Abstract
Young adults had been widely perceived as a low-risk group for COVID-19 severity; therefore, they were deprioritised within the mass vaccination strategies as their prognosis of COVID-19 infection is relatively more favourable than older age groups. On the other hand, vaccination of this demographic group is indispensable to achieve herd immunity. A cross-sectional survey-based study was used to evaluate the side effects of mRNA-based COVID-19 vaccines among university students in the Czech Republic. The validated questionnaire was delivered in a digital form, and it consisted of demographic data; COVID-19 vaccine-related anamnesis; and local, systemic, orofacial, and skin-related side effects’ prevalence, onset, and duration. Out of the 539 included participants, 70.1% were females and 45.8% were <23 years old. The vast majority (95.2%) reported at least one side effect. The most common side effect was injection site pain (91.8%), followed by fatigue (62.5%), headache (36.4%), and muscle pain (34.9%). The majority of local side effects occurred after both doses (74.4%), while most systemic side effects occurred after the second dose only (56.2%). Most local (94.2%) and systemic (93.3%) side effects resolved within three days after vaccination. Females participants’ adjusted odds ratio (AOR) showed they were 2.566 (CI 95%: 1.103–5.970) times more likely to experience post-vaccination side effects, and the participants who received two doses reported an increased AOR of 1.896 (0.708–5.077) for experiencing side effects. The results of this study imply that mRNA-based COVID-19 vaccines are highly probably safe for young adults, and further studies are required to investigate the role of medical anamnesis, prior COVID-19 infection, and gender in side effects incidence.
1. Introduction
The outbreak of the novel coronavirus diseases (COVID-19) has imposed unprecedented challenges to health systems worldwide that has led to disrupted services provision, delayed diagnoses, and increased severity and morbidity of major killers, the non-communicable diseases (NCDs) [1,2,3,4,5,6]. Therefore, mass vaccination strategies are strongly mandated to achieve substantial levels of community immunity that can guarantee the vulnerable with NCDs are protected [7,8]. Apprehension of post-vaccination side effects has been depicted as a key barrier for vaccination by the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) [9,10]. This proposition has been repeatedly confirmed in the context of COVID-19 vaccines, especially among young adults. Khuc et al. (2021) found that concerns about potential side effects were significantly associated with COVID-19 vaccine hesitancy and rejection among Vietnamese youth [11]. Similarly, studies from the United States (US), Egypt, Portugal, China, and Japan concluded that aversion to side effects was associated with an increased risk of vaccine hesitancy among the youth population [12,13,14,15,16,17]. A recent global cross-sectional study of healthcare students (n = 6639) found that the low confidence in COVID-19 vaccines safety was a significant promoter of vaccine hesitancy [18].
In March 2020, Liao et al. published the first epidemiologic evidence of COVID-19’s impact on young adults (≤35 years old), where the vast majority of included cases exhibited mild forms of clinical severity and some of them were asymptomatic [19]. Since that time, asymptomatic young adults have been known to be able to transmit the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection to their households [19]. Therefore, the nonpharmacologic measures in universities, other higher education institutions, and workplaces were deemed necessary to control the community transmission. Several studies emerged recently to demonstrate the negative impact of COVID-19 on the mental health of young adults, especially university students, who were dramatically shifted from campus education to remote learning with very minimal interpersonal communication and support [20,21,22,23,24].
The young adults have faced another challenge in trying return to the normal setting, which is why the WHO guideline recommends prioritising certain population groups to receive COVID-19 vaccines based on their empirically determined risk. Frontline healthcare workers, essential workers, older adults, and individuals with comorbidities were widely accepted as the priority groups in most countries, including the European Union (EU) member states [7]. However, though this policy has proven to be effective so far, it may have increased the levels of vaccine hesitancy among young adults inadvertently by giving them a false sense of protection as a low-risk group. Additionally, this policy led to an increased strain on the young adults’ return to normal settings due to long waits for their vaccinations. For example, the COVID-19 vaccine rollout began in the Czech Republic on 27 December 2020, and young adults (≤30 years old) had to wait over five months to start to register for vaccination on 4 June 2021 [25].
Though young adults have an empirically confirmed low risk of COVID-19 severity for known virus variants, they were found to be at increased risk of long-standing complications following the mild course of COVID-19 infection, which are referred to as (long COVID) [26]. Moreover, young adults represent a critical demographic group for achieving herd immunity through vaccination. Therefore, their attitudes towards receiving COVID-19 vaccines are of practical value for our battle against SARS-CoV-2. Heretofore, we identified a lack of evidence on the post-vaccination side effects of this particular group as they were conventionally combined with middle-aged adults in one cohort, which was consequently compared against the senior adults.
The overarching aim of this study was to evaluate the safety of the mRNA-based COVID-19 vaccines among the young adult population. Therefore, the primary objective was to estimate the prevalence, onset, and duration of the self-reported side effects following mRNA-based vaccine administration. The secondary objective was to evaluate the association between the post-vaccination reported side effects and their potential risk factors among the target population.
2. Materials and Methods
2.1. Design
This post-marketing (phase IV) trial was designed as a cross-sectional study targeting university students in the Czech Republic who received mRNA-based COVID-19 vaccines in the early months of 2021.
The study utilised a validated questionnaire created in a digital form and disseminated using KoBoToolbox version 2.021.03 (Harvard Humanitarian Initiative, Cambridge, MA, USA, 2021). The protocol of this study was registered a priori in the US National Library of Medicine (NLM) with the title (COVID-19 Vaccines Safety Tracking—CoVaST) and under the identifier NCT04834869 [27,28].
2.2. Participants
The target population was young adults aged between 18 and 30 years old; therefore, the full-time students enrolled in Czech universities were approached. Non-random sampling through the snowballing technique was used to recruit the study participants. The digital questionnaire was circulated through the students’ organisations, the students’ representatives of the universities’ academic senates, and the students’ representatives at the Council of Higher Education Institutions [29].
The recruitment took place from 21 April to 15 June 2021; at that time, people < 60 years old were not freely permitted to be vaccinated; however, the healthcare and social care workers, including the volunteering students, had been already halfway through their vaccination phase [30]. Therefore, the students who joined this study were primarily from these volunteering groups.
