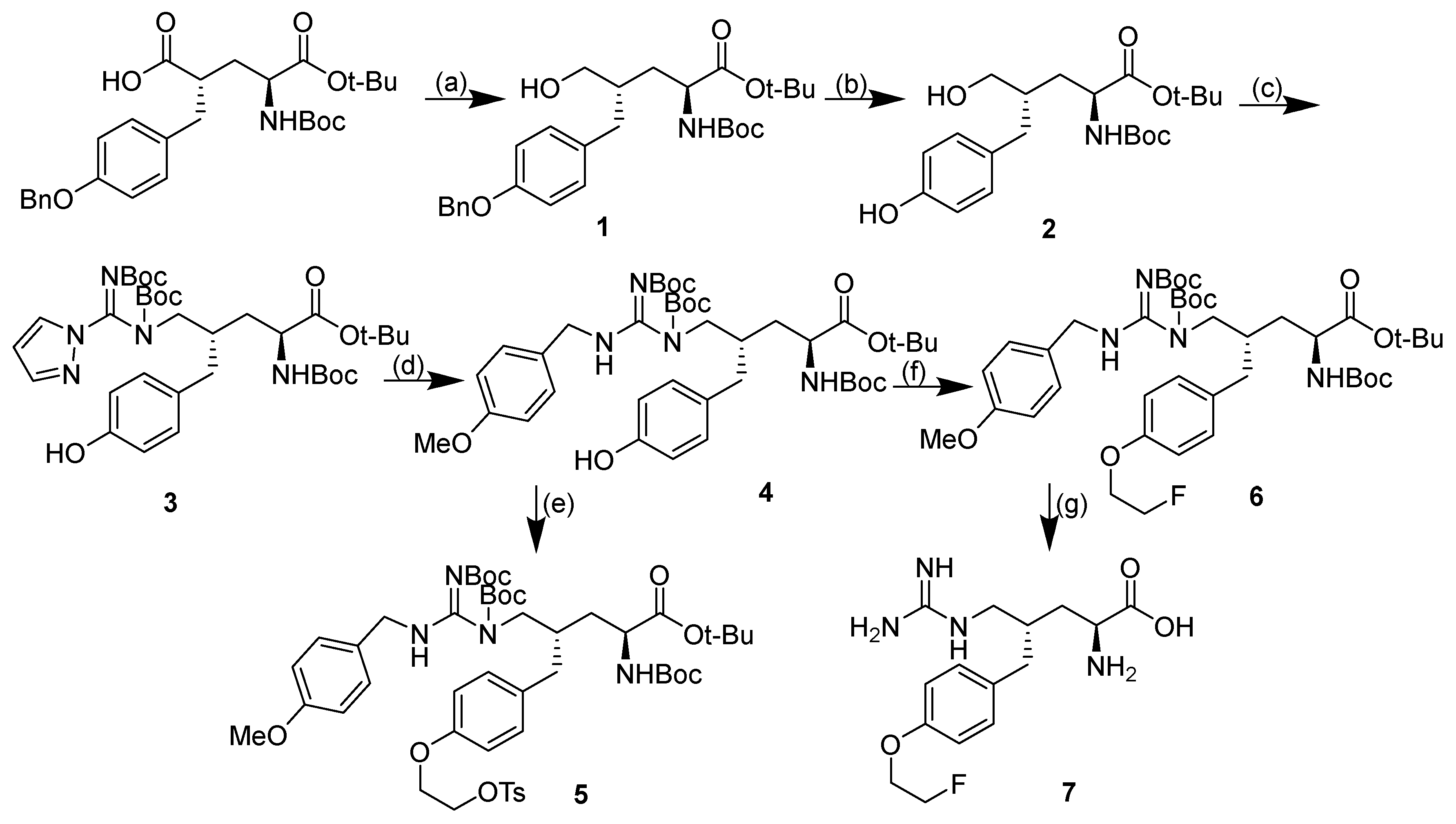
2.1. Design and Synthesis of Compound 7
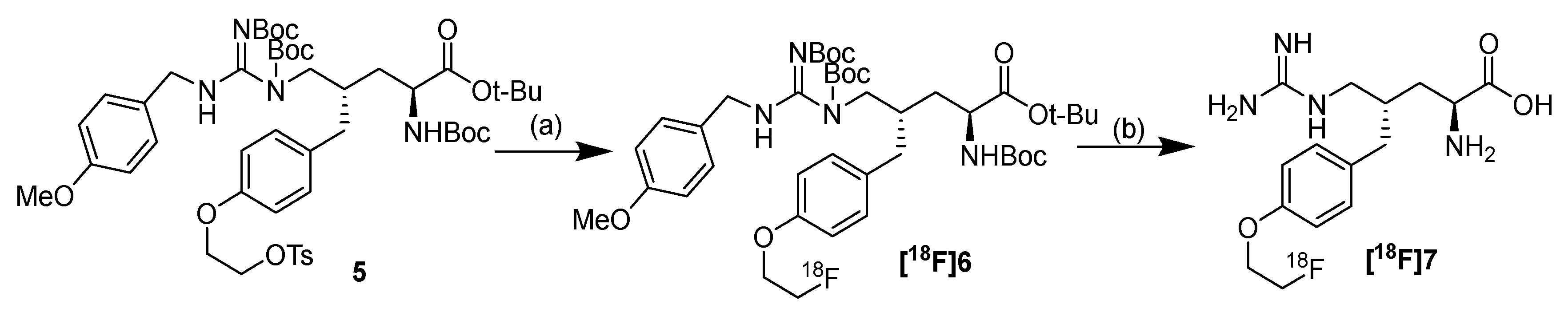
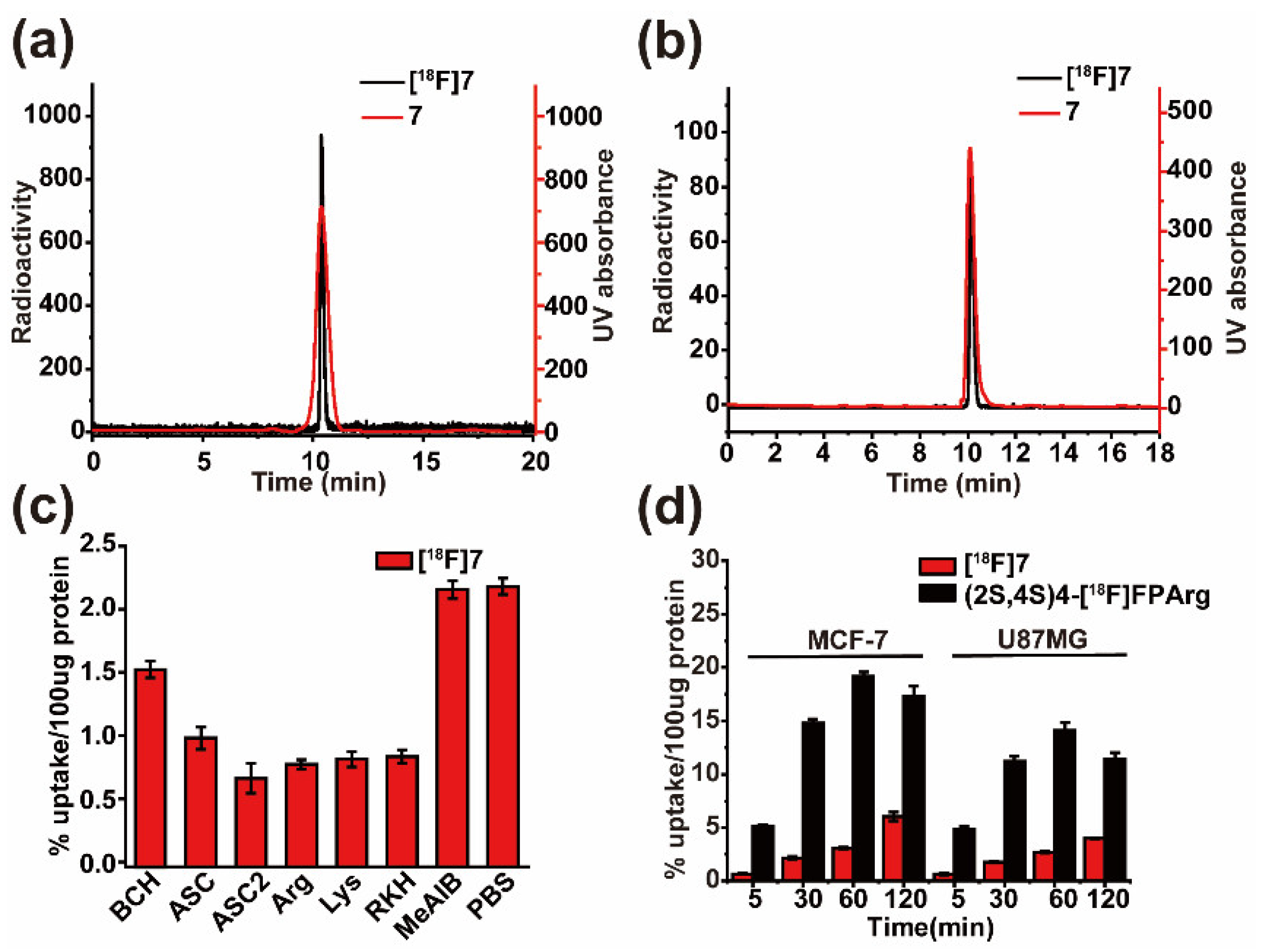
2.2. Radiolabeling
2.3. Cell Uptake Assays and Inhibition Studies
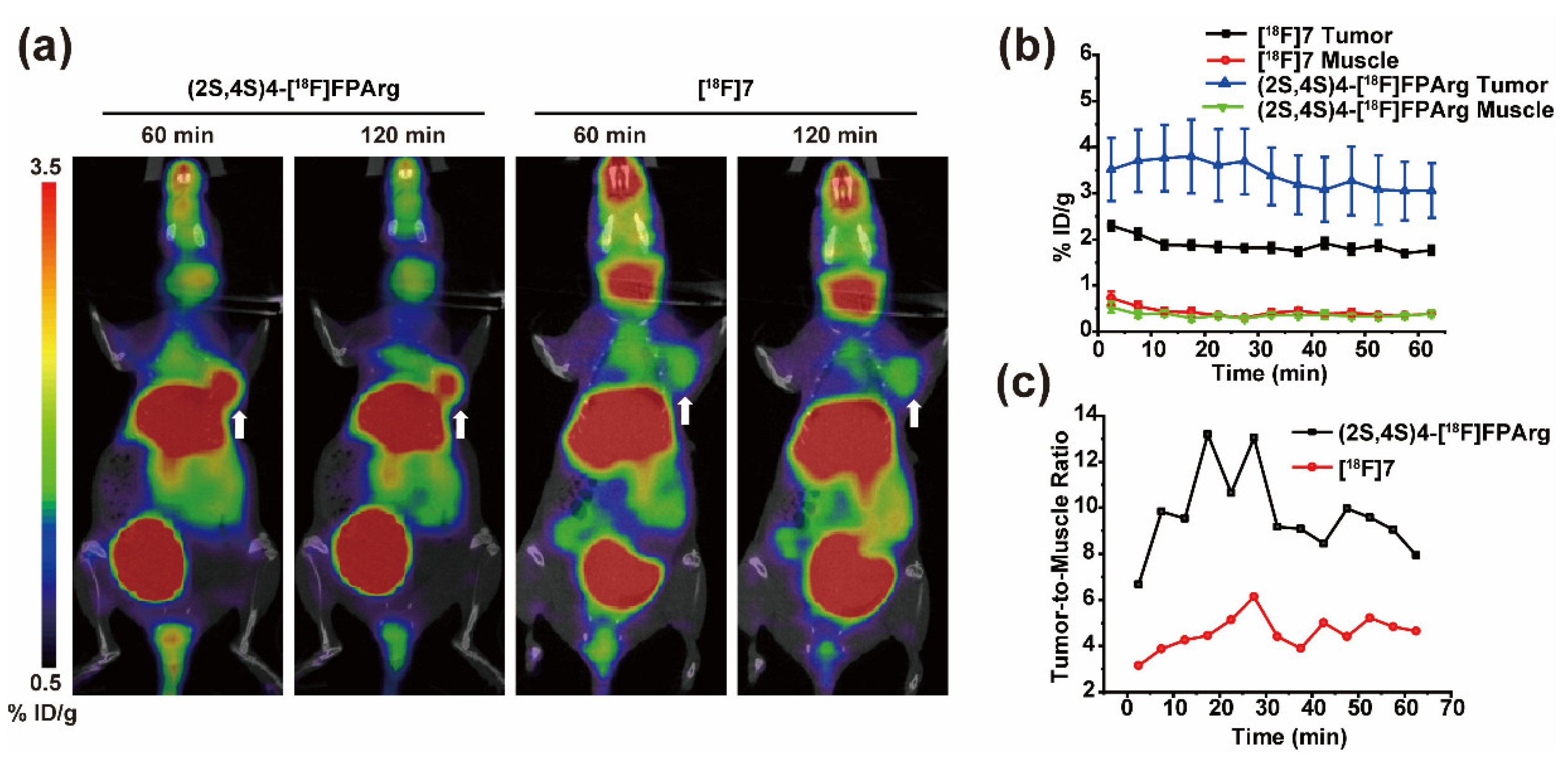
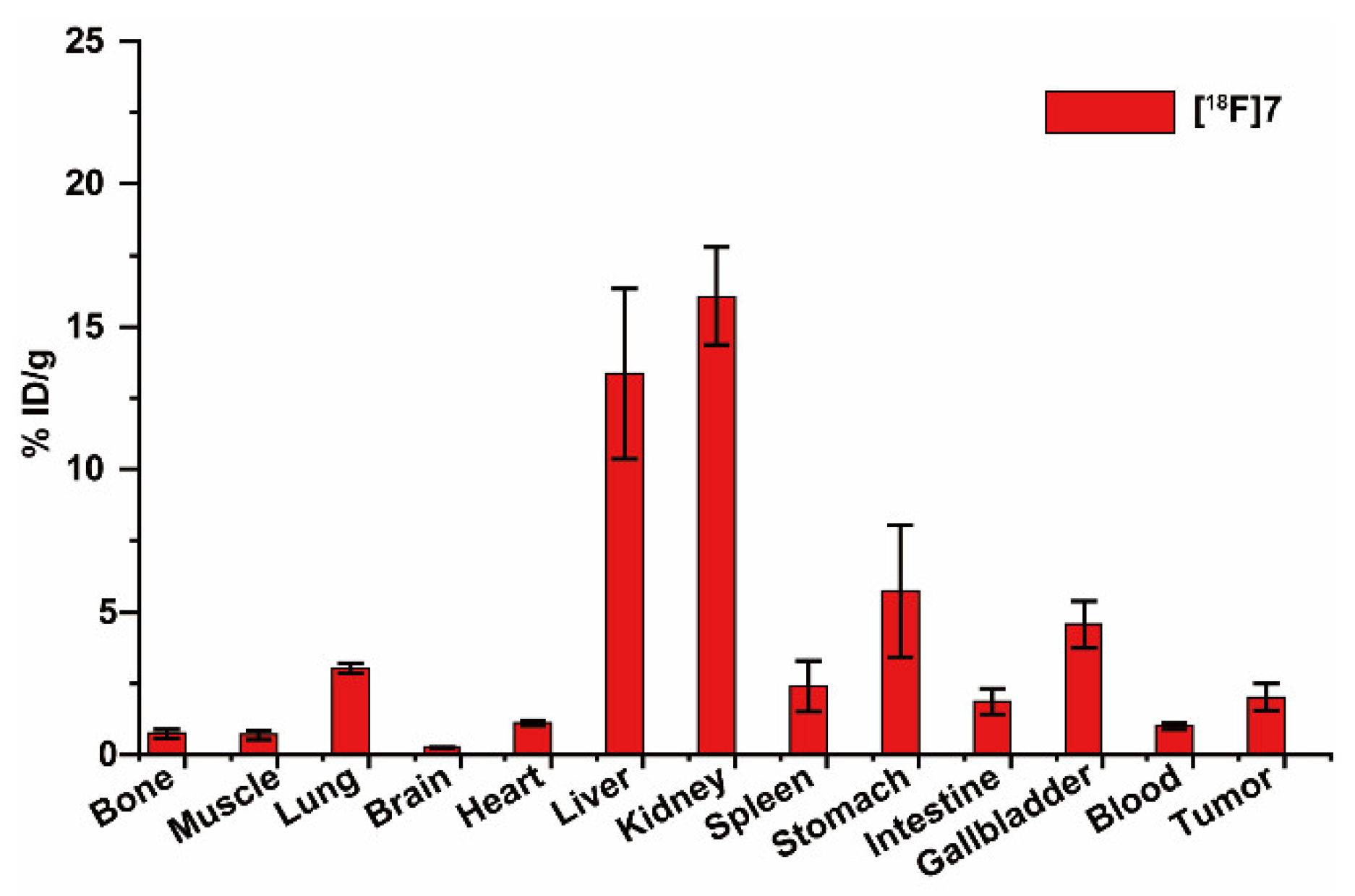
2.4. Small Animal PET-CT Imaging and Biodistribution in MCF-7 Subcutaneous Tumors
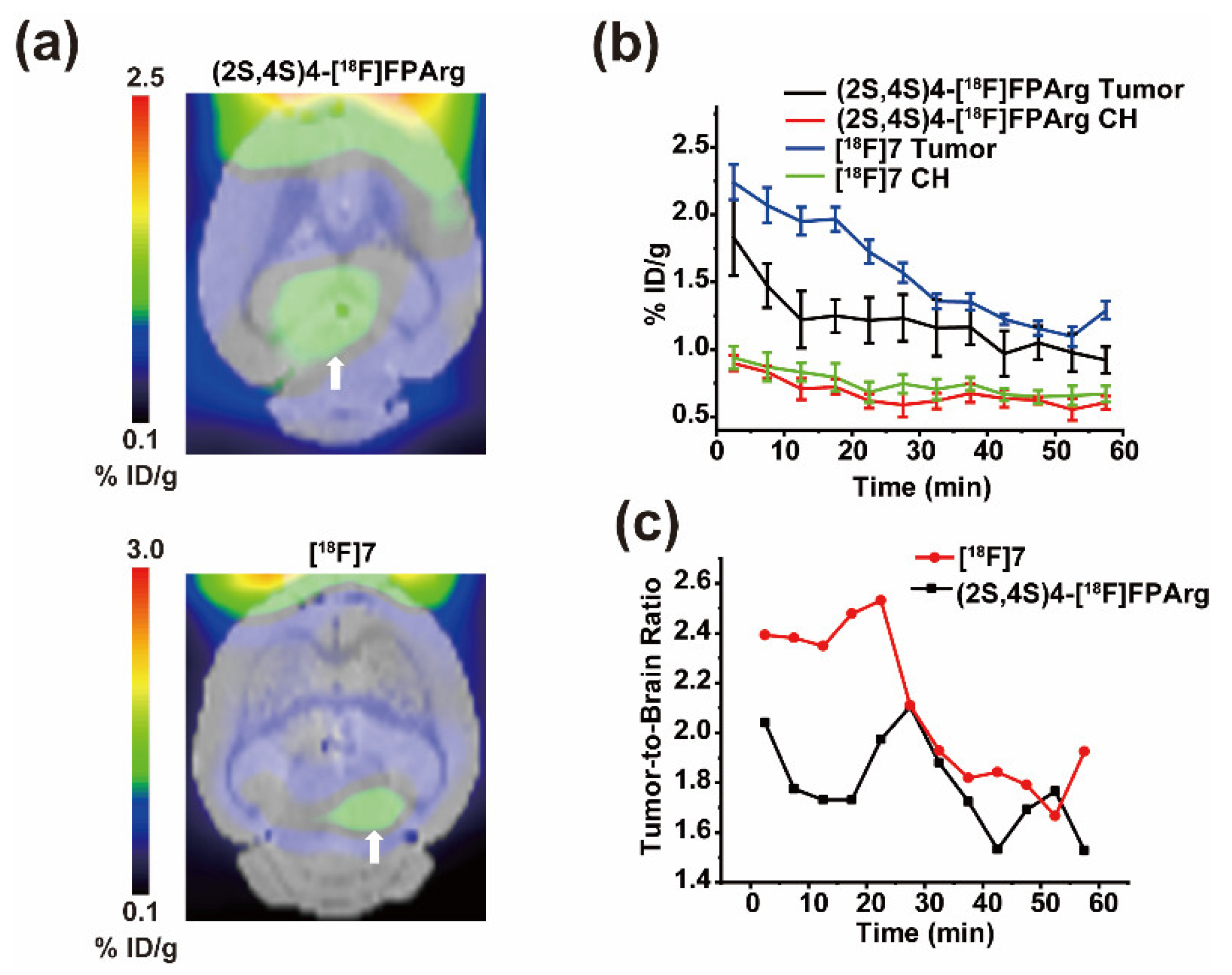
2.5. Small Animal PET-CT Imaging in U87MG Intracranial Tumor Mouse Model
3. Discussion
4. Materials and Methods
4.1. Synthesis
- tert-butyl (2S,4S)-4-(4-(benzyloxy)benzyl)-2-((tert-butoxycarbonyl)amino)-5 -hydroxypentanoate (1)
- tert-butyl (2S,4S)-2-((tert-butoxycarbonyl)amino)-5-hydroxy-4-(4-hydroxybenzyl) pentanoate (2)
- tert-butyl (2S,4S)-5-((Z)-N,N′-bis(tert-butoxycarbonyl)-1H-pyrazole-1- carboximidamido)-2-((tert-butoxycarbonyl)amino)-4-(4-hydroxybenzyl)pentanoate (3)
- tert-butyl (2S,4S)-5-((E)-1,2-bis(tert-butoxycarbonyl)-3-(4-methoxybenzyl)guanidino) -2-((tert-butoxycarbonyl)amino)-4-(4-hydroxybenzyl)pentanoate (4)
- tert-butyl (2S,4S)-5-((E)-1,2-bis(tert-butoxycarbonyl)-3-(4-methoxybenzyl)guanidino) -2-((tert-butoxycarbonyl)amino)-4-(4-(2-(tosyloxy)ethoxy)benzyl)pentanoate (5)
- tert-butyl (2S,4S)-5-((E)-1,2-bis(tert-butoxycarbonyl)-3-(4-methoxybenzyl)guanidino) -2-((tert-butoxycarbonyl)amino)-4-(4-(2-fluoroethoxy)benzyl)pentanoate (6)
- (2S,4S)-2-amino-4-(4-(2-fluoroethoxy)benzyl)-5-guanidinopentanoic acid (7)
4.2. Radiolabeling
