Abstract
Transcranial magnetic stimulation (TMS) is a noninvasive technique mainly used for the assessment of corticospinal tract integrity and excitability of the primary motor cortices. Motor evoked potentials (MEPs) play a pivotal role in TMS studies. TMS clinical guidelines, concerning the use and interpretation of MEPs in diagnosing and monitoring corticospinal tract integrity in people with multiple sclerosis (pwMS), were established almost ten years ago and refer mainly to the use of TMS implementation; this comprises the magnetic stimulator connected to a standard EMG unit, with the positioning of the coil performed by using the external landmarks on the head. The aim of the present work was to conduct a narrative literature review on the MEP assessment and outcome measures in clinical and research settings, assessed by TMS Methodological characteristics of different TMS system implementations (TMS without navigation, line-navigated TMS and e-field-navigated TMS); these were discussed in the context of mapping the corticospinal tract integrity in MS. An MEP assessment of two case reports, by using an e-field-navigated TMS, was presented; the results of the correspondence between the e-field-navigated TMS with MRI, and the EDSS classifications were presented. Practical and technical guiding principles for the improvement of TMS studies in MEP assessment for MS are discussed, suggesting the use of e-field TMS assessment in the sense that it can improve the accuracy of corticospinal tract integrity testing by providing a more objective correspondence of the neurophysiological (e-field-navigated TMS) and clinical (Expanded Disability Status Scale—EDSS) classifications.
1. Introduction
Multiple sclerosis (MS) is an inflammatory autoimmune disease of the central nervous system (CNS) of an unknown cause, characterized by demyelinating white matter lesions and neuronal degeneration [1]. The prevalence of MS in the world ranges from 5 to 300 per 100,000 people, and affects women more often [2]. Relapsing–remitting form of the disease (RRMS) is the most common form. The primary progressive form of the disease (PPMS) is significantly less common and occurs in 10% of people with MS (pwMS), while the further progression of the disease indicates the transition from the relapsing–remitting form to the secondary progressive form (SPMS).
The diagnosis of MS is based on laboratory findings (e.g., cerebrospinal fluid-specific bands), oligoclonal bands, and radiologic findings (e.g., magnetic resonance imaging [MRI ≥ 1.5 T or 3T] T2 lesions of the brain and spinal cord, lesions that increase gadolinium), including the application of the 2017 McDonald criteria and the 2021 MAGNIMS-CMSC-NAIMS recommendations [3,4]. The clinical status of disability is expressed through the Expanded Disability Status Scale (EDSS) [5,6], which assesses the status of functional systems including the pyramidal–corticospinal pathway (muscle strength, limb movement), cerebellum (balance, coordination), brainstem (speech, swallowing, nystagmus), sensory pathway (sensation), visual pathway (sight), bladder and bowel function, cognitive functions (memory), and ambulation (walking measured in meters). The key functional components of the EDSS, correlating with sustained disability progression, appear to be mostly pyramidal, followed by cerebellar and sensory functional systems [7].
Various quantitative measures (i.e., the number and volume of contrast-enhancing, the volumes of T2-hyperintense and T1-hypointense lesions, and brain volume changes), derived from conventional and advanced MRI methods, have been proposed as prognostic biomarkers for MS. However, correlations between different MRI indicators and EDSS are not satisfactory, and no specific MRI measure is used as a comprehensive prognostic imaging biomarker for MS [8,9,10].
Evoked potentials (EP) represent neurophysiological measures of signal conduction in the CNS in vivo, and are used to measure the impact of MS pathology on CNS function pathways correlating with clinical status [11]. Multimodal Eps, such as somatosensory evoked potentials (SEPs), visual evoked potentials (VEPs), and motor evoked potentials (MEPs), recorded as baseline (at diagnosis), have been shown to correlate with EDSS [12]. Recent findings suggest the likely application of TMS as a subclinical MEP test that could represent a biomarker of the degree of MS disability [13,14]. Current data suggest a connection between the pathophysiological mechanisms of MS (demyelination and loss of axons) and TMS neurophysiological measures (e.g., lower amplitudes and longer latencies of MEP responses from upper and lower limb muscles, elevated resting motor threshold (RMT), and changes in specific neurophysiological measures of excitation and inhibition) [15]. Furthermore, changes in cortical excitatory and inhibitory processes in MS, assessed with TMS, appear to be evident in early disease progression, during relapse, and later during disease progression [11,15,16]. In addition, changes in neurophysiological TMS measures are associated with the clinical characteristics of MS [14,15]. It has to be noted that MEPs acquired in TMS studies in MS subjects, mainly represent the marker of the integrity of the corticospinal tract (lateral funicle of the cord known as the lateral corticospinal tract) [17] and primary motor cortices (M1). Motor mapping can also demonstrate the presence of the ipsilateral MEP corticospinal tract projections reported in congenital pathologies, including hemiplegic cerebral palsy [18,19,20,21,22] and congenital mirror movements [18,23,24,25]; this is evident in progressive immune-mediated Rasmussen encephalitis, leading to unihemispheric brain atrophy [26] during intraoperative neurosurgical monitoring in patients [27], and in acquired lesions, such as during a cerebral stroke [28,29] or following hemispherectomy [28]. MEPs can also be recorded in the ipsilateral muscles of the upper extremities in healthy subjects [30]. Ipsilateral MEPs are thought to reflect the functional activity of the uncrossed lateral corticospinal tract from the ipsilateral hemisphere [31], may reflect the activation of the cortical–subcortical–spinal pathways [32], or may be due to the activation of the crossed corticospinal tract from the hemisphere contralateral to the target limb; this is due to the proximity of the M1 cortices for lower extremity muscle representation. The functional role of ipsilateral M1 areas in MS has been associated with an adaptive response to chronic CNS injury [33,34,35]. Overall, the TMS investigation of ipsilateral MEPs in MS has not been widely considered, due to the neurophysiological mechanisms still being unknown.
Concerning the clinical use and interpretation of MEPs in diagnosing and monitoring pwMS, TMS guidelines were established by Fernández et al. in 2013 [36], referring to the TMS. This mainly included the magnetic stimulator connected to a standard EMG unit, and was less connected to linenavigated TMS implementations.
Therefore, this paper aims to review the current literature state of MEP assessment in MS. The article is organized as follows: The Section 2 examines the MEP assessment and outcome measures in MS research, assessed by TMS without navigation, TMS with line navigation, and e-field navigation TMS techniques. The Section 3 presents a two case report on MS research in MEP assessment using an e-field-navigated TMS; this is performed by also testing the correspondence of e-field-navigated TMS testing with MRI and EDSS classifications. The Section 4 presents practical and technical guiding principles for improvements to TMS studies in MEP assessment in MS.
2. Assessment of MEPs in Multiple Sclerosis
2.1. Targeting M1 with TMS without Navigation, Line-Navigated TMS and e-Field-Navigated TMS
TMS is a noninvasive technique mainly used for the evaluation of corticospinal tract integrity and the excitability of M1 cortices in MS. The basic principle of TMS can be explained by electromagnetic induction, generating a suprathreshold current in the brain. TMS devices consist of a few circular turns of copper wire, connected to the terminals of a large electrical capacitance via a switch. A large current (monophasic or biphasic pulse configuration) of several thousand Amps flows briefly through the wire coil for less than one millisecond. The current pulse produces a rapidly changing and brief magnetic field, with a field strength similar to the static field in an MRI scanner (1–2 T). Magnetic fields generate current in the brain tissue, according to Faraday’s law of electromagnetic induction (Figure 1).
