Highlights
What are the main findings?
- A novel precipitation method via solution blending and centrifugation was developed for the facile preparation of conductive polymer composite hydrogels (e.g., PEDOT/PAA/PVA).
- The prepared hydrogels exhibit excellent electrical (conductivity: 4.065 S/m) and mechanical (Young’s modulus: 311.6 kPa) properties, and perform well as strain sensors (sensitivity: 1.86; response time: 400 ms) and bioelectrodes (lower contact impedance than commercial electrodes, and showed no signs of skin irritation under tested conditions).
- The method shows universal applicability for different conductive polymers (e.g., PANI, PPy) and hydrogel substrates (e.g., PVA, PAAm).
What is the implication of the main finding?
- It provides a universal, simple, and low-cost strategy for rapid synthesis of customizable conductive hydrogels, overcoming limitations of traditional complex methods.
Abstract
Conductive polymer hydrogels have attracted extensive attention in wearable devices, soft machinery, and energy storage due to their excellent mechanical and conductive properties. However, their preparation is often complex, expensive, and time-consuming. Herein, we report a facile precipitation method to prepare conductive polymer composite hydrogels composed of poly(acrylic acid) (PAA), poly(vinyl alcohol) (PVA), and poly(3,4-ethylenedioxythiophene) (PEDOT) via straightforward solution blending and centrifugation. During the preparation, PEDOT, grown along the PAA template, is uniformly dispersed in the hydrogel matrix. After shaping and rinsing, the PEDOT/PAA/PVA hydrogel shows good mechanical and electrical properties, with a conductivity of 4.065 S/m and a Young’s modulus of 311.6 kPa. As a strain sensor, it has a sensitivity of 1.86 within 0–100% strain and a response time of 400 ms. As a bioelectrode, it exhibits lower contact impedance than commercially available electrodes and showed no signs of skin irritation in the test. The method’s versatility is confirmed by the observation of similar performance of hydrogels with different compositions (e.g., polyaniline (PANI)/PAA/PVA). These results demonstrate the broad applicability of the method.
1. Introduction
With rising health awareness, the traditional rigid medical equipment is no longer adequate to address the diverse and evolving demands of people. Wearable flexible electronic devices are gradually being more widely concerned [1,2]. These newly developed flexible wearable electronics are like a bridge between humans and machines, and they can be applied to the skin surface as sensors or patch electrodes to monitor physiological parameters and treatment effects of patients, providing an important auxiliary means for medical diagnosis and treatment [3,4,5].
It is noteworthy that conductive polymer composite hydrogels show great advantages in the manufacture of bioflexible electronic devices because of their excellent conductivity and biocompatibility [6,7,8]. The formation of soft conductive hydrogels is achieved through the incorporation of conductive fillers or conductive polymers within an insulating hydrogel substrate, which can be facilitated by the introduction of multivalent metal ions or through post-processing of the resultant polymers [9,10,11,12,13,14,15,16,17,18,19,20,21,22]. These conductive hydrogels are tunable and designable, allowing their properties (e.g., mechanical properties) to be adapted to the environment in which they are to be used through the adjustment of formulations and the choice of materials [20,21,23,24]. In a notable study, Wei et al. constructed a conductive polymer composite hydrogel by in situ polymerization of aniline monomers in polyvinyl alcohol (PVA), followed by cross-linking of PVA by glutaraldehyde (GA) as a cross-linking agent to act as a wearable sensor. This sensor enabled the detection of different movements of the human body and even the differentiation of speech content [20]. The distinctive characteristics of conductive hydrogels make them a promising class of materials for a wide range of applications.
At present, the preparation of conductive polymer hydrogels can be achieved through a variety of methodologies, including electrochemical preparation, soaking preparation, dispersion preparation, and solution processing. Electrochemical polymerization involves the infiltration of conductive polymer monomers into a system of hydrophilic hydrogels through the use of electrodes. It was reported that a sulfhydryl self-assembly layer was grafted onto the surface of titanium using gelatin methacrylate, and subsequently, a conductive polypyrrole layer was introduced through the electrochemical method. The conductive hydrogel coating prepared by this method has been shown to exhibit excellent electrochemical properties and biocompatibility [25,26,27,28]. Immersion preparation method is one of the most popular methods for preparing conductive polymer hydrogels. Conductive polymer monomers and oxidants are mixed together and infiltrated into the prepared hydrogel network by means of immersion. Wang et al. introduced aniline into conductive polymer hydrogel prepared in PAA hydrogel system by immersion method to prepare strain sensor for detecting human behavior [21,29]. The dispersion preparation method involves the dispersion of a conductive polymer, which was prepared in advance, in a hydrogel precursor solution by means of ultrasound, followed by the initiation of hydrogel network polymerization by heat curing to prepare the conductive polymer composite hydrogel [30]. The solution processing method is a new synthesis method. Firstly, the hydrogel polymer, the conductive polymer monomer and the oxidizer are mixed. Thereafter, the conductive polymer grows along the long chain of the hydrogel polymer. Subsequently, hydrogel polymer chains are crosslinked to synthesize the conductive polymer composite hydrogel by post-crosslinking [30].
The fundamental principle underlying these methods lies in the sequence of conductive polymer synthesis relative to hydrogel network formation. Conductive polymers are either grown along pre-existing hydrogel templates or dispersed within the precursor solution. However, each method presents certain limitations. For instance, the soaking process is often time-consuming and may lead to hydrogel swelling, while dispersion methods can result in inhomogeneous distribution of the conductive polymer. Increasing the concentration of conductive polymers within the hydrogel remains challenging. These issues complicate the fabrication process and may restrict the practical applications of such hydrogels. Thus, developing a simple, low-cost, and efficient preparation strategy that balances mechanical and electrical properties continues to be a significant challenge [31,32,33,34].
In this study, we reported a low-cost and simple preparation method of conductive polymer composite hydrogels. The conductive polymer composite hydrogels, based on polyacrylic acid (PAA) and polyvinyl alcohol (PVA), were successfully prepared by means of simple solution blending and deposition. The conductive polymer composite hydrogel was formed by growing the conductive polymer along the long chain of the hydrogel polymer and precipitating and concentrating together with the hydrogel polymer. This method of preparation was designed to avert the occurrence of conductive polymer aggregation, which could result from an excess of conductive polymer monomer during the polymerization process. Compared to conventional multi-step methods such as soaking or dispersion preparation, which often require hours or specialized equipment, our precipitation strategy offers a remarkably facile and rapid route to functional conductive hydrogels. Additionally, it ensured the incorporation of conductive polymer into the concentrated hydrogel. The prepared hydrogels were successfully packaged as strain sensors, which could be used to detect movements. The hydrogels could also be used as bioelectrodes to detect electrocardiogram (ECG) or electroencephalogram (EEG) signals. This method was proved to be universally applicable and could be employed in a variety of conductive polymer materials and hydrogel substrates.
