Abstract
Breast cancer is the most commonly diagnosed cancer in women. Resveratrol, a naturally occurring phytochemical, shows great promise in developing novel anti-cancer therapies. This study hypothesized that the mitochondria-targeted delivery of resveratrol would increase its potency and induce mitochondria-mediated apoptosis. The targeted delivery of resveratrol was achieved by conjugating resveratrol to triphenylphosphonium (TPP). The anti-cancer effects of TPP-resveratrol were studied in the murine breast cancer 4T1 and the human breast cancer MDA-MB-231 cell lines. Flow cytometry was used to study apoptosis induction, cell cycle arrest, and mitochondrial membrane potential loss. The morphological changes in the mitochondria in MDA-MB-231 cells after TPP-resveratrol treatments were examined using transmission electron microscopy. Moreover, the changes in MDA-MB-231 cell metabolism after resveratrol and TPP-resveratrol treatments were studied using metabolomic analysis. We demonstrate that TPP-resveratrol significantly improved cytotoxicity in 4T1 cells and MDA-MB-231 cells by inducing apoptosis and mitochondrial membrane potential loss. Swollen and vacuolated mitochondria were observed after the TPP-resveratrol treatment. Meanwhile, TPP-resveratrol treatment down-regulated amino acid and energy metabolism and caused the dysfunction of purine and pyrimidine metabolism. Our results provide evidence supporting the targeted delivery of resveratrol to mitochondria and suggest that TPP-resveratrol may be an effective agent for breast cancer treatment.
1. Introduction
Breast cancer is one of the most life-threatening diseases with a high mortality rate among women [1]. Traditional breast cancer therapies, including surgery, chemotherapy, and radiotherapy, have been used to treat a variety of cancers and improve patients’ survival rates [2]. Although these treatments have been successful to some extent, they are not sufficient to eliminate all cancer cells and are associated with multiple adverse effects. Surgery, for example, does not prevent tumor recurrence and is usually followed by adjuvant therapy to reduce the risk of metastasis [3]. In addition, chemotherapy and radiotherapy treatments are often limited by cytotoxicity, multi-drug resistance, and poor selectivity [4]. Therefore, the development of novel, effective alternatives is imperative to reduce the side effects and improve the therapeutic efficacy of current breast cancer therapies.
In recent years, organelle-targeted drug delivery systems have attracted increasing attention as a means to reduce toxicity, lower drug dosages, improve therapeutic efficiency, and prevent drug resistance in cancer patients [5,6,7]. Mitochondria are crucial subcellular organelles that play an important role in cellular energy production, metabolism, and signaling. It has emerged as one of the most important targets for new drug design in cancer treatment [8,9]. Mitochondrial targeting can be classified into three types: lipophilic cation moieties, penetrating peptides, and mitochondrial targeting signal proteins [10]. One of the most widely used lipophilic cations is triphenylphosphonium (TPP). TPP conjugated with drugs, such as doxorubicin, coenzyme Q10, and curcumin, has been suggested as an alternative approach to cancer therapy [11,12,13].
Resveratrol, a natural polyphenolic phytoalexin, exhibits promising anti-cancer properties [14]. The anti-cancer effect may be due to apoptosis induction through the mitochondrial pathway. Resveratrol is also an inhibitor of mitochondrial complex III [9]. It could inhibit the activity of the adenine nucleotide transporter and decrease mitochondrial oxygen consumption [15]. Lucia Biasutto reported that TPP-resveratrol conjugates could significantly increase the cytotoxicity of colon cancer cells [16]. However, the specific mechanism of the TPP-resveratrol complex in cancer treatment remains poorly understood and needs further investigation.
Currently, metabolomics provides a promising analytical tool for identifying biomarkers and metabolic pathways altered in cancer and allows an in-depth investigation of the mechanisms associated with medical exposures [17]. It has been successfully applied to evaluate the effects of various drugs on breast cancer cells, lung adenocarcinoma cells, hepatocellular carcinoma cells, and prostate cancer cells [18,19,20,21]. At present, nuclear magnetic resonance (NMR), gas chromatography (GC), and liquid chromatography (LC) coupled with mass spectrometry (MS) are the most commonly used methods for metabolite detection [22]. In contrast to the other approaches, LC/MS offers higher efficiency in metabolic profiling, which is important for untargeted metabolomic research [19]. Therefore, in this study, we used LC/MS-based global metabolic profiling to investigate the effects of resveratrol and TPP-resveratrol on breast cancer cells.
2. Results
2.1. Cytotoxicity
To evaluate the anti-cancer effects of resveratrol and TPP-resveratrol, murine 4T1 and human MDA-MB-231 breast cancer cell line models were used. In the 4T1 cell cells, the IC50 values of resveratrol and TPP-resveratrol were 21.067 ± 3.7 and 16.216 ± 1.85 µM, respectively. The IC50s of resveratrol and TPP-resveratrol on the MDA-MB-231 cells were 29.97 ± 1.25 and 11.82 ± 1.46 µM, respectively. TPP did not show significant cytotoxicity to 4T1 and MDA-MB-231. Compared with resveratrol, the TPP-resveratrol conjugate significantly enhanced the anti-cancer effect of resveratrol (p < 0.05).
2.2. Mitochondrial Membrane Potential Loss
Mitochondrial membrane potential loss was assessed by flow cytometry. Rhodamine 123 (Rh-123) could penetrate the cell membrane and accumulate in the mitochondria of living cells, emitting yellow-green fluorescence. In this study, 4T1 and MDA-MB-231 retained 92.73 ± 0.28% and 95.56 ± 0.05% fluorescence, respectively. When 4T1 and MDA-MB-231 cells were treated with 50 µM resveratrol for 6 h, the fluorescence decreased to 13.46 ± 0.55% and 5.78 ± 0.04%, respectively. After the TPP-resveratrol treatment (50 µM), the fluorescence was reduced to 40.33 ± 0.38% and 19.33 ± 0.25% in the 4T1 and MDA-MB-231 cell lines, respectively (Figure 1).
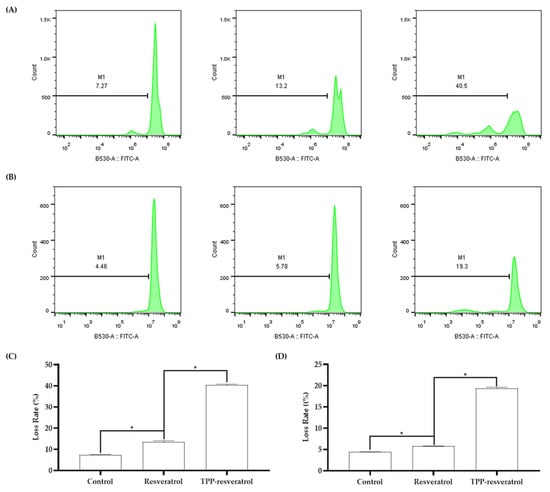
Figure 1.
