Drug Conjugates for Targeting Eph Receptors in Glioblastoma
Abstract
1. Introduction
2. Results
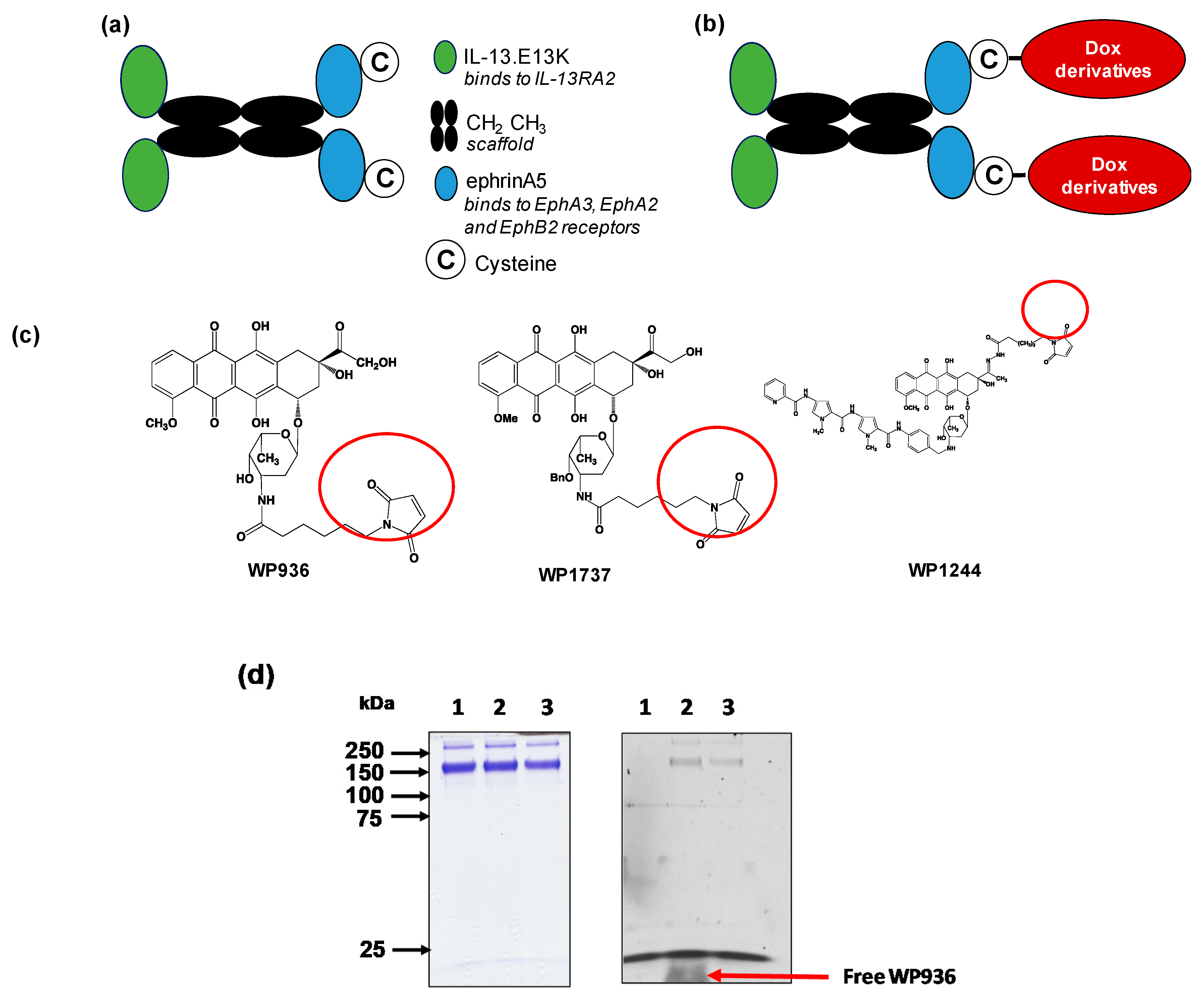
2.1. QUAD 3.0 Was Successfully Conjugated to Dox Derivatives
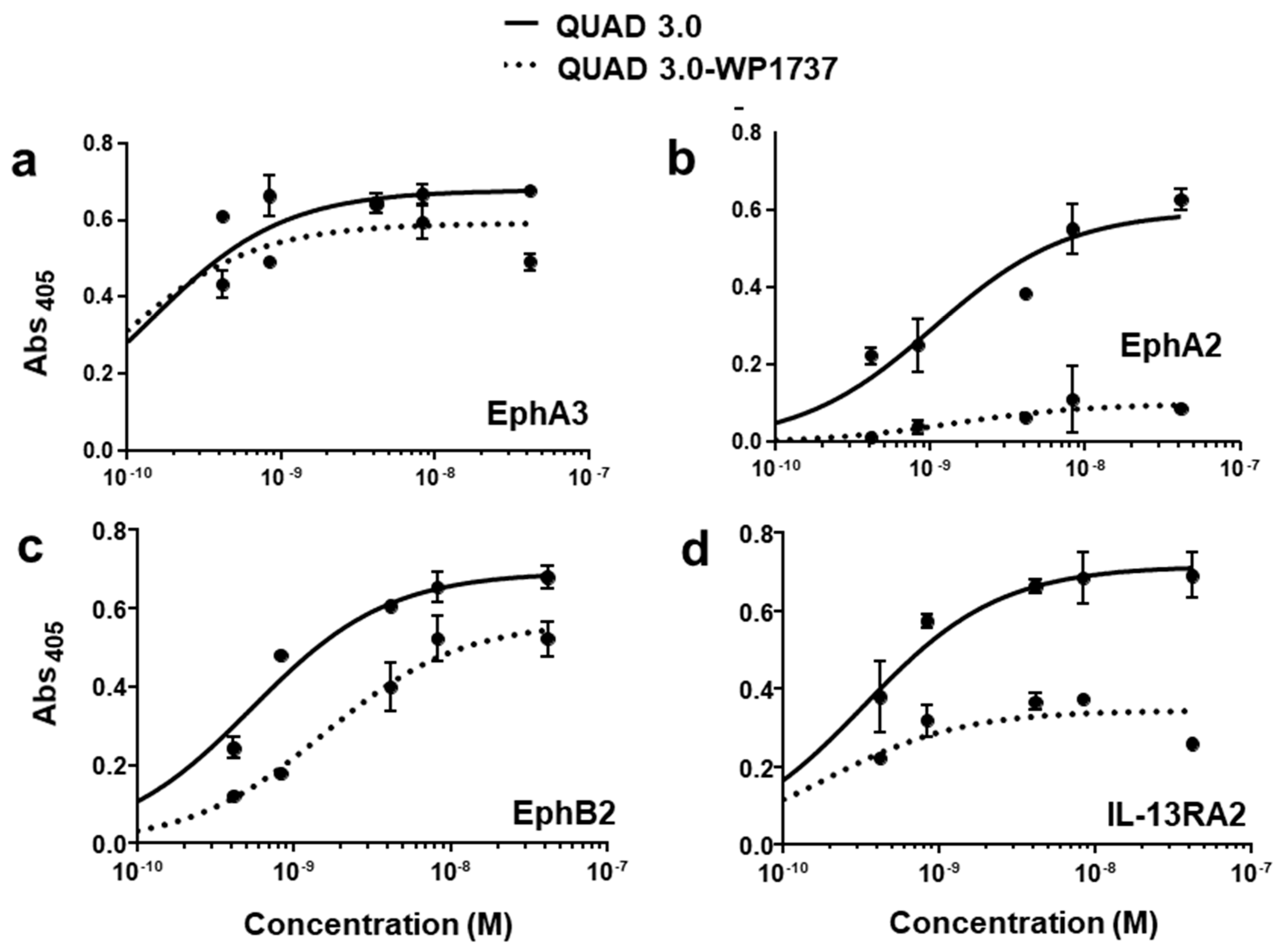
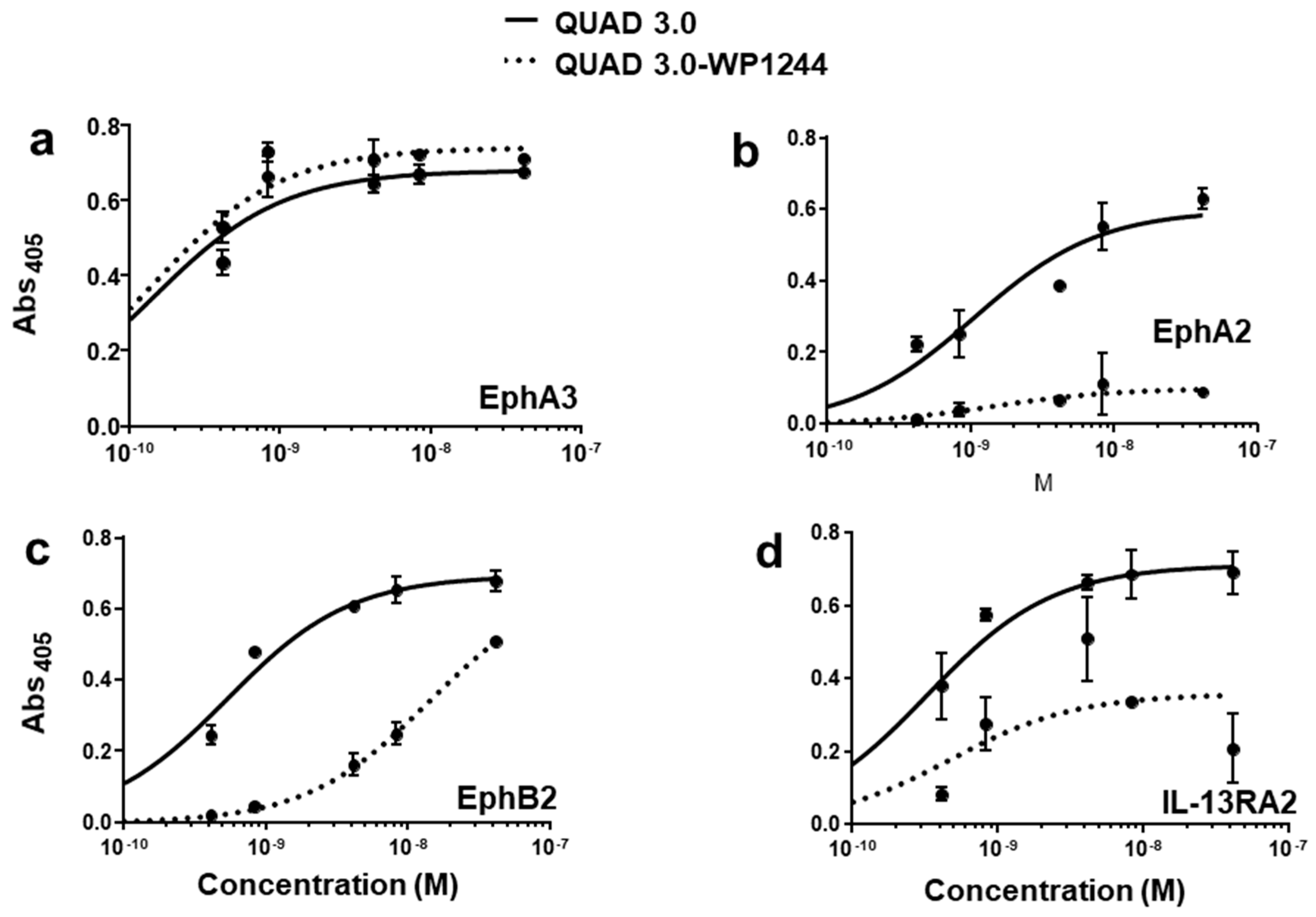
2.2. QUAD 3.0-Dox Derivative Conjugates Bind to EphA3, EphA2, EphB2, and IL-13RA2 Receptors
2.3. QUAD 3.0-WP936 Conjugate Binds and Is Internalized by the GBM Cells
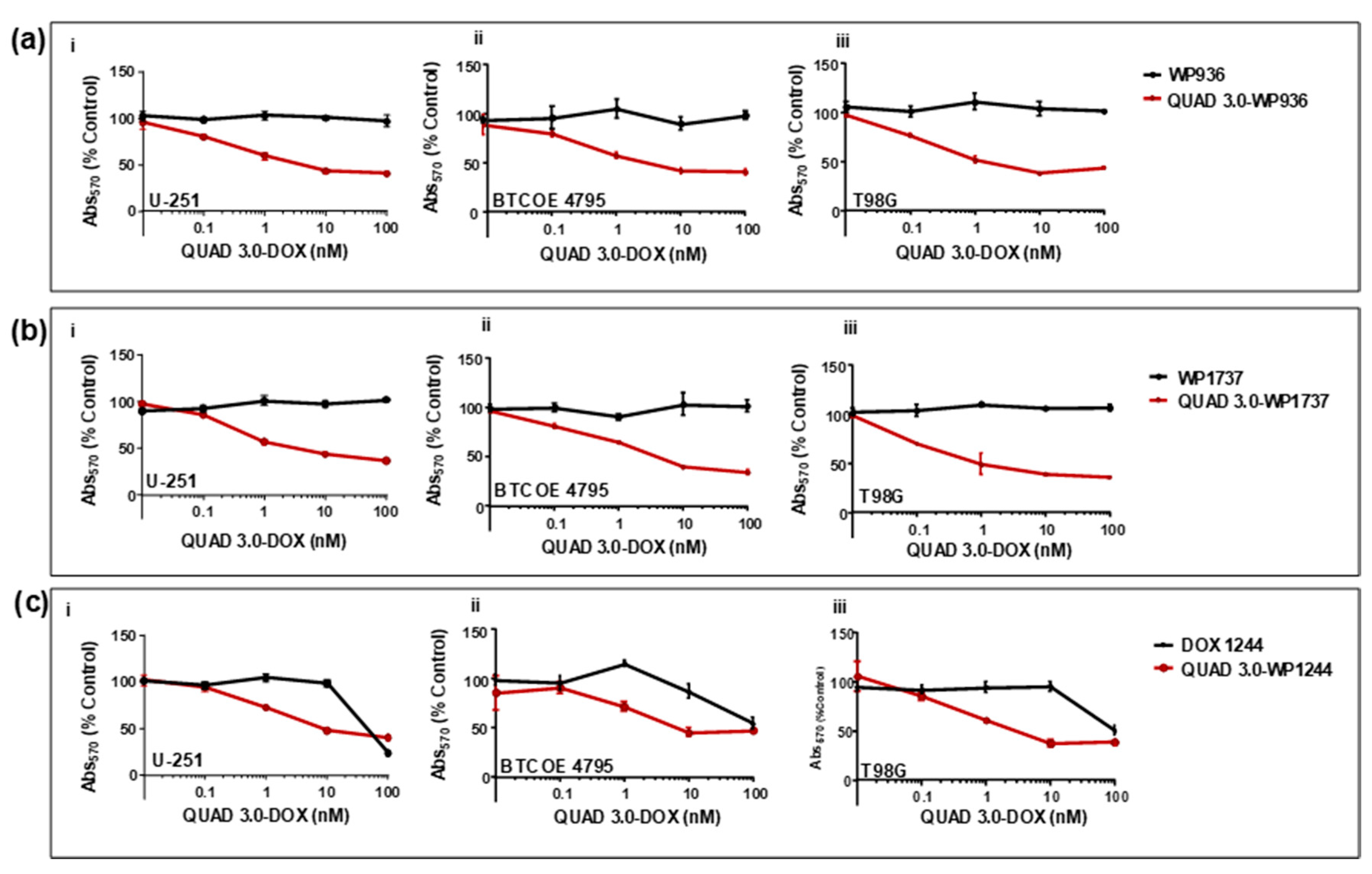
2.4. QUAD 3.0-Dox Conjugates Are Cytotoxic to Established and Patient-Derived GBM Cells
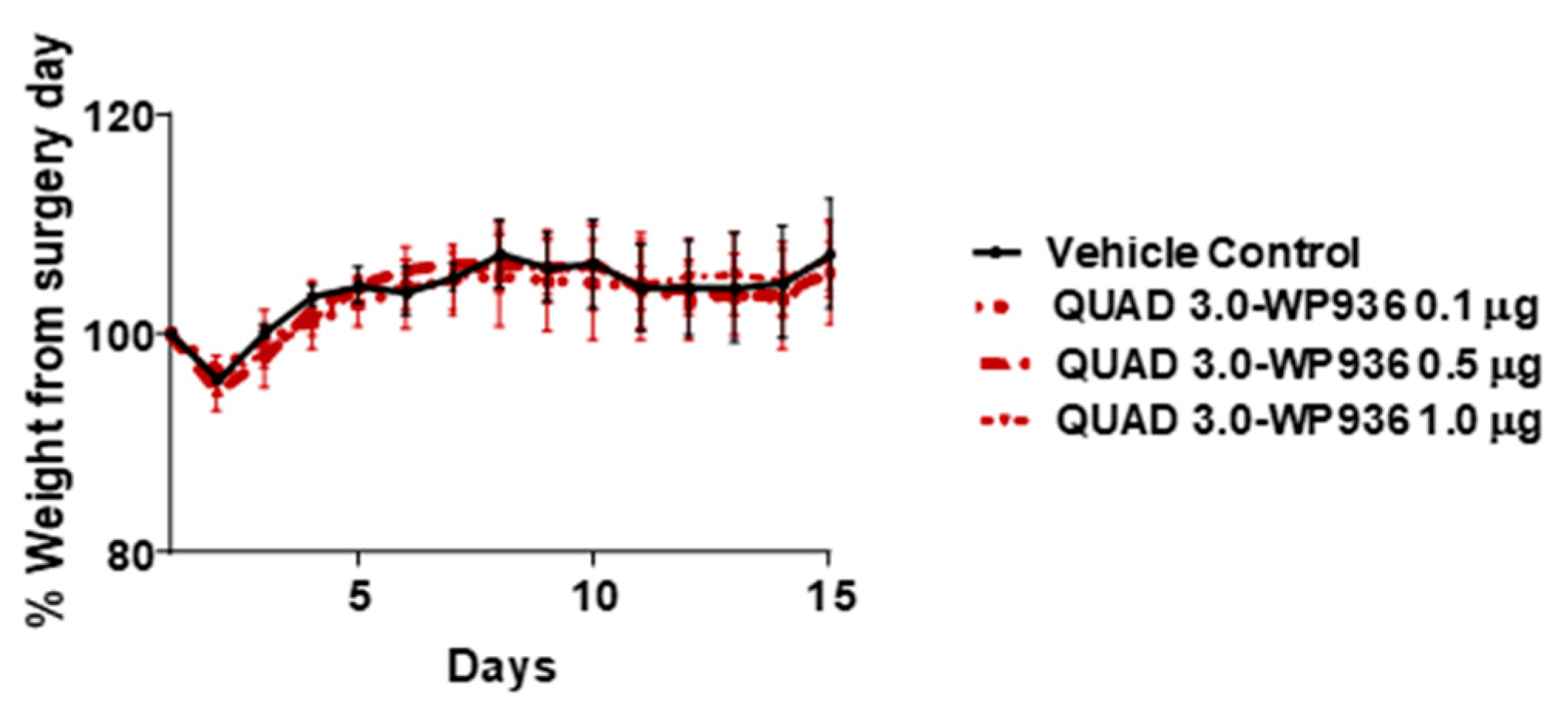
2.5. Intracranial Injections of QUAD 3.0-WP 936 Conjugate Are Safe in Mice
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Production of the Multivalent Protein, QUAD 3.0
4.3. Chemical Conjugation and Purification of Conjugates
4.4. ELISA Binding Assays
4.5. Drug to Vector (DAR) Ratio Determination
4.6. Cell Binding and Internalization of a QUAD 3.0-WP936 Conjugate
4.7. Cell Viability Assays
4.8. IC50 Value Determination
- ‘a’ is the maximum value of y (response at 0 dose)
- ‘b’ is the slope factor or Hill Coefficient
- ‘c’ is the point of inflection (the point halfway between a and d, or IC50)
- ‘d’ is the minimum value of y (response at maximum dose).
4.9. In Vivo Toxicity
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lim, S.K.; Llaguno, S.R.A.; McKay, R.M.; Parada, L.F. Glioblastoma multiforme: A perspective on recent findings in human cancer and mouse models. BMB Rep. 2011, 44, 158–164. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; de Blank, P.M.; Finlay, J.L.; Gurney, J.G.; McKean-Cowdin, R.; Stearns, D.S.; Wolff, J.E.; Liu, M.; Wolinsky, Y.; et al. American brain tumor association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro. Oncol. 2016, 18, i1–i50. [Google Scholar] [CrossRef]
- Bi, W.L.; Beroukhim, R. Beating the odds: Extreme long-term survival with glioblastoma. Neuro. Oncol. 2014, 16, 1159–1160. [Google Scholar] [CrossRef]
- Debinski, W.; Slagle, B.; Gibo, D.M.; Powers, S.K.; Gillespie, G.Y. Expression of a restrictive receptor for interleukin 13 is associated with glial transformation. J. Neurooncol. 2000, 48, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D.J. Cancer genetics: Initially complex, always heterogeneous. Nat. Rev. Cancer 2011, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Ellis, H.P.; Greenslade, M.; Powell, B.; Spiteri, I.; Sottoriva, A.; Kurian, K.M. Current challenges in glioblastoma: Intratumour heterogeneity, residual disease, and models to predict disease recurrence. Front. Oncol. 2015, 5, 251. [Google Scholar] [CrossRef]