2. Materials and Methods
2.1. Materials
Sodium polyacrylate (PAAS, Mw 3000–7000 kDa) was purchased from Shanghai Xianding Biotechnology Co., Ltd. (Shanghai, China). Polyvinyl alcohol (PVA-224), 3,4-ethylenedioxythiophene (EDOT, 99%), aniline (≥99.5%), glutaraldehyde (GA, 50%), dopamine hydrochloride (98%), ammonium persulfate (APS, 98.5%), ferric trichloride hexahydrate (99%), pyrrole (Py, 99%), polyacrylamide (PAAm, Mw 7000 kDa) were all purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Sodium hydroxide (AR) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Urea (99%, note: original text incorrectly wrote “urea nitrogen”) was purchased from Shanghai Tengzhun Biotechnology Co., Ltd. (Shanghai, China). Citric acid (AR, ≥99.5%) was purchased from Shanghai Boer Chemical Reagent Co., Ltd. (Shanghai, China). Anhydrous sodium citrate (≥99%) was purchased from Shanghai Dibo Biotechnology Co., Ltd. (Shanghai, China). Sodium chloride (99.8%) was purchased from Shanghai Aladdin Biochemical Technology Co. Ltd. (Shanghai, China).
2.2. Preparation of PEDOT/PAA/PVA Hydrogels
A FeCl3-HCl solution was prepared by mixing 20 mL of pH 0 HCl solution with 1 mL of 10% (w/w) FeCl3 solution. After thorough mixing of the FeCl3-HCl solution with 1% (w/v) PAA solution, 10% (w/w) APS and EDOT monomer were added. The mixture was shaken at room temperature for 24 h using a shaking machine to obtain PEDOT/PAA solution. 6 mL of PEDOT/PAA solution was mixed with 2.3 mL of 1% (w/v) PVA solution, thoroughly blended, and centrifuged for 3 min. The precipitate was collected as PEDOT/PAA/PVA hydrogel. After absorbing surface water with non-woven cloth, the hydrogel was placed into a glass fixture with a 300 μm spacing and oven-dried at 60 °C for 15 min for dehydration and solidification. Subsequently, 8 μL of an aqueous glutaraldehyde (GA) solution (5% w/w) was evenly drop-cast onto the surface of the hydrogel to facilitate chemical cross-linking, which was allowed to proceed at room temperature for 10 min. After the reaction was complete, the hydrogel was retrieved. A cleaning solution was prepared by dissolving 50.4 g FeCl3, 36 g NaCl, 360 g urea, 17.5 g citric acid (CA), and 100.3 g sodium citratein 2.4 L deionized (DI) water with thorough stirring. The PEDOT/PAA/PVA hydrogel cross-linked with glutaraldehyde was repeatedly immersed in 50 mL of this cleaning solution.
2.3. Preparation of PAA/PVA Hydrogels
6 mL of FeCl3-HCl solution was mixed with 2.3 mL of PAA solution, thoroughly agitated, and centrifuged for 3 min. The precipitate was collected as PAA/PVA hydrogel, which was then shaped, soaked, and cleaned to obtain the final product.
2.4. Material Characterization
Fourier transform infrared (FT-IR) spectroscopy: Performed using a Thermo Scientific Nicolet IS50 (Waltham, MA, USA) in the wavelength range of 4000~400 cm−1.
X-ray photoelectron spectroscopy (XPS): Acquired using a Thermo Scientific ESCALAB Xi+ instrument (Waltham, MA, USA) with scanning in the 1350~0 eV interval for compositional analysis of N, C, and S elements.
Samples (PEDOT/PAA/PVA and PAA/PVA hydrogels) were lyophilized using a BIOCOOL vacuum freeze dryer FD-1A-80 (BIOCOOL, Beijing, China) before characterization.
2.5. Conductivity Test
PEDOT/PAA solutions were prepared as per Table 1. Hydrogels were cut into 10 mm × 10 mm × 0.3 mm squares, sandwiched between two electrode pads, and subjected to alternating current (AC) impedance testing using a CHI660E electrochemical workstation (Shanghai Chenhua Co., Ltd., Shanghai, China) in the 10−1 to 104 Hz frequency range.

Table 1.
Experimental formulation utilizing PEDOT concentration control.
Sheet resistance was measured using a four-probe tester (RTS-9, 4Probes Tech Ltd., Guangzhou, China). Conductivity (κ) was calculated as:
where R□ is the sheet resistance (Ω/□) and
d is the hydrogel thickness (m).
κ = 1/(d × R□)
2.6. Skin Contact Impedance Measurement of Hydrogels
Three 10 mm × 10 mm × 0.3 mm PEDOT/PAA/PVA
hydrogels were applied to the inner side of the forearm at 10 cm intervals,
connected to the working, reference, and counter electrodes of an
electrochemical workstation, respectively, and subjected to AC impedance
testing in the 10−1–104 Hz range.
2.7. Mechanical Property Test
Hydrogels were trimmed into 30 mm × 10 mm × 0.3 mm
strips, fixed to a universal testing machine ZHIQU ZQ-990B (Dongguan, China)
clamps, and subjected to uniaxial cyclic tensile tests at 100 mm/min within
0–20%, 0–40%, 0–60%, 0–80%, and 0–100% strain.
where F is the load (N) and S is the
cross-sectional area (mm2).
where L is the elongated length (mm) and L0
is the initial length (mm).
Stress (σ): σ = F/S
Strain (ε): ε = (L − L0)/L0 × 100%
Young’s modulus (E): E = σ/ε
Calculated using two points in the linear region of
the stress–strain curve.
2.8. Strain Sensor Performance Test
A 30 mm × 10 mm × 0.3 mm hydrogel strip was fixed
to a universal testing machine, with copper tape at both ends connected to a
portable precision resistance/capacitance measuring device (TruEbox 01RC,
LinkZill, Hangzhou, China) at 250 kHz.
The gauge factor (GF) can be expressed as the rate
of change in resistance (ΔR/R0) in the range of 0 to 200% for
samples tested at 5% tensile deformation on each occasion.
Gauge factor (GF) was calculated as:
GF = (ΔR/R0)/(ΔL/L0)
ΔR = |R − R0|
ΔL = |L − L0|
R is real-time resistance, R0 is initial
resistance, L is real-time length, and L0 is initial length. Tests
included variable amplitude stretching (5%, 15% strain at 1 Hz), variable
frequency stretching (10% strain at 0.25 Hz and 0.5 Hz), and 100 cyclic loading
tests (10% strain at 1 Hz).
