Abstract
Heart rate variability (HRV) is the physiological variation in the intervals between consecutive heartbeats that reflects the activity of the autonomic nervous system. This parameter is traditionally evaluated based on electrocardiograms (ECG signals). Seismocardiography (SCG) and/or gyrocardiography (GCG) are used to monitor cardiac mechanical activity; therefore, they may be used in HRV analysis and the evaluation of valvular heart diseases (VHDs) simultaneously. The purpose of this study was to compare the time domain, frequency domain and nonlinear HRV indices obtained from electrocardiograms, seismocardiograms (SCG signals) and gyrocardiograms (GCG signals) in healthy volunteers and patients with valvular heart diseases. An analysis of the time domain, frequency domain and nonlinear heart rate variability was conducted on electrocardiograms and gyrocardiograms registered from 29 healthy male volunteers and 30 patients with valvular heart diseases admitted to the Columbia University Medical Center (New York City, NY, USA). The results of the HRV analysis show a strong linear correlation with the HRV indices calculated from the ECG, SCG and GCG signals and prove the feasibility and reliability of HRV analysis despite the influence of VHDs on the SCG and GCG waveforms.
1. Introduction
Cardiovascular diseases remain the most common cause of death in the world and constitute a significant concern for public health due to the economic burden (expected to reach 47 trillion USD by 2030) and the overload on medical personnel (17.9 million deaths worldwide in 2016) despite progress in prevention, diagnosis and therapy [1,2,3,4,5]. Due to its growing prevalence and significant impact on quality of life [6], we consider valvular heart disease (VHD) in this study.
Valvular heart disease is any cardiovascular disease that affects any heart valve (the aortic valve, mitral valve, pulmonic valve and tricupsid valve) [7,8]. The main causes of VHDs are rheumatic heart disease and ageing [6,8,9,10]. The most prevalent VHD is aortic stenosis (AS), which is the third most common cardiovascular disease after hypertension and coronary artery disease, and is usually caused either by degenerative calcification of the aortic valve or progressive stenosis of a congenital bicuspid valve [8].
VHDs are usually diagnosed by echocardiography, computed tomography or magnetic resonance imaging [9], which are not feasible in outpatient monitoring [11]. This problem has been addressed by applying various methods [12,13], including exercise electrocardiography (ECG) [14], seismocardiography (SCG) or gyrocardiography (GCG), which register the current mechanical function of the heart with an inertial measurement unit (IMU) placed on the chest wall [11,15,16].
Seismocardiography (SCG) and gyrocardiography (GCG) are two complementary techniques [15,17,18]; seismocardiography is a technique for registering low-frequency precordial acceleration, invented by Bozhenko in 1961 [19,20], whereas gyrocardiography registers the rotational component of cardiac vibrations and was invented by Meriheinä et al. in 2015 [15,16,18,21,22].
Seismocardiographic (SCG) and gyrocardiographic (GCG) signals are non-stationary signals with distinct quasiperiodic features known as waves, e.g., the mitral valve opening wave (MO), the mitral valve closure wave (MC), the isovolumetric contraction wave, the rapid ejection wave, the aortic valve opening wave (AO), the aortic valve closure (AC) wave and the cardiac filling wave [18,22,23,24,25,26,27,28]. Figure 1 presents an annotation of concurrent ECG, SCG and GCG signals in a healthy subject.
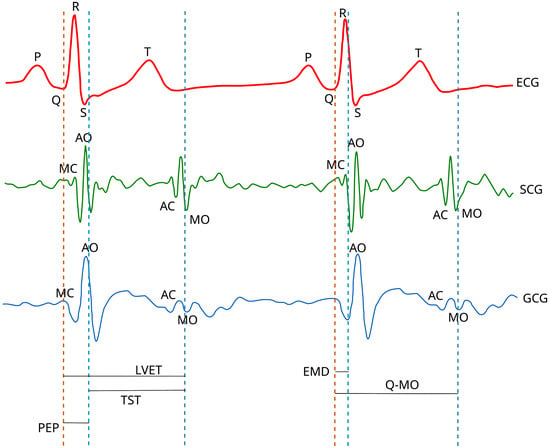
Figure 1.
The annotation of ECG, SCG and GCG waveforms in healthy subjects. Based on the diagrams published in [15,18,26,29] under the CC-BY 4.0 license.
SCG and GCG have found applications in the diagnosis of several cardiovascular diseases, such as aortic stenosis [30,31,32], aortic valve disease (AVD) [33], coronary artery disease [34], myocardial infarction [35,36], atrial fibrillation [37,38,39,40], the effects of cardiac resynchronization therapy [41] and heart failure [35,42]. This has usually involved the use of computational intelligence techniques, such as artificial neural networks (including deep and convolutional neural networks), random forests, extreme gradient boosting and support vector machines [28,30,32,35,36,37,38,39,40].
One of the most prominent applications of seismocardiography and gyrocardiography is heart rate variability (HRV) analysis [16,18,22,31,43,44,45,46,47,48,49,50,51,52,53,54,55]. Heart rate variability is defined as the physiological variation in the intervals between consecutive heartbeats (inter-beat interval) and reflects the activity of the autonomic nervous system [56,57,58].
HRV analysis has traditionally been performed on interbeat intervals obtained from electrocardiograms (ECG signals) [22,43,45,50,51,55,56,59]. The first attempt of HRV analysis based on cardiac mechanical signals (mechanocardiograms) was performed by Friedrich et al. in 2010 [60] on ballistocardiograms. In 2012, Ramos-Castro et al. performed the first HRV analysis on seismocardiograms [43], and the first HRV analysis on gyrocardiograms was performed by Lahdenoja et al. [61] in 2016. The validity of HRV indices obtained from the SCG signal was first demonstrated by Laurin et al. [62] in 2013 and then in later studies [22,45,46,51,52,53,54,55].
The advantages of using seismocardiography and/or gyrocardiography over electrocardiography for cardiac diagnosis are the simpler measurement setup (using only one sensor) and the availability of information on cardiac intervals, contractility and the state of heart valves at the same time [11,16,22,27,43,44,45,51,53,56,59,63]. However, the limitations of SCG and GCG include the inter-subject variability of signal morphology that can be significantly affected by cardiac diseases or sensor placement and susceptibility to motion artifacts [11,16,23,27,55,64].
The purpose of this study is to evaluate the differences between the time domain, frequency domain and nonlinear HRV indices derived from electrocardiograms, seismocardiograms and gyrocardiograms in healthy volunteers and patients with valvular heart diseases. This study is an extended version of [54] and is based on two publicly available datasets obtained from healthy people and patients with VHDs.
2. Materials and Methods
2.1. Datasets
This study was carried out on two publicly available datasets with concurrent electrocardiograms, seismocardiograms and gyrocardiograms. The first dataset if “Mechanocardiograms with ECG reference” published by M. Kaisti et al. [65,66] containing signals acquired from twenty-nine healthy volunteers and the second contains thirty signals derived from “An Open-access Database for the Evaluation of Cardio-mechanical Signals from Patients with Valvular Heart Diseases” published by C. Yang et al. in [11,67].
