Abstract
This paper presents recent progress (2019–2025) in the role of polymer-based sensors implemented for heart and blood vessel monitoring. The existing variety of polymers, of synthetic and natural origin, allows the creation of sensors tailored to specific needs, to monitor heart health status for invasive cardiovascular surgery. Polymers, in combination with nanomaterials, nanostructures, or nanostructured materials, enhance the characteristics of force sensors. The review discusses implantable sensors applied in healthcare, especially for cardiovascular system monitoring, which provide the possibility to prevent the development of pathology or to control existing pathology. Additionally, the emerging need for biodegradable devices requires a review of the polymers already used. The quality and accuracy requirements of sensors for self-monitoring and health status control in medical institutions vary; yet needing a variety of sensors does not reduce the importance of finding sensors that are more accurate or more comfortable to wear. Sensors suitable for short-term use become important in the postoperative period, with the need for biodegradable polymers. This review focuses on publications that provide an analysis of the sensors as well as their potential for medical purposes. Our review focuses on polymers applied to force sensors for cardiovascular system monitoring. Overall, this review explores the paths of innovations in the field of novel technologies for self-monitoring of health. Finally, future research directions reported in the selected articles for cardiovascular care sensors are discussed.
1. Introduction
Cardiovascular disease (CVD) has become a significant health problem globally []. While cardiovascular diseases (CVDs) have become one of the leading causes of mortality worldwide over the last few decades [,], some sensors can help to monitor this health problem. The main pathology of cardiovascular diseases is atherosclerosis or the hardening of blood vessels, wherein the narrowing of the arteries disrupts the blood flow and regular supply of oxygen and nutrients, leading to heart attack and stroke []. The common treatment today, with the advancements of modern medical sciences, is stenting [,]. Cardiovascular stent coatings require much investigation, to support the multifunctional properties of stents []. Although most smart stent technologies do not have an integrated sensor, there is potential to integrate microsensors to monitor situations in real time for precise drug delivery or to adjust the therapeutic effects []. J. Park [] reviewed the latest medical tools to incorporate sensors. The limitations of metallic stents have led to the development of polymer stents, which, in turn, opened up the field of biodegradable shape-memory nanocomposites for controlling heart diseases []. One possible way to control the disease is with implantable stents equipped with sensors. Polymer-based medical implantable devices require biocompatibility over the entire lifecycle. The capabilities of developing cardiovascular monitoring and healthcare devices are based on the unique properties of polymers []. Another decision involves the strut patterns of various stents, which can cause side effects from non-biodegradable metal []. Self-powered mechanical sensors can enable long-term real-time monitoring by cardiovascular devices to detect abnormalities, thus allowing early intervention []. The diversity of medical equipment is a huge achievement, allowing one to choose the most appropriate solution for a specific patient; however, the abundance of information can complicate knowledge management and information accessibility.
This review focuses on an analysis of the achievements in cardiovascular system stents containing biometric parameters’ polymer-based force sensors, incorporated into the stent structure, without delving into the polymer synthesis or physicochemical properties of polymer manufacturing and without considering additional functions such as drug delivery. On the other hand, we mention bioabsorbable smart stents and future perspectives that may evolve this area of research. This review focuses solely on the structural function of cardiovascular stents with integrated sensors, depending on the selected polymer. This research is presented to demonstrate the critical role of polymers in the development of stents with embedded sensors, using relevant examples from publications that illustrate a variety of solutions. The presented statistics on publications related to this topic may be useful for developers to assess which path is most promising for developing a new generation of stents with polymer-based sensors. As in most medical device development, polymers play a crucial role. Even well-known designs can be significantly improved with the use of new-generation polymers.
The main goal of this study is to inform the scientific community and physicians about the innovative equipment, polymer developers about which products have been successful, and sensor manufacturers about which materials provide better results, in order to produce a novel sensor. The topic of microelectronics is also discussed, where the essential interaction between molecules and nanostructures takes place.
For clarity, the aim can be expressed by these three objectives:
- To analyze the achievements in cardiovascular system stents containing biometric parameter polymer sensors;
- To demonstrate the critical role of polymers in the development of stents;
- To inform the scientific community, physicians, and polymer developers about the innovative equipment.
The remaining part of this paper is organized as follows: Section 2 presents the method of article selection, indicating the main ideas of the investigation; Section 3 presents the fundamentals of force sensors integrated in cardiovascular stents with polymeric structures, and Section 4 discusses polymer-based smart stents with integrated sensor technologies. The paper concludes with the results obtained and highlights the observed trends in the researched area.
2. Methods of Investigation
This research analyzes interdisciplinary data on the role and influence of polymers in the development of cardiac stents containing an integrated mechanical sensor, published between 2019 and October 2025. The selected data underwent a three-step procedure: sorting and searching by keywords, screening by title, and detailed reading, with a focus on polymer application in force sensors used for cardiovascular system monitoring. Statistical data are used to identify trends that may lead to promising future research. The statistical analysis was performed according to the PRISMA procedure []. The results of the screening are presented in Figure 1. After statistical analysis of the selected articles, the results were presented in the following topic groups:
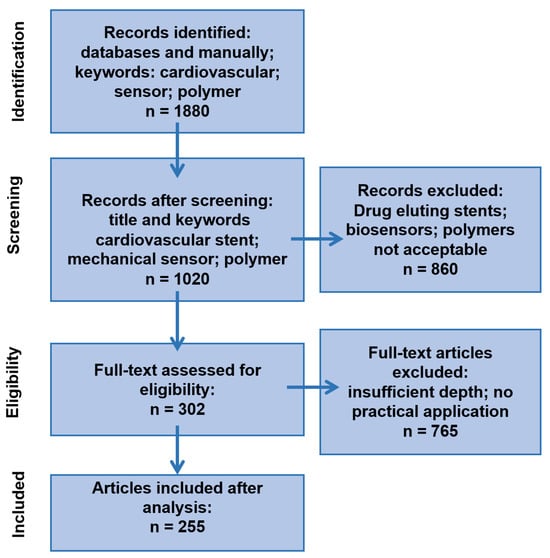
Figure 1.
PRISMA-style diagram illustrating the number of articles at each stage of investigation/reviewing.
- Polymers applied in force sensor manufacturing.
- Materials to improve the properties of polymer-based force sensors.
- Polymers’ role in “smart stent” elaboration.
- Polymer-based microsensors for cardiovascular monitoring.
- Biodegradable polymers used in force sensor manufacturing for “smart stents”.
The search was performed in several stages. For example, during the first search stage, articles with the mentioned keyword “polymer” were selected, and after this selection, the review was performed using the specific name of the polymer. An additional analysis was performed using the name of the particular polymer and including other keywords important for the review, so that the content corresponded to the selected topic: the polymer is used in the structure of the force sensor, and the sensor itself is integrated into the cardiovascular stent. For articles with the keywords ‘Polymer’ and ‘Cardiovascular sensor’, it is observed that the number of publications is increasing annually, and in 2024, it exceeded two and a half thousand. However, upon examining the specific name of the polymer, no dominant polymer was found, and the numbers of publications on the chosen polymer names are presented in Figure 2.
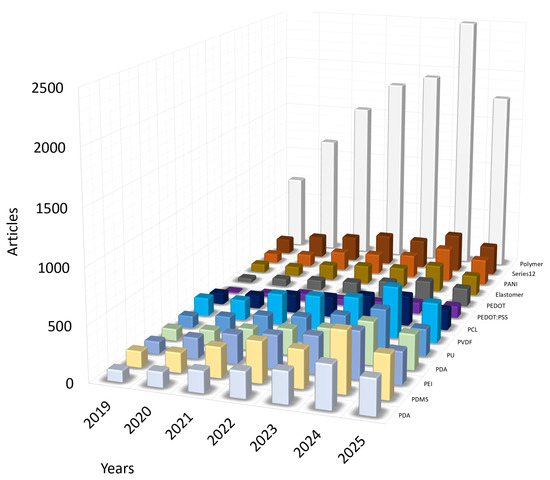
Figure 2.
Statistics of published articles 2019–2025 on keywords. Visual representation of the distribution of articles by polymer names in articles on cardiovascular stent manufacturing.
Luminescent molecularly imprinted polymers or other polymers with wide applications in biosensors are not discussed in this work. One of the aims of this work is to evaluate the evolution of “smart polymers”, those that can change their mechanical properties in response to an electric field and vice versa. The quality and suitability of polymers in sensor manufacturing are already well-established. The application of polymers in sensors for cardiology has been reviewed from several aspects. Polymers play a scaffold and frame role, and electrically conductive polymers become the main part of the sensor, converting mechanical energy into an electrical current. Biomedical polymers are either of natural origin or synthetic varieties, which are friendly to human health, biocompatible, and do not cause mutagenic, carcinogenic, or immunogenic reactions. Natural biomedical polymers, such as polysaccharides, are promising in the manufacturing of biodegradable devices. Synthetic biopolymers have more stable performance and are easier to produce. The discussion of biomedical polymers is intended to serve as a source to develop innovative polymers and apply known research in clinical practice. In this research, Google Scholar, ScienceDirect, IEEE Xplore, and PubMed were utilized as research engines. The analyzed publications were selected from the ScienceDirect, Springer, Google Scholar, IEEE Xplore, Research Gate, Web of Sciences, MDPI, and Wiley databases using the following keywords: electroactive polymer, conductive polymer, piezoelectric polymer, biodegradable polymers, carbon nanomaterial, cardiovascular, conducting polymers, sensor, force sensor, Kapton, MEMS, microsensors, nanostructures, natural polymers, polymer, Polydimethylsiloxane (PDMS), Polyaniline (PANI), Poly(acryl amide) (PAAm), polylactic acid (PLA), polydopamine, polypyrrole (Ppy), poly(ether-b-amide), polyimide (PI), poly(ɛ-caprolactone), poly(vinyl alcohol) (PVA), poly(vinylidene fluoride) (PVDF), Polyvinyl chloride (PVC), Silk fibroin, thermoplastic polyurethane (PU), strain sensor, sensor in stent, smart stent, TENG, B-TENG. Some of the numbers of references are shown in tables to demonstrate the novel decisions and trends in sensor manufacturing for cardiovascular system monitoring. The statistical data were extracted from the ScienceDirect database. It is evident that the specifics of the chosen topic currently limit the use of certain polymer classes in the production of implantable cardiovascular stent parameter sensors; however, the situation may change in the future, as new solutions emerge.
3. Principles and Fundamentals
Medical devices are facing increasingly diverse performance requirements. Y. Wu [] presented new bioresorbable mechanical sensors for vascular stents and the fundamental design principles of mechanical sensors.
Printed electronic vascular stents with integrated sensors can monitor pressure, blood flow, and arterial stiffness []. Although the variety of modern stents produced for cardiovascular system surgery is in the hundreds, their functionality is determined by the sensors built into them and the polymers employed [].
Conductive polymers demonstrate the ability to conduct electrons through their conjugated backbone [,,]. In some cases, molecular imprinted polymers have been applied for stent surface modification, making the stent a multifunctional device [,,]. The application of conjugated polymers in stents has opened up drug-delivery functions []. Shape memory polymers are a promising material for stent manufacturing []. Personalized stents with drug-eluting properties and bioresorbable stents have received attention due to novel polymeric materials []. Shape memory polymers have a role in stent manufacturing []. Shape memory polymers (SMPs) utilizing magnetic fields represent another family of smart materials applicable for medical device manufacturing, where SMPs utilize thermal energy to activate their shape memory [,,]. The thermosetting polymers have had successful applications in a variety of stents [,,,,,]. To avoid the complications of in-stent restenosis, inflammations caused by metal structures, stents have experienced various forms of improvement, including smart sensor-based devices [].
Modern sensors allow implantable medical devices to perform an increasing number of monitoring functions. The sensors integrated into stents enable the detection of blood clots by impedance monitoring []. The elaboration of multifunctional stents with integrated sensors and biosensors has become an interdisciplinary research area requiring collaboration between medical doctors and engineers, adapting the latest sensor technologies for real clinical applications []. With the rapid development of flexible and stretchable electronics, the mechanical compatibility and biocompatibility of electronic devices have been significantly improved, and a series of new conductive polymer materials or semiconductors with excellent stretchability have been adapted [,,,].
Developers of modern sensors take into account not only the standard requirements for flexible wearable or implantable sensors but also features such as the product’s antibacterial function, electromagnetic shielding, waterproofing, and self-healing [,]. The variety of synthetic and natural polymers is currently quite large, but the suitability of these polymers for implantable wireless, self-charging, or biodegradable devices still requires better solutions [,,,,]. The manufacturing of polymers for medical purposes is a complex and responsible field of science, as the final product must meet extremely high standards [,]. Electroactive polymers exhibit shape changes in an electrical field and are useful for tissue regeneration. The application possibilities are expanded when used in conjunction with pressure, strain, or tactile sensors []. Polymers for implantable devices have the requirement of biocompatibility and biodegradability in some cases, with appropriate biochemical reactions to surrounding tissues, and useful physical properties [].
Conductive hydrogels with ionic–electronic conduction have application in tissue regeneration, biomedical applications, and stents []. Conductive polymers have a tremendous impact on implantable devices [,]. Biodegradable polymeric stents are rightly called a new step in cardiovascular disease monitoring and treatment [,,]. The collection of smart and intelligent materials for cardiovascular healthcare is rapidly growing []. With the application of stents as a treatment, there is a need for accessible and cost-effective methods for restenosis detection or smart stents with microelectromechanical systems (MEMS) []. Recently identified as a new technology, they are already being applied in medical equipment. R. Herbert presented the aerosol jet printing system [], where a ‘smart’ stent can measure the pulse rate and blood pressure. Although many stent implantation operations are performed every year, the most common complication of restenosis still poses a significant risk to patients [,]. Recently, wireless communication for medical implants has become crucial to real-time health monitoring. However, existing electromagnetic wave-based methods of communication face some limitations due to their specific signal attenuation, and ultrasound methods require specific integrated circuits or special metamaterial-based sensors []. Electromagnetic-based communication methods have high attenuation and limited penetration into biological tissues, but ultrasound connections can address these shortcomings, typically requiring highly customized and complex designs of implant electronics or physical changes to the implanted metamaterials []. Differences in the area of the inferior vena cava (IVC) and folding are early markers of overload and predict the risk of heart failure (HF) []. Despite the availability of advanced medical solutions for treating cardiovascular diseases, prevention programs remain equally relevant. Hence, continuous and efficient monitoring of the cardiovascular system using pressure sensors becomes essential for the prevention of heart failure. Constant monitoring of the vascular system by devices attached to the human skin has attracted attention to determine the early stage of cardiovascular diseases to prevent mortality [,,,,]. A single-device platform for cardiac diseases is desirable [,]. Mechanical energy converting to electrical energy, or triboelectric sensors, can be applied for blood pressure monitoring [,,]. Recently, highly elastic fiber sensors have attracted great interest due to their applications in wearable electronics, human–machine interfaces, and implantable biomedical devices []. With advanced material processing techniques, the new generation of physical sensor manufacturing has begun.
The quality of sensors is improving significantly with the use of carbon materials and nanostructures in their production []. Materials, such as metals, metal films, oxides in nanostructured form, nanostructured polymer surfaces, hydrogels, and foam-like structures, have opened a new era in the manufacturing of health monitoring devices [,,,].
Another essential issue in wearable sensor system manufacturing is the biodegradability of these products and the use of human-friendly technologies in all steps of manufacturing [,,,]. For example, some nanomaterial production processes result in toxic byproducts, which are unacceptable due to their negative environmental impact, as they release toxic gases or chemicals in other physical states [,]. The processing, storage, and transportation of toxic waste increase the cost of the final product and are unattractive to the workers producing it, as well as to organizations concerned with environmental protection, and to consumers who are ultimately responsible for its consumption. With advanced material processing techniques, a new generation of physical sensor manufacturing has begun. The quality of sensors is improving significantly with the use of carbon materials and nanostructures in their production. Patterned structures of polymers give the surface exceptional biological, adhesive, wettable, and optoelectronic properties, which are applicable for sensing applications [,]. Ideas of sustainability and environmental protection also drive the search for new solutions. When developing implantable medical devices [], the scientists and engineers prioritize wireless connectivity, biocompatibility, and structural comfort. Hence, a power supply becomes essential to provide longevity in the human body. Some of the latest data on several different types of sensors and sensing systems integrated into endovascular catheters are presented by C. Kaminski []. The magnetic sensors, which are independent of light transparency, interact better with the skin surface and maintain accuracy during movement, making them suitable for future health status monitoring devices []. Optical and magnetic sensors are critical to advancing heart health monitoring. Their integration will improve our ability to monitor, diagnose, and manage cardiovascular health, promising a more proactive and patient-centered approach to care [].