2.9. Bioelectrode Performance Test
Skin irritation test: Hydrogel electrodes and
commercial ECG electrodes were attached to the inner side of the forearm for 4
h, then removed to check for redness, swelling, or allergies. This test was
conducted to preliminarily assess the short-term skin compatibility of the
hydrogel electrodes.
ECG signal acquisition: Hydrogel electrodes (0.3 mm
thick, 10 mm diameter) and commercially available ECG electrodes were attached
to the inner side of the forearm using PU tape and connected to an ECG signal
collector. Signals were recorded synchronously. The cosine similarity of
signals was calculated to verify reliability:
where Ai and Bi are voltage
values of commercial and hydrogel electrodes at the i-th sampling point,
respectively.
EEG signal acquisition: A hydrogel electrode (1 mm
thick, 10 mm diameter) was placed at the right prefrontal (Fp2), and the EEG
paste was injected into the left prefrontal (Fp1). EEG signals were recorded
synchronously, and cosine similarity was calculated. The impedances of the
electrodes were recorded hourly to monitor the long-term performance.
2.10. Universality of the Preparation Methods
PANI/PAA/PVA and PPy/PAA/PVA hydrogels: Conductive
polymer monomers (aniline or pyrrole), PAA, HCl, FeCl3, and APS were
mixed, stirred at 4 °C until reaction completion to obtain PANI/PAA or PPy/PAA
solutions, which were then mixed with PVA solution. Precipitates were collected
by centrifugation.
PEDOT/PAA/PAAm hydrogels: 6 mL PEDOT/PAA solution
was mixed with 2.3 mL 1% (w/v) PAAm solution, centrifuged, and
the precipitate was collected.
PDA/PVA/PAA hydrogels: A 0.5% (w/v)
PVA solution (pH 11) with 20 mg dopamine hydrochloride was shaken at room
temperature for 12 h to obtain PDA/PVA solution. 2.5 mL 1% (w/v)
PAA solution was mixed with 125 μL pH 0 HCl and 1 mL FeCl3 solution
to form FeCl3-PAA solution. The two solutions were mixed, and the
precipitate was collected by centrifugation.
PANI/PAA/PVA hydrogels were tested as ECG
electrodes similarly to PEDOT/PAA/PVA, with cosine similarity calculated for
signals.
3. Results and Discussion
3.1. Preparation of PEDOT/PAA/PVA Hydrogels
The precipitation method of preparing conductive
polymer hydrogels was illustrated in Figure 1a
and PEDOT was utilized first as a model conductive polymer for this universal
method. Initially, EDOT and PAA were combined at room temperature with
continuous stirring initiated subsequent to the addition of Ammonium Persulfate
(APS), HCl and FeCl3. EDOT was then grown into PEDOT/PAA with good
solubility using PAA as the template and the dopant under the oxidation of FeCl3
and APS. The incorporation of HCl provided an acidic condition for the
polymerization of EDOT, lowered the viscosity of the PAA solution, and
prevented PAA from precipitation due to the liganding effect of Fe3+.
Subsequently, the prepared PEDOT/PAA solution was mixed with the PVA solution,
leading to the interaction between PEDOT/PAA and PVA through hydrogen bonding
and their entanglement, and ultimately yielding a blue-black, fluffy
precipitate. On the other hand, the solution itself became yellowish
transparent, indicating that the majority of the PEDOT precipitated together
with the hydrogel network, rather than remaining in the solution. The
precipitate was collected by centrifugation and allowed to dehydrate further,
resulting in the formation of a PEDOT/PAA/PVA hydrogel. The hydrogel underwent
a heating and dehydration process, with the hydrogel sandwiched between two
glass slides separated by a distance of 300 μm. The dehydrated hydrogel was
further treated with GA for a quick crosslinking followed by the immersion in
cleaning solution, resulting in a transition from plasticity to elasticity. Figure 1b illustrates the proposed interactions
between the components of the hydrogel. This uniform network is achieved
because PEDOT grows in situ along the molecularly dispersed PAA chains. This
result aligns with accounts found in most published studies [35,36,37]. Subsequently, the strong hydrogen bonding
between PAA and PVA, synergizing with the Fe3+ ionic cross-linking,
ensures the integrated co-precipitation and homogeneous distribution of the
conductive polymer within the resulting hydrogel matrix. The ternary network
structure and intricate interactions endowed the hydrogel with excellent structural
stability.
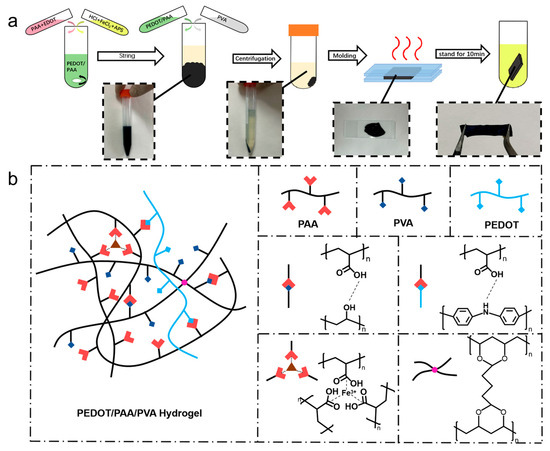
Figure 1.
Schematic diagram of (a) PEDOT/PAA/PVA hydrogel preparation process and (b) iteraction of components of PEDOT/PAA/PVA hydrogels.
Fourier transform infrared (FTIR) and X-ray
photoelectron spectroscopy (XPS) spectra of PEDOT/PAA/PVA hydrogels and PAA/PVA
hydrogels after lyophilization were collected to gain insight into the
components present in the hydrogel and the interactions between them. As
illustrated in Figure 2a, distinctive
absorption peaks were observed at 1716 cm−1 and 1385 cm−1
in the FTIR spectra of both PEDOT/PAA/PVA and PAA/PVA hydrogels, attributed to
-COOH and PAA-Fe, respectively, supporting that PAA was physically crosslinked
by Fe3+. The characteristic peaks of C-O-C at 1141 cm−1
and C-S at 977 cm−1 and 840 cm−1 in the spectrum of
PEDOT/PAA/PVA hydrogels indicated the formation of PEDOT [36,38,39,40,41,42]. XPS analysis (Figure 2b–d) revealed the presence of S 2p, C
1s, O 1s, and Na 1s peaks in the hydrogel. The XPS spectra of C 1s were
convolved to three peaks that could correspond to 285.0 eV (C-C), 286.4 eV
(C-S), and 288.7 eV (-COO-), respectively. Furthermore, the XPS spectra of O 1s
were convolved to peaks that could correspond to 531.5 eV (C=O) and 533 eV
(-OH), indicating the presence of PEDOT, PAA, and PVA [43]. The
predominant source of sodium was inferred to be NaCl in the cleaning solution,
while the source of sulfur was identified as PEDOT in the hydrogel.