Representative flow cytometry histograms of 4T1 and MDA-MB-231 mitochondrial membrane potential loss after 50 μM resveratrol or TPP-resveratrol treatment. (A) The treatments induced membrane potential loss in 4T1 cells. (B) Membrane potential loss in MDA-MB-231 cells after the treatments. (C) Statistical analysis of the potential loss in 4T1 cells. (D) Statistical analysis of the potential loss in MDA-MB-231 cells (* p < 0.05).
2.3. Cell Apoptosis Induction
Apoptosis induction was studied using an Annexin V-FITC Apoptosis Detection Kit via flow cytometry. Propidium iodide (PI) is a nucleic acid dye that can only penetrate the membrane of late apoptotic and dead cells. Annexin V and PI were combined to distinguish late apoptotic cells from dead cells [23]. After the 4T1 cells were treated with 50 µM resveratrol or TPP-resveratrol, the total apoptotic cells were 16.6 ± 0.47% and 36.6 ± 0.45%, respectively. In the MDA-MB-231 cell line, the apoptotic cell populations of resveratrol and TPP-resveratrol treatments were 10.4 ± 0.27% and 23.6 ± 0.62%, respectively (Figure 2).
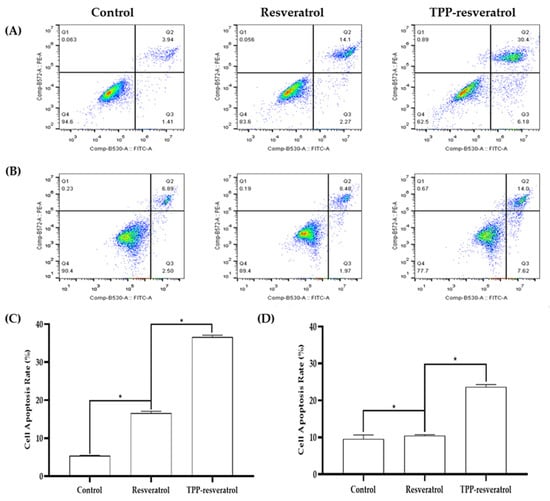
Figure 2.
Representative results of apoptosis for resveratrol and TPP-resveratrol treatments of 4T1 and MDA-MB-231 breast cancer cells. Resveratrol and TPP-resveratrol treatments induced 4T1 (A) and MDA-MB-231 (B) cell apoptosis. Statistical analysis of apoptosis in 4T1 cancer cells (C) and MDA-MB-231 cancer cells (D) by flow cytometry (* p < 0.05).
2.4. Cell Cycle Arrest
Cell cycle arrest was analyzed using flow cytometry to determine if resveratrol or TPP-resveratrol induced apoptosis-altered cell cycle distribution. The fluorescent dye PI could enter cells with damaged membranes and bind to their DNA. This strategy allowed the proportion of cells at each stage to be assessed according to their DNA content. In 4T1 cells, resveratrol arrested the cell cycle in the G1 phase (47.69%) and TPP-resveratrol arrested the cell cycle in the G1 (38.94%) and G2 (19.29%) phases. On the other hand, MDA-MB-231 cells were arrested in the G1 phase after resveratrol and TPP-resveratrol treatments (Figure 3).
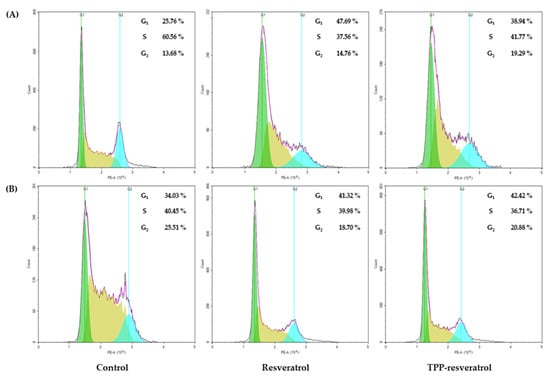
Figure 3.
Representative results of cell cycle analysis for the resveratrol and TPP-resveratrol treatments of 4T1 and MDA-MB-231 breast cancer cells. (A) Both resveratrol and TPP-resveratrol induced 4T1 cell cycle arrest in the G1 phase. (B) The cell cycle was arrested in the G1 phase following resveratrol or TPP-resveratrol treatment in MDA-MB-231 breast cancer cells.
2.5. Mitochondrial Morphology
Transmission electron microscopy (TEM) was used to evaluate the morphological changes in mitochondria after resveratrol and TPP-resveratrol treatments. In MDA-MB-231 cells, mitochondrial cristae were partly lost after resveratrol treatment. Furthermore, after TPP-resveratrol treatment, swollen and vacuolated mitochondria were observed (Figure 4).
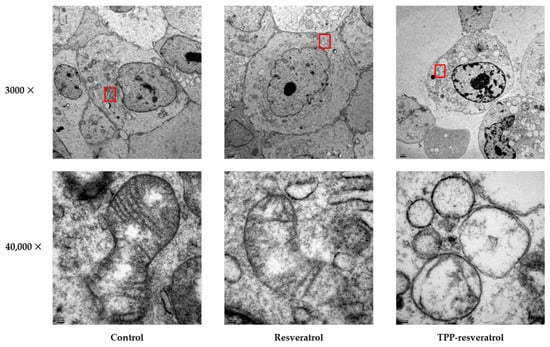
Figure 4.
Transmission electron microscopy of MDA-MB-231 cancer cells treated with resveratrol or TPP-resveratrol at 50 μM. The selected higher-magnification regions are labeled in red squares.
2.6. Metabolic Profiling Using Multivariate Analysis
The effects of resveratrol and the TPP-resveratrol conjugate on the human breast cancer MDA-MB-231 cells were analyzed using a metabolomics approach. The Pearson correlation coefficients between the quality control (QC) samples were calculated to assure data reliability [24]. As shown in Figure 5, the values were close to 1 in both the positive- and negative-ion modes, demonstrating that the obtained data were reliable. An unsupervised multivariate analysis method, principal component analysis (PCA), was carried out to obtain an overview of the metabolic pattern changes. As shown in Figure 5C,D, the metabolic patterns in the control, resveratrol, and TPP-resveratrol groups were primarily separated based on intracellular metabolites in both the positive and negative modes.
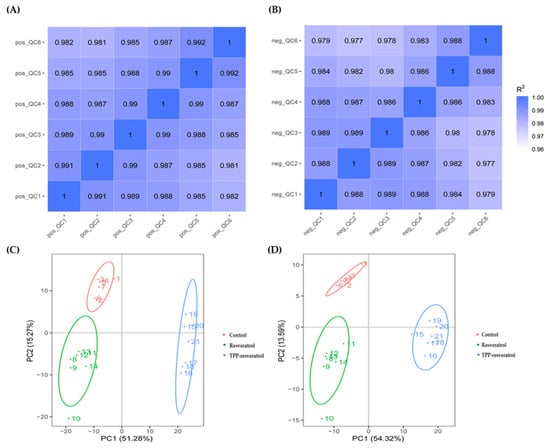
Figure 5.
