4.1. Chemistry
All reagents purchased from commercial sources were used without further purification. 1H NMR spectra were recorded on a JEOL JNM-EX400 (400 MHz). Chemical shifts were reported in ppm from tetramethylsilane (TMS) with the solvent resonance resulting from incomplete deuteration as the internal reference (CDCl3: 7.26 ppm) or relative to TMS (δ = 0.0). 13C NMR spectra were recorded on a JEOL JNM-EX400 (100 MHz) with complete proton decoupling. Chemical shifts were reported in ppm from tetramethylsilane with the solvent as the internal reference (CDCl3: 77.16 ppm). High-resolution mass spectra (HRMS) were expressed in m/z using the ESI-MS Agilent 6530 Accurate-Mass Quadruple Time-of-Flight (Q-TOF) method at the Metabolomics Research Center for Functional Materials, Kyungsung University, Republic of Korea.
Methyl (1
H-indole-2-carbonyl)-L-serinate (
5). To an anhydrous CH
2Cl
2 solution of indole-2-carboxylic acid (
3) (389 mg, 2.41 mmol), HOBt (261 mg, 1.93 mmol) and L-serine methyl ester (
4) (300 mg. 1.93 mmol), EDC (430 mL, 2.41 mmol) were added and cooled to 0 °C. Triethylamine was added dropwise for 30 min and then the reaction mixture was warmed to ambient temperature. After stirring for 18 h, the reaction mixture was concentrated under reduced pressure. The crude mixture was purified by flash column chromatography on silica gel (THF/DCM = 1/50) to afford the compound
5 (yield: 98%) [
40].
1H NMR (400 MHz, CDCl
3)
δ 9.81 (s, 1H, NH), 7.59 (dd,
J = 8.0, 0.8 Hz, 1H), 7.42 (d,
J = 7.6 Hz, 1H, NH), 7.38 (dd,
J = 8.3, 0.8 Hz, 1H), 7.26 (td,
J = 7.0, 1.0 Hz, 1H), 7.11 (td,
J = 7.0, 1.0 Hz, 1H), 6.99 (dd,
J = 2.1, 0.8 Hz, 1H), 4.89 (dt,
J = 7.3, 3.5 Hz, 1H,), 4.04 (m, 2H), 3.75 (s, 3H).
Methyl 1-oxo-1,2,3,4-tetrahydropyrazino[1,2-
a]indole-3-carboxylate (
6). A mixture of methyl (1
H-indole-2-carbonyl)-L-serinate (
5) (504 mg, 1.92 mmol), triphenylphosphine (655 mg, 2.50 mmol), DEAD (1.10 mL, 2.50 mmol) and anhydrous THF (25 mL) was stirred for 24 h. After stirring for 24 h, the solvent was evaporated, then purification via flash column chromatography (2% THF in CH
2Cl
2) provided compound
6 (yield: 54%) [
40].
1H NMR (400 MHz, CDCl
3)
δ 9.10 (s, 1H, NH), 7.67 (dd,
J = 8.0, 1.0 Hz, 1H), 7.39 (dd,
J = 8.3, 0.9 Hz, 1H), 7.29 (td,
J = 7.0, 1.0 Hz, 1H), 7.14 (td,
J = 7.0, 0.9 Hz, 1H), 7.09 (m, 1H), 4.98 (dd,
J = 10.5, 7.9 Hz, 1H), 4.74 (dd,
J = 8.6, 7.9 Hz, 1H), 4.65 (dd,
J = 10.5, 8.6 Hz, 1H), 3.84 (s, 3H).
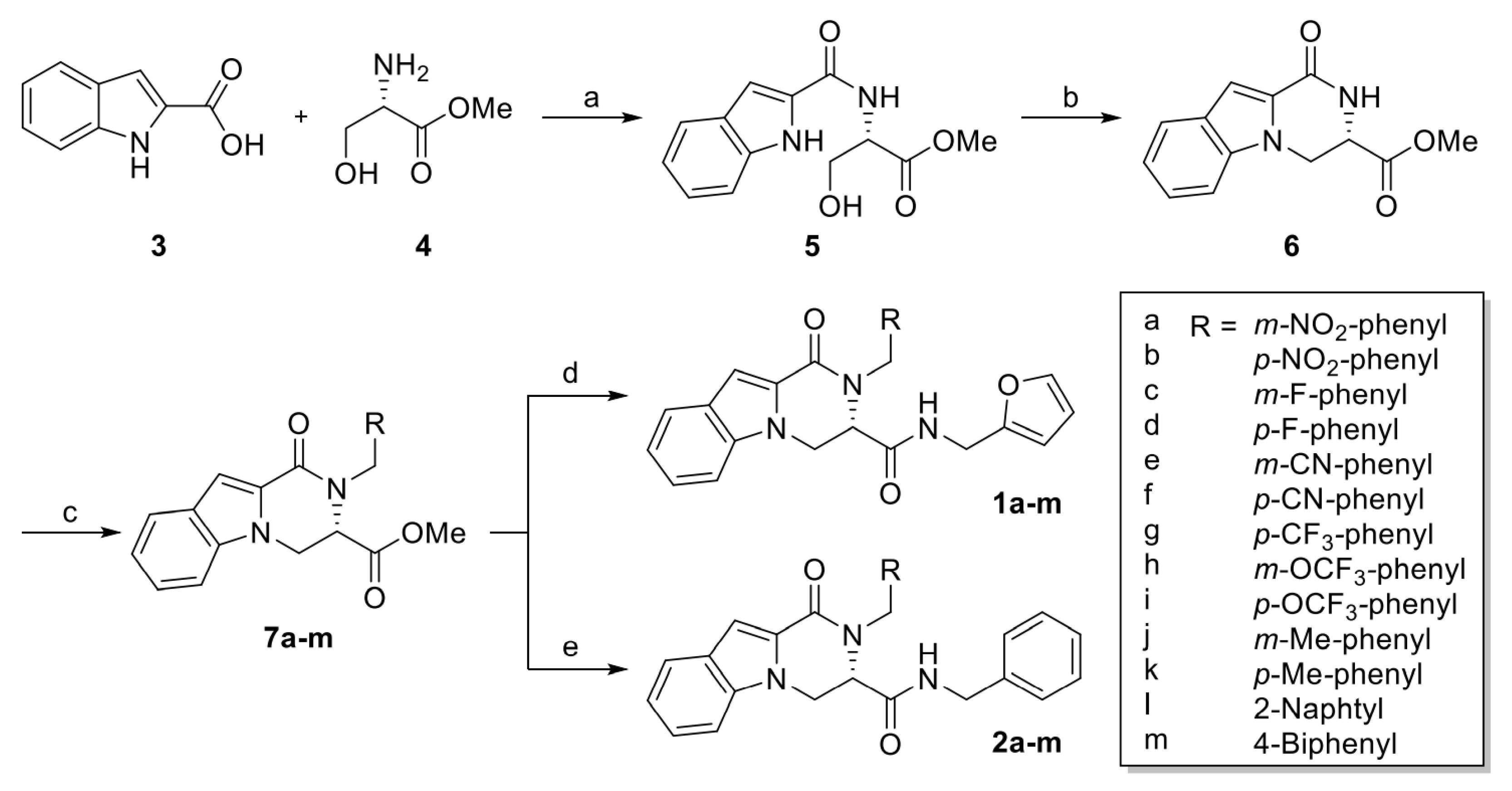
4.1.1. General Procedure of Methyl (S)-2-Arylmethyl-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7a–m)
A mixture of methyl (S)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (6) (140 mg, 0.573 mmol), K2CO3 (119 mg, 0.860 mmol) and anhydrous DMF (5 mL) was stirred, and then substituted arylmethyl bromide (4.01 mmol) was added. After stirring for 5 h, the solvent was evaporated, then purification via flash column chromatography provided compound 7a–m.
