Abstract
In this study, we designed a new biosensing membrane for the development of an electrochemical glucose biosensor. To proceed, we used a chitosan-based hydrogel that entraps glucose oxidase enzyme (GOx), and we crosslinked the whole matrix using glutaraldehyde, which is known for its quick and reactive crosslinking behavior. Then, the stability of the designed biosensors was investigated over time, according to different storage conditions (in PBS solution at temperatures of 4 °C and 37 °C and in the presence or absence of glucose). In some specific conditions, we found that our biosensor is capable of maintaining its stability for more than six months of storage. We also included catalase to protect the biosensing membranes from the enzymatic reaction by-products (e.g., hydrogen peroxide). This design protects the biocatalytic activity of GOx and enhances the lifetime of the biosensor.
1. Introduction
Electrochemical enzymatic glucose biosensors are the most commonly used devices to monitor blood glucose levels in diabetic patients [1,2,3]. Some of these devices can be implanted subcutaneously in diabetic patients to monitor glucose continuously for up to several weeks, and are called continuous glucose monitoring systems (CGMs). They often contain an electrode and a porous polymer, or hydrogel, encapsulating an enzyme, such as glucose oxidase (GOx), that catalyzes glucose oxidation.
However, commercially available biosensors suffer from low stability for long-time operation and a gradual decrease in sensitivity with storage. Due to the enzyme’s fragility, enzyme-based biosensors typically show a loss of sensitivity over time and consequently, express short lifetime. The loss of sensitivity implies either a replacement or a regular calibration of the sensors [4,5]. Biosensor lifetime is also limited by inflammatory reactions, or foreign body reactions that isolate the device from healthy interstitial fluid. However, providing sensors with enhanced lifetime would improve patient’s quality of life [6,7]. Therefore, it is interesting to develop new methods for stabilizing the enzyme and enhancing glucose biosensors lifetime.
Several strategies have been proposed to enhance enzymatic biosensor lifetime, such as enzyme immobilization and enzyme engineering [8,9]. Indeed, the first strategy remains the most popular, due to its simplicity. There are different enzyme immobilization techniques, including physical adsorption, surface binding by covalent and enzyme encapsulation, and/or entrapment in polymeric matrix [10,11]. In recent years, enzyme encapsulation in hydrogel or polymers has attracted great interest because they adsorb water and provide a microenvironment favorable for enzyme stability over time. However, physical and chemical proprieties of these matrixes (i.e., surface area and roughness, pores size and distribution, membrane thickness and composition, etc.) play a key role in the stability of encapsulated or entrapped enzymes [12]. Depending on those properties, diffusion of glucose through membranes and then the enzyme accessibility may be affected, which influences the biocatalytic activity of the enzymes. Therefore, the choice of polymers or hydrogel-based matrixes and their nature and composition are of great importance.
Many materials were reported as potential candidates for the development of biomembranes such as cellulose [13,14], polycarbonate [15], hyaluronic acid [16], collagen [17,18] and chitosan [19,20]. However, chitosan-based materials are of particular interest due to their particular structural and functional characteristics, i.e., biocompatibility, high permeability, antibacterial and antimicrobial properties, adsorptive characteristics, and low cost [21]. Minteer et al. suggested that enzyme encapsulation within the pores/pockets of the hydrophobically modified micellar polymers, such as Nafion® and chitosan, drastically improves enzymes lifetime [22]. S El ICHI et al. reported that laccase encapsulation in chitosan multi-walled carbon nanotubes composite allows designing a biocathode with several months’ stability [23,24]. Gorski et al. showed that modifying chitosan with a permeability-controlling agent, Acetyl Yellow 9 (AY9), decreases the sensing potential and allows for the interference-free determination of the enzyme’s substrate [25,26]. He also demonstrated in a different work that chitosan could be incorporated in a carbon nanotube powder allowing for the designing of a second-generation biosensor using different enzyme substrates [27,28]. Similarly, in a previous work, we investigated the stability of glucose dehydrogenase (GDH) in a chitosan-MWCNTs pellet and showed enhanced GDH stability for more than one year [29]. In these studies, the introduction of crosslinkers has been proposed to enhance chitosan stability. Indeed, chitosan is a biodegradable polymer that suffers from a loss of stability in an aqueous solution over time, which requires the use of a crosslinker such as genipin or glutaraldehyde. Conversely to genipin, the crosslinking process of chitosan by glutaraldehyde does not demand heating, and can be achieved at room temperature, which prevents enzyme denaturation [30,31]. This makes glutaraldehyde an option of choice for the development of a chitosan-based biomembrane.