Pearson correlation coefficients of the quality control (QC) samples in the positive-ion mode (A) and the negative-ion mode (B). PCA score plots of the positive-ion mode (C) and the negative-ion mode (D).
Orthogonal projections to latent structures–discriminant analysis (OPLS-DA) models were established to investigate the metabolite profiles in different groups (Figure 6 and Figure 7). Figure 6A,D and Figure 7A,D showed the score plots between the control/resveratrol groups and control/TPP-resveratrol groups in the positive- and negative-ion modes. The results indicated that the cancer cell metabolites were significantly altered after resveratrol and TPP-resveratrol treatments. Meanwhile, the permutation test was used to evaluate the OPLS-DA models’ fitting abilities and prediction abilities (Figure 6B,E and Figure 7B,E). The related parameters showed that R2 was higher than Q2, and the regression line of the Q2 points intersected the vertical at below zero, demonstrating that the models did not over-fit [19]. These results in our study followed the above standards, indicating that the OPLS-DA models were reliable.
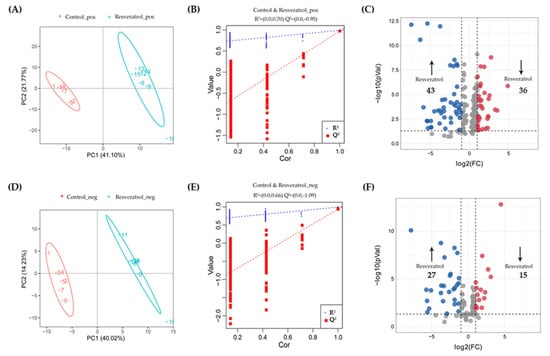
Figure 6.
Differential metabolites selection in the MDA-MB-231 cells after resveratrol treatment. Score plots of OPLS-DA and permutation tests in the positive-ion mode (A,B) and negative-ion mode (D,E). The OPLS-DA models’ statistical parameters are as follows: (A) R2Y = 0.99, Q2Y = 0.98 and (D) R2Y = 0.99, Q2Y = 0.94. Volcano plots of resveratrol treatments compared with controls in the positive-ion mode (C) and negative-ion mode (F).
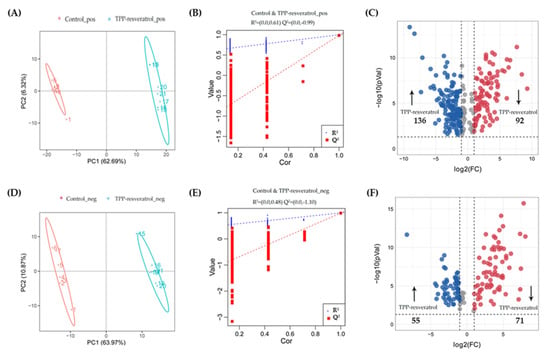
Figure 7.
Differential metabolites selection in the MDA-MB-231 cells after TPP-resveratrol treatment. Score plots of OPLS-DA and permutation tests in the positive-ion mode (A,B) and negative-ion mode (D,E). The OPLS-DA models’ statistical parameters are as follows: (A) R2Y = 1 Q2Y = 0.98 and (D) R2Y = 1 Q2Y = 0.99. Volcano plots of TPP-resveratrol treatments compared with controls in the positive-ion mode (C) and negative-ion mode (F).
2.7. Identification of Significantly Altered Metabolites
The criteria for identifying differential metabolites were as follows: VIP > 1, p-value < 0.05, and fold change >2 or <0.5. Among the differential metabolites in MDA-MB-231 cells, 70 for resveratrol and 191 for TPP-resveratrol treatment were significantly higher than those in the control group, while 51 for resveratrol and 163 for TPP-resveratrol treatment were significantly lower (Figure 6C,F and Figure 7C,F). This demonstrates that TPP-resveratrol treatment had a more pronounced effect on cellular metabolism than the resveratrol group.
The significantly altered metabolites included glycerophospholipids, organoheterocyclic compounds, nucleosides, nucleotides and analogs, organic acids and derivatives, organic oxygen compounds, lipids and lipid-like molecules, phenylpropanoids and polyketides, benzenoids, and organic nitrogen compounds (Figure 8A,B). In the Venn diagram analysis, 25 features were identified as common to both groups, while 329 and 96 were unique features to the TPP-resveratrol and resveratrol groups, respectively (Figure 8C). In addition, these differential metabolites were selected for further analysis.
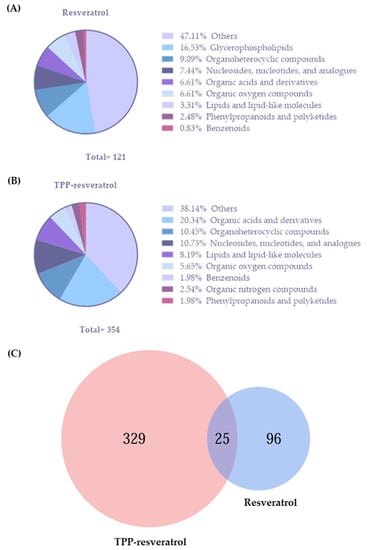
Figure 8.
Classes of metabolites identified in the resveratrol group (A) and the TPP-resveratrol group (B). (C) Venn diagram showing the common and unique features between the resveratrol and TPP-resveratrol treatments.
The predominant compounds detected in the resveratrol and TPP-resveratrol groups were glycerophospholipids (16.53%, Figure 8A) and organic acids and derivatives (20.34%, Figure 8B), respectively. The up-regulated glycerophospholipids in the MDA-MB-231 cells are listed in Table 1. Table 2 shows biologically relevant metabolites and pathways that changed significantly in the cells after treatment with resveratrol and TPP-resveratrol. Among them, 11 metabolites belonging to organic oxygen compounds, organic acids and their derivatives, organoheterocyclic compounds, nucleosides, nucleotides and analogs, and organoheterocyclic compounds were increased. Thirty-eight metabolites associated with organic acids and derivatives, nucleosides, nucleotides and analogs, organic nitrogen compounds, and organoheterocyclic compounds were decreased compared with the control group.

Table 1.
Significantly altered glycerophospholipids in the MDA-MB-231 cells after resveratrol treatment.

Table 2.
Biologically relevant metabolites and pathways that changed significantly in the cells after resveratrol and TPP-resveratrol treatments.
