Abstract
This review is dedicated to the development of molecularly imprinted polymers (MIPs) and the application of MIPs in sensor design. MIP-based biological recognition parts can replace receptors or antibodies, which are rather expensive. Conducting polymers show unique properties that are applicable in sensor design. Therefore, MIP-based conducting polymers, including polypyrrole, polythiophene, poly(3,4-ethylenedioxythiophene), polyaniline and ortho-phenylenediamine are frequently applied in sensor design. Some other materials that can be molecularly imprinted are also overviewed in this review. Among many imprintable materials conducting polymer, polypyrrole is one of the most suitable for molecular imprinting of various targets ranging from small organics up to rather large proteins. Some attention in this review is dedicated to overview methods applied to design MIP-based sensing structures. Some attention is dedicated to the physicochemical methods applied for the transduction of analytical signals. Expected new trends and horizons in the application of MIP-based structures are also discussed.
1. Introduction
In order to simplify analysis and to reduce costs, various affinity sensors are currently applied for various practical needs. In these sensors, a variety of analytical signal transduction methods are used to achieve sufficient sensitivity. However, the achievement of reliable selectivity is a much more complicated task; therefore, to resolve this challenge, many new and/or chemically advanced materials are designed [1,2]. Saliva, blood, blood serum, urine and other biological liquids contain some markers that are important for biomedical purposes. Currently, catalytic and affinity-based biosensors are most frequently used for the determination of some biologically active compounds that are present in biological samples [3,4].
To improve the selectivity of chemical sensors, many different semiconducting structures are designed [5,6]. It should be noted that, rather frequently, conducting polymers (CPs) are applied in the design of these sensing structures. Conducting polymers can be formed in many different ways; therefore, they are applied for the design of sensing structures, which increases the selectivity of the analytical method towards target compounds [1]. Conducting polymers are electrically conducting [7], have great electrical capacitance [8,9,10], can be well adhered to surface electrodes and form mechanically stable layers [11,12]. Some CPs demonstrate a great ability to transfer electrical charges and are used for electron transfer from some redox proteins and other biological structures [13]. Due to the above-mentioned properties, CPs are applied in the design of sensing layers used together with different signal transducers. In sensors and biosensors, the most frequently applied CPs are polypyrrole (Ppy), polyaniline (PANI), polythiophene (PTH) and poly(3,4-ethylenedioxythiophene) (PEDOT) [14,15,16,17,18]. Many different polymerization methods are used to design sensing structures based on conducting polymers, which are divided into chemical synthesis [19], electrochemical deposition [8], enzymatic formation [20] and/or microorganism assisted polymerization [21,22,23]. Moreover, CPs can serve as matrices for the immobilization of biomaterials, which are selectively binding targeted analytes, namely DNA [24], antibodies [1] receptors [25], antigens [26], antibodies [20] and enzymes [27,28,29]. These immobilized biomolecules provide specific selectivity to CP-based sensors. However, these biomolecules are not very stable and are very expensive; therefore, suitable replacements of these compounds are demanded. One of the most attractive alternatives to natural biological recognition compounds is based on the application of ‘synthetic receptors, mimics of antibodies and/or molecularly imprinted polymers (MIPs) [30,31], where conducting polymers are finding very great applicability [30,31,32,33,34,35]. In numerous researches, it was demonstrated that MIPs can be applied in the development of sensors for the diagnosis of infectious [36] and some other diseases.
Herein, we overview methods applied for the design of conducting polymer-based sensors and the modification of these polymers by molecular imprints of different molecules.
2. Chemical Formation of Conducting Polymers Based on Redox Processes
The correct chemical formation is required for the development of conducting polymer-based nano- or/and micro-particles suitable for the design of molecularly imprinted polymer-based layers used for chemical sensors and chromatographic systems, and many other purposes [37,38]. Electroactive polymers are used not only for sensor design [39,40] but also for tissue regeneration and some other biomedical purposes [41]. Many approaches for the formation of conducting polymers are elaborated in order to fulfill technological demands. Chemical methods are very frequently used for the synthesis of large quantities of CPs, where polymerization is initiated by oxidators, namely FeCl3, H2O2 etc. [42,43,44]. The application of H2O2 enables the formation of very pure conducting polymers because the unreacted H2O2 rather quickly degrades into oxygen and water. Figure 1 represent the enzymatic synthesis of conducting polymer polypyrrole [19,44]. Many others conducting polymers, including polythiophene [44,45], poly-phenanthrenequinone [46], poly(pyrrole-2-carboxylic acid) [47], polyphenanthroline [13], azobenzene [48], carbazole [49], were designed using approaches similar to that represented in Figure 1.
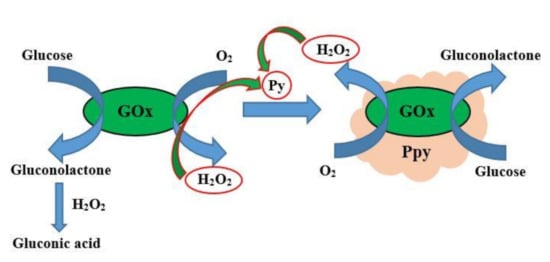
Figure 1.
Chemical synthesis of polypyrrole, which was performed by glucose oxidase (GOx) assisted polymerization, where H2O2 is formed by GOx and acts as an initiator of the polymerization reaction. Figure adapted from [50].
In several of our researches, we have shown that some conducting polymers (e.g., polypyrrole) possess very good compatibility with stem cells and retain their biological activity [51,52]. A very important finding demonstrated by our research team illustrated that polypyrrole particles injected into the peritoneum of mice do not irritate the immune system of these animals [53]. Simple chemical polymerization procedures enable the synthesis of large quantities of various conducting polymers, which in addition can be additionally functionalized by entrapped molecules and other structures. Hence, various biomolecules, nanostructures, organic molecules, inorganic compounds and even ions can be incorporated within chemically formed conducting polymers [29,47,54,55].
Various redox enzymes, including oxidases, can be applied to perform the synthesis of conducting polymers. Glucose oxidase is the most frequently used for the formation of conducting polymers [20,29,47,54,55]. A very important fact is that such enzymatic synthesis can be pursued in an aqueous environment at ambient pH and temperature [56]. During the enzyme-assisted synthesis of conducting polymers, dissolved [54] and immobilized [27,29,47,55] enzymes (e.g.: glucose oxidase) can be used as producers of hydrogen peroxide and after theself-encapsulation within the formed conducting-polymer matrix these enzymes retain their catalytic activity. This method is well suited for the tuning of catalytic characteristics (especially for the adjustment of apparent Michaelis constant, which is mainly affected by the reduced diffusion rate of the substrate through a formed polymer layer) of enzymes immobilized in such conducting polymer-based structures. Such composite structures, which are based on immobilized enzymes, can be applied in the development of biosensors and biofuel cells [1,4,57,58].
It should be noted that conducting polymer (e.g., polypyrrole) formation can be induced by other oxidizing compounds, e.g., Fe3+ of [Fe(CN)6]3− ions [59]. Therefore, metabolic redox reactions, which are running in microorganisms, can be involved in redox cycling of [Fe(CN)6]3−/[Fe(CN)6]4− ions and in such a way can be exploited for the initiation of conducting polymer formation [21,22,23]. In some particular cases, nano-composite-based structures based on conducting polymers (e.g., PANI, Ppy, etc.) with embedded gold nanoparticles (AuNPs) and glucose oxidase (GOx) PANI/AuNPs-GOx can be designed [60]. Chemical polymerization is a rather basic procedure, which is suitable for the production of large quantities of MIPs [61,62]. Chemically formed conducting polymers can be deposited on transducer surfaces by spin coatings, solvent casting and other surface modification techniques [63]. However, most conducting polymers are not soluble in conventional solvents; therefore, technologically, it is not easy to deposit a sensing layer on the surface of the transducer. This challenge can be overcome using electrochemical conducting polymer formation methods.
3. Formation of Conducting Polymers by Electrochemical Methods
There are many methods used for the electrochemical formation of conducting polymers [64]; therefore, there is plenty of room to perform various modifications of CP-based layers during the deposition procedure. Characteristics of formed CP-based layers and other structures depend on parameters applied during the deposition procedure. The most important electrochemical deposition parameters are: (i) applied voltage, (ii) potential sweep rate used when potential cycling is applied, (iii) the duration of applied potential pulses and periods between them when potential pulse techniques are used, (iv) the control of charge, which is passing through the cell, [65,66], (v) the variation of ion and material concentrations that are present in the polymerization-bulk solution [67,68,69], (vi) treatment by ultrasound and some other external factors [70]. Thickness, density, ion permeability and many other characteristics of electrochemically deposited films are mostly affected by these conditions [26,71,72]. The porosity of the chemically sensitive layer, which is one of the most important factors affecting the analytical performance of the sensing layer, can also be changed by the aforementioned parameters [73,74,75]. Therefore, the adaptation of the above-listed parameters paves a way to design CP-modified electrodes with different electrochemical and analytical properties. In addition, the electrochemical modification of electrodes by conducting polymers can be instantly controlled by measuring/calculating the resistance and capacitance of the formed layer [26]. Polypyrrole [8,11,14,15,26,30,76], polyaniline [60], poly-9,10-phenanthrenequinone [46] and polythiophene derivatives [42], are among the most frequently electrochemically formed conducting polymers.
Various nanostructures (e.g., carbon nanotubes [77], molecules [35] and/or ions) can be entrapped within the formed conducting polymer layer, see Figure 2 [50]. It is very remarkable that these molecules and/or ions can be extracted from the polymer matrix using various procedures and in such a way that molecularly imprinted polymers are designed. Many polymerization approaches (including the above-mentioned electrochemical and chemical procedures) are used for the formation of MIPs. However, we believe that electrochemical deposition of MIP-based layers is more advantageous [78,79] when compared to chemical methods because electrochemical deposition provides much more options for variations in thickness, morphology and doping/de-doping of MIP-based structures. In addition, many other electrochemically performed procedures can be applied to advance these formed structures. One very useful procedure is overoxidation, which can be performed by the electrochemical treatment of film by positive electrode potentials that are much higher than those required for the initiation of a polymerization reaction [50]. Sometimes overoxidation occurs spontaneously when dissolved oxygen is present in the solution of polymerization-bulk and/or very active oxygen forms are electrochemically formed at the anode of the electrochemical cell.
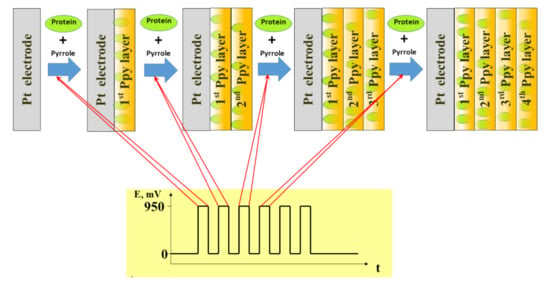
Figure 2.
Electrochemical deposition of polypyrrole with simultaneously entrapped proteins using potential pulse-based technique, figure adapted from [50].
It should be noted that, in some cases, overoxidation can terminate the polymerization reaction and/or destroy the conjugated π–π system, which is responsible for the good electrical conductivity of conducting polymers.
Overoxidation is very advantageous for the design of MIPs because groups (-COOH (carboxyl), >C=O (carbonyl), -OH (hydroxyl)), which are capable of hydrogen bond formation and the establishment of other electrostatic interactions, are formed and arranged in proximity with entrapped molecules. After the extraction of imprinted molecules, these carboxyl, carbonyl and hydroxyl groups form a recognition-able structure, which provides advanced selectivity towards the imprinted molecule. Therefore, overoxidation performed after the electrochemical formation of the conducting polymer layer is a very advantageous procedure. In some particular cases, overoxidation can help to remove imprinted molecules [80]. Overoxidized polypyrrole formed on a glassy carbon electrode was applied to detect Adefovir [81], and overoxidized polypyrrole formed on a glassy carbon electrode functionalized by a carboxylic acid multi-walled carbon nanotube layer was applied for the detection of pemetrexed [82].