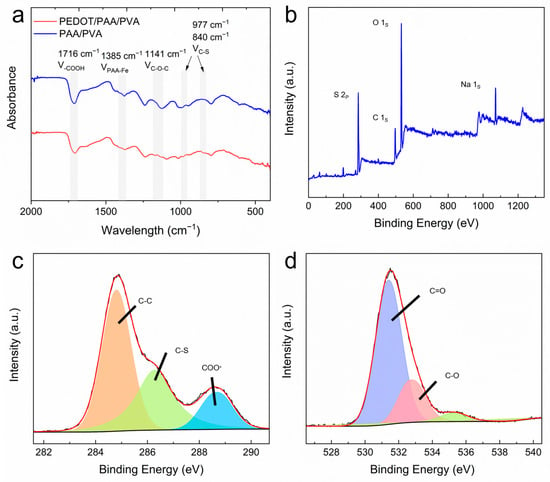
Figure 2.
(a) FTIR spectra of dried hydrogels. XPS survey spectrum (b) and high-resolution XPS spectra of C 1s (c) and O 1s (d) of PEDOT/PAA/PVA.
3.2. The Characteristics of PEDOT/PAA/PVA Hydrogels
Electrical properties are an important index for
the evaluation of bioelectrodes. The impedance of PEDOT/PAA/PVA hydrogels was
tested by sandwiching the hydrogel sheets with varying EDOT dosages between
copper tapes within the frequency range of 10−1 Hz to 104
Hz, utilizing an electrochemical workstation. As illustrated in Figure 3a, the impedance of PEDOT/PAA/PVA
hydrogels decreased as the amount of EDOT fed increased, until it reached 7.5
μL (EDOT7.5). However, when the EDOT dosage further increased to 10 μL, the
impedance increased instead, which is speculated to be resulted from the
aggregation of PEDOT. Furthermore, to assess the electrical property of
PEDOT/PAA/PVA hydrogels, the sheet resistance was measured using the four-probe
method. Similarly, PEDOT/PAA/PVA hydrogels exhibited the lowest sheet
resistance value of 820 Ω/□ when the EDOT dosage was 7.5 μL and the
conductivity is calculated to be 4.065 S/m (Figure
3b). Beyond this point, the impedance increased, likely due to the
aggregation of PEDOT chains at higher concentrations, which can hinder charge
transport by creating discontinuous conductive domains, a common challenge in
conductive polymer composites [44,45]. Accordingly,
the PEDOT/PAA/PVA hydrogel with EDOT dosage of 7.5 μL was selected for all
subsequent investigation.
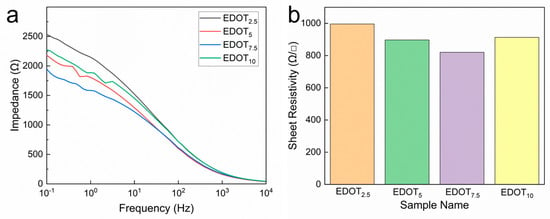
Figure 3.
Impedance spectra (a) and sheet resistance (b) of PEDOT/PAA/PVA hydrogels with different EDOT dosage.
The conductive hydrogel material is anticipated to
demonstrate resilience in the face of repeated loading cycles to cope with a
variety of application scenarios. In order to assess the mechanical properties
and stability of the hydrogel, it was affixed to the tensile tester fixture for
uninterrupted stretching-unloading experiments. The amplitude of stretching was
set at 20%, 40%, 60%, 80%, and 100%, respectively. The continuous cyclic
tensile loading-unloading curves of PEDOT/PAA/PVA hydrogels at different
strains demonstrate that the mechanical properties of the hydrogels within this
interval exhibit good linearity and reproducibility. Furthermore, the hydrogels
can be rapidly restored to their original state without any damage (Figure S1). The Young’s modulus of the hydrogel
was determined to be 311.6 kPa.
It should be noted that the concentration of FeCl3
was found to be critical for effective precipitation and initial gel formation.
Although not the main focus of this study, it was qualitatively observed that
the mechanical properties of the resulting hydrogel were modulated by altering
the FeCl3 concentration, as this parameter controls the physical
cross-linking density of PAA. These observations underscore the role of Fe3+
in the network formation.
The PEDOT/PAA/PVA hydrogel, with a Young’s modulus
of approximately 311.6 kPa and a conductivity of 4.065 S/m, performs
competitively against recently reported conductive hydrogels for wearable
sensing [35,46,47]. This balanced performance
was achieved through a simple and rapid precipitation method, contrasting with
the more complex synthesis routes typically required for such materials.
3.3. The Application of PEDOT/PAA/PVA Hydrogels
Due to its excellent resistance to pressure, the
hydrogel has been selected as a candidate for the fabrication of sensors. A
piece of PEDOT/PAA/PVA hydrogel with dimensions of 30 mm in length, 10 mm in
width, and 0.3 mm in thickness was extracted and affixed with copper tape at
both extremities as electrodes. It was then encased in PU tape, thus creating a
rudimentary sensor. The sensors were secured on a horizontal tensile stage and
subjected to examination of their intrinsic electrical characteristics. Figure 4a demonstrated the resistance
alteration rate of the hydrogel in the range of 0–100% strain corresponding to
varying strains of the hydrogel. The hydrogel exhibited a sensitivity of 1.82
within this interval, which encompasses a broad detection range, high
sensitivity, and robust stability. The linear response within the 0–100% strain
range defines the effective sensing window for this sensor, which amply covers
the requirements for detecting most human physiological activities and joint
movements.
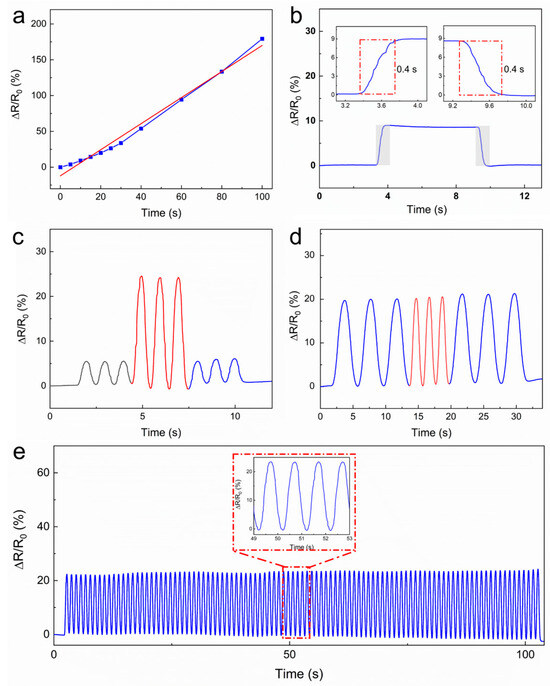
Figure 4.