4.3. Partition Coefficient and in Vivo Stability
4.4. In Vitro Cell Uptake Studies
4.5. MicroPET-CT Imaging
4.6. Biodistribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Vandamme, M.; Bormans, G.; Cleeren, F. Design and Challenges of Radiopharmaceuticals. Semin. Nucl. Med. 2019, 49, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Allott, L.; Aboagye, E.O. Chemistry Considerations for the Clinical Translation of Oncology PET Radiopharmaceuticals. Mol. Pharm. 2020, 17, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Piel, M.; Vernaleken, I.; Rösch, F. Positron Emission Tomography in CNS drug discovery and drug monitoring. J. Med. Chem. 2014, 57, 9232–9258. [Google Scholar] [CrossRef]
- Ricard, F.; Cheson, B.; Barrington, S.; Trotman, J.; Schmid, A.; Brueggenwerth, G.; Salles, G.; Schwartz, L.; Goldmacher, G.; Jarecha, R.; et al. Application of the Lugano Classification for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The PRoLoG Consensus Initiative (Part 1-Clinical). J. Nucl. Med. 2023, 64, 102–108. [Google Scholar] [CrossRef]
- Zanoni, L.; Bezzi, D.; Nanni, C.; Paccagnella, A.; Farina, A.; Broccoli, A.; Casadei, B.; Zinzani, P.L.; Fanti, S. PET/CT in Non-Hodgkin Lymphoma: An Update. Semin. Nucl. Med. 2023, 53, 320–351. [Google Scholar] [CrossRef]
- Farolfi, A.; Calderoni, L.; Mattana, F.; Mei, R.; Telo, S.; Fanti, S.; Castellucci, P. Current and Emerging Clinical Applications of PSMA PET Diagnostic Imaging for Prostate Cancer. J. Nucl. Med. 2021, 62, 596–604. [Google Scholar] [CrossRef]