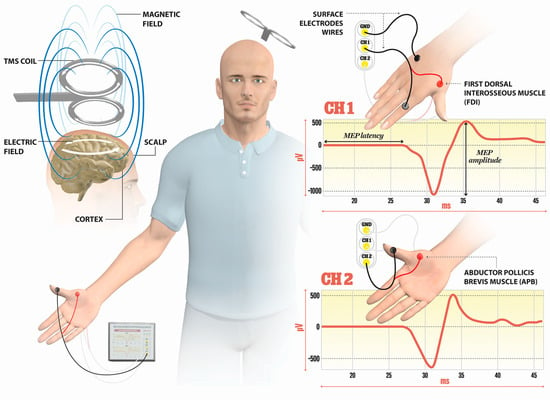
Figure 1.
Illustration of the direction of current flows in a magnetic coil and the induced current in the brain tissue. An electric field is induced perpendicularly to the magnetic field. The magnetic coil is positioned over the M1 cortex, and surface electrodes are on the target muscles, here shown for the first dorsal interosseus (FDI) and abductor pollicis brevis (APB). Elicited MEP responses are detected at channels Ch1 for FDI, and Ch2 for APB muscle. The illustration is the property of the School of the Medicine University of Split, Department of Neuroscience, Laboratory for Human and Experimental Neurophysiology).
The TMS includes the magnetic stimulator connected to a standard EMG unit, with the positioning of the coil performed by using the external landmarks on the head. The determination of M1 representation for upper muscle is performed by the coil positioning; the coil is placed 5 cm lateral to the vertex along the auricular line and positioned by turning the coil approximately 45° to the parasagittal plane. In mapping the M1 representation for leg muscles, the coil is recommended to be placed over the vertex. Cervical stimulation is agreed upon by placing the coil above the C7 spinous process at the midline, or 2 cm lateral to the midline, while for the stimulation of lumbosacral roots, the coil is placed along the midline over the target vertebral body.
Line-navigated TMS is performed by placing a magnetic coil over the target area on the basis of the individual MRI image, with the maximal activation supposed to be located on the line that passes through the center of the coil perpendicular to the surface of the bottom of the coil; this is without the visualization of the spot of maximal stimulation if there is slight coil tilt [37]. Line-navigated TMS is susceptible to errors when the coil is not held continuously tangentially against the head.
E-field-navigated TMS computes the e-field maximum, where the cortex is best stimulated, online; it considers the geometry of the head, the magnetic coil shape, location, orientation, individual head shape, size, and the orientation of the cortical folds [37]. Navigated TMS combines TMS with 3D brain imaging, approximated with the spherical models, and comprises a magnetic stimulator, stereotactic camera, and integrated EMG system, including tracking tools (head tracker, coil tracker, digitizing pen). Prior to mapping M1 with navigated TMS, an MRI of the head for the subject is performed, including the MRI of the head and visible ears. After the co-registration of the subject, the reference anatomical spot for M1 for upper extremity representation is determined by the ‘‘omega knob’’ on axial MRI images, or a ‘‘hook structure’’ at the sagittal MRI [38]. The central sulcus is used as a landmark, while moving the coil in the anterior–posterior direction, to map the hot spot for M1 for the upper extremity muscle (i.e., abductor pollicis brevis, APB). When mapping the M1 for lower extremity muscles, the central sulcus is again followed as a landmark, with the posterior-to-anterior direction of the coil positioned medially over the vertex of the target hemisphere.
Line-navigated TMS and e-field-navigated TMS methods were compared in studies investigating MEPs; this was performed by stimulating the M1 area in tumor patients in preoperative settings [39], resulting in only a partial overlap in MEP maps while mapping M1 representation of upper and lower extremity muscles. The distances between the M1 motor hotspots between the two methods were 8.6 ± 4.5 mm on the contralesional hemisphere. Further, motor positive spots eliciting MEPs were significantly higher for e-field-navigated TMS, compared to line-navigated TMS. The lower rate of the positive motor hot spots detected with line-navigated TMS is probably due to a nonoptimal coil orientation and tilting with the decreased electric field at the cortex. In addition, the manual placing of the coil is more time-consuming in line-navigated TMS. Likewise, an e-field-navigated TMS can calculate and visualize the electric field online during the mapping procedure with its orientation and dose, allowing the continuous optimization of the coil positioning [39]. The final conclusions regarding the accuracy of the e-field-navigated TMS and line-navigated TMS methods are to be tested against the intraoperative golden standard direct electrical stimulation (DES) technique. Currently, e-field-navigated TMS systems have been evaluated in patients with tumors undergoing preoperative mapping of the M1 area and intraoperative DES procedures, showing a correlation between e-field-navigated TMS and DES [37].
Lastly, it is important to emphasize the variability in corticospinal excitability by mapping the M1 due to physical (tilt, location, intensity, and orientation of the coil) and physiological factors, in addition, interindividual anatomical differences in M1 that can be controlled by e-field-navigated TMS, including online calculation and visualization of an electric field [40,41], are mapped. The spatial accuracy of e-field-navigated TMS is approximately 2 mm [41], with location changes larger than 2 mm resulting in a variability of corticospinal excitability (i.e., changes in peak-to-peak MEP amplitude values), pointing to the fact that mapping of the integrity of the corticospinal tract is susceptible to small changes in physical parameters.
2.2. Neurophysiological Changes in the Central and Peripheral System in pwMS Investigated with TMS
The single-pulse TMS is applied for mapping the M1 and the integrity of the corticospinal tract by examining MEP outcome measures; this includes MEP latency (the transmission duration from the stimulating cortex to the onset of MEP in the EMG of the target muscle), MEP amplitude (peak-to-peak difference in MEP signal), the MEP input–output curve (I/O) (a sigmoid-shaped relation between the MEP amplitude at incremented TMS intensities), the central motor conduction time (CMCT) (the time it takes for the action potentials to travel from the site of cortical stimulation to the spinal neuron), the cortical silent period (CSP) (intracortical inhibition measure), or the resting motor threshold (RMT) (minimum intensity of stimulator output eliciting MEPs of 50 µV in at least ten trials in relaxing muscle) [17,42]. Further, short-interval intracortical inhibition (SICI), intracortical facilitation (ICF), and short-interval intracortical facilitation (SICF) can be explored if a paired-pulse TMS protocol is applied.
Recommendations for the clinical use of MEPs in MS are reported by Fernández et al. [36], and mainly discuss the application of TMS with no navigation for the use of MEP assessment in pwMS. The majority of reported studies (Table 1), assessing the MEP in pwMS, used TMS apparatus with no navigation. Table 1 presents an overview of the neurophysiological changes in the central and peripheral nervous system in pwMS when compared to healthy controls.
