Methanolic Extract of the Herb Ononis spinosa L. Is an Antifungal Agent with no Cytotoxicity to Primary Human Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Phenolic Compounds
2.2. Antifungal Activity of O. spinosa Methanolic Extract
2.3. Antibiofilm Activity of O. spinosa
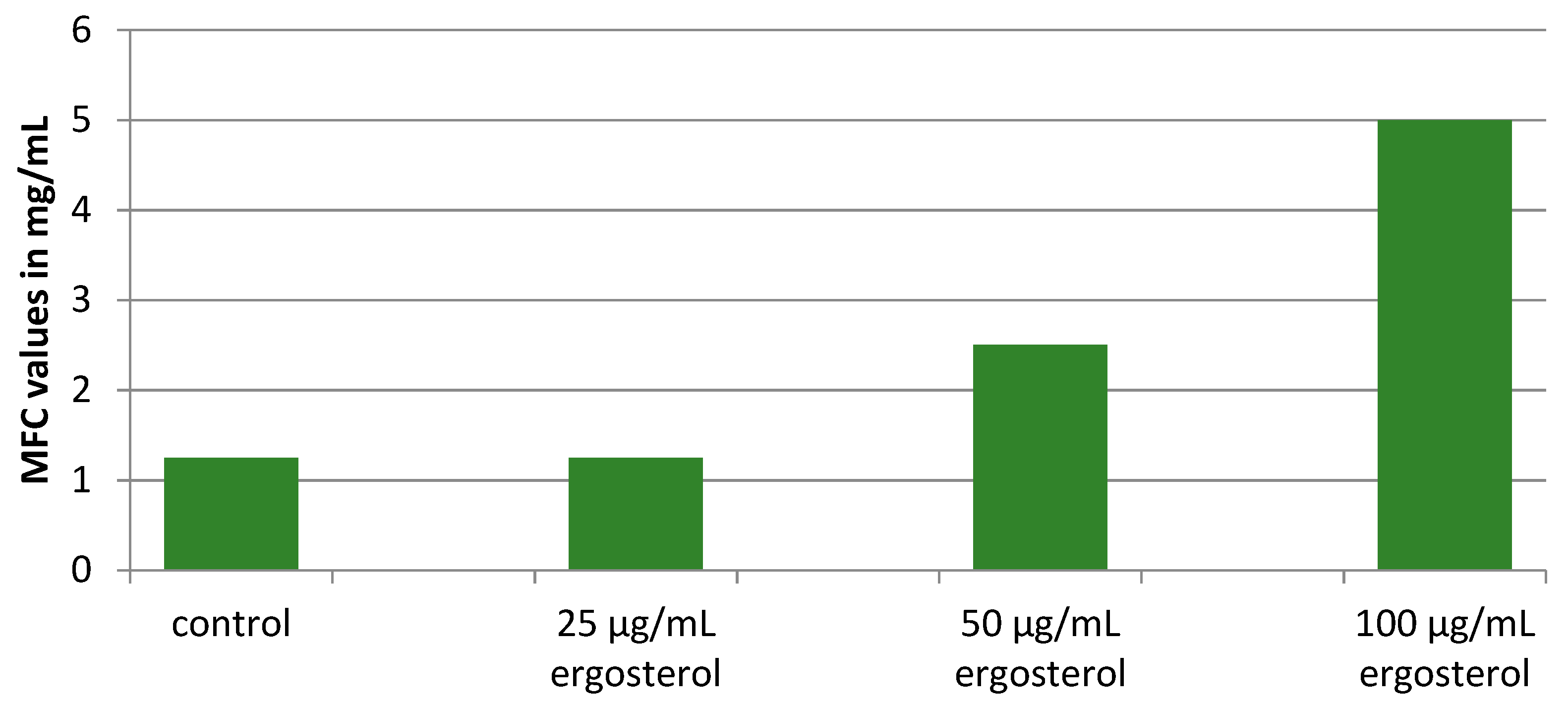
2.4. Insights into the Modes of Antifungal Action
2.5. Evaluation of Cytotoxicity of the O. spinosa Methanolic Extract on Primary Human Gingival Fibroblast Cells
3. Materials and Methods
3.1. Standards and Reagents
3.2. Collection and Extraction of O. spinosa
3.3. Analysis of Phenolic Compounds
3.4. Antifungal Activity Assay
3.5. Biofilm Inhibition Assay on Candida Strains
3.6. Insights into the mode of Antifungal Action: Ergosterol Binding and Membrane Permeability Assays
3.7. Investigation of O. spinosa Methanolic Extract Cytotoxic Activity
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Donlan, M.R.; Costerton, W.J. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Altuner, E.M.; Çeter, T.; Lşlek, C. Investigation of antifungal activity of Ononis spinosa L. ASH used for the therapy of skin infections as folk remedies. Mikrobiyoloji Bul. 2010, 44, 633–639. [Google Scholar]
- Diklić, N. Rod Ononis L. In Flora SR Srbije, 1st ed.; Josifović, M., Ed.; Srpska Akademija nauke i umetnosti: Beograd, Srbija, 1992; Volume 4, pp. 392–396. [Google Scholar]
- Gampe, N.; Darcsi, A.; Kursinszki, L.; Béni, S. Separation and characterization of homopipecolic acid isoflavonoid ester derivatives isolated from Ononis spinosa L. root. J. Chromatogr. B 2018, 1091, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, M.L.; Ivancheva, S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 2003, 87, 123–142. [Google Scholar] [CrossRef]
- Mamedov, N.; Gardner, Z.; Craker, L.E. Medicinal plants used in Russia and Central Asia for the treatment of selected skin conditions. J. Herbs Spices Med. Plants 2005, 11, 191–222. [Google Scholar] [CrossRef]
- Altanlar, N.; Saltan Çitoğlu, G.; Sever Yılmaz, B. Antilisterial activity of some plants used in folk medicine. Pharm. Biol. 2006, 44, 91–94. [Google Scholar] [CrossRef]