Polypyrrole can be deposited electrochemically from pyrrole aqueous solutions by different electrochemical approaches [83] that can be easily controlled by computerized software [26]. Ppy-based MIPs were implemented in sensors dedicated for the detection of low molecular mass analytes such as: dopamine [84,85], theophylline [30,86], caffeine [14,15,87], histamine [88], quercetin [89], gallic acid [90], bilirubin [91], sarcosine [92], tetracycline [83], microcystin-LR [93], sulfanilamide [94], ganciclovir [95], uric acid [96], serotonin [97], L-aspartic acid [98], cysteine enantiomers [99], kanamycin [100], dibutyl phthalate [101], epinephrine (adrenaline) [102,103], coccidiostat clopidol [104], tryptamine [105], testosterone [106], fenvalerate [107] and NO3− ions [108]. A summary of conducting polymer-based MIPs implemented in electrochemical sensors and dedicated to the detection of some low molecular mass analytes is listed in Table 1.

Table 1.
A summary of conducting polymer-based MIPs implemented in electrochemical sensors and dedicated to the detection of some low molecular mass analytes.
Phenylenediamine-derivatives [120,121,122] are also often used in the design of MIPs, which are applied for pharmaceutical and bioanalytical needs. Ortho-phenylenediamine was imprinted by anticancer drugs such as pemetrexed [123] and butyrylcholinesterase [124]. Electrochemically formed poly-meta-phenylenediamine MIP imprinted by erythromycin was used for the detection of erythromycin in real water-based aliquots [120]. Electrochemical dopamine sensors on poly-nicotinamide [125], sulphanilamide imprinted polyresorcinol [126], poly(1-naphthylamine), triphenylamine-based molecularly imprinted polythionine [127] or copolymer imprinted by azorubine [128] were previously reported. Recently, MIPs were used for the detection of some newly developed anticancer drugs [129].
Structures based on conducting polymers are environmentally stable and can be easily modified in many different ways [27]. Conducting polymers can be involved in the formation of composite structures modified by organic materials [130], inorganic structures [29,47,54,55] and biomolecules [44,54,60,131,132]. For this reason, they are often applied in the development of transducers for sensing and biosensing devices [133]. The doping of conducting polymers by particular ions and/or materials can significantly increase the electrical conductivity of conducting polymers [134].
4. MIPs Structures Imprinted by Biomolecules of High Molecular Weight
A variety of protein-based affinity sensors were designed via the immobilization of proteins within various polymer-based structures. These sensors are called immunosensors. The action of immunosensors is based on immobilized proteins that recognize analytes, whih are mostly other proteins or polypeptides, and form an immune complex, which induces the measurable signal of a particular signal transducer [135]. The orientation of immobilized protein molecules is also a key issue during the development of affinity sensors [25,136] because it determines the efficiency of analyte binding to the protein-modified surface [136]. Therefore, analytical characteristics of affinity sensors can be advanced by the proper orientation of immobilized receptors [25], whole antibodies [137], or fragments of chemically-split antibodies [136]. The electrochemical formation of conducting polymers is suitable for the entrapment of proteins in a formed polymer layer [26]. However, the successful application of molecular imprinting within polymers enables us to design MIPs that bind the imprinted analyte molecules [96,138,139].
The imprinting of polymers by proteins is a promising direction of MIP technology [140,141] (Figure 3) and can replace less stable proteins (such as antibodies [26] and receptors [25]), which are used in the design of affinity biosensors. It should be noted that the development of protein imprinted MIPs is a very promising direction of bioanalytical chemistry [142,143,144,145], some conformational variations of protein structure when protein is entrapped within a polymer [146], or proper protein orientation within polymers [147]. Molecularly imprinted polymers are sometimes called‘synthetic receptors’, ‘artificial receptors’ [143] or ‘plastic antibodies’ [148,149].
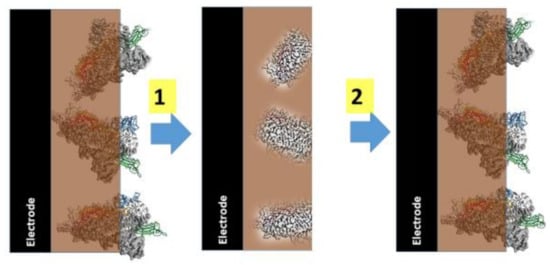
Figure 3.
(1) Formation of molecularly imprinted polymer-based sensor, (2) MIP based layer in action. Figure adapted from [1].
Bovine leukemia virus glycoprotein imprinted polypyrrole was applied in a MIP-based sensor design [143]. Electrochemically deposited poly o-phenylenediamine/hydroquinone imprinted by human serum albumin (HSA) was used for the detection of HSA in urine [150]. Polydopamine was surface-imprinted by immunoglobulin G [151]. Surface-imprinted PEDOT/PSS-based structures were also applied for the binding of proteins [147]. Synthetic receptors based on electrodeposited dopamine were applied for the detection of a prostate-specific antigen in samples of human blood plasma [152]. Electrochemically deposited polypyrrole/(carbon nanotube) composite was imprinted by S-ovalbumin and used for the determination of this albumin in egg white [153]. Electrochemically formed poly-o-phenylenediamine was imprinted by myoglobin [154]. Electrochemically formed MIP based on polyscopoletin was applied for the determination of HSA [155]. Poly-scopoletin was imprinted by cytochrome c [156]. A copolymer based on hydroxyethyl acrylate and ethylene glycol dimethacrylate imprinted by lysozyme was developed [157]. Poly(2-hydroxyethyl methacrylate-N-methacryloyl-(L)-histidin-Cu(II)) was formed using radical polymerization and imprinted by ceruloplasmin [158]. SARS-CoV-2 spike glycoprotein was imprinted into polypyrrole and deposited on a platinum electrode [159]. An electrochemical sensor based on poly-m-phenylenediamine imprinted by SARS-CoV-2 protein was applied for the diagnosis of COVID-19 [122]. Hexagonally packed macroporous molecularly imprinted polymers for chemosensing of follicle-stimulating hormone protein were developed [160].
Electrochemically deposited ortho-polydopamine was imprinted by alpha-fetoprotein, which before deposition of ortho-polydopamine was temporarily covalently immobilized on a gold nano-particle covered substrate [161]. Acrylamide/N,N0-methylenebisacrylamide copolymers were imprinted by two compounds: prostate-specific antigen and myoglobin [162]. Polyacrylamide imprinted by hemoglobin was designed [163]. O-phenylenediamine was used for the detection of imprinted troponin T that is an important biomarker for the diagnosis of early cardiac disease [164]. Conducting polymer polyaniline (PANI) is very often used in the design of sensors and biosensors [1], but despite the design of molecularly imprinted polymers, only very few research reports on PANI imprinted by antibiotic azithromycin [165] and by some saccharides and hydroxy acids exist [166]. It is remarkable that even titanium dioxide (TiO2) was imprinted by the enzyme urease, and it was applied in the sensor for the potentiometric determination of urea [167]. Very recently, it was demonstrated that the epitope imprinting approach applies exposed peptides as templates to synthesize electrochemically Molecularly Imprinted Polymers (MIPs) for the recognition of the parent protein [168]. Using this technology, epitope (N-terminal pentapeptide VHLTP-amide of human hemoglobin (HbA)) imprinted polymers were electrochemically deposited on an electrode surface.
MIP-formation is a highly multidisciplinary approach, which involves polymer and organic chemistry and nanotechnology [169,170]. It should be noted that DNA [27] can also be entrapped [24] or molecularly imprinted [171,172,173] within conducting polymers and applied for bioanalytical purposes. Therefore, a lot of researchers are targeted towards the replacement of biomolecules, such as receptors, antibodies, DNA-based sequences and DNA-aptamers, with molecularly imprinted polymer-based structures or by artificial receptors [174].
Even larger structures such as bacteria and other microorganisms [175,176] (e.g., bacteria Escherichia coli [177] or spores of bacillus cereus [178]) were imprinted within electrochemically deposited polypyrrole. Some more MIPs imprinted by viruses [179], spores [178], bacteria [177,180,181,182,183] and even some other living cells [184] were designed. Bacteria imprinted polymers can be used for the diagnosis of bacteria-induced infections and/or some bacterial toxin-based diseases [36,185].
5. Physicochemical Methods Used in Molecularly Imprinted Polymer Based Analytical Systems
MIP-based structures can selectively interact and bind various molecules. Moreover, in comparison to bio-receptors and enzymes, MIPs are better resistant to harmful environmental factors such as pH, temperature, etc. Some molecularly imprinted polymers are very stable at room conditions. Therefore, molecularly imprinted polymers seem very promising in the design of more stable and cheap sensing layers [186,187]. Due to the abovementioned reasons, the application of molecularly imprinted polymers in the design of affinity sensors is a rather new and promising direction of sensorics [188]. The highest stability is observed for MIPs based on acrylamide, methacrylic acid and acrylic acid [113,189,190,191,192].
The application of a suitable polymer for the design of a molecularly imprinted polymer plays a significant role in the design of efficient MIPs [193], which interact with analytes by the establishment of hydrogen bonds and π–π interactions, electrostatically, through van der Waals forces, and/or by hydrophobic-based interactions [194]. All these interactions are capable of dissociation; therefore, MIP-based sensors can be easily dissociated [2,191,195]. The formation and mechanism of action of molecularly imprinted polymers can be explained on the background of the phenomenological thermodynamic model, which explains the shape-memory effect of MIPs by solution theories derived by Flory and Huggins [196,197], Hansen [198] and Hildebrand [199]. Molecularly imprinted polymer shape changes are very often induced by swelling [200], which can significantly influence the ‘shape memory’ induced behaviour of these polymers [201]. In order to design molecularly imprinted polymers with desired analytical properties, molecular dynamics [202] and Density-Functional-Theory (DFT) [203,204] based calculations were recently performed.
Therefore, electrochemical methods are very frequently used for the registration of analytical signals by sensors based on MIPs (Figure 4). Direct and indirect electrochemical detection methods are applied for the determination of analyte binding with MIPs. During direct measurements, the quantification of analyte binding to molecularly imprinted polymers is determined by different action explanations: (i) The most simple effect is derived from the so-called ‘gate effect’ and is based on rearrangements of MIP-based layer structures by swelling or shrinking that can be induced by analyte binding with an imprinted cavity. These variations of polymer structure affect the ion diffusion rate within the MIP-based layer and can be sensitively determined by a variety of potentiodynamic electrochemical methods [205,206]; (ii) Another effect is based on the charges of molecularly imprinted polymers, which are interacting oppositely charged ions and/or restrict the diffusion of some ions. Moreover, when analytes bind to molecularly imprinted polymers their electronic structure can be rearranged, which induces variations in the electrical conductivity of the sensing layer [205]. On the basis of the aforementioned effects, organic electrochemical transistors based on MIPs can be designed [207]. Dependent on doping conducting polymers, namely polyaniline (PANI) [208], polypyrrole (Ppy) [26] and poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) (PEDOT/PSS) [209] are p- or n-type semiconductors. Doping and/or ‘un-doping’ of conducting polymers by some ions and materials is largely a reversible process responsible for the appearance of easily detectable variations of physical properties such as conductivity, light absorption or photoluminescence ability.
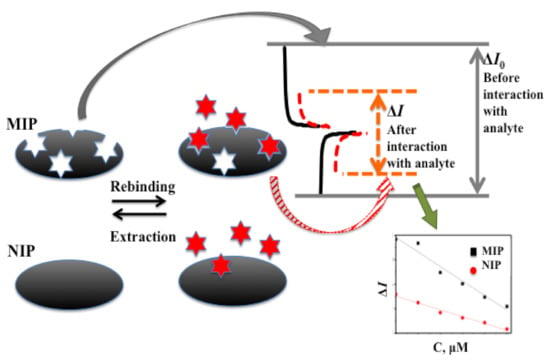
Figure 4.
The principle of operation of molecularly imprinted and non-imprinted polymer-based sensors.