The findings for the neurophysiological assessment in MS, compared to healthy controls, include a prolongation in the MEP latency, an increase in the CMCT, and a decrease in the MEP amplitude, with still nonconclusive results related to RMT (findings point to be increased), CSP (findings point to be prolonged), and SICI (probably decreased) (Table 1) [17]. Two studies by Neva et al. [14] and Nantes et al. [43] used the TMS system with line navigation (neuronavigation software package by Rogue Research Inc., Canada), and a single group by Rogić Vidaković [44] used the e-field-navigated TMS to localize the M1 representation for upper and lower extremity muscles. So far, most of the MEP studies in MS have been conducted via TMS with no navigation, such as the study by Magstim, reporting the use of different coil types (circular, double-cone, figure-of-eight) (Table 1). Most of the studies included healthy controls (i.e., Pisa et al. [45]), or included the results of clinical samples of healthy controls in previously published studies. Recent reports tend to report the results of multimodal measures, including neurophysiological (MEP) assessment data, combined with MRI data on lesions, disease-related information, and clinical results of the neurological assessment (EDSS) [46].

Table 1.
An overview of TMS studies examining the central and peripheral systems in pwMS.
Table 1.
An overview of TMS studies examining the central and peripheral systems in pwMS.
| Author (Year) (Reference Number) | TMS Device, Coil Type, M1 Target Location (TMS without Navigation, e-Field Navigation TMS, Navigate TMS) | Number of pwMS/HC | MEP Latency/INVESTIGATED Muscles | MEP Amplitude | RMT | CMCT | CSP | SICI |
|---|---|---|---|---|---|---|---|---|
| Yperman et al. (2022) [47] | Magstim 2002, round coil, TMS without navigation | 963/ | The study includes a dataset of 100,000 MEP signals in MS Metacarpal I/II, APB, metatarsal I, AH | The study includes a dataset of 100,000 MEP signals in MS | - | - | - | - |
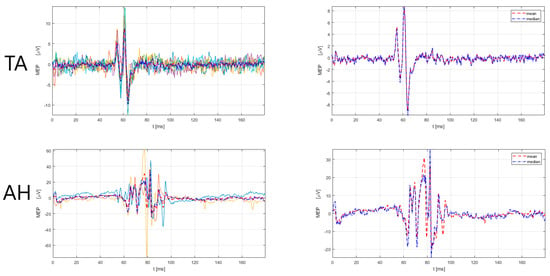
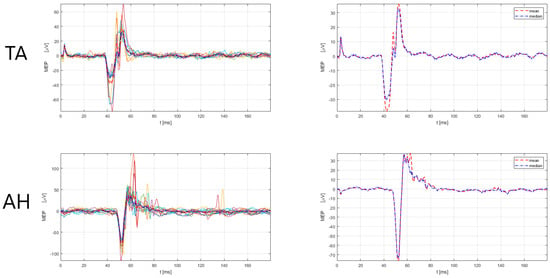
| Rogić Vidaković et al. (2022) [44] | NBS navigation system (Nexstim Plc., Helsinki, Finland), figure-of-eight coil, navigated TMS, biphasic stimulation | single pwMS case report/ | Prolonged MEP latencies in upper and lower extremity muscles APB, ADM, TA, AH | ns | ns | ns | ns | ns |
| Rogić Vidaković et al. (in review, unpublished) [48] | NBS navigation system (Nexstim Plc., Helsinki, Finland), figure-of-eight coil), navigated TMS, biphasic stimulation | 23/clinical samples of healthy subjects | Prolonged MEP latencies in pwMS compared to clinical samples of HC (p < 0.001) APB, ADM, TA, AH | ns | ns | ns | ns | ns |
| Mamoei et al. (2021) [46] | Dantec Magnetic Primer TwinTop TMS & MagLite (Berlin, Germany), r-25 magnetic stimulator, circular coil, TMS without navigation | 41/longitudinal study testing Fampridine responsiveness | ns VM, TA | Decreased MEP amplitude after 1 year (p < 0.035) | ns | CMCT prolonged after 1 year | ns | ns |
| Stampanon and Basssi et al. (2020) [15] | Magstim 2002 (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, TMS without navigation | 18/18 | ns FDI | ns | RMT increased in pwMS compared to HC (p = 0.009) | ns | ns | SICI decreased in pwMS compared to HC (p = 0.007) |
| Pisa et al. (2021) [49] | Magstim 200 (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, TMS without navigation | 30/15 | Prolonged MEP latencies compared to HC (p > 0.05) (posterior-anterior coil orientation) FDI | Decreased MEP amplitude compared to HC (p < 0.05) (posterior-anterior coil orientation) | RMT increased in pwMS compared to HC (p < 0.05) (posterior-anterior coil orientation) | |||
| Pisa et al. (2020) [45] | Magstim 200 (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, TMS without navigation | 50/ | Delayed or absent MEP to the upper limbs. MEPs bilaterally absent in the lower limbs 74% (PPMS) FDI, TA | ns | ns | ns | ns | ns |
| Mordillo-Mateos et al. (2019) [50] | Magstim 200 (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, TMS without navigation, monophasic stimulation | 17/16 | ns FDI | Decreased MEP amplitude after abductions of FDI in pwMS compared to HC | RMT increased in pwMS compared to HC (p = 0.0139) | CMCT increased in pwMS (p = 0.009) | ns | ns |
| Zipser et al. (2018) [51] | Magstim 200 (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, TMS without navigation, monophasic stimulation | 13/16 | ns APB | ns | RMT increased in pwMS compared to HC (p < 0.05) | ns | ns | ns |
| Neva et al. (2016) [14] | Magstim 2002 (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, BrainsightTM neuronavigation software package (Rogue Research Inc., Montréal, Canada), TMS with e-field navigation | 26/11 | MEP latency prolonged in pwMS compared to HC (p = 0.001) extensor carpi radialis | ns | RMT increased in pwMS compared to HC (p = 0.022) | ns | CSP onset prolonged in pwMS compared to HC (p = 0.011) | ns |
| Nantes et al. (2016) (2017) [43] | Magstim 2002 (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, BrainsightTM neuronavigation software package (Rogue Research Inc., Montréal, Canada), TMs with e-field navigation | 43/29 | MEP latency prolonged in pwMS compared to HC (p < 0.001) FDI | Decreased MEP amplitude during rest in pwMS compared to HC (p < 0.001) | No difference between pwMS and HC (p > 0.05) | ns | CSP increased in pwMS (p < 0.01) | No difference between pwMS and HC (p > 0.05) |
| Cabib et al. (2015) [52] | ns, figure-of-eight coil, TMS without navigation | 20/13 | MEP latency prolonged in pwMS compared to HC (p = 0.005) FDI | ns | ns | ns | ns | ns |
| Bridoux et al. (2015) [53] | - | 12/12 | ns extensor carpi radialis | Decreased MEP amplitude in pwMS (p = 0.03) | ns | ns | ns | |
| Di Sapio et al. (2014) [54] | Magstim Rapid2 Device (Magstim Company Ltd., Spring Gardens, Whitland, UK), double- cone coil, TMS, without navigation | 28/28 | ns VM, flexor hallucis brevis, TA | ns | No difference between pwMS and HC | CMCT increased in pwMS (p < 0.001) | ns | ns |
| Von Mayenburg et al. (2013) [55] | Magstim 200 (Magstim Company Ltd., Spring Gardens, Whitland, UK), circular coil, TMS without navigation, biphasic stimulation | 41/28 | ns ADM, TA | ns | ns | CMCT increased in pwMS (p = 0.002) | ns | ns |
| Conte et al. (2009) [56] | Magstim (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, TMS without navigation | 30/17 | MEP latency prolonged in pwMS FDI | Decreased MEP amplitude in pwMS (p = 0.001) | ns | CMCT increased in pwMS (p = 0.002) | ns | SICI decreased in pwMS |
| Firmin et al. (2012) [57] | Bistim 200 (Magstim Company Ltd., Spring Gardens, Whitland, UK), circular coil, TMS without navigation | 16/29 | ns ADM | ns | ns | No difference in CMCT between pwMS and HC | ns | ns |