- Çakilcioğlu, U.; Türkoğlu, I. An ethnobotanical survey of medicinal plants in Sivrice (Elazığ-Turkey). J. Ethnopharmacol. 2010, 132, 165–175. [Google Scholar] [CrossRef]
- Benedec, D.; Vlase, L.; Oniga, I.; Toiu, A.; Tamas, M.; Tiperciuc, B. Isoflavonoids from Glycyrrhiza sp. and Ononis spinosa. Farmacia 2012, 60, 615–620. [Google Scholar]
- Daruhazi, A.E.; Szarka, S.; Hethelyi, E.; Simandi, B.; Gyurjan, I.; Laszlo, M.; Szoke, E. GC-MS identification and GC-FID quantitation of terpenoids in Ononidis spinosae Radix. Chromatographia 2008, 68, 71–76. [Google Scholar] [CrossRef]
- Jimenez-Gonzales, L.; Alvarez-Corral, M.; Munoz-Dorado, M.; Rodriguez-Garcia, I. Pterocarpans: Interesting natural products with antifungal activity and other biological properties. Phytochem. Rev. 2008, 7, 125–154. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Al-Masri, M.; Zaid, A.N.; Hussein, F.; Al-Rimawi, F.; Mokh, A.A.; Mokh, J.A.; Ghonaim, S. Phytochemical, antimicrobial and antioxidant preliminary screening of a traditional Palestinian medicinal plant, Ononis pubescens L. Eur. J. Integr. Med. 2017, 14, 46–51. [Google Scholar] [CrossRef]
- Dénes, T.; Bartha, S.G.; Kerényi, M.; Varga, E.; Balázs, V.L.; Csepregi, R.; Papp, N. Histological and antimicrobial study of Ononis arvensis L. Acta Biol. Hung. 2017, 68, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Tunde, D.; Papp, N.; Marton, K.; Kaszas, A.; Felinger, A.; Varga, E.; Boros, B. Polyphenol Content of Ononis arvensis L. and Rhinanthus serotinus (Schönh. ex Halácsy & Heinr. Braun) Oborny Used in the Transylvanian Ethnomedicine. Int. J. Pharmacogn. Phytochem. 2015, 30, 2051–2058. [Google Scholar]
- Mezrag, A.; Malafronte, N.; Bouheroum, M.; Travaglino, C.; Russo, D.; Milella, L.; Severino, L.; De Tommasi, N.; Braca, A.; Dal Piaz, F. Phytochemical and antioxidant activity studies on Ononis angustissima L. aerial parts: Isolation of two new flavonoids. Nat. Prod. Res. 2017, 31, 507–514. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Pinela, J.; Carvalho, A.M.; Buelga, C.S.; Ferreira, I.C.F.R. Characterization and Quantification of Phenolic Compounds in Four Tomato (Lycopersicon esculentum L.) Farmers’ Varieties in Northeastern Portugal Homegardens. Plant Food Hum. Nutr. 2012, 67, 229–234. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Wang, X.; Yang, J.; Wang, Q. Qualitative analysis and simultaneous quantification of phenolic compounds in the aerial parts of Salvia miltiorrhiza by HPLC-DAD and ESI/MSn. Phytochem. Anal. 2011, 22, 247–257. [Google Scholar] [CrossRef]