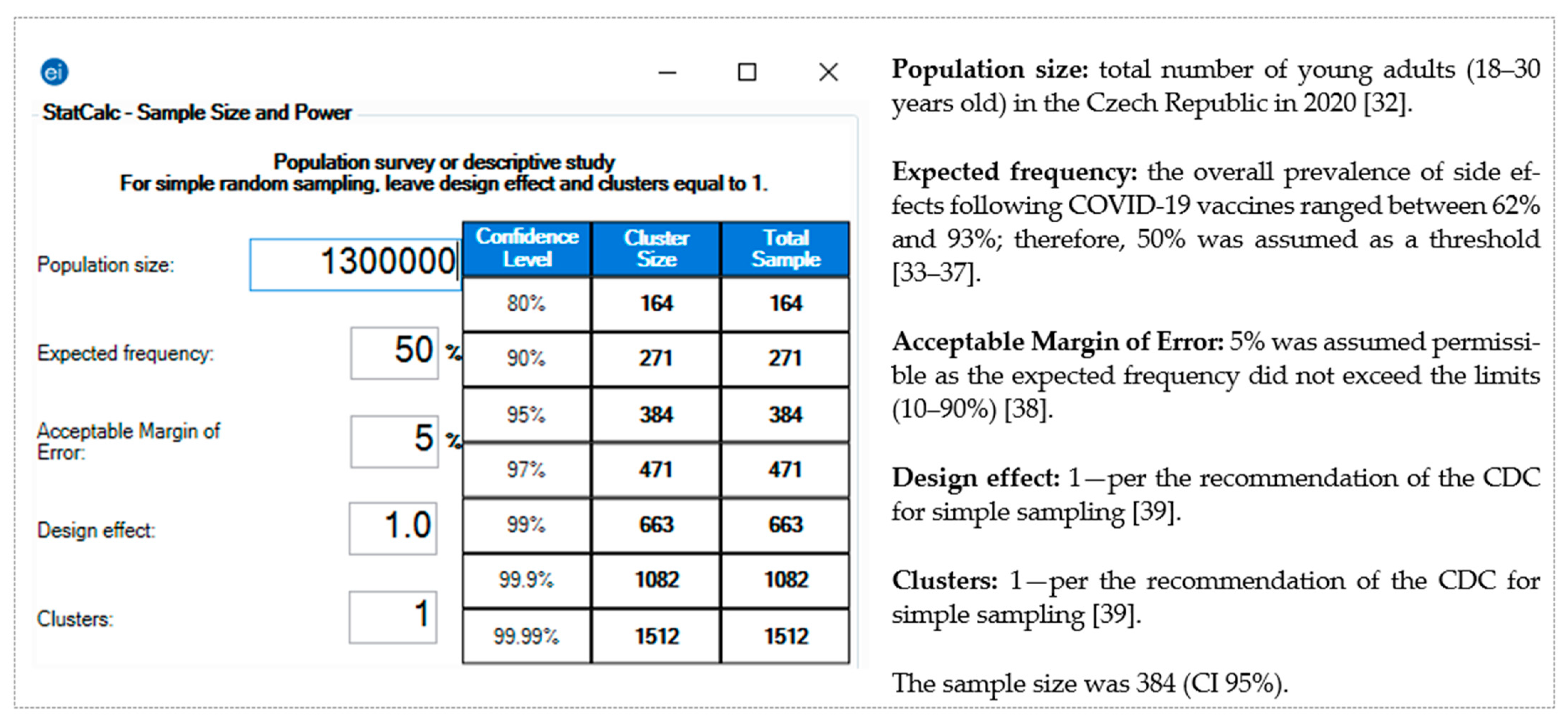
The pragmatic sample size for this study was calculated using Epi-InfoTM version 7.2.4 (CDC, Atlanta, GA, USA, 2020) [31]. The following assumptions were used according to the total population size [32]: expected frequency, 50% [33,34,35,36,37]; margin of error, 5% [38]; confidence interval (CI), 95%; and the required sample was 384 [39] (Figure 1).
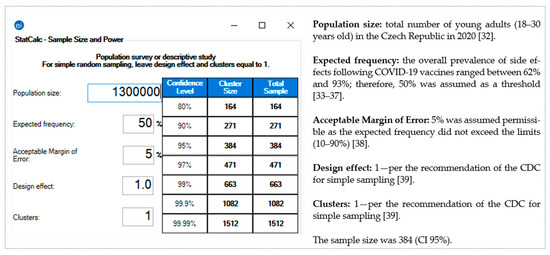
Figure 1.
Sample size of the young adults (18–30 years old) in the Czech Republic—Epi-Info TM version 7.2.4.
2.3. Instrument
The self-administered questionnaire consisted of sixteen closed-ended items, which were stratified into three categories: (a) demographic data, including gender, age, nationality, study year, field, and university; (b) COVID-19 vaccine-related anamnesis, including the type of vaccine, number of doses, and willingness to get the following doses; (c) post-vaccination side effects, including local, systemic, orofacial, and skin-related ones [25].
The questionnaire items were adapted from previous instruments that had been validated [33,34,35,36,37]. The results of the validation and reliability testing process have been published in detail elsewhere [33]. Two independent forward translators translated the instrument from English to Czech, then a panel of experts was appointed to evaluate the two Czech versions to resolve any discrepancies between them and create a common working version.
2.4. Ethics
The study was carried out in accordance with the Declaration of Helsinki, and it was reported according to the STROBE guidelines for cross-sectional studies [40,41]. The ethical approval was obtained from the ethics committee of the Faculty of Medicine, Masaryk University, with Ref No. 26/2021.
The participants had to provide their informed consent digitally before filling in the questionnaire, and they were able to withdraw at any time from the study without the need to justify their decision. The participants did not receive financial compensation or any other form of incentives to minimise both selection and information biases. The study data were stored and processed according to the European Union (EU) General Data Protection Directive (GDPR); therefore, no identifying personal data were collected from the participants [42].
2.5. Analysis
The Statistical Package for the Social Sciences (SPSS) version 27.0 (SPSS Inc., Chicago, IL, USA, 2020) was used to analyse the obtained dataset [43]. Before running any inferential tests, the normal distribution of the dependent variables was tested using Shapiro–Wilk test with a significance level (Sig.) of ≤0.05.
Primarily, descriptive statistics were carried out to present the demographic variables (gender, age, study year, field, and university), COVID-19 vaccine-related anamnesis (number of doses, duration between doses, and vaccine type), and post-vaccination side effects (prevalence, onset, and duration) using frequencies (n), percentages (%), mean (μ), and standard deviation (SD).
Consequently, inferential statistics were carried out to estimate the association between post-vaccination side effects and potential risk factors using the Chi-squared test (χ2), Fisher’s exact test if the expected frequency was less than 5, and the Mann–Whitney test (U). Binary logistic regression was used to evaluate the strength of association between the proposed predictors and the post-vaccination side effects. All the inferential tests were conducted with a significance level (Sig.) of ≤0.05.
3. Results
3.1. Demographic Characteristics
Five hundred and eighty-six students responded to the questionnaire, of which 15 were excluded because they were >30 years old. Further, thirty-two participants received viral vector-based vaccines; therefore, they were excluded from the downstream analysis while their data were pooled and analysed in [25].
Out of the remaining 539 included participants, 378 (70.1%) were females, and 360 (66.8%) were Czech nationals (66.8%). Their mean age was 22.86 ± 2.05 years; therefore, the age of 23 years was used as a cut-off in the downstream analyses. The most represented study field was medical and healthcare sciences (84%), followed by social sciences (5%) and arts and humanities (3.5%). The majority of participants were from Masaryk University (59.9%) and Charles University (30.6%). (Table 1).

Table 1.
Demographic characteristics of young adults (18–30 years old) receiving mRNA-based COVID-19 vaccines, Czech Republic, April–June 2021 (n = 539).
3.2. COVID-19 Vaccine-Related Anamnesis
At the time of filling in the questionnaire, 86.3% of the participants had received two doses of the mRNA-based COVID-19 vaccines, and 13.7% received the first dose only. All the students (100%) who received the first dose were willing to receive the second dose. The mean duration between the first and second dose was 28.84 ± 15.17 days. While 92% received BTN162b2 COVID-19 vaccine, 8% received the mRNA-1273 COVID-19 vaccine. No significant differences between females and males were found in terms of the number of doses, duration between doses, and vaccine type. (Table 2).

Table 2.
mRNA-based COVID-19 vaccine-related anamnesis of young adults (18–30 years old), Czech Republic, April–June 2021 (n = 539).