- Debinski, W. Drug cocktails for effective treatment of glioblastoma multiforme. Expert Rev. Neurother. 2008, 8, 515–517. [Google Scholar] [CrossRef][Green Version]
- Dutoit, V.; Herold-Mende, C.; Hilf, N.; Schoor, O.; Beckhove, P.; Bucher, J.; Dorsch, K.; Flohr, S.; Fritsche, J.; Lewandrowski, P.; et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain 2012, 135, 1042–1054. [Google Scholar] [CrossRef]
- Malpass, K. Identification of novel glioblastoma-associated antigens reveals targets for immunotherapy. Nat. Rev. Neurol. 2012, 8, 240. [Google Scholar] [CrossRef]
- Sharma, P.; Debinski, W. Receptor-targeted glial brain tumor therapies. Int. J. Mol. Sci. 2018, 19, 3326. [Google Scholar] [CrossRef]
- Sonawane, P.; Choi, Y.A.; Pandya, H.; Herpai, D.M.; Fokt, I.; Priebe, W.; Debinski, W. Novel molecular multilevel targeted antitumor agents. Cancer Transl. Med. 2017, 3, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ferluga, S.; Tomé, C.M.L.; Herpai, D.M.; D’Agostino, R.; Debinski, W.; Ferluga, S.; Tomé, C.M.L.; Herpai, D.M.; D’Agostino, R.; Debinski, W. Simultaneous targeting of Eph receptors in glioblastoma. Oncotarget 2016, 7, 59860–59876. [Google Scholar] [CrossRef]
- Wykosky, J.; Gibo, D.M.; Debinski, W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol. Cancer Ther. 2007, 6, 3208–3218. [Google Scholar] [CrossRef]
- Fukai, J.; Nishio, K.; Itakura, T.; Koizumi, F. Antitumor activity of cetuximab against malignant glioma cells overexpressing EGFR deletion mutant variant III. Cancer Sci. 2008, 99, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Peng, Y.; Liao, Y.; Jiang, W.; Wei, R.; Huo, L.; Han, Z.; Duan, C.; Zhong, M. Nimotuzumab prolongs survival in patients with malignant gliomas: A phase I/II clinical study of concomitant radiochemotherapy with or without nimotuzumab. Exp. Ther. Med. 2012, 4, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Madhankumar, A.B.; Slagle-Webb, B.; Mintz, A.; Sheehan, J.M.; Connor, J.R. Interleukin-13 receptor-targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol. Cancer Ther. 2006, 5, 3162–3169. [Google Scholar] [CrossRef]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef]
- Phuphanich, S.; Wheeler, C.J.; Rudnick, J.D.; Mazer, M.; Wang, H.; Nuño, M.A.; Richardson, J.E.; Fan, X.; Ji, J.; Chu, R.M.; et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol. Immunother. 2013, 62, 125–135. [Google Scholar] [CrossRef]
- Kong, S.; Sengupta, S.; Tyler, B.; Bais, A.J.; Ma, Q.; Doucette, S.; Zhou, J.; Sahin, A.; Carter, B.S.; Brem, H.; et al. Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T Cells. Clin. Cancer Res. 2012, 18, 5949–5960. [Google Scholar] [CrossRef]