Methyl (S)-2-(3-nitrobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7a). Compound 7a was synthesized according to the synthetic procedure given above, with a yield of 54%. 1H NMR (400 MHz, CDCl3) δ 8.12–8.04 (m, 2H), 7.71 (dt, J = 8.0, 0.9 Hz, 1H), 7.43–7.27 (m, 4H), 7.26 (s, 1H), 7.17 (ddd, J = 8.0, 5.0, 3.0 Hz, 1H), 6.10, 6.03 (ABq, J = 16.3 Hz, 2H), 4.95 (dd, J = 10.4, 7.6 Hz, 1H), 4.59 (dd, J = 8.6, 7.6 Hz, 1H), 4.52 (dd, J = 10.4, 8.6 Hz, 1H), 3.74 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 171.6, 160.5, 148.5, 140.7, 139.2, 133.0, 129.6, 126.8, 125.4, 125.2, 122.6, 122.4, 122.2, 121.2, 110.3, 109.9, 69.1, 68.7, 53.6, 47.6.
Methyl (S)-2-(4-nitrobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7b). Compound 7b was synthesized according to the synthetic procedure given above, with a yield of 28%. 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 8.7 Hz, 2H), 7.71 (d, J = 8.0 Hz, 1H), 7.29 (td, J = 7.5, 1.0 Hz, 1H), 7.25 (m, 1H), 7.21 (d, J = 8.7 Hz, 2H), 7.18 (td, J = 7.5, 1.0 zHz, 1H), 6.12, 6.03 (ABq, J = 16.8 Hz, 2H), 4.92 (dd, J = 10.4, 7.5 Hz, 1H), 4.57 (dd, J = 8.6, 7.5 Hz, 1H), 4.50 (dd, J = 10.4, 8.6 Hz, 1H), 3.73 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.6, 160.5, 147.2, 146.0, 139.2, 127.6, 126.8, 125.4, 125.2, 123.9, 122.6, 121.2, 110.3, 109.9, 69.1, 68.7, 52.8, 47.8.
Methyl (S)-2-(3-fluorobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7c). Compound 7c was synthesized according to the synthetic procedure given above, with a yield of 51%. 1H NMR (400 MHz, CDCl3) δ 7.69 (dt, J = 8.0, 1.0 Hz, 1H), 7.33–7.30 (m, 1H), 7.29 (dd, J = 6.5, 1.2 Hz, 1H), 7.24 (d, J = 0.8 Hz, 1H), 7.20 (td, J = 7.9, 5.9 Hz, 1H), 7.16 (ddd, J = 8.0, 6.4, 1.5 Hz, 1H), 6.93–6.85 (m, 2H), 6.85–6.80 (m, 1H), 6.00, 5.95 (ABq, J = 16.3 Hz, 2H), 4.96 (dd, J = 10.4, 7.4 Hz, 1H), 4.60 (dd, J = 8.6, 7.4 Hz, 1H), 4.50 (dd, J = 10.4, 8.6 Hz, 1H), 3.76 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.7, 163.1 (JFC = 245.8 Hz), 160.6, 141.1 (JFC = 7.1 Hz), 139.3, 130.0 (JFC = 8.3 Hz), 126.7, 125.5, 124.9, 122.5 (JFC = 2.9 Hz), 122.4, 120.9, 114.1 (JFC = 21.1 Hz), 114.0 (JFC = 22.0 Hz), 110.7, 109.6, 69.1, 68.5, 52.8, 47.7 (JFC = 2.0 Hz).
Methyl (S)-2-(4-fluorobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7d). Compound 7d was synthesized according to the synthetic procedure given above, with a yield of 56%. 1H NMR (400 MHz, CDCl3) δ 7.69 (dt, J = 8.0, 1.0 Hz, 1H), 7.35–7.30 (m, 1H), 7.30–7.25 (m, 1H), 7.23 (d, J = 0.7 Hz, 1H), 7.15 (ddd, J = 7.9, 6.7, 1.3 Hz, 1H), 7.11–7.06 (m, 2H), 6.95–6.88 (m, 2H), 5.98, 5.91 (ABq, J = 16.2 Hz, 2H), 4.96 (dd, J = 10.4, 7.5 Hz, 1H), 4.59 (dd, J = 8.6, 7.5 Hz, 1H), 4.50 (dd, J = 10.4, 8.6 Hz, 1H), 3.76 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.7, 162.0 (JFC = 244.9 Hz), 160.7, 139.3, 134.1 (JFC = 3.1 Hz), 128.6 (JFC = 8.0 Hz), 126.8, 125.5, 124.8, 122.4, 120.8, 115.4 (JFC = 21.5 Hz), 110.8, 109.5, 69.1, 68.5, 52.7, 47.5 (JFC = 0.8 Hz).
Methyl (S)-2-(3-cyanobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7e). Compound 7e was synthesized according to the synthetic procedure given above, with a yield of 44%. 1H NMR (400 MHz, CDCl3) δ 7.71 (dt, J = 8.0, 1.0 Hz, 1H), 7.51–7.47 (m, 1H), 7.44–7.43 (m, 1H), 7.35–7.33 (m, 2H), 7.30–7.27 (m, 2H), 7.25 (d, J = 0.7 Hz, 1H), 7.17 (ddd, J = 8.0, 6.3, 1.7 Hz, 1H), 6.01, 5.97 (ABq, J = 16.3 Hz, 2H), 4.95 (dd, J = 10.4, 7.5 Hz, 1H), 4.59 (dd, J = 8.6, 7.5 Hz, 1H), 4.51 (dd, J = 10.4, 8.6 Hz, 1H), 3.77 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.6, 160.5, 140.1, 139.2, 131.4, 131.0, 130.7, 129.4, 126.8, 125.3, 125.2, 122.6, 121.1, 118.9, 112.6, 110.3, 109.9, 69.1, 68.6, 52.8, 47.5.
Methyl (S)-2-(4-cyanobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7f). Compound 7f was synthesized according to the synthetic procedure given above, with a yield of 45%. 1H NMR (400 MHz, CDCl3) δ 7.70 (dt, J = 8.0, 1.0 Hz, 1H), 7.54–7.50 (m, 2H), 7.29 (ddd, J = 8.1, 6.7, 1.2 Hz, 1H), 7.25 (d, J = 0.9 Hz, 1H), 7.25–7.22 (m, 1H), 7.17 (ddd, J = 7.9, 6.7, 1.2 Hz, 1H), 7.16–7.13 (m, 2H), 6.08, 5.99 (ABq, J = 16.7 Hz, 2H), 4.92 (dd, J = 10.4, 7.4 Hz, 1H), 4.57 (dd, J = 8.7, 7.4 Hz, 1H), 4.49 (dd, J = 10.4, 8.6 Hz, 1H), 3.74 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.5, 160.5, 144.0, 139.2, 132.4, 128.5, 127.5, 126.8, 125.4, 125.1, 122.6, 121.1, 111.0, 110.4, 109.8, 69.0, 68.6, 52.7, 47.9.
Methyl (S)-2-(4-trifluoromethylbenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7g). Compound 7g was synthesized according to the synthetic procedure given above, with a yield of 33%. 1H NMR (400 MHz, CDCl3) δ 7.70 (dt, J = 8.0, 1.0 Hz, 1H), 7.49 (d, J = 8.1 Hz, 2H), 7.29–7.26 (m, 2H), 7.25 (s, 1H), 7.18–7.14 (m, 3H), 6.09, 5.99 (ABq, J = 16.5 Hz, 2H), 4.93 (dd, J = 10.4, 7.4 Hz, 1H), 4.58 (dd, J = 8.6, 7.4 Hz, 1H), 4.50 (dd, J = 10.4, 8.6 Hz, 1H), 3.72 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.6, 160.6, 142.6 (JFC = 1.4 Hz), 139.3, 129.4 (JFC = 32.4 Hz), 127.0, 126.8, 125.54 (JFC = 3.8 Hz), 125.53, 124.1 (JFC = 273 Hz), 125.1, 122.5, 121.0, 110.6, 109.7, 69.09, 68.59, 52.74, 47.84.