- Mori, Y.; Dendl, K.; Cardinale, J.; Kratochwil, C.; Giesel, F.L.; Haberkorn, U. FAPI PET: Fibroblast Activation Protein Inhibitor Use in Oncologic and Nononcologic Disease. Radiology 2023, 306, e220749. [Google Scholar] [CrossRef]
- Van den Hoven, A.F.; Keijsers, R.G.M.; Lam, M.G.E.H.; Glaudemans, A.W.J.M.; Verburg, F.A.; Vogel, W.V.; Lavalaye, J. Current research topics in FAPI theranostics: A bibliometric analysis. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1014–1027. [Google Scholar] [CrossRef]
- Herrmann, K.; Schwaiger, M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Hricak, H.; Weissleder, R. Radiotheranostics: A roadmap for future development. Lancet Oncol. 2020, 21, e146–e156. [Google Scholar] [CrossRef]
- Bodei, L.; Herrmann, K.; Schöder, H.; Scott, A.M.; Lewis, J.S. Radiotheranostics in oncology: Current challenges and emerging opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 534–550. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Bögeman, M.; Yordanova, A.; Eveslage, M.; Schäfers, M.; Essler, M.; Ahmadzadehfar, H. Delayed response after repeated 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.-E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjugate Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.B.; Wang, C.; Wong, K.K.; Frey, K.A.; Muzik, O.; Schipper, M.J.; Dewaraja, Y.K. 177Lu-DOTATATE Theranostics: Predicting Renal Dosimetry from Pretherapy 68Ga-DOTATATE PET and Clinical Biomarkers. Clin. Nucl. Med. 2023, 48, 393–399. [Google Scholar] [CrossRef]