- Hegde, M.; Mukherjee, M.; Grada, Z.; Pignata, A.; Landi, D.; Navai, S.A.; Wakefield, A.; Fousek, K.; Bielamowicz, K.; Chow, K.K.H.; et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Investig. 2016, 126, 3036–3052. [Google Scholar] [CrossRef]
- Choi, B.D.; O’Rourke, D.M.; Maus, M.V. Engineering chimeric antigen receptor t cells to treat glioblastoma. J. Target. Ther. Cancer 2017, 6, 22–25. [Google Scholar] [PubMed]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific chimeric antigen receptor–modified virus-specific T cells for progressive glioblastoma. JAMA Oncol. 2017, 3, 1094. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F.; Jakacki, R.I.; Butterfield, L.H.; Hamilton, R.L.; Panigrahy, A.; Normolle, D.P.; Connelly, A.K.; Dibridge, S.; Mason, G.; Whiteside, T.L.; et al. Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas. Neuro. Oncol. 2016, 18, 1157–1168. [Google Scholar] [CrossRef]
- Okada, H.; Butterfield, L.H.; Hamilton, R.L.; Hoji, A.; Sakaki, M.; Ahn, B.J.; Kohanbash, G.; Drappatz, J.; Engh, J.; Amankulor, N.; et al. Induction of robust type-I CD8+ T-cell responses in WHO grade 2 low-grade glioma patients receiving peptide-based vaccines in combination with poly-ICLC. Clin. Cancer Res. 2015, 21, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.; Lai, R.K.; Recht, L.D.; Reardon, D.A.; Paleologos, N.A.; Groves, M.D.; Mrugala, M.M.; Jensen, R.; Baehring, J.M.; Sloan, A.; et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: The ACT III study. Neuro. Oncol. 2015, 17, 854–861. [Google Scholar] [CrossRef]
- Hunter, P. The fourth pillar. EMBO Rep. 2017, 18, 1889–1892. [Google Scholar] [CrossRef]
- Lisabeth, E.M.; Falivelli, G.; Pasquale, E.B. Eph receptor signaling and ephrins. Cold Spring Harb. Perspect. Biol. 2013, 5, a009159. [Google Scholar] [CrossRef]
- Holmberg, J.; Armulik, A.; Senti, K.A.; Edoff, K.; Spalding, K.; Momma, S.; Cassidy, R.; Flanagan, J.G.; Frisén, J. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005, 19, 462–471. [Google Scholar] [CrossRef]
- Genander, M.; Holmberg, J.; Frisén, J. Ephrins negatively regulate cell proliferation in the epidermis and hair follicle. Stem Cells 2010. [Google Scholar] [CrossRef]
- Giniger, E. How do Rho family GTPases direct axon growth and guidance? A proposal relating signaling pathways to growth cone mechanics. Differentiation 2002, 70, 385–396. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Galea, M.P.; Wise, G.; Bartlett, P.F.; Turnley, A.M. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J. Neurosci. 2004, 24, 10064–10073. [Google Scholar] [CrossRef] [PubMed]
- Dickson, B.J. Rho GTPases in growth cone guidance. Curr. Opin. Neurobiol. 2001, 11, 103–110. [Google Scholar] [CrossRef]