However, direct electrochemical methods have a significant disadvantage because all nonspecific interactions influence analytical signals. Therefore, in some particular cases, various redox-active probes are applied in order to improve the selectivity and sensitivity of MIP-based sensors [210,211]. In some cases, enzymatic activity is utilized for the enhancement of the analytical response in the design of such assays where tyrosinase [212], glucose oxidase [213], acetylcholinesterase [214], creatine kinase [215], cytochrome P450 [216], hexameric heme protein [217], laccase [218], microperoxidase [219], horseradish peroxidase [219,220,221], lactoperoxidase [219] are exploited. Sometimes enzyme-like activities, Pt/Cu bimetallic nanoparticles [222] and the inhibition of enzymatic activity [223] can be exploited amplifiy the analytical signal of MIP-based sensors.
Quartz crystal microbalance (QCM) with MIP-modified resonators [224] were used for the detection of: (i) some rather low molecular weight compounds [224,225], such as naproxen [226], histamine [227], S-propranolol [228], uric acid (Figure 5) [96], and ibuprofen [229]; (ii) proteins [176,230,231,232], such as trypsin [233], ribonuclease A [234] and oxidized-low-density lipoprotein [235]; and (iii) DNA [232,236]. QCM-based determination of the changes of the mass of the formed MIP layer can be simultaneously performed with electrochemical methods and can be used as an electrochemical-QCM (EQCM) method [15,96,237]. A more advantageous QCM-based technique, QCM with dissipation (QCM-D), was also used for the registration of analytical signal generated by MIP-modified resonators [238].
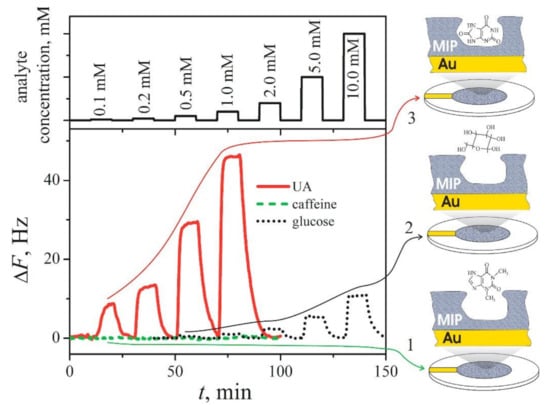
Figure 5.
Variations of resonant frequency of an EQCM-resonator modified by uric acid-imprinted MIP(UA)-Ppy to the addition of different concentrations of uric acid (solid line 3), caffeine (dashed line 2) and glucose (dotted line 1) [96]. Copyright 2021 by Elsevier. Reprinted with permission.
Various optical analytical signal registration methods, including photoluminescence [208,239] surface plasmon resonance (SPR) [240], can also be applied in MIP-based sensors. Remarkable optical characteristics of CPs can be well applied in the design of sensors based on optical transducers [241,242] and photoluminescence sensors [208,243,244]. We have demonstrated that conducting polymer polypyrrole has a great photoluminescence quenching ability [208,243], which can be exploited in the design of sensing devices and advance sensitivity and selectivity of biosensors [245]. Molecularly imprinted polymers can also be utilized for the detection of some organics, e.g., estradiol-derivatives [246,247,248]. MIP-based on quantum dot nanoparticles modified by poly(ethylene-co-vinyl alcohol) heterocomposite was used for optical detection of some salivary proteins [249]. MIP-based on a Cu2+-metalorganic-framework imprinted by tetrabromobisphenol A exhibited enzyme-like catalytic activity towards the oxidation of tetrabromobisphenol A by hydrogen peroxide [250]. Microarrays based on poly-scopoletin imprinted by ferritin were electro-spotted on a gold-modified substrate and applied in surface plasmon resonance (SPR)-based ferritin detection [251]. To advance optical capabilities, MIPs can be combined with photonic crystals [252] and liquid crystals [253]. A very useful optoelectrochemical property of some conducting polymers is their electrochromic effect, which can be exploited in the development of sensing devices.
6. Conclusions
MIPs are frequently used in the design of chemical sensors and biosensors, as well as for many other technological approaches. Some sensors based on MIPs provide fast analytical responses, operate at ambient conditions and are characterized by good sensitivity and selectivity. Some conducting polymers are well suited for the formation of MIPs, and these polymers can be designed using different polymerization methods. The electrochemical formation of CP-based structures can be controlled in many different ways and enables the ability to design of very different CP-based structures even from the same composition of polymerization-bulk solution; therefore, they are very well suited to the development of a great variety of MIPs. Some conducting polymers can be overoxidized after formation; this treatment is especially suited to the development of MIP-based sensors because it can be applied for (i) the formation of oxidized radicals, which increase sensitivity/selectivity towards imprinted target molecules, within MIP-based structures, (ii) the facilitation of template removal and/or regeneration of MIP-based layers.
Polypyrrole is the most used conducting polymer, and it is often applied in the formation of MIPs. Moreover, the advantages of the overoxidation of conducting polymer polypyrrole are the most frequently reported. However, in our opinion, this application of overoxidized polypyrrole still has a lot of room for improvement and extension in the application of polypyrrole based MIPs because polypyrrole can be easily synthesized by chemical and electrochemical methods from various solutions based on the most frequently used solvents. In addition, overoxidation of polypyrrole can be easily performed during the synthesis and/or after the formation of a Ppy-based layer. Moreover, polypyrrole shows great compatibility with various biological compounds and does not irritate the immune system of mammalians; therefore, it is suitable for the development of implantable biomedical tools, such as sensors, biosensors, biofuel cells, etc.
Author Contributions
S.R. performed literature research, analysis and drafted the paper. U.S.-B., V.R., I.M.-V., I.P. and R.V. performed writing—review & editing. A.R., initiated and supervised the work and provided insights. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted under the Lithuanian-Latvian-Taiwan project, and it has received funding according to agreement No S-LLT-21-3 with the Research Council of Lithuania (LMTLT). BioRender.com was used to create the schematic illustrations by Habil Arunas Ramanavicius.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Progress and Insights in the Application of MXenes as New 2D Nano-Materials Suitable for Biosensors and Biofuel Cell Design. Int. J. Mol. Sci. 2020, 21, 9224. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Insights in the Application of Stoichiometric and Non-Stoichiometric Titanium Oxides for the Design of Sensors for the Determination of Gases and VOCs (TiO2−x and TinO2n−1 vs. TiO2). Sensors 2020, 20, 6833. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Fotouhi, L.; Ehsani, A. Recent Progress in the Development of Conducting Polymer-Based Nanocomposites for Electrochemical Biosensors Applications: A Mini-Review. Chem. Rec. 2018, 18, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Pontes, K.; Indrusiak, T.; Soares, B.G. Poly(vinylidene fluoride-co-hexafluorpropylene)/polyaniline conductive blends: Effect of the mixing procedure on the electrical properties and electromagnetic interference shielding effectiveness. J. Appl. Polym. Sci. 2021, 138, 49705. [Google Scholar] [CrossRef]
- Samukaite-Bubniene, U.; Valiūnienė, A.; Bucinskas, V.; Genys, P.; Ratautaite, V.; Ramanaviciene, A.; Aksun, E.; Tereshchenko, A.; Zeybek, B.; Ramanavicius, A. Towards supercapacitors: Cyclic voltammetry and fast Fourier transform electrochemical impedance spectroscopy based evaluation of polypyrrole electrochemically deposited on the pencil graphite electrode. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125750. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, T.; Miao, Y.; Zhao, X. Chloride ion-doped polyaniline/carbon nanotube nanocomposite materials as new cathodes for chloride ion battery. Electrochim. Acta 2018, 270, 30–36. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Liu, Y.; Liu, W.; Zhao, P.; Li, Y.; Dong, Y.; Wang, H.; Yang, J. Urchin-like Ni1/3Co2/3(CO3)0.5OH·0.11H2O anchoring on polypyrrole nanotubes for supercapacitor electrodes. Electrochim. Acta 2019, 295, 989–996. [Google Scholar] [CrossRef]
- Ratautaite, V.; Ramanaviciene, A.; Oztekin, Y.; Voronovic, J.; Balevicius, Z.; Mikoliunaite, L.; Ramanavicius, A. Electrochemical stability and repulsion of polypyrrole film. Colloids Surf. A Physicochem. Eng. Asp. 2013, 418, 16–21. [Google Scholar] [CrossRef]
- Iroh, J.O.; Su, W. Corrosion performance of polypyrrole coating applied to low carbon steel by an electrochemical process. Electrochim. Acta 2000, 46, 15–24. [Google Scholar] [CrossRef]
- Oztekin, Y.; Ramanaviciene, A.; Yazicigil, Z.; Solak, A.O.; Ramanavicius, A. Direct electron transfer from glucose oxidase immobilized on polyphenanthroline-modified glassy carbon electrode. Biosens. Bioelectron. 2011, 26, 2541–2546. [Google Scholar] [CrossRef] [PubMed]
- Emir, G.; Dilgin, Y.; Ramanaviciene, A.; Ramanavicius, A. Amperometric nonenzymatic glucose biosensor based on graphite rod electrode modified by Ni-nanoparticle/polypyrrole composite. Microchem. J. 2021, 161, 105751. [Google Scholar] [CrossRef]
- Ratautaite, V.; Plausinaitis, D.; Baleviciute, I.; Mikoliunaite, L.; Ramanaviciene, A.; Ramanavicius, A. Characterization of caffeine-imprinted polypyrrole by a quartz crystal microbalance and electrochemical impedance spectroscopy. Sens. Actuators B Chem. 2015, 212, 63–71. [Google Scholar] [CrossRef]
- Holguín, M.; Rojas Álvarez, O.E.; Arizabaleta, C.A.; Torres, W. Molecular dynamics of the interaction of l-tryptophan with polypyrrole oligomers. Comput. Theor. Chem. 2019, 1147, 29–34. [Google Scholar] [CrossRef]
- Kumar, V.; Mirzaei, A.; Bonyani, M.; Kim, K.-H.; Kim, H.W.; Kim, S.S. Advances in electrospun nanofiber fabrication for polyaniline (PANI)-based chemoresistive sensors for gaseous ammonia. TrAC Trends Anal. Chem. 2020, 129, 115938. [Google Scholar] [CrossRef]
- Tekbaşoğlu, T.Y.; Soganci, T.; Ak, M.; Koca, A.; Şener, M.K. Enhancing biosensor properties of conducting polymers via copolymerization: Synthesis of EDOT-substituted bis(2-pyridylimino)isoindolato-palladium complex and electrochemical sensing of glucose by its copolymerized film. Biosens. Bioelectron. 2017, 87, 81–88. [Google Scholar] [CrossRef]
- Leonavicius, K.; Ramanaviciene, A.; Ramanavicius, A. Polymerization Model for Hydrogen Peroxide Initiated Synthesis of Polypyrrole Nanoparticles. Langmuir 2011, 27, 10970–10976. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, A.; Andriukonis, E.; Stirke, A.; Mikoliunaite, L.; Balevicius, Z.; Ramanaviciene, A. Synthesis of polypyrrole within the cell wall of yeast by redox-cycling of [Fe(CN)6]3−/[Fe(CN)6]4−. Enzyme Microb. Technol. 2016, 83, 40–47. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Carac, G.; Bahrim, G.; Ramanaviciene, A.; Ramanavicius, A. Modification of Aspergillus niger by conducting polymer, Polypyrrole, and the evaluation of electrochemical properties of modified cells. Bioelectrochemistry 2018, 121, 46–55. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Carac, G.; Ramanaviciene, A.; Bahrim, G.; Tanase, C.; Ramanavicius, A. Cell-assisted synthesis of conducting polymer—Polypyrrole—For the improvement of electric charge transfer through fungal cell wall. Colloids Surf. B Biointerfaces 2019, 175, 671–679. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Pulsed amperometric detection of DNA with an ssDNA/polypyrrole-modified electrode. Anal. Bioanal. Chem. 2004, 379, 287–293. [Google Scholar] [CrossRef]