- Addotey, J.N.; Lengers, I.; Jose, J.; Gampe, N.; Béni, S.; Petereit, F.; Hensel, A. Isoflavonoids with inhibiting effects on human hyaluronidase-1 and norneolignan clitorienolactone B from Ononis spinosa L. root extract. Fitoterapia 2018, 130, 169–174. [Google Scholar] [CrossRef]
- Öz, B.E.; İşcan, G.S.; Akkol, E.K.; Süntar, İ.; Acıkara, Ö.B. Isoflavonoids as wound healing agents from Ononidis Radix. J. Ethnopharmacol. 2018, 211, 384–393. [Google Scholar]
- Gampe, N.; Darcsi, A.; Lohner, S.; Béni, S.; Kursinszki, L. Characterization and identification of isoflavonoid glycosides in the root of Spiny restharrow (Ononis spinosa L.) by HPLC-QTOF-MS, HPLC-MS/MS and NMR. J. Pharm. Biomed. 2016, 123, 74–81. [Google Scholar] [CrossRef]
- Pietta, P.; Calatroni, A.; Zio, C. High-performance liquid chromatographic analysis of flavonoids from Ononis spinosa L. J. Chromatogr. A 1983, 280, 172–175. [Google Scholar] [CrossRef]
- McCue, P.; Shetty, K. Health benefits of soy isoflavonoids and strategies for enhancement: A review. Crit. Rev. Food Sci. 2004, 44, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, K.; Humayoun Akhtar, M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. 2017, 57, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Deliorman Orhan, D.; Özçelik, B.; Hoşbaş, S.; Vural, M. Assessment of antioxidant, antibacterial, antimycobacterial, and antifungal activities of some plants used as folk remedies in Turkey against dermatophytes and yeast-like fungi. Turk. J. Biol. 2012, 36, 672–686. [Google Scholar]
- Smiljković, M.; Kostić, M.; Stojković, D.; Glamočlija, J.; Soković, M. Could flavonoids compete with synthetic azoles in diminishing Candida albicans infections? A comparative review based on in vitro studies. Curr. Med. Chem. 2019, 26, 2536–2554. [Google Scholar] [CrossRef]
- Ernst, E.; Pittler, M.H.; Stevinson, C.; White, A. The Desktop Guide to Complementary and Alternative Medicine; Mosby: Edinburgh, UK, 2001. [Google Scholar]
