1,2-Dihydroxyxanthone: Effect on A375-C5 Melanoma Cell Growth Associated with Interference with THP-1 Human Macrophage Activity
Abstract
1. Introduction
2. Results
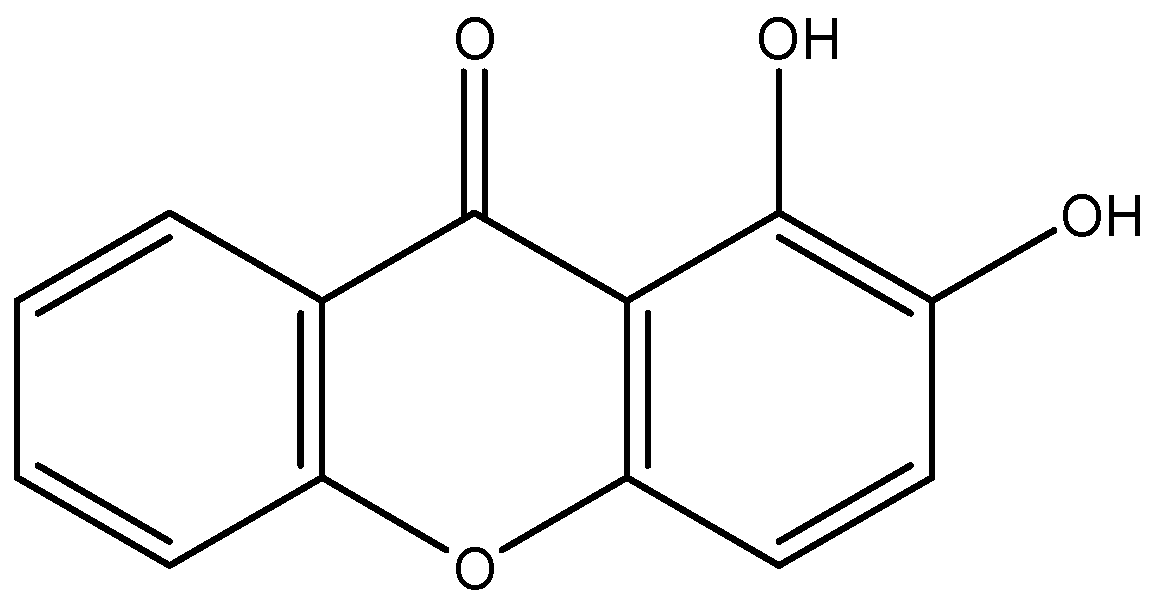
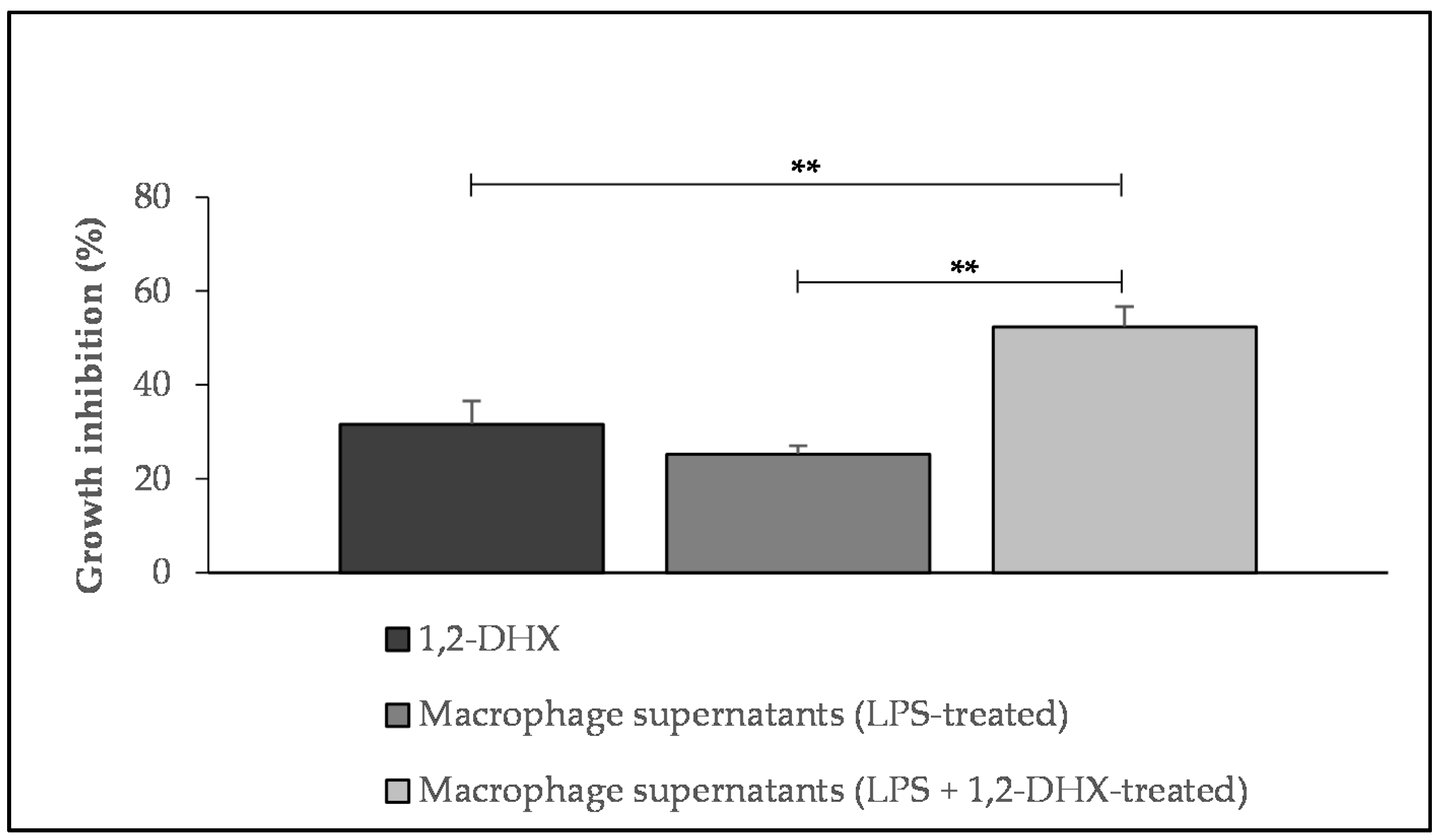
2.1. 1,2-DHX and Supernatants from 1,2-DHX-Treated Macrophage Cultures Inhibited A375-C5 Growth
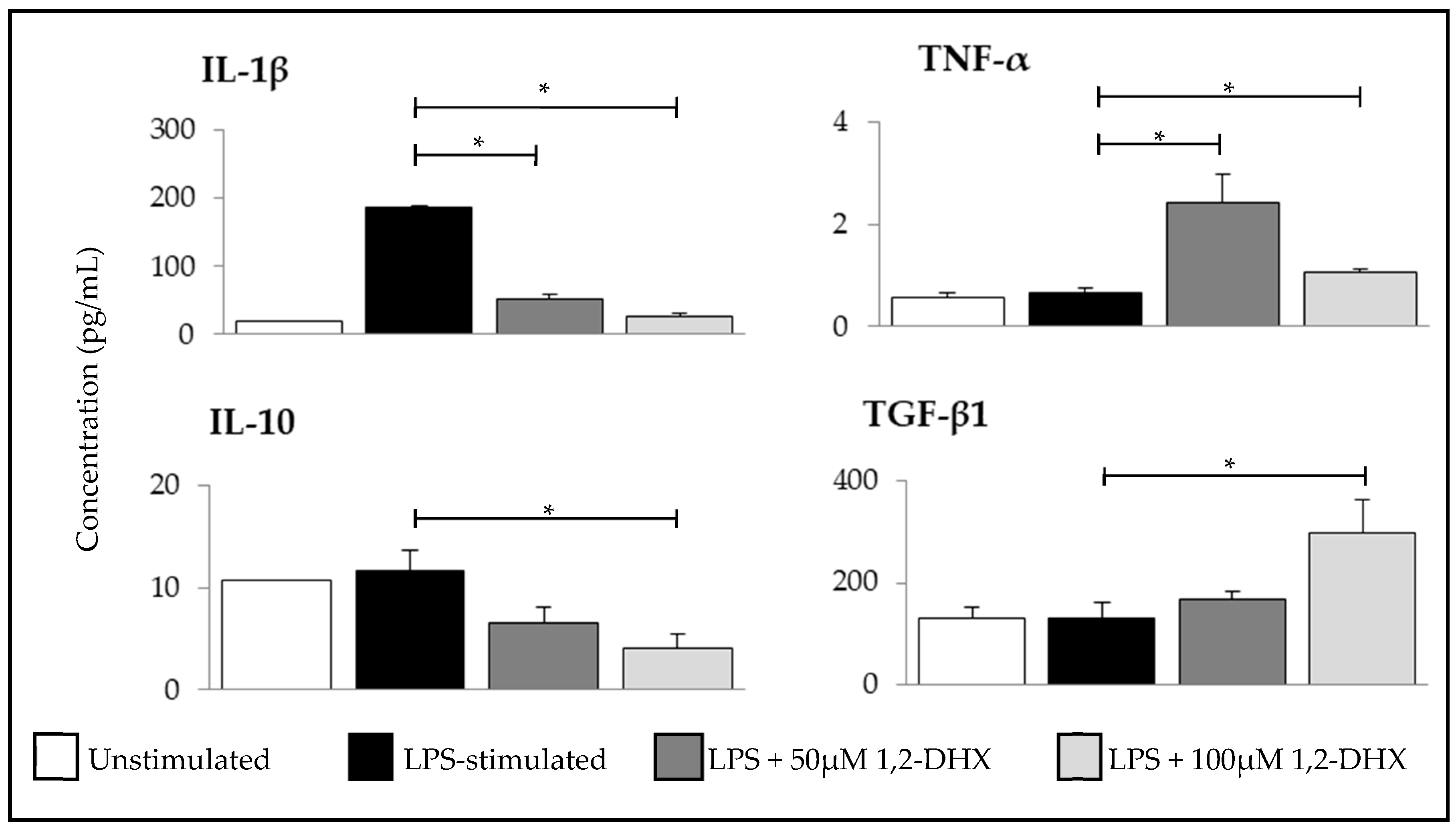
2.2. 1,2-DHX Modulated Cytokine Production by Macrophages
2.3. 1,2-DHX Inhibited NO Production by Macrophages
3. Discussion
4. Materials and Methods
4.1. Xanthone
4.2. Cell Lines
4.3. Sulphorodamine B (SRB) Growth Inhibition Assay
4.4. Antitumor Effect of Conditioned Macrophage Culture Medium
4.5. Cytokine Quantification
4.6. NO Production Assays
4.7. NO Scavenging Assays
4.8. MTT-Viability Assay
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hussein, M.R. Genetic pathways to melanoma tumorigenesis. J. Clin. Pathol. 2004, 57, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, L.; Wang, D.; Zhang, Q.; Zhang, L. Pro-tumor activities of macrophages in the progression of melanoma. Hum. Vaccin Immunother. 2017, 13, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, G. Targets of protective tumor immunity. Ann. N. Y. Acad. Sci. 2009, 1174, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, M.I.; Rozati, S.; Goldinger, S.M.; Widmer, D.S.; Dummer, R.; Levesque, M.P. Melanoma immunotherapy: Historical precedents, recent successes and future prospects. Immunotherapy 2013, 5, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Tentori, L.; Navarra, P. Ipilimumab: A novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol. Res. 2012, 65, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Solit, D.B.; Rosen, N. Resistance to BRAF Inhibition in Melanomas. N. Engl. J. Med. 2011, 364, 772–774. [Google Scholar] [CrossRef]
- Gast, A.; Bermejo, J.L.; Claus, R.; Brandt, A.; Weires, M.; Weber, A.; Plass, C.; Sucker, A.; Hemminki, K.; Schadendorf, D.; et al. Association of inherited variation in Toll-like receptor genes with malignant melanoma susceptibility and survival. PLoS ONE 2011, 6, e24370. [Google Scholar] [CrossRef]
- Kuphal, S.; Bosserhoff, A. Recent progress in understanding the pathology of malignant melanoma. J. Pathol. 2009, 219, 400–409. [Google Scholar] [CrossRef]
- Barbosa, J.; Lima, R.T.; Sousa, D.; Gomes, A.S.; Palmeira, A.; Seca, H.; Choosang, K.; Pakkong, P.; Bousbaa, H.; Pinto, M.M.; et al. Screening a Small Library of Xanthones for Antitumor Activity and Identification of a Hit Compound which Induces Apoptosis. Molecules 2016, 21, 81. [Google Scholar] [CrossRef]