The performance of the PEDOT/PAA/PVA hydrogel as a strain sensor. (a) The electrical resistance and strain curve. The original data is illustrated by a blue line, while the linear-fitted is shown as a red dashed line. (b) The response and recovery times of the hydrogel. (c) The real-time response curve measured at 5% and 15% strains. (d) The real-time response curve measured at 0.25 and 0.5 Hz. (e) The cycling durability test of the hydrogel strain sensor.
As illustrated in Figure 4b, the response time and relaxation time of the hydrogel were both 400
ms. The uniform response speed is further accompanied by consistent resistance
after relaxation, suggesting a stable electrical property. As illustrated in Figure 4c,d, the hydrogel sensor exhibited
stable and rapid responsiveness to cycle stretching at 5% and 15% strains with
a frequency of 1 Hz, as well as to cycle stretching at a 10% strain with
variable frequencies of 0.25 Hz and 0.5 Hz. Furthermore, the sensor exhibited
consistent and effective response to cyclic tensile strain throughout 100
cycles of stretching at 10% strain (Figure 4e)
with minimal signal degradation. The stability of over 100 cycles demonstrates
promising durability for typical wearable applications. Investigation into
ultra-long-term reliability (e.g., >1000 cycles) will be an important aspect
of future work aimed at commercialization.
The hydrogels were then affixed to the joints of
the right forefinger using PU tape encapsulation, and the resistance were
altered corresponding to the movement of the finger in a consistent manner (Figure 5). The resistance change curve
displayed stability and smoothness, and the absence of discernible drift
phenomena.
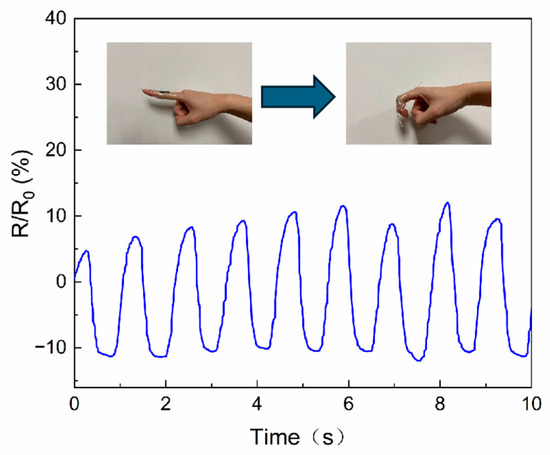
Figure 5.
Finger joint motion measured by PEDOT/PAA/PVA hydrogel.
PEDOT/PAA/PVA hydrogels were assessed for their
capacity to function as bioelectrodes. The electrode-skin contact impedance was
tested within the 100–105 Hz range (Figure 6a). The results demonstrated that the
contact impedance of the hydrogel electrodes was markedly lower than that of
the commercial electrodes (Figure 6b).
This discrepancy could be attributed to two factors: the exceptional
conductivity of the hydrogel itself and the substantial quantity of keratinous
penetrant present in the hydrogel. The stratum corneum, the outermost layer of
the skin, exhibits a high impedance, which increases the contact impedance and
affects the acquisition of bioelectrical signals. The presence of the penetrant
effectively reduced the impact of the high impedance of the stratum corneum on
the measurement. As a bioelectrode, hydrogel should not cause allergic skin
redness and swelling. The PEDOT/PAA/PVA hydrogel electrodes and the
commercially available ECG electrodes were attached to the skin on the inner
side of the forearm for a period of four hours. Then, the electrode sheet was
removed, and the condition of the skin was examined to ascertain the presence
of any irritation and to determine the contact impedance of the hydrogel. The
results demonstrated that the area where the commercial electrodes and hydrogel
electrodes were attached exhibited no evidence of allergic reactions, redness,
or swelling, with the exception of the indentation caused by adhesion.
Therefore, under the conditions of this 4 h test, the hydrogel showed no
observable risk of causing acute skin irritation or allergies, suggesting its
potential suitability for bioelectricity acquisition (Figure S2).
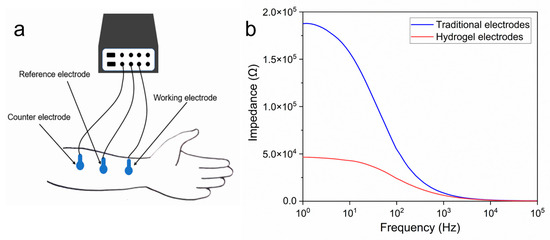
Figure 6.
(a) Measurement of skin contact impedance; (b) Comparison of contact impedance between the PEDOT/PAA/PVA hydrogel electrode and commercial ECG electrodes.
The PEDOT/PAA/PVA hydrogel exhibited conductivity
and sensitivity, and it did not cause skin redness or swelling or allergic
reactions, rendering it suitable for use as a skin surface electrode. In a pair
of commercially available ECG electrodes attached adjacent to the PEDOT/PAA/PVA
hydrogel electrodes as a control group of ECG acquisition signals, synchronized
processing of their acquired ECG signals of the subjects revealed that both
electrodes acquired clear ECG signal images (Figures S3 and S4), with a cosine similarity of 0.9928 between the two curves.
These findings suggested that PEDOT/PAA/PVA hydrogels could be used as ECG
electrodes with a high degree of safety and reliability.
This approach was expected to enhance signal
quality and mitigate the impact of motion artifacts on the measurements. In
order to guarantee the uninterrupted procurement of EEG data over an extended
timeframe, it is imperative to preserve low impedance across the EEG electrode
over the course of time. The commercially available electrode was attached to
the forehead in conjunction with the PEDOT/PAA/PVA hydrogel electrode, and the
impedance data were continuously monitored. It has been demonstrated that the
hydrogel electrode maintained a Direct Current (DC) contact impedance of less
than 10 kΩ for a duration of five hours during continuous measurement (Figure 7), thereby indicating the feasibility
of prolonged EEG monitoring.
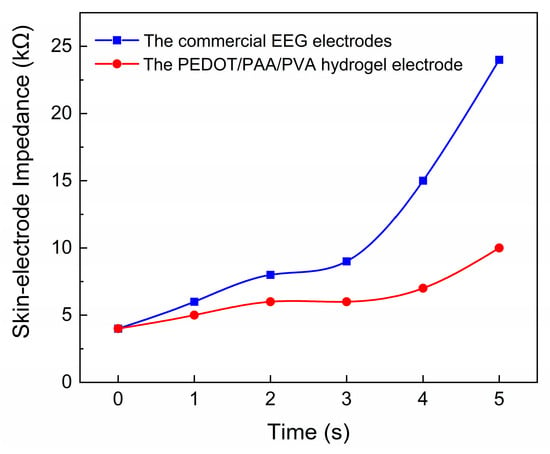
Figure 7.