Biodegradable energy sources have their own benefits in contrast to retractable non-biodegradable ones, whose removal can cause health risks []. For example, B-TENG made from PLA in a health monitoring device can ensure healthcare and bio-resorb at the scheduled time []. Biodegradable materials from renewable sources and synthetic materials can be hydrolyzed or can be modified with additives like antioxidants []. Since both temperature and pulse signal monitoring are complementary diagnostic tools for cardiovascular diseases, the incidence of certain coronary diseases, such as hypertension, tachycardia, and bradycardia, can be predicted using strain sensors [,,,,,]. Hence, the increase in pulse rate shows a strong positive correlation with the prevalence of coronary heart disease, where tachycardia denotes a pulse rate exceeding 100, while bradycardia is defined as a pulse rate of 60 beats per minute []. Thus, pulse rate measurement has become an indispensable diagnostic procedure for patients, just as force sensors have become essential. Self-powered wearable devices for health monitoring have made significant progress in the last few years [,]. The operating mechanisms of self-powered sensors are piezoelectric, electrostatic, and electromagnetic [,]. Biodegradable devices, especially triboelectric nanogenerators (B-TENGS), have emerged as an innovative technology in the field of self-powered implantable devices field [,,]. Biodegradable nano-sensors are critical devices for the detection of postoperative complications [,,,,]. Biomedical polymers can be identified as key materials at the forefront of medical advances, offering innovative solutions for disease prevention, diagnosis, treatment, and clinical use due to their exceptional physical and chemical properties. Innovations in healthcare are constantly discussed in reviews []. However, the abundance of information is so great that it is necessary to frequently systematize the new messages that appear. The latest trend in cardiovascular system monitoring is heart health apps, which are accessible on the Apple App Store and Google Play stores or other sources locally []. This situation entitles a separate area of health self-monitoring where sensors are fully integrated into consumer products. A machine learning approach with non-invasive sensors was presented by P. Arpaia []. Machine learning is promising for the future, with two key benefits: it will enable the real-time investigation of patients’ cardiovascular risk and provide a database for future research. There is a vast supply of devices and health monitoring sensors, suitable for specific operating conditions [,].
4. Polymers Applied in Force Sensor Applications
The specific structure of natural tissues necessitates the development of an artificial system that functions similarly to well-adapted natural tissues, enabling effective force sensors to record the physiological properties of the human body. Thin film device manufacturing arises with emerging carbon nano-materials and conductive polymers []. Conductive polymers can be synthesized directly or be produced, for example, by doping or some chemical treatment: electrochemical polymerization or potentiodynamic method [,]. The basic information about conductive polymers is presented in Figure 3 [].
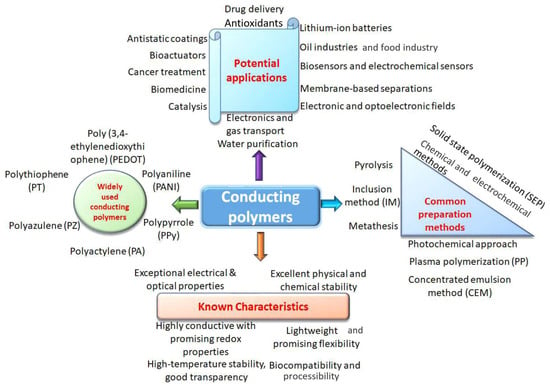
Figure 3.
Known characteristics, potential applications, and common synthesis methods for CPs production [].
The low detection limit and high sensitivity issues require the employment of conductive polymer composites with nanomaterials or nanostructured [] and wrinkled surfaces [,,,,]. Flexible substrates that replace rigid materials enable sensors to optimize their best features for the requirements of wearable devices. Polymer films are the desired material for developers of human body parameter monitoring systems. Conducting polymers, with their unique material properties due to their electrical, optical, and biocompatible properties, are becoming a promising material for medical purposes. Z. Zhou [] proposed PANI nanowires using interfacial polymerization for wrinkled microstructure through repetitive stretching for a pressure sensor to track dynamic and static activities. The polymer sensor surface structure is essential for sensor functioning [].
4.1. Materials to Improve Polymer-Based Force Sensors Properties
Modern sensors are typically made of polymers enriched with nanostructures or microstructures. Nanomaterials play a crucial role in the applications of the new generation of wearable electronics [,,], but nanomaterial-based actuators are still in the early stages of successful development []. To produce micrometric-size sensors, it is essential to understand sensor physics and processes at the molecular level. The synthesis of nanomaterials with predefined structures, such as 0D [], 1D [], 2D [], or 3D [], has shown promising results in the development of these sensors []. Developers often point out that, despite the sensor’s functionality, the existing methods have shortcomings that prevent the production of high-quality and defect-free sensing layers [,,]. To resolve this issue, the development of suitable 2D materials with appropriate features, low cost, and a simple method for mass production is an area of focus for future material scientists []. The application of nanoparticles [,,] in sensor manufacturing has led to the development of novel technologies, such as pharmaceutical nanotechnology, enabling multifunctional implanted devices, biocompatible [] and biodegradable devices [], as well as antimicrobial nanocoatings [] and hydrogels [].
The microstructure-frame-supported organic thermoelectric (MFSOTE) material-based devices are promising for health monitoring. MFSOTE materials are, for example, poly(3,4-ethylenedioxythiophene), poly-(styrenesulfonate), (PEDOT: PSS), and PU []. Recently, flexible electronic devices have attracted significant interest as an alternative to traditional rigid metallic conductors for personal healthcare, as traditional conductors have weak properties: poor stretchability, sensitivity, and strength, as well as low conductivity and a single sensing function. The application of metals, ceramics, and other solid materials in sensor manufacturing was renewed with the discovery of the significance of cracks, micropores, and rough surfaces in the development of such sensors [].
4.2. Polymer Role in “Smart Stent” Elaboration
As stated by many authors on the cardiovascular stent topic, cardiovascular stent angioplasty is the best option for treating coronary artery diseases. Despite successful developments in this field, when drug-eluting stents, short-term stents, and bioabsorbable stents are elaborated and introduced into cardiovascular healthcare routine, several clinical complications related to stent applications, such as thrombosis and restenosis, have occurred []. Thus, it is first necessary to identify what and how adverse effects occur when using stents in the cardiovascular system, as well as the challenges for developers. The corresponding “smart stent” systems with sensors are receiving research interest, given their potential to mitigate/reduce several undesirable stent-related consequences []. Smart stents focus on integrated pressure sensors [].
The technical evaluation of stents can be performed using optical polymers and technologies such as optical coherence tomography (OCT), which allows obtaining high-resolution cross-sectional images of the coronary arteries, allowing the evaluation of the results of stent implantation []; however, polymers with optical properties are not the subject of implantable sensors in stents.
Figure 4 schematically represents stent-related complications. Y.X. Ang [] presented restenosis treatment stages. Therefore, it is of extraordinary importance in the development of technologies to reduce the health risk and improve the clinical efficiency in cardiovascular surgery.
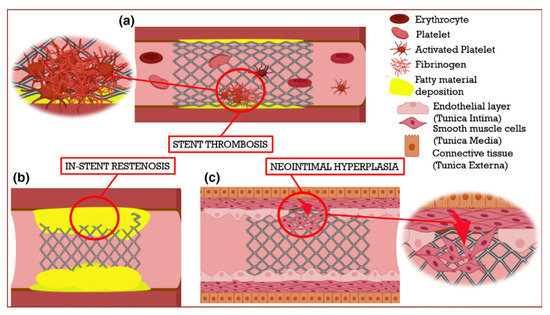
Figure 4.
Schematic representation of the significant limitations associated with cardiovascular stents []. (a) Stent thrombosis occurs due to the formation of a blood clot inside the stent region. (b) In-stent restenosis develops with the re-occurrence of fatty deposition (as shown in yellow) in the stenotic region. (c) After a stent is implanted, delayed endothelialization or endothelial denudation can lead to the migration of smooth muscle cells from the middle layer of the blood vessel, resulting in smooth muscle cell proliferation and neointimal hyperplasia.
A silicon-based self-rollable polymer stent with integrated pressure sensor (Figure 5) was presented by N.E. Oyunbaatar [], using the MEMS technique to monitor blood pressure inside the arteries.
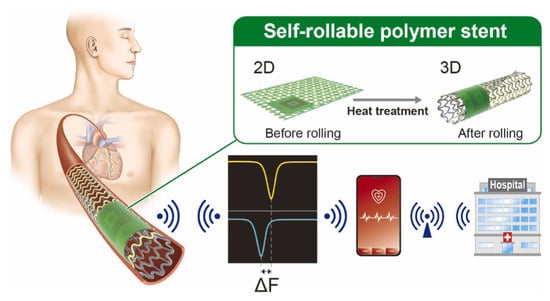
Figure 5.
Schematic representation of a self-rollable polymer stent integrated with an LC pressure sensor [].
A serpentine-shaped wireless biodegradable polymer-based self-reported stent structure was presented by N.E. Oyunbaatar [], which is capable of monitoring vascular abnormalities due to the incorporated capacitive pressure sensor. The authors reported successful stent implantation into the arteries of a three-dimensional phantom system, operating in various environments, including in vivo conditions. The sensor demonstrated a pressure sensitivity of 0.15 mm/Hg.
The PDMS is valuable due to its biocompatibility and elasticity. PDMS has a low elastic modulus and high deformability, enabling dynamic surface compliance that makes it ideal for biological sensor devices []. The most common application of PDMS is to cover the functional layer of the sensor, thereby obtaining a flexible strain sensor. P. Sharma [] proposed graphene nanoplatelets (GNP) and PDMS to fabricate a strain sensor that is widely applicable, from health status monitoring to monitoring of climate change effects. The construction of conductive polymers using PDMS has resulted in effective sensing functions, as supported by numerous examples. A simple shark skin-inspired pulse sensor made from PEDPT: PSS with PDMS was proposed by H.-H. Jang []. The PDMS-MXene, MoS2-based on a silicon substrate, for heart rate and UV detection, a self-powered and integrated triboelectric nanogenerator (TENG) was presented by A. Mirsepah []. S. Chen [] developed a self-powered pressure sensor implemented for hierarchical elastomer, based on electrostatic nanogenerators designed for real-time blood pressure measurements. H. Fang [] proposed a Real-time Health Evaluation System (RHES) using arch piezoelectric sensors for capturing pulse signals, produced using poly(vinylidene fluoride)–polydimethylsiloxane (PVDF–PDMS). A porous PDMS was applied in a pulse-wave hair cell structured sensor by M. Seok [], which contains a 100/10 nm thick Au/Cr layer and PI. A flexible capacitive pressure sensor BaTiO3-doped PVDF electrospun fiber with PDMS microcylindrical structures elaborated as a dielectric layer was proposed by C.-R. Yang [], with a sensitivity of 5 kPa−1. For non-conductive polymers, their conductivity can be improved by incorporating specific functional groups. The most popular conductive polymers are polyacetylene, Ppy, poly(p-phenylene), polythiophene, and PANI, all of which possess delocalized electrons in their π orbitals, facilitating electrical conduction []. In Figure 6, a 3D-printed patterned PDMS capacitive sensor integrated into a stent [] is depicted, which successfully recorded data for 2 months and continued to function for an additional 3 months after implantation. The skin-conformable polymer/oxide p-type semiconductor phototransistor, used as a photoplethysmogram sensor for cardiovascular monitoring, was presented in the work by B.H. Kang [].
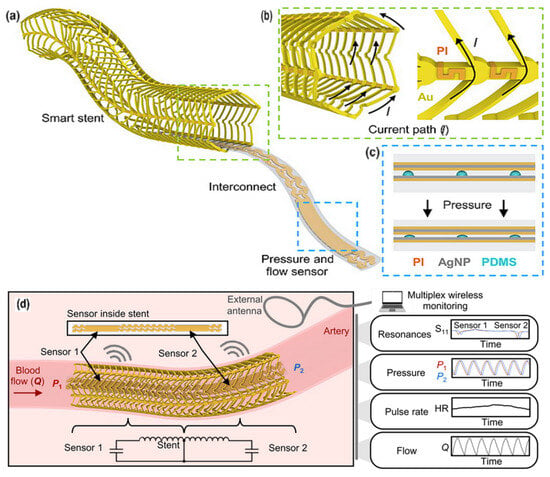
Figure 6.
Fully implantable wireless vascular electronic system. (a) Illustration of the implantable electronic components; (b) inductive stent design using conductive Au loops and nonconductive PI connectors to achieve a current path resembling a solenoid (left) and a scanning electron microscopy (SEM) image of the stent (right). (c) Layers of the soft pressure sensor using a printed dielectric layer (left) and a photo of an index finger holding a simultaneous flow and pressure sensor (right). AgNP, silver nanoparticle; PDMS. (d) Illustration of the wireless design and sensing scheme to simultaneously monitor pressure, heart rate (HR), and flow [].
PDMS is known for its high dielectric constant value, flexibility, and as a biocompatible polymer with wide application [,]. Some successful solutions were obtained by combining several polymers. Illustrations of the successful combinations of one selected polymer, PDMS, with other polymers in sensor manufacturing are presented in Table 1.

Table 1.
PDMS in healthcare sensors.
The fractional flow reserve (FFR) guidewire has a diameter of less than 200 μm due to its need to enter constricted blood vessels; the MEMS was elaborated and presented in K.-J. Moon [], as shown in Figure 7. The sensor must transmit the collected data from its location. The sensor should be electrically connected, waterproof, and coated to withstand the pressure inside the blood vessel [,].
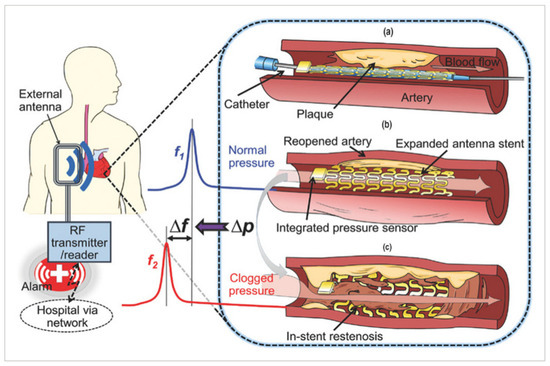
Figure 7.
Conceptual schematic of the smart stent and its functions []: (a) a pressure-microsensor-integrated wireless stent crimped on the balloon catheter is positioned at the targeted stenosis site in the artery; (b) the smart stent is deployed by balloon inflation to start self-diagnosing while scaffolding the narrowed artery after removal of the catheter; the stent’s resonant frequency is at its nominal level (f1); (c) in-stent restenosis changes local blood pressure and shifts the stent’s frequency (to f2) as a sign of the problem; the implant is continuously monitored through a handheld wireless reader (GORE-TEX Stretch Vascular Graft, W. L. Gore & Associates, Inc., Flagstaff, AZ, USA) that sends out a warning of restenosis upon occurrence.
U.-V. Romero [] introduced the nitinol and Ni-Ti alloy-based stent sensor, which has been successfully used for medical purposes, due to its pseudo-elasticity and strong corrosion resistance. Additionally, it features the ability to measure several physiological parameters, utilizing PVDF and PDMS. The nitinol-based health monitoring sensor (NHMS) contains an integrated TENG and has the ability to charge a commercial capacitor, enabling it to function as a self-powered sensor. It was developed to measure the heart rate, blood pressure, and breathing patterns. The PVDF is highly favorable due to its high residual polarization and excellent thermal stability, making it a good option for smart stent manufacturing. The PVDF presents as five different crystalline polymorphs: α, β, γ, δ, and ε phases [], where β-phase PVDF generates the best piezoelectric properties []. The most negatively charged and, therefore, the most preferred material for triboelectric device manufacturing is polymer polytetrafluoroethylene (PTFE) []. L. Wang [,] proposed a hybrid Co/Cr-Poly(ɛ-caprolactone) (PCL)–Co/Cr-based stent with an LC wireless pressure sensor, which can provide an intravascular signal. The biodegradability of a PCL and PLA polymer stent with a wireless MEMS-based LC-type sensor was presented by J.L. Wei []. PAA/Fe hydrogel demonstrates an accuracy and sensitivity of 12 nF/kPa for pressure detection []. PU is an excellent and widely used polymer with good biocompatibility and mechanical properties, making it especially useful in orthopedic, skin sensor manufacturing, and cardiovascular system monitoring devices []. PPy is a conducting polymer that, when blended with natural directing agents, can be suitable for improving sensor performance []. Conductive polymer (CP) hydrogels are desirable materials in sensor manufacturing; however, hydrophilic CPs and hydrophobic CP networks often exhibit inadequate bonding in matrices, resulting in the loss of desirable mechanical and electrical properties required for sensor applications. Z. Sun [] investigated the mechanism of supramolecular interactions and stated that the impact of external conditions for many conducting polymer hydrogels remained largely unexplored. Hydrogels are 3D polymer networks, a promising material in sensor manufacturing []. Hence, scenarios for the successful application of supramolecular conductive polymer hydrogels are a challenge for the future []. The application of nanotechnologies and nanomaterials in cardiology is a promising frontier in patient care []. With the development of novel nanomaterials and nanostructures, combined with novel tissue technologies, clinicians have gained tools for innovative methods of diagnostics, treatment, and prevention of cardiovascular diseases []. PANI nanofibers with a high thermal expansion coefficient exhibit a short-term response to temperature changes, as the charge carrier hopping rate between fibers depends on the temperature []. Hence, the special role of fibers in the charge transfer pathway is a critical aspect in sensor development []. The use of bioresorbable stents with SMPs and additive manufacturing is changing the healthcare sector []. The application of dry electrodes for long-term ECG monitoring is a promising technology [], as wet electrodes for long-term health monitoring may cause skin disorders. The application of polymer-based sensors with nanostructures or nanomaterials is presented in Table 2.

Table 2.
Polymers in healthcare sensors.
As presented in the table above, most publications dedicated to pressure and temperature sensors can be divided into two categories: one focuses on the manufacturer creating and testing the sensor for medical suitability, while the other focuses on a careful investigation of the technical parameters of the developed product. Thus, the development of the modern sensor is not solely in the hands of specialists with knowledge of nanotechnologies or polymers. To improve medical sensors more effectively, collaboration between interdisciplinary laboratories and the availability of simulation and modeling equipment is necessary. Hence, every well-designed sensor can be included in medical suitability studies; however, there is a delay, due to the lack of medical assessment. Due to the unique properties of natural-origin biopolymers, such as starch in combination with other polymers, a new era of sensor manufacturing has been opened. Starch-based gels and soft starch-based composites have been applied in resistive, capacitive, piezoelectric, and triboelectric sensors to measure temperature, humidity, fluids, and enzymes, to detect a wide range of stimuli, and to monitor human body movements and physiological signals [,]. A natural polymer reinforced with suitable compounds in a nanocomposite opens up promising multidisciplinary applications in sensor manufacturing. The gelatin and oxidized sodium O-(carboxymethyl) cellulose hydrogel demonstrates good mechanical strength []. Methylcellulose was used as a stabilizer in J. Gao’s [] investigation of stretchable sensors.