- Genovese, S.; Fiorito, S.; Taddeo, V.; Epifano, F. Recent developments in the pharmacology of prenylated xanthones. Drug Discov. Today 2016, 21, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Castanheiro, R. Natural Prenylated Xanthones: Chemistry and Biological Activities. In Natural Products: Chemistry, Biochemistry and Pharmacology; Brahmachari, G., Ed.; Alpha Science International: Oxford, UK, 2009. [Google Scholar]
- Pinto, M.M.; Sousa, M.E.; Nascimento, M.S. Xanthone derivatives: New insights in biological activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Masawang, K.; Tiritan, M.E.; Sousa, E.; de Lima, V.; Afonso, C.; Bousbaa, H.; Sudprasert, W.; Pedro, M.; Pinto, M.M. New chiral derivatives of xanthones: Synthesis and investigation of enantioselectivity as inhibitors of growth of human tumor cell lines. Bioorg. Med. Chem. 2014, 22, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Pedro, M.; Cerqueira, F.; Sousa, M.E.; Nascimento, M.S.J.; Pinto, M. Xanthones as inhibitors of growth of human cancer cell lines and their effects on the proliferation of human lymphocytes in vitro. Bioorg. Med. Chem. 2002, 10, 3725–3730. [Google Scholar] [CrossRef]
- Azevedo, C.; Pinto, C.M.M.; Pinto, M.M.M. Routes to Xanthones: An Update on the Synthetic Approaches. Curr. Org. Chem. 2012, 16, 2818–2867. [Google Scholar] [CrossRef]
- Azevedo, C.; Afonso, C.; Soares, J.; Reis, S.; Sousa, S.; Lima, R.; Vasconcelos, M.H.; Pedro, M.; Barbosa, J.; Gales, L.; et al. Pyranoxanthones: Synthesis, growth inhibitory activity on human tumor cell lines and determination of their lipophilicity in two membrane models. Eur. J. Med. Chem. 2013, 69, 798–816. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.E.; Pinto, M.M.M. Synthesis of Xanthones: An Overview. Curr. Med. Chem. 2005, 12, 2447–2479. [Google Scholar] [CrossRef] [PubMed]
- Vermes, B.; Seligmann, O.; Wagner, H. Synthesis of xanthone O-glycosides. Part III. Synthesis of 1- and 8-O-β-D-glycosides of 5-O-methyl- and de-O-methylbellidifolin. Helv. Chim Acta 1985, 68, 2359–2366. [Google Scholar] [CrossRef]
- Sousa, E.P.; Silva, A.M.S.; Pinto, M.M.M.; Pedro, M.M.; Cerqueira, F.A.M.; Nascimento, M.S.J. Isomeric Kielcorins and Dihydroxyxanthones: Synthesis, Structure Elucidation, and Inhibitory Activities of Growth of Human Cancer Cell Lines and on the Proliferation of Human Lymphocytes In Vitro. Helv. Chim Acta 2002, 85, 2862–2876. [Google Scholar] [CrossRef]