In the case of first-generation enzymatic glucose biosensor where the enzymatic oxidation of glucose by GOx leads to the production hydrogen peroxide (H2O2) and gluconic acid, the chitosan matrix should be capable of maintaining its stability in the presence of H2O2 and in an acidic microenvironment resulting from the accumulation of gluconic acid. The aim of the present work is to design a chitosan-based enzymatic glucose biosensor and the study of its stability over time. Our strategy consisted of crosslinking and encapsulating the enzyme GOx into/with the chitosan matrix. Here, we investigated the adhesion of chitosan on the surface of different electrode materials (gold, platinum, and carbon). The stability of the biosensors over time according to different storage conditions (in PBS at 4 °C/37 °C and in the presence or absence of glucose) was also investigated. We also investigated the stability of chitosan-based membranes under dry storage conditions at various temperatures of 4 °C, 37 °C, and at room temperature.
2. Materials and Methods
2.1. Chemicals
Glutaraldehyde solution—grade 1, 70% in water (C5H8O2, CAS No: 111-30-8, ref. G7776), Thiol-NH2 powder (HS-PEG2K-NH2, ref. JKA5143), collagen from bovine Achilles tendon (CAS No: 9007-34-5, ref. C9879), chitosan high molecular weight (310–375 kDa, >75% deacetylated, CAS No: 9007-34-5, ref. 419419), D-Glucose powder (purity > 99.5%, CAS No: 50-99-7, ref. G8270), GOx from Aspergillus Niger (type X-S lyophilized powder, CAS No: 9001-37-0, ref. G7141-50KU), GOx from Aspergillus niger recombinant (CAS No: 9001-37-0, ref. 345386-10KU) and catalase from bovine liver (CAS No: 9001-05-2, ref. C1345) were purchased from Sigma-Aldrich. Stock solution of phosphate-buffered saline (PBS 10x) was also purchased from Sigma-Aldrich and utilized for the preparation of the working solutions (PBS 1x pH 7.4). All reagents were of analytical grade, and milli-Q water was used to prepare solutions.
2.2. Preparation of Hydrogels
Collagen solution (1 wt%) was prepared by dissolving collagen powder in acetic acid solution (0.1 v/v %). The obtained emulsion was stirred at room temperature for 24 h until the collagen powder was fully dissolved. The obtained gel was stored in the fridge at 4 °C when not in use.
Chitosan solution (1 wt%) was prepared by dissolving chitosan powder (high molecular weight) in acetic acid solution (0.5 v/v %). The mixture was left to stir at 70 °C for 1 h to fully dissolve the chitosan. The obtained gel was then placed at room temperature to cool down, and stored in the fridge at 4 °C when not in use. The 1% concentration of chitosan was chosen to limit acetic acid accumulation as much as possible. A higher concentration of acetic acid is required to dissolve chitosan powder when the chitosan concentration increases.
2.3. Electrochemical Apparatus and Electrodes
An SP-300 potentiostat (Biologic Instruments, Grenoble–France) was used for all electrochemical experiments. Screen-printed gold electrodes (auxiliary electrode: Pt and reference electrode: Ag, ref. DRP-250AT-U75) were purchased from METROHM (Villebon-sur-Yvette, France). The working electrodes have a 4 mm diameter characteristic which represents a surface area of 12.56 mm2.
2.4. Electrode Design
For the chitosan-based materials, it was necessary to crosslink the chitosan-hydrogel by adding glutaraldehyde in order to ensure its stability. Our strategy consisted of combining crosslinking and encapsulating the GOx into/within the chitosan matrix. This allows for large amounts of enzymes to be immobilized and stabilizes the GOx enzyme being used [32]. Indeed, some trials conducted in our lab showed that the chitosan matrixes require the use of an adhesion layer specifically dedicated to the commercial gold-based electrodes (METROHM, DRP-250AT). For this purpose, a collagen solution (1 wt%) was used in the presence of glutaraldehyde (added at 2 wt% vs. collagen) and mixed. Five minutes later, 10 µL of the mixture was pipetted and deposed by drop-casting on the working Au-based electrodes and maintained at room temperature for complete drying (approx. 1 h) to provide an intermediate layer that avoided the shrinkage of the chitosan matrix on the working Au-based electrodes [33].
For the preparation of the chitosan matrix: 10 mg of GOx was added to 1 mL chitosan solution (1 wt%), and the mixture was gently shaken for a few seconds (IKA® Vortex). Glutaraldehyde solution (2 wt% related to chitosan) was then added after 5 min, and 10 µL from the prepared mixture was deposed by drop-casting as a second layer on the collagen layer. The electrodes were kept at room temperature until complete drying was achieved (approx. 1 h). Finally, the sensors were rinsed with distilled water (Figure 1).
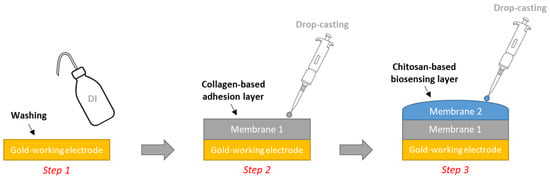
Figure 1.
Illustration of the modification process of the working gold-based screen-printed electrode for the fabrication of glucose biosensors.