2.8. Perturbed Metabolic Pathways Caused by Resveratrol and TPP-Resveratrol Exposure
A comprehensive metabolic network analysis was performed using MetabolAnalyst 5.0, revealing the most relevant pathways affected by resveratrol and TPP-resveratrol. Compared with the control group, alanine, aspartate and glutamate metabolism, purine metabolism, arginine biosynthesis, pyrimidine metabolism, arginine and proline metabolism, and nicotinate and nicotinamide metabolism were the significantly altered pathways in the TPP-resveratrol group (Figure 9A). In the resveratrol group, the impact value was low, even though the most significantly altered pathway was the pentose phosphate pathway (Figure 9B). Information regarding the relevant metabolites in these pathways and the tricarboxylic acid cycle (TCA cycle) is shown in Table 2.
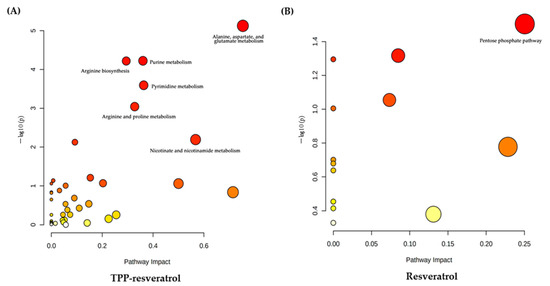
Figure 9.
Summary of pathway analysis using MetaboAnalyst 5.0. (A) TPP-resveratrol treatment group. (B) Resveratrol treatment group.
3. Discussion
Previous studies have shown that resveratrol has an anti-cancer effect against breast cancer [25,26]. Our study formulated a mitochondria-targeted TPP-resveratrol conjugate and studied its efficacy on 4T1 and MDA-MB-231 cell lines. We showed that the conjugate could enhance the anti-cancer effect of resveratrol. In cytotoxicity assays, TPP-resveratrol reduced the IC50 of human MDA-MB-231 cells by nearly three-fold. The IC50 of murine 4T1 cells was also significantly reduced by the TPP-resveratrol formulation. These results suggest that the therapeutic effects of TPP-resveratrol at lower doses are comparable to those of resveratrol at higher levels.
Mitochondria, the sites for producing adenosine triphosphate (ATP), play important roles in regulating important signal transduction and metabolic processes associated with calcium homeostasis, reduction–oxidation (redox) potentials, and steroid hormone biosynthesis [27]. The mitochondrial dysfunction induced by growth factor changes, DNA damage, calcium overload, and cytoskeleton alterations would disrupt the mitochondrial homeostasis and induce type I programmed cell death (mitochondria-mediated apoptosis) [28,29,30]. Our results showed a high apoptotic rate by flow cytometry in these two cell lines after treatment with resveratrol or TPP-resveratrol. We also evaluated mitochondrial functioning via changes in the mitochondrial membrane potential. The results were consistent with those of apoptosis analysis. Moreover, we observed significant morphological changes in mitochondria after treatment with TPP-resveratrol. Therefore, TPP-resveratrol exhibited mitochondrial targeting and higher anti-cancer activity. Previous studies showed that resveratrol could arrest MDA-MB-231 cells in the G1 phase. These results are consistent with our data and indicate that the same signaling pathways were regulated [31,32].
Resveratrol has the ability to alter membrane lipids [33]. It has been reported that resveratrol-mediated lipidomic alterations can significantly affect glycerophospholipid metabolism [34]. Luciana Gomes found that phosphatidylcholine (PC) and phosphatidylethanolamine (PE) were decreased in MDA-MB-231 cells after resveratrol treatment [35]. Our study observed an increasing tendency in glycerophospholipids, including lysophosphatidylcholine (LPC) and lysophosphatidylethanolamine (LPE), as shown in Table 1. In apoptotic cells, the progressive degradation of PC is likely to result in LPC generation [36]. Interestingly, TPP-resveratrol exerted a greater impact on MDA-MB-231 cells’ metabolism than resveratrol, which had fundamentally different metabolic phenotypes. TPP, as a lipophilic cation, has been widely used in mitochondrial-targeting cancer therapeutics [37,38]. A recent study reported that resveratrol-encapsulated TPP liposomes enhanced the anti-cancer efficacy and mitochondrial depolarization in B16F10 cells [39]. However, the actual effect of TPP-resveratrol on the metabolism of breast cancer cells is still unclear. Cell metabolomics based on LC-MS/MS were used to investigate the metabolic mechanism of MDA-MB-231 cells after resveratrol or TPP-resveratrol treatment. The results indicated that TPP-resveratrol-induced apoptosis perturbed the amino acid and base metabolic networks the most.
In addition to providing substrates for the tricarboxylic acid (TCA) cycle, amino acids contribute to a broad array of processes essential for cell proliferation [40]. In the TPP-resveratrol treatment group, alterations in 7 metabolites involved in arginine biosynthesis and 10 metabolites involved in arginine and proline metabolism were observed. According to Table 2, the contents of the majority of metabolites in these pathways decreased, except for ornithine, which showed the opposite trend compared with the control group. Ornithine is a non-essential amino acid synthesized in the cytosol and transported into the mitochondria to perform its biological function [41]. It is produced by the enzymatic action of arginase on arginine and is a crucial substrate for proline synthesis [42]. It was revealed that TPP-resveratrol treatment decreased ornithine utilization and blocked the synthesis of arginine and proline. In terms of alanine, aspartate, and glutamate metabolism, the levels of 11 metabolites were decreased in the TPP-resveratrol group (Table 2). The biosynthesis of amino acids, such as aspartate and glutamate, is an important intermediate of the TCA cycle in the mitochondria [43]. The conjugation of a TPP group to various small molecules can facilitate mitochondrial targeting [44]. Our study also observed a significant perturbation of the TCA cycle in the TPP-resveratrol-treated cells. As shown in Table 2, the contents of TCA cycle-related metabolites (succinic acid, cis-aconitic acid, fumaric acid, citric acid, and malic acid) were decreased compared with those in the control group. Furthermore, a downward trend was observed in nicotinate and nicotinamide metabolism, which are also closely related to energy metabolism [45]. In this pathway, the levels of four metabolites, nicotinamide adenine dinucleotide (NAD), nicotinamide, 1-Methylnicotinamide, and nicotinamide adenine dinucleotide phosphate (NADP), decreased, as shown in Table 2, revealing that energy metabolism was down-regulated after TPP-resveratrol exposure.