- Cidade, H.; Rocha, V.; Palmeira, A.; Marques, C.; Tiritan, M.E.; Ferreira, H.; Lobo, J.S.; Almeida, I.F.; Sousa, M.E.; Pinto, M. In silico and in vitro antioxidant and cytotoxicity evaluation of oxygenated xanthone derivatives. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Kasemwattanaroj, P.; Moongkarndi, P.; Pattanapanyasat, K.; Mamgmool, S.; Rodpai, E.; Samer, J.; Konlata, J.; Sukapirom, K. Immunomodulatory activities of alpha-mangostin on peripheral Blood mononuclear cells. Nat. Prod. Commun. 2013, 8, 1257–1260. [Google Scholar] [PubMed]
- Ngoupayo, J.; Tabopda, T.; Ali, M. Antimicrobial and immunomodulatory properties of prenylated xanthones from twigs of Garcinia staudtii. Bioorg. Med. Chem. 2009, 17, 5688–5695. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.; Cerqueira, F.; Barbosa, C.M.; Nascimento, M.S.; Pinto, M. Improvement of the inhibitory effect of xanthones on NO production by encapsulation in PLGA nanocapsules. J. Drug Target. 2005, 13, 129–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, L.; Shao, M.; Yang, B.; Bai, W. Immunomodulatory and anticancer activities of phenolics from Garcinia mangostana fruit pericarp. Food Chem. 2009, 116, 969–973. [Google Scholar] [CrossRef]
- Allavena, P.; Sica, A.; Solinas, G.; Porta, C.; Mantovani, A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol. 2008, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Allavena, P.; Mantovani, A. Tumor-associated macrophages: Functional diversity, clinical significance, and open questions. Semin. Immunopathol. 2013, 35, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Redente, E.; Dwyer-Nield, L.D.; Merrick, D.T.; Raina, K.; Agarwal, R.; Pao, W.; Rice, P.L.; Shroyer, K.R.; Malkinson, A.M. Tumor progression stage and anatomical site regulate tumor-associated macrophage and bone marrow-derived monocyte polarization. Am. J. Pathol. 2010, 176, 2972–2985. [Google Scholar] [CrossRef] [PubMed]
- Zaynagetdinov, R.; Sherrill, T.P.; Polosukhin, V.V.; Han, W.; Ausborn, J.A.; McLoed, A.G.; McMahon, F.B.; Gleaves, L.A.; Degryse, A.L.; Stathopoulos, G.T.; et al. A critical role for macrophages in promotion of urethane-induced lung carcinogenesis. J. Immunol. 2011, 187, 5703–5711. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Mantovani, A. Diversity and plasticity of mononuclear phagocytes. Eur. J. Immunol. 2011, 41, 2470–2472. [Google Scholar] [CrossRef]