2.5. Stability Test of Biosensing Membranes
We investigated the stability of the GOx-chitosan-based glucose biosensor under different storage conditions. The biosensors were first tested in PBS (1X) medium by chronoamperometry. Thereby, the sensors were polarized at + 0.7 V vs. Ag/AgCl via the reference electrode (Figure S1). The electrochemical response was then recorded in presence of an increasing concentration of glucose in the bulk solution under a dynamic condition of 200 rpm. This allows for the establishment of the calibration curve (iglucose = f ([Glucose]) and the determination of the sensitivity of the biosensors at the first day. Herein, the sensitivity corresponds to the slope value of the calibration curve (expressed in nA/mM).
3. Results and Discussion
In order to highlight the immobilization process, it was necessary to understand the reaction that could occur during the fabrication of the bio-based membranes. Indeed, the Gox, as well as the chitosan polymer, are well-known to contain many amine groups in their structures. This feature facilitates the derivatization process. Thus, in the presence of the glutaraldehyde crosslinker (added at 2 wt%), a non-selective reaction leads to obtain three reaction byproducts, as represented in Figure 2. The best scenario is reached when the chitosan is crosslinked and bonded to the GOx enzyme through the glutaraldehyde chain. This configuration specifically prevents from enzyme’s release from the chitosan matrix into solution. However, a chitosan polymer could be crosslinked to another chitosan polymer chain, and the GOx could be also crosslinked and bonded to another GOx enzyme through a glutaraldehyde chain. This reaction occurs randomly; thus, the homogeneity of the GOx/chitosan mixture is of great importance in order to obtain reproducible results.
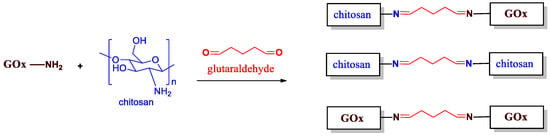
Figure 2.
Immobilization process of the enzyme GOx into the chitosan polymer using the glutaraldehyde crosslinker (added at 2 wt%).
Figure 3 shows the amperometric response of GOx-chitosan biosensor to successive additions of glucose. We can see that we have a typical amperometric response as a function of glucose concentration showed a linear relationship (r2 > 0.996) in a concentration range of glucose of 0.25–1.5 mM (Figure 3 inset). The apparent Michaelis–Menten constant (Kmapp) of the GOx was determined, and it is near 1 mM. Initially, the biosensors were kept and stored in a phosphate buffer solution (PBS 1X) in the absence or presence of glucose (2 mM) in the solution. After that, the biosensors are retested to investigate the variation of their sensitivities at different times. The results are always normalized according to the value of the sensors’ sensitivities obtained at day 1 for each biosensor.
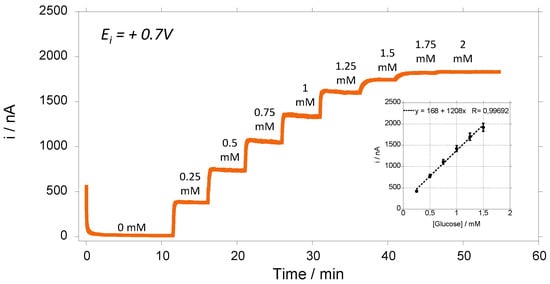
Figure 3.
Amperometric responses at + 0.7 V vs. Ag/AgCl of glucose biosensing) in PBS(1X) medium under hydrodynamic conditions of 200 rpm. Inset: calibration curve of glucose during electrochemical oxidation (average value and standard deviation on 3 electrodes).
The changes in biosensors sensitivity as a function of the storage day in PBS (1X) in the presence of glucose at 2 mM at 37 °C (Figure 4, grey plot) show a stable sensitivity in the first 7 days of storage, with only a 10% decrease in sensitivity after 1 week of storage. The sensitivity drastically decreased after 2 weeks of storage, with a 90% loss of sensitivity (Figure 4), probably due to the degradation of the chitosan-based membrane. Indeed, in the presence of glucose in the storage solution, GOx continuously catalyzes the oxidation of glucose into hydrogen peroxide (H2O2) and gluconic acid, according to the following reaction [2,34]:
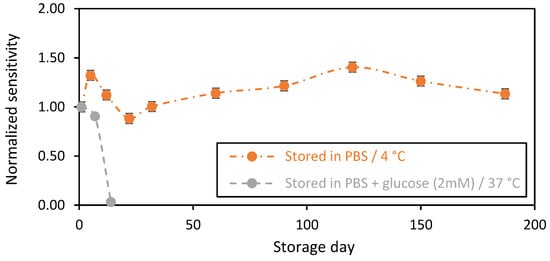
Figure 4.
Stability in function of time of the chitosan-based biosensing membranes deposed on gold screen-printed electrodes. Orange plot: biosensors stored in PBS at 4 °C, and grey plot: PBS + glucose (2 mM) at 37 °C (average value and standard deviation on 2 sensors).