- Plikusiene, I.; Balevicius, Z.; Ramanaviciene, A.; Talbot, J.; Mickiene, G.; Balevicius, S.; Stirke, A.; Tereshchenko, A.; Tamosaitis, L.; Zvirblis, G.; et al. Evaluation of affinity sensor response kinetics towards dimeric ligands linked with spacers of different rigidity: Immobilized recombinant granulocyte colony-stimulating factor based synthetic receptor binding with genetically engineered dimeric analyte d. Biosens. Bioelectron. 2020, 156, 112112. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Oztekin, Y.; Ramanaviciene, A. Electrochemical formation of polypyrrole-based layer for immunosensor design. Sens. Actuators B Chem. 2014, 197, 237–243. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A. Self-encapsulation of oxidases as a basic approach to tune the upper detection limit of amperometric biosensors. Analyst 2008, 133, 1083. [Google Scholar] [CrossRef] [PubMed]
- Lakard, B.; Magnin, D.; Deschaume, O.; Vanlancker, G.; Glinel, K.; Demoustier-Champagne, S.; Nysten, B.; Jonas, A.M.; Bertrand, P.; Yunus, S. Urea potentiometric enzymatic biosensor based on charged biopolymers and electrodeposited polyaniline. Biosens. Bioelectron. 2011, 26, 4139–4145. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Ramanavicius, A.; Voronovic, J.; Ramanaviciene, A. Glucose biosensor based on glucose oxidase and gold nanoparticles of different sizes covered by polypyrrole layer. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 224–230. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors (Review). Polymers 2021, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Baleviciute, I.; Ratautaite, V.; Ramanaviciene, A.; Balevicius, Z.; Broeders, J.; Croux, D.; McDonald, M.; Vahidpour, F.; Thoelen, R.; de Ceuninck, W.; et al. Evaluation of theophylline imprinted polypyrrole film. Synth. Met. 2015, 209, 206–211. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Sangiorgi, A.; Tarterini, F.; Sanson, A. Molecularly imprinted polypyrrole counter electrode for gel-state dye-sensitized solar cells. Electrochim. Acta 2019, 305, 322–328. [Google Scholar] [CrossRef]
- Syritski, V.; Reut, J.; Öpik, A.; Idla, K. Environmental QCM sensors coated with polypyrrole. Synth. Met. 1999, 102, 1326–1327. [Google Scholar] [CrossRef]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef] [PubMed]
- Keçili, R.; Yılmaz, E.; Ersöz, A.; Say, R. Imprinted materials: From green chemistry to sustainable engineering. In Sustainable Nanoscale Engineering; Szekely, G., Livingston, A.B.T.-S.N.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 317–350. ISBN 978-0-12-814681-1. [Google Scholar]
- Cui, F.; Zhou, Z.; Zhou, H.S. Molecularly Imprinted Polymers and Surface Imprinted Polymers Based Electrochemical Biosensor for Infectious Diseases. Sensors 2020, 20, 996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.-L.; Wang, Y.; Hjertén, S. A novel support with artificially created recognition for the selective removal of proteins and for affinity chromatography. Chromatographia 1996, 42, 259–262. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Batista, A.D.; Cárdenas, S. Molecularly Imprinted Polymer Micro- and Nano-Particles: A Review. Molecules 2020, 25, 4740. [Google Scholar] [CrossRef] [PubMed]
- Bakirhan, N.K.; Ozcelikay, G.; Ozkan, S.A. Recent progress on the sensitive detection of cardiovascular disease markers by electrochemical-based biosensors. J. Pharm. Biomed. Anal. 2018, 159, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Sankiewicz, A.; Romanowicz, L.; Pyc, M.; Hermanowicz, A.; Gorodkiewicz, E. SPR imaging biosensor for the quantitation of fibronectin concentration in blood samples. J. Pharm. Biomed. Anal. 2018, 150, 1–8. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Damaskaite, A.; Plikusiene, I.; Ramanavicius, A.; Ramanaviciene, A. Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of PANI–PEDOT Film Deposited on Transparent Electrode. Polymers 2020, 12, 2778. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of enzymatic formation of polyaniline nanoparticles. Polymer 2017, 115, 211–216. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Enzymatic Formation of Polyaniline, Polypyrrole, and Polythiophene Nanoparticles with Embedded Glucose Oxidase. Nanomaterials 2019, 9, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krikstolaityte, V.; Kuliesius, J.; Ramanaviciene, A.; Mikoliunaite, L.; Kausaite-Minkstimiene, A.; Oztekin, Y.; Ramanavicius, A. Enzymatic polymerization of polythiophene by immobilized glucose oxidase. Polymer 2014, 55, 1613–1620. [Google Scholar] [CrossRef]
- Genys, P.; Aksun, E.; Tereshchenko, A.; Valiūnienė, A.; Ramanaviciene, A.; Ramanavicius, A. Electrochemical Deposition and Investigation of Poly-9,10-Phenanthrenequinone Layer. Nanomaterials 2019, 9, 702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kausaite-Minkstimiene, A.; Glumbokaite, L.; Ramanaviciene, A.; Ramanavicius, A. Reagent-less amperometric glucose biosensor based on nanobiocomposite consisting of poly(1,10-phenanthroline-5,6-dione), poly(pyrrole-2-carboxylic acid), gold nanoparticles and glucose oxidase. Microchem. J. 2020, 154, 104665. [Google Scholar] [CrossRef]
- Gicevicius, M.; Bagdziunas, G.; Abduloglu, Y.; Ramanaviciene, A.; Gumusay, O.; Ak, M.; Soganci, T.; Ramanavicius, A. Experimental and Theoretical Investigations of an Electrochromic Azobenzene and 3,4-Ethylenedioxythiophene-based Electrochemically Formed Polymeric Semiconductor. ChemPhysChem 2018, 19, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Olgac, R.; Soganci, T.; Baygu, Y.; Gök, Y.; Ak, M. Zinc(II) phthalocyanine fused in peripheral positions octa-substituted with alkyl linked carbazole: Synthesis, electropolymerization and its electro-optic and biosensor applications. Biosens. Bioelectron. 2017, 98, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef]
- Vaitkuviene, A.; Kaseta, V.; Voronovic, J.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of cytotoxicity of polypyrrole nanoparticles synthesized by oxidative polymerization. J. Hazard. Mater. 2013, 250–251, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Vaitkuviene, A.; Ratautaite, V.; Mikoliunaite, L.; Kaseta, V.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Some biocompatibility aspects of conducting polymer polypyrrole evaluated with bone marrow-derived stem cells. Colloids Surf. A Physicochem. Eng. Asp. 2014, 442, 152–156. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Kausaite, A.; Tautkus, S.; Ramanavicius, A. Biocompatibility of polypyrrole particles: An in-vivo study in mice. J. Pharm. Pharmacol. 2010, 59, 311–315. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation of Polyaniline and Polypyrrole Nanocomposites with Embedded Glucose Oxidase and Gold Nanoparticles. Polymers 2019, 11, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazeiko, V.; Kausaite-Minkstimiene, A.; Ramanaviciene, A.; Balevicius, Z.; Ramanavicius, A. Gold nanoparticle and conducting polymer-polyaniline-based nanocomposites for glucose biosensor design. Sens. Actuators B Chem. 2013, 189, 187–193. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Immobilizing Enzymes: How to Create More Suitable Biocatalysts. Angew. Chem. Int. Ed. 2003, 42, 3336–3337. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [Green Version]
- Miletić, N.; Nastasović, A.; Loos, K. Immobilization of biocatalysts for enzymatic polymerizations: Possibilities, advantages, applications. Bioresour. Technol. 2012, 115, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andriukonis, E.; Ramanaviciene, A.; Ramanavicius, A. Synthesis of Polypyrrole Induced by [Fe(CN)6]3− and Redox Cycling of [Fe(CN)6]4−/[Fe(CN)6]3−. Polymers 2018, 10, 749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation and Electrochemical Evaluation of Polyaniline and Polypyrrole Nanocomposites Based on Glucose Oxidase and Gold Nanostructures. Polymers 2020, 12, 3026. [Google Scholar] [CrossRef]
- Ye, L.; Mosbach, K. Molecularly imprinted microspheres as antibody binding mimics. React. Funct. Polym. 2001, 48, 149–157. [Google Scholar] [CrossRef]
- Peeters, M.; Kobben, S.; Jiménez-Monroy, K.L.; Modesto, L.; Kraus, M.; Vandenryt, T.; Gaulke, A.; van Grinsven, B.; Ingebrandt, S.; Junkers, T.; et al. Thermal detection of histamine with a graphene oxide based molecularly imprinted polymer platform prepared by reversible addition–fragmentation chain transfer polymerization. Sens. Actuators B Chem. 2014, 203, 527–535. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Mosbach, K.; Haupt, K. A Simple Method for Spin-Coating Molecularly Imprinted Polymer Films of Controlled Thickness and Porosity. Adv. Mater. 2004, 16, 719–722. [Google Scholar] [CrossRef]
- Lete, C.; Lakard, B.; Hihn, J.-Y.; del Campo, F.J.; Lupu, S. Use of sinusoidal voltages with fixed frequency in the preparation of tyrosinase based electrochemical biosensors for dopamine electroanalysis. Sens. Actuators B Chem. 2017, 240, 801–809. [Google Scholar] [CrossRef]
- Long, Y.-Z.; Li, M.-M.; Gu, C.; Wan, M.; Duvail, J.-L.; Liu, Z.; Fan, Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Patois, T.; Lakard, B.; Martin, N.; Fievet, P. Effect of various parameters on the conductivity of free standing electrosynthesized polypyrrole films. Synth. Met. 2010, 160, 2180–2185. [Google Scholar] [CrossRef]
- Rahman, M.A.; Kumar, P.; Park, D.-S.; Shim, Y.-B. Electrochemical Sensors Based on Organic Conjugated Polymers. Sensors 2008, 8, 118–141. [Google Scholar] [CrossRef] [PubMed]
- Bredas, J.L.; Street, G.B. Polarons, bipolarons, and solitons in conducting polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Srilalitha, S.; Jayaveera, K.N.; Madhvendhra, S.S. The Effect of Dopant, Temperature and Band Gap on Conductivity of Conducting Polymers. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 2694–2696. [Google Scholar]
- Lakard, B.; Ploux, L.; Anselme, K.; Lallemand, F.; Lakard, S.; Nardin, M.; Hihn, J.Y. Effect of ultrasounds on the electrochemical synthesis of polypyrrole, application to the adhesion and growth of biological cells. Bioelectrochemistry 2009, 75, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-H.H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Hu, Q.; Zeng, Q.; Wang, M.; Wang, L. Variations in Surface Morphologies, Properties, and Electrochemical Responses to Nitro-Analyte by Controlled Electropolymerization of Thiophene Derivatives. ACS Appl. Mater. Interfaces 2018, 10, 11319–11327. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Xu, Y.; Guo, Z.; Jiang, D. Supercapacitive Energy Storage and Electric Power Supply Using an Aza-Fused π-Conjugated Microporous Framework. Angew. Chem. Int. Ed. 2011, 50, 8753–8757. [Google Scholar] [CrossRef]