- Liao, Q.; Zeng, Z.; Guo, X.; Li, X.; Wei, F.; Zhang, W.; Li, X.; Chen, P.; Liang, F.; Xiang, B.; et al. LPLUNC1 suppresses IL-6-induced nasopharyngeal carcinoma cell proliferation via inhibiting the Stat3 activation. Oncogene 2014, 33, 2098–2109. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: Role in tumour progression. Eur. J. Cancer 2004, 40, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Schioppa, T.; Mantovani, A.; Allavena, P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur. J. Cancer 2006, 42, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Bernengo, M.G.; Quaglino, P.; Cappello, N.; Lisa, F.; Osella-Abate, S.; Fierro, M.T. Macrophage-mediated immunostimulation modulates therapeutic efficacy of interleukin-2 based chemoimmunotherapy in advanced metastatic melanoma patients. Melanoma Res. 2000, 10, 55–65. [Google Scholar] [CrossRef]
- Bröcker, E.B.; Zwadlo, G.; Holzmann, B.; Macher, E.; Sorg, C. Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int. J. Cancer 1988, 41, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Mäkitie, T.; Summanen, P.; Tarkkanen, A.; Kivelä, T. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1414–1421. [Google Scholar]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef]
- Porta, C.; Kumar, B.S.; Larghi, P.; Rubino, L.; Mancino, A.; Sica, A. Tumor Promotion by Tumor-Associated Macrophages. In Advances in Molecular Oncology. Advances in Experimental Medicine and Biology; Fagagna, F., Chiocca, S., McBlane, F., Cavallaro, U., Eds.; Springer: Boston, MA, USA, 2007; Volume 604, pp. 67–86. [Google Scholar]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef]
- Varney, M.L.; Johansson, S.L.; Singh, R.K. Tumour-associated macrophage infiltration, neovascularization and aggressiveness in malignant melanoma: Role of monocyte chemotactic protein-1 and vascular endothelial growth factor-A. Melanoma Res. 2005, 15, 417–425. [Google Scholar] [CrossRef]
- Jacobs, A.; Ignarro, L. Lipopolysaccharide-induced Expression of Interferon-β Mediates the Timing of Inducible Nitric-oxide Synthase Induction in RAW 264. 7 Macrophages. J. Biol. Chem. 2001, 276, 47950–47957. [Google Scholar] [CrossRef]
- Shinoda, J.; Mclaughlin, K.E.; Bell, H.S.; Swaroop, G.R.; Yamaguchi, S.-I.; Holmes, M.C.; Whittle, A.R. Molecular mechanisms underlying dexamethasone inhibition of iNOS expression and activity in C6 glioma cells. Glia 2003, 42, 68–76. [Google Scholar] [CrossRef]