Methyl (S)-2-(3-trifluoromethoxylbenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7h). Compound 7h was synthesized according to the synthetic procedure given above, with a yield of 41%. 1H NMR (400 MHz, CDCl3) δ 7.69 (dt, J = 8.0, 1.0 Hz, 1H), 7.32–7.28 (m, 2H), 7.25–7.21 (m, 2H), 7.16 (ddd, J = 8.0, 5.8, 2.1 Hz, 1H), 7.05 (d, J = 7.6 Hz, 1H), 7.04 (s, 1H), 6.96 (d, J = 8.0 Hz, 1H), 6.01, 5.96 (ABq, J = 16.3 Hz, 2H), 4.95 (dd, J = 10.4, 7.5 Hz, 1H), 4.59 (dd, J = 8.6, 7.5 Hz, 1H), 4.50 (dd, J = 10.4, 8.6 Hz, 1H), 3.75 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.7, 160.6, 149.5 (JFC = 1.8 Hz), 140.9, 139.3, 129.9, 126.8, 125.5, 125.2, 125.0, 122.5, 121.0, 120.5 (JFC = 258 Hz), 119.8 (JFC = 0.9 Hz), 119.5 (JFC = 0.9 Hz), 110.6, 109.7, 69.1, 68.6, 52.7, 47.7.
Methyl (S)-2-(4-trifluoromethoxylbenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7i). Compound 7i was synthesized according to the synthetic procedure given above, with a yield of 41%. 1H NMR (400 MHz, CDCl3) δ 7.70 (dt, J = 8.0, 1.0 Hz, 1H), 7.32–7.27 (m, 2H), 7.24 (s, 1H), 7.16 (ddd, J = 8.0, 6.0, 2.0 Hz, 1H), 7.12 (d, J = 9.0 Hz, 2H), 7.08 (d, J = 9.0 Hz, 2H), 6.01, 5.95 (ABq, J = 16.2 Hz, 2H), 4.95 (dd, J = 10.4, 7.5 Hz, 1H), 4.59 (dd, J = 8.6, 7.5 Hz, 1H), 4.50 (dd, J = 10.4, 8.6 Hz, 1H), 3.74 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.7, 160.6, 148.3 (JCF = 1.8 Hz), 139.3, 137.2, 128.3, 126.8, 125.5, 125.0, 122.5, 121.1 (JCF = 1.0 Hz), 120.9, 120.6 (JCF = 257 Hz), 110.7, 109.6, 69.1, 68.6, 52.7, 47.5.
Methyl (S)-2-(3-methylbenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7j). Compound 7j was synthesized according to the synthetic procedure given above, with a yield of 45%. 1H NMR (400 MHz, CDCl3) δ 7.68 (d, J = 8.0 Hz, 1H), 7.32 (dd, J = 8.3, 0.8 Hz, 1H), 7.26 (dd, J = 8.3, 1.3 Hz, 1H), 7.24 (d, J = 0.6 Hz, 1H), 7.15 (dd, J = 7.9, 1.0 Hz, 1H), 7.11 (d, J = 7.7 Hz, 1H), 7.00 (d, J = 7.7 Hz, 1H), 6.94 (s, 1H), 6.86 (d, J = 7.7 Hz, 1H), 5.89 (ABq, J = 16.2 Hz, 2H), 4.96 (dd, J = 10.4, 7.6 Hz, 1H), 4.60 (dd, J = 8.6, 7.6 Hz, 1H), 4.50 (dd, J = 10.4, 8.6 Hz, 1H), 3.75 (s, 3H), 2.26 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.7, 160.7, 139.4, 138.3, 138.1, 128.4, 127.9, 127.4, 126.7, 126.0, 124.7, 123.8, 122.2, 120.6, 111.0, 109.2, 69.1, 68.5, 52.7, 48.2, 21.6.
Methyl (S)-2-(4-methylbenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7k). Compound 7k was synthesized according to the synthetic procedure given above, with a yield of 58%. 1H NMR (400 MHz, CDCl3) δ 7.67 (dt, J = 8.0, 0.8 Hz, 1H), 7.33 (dd, J = 8.3, 0.8 Hz, 1H), 7.25 (ddd, J = 8.3, 6.9, 1.2 Hz, 1H), 7.22 (d, J = 0.7 Hz, 1H), 7.13 (ddd, J = 7.9, 6.9, 1.1 Hz, 1H), 7.04 (d, J = 8.1 Hz, 2H), 6.99 (d, J = 8.1 Hz, 2H), 6.01, 5.88 (ABq, J = 16.1 Hz, 2H), 4.96 (dd, J = 10.4, 7.5 Hz, 1H), 4.59 (dd, J = 8.6, 7.5 Hz, 1H), 4.49 (dd, J = 10.4, 8.6 Hz, 1H), 3.76 (s, 3H), 2.27 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.8, 160.8, 139.4, 136.7, 135.4, 129.2, 126.8, 126.8, 125.7, 124.7, 122.3, 120.7, 111.0, 109.3, 69.2, 68.5, 52.7, 47.9, 21.2.
Methyl (S)-2-(naphthalen-2-ylmethyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7l). Compound 7l was synthesized according to the synthetic procedure given above, with a yield of 55%. 1H NMR (400 MHz, CDCl3) δ 7.80–7.67 (m, 4H), 7.48 (s, 1H), 7.44–7.39 (m, 2H), 7.36 (dd, J = 8.4, 1.0 Hz, 1H), 7.29–7.22 (m, 3H), 7.15 (ddd, J = 8.0, 6.9, 1.0 Hz, 1H), 6.21, 6.10 (ABq, J = 16.4 Hz, 1H), 4.95 (dd, J = 10.4, 7.4 Hz, 1H), 4.59 (dd, J = 8.6, 7.4 Hz, 1H), 4.49 (dd, J = 10.4, 8.6 Hz, 1H), 3.67 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.7, 160.8, 139.5, 136.0, 133.4, 132.7, 128.3, 127.9, 127.7, 126.8, 126.1, 125.8, 125.8, 125.3, 125.1, 124.8, 122.3, 120.8, 111.0, 109.4, 69.2, 68.6, 52.7, 48.4.
Methyl (S)-2-([1,1′-biphenyl]-4-ylmethyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxylate (7m). Compound 7m was synthesized according to the synthetic procedure given above, with a yield of 26%. 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 7.9 Hz, 1H), 7.51 (d, J = 7.4 Hz, 2H), 7.45 (d, J = 8.2 Hz, 2H), 7.42–7.34 (m, 3H), 7.33–7.27 (m, 2H), 7.25 (s, 1H), 7.19–7.12 (m, 3H), 6.09, 5.97 (ABq, J = 16.2 Hz, 2H), 4.96 (dd, J = 10.4, 7.5 Hz, 1H), 4.60 (dd, J = 8.6, 7.5 Hz, 1H), 4.50 (dd, J = 10.4, 8.6 Hz, 1H), 3.74 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.7, 160.8, 141.0, 140.1, 139.5, 137.5, 128.9, 127.32, 127.31, 127.28, 127.1, 126.8, 125.7, 124.8, 122.4, 120.8, 111.0, 109.4, 69.2, 68.6, 52.8, 48.0.
4.1.2. General Procedure of (S)-N-(Furan-2-ylmethyl)-2-arylmethyl-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indol-3-carboxamide (1a–m)
To a solution of (S)-methyl 2-arylmethyl-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]-indole-3-carboxylate (7a–m) (1.00 equiv.) in absolute methanol, furfurylamine (5.00 equiv.) was added and stirred for 15h at r.t. After evaporation, the residue was purified by column chromatography (EtOAc/hexanes = 1:2).
(S)-N-(Furan-2-ylmethyl)-2-(3-nitrobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1a). Compound 1a was synthesized according to the synthetic procedure given above, with a yield of 41%. 1H NMR (400 MHz, CDCl3) δ 8.07–8.04 (m, 1H), 7.98 (s, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.36 (t, J = 8.0 Hz, 1H), 7.34–7.30 (m, 2H), 7.27–7.25 (m, 2H), 7.22–7.18 (m, 2H), 6.32 (t, J = 5.9 Hz, 1H, NH), 6.27 (dd, J = 3.2, 1.9 Hz, 1H), 6.21 (d, J = 16.9 Hz, 1H), 6.08 (dd, J = 3.2, 0.8 Hz, 1H), 5.83 (d, J = 16.9 Hz, 1H), 4.85 (dd, J = 11.0, 8.6 Hz, 1H), 4.64 (dd, J = 11.0, 8.6 Hz, 1H), 4.53 (t, J = 8.6 Hz, 1H), 4.36 (dd, J = 15.5, 5.9 Hz, 1H), 4.15 (dd, J = 15.6, 5.9 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.2, 160.4, 150.7, 148.6, 142.3, 140.8, 139.3, 131.9, 129.8, 126.7, 125.5, 125.2, 122.8, 122.4, 121.4, 121.1, 110.5, 110.2, 110.0, 107.6, 69.5, 69.4, 47.6, 36.1; HRMS (ESI-QTOF) m/z calcd for C24H21N4O5+ [M+H]+ 445.1506, found 445.1507.