However, it is well known that the accumulation of the enzymatic reaction by-products, in particular, H2O2 at high concentration levels, could contribute to the change in the electrode material properties [35,36,37]. Herein, the pH of the storage solution was investigated and followed over time. A slight acidification of the medium was detected (ΔpH ≈ 0.2), despite the presence of phosphate buffer, confirming the accumulation of acidic byproducts of the enzymatic reaction. Thus, this observation could help to confirm our hypothesis. It is important that we note that the pH value can be lower inside the matrix due to a local acidification of the nearby chitosan GOxs sites. Indeed, the activity of the immobilized GOx is pH-dependent. Thus, the local acidification in chitosan matrixes negatively affects the catalytic activity of the GOxs and acts to decrease the sensor’s sensitivities over time [38]. On the other hand, the continuous measurement of glucose for 24 h was studied (Figure S2). We observed a significant drift, compared to that in the storage condition. We believe here that this drift is more related to the degradation of the electrode under H2O2 electrooxidation, which is capable of passivating the electrode surface. This is a typical behavior of the gold-based sensors (signal drift) that should be considered and corrected. For the biosensors that were stored in PBS without any glucose at 4 °C (orange plot), functional stability was observed for more than 6 months of storage. The changes in the biosensors sensitivities over time may be due to the conditioning of the chitosan-based matrix. For instance, chitosan material is well known for its high swelling capacity, which could affect the enzyme stability over time [39]. Indeed, it is noteworthy to highlight that the apparent Michaelis–Menten constant (Kmapp) of the GOx was not affected during the long storage period, and it expressed the same value over time of ~1 mM.
Until now, and for all these samples, GOx and chitosan crosslinking was performed in a single step and that leads to a chitosan matrix where a single GOx may be crosslinked to chitosan. As mentioned above, one step crosslinking leads to the random distribution and crosslinking of GOx. It was interesting to study the effect of the crosslinking order on the stability of the biosensing membranes. To do so, we first crosslinked GOx molecules and subsequently added them to chitosan. In contrast to the first membrane design, where the crosslinking was realized non-selectively between the GOx and the chitosan, herein, only one reaction product was expected, considering that the GOx molecules are crosslinked and bonded together. In the latter design, the GOx molecules are entrapped without any covalent bonds within the chitosan polymer. Here, we assumed that all the glutaraldehyde was consumed because of its high reactivity and thus, we supposed that no more glutaraldehyde was left to react and crosslink the chitosan polymer.
This new enzyme immobilization design displayed higher stability (Figure 5, grey plot). The biosensors showed an increased normalized sensitivity of ~ 1.18 for the first week of storage in PBS in the presence of glucose at 2 mM (normalized with respect to first day of operation). This is probably due to the swelling capacity of the chitosan matrixes that increased the glucose diffusion to the GOx active sites. Afterwards, the biosensor sensitivity decreased progressively over time, probably due to a decrease in the biocatalytic activity of the GOx. After one month of storage, the sensors expressed a normalized sensitivity near 0.25. During this period, the sensors were stored in the presence of glucose with continuous enzymatic activity. Despite this decrease in the biocatalytic activity of the GOx, this immobilization method stabilized the redox enzyme that was active for more than one month.
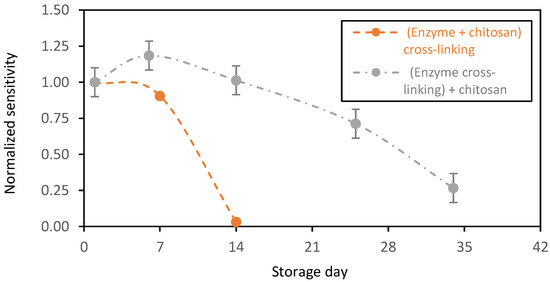
Figure 5.
Stability in function of time of the chitosan-based biosensing membranes deposed on gold screen-printed electrodes and stored in PBS + glucose (2 mM) at 37 °C. Orange plot: GOx entrapped and crosslinked, and grey plot: GOx entrapped (average value and standard deviation on 2 sensors).
We then compared the performance of our GOx-chitosan based glucose biosensors to recombinant GOx (rGOx)-chitosan based glucose biosensors. Indeed, the rGOx is a structurally modified enzyme on a nanoscopic scale. The recombinant enzyme displays similar properties to the native enzyme, but exhibits more advantageous properties for glucose oxidation, including the specificity to the substrate (glucose) and an enhanced stability over time [40,41]. Additionally, rGOx might contain more impurities, including enzymatic impurities. In particular, rGOx contains catalase as an enzymatic impurity at a higher percentage compared to the native GOx from Aspergillus niger. Here, it should be noted that catalase, an oxidoreductase, catalyzes the decomposition of H2O2 into O2 and water as follows [42]:
According to this reaction, H2O2 produced by the GOx or rGOx should be consumed/decomposed locally by the catalase into O2 and H2O. It is noteworthy to highlight that this decomposition should be partial to allow for the detection and oxidization of H2O2 on the surface of the electrode. In contrast, the complete decomposition of H2O2 leads to the elimination of the electrochemical response related to H2O2 oxidation.