3.3. Local Side Effects of mRNA-Based COVID-19 Vaccines
Overall, 92.4% of the participants reported at least one local side effect related to the injection site. Female participants (94.4%) had a significantly (χ2 = 7.957; Sig. = 0.005) higher level of local side effects prevalence compared to male participants (87.3%). The most common local side effect was injection site pain (91.8%), followed by injection site swelling (17.4%) and injection site redness (13.4%). Females (1.31 ± 0.77) had a significantly (U = 24,682; Sig. < 0.001) higher level of local side effects intensity compared to males (1.03 ± 0.59). The intensity was defined as the number of side effects reported by an individual, and it ranged between 0 and 3.
The ≥23-years-old participants (92.5%) had a similar level of local side effects compared to the <23-years-old participants (92.3%). Slovak students (95.5%) reported a higher level of local side effect prevalence than Czechs (90.8%). The healthcare students (94.3%) and the students who received two doses (93.5%) reported significantly (χ2 = 14.084 and 6.430; Sig. < 0.001 and =0.011) higher levels of local side effects compared to the non-healthcare students (82.6%) and the students who received one dose (85.1%).
While 74.4% of the participants who experienced local side effects reported that they occurred after both doses, 19.6% reported them after the first dose only, and 6% reported them after the second dose only. No significant differences were found in the onset of local side effects between females and males. The vast majority (94.2%) of local side effects resolved within three days after the vaccination—28.2% after the first day, 43.8% after the second day, and 22.2% after the third day. In general, the mean duration of local side effects was significantly different (U = 21,219.5; Sig. = 0.017) between females (2.15 ± 0.96) and males (1.93 ± 0.88). (Table 3).

Table 3.
Local side effects of young adults (18–30 years old) receiving mRNA-based COVID-19 vaccines, Czech Republic, April–June 2021 (n = 539).
The mean duration of local side effects was not significantly (U = 30,708 and 25,165.5; Sig. = 0.906 and 0.083) different among the ≥23-years-old participants (2.11 ± 1.01) vs. the <23-years-old participants (2.05 ± 0.86), and Czech students (2.15 ± 0.99) vs. Slovak students (1.96 ± 0.84). The mean duration of local side effects was significantly (U = 17,257.5 and 11,553; Sig. = 0.025 and 0.037) different among the healthcare students (2.05 ± 0.94) vs. the non-healthcare students (2.30 ± 0.97), and the students with two doses (2.06 ± 0.96) vs. the students with one dose (2.25 ± 0.82).
3.4. Systemic Side Effects of mRNA-Based COVID-19 Vaccines
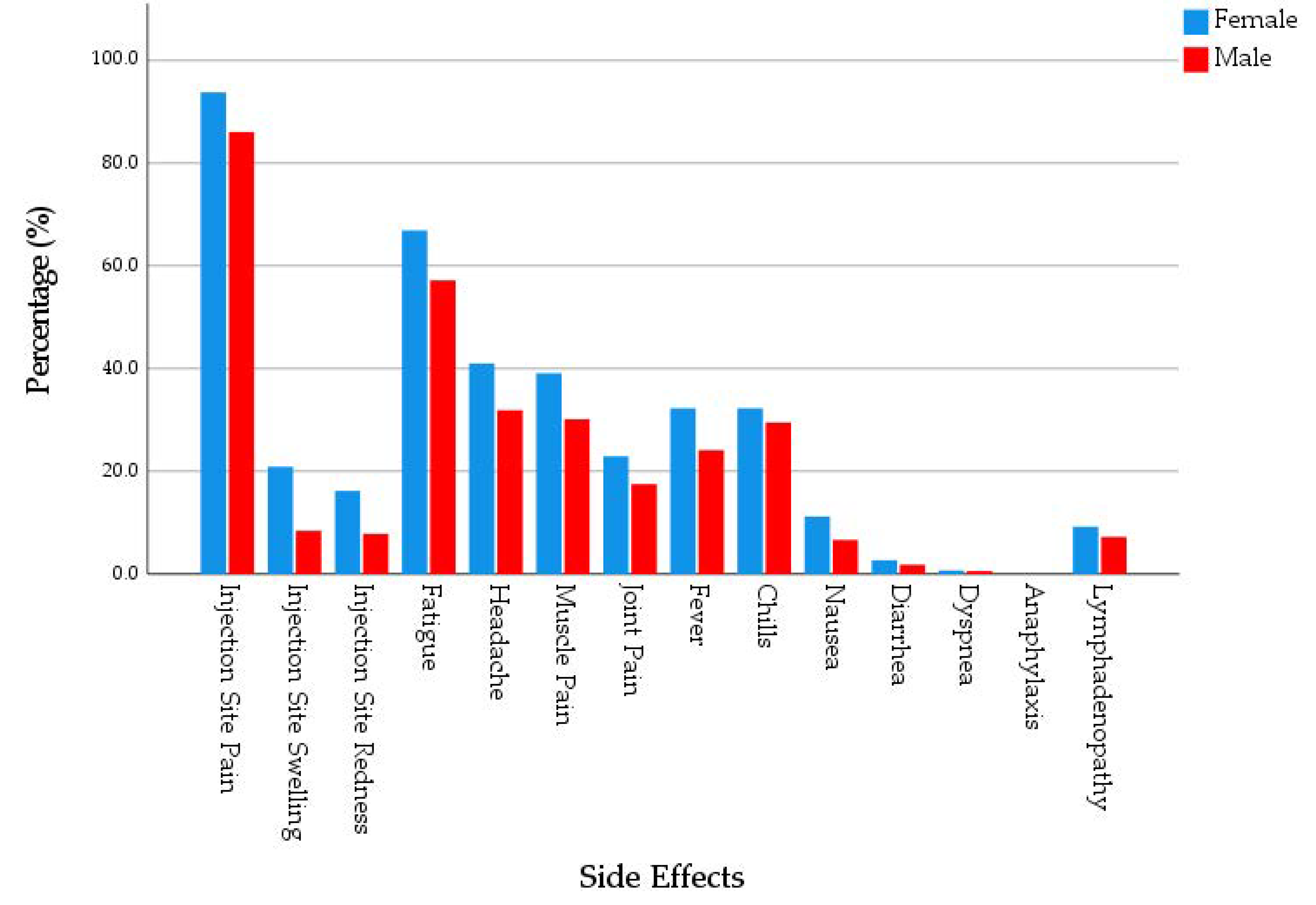
Overall, 72.5% of the participants reported at least one systemic side effect. Female participants (74.6%) had a slightly higher level of systemic side effects prevalence compared to male participants (67.7%). The most common systemic side effect was fatigue (62.5%), followed by headache (36.4%), muscle pain (34.9%), chills (29.9%), fever (27.3%), and joint pain (20.4%). Four participants, three of them were females, reported dyspnoea, and no participants reported anaphylaxis. Females had significantly higher prevalence of fatigue (65.6% vs. 55.7%), headache (39.2% vs. 29.7%), fever (29.6% vs. 21.5%), and nausea (11.1% vs. 5.1%) compared to males (χ2 = 4.680, 4.260, 3.698 and 4.818; Sig. = 0.031, 0.039, 0.054 and 0.028, respectively). Females (2.48 ± 2.19) had a significantly (U = 25,498.5; Sig. = 0.007) higher level of systemic side effects intensity compared to males (1.94 ± 2.08). The intensity was defined as the number of side effects reported by an individual, and it ranged between 0 and 11. (Figure 2).