(S)-N-(Furan-2-ylmethyl)-2-(4-nitrobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1b). Compound 1b was synthesized according to the synthetic procedure given above, with a yield of 73%. 1H NMR (400 MHz, CDCl3) δ 8.07 (d, J = 8.8 Hz, 2H), 7.74 (d, J = 7.9 Hz, 1H), 7.32 (ddd, J = 8.5, 6.8, 1.1 Hz, 1H), 7.30 (d, J = 0.9 Hz, 1H), 7.29 (dd, J = 1.9, 0.9 Hz, 1H), 7.24 (dd, J = 8.5, 0.9 Hz, 1H), 7.21 (ddd, J = 7.9, 6.8, 1.1 Hz, 1H), 7.09 (d, J = 8.8 Hz, 2H), 6.27 (dd, J = 3.2, 1.9 Hz, 1H), 6.26 (d, J = 17.3 Hz, 1H), 6.23 (s, 1H, NH) 6.07 (dd, J = 3.2, 0.9 Hz, 1H), 5.79 (d, J = 17.3 Hz, 1H), 4.84 (dd, J = 11.0, 8.6 Hz, 1H), 4.63 (dd, J = 11.0, 8.6 Hz, 1H), 4.51 (t, J = 8.6 Hz, 1H), 4.31 (dd, J = 15.5, 5.9 Hz, 1H), 4.17 (dd, J = 15.5, 5.9 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.2, 160.4, 150.6, 147.2, 146.1, 142.4, 139.4, 126.7, 126.6, 125.5, 125.2, 124.1, 122.8, 121.4, 110.5, 110.2, 110.0, 107.6, 69.5, 69.4, 47.8, 36.0; HRMS (ESI-QTOF) m/z calcd for C24H21N4O5+ [M+H]+ 445.1506, found 445.1510.
(S)-N-(Furan-2-ylmethyl)-2-(3-fluorobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1c). Compound 1c was synthesized according to the synthetic procedure given above, with a yield of 54%. 1H NMR (400 MHz, CDCl3) δ 7.73 (dt, J = 8.0, 1.0 Hz, 1H), 7.33–7.29 (m, 3H), 7.28 (s, 1H), 7.22–7.17 (m, 2H), 6.87 (td, J = 8.4, 2.6 Hz, 1H), 6.75 (ddd, J = 7.7, 1.7, 0.9 Hz, 1H), 6.62 (dt, J = 9.7, 2.1 Hz, 1H), 6.35 (t, J = 5.9 Hz, 1H, NH), 6.29 (dd, J = 3.2, 1.8 Hz, 1H), 6.16 (d, J = 16.8 Hz, 1H), 6.09 (dd, J = 3.2, 0.9 Hz, 1H), 5.71 (d, J = 16.8 Hz, 1H), 4.85 (dd, J = 11.0, 8.4 Hz, 1H), 4.62 (dd, J = 11.0, 8.4 Hz, 1H), 4.50 (t, J = 8.4 Hz, 1H), 4.35 (dd, J = 15.6, 5.9 Hz, 1H), 4.11 (dd, J = 15.6, 5.9 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.5, 163.3 (JFC = 247 Hz), 160.3, 151.0, 142.2, 141.4 (JFC = 6.9 Hz), 139.6, 130.4 (JFC = 8.2 Hz), 126.5, 125.3, 125.2, 122.6, 121.4 (JFC = 2.9 Hz), 121.1, 114.2 (JFC = 21.1 Hz), 112.9 (JFC = 22.2 Hz), 110.5, 110.3, 109.9, 107.4, 69.5, 69.4, 47.7 (JFC = 2.0 Hz), 36.1; HRMS (ESI-QTOF) m/z calcd for C24H21FN3O3+ [M+H]+ 418.1561, found 418.1566.
(S)-N-(Furan-2-ylmethyl)-2-(4-fluorobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1d). Compound 1d was synthesized according to the synthetic procedure given above, with a yield of 67%. 1H NMR (400 MHz, CDCl3) δ 7.72 (d, J = 8.1 Hz, 1H), 7.33 – 7.29 (m, 3H), 7.26 (s, 1H), 7.18 (dt, J = 8.0, 4.0 Hz, 1H), 6.95–6.85 (m, 4H), 6.35 (t, J = 6.0 Hz, 1H, NH), 6.30 (dd, J = 3.3, 1.9 Hz, 1H), 6.18–6.06 (m, 2H), 5.66 (d, J = 16.5 Hz, 1H), 4.86 (dd, J = 11.0, 8.4 Hz, 1H), 4.62 (dd, J = 11.0, 8.4 Hz, 1H), 4.50 (t, J = 8.4 Hz, 1H), 4.35 (dd, J = 15.5, 6.0 Hz, 1H), 4.14 (dd, J = 15.5, 6.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.5, 161.8 (JFC = 245 Hz), 160.4, 150.9, 142.4, 139.6, 134.3 (JFC = 3.1 Hz), 127.4 (JFC = 7.9 Hz), 126.6, 125.3, 125.2, 122.6, 121.1, 115.8 (JFC = 21.5 Hz), 110.5, 110.4, 109.8, 107.5, 69.5, 69.4, 47.5, 36.1; HRMS (ESI-QTOF) m/z calcd for C24H21FN3O3+ [M+H]+ 418.1561, found 418.1565.
(S)-N-(Furan-2-ylmethyl)-2-(3-cyanobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1e). Compound 1e was synthesized according to the synthetic procedure given above, with a yield of 44%. 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 7.9 Hz, 1H), 7.48 (d, J = 7.6 Hz, 1H), 7.35–7.32 (m, 2H), 7.32–7.28 (m, 2H), 7.26–7.23 (m, 2H), 7.23–7.17 (m, 2H), 6.34–6.29 (m, 1H), 6.28 (t, J = 5.9 Hz, 1H, NH), 6.12 (d, J = 16.9 Hz, 1H), 6.12 (dt, J = 3.2, 0.8 Hz, 2H), 5.80 (d, J = 16.9 Hz, 1H), 4.84 (dd, J = 11.0, 8.6 Hz, 1H), 4.63 (dd, J = 11.0, 8.6 Hz, 1H), 4.52 (t, J = 8.6 Hz, 1H), 4.37 (dd, J = 15.6, 5.9 Hz, 1H), 4.17 (dd, J = 15.6, 5.9 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.3, 160.4, 150.8, 142.4, 140.3, 139.4, 131.1, 130.3, 129.6, 129.4, 126.7, 125.6, 125.2, 122.8, 121.4, 118.7, 113.1, 110.6, 110.2, 110.1, 107.7, 69.5, 69.4, 47.6, 36.1; HRMS (ESI-QTOF) m/z calcd for C25H21N4O3+ [M+H]+ 425.1608, found 425.1610.