- Xie, Q.W.; Kashiwabara, Y.; Nathan, C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994, 269, 4705–4708. [Google Scholar]
- Castanheiro, R.A.; Pinto, M.M.M.; Silva, A.M.S.; Cravo, S.M.; Gales, L.; Damas, A.M.; Nazareth, N.; Nascimento, M.S.; Eaton, G. Dihydroxyxanthones prenylated derivatives: Synthesis, structure elucidation, and growth inhibitory activity on human tumor cell lines with improvement of selectivity for MCF-7. Bioorg. Med. Chem. 2007, 15, 6080–6088. [Google Scholar] [CrossRef]
- Gutierrez-Orozco, F.; Failla, M.L. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Zhang, Z.; Zhang, S.; Yang, S.; Zhang, A.; Yin, L.; Swarts, S.; Vidyasagar, S.; Zhang, L.; et al. Synthesis and anticancer potential of novel xanthone derivatives with 3,6-substituted chains. Bioorg. Med. Chem. 2016, 24, 4263–4271. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef]
- Sukandar, E.; Ersam, T.; Fatmawati, S.; Siripong, P.; Aree, T.; Tip-pyang, S. Cylindroxanthones A-C, three new xanthones and their cytotoxicity from the stem bark of Garcinia cylindrocarpa. Fitoterapia 2016, 198, 62–65. [Google Scholar] [CrossRef]
- Schildberger, A.; Rossmanith, E.; Eichhorn, T.; Strassl, K.; Weber, V. Monocytes, Peripheral Blood Mononuclear Cells, and THP-1 Cells Exhibit Different Cytokine Expression Patterns following Stimulation with Lipopolysaccharide. Mediat. Inflamm. 2013, 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Kouklakis, G.; Efremidou, E.I.; Pitiakoudis, M.; Lyratzopoulos, N.; Polychronidis, A. Development of primary malignant melanoma during treatment with a TNF-alpha antagonist for severe Crohn’s disease: A case report and review of the hypothetical association between TNF-alpha blockers and cancer. Drug Des. Dev. Ther. 2013, 7, 195–199. [Google Scholar] [CrossRef]
- Mariette, X.; Matucci-Cerinic, M.; Pavelka, K.; Taylor, P.; van Vollenhoven, R.; Heatley, R.; Walsh, C.; Lawson, R.; Reynolds, A.; Emery, P. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: A systematic review and meta-analysis. Ann. Rheum. Dis. 2011, 70, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Mercer, L.K.; Askling, J.; Raaschou, P.; Dixon, W.; Dreyer, L.; Hetland, M.; Strangfeld, A.; Zink, A.; Mariette, X.; Finckh, A.; et al. Risk of invasive melanoma in patients with rheumatoid arthritis treated with biologics: Results from a collaborative project of 11 European biologic registers. Ann. Rheum. Dis. 2017, 76, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Sanchez-Laorden, B.; O’Brien, K.; Brunton, H.; Ferguson, J.; Young, H.; Dhomen, N.; Flaherty, K.; Frederick, D.; Cooper, Z.; et al. The immune-microenvironment confers resistance to MAP kinase pathway inhibitors through macrophage-derived TNFα. Cancer Discov. 2014, 4, 1214–1229. [Google Scholar] [CrossRef] [PubMed]