The first dataset consists of 29 recordings of concurrent ECG, SCG and GCG signals acquired from 29 healthy male volunteers that were registered with sensors attached to the chest wall over the sternum with a double-sided tape and with a sampling frequency of 800 Hz. The subjects were lying either in the supine position or on their left or right side [61,65,66].
Electrocardiograms were acquired with an ADS1293 (Texas Instruments, Dallas, TX, USA), seismocardiograms were recorded with a triaxial capacitive digital accelerometer (MMA8451Q from Freescale Semiconductor, Austin, TX, USA) and gyrocardiograms were acquired using a 3-axial MAX21000 gyroscope (Maxim Integrated, San Jose, CA, USA) [15,65].
The rotation and translation axes in seismocardiography and gyrocardiography were defined for both datasets as follows: the x axis was oriented laterally from left to right, the y axis was oriented from head to foot and the z axis was oriented from back to chest [65].
The second dataset consists of 100 simultaneous recordings of raw ECG, SCG and GCG signals with annotated heartbeats acquired from 100 patients with valvular diseases admitted to two different clinical sites: 30 patients were admitted to Columbia University Medical Center (New York City, NY, USA) and 70 patients were admitted to the First Affiliated Hospital of Nanjing Medical University (Nanjing, Jiangsu Province, People’s Republic of China). ECG, SCG and GCG signals were recorded before any treatment in both populations of patients [11].
To balance the number of healthy volunteers and patients with valvular heart disease in this comparison study, we took only 30 patients (14 female and 16 male subjects) who were admitted to the Columbia University Medical Center (New York City, NY, USA). A total of thirty patients had aortic stenosis, nine patients had tricupsid valve regurgitation (TR), five had mitral valve stenosis (MS), four had mitral valve regurgitation (MR) and no patients had aortic valve regurgitation. During registration, each subject was asked to be awake and stay in the supine position, breathing normally.
The ECG, SCG and GCG signals were recorded with Shimmer 3 ECG module (Shimmer Sensing, Dublin, Ireland) with a sampling frequency of 256 Hz (recordings UP-01 to UP-21) and 512 Hz (recordings UP-22 to UP-30) [11,67]. The shimmer 3 device contains a 3-axial inertial measurement unit that contains an accelerometer, a gyroscope and a magnetometer (ICM-20948 from TDK InvenSense, San Jose, CA, USA) and a separate low-noise 3-axial Kionix KXTC9-2050 accelerometer (Kionix, Inc., Ithaca, NY, USA) [68]. Before the measurements, each subject gave informed consent by signing a consent form. All metadata were deidentified before publication [11,67].
The basic characteristics of both datasets are shown in Table 1. Figure 2 and Figure 3 present a 15-second and 16-second fragment of raw ECG, SCG and GCG signals in subject 10 from the first dataset and UP-13 of the second dataset, respectively. More details are revealed in Appendix A.

Table 1.
Basic characteristics of the datasets. Aggregated values are expressed as the range (min–max) and mean ± SD.
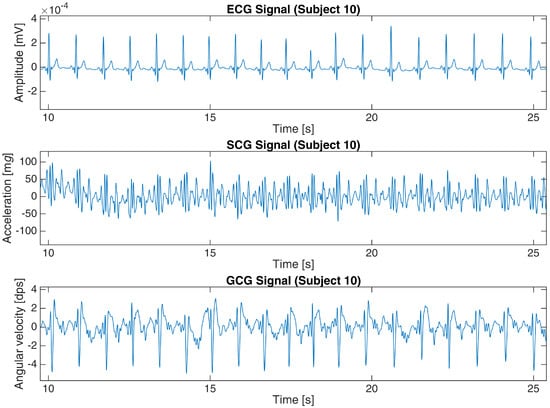
Figure 2.
ECG, SCG and GCG signals from subject 10 in the first dataset (25-s fragment).
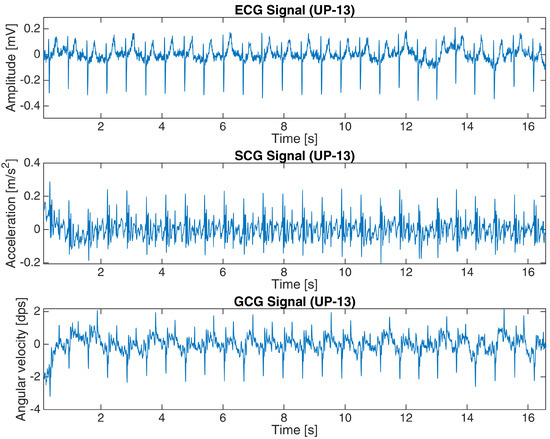
Figure 3.
ECG, SCG and GCG signals from subject UP-13 in the second dataset (16-s fragment).
2.2. Signal Processing
Signal processing started with importing the data into MATLAB R2022b (MathWorks, Inc., Natick, MA, USA); data from [67] were directly loaded into the MATLAB workspace, while data from [66] required importing each line of the text files containing signal samples for subjects 1–8 and discarding the first sample (an artifact) for subjects 9–29. In both datasets, each file represented one subject [66,67].
Heartbeat detection in SCG signals and GCG signals for both datasets was based on the approach presented in [11,22,45,53,54,69], which consists of the following steps: The first step was to apply the Pan–Tompkins algorithm (introduced in [70]) to ECG signals. The next step was to find local maxima in the SCG and GCG signals within 100 ms of the closest R waves in the ECG signals based on the observations published in [45,71], and the final step was to calculate the intervals between each consecutive heartbeat in the ECG, SCG and GCG signals [11,69]. An example of a tachogram for a healthy subject is shown in Figure 4, and Figure 5 presents a tachogram of a patient with VHD.
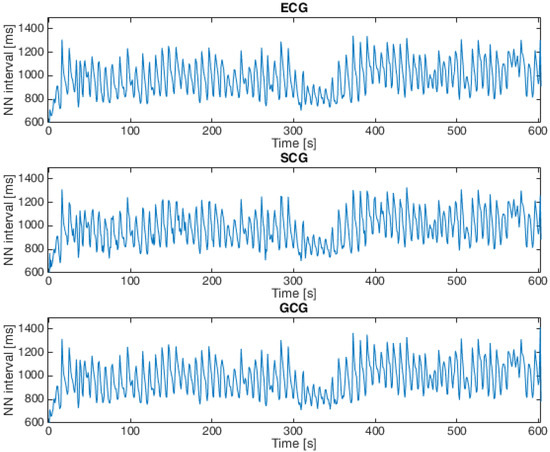
Figure 4.
Tachogram derived from ECG, SCG and GCG signals taken from subject 9 in the first dataset (15-second fragment).