(S)-N-(Furan-2-ylmethyl)-2-(4-cyanobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1f). Compound 1f was synthesized according to the synthetic procedure given above, with a yield of 33%. 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 8.0 Hz, 1H), 7.47 (d, J = 8.2 Hz, 2H), 7.37–7.34 (m, 1H), 7.32 (ddd, J = 8.4, 6.9, 1.2 Hz, 1H), 7.29 (s, 1H), 7.24 (d, J = 8.0 Hz, 1H), 7.22–7.17 (m, 1H), 7.04 (d, J = 8.2 Hz, 2H), 6.35 (dd, J = 3.1, 1.9 Hz, 1H), 6.26 (t, J = 5.8 Hz, 1H, NH), 6.21 (d, J = 17.2 Hz, 1H), 6.15 (d, J = 3.1 Hz, 1H), 5.75 (d, J = 17.2 Hz, 1H), 4.84 (dd, J = 11.0, 8.6 Hz, 1H), 4.63 (dd, J = 11.0, 8.6 Hz, 1H), 4.51 (t, J = 8.6 Hz, 1H), 4.35 (dd, J = 15.5, 5.8 Hz, 1H), 4.17 (dd, J = 15.5, 5.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.3, 160.4, 150.7, 144.2, 142.5, 139.4, 132.7, 126.7, 126.6, 125.5, 125.2, 122.8 121.4, 118.7, 111.3, 110.7, 110.12, 110.08, 107.8, 69.5, 69.4, 48.0, 36.1; HRMS (ESI-QTOF) m/z calcd for C25H21N4O3+ [M+H]+ 425.1608, found 425.1607.
(S)-N-(Furan-2-ylmethyl)-2-(4-trifluoromethylbenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1g). Compound 1g was synthesized according to the synthetic procedure given above, with a yield of 77%. 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 7.8 Hz, 1H), 7.49 (d, J = 8.0 Hz, 2H), 7.34–7.29 (m, 3H), 7.28 (s, 1H), 7.23–7.16 (m, 1H), 7.07 (d, J = 8.0 Hz, 2H), 6.34 (t, J = 6.0 Hz, 1H, NH), 6.29 (dd, J = 3.2, 1.9 Hz, 1H), 6.25 (d, J = 16.9 Hz, 1H), 6.10 (d, J = 3.2 Hz, 1H), 5.76 (d, J = 16.9 Hz, 1H), 4.85 (dd, J = 11.0, 8.5 Hz, 1H), 4.62 (dd, J = 11.0, 8.5 Hz, 1H), 4.51 (t, J = 8.5 Hz, 1H), 4.35 (dd, J = 15.6, 6.4 Hz, 1H), 4.09 (dd, J = 15.6, 5.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.4, 160.4, 150.9, 142.8 (JFC = 1.4 Hz), 142.4, 139.5, 129.6 (JFC = 32.5 Hz), 126.7, 126.2, 125.9 (JFC = 3.8 Hz), 125.4, 125.3, 124.1 (JFC = 272 Hz), 122.7, 121.3, 110.5, 110.2, 110.0, 107.5, 69.5, 69.4, 47.9, 36.0; HRMS (ESI-QTOF) m/z calcd for C25H21F3N3O3+ [M+H]+ 468.1530, found 468.1537.
(S)-N-(Furan-2-ylmethyl)-2-(3-trifluoromethoxybenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1h). Compound 1h was synthesized according to the synthetic procedure given above, with a yield of 62%. 1H NMR (400 MHz, CDCl3) δ 7.73 (dt, J = 8.3, 0.9 Hz, 1H), 7.34–7.29 (m, 3H), 7.28 (s, 1H), 7.23–7.16 (m, 2H), 7.08–7.02 (m, 1H), 6.88 (s, 1H), 6.83 (ddd, J = 7.7, 1.7, 0.9 Hz, 1H), 6.35 (t, J = 5.9 Hz, 1H, NH), 6.28 (dd, J = 3.2, 1.9 Hz, 1H), 6.13 (d, J = 16.9 Hz, 1H), 6.09 (dd, J = 3.2, 0.8 Hz, 1H), 5.76 (d, J = 16.9 Hz, 1H), 4.85 (dd, J = 11.0, 8.5 Hz, 1H), 4.62 (dd, J = 11.0, 8.5 Hz, 1H), 4.51 (t, J = 8.5 Hz, 1H), 4.35 (dd, J = 15.6, 5.9 Hz, 1H), 4.14 (dd, J = 15.6, 5.9 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.4, 160.4, 151.0, 149.8 (JFC = 1.8 Hz), 142.3, 141.2, 139.5, 130.3, 126.6, 125.4, 125.3, 124.1, 122.6, 121.2, 120.5 (JFC = 258 Hz), 119.4, 118.5, 110.5, 110.3, 110.0, 107.4, 69.5, 69.4, 47.8, 36.1; HRMS (ESI-QTOF) m/z calcd for C25H21F3N3O4+ [M+H]+ 484.1479, found 484.1494.
(S)-N-(Furan-2-ylmethyl)-2-(4-trifluoromethoxybenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1i). Compound 1i was synthesized according to the synthetic procedure given above, with a yield of 66%. 1H NMR (400 MHz, CDCl3) δ 7.72 (d, J = 8.0 Hz, 1H), 7.35–7.29 (m, 3H), 7.28 (s, 1H), 7.19 (ddd, J = 8.0, 5.8, 2.0 Hz, 1H), 7.07 (d, J = 8.5 Hz, 2H), 6.99 (d, J = 8.5 Hz, 2H), 6.41 (t, J = 5.8 Hz, 1H, NH), 6.30 (dd, J = 3.2, 1.9 Hz, 1H), 6.17 (d, J = 16.7 Hz, 1H), 6.13 (d, J = 3.2 Hz, 1H), 5.72 (d, J = 16.7 Hz, 1H), 4.86 (dd, J = 11.0, 8.5 Hz, 1H), 4.63 (dd, J = 11.0, 8.5 Hz, 1H), 4.52 (t, J = 8.5 Hz, 1H), 4.38 (dd, J = 15.6, 5.8 Hz, 1H), 4.13 (dd, J = 15.6, 5.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.4, 160.4, 150.9, 149.7 (JFC = 1.4 Hz), 142.3, 141.2, 139.5, 130.2, 126.6, 125.4, 125.3, 124.0, 122.6, 121.2, 120.5 (JFC = 257 Hz), 119.4, 118.5, 110.5, 110.3, 109.9, 107.4, 69.5, 69.4, 47.8, 36.1; HRMS (ESI-QTOF) m/z calcd for C25H21F3N3O4+ [M+H]+ 484.1479, found 484.1491.
(S)-N-(Furan-2-ylmethyl)-2-(3-methylbenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1j). Compound 1j was synthesized according to the synthetic procedure given above, with a yield of 49%. 1H NMR (400 MHz, CDCl3) δ 7.73 (dd, J = 8.0, 1.0 Hz, 1H), 7.38–7.28 (m, 3H), 7.27 (s, 1H), 7.18 (ddd, J = 8.0, 6.4, 1.4 Hz, 1H), 7.09 (t, J = 7.6 Hz, 1H), 6.99 (d, J = 7.6 Hz, 1H), 6.77 (s, 1H), 6.71 (d, J = 7.6 Hz, 1H), 6.28 (dd, J = 3.2, 1.9 Hz, 1H), 6.26 (s, 1H, NH), 6.14 (d, J = 16.7 Hz, 1H), 6.06 (dd, J = 3.2, 0.9 Hz, 1H), 5.69 (d, J = 16.7 Hz, 1H), 4.84 (dd, J = 11.0, 8.3 Hz, 1H), 4.60 (dd, J = 11.0, 8.3 Hz, 1H), 4.50 (t, J = 8.3 Hz, 1H), 4.28 (dd, J = 15.6, 6.3 Hz, 1H), 4.02 (dd, J = 15.7, 6.3 Hz, 1H), 2.22 (s, 3H); 13C NMR (100 MHz, CDCl3) δ171.8, 160.4, 151.3, 142.0, 139.8, 138.8, 138.7, 128.7, 128.0, 126.5, 126.3, 125.4, 125.1, 122.7, 122.5, 120.9, 110.47, 110.46, 109.5, 107.1, 69.5, 69.4, 48.2, 36.2, 21.6; HRMS (ESI-QTOF) m/z calcd for C25H24N3O3+ [M+H]+ 414.1812, found 414.1818.