| Steens et al. (2012) [58] | - | 20/20 | ns FDI | ns | No RMT difference between pwMS and HC (p = 0.18) | CMCT increased in pwMS (p = 0.02) | ns | ns |
| Morgante et al. (2011) [59] | Magstim 200 (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, TMS without navigation, biphasic stimulation | 33/12 | ns FDI, APB | Decreased MEP amplitude in pwMS compared to HC (p = 0.001) | No RMT difference between pwMS and HC | CMCT increased in pwMS (p = 0.003) | ns | No SICI difference between pwMS and HC (p = 0.04) |
| Thickbroom et al. (2008) [60] | Magstim 200 (Magstim Company Ltd., Spring Gardens, Whitland, UK), double-cone coil, TMS without navigation | 10/13 | MEP latency prolonged in pwMS (p < 0.05) TA | No MEP amplitude difference between pwMS and HC (p < 0.05) | ns | CMCT increased in pwMS | ns | ns |
| Gagliardo et al. (2007) [61] | Magstim 200 (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, TMS without navigation, monophasic stimulation | 32/20 | ns TA | Decreased MEP amplitude in pwMS compared to HC (p < 0.001) | RMT increased in pwMS compared to HC (p = 0.001) | CMCT increased in pwMS (p = 0.001) | ns | ns |
| Thickroom et al. (2006) [62] | Magstim 200 (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, TMS without navigation | 23/15 | No MEP latency difference between pwMS and HC FDI | Decreased MEP amplitude in pwMS (p < 0.01) | ns | No CSP difference between pwMS and HC (p > 0.05) | ns | |
| Liepert et al. (2005) [63] | Magstim (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, TMS without navigation | 16/6 | ns superficial flexor digitorum | No MEP amplitude difference between pwMS and HC | ns | ns | ns | SICI decreased in pwMS (p < 0.01) |
| Mainero et al. (2004) [64] | Magstim (Magstim Company Ltd., Spring Gardens, Whitland, UK), figure-of-eight coil, TMS without navigation | 12/12 | ns FDI | ns | ns | CMCT increased in pwMS (p < 0.001) | ns | ns |
| Schubert et al. (1998) [65] | - | 11/10 | ns flexor hallucis brevis, TA | MEP area reduced in pwMS compared to HC | No RMT difference between pwMS and HC | CMCT increased in pwMS | ns | ns |
| Sheean et al. (1997) [66] | Magstim 200 (Magstim Company Ltd., Spring Gardens, Whitland, UK), circular coil, TMS without navigation | 21/19 | MEP latency prolonged in pwMS compared to HC (p < 0.05) adductor pollicis | No MEP amplitude difference between pwMS and HC | No RMT difference between pwMS and HC | CMCT increased in pwMS (p < 0.01) | ss | ns |
Abbreviations: pwMS, people with multiple sclerosis; HC, healthy controls; MEP, motor evoked potentials; CMCT, central motor conduction time; RMT, resting motor threshold; CSP, cortical silent period; SICI, short intracortical inhibition; APB, abductor pollicis brevis; ADM, aductor digiti minimi; AH, abductor hallucis; FDI, first dorsal interosseus; TA, tibialis anterior; VS, vastus medialis; ns, not specified; -, information missing due to technical reasons; clinical samples of healthy subjects refers to samples of healthy controls from previous studies.
4. Discussion on Some Practical and Technical Guidelines for Improvements of TMS Studies in MEP Assessment in Multiple Sclerosis
According to recommendations from Fernández et al. [36], MEP studies are considered the first choice in patients with symptoms that are compatible with the first episode of demyelinating diseases, in patients with a clinical diagnosis of MS, and normal or inconclusive results from a brain MRI. Further, since the functional pyramidal EDSS score was shown to be the most frequently associated with sustained disability progression, it might be relevant to report functional pyramidal EDSS scores together with the overall EDSS score. In addition, with the 2017 McDonald criteria and the 2021 MAGNIMS-CMSC-NAIMS recommendations [3,4] on MRI lesion reporting, the additional inspection of corticospinal tract lesions would be suggested. It would also be recommended for TMS studies, assessing MEP in MS, to consistently report technical specifications such as EMG sampling frequency per channel, peak-to-peak amplitude, TMS pulse type, pulse width, stimulation intensity (presented as intensity value of maximal stimulator output and/or expressed in percentage in relation to RMT), and magnetic coil type. Studies using line-navigated versus e-field-navigated systems are welcomed in future studies of corticospinal tract integrity in MS, to test the accuracy of both techniques in MS research.
In research and clinical medical practice, MEP latency is a relevant neurophysiological parameter to determine the conduction time for neural impulses from the cortex to peripheral muscles. The manual latency assessment requires extensive resources and time. Thus, the importance of automatic latency estimation is emphasized. The automated latency estimation algorithms could be divided into the following categories: algorithms based on the absolute hard threshold estimator (AHTE), which are based on using hard threshold and so-called magic number [69], and algorithms that are based on statistical measures (SM), which use a standard deviation metric to find a magic number to determine the onset of the MEP [70,71,72]. Further, there is an algorithm based on the squared hard thresholded estimator (SHTE), where the coefficients of the MEP are squared and then thresholded to obtain a magic number and, thus, obtain the onset of the MEP [68]. Finally, there is an algorithm based on approximating the first derivative to find the time point of the initial deflection to determine the onset of the MEP [73]. According to the current research state, all algorithms show an accurate estimation of the latency for the peak-to-peak amplitudes greater than 100 µV. However, for the peak-to-peak amplitudes between 50 and 100 µV, the SHTE algorithm shows a slightly better accurate latency estimation than AHTE and SM, which is validated by performing a robustness test and calculating the percentage of the deviation index (PDI) [68]. We believe that there is room for improvement in latency estimation for the MEPs with peak-to-peak amplitudes of less than 100 µV; this is generally for research and clinical purposes, in order to evaluate corticospinal tract integrity. The development of such automatic algorithms would also be useful in MS due to the fact that low peak-to-peak amplitude MEPs, of less than 100 µV or less than 50 µV, could be recorded in these patients when mapping the lower extremity muscles. Still, many clinicians and researchers choose to manually determine the MEP latency and even the peak-to-peak MEP amplitude, which may lead to errors, especially if there is a larger sample of MEPs in trials.