- Huang, C.; McConathy, J. Radiolabeled Amino Acids for Oncologic Imaging. J. Nucl. Med. 2013, 54, 1007–1010. [Google Scholar] [CrossRef]
- McConathy, J.; Goodman Mark, M. Non-natural amino acids for tumor imaging using positron emission tomography and single photon emission computed tomography. Cancer Metastasis Rev. 2008, 27, 555–573. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Li, C.; Li, Z.; Chen, H.; Zhang, L.; Liang, Y.; Wu, Z. Evaluation of (2S,4S)-4-[18F]FEBGln as a Positron Emission Tomography Tracer for Tumor Imaging. Mol. Pharm. 2023, 20, 5195–5205. [Google Scholar] [CrossRef]
- Baek, S.; Mueller, A.; Lim, Y.S.; Lee, H.C.; Lee, Y.J.; Gong, G.; Kim, J.S.; Ryu, J.-S.; Oh, S.J.; Lee, S.J.; et al. (4S)-4-(3-18F-fluoropropyl)-L-glutamate for imaging of xC transporter activity in hepatocellular carcinoma using PET: Preclinical and exploratory clinical studies. J. Nucl. Med. 2013, 54, 117–123. [Google Scholar] [CrossRef]
- Bouhlel, A.; Zhou, D.; Li, A.; Yuan, L.; Rich, K.M.; McConathy, J. Synthesis, Radiolabeling, and Biological Evaluation of (R)- and (S)-2-Amino-5-[18F]fluoro-2-methylpentanoic Acid ((R)-, (S)-[18F]FAMPe) as Potential Positron Emission Tomography Tracers for Brain Tumors. J. Med. Chem. 2015, 58, 3817–3829. [Google Scholar] [CrossRef]