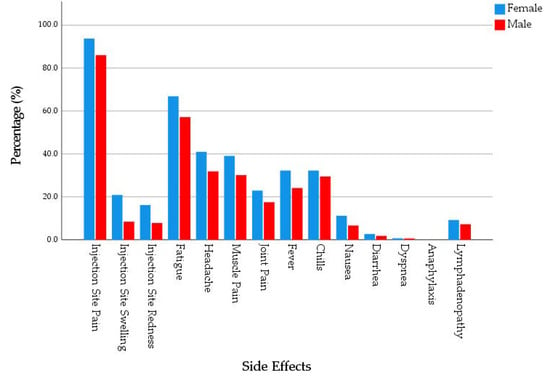
Figure 2.
Side Effects of mRNA-based COVID-19 Vaccines Experienced by Young Adults (18–30 years old), Czech Republic, April–June 2021 (n = 539).
The ≥23-years-old participants (73.6%), Czech students (71.7), and the healthcare students (72.4) had a similar level of systemic side effects prevalence compared to the <23-years-old participants (71.3%), Slovak students (74.3%), and the non-healthcare students (73.3). The students who received two doses (74.6%) reported a significantly (χ2 = 7.370; Sig. = 0.007) higher level of systemic side effects prevalence compared to the students who received one dose (59.5%).
While 56.2% of the participants who experienced systemic side effects reported that they occurred after the second dose only, 16.4% reported them after the first dose only and 27.4% reported them after both doses. The vast majority (93.3%) of systemic side effects resolved within three days after the vaccination—46.9% after the first day, 33.6% after the second day, and 12.8% after the third day. In general, the mean duration of systemic side effects was significantly longer (U = 13,160; Sig. = 0.050) among females (1.91 ± 1.13) compared to males (1.70 ± 1.03). (Table 4).

Table 4.
Systemic side effects of young adults (18–30 years old) receiving mRNA-based COVID-19 vaccines, Czech Republic, April–June 2021 (n = 539).
Only four participants, three females and one male, reported that their systemic side effects lasted for over a month, and their side effects included fatigue (75%), headache (25%), muscle pain (25%), and lymphadenopathy (50%). The onset of their systemic side effects was either after the second dose only (50%) or after both doses (50%).
The mean duration of systemic side effects was not significantly (U = 17,335, 15,894, and 6572.5; Sig. = 0.149, 0.245, and 0.169) different among the ≥23-years-old participants (1.78 ± 1.04) vs. the <23-years-old participants (1.94 ± 1.18), Czech students (1.89 ± 1.11) vs. Slovak students (1.78 ± 1.09), and the students who received two doses (1.84 ± 1.12) vs. those who received one dose (1.95 ± 0.925). The mean duration of local side effects was significantly (U = 12,378; Sig. = 0.003) different among the healthcare students (1.80 ± 1.10) vs. the non-healthcare students (2.15 ± 1.10).
3.5. Orofacial and Skin-Related Side Effects of mRNA-Based COVID-19 Vaccines
Overall, 3.5% of the participants reported at least one orofacial or skin-related side effect. Oral paraesthesia (1.3%) was the most common side effect, followed by oral ulcers (1.1%), taste disturbance (0.4%), skin rash (0.4%), and skin eruptions (0.4%). Only one female student (22 years old) reported Bell’s palsy following receiving BNT162b2. There was no significant difference between females and males in terms of orofacial and skin-related side effects prevalence or intensity. (Table 5).

Table 5.
Orofacial and skin-related side effects of young adults (18–30 years old) receiving mRNA-based COVID-19 vaccines, Czech Republic, April–June 2021 (n = 539).
3.6. Analgesic Drugs after mRNA-Based COVID-19 Vaccines
Out of the 539 participants, 165 (30.6%) reported using analgesic drugs after the vaccination to relieve their post-vaccination side effects. Females (34.7%) had a significantly (χ2 = 10.922; Sig. = 0.001) higher level of analgesics consumption compared to males (20.3%).
The most frequently used drug was acetaminophen (69.1%), through its common brand names Paralen (Opella Healthcare Czech s.r.o., Prague, Czech Republic) and Panadol (GlaxoSmithKline Consumer Healthcare Czech Republic s.r.o., Prague, Czech Republic). About one quarter of the participants consumed ibuprofen (25.5%) through its common brand names Ibalgin (Opella Healthcare Czech s.r.o., Prague, Czech Republic) and Ibuprofen (STADA PHARMA CZ s.r.o., Prague, Czech Republic) (Table 6).

Table 6.
Analgesics used by young adults (18–30 years old) receiving mRNA-based COVID-19 vaccines, Czech Republic, April–June 2021 (n = 539).
Most side effects, both locally and systemically, were significantly associated with the use of analgesics, including injection site pain (33.1% vs. 2.3%; χ2 = 18.115; Sig. < 0.001), injection site swelling (39.4% vs. 28.8%; χ2 = 4.103; Sig. = 0.001), fatigue (43.3% vs. 9.4%; χ2 = 68.401; Sig. < 0.001), headache (55.6% vs. 16.3%; χ2 = 90.626; Sig. < 0.001), muscle pain (51.6% vs. 19.4%; χ2 = 59.844; Sig. < 0.001), joint pain (59.1% vs. 23.3%; χ2 = 52.770; Sig. < 0.001), fever (70.1% vs. 15.8%; χ2 = 148.137; Sig. < 0.001), chills (55.8% vs. 20.4%; χ2 = 65.411; Sig. < 0.001), nausea (56% vs. 28%; χ2 = 16.723; Sig. < 0.001), and lymphadenopathy (47.9% vs. 28.9%; χ2 = 7.428; Sig. = 0.006).
The use of analgesics was significantly (U = 35,768 and 51,414.5; Sig. < 0.001 and <0.001) associated with higher intensity levels of local side effects (1.39 ± 0.71 vs. 1.16 ± 0.72) and systemic side effects (4.05 ± 1.90 vs. 1.56 ± 1.82). Similarly, the use of analgesics was significantly (U = 31,547 and 21,385.5; Sig. = 0.003 and 0.005) associated with longer duration of local (2.25 ± 0.95 vs. 2.00 ± 0.93) and systemic side effects (1.95 ± 1.01 vs. 1.78 ± 1.16).
3.7. Risk Factors of Post-Vaccination Side Effects
Binary logistic regression revealed that females with an adjusted odds ratio (AOR) were 2.566 (CI 95%: 1.103–5.970; Sig. = 0.029) times more likely to experience post-vaccination side effects compared to males. The ≥23-years-old participants had an AOR of 1.791 (CI 95%: 0.775–4.139; Sig. = 0.173) for experiencing post-vaccination side effects compared to the <23-years-old participants. Similarly, Slovak students (AOR: 2.592; CI 95%: 0.842–7.979), healthcare students (AOR: 2.933; CI 95%: 1.100–7.825), the participants who received two doses (AOR: 1.896; CI 95%: 0.708–5.077), and the participants who received BNT16b2 (AOR: 1.389; CI 95%: 0.377–5.110) had higher adjusted ratios of experiencing post-vaccination side effects. (Table 7).

Table 7.
Predictors of mRNA-based COVID-19 vaccines side effects (general side effects) experienced by young adults (18–30 years old), Czech Republic, April–June 2021 (n = 539).