Further, another parameter that can play the role of the biomarker of corticospinal tract integrity [74,75,76,77], and is closely related to peak-to-peak amplitudes, is the MEP I/O recruitment curve [78]. The MEP recruitment curve, or I/O curve, is a peak-to-peak amplitude versus. signal strength function. It represents the average increase in MEP amplitude (from a region of nondetectable MEPs at low stimulation strength) to an upper saturation level that, with an increase in the stimulus, can no longer increase the response [79,80]. Studies of corticospinal excitability in MS generally have not utilized the MEP I/O curves. A single study by Neva et al. [14] assessed the MEP I/O recruitment curve in 22 subjects with MS, utilizing line-navigated TMS (Magstim 2002 stimulator, Magstim Co., by means of Brainsight™ neuronavigation software package from Rogue Research Inc., Montreal, QC, Canada) and reported the linear slope of the MEP amplitude I/O curve correlation with EDSS. It is suggested that future studies could assess the MEP I/O recruitment curve in MS to convey a more comprehensive evaluation of the overall corticospinal excitability, in comparison to motor thresholds, MEP amplitude, or latency. Further, it is well known that for any given TMS strength, the generated MEPs could vary due to known [81,82,83] and still unknown reasons [84]. We believe that e-field-navigated TMS provides better options in the localization of the target region, which is important for monitoring the clinical status of the pwMS, especially in the context of mapping the M1 and functional integrity of the corticospinal pathway. In addition, MEP variability, especially related to peak-to-peak amplitude, could be reduced by using e-field-navigated TMS in MS research and clinical settings.
Finally, combining e-field-navigated TMS with electroencephalography (EEG) would enable the measurement of the brain-wide cortical reactivity to TMS in MS. The quantification of TMS-induced changes in oscillatory power, and the phase of EEG with event-related spectral perturbation and inter-trial coherence, could be investigated as an example of a measure of the cortical excitability threshold in M1 [74]. TMS–EEG is feasible for testing excitability and connectivity in cortical neural networks in MS patients, complementary to conventional EPs [50]. Still, we believe that, from the clinical point of view, combining an e-field-navigated TMS and EEG would be rather time-consuming at this point.
In addition, there are potential limits to e-field-navigated TMS, such as the accuracy and precision of the navigated transcranial magnetic stimulation [85]. When performing stimulation, possible navigation errors can occur due to distortions in image MRIs, head-to-MRI registration [86,87], localization and movement of the head tracker [88], and localization of the coil tracker [41]. Nieminen et al. [85] concluded that the head-image coordinates’ coil localization accuracy and precision, and their effect on the e-field estimation, depends on the navigation method. For example, they have found that the average coregistration accuracies were in the range of 2.2–3.6 mm and 1°, and the precision values were approximately half of the accuracy values. They recommended utilizing the surface-based approach for head-to-MRI registration and realistic e-field model computations to ensure a better navigation.
5. Conclusions
The functional integrity of the corticospinal pathway in MS can be investigated in two directions: medical and technical. The reason for recommending the use of an e-field-navigated TMS is to reduce technical errors [85]. By reducing technical errors, medical research that is as relevant as possible is achieved; this can ultimately result in new insights into the neurophysiological mechanisms in MS. The functional systems in MS are clinically evaluated by EDSS, with the functional pyramidal component highly correlating with sustained disability progression [7]. In addition to TMS becoming an important tool for detecting the degree of disability, by assessing the markers of corticospinal excitability in MS [14,15,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65], the introduction of e-field-navigated TMS reduces errors and reproducibility, and enables a more objective testing of the correspondence with clinical EDSS and MRI data. A neurological EDSS assessment of the functional pyramidal system could be functionally verified or tested via neurophysiological e-field-navigated TMS assessment; this is because it can improve the assessment’s accuracy, leading to more the objective correspondence testing of corticospinal tract integrity by TMS and EDSS. Ultimately, the technical advantages of e-field-navigated TMS should be considered in MS research and clinical settings to improve the reliability of MEPs, especially in monitoring disease progression.
Author Contributions
Conceptualization, J.Š., M.R.V., I.V., S.P.; M.R.V. and J.Š. methodology; formal analysis, M.R.V. and J.Š.; investigation, M.R.V. and J.Š.; data curation, M.R.V. and S.P.; writing—original draft preparation, M.R.V., J.Š.; writing—review and editing, I.V. and S.P.; visualization, M.R.V. and J.Š.; supervision, I.V. and S.P.; project administration, M.R.V., J.Š., S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the University of Split, School of Medicine (Class: 003-08/21-03/0003, No: 2181-198-03-04-21-0039- April 2021 annex, and second annex Class: 003-08/22-03/003, No: 2181-198-03-04-22-0021) approved all aspects of the study protocol.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Henri Hannula (Nexstim Plc.) for his selfless help and support in sending the necessary literature regarding the comparison of line and e-field-navigated TMS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goodin, D.S.; Khankhanian, P.; Gourraud, P.-A.; Vince, N. The nature of genetic and environmental susceptibility to multiple sclerosis. PLoS ONE 2021, 16, e0246157. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Wattjes, M.P.; Ciccarelli, O.; Reich, D.S.; Banwell, B.; de Stefano, N.; Enzinger, C.; Fazekas, F.; Filippi, M.; Frederiksen, J.; Gasperini, C.; et al. 2021 MAGNIMS–CMSC–NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021, 20, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L. Definitions for a Standardised, Quantified Neurological Examination and Assessment of Kurtzke’s Functional Systems and Expanded Disability Status Scale in Multiple Sclerosis; Version 03/09; University Hospital Basel: Basel, Switzerland, 2009; Available online: https://www.neurostatus.net/media/specimen/Definitions_0309_specimen.pdf (accessed on 15 November 2022).