- Cissé, M.; Checler, F. Eph receptors: New players in Alzheimer’s disease pathogenesis. Neurobiol. Dis. 2015, 73, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, A.; Schoonaert, L.; Lemmens, R.; Timmers, M.; Staats, K.A.; Laird, A.S.; Peeters, E.; Philips, T.; Goris, A.; Dubois, B.; et al. EPHA4 is a disease modifier of amyotrophic lateral sclerosis in animal models and in humans. Nat. Med. 2012, 18, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Wykosky, J.; Debinski, W. The EphA2 receptor and ephrinA1 ligand in solid tumors: Function and therapeutic targeting. Mol. Cancer Res. 2008, 6, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Regulation of tumor initiation and metastatic progression by Eph receptor tyrosine kinases. In Advances in Cancer Research; Academic Press Inc.: Cambridge, MA, USA, 2012; Volume 114, pp. 1–20. [Google Scholar]
- Shiuan, E.; Chen, J. Eph receptor tyrosine kinases in tumor immunity. Cancer Res. 2016, 76, 6452–6457. [Google Scholar] [CrossRef] [PubMed]
- Wykosky, J.; Gibo, D.M.; Stanton, C.; Debinski, W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol. Cancer Res. 2005, 3, 541–551. [Google Scholar] [CrossRef]
- Liu, F.; Park, P.J.; Lai, W.; Maher, E.; Chakravarti, A.; Durso, L.; Jiang, X.; Yu, Y.; Brosius, A.; Thomas, M.; et al. A genome-wide screen reveals functional gene clusters in the cancer genome and identifies Epha2 as a mitogen in glioblastoma. Cancer Res. 2006, 66, 10815–10823. [Google Scholar] [CrossRef]
- Debinski, W.; Priebe, W.; Tatter, S.B. Maximizing Local Access to Therapeutic Deliveries in Glioblastoma. Part I: Targeted Cytotoxic Therapy; Codon Publications: Brisbane, Australia, 2017; ISBN 9780994438126. [Google Scholar]
- Nakada, M.; Niska, J.A.; Miyamori, H.; McDonough, W.S.; Wu, J.; Sato, H.; Berens, M.E. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004, 64, 3179–3185. [Google Scholar] [CrossRef]
- Wang, S.D.; Rath, P.; Lal, B.; Richard, J.-P.; Li, Y.; Goodwin, C.R.; Laterra, J.; Xia, S. EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene 2012, 31, 5132–5143. [Google Scholar] [CrossRef]
- Rossmeisl, J.H.; Herpai, D.; Robertson, J.L.; Dickinson, P.J.; Tatter, S.B.; Debinski, W. P08.12 Tolerability and initial efficacy of convection-enhanced delivery of combinatorial IL-13RA2 and EphA2 targeted cytotoxins to dogs with spontaneous intracranial malignant gliomas. Neuro. Oncol. 2017, 19, iii56. [Google Scholar] [CrossRef]
- Sattiraju, A.; Sai, K.K.S.; Xuan, A.; Pandya, D.N.; Almaguel, F.G.; Wadas, T.J.; Herpai, D.M.; Debinski, W.; Mintz, A. IL13RA2 targeted alpha particle therapy against glioblastomas. Oncotarget 2017, 8, 42997–43007. [Google Scholar] [CrossRef]