- Giavazzi, R.; Garofalo, A.; Bani, M.R.; Abbate, M.; Ghezzi, P.; Boraschi, D.; Mantovani, A.; Dejana, E. Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer Res. 1990, 50, 4771–4775. [Google Scholar] [PubMed]
- Meyer, C.; Sevko, A.; Ramacher, M.; Bazhin, A.V.; Falk, C.S.; Osen, W.; Borrello, I.; Kato, M.; Schadendorf, D.; Baniyash, M.; et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc. Natl. Acad. Sci. USA 2011, 108, 17111–17116. [Google Scholar] [CrossRef]
- Apte, R.N.; Voronov, E. Is interleukin-1 a good or bad ‘guy’ in tumor immunobiology and immunotherapy? Immunol. Rev. 2008, 222, 222–241. [Google Scholar] [CrossRef]
- Fairweather, D.; Cihakova, D. Alternatively activated macrophages in infection and autoimmunity. J. Autoimmun. 2009, 33, 222–230. [Google Scholar] [CrossRef]
- Mimnaugh, E.; Monti, E.; Sebers, S.; Stetler-Stevenson, M.; Sinha, B. Synergistic Antiproliferative Effects of the Combination of Interleukin-1a and Doxorubicin against Human Melanoma Cells. Oncol. Res. 1992, 4, 401–412. [Google Scholar]
- Morinaga, Y.; Suzuki, H.; Takatsuki, F.; Akiyama, Y.; Taniyama, T.; Matsushima, K.; Onozaki, K. Contribution of Il-6 to the antiproliferative effect of IL-1 and tumor necrosis factor on tumor cell lines. J. Immunol. 1989, 143, 3538–3542. [Google Scholar] [PubMed]
- Usui, N.; Mimnaugh, E.; Sinha, B. A role for the Interleukin 1 Receptor in the Synergistic Antitumor Effects on Human Interleukin 1a and Etoposide against Human Melanoma Cells. Cancer Res. 1991, 51, 769–774. [Google Scholar] [PubMed]
- García-Hernández, M.L.; Hernández-Pando, R.; Gariglio, P.; Berumen, J. Interleukin-10 promotes B16-melanoma growth by inhibition of macrophage functions and induction of tumour and vascular cell proliferation. Immunology 2002, 105, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Itakura, E.; Huang, R.R.; Wen DRPaul, E.; Wünsch, P.H.; Cochran, A.J. IL-10 expression by primary tumor cells correlates with melanoma progression from radial to vertical growth phase and development of metastatic competence. Mod. Pathol. 