Purine and pyrimidine metabolism are critical metabolic pathways in cell development, proliferation, and repair [46]. In this study, among the purine-related metabolites, the levels of 10 metabolites (guanosine 5′-diphosphate, l-glutamine, adenosine 3′5′-cyclic monophosphate, adenosine diphosphate, adenosine 5′-monophosphate, adenylosuccinate acid, inosine-5′-monophosphate, guanosine monophosphate, inosine, and adenine) were decreased while those of 5 metabolites (xanthine, deoxyinosine, guanine, deoxyguanosine, and guanosine) were increased (Table 2). In pyrimidine metabolism, the up-regulated metabolites included uridine monophosphate (UMP), cytidine-5′-monophosphate (CMP), and uracil, and the down-regulated metabolites included uridine diphosphate (UDP), L-glutamine, uridine, cytidine-5′-diphosphate (CDP), cytidine, dCDP, and dCMP. These results suggest that TPP-resveratrol exposure disrupted the homeostasis of purine and pyrimidine metabolism in breast cancer cells. The intermediate metabolites of these pathways are the crucial materials for the cell cycle, cell energy metabolism, and signal transduction [47,48,49]. Cancer cells with high proliferation and progression rates result in continuous high demand for energy and nutrients [50]. Therefore, our results demonstrate that TPP-resveratrol treatment might induce cell apoptosis by the down-regulation of amino acid and energy metabolism and the dysfunction of purine and pyrimidine metabolism.
4. Materials and Methods
4.1. Materials
RPMI-1640 medium and fetal bovine serum (FBS) were purchased from Corning (Shanghai, China). Penicillin–streptomycin was obtained from Gibco (Shanghai, China). Dojindo Molecular Technologies (Shanghai, China) provided the Cellstain® Rhodamine 123 (Rh-123), Annexin V-FITC Apoptosis Detection, and PI/RNase Staining kits. Cell Counting Kit-8 (CCK-8) was purchased from GlpBio (Shanghai, China). Breast cancer cell lines 4T1 and MDA-MB-231 were commercially purchased from ProCell Life Science & Technology (CL-007) (Wuhan, Hubei, China) and the Peking Union Medical College Cell Culture Center (1101HUM-PUMC000014) (Beijing, China), respectively.
4.2. Synthesis of TPP-Resveratrol
The synthesis of the TPP-resveratrol conjugate is shown in Scheme 1. Triphenylphosphine (2.623 g, 10 mL, 1.0 eq), 4-bromobutyric acid (1.670 g, 10 mmol, 1.0 eq), and 40 mL of acetonitrile were added to a 100 mL round-bottomed flask. The reaction was then allowed to continue for 48 h at 80 °C. Upon completion, the reaction mixture was filtered to obtain a solid, which was washed with 40 mL × 3 filtrate and dried under a vacuum at room temperature overnight to produce the desired compound, TPP-COOH (2.446 g, 70% yield). 1H NMR (300 MHz, DMSO) δ 12.50 (s, 1H), 7.79 (s, 6H), 7.67 (d, J = 1.9 Hz, 1H), 7.54 (d, J = 8.7 Hz, 1H), 6.88 (d, J = 8.6 Hz, 1H), 6.40 (s, 1H), 6.18 (s, 1H), 5.58 (d, J = 7.7 Hz, 4H), 2.55 (s, 2H), 1.74 (s, 2H), and 1.24 (s, 2H).
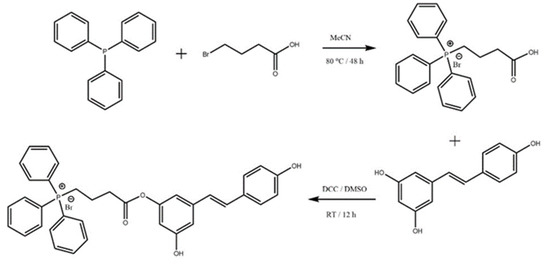
Scheme 1.
Synthesis of triphenylphosphonium–resveratrol conjugate.
To a 100 mL round-bottomed flask, TPP-COOH (0.856 g, 2 mmol, 1.0 eq), dicyclohexyl carbodiimide (0.619 g, 3 mmol, 1.5 eq), and 10 mL of dimethyl sulfoxide (DMSO) were added. Then, the reaction proceeded at room temperature for 2 h. The reaction liquid was filtered to remove the solid, and resveratrol (0.6847 g, 3 mmol, 1.5 eq) was added to the solution, followed by stirring at room temperature for 10 h. Upon completion, the reaction mixture was poured into 100 mL of ultrapure water to form a precipitate, which was filtered, washed with 40 mL of acetonitrile, and dried under a vacuum at room temperature overnight to produce the desired compound (0.9874 g, 77% yield). 1H NMR (300 MHz, DMSO) δ 9.21 (s, 1H), 8.25 (d, J = 7.7 Hz, 1H), 7.92–7.89 (m, 2H), 7.84–7.77 (m, 10H), 7.41 (s, 1H), 7.38 (s, 1H), 6.90 (s, 1H), 6.84 (s, 1H), 6.77 (s, 1H), 6.74 (s, 1H), 6.38 (d, J = 2.0 Hz, 2H), 6.11 (s, 1H), 5.59 (d, J = 8.0 Hz, 4H), 2.55 (s, 2H), 1.74 (s, 2H), and 1.24 (s, 2H).
4.3. Cytotoxicity
4T1 and MDA-MB-231 cells were cultured in RPMI-1640 medium containing 10% FBS and 1% penicillin–streptomycin. Both cell lines were maintained in a 37 °C incubator with 5% CO2. Cell Counting Kit-8 was used to test the cytotoxicity. 4T1 cells (2 × 103 cells/well) and MDA-MB-231 cells (8 × 103 cells/well) were seeded in 96-well plates overnight, followed by the addition of 10 μL of different concentrations of resveratrol (1, 5, 10, 50, 100, and 200 μM) and TPP-resveratrol (0.001, 0.01, 0.1, 1, 5, and 50 μM). After 48 h, 10 μL of CCK-8 solution was added to each well. Subsequently, the plate was incubated at 37 °C for 4 h. Finally, the optical density was measured at 450 nm and 650 nm using a microplate reader (VarioskanFlash-4.00.53, Thermo Scientific Inc., Shanghai, China).
4.4. Mitochondrial Membrane Potential Loss
A Cellstain® Rhodamine 123 (Rh-123) kit was used to study mitochondrial functioning in the cells. 4T1 cells (1 × 106 cells/well) and MDA-MB-231 cells (1 × 106 cells/well) were seeded in 6-well plates overnight. After resveratrol and TPP-resveratrol (both 50 μM) treatment for 6 h at 37 °C, the cells were collected (>106 cells) and washed. The cells were then resuspended in 1 mL of RPMI-1640 medium containing 20 μM of Rh-123 and incubated at 37 °C for 30 min. The cells were washed with phosphate buffer (PBS) once and analyzed by flow cytometry (NovoCyte 3000, Agilent; Software: Flowjo_V10, Novoexpress 1.5.0, Agilent, CA, USA).