- Chiotellis, A.; Muller Herde, A.; Rossler, S.L.; Brekalo, A.; Gedeonova, E.; Mu, L.; Keller, C.; Schibli, R.; Krämer, S.D.; Ametamey, S.M. Synthesis, Radiolabeling, and Biological Evaluation of 5-Hydroxy-2-[18F]fluoroalkyl-tryptophan Analogues as Potential PET Radiotracers for Tumor Imaging. J. Med. Chem. 2016, 59, 5324–5340. [Google Scholar] [CrossRef] [PubMed]
- Minn, H.; Kauhanen, S.; Seppanen, M.; Nuutila, P. 18F-FDOPA: A multiple-target molecule. J. Nucl. Med. 2009, 50, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Sai, K.K.; Huang, C.; Yuan, L.; Zhou, D.; Piwnica-Worms, D.; Garbow, J.R.; Engelbach, J.A.; Mach, R.H.; Rich, K.M.; McConathy, J. 18F-AFETP, 18F-FET, and 18F-FDG imaging of mouse DBT gliomas. J. Nucl. Med. 2013, 54, 1120–1126. [Google Scholar]
- Morris, S.M., Jr. Arginine Metabolism Revisited. J. Nutr. 2016, 146, 2579S–2586S. [Google Scholar] [CrossRef]
- Chantranupong, L.; Scaria, S.M.; Saxton, R.A.; Gygi, M.P.; Shen, K.; Wyant, G.A.; Wang, T.; Harper, J.W.; Gygi, S.P.; Sabatini, D.M. The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway. Cell 2016, 165, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Hatzoglou, M.; Fernandez, J.; Yaman, I.; Closs, E. Regulation of cationic amino acid transport: The story of the CAT-1 transporter. Annu. Rev. Nutr. 2004, 24, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chung, S.-F.; Tam, S.-Y.; Leung, Y.-C.; Guan, X. Arginine deprivation as a strategy for cancer therapy: An insight into drug design and drug combination. Cancer Lett. 2021, 502, 58–70. [Google Scholar] [CrossRef]
- Fung, M.K.L.; Chan, G.C.-F. Drug-induced amino acid deprivation as strategy for cancer therapy. J. Hematol. Oncol. 2017, 10, 144. [Google Scholar] [CrossRef]
- Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; Clark, M.A. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002, 62, 5443–5450. [Google Scholar]
- Morais, M.; Ferreira, V.F.C.; Figueira, F.; Mendes, F.; Raposinho, P.; Santos, I.; Oliveira, B.L.; Correia, J.D.G. Technetium-99m complexes of l-arginine derivatives for targeting amino acid transporters. Dalton Trans. 2017, 46, 14537–14547. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Zhu, S.; Hu, K.; Yao, S.; Tang, C.; Yang, C.; Wang, Y.; Li, J.; Pan, X.; et al. Semi-automated radiosynthesis of 18F-labeled l-arginine derivative as a potential PET tracer for lung cancer imaging. J. Radioanal. Nucl. Chem. 2016, 309, 1257–1264. [Google Scholar] [CrossRef]
- Wu, R.; Liu, S.; Liu, Y.; Sun, Y.; Cheng, X.; Huang, Y.; Yang, Z.; Wu, Z. Synthesis and biological evaluation of [18F](2S,4S)4-(3-fluoropropyl) arginine as a tumor imaging agent. Eur. J. Med. Chem. 2019, 183, 111730. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Wang, M.; Li, C.; Zheng, W.; Chen, H.; Liang, Y.; Wu, Z. Optimization of Precursor Synthesis Conditions of (2S,4S)4-[18F]FPArg and Its Application in Glioma Imaging. Pharmaceuticals 2022, 15, 946. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, S.; Wu, R.; Zhang, L.; Zhang, Y.; Hong, H.; Zhang, A.; Xiao, H.; Liu, Y.; Wu, Z.; et al. Synthesis and preliminary evaluation of a novel glutamine derivative: (2S,4S)4-[18F]FEBGln. Bioorganic