- Pandya, H.; Gibo, D.M.; Garg, S.; Kridel, S.; Debinski, W. An interleukin 13 receptor α 2–specific peptide homes to human Glioblastoma multiforme xenografts. Neuro. Oncol. 2012, 14, 6–18. [Google Scholar] [CrossRef]
- Sharma, P.; Herpai, D.; Rossmeisl, J.; Tatter, S.; Debinski, W. EXTH-54. Multivalent Targeted Proteins For Glioblastoma TreatmenT. Neuro. Oncol. 2019, 21, vi93–vi94. [Google Scholar] [CrossRef]
- Sai, K.K.S.; Sattiraju, A.; Almaguel, F.G.; Xuan, A.; Rideout, S.; Krishnaswamy, R.S.; Zhang, J.; Herpai, D.M.; Debinski, W.; Mintz, A. Peptide-based PET imaging of the tumor restricted IL13RA2 biomarker. Oncotarget 2017, 8, 50997–51007. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.; Marino, M.P.; Suzuki, A.; Joshi, B.; Husain, S.R.; Maisner, A.; Galanis, E.; Puri, R.K.; Reiser, J. Specific targeting of human interleukin (IL)-13 receptor α2-positive cells with lentiviral vectors displaying IL-13. Hum. Gene Ther. Methods 2012, 23, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Roizman, B. Construction and properties of a herpes simplex virus 1 designed to enter cells solely via the IL-13alpha2 receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 5508–5513. [Google Scholar] [CrossRef]
- Candolfi, M.; Xiong, W.; Yagiz, K.; Liu, C.; Muhammad, A.K.M.G.; Puntel, M.; Foulad, D.; Zadmehr, A.; Ahlzadeh, G.E.; Kroeger, K.M.; et al. Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics. Proc. Natl. Acad. Sci. USA 2010, 107, 20021–20026. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zhao, J.; Liu, X.; Bu, J.; Yan, X.; Huang, R. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 2013, 135, 4799–4804. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.-C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and safety of IL13R 2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef]
- Debinski, W.; Obiri, N.I.; Pastan, I.; Puri, R.K. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J. Biol. Chem. 1995, 270, 16775–16780. [Google Scholar] [CrossRef] [PubMed]
- Debinski, W.; Gibo, D.M.; Hulet, S.W.; Connor, J.R.; Gillespie, G.Y. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin. Cancer Res. 1999, 5, 985–990. [Google Scholar]
- Debinski, W.; Miner, R.; Leland, P.; Obiri, N.I.; Puri, R.K. Receptor for interleukin (IL) 13 does not interact with IL4 but receptor for IL4 interacts with IL13 on human glioma cells. J. Biol. Chem. 1996, 271, 22428–22433. [Google Scholar] [CrossRef] [PubMed]
- Debinski, W.; Gibo, D.; Wykosky, J.; Stanton, C.; Rossmeisl, J.; Robertson, J. Canine gliomas over-express IL-13Ealpha2, EphA2 and Fra-1 in common with human high-grade astrocytomas. Neuro. Oncol. 2007, 9, 535–536. [Google Scholar]