4.3. Biodegradable Polymer Used in Force Sensors for “Smart Stent”
Biodegradable materials enable customized medical devices that improve personalized medicine, which are increasingly produced from environmentally friendly polymers, plant-based, such as polysaccharides [], alginate [], cellulose [,], and starch [], and protein-based [], derived from animal tissues, such as collagen [], chitosan, fibrin, silk, and gelatin []. Prototyping biodegradable compositions for stents, E. Hasanpur [] investigated the composition of PLA/Mg and noticed that Mg particles act as nucleation sites and support the hydrolysis-induced degradation of PLA. Due to its good biocompatibility and relatively large piezoelectric coefficients, as well as its non-pyroelectric properties, PLA has applications in various sensors and energy harvesting devices. A PLA film-based stethoscope and pulse sensor was presented by J. Tian []. A zero-power consumption and implantable bias-free cardiac monitoring capsule working on the triboelectric effect was proposed by X. Qu []. It is plant-based, 3D-printed, and incorporates near-field communication technology, featuring an integrated acquisition/transmission module that meets many modern requirements for this type of equipment. Biodegradable and bioresorbable devices eliminate the need for the surgical removal of implantable devices, reducing the risk and associated healthcare costs []. The sensors, made from natural-origin polymers, can naturally decompose within the body, making them suitable for creating temporary and less intrusive medical sensors. Developed pressure sensors, biosensors, and chemical sensors based on biodegradable PLA with biodegradable magnesium metallic electrodes are presented in R. Omar’s [] article. Electrospinning, using natural proteins, such as collagen, elastin, gelatine, or synthetic polymers, such as PCL, is applied in tissue engineering []. The electrospun nanofibers proposed by B. Oh [] were used for tissue engineering. Polymers implemented in biological parameter measuring sensors are presented in Table 3.

Table 3.
Polymers in biological parameter measuring sensors.
High-density lipoprotein (HDL) natural nanoparticles are 10 nm in size []. M. Zahran [] conducted a study on the suitability of natural biopolymers, such as carbohydrate polymers for the production of sensors: alginate, cellulose, chitosan, dextran, gelatin, gum, pectin, and starch and their compatibility with nanostructures of different metal oxides: Ag, Au, Cu, Fe, Ti, Bi, Co, presenting careful systematization. A functional flexible sensor alginate–gelatin sponge was introduced for human motion monitoring by Y. Fu []. It can be used in multi-field sensing systems and human–machine interface systems. Table 4 presents examples of natural polymers used in conjunction with synthetic ones in healthcare sensors.

Table 4.
Combinations of natural and synthetic polymers suitable for healthcare sensors.
Additionally, as a proof-of-concept, the sensor was integrated into a wound dressing and functioned as a smart wound dressing. The hydrogen-bonding network based on carboxyl styrene butadiene rubber and hydrophilic sericin non-covalent carbon nanotubes was employed as a multifunctional sensor to obtain weak and large deformations with a detection limit of 1% of strain and high sensitivity to thermal change in 0.01 °C; it was proposed for an intelligent health tracking system by M. Lin []. Self-healing hydrogels for bioelectronic devices have enabled the realization of the tissue-like mechanical device era, due to their biocompatibility and good adhesiveness. Despite some of the hydrogel’s poor parameters, sensors based on hydrogels are very popular. The importance of sensors and actuators in wearable and portable electronics is growing, driven by new applications and innovative solutions for equipment. Proposing a well-working sensor is essential to avoid the influences of environmental temperature, humidity, and light on sensor operation. Problems cannot always be solved by encapsulation or be compensated for by additional temperature sensors, feedback circuits, or other improvements []. Hydrogels from natural-origin materials demonstrate good adhesion, biodegradability, and conductivity. Silk fibroin exhibits properties beneficial in postoperative surgery, particularly when it is crucial to minimize secondary damage []. Cardio tissue engineering, combined with biodegradable sensors for short-term monitoring, has great potential in regenerative medicine. Silk fibroin, in conjunction with graphene oxide (GO), plays an important role, as this composition can form flexible carbon monoliths [].
4.4. The Polymer-Based Microsensors for Cardiovascular Monitoring
Microsensors play a key role in converting mechanical signals into electrical signals. In recent decades, significant progress has been made in sensing modalities, including temperature, pressure, inertial forces, magnetic fields, radiation, and other measurements, which are realized through various sensing modalities. Micromechanical systems (MEMS) are a group of devices, whose length is less than 1 mm []. The first successful single-crystal-based MEMS was introduced in 1954 by Smith []. The typical MEMS consists of a power supply, a signal transduction and processing unit, a micro-actuating element, a sensor, and an output action element []. The CardioMEMS™ HF system micromechanical sensor (Champion, CardioMEMS, Atlanta, GA, USA) is designed to assess pulmonary artery pressure []. N.-E. Oyubbaatar [] presented a self-rollable polymer (SU-8) stent integrated with a passive inductor–capacitor resonator (LC) pressure sensor. The studies of other scientific groups were focused on the integration of sensors with stents using different techniques, such as laser welding, gluing, and bonding [,]. A stainless-steel (SS) chip of a capacitive pressure sensor (Figure 8) integrated by a micro-welding process for MEMS, proposed by X. Chen [], has been found to offer both mechanical and electrical advantages over conventional conductive epoxy bonding. Fabricated sensors exhibit an average sensitivity of 100 ppm/mmHg over a gauge pressure of 250 mmHg.
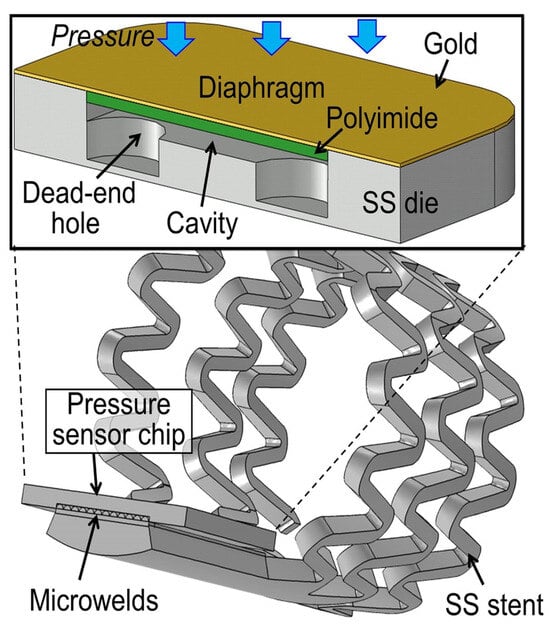
Figure 8.
The cross-sectional structure of the developed capacitive pressure sensor chip and its micro-welding integration are illustrated with an example of the smart stent [].
The capacitive transducers used as pressure sensors in MEMS have become popular, especially in stents. X. Chen [,] presented a smart stent with a micro-sensor and a wireless interface to monitor restenosis through an implanted stent, working as a radio frequency wireless pressure transducer for tracking local hemodynamic changes upon narrowing conditions and expected to be used in clinical practice.
The principle of operation of a capacitive sensor in deep soft tissues, elaborated by U.-C. Yener [], is presented in Figure 9, where a piezoceramic US antenna connected to a capacitive sensor was used.
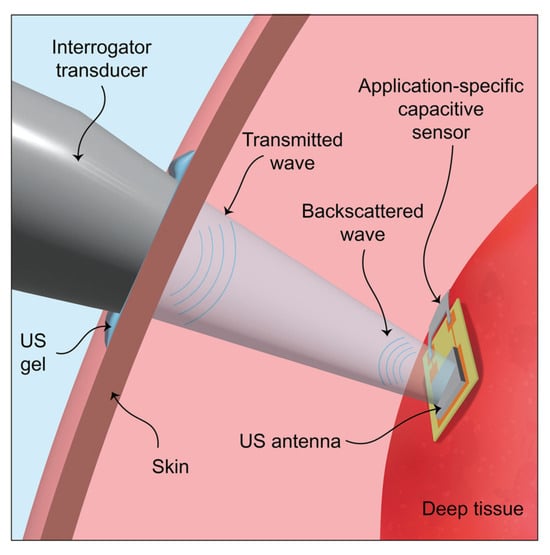
Figure 9.
Schematic of the passive ultrasonic communication method. The ultrasonic antenna is placed on deep tissue, and the wireless communication link is established between the device and the external interrogator transducer [].
An inferior vena cava (IVC) implantable sensor with ultrasound data collection principle, operating controlled by computed tomography, providing daily self-assessment by patients was presented by P.R. Karla []. The FIRE1 system-type crown-shaped implant, incorporated in a belt antenna, deployed in the IVC between the hepatic and renal veins, was presented by W.S. Sheridan []. Triboelectric sensors demonstrate a fast response time, high sensitivity, and versatility, which can help to improve the functionality of cardiovascular monitoring devices []. Triboelectric nanogenerators (TENGs) have been considered an effective method for self-powered systems for a decade.
Triboelectric sensors (Figure 10) can be successfully applied for monitoring the status of the heart and blood vessels. They serve as an alternative to large-scale monitoring equipment, enabling the implementation of personalized medicine.
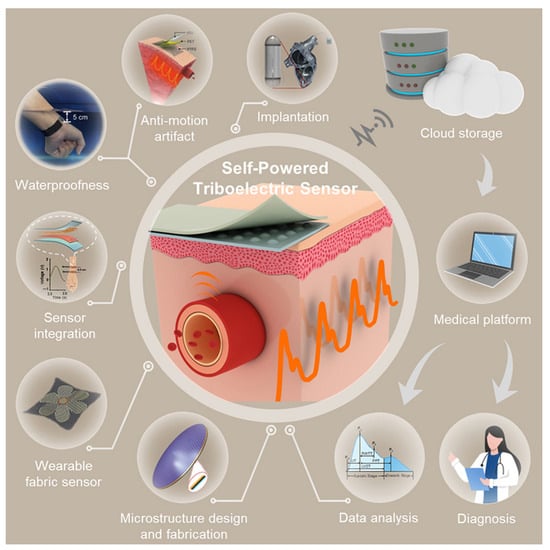
Figure 10.
Self-powered triboelectric sensors [].
The triboelectric nanogenerator (TENG) used in medical devices is unique in its ability to utilize energy that would otherwise be wasted. It can harness energy generated by the contraction of arteries to sense abnormal heart rhythms and to power itself. The self-powered endocardial pressure sensor (SEPS) based on TENG, which is integrated with a surgical catheter for minimally invasive implantation, was presented by Zh. Liu [], where nano-PTFE film with ultrathin gold (Au) layer (50 nm) was deposited on the back; then, it was treated by the inductively coupled plasma (ICP) method, and 3D ethylene-vinyl acetate (EVA) copolymer film (500 µm) was employed as the spacer layer. According to U.V. Romero’s [] investigation, the stent-TENG operating mechanism can be divided into few steps: in the stent, when there is no arterial pressure and during vasodilation, no electrons are generated; with occurring arterial constriction (vasoconstriction), electrons are coming from PDMS to PVDF, and a negative charge is created on PVDF and a positive charge on PDMS. A potential difference is generated between the electrodes, and then the electrode layers are separated. An external circuit is used to allow the electrical current to flow. The potential obtains higher values, the current returns to zero, and TENG reaches the released condition. Macro, micro, and nano wrinkles have been fabricated to provide electrodes and active tribo-layers with high tensile ability for sensors based on PEDOT: PSS with rGO, MXene for TENGs (MW-STENGs), with stable MW-carbon-based electrodes/PDMS (MW-rGO/PDMS, MW-SWCNTs/PDMS, and MW-MXene/PDMS) to form the other tribo-electrode layer []. Miniaturized SEPS can reduce damage to the implanting tissues, resulting in a minimally invasive surgical method. With such a device, life-threatening arrhythmias can also be detected in time, based on sudden changes in endocardial pressure (EP). Implantable electronic devices have some disadvantages, including a limited battery life. A surgical procedure is performed periodically to replace the battery, causing patients to suffer and increasing the healthcare costs. A self-powered single-cell implantable triboelectric active sensor (iTEAS) that can continuously monitor a wide range of physiological parameters with efficient use of internal mechanical energy was presented by Y. Ma [] for cardiac arrhythmias such as atrial fibrillation and premature ventricular contraction. The applied polymers for this sensor are Kapton PI, PDMS, and n-PTFE as the triboelectric layer, with metals such as Au, Ti, and Al. As with most implantable products, the biocompatibility of the device was tested in vivo after 2 weeks of implantation, demonstrating its suitability for practical use. Elastic fiber sensors have attracted interest due to their easy application in wearable sensors with human–machine interfaces and implantable devices. A fiber sensor proposed by Y. Zhang [] achieved GF ≈ 1950, a broad strain-sensing range (400%), and high durability over 1000 stretching and bending cycles. The endocardial pressure has significant clinical implications for patients with heart failure. In vivo biomechanical energy harvesting is a solution for obtaining sustainable electrical energy to power implanted devices. The presented triboelectric nanogenerator was made using Kapton, PTFE, and PDMS with parylene encapsulation, as described by Q. Zheng []; this device can directly indicate the physiological heartbeat behavior. The conductive micro-nano carbon ink, printed on an elastomer, is fabricated as a force sensor proposed for use in smart gloves for human–machine interfaces and rehabilitation treatment. The product responds to different gestures, for example, Morse code by finger bending []. An indium tin oxide single electrode was applied as a pulse sensor, as proposed by X. Cui [], working as a triboelectric nanogenerator, where incident waves and reflected waves are observed successfully.
5. Discussion and Conclusions
By evaluating the collected information and data from the discussed publications, the following conclusions can be stated for force sensors integrated into cardiovascular stents, as well as based on successful applications in surgery provided by the authors of the selected articles.
Sensors implanted in cardiovascular stents perform the following functions:
- Pulse registration,
- Blood pressure measurement,
- Tissue movement measurement,
- Micro-deformation detection,
- ECG and EMG data collection.
Improvements are needed for implantable cardiovascular stents with integrated force sensors, as follows:
- Implantable devices are a potential unwanted source of health status complications;
- Short-term postoperative monitoring requires temporary implantable devices that are subsequently removed from the body and pose additional risks.
The development of devices poses certain requirements, as follows:
- Biodegradable devices for short-term monitoring;
- Long-term devices with reliable long-term functioning;
- Supporting equipment for implantable health monitoring devices.
Polymers for implantable device application can be selected to improve the physical properties:
- Biocompatible polymers;
- A combination of polymers resulting in better application and device performance;
- A combination with nanostructures to improve device performance.
Hence, polymers have become an integral part of modern medical equipment. The polymer-based force sensors in cardiology are being developed in several directions:
- For personal self-monitoring systems, which can be implanted or integrated into wearable gadgets, with data collection and monitoring from the medical institution;
- systems utilizing AI and ML, which do not require exceptional accuracy, due to recommendation-type devices at the level of monitoring required by the health condition;
- Ultrasonic communication for data collection;
- Other types of sensors incorporated into cardiovascular stents or devices for healthcare personnel, dependent on health status.
The following solutions have been chosen for force sensor data collection and servicing:
- Machine learning algorithms, AI, and IoT are essential tools being analyzed to support long-term cardiovascular care through real-time monitoring and database collection for further research;
- The health status information collected from implanted devices with sensors, whose parameters can be read remotely.
The variety of solutions and new devices available promises greater flexibility in using equipment that is most suitable for a given patient. Hence, one of the currently relevant topics of sensor development is the reliability over the broadest possible operating range in various environmental conditions.
To assess which type of sensor is in higher demand or which kind of sensor needs improvement, studies should be conducted based on regional or traditional medicine requirements with specific needs for sensors:
- Implanted biodegradable sensors to monitor postoperative conditions in the short term;
- Long-term or lifelong stents with force sensors that maintain the quality of parameter recording throughout the entire period.
Three-dimensional printing technologies are increasingly being chosen for the production of force sensors integrated into stents, which require smaller production facilities and are more environmentally friendly.
Despite the wide variety of polymers suitable for contact with internal organs, most publications have used PDMS in combination with other polymers.
When discussing nanostructures used for sensors, two trends stand out. When a stent with a sensor is implanted for a long time, the aim is usually to use metallic nanostructures, and their toxicity is addressed by other technological solutions that protect against the toxic effects. When a sensor with a stent is implanted for a short time, in order to collect data on the success of invasive treatment procedures, materials that are harmless to the body are chosen, which naturally decompose and are removed from the body without additional surgical interventions.
Thus, the summary of the entire study is as follows.
With the successful application of hydrogels in sensors, the need arises for implanted biodegradable sensors to monitor postoperative conditions in the short term, which can be utilized naturally without ancillary surgery. As mentioned in some of the discussed publications, one of the main problems with all implantable devices is the potential for undesirable side effects. The quality of cardiovascular stents with integrated sensors, as well as becoming a safe “friendly” device for the human body, is still in the hands of developers. The reliability and quality of cardiovascular stents, as well as the diversity of patient needs, can be enhanced by using a combination of materials, both newly developed and established, in conjunction with nanomaterials, nanostructures, or emerging technologies. One of the most critical directions for increasing the diversity of polymer sensor compositions is the use of nanostructures, which can be achieved by incorporating additional materials and by forming nanostructures directly from the polymers themselves. Among the nanomaterials used in the production of polymer force sensors, there is a clear leader—carbon nanomaterials. Among nanostructures formed from the polymer itself, the most commonly referred to are nano wrinkles and nano threads. Mechano-nanomedicine is not addressed in this research, but it is impossible not to mention this promising field of polymer application in healthcare.