- Jiang, J.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z.; et al. Conjugated Microporous Poly(aryleneethynylene) Networks. Angew. Chem. Int. Ed. 2007, 46, 8574–8578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, S.; Wang, Q.; Xiao, Q.; Yue, Y.; Ren, S. Fluorene-Based Conjugated Microporous Polymers: Preparation and Chemical Sensing Application. Macromol. Rapid Commun. 2017, 38, 1700445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, S.; Füser, M.; Bolte, M.; Terfort, A. Self-assembled monolayers of aromatic pyrrole derivatives: Electropolymerization and electrocopolymerization with pyrrole. Electrochim. Acta 2017, 246, 853–863. [Google Scholar] [CrossRef]
- Wu, Y.; Li, G.; Tian, Y.; Feng, J.; Xiao, J.; Liu, J.; Liu, X.; He, Q. Electropolymerization of molecularly imprinted polypyrrole film on multiwalled carbon nanotube surface for highly selective and stable determination of carcinogenic amaranth. J. Electroanal. Chem. 2021, 895, 115494. [Google Scholar] [CrossRef]
- Peng, Y.; Su, H. Recent Innovations of Molecularly Imprinted Electrochemical Sensors Based on Electropolymerization Technique. Curr. Anal. Chem. 2015, 11, 307–317. [Google Scholar] [CrossRef]
- Moreira Gonçalves, L. Electropolymerized molecularly imprinted polymers: Perceptions based on recent literature for soon-to-be world-class scientists. Curr. Opin. Electrochem. 2021, 25, 100640. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Hudson, A.; Foster, C.W.; Eersels, K.; van Grinsven, B.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent Advances in Electrosynthesized Molecularly Imprinted Polymer Sensing Platforms for Bioanalyte Detection. Sensors 2019, 19, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaabal, M.; Bakirhan, N.K.; Doulache, M.; Kaddour, S.; Saidat, B.; Ozkan, S.A. A New Approach on Sensitive Assay of Adefovir in Pharmaceutical and Biological Fluid Samples Using Polypyrrole Modified Glassy Carbon Electrode. Sens. Actuators B Chem. 2020, 323, 128657. [Google Scholar] [CrossRef]
- Karadas, N.; Ozkan, S.A. Electrochemical preparation of sodium dodecylsulfate doped over-oxidized polypyrrole/multi-walled carbon nanotube composite on glassy carbon electrode and its application on sensitive and selective determination of anticancer drug: Pemetrexed. Talanta 2014, 119, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Devkota, L.; Nguyen, L.T.; Vu, T.T.; Piro, B. Electrochemical determination of tetracycline using AuNP-coated molecularly imprinted overoxidized polypyrrole sensing interface. Electrochim. Acta 2018, 270, 535–542. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, F.; Kan, X. Voltammetric dopamine sensor based on three-dimensional electrosynthesized molecularly imprinted polymers and polypyrrole nanowires. Microchim. Acta 2017, 184, 2515–2522. [Google Scholar] [CrossRef]
- Li, Y.; Song, H.; Zhang, L.; Zuo, P.; Ye, B.; Yao, J.; Chen, W. Supportless electrochemical sensor based on molecularly imprinted polymer modified nanoporous microrod for determination of dopamine at trace level. Biosens. Bioelectron. 2016, 78, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Ratautaite, V.; Janssens, S.D.; Haenen, K.; Nesládek, M.; Ramanaviciene, A.; Baleviciute, I.; Ramanavicius, A. Molecularly Imprinted Polypyrrole Based Impedimentric Sensor for Theophylline Determination. Electrochim. Acta 2014, 130, 361–367. [Google Scholar] [CrossRef]
- Betlem, K.; Mahmood, I.; Seixas, R.D.; Sadiki, I.; Raimbault, R.L.D.; Foster, C.W.; Crapnell, R.D.; Tedesco, S.; Banks, C.E.; Gruber, J.; et al. Evaluating the temperature dependence of heat-transfer based detection: A case study with caffeine and Molecularly Imprinted Polymers as synthetic receptors. Chem. Eng. J. 2019, 359, 505–517. [Google Scholar] [CrossRef] [Green Version]
- Ratautaite, V.; Nesladek, M.; Ramanaviciene, A.; Baleviciute, I.; Ramanavicius, A. Evaluation of Histamine Imprinted Polypyrrole Deposited on Boron Doped Nanocrystalline Diamond. Electroanalysis 2014, 26, 2458–2464. [Google Scholar] [CrossRef]
- Zhang, W.; Zong, L.; Geng, G.; Li, Y.; Zhang, Y. Enhancing determination of quercetin in honey samples through electrochemical sensors based on highly porous polypyrrole coupled with nanohybrid modified GCE. Sens. Actuators B Chem. 2018, 257, 1099–1109. [Google Scholar] [CrossRef]
- Ye, C.; Chen, X.; Xu, J.; Xi, H.; Wu, T.; Deng, D.; Zhang, J.; Huang, G. Highly sensitive detection to gallic acid by polypyrrole-based MIES supported by MOFs-Co2+@Fe3O4. J. Electroanal. Chem. 2020, 859, 113839. [Google Scholar] [CrossRef]
- Zhang, C.; Bai, W.; Yang, Z. A novel photoelectrochemical sensor for bilirubin based on porous transparent TiO2 and molecularly imprinted polypyrrole. Electrochim. Acta 2016, 187, 451–456. [Google Scholar] [CrossRef]
- Nguy, T.P.; van Phi, T.; Tram, D.T.N.; Eersels, K.; Wagner, P.; Lien, T.T.N. Development of an impedimetric sensor for the label-free detection of the amino acid sarcosine with molecularly imprinted polymer receptors. Sens. Actuators B Chem. 2017, 246, 461–470. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Z.; Chen, Z. Ultrasensitive electrochemical microcystin-LR immunosensor using gold nanoparticle functional polypyrrole microsphere catalyzed silver deposition for signal amplification. Sens. Actuators B Chem. 2017, 246, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Tadi, K.K.; Motghare, R.V.; Ganesh, V. Electrochemical Detection of Sulfanilamide Using Pencil Graphite Electrode Based on Molecular Imprinting Technology. Electroanalysis 2014, 26, 2328–2336. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Karimian, N. Fabrication of a highly selective and sensitive voltammetric ganciclovir sensor based on electropolymerized molecularly imprinted polymer and gold nanoparticles on multiwall carbon nanotubes/glassy carbon electrode. Sens. Actuators B Chem. 2015, 215, 471–479. [Google Scholar] [CrossRef]
- Plausinaitis, D.; Sinkevicius, L.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Evaluation of electrochemical quartz crystal microbalance based sensor modified by uric acid-imprinted polypyrrole. Talanta 2020, 220, 121414. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. A novel detection approach for serotonin by graphene quantum dots/two-dimensional (2D) hexagonal boron nitride nanosheets with molecularly imprinted polymer. Appl. Surf. Sci. 2018, 458, 648–655. [Google Scholar] [CrossRef]
- Syritski, V.; Reut, J.; Menaker, A.; Gyurcsányi, R.E.; Öpik, A. Electrosynthesized molecularly imprinted polypyrrole films for enantioselective recognition of l-aspartic acid. Electrochim. Acta 2008, 53, 2729–2736. [Google Scholar] [CrossRef]
- Gu, J.; Dai, H.; Kong, Y.; Tao, Y.; Chu, H.; Tong, Z. Chiral electrochemical recognition of cysteine enantiomers with molecularly imprinted overoxidized polypyrrole-Au nanoparticles. Synth. Met. 2016, 222, 137–143. [Google Scholar] [CrossRef]
- Işık, D.; Şahin, S.; Caglayan, M.O.; Üstündağ, Z. Electrochemical impedimetric detection of kanamycin using molecular imprinting for food safety. Microchem. J. 2021, 160, 105713. [Google Scholar] [CrossRef]
- Bolat, G.; Yaman, Y.T.; Abaci, S. Molecularly imprinted electrochemical impedance sensor for sensitive dibutyl phthalate (DBP) determination. Sens. Actuators B Chem. 2019, 299, 127000. [Google Scholar] [CrossRef]
- Zaidi, S.A. Utilization of an environmentally-friendly monomer for an efficient and sustainable adrenaline imprinted electrochemical sensor using graphene. Electrochim. Acta 2018, 274, 370–377. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, G.; Zhu, A.; Zhao, Z.; Ren, C.; Nie, L.; Kan, X. A multiporous electrochemical sensor for epinephrine recognition and detection based on molecularly imprinted polypyrrole. RSC Adv. 2012, 2, 7803. [Google Scholar] [CrossRef]
- Radi, A.-E.; El-Naggar, A.-E.; Nassef, H.M. Determination of coccidiostat clopidol on an electropolymerized-molecularly imprinted polypyrrole polymer modified screen printed carbon electrode. Anal. Methods 2014, 6, 7967–7972. [Google Scholar] [CrossRef]
- Xing, X.; Liu, S.; Yu, J.; Lian, W.; Huang, J. Electrochemical sensor based on molecularly imprinted film at polypyrrole-sulfonated graphene/hyaluronic acid-multiwalled carbon nanotubes modified electrode for determination of tryptamine. Biosens. Bioelectron. 2012, 31, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, Y.; Sun, G.; Wang, S.; Deng, J.; Wei, H. Molecularly imprinted polymers on graphene oxide surface for EIS sensing of testosterone. Biosens. Bioelectron. 2017, 92, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Nezhadali, A.; Feizy, J.; Beheshti, H.R. A Molecularly Imprinted Polymer for the Selective Extraction and Determination of Fenvalerate from Food Samples Using High-Performance Liquid Chromatography. Food Anal. Methods 2015, 8, 1225–1237. [Google Scholar] [CrossRef]
- Agrisuelas, J.; Gabrielli, C.; García-Jareño, J.J.; Perrot, H.; Sanchis-Gual, R.; Sel, O.; Vicente, F. Evaluation of the electrochemical anion recognition of NO3−-imprinted poly(Azure A) in NO3−/Cl− mixed solutions by ac-electrogravimetry. Electrochim. Acta 2016, 194, 292–303. [Google Scholar] [CrossRef]
- Zang, Y.; Nie, J.; He, B.; Yin, W.; Zheng, J.; Hou, C.; Huo, D.; Yang, M.; Liu, F.; Sun, Q.; et al. Fabrication of S-MoSe2/NSG/Au/MIPs imprinted composites for electrochemical detection of dopamine based on synergistic effect. Microchem. J. 2020, 156, 104845. [Google Scholar] [CrossRef]
- Kang, Q.; Zhang, Q.; Zang, L.; Zhao, M.; Chen, X.; Shen, D. Enhancement anti-interference ability of photoelectrochemical sensor via differential molecularly imprinting technique demonstrated by dopamine determination. Anal. Chim. Acta 2020, 1125, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Zhou, H.; Li, C.; Zhu, A.; Xing, Z.; Zhao, Z. Imprinted electrochemical sensor for dopamine recognition and determination based on a carbon nanotube/polypyrrole film. Electrochim. Acta 2012, 63, 69–75. [Google Scholar] [CrossRef]
- Peeters, M.; Troost, F.J.; van Grinsven, B.; Horemans, F.; Alenus, J.; Murib, M.S.; Keszthelyi, D.; Ethirajan, A.; Thoelen, R.; Cleij, T.J.; et al. MIP-based biomimetic sensor for the electronic detection of serotonin in human blood plasma. Sens. Actuators B Chem. 2012, 171–172, 602–610. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Wu, G.; Zhu, L.; Lu, X. Development and Application of the Serotonin Voltametric Sensors Based on Molecularly Imprinting Technology. J. Electrochem. Soc. 2015, 162, B201–B206. [Google Scholar] [CrossRef]