- Scott, T.; Wang, P.; You, X.; Mann, M.; Sperling, B. Relationship between sustained disability progression and functional system scores in relapsing-remitting multiple sclerosis: Analysis of placebo data from four randomized clinical trials. Neuroepidemiology 2015, 44, 16–23. [Google Scholar] [CrossRef]
- Cocozza, S.; Pontillo, G.; Lanzillo, R.; Russo, C.; Petracca, M.; Di Stasi, M.; Paolella, C.; Vola, E.A.; Criscuolo, C.; Moccia, M.; et al. MRI features suggestive of gadolinium retention do not correlate with Expanded Disability Status Scale worsening in Multiple Sclerosis. Neuroradiology 2019, 61, 155–162. [Google Scholar] [CrossRef]
- Barreiro-González, A.; Sanz, M.T.; Carratalà-Boscà, S.; Pérez-Miralles, F.; Alcalá, C.; Carreres-Polo, J.; España-Gregori, E.; Casanova, B. Design and Validation of an Expanded Disability Status Scale Model in Multiple Sclerosis. Eur. Neurol. 2022, 85, 112–121. [Google Scholar] [CrossRef]
- Valizadeh, A.; Moassefi, M.; Barati, E.; Sahraian, M.A.; Aghajani, F.; Fattahi, M. Correlation between the clinical disability and T1 hypointense lesions’ volume in cerebral magnetic resonance imaging of multiple sclerosis patients: A systematic review and meta-analysis. CNS Neurosci. Ther. 2021, 27, 1268–1280. [Google Scholar] [CrossRef]
- Hardmeier, M.; Schindler, C.; Kuhle, J.; Fuhr, P. Validation of Quantitative Scores Derived From Motor Evoked Potentials in the Assessment of Primary Progressive Multiple Sclerosis: A Longitudinal Study. Front. Neurol. 2020, 11, 735. [Google Scholar] [CrossRef]
- Schlaeger, R.; Schindler, C.; Grize, L.; Dellas, S.; Radue, E.W.; Kappos, L.; Fuhr, P. Combined visual and motor evoked potentials predict multiple sclerosis disability after 20 years. Mult. Scler. 2014, 20, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Chalah, M.A.; Palm, U.; Ayache, S.S. Editorial: Corticospinal Excitability in Patients With Multiple Sclerosis. Front. Neurol. 2021, 11, 635612. [Google Scholar] [CrossRef] [PubMed]
- Neva, J.; Lakhani, B.; Brown, K.; Wadden, K.; Mang, C.; Ledwell, N.; Borich, M.; Vavasour, I.; Laule, C.; Traboulsee, A.; et al. Multiple measures of corticospinal excitability are associated with clinical features of multiple sclerosis. Behav. Brain Res. 2016, 297, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.S.; Buttari, F.; Gilio, L.; De Paolis, N.; Fresegna, D.; Centonze, D.; Iezzi, E. Inflammation and Corticospinal Functioning in Multiple Sclerosis: A TMS Perspective. Front. Neurol. 2020, 11, 566. [Google Scholar] [CrossRef]
- Mamoei, S.; Hvid, L.G.; Jensen, H.B.; Zijdewind, I.; Stenager, E.; Dalgas, U. Neurophysiological impairments in multiple sclerosis—Central and peripheral motor pathways. Acta Neurol. Scand. 2020, 142, 401–417. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.F.; Harrison, L.M.; Ingram, D.A.; Stephens, J.A. Plasticity of central motor pathways in children with hemiplegic cerebral palsy. Neurology 1991, 41, 1505. [Google Scholar] [CrossRef]
- Carr, L.J.; Harrison, L.M.; Evans, A.L.; Stephens, J.A. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain 1993, 116, 1223–1247. [Google Scholar] [CrossRef]
- Maegaki, Y.; Maeoka, Y.; Ishii, S.; Shiota, M.; Takeuchi, A.; Yoshino, K.; Takeshita, K. Mechanisms of central motor reorganization in pediatric hemiplegic patients. Neuropediatrics 1997, 28, 168–174. [Google Scholar] [CrossRef]
- Staudt, M.; Grodd, W.; Gerloff, C.; Erb, M.; Stitz, J.; Krägeloh-Mann, I. Two types of ipsilateral reorganization in congenital hemiparesis: A TMS and fMRI study. Brain 2002, 125 Pt 10, 2222–2237. [Google Scholar] [CrossRef]
- Kowalski, J.L.; Nemanich, S.T.; Nawshin, T.; Chen, M.; Peyton, C.; Zorn, E.; Hickey, M.; Rao, R.; Georgieff, M.; Rudser, K.; et al. Motor Evoked Potentials as Potential Biomarkers of Early Atypical Corticospinal Tract Development in Infants with Perinatal Stroke. J. Clin. Med. 2019, 8, 1208. [Google Scholar] [CrossRef] [PubMed]
- Konagaya, Y.; Mano, Y.; Konagaya, M. Magnetic stimulation study in mirror movements. J. Neurol. 1990, 237, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Nezu, A.; Kimura, S.; Takeshita, S.; Tanaka, M. Functional recovery in hemiplegic cerebral palsy: Ipsilateral electromyographic responses to focal transcranial magnetic stimulation. Brain Dev. 1999, 21, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Rich, T.L.; Nemanich, S.; Chen, C.-Y.; Sutter, E.N.; Feyma, T.; Krach, L.; Gillick, B.T. Ipsilateral Corticospinal Tract Excitability Contributes to the Severity of Mirror Movements in Unilateral Cerebral Palsy: A Case Series. Clin. EEG Neurosci. 2020, 51, 185–190. [Google Scholar] [CrossRef]
- Nardone, R.; Langthaler, P.B.; Orioli, A.; Versace, V.; Scarano, G.I.; Brigo, F.; Saltuari, L.; Carnicelli, L.; Trinka, E.; Sebastianelli, L. Ipsilateral motor evoked potentials in a patient with unihemispheric cortical atrophy due to Rasmussen encephalitis. Neural Regen. Res. 2019, 14, 1025–1028. [Google Scholar] [CrossRef]
- Lo, Y.L.; Dan, Y.F.; Tan, Y.E.; Fook-Chong, S.; Tan, S.B.; Tan, C.T.; Raman, S. Intraoperative monitoring study of ipsilateral motor evoked potentials in scoliosis surgery. Eur. Spine J. 2006, 15 (Suppl. S5), 656–660. [Google Scholar] [CrossRef]
- Benecke, R.; Meyer, B.-U.; Freund, H.-J. Reorganisation of descending motor pathways in patients after hemispherectomy and severe hemispheric lesions demonstrated by magnetic brain stimulation. Exp. Brain Res. 1991, 83, 419–426. [Google Scholar] [CrossRef]
- Trunk, B.H.; Ziegler, L.; Gharabaghi, A. Ipsilateral corticospinal maps correspond to severe poststroke motor impairment. Brain Stimul. 2022, 15, 758–760. [Google Scholar] [CrossRef]
- Ziemann, U.; Ishii, K.; Borgheresi, A.; Yaseen, Z.; Battaglia, F.; Hallett, M.; Cincotta, M.; Wassermann, E.M. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J. Physiol. 1999, 518, 895–906. [Google Scholar] [CrossRef]
- Jankowska, E.; Edgley, S.A. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist 2006, 12, 67–79. [Google Scholar] [CrossRef]
- Brum, M.; Cabib, C.; Valls-Solé, J. Clinical value of the assessment of changes in MEP duration with voluntary contraction. Front. Neurosci. 2015, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Pantano, P. Contribution of corticospinal tract damage to cortical motor reorganization after a single clinical attack of multiple sclerosis. NeuroImage 2002, 17, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Zeller, D.; Dang, S.-Y.; Stefan, K.; Biller, A.; Bartsch, A.; Saur, D.; Bendszus, M.; Rieckmann, P.; Toyka, K.V.; Classen, J. Functional role of ipsilateral motor areas in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2010, 82, 578–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peterson, D.S.; Fling, B.W. How changes in brain activity and connectivity are associated with motor performance in people with MS. NeuroImage Clin. 2017, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, V.; Valls-Sole, J.; Relova, J.; Raguer, N.; Miralles, F.; Dinca, L.; Taramundi, S.; Costa-Frossard, L.; Ferrándiz, M.; Ramió-Torrentà, L.; et al. Recommendations for the clinical use of motor evoked potentials in multiple sclerosis. Neurologia 2013, 28, 408–416. [Google Scholar] [CrossRef]