The diversity of materials, combined with the unique properties of polymers, enables a more reliable replacement of metal stents with sensors and opens up the possibility of stent resorption when its functions are no longer needed. This is especially useful when stents are used in the postoperative period, and the absence of a stent removal procedure reduces the risk to the patient’s health. Thus, polymers are an integral part of healthcare product manufacturing, the influence of which is becoming increasingly significant every year.
Further development of polymer-based sensors for heart and blood vessel monitoring can lead to the development of new designs of these sensors, which now are based on a metal-inspired form. The appearance of new polymers, like biopolymers, suitable for 3D printing leads to personalized medicine development and broader accessibility.
Author Contributions
Conceptualization, V.B. and A.D.; methodology, J.J.P.; software, N.Š.; validation, G.V., N.Š. and J.J.P.; formal analysis, G.V.; investigation, J.J.P.; resources, A.D.; data curation, J.J.P. and G.V.; writing—original draft preparation, J.J.P.; writing—review and editing, V.B. and A.D.; visualization, J.J.P.; supervision, V.B.; project administration, A.D.; funding acquisition, V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AA | Acrylic acid |
| AAO | Alumina template |
| ADSP | Acetylated di-starch phosphate |
| AI | Artificial intelligence |
| AlN | Aluminum nitride |
| Ag@OH-f MWCNTs | Nanosilver (Ag)-coated hydroxyl-functionalized multi-walled carbon nanotubes |
| AgNWs | Silver nanowires |
| BCMC | Bias-free cardiac monitoring capsule |
| BPTT | Brachial artery transit time |
| CB | Carbon black |
| CIPs | Carbonyl iron particles |
| CNDs | Carbon nanodots |
| CPI | Colorless polyimide |
| CuNPs | Copper metal nanoparticles |
| CYTOP | Fluorinated polymer passivation |
| CVD | Cardiovascular diseases |
| DES | Ionic deep eutectic solvents |
| DBRPTT | Transit time difference between the radial and the brachial artery |
| EB | Elastic band |
| ECG | Electrocardiogram |
| EMG | Electromyography |
| [Emim]Ac | 1-ethyl-3-methyl imidazolium acetate |
| FEP | Fluorinated ethylene propylene |
| FSS | Functionalized silk sericin |
| GF | Gauge factor |
| G | Graphene |
| GMA | Glycidyl methacrylate |
| GNPs | Graphene nanoplatelets |
| GO | Graphene oxide |
| GPANI/PVB | Polyaniline-graphene/polyvinyl butyral film |
| GOPS | (3-glycidyl oxy propyl) trimethoxysilane |
| HG-TENG | Hydroxyethyl cellulose (HEC) and gelatin-based TENG |
| HF | Heart failure |
| HDL | High-density lipoprotein |
| HPFS | Hydrogel-based polymer fiber strain |
| HSPS | Hierarchical self-powered pressure sensor |
| ISFET | Ion-sensitive field-effect transistor |
| ITO | Indium tin oxide |
| IVC | Inferior vena cava |
| Kapton (PI) | Kapton poliimide |
| LBG | Locust bean gum |
| MC | Methylcellulose |
| MW-STENGs | Macro-micro-wrinkled stretchable TENGs |
| MEMS | Micro-electromechanical systems |
| MWCNTs | Multi-walled carbon nanotubes |
| PTFE | Polytetrafluoroethylene |
| PPy/MWCNT/PU | Polypyrrole/multi-walled carbon nanotube/polyurethane |
| NHMS | Nitinol health monitor sensor |
| NFs | Nanofibers |
| NPs | Metal oxide nanoparticles |
| OCMC | Oxidized sodium carboxymethyl cellulose |
| PAAm | Poly(acryl amide) |
| PANI | Polyaniline |
| PCL | Poly(ɛ-caprolactone) |
| PDA | Polydopamine |
| [P(DPP2ODT2-T)] | Poly{3-([2,2′:5′,2″-terthiophen]-5-yl)-2,5-bis(2-octyldodecyl)-2,5-dihydropyrrolo [3,4-c]pyrrole-1,4-dione-6,5″-diyl} |
| PDMS | Polydimethylsiloxane |
| PEDOT: PSS | Poly(3,4-ethylenedioxythiophene): poly (styrene-sulfonate) |
| PMMA | Poly (methyl methacrylate) |
| Pebax | Poly-ether-b-amide |
| PEI | Polyethyleneimine |
| Pebax | Poly(ether-b-amide) |
| PEN | Polyethylenenaphthalene |
| PET | Polyethylene terephthalate |
| PI | Polyimide |
| PHMG | Poly(hexamethylene biguanide) hydrochloride |
| PLA | Polylactic acid |
| PP | Polypropylene |
| PU | Polyurethane |
| PVA | Poly(vinyl alcohol) |
| PVDF | Poly(vinylidene fluoride) |
| PVC | Polyvinyl chloride |
| rGO | Reduced graphene oxide |
| RPTT | Radial artery transit time |
| SA | Sodium alginate |
| SGS | Sodium alginate (SA)/gelatin (GE) sponge |
| SLG | Single layer graphene |
| SMS | Single-mode–multi-mode–single-mode |
| SCMC | Sodium carboxymethyl cellulose |
| SPB | Starch/PVA/borax |
| SPOFs | Stretchable polymer-based optical fibers |
| SBMA | 2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl) ammonium hydroxide |
| SF | Silk fibroin |
| SWCNT | Single-walled carbon nanotubes |
| SF@MXene | Silk fibroin@Ti3C2Tx MXene |
| TA@CNCs | Tannic acid-coated cellulose nanocrystal |
| TPU | Thermoplastic polyurethane |
| UCNPs | Thermal-sensitive upconverted nanoparticles |
| WVTR | Water vapor transmission rate |
References
- Sajjad, M.W.; Muzamil, F.; Sabir, M.; Ashfaq, U.A. Regenerative Medicine and Nanotechnology Approaches against Cardiovascular Diseases: Recent Advances and Future Prospective. Curr. Stem Cell Res. Ther. 2025, 20, 50–71. [Google Scholar] [CrossRef]
- Woodruff, R.C.; Tong, X.; Khan, S.S.; Shah, N.S.; Jackson, S.L.; Loustalot, F.; Vaughan, A.S. Trends in Cardiovascular Disease Mortality Rates and Excess Deaths, 2010–2022. Am. J. Prev. Med. 2024, 66, 582–589. [Google Scholar] [CrossRef]
- Zhao, B.; Gan, L.; Graubard, B.I.; Männistö, S.; Fang, F.; Weinstein, S.J.; Liao, L.M.; Sinha, R.; Chen, X.; Albanes, D.; et al. Plant and Animal Fat Intake and Overall and Cardiovascular Disease Mortality. JAMA Intern. Med. 2024, 184, 1234–1245. [Google Scholar] [CrossRef]
- Attiq, A.; Afzal, S.; Ahmad, W.; Kandeel, M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur. J. Pharmacol. 2024, 966, 176338. [Google Scholar] [CrossRef]
- Oyunbaatar, N.E.; Kim, D.S.; Shanmugasundaram, A.; Kim, S.H.; Jeong, Y.J.; Jo, J.; Kwon, K.; Choi, E.; Lee, D.W. Implantable Self-Reporting Stents for Detecting In-Stent Restenosis and Cardiac Functional Dynamics. ACS Sens. 2023, 8, 4542–4553. [Google Scholar] [CrossRef]
- Udris, A.S.; Niculescu, A.-G.; Grumezescu, A.M.; Bădilă, E.; Vitoria, B.; Gay, J.C.; Mendiguren, A.E.; Carrillo, L.J.Q. Cardiovascular Stents: A Review of Past, Current, and Emerging Devices. Materials 2021, 14, 2498. [Google Scholar] [CrossRef] [PubMed]
- Udriște, A.S.; Burdușel, A.C.; Niculescu, A.G.; Rădulescu, M.; Grumezescu, A.M. Coatings for Cardiovascular Stents—An Up-to-Date Review. Int. J. Mol. Sci. 2024, 25, 1078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liang, W.; Chen, X.-B.; Mang, J. Smart Materials Strategy for Vascular Challenges Targeting In-Stent Restenosis: A Critical Review. Regen. Biomater. 2025, 12, rbaf020. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Seo, B.; Jeong, Y.; Park, I. A Review of Recent Advancements in Sensor-Integrated Medical Tools. Adv. Sci. 2024, 11, 2307427. [Google Scholar] [CrossRef]
- Hashemi, M.; Ghasemi, I.; Omrani, A.; Rostami, A.; Durán-Valle, C.J.; Qandalee, M. Biodegradable Shape Memory Nanocomposites Based on PCL/PPC/Graphene: As a Proposal Material for Cardiovascular Stent. J. Polym. Environ. 2025, 33, 2464–2479. [Google Scholar] [CrossRef]
- Jalandhra, G.K.; Srethbhakdi, L.; Davies, J.; Nguyen, C.C.; Phan, P.T.; Och, Z.; Ashok, A.; Lim, K.S.; Phan, H.-P.; Do, T.N.; et al. Materials Advances in Devices for Heart Disease Interventions. Adv. Mater. 2025, 37, 2420114. [Google Scholar] [CrossRef]
- Im, S.H.; Im, D.H.; Park, S.J.; Jung, Y.; Kim, D.H.; Kim, S.H. Current status and future direction of metallic and polymeric materials for advanced vascular stents. Prog. Mater. Sci. 2022, 126, 100922. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Z.; Li, L. Mechanical Sensors for Cardiovascular Monitoring: From Battery-Powered to Self-Powered. Biosensors 2022, 12, 651. [Google Scholar] [CrossRef]
- Amobonye, A.; Lalung, J.; Mheta, G.; Pillai, S. Writing a Scientific Review Article: Comprehensive Insights for Beginners. Sci. World J. 2024, 2024, 7822269. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Z.; Fan, Y. Biomedical Applications of Bioresorbable Mechanical Sensors. Adv. Funct. Mater. 2025, e13422. [Google Scholar] [CrossRef]
- Herbert, R. Wireless vascular bioelectronic systems with printed soft sensors and flexible electronic stents. In Soft Mechatronics and Wearable Systems, Proceedings of the SPIE Smart Structures + Nondestructive Evaluation 2024, Long Beach, CA, USA, 25–28 March 2024; SPIE Digital Library: Washington, DC, USA, 2024; Volume 12948C, pp. 56–62. [Google Scholar] [CrossRef]
- Amreen, K.; Goel, S. Smart Polymers in Flexible Devices. In Specialty Polymers; CRC Press: Boca Raton, FL, USA, 2023; pp. 459–472. [Google Scholar] [CrossRef]
- Ban, S.; Lee, H.; Chen, J.; Kim, H.S.; Hu, Y.; Cho, S.J.; Yeo, W.H. Recent advances in implantable sensors and electronics using printable materials for advanced healthcare. Biosens. Bioelectron. 2024, 257, 116302. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, W.; Yang, D.; Ma, J.; Li, X.; Tian, G. The latest advances in two-way shape memory polymers: Fabrication strategies, programming mechanisms, and emerging applications. Chem. Eng. J. 2025, 521, 166372. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, Z.; Wu, Y.; Song, Y.; He, Y.; Liu, Z.; Fu, X.; Wang, L. The cardiac electrophysiology-inspired patches for repairing myocardial infarction: A review. Smart Mater. Med. 2025, 6, 108–119. [Google Scholar] [CrossRef]
- Sareło, P.; Sobieszczańska, B.; Wysokińska, E.; Gąsior-Głogowska, M.; Kałas, W.; Podbielska, H.; Wawrzyńska, M.; Kopaczyńska, M. In vitro examinations of the anti-inflammatory interleukin functionalized polydopamine based biomaterial as a potential coating for cardiovascular stents. Biocybern. Biomed. Eng. 2023, 43, 369–385. [Google Scholar] [CrossRef]
- Hertault, A.; Chai, F.; Maton, M.; Sobocinski, J.; Woisel, P.; Maurel, B.; Lyskawa, J.; Blanchemain, N. In vivo evaluation of a pro-healing polydopamine coated stent through an in-stent restenosis rat model. Biomater. Sci. 2021, 9, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, B.; Yin, G.; Liu, L.; Yang, P.; Huang, N.; Zhao, A. Design and Fabrication of Environmentally Responsive Nanoparticles for the Diagnosis and Treatment of Atherosclerosis. ACS Biomater. Sci. Eng. 2024, 10, 1190–1206. [Google Scholar] [CrossRef]
- Arora, R.; Mohanta, M.; Jaiswal, S.; Thirugnanam, A.; Mohanta, P. An overview of polymer-based-bioresorbable drug-eluting stents. Innov. Emerg. Technol. 2024, 11, 2430002. [Google Scholar] [CrossRef]
- Yeazel, T.R.; Becker, M.L. Advancing Toward 3D Printing of Bioresorbable Shape Memory Polymer Stents. Biomacromolecules 2020, 21, 3957–3965. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Vafadar, A.; Tolouei-Rad, M. Application of Additive Manufacturing in the Development of Polymeric Bioresorbable Cardiovascular Stents: A Review. Adv. Mater. Technol. 2025, 10, 2400210. [Google Scholar] [CrossRef]
- Joseph, T.M.; Thomas, M.G.; Mahapatra, D.K.; Unni, A.B.; Kianfar, E.; Haponiuk, J.T.; Thomas, S. Adaptive and intelligent polyurethane shape-memory polymers enabling next-generation biomedical platforms. Case Stud. Chem. Environ. Eng. 2025, 11, 101165. [Google Scholar] [CrossRef]
- Amin, M.; Farsangi, A.; Reza, M.; Ahari, M.F.; Mirsayar, M. Magnetic shape memory polymers: A state-of-the-art review. Smart Mater. Struct. 2025, 34, 053001. [Google Scholar] [CrossRef]
- Kim, Y.B.; Song, H.; Kim, S.; Chun, H.J. 4D printing of magneto-responsive shape memory nano-composite for stents. Smart Mater. Struct. 2024, 33, 105004. [Google Scholar] [CrossRef]
- Yeazel, T.R.; Davis, A.G.; Becker, L.M. Thiol-ene-Based 3D Printing of Bi oresorbable Fumarate-Based ABA Triblock Copolyester Elastomers. Adv. Mater. Technol. 2023, 8. [Google Scholar] [CrossRef]
- Kumar, V.; Babbar, A.; Sharma, A.; Kumar, R.; Tyagi, A. Polymer 3D Bioprinting for Bionics and Tissue Engineering Applications. In Additive Manufacturing of Polymers for Tissue Engineering; CRC Press: Boca Raton, FL, USA, 2022; pp. 17–39. [Google Scholar] [CrossRef]
- Babbar, A.; Kumar, R.; Dhavan, V.; Ranjan, N.; Sharma, A. Additive Manufacturing of Polymers for Tissue Engineering—Fundamentals, Application and Future Advancements. CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Parisi, O.I.; Curcio, M.; Puoci, F. Polymer Chemistry and Synthetic Polymers. In Advanced Polymers in Medicine; Springer: Cham, Switzerland, 2015; pp. 1–31. [Google Scholar] [CrossRef]
- Mansuri, S.S.; Patil, H.S.; Nunse, D.K.; Jumde, S.M.; Gupta, P.R.; Talele, S.G.; Borse, L.B. Polymer and Plastic Fundamentals and Its Connecting with Current Environmental, Biomedical, and Pharmaceutical Engineering. In Sustainability in Polymer Technology and Plastic Engineering; Apple Academic Press: Burlington, ON, Canada, 2025; pp. 117–255. [Google Scholar] [CrossRef]
- Sajjad, R.; Chauhdary, S.T.; Anwar, M.T.; Zahid, A.; Khosa, A.A.; Imran, M.; Sajjad, M.H. A review of 4D printing—Technologies, shape shifting, smart polymer based materials, and biomedical applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 20–36. [Google Scholar] [CrossRef]
- Hasirci, V.; Hasirci, N.; Biomaterials, P.A. Fundamentals of Biomaterials; Springer: Cham, Switzerland, 2024; pp. 83–101. [Google Scholar] [CrossRef]
- Wang, L.; Oyunbaatar, N.E.; Jeong, Y.J.; Lee, H.; Won, Y.; Jeong, I.S.; Kayumov, M.; Obiweluozor, F.O.; Kim, D.S.; Lee, D.W. The PolyCraft Polymer–Metal Hybrid Smart Stent System: The Future of Cardiovascular Blood Pressure Management. Adv. Funct. Mater. 2024, 34, 2408022. [Google Scholar] [CrossRef]
- Kirimi, M.T.; Hoare, D.; Holsgrove, M.; Czyzewski, J.; Mirzai, N.; Mercer, J.R.; Neale, S.L.; Kirimi, M.T.; Neale, S.L.; Hoare, D.; et al. Detection of Blood Clots Using a Whole Stent as an Active Implantable Biosensor. Adv. Sci. 2024, 11, 2304748. [Google Scholar] [CrossRef] [PubMed]
- Cricco, R.; Segreti, A.; Ferro, A.; Beato, S.; Castaldo, G.; Ciancio, M.; Sacco, F.M.; Pennazza, G.; Ussia, G.P.; Grigioni, F. Advancements in Sensor Technology for Monitoring and Management of Chronic Coronary Syndrome. Sensors 2025, 25, 4585. [Google Scholar] [CrossRef]
- Green, R.A.; Lovell, N.H.; Wallace, G.G.; Poole-Warren, L.A. Conducting polymers for neural interfaces: Challenges in developing an effective long-term implant. Biomaterials 2008, 29, 3393–3399. [Google Scholar] [CrossRef]