We tested the stability of the biosensing membranes designed with the GOx or rGOx, and found increased rGOx stability over time (Figure 6, grey plot). Nevertheless, after 2 weeks of storage in PBS in the presence of glucose (at 2 mM), a 30% decrease in sensitivity was observed, with a 50% decrease after 3 weeks. This relatively decreases the level of the catalytic current, but it probably protects the chitosan-based membrane from intoxication by the local and continuous accumulation of H2O2 and prevents the biocatalytic activity of GOx from drastically decreasing.
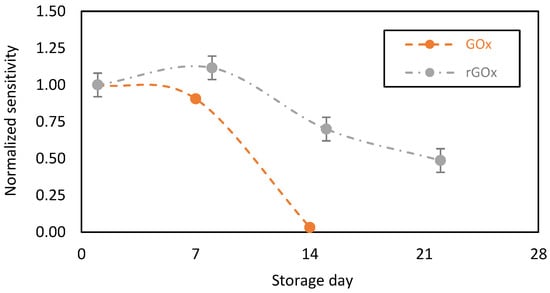
Figure 6.
Stability in function of time of the chitosan-based biosensing membranes deposed on gold screen-printed electrodes and stored in PBS + glucose (2 mM) at 37 °C. Orange plot: normal Gox, and grey plot: recombinant rGOx (average value and standard deviation on 2 sensors).
To verify this hypothesis, we prepared a GOx biosensor in which we added catalase to the native GOx in 2/10 ratio (2 catalase units per 10 GOx units). Each GOx unit oxidizes 1 µmole of glucose, while each unit of catalase theoretically decomposes 1 µmole of H2O2 per minute. Therefore, mixing catalase with GOx in a 2/10 ratio was expected to decrease the local H2O2 concentration within the membrane, while preserving glucose detection through H2O2 reoxidation at the electrode surface.
Unsurprisingly, the catalytic current observed at day 1 in the presence of catalase was lower than that observed in the absence of catalase (Figure 7a). This could be attributed to the partial decomposition of H2O2 by catalase, which automatically prevents H2O2 diffusion to the electrode surface and reduces the catalytic current by cascade. Additionally, the effect of the addition of catalase on the limit of detection (LoD) of the biosensors was also studied. Unsurprisingly, LoD was slightly affected by the presence of catalase (10.8 µM vs. 7.1 µM, respectively, in the absence and in presence of catalase in the biosensing membrane). However, the biosensing membranes containing catalase were also more stable over time (Figure 7b, grey plot). Indeed, even in the presence of glucose (2 mM), there was a remarkable increase in the sensors’ sensitivity that peaked at ~ 3 times its initial sensitivity after 2 weeks of storage. This can be attributed to the swelling capacity of chitosan, as well as to the change in the physicochemical properties of the biomembranes due to the presence of catalase. Indeed, the Kmapp of the GOx was not affected by the presence of catalase. This aspect is of high interest, as it supports that the GOx preserved similar properties in the latter conditions.
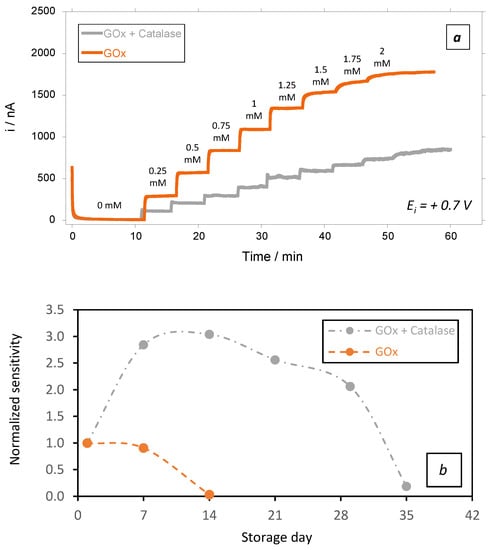
Figure 7.
(a) Amperometric responses at + 0.7 V vs. Ag/AgCl in PBS(1X) medium under hydrodynamic conditions of 200 rpm for testing of biosensors at day 1 in presence of an increased concentration of glucose, and (b) stability in function of time of the chitosan-based biosensing membranes deposed on gold screen-printed electrodes and stored in PBS + glucose (2 mM) at 37 °C: orange plots: GOx, and grey plots: GOx + added catalase (average value and standard deviation on 2 sensors).
These results confirm that the catalase added in the vicinity of GOx firstly, protects the chitosan-based membrane from the intoxication by the locally accumulated H2O2 and secondly, improves/protects the biocatalytic activity of the GOx.
On the other hand, in addition to the wet storage in PBS(1X) (with or without glucose), it is considerably interesting to investigate the enzymatic biosensor’s stability under dry storage conditions. In fact, for logistical reasons, there is a need to understand the impact of temperature variation on the enzymatic membrane’s stability. For this purpose, the stability of the biosensing membranes was investigated under dry storage conditions at various temperatures of 4 °C (realized in the fridge), 37 °C (realized in the oven), and at room temperature. In the cases where the biosensors were stored at 4 °C and at 37 °C, they maintained stable temperatures. However, for the storage at room temperature, the biosensors suffered from humidity and the change in temperature between day and night. The storage at 4 °C ensures a high humidity level in contrast to the storage in the oven (at 37 °C), where the humidity is relatively low. For all the sensors, the enzymatic reaction is stopped during the dry storage, while the sensors’ sensitivity was evaluated at room temperature by establishing a calibration curve for each sensor and comparing their sensitivities on a normalized scale at different times.