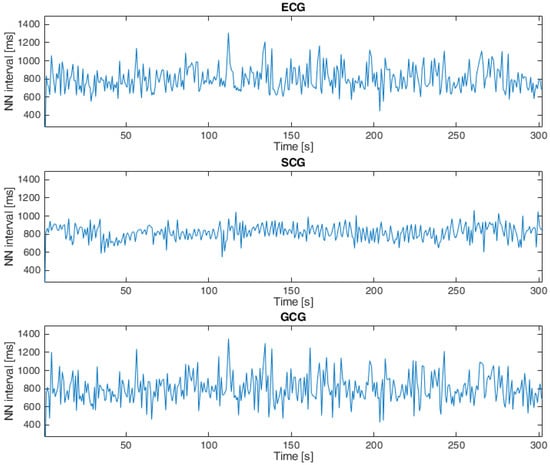
Figure 5.
Tachogram ECG, SCG and GCG signals taken from subject UP-09 in the second dataset.
Local maxima that occur within 100 ms of the R wave in concurrent ECG signals are associated with the aortic valve opening waves that are single sharp peaks on the z-axis of the SCG signal and the y-axis of the GCG signal [15,18,20,45,71]. Taking into account only the z-axis of the SCG signal and the y-axis of the GCG signal for analyses was based on the higher signal-to-noise ratio compared to the other axes [15,22,23,65].
2.3. HRV Analysis
HRV analysis was carried out according to the recommendations published in [56,59] and consisted of the following time and frequency domain indices: mean interbeat interval (AVNN); standard deviation of the interbeat interval (SDNN); root mean square of the differences of successive interbeat intervals (RMSSD); the ratio of successive differences greater than 50 ms in all interbeat intervals (RMSSD); the power of the HRV signal in the very low-frequency band (VLF), in the low-frequency band (LF) and in the high-frequency band (HF); and the LF/HF ratio (LF/HF).
The frequency bands of the HRV spectrum were defined as follows: the very low-frequency band was defined as 0.0033–0.04 Hz, the low frequency band was defined as 0.04–0.15 Hz and the high-frequency band was defined as 0.15–0.4 Hz [56,72]. The analyses were performed with the CardioNet Cardiovascular Signal Toolbox and MATLAB R2022b. HRV indices in the frequency domain were based on spectral power estimates calculated as 1024-sample Lomb periodograms [72,73]. The Lomb periodogram for frequency is expressed as:
where is the time delay defined in Equation (2), is the value of the j-th sample and is the time of the j-th sample [74,75].
The non-linear analysis of heart rate variability was based on three indices derived from the geometrical features of Poincaré maps: SD1, SD2 and SD1/SD2.
SD1 is a measure of the short-term heart rate variability that is defined as the width of an ellipse fitted to scatter points of a Poincaré map and may be expressed as the standard deviation of the distances from the identity line ( axis) of the Poincaré plot [76,77]:
where is the i-th inter-beat interval series for , is the next inter-beat interval and denotes the standard deviation (SD) [78,79,80,81].
SD2 is the length of an ellipse fitted to the scatter points of a Poincaré map that reflects the long-term heart rate variability and is calculated as the standard deviation of the distance of points from the axis:
SD1/SD2 is calculated as the ratio between SD1 and SD2 and reflects the unpredictability of the heart rate [82].
3. Results
The results of HRV analyses on electrocardiograms, seismocardiograms and gyrocardiograms obtained from healthy volunteers and patients with VHDs were expressed as the mean and standard deviation (SD) values and are shown in Table 2, Table 3 and Table 4, respectively. The HRV indices calculated for patients with VHD were derived from [53], except for SD1, SD2 and SD1/SD2.

Table 2.
HRV indices derived from ECG signals from both datasets.

Table 3.
HRV indices derived from SCG signals from both datasets.

Table 4.
HRV indices derived from GCG signals from both datasets.
The mean and standard deviation values of most HRV indices for patients with VHD are significantly different from those of healthy volunteers, except for AVNN and VLF. These differences were further evaluated by applying the Student’s t-test for the significance level of 0.05. The results of the t-test are shown in Table 5.

Table 5.
Results of Student’s t-tests between healthy subjects and VHD subjects.
The differences between the HRV indices in healthy volunteers and in patients with VHD shown in Table 5 are statistically significant for all analyzed HRV indices except for AVNN, SDNN, pNN50 (ECG), VLF (GCG), LF and SD2. This proves a significant influence of ventricular heart diseases on the results of heart rate variability in time domain, frequency domain and nonlinear analyses. These results confirm the findings related to Table 3.
The findings reported in [17,22,45,51] for HRV indices obtained from ECG, SCG and GCG signals in healthy volunteers and patients with VHDs were verified with the Pearson’s linear correlation that were expressed as Pearson’s linear correlation coefficient () for healthy volunteers and VHD patients. A linear correlation coefficient larger than 0.7 was considered as a strong linear correlation between two given datasets [83].
The results presented in Table 6 indicate a strong linear correlation for the p-value under 0.001, except for SD1/SD2 between ECG and SCG signals from VHD patients. The correlation between the analyzed HRV indices obtained from ECG and GCG signals (as shown in Table 7) is weaker than between the ECG and SCG signals, but remains strong for all HRV indices except for VLF, SD2 and SD1/SD2. The strongest correlation is observed for HF, pNN50, RMSSD and SD1, and the weakest correlation is observed for VLF (−0.0663).

Table 6.
Pearson’s linear correlation coefficient of HRV indices obtained from ECG and SCG signals.

Table 7.
Pearson’s linear correlation coefficient of HRV indices obtained from ECG and GCG signals.
4. Discussion
We have performed HRV analysis on electrocardiograms, seismocardiograms and gyrocardiograms from healthy volunteers and patients with VHD based on publicly available datasets.
The mean and standard deviation values of HRV indices obtained from healthy subjects are similar to those reported by Siecinski et al. in [22,50,52,53], except for the LF/HF and frequency domain indices in [22,52], and also similar to the results reported by Ramos-Castro et al. in [43] and Tadi et al. in [45]. The discrepancies in the mean HRV indices are within the standard deviation for each signal (ECG, SCG and GCG) and may be related to inter-subject variations.
The mean and standard deviation values of most HRV indices for patients with VHD are significantly different from those of healthy volunteers, except for AVNN, LF and SD2. This observation was confirmed by a Student’s t-test. Despite the fact that RMSSD and SD1 should be identical [76], there were significant differences between these indices in each case.
We have shown the significant influence of valvular heart disease on HRV indices, except for AVNN, SDNN (ECG and SCG), pNN50 (ECG), VLF (GCG), LF, HF (ECG) and SD2, which was in line with [84,85]. The similarities between the results of the HRV analysis in patients with VHD in our study and those reported in [84] prove that the HRV indices obtained from seismocardiograms are valid both for healthy subjects, patients with aortic stenosis and also for other VHD patients as long as heartbeats were reliably detected [45,53,54,62].
Despite the significant influence of VHDs on HRV indices, the correlation between the HRV indices obtained from ECG and SCG signals is strong, except for SD2. Obtained values of are similar to those reported by Siecinski et al. in [50] and Charlier et al. in [86]. However, the correlations of analyzed pairs of signals are weaker for VHD patients than for healthy volunteers, especially the correlations of HRV indices from ECG and GCG signals [22,43,45,52].