- Liu, Y.; Feig, V.R.; Bao, Z. Conjugated Polymer for Implantable Electronics toward Clinical Application. Adv. Healthc. Mater. 2021, 10, 2001916. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, B.; Du, P.; Travas-Sejdic, J. Electrochemical and Electrical Biosensors for Wearable and Implantable Electronics Based on Conducting Polymers and Carbon-Based Materials. Chem. Rev. 2024, 124, 722–767. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Li, Y.; Wang, P.; Hu, D. Recent development of flexible force sensors with multiple environmental adaptations. Nano Energy 2024, 124, 109443. [Google Scholar] [CrossRef]
- Gao, W.; Yu, C. Wearable and Implantable Devices for Healthcare. Adv. Healthc. Mater. 2021, 10, 2101548. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Cui, J.; Yu, F.; Liu, M.; Chen, Y.; Wu, J.; Sun, B.; Mo, X. Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules 2022, 12, 1245. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; He, Q.; Liu, Y.; Qiu, M.; Wu, J.; Hu, B. Advances in the development of biodegradable coronary stents: A translational perspective. Mater. Today Bio 2022, 16, 100368. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.K.; Park, S.A.; Lee, D.W. Biodegradable polymer material based smart stent: Wireless pressure sensor and 3D printed stent. Microelectron. Eng. 2019, 206, 1–5. [Google Scholar] [CrossRef]
- Tetali, S.S.V.; Fricker, A.T.R.; van Domburg, Y.A.; Roy, I. Intelligent biomaterials for cardiovascular applications. Curr. Opin. Biomed. Eng. 2023, 28, 100474. [Google Scholar] [CrossRef]
- Udriște, A.S.; Burdușel, A.C.; Niculescu, A.G.; Rădulescu, M.; Grumezescu, A.M. Metal-based nanoparticles for cardiovascular diseases. Int. J. Mol. Sci. 2024, 25, 1001. [Google Scholar] [CrossRef]
- Mondal, S. Additive Manufacturing of Polymers for Biomedical Applications. In Additive Manufacturing Processes in Biomedical Engineering; CRC Press: Boca Raton, FL, USA, 2022; pp. 99–115. [Google Scholar] [CrossRef]
- Shakibania, S.; Ghazanfari, L.; Raeeszadeh-Sarmazdeh, M.; Khakbiz, M. Medical application of biomimetic 4D printing. Drug Dev. Ind. Pharm. 2021, 47, 521–534. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef]
- Ershad-Langroudi, A.; Babazadeh, N.; Alizadegan, F.; Mousaei, S.M.; Moradi, G. Polymers for implantable devices. J. Ind. Eng. Chem. 2024, 137, 61–86. [Google Scholar] [CrossRef]
- Liu, J.; Garcia, J.; Leahy, L.M.; Song, R.; Mullarkey, D.; Fei, B.; Dervan, A.; Shvets, I.V.; Stamenov, P.; Wang, W.; et al. 3D Printing of Multifunctional Conductive Polymer Composite Hydrogels. Adv. Funct. Mater. 2023, 33, 2214196. [Google Scholar] [CrossRef]
- Baker, C.; Wagner, K.; Wagner, P.; Officer, D.L.; Mawad, D. Biofunctional conducting polymers: Synthetic advances; challenges, and perspectives towards their use in implantable bioelectronic devices. Adv. Phys. X 2021, 6, 1899850. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, C.; Sun, B.; Zhang, Y.; Sun, X.; El-Newehy, M.; Mo, X. 3D printed personalized, heparinized and biodegradable coronary artery stents for rabbit abdominal aorta implantation. Chem. Eng. J. 2022, 450, 138202. [Google Scholar] [CrossRef]
- Sun, J.; Sun, K.; Bai, K.; Chen, S.; Wang, F.; Zhao, F.; Hu, H. A novel braided biodegradable stent for use in congenital heart disease: Short-term results in porcine iliac artery. J. Biomed. Mater. Res. Part A 2022, 107, 1667–1677. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.K.; Kim, D.S.; Shanmugasundaram, A.; Park, S.A.; Kang, S.; Kim, S.H.; Jeong, M.H.; Lee, D.W. Wireless pressure sensor integrated with a 3D printed polymer stent for smart health monitoring. Sens. Actuators B Chem. 2019, 280, 201–209. [Google Scholar] [CrossRef]
- Suhad, D. Biomaterials for Cardiovascular Applications. In Handbook of Biomaterials for Medical Applications, Volume 2: Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 105–139. [Google Scholar] [CrossRef]
- Drexler, P.; Steinbauer, M.; Alsaleem, F.; Ciotola, F.; Pyxaras, S.; Rittger, H.; Buia, V. MEMS Technology in Cardiology: Advancements and Applications in Heart Failure Management Focusing on the CardioMEMS Device. Sensors 2024, 24, 2922. [Google Scholar] [CrossRef]
- Herbert, R.; Lim, H.R.; Rigo, B.; Yeo, W.H. Fully implantable wireless batteryless vascular electronics with printed soft sensors for multiplex sensing of hemodynamics. Sci. Adv. 2022, 8, eabm1175. [Google Scholar] [CrossRef] [PubMed]
- Bajeu, I.T.; Niculescu, A.G.; Scafa-Udriște, A.; Andronescu, E. Intrastent Restenosis: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 1715. [Google Scholar] [CrossRef]
- Herbert, R. Abstract 4144093: Stent-Based Sensor System for Wirelessly Monitoring Arterial Stiffness and Restenosis. Circulation 2024, 150, A4144093. [Google Scholar] [CrossRef]
- Yener, U.C.; Toymus, A.T.; Esat, K.; Alem, M.; Beker, L. Passive ultrasonic communication link for deep-tissue sensor implants. Device 2025, 3, 100755. [Google Scholar] [CrossRef]
- Kalra, P.R.; Gogorishvili, I.; Khabeishvili, G.; Málek, F.; Toman, O.; Critoph, C.; Flett, A.S.; Cowburn, P.J.; Mehra, M.R.; Sheridan, W.S.; et al. First-in-Human Implantable Inferior Vena Cava Sensor for Remote Care in Heart Failure: FUTURE-HF. JACC Heart Fail. 2025, 13, 1000–1010. [Google Scholar] [CrossRef]
- Tang, L.; Yang, J.; Wang, Y.; Deng, R. Recent Advances in Cardiovascular Disease Biosensors and Monitoring Technologies. ACS Sens. 2023, 8, 956–973. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Y.; Song, C.; Sheng, S.; Yang, L.; Yan, Z.; Hu, D.J.J.; Sun, Q. Wearable Alignment-Free Microfiber-Based Sensor Chip for Precise Vital Signs Monitoring and Cardiovascular Assessment. Adv. Fiber Mater. 2022, 4, 475–486. [Google Scholar] [CrossRef]
- Chun, K.-Y.; Seo, S.; Han, C.-S.; Chun, K.-Y.; Han, C.-S.; Seo, S. A Wearable All-Gel Multimodal Cutaneous Sensor Enabling Simultaneous Single-Site Monitoring of Cardiac-Related Biophysical Signals. Adv. Mater. 2022, 34, 2110082. [Google Scholar] [CrossRef]
- Kwon, S.H.; Dong, L. Flexible sensors and machine learning for heart monitoring. Nano Energy 2022, 102, 107632. [Google Scholar] [CrossRef]
- Mao, P.; Li, H.; Yu, Z. A Review of Skin-Wearable Sensors for Non-Invasive Health Monitoring Applications. Sensors 2023, 23, 3673. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.C.; Kim, M.; Kim, S.; Seo, H.; An, S.; Jang, E.H.; Han, S.Y.; Kim, M.J.; Kim, N.K.; Cho, S.W.; et al. In situ diagnosis and simultaneous treatment of cardiac diseases using a single-device platform. Sci. Adv. 2022, 8, 897. [Google Scholar] [CrossRef] [PubMed]
- Presti, D.L.; Bianchi, D.; Massaroni, C.; Gizzi, A.; Schena, E. A Soft and Skin-Interfaced Smart Patch Based on Fiber Optics for Cardiorespiratory Monitoring. Biosensors 2022, 12, 363. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Wang, J.; Li, K.; Liu, Y.; Ji, X.; Dong, Y.; Zhang, D. A multi-indicator pulse monitoring system based on an ultra-sensitive and stable self-powered wearable triboelectric sensor with assistance of personalized deep learning. Nano Energy 2025, 140, 111039. [Google Scholar] [CrossRef]
- Kaur, A.; Jadaun, S.; Sharma, M.; Gupta, A.; Sapra, G. Single electrode Triboelectric Nanogenerator integrated pacemaker lead for cardiac energy harvesting. Sens. Actuators A Phys. 2025, 390, 116606. [Google Scholar] [CrossRef]
- Park, Y.J.; Kwak, M.S.; Kim, Y.; Na, S.; Chang, Y.; Kim, Y.R.; Cho, H.; Lee, S.; Kim, J.J.; Ko, H. Biodegradable, stretchable, and high-performance triboelectric nanogenerators through interfacial polarization in bilayer structure. Nano Energy 2024, 132, 110411. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Kim, J.; Tong, Y.; Thompson, E.G.; Jiang, S.; Feng, Z.; Yu, L.; Wang, J.; Ha, D.S.; et al. Thermally Drawn Stretchable Electrical and Optical Fiber Sensors for Multimodal Extreme Deformation Sensing. Adv. Opt. Mater. 2021, 9, 2001815. [Google Scholar] [CrossRef]
- Paramasivam, G.; Palem, V.V.; Meenakshy, S.; Suresh, L.K.; Gangopadhyay, M.; Antherjanam, S.; Sundramoorthy, A.K. Advances on carbon nanomaterials and their applications in medical diagnosis and drug delivery. Colloids Surf. B Biointerfaces 2024, 241, 114032. [Google Scholar] [CrossRef]
- Cheng, M.; Zhu, G.; Zhang, F.; Tang, W.L.; Jianping, S.; Yang, J.Q.; Zhu, L.Y. A review of flexible force sensors for human health monitoring. J. Adv. Res. 2020, 26, 53–68. [Google Scholar] [CrossRef]
- Petronienė, J.J.; Dzedzickis, A.; Morkvėnaitė-Vilkončienė, I.; Bučinskas, V. Flexible strain sensors: Recent progress 2016–2023. Sens. Actuators A Phys. 2024, 366, 114950. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Xia, Z.; Cai, K. A review of electronic skin: Soft electronics and sensors for human health. J. Mater. Chem. B 2020, 8, 852–862. [Google Scholar] [CrossRef]
- Sun, X.; Yao, F.; Li, J. Nanocomposite hydrogel-based strain and pressure sensors: A review. J. Mater. Chem. A Mater. 2020, 8, 18605–18623. [Google Scholar] [CrossRef]
- Alam, F.; Ahmed, M.A.; Jalal, A.H.; Siddiquee, I.; Adury, R.Z.; Hossain, G.M.M.; Pala, N. Recent Progress and Challenges of Implantable Biodegradable Biosensors. Micromachines 2024, 15, 475. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.; Saliba, W.; Khatib, M.; Zheng, Y.; Pieters, C.; Oved, H.; Silberman, E.; Zohar, O.; Hu, Z.; Kloper, V.; et al. Biodegradable, Biocompatible, and Implantable Multifunctional Sensing Platform for Cardiac Monitoring. ACS Sens. 2024, 9, 126–138. [Google Scholar] [CrossRef]
- Huang, C.; Xiao, M.; Li, Z.; Fu, Z.; Shi, R. Bioinspired breathable biodegradable bioelastomer-based flexible wearable electronics for high-sensitivity human-interactive sensing. Chem. Eng. J. 2024, 486, 150013. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, J.Y.; Kim, Y.N.; Chae, M.; Lee, W.J.; Lee, J.; Kim, I.D.; Hyun, J.K.; Lee, K.S.; Kang, D.; et al. A Fully Biodegradable and Ultra-Sensitive Crack-Based Strain Sensor for Biomechanical Signal Monitoring. Adv. Funct. Mater. 2024, 34, 2406035. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, K.S.; Dip, T.M.; Chowdhury, M.F.M.; Debnath, S.R.; Hasan, S.M.M.; Sakib, M.S.; Saha, T.; Padhye, R.; Houshyar, S. A review on nanomaterial-based additive manufacturing: Dynamics in properties, prospects, and challenges. Prog. Addit. Manuf. 2024, 9, 1197–1224. [Google Scholar] [CrossRef]
- Salari, S.; Sadeghi-Yarandi, M.; Golbabaei, F. An integrated approach to occupational health risk assessment of manufacturing nanomaterials using Pythagorean Fuzzy AHP and Fuzzy Inference System. Sci. Rep. 2024, 14, 180. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Ma, X.; Su, Z.; Yin, J.; Jiang, X. Light-Induced Programmable 2D Ordered Patterns Based on a Hyperbranched Poly(ether amine) (hPEA)-Functionalized Graphene Film. ACS Appl. Mater. Interfaces 2021, 13, 1704–1713. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Y.; Li, Z.; Zhao, H.; Yu, Q.; Huang, Y.; Yang, J.; Huang, L.; Interfaces, S.W. Morphing, and Coding of Polymer Surfaces by Dynamic Anisotropic Wrinkling. Langmuir 2024, 40, 18837–18856. [Google Scholar] [CrossRef]
- Lin, J.; Fu, R.; Zhong, X.; Yu, P.; Tan, G.; Li, W.; Zhang, H.; Li, Y.; Zhou, L.; Ning, C. Wearable sensors and devices for real-time cardiovascular disease monitoring. Cell Rep. Phys. Sci. 2021, 2, 100541. [Google Scholar] [CrossRef]
- Kaminski, C.; Beardslee, L.A.; Rajani, R. Sensorized Endovascular Technologies: Additional Data to Enhance Decision-Making. Ann. Vasc. Surg. 2024, 99, 105–116. [Google Scholar] [CrossRef]
- Khan, M.S.Z.; Khan, S.U.; Alrumaihi, F.; Alwanian, W.M.; Alharbi, H.O.; Alfifi, S.M.; Makki, L.K.; Sahli, M.; AL-Nafjan, A.A.; Jackson, M. Future of magnetic sensors applications in early prediction of cardiac health status. Curr. Probl. Cardiol. 2025, 50, 103022. [Google Scholar] [CrossRef]
- Sunwoo, S.-H.; Han, S.I.; Park, C.S.; Kim, J.H.; Georgiou, J.S.; Lee, S.-P.; Kim, D.-H.; Hyeon, T. Soft bioelectronics for the management of cardiovascular diseases. Nat. Rev. Bioeng. 2023, 2, 8–24. [Google Scholar] [CrossRef]
- Baburaj, A.; Banerjee, S.; Aliyana, A.K.; Shee, C.; Banakar, M.; Bairagi, S.; Kumar, S.K.N.; Ali, S.W.; Stylios, G.K. Biodegradable based TENGs for self-sustaining implantable medical devices. Nano Energy 2024, 127, 109785. [Google Scholar] [CrossRef]
- Mallegni, N.; Cicogna, F.; Passaglia, E.; Gigante, V.; Coltelli, M.B.; Coiai, S. Natural Antioxidants: Advancing Stability and Performance in Sustainable Biobased and Biodegradable Plastics. Compounds 2025, 5, 4. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Wu, X.; Yin, M.; Yin, L.; Qu, S.; Zhang, F.; Li, K.; Huang, Y.A. Flexible Strain Sensors with Ultra-High Sensitivity and Wide Range Enabled by Crack-Modulated Electrical Pathways. Nano-Micro Lett. 2025, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Xiao, Y.; Huang, S.; Hu, X.; Chen, W.; Chen, X.; Chen, H.; Zhao, N. A temperature-insensitivity multi-channel strain sensor based on chirped fiber grating loop ring-down structure. Opt. Laser Technol. 2025, 181, 111729. [Google Scholar] [CrossRef]
- Xie, T.; Chen, H.; Xu, Z.; Huang, W.; Fu, H. High Precision Strain Sensing System Based on Optoelectronic Oscillator for Human Pulse Monitoring. IEEE Sens. J. 2025, 25, 6355–6362. [Google Scholar] [CrossRef]
- Abbas, Z.; Hassan, G.; Khan, M.U.; Abbas, H.; Ahmad, B.; Shuja, A.; Sajid, M.; Bae, J.; Choi, C. Polyurethane packed graphene-coated spider silk by dip-casting for a highly stretchable strain sensor. J. Mater. Chem. B 2025, 13, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, Y.; Park, C.; Ro, Y.G.; Kwak, M.S.; Jeong, G.; Park, J.; Lee, H.; Kim, P.K.; Chung, S.I.; et al. Shape-Reconfigurable Crack-Based Strain Sensor with Ultrahigh and Tunable Sensitivity. Adv. Funct. Mater. 2025, 35, 2421812. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Chen, Z.; Shen, T.; Wang, Y.; Yin, R.; Liu, H.; Liu, C.; Shen, C. Ultrasensitive electrospinning fibrous strain sensor with synergistic conductive network for human motion monitoring and human-computer interaction. J. Mater. Sci. Technol. 2025, 213, 213–222. [Google Scholar] [CrossRef]
- Gillum, R.F.; Makuc, D.M.; Feldman, J.J. Pulse rate, coronary heart disease, and death: The NHANES I Epidemiologic Follow-up Study. Am. Heart J. 1991, 121, 172–177. [Google Scholar] [CrossRef]
- Javaid, S.; Fahim, H.; Zeadally, S.; He, B.; Sensors, S.-P. Challenges, Solutions. IEEE Sens. J. 2023, 23, 20483–20509. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, M.; Jiang, Y.; Yuan, Z.; Tai, H. Emerging electrochemical humidity sensors for zero power consumption and self-powered humidity detection: A perspective. J. Mater. Chem. A Mater. 2024, 12, 14975–14985. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, X.; Wan, J.; Xu, C.; Han, M. Recent Progress in Wearable Self-Powered Biomechanical Sensors: Mechanisms and Applications. Adv. Mater. Technol. 2024, 9, 2301895. [Google Scholar] [CrossRef]