- Aleksić, M.; Stanisavljević, D.; Smiljković, M.; Vasiljević, P.; Stevanović, M.; Soković, M.; Stojković, D. Pyrimethanil: Between efficient fungicide against Aspergillus rot on cherry tomato and cytotoxic agent on human cell lines. Ann. Appl. Biol. 2019, 175, 228–235. [Google Scholar] [CrossRef]
- Nicola, A.M.; Albuquerque, P.; Paes, H.C.; Fernandes, L.; Costa, F.F.; Kioshima, E.S.; Abadio, A.K.R.; Bocca, A.L.; Felipe, M.S. Antifungal drugs: New insights in research & development. Pharmacol. Therapeut. 2019, 195, 21–38. [Google Scholar]
- Chauhan, N.; Calderone, R. Two-Component Signal Transduction Proteins as Potential Drug Targets in Medically Important Fungi. Infect. Immun. 2008, 76, 4795–4803. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Ćirić, A.; Kruljević, I.; Stojković, D.; Fernandes, Â.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R.; Soković, M.; Glamočlija, J. Comparative investigation on edible mushrooms Macrolepiota mastoidea, M. rhacodes and M. procera: Functional foods with diverse biological activities. Food Funct. 2019, 10, 7678–7686. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crop Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Soković, M.; Van Griensven, L.J.L.D. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- CLSI. Clinical and Laboratory Standards Institute Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 8th ed.; CLSI Publication M07-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Popović, V.; Stojković, D.; Nikolić, M.; Heyerick, A.; Petrović, S.; Soković, M.; Niketić, M. Extracts of three Laserpitium L. species and their principal components laserpitine and sesquiterpene lactones inhibit microbial growth and biofilm formation by oral Candida isolates. Food Funct. 2015, 6, 1205–1211. [Google Scholar] [CrossRef]
- Stojković, D.S.; Živković, J.; Soković, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Janković, T.; Maksimović, Z. Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem. Toxicol. 2013, 55, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 4, 087379. [Google Scholar] [CrossRef] [PubMed]
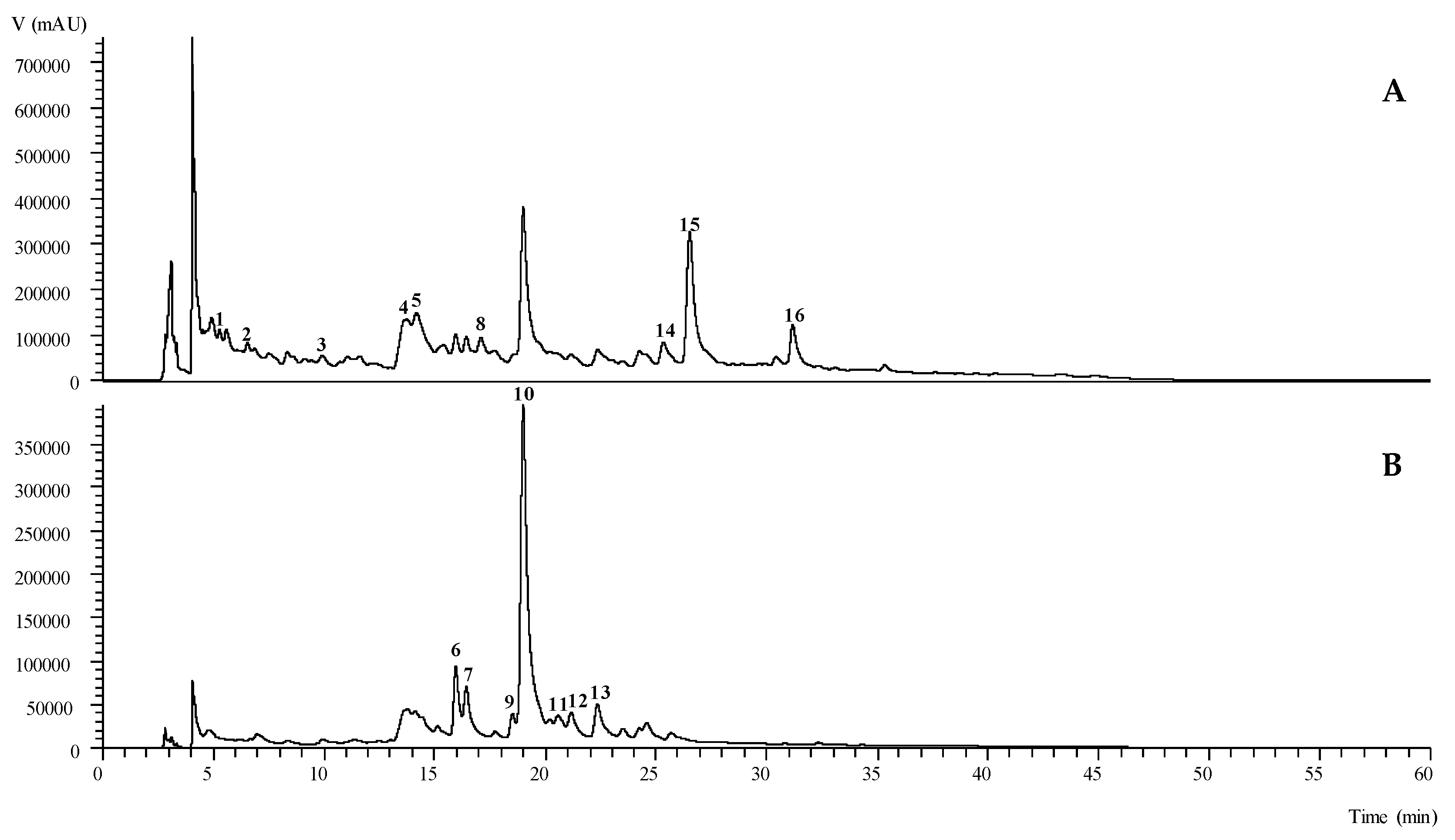