- Brown, C.E.; Warden, C.D.; Starr, R.; Deng, X.; Badie, B.; Yuan, Y.-C.; Forman, S.J.; Barish, M.E. Glioma IL13Rα2 is associated with mesenchymal signature gene expression and poor patient prognosis. PLoS ONE 2013, 8, e77769. [Google Scholar] [CrossRef]
- Thaci, B.; Brown, C.E.; Binello, E.; Werbaneth, K.; Sampath, P.; Sengupta, S. Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro. Oncol. 2014, 16, 1304–1312. [Google Scholar] [CrossRef]
- Nguyen, V.; Conyers, J.M.; Zhu, D.; Gibo, D.M.; Dorsey, J.F.; Debinski, W.; Mintz, A. IL-13Rα2-targeted therapy escapees: Biologic and therapeutic implications. Transl. Oncol. 2011, 4, 390–400. [Google Scholar] [CrossRef]
- Coats, S.; Williams, M.; Kebble, B.; Dixit, R.; Tseng, L.; Yao, N.S.; Tice, D.A.; Soria, J.C. Antibody-drug conjugates: Future directions in clinical and translational strategies to improve the therapeutic index. Clin. Cancer Res. 2019, 25, 5441–5448. [Google Scholar] [CrossRef]
- Bross, P.F.; Beitz, J.; Chen, G.; Chen, X.H.; Duffy, E.; Kieffer, L.; Roy, S.; Sridhara, R.; Rahman, A.; Williams, G.; et al. Approval summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 2001, 7, 1490–1496. [Google Scholar]
- Scott, L.J. Brentuximab vedotin: A review in CD30-positive hodgkin lymphoma. Drugs 2017, 77, 435–445. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Inotuzumab ozogamicin: First global approval. Drugs 2017, 77, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Kim, M. Inotuzumab ozogamicin in relapsed or refractory B-Cell acute lymphoblastic leukemia. J. Adv. Pract. Oncol. 2018, 9, 670–676. [Google Scholar] [PubMed]
- Gymnopoulos, M.; Betancourt, O.; Blot, V.; Fujita, R.; Galvan, D.; Lieuw, V.; Nguyen, S.; Snedden, J.; Stewart, C.; Villicana, J.; et al. TR1801-ADC: A highly potent cMet antibody-drug conjugate with high activity in patient-derived xenograft models of solid tumors. Mol. Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Polatuzumab vedotin: First global approval. Drugs 2019, 79, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Breij, E.C.W.; De Goeij, B.E.C.G.; Verploegen, S.; Schuurhuis, D.H.; Amirkhosravi, A.; Francis, J.; Miller, V.B.; Houtkamp, M.; Bleeker, W.K.; Satijn, D.; et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res. 2014, 74, 1214–1226. [Google Scholar] [CrossRef]
- Cocco, E.; Varughese, J.; Buza, N.; Bellone, S.; Glasgow, M.; Bellone, M.; Todeschini, P.; Carrara, L.; Silasi, D.A.; Azodi, M.; et al. Expression of tissue factor in adenocarcinoma and squamous cell carcinoma of the uterine cervix: Implications for immunotherapy with hI-con1, a factor VII-IgGFaacchimeric protein targeting tissue factor. BMC Cancer 2011, 11. [Google Scholar] [CrossRef]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The latest research and development into the antibody–drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem. Pharm. Bull. 2019, 67, 173–185. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2019. [Google Scholar] [CrossRef]