Med. Chem. Lett. 2019, 29, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Huang, Y.; Chen, H.; Jiang, Z.; Yu, Z.; Yang, T.; Zhang, L.; Cheng, X.; Liu, Y.; Liu, Q.; et al. Synthesis and In Vitro and In Vivo Evaluation of 18F-Labeled Positron Emission Tomography Tracers for Imaging Aβ Plaques. ACS Chem. Neurosci. 2023, 14, 988–1003. [Google Scholar] [CrossRef] [PubMed]
- Mirrione, M.M.; Schiffer, W.K.; Fowler, J.S.; Alexoff, D.L.; Dewey, S.L.; Tsirka, S.E. A novel approach for imaging brain–behavior relationships in mice reveals unexpected metabolic patterns during seizures in the absence of tissue plasminogen activator. NeuroImage 2007, 38, 34–42. [Google Scholar] [CrossRef] [PubMed]
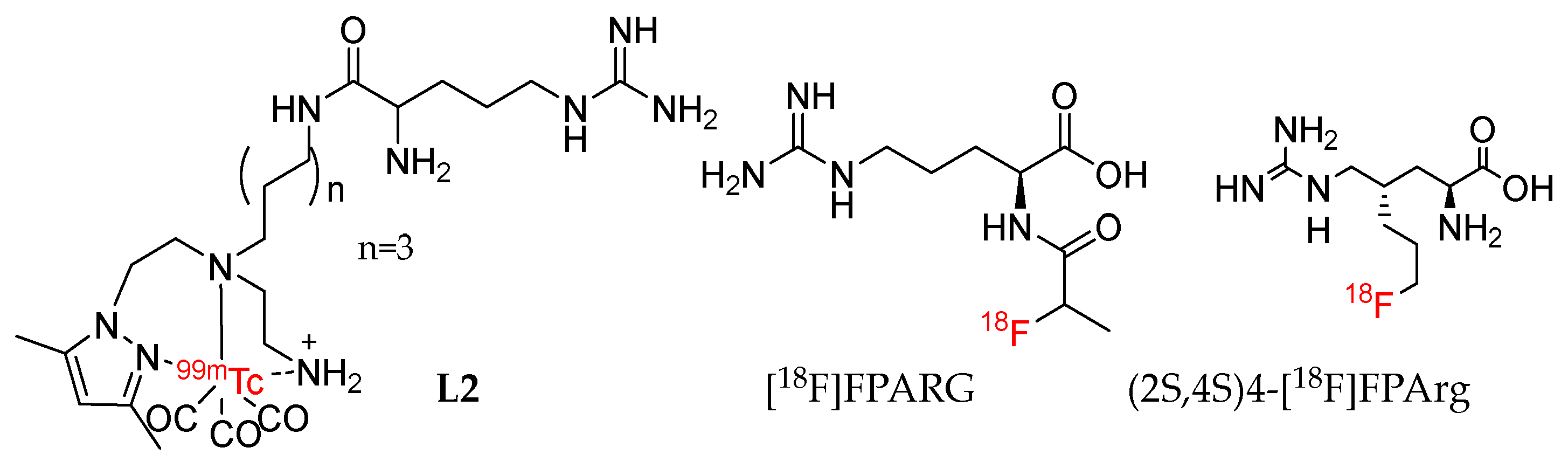
| Tracer | Organic Synthesis | Radiolabeling | Evaluation |
|---|---|---|---|
| (2S,4S)4-[18F]F PArg [32,33] | 10 step, Configuration determination | 2 step, yield < 6% | Targeting CAT transporters; PET imaging, MCF-7 (T/M = 6) and U87MG (T/M = 6) subcutaneous tumor, U87MG (T/B =1.5) and HS683 (T/B = 6.5) intracranial tumor, at 60 min p.i. |
| [18F]7 | 10 step, Configuration determination | 2 step, yield = 8% | Targeting CAT, ASC, ASC2 transporters, PET imaging, MCF-7 Tumor (T/M = 4), intracranial tumor (T/B = 2) at 60 min p.i. |
| L2 [30] | 3 step, Configuration determination | 1 step, yield = 100% | Targeting y+ transporters, no PET Imaging |
| [18F]FPARG [31] | 4 step, Configuration undetermined | 5 step, yield = 15 ± 3% | No testing of transport mechanism, PET imaging, NCI-H460 Tumor (T/M = 2) at 60 min p.i. |