- Tian, G.; Deng, W.; Yang, T.; Zhang, J.; Xu, T.; Xiong, D.; Lan, B.; Wang, S.; Sun, Y.; Ao, Y.; et al. Hierarchical Piezoelectric Composites for Noninvasive Continuous Cardiovascular Monitoring. Adv. Mater. 2024, 36, 2313612. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, X.; Xie, H.; Luo, Y.; Li, Z.; Peng, X.; Tao, G.; Wang, Z.L.; Dong, K. Revolutionizing wearable sustainable energy enabled by mechano-electric conversion fibers. Energy Environ. Sci. 2025, 18, 3955–3985. [Google Scholar] [CrossRef]
- Dong, F.; Zhu, M.; Wang, Y.; Chen, Z.; Dai, Y.; Xi, Z.; Du, T.; Xu, M. AI-enabled rolling triboelectric nanogenerator for bearing wear diagnosis aiming at digital twin application. Nano Energy 2025, 134, 110550. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Kumar, R.R.; Kumar, K.U.; Borrás, A.; Mishra, Y.K.; Kim, H.J. Nanostructures for energy harvesting. In Advances in Nanostructures: Processing and Methodology to Grow Nanostructures; Elsevier: Amsterdam, The Netherlands, 2025; pp. 251–323. [Google Scholar] [CrossRef]
- Mirzajani, H.; Zolfaghari, P.; Nakhjavani, S.A.; Koca, Y.; Khodapanahandeh, M.; Urey, H.; Mirzajani, H.; Zolfaghari, P.; Nakhjavani, S.A.; Koca, B.Y.; et al. Transient Implantable Electronics for Postsurgery Preventive Medicine. Adv. Funct. Mater. 2025, 35, 2413324. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Zhou, Q.; Yu, Z.; Liu, X.; Xu, R.; Sung, H.-K.; Chernogor, L.; Sun, T.; Yao, Z.; et al. Biocompatible, biodegradable, and high-performance flexible pressure sensors for severity grading and rehabilitation assessment in Parkinson’s disease management. Nano Energy 2025, 140, 111030. [Google Scholar] [CrossRef]
- Rich, A.M.; Rubin, W.; Rickli, S.; Akhmetshina, T.; Cossu, J.; Berger, L.; Magno, M.; Nuss, K.M.; Schaller, B.; Löffler, J.F. Development of an implantable sensor system for in vivo strain, temperature, and pH monitoring: Comparative evaluation of titanium and resorbable magnesium plates. Bioact. Mater. 2025, 43, 603–618. [Google Scholar] [CrossRef]
- Yang, G.; Lin, R.; Li, H.; Chen, Y.; Liu, M.; Luo, Z.; Wang, K.; Tu, J.; Xu, Y.; Fan, Z.; et al. Implantable wireless suture sensor for in situ tendon and ligament strain monitoring. Sci. Adv. 2025, 11, 3811. [Google Scholar] [CrossRef]
- Zhang, J.; Li, R.; Dong, L.; Ke, Y.; Liu, C.; Pei, M.; Hu, K.; Ruan, J.; Li, J.; Yang, F. Ultrasensitive biodegradable piezoelectric sensors with localized stress concentration strategy for real-time physiological monitoring. Chem. Eng. J. 2025, 507, 160521. [Google Scholar] [CrossRef]
- Miao, F.; Wu, D.; Liu, Z.; Zhang, R.; Tang, M.; Li, Y. Wearable sensing, big data technology for cardiovascular healthcare: Current status and future prospective. Chin. Med. J. 2023, 136, 1015–1025. [Google Scholar] [CrossRef]
- Chauhan, G.K.; Vavken, P.; Jacob, C. Mobile Apps and Wearable Devices for Cardiovascular Health: Narrative Review. JMIR Mhealth Uhealth 2025, 13, e65782. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, P.; Cuocolo, R.; Donnarumma, F.; Esposito, A.; Moccaldi, N.; Natalizio, A.; Prevete, R. Conceptual design of a machine learning-based wearable soft sensor for non-invasive cardiovascular risk assessment. Measurement 2021, 169, 108551. [Google Scholar] [CrossRef]
- Williams, G.J.; Al-Baraikan, A.; Rademakers, F.E.; Ciravegna, F.; van de Vosse, F.N.; Lawrie, A.; Rothman, A.; Ashley, E.A.; Wilkins, M.R.; Lawford, P.V.; et al. Wearable technology and the cardiovascular system: The future of patient assessment. Lancet Digit. Health 2023, 5, e467–e476. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, C.; Nocchiero, G.; Ville, J. The future of 3D printing in instrumented implantable polymer meta-stents. Ann. 3D Print. Med. 2025, 19, 100211. [Google Scholar] [CrossRef]
- Camlibel, N.O.; Kandola, B.K. Highly sensitive textile pressure sensors with novel hierarchical architecture based on conductive polymers, silver nanoparticles and carbon nanotubes. Sens. Actuators A Phys. 2025, 382, 116166. [Google Scholar] [CrossRef]
- Gospodinova, N.; Terlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Prog. Polym. Sci. 1998, 23, 1443–1484. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lee, Y.H.; Park, H.; Jin, S.W.; Jeong, Y.R.; Yun, J.; You, I.; Zi, G.; Ha, J.S. Stretchable Active Matrix Temperature Sensor Array of Polyaniline Nanofibers for Electronic Skin. Adv. Mater. 2016, 28, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Malode, S.J.; Alodhayb, A.N.; Mondal, K.; Shetti, N.P. Conducting polymer-based electrochemical sensors: Progress, challenges, future perspectives. Talanta Open 2025, 11, 100395. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, X.; Liang, Z.X.; Zhang, J.; Li, G.X.; He, X.Z.; Tan, Z.W.; Tang, Y.L.; Lv, J.C.; Zhang, S.H. Wrinkle-like polyaniline nanowires thin-film-based flexible pressure sensors for dynamic and static activity tracking. IEEE Sens. J. 2024, 25, 3484–3489. [Google Scholar] [CrossRef]
- Guo, H.; Chu, Z.; Fu, L.; Lv, Y.; Liu, X.; Fan, X.; Zhang, W. Thickness-induced gradient micro-wrinkle PDMS/MXene/rGO wearable strain sensor with high sensitivity and stretchability for human motion detection. Chem. Eng. J. 2024, 495, 153684. [Google Scholar] [CrossRef]
- Jeon, S.H.; Min, H.; Son, J.; Ahn, T.K.; Pang, C. Highly Sensitive Stretchable Electronic Skin with Isotropic Wrinkled Conductive Network. J. Sens. Sci. Technol. 2024, 33, 7–11. [Google Scholar] [CrossRef]
- Haghayegh, M.; Bagherzadeh, R.; Cao, R.; Zabihi, F.; Miao, Y.-E.; Yang, S.; Zhu, M. Macro- and micro-wrinkled conductors and multi-scale wrinkled nanofibers for omnidirectional stretchable wearable triboelectric nanogenerators. Sens. Actuators B Chem. 2025, 439, 137813. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Zhou, H.; Li, Z.; Wang, R.; Jin, B.; Liu, H.; Fan, Q.; Fang, Y.; Liu, N.; et al. Wrinkled and Fibrous Conductive Bandages with Tunable Mechanoelectrical Response Toward Wearable Strain Sensors. Adv. Fiber Mater. 2024, 6, 1174–1187. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Jafarizadeh, B.; Baboukani, A.R.; Pala, N.; Wang, C. Monitoring and analysis of cardiovascular pulse waveforms using flexible capacitive and piezoresistive pressure sensors and machine learning perspective. Biosens. Bioelectron. 2023, 237, 115449. [Google Scholar] [CrossRef]
- Lv, K.; Tian, G.; Yan, Y.; Zhou, H.; Fan, Q.; Liang, L.; Liu, N.; Wang, D.; Song, Z.; Xu, F.; et al. Stretchable carbon nanotube/Ecoflex conductive elastomer films toward multifunctional wearable electronics. Chem. Eng. J. 2024, 500, 157534. [Google Scholar] [CrossRef]
- Sagar, P.; Sinha, N.; Shukla, M.; Yadav, T.; Kumar, B. Flexible piezoelectric nanogenerator based on Nd-ZnS nanoplates for human body movements detection and wearable electronics. J. Alloys Compd. 2025, 1010, 178035. [Google Scholar] [CrossRef]
- Zheng, M.; Li, A.; He, X.; Wang, L.; Qin, X. Liquid metals and electrospun nanofibers: A magical marriage for wearable electronics. Nano Energy 2024, 129, 110078. [Google Scholar] [CrossRef]
- Jayathilaka, W.A.D.M.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano-García, W.; Baskar, C.; Wang, H.; He, J.; Cui, S.; Thomas, S.W.; et al. Significance of Nanomaterials in Wearables: A Review on Wearable Actuators and Sensors. Adv. Mater. 2019, 31, 1805921. [Google Scholar] [CrossRef]
- Aseri, V.; Kumari, P.; Nagar, V.; Godara, V.; Verma, R.K.; Awasthi, G.; Awasthi, K.K.; Sankhla, M.S.; Aseri, V.; Kumari, P.; et al. Synthetization and Functionalization of Natural, Polymer, and Quantum-Based Carbon Nanodots and Their Application in Biomedicine. Macromol. Symp. 2024, 413, 2300052. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, L.; Yang, G.; Zhuang, X.; Cheng, B. 3D fibrous aerogels from 1D polymer nanofibers for energy and environmental applications. J. Mater. Chem. A Mater. 2023, 11, 512–547. [Google Scholar] [CrossRef]
- Chauhan, N.; Chawla, S.; Pundir, C.S.; Jain, U. An electrochemical sensor for detection of neurotransmitter-acetylcholine using metal nanoparticles, 2D material and conducting polymer modified electrode. Biosens. Bioelectron. 2017, 89, 377–383. [Google Scholar] [CrossRef]
- Rajan, S.P.; Paduvilan, J.K.; Velayudhan, P.; Sidharthan, S.K.; Simon, S.M.; Thomas, S. Progress in 2D/3D nanomaterials incorporated polymer thin films for refractive index engineering: A critical review. J. Polym. Res. 2024, 31, 124. [Google Scholar] [CrossRef]
- Zhang, J.H.; Sun, X.; Wang, H.; Li, J.; Guo, X.; Li, S.; Wang, Y.; Cheng, W.; Qiu, H.; Shi, Y.; et al. From 1D to 2D to 3D: Electrospun Microstructures towards Wearable Sensing. Chemosensors 2023, 11, 295. [Google Scholar] [CrossRef]
- Sun, M.; Wang, S.; Zhang, Y.; Zhang, Z.; Wang, S.; Wang, Z.; Chen, X.; Liu, H.; Zhang, Y.; Han, L. An ultrasensitive flexible biosensor enabled by high-performance graphene field-effect transistors with defect-free van der Waals contacts for breast cancer miRNA fast detection. Talanta 2025, 287, 127637. [Google Scholar] [CrossRef]
- Kim, D.S.; Jeong, J.M.; Park, H.J.; Kim, Y.K.; Lee, K.G.; Choi, B.G.; Concentrated, H. Conductive, Defect-free Graphene Ink for Screen-Printed Sensor Application. Nanomicro Lett. 2021, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Somarathna, U.S.; Garakani, B.; Weerawarne, D.L.; Alhendi, M.; Poliks, M.D.; Misner, M.; Burns, A.; Khinda, G.S.; Alizadeh, A. Reliability of Screen-printed Water-based Carbon Resistors for Sustainable Wearable Sensors. IEEE Sens. J. 2025, 25, 6449–6463. [Google Scholar] [CrossRef]
- Ismail, S.N.A.; Nayan, N.A.; Haniff, M.A.S.M.; Jaafar, R.; May, Z. Wearable Two-Dimensional Nanomaterial-Based Flexible Sensors for Blood Pressure Monitoring: A Review. Nanomaterials 2023, 13, 852. [Google Scholar] [CrossRef]
- Abid, J.; Khalil, F.M.A.; Saeed, S.; Khan, S.U.; Iqbal, I.; Khan, S.U.; Anthony, S.; Shahzad, R.; Koerniati, S.; Naz, F. Nano revolution in cardiovascular health: Nanoparticles (NPs) as tiny titans for diagnosis and therapeutics. Curr. Probl. Cardiol. 2024, 49, 102466. [Google Scholar] [CrossRef]
- Arshad, I.; Kanwal, A.; Zafar, I.; Unar, A.; Mouada, H.; Razia, I.T.; Arif, S.; Ahsan, M.; Kamal, M.A.; Rashid, S.; et al. Multifunctional role of nanoparticles for the diagnosis and therapeutics of cardiovascular diseases. Environ. Res. 2024, 242, 117795. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhu, S.; Lv, M.; Gu, Z.; Hu, H. Harnessing nanotechnology for cardiovascular disease applications—A comprehensive review based on bibliometric analysis. Nano Today 2022, 44, 101453. [Google Scholar] [CrossRef]
- Tavakoli, M. Surface modification of polymers to enhance biocompatibility. In Surfaces and Interfaces for Biomaterials; Woodhead Publishing: Cambridge, UK, 2005; pp. 719–744. [Google Scholar] [CrossRef]
- Tyagi, Y.; Kumar, R.; Sharma, A. Development of biodegradable polymeric nanoparticles–based nanobiosystems. In Intelligent Nanobiosystems in Medicine and Healthcare, Volume 1: Fundamentals, Fabrication and Commercialization; Academic Press: Cambridge, MA, USA, 2025; pp. 241–302. [Google Scholar] [CrossRef]
- Benjamin, S.R.; Júnior, E.J.M.R. Carbon-based nanomaterials as antimicrobial nanocoatings for medical devices and implants. In Next-Generation Antimicrobial Nanocoatings for Medical Devices and Implants; Woodhead Publishing: Cambridge, UK, 2024; pp. 205–230. [Google Scholar] [CrossRef]
- Menaga, S.; Kumari, S. Clinically used hydrogels for biomedical applications. In Hydrogel Tissue Analogues; Woodhead Publishing: Cambridge, UK, 2025; pp. 515–527. [Google Scholar] [CrossRef]
- Zhang, F.; Zang, Y.; Huang, D.; Di, C.A.; Zhu, D. Flexible and self-powered temperature–pressure dual-parameter sensors using microstructure-frame-supported organic thermoelectric materials. Nat. Commun. 2015, 6, 8356. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.I.; Choi, K.S.; Chang, S.P. A novel means of fabricating microporous structures for the dielectric layers of capacitive pressure sensor. Microelectron. Eng. 2017, 179, 60–66. [Google Scholar] [CrossRef]
- Vishnu, J.; Manivasagam, G. Perspectives on smart stents with sensors: From conventional permanent to novel bioabsorbable smart stent technologies. Med. Devices Sens. 2020, 3, e10116. [Google Scholar] [CrossRef]
- Chaparro-Rico, B.D.M.; Sebastiano, F.; Cafolla, D. A Smart Stent for Monitoring Eventual Restenosis: Computational Fluid Dynamic and Finite Element Analysis in Descending Thoracic Aorta. Machines 2020, 8, 81. [Google Scholar] [CrossRef]
- Pang, W.; Yuan, C.; Zhong, T.; Huang, X.; Pan, Y.; Qu, J.; Nie, L.; Zhou, Y.; Lai, P. Diagnostic and therapeutic optical imaging in cardiovascular diseases. iScience 2024, 27, 111216. [Google Scholar] [CrossRef]
- Ang, Y.X.; Ghazali, F.A.M.; Ali, M.S.M. Micromachined Shape Memory Alloy Active Stent with Wireless Monitoring and Re-Expansion Features. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 396–399. [Google Scholar] [CrossRef]
- Oyunbaatar, N.E.; Kim, D.S.; Prasad, G.; Jeong, Y.J.; Lee, D.W. Self-rollable Polymer Stent Integrated with Wireless Pressure Sensor for Real-time Monitoring of Cardiovascular Pressure. Sens. Actuators A Phys. 2022, 346, 113869. [Google Scholar] [CrossRef]
- Oyunbaatar, N.E.; Shanmugasundaram, A.; Kwon, K.; Lee, D.W. Continuous monitoring of cardiovascular function with a smart stent incorporating a flexible and stretchable wireless pressure sensor. J. Micromechanics Microengineering 2023, 33, 115001. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Song, H.; Huang, H.; Gou, J. Highly Stretchable and Wearable Strain Sensor Based on Printable Carbon Nanotube Layers/Polydimethylsiloxane Composites with Adjustable Sensitivity. ACS Appl. Mater. Interfaces 2018, 10, 7371–7380. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, R.; Janyani, V.; Verma, D. Development of a multi-modal graphene nanoparticles (GNP)—Polydimethylsiloxane (PDMS) flexible sensor for human activity monitoring and health assessment. Int. J. Electrochem. Sci. 2023, 18, 100236. [Google Scholar] [CrossRef]
- Jang, H.H.; Park, J.S.; Choi, B. Flexible piezoresistive pulse sensor using biomimetic PDMS mold replicated negatively from shark skin and PEDOT:PSS thin film. Sens. Actuators A Phys. 2019, 286, 107–114. [Google Scholar] [CrossRef]
- Mirsepah, A.; Shooshtari, L.; Mohammadpour, R.; Esfandiar, A.; Irajizad, A. Wearable broadband MoS2 photodetector for dual heart rate and UV detection powered by PDMS-MXene TENG. Chem. Eng. J. 2024, 499, 155953. [Google Scholar] [CrossRef]