(S)-N-(Furan-2-ylmethyl)-2-(4-methylbenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1k). Compound 1k was synthesized according to the synthetic procedure given above, with a yield of 45%. 1H NMR (400 MHz, CDCl3) δ 7.72 (dt, J = 8.0, 1.0 Hz, 1H), 7.38–7.28 (m, 3H), 7.26 (d, J = 0.8 Hz, 1H), 7.18 (ddd, J = 8.0, 6.7, 1.2 Hz, 1H), 7.01 (d, J = 8.0 Hz, 2H), 6.83 (d, J = 8.0 Hz, 2H), 6.34 (t, J = 5.8 Hz, 1H, NH), 6.29 (dd, J = 3.2, 1.8 Hz, 1H), 6.14 (d, J = 16.6 Hz, 1H), 6.06 (dd, J = 3.2, 0.9 Hz, 1H), 5.68 (d, J = 16.6 Hz, 1H), 4.84 (dd, J = 11.0, 8.0 Hz, 1H), 4.60 (dd, J = 11.0, 8.6 Hz, 1H), 4.49 (dd, J = 8.6, 8.0 Hz, 1H), 4.30 (dd, J = 15.7, 5.8 Hz, 1H), 4.02 (dd, J = 15.7, 5.8 Hz, 1H), 2.25 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.8, 160.4, 151.3, 142.0, 139.8, 136.75, 135.72, 129.6, 126.5, 125.7, 125.3, 125.1, 122.5, 120.9, 110.48, 110.46, 109.5, 107.1, 69.5, 69.4, 48.0, 36.1, 21.1; HRMS (ESI-QTOF) m/z calcd for C25H24N3O3+ [M+H]+ 414.1812, found 414.1817.
(S)-N-(Furan-2-ylmethyl)-2-(naphthalen-1-ylmethyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1l). Compound 1l was synthesized according to the synthetic procedure given above, with a yield of 48%. 1H NMR (400 MHz, CDCl3) δ 7.79–7.74 (m, 2H), 7.72 (d, J = 8.5 Hz, 1H), 7.65–7.60 (m, 1H), 7.45–7.35 (m, 3H), 7.34–7.28 (m, 2H), 7.25 (s, 1H), 7.23–7.17 (m, 2H), 7.16 (dd, J = 1.9, 0.9 Hz, 1H), 6.36 (d, J = 16.9 Hz, 1H), 6.16 (dd, J = 3.2, 1.9 Hz, 1H), 6.13 (d, J = 6.3 Hz, 1H, NH), 5.86 (d, J = 16.9 Hz, 1H), 5.81 (dd, J = 3.2, 0.9 Hz, 1H), 4.82 (dd, J = 10.9, 7.9 Hz, 1H), 4.59 (dd, J = 10.9, 8.7 Hz, 1H), 4.48 (dd, J = 8.7, 7.9 Hz, 1H), 3.98 (dd, J = 15.6, 6.3 Hz, 1H), 3.55 (dd, J = 15.6, 6.3 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.6, 160.4, 151.0, 142.0, 139.8, 136.3, 133.4, 132.6, 128.8, 127.9, 127.8, 126.6, 126.5, 126.0, 125.5, 125.2, 124.1, 124.0, 122.6, 121.0, 110.5, 110.3, 109.7, 106.9, 69.5, 69.4, 48.4, 35.8; HRMS (ESI-QTOF) m/z calcd for C28H24N3O3+ [M+H]+ 450.1812, found 450.1818.
(S)-N-(Furan-2-ylmethyl)-2-([1,1′-biphenyl]-4-ylmethyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (1m). Compound 1m was synthesized according to the synthetic procedure given above, with a yield of 69%. 1H NMR (400 MHz, CDCl3) δ 7.74 (dt, J = 8.0, 1.0 Hz, 1H), 7.50–7.47 (m, 2H), 7.47–7.43 (m, 2H), 7.43–7.39 (m, 2H), 7.38–7.36 (m, 1H), 7.35–7.30 (m, 2H), 7.29 (d, J = 0.8 Hz, 1H), 7.22 (dd, J = 1.9, 0.8 Hz, 1H), 7.24–7.14 (m, 1H), 7.02 (d, J = 8.5 Hz, 2H), 6.40 (t, J = 6.1 Hz, 1H, NH), 6.24 (d, J = 16.8 Hz, 1H), 6.19 (dd, J = 3.2, 1.9 Hz, 1H), 5.95 (dd, J = 3.2, 0.9 Hz, 1H), 5.76 (d, J = 16.8 Hz, 1H), 4.87 (dd, J = 11.0, 8.0 Hz, 1H), 4.62 (dd, J = 11.0, 8.7 Hz, 1H), 4.51 (dd, J = 8.7, 8.0 Hz, 1H), 4.28 (dd, J = 15.6, 6.1 Hz, 1H), 3.97 (dd, J = 15.6, 6.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.7, 160.5, 142.1, 140.5, 140.0, 139.7, 129.0, 128.9, 127.6, 127.5, 127.1, 127.0, 126.6, 126.2, 125.4, 125.2, 122.5, 121.0, 110.5, 110.4, 109.7, 107.2, 69.5, 69.4, 48.0, 36.1; HRMS (ESI-QTOF) m/z calcd for C30H26N3O3+ [M+H]+ 476.1969, found 476.1977.
4.1.3. General Procedure of N-Benzyl (S)-2-Arylmethyl-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indol-3-carboxamide (2a–m)
To a solution of (S)-methyl 2-arylmethyl-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]-indole-3-carboxylate (7a–m) (1.00 equiv.) in absolute methanol, benzylamine (5.00 equiv.) was added and stirred for 15h at r.t. After evaporation, the residue was purified by column chromatography (EtOAc/hexanes = 1:2).
(S)-N-Benzyl-2-(3-nitrobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2a). Compound 2a was synthesized according to the synthetic procedure given above, with a yield of 73%. 1H NMR (400 MHz, CDCl3) δ 7.91 (s, 1H), 7.86 (dt, J = 7.1, 2.3 Hz, 1H), 7.74 (d, J = 7.9 Hz, 1H), 7.34–7.26 (m, 5H), 7.25–7.19 (m, 2H), 7.18–7.11 (m, 2H), 7.09–7.04 (m, 2H), 6.33 (t, J = 6.3 Hz, 1H, NH), 6.19, 5.79 (ABq, J = 16.9 Hz, 2H), 4.90 (dd, J = 11.0, 8.4 Hz, 1H), 4.68 (dd, J = 11.0, 8.8 Hz, 1H), 4.57 (t, J = 8.6 Hz, 1H), 4.38 (dd, J = 15.0, 6.4 Hz, 1H), 4.20 (dd, J = 15.0, 5.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.4, 160.5, 148.5, 140.8, 139.4, 137.7, 131.8, 129.7, 128.8, 127.7, 127.5, 126.8, 125.6, 125.3, 122.8, 122.4, 121.4, 121.0, 110.3, 110.1, 69.59, 69.57, 47.7, 43.1; HRMS (ESI-QTOF) m/z calcd for C26H23N4O4+ [M+H]+ 455.1714, found 455.1719.
(S)-N-Benzyl-2-(4-nitrobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2b). Compound 2b was synthesized according to the synthetic procedure given above, with a yield of 52%. 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.8 Hz, 2H), 7.76–7.72 (m, 1H), 7.33–7.26 (m, 5H), 7.23–7.18 (m, 2H), 7.07–7.03 (m, 2H), 7.00 (d, J = 8.8 Hz, 2H), 6.30–6.23 (m, 2H), 5.75 (d, J = 17.3 Hz, 1H), 4.89 (dd, J = 11.0, 8.4 Hz, 1H), 4.67 (dd, J = 11.0, 8.8 Hz, 1H), 4.56 (t, J = 8.6 Hz, 1H), 4.36 (dd, J = 15.0, 6.4 Hz, 1H), 4.16 (dd, J = 15.0, 5.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.4, 160.4, 147.1, 146.0, 139.4, 137.6, 128.9, 127.8, 127.3, 126.7, 126.5, 125.6, 125.2, 124.1, 122.8, 121.5, 110.2, 110.1, 69.58, 69.54, 47.8, 43.1; HRMS (ESI-QTOF) m/z calcd for C26H23N4O4+ [M+H]+ 455.1714, found 455.1717.