On analysing the potential risk factors of local side effects, the AOR for female participants showed that they were2.903 (CI 95%: 1.473–5.722; Sig. = 0.002) times more likely to experience local side effects compared to males. Similarly, the AOR of healthcare students showed that they were 3.542 (CI 95%: 1.545–7.712; Sig. = 0.003) times more likely to experience local side effects compared to non-healthcare students.
On analysing the potential risk factors of systemic side effects, the participants who received two doses had an AOR showing that they were 2.237 (CI 95%: 1.261–3.969; Sig. = 0.006) times more likely to experience systemic side effects than the participants who received one dose. (Table 8).

Table 8.
Predictors of mRNA-based COVID-19 vaccines side effects (local and systemic side effects) experienced by young adults (18–30 years old), Czech Republic, April–June 2021 (n = 539).
4. Discussion
In total, 95.2% of the participating young adults (18–30 years old) reported at least one side effect after vaccination against COVID-19 with mRNA-based vaccines. Although we were also collecting data about viral vector-based vaccines in the Czech Republic, we collected a very small sample size that would not contribute to the statistical analyses. The most common side effects of mRNA-based vaccines were injection site pain (91.8%), fatigue (62.5%), headache (36.4%), and muscle pain (34.9%). The majority of local side effects occurred after both doses (74.4%), while most systemic side effects occurred after the second dose only (56.2%). Most local (94.2%) and systemic (93.3%) side effects resolved within three days after vaccination.
All prior active surveillance studies of COVID-19 vaccines concluded that younger age groups had an increased risk of side effects incidence [33,34,35,36,37,44,45,46,47,48,49]. Mathioudakis et al. (2021) surveyed a sample of recently vaccinated individuals, mainly from the United Kingdom (UK) and Greece, using 60 years as a cut-off for their age-related analysis [45]. Their multivariate analyses confirmed a strong negative relationship between age and the self-reported side effects [45]. In a national cross-sectional study in the UK, Menni et al., 2021 found that the ≤55-years-old individuals had significantly higher levels of side effects prevalence, including injection site pain, headache, and fatigue, compared to the >55-years-old individuals [49]. This trend was found in both the mRNA-based (BNT162b2) and the viral vector-based vaccine (ChAdOx1 nCoV-19) [49].
Riad et al., (2021) examined a sample of healthcare workers from the Czech Republic who received the BNT162b2 vaccine and found that the ≤43-years-old group had significantly higher levels of general side effects [33]. Similar results were found in Jordan by Abu-Hammad et al., 2021 and in Malta by Cuschieri et al., (2021) among healthcare workers while using 45 years of age as a cut-off point [47,50].
In a randomised phase IV trial of mRNA-1273, young adults (18–30 years old) represented only 6.02% of the entire sample, thus indicating that this cohort was not an interesting population group for the investigators [44]. In the rest of the published post-marketing studies, there is a lack of age-stratified analyses; therefore, it is not possible to evaluate the safety profile of COVID-19 vaccines for young adults based on these studies [33,34,35,36,37,45,46,47,48,49].
On evaluating the phase III results of the BNT162b2 vaccine, published by the US Centre for Disease Control and Prevention (CDC), injection site pain (80.5%) among young and middle-aged adults (18–55 years old) was significantly (χ2 = 38.568; Sig. < 0.001) less prevalent than what was found in our participants (91.9%) who received the BNT162b2 vaccine [51]. Similarly, injection site swelling and injection site redness were significantly (χ2 = 77.591 and 49.899; Sig. < 0.001 and <0.001) more prevalent among our sample (16.7% and 13.1%, respectively) than in the manufacturer’s report (6% and 5.2%, respectively) [51].
Fatigue was significantly (χ2 = 13.775; Sig. < 0.001) more prevalent among our sample (61.9%) than in the manufacturer’s report (53.1%) [51]. Similarly, muscle pain (34.7% vs. 28.9%), fever (26% vs. 9.5%), chills (27.4% vs. 24.1%), and joint pain (20.2% vs. 16.2%) were more prevalent in our sample compared to the manufacturer’s report [51]. Contrarily, headache (46.6% vs. 36.5%) and diarrhoea (10.8% vs. 2.8%) were more prevalent in the manufacturer’s report than in our sample [51]. The analgesics consumption was slightly lower among our sample (29.8%) compared to the manufacturer’s report (30.1%) [51].
In our sample, females were at greater risk of experiencing post-vaccination side effects. In February 2021, the CDC published a report on the side effects of COVID-19 vaccines, where 72% of the reports were of females, while only 61% of the vaccine doses were administered to females [52]. This result is in agreement with the findings of Di Resta et al. (2021), where post-vaccination side effects were more frequent among female healthcare workers in Italy compared to their male colleagues [53]. They also found that females had significantly higher serological values, thus suggesting that the more frequent and more severe side effects experienced by females could be related to the more vigorous immune response they had developed [53].
While testosterone generally decreases the immune functions and increases, in particular, males’ susceptibility to viral infections, the physiological levels of oestrogen stimulate humoral responses to viral infections by activating antibody-producing cells [54,55]. The more potent immune response and the lower pain threshold of females are among the suggested propositions attempting to explain the gender-based differences in self-reported COVID-19 vaccine side effects [56,57]. Moreover, the sociocultural structure of femininity and masculinity may play another role in this issue, as females are more inclined to seek medical care than males, who may have several barriers to help-seeking behaviours [58].
In the past, the female gender was reportedly associated with a higher level of side effects prevalence after various viral vaccines, e.g., influenza, attenuated Japanese encephalitis, measles–mumps–rubella combination vaccine (MMR), and attenuated Dengue vaccines [56,59]. Halsey et al. (2013) found that females were four times more likely to report allergic reactions following H1N1 vaccination than males, and this difference was only prominent during the childbearing age and disappeared in the other age groups [60].
Di Resta et al. (2021) also found that antibody titre and side effects were decreasing with age, thus placing the young adults at a greater risk of more frequent and more severe side effects, especially the female youth [53]. On comparing adolescents (12–15 years old) and young adults (18–25 years old), the BNT162b2 vaccine was found to induce greater immune response among adolescents and almost the same safety profile and side effects prevalence [61].
Oral paraesthesia (1.3%) and oral ulcers (1.1%) were rarely reported by our participants, thus indicating that oral side effects among young adults might have a low prevalence. While the COVID-19 infection-related oral manifestations were reported by young adults as well as middle-aged and senior adults, all the reported cases of oral side effects following BNT162b2 and ChAdOx1 nCoV-19 vaccines belonged to middle-aged adults [62,63,64,65,66,67,68,69,70,71,72]. Skin rash (0.4%) and skin eruptions (0.4%) were also reported rarely by our participants and evidence on the predicted prevalence of the rare orofacial and skin-related side effects is still lacking [73].
4.1. Strengths
To the best of our knowledge, this is the first study to evaluate the self-reported side effects of young adults (18–30 years old) following COVID-19 vaccination in the post-marketing phase. The recruited sample of this study were university students (84% in healthcare) with a likely higher level of health literacy and scientific background that predisposes them to understand and fill out this kind of questionnaire reliably and properly.
The proportion of the participants who had received one dose was small and all of them expressed their interest to get the second dose regardless of the side effects they had experienced. Another strong point of this study is that it is one of the few studies that investigated the use of analgesics to manage the post-vaccination side effects.