The results comparing the stability of the biosensing membranes that were stored dry at the various temperatures for up to 5 months are presented in Figure 8. It is clearly shown that the stability of sensors stored at room temperature (blue plot) was maximized for all the measurements. They expressed stable responses during the first 3 weeks. After that, the sensitivity of the biosensing membranes decreased by 15 and 60% at 2 and 5 months, respectively. However, for the sensors stored at 37 °C (orange plot), the sensitivity increased by 20% after 2 weeks of storage and then fell to 25% and 55% after 3 and 5 months, respectively. The sensors stored at 4 °C (grey plot) showed stable responses in the first week of dry storage, following by a decrease of 23%, 48%, and 63% after 20, 90, and 150 days, respectively. These results confirm that the storage temperature (under dry conditions) impacts the catalytic activity of the GOx and affects the chitosan biomembrane microenvironment. This should be considered in the future for designing long-term in vivo sensor systems.
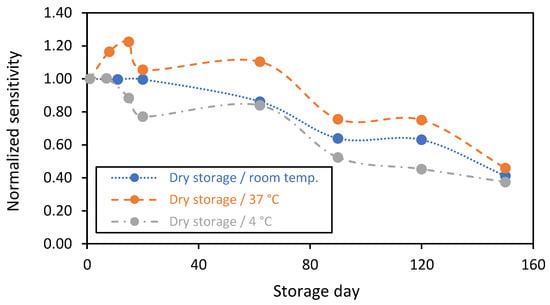
Figure 8.
Stability in function of time of the chitosan-based biosensing membranes deposed on gold screen-printed electrodes and stored dry at various temperatures: (blue plot) room temperature, (orange plot) 37 °C, and (grey plot) 4 °C (n = 1 sensor).
4. Conclusions
A GOx-based first generation glucose biosensor has been successfully designed and its stability over time under different storage and operation conditions has been investigated. Our results show that GOx encapsulation in a chitosan matrix is capable of providing high catalytic activity for at least six months.
The stability of the biosensors decreased dramatically when they were stored in glucose 2 mM, but prior crosslinking of GOx before its encapsulation into chitosan significantly improved the stability of the biosensors. In addition, we demonstrated that the addition of catalase enhanced and protected the chitosan-based membrane from intoxication by the local accumulation of H2O2. Overall, our GOx-chitosan-based glucose biosensors could remain functional for up to one month, thereby showing a high potential for industrial developments in continuous glucose monitors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/s23010465/s1, Figure S1: Stability in function of time of the chitosan-based biosensing membranes deposed on gold screen-printed electrodes during continuous measurement of glucose for 24 h. Experimental conditions: PBS(1X) medium, [Glucose] = 0.5 mM (stable), under stirring (200 rpm) and current sampling frequency of 10 s and; Figure S2: Study by cyclic voltammetry at various scan rates between 0–1 V of the chitosan-based biosensing membranes deposed on gold screen-printed electrodes, [Glucose] = 1 mM.
Author Contributions
Conceptualization, A.C. and I.S.; methodology, A.C. and A.Z; validation, A.C.; formal analysis, A.C., A.Z. and S.M.; investigation, A.C.; resources, A.C.; writing—original draft preparation, A.C.; writing—review and editing, A.C., I.S., A.Z. and S.M.; visualization, A.C. and A.Z.; supervision, A.Z.; project administration, A.Z.; funding acquisition, S.M. and A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the generosity of the following institutions that supported our research work: CNRS (MITI 2022 project), Institut Carnot LSI (BIOEPC 2022 project), and Campus France (PHC POLONIUM 2021 program).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent Advances in Electrochemical Glucose Biosensors: A Review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Soranzo, T.; ben Tahar, A.; Chmayssem, A.; Zelsmann, M.; Vadgama, P.; Lenormand, J.-L.; Cinquin, P.K.; Martin, D.; Zebda, A. Electrochemical Biosensing of Glucose Based on the Enzymatic Reduction of Glucose. Sensors 2022, 22, 7105. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.D.; Turner, A.P.F. Home Blood Glucose Biosensors: A Commercial Perspective. Biosens. Bioelectron. 2005, 20, 2435–2453. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Lopez, D.A.; Lattanzi, G.; Schreiber, L.H.; Wallace, E.J.; Wylie, R.; O’Sullivan, J.; Dolan, E.B.; Duffy, G.P. Medical Devices, Smart Drug Delivery, Wearables and Technology for the Treatment of Diabetes Mellitus. Adv. Drug Deliv. Rev. 2022, 185, 114280. [Google Scholar] [CrossRef]