(S)-N-Benzyl-2-(3-fluorobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2c). Compound 2c was synthesized according to the synthetic procedure given above, with a yield of 44%. 1H NMR (400 MHz, CDCl3) δ 7.72 (d, J = 8.0 Hz, 1H), 7.32–7.24 (m, 6H), 7.18 (ddd, J = 7.9, 5.6, 2.3 Hz, 1H), 7.12–7.08 (m, 2H), 7.00 (td, J = 7.9, 5.8 Hz, 1H), 6.74 (td, J = 8.2, 2.3 Hz, 1H), 6.69 (d, J = 7.7 Hz, 1H), 6.59 (d, J = 9.2 Hz, 1H), 6.41 (t, J = 6.4 Hz, 1H, NH), 6.15, 5.69 (ABq, J = 16.8 Hz, 2H), 4.88 (dd, J = 10.9, 8.1 Hz, 1H), 4.64 (dd, J = 10.9, 8.7 Hz, 1H), 4.52 (dd, J = 8.7, 8.1 Hz, 1H), 4.37 (dd, J = 15.0, 6.7 Hz, 1H), 4.12 (dd, J = 15.0, 5.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.7, 163.2 (JFC = 247 Hz), 160.3, 141.5 (JFC = 6.9 Hz), 139.5, 137.9, 130.3 (JFC = 8.2 Hz), 128.7, 127.5, 127.4, 126.6, 125.3 (JFC = 2.4 Hz), 122.6, 121.2 (JFC = 2.6 Hz), 121.1, 114.1 (JFC = 21.1 Hz), 112.8 (JFC = 22.1 Hz), 110.2, 109.8, 69.5, 69.5, 47.7, 42.9; HRMS (ESI-QTOF) m/z calcd for C26H23FN3O2+ [M+H]+ 428.1769, found 428.1775.
(S)-N-Benzyl-2-(4-fluorobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2d). Compound 2d was synthesized according to the synthetic procedure given above, with a yield of 67%. 1H NMR (400 MHz, CDCl3) δ 7.72 (dt, J = 8.0, 1.0 Hz, 1H), 7.33–7.26 (m, 6H), 7.17 (ddd, J = 8.0, 5.2, 2.8 Hz, 1H), 7.14–7.10 (m, 2H), 6.87–6.81 (m, 2H), 6.77–6.69 (m, 2H), 6.33 (d, J = 6.5 Hz, 1H, NH), 6.13, 5.62 (ABq, J = 16.6 Hz, 2H), 4.89 (dd, J = 10.9, 8.1 Hz, 1H), 4.64 (dd, J = 10.9, 8.7 Hz, 1H), 4.53 (dd, J = 8.7, 8.1 Hz, 1H), 4.38 (dd, J = 15.0, 6.7 Hz, 1H), 4.11 (dd, J = 15.0, 5.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.7, 161.8 (JFC = 245 Hz), 160.4, 139.6, 137.9 134.3 (JFC = 3.1 Hz), 128.8, 127.6, 127.5, 127.3 (JFC = 7.9 Hz), 126.6, 125.3, 125.2, 122.6, 121.1, 115.7 (JFC = 21.5 Hz), 110.3, 109.8, 69.6, 69.5, 47.5, 43.0; HRMS (ESI-QTOF) m/z calcd for C26H23FN3O2+ [M+H]+ 428.1769, found 428.1778.
(S)-N-Benzyl-2-(3-cyanobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2e). Compound 2e was synthesized according to the synthetic procedure given above, with a yield of 67%. 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 7.9 Hz, 1H), 7.35–7.26 (m, 6H), 7.24–7.17 (m, 3H), 7.13–7.06 (m, 4H), 6.29 (t, J = 5.8 Hz, 1H, NH), 6.10, 5.75 (ABq, J = 16.9 Hz, 2H), 4.89 (dd, J = 11.0, 8.4 Hz, 1H), 4.66 (dd, J = 11.0, 8.7 Hz, 1H), 4.56 (t, J = 8.5 Hz, 1H), 4.41 (dd, J = 15.1, 6.4 Hz, 1H), 4.20 (dd, J = 15.0, 5.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.4, 160.4, 140.2, 139.3, 137.7, 130.9, 130.1, 129.5, 129.2, 128.9, 127.7, 127.5, 126.7, 125.5, 125.1, 122.8, 121.4, 118.6, 112.9, 110.2, 110.0, 69.52, 69.50, 47.5, 43.1; HRMS (ESI-QTOF) m/z calcd for C27H23N4O2+ [M+H]+ 435.1816, found 435.1823.
(S)-N-Benzyl-2-(4-cyanobenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2f). Compound 2f was synthesized according to the synthetic procedure given above, with a yield of 70%. 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 7.9 Hz, 1H), 7.37–7.27 (m, 5H), 7.24 (d, J = 8.6 Hz, 2H), 7.22–7.17 (m, 2H), 7.13 (dd, J = 7.7, 1.8 Hz, 2H), 6.93 (d, J = 8.3 Hz, 2H), 6.25 (t, J = 6.1 Hz, 1H, NH), 6.20, 5.69 (ABq, J = 17.2 Hz, 2H), 4.88 (dd, J = 10.9, 8.3 Hz, 1H), 4.65 (dd, J = 11.0, 8.8 Hz, 1H), 4.55 (t, J = 8.5 Hz, 1H), 4.39 (dd, J = 15.0, 6.4 Hz, 1H), 4.15 (dd, J = 15.0, 5.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.4, 160.4, 144.1, 139.4, 137.7, 132.6, 129.0, 127.8, 127.5, 126.6, 126.4, 125.5, 125.2, 122.7, 121.4, 118.6, 111.1, 110.1, 110.0, 69.52, 69.49, 47.9, 43.1; HRMS (ESI-QTOF) m/z calcd for C27H23N4O2+ [M+H]+ 435.1816, found 435.1821.
(S)-N-Benzyl-2-(4-trifluoromethylbenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2g). Compound 2g was synthesized according to the synthetic procedure given above, with a yield of 72%. 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 8.0 Hz, 1H), 7.35 (d, J = 8.1 Hz, 2H), 7.33–7.26 (m, 5H), 7.26–7.23 (m, 1H), 7.19 (ddd, J = 7.9, 6.7, 1.1 Hz, 1H), 7.14–7.10 (m, 2H), 7.00 (d, J = 8.0 Hz, 2H), 6.38 (t, J = 6.3 Hz, 1H, NH), 6.24, 5.73 (ABq, J = 16.9 Hz, 2H), 4.89 (dd, J = 11.0, 8.3 Hz, 1H), 4.65 (dd, J = 11.0, 8.7 Hz, 1H), 4.55 (t, J = 8.5 Hz, 1H), 4.40 (dd, J = 15.1, 6.8 Hz, 1H), 4.07 (dd, J = 15.1, 5.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.5, 160.4, 142.8 (JFC = 1.3 Hz), 139.5, 137.9, 129.5 (JFC = 32.5 Hz), 128.8, 127.6, 127.4, 126.7, 126.2, 125.90 (JFC = 3.8 Hz), 125.4, 125.3, 124.1 (JFC = 272 Hz), 122.7, 121.3, 110.2, 110.0, 69.6, 69.5, 47.9, 42.9; HRMS (ESI-QTOF) m/z calcd for C27H23F3N3O2+ [M+H]+ 478.1737, found 478.1746.
(S)-N-Benzyl-2-(3-trifluoromethoxybenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2h). Compound 2h was synthesized according to the synthetic procedure given above, with a yield of 85%. 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 8.0 Hz, 1H), 7.34–7.26 (m, 6H), 7.19 (ddd, J = 8.0, 6.5, 1.4 Hz, 1H), 7.12–7.08 (m, 2H), 7.01 (t, J = 7.9 Hz, 1H), 6.92 (d, J = 8.2 Hz, 1H), 6.84 (s, 1H), 6.76 (d, J = 7.6 Hz, 1H), 6.36 (t, J = 5.7 Hz, 1H, NH), 6.13, 5.72 (ABq, J = 16.8 Hz, 2H), 4.88 (dd, J = 11.0, 8.3 Hz, 1H), 4.65 (dd, J = 11.0, 8.7 Hz, 1H), 4.54 (t, J = 8.5 Hz, 1H), 4.37 (dd, J = 15.1, 6.6 Hz, 1H), 4.15 (dd, J = 15.1, 5.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.6, 160.4, 149.7 (JFC = 1.7 Hz), 141.2, 139.5, 137.9, 130.2, 128.8 127.6, 127.5, 126.6, 125.4, 125.3, 123.9, 122.6, 121.2, 120.5 (JFC = 258 Hz), 119.3 (JFC = 0.8 Hz), 118.4 (JFC = 0.8 Hz), 110.2, 109.9, 69.6, 69.5, 47.8, 43.0; HRMS (ESI-QTOF) m/z calcd for C27H23F3N3O3+ [M+H]+ 494.1686, found 494.1699.