- Chen, S.; Wu, N.; Lin, S.; Duan, J.; Xu, Z.; Pan, Y.; Zhang, H.; Xu, Z.; Huang, L.; Hu, B.; et al. Hierarchical elastomer tuned self-powered pressure sensor for wearable multifunctional cardiovascular electronics. Nano Energy 2020, 70, 104460. [Google Scholar] [CrossRef]
- Fang, H.; Ji, Y.; Li, S.; Liu, H.; Wang, D. RHES: Development of real-time health evaluation system based on human pulse signal utilizing PVDF/PDMS arch-type piezoelectric sensor. Measurement 2024, 224, 113856. [Google Scholar] [CrossRef]
- Seok, M.; Yoon, S.; Kim, M.; Cho, Y.H. A porous PDMS pulsewave sensor with haircell structures for water vapor transmission rate and signal-to-noise ratio enhancement. Nanoscale Adv. 2021, 3, 4843–4850. [Google Scholar] [CrossRef]
- Yang, C.R.; Lin, M.F.; Huang, C.K.; Huang, W.C.; Tseng, S.F.; Chiang, H.H. Highly sensitive and wearable capacitive pressure sensors based on PVDF/BaTiO3 composite fibers on PDMS microcylindrical structures. Measurement 2022, 202, 111817. [Google Scholar] [CrossRef]
- Mirzajani, H.; Kraft, M. Soft Bioelectronics for Heart Monitoring. ACS Sens. 2024, 9, 4363. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Park, K.; Hambsch, M.; Hong, S.; Kim, H.T.; Choi, D.H.; Lee, J.H.; Kim, S.; Kim, H.J. Skin-conformable photoplethysmogram sensors for energy-efficient always-on cardiovascular monitoring systems. Nano Energy 2022, 92, 106773. [Google Scholar] [CrossRef]
- Papavassiliou, D.; Lima, R.A. The Impact of Polydimethylsiloxane (PDMS) in Engineering: Recent Advances and Applications. Fluids 2025, 10, 41. [Google Scholar] [CrossRef]
- Alam, S.T.; Urooj, S.; Ansari, A.Q.; Arif, A. Design and Performance Assessment of Biocompatible Capacitive Pressure Sensors with Circular and Square Geometries Using ANSYS Workbench. Sensors 2025, 25, 2423. [Google Scholar] [CrossRef]
- Trung, T.Q.; Ramasundaram, S.; Hwang, B.-U.; Lee, N.-E.; Trung, T.Q.; Hwang, B.-U.; Ramasundaram, S.; Lee, E.N. An All-Elastomeric Transparent and Stretchable Temperature Sensor for Body-Attachable Wearable Electronics. Adv. Mater. 2016, 28, 502–509. [Google Scholar] [CrossRef]
- Yuan, Z.; Pei, Z.; Shahbaz, M.; Zhang, Q.; Zhuo, K.; Zhao, C.; Zhang, W.; Ma, X.; Sang, S. Wrinkle Structured Network of Silver-Coated Carbon Nanotubes for Wearable Sensors. Nanoscale Res. Lett. 2019, 14, 356. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Li, A.; Chen, J. Multi-dimensional synergistic force sensor for pulsation signal detection. Int. J. Mech. Sci. 2025, 295, 110274. [Google Scholar] [CrossRef]
- Liu, Q.; Tai, H.; Yuan, Z.; Zhou, Y.; Su, Y.; Jiang, Y.; Liu, Q.; Tai, H.; Yuan, Z.; Zhou, Y.; et al. A High-Performances Flexible Temperature Sensor Composed of Polyethyleneimine/Reduced Graphene Oxide Bilayer for Real-Time Monitoring. Adv. Mater. Technol. 2019, 4, 1800594. [Google Scholar] [CrossRef]
- Pang, Y.N.; Liu, B.; Liu, J.; Wan, S.P.; Wu, T.; Yuan, J.; Xin, X.; He, X.D.; Wu, Q. Singlemode-Multimode-Singlemode Optical Fiber Sensor for Accurate Blood Pressure Monitoring. J. Light. Technol. 2022, 40, 4443–4450. [Google Scholar] [CrossRef]
- Li, S.; Dong, K.; Li, R.; Huang, X.; Chen, T.; Xiao, X. Capacitive pressure sensor inlaid a porous dielectric layer of superelastic polydimethylsiloxane in conductive fabrics for detection of human motions. Sens. Actuators A Phys. 2020, 312, 112106. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, K.; Fernando, A.; Gao, Y.; Li, G.; Jin, L.; Zhai, H.; Yi, Y.; Xu, L.; Zheng, Y.; et al. Permeable graphited hemp fabrics-based, wearing-comfortable pressure sensors for monitoring human activities. Chem. Eng. J. 2021, 403, 126191. [Google Scholar] [CrossRef]
- Pruvost, M.; Smit, W.J.; Monteux, C.; Poulin, P.; Colin, A. Polymeric foams for flexible and highly sensitive low-pressure capacitive sensors. Npj Flex. Electron. 2019, 3, 7. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, B.; Yang, C.; Dai, Q.; Kong, L.; Guo, J.; Zhou, B.; Yang, C.; Kong, L.; Dai, Q. Stretchable and Temperature-Sensitive Polymer Optical Fibers for Wearable Health Monitoring. Adv. Funct. Mater. 2019, 29, 1902898. [Google Scholar] [CrossRef]
- Lin, M.; Zheng, Z.; Yang, L.; Luo, M.; Fu, L.; Lin, B.; Xu, C.; Lin, M.; Zheng, Z.; Yang, L.; et al. High-Performance, Sensitive, Wearable Multifunctional Sensor Based on Rubber/CNT for Human Motion and Skin Temperature Detection. Adv. Mater. 2022, 34, 2107309. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Y.; Cheng, S.; Gao, Q.; Yuan, Y.; Li, A.; Guan, S. PDMS-based conductive elastomeric composite with 3D reduced graphene oxide conductive network for flexible strain sensor. Compos. Part. A Appl. Sci. Manuf. 2022, 161, 107113. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Du, M.; Song, A.; Tan, Y.; Li, T. Hydrogel-Based Polymer Fiber Strain Sensor for Human Body Monitoring. In Proceedings of the 2024 Academic Conference of China Instrument and Control Society (ACCIS), Chengdu, China, 28–31 July 2024; pp. 195–198. [Google Scholar] [CrossRef]
- Liu, Z.; Xiang, C.; Tong, Y.; Li, K.H.; Guan, X. Transfer Learning Enhanced Blood Pressure Monitoring Based on Flexible Optical Pulse Sensing Patch. ACS Sens. 2025, 10, 2732–2742. [Google Scholar] [CrossRef]
- Xu, M.; Peng, Q.; Song, Z.; Qin, M.; Sun, Y.; Du, Z.; Zheng, K.; Wang, X.; Yang, B.; Liu, J. Multimodal MEMS Microwrinkle Electronics for Cardiac Pulsed Field Ablation and Sensing. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Kaohsiung, Taiwan, 19–23 January 2025; pp. 484–487. [Google Scholar] [CrossRef]
- Ding, S.; Pichon, L.; Chen, Y. A Low-cost Microwave Stentenna for In-stent Restenosis Detection. IEEE Trans. Antennas Propag. 2025, 73, 8681–8692. [Google Scholar] [CrossRef]
- Abdin, Z.U.; Shah, S.A.A.; Shah, I.A.; Iman, U.R.; Mian, S.H.; Yoo, H. A Novel Dual-Band MIMO-Enabled Biotelemetric Endovascular Stent IoMT System. IEEE Trans. Instrum. Meas. 2025, 74, 5505215. [Google Scholar] [CrossRef]
- Bateman, A.; He, Y.; Cherono, C.; Lee, J.; Ghalichechian, N.; Yeo, W.H. Implantable Membrane Sensors and Long-Range Wireless Electronics for Continuous Monitoring of Stent Edge Restenosis. ACS Appl. Mater. Interfaces 2025, 17, 42781–42790. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.-J.; Dong, C.-W.; Ham, D.-H.; Park, W.-T. Ultra-Miniature MEMS Sensor Packaging Process for Intravascular Medical Device. Int. J. Precis. Eng. Manuf. 2025, 2025, 2739–2748. [Google Scholar] [CrossRef]
- Chen, X.; Assadsangabi, B.; Hsiang, Y.; Takahata, K.; Chen, X.; Assadsangabi, B.; Takahata, K.; Hsiang, Y.; Angioplasty-Ready, E. “Smart” Stents to Detect In-Stent Restenosis and Occlusion. Adv. Sci. 2018, 5, 1700560. [Google Scholar] [CrossRef]
- Romero, U.V.; Abir, S.S.H.; Karam, N.; Torres, M.; Ahmed, S.; Rahman, M.W.; Azimi, B.; Danti, S.; Li, J.; Uddin, M.J. A biocompatible nitinol based triboelectric stent sensor for prospective cardiovascular health monitoring. Hybrid Adv. 2025, 10, 100484. [Google Scholar] [CrossRef]
- Sappati, K.K.; Bhadra, S. Piezoelectric Polymer and Paper Substrates: A Review. Sensors 2018, 18, 3605. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and applications of the β phase poly(vinylidene fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Tanguy, N.R.; Rana, M.; Khan, A.A.; Zhang, X.; Tratnik, N.; Chen, H.; Ban, D.; Yan, N. Natural lignocellulosic nanofibrils as tribonegative materials for self-powered wireless electronics. Nano Energy 2022, 98, 107337. [Google Scholar] [CrossRef]
- Wang, L.; Jiao, L.; Pang, S.; Yan, P.; Wang, X.; Qiu, T. The development of design and manufacture techniques for bioresorbable coronary artery stents. Micromachines 2021, 12, 990. [Google Scholar] [CrossRef]
- Wei, J.-L.; Oyunbaatar, N.-E.; Kim, D.-S.; Lee, D.-W. Hybrid Biodegradable Polymer Stent Fabrication Using 3D Printers and Integration with Wireless Sensors for Real-Time Pressure Monitoring in Blood Vessels. In Proceedings of the 22nd International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Kyoto, Japan, 25–29 June 2023; Available online: https://ieeexplore.ieee.org/abstract/document/10516841 (accessed on 12 November 2025).
- Xue, Y.-H.; Lv, T.-R.; Zhang, H.; Yang, Y.-Q.; Yin, M.-J.; An, Q.-F. Bioinspired hydrogel tactile sensor via pressure-intensified ion signals with self-healing and anti-frozen capability. Chem. Eng. J. 2025, 515, 163485. [Google Scholar] [CrossRef]
- Cui, M.; Chai, Z.; Lu, Y.; Zhu, J.; Chen, J. Developments of polyurethane in biomedical applications: A review. Resour. Chem. Mater. 2023, 2, 262–276. [Google Scholar] [CrossRef]
- Pavithra, S.; Thejas, R.; Rao, H.N.A.; Krishna, B.S.; Nagaraju, G. Preparation of polypyrrole by chemical oxidation: Applications for sensor studies. Macromol. Res. 2024, 32, 23–33. [Google Scholar] [CrossRef]
- Sun, Z.; Ou, Q.; Dong, C.; Zhou, J.; Hu, H.; Li, C.; Huang, Z. Conducting polymer hydrogels based on supramolecular strategies for wearable sensors. Exploration 2024, 4, 20220167. [Google Scholar] [CrossRef]
- Guo, Z.; Liao, G.; Ren, L.; Qiao, H.; Huang, Z.; Wang, Z.; Qi, X. Non-invasive flexible sensor based on liquid metal for human physiological detection. Next Nanotechnol. 2024, 5, 100042. [Google Scholar] [CrossRef]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Amaechi, E.C.; Elendu, I.D.; Omeludike, J.C.; Omeludike, E.K.; Onubogu, N.C.; Ogelle, E.C.; Meduoye, O.O.M.; et al. Essential information about nanotechnology in cardiology. Ann. Med. Surg. 2025, 87, 748–779. [Google Scholar] [CrossRef]
- Ge, G.; Lu, Y.; Qu, X.; Zhao, W.; Ren, Y.; Wang, W.; Wang, Q.; Huang, W.; Dong, X. Muscle-Inspired Self-Healing Hydrogels for Strain and Temperature Sensor. ACS Nano 2020, 14, 218–228. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.N.; Tao, X.M.; Ding, X. Review of Flexible Temperature Sensing Networks for Wearable Physiological Monitoring. Adv. Healthc. Mater. 2017, 6, 1601371. [Google Scholar] [CrossRef]
- Yasmin, F.; Zaidi, S.F.; Moeed, A.; Shahzad, M.; Asghar, M.S.; Sadiq, M.; Iqbal, J.; Surani, S.; Alraies, M.C. Clinical Outcomes of Immediate Versus Staged Revascularization of Nonculprit Arteries in Patients With Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. Clin. Cardiol. 2025, 48, e70105. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Duggal, B.; Singh, J.P.; Shrivastava, Y. Efficacy of Various Dry Electrode-Based ECG Sensors: A Review. J. Biomed. Mater. Res. A 2025, 113, e37845. [Google Scholar] [CrossRef]
- Oyunbaatar, N.-E.; Wei, J.; Wang, L.; Kim, S.-H.; Lee, H.; Kwon, K.; Won, Y.; Lee, D.-W. 3D-Printed CNT-Reinforced Bioresorbable Vascular Scaffold with Enhanced Mechanical Stability and Integrated Wireless Pressure Sensor for Continuous Hemodynamic Monitoring. ACS Sens. 2025, 10, 5735. [Google Scholar] [CrossRef]
- Wang, L.; Oyunbaatar, N.E.; Kim, D.S.; Wei, J.; Jeong, Y.J.; Lee, H.; Kimv, S.H.; Won, Y.; Kwon, K.; Jeong, I.S.; et al. Ultra-Sensitive Wireless Pressure Sensor for Real-Time Cardiovascular Restenosis Monitoring in Smart Stents. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Kaohsiung, Taiwan, 19–23 January 2025; pp. 12–15. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, Y.; Zhou, Y.; Wang, Y.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Ultra-stretchable, sensitive and durable strain sensors based on polydopamine encapsulated carbon nanotubes/elastic bands. J. Mater. Chem. C Mater. 2018, 6, 8160–8170. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, R.; Li, G. Temperature and Strain Compensation for Flexible Sensors Based on Thermosensation. ACS Appl. Mater. Interfaces 2020, 12, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Arie, T.; Akita, S.; Takei, K. Wearable, Flexible, and Multifunctional Healthcare Device with an ISFET Chemical Sensor for Simultaneous Sweat pH and Skin Temperature Monitoring. ACS Sens. 2017, 2, 443–448. [Google Scholar] [CrossRef]
- Ma, Z.; Wei, A.; Li, Y.; Shao, L.; Zhang, H.; Xiang, X.; Wang, J.; Ren, Q.; Kang, S.; Dong, D.; et al. Lightweight, flexible and highly sensitive segregated microcellular nanocomposite piezoresistive sensors for human motion detection. Compos. Sci. Technol. 2021, 203, 108571. [Google Scholar] [CrossRef]
- Guan, L.; Yan, S.; Liu, X.; Li, X.; Gao, G. Wearable strain sensors based on casein-driven tough, adhesive and anti-freezing hydrogels for monitoring human-motion. J. Mater. Chem. B 2019, 7, 5230–5236. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, J.; Yang, J.; Huang, Y.; Zhang, Y.; Wang, Y.; Zhang, J.; Wang, Y.; Yuan, L.; Cai, M.; et al. Highly Sensitive Flexible Iontronic Pressure Sensor for Fingertip Pulse Monitoring. Adv. Healthc. Mater. 2020, 9, 2001023. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Liu, S.; Zhang, H.; Lu, J. A Flexible Temperature Sensor Array with Polyaniline/Graphene–Polyvinyl Butyral Thin Film. Sensors 2019, 19, 4105. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Liu, T.; Wu, F.; Wang, S.; Ge, S.; Li, Y.; Liu, J.; Ye, H.; Lei, R.; Wang, C.; et al. An Antisweat Interference and Highly Sensitive Temperature Sensor Based on Poly(3,4-ethylenedioxythiophene)-Poly(styrenesulfonate) Fiber Coated with Polyurethane/Graphene for Real-Time Monitoring of Body Temperature. ACS Nano 2023, 17, 21073–21082. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Lee, Y.; Lee, H.S.; Ko, H. Nanomaterials: Fingertip skin-inspired microstructured ferroelectric skins discriminate static/dynamic pressure and temperature stimuli. Sci. Adv. 2015, 1, e1500661. [Google Scholar] [CrossRef]
- Tan, J.Y.; Kim, C.; Mou, T.D.; Kim, A.; Kim, J.J.K. Wireless Pressure Sensing Smart Stent for Enhanced Post-Endovascular Aneurysm Repair (EVAR) Surveillance. IEEE Access 2024, 12, 198123–198131. [Google Scholar] [CrossRef]
- Oyunbaatar, N.E.; Lee, D.W. Carbon Nano Tubes-Incorporated Smart Stents to Improve Mechanical Strength and Sensor Reliability. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Austin, TX, USA, 21–25 January 2024; pp. 386–389. [Google Scholar] [CrossRef]
- Qu, X.; Cheng, S.; Liu, Y.; Hu, Y.; Shan, Y.; Luo, R.; Weng, S.; Li, H.; Niu, H.; Gu, M.; et al. Bias-Free Cardiac Monitoring Capsule. Adv. Mater. 2024, 36, 2402457. [Google Scholar] [CrossRef]
- Palwai, S.; Batra, A.; Kotru, S.; Vaseashta, A. Electrospun Polyvinylidene Fluoride Nanofiber Membrane-Based Flexible Capacitive Tactile Sensors for Biomedical Applications. Surf. Eng. Appl. Electrochem. 2022, 58, 194–201. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, H.; Xie, G.; Jiang, Y.; Chen, C.; Su, Y.; Wang, Y.; Tai, H. Flexible piezoelectric pressure sensor based on polydopamine-modified BaTiO3/PVDF composite film for human motion monitoring. Sens. Actuators A Phys. 2020, 301, 111789. [Google Scholar] [CrossRef]