4.2. Limitations
The first limitation of this study is the lack of information about the medical anamnesis of the participants, including chronic diseases and regular medications; nevertheless, this can be justified by the fact that the prevalence of chronic illnesses among this particular age group is supposedly very low and it would not have yielded a comparable sample size to explore the impact of pre-existing conditions and medications on the post-vaccination side effects.
The second limitation is the lack of information about any prior COVID-19 infection of the participants, though this can be justified by the fact that clinical presentation of COVID-19 in young adults tends to be mild or even asymptomatic, which may lead to underestimation of the impact of prior COVID-19 infection on the post-vaccination side effects [19]. Nevertheless, mild COVID-19 in young adults can lead to prolonged complications referred to as “long COVID”, which requires further investigation to establish the impact of COVID-19 vaccination on these complications [26].
The third limitation is the minuscule proportion of the participants who received the mRNA-1273 vaccine and viral vector-based COVID-19 vaccines in this sample; however, this can be justified by the fact that 81.95% of the administered shots in the Czech Republic were of BNT162b2 vaccine, 8.45% were of mRNA-1273, 8.44% were of ChAdOx1 nCoV-19, and 1.15% were of Ad26.COV2.S, as of 22 July 2021 [74]. Therefore, our sample was deemed to represent the actual situation in the Czech Republic, even if a non-random sampling technique was used.
The fourth limitation is the gender imbalance in our sample, as 70.1% of the participants were females. The latest report of the Czech Statistical Office (ČSÚ) revealed that 55.6% of public university students and 57.1% of private university students were females [32].
The fifth limitation is due to the snowballing technique (non-random sampling) that was used in recruiting the participants, as it may have led to self-selection bias, thus causing overestimation of the side effects prevalence. The students who experienced post-vaccination side effects may have been more inclined to respond to the questionnaire and pass it to their colleagues than those who did not experience post-vaccination side effects.
The sixth limitation is the lack of information about the severity of the solicited side effects in this survey, which had been omitted from this study because this type of question is subjected to a high risk of recall bias; therefore, future research on young adults’ side effects is recommended to investigate the severity of the mRNA-based COVID-19 vaccine side effects.
4.3. Implications
The findings of this study confirmed that immunisation of young adults against COVID-19 using mRNA-based vaccines is highly probably a safe process that needs to be accelerated to reach substantial levels of collective (herd) immunity. Future studies should evaluate the role of medical anamnesis and prior COVID-19 infection as they may have a role in the incidence and intensity of post-vaccination side effects among young adults, as the healthy young adults may have a stronger immune response, thus yielding more burdening side effects.
Future research needs to investigate the impact of COVID-19 vaccination on long COVID-19 complications among young adults. The gender-based differences of COVID-19 vaccine side effects require further investigation, where the female-related confounding variables like the menstrual cycle, pregnancy, and contraceptive consumption should be controlled. In addition, future research on COVID-19 vaccine safety should carry out age-stratified analyses, with a highlight on the young adult group (18–30 years old).
5. Conclusions
To the best of our knowledge, this is the first study to focus on the side effects of COVID-19 vaccines among young adults. In total, 95.2% of the participants reported at least one side effect after vaccination against COVID-19 with mRNA-based vaccines. The most common side effect was injection site pain (91.8%), followed by fatigue (62.5%), headache (36.4%), and muscle pain (34.9%).
The majority of local side effects occurred after both doses (74.4%), while most systemic side effects occurred after the second dose only (56.2%). Most local (94.2%) and systemic (93.3%) side effects resolved within three days after vaccination. The AOR of females participants showed that they were 2.566 (CI 95%: 1.103–5.970) times more likely to experience post-vaccination side effects, and the participants who received two doses had an increased AOR of 1.896 (0.708–5.077) for experiencing side effects.
The results of this study imply that mRNA-based COVID-19 vaccines are highly probably safe for young adults, and further studies are required to investigate the role of medical anamnesis, prior COVID-19 infection, and gender in side effects incidence.
Author Contributions
Conceptualisation, A.R.; methodology, A.R. and M.K. (Miloslav Klugar); validation, M.K. (Michal Koščík), J.K. and L.K.; formal analysis, A.R.; investigation, N.A. and M.K. (Michal Koščík); writing—original draft preparation, A.R.; writing—review and editing, A.P., J.K., L.K. and M.K. (Miloslav Klugar); project administration, A.R.; funding acquisition, M.K. (Miloslav Klugar) and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Masaryk University, grant numbers MUNI/IGA/1543/2020, MUNI/A/1608/2020, and MUNI/IGA/1068/2020. The work of A.R., A.P., J.K. and M.K. (Miloslav Klugar) was supported by the INTER-EXCELLENCE grant number LTC20031—”Towards an International Network for Evidence-based Research in Clinical Health Research in the Czech Republic”.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of the Faculty of Medicine, Masaryk University Ref No. 3/2021 on 20 January 2021.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
This work is dedicated to the more than thirty thousand fatalities and their families who have fallen victim to COVID-19 in the Czech Republic. The authors would like to thank Zuzana Derflerová Brázdová for her support in conducting this study and collecting data from the target population.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mercier, G.; Arquizan, C.; Roubille, F. Understanding the effects of COVID-19 on health care and systems. Lancet Public Health 2020, 5, e524. [Google Scholar] [CrossRef]
- Chang, A.Y.; Cullen, M.R.; Harrington, R.A.; Barry, M. The impact of novel coronavirus COVID-19 on noncommunicable disease patients and health systems: A review. J. Intern. Med. 2021, 289, 450–462. [Google Scholar] [CrossRef]
- Richards, M.; Anderson, M.; Carter, P.; Ebert, B.L.; Mossialos, E. The impact of the COVID-19 pandemic on cancer care. Nat. Cancer 2020, 1, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Bojdani, E.; Rajagopalan, A.; Chen, A.; Gearin, P.; Olcott, W.; Shankar, V.; Cloutier, A.; Solomon, H.; Naqvi, N.Z.; Batty, N.; et al. COVID-19 Pandemic: Impact on psychiatric care in the United States. Psychiatry Res. 2020, 289, 113069. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, F.; Gualano, G.; Timelli, L.; Vittozzi, P.; Di Bari, V.; Libertone, R.; Cerva, C.; Pinnarelli, L.; Nisii, C.; Ianniello, S.; et al. Increase in Tuberculosis Diagnostic Delay during First Wave of the COVID-19 Pandemic: Data from an Italian Infectious Disease Referral Hospital. Antibiotics 2021, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Boccuzzi, M.; Pold, A.; Krsek, M. The alarming burden of non-communicable diseases in COVID-19 new normal: Implications on oral health. Oral Dis. 2020, 27, 791–792. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization (PAHO) of the World Health Organization (WHO). Introducing COVID-19 Vaccination: Guidance for Determining Priority Groups and Microplanning. Version 1; 18 January 2021. Available online: https://iris.paho.org/handle/10665.2/53318 (accessed on 13 October 2021).
- Frederiksen, L.S.F.; Zhang, Y.; Foged, C.; Thakur, A. The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies. Front. Immunol. 2020, 11, 1817. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, N.E.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B.; Goldstein, S.; Larson, H.; Manzo, M.L.; Reingold, A.; et al. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Strategic Advisory Group of Experts on Immunization (SAGE). Vaccine Hesitancy Survey Questions Related to SAGE Vaccine Hesitancy Matrix; WHO: Geneva, Switzerland.