| Peak | Rt (min) | λmax ((nm)) | Molecular Ion [M − H]− (m/z) | MS2 (m/z) | Tentative Identification | Quantification (mg/g Extract) |
|---|---|---|---|---|---|---|
| 1 | 5.31 | 310 | 341 | 179(100),135(5) | Caffeic acid hexosideA | tr |
| 2 | 6.55 | 287 | 355 | 193(100),179(5),149(5) | Ferulic acid hexosideB | 0.078 ± 0.001 |
| 3 | 9.93 | 310 | 179 | 135(100) | Caffeic acidA | 0.020 ± 0.001 |
| 4 | 13.66 | 328 | 473 | 311(100),293(92),267(5),179(5),149(8),135(5) | cis Chicoric acid A | 0.81 ± 0.02 |
| 5 | 14.17 | 328 | 473 | 311(100),293(90),267(5),179(5),149(10),135(5) | trans Chicoric acidA | 0.76 ± 0.01 |
| 6 | 15.96 | 348 | 595 | 301(100) | Quercetin-O-hexoside-pentosideC | 0.824 ± 0.001 |
| 7 | 16.43 | 341 | 609 | 285(100) | Kaempherol-O-dihexosideC | 0.732 ± 0.002 |
| 8 | 17.11 | 305 | 459 | 297(100) | Spinonin-O-hexosideE | tr |
| 9 | 18.5 | 328 | 463 | 301(100) | Quercetin-3-O-glucosideD | 0.61 ± 0.01 |
| 10 | 19.07 | 346 | 579 | 285(100) | Kaempherol-O-hexoside-pentosideC | 5.1 ± 0.2 |
| 11 | 20.58 | 348 | 505 | 463(70),301(100) | Acetylquercetin-O-hexosideD | 0.566 ± 0.002 |
| 12 | 21.18 | 337 | 447 | 285(100) | Kaempherol-O-hexosideD | 0.58 ± 0.01 |
| 13 | 22.37 | 340 | 447 | 285(100) | Kaempherol-3-O-glucosideD | 0.71 ± 0.02 |
| 14 | 25.34 | 262/291 | 489 | 281(100) | Pseudobaptigenin-O-hexosideE | 0.134 ± 0.003 |
| 15 | 26.56 | 261/310 | 475 | 267(100) | Formononetin derivativeF | 1.28 ± 0.02 |
| 16 | 31.18 | 260/310 | 515 | 471(15),429(5),267(100) | Formononetin-O-malonyl-hexosideF | tr |
| Total phenolic acids | 1.66 ± 0.03 | |||||
| Total isoflavonoids | 1.41 ± 0.02 | |||||
| Total flavonoids | 9.1 ± 0.1 | |||||
| Total phenolic compounds | 12.2 ± 0.1 |
| Microfungi | O. spinosa | Ketoconazole | Bifonazole | |
|---|---|---|---|---|
| A. fumigatus (ATCC 9197) | MIC | 0.08 b | 0.20 a | 0.15 b |
| MFC | 0.08 b | 0.50 b | 0.20 a | |
| A. versicolor (ATCC 11730) | MIC | 0.62 d | 0.20 a | 0.10 a |
| MFC | 1.25 d | 0.50 b | 0.20 a | |
| A. niger (ATCC 6275) | MIC | 0.62 d | 0.20 a | 0.15 b |
| MFC | 1.25 d | 0.50 b | 0.20 a | |
| A. ochraceus (ATCC 12066) | MIC | 2.50 e | 1.50 e | 0.15 b |
| MFC | 2.50 e | 2.00 e | 0.20 a | |
| Trichoderma viride (IAM 5061) | MIC | 0.62 d | 1.00 d | 0.15 b |
| MFC | 1.25 d | 1.00 c | 0.20 a | |