The long-term monitoring of impedance for the electrode filled with EEG paste and the PEDOT/PAA/PVA hydrogel electrode.
In the context of EEG testing, a circular piece of
hydrogel electrode, measuring 10 mm in diameter and 1 mm in height, was
positioned at the subject’s right prefrontal test point, Fp2. Similarly, a
commercially available conductive paste was instilled at the left prefrontal
test point, Fp1. The EEG signals acquired by the two types of EEG electrodes
were recorded simultaneously at Fp1 and Fp2. Figure 8a illustrates the EEG signals of the subject while maintaining a
resting state. It can be observed that the commercial EEG electrodes and the
PEDOT/PAA/PVA hydrogel electrodes yielded high-frequency signals with
comparable shapes. The cosine similarity was calculated to be 0.9967, thereby
substantiating the assertion that the PEDOT/PAA/PVA hydrogel possessed the
capacity to measure high-frequency signals. The analysis of the collected EEG
signals during regular blinking and eye rotation of the subjects revealed that
the cosine similarity of the signals acquired by hydrogel electrodes and
commercial EEG electrodes was 0.9970 and 0.9994, respectively (Figure 8b,c). These findings indicate that
hydrogel can be used as an EEG electrode without causing skin irritation and
that it possesses a reliable ability to acquire bioelectric signals.
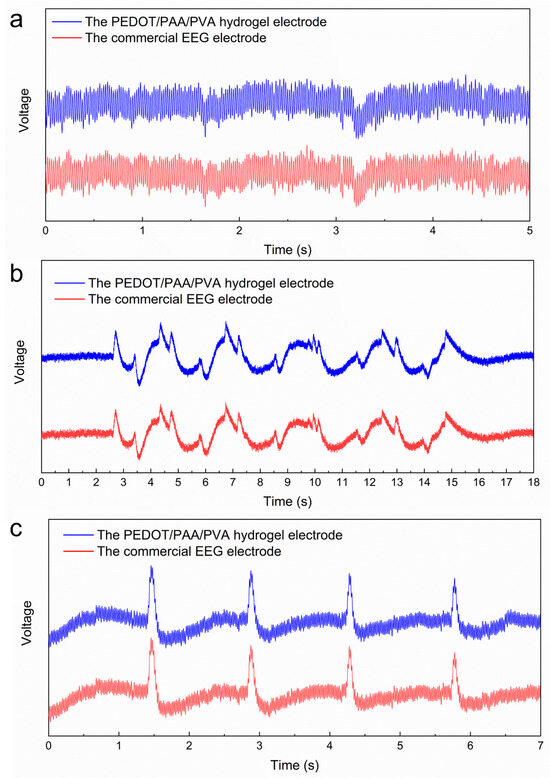
Figure 8.
EEG signals in different states: (a) the signal of closing the eyes and relaxing, (b) the signal of blinking and (c) rolling the eyes.
3.4. Universal Applicability for the Preparation of Conductive Hydrogels
Subsequent to the substitution of various hydrogel
substrates and conductive polymers in the procedure, an endeavor was undertaken
to fabricate conductive hydrogels with disparate compositions employing the
proposed method. The black-green Polypyrrole (PPy)/PAA and dark brown PANI/PAA
solutions was obtained by subjecting pyrrole or aniline into a mixture of PAA,
HCl, FeCl3, and APS at a low-temperature environment. The resulting
PPy/PAA and PANI/PAA solutions were subsequently amalgamated with the PVA
solution. On the other hand, the PEDOT/PAA solution was mixed with the
Polyacrylamide (PAAm) to substitute PVA. It was observed that the mixing
process resulted in precipitations, and the solution adopted a light yellow hue
and became transparent, indicating the integration of conductive polymers in
precipitates. The precipitates resulting from the aforementioned mixing were
collected, subjected to centrifugation, dried, and shaped to yield PPy/PAA/PVA,
PANI/PAA/PVA, and PEDOT/PAA/PAAm hydrogels. There was a little difference in
preparing the polydopamine composite hydrogel. The PVA solution was prepared as
an alkaline solution and combined with dopamine to form a Polydopamine
(PDA)/PVA mixture, followed by the mixing with a blend of PAA, HCl, and FeCl3.
The resultant mixture produced a black-brown precipitate. This precipitate was
then subjected to the same procedures as other formulars. All these hydrogels
exhibited favorable mechanical properties. Additionally, PANI/PAA/PVA was
selected as an example to function as an ECG electrode using the same method
used for PEDOT/PAA/PVA (Figure S5). The
cosine similarity of the two signal curves obtained from the PANI/PAA/PVA
hyrogel electrode and the commercial ECG electrode reached 0.9934, thereby
demonstrating that the excellent electrical properties of the PANI/PAA/PVA
hydrogel are sufficient for use as ECG electrodes. The experimental findings
demonstrated the efficacy of the precipitation method in replacing both the
hydrogel network and the conductive polymer in the fabrication of conductive
polymer hydrogel composites. This approach not only provided new possibilities
for customizing hydrogel properties but also significantly expanded the
application scenarios of the precipitation method.
4. Conclusions
In conclusion, a novel method for the facile
preparation of conductive polymer composite hydrogels was developed through a
straightforward solution blending and centrifugation process. The PEDOT/PAA/PVA
hydrogels exhibited excellent electrical and mechanical properties with a
conductivity of 4.065 S/m. The hydrogel strain sensor exhibited a response
speed of 400 ms, a sensitivity of 1.86, and good stability and repeatability.
Furthermore, in a preliminary skin irritation test, the hydrogel demonstrated no
signs of skin irritation under the tested conditions, and exhibited a skin
contact impedance that was much smaller than that of commercial ECG electrodes.
The ECG and EEG signals acquired by hydrogel electrodes and commercial
electrodes showed a very high degree of similarity, thus proving the
reliability of PEDOT/PAA/PVA hydrogels as bioelectrodes. Furthermore, the
replacement of the hydrogel substrate and conductive polymer materials,
respectively, was also successfully achieved. Consequently, this study establishes
a versatile precipitation strategy for the rapid synthesis of high-performance
conductive hydrogels, thereby overcoming the long-standing limitations of
complex and time-consuming fabrication methods and providing a scalable
platform for on-demand conductive hydrogel design.