4.5. Cell Apoptosis Induction
An Annexin V-FITC Apoptosis Detection Kit was used to examine the apoptotic induction of resveratrol and TPP-resveratrol (both 50 μM) in 4T1 and MDA-MB-231 cells. After 6 h of treatment, the cells collected by centrifugation (1000 rpm, 5 min) were washed in pre-cooled PBS and then suspended in 1 × Annexin V Binding Solution to prepare a cell suspension with a final concentration of 1 × 106 cells/mL. Later, 5 μL of Annexin V-FITC conjugate was added to the cell suspension, followed by 5 μL of PI solution. The mixture was incubated at room temperature for 15 min in the dark. Finally, 400 μL of 1 × Annexin V Binding Solution was added, and the cells were detected by flow cytometry.
4.6. Cell Cycle Arrest
Cell cycle analysis was performed using a PI/RNase staining kit. The cells were treated with resveratrol or TPP-resveratrol (both 50 μM). After 6 h of treatment, the cells (>106 cells/mL) were fixed with 70% ethanol at 4 °C for 2 h. Then, the cells were washed with 1 mL of PBS and centrifuged at 1000 rpm for 3 min. The supernatant was removed and 0.5 mL of working solution was added to the cell pellet. The mixture was then incubated for 30 min at 37 °C in the dark, followed by another 30 min at 4 °C. Finally, the cells were filtered through a nylon mesh to remove cell clumps from the sample. The distribution of cells in distinct cell cycle phases was determined by flow cytometry.
4.7. Transmission Electron Microscopy (TEM)
MDA-MB-231 cells were treated with resveratrol or TPP-resveratrol (both 50 μM) for 24 h and then collected in 2 mL micro-centrifuge tubes (>106 cells). After centrifugation at 3000 rpm for 10 min, the supernatant was discarded and a pre-cooled 2.5% glutaraldehyde solution was slowly added. The samples were stored at 4 °C for 12 h. Then, the supernatant was discarded and the samples were washed with PBS and fixed with a 1% osmic acid solution for 1–2 h. Subsequently, the osmic acid waste solution was carefully removed. The samples were washed with PBS and dehydrated with an ethanol solution with gradient concentrations (including 30%, 50%, 70%, 80%, 90%, and 95%). Finally, the samples were treated with 100% ethanol for 20 min. Next, the samples were treated with pure acetone for 20 min, a mixed solution of embedding medium and acetone (V/V = 1/1) for 1 h, a mixed solution of embedding medium and acetone (V/V = 3/1) for 3 h, and pure embedding medium overnight. The embedded samples were obtained by heating the infiltrated samples at 70 °C overnight. The samples were sectioned using a LEICA EM UC7 ultramicrotome to obtain 70–90 nm sections. The sliced samples were then stained with lead citrate solution and 50% uranyl acetate-saturated ethanol for 10 min. After drying, TEM images were captured using a HITACHI H-7650 electron microscope to observe the mitochondrial morphology.
4.8. Intracellular Metabolite Extraction
The methanol–chloroform–water extraction method was used to extract intracellular metabolites [51]. For intracellular samples, the cells were rapidly washed three times with 10 mL of ice-cold PBS buffer. The cells were then trypsinized and centrifuged at 2000 rpm for 10 min at 4 °C. The supernatant was discarded, and 10 mL of PBS was used to wash the cell mass. The cell suspension was centrifuged at 2000 rpm for 5 min at 4 °C. The supernatant was discarded and the cell mass was bounced. Then, 450 uL of ice-cold methanol/chloroform (2:1, v/v) was added and sonicated on ice for 30 min. After that, 450 uL of ice-cold chloroform/water (1:1, v/v) was added, vortexed for 1 min, and incubated on ice for 15 min. Finally, the mixture was centrifuged at 12,000 rpm for 20 min at 4 °C. The supernatant was lyophilized and stored at −80 °C until it was analyzed.
4.9. UHPLC-MS Analysis
Metabolite profiling was carried out using a Vanquish UHPLC system (ThermoFisher, Hannover, Germany) coupled with an Orbitrap Q Exactive TMHF-X mass spectrometer (ThermoFisher, Hannover, Germany). All chromatographic separations were performed using a Hypesil Gold Column (C18, 100 × 2.1 mm, 1.9 μm) with a flow rate of 0.2 mL/min. The mobile phase for the positive polarity mode consisted of 0.1% formic acid in water (A) and methanol (B). The negative polarity mode’s eluent A was 5 mM of ammonium acetate buffer (pH 9.0) and eluent B was methanol. The solvent gradient program was as follows: (i) 1.5 min with 2% B, (ii) 1.5 min with a linear gradient from 2% to 85% B, (iii) 7 min with a linear gradient from 85% to 100% B, and (iv) 2 min with 2% B. Separated metabolites were analyzed under both positive- and negative-ionization modes with a spray voltage of 3.5 kV, capillary temperature of 320 °C, sheath gas flow rate of 35 psi, aux gas flow rate of 10 L/min, S-lens RF level of 60, and aux gas heater temperature of 350 °C.
4.10. Data Analysis and Metabolite Identification
Raw LC-MS/MS data were processed using a Compound Discoverer 3.1 (CD3.1, ThermoFisher) for peak alignment, peak picking, and quantitation of each metabolite. The main parameters were set as follows: retention time tolerance of 0.2 min, actual mass tolerance of 5 ppm, signal intensity tolerance of 30%, signal–noise ratio of 3, and minimum intensity of 100,000. After that, the peak intensities were normalized and the data were used to predict the molecular formula based on additive ions, molecular ion peaks, and fragment ions. Then, the peaks were matched with the mzCloud (https://www.mzcloud.org/, accessed on 10 June 2022), mzVault, and MassList databases to obtain accurate qualitative and quantitative results. A pooled quality control (QC) sample was prepared by mixing aliquots of all samples to assess the performance of the LC-MS instrument. The coefficient of variability (CV) was calculated, and compounds with CVs greater than 30% in the QC samples were removed [52].
Metabolites were annotated using the online Kyoto Encyclopedia of Genes and Genomes (KEGG), the Human Metabolome Database (HMDB), and the Lipid Metabolites and Pathways Strategy (LIPID Maps). Multivariate analysis, including principal components analysis (PCA) and orthogonal projection to latent structures–discriminant analysis (OPLS-DA), was conducted by metaX. Seven-fold cross-validation was applied to assess the performance of OPLS-DA. The fitness of model (R2) and predictive ability (Q2) values were calculated to monitor the models’ robustnesses. The values of the two parameters being close to 1.0 indicated an acceptable model. Important metabolites responsible for the class separation between the compared groups in the OPLS-DA scores plot were identified by the variable importance in the projection (VIP) scores. Volcano plots were used to filter metabolites of interest based on the fold changes (log2) and p-values (−log10). Metabolic pathway analysis was carried out by using Metaboanalyst 5.0 (https://www.metaboanalyst.ca/, accessed on 1 July 2022).