- Van Khuc, Q.; Nguyen, T.; Nguyen, T.; Pham, L.; Le, D.-T.; Ho, H.-H.; Truong, T.-B.; Tran, Q.-K. Young Adults’ Intentions and Rationales for COVID-19 Vaccination Participation: Evidence from a Student Survey in Ho Chi Minh City, Vietnam. Vaccines 2021, 9, 794. [Google Scholar] [CrossRef]
- Lucia, V.C.; Kelekar, A.; Afonso, N.M. COVID-19 vaccine hesitancy among medical students. J. Public Health 2020, 43, 445–449. [Google Scholar] [CrossRef]
- Tam, C.C.; Qiao, S.; Li, X. Factors associated with decision making on COVID-19 vaccine acceptance among college students in South Carolina. medRxiv 2020, 1–12. [Google Scholar] [CrossRef]
- Saied, S.M.; Saied, E.M.; Kabbash, I.A.; Abdo, S.A.E. Vaccine hesitancy: Beliefs and barriers associated with COVID-19 vaccination among Egyptian medical students. J. Med. Virol. 2021, 93, 4280–4291. [Google Scholar] [CrossRef]
- Soares, P.; Rocha, J.V.; Moniz, M.; Gama, A.; Laires, P.A.; Pedro, A.R.; Dias, S.; Leite, A.; Nunes, C. Factors Associated with COVID-19 Vaccine Hesitancy. Vaccines 2021, 9, 300. [Google Scholar] [CrossRef]
- Yoda, T.; Katsuyama, H. Willingness to Receive COVID-19 Vaccination in Japan. Vaccines 2021, 9, 48. [Google Scholar] [CrossRef]
- Wang, J.; Jing, R.; Lai, X.; Zhang, H.; Lyu, Y.; Knoll, M.D.; Fang, H. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines 2020, 8, 482. [Google Scholar] [CrossRef]
- Riad, A.; Abdulqader, H.; Morgado, M.; Domnori, S.; Koščík, M.; Mendes, J.J.; Klugar, M.; Kateeb, E. Global Prevalence and Drivers of Dental Students’ COVID-19 Vaccine Hesitancy. Vaccines 2021, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Fan, S.; Chen, J.; Wu, J.; Xu, S.; Guo, Y.; Li, C.; Zhang, X.; Wu, C.; Mou, H.; et al. Epidemiological and Clinical Characteristics of COVID-19 in Adolescents and Young Adults. Innovation 2020, 1, 100001. [Google Scholar] [CrossRef]
- Elmer, T.; Mepham, K.; Stadtfeld, C. Students under lockdown: Comparisons of students’ social networks and mental health before and during the COVID-19 crisis in Switzerland. PLoS ONE 2020, 15, e0236337. [Google Scholar] [CrossRef] [PubMed]
- Fawaz, M.; Samaha, A. E-learning: Depression, anxiety, and stress symptomatology among Lebanese university students during COVID-19 quarantine. Nurs. Forum 2021, 56, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Alawia, R.; Riad, A.; Kateeb, E. Risk perception and readiness of dental students to treat patients amid COVID-19: Implication for dental education. Oral Dis. 2020. [Google Scholar] [CrossRef]
- Marotta, C.; Nacareia, U.; Estevez, A.; Tognon, F.; Genna, G.; De Meneghi, G.; Occa, E.; Ramirez, L.; Lazzari, M.; Di Gennaro, F.; et al. Mozambican Adolescents and Youths during the COVID-19 Pandemic: Knowledge and Awareness Gaps in the Provinces of Sofala and Tete. Healthcare 2021, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Alawia, R.; Riad, A.; Kateeb, E. Knowledge and attitudes among dental students about COVID-19 and its precautionary measures: A cross-sectional study. J. Oral Med. Oral Surg. 2021, 27, 17. [Google Scholar] [CrossRef]
- Riad, A.; Pokorná, A.; Antalová, N.; Krobot, M.; Zviadadze, N.; Serdiuk, I.; Koščík, M.; Klugar, M. Prevalence and Drivers of COVID-19 Vaccine Hesitancy among Czech University Students: National Cross-Sectional Study. Vaccines 2021, 9, 948. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, B.; Mohn, K.G.-I.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.-A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Klugar, M.; Riad, A. COVID-19 Vaccines Safety Tracking (CoVaST). Available online: https://clinicaltrials.gov/ct2/show/NCT04834869 (accessed on 15 July 2021).
- Riad, A.; Schünemann, H.; Attia, S.; Peričić, T.P.; Žuljević, M.F.; Jürisson, M.; Kalda, R.; Lang, K.; Morankar, S.; Yesuf, E.A.; et al. COVID-19 Vaccines Safety Tracking (CoVaST): Protocol of a Multi-Center Prospective Cohort Study for Active Surveillance of COVID-19 Vaccines’ Side Effects. Int. J. Environ. Res. Public Health 2021, 18, 7859. [Google Scholar] [CrossRef] [PubMed]
- Masaryk University Clubs & Associations at the Faculty of Medicine. Available online: https://www.muni.cz/en/students/student-clubs-associations/contact-1/faculty-of-medicine (accessed on 20 June 2021).
- Ministerstvo Vnitra Časová osa Očkování. Available online: https://covid.gov.cz/situace/registrace-na-ockovani/casova-osa-ockovani (accessed on 21 July 2021).
- Centers for Disease Control and Prevention, (CDC) Epi InfoTM for Windows. Available online: https://www.cdc.gov/epiinfo/pc.html (accessed on 25 December 2020).
- Czech Statistical Office Statistical Yearbook of the Czech Republic. 2020. Available online: https://www.czso.cz/csu/czso/statistical-yearbook-of-the-czech-republic-2020 (accessed on 1 July 2021).
- Riad, A.; Pokorná, A.; Attia, S.; Klugarová, J.; Koščík, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef]
- Riad, A.; Pokorná, A.; Mekhemar, M.; Conrad, J.; Klugarová, J.; Koščík, M.; Klugar, M.; Attia, S. Safety of ChAdOx1 nCoV-19 Vaccine: Independent Evidence from Two EU States. Vaccines 2021, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Klugar, M.; Riad, A.; Mekhemar, M.; Conrad, J.; Buchbender, M.; Howaldt, H.-P.; Attia, S. Side Effects of mRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers. Biology 2021, 10, 752. [Google Scholar] [CrossRef]
- Riad, A.; Sağıroğlu, D.; Üstün, B.; Pokorná, A.; Klugarová, J.; Attia, S.; Klugar, M. Prevalence and Risk Factors of CoronaVac Side Effects: An Independent Cross-Sectional Study among Healthcare Workers in Turkey. J. Clin. Med. 2021, 10, 2629. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Hocková, B.; Kantorová, L.; Slávik, R.; Spurná, L.; Stebel, A.; Havriľak, M.; Klugar, M. Side Effects of mRNA-Based COVID-19 Vaccine: Nationwide Phase IV Study among Healthcare Workers in Slovakia. Pharmaceuticals 2021, 14, 873. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Vahedi, M.; Rahimzadeh, M. Sample size calculation in medical studies. Gastroenterol. Hepatol. From Bed Bench 2013, 6, 14. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Population Survey or Descriptive Study. Available online: https://www.cdc.gov/epiinfo/user-guide/statcalc/samplesize.html (accessed on 19 May 2021).