| Penicillium funiculosum (ATCC 36839) | MIC | 0.62 d | 0.20 a | 0.20 c |
| MFC | 1.25 d | 0.50 b | 0.25 b | |
| P. aurantiogriseum (food isolate) | MIC | 0.02 a | 0.20 a | 0.10 a |
| MFC | 0.04 a | 0.30 a | 0.20 a | |
| P. ochrochloron (ATCC 9122) | MIC | 5.00 f | 1.00 d | 0.20 c |
| MFC | 10.00 f | 1.50 d | 0.25 b | |
| Candida albicans (ATCC 10231) | MIC | 0.62 d | 0.50 c | 0.15 b |
| MFC | 1.25 d | 1.00 c | 0.30 c | |
| C. krusei (clinical isolate) | MIC | 0.62 d | 0.50 c | 0.25 d |
| MFC | 1.25 d | 1.00 c | 0.50 d | |
| C. tropicalis (ATCC 750) | MIC | 0.31 c | 0.30 b | 0.25 d |
| MFC | 0.62 c | 0.50 b | 0.50 d | |
| Fungi | O. spinosa Methanolic Extract | Fluconazole | ||
|---|---|---|---|---|
| MIC | MFC | MIC | MFC | |
| C. albicans (ATCC 10231) | 5.00 b | 10.00 b | 8.00 c | 9.00 c |
| C. krusei (clinical isolate) | 2.50 a | 5.00 a | 2.00 a | 3.00 a |
| C. tropicalis (ATCC 750) | 5.00 b | 10.00 b | 3.00 b | 6.00 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojković, D.; Dias, M.I.; Drakulić, D.; Barros, L.; Stevanović, M.; C. F. R. Ferreira, I.; D. Soković, M. Methanolic Extract of the Herb Ononis spinosa L. Is an Antifungal Agent with no Cytotoxicity to Primary Human Cells. Pharmaceuticals 2020, 13, 78. https://doi.org/10.3390/ph13040078
Stojković D, Dias MI, Drakulić D, Barros L, Stevanović M, C. F. R. Ferreira I, D. Soković M. Methanolic Extract of the Herb Ononis spinosa L. Is an Antifungal Agent with no Cytotoxicity to Primary Human Cells. Pharmaceuticals. 2020; 13(4):78. https://doi.org/10.3390/ph13040078
Chicago/Turabian StyleStojković, Dejan, Maria Inês Dias, Danijela Drakulić, Lillian Barros, Milena Stevanović, Isabel C. F. R. Ferreira, and Marina D. Soković. 2020. "Methanolic Extract of the Herb Ononis spinosa L. Is an Antifungal Agent with no Cytotoxicity to Primary Human Cells" Pharmaceuticals 13, no. 4: 78. https://doi.org/10.3390/ph13040078
APA StyleStojković, D., Dias, M. I., Drakulić, D., Barros, L., Stevanović, M., C. F. R. Ferreira, I., & D. Soković, M. (2020). Methanolic Extract of the Herb Ononis spinosa L. Is an Antifungal Agent with no Cytotoxicity to Primary Human Cells. Pharmaceuticals, 13(4), 78. https://doi.org/10.3390/ph13040078