5. Conclusions
In this study, a mitochondrial-targeted TPP-resveratrol was formulated. The results showed that TPP-resveratrol could trigger morphological changes in mitochondria and improve cytotoxicity by inducing apoptosis and mitochondrial membrane potential loss. Moreover, TPP-resveratrol could induce cell apoptosis by downregulating amino acid and energy metabolism, as well as by impairing purine and pyrimidine metabolism. Our results suggest that TPP-resveratrol may be an effective agent for breast cancer treatment.
Author Contributions
Conceptualization, Y.Z. and S.D.; methodology, Y.Z.; validation, H.Y.; formal analysis, L.J. and H.Y.; investigation, L.J., H.Y., C.W., F.H., Z.S., H.T. and N.N.; resources, Y.Z. and S.D.; data curation, Y.Z.; writing—original draft preparation, L.J., H.Y. and C.W.; writing—review and editing, Y.Z. and S.D.; visualization, L.J. and H.Y.; supervision, Y.Z. and S.D.; project administration, Y.Z. and S.D.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Department of Education of Zhejiang Province General Program, Y202250083; Wenzhou Municipal Science and Technology Research Program, Y20220901; and Wenzhou-Kean University Student Partnering with Faculty Research Program, WKUSPF202226. The APC was funded by the Wenzhou Municipal Key Lab of Applied Biomedical and Biopharmaceutical Informatics, Wenzhou-Kean University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- Nounou, M.I.; ElAmrawy, F.; Ahmed, N.; Abdelraouf, K.; Goda, S.; Syed-Sha-Qhattal, H. Breast Cancer: Conventional Diagnosis and Treatment Modalities and Recent Patents and Technologies. Breast Cancer Auckl. 2015, 9, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Dhankhar, R.; Vyas, S.P.; Jain, A.K.; Arora, S.; Rath, G.; Goyal, A.K. Advances in novel drug delivery strategies for breast cancer therapy. Artif. Cells. Blood Substit. Immobil. Biotechnol. 2010, 38, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Han, Y.H.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Subcellular Performance of Nanoparticles in Cancer Therapy. Int. J. Nanomed. 2020, 15, 675–704. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, Y.; Khan, A.R.; Ji, J.; Yu, A.; Zhai, G. A novel progress of drug delivery system for organelle targeting in tumour cells. J. Drug Target. 2021, 29, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Pan, W.; Li, N.; Tang, B. Boosting Cancer Therapy with Organelle-Targeted Nanomaterials. ACS Appl. Mater. Interfaces 2019, 11, 26529–26558. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Sun, Z.; Li, C.; Jiang, B. Recent progress on mitochondrial targeted cancer therapy based on inorganic nanomaterials. Mater. Today Chem. 2019, 12, 240–260. [Google Scholar] [CrossRef]
- Jeena, M.T.; Kim, S.; Jin, S.; Ryu, J.H. Recent Progress in Mitochondria-Targeted Drug and Drug-Free Agents for Cancer Therapy. Cancers 2019, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ning, N.; Meng, X.; Chittasupho, C.; Jiang, L.; Zhao, Y. Sequential Drug Delivery in Targeted Cancer Therapy. Pharmaceutics 2022, 14, 573. [Google Scholar] [CrossRef]
- Han, M.; Vakili, M.R.; Soleymani Abyaneh, H.; Molavi, O.; Lai, R.; Lavasanifar, A. Mitochondrial delivery of doxorubicin via triphenylphosphine modification for overcoming drug resistance in MDA-MB-435/DOX cells. Mol. Pharm. 2014, 11, 2640–2649. [Google Scholar] [CrossRef] [PubMed]
- Sena Ozbay, H.; Yabanoglu-Ciftci, S.; Baysal, I.; Gultekinoglu, M.; Can Eylem, C.; Ulubayram, K.; Nemutlu, E.; Topaloglu, R.; Ozaltin, F. Mitochondria-targeted CoQ10 loaded PLGA-b-PEG-TPP nanoparticles: Their effects on mitochondrial functions of COQ8B−/− HK-2 cells. Eur. J. Pharm. Biopharm. 2022, 173, 22–33. [Google Scholar] [CrossRef]
- Reddy, C.A.; Somepalli, V.; Golakoti, T.; Kanugula, A.K.; Karnewar, S.; Rajendiran, K.; Vasagiri, N.; Prabhakar, S.; Kuppusamy, P.; Kotamraju, S.; et al. Mitochondrial-targeted curcuminoids: A strategy to enhance bioavailability and anticancer efficacy of curcumin. PLoS ONE 2014, 9, e89351. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, K.; Cheng, L.; Yan, B.; Qian, W.; Cao, J.; Li, J.; Wu, E.; Ma, Q.; Yang, W. Resveratrol and cancer treatment: Updates. Ann. N. Y. Acad. Sci. 2017, 1403, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Wenzel, U.; Daniel, H.; Planas, J.M. Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J. Agric. Food Chem. 2008, 56, 4813–4818. [Google Scholar] [CrossRef]
- Biasutto, L.; Mattarei, A.; Marotta, E.; Bradaschia, A.; Sassi, N.; Garbisa, S.; Zoratti, M.; Paradisi, C. Development of mitochondria-targeted derivatives of resveratrol. Bioorg. Med. Chem. Lett. 2008, 18, 5594–5597. [Google Scholar] [CrossRef] [PubMed]
- Beger, R.D. A review of applications of metabolomics in cancer. Metabolites 2013, 3, 552–574. [Google Scholar] [CrossRef] [PubMed]
- Dahabiyeh, L.A.; Mahmoud, N.N.; Al-Natour, M.A.; Safo, L.; Kim, D.H.; Khalil, E.A.; Abu-Dahab, R. Phospholipid-Gold Nanorods Induce Energy Crisis in MCF-7 Cells: Cytotoxicity Evaluation Using LC-MS-Based Metabolomics Approach. Biomolecules 2021, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Guan, S.; Jin, Y.; Sun, W.; Wang, Q.; Du, Y.; Zhang, R. Cell metabolomics to study the cytotoxicity of carbon black nanoparticles on A549 cells using UHPLC-Q/TOF-MS and multivariate data analysis. Sci. Total Environ. 2020, 698, 134122. [Google Scholar] [CrossRef] [PubMed]
- Lindeque, J.Z.; Matthyser, A.; Mason, S.; Louw, R.; Taute, C.J.F. Metabolomics reveals the depletion of intracellular metabolites in HepG2 cells after treatment with gold nanoparticles. Nanotoxicology 2018, 12, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, A.H.; Liu, S.B.; Qiu, S.; Li, X.N.; Zhang, T.L.; Liu, L.; Wang, X.J. Cell metabolomics identify regulatory pathways and targets of magnoline against prostate cancer. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1102–1103, 143–151. [Google Scholar] [CrossRef]