- Huang, P.; Wei, Q.; Wang, B.; Li, B.; Wu, Z.; Xing, Y. Continuous blood pressure monitoring based on improved multi-scale U-net assisted with piezoelectric polymer nanocomposite sensors. Biomed. Signal Process Control 2025, 105, 107598. [Google Scholar] [CrossRef]
- Zhang, M.; Yeow, J.T.W. A flexible, scalable, and self-powered mid-infrared detector based on transparent PEDOT: PSS/graphene composite. Carbon 2020, 156, 339–345. [Google Scholar] [CrossRef]
- Zhao, S.; Ran, W.; Wang, D.; Yin, R.; Yan, Y.; Jiang, K.; Lou, Z.; Shen, G. 3D Dielectric Layer Enabled Highly Sensitive Capacitive Pressure Sensors for Wearable Electronics. ACS Appl. Mater. Interfaces 2020, 12, 32023–32030. [Google Scholar] [CrossRef]
- Yin, R.; Yang, S.; Li, Q.; Zhang, S.; Liu, H.; Han, J.; Liu, C.; Shen, C. Flexible conductive Ag nanowire/cellulose nanofibril hybrid nanopaper for strain and temperature sensing applications. Sci. Bull. 2020, 65, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qi, J.; Fan, S.; Qiao, Z.; Yeo, J.C.; Lim, C.T. Flexible wearable sensors for cardiovascular health monitoring. Adv. Healthc. Mater. 2021, 10, e2100116. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kwon, D.; Kim, K.; Park, J.; Del Orbe, D.; Gu, J.; Ahn, J.; Cho, I.; Jeong, Y.; Oh, Y.; et al. Synergetic Effect of Porous Elastomer and Percolation of Carbon Nanotube Filler toward High Performance Capacitive Pressure Sensors. ACS Appl. Mater. Interfaces 2020, 12, 1698–1706. [Google Scholar] [CrossRef]
- Wang, Y.F.; Sekine, T.; Takeda, Y.; Yokosawa, K.; Matsui, H.; Kumaki, D.; Shiba, T.; Nishikawa, T.; Tokito, S. Fully Printed PEDOT:PSS-based Temperature Sensor with High Humidity Stability for Wireless Healthcare Monitoring. Sci. Rep. 2020, 10, 2467. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Xiao, S.; Meng, Z.; Liu, H.; Wan, G.; He, Y. High-performance flexible pressure sensor based on ordered double-level nanopillar array films: Design, development, and modeling. Compos. Sci. Technol. 2023, 241, 110157. [Google Scholar] [CrossRef]
- Peng, H.K.; Shi, Y.Y.; Yu, Y.; Li, T.T.; Zhang, X.Y.; Fan, X.X.; Lin, J.H. Temperature/Pressure Dual-Mode Flexible Sensors: PP Nonwoven-Based and Low-Temperature Polymerized with Pyrrole. Fibers Polym. 2024, 25, 901–912. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, K.; Bu, F.; Meng, R.; Xie, G.; Guo, K.; Cao, A.; Tu, L. Low-cost, reliable and flexible piezoresistive pressure sensors coated with single layer graphene and silver nanowires on three-dimensional polyurethane sponge. Sens. Actuators A Phys. 2024, 375, 115524. [Google Scholar] [CrossRef]
- Xie, F. Natural polymer starch-based materials for flexible electronic sensor development: A review of recent progress. Carbohydr. Polym. 2024, 337, 122116. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.; Chakroborty, S.; Vishwakarma, D.P.; Goga, G.; Yadav, A.S.; Mohan, R. Recent advances in sustainable nature-based functional materials for biomedical sensor technologies. Environ. Sci. Pollut. Res. 2023, 31, 57289–57313. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhao, Z.; Deng, J.; Zhao, Y.; Liang, S.; Yi, Y.; Li, J.; Wei, Y. Tough, conductive hydrogels based on gelatin and oxidized sodium carboxymethyl cellulose as flexible sensors. Carbohydr. Polym. 2024, 335, 121920. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, X.; Xu, L.; Yan, M.; Bi, H.; Wang, Q. Transparent multifunctional cellulose-based conductive hydrogel for wearable strain sensors and arrays. Carbohydr. Polym. 2024, 329, 121784. [Google Scholar] [CrossRef]
- Li, N.; Yu, X.; Yang, D.P.; He, J. Natural polysaccharides-based smart sensors for health monitoring, diagnosis and rehabilitation: A review. Int. J. Biol. Macromol. 2025, 304, 140966. [Google Scholar] [CrossRef]
- Tordi, P.; Ridi, F.; Samorì, P.; Bonini, M.; Tordi, P.; Ridi, F.; Bonini, M.; Samorì, P. Cation-Alginate Complexes and Their Hydrogels: A Powerful Toolkit for the Development of Next-Generation Sustainable Functional Materials. Adv. Funct. Mater. 2025, 35, 2416390. [Google Scholar] [CrossRef]
- Saleh, A.K.; El-Sayed, M.H.; El-Sakhawy, M.A.; Alshareef, S.A.; Omer, N.; Abdelaziz, M.A.; Jame, R.; Zheng, H.; Gao, M.; Du, H. Cellulose-based Conductive Materials for Bioelectronics. ChemSusChem 2025, 18, e202401762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Lei, Q.Y.; Liu, R.J.; Zhang, L.; Lyu, B.; Liu, L.P.; Ma, J.Z. Self-healing cellulose-based flexible sensor: A review. Ind. Crops Prod. 2023, 206, 117724. [Google Scholar] [CrossRef]
- Dong, M.; Soul, A.; Li, Y.; Bilotti, E.; Zhang, H.; Cataldi, P.; Papageorgiou, D.G.; Dong, M.; Soul, A.; Li, Y.; et al. Transient Starch-Based Nanocomposites for Sustainable Electronics and Multifunctional Sensing. Adv. Funct. Mater. 2025, 35, 2412138. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, C.; Liu, Y. Progress in the Development of Flexible Devices Utilizing Protein Nanomaterials. Nanomaterials 2025, 15, 367. [Google Scholar] [CrossRef] [PubMed]
- PereiraTavares, A.J.; Aranda-Michel, L.; Hahn, S.; Coffin, B.D.; Ashraf, S.F.; Szafron, J.M.; Straub, A.C.; Shiwarski, D.J. Development of an Open-source Low-cost Pressure Myography and Cardiac Flow Simulator, HemoLens, for Mechanical Characterization of Native and Engineered Blood Vessels. BioRxiv 2025. [Google Scholar] [CrossRef]
- Sharma, R.; Malviya, R.; Patel, M.; Raval, D. Biodegradable Materials for Customized Medical Devices Improve Personalized Medicine. In Sustainable Nanocomposites with Green Biomaterials; Springer: Cham, Switzerland, 2025; pp. 357–375. [Google Scholar] [CrossRef]
- Hasanpur, E.; Ghazavizadeh, A.; Sadeghi, A.; Haboussi, M. In vitro corrosion study of PLA/Mg composites for cardiovascular stent applications. J. Mech. Behav. Biomed. Mater. 2021, 124, 104768. [Google Scholar] [CrossRef]
- Tian, J.; Jiang, F.; Zeng, Q.; Pourhosseiniasl, M.J.; Han, C.; Ren, K. Biocompatible Piezoelectric Polymer Poly(Lactic Acid)-Based Stethoscope for Wearable Health Monitoring Devices. IEEE Sens. J. 2023, 23, 6264–6271. [Google Scholar] [CrossRef]
- Natesan, T.; Nangan, S.; Ramasamy, R. Bioresorbable polymer-based sensors for medical applications. In Bioresorbable Polymers and Their Composites: Characterization and Fundamental Processing for Pharmaceutical and Medical Device Development; Woodhead Publishing: Cambridge, UK, 2024; pp. 469–494. [Google Scholar] [CrossRef]
- Heydarkhan-Hagvall, S.; Schenke-Layland, K.; Dhanasopon, A.P.; Rofail, F.; Smith, H.; Wu, B.M.; Shemin, R.; Beygui, R.E.; MacLellan, W.R. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials 2008, 29, 2907–2914. [Google Scholar] [CrossRef]
- Oh, B.; Lee, C.H. Nanofiber for cardiovascular tissue engineering. Expert. Opin. Drug Deliv. 2013, 10, 1565–1582. [Google Scholar] [CrossRef]
- Zhao, Z.; Ji, J.; Zhang, Y.; Liu, J.; Yu, R.; Yang, X.; Zhao, X.; Huang, W.; Zhao, W. Ultra-elastic conductive silicone rubber composite foams for durable piezoresistive sensors via direct ink writing three-dimensional printing. Chem. Eng. J. 2025, 504, 158733. [Google Scholar] [CrossRef]
- Badawi, M.N.; Batoo, K.M. Recent advances incorporating conductive cotton for applications in Sensors, Optoelectronics, and energy Harvesting: A review. Inorg. Chem. Commun. 2025, 173, 113876. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Lou, Z.; Zheng, Y.; Wang, K.; Zhao, L.; Han, W.; Jiang, K.; Shen, G. Biomimetic, biocompatible and robust silk Fibroin-MXene film with stable 3D cross-link structure for flexible pressure sensors. Nano Energy 2020, 78, 105252. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Q.; Wei, J.; Zhu, C.; Wang, Y.; Yu, A.; Wang, W.; Zou, J.; Xie, J.; Fu, Z. Biocomposite silk fibroin hydrogel with stretchability, conductivity and biocompatibility for wireless strain sensor. J. Mater. Sci. Technol. 2025, 210, 195–203. [Google Scholar] [CrossRef]
- Chen, Y.; Pötschke, P.; Pionteck, J.; Voit, B.; Qi, H. Smart cellulose/graphene composites fabricated by in situ chemical reduction of graphene oxide for multiple sensing applications. J. Mater. Chem. A Mater. 2018, 6, 7777–7785. [Google Scholar] [CrossRef]
- Yin, H.; Liu, F.; Abdiryim, T.; Chen, J.; Liu, X. Sodium carboxymethyl cellulose and MXene reinforced multifunctional conductive hydrogels for multimodal sensors and flexible supercapacitors. Carbohydr. Polym. 2024, 327, 121677. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, S.; Wan, Z.; Tian, Y.; Wang, D.; Ma, Y.; Yang, L.; Wei, Z. Functional magnetic alginate/gelatin sponge-based flexible sensor with multi-mode response and discrimination detection properties for human motion monitoring. Carbohydr. Polym. 2024, 324, 121677. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Wang, Y.; Cheng, J.; Chen, G.; Xing, T. Robust and flexible smart silk/PEDOT conductive fibers as wearable sensor for personal health management and information transmission. Int. J. Biol. Macromol. 2023, 248, 125870. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, J.; Liu, H.; Zhang, L. Silk nanofibrous iontronic sensors for accurate blood pressure monitoring. Chem. Eng. J. 2023, 453, 139815. [Google Scholar] [CrossRef]
- Muhammad, U.; Cao, X.; Zhang, T.; Ji, W.; Lv, R.; Chen, J.; Wei, Y. Fabrication of highly tough, self-healing sodium alginate/polyacrylamide and copper based nanocomposite hydrogel and its application as strain and pressure sensor for human health monitoring and signature recognition. Int. J. Biol. Macromol. 2025, 311, 143734. [Google Scholar] [CrossRef]
- Julius, A.; Malakondaiah, S.; Pothireddy, R.B. Polymer and nanocomposite fillers as advanced materials in biomedical applications. Nano Trends 2025, 9, 100087. [Google Scholar] [CrossRef]
- Zahran, M. Carbohydrate polymer-supported metal and metal oxide nanoparticles for constructing electrochemical sensors. Mater. Adv. 2024, 5, 68–82. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Y.; Zhang, Z.; Jia, F.; Gao, G. Acetylated Distarch Phosphate-Mediated Tough and Conductive Hydrogel for Antibacterial Wearable Sensors. ACS Appl. Mater. Interfaces 2022, 14, 51420–51428. [Google Scholar] [CrossRef]
- Lu, L.; Huang, Z.; Li, X.; Li, X.; Cui, B.; Yuan, C.; Guo, L.; Liu, P.; Dai, Q. A high-conductive, anti-freezing, antibacterial and anti-swelling starch-based physical hydrogel for multifunctional flexible wearable sensors. Int. J. Biol. Macromol. 2022, 213, 791–803. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Wu, J.; Han, L.; Yang, Z.; Jiang, Z.; Wang, R.; Huang, Z.; Xu, M.; Self-Healing, U. Reusable, and Conductive Polysaccharide-Based Hydrogels for Sensitive Ionic Sensors. ACS Sustain. Chem. Eng. 2020, 8, 18506–18518. [Google Scholar] [CrossRef]
- Hu, X.; Chen, J.; Yan, Z.; Nie, D.; Guan, F.; Shi, C.; Lin, N. Multifunctional Silk Fibroin Hydrogels with Strong Adhesion for Tissue Sealing and Wearable Electronic Sensors. ACS Appl. Mater. Interfaces 2025, 17, 16453–16467. [Google Scholar] [CrossRef]
- Lv, Q.; Chen, S.; Luo, D.; Liu, H.; Song, Y.; Liu, M.; Xiao, F.; Wang, Z.; Wang, L. An Implantable and Degradable Silk Sericin Protein Film Energy Harvester for Next-Generation Cardiovascular Electronic Devices. Adv. Mater. 2025, 37, 2413610. [Google Scholar] [CrossRef]
- Luo, Y.; Abidian, M.R.; Ahn, J.-H.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.; Berggren, M.; Berkey, C.A.; Bettinger, C.J.; et al. Technology Roadmap for Flexible Sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, X.; Li, B.; Huang, G.; Xu, F.; Zhang, X. Solvent-Free Fabrication of Carbon Nanotube/Silk Fibroin Electrospun Matrices for Enhancing Cardiomyocyte Functionalities. ACS Biomater. Sci. Eng. 2020, 6, 1630–1640. [Google Scholar] [CrossRef]
- Gad-el-Hak, M. MEMS: Design and Fabrication; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Hsu, T.R. MEMS and Microsystems: Design; Manufacture; Nanoscale Engineering; John Wiley and Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Brox, D.S.; Chen, X.; Mirabbasi, S.; Takahata, K. Wireless Telemetry of Stainless-Steel-Based Smart Antenna Stent Using a Transient Resonance Method. IEEE Antennas Wirel. Propag. Lett. 2016, 15, 754–757. [Google Scholar] [CrossRef]
- Chen, X.; Brox, D.; Assadsangabi, B.; Ali, M.S.M.; Takahata, K. A stainless-steel-based implantable pressure sensor chip and its integration by microwelding. Sens. Actuators A Phys. 2017, 257, 134–144. [Google Scholar] [CrossRef]
- Chen, X.; Brox, D.; Assadsangabi, B.; Hsiang, Y.; Takahata, K. Intelligent telemetric stent for wireless monitoring of intravascular pressure and its in vivo testing. Biomed. Microdevices 2014, 16, 745–759. [Google Scholar] [CrossRef]
- Sheridan, W.S.; Wetterling, F.; Testani, J.M.; Borlaug, B.A.; Fudim, M.; Damman, K.; Gray, A.; Gaines, P.; Poloczek, M.; Madden, S.; et al. Safety and performance of a novel implantable sensor in the inferior vena cava under acute and chronic intravascular volume modulation. Eur. J. Heart Fail. 2023, 25, 754–763. [Google Scholar] [CrossRef]
- Lu, Y.; Mi, Y.; Wu, T.; Cao, X.; Wang, N. From Triboelectric Nanogenerator to Polymer-Based Biosensor: A Review. Biosensors 2022, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zou, M.; Lu, Y.; Deng, Y.; Nie, S.; Yang, J.; Guo, H. Device design and data processing strategies for self-powered cardiovascular sensors. Device 2025, 3, 100726. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Y.; Ouyang, H.; Shi, B.; Li, N.; Jiang, D.; Xie, F.; Qu, D.; Zou, Y.; Huang, Y.; et al. Transcatheter Self-Powered Ultrasensitive Endocardial Pressure Sensor. Adv. Funct. Mater. 2019, 29, 1807560. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, Q.; Liu, Y.; Shi, B.; Xue, X.; Ji, W.; Liu, Z.; Jin, Y.; Zou, Y.; An, Z.; et al. Self-Powered, One-Stop, and Multifunctional Implantable Triboelectric Active Sensor for Real-Time Biomedical Monitoring. Nano Lett. 2016, 16, 6042–6051. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, H.; Shi, B.; Xue, X.; Liu, Z.; Jin, Y.; Ma, Y.; Zou, Y.; Wang, X.; An, Z.; et al. In Vivo Self-Powered Wireless Cardiac Monitoring via Implantable Triboelectric Nanogenerator. ACS Nano 2016, 10, 6510–6518. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, L.; Liu, X.; Zhou, Y.; Zhang, M.; Wang, Y.; Yang, L.; Wei, D. Wide linear range and highly sensitive flexible pressure sensor based on multistage sensing process for health monitoring and human-machine interfaces. Chem. Eng. J. 2021, 412, 128649. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, C.; Liu, W.; Zhang, Y.; Zhang, J.; Li, X.; Geng, L.; Wang, X. Pulse sensor based on single-electrode triboelectric nanogenerator. Sens. Actuators A Phys. 2018, 280, 326–331. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).