2011, 24, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Javelaud, D.; Alexaki, V.I.; Mauviel, A. Transforming growth factor-beta in cutaneous melanoma. Pigment Cell Melanoma Res. 2008, 21, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Krasagakis, K.; Tholke, D.; Farthmann, B.; Eberle, J.; Mansmann, U.; Orfanos, C.E. Elevated plasma levels of transforming growth factor (TGF)-beta1 and TGF-beta2 in patients with disseminated malignant melanoma. Br. J. Cancer 1998, 77, 1492–1494. [Google Scholar] [CrossRef]
- Wink, D.A.; Vodovotz, Y.; Laval, J.; Laval, F.; Dewhirst, M.W.; Mitchell, J.B. The multifaceted roles of nitric oxide in cancer. Carcinogenesis 1998, 19, 711–721. [Google Scholar] [CrossRef]
- Choi, B.M.; Pae, H.O.; Jang, S.I.; Kim, Y.M.; Chung, H.T. Nitric oxide as a pro-apoptotic as well as anti-apoptotic modulator. J. Biochem. Mol. Biol. 2002, 35, 116–126. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L. Nitric oxide and angiogenesis. J. Neurooncol. 2000, 50, 139–148. [Google Scholar] [CrossRef]
- Wink, D.A.; Kasprzak, K.S.; Maragos, C.M.; Elespuru, R.K.; Misra, M.; Dunams, T.M.; Cebula, T.A.; Koch, W.H.; Andrews, A.W.; Allen, J.S.; et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science 1991, 254, 1001–1003. [Google Scholar] [CrossRef]
- Lala, P.K.; Orucevic, A. Role of nitric oxide in tumor progression: Lessons from experimental tumors. Cancer Metastasis Rev. 1998, 17, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, S.K.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World, J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Hofseth, L.J. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007, 67, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- McCall, T.B.; Feelisch, M.; Palmer, R.M.; Moncada, S. Identification of N-iminoethyl-l-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br. J. Pharmacol. 1991, 102, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Hämäläinen, M.; Kankaanranta, H.; Moilanen, E. Dexamethasone inhibits inducible nitric-oxide synthase expression and nitric oxide production by destabilizing mRNA in lipopolysaccharide-treated macrophages. Mol. Pharmacol. 2002, 62, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rodrigues, L.M.; Esteves, A.P.; Oliveira-Campos, A.M.; Nascimento, M.S.; Nazareth, N.; Cidade, H.; Neves, M.P.; Fernandes, E.; Pinto, M.; et al. Synthesis of N-aryl-5-amino-4-cyanopyrazole derivatives as potent xanthine oxidase inhibitors. Eur. J. Med. Chem. 2008, 43, 771–780. [Google Scholar] [CrossRef]