- Lee, K.M.; Jeon, J.Y.; Lee, B.J.; Lee, H.; Choi, H.K. Application of Metabolomics to Quality Control of Natural Product Derived Medicines. Biomol. Ther. 2017, 25, 559–568. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087288. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Sui, J.; Zhang, J. Metabolomics reveals significant variations in metabolites and correlations regarding the maturation of walnuts (Juglans regia L.). Biol. Open 2016, 5, 829–836. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef]
- Chin, Y.T.; Hsieh, M.T.; Yang, S.H.; Tsai, P.W.; Wang, S.H.; Wang, C.C.; Lee, Y.S.; Cheng, G.Y.; HuangFu, W.C.; London, D.; et al. Anti-proliferative and gene expression actions of resveratrol in breast cancer cells in vitro. Oncotarget 2014, 5, 12891–12907. [Google Scholar] [CrossRef]
- Cho, H.; Cho, Y.Y.; Shim, M.S.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondria-targeted drug delivery in cancers. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165808. [Google Scholar] [CrossRef]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Marzetti, E. Cell Death and Inflammation: The Role of Mitochondria in Health and Disease. Cells 2021, 10, 537. [Google Scholar] [CrossRef]
- Gibellini, L.; Moro, L. Programmed Cell Death in Health and Disease. Cells 2021, 10, 1765. [Google Scholar] [CrossRef]
- Yang, M.F.; Yao, X.; Chen, L.M.; Gu, J.Y.; Yang, Z.H.; Chen, H.F.; Zheng, X.; Zheng, Z.T. Synthesis and biological evaluation of resveratrol derivatives with anti-breast cancer activity. Arch. Pharm. 2020, 353, e2000044. [Google Scholar] [CrossRef] [PubMed]
- Bartolacci, C.; Andreani, C.; Amici, A.; Marchini, C. Walking a Tightrope: A Perspective of Resveratrol Effects on Breast Cancer. Curr. Protein Pept. Sci. 2018, 19, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Brittes, J.; Lucio, M.; Nunes, C.; Lima, J.L.; Reis, S. Effects of resveratrol on membrane biophysical properties: Relevance for its pharmacological effects. Chem. Phys. Lipids 2010, 163, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, C.; Lin, C.; Zhang, L.; Zheng, H.; Zhou, Y.; Li, X.; Li, C.; Zhang, X.; Yang, X.; et al. Lipidomic Alterations and PPARalpha Activation Induced by Resveratrol Lead to Reduction in Lesion Size in Endometriosis Models. Oxid. Med. Cell Longev. 2021, 2021, 9979953. [Google Scholar] [CrossRef]
- Gomes, L.; Viana, L.; Silva, J.L.; Mermelstein, C.; Atella, G.; Fialho, E. Resveratrol Modifies Lipid Composition of Two Cancer Cell Lines. Biomed Res. Int. 2020, 2020, 5393041. [Google Scholar] [CrossRef]
- Fuchs, B.; Schiller, J.; Cross, M.A. Apoptosis-associated changes in the glycerophospholipid composition of hematopoietic progenitor cells monitored by 31P NMR spectroscopy and MALDI-TOF mass spectrometry. Chem. Phys. Lipids 2007, 150, 229–238. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Mallick, A.; More, P.; Ghosh, S.; Chippalkatti, R.; Chopade, B.A.; Lahiri, M.; Basu, S. Dual drug conjugated nanoparticle for simultaneous targeting of mitochondria and nucleus in cancer cells. ACS Appl. Mater. Interfaces 2015, 7, 7584–7598. [Google Scholar] [CrossRef]
- Kang, J.H.; Ko, Y.T. Enhanced Subcellular Trafficking of Resveratrol Using Mitochondriotropic Liposomes in Cancer Cells. Pharmaceutics 2019, 11, 423. [Google Scholar] [CrossRef]
- Lukey, M.J.; Katt, W.P.; Cerione, R.A. Targeting amino acid metabolism for cancer therapy. Drug Discov. Today 2017, 22, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Pita, A.M.; Fernandez-Bustos, A.; Rodes, M.; Arranz, J.A.; Fisac, C.; Virgili, N.; Soler, J.; Wakabayashi, Y. Orotic aciduria and plasma urea cycle-related amino acid alterations in short bowel syndrome, evoked by an arginine-free diet. J. Parenter. Enter. Nutr. 2004, 28, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugam, M.; Jaidev, J.; Umashankar, V.; Sulochana, K.N. Ornithine and its role in metabolic diseases: An appraisal. Biomed. Pharmacother. 2017, 86, 185–194. [Google Scholar] [CrossRef]
- Engskog, M.K.; Ersson, L.; Haglof, J.; Arvidsson, T.; Pettersson, C.; Brittebo, E. beta-N-Methylamino-L-alanine (BMAA) perturbs alanine, aspartate and glutamate metabolism pathways in human neuroblastoma cells as determined by metabolic profiling. Amino Acids 2017, 49, 905–919. [Google Scholar] [CrossRef]
- Biswas, S.; Dodwadkar, N.S.; Piroyan, A.; Torchilin, V.P. Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria. Biomaterials 2012, 33, 4773–4782. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, R.S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, S.; Aa, N.; Zhang, Y.; Zhang, R.; Xu, C.; Zhang, S.; Kong, X.; Wang, G.; Aa, J.; et al. Quantitative analysis of 20 purine and pyrimidine metabolites by HILIC-MS/MS in the serum and hippocampus of depressed mice. J. Pharm. Biomed. Anal. 2022, 219, 114886. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chmela, V.; Green, N.J.; Russell, D.A.; Janicki, M.J.; Gora, R.W.; Szabla, R.; Bond, A.D.; Sutherland, J.D. Selective prebiotic formation of RNA pyrimidine and DNA purine nucleosides. Nature 2020, 582, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Oizel, K.; Tait-Mulder, J.; Fernandez-de-Cossio-Diaz, J.; Pietzke, M.; Brunton, H.; Lilla, S.; Dhayade, S.; Athineos, D.; Blanco, G.R.; Sumpton, D.; et al. Formate induces a metabolic switch in nucleotide and energy metabolism. Cell Death Dis. 2020, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Introduction to Purinergic Signaling. Methods Mol. Biol. 2020, 2041, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Leal-Esteban, L.C.; Fajas, L. Cell cycle regulators in cancer cell metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165715. [Google Scholar] [CrossRef]
- Cai, A.; Zheng, H.; Chen, Z.; Lin, X.; Li, C.; Yao, Q.; Bhutia, Y.D.; Ganapathy, V.; Chen, R.; Kou, L. Synergism between SLC6A14 blockade and gemcitabine in pancreactic cancer: A 1H-NMR-based metabolomic study in pancreatic cancer cells. Biochem. J. 2020, 477, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.; Zhu, Y.; Guo, L.; Zhang, Y.; et al. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).