- Hannula, H.; Ilmoniemi, R.J. Basic Principles of navigated TMS. In Navigated Transcranial Magnetic Stimulation in Neurosurgery; Krieg, S.M., Ed.; Springer International Publishing AG: Helsinki, Finland, 2017; pp. 3–29. [Google Scholar]
- Yousry, T. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 1997, 120, 141–157. [Google Scholar] [CrossRef]
- Sollmann, N.; Goblirsch-Kolb, M.F.; Ille, S.; Butenschoen, V.M.; Boeckh-Behrens, T.; Meyer, B.; Ringel, F.; Krieg, S.M. Comparison between electric-field-navigated and line-navigated TMS for cortical motor mapping in patients with brain tumors. Acta Neurochir. 2016, 158, 2277–2289. [Google Scholar] [CrossRef]
- Danner, N.; Julkunen, P.; Könönen, M.; Säisänen, L.; Nurkkala, J.; Karhu, J. Navigated transcranial magnetic stimulation and computed electric field strength reduce stimulator-dependent differences in the motor threshold. J. Neurosci. Methods 2008, 174, 116–122. [Google Scholar] [CrossRef]
- Schmidt, S.; Bathe-Peters, R.; Fleischmann, R.; Rönnefarth, M.; Scholz, M.; Brandt, S.A. Nonphysiological factors in navigated TMS studies; Confounding covariates and valid intracortical estimates. Hum. Brain Mapp. 2015, 36, 40–49. [Google Scholar] [CrossRef]
- Siebner, H.R.; Funke, K.; Aberra, A.S.; Antal, A.; Bestmann, S.; Chen, R.; Classen, J.; Davare, M.; Di Lazzaro, V.; Fox, P.T.; et al. Transcranial magnetic stimulation of the brain: What is stimulated?—A consensus and critical position paper. Clin. Neurophysiol. 2022, 140, 59–97. [Google Scholar] [CrossRef]
- Nantes, J.C.; Zhong, J.; Holmes, S.A.; Whatley, B.; Narayanan, S.; Lapierre, Y.; Arnold, D.L.; Koski, L. Intracortical inhibition abnormality during the remission phase of multiple sclerosis is related to upper limb dexterity and lesions. Clin. Neurophysiol. 2016, 127, 1503–1511, Erratum in Clin. Neurophysiol. 2017, 128, 393. [Google Scholar] [CrossRef]
- Vidaković, M.R.; Katić, A.Ć.; Jerković, A.; Šoda, J.; Kosta, V.; Mužinić, N.R.; Mastelić, A.; Benzon, B.; Poljičanin, A.; Buljan, I.; et al. Abstracts from the IFESS 2021 conferences (Abstract—45 Neurophysiological impairment in multiple sclerosis patient confirmed by transcranial magnetic stimulation of the central nervous system but not with electrical stimulation of peripheral nervous system). Artif. Organs. 2022, 46, E33–E210. [Google Scholar] [CrossRef]
- Pisa, M.; Chieffo, R.; Giordano, A.; Gelibter, S.; Comola, M.; Comi, G.; Leocani, L. Upper limb motor evoked potentials as outcome measure in progressive multiple sclerosis. Clin. Neurophysiol. 2020, 131, 401–405. [Google Scholar] [CrossRef]
- Mamoei, S.; Jensen, H.B.; Pedersen, A.K.; Nygaard, M.K.E.; Eskildsen, S.F.; Dalgas, U.; Stenager, E. Clinical, Neurophysiological, and MRI Markers of Fampridine Responsiveness in Multiple Sclerosis—An Explorative Study. Front. Neurol. 2021, 12, 758710. [Google Scholar] [CrossRef]
- Yperman, J.; Popescu, V.; Van Wijmeersch, B.; Becker, T.; Peeters, L.M. Motor evoked potentials for multiple sclerosis, a multiyear follow-up dataset. Sci. Data 2022, 9, 207. [Google Scholar] [CrossRef]
- Rogić Vidaković, M.; Ćurković Katić, A.; Pavelin, S.; et al. Corticospinal excitability assessment with navigated TMS corresponds to MRI and the EDSS classifications in relapsing-remitting multiple sclerosis. Eur. J. Neurol. 2022. submitted (in review). [Google Scholar]
- Pisa, M.; Chieffo, R.; Congiu, M.; Costa, G.D.; Esposito, F.; Romeo, M.; Comola, M.; Comi, G.; Leocani, L. Intracortical motor conduction is associated with hand dexterity in progressive multiple sclerosis. Mult. Scler. 2021, 27, 1222–1229. [Google Scholar] [CrossRef]
- Mordillo-Mateos, L.; Soto-Leon, V.; Torres-Pareja, M.; Peinado-Palomino, D.; Mendoza-Laiz, N.; Alonso-Bonilla, C.; Dileone, M.; Rotondi, M.; Aguilar, J.; Oliviero, A. Fatigue in multiple sclerosis: General and perceived fatigue does not depend on corticospinal tract dysfunction. Front. Neurol. 2019, 10, 339. [Google Scholar] [CrossRef]
- Zipser, C.M.; Premoli, I.; Belardinelli, P.; Castellanos, N.; Rivolta, D.; Heidegger, T.; Müller-Dahlhaus, F.; Ziemann, U. Cortical excitability and interhemispheric connectivity in early relapsing–remitting multiple sclerosis studied with TMS-EEG. Front. Neurosci. 2018, 12, 393. [Google Scholar] [CrossRef]
- Cabib, C.; Llufriu, S.; Casanova-Molla, J.; Saiz, A.; Valls-Solé, J. Defective sensorimotor integration in preparation for reaction time tasks in patients with multiple sclerosis. J. Neurophysiol. 2015, 113, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Bridoux, A.; Créange, A.; Sangare, A.; Ayache, S.; Hosseini, H.; Drouot, X.; Lefaucheur, J.-P. Impaired sleep-associated modulation of post-exercise corticomotor depression in multiple sclerosis. J. Neurol. Sci. 2015, 354, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Di Sapio, A.; Bertolotto, A.; Melillo, F.; Sperli, F.; Malucchi, S.; Troni, W. A new neurophysiological approach to assess central motor conduction damage to proximal and distal muscles of lower limbs. Clin. Neurophysiol. 2014, 125, 133–141. [Google Scholar] [CrossRef] [PubMed]
- von Meyenburg, J.; Wilm, B.J.; Weck, A.; Petersen, J.; Gallus, E.; Mathys, J.; Schaetzle, E.; Schubert, M.; Boesiger, P.; von Meyenburg, K.; et al. Spinal cord diffusion-tensor imaging and motor-evoked potentials in multiple sclerosis patients: Microstructural and functional asymmetry. Radiology 2013, 267, 869–879. [Google Scholar] [CrossRef]
- Conte, A.; Lenzi, D.; Frasca, V.; Gilio, F.; Giacomelli, E.; Gabriele, M.; Bettolo, C.M.; Iacovelli, E.; Pantano, P.; Pozzilli, C.; et al. Intracortical excitability in patients with relapsing–remitting and secondary progressive multiple sclerosis. J. Neurol. 2009, 256, 933–938. [Google Scholar] [CrossRef]
- Firmin, L.; Müller, S.; Rösler, K.M. The latency distribution of motor evoked potentials in patients with multiple sclerosis. Clin. Neurophysiol. 2012, 123, 2414–2421. [Google Scholar] [CrossRef]
- Steens, A.; Heersema, D.; Maurits, N.; Renken, R.; Zijdewind, I. Mechanisms underlying muscle fatigue differ between multiple sclerosis patients and controls: A combined electrophysiological and neuroimaging study. Neuroimage 2011, 59, 3110–3118. [Google Scholar] [CrossRef]
- Morgante, F.; Dattola, V.; Crupi, D.; Russo, M.; Rizzo, V.; Ghilardi, M.F.; Terranova, C.; Girlanda, P.; Quartarone, A. Is central fatigue in multiple sclerosis a disorder of movement preparation? J. Neurol. 2011, 258, 263–272. [Google Scholar] [CrossRef]