- Hovorka, R. Continuous Glucose Monitoring and Closed-Loop Systems. Diabet. Med. 2006, 23, 1–12. [Google Scholar] [CrossRef]
- Freckmann, G. Basics and Use of Continuous Glucose Monitoring (CGM) in Diabetes Therapy. J. Lab. Med. 2020, 44, 71–79. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme Immobilization: An Update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, G.; Ahmad, T.; Mansoor, S.; Kaur, B. Enzyme Engineering: Current Trends and Future Perspectives. Food Rev. Int. 2021, 37, 121–154. [Google Scholar] [CrossRef]
- Reyes-De-Corcuera, J.I.; Olstad, H.E.; García-Torres, R. Stability and Stabilization of Enzyme Biosensors: The Key to Successful Application and Commercialization. Annu. Rev. Food Sci. Technol. 2018, 9, 293–322. [Google Scholar] [CrossRef]
- Chmayssem, A.; Petit, L.; Verplanck, N.; Mourier, V.; Vignoud, S.; Vrana, N.E.; Mailley, P. Characterization of the Impact of Classical Cell-culture Media on the Response of Electrochemical Sensors. Electroanalysis 2022, 34, 1201–1211. [Google Scholar] [CrossRef]
- van den Hurk, R.; Evoy, S. A Review of Membrane-Based Biosensors for Pathogen Detection. Sensors 2015, 15, 14045–14078. [Google Scholar] [CrossRef] [PubMed]
- Rauf, S.; Ihsan, A.; Akhtar, K.; Ghauri, M.A.; Rahman, M.; Anwar, M.A.; Khalid, A.M. Glucose Oxidase Immobilization on a Novel Cellulose Acetate- Polymethylmethacrylate Membrane. J. Biotechnol. 2006, 121, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; He, J.; Chen, X.; Qiao, Y.; Wang, F.; Xia, Q.; Yu, L.; Lu, Z. A Wearable, Cotton Thread/Paper-Based Microfluidic Device Coupled with Smartphone for Sweat Glucose Sensing. Cellulose 2019, 26, 4553–4562. [Google Scholar] [CrossRef]
- Zahran, E.M.; Prodromidis, M.I.; Bhattacharyya, D.; Bachas, L.G. Palladium Nanoparticle-Decorated Iron Nanotubes Hosted in a Polycarbonate Porous Membrane: Development, Characterization, and Performance as Electrocatalysts of Ascorbic Acid. Anal. Bioanal. Chem. 2012, 404, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/Chitosan/Hyaluronic Acid–Based Injectable Hydrogels for Tissue Engineering Applications – Design, Physicochemical and Biological Characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef]
- Coulet, P.R.; Gautheron, D.C. Enzymes Immobilized on Collagen Membranes: A Tool for Fundamental Research and Enzyme Engineering. J. Chromatogr. A 1981, 215, 65–72. [Google Scholar] [CrossRef]
- Chaubaroux, C.; Vrana, E.; Debry, C.; Schaaf, P.; Senger, B.; Voegel, J.C.; Haikel, Y.; Ringwald, C.; Hemmerlé, J.; Lavalle, P.; et al. Collagen-Based Fibrillar Multilayer Films Cross-Linked by a Natural Agent. Biomacromolecules 2012, 13, 2128–2135. [Google Scholar] [CrossRef]
- Song, M.; Lin, X.; Peng, Z.; Xu, S.; Jin, L.; Zheng, X.; Luo, H. Materials and Methods of Biosensor Interfaces With Stability. Front. Mater. 2021, 7, 583739. [Google Scholar] [CrossRef]
- Bahar, T.; Yazici, M.S. Assessment of Glucose Oxidase Based Enzymatic Fuel Cells Integrated With Newly Developed Chitosan Membranes by Electrochemical Impedance Spectroscopy. Electroanalysis 2020, 32, 1304–1314. [Google Scholar] [CrossRef]
- Annu; Raja, A.N. Recent Development in Chitosan-Based Electrochemical Sensors and Its Sensing Application. Int. J. Biol. Macromol. 2020, 164, 4231–4244. [Google Scholar] [CrossRef] [PubMed]
- Klotzbach, T.L.; Watt, M.; Ansari, Y.; Minteer, S.D. Improving the Microenvironment for Enzyme Immobilization at Electrodes by Hydrophobically Modifying Chitosan and Nafion® Polymers. J. Memb. Sci. 2008, 311, 81–88. [Google Scholar] [CrossRef]
- el Ichi-Ribault, S.; Zebda, A.; Tingry, S.; Petit, M.; Suherman, A.L.; Boualam, A.; Cinquin, P.; Martin, D.K. Performance and Stability of Chitosan-MWCNTs-Laccase Biocathode: Effect of MWCNTs Surface Charges and Ionic Strength. J. Electroanal. Chem. 2017, 799, 26–33. [Google Scholar] [CrossRef]