| Synthesis | Radiolabeling | Evaluation | ||
|---|---|---|---|---|
| In Vitro | In Vivo | |||
| Compound 1–7 | Two-step radiolabeling method follow | Partition Coefficient | PET-CT imaging compared with (2S,4S)4-[18F]FPArg | MCF-7 subcutaneous tumor |
| Stability in PBS and Plasma | U87MG intracranial tumors | |||
| Cell uptake | Biodistribution | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, C.; Li, Z.; Xie, Y.; Chen, H.; Li, S.; Liang, Y.; Wu, Z. Design, Synthesis, and Biological Evaluation of a Novel [18F]-Labeled Arginine Derivative for Tumor Imaging. Pharmaceuticals 2023, 16, 1477. https://doi.org/10.3390/ph16101477
Huang Y, Li C, Li Z, Xie Y, Chen H, Li S, Liang Y, Wu Z. Design, Synthesis, and Biological Evaluation of a Novel [18F]-Labeled Arginine Derivative for Tumor Imaging. Pharmaceuticals. 2023; 16(10):1477. https://doi.org/10.3390/ph16101477
Chicago/Turabian StyleHuang, Yong, Chengze Li, Zhongjing Li, Yi Xie, Hualong Chen, Shengli Li, Ying Liang, and Zehui Wu. 2023. "Design, Synthesis, and Biological Evaluation of a Novel [18F]-Labeled Arginine Derivative for Tumor Imaging" Pharmaceuticals 16, no. 10: 1477. https://doi.org/10.3390/ph16101477
APA StyleHuang, Y., Li, C., Li, Z., Xie, Y., Chen, H., Li, S., Liang, Y., & Wu, Z. (2023). Design, Synthesis, and Biological Evaluation of a Novel [18F]-Labeled Arginine Derivative for Tumor Imaging. Pharmaceuticals, 16(10), 1477. https://doi.org/10.3390/ph16101477