- He, X.; Shu, J.; Xu, L.; Lu, C.; Lu, A. Inhibitory effect of Astragalus polysaccharides on lipopolysaccharide-induced TNF-a and IL-1beta production in THP-1 cells. Molecules 2012, 17, 3155–3164. [Google Scholar] [CrossRef]
- Chanput, W.; Reitsma, M.; Kleinjans, L.; Mes, J.J.; Savelkoul, H.F.; Wichers, H.J. β-Glucans are involved in immune-modulation of THP-1 macrophages. Mol. Nutr. Food Res. 2012, 56, 822–833. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Cerqueira, F.; Cidade, H.; Van Ufford, L.; Beukelman, C.; Kijjoa, A.; Nascimento, M.S. The natural prenylated flavone artelastin is an inhibitor of ROS and NO production. Int. Immunopharmacol. 2008, 8, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.; Cordeiro-da-Silva, A.; Araujo NCidade HKijjoa, A. Nascimento MS Inhibition of lymphocyte proliferation by prenylated flavones: Artelastin as a potent inhibitor. Life Sci. 2003, 73, 2321–2334. [Google Scholar] [CrossRef]
| Compound | Growth inhibition (GI50) |
|---|---|
| 1,2-DHX | 55.4 ± 2.0 µM |
| Doxorubicin | 1.8 × 10−2 ± 0.4 × 10−2 µM |
| Compound | NO Inhibition (% of Control) | ||
|---|---|---|---|
| 0 h | 6 h | 14 h | |
| 1,2-DHX (25 μM) | 56.6 ± 1.8 | 25.0 ± 2.8 * | n.i. |
| L-NAME (62.5 μM) | 52.8 ± 5.0 | 50.7 ± 3.8 | 25.9 ± 2.7 ** |
| Dexamethasone (6.25 μM) | 55.9 ± 2.3 | 15.7 ± 4.9 * | n.i. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.; Cerqueira, F.; Nazareth, N.; Medeiros, R.; Sarmento, A.; Sousa, E.; Pinto, M. 1,2-Dihydroxyxanthone: Effect on A375-C5 Melanoma Cell Growth Associated with Interference with THP-1 Human Macrophage Activity. Pharmaceuticals 2019, 12, 85. https://doi.org/10.3390/ph12020085
Silva V, Cerqueira F, Nazareth N, Medeiros R, Sarmento A, Sousa E, Pinto M. 1,2-Dihydroxyxanthone: Effect on A375-C5 Melanoma Cell Growth Associated with Interference with THP-1 Human Macrophage Activity. Pharmaceuticals. 2019; 12(2):85. https://doi.org/10.3390/ph12020085
Chicago/Turabian StyleSilva, Viviana, Fátima Cerqueira, Nair Nazareth, Rui Medeiros, Amélia Sarmento, Emília Sousa, and Madalena Pinto. 2019. "1,2-Dihydroxyxanthone: Effect on A375-C5 Melanoma Cell Growth Associated with Interference with THP-1 Human Macrophage Activity" Pharmaceuticals 12, no. 2: 85. https://doi.org/10.3390/ph12020085
APA StyleSilva, V., Cerqueira, F., Nazareth, N., Medeiros, R., Sarmento, A., Sousa, E., & Pinto, M. (2019). 1,2-Dihydroxyxanthone: Effect on A375-C5 Melanoma Cell Growth Associated with Interference with THP-1 Human Macrophage Activity. Pharmaceuticals, 12(2), 85. https://doi.org/10.3390/ph12020085