Such results are influenced by age (the population of healthy volunteers is significantly younger than those of patients with VHD), comorbidities, signal quality and the use of different accelerometers and gyroscopes operating with different sampling frequency and accuracy, according to the available datasheets [68,87,88].
The differences between RMSSD and SD1 values did not result in significantly different values. This indicates the lower accuracy of automatic heartbeat detection in SCG and GCG signals of patients with VHDs that was affected by morphological changes caused by VHDs and/or ageing [53,54].
The limitations of the study include the use of only one type of heartbeat detector for seismocardiograms and gyrocardiograms that depends on a concurrent electrocardiogram, the influence of specific cardiovascular conditions or medication on the calculated HRV indices was not considered and the morphological changes in SCG and GCG signals due to valvular heart disease was not evaluated.
In future studies, we will consider evaluating the influence of various cardiovascular conditions on HRV indices derived from ECG, SCG and GCG signals; indices derived from larger and more diverse groups, including the analysis of SCG and GCG signal morphology; and indices derived from other detectors of SCG and GCG signals. In this study, we proved that a heart rate variability analysis based on cardiac mechanical signals [30,31,89] may be useful for a more cost-effective and convenient diagnosis and monitoring of patients with cardiovascular disease.
5. Conclusions
The results of the heart rate variability analysis based on mechanocardiograms (SCG and GCG signals) in both a healthy population and patients with VHD remain valid as long as heartbeats are correctly detected. Valvular heart disease significantly affects RMSSD, pNN50 (only SCG and GCG signals), VLF (only ECG and SCG signals), HF (only SCG and GCG signals), LF/HF, SD1 and SD1/SD2. The linear correlation between the HRV indices obtained from the ECG and the mechanocardiograms is strong both in healthy volunteers and in patients with VHD, except for SD2.
Future studies should include an evaluation of other cardiovascular conditions, larger and more diverse groups and other heartbeat detection methods for mechanocardiograms, and an analysis of the morphology of cardiac mechanical signals. We showed that mechanocardiogram-based heart rate variability analyses can be used in the diagnosis and monitoring of cardiovascular disease, which could be more cost-effective and convenient for patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/s23042152/s1.
Author Contributions
Conceptualization, S.S.; methodology, S.S.; software, S.S.; validation, S.S. and P.S.K.; formal analysis, S.S.; investigation, S.S.; resources, S.S.; data curation, S.S.; writing—original draft preparation, S.S.; writing—review and editing, S.S., E.J.T. and P.S.K.; visualization, S.S.; supervision, E.J.T. and P.S.K.; project administration, E.J.T. and P.S.K.; funding acquisition, E.J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The research was based on publicly available data published in [66,67]. Supplementary Data (source code and full results) are available online. However, the data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VHD | Valvular heart disease |
| HRV | Heart rate variability |
| ECG | Electrocardiography, electrocardiogram |
| SCG | Seismocardiography |
| GCG | Gyrocardiography |
| MEMS | Microelectromechanical systems |
| MRI | Magnetic resonance imaging |
| EMD | Empirical mode decomposition |
| CABG | Coronary artery bypass graft surgery |
| F | Female |
| MR | Mitral valve regurgitation |
| MS | Mitral valve stenosis |
| M | Male |
| MI | Myocardial infarction |
| NN | The interval between consecutive normal heartbeats |
| FIR | Fininte impulse response (filter) |
| AO | Aortic valve opening (wave) |
| RSA | Respiratory sinus arrhythmia |
| SNR | Signal-to-noise (ratio) |
| AVNN | Mean inter-beat interval |
| SDNN | Standard deviation of all interbeat intervals |
| RMSSD | Root mean square of differences (RMSSD) of successive inter-beat intervals |
| pNN50 | The proportion of the number of pairs of successive differences greater than 50 ms divided by total number of normal inter-beat intervals |
| VLF | The power of very low frequency band (0.0033–0.04 Hz) of HRV spectrum |
| LF | The power of low frequency band (0.04–0.15 Hz) of HRV spectrum |
| HF | The power of high frequency band (0.15–0.4 Hz) of HRV spectrum |
| LF/HF | LF/HF ratio |
| The width of the ellipse which containes the scatter points of Poincaré map | |
| The length of the ellipse which containes the scatter points of Poincaré map | |
| to ratio | |
| AVD | Aortic valve disease |
| AC | Aortic valve closure |
| AO | Aortic valve opening |
| MC | Mitral valve closure |
| MO | Mitral valve opening |
| PCI | Percutaneous coronary intervention |
| AS | Aortic valve stenosis |
| AR | Aortic valve regurgitation |
| TR | Tricupsid valve regurgitation |
| Pearson’s linear correlation coefficient |
Appendix A. Recording Descriptions in Datasets
This appendix presents a complete description of the recordings in both analyzed datasets based on available metadata [66,67]; the recordings from the healthy volunteers dataset (Mechanocardiograms with ECG reference) are shown in Table A1, while the full description of the recordings in the VHD patients dataset is presented in Table A2.

Table A1.
Recording description in the “Mechanocardiograms with ECG reference” dataset.
Table A1.
Recording description in the “Mechanocardiograms with ECG reference” dataset.
| Subject | Length of Recording | Position | Breathing | Remarks |
|---|---|---|---|---|
| 1 | 3 min | Left or right side | 2 min normal, | |
| 30 s holding a breath, | ||||
| 30 s normal | ||||
| 2 | 3 min | Left or right side | 2 min normal, | |
| 30 s holding a breath, | ||||
| 30 s normal | ||||
| 3 | 3 min | Left or right side | 2 min normal, | |
| 30 s holding a breath, | ||||
| 30 s normal | ||||
| 4 | 3 min | Left or right side | 2 min normal, | |
| 30 s holding a breath, | ||||
| 30 s normal | ||||
| 5 | 3 min | Left or right side | 2 min normal, | |
| 30 s holding a breath, | ||||
| 30 s normal | ||||
| 6 | 3 min | Left or right side | 2 min normal, | Sensor not strictly secured |
| 30 s holding a breath, | on chest because of body hair. | |||
| 30 s normal | ||||
| 7 | 3 min | Left or right side | 2 min normal, | |
| 30 s holding a breath, | ||||
| 30 s normal | ||||
| 8 | 3 min | Supine | Normal | |
| 9 | 10 min | Supine | Normal | |
| 10 | 10 min | Supine | Normal | |
| 11 | 30 min | Supine | Normal | |
| 12 | 10 min | Supine | Normal | |
| 13 | 10 min | Supine | Normal | |
| 14 | 10 min | Supine | Normal | |
| 15 | 10 min | Supine | Normal | |
| 16 | 10 min | Supine | Normal | |
| 17 | 10 min | Supine | Normal | |
| 18 | 10 min | Supine | Normal | |
| 19 | 10 min | Supine | Normal | |
| 20 | 10 min | Supine | Normal | |
| 21 | 10 min | Supine | Normal | |
| 22 | 10 min | Supine | Normal | Sensor loose in the end. |
| 23 | 10 min | Left or right side | Normal | |
| 24 | 10 min | Supine | Normal | |
| 25 | 9 min | Supine | Normal | |
| 26 | 10 min | Supine | Normal | |
| 27 | 10 min | Left or right side | Normal | |
| 28 | 10 min | Supine | Normal | |
| 29 | 10 min | Supine | Normal |

Table A2.