- Thickbroom, G.W.; Sacco, P.; Faulkner, D.L.; Kermode, A.G.; Mastaglia, F.L. Enhanced corticomotor excitability with dynamic fatiguing exercise of the lower limb in multiple sclerosis. J. Neurol. 2008, 255, 1001–1005. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gagliardo, A.; Galli, F.; Grippo, A.; Amantini, A.; Martinelli, C.; Amato, M.P.; Borsini, W. Motor evoked potentials in multiple sclerosis patients without walking limitation: Amplitude vs. conduction time abnormalities. J. Neurol. 2007, 254, 220–227. [Google Scholar] [CrossRef]
- Thickbroom, G.W.; Sacco, P.; Kermode, A.G.; Archer, S.A.; Byrnes, M.L.; Guilfoyle, A.; Mastaglia, F.L. Central motor drive and perception of effort during fatigue in multiple sclerosis. J. Neurol. 2006, 253, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Mingers, D.; Heesen, C.; Bäumer, T.; Weiller, C. Motor cortex excitability and fatigue in multiple sclerosis: A transcranial magnetic stimulation study. Mult. Scler. 2005, 11, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Mainero, C.; Inghilleri, M.; Pantano, P.; Conte, A.; Lenzi, D.; Frasca, V.; Bozzao, L.; Pozzilli, C. Enhanced brain motor activity in patients with MS after a single dose of 3,4-diaminopyridine. Neurology 2004, 62, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Wohlfarth, K.; Rollnik, J.D.; Dengler, R. Walking and fatigue in multiple sclerosis: The role of the corticospinal system. Muscle Nerve 1998, 21, 1068–1070. [Google Scholar] [CrossRef]
- Sheean, G.L.; Murray, N.M.; Rothwell, J.; Miller, D.H.; Thompson, A. An electrophysiological study of the mechanism of fatigue in multiple sclerosis. Brain 1997, 120 Pt 2, 299–315. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Berger, M.S.; Tarapore, P.E. Preoperative Applications of Navigated Transcranial Magnetic Stimulation. Front. Neurol. 2021, 11, 628903. [Google Scholar] [CrossRef]
- Soda, J.; Vidakovic, M.R.; Lorincz, J.; Jerkovic, A.; Vujovic, I. A Novel Latency Estimation Algorithm of Motor Evoked Potential Signals. IEEE Access 2020, 8, 193356–193374. [Google Scholar] [CrossRef]
- Giridharan, S.R.; Gupta, D.; Pal, A.; Mishra, A.M.; Hill, N.J.; Carmel, J.B. Motometrics: A toolbox for annotation and efficient analysis of motor evoked potentials. Front. Neuroinform. 2019, 13, 8. [Google Scholar] [CrossRef]
- Harquel, S.; Beynel, L.; Guyader, N.; Marendaz, C.; David, O.; Chauvin, A. CortExTool: A Toolbox for Processing Motor Cortical Excitability Measurements by Transcranial Magnetic Stimulation. 2016. Available online: https://hal.archives-ouvertes.fr/hal-01390016 (accessed on 15 November 2022).
- Harquel, S. Robotized Transcranial Magnetic Stimulation: From Automatized Protocols towards New Approaches in Functional Neu Roimaging. Ph.D. Dissertation, Grenoble Institut des Neurosciences, Laboratoire de Psychologie et NeuroCognition, Neurosciences, Université Greno ble-Alpes, Grenoble, France, 2017. Available online: https://tel.archives-ouvertes.fr/tel-01504993 (accessed on 15 November 2022).
- MEPHunter, a Free Software for Signal Visualization and Analysis; NeuroMat: Paulo, Brazil, 2014.
- Bigoni, C.; Cadic-Melchior, A.; Vassiliadis, P.; Morishita, T.; Hummel, F.C. An automatized method to determine latencies of motor-evoked potentials under physiological and pathophysiological conditions. J. Neural Eng. 2022, 19, 024002. [Google Scholar] [CrossRef]
- Saari, J.; Kallioniemi, E.; Tarvainen, M.; Julkunen, P. Oscillatory TMS-EEG-Responses as a Measure of the Cortical Excitability Threshold. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 383–391. [Google Scholar] [CrossRef]
- Pearce, A.J.; Clark, R.; Kidgell, D. A Comparison of two methods in acquiring stimulus–response curves with transcranial magnetic stimulation. Brain Stimul. 2013, 6, 306–309. [Google Scholar] [CrossRef]
- Houdayer, E.; Degardin, A.; Cassim, F.; Bocquillon, P.; Derambure, P.; Devanne, H. The effects of low- and high-frequency repetitive TMS on the input/output properties of the human corticospinal pathway. Exp. Brain Res. 2008, 187, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, L.; Meyer, B.-U.; Weyh, T. Influence of pulse configuration and direction of coil current on excitatory effects of magnetic motor cortex and nerve stimulation. Clin. Neurophysiol. 2000, 111, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Devanne, H.; Lavoie, B.A.; Capaday, C. Input-output properties and gain changes in the human corticospinal pathway. Exp. Brain Res. 1997, 114, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Goetz, S.M.; Luber, B.; Lisanby, S.H.; Peterchev, A. A Novel Model Incorporating Two Variability Sources for Describing Motor Evoked Potentials. Brain Stimul. 2014, 7, 541–552. [Google Scholar] [CrossRef]
- Goetz, S.M.; Alavi, S.M.M.; Deng, Z.-D.; Peterchev, A.V. Statistical Model of Motor-Evoked Potentials. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, P.; Ferreri, F. W14.4 Amplitude values of motor evoked potentials: Statistical properties and neurophysiological implications. Clin. Neurophysiol. 2011, 122 (Suppl. S1), S44–S45. [Google Scholar] [CrossRef]
- Fadiga, L.; Fogassi, L.; Pavesi, G.; Rizzolatti, G. Motor facilitation during action observation: A magnetic stimulation study. J. Neurophysiol. 1995, 73, 2608–2611. [Google Scholar] [CrossRef]
- Kiers, L.; Cros, D.; Chiappa, K.H.; Fang, J. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1993, 89, 415–423. [Google Scholar] [CrossRef]
- Amassian, V.E.; Cracco, R.Q.; Maccabee, P.J. Focal stimulation of human cerebral cortex with the magnetic coil: A comparison with electrical stimulation. Electroencephalogr. Clin. Neurophysiol. 1989, 74, 401–416. [Google Scholar] [CrossRef]
- Nieminen, E.A.; Nieminen, J.O.; Stenroos, M.; Novikov, P.; Nazarova, M.; Vaalto, S.; Nikulin, V.; Ilmoniemi, R.J. Accuracy and precision of navigated transcranial magnetic stimulation. J. Neural Eng. 2022, 19, 066037, accepted. [Google Scholar] [CrossRef] [PubMed]
- Spetzger, U.; Hubbe, U.; Struffert, T.; Reinges, M.H.T.; Krings, T.; Krombach, G.A.; Zentner, J.; Gilsbach, J.M.; Stiehl, H.S. Error analysis in cranial neuronavigation. Minim Invasive Neurosurg. 2002, 45, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Widmann, G.; Schullian, P.; Ortler, M.; Bale, R. Frameless stereotactic targeting devices: Technical features, targeting errors and clinical results. Int. J. Med. Robot. 2011, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, G.B.; Thielscher, A.; Madsen, K.H.; Knösche, T.R.; Weise, K. A principled approach to conductivity uncertainty analysis in electric field calculations. Neuroimage 2018, 188, 821–834. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).