- Akhoundian, M.; Rüter, A.; Shinde, S. Ultratrace Detection of Histamine Using a Molecularly-Imprinted Polymer-Based Voltammetric Sensor. Sensors 2017, 17, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, S.; Yeung, C.-C.; Li, T.; Lau, S.C.; Sun, Q.-J.; Li, L.-Y.; Li, J.H.; Lam, M.H.W.; Roy, V.A.L. Portable molecularly imprinted polymer-based platform for detection of histamine in aqueous solutions. J. Hazard. Mater. 2021, 410, 124609. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhong, T.; Long, Q.; Huang, C.; Chen, L.; Lu, D.; Li, X.; Zhang, Z.; Shen, G.; Hou, X. A gold nanoparticles-based molecularly imprinted electrochemical sensor for histamine specific-recognition and determination. Microchem. J. 2021, 171, 106844. [Google Scholar] [CrossRef]
- Kim, J.M.; Yang, J.C.; Park, J.Y. Quartz crystal microbalance (QCM) gravimetric sensing of theophylline via molecularly imprinted microporous polypyrrole copolymers. Sens. Actuators B Chem. 2015, 206, 50–55. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Lu, Z.; Liu, T.; Zhao, L.; Ding, F.; Zou, P.; Wang, X.; Zhao, Q.; Rao, H. Molecularly imprinted polydopamine modified with nickel nanoparticles wrapped with carbon: Fabrication, characterization and electrochemical detection of uric acid. Microchim. Acta 2019, 186, 414. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Deng, P.; Wu, Y.; Ding, Z.; Li, G.; Liu, J.; He, Q. A Simple and Efficient Molecularly Imprinted Electrochemical Sensor for the Selective Determination of Tryptophan. Biomolecules 2019, 9, 294. [Google Scholar] [CrossRef] [Green Version]
- Ayankojo, A.G.; Reut, J.; Ciocan, V.; Öpik, A.; Syritski, V. Molecularly imprinted polymer-based sensor for electrochemical detection of erythromycin. Talanta 2020, 209, 120502. [Google Scholar] [CrossRef]
- Bozal-Palabiyik, B.; Erkmen, C.; Uslu, B. Molecularly Imprinted Electrochemical Sensors: Analytical and Pharmaceutical Applications Based on Ortho-Phenylenediamine Polymerization. Curr. Pharm. Anal. 2020, 16, 350–366. [Google Scholar] [CrossRef]
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029. [Google Scholar] [CrossRef] [PubMed]
- Ozcelikay, G.; Karadas-Bakirhan, N.; Taskin-Tok, T.; Ozkan, S.A. A selective and molecular imaging approach for anticancer drug: Pemetrexed by nanoparticle accelerated molecularly imprinting polymer. Electrochim. Acta 2020, 354, 136665. [Google Scholar] [CrossRef]
- Ozcelikay, G.; Kurbanoglu, S.; Zhang, X.; Kosak Soz, C.; Wollenberger, U.; Ozkan, S.A.; Yarman, A.; Scheller, F.W. Electrochemical MIP Sensor for Butyrylcholinesterase. Polymers 2019, 11, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Li, Y.; Liu, T.; Wang, G.; Sun, M.; Jiang, Y.; He, H.; Wang, Y.; Zou, P.; Wang, X.; et al. A dual-template imprinted polymer electrochemical sensor based on AuNPs and nitrogen-doped graphene oxide quantum dots coated on NiS2/biomass carbon for simultaneous determination of dopamine and chlorpromazine. Chem. Eng. J. 2020, 389, 124417. [Google Scholar] [CrossRef]
- Kumar Prusty, A.; Bhand, S. Molecularly Imprinted Polyresorcinol Based Capacitive Sensor for Sulphanilamide Detection. Electroanalysis 2019, 31, 1797–1808. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Y.; Li, Y. Molecularly imprinted polymer-decorated signal on-off ratiometric electrochemical sensor for selective and robust dopamine detection. Biosens. Bioelectron. 2019, 135, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Piri, S.; Piri, F.; Yaftian, M.R.; Zamani, A. Imprinted Azorubine electrochemical sensor based upon composition of MnO2 and 1-naphthylamine on graphite nanopowder. J. Iran. Chem. Soc. 2018, 15, 2713–2720. [Google Scholar] [CrossRef]
- Yarman, A.; Kurbanoglu, S.; Jetzschmann, K.J.; Ozkan, S.A.; Wollenberger, U.; Scheller, F.W. Electrochemical MIP-Sensors for Drugs. Curr. Med. Chem. 2018, 25, 4007–4019. [Google Scholar] [CrossRef] [PubMed]
- Gokoglan, T.C.; Soylemez, S.; Kesik, M.; Unay, H.; Sayin, S.; Yildiz, H.B.; Cirpan, A.; Toppare, L. A novel architecture based on a conducting polymer and calixarene derivative: Its synthesis and biosensor construction. RSC Adv. 2015, 5, 35940–35947. [Google Scholar] [CrossRef]
- Ahuja, T.; Mir, I.; Kumar, D. Biomolecular immobilization on conducting polymers for biosensing applications. Biomaterials 2007, 28, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Neo, W.T.; Ye, Q.; Chua, S.-J.; Xu, J. Conjugated polymer-based electrochromics: Materials, device fabrication and application prospects. J. Mater. Chem. C 2016, 4, 7364–7376. [Google Scholar] [CrossRef]
- Soylemez, S.; Hacioglu, S.O.; Kesik, M.; Unay, H.; Cirpan, A.; Toppare, L. A Novel and Effective Surface Design: Conducting Polymer/β-Cyclodextrin Host–Guest System for Cholesterol Biosensor. ACS Appl. Mater. Interfaces 2014, 6, 18290–18300. [Google Scholar] [CrossRef]
- Wang, B.; Tang, J.; Wang, F. Electrochemical polymerization of aniline. Synth. Met. 1987, 18, 323–328. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makaraviciute, A.; Ramanaviciene, A. Site-directed antibody immobilization techniques for immunosensors. Biosens. Bioelectron. 2013, 50, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Baleviciute, I.; Balevicius, Z.; Makaraviciute, A.; Ramanaviciene, A.; Ramanavicius, A. Study of antibody/antigen binding kinetics by total internal reflection ellipsometry. Biosens. Bioelectron. 2013, 39, 170–176. [Google Scholar] [CrossRef]
- Rattanarat, P.; Suea-Ngam, A.; Ruecha, N.; Siangproh, W.; Henry, C.S.; Srisa-Art, M.; Chailapakul, O. Graphene-polyaniline modified electrochemical droplet-based microfluidic sensor for high-throughput determination of 4-aminophenol. Anal. Chim. Acta 2016, 925, 51–60. [Google Scholar] [CrossRef]
- Gurudatt, N.G.; Chung, S.; Kim, J.-M.; Kim, M.-H.; Jung, D.-K.; Han, J.-Y.; Shim, Y.-B. Separation detection of different circulating tumor cells in the blood using an electrochemical microfluidic channel modified with a lipid-bonded conducting polymer. Biosens. Bioelectron. 2019, 146, 111746. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Tsai, W.-B.; Garrison, M.D.; Ferrari, S.; Ratner, B.D. Template-imprinted nanostructured surfaces for protein recognition. Nature 1999, 398, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Jeans, C.W.; Brain, K.R.; Allender, C.J.; Hlady, V.; Britt, D.W. From 3D to 2D: A review of the molecular imprinting of proteins. Biotechnol. Prog. 2006, 22, 1474–1489. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, S.; Whitcombe, M.J.; Piletsky, S.A. Size matters: Challenges in imprinting macromolecules. Prog. Polym. Sci. 2014, 39, 145–163. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Molecularly imprinted polypyrrole-based synthetic receptor for direct detection of bovine leukemia virus glycoproteins. Biosens. Bioelectron. 2004, 20, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Hishiya, T. Molecular imprinting of proteins emerging as a tool for protein recognition. Org. Biomol. Chem. 2008, 6, 2459. [Google Scholar] [CrossRef]
- Erdőssy, J.; Horváth, V.; Yarman, A.; Scheller, F.W.; Gyurcsányi, R.E. Electrosynthesized molecularly imprinted polymers for protein recognition. Trends Anal. Chem. 2016, 79, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Kryscio, D.R.; Fleming, M.Q.; Peppas, N.A. Protein Conformational Studies for Macromolecularly Imprinted Polymers. Macromol. Biosci. 2012, 12, 1137–1144. [Google Scholar] [CrossRef]
- Menaker, A.; Syritski, V.; Reut, J.; Öpik, A.; Horváth, V.; Gyurcsányi, R.E. Electrosynthesized Surface-Imprinted Conducting Polymer Microrods for Selective Protein Recognition. Adv. Mater. 2009, 21, 2271–2275. [Google Scholar] [CrossRef]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.V.; Turner, A.P.F.; Piletsky, S.A. Solid-Phase Synthesis of Molecularly Imprinted Polymer Nanoparticles with a Reusable Template-“Plastic Antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosini, S.; Beyazit, S.; Haupt, K.; Tse Sum Bui, B. Solid-phase synthesis of molecularly imprinted nanoparticles for protein recognition. Chem. Commun. 2013, 49, 6746. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Y.; Guo, M.; Lin, B.; Zhang, L. A sensitive determination of albumin in urine by molecularly imprinted electrochemical biosensor based on dual-signal strategy. Sens. Actuators B Chem. 2019, 288, 564–570. [Google Scholar] [CrossRef]
- Tretjakov, A.; Syritski, V.; Reut, J.; Boroznjak, R.; Volobujeva, O.; Öpik, A. Surface molecularly imprinted polydopamine films for recognition of immunoglobulin G. Microchim. Acta 2013, 180, 1433–1442. [Google Scholar] [CrossRef]
- Tamboli, V.K.; Bhalla, N.; Jolly, P.; Bowen, C.R.; Taylor, J.T.; Bowen, J.L.; Allender, C.J.; Estrela, P. Hybrid Synthetic Receptors on MOSFET Devices for Detection of Prostate Specific Antigen in Human Plasma. Anal. Chem. 2016, 88, 11486–11490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Q. A Molecularly Imprinted Electrochemical Sensor Based on Polypyrrole/Carbon Nanotubes Composite for the Detection of S-ovalbumin in Egg White. Int. J. Electrochem. Sci. 2017, 12, 3965–3981. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Sigolaeva, L.V.; Kuzikov, A.V.; Archakov, A.I. Electrosynthesis and binding properties of molecularly imprinted poly-o-phenylenediamine for selective recognition and direct electrochemical detection of myoglobin. Biosens. Bioelectron. 2016, 86, 330–336. [Google Scholar] [CrossRef]
- Stojanovic, Z.; Erdőssy, J.; Keltai, K.; Scheller, F.W.; Gyurcsányi, R.E. Electrosynthesized molecularly imprinted polyscopoletin nanofilms for human serum albumin detection. Anal. Chim. Acta 2017, 977, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarman, A.; Dechtrirat, D.; Bosserdt, M.; Jetzschmann, K.J.; Gajovic-Eichelmann, N.; Scheller, F.W. Cytochrome c-Derived Hybrid Systems Based on Moleculary Imprinted Polymers. Electroanalysis 2015, 27, 573–586. [Google Scholar] [CrossRef]
- Qian, L.-W.; Hu, X.-L.; Guan, P.; Gao, B.; Wang, D.; Wang, C.-L.; Li, J.; Du, C.-B.; Song, W.-Q. Thermal preparation of lysozyme-imprinted microspheres by using ionic liquid as a stabilizer. Anal. Bioanal. Chem. 2014, 406, 7221–7231. [Google Scholar] [CrossRef] [PubMed]
- Dolak, I.; Canpolat, G.; Ersöz, A.; Say, R. Metal chelate based site recognition of ceruloplasmin using molecularly imprinted polymer/cryogel system. Sep. Sci. Technol. 2020, 55, 199–208. [Google Scholar] [CrossRef]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Ramanaviciene, A.; Ciplys, E.; Juozapaitis, M.; Slibinskas, R.; Bechelany, M.; Ramanavicius, A. Molecularly imprinted polypyrrole based sensor for the detection of SARS-CoV-2 spike glycoprotein. Electrochim. Acta 2022, 403, 139581. [Google Scholar] [CrossRef] [PubMed]
- Kalecki, J.; Cieplak, M.; Dąbrowski, M.; Lisowski, W.; Kuhn, A.; Sharma, P.S. Hexagonally Packed Macroporous Molecularly Imprinted Polymers for Chemosensing of Follicle-Stimulating Hormone Protein. ACS Sens. 2020, 5, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Zhang, C.; Deng, Y.; Yang, G.; Li, S.; Tang, C.; He, N. A novel α -fetoprotein-MIP immunosensor based on AuNPs/PTh modified glass carbon electrode. Chin. Chem. Lett. 2018, 30, 160–162. [Google Scholar] [CrossRef]