Recording description in “An Open-access Database for the Evaluation of Cardio-mechanical Signals from Patients with Valvular Heart Diseases” dataset.
Table A2.
Recording description in “An Open-access Database for the Evaluation of Cardio-mechanical Signals from Patients with Valvular Heart Diseases” dataset.
| Subject Number | Length of Recording | Age (Years) | Gender | Height (cm) | Weight (kg) | History of | MS | MR | AR | AS | TR | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI | CABG | PCI | |||||||||||
| UP-01 | 6 min 8 s | 89 | M | 154.9 | 49.0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| UP-02 | 6 min 10 s | 89 | M | 170.2 | 82.0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| UP-03 | 6 min 1 s | 96 | M | 162.5 | 66.0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| UP-04 | 5 min 36 s | 84 | M | 152.4 | 65.0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| UP-05 | 6 min 35 s | 70 | F | 162.5 | 79.0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| UP-06 | 5 min 39 s | 90 | F | 160 | 48.0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| UP-07 | 6 min 3 s | 84 | M | 162.5 | 79.0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| UP-08 | 5 min 11 s | 95 | F | 152.4 | 44.0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| UP-09 | 5 min 15 s | 89 | M | 182.8 | 90.7 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| UP-10 | 5 min 2 s | 80 | F | 157.4 | 74.0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| UP-11 | 5 min 6 s | 68 | M | 177.8 | 79.0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| UP-12 | 5 min 10 s | 79 | F | 154.9 | 78.0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| UP-13 | 5 min 18 s | 95 | F | 160 | 73.0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
| UP-14 | 5 min 38 s | 85 | F | 152 | 82.0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| UP-15 | 5 min 34 s | 84 | F | 175 | 76.0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| UP-16 | 5 min 44 s | 97 | M | 157 | 77.0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| UP-17 | 5 min 54 s | 80 | M | 182.8 | 86.0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| UP-18 | 5 min 9 s | 90 | F | 152.4 | 92.0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| UP-19 | 5 min 17 s | 78 | M | 170.1 | 78.0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| UP-20 | 7 min 49 s | 92 | F | 139.7 | 53.0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
| UP-21 | 5 min 10 s | 72 | M | 172.7 | 68.0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| UP-22 | 10 min 3 s | 77 | F | 165.1 | 51.3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| UP-23 | 9 min 59 s | 84 | F | 139.7 | 70.3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| UP-24 | 5 min | 80 | F | 155 | 67.6 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| UP-25 | 9 min 5 s | 87 | F | 155.0 | 54.0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| UP-26 | 5 min 8 s | 80 | M | 175.3 | 85.7 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| UP-27 | 5 min 5 s | 82 | M | 180.0 | 118.0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| UP-28 | 5 min 2 s | 71 | M | 175.0 | 117.0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| UP-29 | 4 min 58 s | 80 | M | 168.9 | 65.8 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| UP-30 | 4 min 59 s | 71 | M | 177.8 | 81.6 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
M: male; F: female; MI: myocardial infarction; CABG: coronary artery bypass graft surgery; PCI: percutaneous coronary intervention; MS: mitral valve stenosis; MR: mitral valve regurgitation; AS: aortic valve stenosis; AR: aortic valve regurgitation; TR: tricupsid valve regurgitation; The presence of the cardiac condition: 1—yes; 0—no.
References
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization. The Top 10 Causes of Death. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 3 February 2023).
- World Health Organization. World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2020; 77p. [Google Scholar]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Coffey, S.; Roberts-Thomson, R.; Brown, A.; Carapetis, J.; Chen, M.; Enriquez-Sarano, M.; Zühlke, L.; Prendergast, B.D. Global epidemiology of valvular heart disease. Nat. Rev. Cardiol. 2021, 18, 853–864. [Google Scholar] [CrossRef]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Maganti, K.; Rigolin, V.H.; Sarano, M.E.; Bonow, R.O. Valvular Heart Disease: Diagnosis and Management. Mayo Clin. Proc. 2010, 85, 483–500. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Chen, Z.; Wang, X.; Zhang, L.; Li, S.; Zheng, C.; Kang, Y.; Jiang, L.; Zhu, Z.; et al. Current status and etiology of valvular heart disease in China: A population-based survey. BMC Cardiovasc. Disord. 2021, 21, 339. [Google Scholar] [CrossRef]
- Yang, C.; Fan, F.; Aranoff, N.; Green, P.; Li, Y.; Liu, C.; Tavassolian, N. An Open-Access Database for the Evaluation of Cardio-Mechanical Signals From Patients with Valvular Heart Diseases. Front. Physiol. 2021, 12, 750221. [Google Scholar] [CrossRef]
- Alugubelli, N.; Abuissa, H.; Roka, A. Wearable Devices for Remote Monitoring of Heart Rate and Heart Rate Variability—What We Know and What Is Coming. Sensors 2022, 22, 8903. [Google Scholar] [CrossRef]
- Taoum, A.; Bisiaux, A.; Tilquin, F.; Le Guillou, Y.; Carrault, G. Validity of Ultra-Short-Term HRV Analysis Using PPG—A Preliminary Study. Sensors 2022, 22, 7995. [Google Scholar] [CrossRef]
- Glaveckaite, S.; Petrikonyte, D.; Latveniene, L.; Serpytis, P.; Laucevicius, A. Ocena choroby zastawkowej serca za pomocą elektrokardiografii wysiłkowej i echokardiografii obciążeniowej: Czy te badania są nadal potrzebne? Folia Cardiol. 2018, 13, 318–330. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Saraste, A.; Tuominen, J.; Koskinen, J.; Teräs, M.; Airaksinen, J.; Pänkäälä, M.; Koivisto, T. Gyrocardiography: A New Non-invasive Monitoring Method for the Assessment of Cardiac Mechanics and the Estimation of Hemodynamic Variables. Sci. Rep. 2017, 7, 6823. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Thakkar, H.K.; Rajput, S.S.; Santamaria, J.; Bhatt, C.; Roca, F. A Comprehensive Review on Seismocardiogram: Current Advancements on Acquisition, Annotation, and Applications. Mathematics 2021, 9, 2243. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Pankäälä, M.; Saraste, A.; Vasankari, T.; Terás, M.; Koivisto, T. Gyrocardiography: A new non-invasive approach in the study of mechanical motions of the heart. Concept, method and initial observations. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2034–2037. [Google Scholar] [CrossRef]