Supplementary Materials
The following
supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/s25196032/s1, Figure S1:
Continuous cyclic compression load-unloading curves of PEDOT/PAA/PVA hydrogels
at different strains (20–100%); Figure S2: Skin irritation test: (a) test process (b)
skin condition after test, the upper and lower core electrodes are commercial
core electrodes and hydrogel electrocardiograms; Figure S3: ECG signal comparison test; Figure S4: Comparison of contact impedance
between hydrogel and commercial ECG electrodes; Figure
S5: Comparison of ECG test signals
of PANI/PAA/PVA hydrogel and commercial ECG electrode.
Author Contributions
Conceptualization, H.Z.; methodology, B.W.
and J.L.; validation, B.W., Z.Z., J.L. and H.Z.; formal analysis, H.Z.;
investigation, B.W., Z.Z. and H.Z.; resources, H.Z.; data curation, J.L. and
B.W.; writing—original draft preparation, B.W.; writing—review and editing,
H.Z., B.L. and N.L.; visualization, B.W.; supervision, H.Z. and B.L.; project
administration, H.Z., N.L. and B.L.; funding acquisition, H.Z. All authors have
read and agreed to the published version of the manuscript.
Funding
This study was supported by the Fundamental Research Funds for the Central Universities (DUT24YG150). The authors acknowledge the assistance of DUT Instrumental Analysis Center.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, J.; Cui, Y.; Li, W. Research progress of flexible wearable pressure sensors. Sens. Actuators A Phys. 2021, 330, 112838. [Google Scholar] [CrossRef]
- Feiner, R.; Dvir, T. Tissue–electronics interfaces: From implantable devices to engineered tissues. Nat. Rev. Mater. 2017, 3, 17076. [Google Scholar] [CrossRef]
- Chen, G.; Xiao, X.; Zhao, X.; Tat, T.; Bick, M.; Chen, J. Electronic Textiles for Wearable Point-of-Care Systems. Chem. Rev. 2021, 122, 3259–3291. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Z.; Deng, Z.; Du, Z.; Sun, T.L.; Guo, Z.; Yue, K. A Transparent, Highly Stretchable, Solvent-Resistant, Recyclable Multifunctional Ionogel with Underwater Self-Healing and Adhesion for Reliable Strain Sensors. Adv. Mater. 2021, 33, 2105306. [Google Scholar] [CrossRef]
- Han, Y.; Dai, L. Conducting Polymers for Flexible Supercapacitors. Macromol. Chem. Phys. 2019, 220, 1800355. [Google Scholar] [CrossRef]
- Shi, Y.; Fu, X.; Wang, W.; Yu, D. Stretchable, adhesive and low impedance hydrogel prepared by one-pot method used as ECG electrodes. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130998. [Google Scholar] [CrossRef]
- Hsieh, J.-C.; Alawieh, H.; Li, Y.; Iwane, F.; Zhao, L.; Anderson, R.; Abdullah, S.I.; Tang, K.W.K.; Wang, W.; Pyatnitskiy, I.; et al. A highly stable electrode with low electrode-skin impedance for wearable brain-computer interface. Biosens. Bioelectron. 2022, 218, 114756. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Li, H.; Huo, P.; Teng, P.; Ding, H.; Shen, X. Recent progress in fabrications, properties and applications of multifunctional conductive hydrogels. Eur. Polym. J. 2024, 208, 112895. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Hernandez, H.L.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, W.; Yang, Z.; Wu, Y.; Pi, F. An Overview on Recent Progress of the Hydrogels: From Material Resources, Properties, to Functional Applications. Macromol. Rapid Commun. 2022, 43, 2100785. [Google Scholar] [CrossRef]
- Nele, V.; Wojciechowski, J.P.; Armstrong, J.P.; Stevens, M.M. Tailoring Gelation Mechanisms for Advanced Hydrogel Applications. Adv. Funct. Mater. 2020, 30, 2002759. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, J.; Wang, T.; Peng, X.; Liu, J.; Wang, X.; Wang, J.; Zeng, H. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2020, 2, 843–865. [Google Scholar] [CrossRef]
- Zhao, Y.; Ohm, Y.; Liao, J.; Luo, Y.; Cheng, H.-Y.; Won, P.; Roberts, P.; Carneiro, M.R.; Islam, M.F.; Ahn, J.H.; et al. A self-healing electrically conductive organogel composite. Nat. Electron. 2023, 6, 206–215. [Google Scholar] [CrossRef]
- Yang, X.; Sun, M.; Bian, Y.; He, X. A Room-Temperature High-Conductivity Metal Printing Paradigm with Visible-Light Projection Lithography. Adv. Funct. Mater. 2019, 29, 1807615. [Google Scholar] [CrossRef]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.K.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Zhang, L.; Ma, L.; Chen, J.; Wang, Y. Dual-stimuli sensitive composites based on multi-walled carbon nanotubes and poly(N,N-diethylacrylamide-co-acrylic acid) hydrogels. React. Funct. Polym. 2010, 70, 294–300. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, M.W.; Woo, S.H. Enhanced mechanical strength of chitosan hydrogel beads by impregnation with carbon nanotubes. Carbon 2009, 47, 2933–2936. [Google Scholar] [CrossRef]
- Xiao, Y.; He, L.; Che, J. An effective approach for the fabrication of reinforced composite hydrogel engineered with SWNTs, polypyrrole and PEGDA hydrogel. J. Mater. Chem. 2012, 22, 8076–8082. [Google Scholar] [CrossRef]
- Wei, H.; Kong, D.; Li, T.; Xue, Q.; Wang, S.; Cui, D.; Huang, Y.; Wang, L.; Hu, S.; Wan, T.; et al. Solution-Processable Conductive Composite Hydrogels with Multiple Synergetic Networks toward Wearable Pressure/Strain Sensors. ACS Sens. 2021, 6, 2938–2951. [Google Scholar] [CrossRef]
- Shi, W.; Han, G.; Chang, Y.; Song, H.; Hou, W.; Chen, Q. Using Stretchable PPy@PVA Composites as a High-Sensitivity Strain Sensor To Monitor Minute Motion. ACS Appl. Mater. Interfaces 2020, 12, 45373–45382. [Google Scholar] [CrossRef]
- Wang, T.; Xu, B.; Yu, T.; Yu, Y.; Fu, J.; Wang, Y.; Gao, X.; Xue, Z.; Li, R.; Chang, G. PVA/chitosan-based multifunctional hydrogels constructed through multi-bonding synergies and their application in flexible sensors. Carbohydr. Polym. 2024, 350, 123034. [Google Scholar] [CrossRef]