- Karami, P.; Bagheri, H.; Johari-Ahar, M.; Khoshsafar, H.; Arduini, F.; Afkhami, A. Dual-modality impedimetric immunosensor for early detection of prostate-specific antigen and myoglobin markers based on antibody-molecularly imprinted polymer. Talanta 2019, 202, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tan, W.; Xu, H. Protein molecularly imprinted polyacrylamide membrane: For hemoglobin sensing. Analyst 2010, 135, 2523. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Turner, A.P.F.; Tiwari, A. Electrochemical evaluation of troponin T imprinted polymer receptor. Biosens. Bioelectron. 2014, 59, 160–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, S.; Dehghani, M.; Nasirizadeh, N.; Azimzadeh, M. An azithromycin electrochemical sensor based on an aniline MIP film electropolymerized on a gold nano urchins/graphene oxide modified glassy carbon electrode. J. Electroanal. Chem. 2018, 829, 27–34. [Google Scholar] [CrossRef]
- Nikitina, V.N.; Zaryanov, N.V.; Kochetkov, I.R.; Karyakina, E.E.; Yatsimirsky, A.K.; Karyakin, A.A. Molecular imprinting of boronate functionalized polyaniline for enzyme-free selective detection of saccharides and hydroxy acids. Sens. Actuators B Chem. 2017, 246, 428–433. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Z.; Si, S. Potentiometric urea biosensor based on immobilization of urease onto molecularly imprinted TiO2 film. J. Electroanal. Chem. 2009, 635, 1–6. [Google Scholar] [CrossRef]
- Zhang, X.; Caserta, G.; Yarman, A.; Supala, E.; Waffo, A.F.T.; Wollenberger, U.; Gyurcsányi, R.E.; Zebger, I.; Scheller, F.W. “Out of Pocket” Protein Binding—A Dilemma of Epitope Imprinted Polymers Revealed for Human Hemoglobin. Chemosensors 2021, 9, 128. [Google Scholar] [CrossRef]
- Uzun, L.; Turner, A.P.F. Molecularly-imprinted polymer sensors: Realising their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for Molecular Imprinting and the Evolution of MIP Nanoparticles as Plastic Antibodies—Synthesis and Applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slinchenko, O.; Rachkov, A.; Miyachi, H.; Ogiso, M.; Minoura, N. Imprinted polymer layer for recognizing double-stranded DNA. Biosens. Bioelectron. 2004, 20, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Babamiri, B.; Salimi, A.; Hallaj, R. A molecularly imprinted electrochemiluminescence sensor for ultrasensitive HIV-1 gene detection using EuS nanocrystals as luminophore. Biosens. Bioelectron. 2018, 117, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Muti, M.; Soysal, M.; Nacak, F.M.; Gençdağ, K.; Karagözler, A.E. A Novel DNA Probe Based on Molecularly Imprinted Polymer Modified Electrode for the Electrochemical Monitoring of DNA. Electroanalysis 2015, 27, 1368–1377. [Google Scholar] [CrossRef]
- Zamora-Gálvez, A.; Morales-Narváez, E.; Mayorga-Martinez, C.C.; Merkoçi, A. Nanomaterials connected to antibodies and molecularly imprinted polymers as bio/receptors for bio/sensor applications. Appl. Mater. Today 2017, 9, 387–401. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, Z.; Li, J.; Ma, X.; Chen, L.; Yang, X. Molecular imprinting technology for microorganism analysis. TrAC Trends Anal. Chem. 2018, 106, 190–201. [Google Scholar] [CrossRef]
- Iskierko, Z.; Sharma, P.S.; Bartold, K.; Pietrzyk-Le, A.; Noworyta, K.; Kutner, W. Molecularly imprinted polymers for separating and sensing of macromolecular compounds and microorganisms. Biotechnol. Adv. 2016, 34, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, X.; Zhang, L.; Gao, J.; Ma, Q. Electrochemiluminescence Detection of Escherichia coli O157:H7 Based on a Novel Polydopamine Surface Imprinted Polymer Biosensor. ACS Appl. Mater. Interfaces 2017, 9, 5430–5436. [Google Scholar] [CrossRef]
- Ait Lahcen, A.; Arduini, F.; Lista, F.; Amine, A. Label-free electrochemical sensor based on spore-imprinted polymer for Bacillus cereus spore detection. Sens. Actuators B Chem. 2018, 276, 114–120. [Google Scholar] [CrossRef]
- Hayden, O.; Lieberzeit, P.A.; Blaas, D.; Dickert, F.L. Artificial Antibodies for Bioanalyte Detection—Sensing Viruses and Proteins. Adv. Funct. Mater. 2006, 16, 1269–1278. [Google Scholar] [CrossRef]
- Takátsy, A.; Végvári, Á.; Hjertén, S.; Kilár, F. Universal method for synthesis of artificial gel antibodies by the imprinting approach combined with a unique electrophoresis technique for detection of minute structural differences of proteins, viruses and cells (bacteria). Ib. Gel antibodies against pr. Electrophoresis 2007, 28, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Hayden, O.; Dickert, F.L. Selective Microorganism Detection with Cell Surface Imprinted Polymers. Adv. Mater. 2001, 13, 1480–1483. [Google Scholar] [CrossRef]
- Zaidi, S.A. Bacterial Imprinting Methods and Their Applications: An Overview. Crit. Rev. Anal. Chem. 2020, 51, 609–618. [Google Scholar] [CrossRef]
- Golabi, M.; Kuralay, F.; Jager, E.W.H.; Beni, V.; Turner, A.P.F. Electrochemical bacterial detection using poly(3-aminophenylboronic acid)-based imprinted polymer. Biosens. Bioelectron. 2017, 93, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef]
- Gupta, G.; Singh, P.K.; Boopathi, M.; Kamboj, D.V.; Singh, B.; Vijayaraghavan, R. Molecularly imprinted polymer for the recognition of biological warfare agent staphylococcal enterotoxin B based on Surface Plasmon Resonance. Thin Solid Films 2010, 519, 1115–1121. [Google Scholar] [CrossRef]
- Bartold, K.; Pietrzyk-Le, A.; D’Souza, F.; Kutner, W. Oligonucleotide Analogs and Mimics for Sensing Macromolecular Biocompounds. Trends Biotechnol. 2019, 37, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays—An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef]
- Alexander, C.; Andersson, H.S.; Andersson, L.I.; Ansell, R.J.; Kirsch, N.; Nicholls, I.A.; O’Mahony, J.; Whitcombe, M.J. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. 2006, 19, 106–180. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; Bochenkov, V.E.; Runager, K.; Aslan, H.; Dong, M.; Enghild, J.J.; de Freitas, V.; Ferreira Sales, M.G.; Sutherland, D.S. Molecular Imprinting of Complex Matrices at Localized Surface Plasmon Resonance Biosensors for Screening of Global Interactions of Polyphenols and Proteins. ACS Sens. 2016, 1, 258–264. [Google Scholar] [CrossRef]
- Lin, Z.-T.; DeMarr, V.; Bao, J.; Wu, T. Molecularly Imprinted Polymer-Based Biosensors: For the Early, Rapid Detection of Pathogens, Biomarkers, and Toxins in Clinical, Environmental, or Food Samples. IEEE Nanotechnol. Mag. 2018, 12, 6–13. [Google Scholar] [CrossRef]
- Rachkov, A.E.; Cheong, S.-H.; El’skaya, A.V.; Yano, K.; Karube, I. Molecularly imprinted polymers as artificial steroid receptors. Polym. Adv. Technol. 1998, 9, 511–519. [Google Scholar] [CrossRef]
- Des Azevedo, S.; Lakshmi, D.; Chianella, I.; Whitcombe, M.J.; Karim, K.; Ivanova-Mitseva, P.K.; Subrahmanyam, S.; Piletsky, S.A. Molecularly Imprinted Polymer-Hybrid Electrochemical Sensor for the Detection of β-Estradiol. Ind. Eng. Chem. Res. 2013, 52, 13917–13923. [Google Scholar] [CrossRef]
- Spychalska, K.; Zając, D.; Baluta, S.; Halicka, K.; Cabaj, J. Functional Polymers Structures for (Bio)Sensing Application—A Review. Polymers 2020, 12, 1154. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Tharpa, K.; Dima, Ş.-O. Molecularly Imprinted Membranes: Past, Present, and Future. Chem. Rev. 2016, 116, 11500–11528. [Google Scholar] [CrossRef]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly imprinted polymer nanoparticles in chemical sensing—Synthesis, characterisation and application. Sens. Actuators B Chem. 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Flory, P.J. Thermodynamics of High Polymer Solutions. J. Chem. Phys. 1942, 10, 51–61. [Google Scholar] [CrossRef]
- Lu, H.; Du, S. A phenomenological thermodynamic model for the chemo-responsive shape memory effect in polymers based on Flory-Huggins solution theory. Polym. Chem. 2014, 5, 1155–1162. [Google Scholar] [CrossRef]
- Antipchik, M.; Dzhuzha, A.; Sirotov, V.; Tennikova, T.; Korzhikova-Vlakh, E. Molecularly imprinted macroporous polymer monolithic layers for L-phenylalanine recognition in complex biological fluids. J. Appl. Polym. Sci. 2021, 138, 50070. [Google Scholar] [CrossRef]
- Hildebrand, J.H. The term ‘regular solution’. Nature 1951, 168, 868. [Google Scholar] [CrossRef]
- Menge, H.; Hotopf, S.; Pönitzsch, S.; Richter, S.; Arndt, K.F.; Schneider, H.; Heuert, U. Investigation on the swelling behaviour in poly(dimethylsiloxane) rubber networks using nmr and compression measurements. Polymer 1999, 40, 5303–5313. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Leng, J.; Du, S. Qualitative separation of the physical swelling effect on the recovery behavior of shape memory polymer. Eur. Polym. J. 2010, 46, 1908–1914. [Google Scholar] [CrossRef]
- Zanuy, D.; Fabregat, G.; Ferreira, C.A.; Alemán, C. A molecular dynamics study on glucose molecular recognition by a non-enzymatic selective sensor based on a conducting polymer. Phys. Chem. Chem. Phys. 2019, 21, 8099–8107. [Google Scholar] [CrossRef] [PubMed]
- Boroznjak, R.; Reut, J.; Tretjakov, A.; Lomaka, A.; Öpik, A.; Syritski, V. A computational approach to study functional monomer-protein molecular interactions to optimize protein molecular imprinting. J. Mol. Recognit. 2017, 30, e2635. [Google Scholar] [CrossRef] [PubMed]
- Lach, P.; Sharma, P.S.; Golebiewska, K.; Cieplak, M.; D’Souza, F.; Kutner, W. Molecularly Imprinted Polymer Chemosensor for Selective Determination of an N -Nitroso- l -proline Food Toxin. Chem. A Eur. J. 2017, 23, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Garcia-Cruz, A.; Cieplak, M.; Noworyta, K.R.; Kutner, W. ‘Gate effect’ in molecularly imprinted polymers: The current state of understanding. Curr. Opin. Electrochem. 2019, 16, 50–56. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.-T.; Liu, Y.; Qi, X.-M.; Jin, H.-G.; Yang, C.; Liu, J.; Li, G.-L.; He, Q.-G. Recent advances in black phosphorus-based electrochemical sensors: A review. Anal. Chim. Acta 2021, 1170, 338480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, G.; Wu, D.; Xiong, C.; Zheng, L.; Ding, Y.; Lu, H.; Zhang, G.; Qiu, L. Highly selective and sensitive sensor based on an organic electrochemical transistor for the detection of ascorbic acid. Biosens. Bioelectron. 2018, 100, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Turemis, M.; Zappi, D.; Giardi, M.T.; Basile, G.; Ramanaviciene, A.; Kapralovs, A.; Ramanavicius, A.; Viter, R. ZnO/polyaniline composite based photoluminescence sensor for the determination of acetic acid vapor. Talanta 2020, 211, 120658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.P.; Rahman, M.A.; Srivastava, S.; Kang, J.-S.; McGillivray, D.; Abd-Ellah, M.; Heinig, N.F.; Leung, K.T. Highly Conducting Hybrid Silver-Nanowire-Embedded Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) for High-Efficiency Planar Silicon/Organic Heterojunction Solar Cells. ACS Nano 2018, 12, 9495–9503. [Google Scholar] [CrossRef] [PubMed]