- Hanna, K.S. Clinical overview of enfortumab vedotin in the management of locally advanced or metastatic urothelial carcinoma. Drugs 2019. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Glode, A.; Messersmith, W.A.; Diamond, J. Sacituzumab govitecan: Breakthrough targeted therapy for triple-negative breast cancer. Expert Rev. Anticancer Ther. 2019, 19, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. 2019 FDA drug approvals. Nat. Rev. Drug Discov. 2020. [Google Scholar] [CrossRef]
- Debinski, W. Recombinant cytotoxins specific for cancer cells. Ann. N. Y. Acad. Sci. 1999, 886, 297–299. [Google Scholar] [CrossRef]
- Thompson, J.P.; Debinski, W. Mutants of interleukin 13 with altered reactivity toward interleukin 13 receptors. J. Biol. Chem. 1999, 274, 29944–29950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raucher, D.; Bidwell, G.L.; Priebe, W.; Fokt, I. Thermally-Targeted Delivery of Medicaments, Including Doxorubicin. U.S. Patent 8252740B2, 28 January 2010. [Google Scholar]
- Bidwell, G.L.; Fokt, I.; Priebe, W.; Raucher, D. Development of elastin-like polypeptide for thermally targeted delivery of doxorubicin. Biochem. Pharmacol. 2007, 73, 620–631. [Google Scholar] [CrossRef]
- WO2017049091A1—Dna Binding Agents with a Minor Groove Binding Tail—Google Patents. Available online: https://patents.google.com/patent/WO2017049091A1/en (accessed on 30 December 2019).
- Berubicin—CNS Pharmaceuticals. Available online: https://cnspharma.com/berubicin/ (accessed on 30 December 2019).
- Berubicin Breaches Blood-Brain Barrier to Attack Brain Tumors. Available online: https://www.oncnursingnews.com/web-exclusives/berubicin-breaches-bloodbrain-barrier-to-attack-brain-tumors (accessed on 30 December 2019).
- Wang, M.; Sun, L.; Fokt, I.; Zhang, L.; Jayakumar, A.; Priebe, W. Effect of berubicin, the 4’-o-benzylated doxorubicin analog, on growth inhibition and apoptosis in multiple myeloma. J. Clin. Oncol. 2017, 30. Available online: https://ascopubs.org/doi/abs/10.1200/jco.2012.30.15_suppl.e18557 (accessed on 24 January 2020). [CrossRef]
- Sharma, P.; Herpai, D.; Rossmeisl, J.; Tatter, S.; Debinski, W. EXTH-29. MULTI-RECEPTOR TARGETING IN GBM. Neuro. Oncol. 2018, 20, vi91. [Google Scholar] [CrossRef][Green Version]
- US8252740B2—Thermally-Targeted Delivery of Medicaments Including Doxorubicin—Google Patents. Available online: https://patents.google.com/patent/US8252740B2/en (accessed on 30 December 2019).