- Sieciński, S.; Kostka, P.S.; Tkacz, E.J. Gyrocardiography: A Review of the Definition, History, Waveform Description, and Applications. Sensors 2020, 20, 6675. [Google Scholar] [CrossRef] [PubMed]
- Bozhenko, B. Seismocardiography—A new method in the study of the functional condition of the heart. Ter. Arkhiv 1961, 33, 55–64. [Google Scholar]
- Zanetti, J.M.; Salerno, D.M. Seismocardiography: A technique for recording precordial acceleration. In Proceedings of the [1991] Computer-Based Medical Systems, Proceedings of the Fourth Annual IEEE Symposium, Baltimore, MD, USA, 12–14 May 1991; pp. 4–9. [Google Scholar] [CrossRef]
- Meriheinä, U.; Juppo, M.; Koivisto, T.; Mikko, P.; Sairanen, K.; Grönholm, M. Heart Monitoring System. WIPO Patent WO 2015/036925 A1, 19 March 2015. [Google Scholar]
- Sieciński, S.; Kostka, P.S.; Tkacz, E.J. Heart Rate Variability Analysis on Electrocardiograms, Seismocardiograms and Gyrocardiograms on Healthy Volunteers. Sensors 2020, 20, 4522. [Google Scholar] [CrossRef]
- Zanetti, J.M.; Tavakolian, K. Seismocardiography: Past, present and future. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 7004–7007. [Google Scholar] [CrossRef]
- Inan, O.T.; Migeotte, P.F.; Park, K.S.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G.K.; et al. Ballistocardiography and Seismocardiography: A Review of Recent Advances. IEEE J. Biomed. Health Inform. 2015, 19, 1414–1427. [Google Scholar] [CrossRef]
- Gurev, V.; Tavakolian, K.; Constantino, J.C.; Kaminska, B.; Blaber, A.P.; Trayanova, N. Mechanisms underlying isovolumic contraction and ejection peaks in seismocardiogram morphology. J. Med. Biol. Eng. 2012, 32, 103. [Google Scholar] [CrossRef]
- Dehkordi, P.; Khosrow-Khavar, F.; Di Rienzo, M.; Inan, O.T.; Schmidt, S.E.; Blaber, A.P.; Sørensen, K.; Struijk, J.J.; Zakeri, V.; Lombardi, P.; et al. Comparison of Different Methods for Estimating Cardiac Timings: A Comprehensive Multimodal Echocardiography Investigation. Front. Physiol. 2019, 10, 1057. [Google Scholar] [CrossRef]
- Santucci, F.; Lo Presti, D.; Massaroni, C.; Schena, E.; Setola, R. Precordial Vibrations: A Review of Wearable Systems, Signal Processing Techniques, and Main Applications. Sensors 2022, 22, 5805. [Google Scholar] [CrossRef]
- Wajdan, A.; Jahren, T.S.; Villegas-Martinez, M.; Khan, F.H.; Halvorsen, P.S.; Odland, H.H.; Elle, O.J.; Solberg, A.H.S.; Remme, E.W. Automatic Detection of Aortic Valve Events Using Deep Neural Networks on Cardiac Signals From Epicardially Placed Accelerometer. IEEE J. Biomed. Health Inform. 2022, 26, 4450–4461. [Google Scholar] [CrossRef]
- Taebi, A.; Solar, B.E.; Bomar, A.J.; Sandler, R.H.; Mansy, H.A. Recent Advances in Seismocardiography. Vibration 2019, 2, 64–86. [Google Scholar] [CrossRef]
- Yang, C.; Ojha, B.D.; Aranoff, N.D.; Green, P.; Tavassolian, N. Classification of aortic stenosis using conventional machine learning and deep learning methods based on multi-dimensional cardio-mechanical signals. Sci. Rep. 2020, 10, 17521. [Google Scholar] [CrossRef]
- Shokouhmand, A.; Aranoff, N.D.; Driggin, E.; Green, P.; Tavassolian, N. Efficient detection of aortic stenosis using morphological characteristics of cardiomechanical signals and heart rate variability parameters. Sci. Rep. 2021, 11, 23817. [Google Scholar] [CrossRef]
- Johnson, E.M.; Robinson, J.D.; Rigsby, C.K.; McCarthy, P.M.; Malaisrie, S.C.; Allen, B.D.; Barker, A.J.; Markl, M. Abstract 9903: Artificial Intelligence Driven Wearable 2-minute Seismocardiography Test for Detection of Aortic Valve Stenosis Severity. Circulation 2022, 146, A9903. [Google Scholar] [CrossRef]
- Johnson, E.M.; Barker, A.J.; Robinson, J.D.; Rigsby, C.K.; Markl, M. Abstract 11504: Elevated Seismocardiography-Derived Chest Energy is Associated With Aortic Flow Abnormalities in Patients With Aortic Valve Disease. Circulation 2022, 146, A11504. [Google Scholar] [CrossRef]
- Korzeniowska-Kubacka, I.; Bilinska, M.; Piotrowicz, R. Usefulness of Seismocardiography for the Diagnosis of Ischemia in Patients with Coronary Artery Disease. Ann. Noninvasive Electrocardiol. 2005, 10, 281–287. [Google Scholar] [CrossRef]
- Iftikhar, Z.; Lahdenoja, O.; Tadi, M.J.; Hurnanen, T.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; Koivisto, T.; Pänkäälä, M. Multiclass Classifier based Cardiovascular Condition Detection Using Smartphone Mechanocardiography. Sci. Rep. 2018, 8, 9344. [Google Scholar] [CrossRef]
- Mehrang, S.; Jafari Tadi, M.; Kaisti, M.; Lahdenoja, O.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; Koivisto, T.; Pänkäälä, M. Machine Learning Based Classification of Myocardial Infarction Conditions Using Smartphone-Derived Seismo- and Gyrocardiography. In Proceedings of the 2018 Computing in Cardiology Conference (CinC), Maastricht, The Netherlands, 23–26 September 2018; Volume 45, pp. 1–4. [Google Scholar] [CrossRef]
- Hurnanen, T.; Lehtonen, E.; Tadi, M.J.; Kuusela, T.; Kiviniemi, T.; Saraste, A.; Vasankari, T.; Airaksinen, J.; Koivisto, T.; Pankaala, M. Automated Detection of Atrial Fibrillation Based on Time–Frequency Analysis of Seismocardiograms. IEEE J. Biomed. Health Inform. 2017, 21, 1233–1241. [Google Scholar] [CrossRef]
- Jafari Tadi, M.; Mehrang, S.; Kaisti, M.; Lahdenoja, O.; Hurnanen, T.; Jaakkola, J.; Jaakkola, S.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; et al. Comprehensive Analysis of Cardiogenic Vibrations for Automated Detection of Atrial Fibrillation Using Smartphone Mechanocardiograms. IEEE Sens. J. 2019, 19, 2230–2242. [Google Scholar] [CrossRef]
- Mehrang, S.; Tadi, M.J.; Hurnanen, T.; Knuutila, T.; Lahdenoja, O.; Jaakkola, J.; Jaakkola, S.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; et al. Reliability of Self-Applied Smartphone Mechanocardiography for Atrial Fibrillation Detection. IEEE Access 2019, 7, 146801–146812. [Google Scholar] [CrossRef]