- World Medical Association. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. UroToday Int. J. 2009, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proton Technologies AG General Data Protection Regulation (GDPR) Compliance Guidelines. Available online: https://gdpr.eu/ (accessed on 1 May 2020).
- SPSS Inc. IBM SPSS Statistics 27. Available online: https://www.ibm.com/support/pages/node/3006603 (accessed on 14 March 2021).
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Gajula, V.; Madathala, R.R.; Chennaiahgari, N.; Malayala, S. V Adverse effects of COVID-19 mRNA-1273 vaccine: A randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J. Med. Virol. 2021, 1–10. [Google Scholar] [CrossRef]
- Mathioudakis, A.G.; Ghrew, M.; Ustianowski, A.; Ahmad, S.; Borrow, R.; Papavasileiou, L.P.; Petrakis, D.; Bakerly, N.D. Self-reported real-world safety and reactogenicity of covid-19 vaccines: A vaccine recipient survey. Life 2021, 11, 249. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Harakeh, S.; Badr-Eldin, S.M.; Bagher, A.M.; Eid, B.G.; Almukadi, H.S.; Alghamdi, B.S.; Alahmadi, A.A.; Hassan, N.A.; Sindi, N.; et al. Minor to Moderate Side Effects of Pfizer-BioNTech COVID-19 Vaccine Among Saudi Residents: A Retrospective Cross-Sectional Study. Int. J. Gen. Med. 2021, 14, 1389–1401. [Google Scholar] [CrossRef]
- Abu-Hammad, O.; Alduraidi, H.; Abu-Hammad, S.; Alnazzawi, A.; Babkair, H.; Abu-Hammad, A.; Nourwali, I.; Qasem, F.; Dar-Odeh, N. Side Effects Reported by Jordanian Healthcare Workers Who Received COVID-19 Vaccines. Vaccines 2021, 9, 577. [Google Scholar] [CrossRef]
- Alhazmi, A.; Alamer, E.; Daws, D.; Hakami, M.; Darraj, M.; Abdelwahab, S.; Maghfuri, A.; Algaissi, A. Evaluation of Side Effects Associated with COVID-19 Vaccines in Saudi Arabia. Vaccines 2021, 9, 674. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Cuschieri, S.; Borg, M.; Agius, S.; Souness, J.; Brincat, A.; Grech, V. Adverse reactions to Pfizer-BioNTech vaccination of healthcare workers at Malta’s state hospital. Int. J. Clin. Pract. 2021, e14605. [Google Scholar] [CrossRef]
- Centres for Diseases Control and Prevention (CDC). Reactions and Adverse Events of the Pfizer-BioNTech COVID-19 Vaccine; Centres for Diseases Control and Prevention (CDC): Atlanta, GA, USA, 2021.
- Gee, J. First Month of COVID-19 Vaccine Safety Monitoring—United States, December 14, 2020–January 13, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Di Resta, C.; Ferrari, D.; Viganò, M.; Moro, M.; Sabetta, E.; Minerva, M.; Ambrosio, A.; Locatelli, M.; Tomaiuolo, R. The Gender Impact Assessment among Healthcare Workers in the SARS-CoV-2 Vaccination—An Analysis of Serological Response and Side Effects. Vaccines 2021, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- Trigunaite, A.; Dimo, J.; Jørgensen, T.N. Suppressive effects of androgens on the immune system. Cell. Immunol. 2015, 294, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Ansar Ahmed, S. The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. Front. Immunol. 2016, 6, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. BJA Br. J. Anaesth. 2013, 111, 52. [Google Scholar] [CrossRef] [Green Version]
- Himmelstein, M.S.; Sanchez, D.T. Masculinity impediments: Internalized masculinity contributes to healthcare avoidance in men and women. J. Health Psychol. 2014, 21, 1283–1292. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.L.; Pekosz, A. Sex-based biology and the rational design of influenza vaccination strategies. J. Infect. Dis. 2014, 209, S114. [Google Scholar] [CrossRef] [Green Version]
- Halsey, N.A.; Griffioen, M.; Dreskin, S.C.; Dekker, C.L.; Wood, R.; Sharma, D.; Jones, J.F.; LaRussa, P.S.; Garner, J.; Berger, M.; et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: Reports to VAERS. Vaccine 2013, 31, 6107–6112. [Google Scholar] [CrossRef]
- Robert, W.; Frenck, J.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Azzi, L.; Toia, M.; Stevanello, N.; Maggi, F.; Forlani, G. An episode of oral mucositis after the first administration of the ChAdOx1 COVID-19 vaccine. Oral Dis. 2021. [Google Scholar] [CrossRef]
- Manfredi, M.; Ghidini, G.; Ridolo, E.; Pizzi, S. Oral lesions postinjection of the first administration of Pfizer-BioNTech SARS-CoV-2 (BNT162b2) vaccine. Oral Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Gad, A.; Hockova, B.; Klugar, M. Oral candidiasis in non-severe COVID-19 patients: Call for antibiotic stewardship. Oral Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Klugar, M.; Krsek, M. COVID-19 Related Oral Manifestations, Early Disease Features? Oral Dis. 2020. [Google Scholar] [CrossRef]
- Riad, A.; Kassem, I.; Issa, J.; Badrah, M.; Klugar, M. Angular cheilitis of COVID-19 patients: A case-series and literature review. Oral Dis. 2020. [Google Scholar] [CrossRef]
- Riad, A.; Kassem, I.; Badrah, M.; Klugar, M. The manifestation of oral mucositis in COVID-19 patients: A case-series. Dermatol. Ther. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Kassem, I.; Hockova, B.; Badrah, M.; Klugar, M. Tongue ulcers associated with SARS-CoV-2 infection: A case series. Oral Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Kassem, I.; Stanek, J.; Badrah, M.; Klugarova, J.; Klugar, M. Aphthous Stomatitis in COVID-19 Patients: Case-series and Literature Review. Dermatol. Ther. 2021. [Google Scholar] [CrossRef]
- Al-Khanati, N.M.; Riad, A.; Sahloul, M.E.; Klugar, M. Aphthous-like stomatitis of COVID-19 patients. Braz. J. Oral Sci. 2020, 19, e201354. [Google Scholar] [CrossRef]
- Hocková, B.; Riad, A.; Valky, J.; Šulajová, Z.; Stebel, A.; Slávik, R.; Bečková, Z.; Pokorná, A.; Klugarová, J.; Klugar, M. Oral Complications of ICU Patients with COVID-19: Case-Series and Review of Two Hundred Ten Cases. J. Clin. Med. 2021, 10, 581. [Google Scholar] [CrossRef]
- Riad, A.; Gomaa, E.; Hockova, B.; Klugar, M. Oral Candidiasis of COVID-19 Patients: Case Report and Review of Evidence. J. Cosmet. Dermatol. 2021, 20, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Gronbeck, C.; Grant-Kels, J.M. Attention all anti-vaccinators: The cutaneous adverse events from the mRNA COVID-19 vaccines are not an excuse to avoid them! Clin. Dermatol. 2021. [Google Scholar] [CrossRef]
- COVID-19: Přehled Očkování|Onemocnění Aktuálně od MZČR. Available online: https://onemocneni-aktualne.mzcr.cz/vakcinace-cr (accessed on 22 July 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).