(S)-N-Benzyl-2-(4-trifluoromethoxybenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2i). Compound 2i was synthesized according to the synthetic procedure given above, with a yield of 70%. 1H NMR (400 MHz, CDCl3) δ 7.72 (d, J = 8.0 Hz, 1H), 7.34–7.25 (m, 6H), 7.18 (t, J = 7.0 Hz, 1H), 7.14 (d, J = 6.9 Hz, 2H), 6.91 (m, 4H), 6.42 (t, J = 6.5 Hz, 1H, NH), 6.15, 5.68 (ABq, J = 16.7 Hz, 2H), 4.90 (dd, J = 10.9, 8.2 Hz, 1H), 4.65 (dd, J = 10.9, 8.7 Hz, 1H), 4.54 (t, J = 8.5 Hz, 1H), 4.41 (dd, J = 15.0, 6.7 Hz, 1H), 4.10 (dd, J = 15.0, 5.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.6, 160.4, 148.2 (JFC = 1.8 Hz), 139.5, 137.9, 137.3, 128.8, 127.6, 127.5, 127.2, 126.6, 125.3, 125.2, 122.61, 121.3 (JFC = 1.0 Hz), 121.2, 120.5 (JFC = 257 Hz), 110.3, 109.9, 69.6, 69.2, 47.5, 43.0; HRMS (ESI-QTOF) m/z calcd for C27H23F3N3O3+ [M+H]+ 494.1686, found 494.1695.
(S)-N-Benzyl-2-(3-methylbenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2j). Compound 2j was synthesized according to the synthetic procedure given above, with a yield of 28%. 1H NMR (400 MHz, CDCl3) δ 7.73 (dt, J = 8.0, 1.0 Hz, 1H), 7.33–7.23 (m, 6H), 7.18 (ddd, J = 8.0, 6.2, 1.8 Hz, 1H), 7.09–7.04 (m, 2H), 6.97 (t, J = 7.6 Hz, 1H), 6.88 (d, J = 7.6 Hz, 1H), 6.73 (s, 1H), 6.66 (d, J = 7.6 Hz, 1H), 6.29 (t, J = 6.3 Hz, 1H, NH), 6.16, 5.65 (ABq, J = 16.7 Hz, 2H), 4.87 (dd, J = 10.9, 7.8 Hz, 1H), 4.62 (dd, J = 10.9, 8.7 Hz, 1H), 4.52 (dd, J = 8.7, 7.8 Hz, 1H), 4.30 (dd, J = 15.1, 6.9 Hz, 1H), 4.03 (dd, J = 15.1, 5.9 Hz, 1H), 2.11 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 172.0, 160.4, 139.8, 138.8, 138.6, 138.2, 128.68, 128.65, 127.9, 127.33, 127.26, 126.5, 126.2, 125.4, 125.1, 122.6, 122.5, 120.9, 110.4, 109.5, 69.6, 69.5, 48.2, 42.9, 21.5; HRMS (ESI-QTOF) m/z calcd for C27H26N3O2+ [M+H]+ 424.2020, found 424.2026.
(S)-N-Benzyl-2-(4-methylbenzyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2k). Compound 2k was synthesized according to the synthetic procedure given above, with a yield of 45%. 1H NMR (400 MHz, CDCl3) δ 7.72 (d, J = 8.1 Hz, 1H), 7.35–7.24 (m, 6H), 7.17 (ddd, J = 7.9, 6.5, 1.3 Hz, 1H), 7.09–7.06 (m, 2H), 6.89 (d, J = 7.7 Hz, 2H), 6.79 (d, J = 7.9 Hz, 2H), 6.39 (t, J = 6.4 Hz, 1H, NH), 6.17 (d, J = 16.5 Hz, 1H), 5.64 (d, J = 16.5 Hz, 1H), 4.88 (dd, J = 11.0, 7.9 Hz, 1H), 4.62 (dd, J = 11.0, 8.7 Hz, 1H), 4.51 (dd, J = 8.7, 7.9 Hz, 1H), 4.33 (dd, J = 15.2, 6.8 Hz, 1H), 4.04 (dd, J = 15.2, 5.9 Hz, 1H), 2.16 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.9, 160.4, 139.7, 138.1, 136.7, 135.7, 129.6, 128.7, 127.4, 127.2, 126.5, 125.6, 125.4, 125.1, 122.5, 120.9, 110.5, 109.5, 69.55, 69.56, 47.9, 42.8, 21.1; HRMS (ESI-QTOF) m/z calcd for C27H26N3O2+ [M+H]+ 424.2020, found 424.2021.
(S)-N-Benzyl-2-(naphthalen-1-ylmethyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2l). Compound 2l was synthesized according to the synthetic procedure given above, with a yield of 88%. 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 8.0 Hz, 1H), 7.70 (dd, J = 6.3, 2.8 Hz, 1H), 7.60 (d, J = 6.7 Hz, 1H), 7.57 (d, J = 9.0 Hz, 1H), 7.42–7.38 (m, 2H), 7.38–7.35 (m, 1H), 7.33 (s, 1H), 7.31 (ddd, J = 8.4, 6.9, 1.2 Hz, 1H), 7.22 (d, J = 2.0 Hz, 1H), 7.21–7.13 (m, 5H), 6.83–6.79 (m, 2H), 6.38 (d, J = 16.8 Hz, 1H), 6.16 (t, J = 6.2 Hz, 1H, NH), 5.82 (d, J = 16.8 Hz, 1H), 4.85 (dd, J = 10.9, 7.9 Hz, 1H), 4.61 (dd, J = 10.9, 8.6 Hz, 1H), 4.51 (dd, J = 8.6, 7.9 Hz, 1H), 4.00 (dd, J = 15.0, 7.0 Hz, 1H), 3.50 (dd, J = 15.0, 5.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.8, 160.4, 139.8, 137.9, 136.3, 133.4, 132.5, 128.7, 128.5, 127.8, 127.3, 127.21, 128.20, 126.60, 126.55, 126.0, 125.4, 125.2, 124.0, 123.9, 122.6, 121.0, 110.4, 109.6, 69.52, 69.49, 48.4, 42.6; HRMS (ESI-QTOF) m/z calcd for C30H26N3O2+ [M+H]+ 460.2020, found 460.2022.
(S)-N-Benzyl-2-([1,1′-biphenyl]-4-ylmethyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide (2m). Compound 2m was synthesized according to the synthetic procedure given above, with a yield of 50%. 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 8.2 Hz, 1H), 7.39 (d, J = 4.4 Hz, 4H), 7.37–7.33 (m, 2H), 7.33–7.30 (m, 4H), 7.23–7.16 (m, 4H), 6.97 (d, J = 7.9 Hz, 2H), 6.96–6.93 (m, 2H), 6.39 (t, J = 6.4 Hz, 1H, NH), 6.26 (d, J = 16.7 Hz, 1H), 5.71 (d, J = 16.7 Hz, 1H), 4.91 (dd, J = 10.9, 8.0 Hz, 1H), 4.64 (dd, J = 10.9, 8.8 Hz, 1H), 4.54 (t, J = 8.4 Hz, 1H), 4.29 (dd, J = 15.2, 6.9 Hz, 1H), 3.94 (dd, J = 15.2, 5.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 171.9, 160.4, 140.3, 140.0, 139.7, 138.0, 137.8, 128.9, 128.6, 127.54, 127.49, 127.3, 127.1, 127.0, 126.6, 126.2, 125.4, 125.2, 122.6, 121.0, 110.4, 109.7, 69.56, 69.57, 48.0, 42.8; HRMS (ESI-QTOF) m/z calcd for C32H28N3O2+ [M+H]+ 486.2176, found 486.2179.