- Mehrang, S.; Tadi, M.J.; Knuutila, T.; Jaakkola, J.; Jaakkola, S.; Kiviniemi, T.; Vasankari, T.; Airaksinen, J.; Koivisto, T.; Pänkäälä, M. End-to-end sensor fusion and classification of atrial fibrillation using deep neural networks and smartphone mechanocardiography. Physiol. Meas. 2022, 43, 055004. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.; Søgaard, P.; Emerek, K.; Jensen, A.S.; Struijk, J.J.; Schmidt, S.E. Seismocardiography as a tool for assessment of bi-ventricular pacing. Physiol. Meas. 2022, 43, 105007. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, T.; Lahdenoja, O.; Hurnanen, T.; Koskinen, J.; Jafarian, K.; Vasankari, T.; Jaakkola, S.; Kiviniemi, T.O.; Airaksinen, K.E.J. Mechanocardiography-Based Measurement System Indicating Changes in Heart Failure Patients during Hospital Admission and Discharge. Sensors 2022, 22, 9781. [Google Scholar] [CrossRef]
- Ramos-Castro, J.; Moreno, J.; Miranda-Vidal, H.; García-González, M.A.; Fernández-Chimeno, M.; Rodas, G.; Capdevila, L. Heart rate variability analysis using a seismocardiogram signal. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 5642–5645. [Google Scholar] [CrossRef]
- Choudhary, T.; Das, M.; Sharma, L.; Bhuyan, M. Analyzing seismocardiographic approach for heart rate variability measurement. Biomed. Signal Process. Control 2021, 68, 102793. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Koivisto, T.; Pänkäälä, M.; Paasio, A.; Teräs, M. Seismocardiography: Toward heart rate variability (HRV) estimation. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA) Proceedings, Turin, Italy, 7–9 May 2015; pp. 261–266. [Google Scholar] [CrossRef]
- Landreani, F.; Morri, M.; Martin-Yebra, A.; Casellato, C.; Pavan, E.; Frigo, C.; Caiani, E.G. Ultra-short-term heart rate variability analysis on accelerometric signals from mobile phone. In Proceedings of the 2017 E-Health and Bioengineering Conference (EHB), Sinaia, Romania, 22–24 June 2017; pp. 241–244. [Google Scholar] [CrossRef]
- Landreani, F.; Faini, A.; Martin-Yebra, A.; Morri, M.; Parati, G.; Caiani, E.G. Assessment of Ultra-Short Heart Variability Indices Derived by Smartphone Accelerometers for Stress Detection. Sensors 2019, 19, 3729. [Google Scholar] [CrossRef]
- Londhe, A.N.; Atulkar, M. Heart Rate Variability: A Methodological Survey. In Proceedings of the 2019 International Conference on Intelligent Sustainable Systems (ICISS), Palladam, India, 21–22 February 2019; pp. 57–63. [Google Scholar] [CrossRef]
- Shokouhmand, A.; Yang, C.; Aranoff, N.D.; Driggin, E.; Green, P.; Tavassolian, N. Mean Pressure Gradient Prediction Based on Chest Angular Movements and Heart Rate Variability Parameters. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; pp. 7170–7173. [Google Scholar] [CrossRef]
- Siecinski, S.; Kostka, P.S.; Tkacz, E.J. Heart Rate Variability Analysis on CEBS Database Signals. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Honolulu, HI, USA, 18–21 July 2018; pp. 5697–5700. [Google Scholar] [CrossRef]
- Siecinski, S.; Tkacz, E.J.; Kostka, P.S. Comparison of HRV indices obtained from ECG and SCG signals from CEBS database. BioMedical Eng. OnLine 2019, 18, 69. [Google Scholar] [CrossRef]
- Siecinski, S.; Kostka, P.S.; Tkacz, E.J. Time Domain Furthermore, Frequency Domain Heart Rate Variability Analysis on Gyrocardiograms. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 2630–2633. [Google Scholar] [CrossRef]
- Siecinski, S.; Kostka, P.S.; Tkacz, E.J. Time Domain and Frequency Domain Heart Rate Variability Analysis on Electrocardiograms and Mechanocardiograms from Patients with Valvular Diseases. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 653–656. [Google Scholar] [CrossRef]
- Siecinski, S.; Kostka, P.S.; Tkacz, E.J. Comparison of Heart Rate Variability Indices Based on Seismocardiograms from Healthy Volunteers and Patients with Valvular Heart Diseases. In Proceedings of the Computing in Cardiology Conference, Tampere, Finland, 4–7 September 2022. [Google Scholar]
- Milena, v.; Romano, C.; De Tommasi, F.; Carassiti, M.; Formica, D.; Schena, E.; Massaroni, C. Linear and Non-Linear Heart Rate Variability Indexes from Heart-Induced Mechanical Signals Recorded with a Skin-Interfaced IMU. Sensors 2023, 23, 1615. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart Rate Variability. Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Saykrs, B. Analysis of Heart Rate Variability. Ergonomics 1973, 16, 17–32. [Google Scholar] [CrossRef]
- Montano, N.; Porta, A.; Cogliati, C.; Costantino, G.; Tobaldini, E.; Casali, K.R.; Iellamo, F. Heart rate variability explored in the frequency domain: A tool to investigate the link between heart and behavior. Neurosci. Biobehav. Rev. 2009, 33, 71–80. [Google Scholar] [CrossRef]
- Sassi, R.; Cerutti, S.; Lombardi, F.; Malik, M.; Huikuri, H.V.; Peng, C.K.; Schmidt, G.; Yamamoto, Y.; Gorenek, B.; Lip, G.Y.; et al. Advances in heart rate variability signal analysis: Joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1341–1353. [Google Scholar] [CrossRef]
- Friedrich, D.; Aubert, X.L.; Führ, H.; Brauers, A. Heart rate estimation on a beat-to-beat basis via ballistocardiography—A hybrid approach. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 4048–4051. [Google Scholar] [CrossRef]
- Lahdenoja, O.; Hurnanen, T.; Tadi, M.J.; Pänkäälä, M.; Koivisto, T. Heart Rate Variability Estimation with Joint Accelerometer and Gyroscope Sensing. In Proceedings of the Computing in Cardiology, Vancouver, BC, Canada, 11–14 September 2016; Volume 43, pp. 717–720. [Google Scholar]
- Laurin, A.; Blaber, A.; Tavakolian, K. Seismocardiograms return valid heart rate variability indices. In Proceedings of the Computing in Cardiology 2013, Zaragoza, Spain, 22–25 September 2013; pp. 413–416. [Google Scholar]
- Rienzo, M.D.; Vaini, E.; Bruno, B.; Castiglioni, P.; Lombardi, P.; Parati, G.; Lombardi, C.; Meriggi, P.; Rizzo, F. Wearable Seismocardiography: Towards the beat-to-beat assessment of cardiac mechanics during sleep in microgravity. In Proceedings of the 2014 8th Conference of the European Study Group on Cardiovascular Oscillations (ESGCO), Trento, Italy, 25–28 May 2014; pp. 239–240. [Google Scholar] [CrossRef]
- Munck, K.; Sørensen, K.; Struijk, J.J.; Schmidt, S.E. Multichannel seismocardiography: An imaging modality for investigating heart vibrations. Physiol. Meas. 2020, 41, 115001. [Google Scholar] [CrossRef]
- Kaisti, M.; Tadi, M.J.; Lahdenoja, O.; Hurnanen, T.; Saraste, A.; Pankaala, M.; Koivisto, T. Stand-Alone Heartbeat Detection in Multidimensional Mechanocardiograms. IEEE Sens. J. 2019, 19, 234–242. [Google Scholar] [CrossRef]
- Kaisti, M.; Tadi, M.J.; Lahdenoja, O.; Hurnanen, T.; Pänkäälä, M.; Koivisto, T. Mechanocardiograms with ECG Reference. IEEE DataPort. 2018. Available online: https://ieee-dataport.org/documents/mechanocardiograms-ecg-reference (accessed on 3 February 2023).