- Benachio, I.; Lobato, A.; Gonçalves, L.M. Employing molecularly imprinted polymers in the development of electroanalytical methodologies for antibiotic determination. J. Mol. Recognit. 2021, 34, e2878. [Google Scholar] [CrossRef] [PubMed]
- Yarman, A.; Scheller, F.W. How Reliable Is the Electrochemical Readout of MIP Sensors? Sensors 2020, 20, 2677. [Google Scholar] [CrossRef] [PubMed]
- Yarman, A. Development of a molecularly imprinted polymer-based electrochemical sensor for tyrosinase. Turk. J. Chem. 2018, 42, 346–354. [Google Scholar] [CrossRef]
- Burow, M.; Minoura, N. Molecular Imprinting: Synthesis of Polymer Particles with Antibody-like Binding Characteristics for Glucose Oxidase. Biochem. Biophys. Res. Commun. 1996, 227, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Jetzschmann, K.J.; Jágerszki, G.; Dechtrirat, D.; Yarman, A.; Gajovic-Eichelmann, N.; Gilsing, H.-D.; Schulz, B.; Gyurcsányi, R.E.; Scheller, F.W. Vectorially Imprinted Hybrid Nanofilm for Acetylcholinesterase Recognition. Adv. Funct. Mater. 2015, 25, 5178–5183. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-Y.; Chen, Y.-C.; Sheu, D.-C.; Chou, T.C. Molecularly imprinted polymers for the recognition of sodium dodecyl sulfate denatured creatine kinase. J. Taiwan Inst. Chem. Eng. 2012, 43, 188–194. [Google Scholar] [CrossRef]
- Jetzschmann, K.J.; Yarman, A.; Rustam, L.; Kielb, P.; Urlacher, V.B.; Fischer, A.; Weidinger, I.M.; Wollenberger, U.; Scheller, F.W. Molecular LEGO by domain-imprinting of cytochrome P450 BM3. Colloids Surf. B Biointerfaces 2018, 164, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yarman, A.; Jetzschmann, K.J.; Jeoung, J.-H.; Schad, D.; Dobbek, H.; Wollenberger, U.; Scheller, F.W. Molecularly Imprinted Electropolymer for a Hexameric Heme Protein with Direct Electron Transfer and Peroxide Electrocatalysis. Sensors 2016, 16, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarman, A. Electrosynthesized Molecularly Imprinted Polymer for Laccase Using the Inactivated Enzyme as the Target. Bull. Korean Chem. Soc. 2018, 39, 483–488. [Google Scholar] [CrossRef]
- Bossi, A.; Piletsky, S.A.; Piletska, E.V.; Righetti, P.G.; Turner, A.P.F. Surface-Grafted Molecularly Imprinted Polymers for Protein Recognition. Anal. Chem. 2001, 73, 5281–5286. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Bai, C.; Teng, Y.; Zhang, J.; Peng, J.; Fang, Z.; Xu, W. Study of horseradish peroxidase and hydrogen peroxide bi-analyte sensor with boronate affinity-based molecularly imprinted film. Can. J. Chem. 2019, 97, 833–839. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, R.; Guo, H.; Wei, Y.; Yang, W. A facile horseradish peroxidase electrochemical biosensor with surface molecular imprinting based on polyaniline nanotubes. J. Electroanal. Chem. 2018, 817, 184–194. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, H.; Zhang, C.; Qu, L.; Yu, L. A novel molecularly imprinted sensor based on PtCu bimetallic nanoparticle deposited on PSS functionalized graphene with peroxidase-like activity for selective determination of puerarin. Talanta 2020, 210, 120621. [Google Scholar] [CrossRef] [PubMed]
- Cutivet, A.; Schembri, C.; Kovensky, J.; Haupt, K. Molecularly Imprinted Microgels as Enzyme Inhibitors. J. Am. Chem. Soc. 2009, 131, 14699–14702. [Google Scholar] [CrossRef] [PubMed]
- Emir Diltemiz, S.; Keçili, R.; Ersöz, A.; Say, R. Molecular Imprinting Technology in Quartz Crystal Microbalance (QCM) Sensors. Sensors 2017, 17, 454. [Google Scholar] [CrossRef] [PubMed]
- Merkoçi, A.; Alegret, S. New materials for electrochemical sensing IV. Molecular imprinted polymers. TrAC Trends Anal. Chem. 2002, 21, 717–725. [Google Scholar] [CrossRef]
- Eslami, M.R.; Alizadeh, N. Nanostructured conducting molecularly imprinted polypyrrole based quartz crystal microbalance sensor for naproxen determination and its electrochemical impedance study. RSC Adv. 2016, 6, 9387–9395. [Google Scholar] [CrossRef]
- Pietrzyk, A.; Suriyanarayanan, S.; Kutner, W.; Chitta, R.; D’Souza, F. Selective Histamine Piezoelectric Chemosensor Using a Recognition Film of the Molecularly Imprinted Polymer of Bis(bithiophene) Derivatives. Anal. Chem. 2009, 81, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Noworyta, K.; Kutner, W. Imprinted polymer-based enantioselective acoustic sensor using a quartz crystal microbalance. Anal. Commun. 1999, 36, 391. [Google Scholar] [CrossRef]
- Eslami, M.R.; Alizadeh, N. A dual usage smart sorbent/recognition element based on nanostructured conducting molecularly imprinted polypyrrole for simultaneous potential-induced nanoextraction/determination of ibuprofen in biomedical samples by quartz crystal microbalance sensor. Sens. Actuators B Chem. 2015, 220, 880–887. [Google Scholar] [CrossRef]
- Dabrowski, M.; Lach, P.; Cieplak, M.; Kutner, W. Nanostructured molecularly imprinted polymers for protein chemosensing. Biosens. Bioelectron. 2018, 102, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Boysen, R.I.; Schwarz, L.J.; Nicolau, D.V.; Hearn, M.T.W. Molecularly imprinted polymer membranes and thin films for the separation and sensing of biomacromolecules. J. Sep. Sci. 2017, 40, 314–335. [Google Scholar] [CrossRef]
- Saylan, Y.; Yilmaz, F.; Özgür, E.; Derazshamshir, A.; Yavuz, H.; Denizli, A. Molecular Imprinting of Macromolecules for Sensor Applications. Sensors 2017, 17, 898. [Google Scholar] [CrossRef] [PubMed]
- Karaseva, N.A.; Pluhar, B.; Beliaeva, E.A.; Ermolaeva, T.N.; Mizaikoff, B. Synthesis and application of molecularly imprinted polymers for trypsin piezoelectric sensors. Sens. Actuators B Chem. 2019, 280, 272–279. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, D.; Guo, T. Construction of a novel macroporous imprinted biosensor based on quartz crystal microbalance for ribonuclease Adetection. Biosens. Bioelectron. 2013, 42, 80–86. [Google Scholar] [CrossRef]
- Chunta, S.; Suedee, R.; Boonsriwong, W.; Lieberzeit, P.A. Biomimetic sensors targeting oxidized-low-density lipoprotein with molecularly imprinted polymers. Anal. Chim. Acta 2020, 1116, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bartold, K.; Pietrzyk-Le, A.; Huynh, T.-P.; Iskierko, Z.; Sosnowska, M.; Noworyta, K.; Lisowski, W.; Sannicolò, F.; Cauteruccio, S.; Licandro, E.; et al. Programmed Transfer of Sequence Information into a Molecularly Imprinted Polymer for Hexakis(2,2′-bithien-5-yl) DNA Analogue Formation toward Single-Nucleotide-Polymorphism Detection. ACS Appl. Mater. Interfaces 2017, 9, 3948–3958. [Google Scholar] [CrossRef] [Green Version]
- Mosch, H.L.K.S.; Akintola, O.; Plass, W.; Höppener, S.; Schubert, U.S.; Ignaszak, A. Specific Surface versus Electrochemically Active Area of the Carbon/Polypyrrole Capacitor: Correlation of Ion Dynamics Studied by an Electrochemical Quartz Crystal Microbalance with BET Surface. Langmuir 2016, 32, 4440–4449. [Google Scholar] [CrossRef]
- Hu, Y.; Xing, H.; Li, G.; Wu, M. Magnetic Imprinted Polymer-Based Quartz Crystal Microbalance Sensor for Sensitive Label-Free Detection of Methylene Blue in Groundwater. Sensors 2020, 20, 5506. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Nishitani, S.; Kajisa, T. Molecularly imprinted polymer-based bioelectrical interfaces with intrinsic molecular charges. RSC Adv. 2020, 10, 16999–17013. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, Q.; Wang, Y.; Liu, X.; Wei, T. Surface plasmon resonance sensor for theophylline using a water-compatible molecularly imprinted film. Anal. Methods 2016, 8, 2349–2356. [Google Scholar] [CrossRef]
- Bhunia, S.; Dey, N.; Pradhan, A.; Bhattacharya, S. A conjugated microporous polymer based visual sensing platform for aminoglycoside antibiotics in water. Chem. Commun. 2018, 54, 7495–7498. [Google Scholar] [CrossRef]
- Tan, J.; Chen, W.-J.; Guo, J. Conjugated microporous polymers with distinctive π -electronic properties exhibiting enhanced optical applications. Chin. Chem. Lett. 2016, 27, 1405–1411. [Google Scholar] [CrossRef]
- Zhang, B.; Li, B.; Wang, Z. Creation of Carbazole-Based Fluorescent Porous Polymers for Recognition and Detection of Various Pesticides in Water. ACS Sens. 2020, 5, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Mo, W.; Tang, H.; Cheng, Z.; Chen, Y.; Zhang, S.; Ma, H.; Li, B.; Li, X. Dendrimer-Based, High-Luminescence Conjugated Microporous Polymer Films for Highly Sensitive and Selective Volatile Organic Compound Sensor Arrays. Adv. Funct. Mater. 2020, 30, 1910275. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Kurilcik, N.; Jursenas, S.; Finkelsteinas, A.; Ramanaviciene, A. Conducting polymer based fluorescence quenching as a new approach to increase the selectivity of immunosensors. Biosens. Bioelectron. 2007, 23, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Lahcen, A.A.; Baleg, A.A.; Baker, P.; Iwuoha, E.; Amine, A. Synthesis and electrochemical characterization of nanostructured magnetic molecularly imprinted polymers for 17-β-Estradiol determination. Sens. Actuators B Chem. 2017, 241, 698–705. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, Y.; Bai, J.; Ning, B.; Sun, S.; Hong, X.; Liu, Y.; Liu, Y.; Gao, Z. A novel electrochemical sensor based on electropolymerized molecularly imprinted polymer and gold nanomaterials amplification for estradiol detection. Sens. Actuators B Chem. 2014, 200, 69–75. [Google Scholar] [CrossRef]
- Duan, D.; Si, X.; Ding, Y.; Li, L.; Ma, G.; Zhang, L.; Jian, B. A novel molecularly imprinted electrochemical sensor based on double sensitization by MOF/CNTs and Prussian blue for detection of 17β-estradiol. Bioelectrochemistry 2019, 129, 211–217. [Google Scholar] [CrossRef]
- Lee, M.-H.; Chen, Y.-C.; Ho, M.-H.; Lin, H.-Y. Optical recognition of salivary proteins by use of molecularly imprinted poly(ethylene-co-vinyl alcohol)/quantum dot composite nanoparticles. Anal. Bioanal. Chem. 2010, 397, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Cui, H.; Chao, J.; Huang, K.; Wang, X.; Zhou, Y.; Jing, T. Colorimetric determination of tetrabromobisphenol A based on enzyme-mimicking activity and molecular recognition of metal-organic framework-based molecularly imprinted polymers. Microchim. Acta 2020, 187, 142. [Google Scholar] [CrossRef] [PubMed]
- Bosserdt, M.; Erdőssy, J.; Lautner, G.; Witt, J.; Köhler, K.; Gajovic-Eichelmann, N.; Yarman, A.; Wittstock, G.; Scheller, F.W.; Gyurcsányi, R.E. Microelectrospotting as a new method for electrosynthesis of surface-imprinted polymer microarrays for protein recognition. Biosens. Bioelectron. 2015, 73, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Meng, Z.; Xue, M.; Shea, K.J. Molecular imprinted photonic crystal for sensing of biomolecules. Mol. Impr. 2016, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cieplak, M.; Węgłowski, R.; Iskierko, Z.; Węgłowska, D.; Sharma, P.S.; Noworyta, K.R.; D’Souza, F.; Kutner, W. Protein Determination with Molecularly Imprinted Polymer Recognition Combined with Birefringence Liquid Crystal Detection. Sensors 2020, 20, 4692. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).