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 2017, 32, 42–56.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Pan, Y.; Gutmann, D.H. The power of the few. Genes Dev. 2017, 31, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jun, Y.; Kim, S.H.; Hoang, H.-H.; Jung, Y.; Kim, S.; Kim, J.; Austin, R.H.; Lee, S.; Park, S. Rapid emergence and mechanisms of resistance by U87 glioblastoma cells to doxorubicin in an in vitro tumor microfluidic ecology. Proc. Natl. Acad. Sci. USA 2016, 113, 14283–14288. [Google Scholar] [CrossRef] [PubMed]
- Debinski; Waldemar When better still might not be good enough. Transl. Cancer Res. 2017, 6, S1244–S1247. [CrossRef] [PubMed]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of glioblastoma after chimeric antigen receptor T-Cell therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Herpai, D.; Robertson, J.; Dickinson, P.; Tatter, S.; Debinski, W. EXTH-43. Effective treatment of canine spontaneous gliomas with a cytotoxic cocktail targeting Il-13ra2 And Epha2 receptors. Neuro. Oncol. 2018, 20, vi94. [Google Scholar] [CrossRef]
- Nakada, M.; Hayashi, Y.; Hamada, J.I. Role of Eph/ephrin tyrosine kinase in malignant glioma. Neuro. Oncol. 2011, 13, 1163–1170. [Google Scholar] [CrossRef]
- Darling, T.K.; Lamb, T.J. Emerging roles for Eph receptors and ephrin ligands in immunity. Front. Immunol. 2019, 10, 1473. [Google Scholar] [CrossRef]
- Day, B.W.; Stringer, B.W.; Boyd, A.W. Eph receptors as therapeutic targets in glioblastoma. Br. J. Cancer 2014, 111, 1255–1261. [Google Scholar] [CrossRef]
- Wang, L.-F.; Fokas, E.; Bieker, M.; Rose, F.; Rexin, P.; Zhu, Y.; Pagenstecher, A.; Engenhart-Cabillic, R.; An, H.-X. Increased expression of EphA2 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. Oncol. Rep. 2008, 19, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Binda, E.; Visioli, A.; Giani, F.; Lamorte, G.; Copetti, M.; Pitter, K.L.; Huse, J.T.; Cajola, L.; Zanetti, N.; DiMeco, F.; et al. The EphA2 receptor drives self-renewal and tumorigenicity in stem-like tumor-propagating cells from human glioblastomas. Cancer Cell 2012, 22, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Day, B.W.; Stringer, B.W.; Al-Ejeh, F.; Ting, M.J.; Wilson, J.; Ensbey, K.S.; Jamieson, P.R.; Bruce, Z.C.; Lim, Y.C.; Offenhäuser, C.; et al. EphA3 Maintains tumorigenicity and is a therapeutic target in glioblastoma multiforme. Cancer Cell 2013, 23, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Serwer, L.; Hashizume, R.; Ozawa, T.; David James, C. Systemic and local drug delivery for treating diseases of the central nervous system in rodent models. J. Vis. Exp. 2010. [Google Scholar] [CrossRef] [PubMed]


| EphA3 | EphA2 | EphB2 | IL-13RA2 | |||||
|---|---|---|---|---|---|---|---|---|
| Kd | Bmax | Kd | Bmax | Kd | Bmax | Kd | Bmax | |
| QUAD 3.0 | 0.25 nM | 1.045 | 0.96 nM | 0.67 | 2.2 nM | 0.91 | 0.55 nM | 0.26 |
| QUAD 3.0-WP936 | 0.28 nM | 1.1 | 1.9 nM | 0.94 | 1.1 nM | 0.93 | 2.6 nM | 0.34 |
| QUAD 3.0 | 0.14 nM | 0.68 | 1.1 nM | 0.60 | 0.53 nM | 0.69 | 0.35 nM | 0.72 |
| QUAD 3.0-WP1737 | 0.09 nM | 0.59 | 0.66 nM | 0.20 | 1.6 nM | 0.57 | 0.19 nM | 0.35 |
| QUAD 3.0-WP1244 | 0.13 nM | 0.74 | 1.4 nM | 0.10 | 13.9 nM | 0.68 | 0.47 nM | 0.36 |
| QUAD 3.0-WP936 | QUAD 3.0-WP1737 | QUAD 3.0-WP1244 | |
|---|---|---|---|
| U-251 | 3.1 nM | 2.4 nM | 7.8 nM |
| BTCOE 4795 | 2.2 nM | 3.7 nM | 1.9 nM |
| T98G | 1.1 nM | 0.87 nM | 2.5 nM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Roberts, C.; Herpai, D.; Fokt, I.D.; Priebe, W.; Debinski, W. Drug Conjugates for Targeting Eph Receptors in Glioblastoma. Pharmaceuticals 2020, 13, 77. https://doi.org/10.3390/ph13040077
Sharma P, Roberts C, Herpai D, Fokt ID, Priebe W, Debinski W. Drug Conjugates for Targeting Eph Receptors in Glioblastoma. Pharmaceuticals. 2020; 13(4):77. https://doi.org/10.3390/ph13040077
Chicago/Turabian StyleSharma, Puja, Callie Roberts, Denise Herpai, Izabela D. Fokt, Waldemar Priebe, and Waldemar Debinski. 2020. "Drug Conjugates for Targeting Eph Receptors in Glioblastoma" Pharmaceuticals 13, no. 4: 77. https://doi.org/10.3390/ph13040077
APA StyleSharma, P., Roberts, C., Herpai, D., Fokt, I. D., Priebe, W., & Debinski, W. (2020). Drug Conjugates for Targeting Eph Receptors in Glioblastoma. Pharmaceuticals, 13(4), 77. https://doi.org/10.3390/ph13040077