- Yang, C.; Fan, F.; Aranoff, N.; Green, P.; Li, Y.; Liu, C.; Tavassolian, N. An Open-access Database for the Evaluation of Cardio-mechanical Signals from Patients with Valvular Heart Diseases (1.0). [Data set]. Zenodo. 2021. Available online: https://zenodo.org/record/5279448#.Y-tv3q1BxPY (accessed on 3 February 2023).
- Shimmer. Shimmer3 (IMU) Wireless Sensor Platform Specifications. 2022. Available online: https://shimmersensing.com/wp-content/uploads/2022/04/Shimmer3-IMU-Spec-Sheet.pdf (accessed on 3 February 2023).
- Yang, C.; Tavassolian, N. Combined Seismo- and Gyro-Cardiography: A More Comprehensive Evaluation of Heart-Induced Chest Vibrations. IEEE J. Biomed. Health Inform. 2018, 22, 1466–1475. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef]
- Sørensen, K.; Schmidt, S.E.; Jensen, A.S.; Søgaard, P.; Struijk, J.J. Definition of Fiducial Points in the Normal Seismocardiogram. Sci. Rep. 2018, 8, 15455. [Google Scholar] [CrossRef]
- Vest, A.N.; Da Poian, G.; Li, Q.; Liu, C.; Nemati, S.; Shah, A.J.; Clifford, G.D. An open source benchmarked toolbox for cardiovascular waveform and interval analysis. Physiol. Meas. 2018, 39, 105004. [Google Scholar] [CrossRef]
- Poian, G.D.; Li, Q.; Schwabedal, J. embar. cliffordlab/PhysioNet-Cardiovascular-Signal-Toolbox: PhysioNet-Cardiovascular-Signal-Toolbox 1.0.2. GitHub. 2019. Available online: https://zenodo.org/record/3358559#.Y-tphq1BxPY (accessed on 3 February 2023).
- Lomb, N.R. Least-squares frequency analysis of unequally spaced data. Astrophys. Space Sci. 1976, 39, 447–462. [Google Scholar] [CrossRef]
- Scargle, J.D. Studies in astronomical time series analysis. II—Statistical aspects of spectral analysis of unevenly spaced data. Astrophys. J. 1982, 263, 835. [Google Scholar] [CrossRef]
- Ciccone, A.B.; Siedlik, J.A.; Wecht, J.M.; Deckert, J.A.; Nguyen, N.D.; Weir, J.P. Reminder: RMSSD and SD1 are identical heart rate variability metrics. Muscle Nerve 2017, 56, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Palaniswami, M.; Kamen, P. Do existing measures of Poincare plot geometry reflect nonlinear features of heart rate variability? IEEE Trans. Biomed. Eng. 2001, 48, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- İşler, Y.; Kuntalp, M. Combining classical HRV indices with wavelet entropy measures improves to performance in diagnosing congestive heart failure. Comput. Biol. Med. 2007, 37, 1502–1510. [Google Scholar] [CrossRef]
- Kitlas Golińska, A. Poincaré Plots in Analysis of Selected Biomedical Signals. Stud. Logic Gramm. Rhetor. 2013, 35, 117–127. [Google Scholar] [CrossRef]
- Ruan, X.; Liu, C.; Liu, C.; Wang, X.; Li, P. Automatic detection of atrial fibrillation using R-R interval signal. In Proceedings of the 2011 4th International Conference on Biomedical Engineering and Informatics (BMEI), Shanghai, China, 15–17 October 2011; Volume 2, pp. 644–647. [Google Scholar] [CrossRef]
- Biala, T.; Dodge, M.; Schlindwein, F.S.; Wailoo, M. Heart rate variability using Poincaré plots in 10 year old healthy and intrauterine growth restricted children with reference to maternal smoking habits during pregnancy. In Proceedings of the 2010 Computing in Cardiology, Belfast, UK, 26–29 September 2010; pp. 971–974. [Google Scholar]
- Kozak, M. What is Strong Correlation? Teach. Stat. 2009, 31, 85–86. [Google Scholar] [CrossRef]
- Arslan, U.; Özdemir, M.; Kocaman, S.A.; Balcıoğlu, S.; Cemri, M.; Çengel, A. Heart rate variability and heart rate turbulence in mild-to-moderate aortic stenosis. EP Eur. 2008, 10, 1434–1441. [Google Scholar] [CrossRef]
- Werner, B.; Piorecka-Makula, A.; Bobkowski, W. Heart rate variability in children with aortic valve stenosis—A pilot study. Arch. Med. Sci. 2013, 9, 535–539. [Google Scholar] [CrossRef]
- Charlier, P.; Cabon, M.; Herman, C.; Benouna, F.; Logier, R.; Houfflin-Debarge, V.; Jeanne, M.; Jonckheere, J.D. Comparison of multiple cardiac signal acquisition technologies for heart rate variability analysis. J. Clin. Monit. Comput. 2019, 34, 743–752. [Google Scholar] [CrossRef]
- NXP Semiconductors. MMA8451Q, 3-Axis, 14-bit/8-bit Digital Accelerometer Datasheet. 2017. Available online: https://www.nxp.com/docs/en/data-sheet/MMA8451Q.pdf (accessed on 3 February 2023).
- Maxim Integrated. MAX21000 Ultra-Accurate, Low Power, 3-Axis Digital Output Gyroscope Datasheet. 2013. Available online: https://www.analog.com/media/en/technical-documentation/data-sheets/MAX21000.pdf (accessed on 3 February 2023).
- Yang, C.; Ojha, B.; Aranoff, N.D.; Green, P.; Tavassolian, N. Classification of Aortic Stenosis Before and After Transcatheter Aortic Valve Replacement Using Cardio-mechanical Modalities. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 2820–2823. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).