- el Ichi, S.; Zebda, A.; Alcaraz, J.P.; Laaroussi, A.; Boucher, F.; Boutonnat, J.; Reverdy-Bruas, N.; Chaussy, D.; Belgacem, M.N.; Cinquin, P.; et al. Bioelectrodes Modified with Chitosan for Long-Term Energy Supply from the Body. Energy Environ. Sci. 2015, 8, 1017–1026. [Google Scholar] [CrossRef]
- Wei, X.; Cruz, J.; Gorski, W. Integration of Enzymes and Electrodes: Spectroscopic and Electrochemical Studies of Chitosan−Enzyme Films. Anal. Chem. 2002, 74, 5039–5046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mullens, C.; Gorski, W. Coimmobilization of Dehydrogenases and Their Cofactors in Electrochemical Biosensors. Anal. Chem. 2007, 79, 2446–2450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gorski, W. Amperometric Ethanol Biosensors Based on Chitosan-NAD+-Alcohol Dehydrogenase Films. Electroanalysis 2011, 23, 1856–1862. [Google Scholar] [CrossRef]
- Zhang, M.; Smith, A.; Gorski, W. Carbon Nanotube−Chitosan System for Electrochemical Sensing Based on Dehydrogenase Enzymes. Anal. Chem. 2004, 76, 5045–5050. [Google Scholar] [CrossRef]
- Tahar, A.B.; Szymczyk, A.; Tingry, S.; Vadgama, P.; Zelsmanne, M.; Tsujumura, S.; Cinquin, P.; Martin, D.; Zebda, A. One-Year Stability of Glucose Dehydrogenase Confined in a 3D Carbon Nanotube Electrode with Coated Poly-Methylene Green: Application as Bioanode for a Glucose Biofuel Cell. J. Electroanal. Chem. 2019, 847, 113069. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yoon, H.H. Immobilization of Glucose Oxidase within Chitosan and Sol-Gel Matrix for Biosensors. Mol. Cryst. Liq. Cryst. 2012, 566, 38–44. [Google Scholar] [CrossRef]
- Bahar, T. Development of Reasonably Stable Chitosan Based Proton Exchange Membranes for a Glucose Oxidase Based Enzymatic Biofuel Cell. Electroanalysis 2020, 32, 536–545. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial Applications of Immobilized Enzymes—A Review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Susanto, A.; Susanah, S.; Priosoeryanto, B.P.; Satari, M.H.; Komara, I. The Effect of the Chitosan-Collagen Membrane on Wound Healing Process in Rat Mandibular Defect. J. Indian Soc. Periodontol. 2019, 23, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Glucose Biosensors: 40 Years of Advances and Challenges. Electroanalysis 2001, 13, 983–988. [Google Scholar] [CrossRef]
- Chmayssem, A.; Verplanck, N.; Tanase, C.E.; Costa, G.; Monsalve-Grijalba, K.; Amigues, S.; Alias, M.; Gougis, M.; Mourier, V.; Vignoud, S.; et al. Development of a Multiparametric (Bio)Sensing Platform for Continuous Monitoring of Stress Metabolites. Talanta 2021, 229, 122275. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.; Ren, G.; Li, Y.; Li, Y.; Du, X. Highly Efficient Electrosynthesis of Hydrogen Peroxide on a Superhydrophobic Three-Phase Interface by Natural Air Diffusion. Nat. Commun. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Ahmad, T.; Iqbal, A.; Halim, S.A.; Uddin, J.; Khan, A.; el Deeb, S.; Al-Harrasi, A. Recent Advances in Electrochemical Sensing of Hydrogen Peroxide (H2O2) Released from Cancer Cells. Nanomaterials 2022, 12, 1475. [Google Scholar] [CrossRef]
- Norouzi, P.; Faridbod, F.; Larijani, B.; Ganjali, M.R. Glucose Biosensor Based on MWCNTs-Gold Nanoparticles in a Nafion Film on the Glassy Carbon Electrode Using Flow Injection FFT Continuous Cyclic Voltammetry. Int. J. Electrochem. Sci. 2010, 5, 1213–1224. [Google Scholar]
- Pradhan, A.K.; Rana, P.K.; Sahoo, P.K. Biodegradability and Swelling Capacity of Kaolin Based Chitosan-g-PHEMA Nanocomposite Hydrogel. Int. J. Biol. Macromol. 2015, 74, 620–626. [Google Scholar] [CrossRef]
- Courjean, O.; Mano, N. Recombinant Glucose Oxidase from Penicillium Amagasakiense for Efficient Bioelectrochemical Applications in Physiological Conditions. J. Biotechnol. 2011, 151, 122–129. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, M.; Zhang, X.; Liang, Q.; Yang, M.; Mou, H.; Zhu, C. A Thermostable Glucose Oxidase from Aspergillus Heteromophus CBS 117.55 with Broad PH Stability and Digestive Enzyme Resistance. Protein Expr. Purif. 2020, 176, 105717. [Google Scholar] [CrossRef] [PubMed]
- Goodman, H.G.; Grumbach, M.M.; Kaplan, S.L.; Escamilla, R.F.; Lewis, U.J. Three-Dimensional Structure of the Enzyme Catalase. Nature 1981, 293, 411–412. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).