- Li, S.; Cong, Y.; Fu, J. Tissue adhesive hydrogel bioelectronics. J. Mater. Chem. B 2021, 9, 4423–4443. [Google Scholar] [CrossRef]
- Wang, D.; Qin, L.; Yang, W.; He, Y.; Zhang, S.; Yang, Y.; Xu, K.; Gao, P.; Yu, J.; Cai, K. A Conductive Hydrogel Based on GaIn and PVA/PAA/Fe 3+ for Strain Sensor and Physiological Signal Detection. ACS Appl. Polym. Mater. 2021, 3, 5268–5276. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, G.; Zhou, L. Preparing Titanium Based Electrically Conductive Hydrogel Composite Coating Material Useful for Regeneration, Repair and Integration of Cardiac Muscle, Nerve Tissue and Bones, Comprises e.g., Processing Titanium or Its Alloy, and Depositing. CN105543924-A, CN105543924-B [P/OL], 06 May 2016. [Google Scholar]
- Fu, L.; Yu, A.; Lai, G. Conductive Hydrogel-Based Electrochemical Sensor: A Soft Platform for Capturing Analyte. Chemosensors 2021, 9, 282. [Google Scholar] [CrossRef]
- Dalrymple, A.N.; Robles, A.U.; Huynh, M.; Nayagam, A.B.; Green, A.R.; Poole-Warren, A.L.; Fallon, J.B.; Shepherd, R.K. Electrochemical and biological performance of chronically stimulated conductive hydrogel electrodes. J. Neural Eng. 2020, 17, 026018. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Korin, E.; Soifer, L.; Bettelheim, A. Ion-Conductive and Transparent Resorcinol-Formaldehyde Hydrogels for Electrochemical and Solar Applications. Electrochem. Solid State Lett. 2012, 15, F1. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Lai, J.; Yan, B.; Liu, H.; Jin, X.; Ma, A.; Zhang, G.; Zhao, W.; Chen, W. Extremely stretchable and electrically conductive hydrogels with dually synergistic networks for wearable strain sensors. J. Mater. Chem. C 2018, 6, 9200–9207. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Li, Y.; Wang, X.; Yang, W.; Ren, J. Polypyrrole-Doped Conductive Self-Healing Composite Hydrogels with High Toughness and Stretchability. Biomacromolecules 2021, 22, 1273–1281. [Google Scholar] [CrossRef]
- Zahid, M.; Zych, A.; Dussoni, S.; Spallanzani, G.; Donno, R.; Maggiali, M.; Athanassiou, A. Wearable and self-healable textile-based strain sensors to monitor human muscular activities. Compos. Part B Eng. 2021, 220, 108969. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef]
- Won, D.; Kim, J.; Choi, J.; Kim, H.; Han, S.; Ha, I.; Bang, J.; Kim, K.K.; Lee, Y.; Kim, T.-S.; et al. Digital selective transformation and patterning of highly conductive hydrogel bioelectronics by laser-induced phase separation. Sci. Adv. 2022, 8, eabo3209. [Google Scholar] [CrossRef]
- Gao, Q.; Li, C.; Wang, M.; Zhu, J.; Gao, C. A low-hysteresis, self-adhesive and conductive PAA/PEDOT:PSS hydrogel enabled body-conformable electronics. J. Mater. Chem. C 2023, 11, 9355–9365. [Google Scholar] [CrossRef]
- Zarrin, N.; Tavanai, H.; Abdolmaleki, A.; Bazarganipour, M.; Alihosseini, F. An investigation on the fabrication of conductive polyethylene dioxythiophene (PEDOT) nanofibers through electrospinning. Synth. Met. 2018, 244, 143–149. [Google Scholar] [CrossRef]
- Ramadhoni, B.; Ichsan, M.Z.N. Synthesis and application of PEDOT/PAA as a conductive binder for silicon anode of lithium-ion battery. AIP Conf. Proc. 2023, 2902, 050004. [Google Scholar]
- Peng, Y.; Tang, S.; Wang, X.; Ran, R. A High Strength Hydrogel with a Core–Shell Structure Simultaneously Serving as Strain Sensor and Solar Water Evaporator. Macromol. Mater. Eng. 2021, 306, 2100309. [Google Scholar] [CrossRef]
- Ren, J.; Woo, Y.C.; Yao, M.; Tijing, L.D.; Shon, H.K. Enhancement of nanoscale zero-valent iron immobilization onto electrospun polymeric nanofiber mats for groundwater remediation. Process. Saf. Environ. Prot. 2017, 112, 200–208. [Google Scholar] [CrossRef][Green Version]
- Hryniewicz, B.M.; Winnischofer, H.; Vidotti, M. Interfacial characterization and supercapacitive behavior of PEDOT nanotubes modified electrodes. J. Electroanal. Chem. 2018, 823, 573–579. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Yin, Q.; Jiang, B. Tuning thermoelectric performance of Poly(3,4-ethylenedioxythiophene): Poly (styrene sulfonate)/Polyaniline composite films by nanostructure evolution of polyaniline. Polym. Test. 2021, 94, 107017. [Google Scholar] [CrossRef]
- Chen, K.; Chen, G.; Wei, S.; Yang, X.; Zhang, D.; Xu, L. Preparation and property of high strength and low friction PVA-HA/PAA composite hydrogel using annealing treatment. Mater. Sci. Eng. C 2018, 91, 579–588. [Google Scholar] [CrossRef]
- Khan, M.A.; Armes, S.P.; Perruchot, C.; Ouamara, H.; Chehimi, M.M.; Greaves, S.J.; Watts, J.F. Surface Characterization of Poly(3,4-ethylenedioxythiophene)-Coated Latexes by X-ray Photoelectron Spectroscopy. Langmuir 2000, 16, 4171–4179. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Pfattner, R.; Yan, H.; Jin, L.; Chen, S.; Molina-Lopez, F.; Lissel, F.; Liu, J.; Rabiah, N.I.; et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 2017, 3, e1602076. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yang, M.; Yang, T.; Xu, C.; Ye, Y.; Wu, X.; Zheng, X.; Wang, B.; Wan, Y.; Luo, Z. Highly Conductive PPy–PEDOT:PSS Hybrid Hydrogel with Superior Biocompatibility for Bioelectronics Application. ACS Appl. Mater. Interfaces 2021, 13, 25374–25382. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Guan, S.; Dong, X.; Huang, H.; Qi, M. Ultratough and Freezing-Tolerant PVA–PAA-PANI Hybrid Hydrogel for Supercapacitors and Flexible Sensors. ACS Sustain. Chem. Eng. 2023, 11, 14886–14894. [Google Scholar] [CrossRef]
- Dong, X.; Ge, Y.; Li, K.; Li, X.; Liu, Y.; Xu, D.; Wang, S.; Gu, X. A high-pressure resistant ternary network hydrogel based flexible strain sensor with a uniaxially oriented porous structure toward gait detection. Soft Matter 2022, 18, 9231–9241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).